Abstract
Prediabetes is the preclinical stage of type 2 diabetes mellitus (T2DM) with intermediate state of hyperglycemia. Hyperglycemia results in a state of oxidative stress, which may contribute to the production of insulin resistance, β-cell dysfunction and long-term complications of diabetes. Novel approaches are required for prevention and treatment of diabetes. New biomarkers that can be used in risk stratification and therapy control as supplementary to current parameters are needed. These biomarkers may facilitate a more individualized and sufficient treatment of diabetes. Therefore, the aim of this study was to investigate the levels of oxidatively induced DNA damage products, 8-oxo-2'-deoxyguanosine (8-oxo-dG) (also known as 8-OH-dG), (5'R)- and (5'S)-8,5'-cyclo-2'-deoxyadenosines (R-cdA and S-cdA), and the lipid peroxidation product 8-iso-prostaglandin F2α (8-iso-PGF2α) as reliable oxidative stress markers in patients with prediabetes or T2DM in comparison with healthy volunteers. Urine samples were collected from these subjects. Absolute quantification of 8-oxo-dG, R-cdA, S-cdA and 8-iso-PGF2α was achieved by liquid chromatography-isotope dilution tandem mass spectrometry. The levels of 8-oxo-dG, S-cdA and 8-iso-PGF2a were significantly greater in prediabetes patients than those in healthy volunteers. T2DM patients also had higher levels of 8-oxo-dG than healthy volunteers. No statistically significant difference was observed for R-cdA levels. 8-oxo-dG levels positively correlated with R-cdA and S-cdA levels for prediabetes and newly diagnosed T2DM. S-cdA levels and HbA1c were found negatively correlated in prediabetes patients. Also 8-iso-PGF2α levels and HbA1c were found negatively correlated in prediabetes patients. These results indicate that oxidatively induced macromolecular damage appears before the establishment of T2DM. Thus, our data suggest that oxidatively induced DNA damage and lipid peroxidation products that were found to be elevated in prediabetic stage may be used as early disease markers in patients at risk for T2DM.
Keywords: Oxidatively induced DNA damage; Lipid peroxidation; Prediabetes; 8-oxo-2’-deoxyguanosine; 8,5'-cyclo-2'-deoxyadenosines; 8-iso-prostaglandin F2α
1. Introduction
The leading risk factor for type 2 diabetes mellitus (T2DM) is a condition called prediabetes, which represents an elevation of plasma glucose levels above the normal range but below clinical diabetes [1,2]. Hyperglycemia results in a state of oxidative stress and that reactive oxygen species contribute to the production of insulin resistance, β-cell dysfunction and long-term complications of T2DM. Oxidative stress is defined as an imbalance between the production and elimination of reactive oxygen species, in favor of the former, and subsequently excessive levels of these species can lead to damage in cellular macromolecules, such as proteins, carbohydrates, lipids and DNA [3,4]. Most of these complications appear before the establishment of diabetes which is the prediabetic stage [5]. Until now, the mechanisms which contribute to these changes have not been fully elucidated [6]. Novel approaches are required to prevent and treat T2DM and to reduce the impact of its complications. New biomarkers that can be used in risk stratification and therapy control as alternatives to current parameters are needed. These biomarkers may facilitate an early diagnosis and individualized treatment of T2DM.
As the most reactive free radical, hydroxyl radical (•OH) interacts with genomic DNA via addition to double bonds of DNA bases and abstraction of H atoms from the sugar moiety, leading to the formation of a plethora of products [7]. The reaction of •OH with guanine leads to the formation of 8-oxo-2'-deoxyguanosine (8-oxo-dG) (also known as 8-OH-dG) [8,9], which is widely used as a biomarker for oxidatively induced DNA damage [10-12]. Among the DNA damage products, (5'R)- and (5'S)-8,5'-cyclopurine-2'-deoxynucleosides (R-cdA and S-cdA) are unique tandem lesions, which represent damage to both sugar and base moieties of the same nucleoside [13]. They are formed via H-abstraction by •OH from C5' of the sugar moiety followed by cyclization between C5' and C8 of a purine-2'-deoxynucleoside and then by oxidation [14]. These and other products are removed from DNA by cellular repair mechanisms. If not repaired, oxidatively induced DNA damage may result in a broad range of pathophysiological processes. The elucidation of the mechanisms, cellular repair, and biological consequences of this type of DNA damage is essential for an understanding of its role in disease processes [13,15]. Since DNA damage is involved in pathogenesis of various major diseases, biomarkers of oxidatively induced DNA damage may be used as potential tools for diagnosis and monitoring of disease. Measurement of DNA damage products is essential for understanding the mechanisms of DNA damage and repair, and their effect in disease processes. Recently, a methodology using liquid chromatography-isotope dilution tandem mass spectrometry (LC-MS/MS) has been developed for the simultaneous measurement of 8-oxo-dG, R-cdA and S-cdA in human urine with high sensitivity and accuracy [16-18].
Isoprostanes are indicators of lipid peroxidation resulting from oxidative lipid damage, produced by non-enzymatic peroxidation of arachidonic acid and excreted into the urine [19-21]. A common mechanism of lipid peroxidation is oxidative damage to cellular membranes. 8-Iso-prostaglandin F2α (8-iso-PGF2α) has been recognized as a gold standard biomarker for the quantification of oxidative stress and oxidative damage [22-24]. Several analytical methods such as enzyme-linked immunosorbent assay (ELISA) and gas chromatography–mass spectrometry (GC-MS), have been used for quantification of 8-iso-PGF2α in various biological samples. However, highly specific and sensitive LC-MS/MS methods are favored to distinguish 8-iso-PGF2α from other isomers [21].
It is well known that oxidative stress is elevated in clinical T2DM; however, there is only limited data relating to the degree of oxidative stress in prediabetic stage. Increased DNA damage and lipid peroxidation has been recently shown using 8-oxo-dG and 8-iso-PGF2α in prediabetes but these studies were limited in terms of reliable methods [25]. Most of the studies have been performed by using semi-quantitative immunoassay methods, whereas we used LC-MS/MS, which is the most reliable technique for accurate quantification of these products. To date, R-cdA and S-cdA have not been investigated in prediabetes or T2DM. Furthermore, there is no study that evaluated simultaneously 8-oxo-dG, R-cdA, S-cdA and 8-iso-PGF2α levels in prediabetes or T2DM. In this work, we investigated the levels of these compounds as reliable oxidative stress markers in patients with prediabetes and T2DM in comparison with healthy volunteers.
2. Materials and methods
2.1. Subjects
The study was approved by the Clinical Research Ethics Committee of Dokuz Eylul University School of Medicine (Ethics committee approval date: 22 March 2012, protocol number: 41-SBKAEK), and was performed with the permission of the Ethics and Medical Research Committee of Dokuz Eylul University School of Medicine. Subjects were selected at the Endocrinology and Metabolism Outpatient Clinic of Dokuz Eylul University and informed consent was obtained in all cases. From April 2012 to April 2014, this study included 47 patients with prediabetes (fasting blood glucose (FBG) levels 100 mg/dL to 125 mg/dL, hemoglobin A1c (HbA1c) levels 5.7 % to 6.4 %) and 43 patients with T2DM (FBG > 125 mg/dL, HbA1c levels > 6.5 %) and 37 healthy volunteers (FBG < 100 mg/dL, HbA1c levels < 5.7%). FBG and HbA1c levels for the groups were defined according to the definition criteria of the American Diabetes Association [26]. Nonsmoking patients and healthy volunteers between the ages of 18 years and 70 years were randomly selected. They used no medication, no special diet, no supplements and no alcohol and had no symptoms of any acute or chronic disease. The exclusion criteria were the positive history of stroke, coronary artery disease, neoplastic disease, rheumatic diseases, pregnancy, inflammatory disease and cancer. Demographic and anthropometric data, and the medical history of the subjects were recorded. Blood, morning urine and 24 h-urine samples were collected and stored at −80 °C until use. Routine biochemical and clinical analyses were performed in the central laboratory of Dokuz Eylul University, School of Medicine directly after sample collection. All LC-MS/MS measurements of 8-oxo-dG, R-cdA, S-cdA and 8-iso-PGF2α were performed at the Medical Biochemistry Department of Dokuz Eylul University. No urine samples or no urine extracts have been sent to National Institute of Standards and Technology (NIST) for analysis or any other purpose.
2.2. Materials
Nylon syringe filters (0.22 μm) were purchased from NALGENE Labware Thermo Fisher Scientific (Rochester, New York, USA). Oasis HLB Extraction Cartridges from Waters Corp. (Milford, Massachusetts, USA) were used for solid phase extraction of the urine samples. Nanosep Omega tubes with molecular mass cutoff of 3 kDa were purchased from Sigma, Aldrich (St. Louis, Missouri, USA). Alkaline phosphatase was purchased from Roche Applied Science (Indianapolis, Indiana, USA). Methanol, acetonitrile, formic acid, hydrochloric acid, hexane and ammonium hydroxide were purchased from Merck (Darmstadt, Germany).
2.3. Stable isotope-labeled internal standards
The stable isotope-labeled internal standards 8-oxo-dG-15N5, R-cdA-15N5 and S-cdA-15N5 were received from NIST (Gaithersburg, Maryland, USA). 8-iso-PGF2α-d4 was purchased from Cambridge Isotope Laboratories (Andover, Massachusetts, USA).
2.4. Measurement of 8-oxo-dG, R-cdA and S-cdA by LC-MS/MS
2.4.1. Preparation of urine samples
First morning urine samples were used for the measurement of DNA damage products. An aliquot of 1 mL of urine was mixed with aliquots of stable isotope-labeled internal standards 8-oxo-dG-15N5 (20 pmol), R-cdA-15N5 (0.5 pmol) and S-cdA-15N5 (0.6 pmol) and centrifuged at 1000 g for 10 min. Then supernatant fractions were separated and filtered using nylon syringe filters (0.22 μm). The solid phase extraction procedure was performed by using Oasis HLB 3 mL Extraction Cartridges, which were activated with 1 mL methanol, dried and then washed with 2 mL of water. Filtered supernatant fractions were loaded onto extraction cartridges, which were then washed with 2 mL of water. Retained material was eluted with 1 mL of 30 % methanol. Extracted samples were dried in a SpeedVac™ (Thermo Scientific Marietta, Ohio, USA) and then dissolved in 100 μL Tris buffer (10 mmol/L, pH 7.5). Prior to LC-MS/MS analyses, all samples were treated with 22 units of alkaline phosphatase at 37 °C for 1 h to remove phosphate groups possibly attached to modified nucleosides, and then filtered using Nanosep Omega tubes with molecular mass cutoff of 3 kDa. Aliquots of 20 μL of the filtrates were used for LC-MS/MS analyses [16,17].
2.4.2. LC-MS/MS analyses
LC-MS/MS analyses were performed using an HPLC system (Shimadzu, Kyoto, Japan) coupled to a triple quadrupole ion-trap mass spectrometer (4000QTRAP Applied Biosystems, CA, USA) equipped with a TurboIonSpray™ source in the positive ionization mode. Samples were separated on a Zorbax SB-Aq LC column (2.1 mm x 150 mm, 3.5 μm particle size, Agilent Technologies Santa Clara, California, USA) with an attached C8 guard column (2.1 mm x 12.5 mm, 5 μm particle size). Mobile phases A and B were water containing 0.1% formic acid and acetonitrile containing 0.1 % formic acid, respectively. The flow rate was 0.3 mL/min. A gradient analysis of 3 % of B/min starting from 98 % A/2 % B (vol/vol) was used. After 10 min, B was increased to 70 % in 0.1 min, kept at this level for 1 min and then lowered to 2 % and kept for 15 min to equilibrate the column. The total analysis time was 26 min. The mass spectrometer settings were optimized as follows: turbo ion spray temperature 500 °C, ion spray voltage 5500 V, declustering potential (DP) 30 V, entrance potential (EP) 10 V, collision energy (CE) 25 V, collision cell exit potential (CXP) 10 V, curtain gas (CUR) 10 units, collision gas (CAD) high and dwell time was 100 ms for all the compounds. The multiple reaction monitoring (MRM) transitions for 8-oxo-dG and 8-oxo-dG-15N5 were m/z 284 → m/z 168 and m/z 289 →m/z 173, respectively. The transitions m/z 250 → m/z 164 and m/z 255 → m/z 169 were used for both R- and S-diastereomers of cdA and cdA-15N5, respectively. The Analyst Software Version 1.5 (Applied Biosystems) was used for data analyses. The quantification was performed using integrated peak area ratios of analytes and internal standards. The results were normalized with urinary creatinine concentrations and expressed as nmol/mmol creatinine. The assay reproducibility was assessed by the precision and accuracy of intra-day and inter-day measurements (n = 10). Urine samples were measured 10 times per day for intra-day precision and sequential ten days for inter-day precision. The intra- and inter-day coefficient of variations for 8-oxo-dG and S-cdA were 5.2 %, 4.9 %, and 2.3 %, 9.2 %, respectively.
2.5. Measurement of 8-iso-PGF2α by LC-MS/MS
2.5.1. Preparation of urine samples
Twenty-four-hour urine samples were used for the measurement of 8-iso-PGF2α. An aliquot of 2 mL of urine samples was mixed with an aliquot of 10 ng of the stable isotope-labeled internal standard 8-iso-PGF2α-d4 and 40 μL 1 mol/L HCl, then centrifuged at 1500 g for 10 min. The solid phase extraction procedure was performed by using Oasis HLB 1 mL extraction cartridges which were activated with 3 mL methanol, dried and then washed with 3 mL of water. Supernatant fractions were loaded onto extraction cartridges which were afterwards washed with 5 mL 0.1 mol/L HCl, 1.5 mL 5% methanol and 0.3 mL hexane. Retained material was eluted by 480 μL methanol containing 0.5% NH4OH. Extracted samples were mixed with 300 μL water and transferred to vials for LC-MS/MS analysis [21].
2.5.2. LC-MS/MS analyses
LC-MS/MS conditions were the same as indicated above. Samples were separated on a Zorbax Eclipse LC column (2.1 mm x 50 mm, 3.5 μm particle size, Agilent Technologies Santa Clara, California, USA) with an attached C8 guard column (2.1 mm x 12.5 mm, 5 μm particle size). Mobile phases A and B were water containing 0.15% ammonium hydroxide and acetonitrile containing 0.15 % ammonium hydroxide, respectively. The flow rate was 0.3 mL/min. A gradient analysis starting from 95 % A/5 % B (vol/vol) was used. B was increased to 100% in 2.5 min and kept at this level for 5 min. The column was then equilibrated for 15 min at 5 % of B. Total analysis time was 10 min. The mass spectrometer settings were optimized as follows: turbo ion spray temperature 600 °C, ion spray voltage −4200 V, DP −75 V, EP −10 V, CE −40 V, CXP −17 V, CUR 12 units, CAD high and dwell time was 200 ms. The MRM transitions for 8-iso-PGF2αand 8-iso-PGF2α-d4 were m/z 353 → m/z 193 and m/z 357→ m/z 197, respectively. The data analysis and the quantification were performed as described above. The results were normalized with urinary creatinine concentrations and expressed as ng/mg creatinine. The assay reproducibility was assessed by the precision and accuracy of intra-day and inter-day measurements (n = 10). Urine samples were measured 10 times per day for intra-day precision and sequential ten days for inter-day precision. The intra- and inter-day coefficient of variations for 8-iso-PGF2α were 1.2 % and 4.5 %, respectively.
2.6. Statistical analysis
Statistical analyses were performed using SPSS 22.0 and GraphPad Prism 5. All measurements were performed in triplicate. Each variable was checked for normality of distribution by the Shapiro-Wilk normality test. Student’s t-test was used for normally distributed variables in the analysis of continuous variables. Then variables with non-Gaussian distribution were compared by using Kruskal-Wallis test. Dunnett's test was used for post-hoc two group comparisons. Pearson and Spearman correlation coefficients were used for the analysis of correlation for continuous normally and non-normally distributed variables, respectively. Values with p < 0.05 were considered to indicate a statistically significant difference. The results of oxidative stress markers were evaluated simultaneously with clinical and routine laboratory data of the patients.
3. Results and discussion
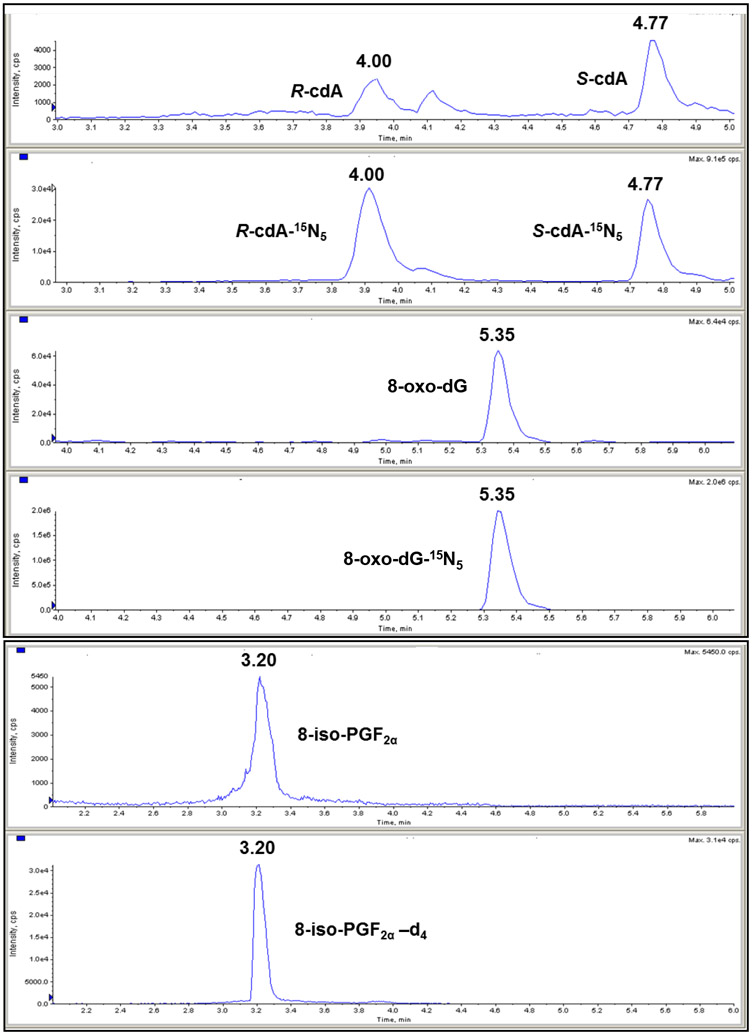
Demographic values and anthropometric measurements of healthy volunteers, and prediabetes and T2DM patients are shown in Table 1. BMI, waist circumstance, HbA1c, FBG, OGTT, ALT and CRP levels were significantly higher in prediabetes and T2DM patients compared to healthy volunteers (Table 1 and 2). Significantly increased HbA1c, ALT, AST and triglyceride levels were found in T2DM patients compared to prediabetes patients (Table 2). Significantly lower HDL levels were found in T2DM patients compared to healthy volunteers, also significantly lower HDL levels in T2DM patients that prediabetes patients (Table 2). Absolute quantification of 8-oxo-dG, R-cdA, S-cdA and 8-iso-PGF2α was achieved by LC-MS/MS. The results were normalized with urinary creatinine concentrations and there was no statistically significant difference between the groups in terms of the urinary creatinine levels. Figure 1 illustrates representative ion-current profiles of the corresponding mass transitions for R-cdA, S-cdA, R-cdA-15N5, S-cdA-15N5, 8-oxo-dG, 8-oxo-dG-15N5, 8-iso-PGF2α and 8-iso-PGF2α-d4, which were recorded during the LC-MS/MS analysis of urine samples. Figures 2 shows the levels of 8-oxo-dG, R-cdA, S-cdA and 8-iso-PGF2α in urine samples of healthy volunteers, and prediabetes and T2DM patients. Statistically significant differences were found between the groups in terms of 8-oxo-dG, S-cdA and 8-iso-PGF2α . The levels of 8-oxo-dG, S-cdA and 8-iso-PGF2α were significantly greater in prediabetes patients than those in healthy volunteers (p < 0.0001, p = 0.011 and p = 0.006, respectively) (Figure 2A,C,D). T2DM patients had greater levels of 8-oxo-dG compared to healthy volunteers (p = 0.031) (Figure 2A). When the R-cdA levels in healthy volunteers and prediabetes patients were compared, a p-value of 0.05 was obtained. Thus, we assumed that there was no statistically significant difference between healthy volunteers and prediabetes patients in terms of the R-cdA levels (Figure 2B). 8-oxo-dG levels were significantly positively correlated with R-cdA and S-cdA levels for prediabetes and T2DM patients (r = 0.549, p < 0.001 and r = 0.599, p < 0.001, r = 0.662, p < 0.001 and r = 0.388, p = 0.011, respectively). HbA1c levels were negatively correlated with S-cdA and 8-iso-PGF2αin prediabetes patients (r = −0.347, p = 0.028 and r = −0.373, p = 0.018, respectively). It should be pointed out that the levels of 8-oxo-dG, R-cdA and S-cdA in urine of healthy volunteers (Figure 2A,B,C) are in excellent agreement with those previously measured in human urine using LC-MS/MS under identical conditions [16,18].
Table 1.
Demographic values and anthropometric measurements for healthy volunteers, prediabetes and T2DM patients. The numbers represent the mean of the ages of individuals or the measured levels. The uncertainties are standard deviations of the healthymean. *p < 0.05 was considered statistically significant between groups. ¥Healthy volunteers compared with prediabetes patients; ¤Healthy volunteers compared with T2DM patients; BMI: body mass index
| Healthy Volunteers (n = 37) |
Prediabetes (n = 47) |
T2DM (n = 43) |
p-value | |
|---|---|---|---|---|
| Number of individuals (women/men) | 27/10 | 34/13 | 20/23 | |
| Age (years) | 41.48 ± 9.32 | 48.04 ± 10.44 | 49.90 ± 9.00 | |
| BMI (kg/m2) | 27.04 ± 4.58 | 31.92 ± 5.09 | 31.87 ± 4.77 |
< 0.001¥
< 0.001¤ |
| Waist circumference (cm) | 87.73 ± 12.22 | 99.04 ± 11.07 | 103.31 ± 13.09 |
< 0.001¥
< 0.001¤ |
Table 2.
Clinical characteristics of healthy volunteers, prediabetes and T2DM patients. The numbers represent the mean of the measured levels. The uncertainties are standard deviations of the mean. *p < 0.05 was considered statistically significant between groups; ¥Healthy volunteers compared with prediabetes patients; ¤Healthy volunteers compared with T2DM patients; £Prediabetes patients compared with T2DM patients. FBG: fasting blood glucose levels, OGTT: glucose tolerance test, AST: aspartate aminotransferase, ALT: alanine aminotransferase, CRP: C reactive protein, BUN: blood urea nitrogen, LDL: low density lipoprotein, HDL: high density lipoprotein
| Healthy Volunteers (n = 37) |
Prediabetes (n = 47) |
T2DM (n = 43) |
p-value | |
|---|---|---|---|---|
| HbA1c (%) | 5.40 ± 0.28 | 5.72 ± 0.33 | 8.54 ± 2.99 | < 0.001¥ < 0.001¤ |
| FBG (mg/dL) | 85.54 ± 6.77 | 106.13 ± 6.37 | 159.72 ± 67.75 | < 0.001¥ < 0.001¤ |
| OGTT (mg/dL) | 113.83 ± 21.49 | 127.19 ± 31.33 | 224.50 ± 105.54 | < 0.001¤ < 0.001¤ |
| ALT (U/L) | 20.29 ± 9.42 | 22.52 ± 10.95 | 35.55 ± 28.46 |
0.005¤
0.011£ |
| AST (U/L) | 19.40 ± 4.49 | 19.93 ± 5.01 | 27.57 ±18.42 | |
| CRP (mg/dL) | 3.69 ± 5.11 | 4.81 ± 4.56 | 8.03 ± 11.25 |
0.019¥
0.018¤ |
| BUN (mg/dL) | 12.17 ± 3.15 | 12.62 ± 4.09 | 13.59 ± 6.93 | |
| Total Cholesterol (mg/dL) | 212.86 ± 39.81 | 211.36 ± 30.95 | 214.16 ± 49.61 | |
| LDL (mg/dL) | 136.86 ± 30.82 | 135.61 ± 28.21 | 133.19 ± 43.95 | |
| HDL (mg/dL) | 53.16 ± 12.25 | 48.40 ± 12.44 | 42.95 ± 8.30 | 0.001 ¤ |
| Triglycerides (mg/dL) | 112.91 ± 62.08 | 141.40 ± 70.74 | 192.76 ± 126.33 |
Fig. 1.
Representative ion-current profiles of the mass transitions m/z 250 → m/z 164 (R-cdA and S-cdA), m/z 255 → m/z 169 (R-cdA-15N5 and S-cdA-15N5), m/z 284 → m/z 168 (8-oxo-dG), m/z 289 → m/z 173 (8-oxo-dG-15N5), m/z 353 → m/z 193 (8-iso-PGF2α ) and m/z 357 → m/z 197 (8-iso-PGF2α -d4), which were recorded during the LC-MS/MS analysis. It should be noted that R-cdA, S-cdA, R-cdA-15N5, S-cdA-15N5, 8-oxo-dG and 8-oxo-dG-15N5 were measured simultaneously, whereas the measurement of 8-iso-PGF2α and 8-iso-PGF2α -d4 was performed in a separate analysis.
Fig. 2.
Levels of 8-oxo-dG (A), R-cdA (B), S-cdA (C) and 8-iso-PGF2α (D) in urine of healthy volunteers (control) (n = 37), prediabetes patients (n = 47) and T2DM patients (n = 43). The uncertainties are standard deviations of the mean. 1 vs. 2: healthy volunteers (control) vs. prediabetes; 1 vs. 3: healthy volunteers (control) vs. T2DM; 2 vs. 3; prediabetes vs. T2DM
Prediabetes is a critical stage that lifestyle changes could modify the risk for progression to T2DM [27]. Biomarkers of oxidative stress are of great interest because of the involvement of this type of damage in disease processes. Products of DNA damage and lipid peroxidation can potentially be used as biomarkers for the early detection of disease, monitoring the progression of disease and determining the efficacy of therapy. Numerous studies have demonstrated oxidative stress in T2DM; however, no unambiguous information on the prediabetic stage has been provided [6]. In the present study, the levels of DNA damage products and a lipid peroxidation product have been examined as reliable oxidative stress markers in patients with prediabetes and T2DM in comparison with healthy volunteers. Patients and healthy volunteers were randomly selected, who were nonsmoking, and used no medication or no supplements to avoid contradictory findings. We found increased levels of oxidatively induced DNA damage and lipid peroxidation in prediabetic stage. Relation between increased oxidative stress and diabetes mellitus has been known for a long time, but there are only a few studies in prediabetic stage [1,28-34]. These studies evaluating oxidative damage during prediabetes have limitations on methods or sample size [25]. Most of the data in the literature have been obtained by methods which provided inconsistent results [35-37]. In contrast, we investigated oxidatively induced DNA damage and lipid peroxidation in prediabetic stage by identification and quantification of reliable biomarkers using LC-MS/MS. This is the first study, in which 8-oxo-dG, R-cdA, S-cdA and 8-iso-PGF2αwere measured simultaneously in patients with prediabetes and T2DM in comparison with healthy volunteers. In early studies, HPLC-ECD, ELISA and mass spectrometry have been used for the measurement of DNA damage and lipid peroxidation products. Our methodology possesses the specificity and sensitivity to measure R-cdA and S-cdA in human urine and permits simultaneous measurement of 8-oxo-dG as well. The purification of urine prior to LC-MS/MS is simple and uses commercially available cartridges for all parameters. The availability of the stable isotope-labeled internal standards enabled accurate measurements. In addition, we analyzed oxidative stress markers in the urine rather than in blood. Urine is obtained more readily and non-invasively than blood, and urinary excretion of damage products reflects the average rate of oxidative stress in the whole body [19,38]. Also, the non-invasive nature of urine collection is a great advantage for large-scale basic research and clinical studies on diabetes.
Over the past two decades, 8-oxo-dG and its free base 8-oxoguanine (8-oxo-Gua) in urine have been mainly used as potential biomarkers [39]. Nevertheless, the origin of 8-oxo-dG and 8-oxo-Gua in urine has been a controversial issue in relation to diet, DNA damage/repair and damage to 2’-deoxytrinucleotides in the nucleotide pool [40-43]. The discovery of R-cdA and S-cdA in human urine offered alternative biomarkers for oxidatively induced DNA damage [16]. Similar to 8-oxo-dG, the origin of R-cdA and S-cdA in urine may not be DNA damage/repair only, but also diet and damage to 2’-deoxytrinucleotides. Unlike 8-oxo-dG (or its free base 8-oxo-Gua) and other measured compounds, R-cdA and S-cdA are repaired by nucleotide excision repair (NER) and are not subject to base excision repair (BER), and possess extraordinary chemical stability [44,45]. These findings also suggested that the measurement of 8-oxo-dG, R-cdA and S-cdA in human urine would result in more reliable data than the measurement of one lesion only. In the present study, greater levels of 8-oxo-dG and S-cdA have been found in prediabetes patients than in healthy volunteers. These findings strongly suggest increased production of oxidatively induced DNA damage before the establishment of T2DM. There are only a few studies that investigated DNA damage in terms of 8-oxo-dG using immunological methods in prediabetes, but they indicate that studies with larger populations should be conducted to define the oxidatively induced DNA damage [5,25,37]. Also to our knowledge, the current study is the first to evaluate R-cdA and S-cdA in prediabetes and T2DM patients. One of the most striking findings of the present study was the presence of a significant negative correlation between S-cdA levels and HbA1c in prediabetes patients, further studies with larger samples are needed for evaluation of S-cdA levels. 8-iso-PGF2α has been used as a reliable standard biomarker for evaluation of lipid peroxidation [46]. Previous studies indicated that 8-iso-PGF2α levels were elevated in T2DM [28,30,32,34]. In the present study, we found significantly increased levels of 8-iso-PGF2α in prediabetic patients compared to healthy volunteers. There is only one previous study that reported 8-iso-PGF2α levels in prediabetic patients and healty volunteers, but no significant difference between the two groups was observed [36].
In conclusion, early disease markers have fundamental importance for understanding the risk for progression to T2DM. This study examined oxidative damage thoroughly by using reliable markers of oxidative stress in patients with prediabetes and newly diagnosed T2DM in comparison with healthy volunteers. Our results indicate that cellular damage appears before the establishment of diabetes. We propose that the simultaneous measurement of 8-oxo-dG, R-cdA, S-cdA and 8-iso-PGF2α in human urine as early disease markers may greatly contribute to risk assessment, diagnosis, testing of drugs, and monitoring and outcome of therapy. Such measurements and the noninvasive nature of urine collection may be of great importance for large scale basic research and clinical studies of T2DM.
Acknowledgements
This research was supported by a grant from Dokuz Eylul University Scientific Research Projects Coordination Unit. Certain commercial equipment or materials are identified in this paper in order to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Abbreviations:
- 8-oxo-dG
8-oxo-2'-deoxyguanosine
- R-cdA and S-cdA
(5'R)- and (5'S)-8,5'-cyclo-2'-deoxyadenosines
- 8-iso-PGF2α
8-iso-prostaglandin F2α
- T2DM
type 2 diabetes mellitus
- LC-MS/MS
liquid chromatography-isotope dilution tandem mass spectrometry
- •OH
hydroxyl radical
- ELISA
enzyme-linked immunosorbent assay
- GC-MS
gas chromatography-mass spectrometry
- HbA1c
Hemoglobin A1c
- FBG
fasting blood glucose
- OGTT
glucose tolerance test
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- CRP
C reactive protein
- BUN
blood urea nitrogen
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- BMI
body mass index
- DP
declustering potential
- EP
entrance potential
- CE
collision energy
- CXP
collision cell exit potential
- CUR
curtain gas
- CAD
collision gas
- MRM
multiple reaction monitoring
- NER
nucleotide excision repair
- BER
base excision repair
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- [1].Song F, Jia W, Yao Y, Hu Y, Lei L, Lin J, Sun X, Liu L, Oxidative stress, antioxidant status and DNA damage in patients with impaired glucose regulation and newly diagnosed Type 2 diabetes, Clin. Sci 112 (2007) 599–606. [DOI] [PubMed] [Google Scholar]
- [2].Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M, Prediabetes: A high-risk state for diabetes development, Lancet 379 (2012) 2279–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hopps E, Noto D, Caimi G, Avema MR, A novel component of the metabolic syndrome: the oxidative stress, Nutr. Metab. Cardiovasc. Dis 20 (2010) 72–77. [DOI] [PubMed] [Google Scholar]
- [4].Maritim AC, Sanders RA, Watkins JB, Diabetes, oxidative stress, and antioxidants: A review, J. Biochem. Mol. Toxicol 17 (2003) 24–38. [DOI] [PubMed] [Google Scholar]
- [5].Pereira CS, Molz P, Palazzo RP, de Freitas TAB, Maluf SW, Horta JA, Pra D, Franke STR, DNA damage and cytotoxicity in adult subjects with prediabetes, Mutat. Res. - Genet. Toxicol. Environ. Mutagen 48 (2013) 581–585. [DOI] [PubMed] [Google Scholar]
- [6].Tabak O, Gelisgen R, Erman H, Erdenen F, Muderrisoglu C, Aral H, Uzun H, Oxidative lipid, protein, and DNA damage as oxidative stress markers in vascular complications of diabetes mellitus, Clin. Invest. Med 34 (2011) E163–171. [DOI] [PubMed] [Google Scholar]
- [7].Friedberg E, DNA damage and repair, Nature 421 (2003) 436–440. [DOI] [PubMed] [Google Scholar]
- [8].Kasai H, Hayami H, Yamaizumi Z, Saito H, Nishimura S, Detection and identification of mutagens and carcinogens as their adducts with guanosine derivatives, Nucleic Acids Res. 12 (1984) 2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Valavanidis A, Vlachogianni T, Fiotakis C, 8-hydroxy-2’-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis, J. Environ. Sci. Health. C. Environ. Carcinog. Ecotoxicol. Rev 27 (2009) 120–139. [DOI] [PubMed] [Google Scholar]
- [10].Kaneko T, Tahara S, Matsuo M, Non-linear accumulation of 8-hydroxy-2’-deoxyguanosine, a marker of oxidized DNA damage, during aging, Mutat. Res. - DNAging Genet. Instab. Aging 316 (1996) 277–285. [DOI] [PubMed] [Google Scholar]
- [11].Cooke MS, Evans MD, Dizdaroglu M, Lunec J, Oxidative DNA damage: mechanisms, mutation, and disease, FASEB J. 17 (2003) 1195–1214. [DOI] [PubMed] [Google Scholar]
- [12].Evans MD, Olinski R, Loft S, Cooke MS, Toward consensus in the analysis of urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine as a noninvasive biomarker of oxidative stress, FASEB J. 24 (2010) 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dizdaroglu M, Oxidatively induced DNA damage: Mechanisms, repair and disease, Cancer Lett. 327 (2012) 26–47. [DOI] [PubMed] [Google Scholar]
- [14].Jaruga P, Dizdaroglu M, 8,5'-Cyclopurine-2'-deoxynucleosides in DNA: Mechanisms of formation, measurement, repair and biological effects, DNA Repair (Amst) 7 (2008) 1413–1425. [DOI] [PubMed] [Google Scholar]
- [15].Dizdaroglu M, Oxidatively induced DNA damage and its repair in cancer, Mutat. Res. Rev. Mutat. Res 763 (2015) 212–245. [DOI] [PubMed] [Google Scholar]
- [16].Jaruga P, Dizdaroglu M, Identification and quantification of (5’R)- and (5’S)-8,5'-cyclo-2'-deoxyadenosines in human urine as putative biomarkers of oxidatively induced damage to DNA, Biochem. Biophys. Res. Commun 397 (2010) 48–52. [DOI] [PubMed] [Google Scholar]
- [17].Dizdaroglu M, Jaruga P, Rodriguez H, Measurement of 8-hydroxy-2'-deoxyguanosine in DNA by high-performance liquid chromatography-mass spectrometry: comparison with measurement by gas chromatography-mass spectrometry, Nucleic Acids Res. 29 (2001) E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jaruga P, Rozalski R, Jawien A, Migdalski A, Olinski R, Dizdaroglu M, DNA damage products (5′R)- and (5′S)-8,5′-cyclo-2′-deoxyadenosines as potential biomarkers in human urine for atherosclerosis, Biochemistry 51 (2012) 1822–1824. [DOI] [PubMed] [Google Scholar]
- [19].Milne GL, Dai Q, Roberts LJ, The isoprostanes-25 years later, Biochim. Biophys. Acta 1851 (2015) 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ, A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism, Proc. Natl. Acad. Sci. U.S.A 87 (1990) 9383–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Teng Y-H, Wang C-W, Liao Y-T, Yang M-W, Liu T-Y, Quantification of urinary 8-iso-prostaglandin F(2 alpha) using liquid chromatography-tandem mass spectrometry during cardiac valve surgery, J. Clin. Lab. Anal 24 (2010) 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska a., Wachsman JT, Ames BN, Basu S, Brot N, FitzGerald G. a., Floyd R. a., George M, Heinecke JW, Hatch GE, Hensley K, Lawson J. a., Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC, Biomarkers of oxidative stress study II. Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning?, Free Radic. Biol. Med 38 (2005) 698–710. [DOI] [PubMed] [Google Scholar]
- [23].Roberts LJ, Morrow JD, The generation and actions of isoprostanes, Biochim. Biophys. Acta - Lipids Lipid Metab 1345 (1997) 121–135. [DOI] [PubMed] [Google Scholar]
- [24].Roberts LJ, Morrow JD, Measurement of F2-isoprostanes as an index of oxidative stress in vivo, Free Radic. Biol. Med 2 (2000) 505–513. [DOI] [PubMed] [Google Scholar]
- [25].Al-Aubaidy HA, Jelinek HF, 8-oxo-2'-deoxyguanosine identifies oxidative DNA damage in a rural prediabetes cohort, Redox Rep. 15 (2010) 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].American Diabetes Association, Diagnosis and classification of diabetes mellitus, Diabetes Care 34 Suppl 1 (2011) S62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Portero McLellan KC, Wyne K, Villagomez ET, Hsueh WA, Therapeutic interventions to reduce the risk of progression from prediabetes to type 2 diabetes mellitus, Ther. Clin. Risk Manag 10 (2014) 173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tabak O, Gelisgen R, Erman H, Erdenen F, Muderrisoglu C, Aral H, Uzun H, Oxidative lipid, protein, and DNA damage as oxidative stress markers in vascular complications of diabetes mellitus, Clin. Invest. Med 34 (2011) E163–E171. [DOI] [PubMed] [Google Scholar]
- [29].Zengi A, Ercan G, Caglayan O, Tamsel S, Karadeniz M, Simsir I, Harman E, Kahraman C, Orman M, Cetinkalp S, Ozgen G, Increased oxidative DNA damage in lean normoglycemic offspring of type 2 diabetic patients, Exp Clin Endocrinol Diabetes 119 (2011) 467–471. [DOI] [PubMed] [Google Scholar]
- [30].Zheng F, Lu W, Jia C, Li H, Wang Z, Jia W, Relationships between glucose excursion and the activation of oxidative stress in patients with newly diagnosed type 2 diabetes or impaired glucose regulation, Endocrine 37 (2010) 201–208. [DOI] [PubMed] [Google Scholar]
- [31].Broedbaek K, Siersma V, Henriksen T, Weimann A, Petersen M, Andersen JT, Jimenez-Solem E, Stovgaard ES, Hansen LJ, Henriksen JE, Bonnema SJ, de F. Olivarius N, Poulsen HE, De Fine Olivarius N, Poulsen HE, Urinary markers of nucleic acid oxidation and long-term mortality of newly diagnosed type 2 diabetic patients, Diabetes Care 34 (2011) 2594–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chang C-M, Hsieh C-J, Huang J-C, Huang I-C, Acute and chronic fluctuations in blood glucose levels can increase oxidative stress in type 2 diabetes mellitus, Acta Diabetol. 49 (2012) 171–177. [DOI] [PubMed] [Google Scholar]
- [33].Al-Aubaidy HA, Jelinek HF, Oxidative DNA damage and obesity in type 2 diabetes mellitus, Eur. J. Endocrinol 164 (2011) 899–904. [DOI] [PubMed] [Google Scholar]
- [34].Siegelaar SE, Barwari T, Kulik W, Hoekstra JB, DeVries HJ, No relevant relationship between glucose variability and oxidative stress in well-regulated type 2 diabetes patients, J. Diabetes Sci. Technol 5 (2011) 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dzięgielewska-ęsiak S, of Lipid Peroxidation Products, Plasma Total Antioxidant Status, and Cu-, Zn-Superoxide Dismutase Activity as Biomarkers of Oxidative Stress in Elderly Prediabetics, Oxidative Med. 2014 (2014) 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Maschirow L, Khalaf K, Al-Aubaidy HA, Jelinek HF, Inflammation, coagulation, endothelial dysfunction and oxidative stress in prediabetes - Biomarkers as a possible tool for early disease detection for rural screening, Clin. Biochem 48 (2015) 581–585. [DOI] [PubMed] [Google Scholar]
- [37].Pereira CS, Molz P, Palazzo RP, de Freitas TAB, Maluf SW, Horta JA, Prá D, Franke SIR, DNA damage and cytotoxicity in adult subjects with prediabetes, Mutat. Res 753 (2013)76–81. [DOI] [PubMed] [Google Scholar]
- [38].Poulsen HE, Oxidative DNA modifications, Exp. Toxicol. Pathol 57 (2005) 161–169. [DOI] [PubMed] [Google Scholar]
- [39].Evans MD, Olinski R, Loft S, Cooke MS, Toward consensus in the analysis of urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine as a noninvasive biomarker of oxidative stress, FASEB J. 24 (2010) 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gackowski D, Rozalski R, Roszkowski K, Jawien A, Foksihski M, Olinski R, 8-Oxo-7,8-dihydroguanine and 8-oxo-7,8-dihydro-2'-deoxyguanosine levels in human urine do not depend on diet, Free Radic. Res 35 (2001) 825–832. [DOI] [PubMed] [Google Scholar]
- [41].Rozalski R, Siomek A, Gackowski D, Foksinski M, Gran C, Klungland A, Olinski R, Substantial decrease of urinary 8-oxo-7,8-dihydroguanine, a product of the base excision repair pathway, in DNA glycosylase defective mice, Int. J. Biochem. Cell Biol 37 (2005) 1331–1336. [DOI] [PubMed] [Google Scholar]
- [42].Cooke MS, Olinski R, Loft S, Measurement and meaning of oxidatively modified DNA lesions in urine, Cancer Epidemiol. Biomarkers Prev 17 (2008) 3–14. [DOI] [PubMed] [Google Scholar]
- [43].Cooke MS, Henderson PT, Evans MD, Sources of extracellular, oxidatively-modified DNA lesions: implications for their measurement in urine, J. Clin. Biochem. Nutr 45 (2009) 255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Brooks PJ, Wise DS, Berry DA, Kosmoski JV, Smerdon MJ, Somers RL, Mackie H, Spoonde AY, Ackerman EJ, Coleman K, Tarone RE, Robbins JH, The oxidative DNA lesion 8,5'-(S)-cyclo-2'-deoxyadenosine is repaired by the nucleotide excision repair pathway and blocks gene expression in mammalian cells, J. Biol. Chem 275 (2000) 22355–22362. [DOI] [PubMed] [Google Scholar]
- [45].Kuraoka I, Bender C, Romieu A, Cadet J, Wood RD, Lindahl T, Removal of oxygen free-radical-induced 5',8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells, Proc. Natl. Acad. Sci. U.S.A 97 (2000) 3832–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Montuschi P, Barnes PJ, Roberts LJ, Isoprostanes: markers and mediators of oxidative stress, FASEB J. 18 (2004) 1791–1800. [DOI] [PubMed] [Google Scholar]