Abstract
Using the simian immunodeficiency virus (SIV)-infected rhesus macaque model, we performed a longitudinal study to determine the effect of antiretroviral therapy on the phenotype and functional potential of CD4+ T cells repopulating intestinal mucosa in human immunodeficiency virus infection. Severe depletion of CD4+ and CD4+ CD8+ T cells occurred in the intestinal mucosa during primary SIV infection. The majority of these cells were of activated memory phenotype. Phosphonate 9-[2-(phosphomethoxypropyl]adenine (PMPA) treatment led to a moderate suppression of intestinal viral loads and repopulation of intestinal mucosa by predominantly activated memory CD4+ T-helper cells. This repopulation was independent of the level of viral suppression. Compared to preinfection values, the frequency of naive CD4+ T cells increased following PMPA therapy, suggesting that new CD4+ T cells were repopulating the intestinal mucosa. Repopulation by CD4+ CD8+ T cells was not observed in either jejunum or colon lamina propria. The majority of CD4+ T cells repopulating the intestinal mucosa following PMPA therapy were CD29hi and CD11ahi. A subset of repopulating intestinal CD4+ T cells expressed Ki-67 antigen, indicating that local proliferation may play a role in the repopulation process. Although the majority of repopulating CD4+ T cells in the intestinal mucosa were functionally capable of providing B- and T-cell help, as evidenced by their expression of CD28, these CD4+ T cells were found to have a reduced capacity to produce interleukin-2 (IL-2) compared to the potential of CD4+ T cells prior to SIV infection. Persistent viral infection may play a role in suppressing the potential of repopulating CD4+ T cells to produce IL-2. Hence, successful antiretroviral therapy should aim at complete suppression of viral loads in mucosal lymphoid tissues, such as intestinal mucosa.
Human immunodeficiency virus (HIV) infection is characterized by a progressive depletion of peripheral CD4+ T cells preceded by CD4+ T-cell dysfunction (7, 10, 24, 26, 31). The depletion of CD4+ T cells contributes to immunodeficiency and to an increased susceptibility to opportunistic infections in HIV-infected patients. Antiretroviral treatment has been used to achieve suppression of viral burden in peripheral blood and has led to various degrees of restoration of CD4+ T-cell numbers and function in HIV-infected patients (14, 15, 19). An immunophenotypic and functional characterization of repopulating CD4+ T cells following antiretroviral therapy has been carried out with peripheral blood lymphocytes and, to a limited extent, peripheral lymph node lymphocytes. The information is limited regarding the repopulation of CD4+ T cells in various lymphoid tissues, such as gut-associated lymphoid tissue, and their functional and immunophenotypic characteristics compared to those of restored CD4+ T cells in the periphery. Therefore, CD4+ T cells repopulating various lymphoid sites need to be evaluated to gain a better understanding of immune reconstitution following antiretroviral therapy.
Numerous studies have demonstrated the potential of the immune system to regenerate CD4+ T cells in HIV-infected patients following antiretroviral therapy (3, 9, 12, 21). An increase in the numbers of memory CD4+ T cells was observed immediately after therapy and was followed by an increase in the numbers of naive CD4+ T cells a few weeks after therapy. However, the increase in naive CD4+ T cells occurred only in patients who had naive CD4+ T cells prior to treatment, indicating that the increase in this subset of T cells was the result of an expansion of already existing naive CD4+ T cells. Increased proliferative responses of repopulating CD4+ T cells to HIV antigens and to recall antigens following antiretroviral therapy were demonstrated (21). Further, a transient improvement in lymphocyte function in patients with advanced HIV disease following antiretroviral therapy was shown (3, 5, 6, 9, 12, 34, 37, 48). Recent studies have attempted to reveal the mechanisms of CD4+ T-cell renewal following antiretroviral therapy. Pakker et al. (36), using highly active antiretroviral therapy, demonstrated that the increase in CD4+ T cells in blood was not characterized by an increase in CD4+ Ki-67+ T cells immediately after treatment, suggesting that this increase was the result of redistribution of CD4+ T cells from other lymphoid tissues. Similarly, other studies have suggested that recirculation of CD4+ T cells may play a significant role in increasing peripheral CD4+ T cells (32, 40). On the other hand, Walker et al. (47) demonstrated that an increase in CD4+ T cells in peripheral blood was the result of an expansion of already existing CD4+ T cells. Most of these studies have used peripheral blood and peripheral lymphoid tissues to evaluate the effects of antiretroviral therapy on immune reconstitution. However, peripheral blood represents only 2% of lymphocytes, whereas the majority of total lymphocytes are found in gut-associated lymphoid tissue. No information is available on the effects of antiretroviral therapy on the regeneration of CD4+ T cells in the gastrointestinal mucosa. Since intestinal tissue is not available from HIV-infected patients early in the infection, a suitable animal model would be extremely valuable for such studies.
Our previous studies have shown that simian immunodeficiency virus (SIV)-infected rhesus macaques are an excellent animal model for studies of HIV-associated enteropathy (17, 18, 42). SIV is a lentivirus that causes simian AIDS in rhesus macaques. The course of pathogenic SIVmac infection includes primary acute, asymptomatic, and terminal stages of disease, as in HIV infection. The intestine has been shown to be an early target organ of SIV, and an SIV-associated enteropathy syndrome was detected in primary SIV infection (17, 18, 42). In contrast to the changes observed in peripheral blood and lymph nodes, a severe depletion of CD4+ and CD4+ CD8+ T cells was observed in the gastrointestinal mucosa during primary SIV infection (29, 39, 46). Numerous studies have evaluated the effects of antiretroviral therapy on viral suppression and CD4+ T-cell repopulation in peripheral blood and peripheral lymph nodes with the SIV model (30, 43–45). A reverse transcriptase inhibitor, phosphonate 9-[2-(phosphomethoxypropyl]adenine (PMPA), was shown to suppress viral loads in SIV-infected infant (45) and adult (43, 44) rhesus macaques. Further short-term treatment with PMPA did not induce any toxicity. Thus, SIVmac-infected macaques are well suited for studying the effects of antiretroviral therapy on immune reconstitution in gastrointestinal lymphoid tissues.
The objective of this study was to determine the phenotype and functional characteristics of CD4+ T cells repopulating gastrointestinal mucosa following antiretroviral therapy of long-term SIV-infected rhesus macaques. The expression of CD45RA, CD69, CD28, CD29, CD11a, and β7-integrin was examined to immunophenotypically characterize the CD4+ T cells repopulating the intestinal mucosa following PMPA therapy. To determine whether local proliferation of CD4+ T cells in the intestinal mucosa contributed to the repopulation process, we determined the expression of Ki-67 antigen in the repopulating CD4+ T cells. The functional potential of CD4+ T cells repopulating the intestinal mucosa was evaluated by using flow cytometry combined with intracellular staining for interleukin-2 (IL-2) following short-term mitogenic stimulation before and after PMPA treatment. Our findings demonstrated that activated memory CD4+ T cells repopulated the intestinal mucosa following PMPA therapy but had decreased functional potential to produce IL-2 compared to preinfection potential.
MATERIALS AND METHODS
Animals, virus, and tissue collection.
Four colony-bred rhesus macaques (Macaca mulatta) from the California Regional Primate Research Center, Davis, were used in this longitudinal study. The animals were housed in accordance with American Association for Accreditation of Laboratory Animal Care guidelines. Animals were seronegative for simian retrovirus type 1 and simian T-cell leukemia virus type 1. Jejunum and colon biopsy samples and peripheral blood samples were obtained prior to SIV infection to establish preinfection baseline values (n = 4). All four animals were infected intravenously with 10 to 100 animal infectious doses of uncloned pathogenic SIVmac251. Samples from jejunum and colon and peripheral blood samples were obtained from all four animals at 4 weeks postinfection (p.i.). PMPA (courtesy of Norbert Bischoffberger, Gilead Inc., Foster City, Calif.) treatment was initiated at 8 weeks p.i. Since the animals were being treated with PMPA over a long period of time, all the animals received PMPA at 10 mg/kg of body weight once daily for 12 weeks. Longitudinal samples from jejunum and colon and peripheral blood were collected at 4 (n = 4) and 12 (n = 2) weeks following the start of PMPA treatment. Two animals were necropsied at 4 weeks after PMPA treatment. Tissue samples were immediately frozen for a branched-DNA (bDNA) assay.
bDNA quantification of SIV RNA.
The bDNA signal amplification assay specific for SIV was used to determine SIV RNA copy number in tissue and plasma samples. This assay was similar to the previously reported Quantiplex HIV RNA assay (35), except for the target probes, which were designed to hybridize with the pol region of SIVmac variants, including SIVmac251 (16). A standard curve for the SIV RNA copies was generated with serial dilutions of SIV-infected tissue culture supernatant containing cell-free SIV. Quantification for this standard curve was obtained by comparison with purified, quantified, in vitro-transcribed SIVmac239 pol RNA. The copy numbers of SIV RNA associated with viral particles in plasma samples were determined by comparison with the standard curve. One milliliter of EDTA-anticoagulated plasma was pelleted at 23,500 × g for 1 h at 4°C and used for the measurement of SIV RNA copy number. The lowest limit for the quantification of SIV RNA in this assay was approximately 1,500 copies per ml of plasma. Jejunum and colon tissue samples were homogenized in guanidine hydrochloride buffer, and SIV RNA copy numbers were quantified by the bDNA assay (16). The SIV RNA burden in tissues was reported as the number of SIV RNA copies per 10 mg of tissue, and that in plasma was reported as the number of SIV RNA copies per ml of plasma. The lowest limit for the quantification of SIV RNA in tissue samples was about 3,000 copies.
Isolation of LPL.
Lamina propria lymphocytes (LPL) were isolated by previously published procedures (29, 39). Jejunum and colon tissue samples were placed in cell isolation medium containing RPMI 1640 (Gibco) supplemented with 100 U of penicillin (Gibco) per ml, 100 U of streptomycin (Gibco) per ml, 5% fetal calf serum (Gibco), and 50 U of collagenase type II (Sigma Chemical Co., St. Louis, Mo.) per 100 ml and subjected to rapid shaking at 37°C for 30 min. This procedure was repeated three times. Cell suspensions were centrifuged, washed, and enriched for mononuclear cells with a 35%:60% (vol/vol) isotonic discontinuous Percoll (Sigma) density gradient. Mononuclear cells were found to band at the interface between the 35% and 60% gradients. More than 98% of the isolated mononuclear cells were viable, as determined by a trypan blue exclusion assay.
Antibodies.
Monoclonal antibodies (MAb) to CD3 (anti-CD3; Pharmingen, San Diego, Calif.), CD4 (anti-CD4; Ortho-Diagnostics Systems, Inc., Raritan, N.J.), CD8 (anti-CD8; Caltag Laboratories, South San Francisco, Calif.), CD45RA (anti-CD45RA; Caltag), CD69 (anti-CD69; Caltag), CD28 (anti-CD28; Coulter Immunotech, Miami, Fla.), CD29 (anti-CD29; Coulter Immunotech), CD11a (anti-CD11a; Coulter Immunotech), β7-integrin (anti–β7-integrin; Pharmingen), Ki-67 (anti–Ki-67; MIB-1 clone; Coulter Immunotech), and IL-2 (Pharmingen) were used in this study. Isotype control MAbs, except for that for IL-2, were obtained from Caltag. The isotype control antibody for IL-2 was obtained from Pharmingen.
Immunophenotypic analysis of CD4+ T cells.
Freshly isolated cells from the jejunum, colon, and peripheral blood were stained with anti-CD3 conjugated to fluorescein isothiocyanate (FITC), anti-CD8 conjugated to tricolor stain TC, and anti-CD4 conjugated to phycoerythrin. To further immunophenotype the CD4+ T cells, cells were also stained with anti-CD4 followed by either anti-CD45RA, anti-CD69, or anti-CD28. To determine the differential expression of adhesion molecules, cells were stained with anti-CD4 and anti-CD29, anti-CD11a, or anti–β7-integrin. Negative control samples were stained with isotype control antibodies. To determine the expression of Ki-67, cells were labeled with anti-CD4 conjugated to phycoerythrin and fixed as described below. These fixed cells were permeabilized and labeled intracellularly with anti–Ki-67 conjugated to FITC. Negative control samples included cells stained with matched isotype control antibodies and cells labeled with anti-CD4 as well as intracellularly with isotype control antibodies.
Flow cytometric detection of IL-2 production by CD4+ T cells.
Intracellular IL-2 production was detected at the single-cell level by methods described previously (29, 39). Briefly, peripheral blood mononuclear cells (PBMC) and LPL were stimulated with 10 ng of phorbol myristate acetate (Sigma) per ml and 500 ng of ionomycin (Calbiochem, La Jolla, Calif.) per ml for 4 h. Monensin (2 μM) was used to disaggregate the Golgi complex to arrest the proteins from being transported. Cells were incubated with monensin only to determine whether cells produce IL-2 in the absence of stimulation. After incubation, the cells were harvested, washed in cytoflow buffer (phosphate-buffered saline [PBS] with 1% bovine serum albumin), and prepared for labeling.
To determine the capacity of PBMC and LPL to produce IL-2, cells were subjected to two-color flow cytometric analysis by methods previously described (11, 29, 39). Briefly, cells were labeled with anti-CD4 conjugated to PE and incubated for 30 min at 4°C. Negative control samples were stained with matched isotype control MAb. After being washed in PBS, cells were fixed (Cell Perm & Fix Kit; Caltag) for 15 min at room temperature in the dark, washed, and labeled with FITC-conjugated anti-human IL-2 resuspended in permeabilizing solution (Cell Perm & Fix Kit) for 15 min. Negative control samples also included samples labeled with CD4 and then with intracellular isotype control MAb suspended in permeabilizing solution. After being washed, cells were resuspended in PBS and prepared for analysis. Cells were also fixed and labeled with anti-human IL-2 and matched isotype control MAb without permeabilization to ensure that only intracellular proteins were being labeled.
Cells prepared for both immunophenotypic analysis and intracellular detection of Ki-67 and IL-2 were analyzed with a FACScan flow cytometer (Becton Dickinson, Mountainview, Calif.). A total of 2,000 to 4,000 events were collected in list mode after simultaneous gating on lymphocytes, based upon their forward- and light-scatter characteristics and FL1 (CD3) or FL2 (CD4) and forward scatter. Collected data were analyzed with Cell Quest software (Becton Dickinson).
RESULTS
Suppression of intestinal tissue viral loads was variable following antiretroviral treatment.
The SIV RNA copy numbers in jejunum and colon tissues and plasma were determined at 4 weeks p.i. and at 4 and 12 weeks after PMPA therapy (Table 1). All four animals had high plasma viremia at 4 weeks p.i. A moderate degree of suppression was observed in the plasma viral loads of only two animals at 4 weeks after PMPA therapy. In one of the animals, the plasma viral loads continued to increase dramatically even after PMPA therapy. This animal did not have any detectable level of antibodies against SIV, suggesting that it may have been a fast progressor. The viral burdens were variable in jejunum and colon tissues at 4 weeks p.i. A moderate degree of viral suppression was observed in the jejunum tissue of all the animals after PMPA therapy. In the colon tissue, however, the viral loads were moderately suppressed in only two of the animals after PMPA therapy.
TABLE 1.
Viral loads and frequency of CD4+ and CD4+ CD8+ T cells in peripheral blood, jejunum, and colon lamina propria of SIV-infected rhesus macaques prior to and following PMPA treatmenta
| Animal | Infection | Peripheral blood
|
Jejunum lamina propria
|
Colon lamina propria
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SIV copy no. (103/ml) | % of cells that were:
|
SIV copy no. (103/10 mg) | % of cells that were:
|
SIV copy no. (103/10 mg) | % of cells that were:
|
|||||
| CD4+ | CD4+ CD8+ | CD4+ | CD4+ CD8+ | CD4+ | CD4+ CD8+ | |||||
| 1 | Preinfection | 0 | 62 | 2 | 0 | 31 | 16 | 0 | 55 | 7 |
| 4 wk p.i. | 57,233 | 57 | 1 | 687 | 6 | <1 | 892 | 1 | <1 | |
| 4 wk post-PMPA therapy | 14,734 | 60 | 1 | 467 | 33 | <1 | 720 | 7 | <1 | |
| 2 | Preinfection | 0 | 51 | 1 | 0 | 30 | 23 | 0 | 47 | 14 |
| 4 wk p.i. | 25,220 | 54 | 1 | 8,799 | 6 | 1 | 264 | 2 | <1 | |
| 4 wk post-PMPA therapy | 194,891 | 56 | 1 | 1,021 | 28 | <1 | 27,583 | 24 | <1 | |
| 3 | Preinfection | 0 | 58 | 1 | 0 | 37 | 10 | 0 | 32 | 9 |
| 4 wk p.i. | 4,266 | 53 | 1 | — | 2 | <1 | <3 | 2 | <1 | |
| 4 wk post-PMPA therapy | 1,889 | 52 | 1 | 267 | — | <1 | — | 29 | <1 | |
| 12 wk post-PMPA therapy | — | 58 | 1 | <3 | 36 | 1 | 474 | 32 | <1 | |
| 4 | Preinfection | 0 | 66 | 1 | 0 | 42 | 13 | 0 | 48 | 16 |
| 4 wk p.i. | 1,055 | 55 | 1 | 62 | 7 | <1 | 438 | 4 | <1 | |
| 4 wk post-PMPA therapy | 3,818 | 42 | 1 | <3 | 10 | <1 | <3 | 12 | 1 | |
| 12 wk post-PMPA therapy | — | 38 | 1 | 42 | 30 | <1 | <3 | 24 | 1 | |
—, no data.
Severe depletion of CD4+ T cells in the gastrointestinal mucosa during primary infection was followed by repopulation of CD4+ T cells after PMPA therapy.
The frequencies of CD3+ CD4+ and CD3+ CD4+ CD8+ T cells in the jejunum, colon, and peripheral blood were determined as percentages of gated CD3+ T cells at 4 weeks p.i. and at 4 and 12 weeks following PMPA treatment and were compared with preinfection values (Table 1). Prior to infection, both the jejunum and the colon lamina propria were found to harbor a high proportion of CD4+ and CD4+ CD8+ T cells. The majority of the CD4+ T cells in peripheral blood were CD4+ T cells only, with fewer than 2% being CD4+ CD8+ T cells. At 4 weeks p.i., a severe depletion of both CD4+ and CD4+ CD8+ T cells was observed in both the jejunum and the colon lamina propria. No major change was observed in peripheral blood. Following PMPA treatment, repopulation of only CD4+ T cells occurred in the jejunum and the colon lamina propria of all the animals, whereas no repopulation of CD4+ CD8+ T cells occurred in either the jejunum or the colon lamina propria (Table 1). In contrast, only minor changes were observed in the frequencies of CD4+ and CD4+ CD8+ T cells in peripheral blood following PMPA treatment.
CD4+ T cells repopulating the gastrointestinal mucosa have an activated memory phenotype.
The frequencies of CD4+ T cells expressing CD45RA, CD69, and CD28 in the jejunum, colon, and peripheral blood before and after PMPA treatment were evaluated (Table 2). Memory CD4+ T cells are CD45RA−, whereas naive CD4+ T cells are CD45RA+. Prior to SIV infection, the majority of CD4+ T cells in both the jejunum and the colon lamina propria were memory CD4+ T cells. In contrast, CD4+ T cells from peripheral blood of uninfected samples were predominantly naive CD4+ T cells (∼41 to 67%).
TABLE 2.
Phenotypes of CD4+ T cells in the jejunum and colon lamina propria of SIV-infected rhesus macaques prior to and following PMPA therapya
| Animal | Infection | % of cells with the indicated phenotype in lamina propria of:
|
|||||
|---|---|---|---|---|---|---|---|
| Jejunum
|
Colon
|
||||||
| CD45RA+ | CD69+ | CD28+ | CD45RA+ | CD69+ | CD28+ | ||
| 1 | Preinfection | 5 | 99 | 99 | 7 | 89 | 91 |
| 2 | 6 | 96 | 96 | 14 | 92 | 96 | |
| 3 | 3 | 92 | 92 | 7 | 93 | 94 | |
| 4 | 5 | 95 | 95 | 5 | 91 | 86 | |
| 1 | 4 wk post-PMPA therapy | 21 | 76 | 97 | 31 | 44 | 96 |
| 2 | 24 | 68 | 98 | 24 | 46 | 99 | |
| 3 | 27 | 72 | 92 | 42 | 52 | 98 | |
| 4 | 35 | 71 | 98 | 33 | 49 | ||
| 1 | 12 wk post-PMPA therapy | 27 | 67 | 94 | 16 | 86 | 97 |
| 2 | 36 | 81 | 96 | 29 | 62 | 98 | |
Due to severe depletion, very few CD4+ T cells could be collected at 4 weeks p.i. to make an accurate analysis.
Following SIV infection, the frequency of naive CD4+ T cells increased in peripheral blood at 4 weeks p.i. (∼78 to 82%). Due to the severe depletion of CD4+ T cells in the jejunum and the colon lamina propria at 4 weeks p.i., very few CD4+ T cells could be collected to accurately determine their phenotype.
Following PMPA treatment, the majority of the CD4+ T cells repopulating the lamina propria of the jejunum and the colon were predominantly memory CD4+ T cells (Table 2). Although naive CD4+ T-cell percentages were low in the intestine prior to SIV infection, PMPA treatment led to a significantly higher prevalence of naive CD4+ T cells in both the jejunum and the colon lamina propria. In peripheral blood, the frequency of naive CD4+ T cells remained high at 4 weeks following PMPA treatment (∼51 to 78%), whereas at 12 weeks following PMPA treatment, no major difference was observed relative to preinfection control values (∼54 to 59%).
To examine the activation status of CD4+ T cells repopulating the intestine, we determined the expression of CD69, an early activation antigen expressed on activated T cells. The expression of CD69 on CD4+ T cells from the jejunum, colon, and peripheral blood at 4 weeks p.i. and at 4 and 12 weeks after PMPA treatment was determined (Table 2) and compared with preinfection baseline values. Most of the CD4+ T cells in the jejunum and the colon lamina propria of uninfected animals expressed CD69 antigen, indicative of an activated phenotype. In contrast, most of the CD4+ T cells in peripheral blood had a resting phenotype, with fewer than 1% of CD4+ T cells expressing CD69. No major change was observed in peripheral blood following SIV infection. Due to severe CD4+ T-cell depletion in the intestine, very few CD4+ T cells could be collected from the jejunum and the colon lamina propria to accurately determine the expression of CD69 antigen. Following PMPA treatment, the majority of CD4+ T cells repopulating the jejunum and the colon lamina propria were found to express CD69 (>69%). In contrast, fewer than 1% of CD4+ T cells in peripheral blood were found to express CD69 prior to or following PMPA treatment.
The majority of CD4+ T cells in the jejunum, colon, and peripheral blood of PMPA-treated animals expressed CD28 at levels comparable to preinfection baseline values (Table 2).
CD4+ T cells repopulating the intestine following PMPA therapy express the CD29hi CD11ahi phenotype.
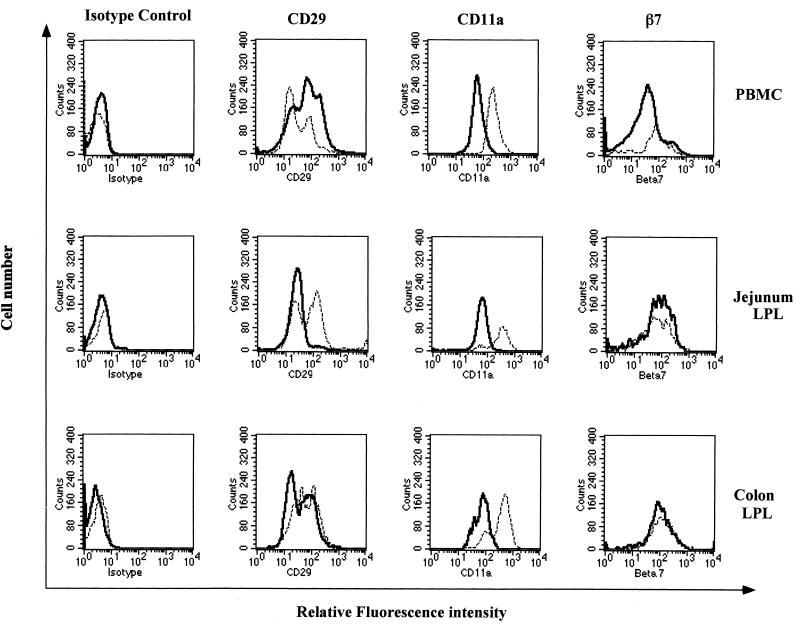
To further characterize the phenotype of CD4+ T cells repopulating the intestinal mucosa following therapy, we determined the expression of CD29, CD11a, and β7-integrin on CD4+ T cells in the jejunum, colon, and peripheral blood following PMPA treatment and compared the values with preinfection baseline values. The majority of CD4+ T cells in the jejunum, colon, and peripheral blood were found to express CD29. However, two subpopulations of CD4+ T cells were detected based on the quantitative expression (density) of CD29. One subset of CD4+ T cells was found to express CD29 at a lower density (CD29lo), with mean fluorescence intensity (MFI) ranging from ∼24 to 32, whereas the other subset of CD4+ T cells was found to express CD29 at a higher density (CD29hi), with an MFI ranging from ∼108 to 207. The relative frequencies of CD4+ CD29lo and CD4+ CD29hi T cells were found to change following PMPA treatment compared to preinfection baseline values, as shown in Fig. 1.
FIG. 1.
Differential expression of cell adhesion molecules (CD29, CD11a, and β7-integrin) on repopulating CD4+ T cells from intestinal mucosa compared to peripheral blood CD4+ T cells. The histograms show the differential expression of CD29 (VLA-4), CD11a (LFA-1), and β7-integrins on CD4+ T cells in jejunum and colon LPL and PBMC from uninfected rhesus macaques (solid line) and SIV-infected rhesus macaques after 4 weeks of PMPA therapy (broken line). Isolated cells were stained for CD4, CD29, CD11a, and β7-integrin and analyzed by flow cytometry. Analysis gates were set to include CD4+ T cells only to determine the relative fluorescence intensity of CD29, CD11a, and β7-integrin expression. Negative control samples were stained with matched isotype control antibodies.
Prior to SIV infection, the majority of CD4+ T cells in both the jejunum and the colon lamina propria had a CD4+ CD29lo phenotype (∼63 to 78%), whereas peripheral blood CD4+ T cells were found to be enriched for the CD29hi phenotype (∼55 to 62%). Due to the severe depletion of CD4+ T cells in the jejunum and the colon lamina propria at 4 weeks p.i., the expression of CD29 could not be determined. In peripheral blood, the frequency of CD4+ CD29hi T cells decreased (∼36 to 39%) at 4 weeks p.i. Following PMPA treatment, the frequency of CD4+ CD29hi T cells increased in the jejunum and the colon lamina propria. The proportions of CD4+ CD29hi T cells in the jejunum lamina propria increased from ∼18 to 23% in uninfected controls to ∼33 to 49% and to ∼48 to 55% at 4 and 12 weeks following PMPA treatment, respectively. A similar trend was observed in the colon lamina propria, where the proportions of CD4+ CD29hi T cells increased at 4 (∼37 to 42%) and 12 (∼49 to 53%) weeks following PMPA treatment relative to preinfection baseline values (∼19 to 27%). In contrast, the proportions of CD4+ CD29hi T cells remained low in peripheral blood at 4 (∼14 to 30%) and 12 (∼22 to 37%) weeks following PMPA treatment relative to preinfection baseline values (∼55 to 62%).
Prior to SIV infection, the majority of CD4+ T cells (>96%) in the jejunum, colon, and peripheral blood expressed CD11a. No significant differences were observed in the frequencies of CD4+ CD11a+ T cells in SIV-infected macaques prior to and following PMPA treatment. However, differences were observed in the density of CD11a expression in SIV-infected macaques and after PMPA treatment compared to preinfection baseline values (Fig. 1). Prior to SIV infection, the MFI of CD11a expression on CD4+ T cells in the jejunum and the colon lamina propria ranged from ∼59 to 117 and from ∼153 to 180, respectively. CD4+ T cells in peripheral blood expressed CD11a at an MFI of ∼58 to 110. The MFI of CD11a expression on peripheral blood CD4+ T cells increased at 4 weeks p.i. (∼188 to 220). Due to severe CD4+ T-cell depletion in the intestine at 4 weeks p.i., the expression of CD11a could not be determined. The CD4+ T cells repopulating the jejunum and the colon lamina propria following PMPA treatment were found to express CD11a at a density higher than preinfection baseline values. CD11a was expressed on CD4+ T cells in the jejunum lamina propria at an MFI of ∼224 to 362 at 4 weeks after PMPA treatment and at an MFI of ∼382 to 401 at 12 weeks after PMPA treatment. Similar increases in the MFI of CD11a expression were observed in the colon at 4 (∼255 to 369) and 12 (∼363 to 391) weeks after PMPA treatment. The MFI of CD11a expression on peripheral blood CD4+ T cells increased at 4 (∼156 to 242) and 12 (∼221 to 263) weeks after PMPA treatment compared to preinfection baseline values.
The majority of CD4+ T cells in the jejunum (∼94 to 95%), colon (∼86 to 92%), and peripheral blood (∼81 to 83%) expressed β7-integrin. No major change in the frequency or density (Fig. 1) of β7-integrin expression following PMPA therapy compared to preinfection baseline values was observed.
Local proliferation of CD4+ T cells in the intestinal mucosa may be a mechanism of CD4+ T-cell repopulation following PMPA therapy.
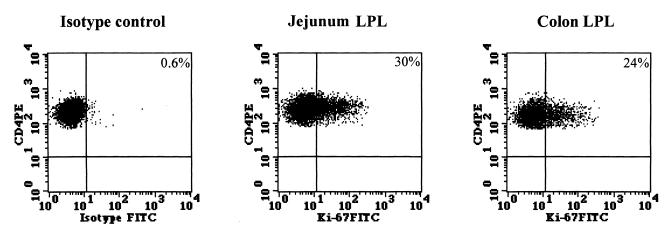
To determine whether the local proliferation of CD4+ T cells in the jejunum and the colon lamina propria may be a potential mechanism for the repopulation of CD4+ T cells in the mucosa, we determined (n = 2) the expression of Ki-67, a nuclear antigen expressed by proliferating cells. A representative dot plot is shown in Fig. 2. The proportions of CD4+ T cells expressing Ki-67 at 4 weeks after PMPA therapy were 22 and 30% in the jejunum lamina propria and 16 and 24% in the colon lamina propria.
FIG. 2.
Proliferation of intestinal CD4+ T cells following PMPA therapy. Cells isolated from jejunum and colon lamina propria after 4 weeks of PMPA therapy were stained for cell surface expression of CD4, fixed, permeabilized, and stained for the intracellular expression of Ki-67 antigen. Analysis gates were set to include CD4+ T cells only to determine the proportion of CD4+ Ki-67+ T cells. Negative control samples were stained with anti-CD4 antibody followed by intracellular labeling with matched isotype control antibody. PE, phycoerythrin; FITC, fluorescein isothiocyanate.
CD4+ T cells repopulating the gastrointestinal mucosa following PMPA therapy exhibit a reduced potential to produce IL-2.
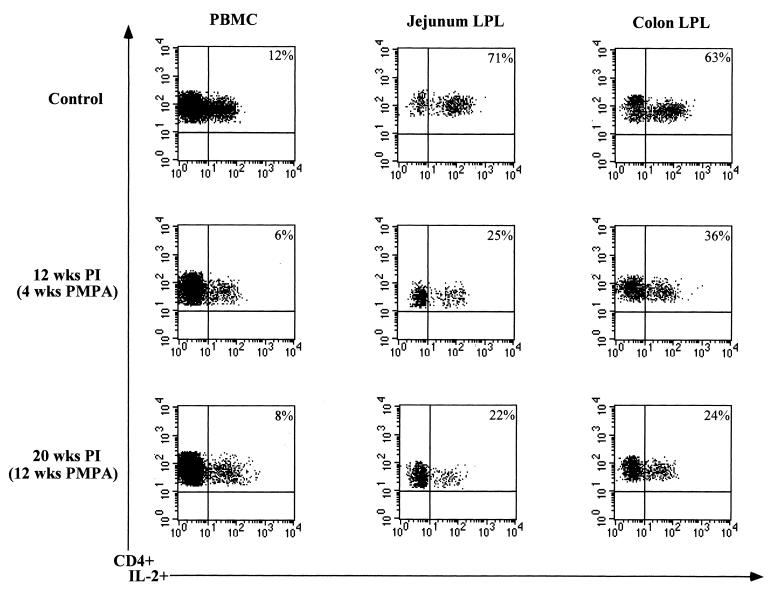
The potential of CD4+ T cells in the jejunum, colon, and peripheral blood (n = 2) to produce IL-2 was determined following PMPA treatment and compared with the preinfection baseline values (Fig. 3). Prior to SIV infection, the frequencies of CD4+ IL-2-producing T cells were 69 and 71% in the jejunum lamina propria and 63 and 76% in the colon lamina propria, whereas in peripheral blood the frequencies of IL-2-producing CD4+ T cells were 9 and 12%. Following PMPA treatment, the frequencies of CD4+ T cells capable of producing IL-2 decreased in the jejunum lamina propria to 22 and 33% at 4 weeks after PMPA treatment and to 22 and 28% at 12 weeks after PMPA treatment. Similarly, the frequencies of IL-2 producing CD4+ T cells in the colon decreased to 18 and 32% at 4 weeks after PMPA treatment and to 28 and 33% at 12 weeks after PMPA treatment. No major differences were observed in the capacity of peripheral blood CD4+ T cells to produce IL-2 following PMPA treatment compared to preinfection baseline values.
FIG. 3.
The CD4+ T cells repopulating the intestinal mucosa following PMPA therapy exhibited a decreased capacity to produce IL-2 compared to uninfected controls. The capacity of CD4+ T cells from jejunum and colon lamina propria and peripheral blood to produce IL-2 was determined following short-term in vitro stimulation with phorbol myristate acetate and ionomycin. Isolated cells were stained for cell surface expression of CD4, fixed, permeabilized, and stained for the intracellular production of IL-2. Analysis gates were set to include CD4+ T cells only to determine the capacity of CD4+ T cells to produce IL-2. Negative controls samples included cells stained with matched isotype control antibodies and cells stained with anti-CD4 antibody followed by intracellular labeling with matched isotype control antibody. PI, postinfection.
DISCUSSION
High viral RNA copy numbers were observed in the gastrointestinal mucosa during primary SIV infection (Table 1). Our previous studies (17, 29, 39) had shown the presence of cells strongly positive for SIV nucleic acids in intestinal tissue during primary SIV infection, indicating active viral replication. The higher viral burden in the intestinal mucosa was accompanied by almost complete depletion of CD4+ and CD4+ CD8+ T cells in jejunum and colon tissues at 4 weeks p.i., as previously reported (29, 39). In contrast, no major depletion of CD4+ T cells was observed in peripheral blood during primary SIV infection. After the start of PMPA treatment, a moderate level of suppression of SIV RNA copy numbers was observed in jejunum tissue, whereas the effects of PMPA treatment on colon tissue viral loads were variable, with two animals not showing a decline (Table 1). Similarly, the plasma viral loads remained variable after PMPA treatment. In one animal, the viral burden increased dramatically even after PMPA treatment. As this animal did not make any detectable level of antibodies against SIV, it may have been a rapid progressor. However, repopulation of CD4+ T cells was observed in both the jejunum and the colon lamina propria of all the animals, irrespective of the level of viral suppression (Table 1). Repopulation of CD4+ T cells in the intestinal mucosa independent of viral loads indicates that PMPA may have an immunomodulatory function in addition to its antiretroviral activity. Zidek et al. (49) reported that an R-enantiomer of PMPA increased the in vitro secretion of tumor necrosis factor alpha by murine peritoneal macrophages, indicating that PMPA may have an immunomodulatory role. Kotler et al. (23) reported that the frequency of CD4+ T cells repopulating the rectal mucosa increased after short-term antiretroviral therapy in HIV-infected patients, which did not directly correlate with changes in HIV loads. Interestingly, no repopulation of CD4+ CD8+ T cells was observed in both the jejunum and the colon lamina propria. It is difficult to determine at this point why PMPA treatment did not lead to the repopulation of this subset of T cells.
The phenotype of repopulating CD4+ T cells in the intestine was found to be similar to that of CD4+ T cells present in uninfected healthy intestinal tissue. The majority of CD4+ T cells repopulating the intestine were predominantly activated (CD69+) and were memory (CD45RA−) T-helper cells (Table 2). However, relative to uninfected control animals, the frequency of naive (CD45RA+) CD4+ T cells increased following PMPA therapy, whereas the frequency of activated CD4+ T cells decreased. About 16 to 30% of the CD4+ T cells repopulating the intestinal mucosa following 4 weeks of PMPA therapy were found to express Ki-67, a nuclear antigen expressed by proliferating cells (Fig. 2). These results suggested that the local proliferation of CD4+ T cells was occurring and may be one of the mechanisms for CD4+ T-cell repopulation in the intestine. The expansion of preexisting mature T cells has been suggested to be a mechanism for the peripheral regeneration of CD4+ T cells in HIV-infected patients following antiretroviral therapy (47). Interestingly, the repopulating CD4+ T cells were found to harbor higher percentages of naive (CD45RA+) CD4+ T cells relative to preinfection baseline values (Table 2). Although the proliferation of existing CD4+ T cells may account for the repopulation of activated memory CD4+ T cells in the intestinal mucosa, the presence of higher percentages of naive CD4+ T cells following PMPA therapy relative to preinfection baseline values suggested that other mechanisms were operative in the intestine. It was difficult to accurately determine the source of these naive CD4+ T cells in the intestinal mucosa, although both thymic and extrathymic sources could potentially contribute to this population. The role of trafficking in increasing the frequency of naive CD4+ T cells cannot be completely ruled out, since naive lymphocytes from the periphery could migrate preferentially through the high endothelial venules located in lymphoid organs such as Peyer’s patches. Previous studies have shown that the redistribution of T cells may be a mechanism of CD4+ T-cell repopulation in peripheral blood of HIV-infected patients undergoing antiretroviral therapy (36). Such a process may also occur in the intestinal mucosa. On the other hand, the increased frequency of naive CD4+ T cells in the intestinal mucosa could result from the local regeneration of CD4+ T cells. We have shown (28) that the gastrointestinal epithelium of rhesus macaques contains a subpopulation of CD34+ Thy-1+ and CD34+ c-Kit+ progenitor cells. The frequency of these progenitor cells increased during primary SIV infection. A potential role for these progenitor cells in the maintenance of tissue homeostasis was suggested. It is possible that these progenitor cells contribute to the repopulation of naive CD4+ T cells in the intestinal mucosa. The intestinal epithelium in mice was shown to be capable of supporting T-cell differentiation and maturation from stem cell progenitors (27, 33). Kanamori et al. (20) have identified tiny clusters of primitive cells in murine intestinal crypts, suggesting that crypts might be an extrathymic site for the development of T- and/or B-cell progenitors and could be the source of extrathymic T cells. Numerous studies have documented an increase in the frequency of naive CD4+ T cells in peripheral blood of HIV-infected patients following antiretroviral therapy (1, 21, 25, 36). However, information on the nature of the CD4+ T cells repopulating the gastrointestinal mucosa is limited.
The majority of CD4+ T cells repopulating the intestinal mucosa were found to express CD29 and CD11a at densities higher than those found in cells present in uninfected healthy mucosa, suggesting that repopulating CD4+ T cells might bind to their ligands at higher affinities (Fig. 2). This could play a role in redirecting CD4+ T cells to the intestinal mucosa. It is possible that a chronic viral infection contributes to increased expression of these adhesion proteins. VLA-4 is a heterodimer that is formed by CD29 and CD49 and binds to vascular cell adhesion molecule 1 (VCAM-1), which is expressed on activated endothelial cells. Similarly, LFA-1 is a heterodimer that is formed by CD11a and CD18, that is expressed by most lymphocytes, and that binds to the intracellular cell adhesion molecules (ICAM) expressed on endothelial cells. The expression of VCAM-1 and ICAM has been found to be increased in the intestinal mucosa of SIV-infected rhesus macaques (41). VLA-4 (38) and LFA-1 (2) have been shown to be expressed by intestinal LPL, suggesting that VLA-4–VCAM-1 and LFA-1–ICAM interactions may play an important role in the migration of lymphocytes to the intestinal mucosa. Higher levels of activated CD4+ HLA-DR+ T cells have been detected in lymph nodes of HIV-infected patients receiving antiretroviral therapy (25). Thus, the potential role of T-cell trafficking in the repopulation process cannot be completely ruled out.
Since the intestinal immune system is severely compromised following SIV infection, it is essential to determine whether antiretroviral treatment would lead to the repopulation of the intestinal mucosa with CD4+ T cells capable of generating functional immune responses. Our results demonstrated that most of the CD4+ T cells repopulating the intestinal mucosa were capable of providing T- and B-cell help, as evidenced by their expression of CD28. CD28 is a costimulatory molecule that is expressed on T cells and plays an important role in T-cell activation. Brinchmann et al. (4) showed that defects in the proliferative responses of peripheral blood CD4+ T cells in HIV-infected patients were due to an increased frequency of CD4+ CD28− subsets. Increased proliferative responses to both recall and HIV antigens were observed in HIV-infected patients undergoing antiretroviral therapy. These responses were shown to be due to increased proportions of CD4+ CD28+ T cells (21). Although the CD4+ T-helper cells repopulating the intestinal mucosa were activated and capable of generating functional immune responses, their potential to produce IL-2 was significantly suppressed relative to preinfection control values (Fig. 3). The suppression of IL-2 production in peripheral blood CD4+ T cells of HIV-infected patients has been demonstrated elsewhere (8, 13, 22). As the level of viral infection in the intestinal mucosa was not completely suppressed even after 12 weeks of PMPA treatment, the low level of persistent viral infection may play a role in suppressing the ability of repopulating CD4+ T cells to produce IL-2. These results suggested that, in contrast to peripheral blood, the functional potential of CD4+ T cells repopulating the intestinal mucosa was not fully restored following antiretroviral therapy.
In conclusion, antiretroviral therapy with PMPA was found to have a significant effect on the repopulation of CD4+ T cells in the intestinal mucosa of SIV-infected rhesus macaques. These CD4+ T cells were found to exhibit an activated memory phenotype, like that found in normal intestinal mucosa. However, unlike those in normal intestinal mucosa, the repopulating CD4+ T cells in infected intestinal mucosa expressed CD29 and CD11a at higher densities. A subset of CD4+ T cells was found to express Ki-67, suggesting that local proliferation may play a role in the repopulation process. The presence of a higher frequency of naive CD4+ T cells in the intestinal mucosa following treatment led us to conclude that new CD4+ T cells were repopulating the intestinal mucosa following PMPA therapy. Although the frequency of activated and functionally capable CD4+ T cells increased in the intestinal mucosa, their potential to produce IL-2 was significantly suppressed, suggesting that chronic viral infection may play a role in this process. These results indicate that in addition to examination of changes in peripheral tissues, the characterization of repopulating CD4+ T cells in the intestinal mucosa will be critical for gaining insights into the efficacy of antiretroviral therapy-associated immune reconstitution and viral suppression.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (R01-DK43183, R01-AI43274, and RR-00169) and the Universitywide AIDS Research Program, University of California (F98-D-097).
We thank Linda Hirst, Ross Tarara, and Don Canfield at the California Regional Primate Research Center for valuable assistance in this project.
REFERENCES
- 1.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 2.Benmerach A N P, Cerf-Bensussan N. Adhesion molecules on mucosal T lymphocytes. In: Kagnoff M, Kiyono H, editors. Essentials of mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1996. pp. 263–274. [Google Scholar]
- 3.Biglino A, Pugliese A, Forno B, Pollono A M, Busso M, Gioannini P. Effects of long-term zidovudine treatment on cell-mediated immune response and lymphokine production. J Acquired Immune Defic Syndr. 1991;4:261–266. [PubMed] [Google Scholar]
- 4.Brinchmann J E, Dobloug J H, Heger B H, Haaheim L L, Sannes M, Egeland T. Expression of costimulatory molecule CD28 on T cells in human immunodeficiency virus type 1 infection: functional and clinical correlations. J Infect Dis. 1994;169:730–738. doi: 10.1093/infdis/169.4.730. [DOI] [PubMed] [Google Scholar]
- 5.Clerici M, Landay A L, Kessler H A, Phair J P, Venzon D J, Hendrix C W, Lucey D R, Shearer G M. Reconstitution of long-term T helper cell function after zidovudine therapy in human immunodeficiency virus-infected patients. J Infect Dis. 1992;166:723–730. doi: 10.1093/infdis/166.4.723. [DOI] [PubMed] [Google Scholar]
- 6.Clerici M, Roilides E, Butler K M, DePalma L, Venzon D, Shearer G M, Pizzo P A. Changes in T-helper cell function in human immunodeficiency virus-infected children during didanosine therapy as a measure of antiretroviral activity. Blood. 1992;80:2196–2202. [PubMed] [Google Scholar]
- 7.Clerici M, Stocks N I, Zajac R A, Boswell R N, Lucey D R, Via C S, Shearer G M. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Investig. 1989;84:1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerici M, Via C S, Lucey D R, Roilides E, Pizzo P A, Shearer G M. Functional dichotomy of CD4+ T helper lymphocytes in asymptomatic human immunodeficiency virus infection. Eur J Immunol. 1991;21:665–670. doi: 10.1002/eji.1830210319. [DOI] [PubMed] [Google Scholar]
- 9.Dadaglio G, Michel F, Langlade-Demoyen P, Sansonetti P, Chevrier D, Vuillier F, Plata F, Hoffenbach A. Enhancement of HIV-specific cytotoxic T lymphocyte responses by zidovudine (AZT) treatment. Clin Exp Immunol. 1992;87:7–14. doi: 10.1111/j.1365-2249.1992.tb06405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolan M J, Clerici M, Blatt S P, Hendrix C W, Melcher G P, Boswell R N, Freeman T M, Ward W, Hensley R, Shearer G M. In vitro T cell function, delayed-type hypersensitivity skin testing, and CD4+ T cell subset phenotyping independently predict survival time in patients infected with human immunodeficiency virus. J Infect Dis. 1995;172:79–87. doi: 10.1093/infdis/172.1.79. [DOI] [PubMed] [Google Scholar]
- 11.Ferrick D A, Schrenzel M D, Mulvania T, Hsieh B, Ferlin W G, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 12.French M A, Mallal S A, Dawkins R L. Zidovudine-induced restoration of cell-mediated immunity to mycobacteria in immunodeficient HIV-infected patients. AIDS. 1992;6:1293–1297. doi: 10.1097/00002030-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Gruters R A, Terpstra F G, De Jong R, Van Noesel C J, Van Lier R A, Miedema F. Selective loss of T cell functions in different stages of HIV infection. Early loss of anti-CD3-induced T cell proliferation followed by decreased anti-CD3-induced cytotoxic T lymphocyte generation in AIDS-related complex and AIDS. Eur J Immunol. 1990;20:1039–1044. doi: 10.1002/eji.1830200514. [DOI] [PubMed] [Google Scholar]
- 14.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, Emini E A, Chodakewitz J A. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 15.Hammer S M, Squires K E, Hughes M D, Grimes J M, Demeter L M, Currier J S, Eron J J, Jr, Feinberg J E, Balfour H H, Jr, Deyton L R, Chodakewitz J A, Fischl M A. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 16.Harris M, Patenaude P, Cooperberg P, Filipenko D, Thorne A, Raboud J, Rae S, Dailey P, Chernoff D, Todd J, Conway B, Montaner J S. Correlation of virus load in plasma and lymph node tissue in human immunodeficiency virus infection. INCAS Study Group. J Infect Dis. 1997;176:1388–1392. doi: 10.1086/517328. [DOI] [PubMed] [Google Scholar]
- 17.Heise C, Miller C J, Lackner A, Dandekar S. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis. 1994;169:1116–1120. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- 18.Heise C, Vogel P, Miller C J, Lackner A, Dandekar S. Distribution of SIV infection in the gastrointestinal tract of rhesus macaques at early and terminal stages of AIDS. J Med Primatol. 1993;22:187–193. [PubMed] [Google Scholar]
- 19.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 20.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelleher A D, Carr A, Zaunders J, Cooper D A. Alterations in the immune response of human immunodeficiency virus (HIV)-infected subjects treated with an HIV-specific protease inhibitor, ritonavir. J Infect Dis. 1996;173:321–329. doi: 10.1093/infdis/173.2.321. [DOI] [PubMed] [Google Scholar]
- 22.Klein S A, Dobmeyer J M, Dobmeyer T S, Pape M, Ottmann O G, Helm E B, Hoelzer D, Rossol R. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS. 1997;11:1111–1118. doi: 10.1097/00002030-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Kotler D P, Shimada T, Snow G, Winson G, Chen W, Zhao M, Inada Y, Clayton F. Effect of combination antiretroviral therapy upon rectal mucosal HIV RNA burden and mononuclear cell apoptosis. AIDS. 1998;12:597–604. doi: 10.1097/00002030-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Krowka J F, Stites D P, Jain S, Steimer K S, George-Nascimento C, Gyenes A, Barr P J, Hollander H, Moss A R, Homsy J M, et al. Lymphocyte proliferative responses to human immunodeficiency virus antigens in vitro. J Clin Investig. 1989;83:1198–1203. doi: 10.1172/JCI114001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landay A L, Bethel J, Schnittman S. Phenotypic variability of lymphocyte populations in peripheral blood and lymph nodes from HIV-infected individuals and the impact of antiretroviral therapy. DATRI 003 Study Group. Division of AIDS Treatment Research Initiative. AIDS Res Hum Retroviruses. 1998;14:445–451. doi: 10.1089/aid.1998.14.445. [DOI] [PubMed] [Google Scholar]
- 26.Lane H C, Masur H, Gelmann E P, Longo D L, Steis R G, Chused T, Whalen G, Edgar L C, Fauci A S. Correlation between immunologic function and clinical subpopulations of patients with the acquired immune deficiency syndrome. Am J Med. 1985;78:417–422. doi: 10.1016/0002-9343(85)90332-8. [DOI] [PubMed] [Google Scholar]
- 27.Maric D, Kaiserlian D, Croitoru K. Intestinal epithelial cell line induction of T cell differentiation from bone marrow precursors. Cell Immunol. 1996;172:172–179. doi: 10.1006/cimm.1996.0230. [DOI] [PubMed] [Google Scholar]
- 28.Mattapallil J J, Smit-McBride Z, Dandekar S. Gastrointestinal epithelium is an early extrathymic site for increased prevalence of CD34+ progenitor cells in contrast to thymus during primary simian immunodeficiency virus infection. J Virol. 1999;73:4518–4523. doi: 10.1128/jvi.73.5.4518-4523.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattapallil J J, Smit-McBride Z, McChesney M, Dandekar S. Intestinal intraepithelial lymphocytes are primed for gamma interferon and MIP-1β expression and display antiviral cytotoxic activity despite severe CD4+ T-cell depletion in primary simian immunodeficiency virus infection. J Virol. 1998;72:6421–6429. doi: 10.1128/jvi.72.8.6421-6429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClure H M, Anderson D C, Ansari A A, Fultz P N, Klumpp S A, Schinazi R F. Nonhuman primate models for evaluation of AIDS therapy. Ann N Y Acad Sci. 1990;616:287–298. doi: 10.1111/j.1749-6632.1990.tb17849.x. [DOI] [PubMed] [Google Scholar]
- 31.Miedema F, Petit A J, Terpstra F G, Schattenkerk J K, de Wolf F, Al B J, Roos M, Lange J M, Danner S A, Goudsmit J, et al. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Investig. 1988;82:1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosier D E. HIV results in the frame. CD4+ cell turnover. Nature. 1995;375:193–194. doi: 10.1038/375193b0. [DOI] [PubMed] [Google Scholar]
- 33.Mosley R L, Klein J R. Peripheral engraftment of fetal intestine into athymic mice sponsors T cell development: direct evidence for thymopoietic function of murine small intestine. J Exp Med. 1992;176:1365–1373. doi: 10.1084/jem.176.5.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nye K E, Knox K A, Pinching A J. Lymphocytes from HIV-infected individuals show aberrant inositol polyphosphate metabolism which reverses after zidovudine therapy. AIDS. 1991;5:413–417. doi: 10.1097/00002030-199104000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Pachl C, Todd J A, Kern D G, Sheridan P J, Fong S J, Stempien M, Hoo B, Besemer D, Yeghiazarian T, Irvine B, et al. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. J Acquired Immune Defic Syndr Hum Retroviral. 1995;8:446–454. doi: 10.1097/00042560-199504120-00003. [DOI] [PubMed] [Google Scholar]
- 36.Pakker N G, Notermans D W, de Boer R J, Roos M T, de Wolf F, Hill A, Leonard J M, Danner S A, Miedema F, Schellekens P T. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 37.Rinaldo C, Huang X L, Piazza P, Armstrong J, Rappocciolo G, Pazin G, McMahon D, Gupta P, Fan Z, Zhang Z, et al. Augmentation of cellular immune function during the early phase of zidovudine treatment of AIDS patients. J Infect Dis. 1991;164:638–645. doi: 10.1093/infdis/164.4.638. [DOI] [PubMed] [Google Scholar]
- 38.Salmi M, Andrew D P, Butcher E C, Jalkanen S. Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J Exp Med. 1995;181:137–149. doi: 10.1084/jem.181.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smit-McBride Z, Mattapallil J J, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J Virol. 1998;72:6646–6656. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprent J, Tough D. HIV results in the frame. CD4+ cell turnover. Nature. 1995;375:194. doi: 10.1038/375194a0. [DOI] [PubMed] [Google Scholar]
- 41.Stone J D, Heise C C, Canfield D R, Elices M J, Dandekar S. Differences in viral distribution and cell adhesion molecule expression in the intestinal tract of rhesus macaques infected with pathogenic and nonpathogenic SIV. J Med Primatol. 1995;24:132–140. doi: 10.1111/j.1600-0684.1995.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 42.Stone J D, Heise C C, Miller C J, Halsted C H, Dandekar S. Development of malabsorption and nutritional complications in simian immunodeficiency virus-infected rhesus macaques. AIDS. 1994;8:1245–1256. doi: 10.1097/00002030-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Tsai C C, Follis K E, Beck T W, Sabo A, Bischofberger N, Dailey P J. Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy on chronic SIV infection in macaques. AIDS Res Hum Retroviruses. 1997;13:707–712. doi: 10.1089/aid.1997.13.707. [DOI] [PubMed] [Google Scholar]
- 44.Tsai C C, Follis K E, Sabo A, Beck T W, Grant R F, Bischofberger N, Benveniste R E, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 45.Van Rompay K K, Cherrington J M, Marthas M L, Berardi C J, Mulato A S, Spinner A, Tarara R P, Canfield D R, Telm S, Bischofberger N, Pedersen N C. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob Agents Chemother. 1996;40:2586–2591. doi: 10.1128/aac.40.11.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veazey R S, DeMaria M, Chalifoux L V, Shvetz D E, Pauley D R, Knight H L, Rosenzweig M, Johnson P R, Desrosiers C R, Lackner A A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 47.Walker R E, Carter C S, Muul L, Natrajan V, Herpin B H, Leitman S F, Klein H G, Mullen C A, Metcalf J A, Baseler M, Falloon J, Davey R T, Jr, Kovacs J A, Polis M A, Masur H, Blaese R M, Lane H C. Peripheral expansion of preexisting mature T cells is an important means of CD4+ T cell regeneration in HIV infected patients. Nat Med. 1998;4:852–856. doi: 10.1038/nm0798-852. [DOI] [PubMed] [Google Scholar]
- 48.Yarchoan R, Mitsuya H, Thomas R V, Pluda J M, Hartman N R, Perno C F, Marczyk K S, Allain J P, Johns D G, Broder S. In vivo activity against HIV and favorable toxicity profile of 2′,3′-dideoxyinosine. Science. 1989;245:412–415. doi: 10.1126/science.2502840. [DOI] [PubMed] [Google Scholar]
- 49.Zidek Z, Holy A, Frankova D. Antiretroviral agent (R)-9-(2-phosphonomethoxypropyl)adenine stimulates cytokine and nitric oxide production. Eur J Pharmacol. 1997;331:245–252. doi: 10.1016/s0014-2999(97)01004-2. [DOI] [PubMed] [Google Scholar]