Abstract
Mesenchymal stem/stromal cells (MSCs) are one of the most widely used cell types in advanced therapies due to their therapeutic potential in the regulation of tissue repair and homeostasis, and immune modulation. However, their use in cancer therapy is controversial: they can inhibit cancer cell proliferation, but also potentially promote tumour growth by supporting angiogenesis, modulation of the immune milieu and increasing cancer stem cell invasiveness. This opposite behaviour highlights the need for careful and nuanced use of MSCs in cancer treatment. To optimize their anti-cancer effects, diverse strategies have bioengineered MSCs to enhance their tumour targeting and therapeutic properties or to deliver anti-cancer drugs. In this review, we highlight the advanced uses of MSCs in cancer therapy, particularly as carriers of targeted treatments due to their natural tumour-homing capabilities. We also discuss the potential of MSC-derived extracellular vesicles to improve the efficiency of drug or molecule delivery to cancer cells. Ongoing clinical trials are evaluating the therapeutic potential of these cells and setting the stage for future advances in MSC-based cancer treatment. It is critical to identify the broad and potent applications of bioengineered MSCs in solid tumour targeting and anti-cancer agent delivery to position them as effective therapeutics in the evolving field of cancer therapy.
Keywords: mesenchymal stem/stromal cells, cell therapy, anti-cancer therapy, targeted therapy, therapeutic vehicles
1. Introduction
Mesenchymal stem/stromal cells (MSCs) are a multipotent adult stem cell heterogeneous population with the ability to both self-renew and differentiate into cells particularly belonging to mesoderm-derived tissues such as bone, adipose tissue and cartilage [1,2]. These cells were first discovered in bone marrow by Friedenstein et al. and were initially called colony forming units–fibroblasts (CFU-Fb) [3], but later they were found in many other adult and extraembryonic tissues including adipose tissue, umbilical cord, dental pulp and amniotic membrane [4,5,6].
The therapeutic potential of MSCs is enormous, particularly because of their ability to modulate immune responses, regulate tissue homeostasis through the secretion of paracrine trophic factors with pleiotropic effects, and maintain tissue health and structure [4,5,7,8,9]. These properties position MSCs as a cornerstone in the development of repairing cell therapies with ongoing clinical trials exploring their potential for the treatment of acute/chronic inflammatory and/or degenerative diseases, such as osteoporosis, osteoarthritis, graft-versus-host disease, systemic sclerosis and myocardial infarction [10,11,12].
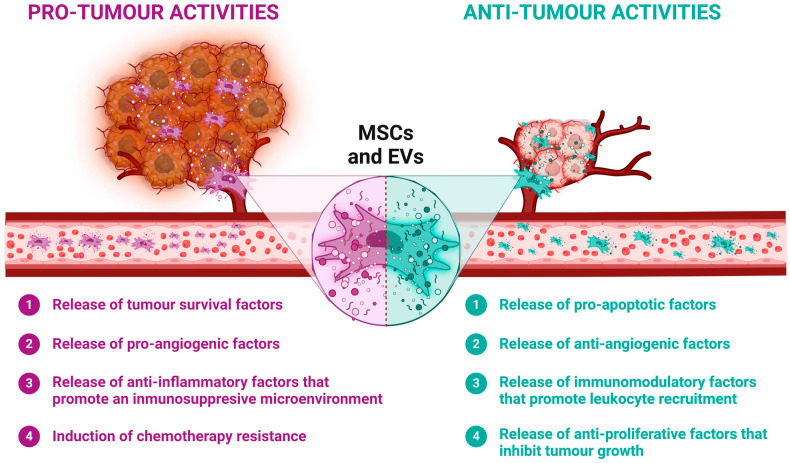
However, the role of MSCs in cancer therapy presents a complex picture. While MSCs can home to tumour sites after their systemic administration, their impact on disease progression can be double-edged. On the one hand, they have been reported to directly fight some types of cancer by suppressing cancer cell proliferation through reducing the expression of positive regulators of the cell cycle [13,14,15,16]. On the other hand, it has also been found that MSCs may contribute to tumour growth by fostering a supporting tumour vascular network which modulates the peritumour immune environment, protecting it from immune attack, and promoting the proliferation and invasiveness of cancer stem cells [17,18,19,20,21] (Figure 1). This paradoxical behaviour of MSCs in tumours highlights the need for cautious and nuanced application in anti-cancer therapeutic approaches [22,23].
Figure 1.
Dual roles of MSCs in tumour dynamics. MSCs possess unique properties that make them promising therapeutic agents. However, these same properties can also influence tumour development. MSCs can release a variety of factors that have both pro- and anti-tumour effects, influencing cellular tumour processes such as survival, proliferation, angiogenesis and chemotherapy resistance. These paracrine factors can be released directly into the tumour microenvironment or transported via extracellular vesicles (EVs). Created using BioRender.
Therefore, the use of MSCs, particularly in cancer therapy, requires a delicate balance. Notwithstanding, several strategies are currently being developed to use MSCs as anti-cancer cell therapeutics. These include bioengineering MSCs to enhance their tumour homing and anti-tumour properties, or to carry as a “Trojan horse” some therapeutic agents that target and eliminate cancer cells more efficiently [24,25,26,27,28]. The development of such strategies requires a deep understanding of the interactions between MSCs, tumour microenvironment and immune system and calls for an interdisciplinary approach combining insights from cell biology, oncology and cell bioengineering to overcome current challenges and fully exploit the therapeutic potential of MSCs in cancer treatment.
2. Cutting-Edge Applications of Mesenchymal Stem/Stromal Cells in Cancer Therapy
2.1. Enhancing MSC Homing to Tumour Sites
Over the last few decades, the ability of MSCs to migrate into the tumour microenvironment has been extensively studied to analyse the effect they have on tumour biology. In preclinical models, systemically administered MSCs have been shown to be able to migrate and infiltrate inflamed or tumour tissues. At these tissues, local elements such as hypoxia, cytokines and chemokines stimulate the MSC infiltration and secretion of a variety of growth factors, thereby accelerating tissue repair [29]. In addition, tumours are able to recruit MSCs from distant tissues, such as adipose tissue or bone marrow, and direct their homing into the tumour microenvironment via inflammatory signals [30]. Several studies have already shown that MSCs are particularly recruited to different tumour types, such as breast cancer, liver cancer and glioma [31,32,33].
The tumour environment is rich in immune cells that, together with cancer cells, release a variety of soluble factors that influence the homing and infiltration of MSCs at sites of injury. Specific cytokines such as IL-6 attract MSCs to tumours, while other studies have reported similar recruitment driven by IL-8 in gliomas [34,35]. In addition, several growth factors have been found to promote MSC migration, including platelet-derived growth factor subunit B (PDGFB), vascular endothelial growth factor (VEGF) and transforming growth factor beta-1 (TGF-β1) [36]. The chemokine receptors CXCR4, CXCR6, CCR1, CCR7 and CCR9 also play an important role in the homing of MSCs to tumours [37]. Once MSCs reach the tumour, they contribute to its stroma by differentiating into fibrovascular cells such as endothelial cells, pericytes and possibly tumour-associated fibroblasts involved in the extracellular matrix remodelling [38]. However, further investigation into the molecular mechanisms that mediate specific migration to tumours may help to improve the efficacy of MSC-based therapeutics.
Given their ability to target and integrate into malignant tissues, coupled with their immune-evasive properties, MSCs are considered an ideal approach for the delivery of anti-cancer therapeutics, potentially improving their efficacy compared to standard anti-cancer treatments [39,40].
Unfortunately, the homing efficiency of systemically administered MSCs is very low, with only a small fraction of these cells reaching their target tissues due to their entrapment in the lungs, liver and spleen vasculature, as demonstrated in several studies [41,42,43], limiting their expected therapeutic efficacy. To overcome this hurdle, the use of a variety of cell bioengineering strategies to improve MSC homing to inflamed and tumour tissues is being widely investigated. Several methodological approaches have been explored to alter the expression of different homing molecules on migrating MSCs, including priming with bioactive molecules, genetic engineering, enzymatic modifications or ligand conjugation techniques [44].
Exposure of MSCs to the pro-inflammatory cytokine TNF-α has been shown to upregulate CXCR4 expression, which may enhance the ability of MSCs to home to specific tissues, including tumours [45,46]. Additionally, priming MSCs with the cytokine TGF-β has been shown to increase their CXCR4-driven homing to glioblastoma, while MSCs stimulated with IL-1β showed an upregulated CXCR4 expression, increased production of metalloproteinases and enhanced migration [47,48]. Furthermore, pre-treating MSCs with valproic acid, erythropoietin, the iron chelator deferoxamine and granulocyte colony-stimulating factor (G-CSF) has been shown to improve their homing to inflamed tissues [49,50,51]. On the other hand, the peritumoural microenvironment that has a persistent inflammatory state, secreting various pro-inflammatory chemokines and cytokines (e.g., MCP-1, TGF-β, CXCL12, TNF-α and various interleukins), could prime MSCs enhancing their migration to tumours.
Another strategy to enhance MSC homing is the genetic modification to achieve permanent overexpression of key homing factors by viral transduction or alternatively transient overexpression by mRNA transfection. Zheng et al. introduced the CXCR4 gene into mouse bone marrow-derived MSCs by lentiviral transduction. Subsequently, mice with colitis-associated tumorigenesis that were injected with these CXCR4-overexpressing MSCs displayed a reduced tumour burden compared to mice treated with unmodified counterparts [52]. Overexpression of other chemokine receptors such as CXCR7 or the α4 chain of VLA-4 integrin, has demonstrated an improved MSC homing to inflamed/injured tissues [53,54]. Levy et al. aimed to enhance the ability of MSCs to tether and roll over endothelium by transfecting these cells with mRNA for PSGL-1 and sialyl Lewis X (sLeX), which are ligands for P- and E-/L-selectin, respectively [55], while Hervás-Salcedo et al. showed that the transient expression of CXCR4 by mRNA transfection improved MSC migration to inflamed tissues [56].
Enzymatic modification to transiently improve MSC homing has also been reported. Treatment of MSCs with an α(1,3)-fucosyltransferase VI or VII in the presence of GDP-fucose has been shown to convert CD44 into the E-selectin ligand HCELL glycovariant on the MSC surface. This modification by exofucosylation significantly enhances rolling contacts on E-selectin-expressing endothelial surfaces in the bone marrow microvasculature and inflamed tissues [57,58]. Notably, other studies have shown that exofucosylated MSCs exhibit enhanced migration towards specific pro-inflammatory chemokines such as CCL5, CCL20 and CXCL16 [59]. In addition, cell surface engineering techniques allow the direct conjugation of desired ligands, rather than modifying existing surface glycoproteins. For example, attaching sLeX to MSC surfaces via a biotin–streptavidin link, coupling E-selectin-targeting peptides to the MSC membrane, or conjugating recombinant CXCR4 to the phospholipid DMPE-PEG enhanced MSC rolling on P- and E-selectin-coated surfaces and migration to inflamed vascular endothelium in vivo [60,61,62,63].
2.2. MSC-Derived Extracellular Vesicles
Extracellular vesicles (EVs) are membrane-derived nanostructures, including microvesicles, exosomes and apoptotic bodies (0.1–2 nm, 40–100 nm, and >1 μm in diameter, respectively) released by cells, including MSCs, under physiological and pathological conditions. Their internal contents depend on the cell of origin and may include proteins, enzymes, growth factors, carbohydrates, lipids and nucleic acids such as double-stranded DNA, mRNAs, long non-coding RNAs or microRNAs [64]. EVs have been shown to be involved in intercellular communication between MSCs and target cells, regulating the immune response and tissue repair [65]. Thus, MSC-derived EVs are considered a promising therapeutic alternative because they recapitulate the biological properties of MSCs themselves, reduce undesirable side effects such as toxicities due to MSC infusion and have potential for use in gene delivery, regenerative medicine and immunomodulation [66].
In the context of cancer, it has been suggested that MSC-derived EVs may facilitate the delivery of their cargo to tumour cells. However, like the MSCs from which they are derived, they can either suppress or promote tumour growth through different mechanisms including modulation of tumour angiogenesis, inhibition of cell proliferation, promotion of apoptosis, and facilitation of tumour growth and metastasis [67,68,69]. MSCs may influence tumour angiogenesis through mechanisms involving increased VEGF secretion, which activates the ERK1/2 pathway [68], or via the transfer of oncogenic miRNAs that affect various processes in tumour cells, such as inhibition of PTEN, inhibition of apoptosis or induction of macrophage type M2 polarization [70,71]. MSC-derived EVs have also been implicated in inducing chemoresistance, particularly in breast and gastric cancers, by transferring miRNAs that modulate distinct cellular signalling pathways and promote a chemotherapy-resisting dormant state [72,73].
On the other hand, some studies have shown that MSC-EVs may contain a diverse array of miRNAs, including but not limited to miR-31, miR-223, miR-205 and miR-21, all of which play critical roles in regulating tumour dormancy, a property of tumours to persist as a small number of undetectable cells following the surgical removal of the primary tumour [72,74]. For example, Wu and colleagues demonstrated the efficacy of EVs derived from human Wharton’s jelly-derived MSCs in halting bladder tumour cell proliferation through inducing G0/G1 phase arrest in a dose-dependent manner [75]. Subsequently, a recent in vitro study unveiled that EVs from bone marrow MSCs can reduce the proliferation, migration and metastatic invasion of osteosarcoma cells by delivery of miR-206, a well-known tumour suppressor [76]. Another study demonstrated the capability of MSC-derived EVs containing miRNA-100 to significantly suppress angiogenesis by modulating the mTOR/HIF-1α/VEGF signalling pathway in breast cancer-derived cells [77], while MSCs carrying miR-23b have been shown to not only reduce the growth and invasion of breast metastatic cancer cells, but also to reduce their sensitivity to the chemotherapy drug docetaxel [74]. Thus, MSC-derived EVs, including EVs derived from modified MSCs, are increasingly being explored for their potential in cancer therapy. These bioengineered EVs can be tailored to enhance their therapeutic efficacy, targeting capabilities and to carry specific therapeutic agents.
EVs can be engineered to carry chemotherapeutic agents and deliver them directly to the tumour site, potentially increasing the efficacy of treatments while reducing systemic toxicity. This targeted approach helps to manage and treat cancer more effectively by ensuring that the drugs are delivered specifically to the cancer cells, minimising the impact on healthy tissue. There are different methods for transferring the desired cargo inside the EVs. Pre-loading methods involve modifying parental cells to package therapeutic cargoes into EVs during their biogenesis. This can be achieved through genetic manipulation, leading to the overexpression of therapeutic molecules, or by incubating drugs with parental cells to produce drug-containing EVs [78]. While pre-loading ensures stable and intact EV membranes, it is time-consuming and has low efficiency. On the other hand, post-loading methods are performed after EV isolation, and the cargoes are encapsulated either passively or actively. Passive loading involves hydrophobic drugs attaching to the EV membrane, while active loading involves physically or chemically permeabilizing the EV membrane to incorporate hydrophilic drugs. Techniques include electroporation, sonication, freeze/thaw cycles and the use of chemical permeabilizers [79,80]. Each strategy has pros and cons, with careful consideration needed to prevent EV membrane damage or EV aggregation [81,82].
Using these manipulation strategies, it is also possible to load MSC-derived EVs with cytotoxic chemotherapeutic agents such as doxorubicin, paclitaxel or gemcitabine. Such EVs have been shown to inhibit cancer cell growth, induce apoptosis and suppress epithelial–mesenchymal transition in oral squamous cell carcinoma and cervical cancer [83,84]. Other approaches include vesicle loading with specific miRNAs, siRNAs, mRNAs, ncRNAs, proteins and peptides that have previously shown anti-tumour activity [69,85,86]. Some studies have reported the use of MSC-EVs to counteract chemoresistance in glioblastoma multiforme cells by delivering anti-miR-9, with promising results in reversing the chemoresistance of this tumour [87]. In addition, MSC-EVs loaded with miR-146b, miR124, miR-145 or miR-122 showed anti-tumour activity in malignant glioma and hepatocellular tumours, respectively [88,89,90]. MSC-EVs containing miR-124a and miR-15a have been shown to reduce cancer stem cell viability and growth in a glioma and multiple myeloma model, respectively [91,92]. Another innovative approach involves engineering MSCs to produce TRAIL-expressing EVs, molecules known to induce cancer cell apoptosis [93].
Despite their promising properties such as stability, target specificity and non-toxicity, challenges remain in the clinical application of MSC-derived EVs. Issues such as the need for large-scale production, standardized manufacturing methods and stringent clinical regulations are critical to maintaining the consistency and functionality of these biological carriers. Current research supports the notion that these EVs can naturally home to cancer sites, enhancing their potential as drug delivery systems. Future developments could include surface modifications to enhance delivery, such as enabling EVs to cross biological barriers, such as the brain–blood barrier, more efficiently and accumulate at tumour sites, thereby increasing the therapeutic impact of their cargo [94]. However, rigorous dosage and efficacy studies are essential to advance the clinical application of EVs and ensure their safe and effective use in cancer therapy.
2.3. MSCs as Therapeutic Cell Vehicles
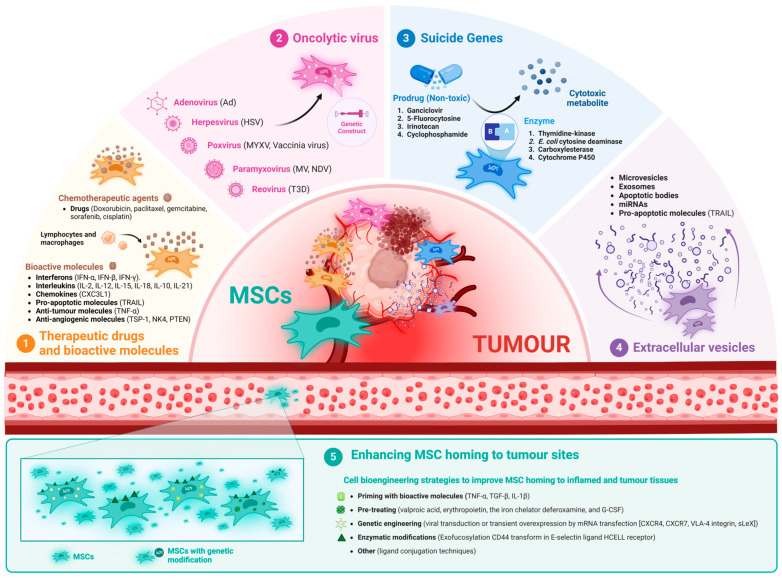
There is considerable interest in using MSCs as carriers for tumour-targeted therapies because of their unique properties, particularly their natural affinity for homing tumours and to infiltrate the tumour environment [95,96,97,98]. Thus, MSCs have shown great promise in delivering drugs and genes in various tumours minimizing side effects of chemotherapeutic drugs and improving clinical outcomes. Therefore, the use of MSCs to directly deliver therapeutic agents to tumours has become a major focus of research. The therapeutic potential of MSCs originates from paracrine factors involving peptides, proteins and hormones, and the transfer of MSC-derived extracellular vesicles (EVs) containing different molecules inside. Remarkably, bioengineering strategies can prepare MSCs for the targeted delivery to tumours of various factors, focusing on a variety of biological approaches. For example, it has been shown that MSCs can be loaded with and subsequently release anti-cancer drugs [99,100,101,102]. However, the ability of MSCs to release a particular drug depends on both the MSC biology and the properties of these therapeutic compounds. Anti-cancer drugs loaded into MSCs have been found in various cellular components, affecting not only tumour cells but also gene expression and cellular functions of the MSCs themselves [101]. In addition, MSCs can be bioengineered to enhance their tumour-killing properties by preloading with suicide genes, oncolytic viruses, cytokines, anti-mitotic or anti-angiogenic factors, among others [98,103,104,105]. Accordingly, a first issue to develop is the analysis of the advanced techniques for bioengineering both MSCs and MSC-derived EVs to promote anti-tumour effects, which hold promise for the development of tumour-targeted therapies (Figure 2).
Figure 2.
Different strategies to enhance the anti-tumour properties of both MSCs and MSC-derived EVs. (1) MSCs can be used to deliver chemotherapeutic drugs or bioactive molecules (e.g., interferons, interleukins, chemokines, and pro-apoptotic, anti-tumour or anti-angiogenic molecules) directly into the tumour microenvironment. (2) MSCs are effective oncolytic virus carriers because they can be easily infected, allowing viral replication and sustained viability until they reach the tumour microenvironment. (3) MSCs can be genetically engineered to carry suicide genes that encode specific enzymes that convert non-toxic prodrugs into cytotoxic metabolites directly in the tumour cells, increasing the specificity and efficacy of therapy while reducing systemic toxicity. (4) Bioengineered MSC-derived EVs can be customized to improve their therapeutic efficacy and capacity to deliver specific anti-cancer agents. (5) Various bioengineering strategies are being investigated to improve MSC homing to tumours, including priming with bioactive molecules, genetic engineering, enzymatic modification and ligand conjugation techniques. Created using BioRender.
2.3.1. Therapeutic Drugs and Bioactive Molecules
MSCs, with their inherent ability to take up a wide range of products including anti-cancer drugs from the culture medium, have emerged as a key tool in cancer therapy. Research has shown that human bone marrow-derived MSCs (hBM-MSCs) can be effectively primed with several chemotherapeutic agents, such as doxorubicin, paclitaxel, gemcitabine and sorafenib, through a simple incubation process [99,100,106,107,108]. This process facilitates the uptake of these drugs by the MSCs, albeit with varying degrees of efficiency. In particular, while hBM-MSCs show significant uptake of paclitaxel and other drugs, their interaction with pemetrexed is less effective, resulting in insufficient drug internalisation to adversely affect tumour cells [107]. Further investigation on MSCs from other sources, such as human adipose tissue (hAd-MSCs) or dental tissues, reveals a similar capacity to absorb several anti-cancer drugs, including cisplatin and paclitaxel [109,110,111]. This uptake is not uniform across MSC types, suggesting a dependence on the cellular properties specific to each MSC type. For example, the amount of paclitaxel absorbed by each hBM-MSC was quantified at approximately 2.7 pg/cell, whereas for other type of cells such as human olfactory bulb stem cells, it was 0.19 pg/cell, indicating a substantial capacity of uptake of MSCs that might involve specific transporters or membrane properties inherent to these cells [100].
The underlying drug uptake mechanisms by MSCs are diverse and include transporter-mediated entry, simple diffusion due to the lipophilic nature of some drugs, and various forms of endocytosis [112,113,114]. Specifically, the hydrophilic nucleoside analogue gemcitabine utilizes nucleoside transporters such as human concentrative nucleoside transporter 1 (hCNT1) and human equilibrative nucleoside transporter 1 (hENT1) for cell entry, suggesting that the efficacy of gemcitabine uptake may be closely linked to the expression levels of these transporters in MSCs [83,99,112]. On the other hand, lipophilic drugs such as paclitaxel can diffuse across the cell membrane, while larger molecules can be internalized via endocytotic pathways mediated by different receptors [111,114].
The therapeutic application of drug-loaded MSCs is two-fold, either through the utilization of MSC-conditioned media or by employing the MSCs as direct drug carriers. The conditioned media from drug-primed MSCs is particularly enriched with a secretome comprising various biologically active molecules, offering a targeted anticancer effect which exceeds that of the drugs alone. This enhanced effect is attributed to a more sophisticated drug release system that possibly influences MSC-EVs for improved drug delivery to cancer cells [115,116]. On the other hand, direct administration of drug-loaded MSCs facilitates an intimate cell-to-cell interaction, allowing a more direct and potent transfer of anti-cancer agents to the tumour cells. This method not only affects the MSC natural tumour-tropic properties but also provides a sustained release of the therapeutic agents, thereby maximizing the therapeutic impact while minimizing systemic side effects.
In vitro and in vivo studies have confirmed the efficacy of MSC-based drug delivery systems. Conditioned media derived from MSCs treated with gemcitabine or paclitaxel exhibit a significant inhibitory effect on the proliferation of several cancer cell lines, including some derived either from pancreatic adenocarcinoma and glioblastoma [100,117]. In addition, direct co-culture of cancer cells with drug-loaded MSCs has shown promising results in reducing tumour cell proliferation and attenuating tumour growth in some in vivo animal models [100,108,118].
In addition to their direct anti-tumour effects, drug-primed MSCs have shown potential for modulating tumour microenvironment-derived factors affecting angiogenesis and metastasis. For example, conditioned media from sorafenib-treated MSCs can inhibit endothelial cell proliferation, thereby affecting tumour vascularization [108]. In addition, the down-regulation of critical adhesion molecules by conditioned media from paclitaxel-loaded MSCs reduces the ability of tumour cells to metastasize [118], highlighting the multifaceted role of MSCs in cancer therapy.
MSCs have also been genetically engineered to produce various bioactive molecules and immunomodulatory cytokines such as interferons (e.g., IFN-α, IFN-β, IFN-γ), interleukins (e.g., IL-2, IL-12, IL-15, IL-18, IL-10, IL-21), chemokines (e.g., CXC3L1), pro-apoptotic molecules (e.g., TRAIL), anti-angiogenic molecules (e.g., alpha-1 anti-trypsin (AAT), NK4, VEGFR1), and molecules with other anti-tumour properties (e.g., TNF-α), enhancing their ability to deliver specific therapeutic gene products, thereby reducing tumour growth, inducing apoptosis or acting as inhibitors of different pro-tumour factors [119,120,121,122,123,124,125,126,127,128,129,130,131,132]. It can be achieved by different methods: genetic modification of MSCs using viral vectors, as well as DNA plasmids or transposons. The choice of the appropriate method for genetic editing depends on the therapeutic goals and the specific targets involved.
Despite the efficacy of engineered MSCs in cancer therapy, their therapeutic outcomes as monotherapy in highly heterogeneous cancers remains limited, prompting the exploration of combined strategies to overcome chemotherapy resistance. For instance, the combination of TRAIL-engineered MSCs with temozolomide has shown greater efficacy in the treatment of glioblastoma than either treatment alone [133]. This synergistic effect that is attributed to the simultaneous induction of apoptosis and inhibition of cancer cell proliferation highlights the potential of engineered MSCs in the treatment of non-Hodgkin’s lymphoma [134]. However, concerns about the long-term safety of viral gene therapy have led to the consideration of non-viral gene transfection methods, despite their lower efficiency. Interferons, known for their anti-tumour properties, have been used in combination with tumour-specific antibodies (e.g., anti-PD-L1) or conventional chemotherapy such as β-cisplatin to control cancer progression in animal models [135,136,137]. Similarly, IL-12 has been recognized for its immunotherapeutic potential, stimulating T and NK cell activation and inhibiting tumour growth in both renal carcinoma and cervical tumour murine models [138,139]. In addition, Zhao et al. showed that MSCs transfected with a recombinant plasmid encoding IL-10 suppressed the proliferation of pancreatic cancer cells, reduced the growth of this xenografted tumour in vivo, and inhibited tumour angiogenesis [130]. MSCs overexpressing IL-21, a pro-inflammatory cytokine naturally produced by Th17 cells that inhibits regulatory T cell differentiation, effectively neutralized disseminated B-cell lymphoma [131]. Chen et al. found that MSC administration suppressed anti-tumour T cell responses and promoted tumour growth, while knocking down PD-L1 with shRNA prevented this effect [140]. Other strategies include the use of zoledronate-primed MSCs, which have a TGF-β-impaired secretion and induce Vδ2 T cell proliferation with anti-tumour properties [141]. These findings provide some explanations on the escape mechanisms that cancer exerts on the immune system. Bioengineered MSCs expressing proteins such as NK4, which inhibits hepatocyte growth factor (HGF), or thrombospondin-1 (TSP-1) variants, which suppress angiogenesis, have shown promising results in reducing tumour growth and vascularization in a lung metastatic and a glioblastoma tumour model, respectively [126,142,143]. However, the safety of such therapies, particularly the risk of teratoma formation, needs to be further evaluated. The bioengineering of MSCs to express other tumour suppressor proteins, such as bone morphogenetic protein 4 (BMP4), or phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase (PTEN), has also explored and further demonstrated the versatility of MSCs in cancer therapy, opening new ways for the development of more effective and targeted cancer treatments [144,145,146].
The anti-neoplastic effects of modified MSCs with different anti-cancer drugs or bioactive/immunomodulatory molecules and the main mechanisms of action in tumour cells are summarized in Table 1.
Table 1.
Anti-tumour mechanisms of drug/biomolecule-loaded MSCs.
| Loaded Drug/Molecule | Target Cancer | Therapeutic Effect | References |
|---|---|---|---|
| Doxorubicin | Lung melanoma metastases | Reduction of tumour cell viability | [106] |
| Oral squamous cell carcinoma | Inhibition of tumour cell growth | [83] | |
| Paclitaxel | Multiple myeloma | Inhibition of tumour cell growth | [99] |
| Prostate, malignant glioma and melanoma cancer cell lines | Inhibition of tumour cell growth | [100] | |
| Oral squamous cell carcinoma | Inhibition of tumour cell growth | [83] | |
| Gemcitabine | Oral squamous cell carcinoma | Inhibition of tumour cell growth | [83] |
| Pancreatic carcinoma | Inhibition of tumour cell growth | [117] | |
| Sorafenib | Glioblastoma multiforme | Inhibition of tumour cell growth and angiogenesis | [108] |
| Cisplatin | Mesothelioma and glioblastoma multiforme | Inhibition of tumour cell growth | [109] |
| IL-18, IFN-β | Intracranial glioma | Inhibition of tumour cell growth | [120,137] |
| IL-2 | Glioma | Increased anti-tumour effects | [122] |
| IL-12, IL-15 | Melanoma, lung cancer, pancreatic cancer, intracranial glioma and hepatoma | Direct anti-tumour effect and activation of cytotoxic T and NK cells | [123,124,125] |
| IL-10 | Pancreatic cancer | Inhibition of tumour cell proliferation and angiogenesis | [130] |
| NK4 | Pancreatic cancer | Inhibition of tumour cell proliferation and migration | [126,142] |
| TRAIL+/− temozolomide |
Malignant glioma | Direct anti-tumour effect and apoptosis of tumour cells |
[127,133] |
| TSP-1 | Glioblastoma multiforme | Inhibition of tumour angiogenesis | [143] |
| BMP4 | Malignant glioma | Increased anti-tumour effects | [144] |
| PTEN | Glioma | Induced cytotoxicity on tumour cells | [145,146] |
2.3.2. Oncolytic Viruses
Oncolytic viruses (OV) are attenuated, non-pathogenic viruses designed to selectively recognize, infect and destroy tumour cells without affecting the rest of the healthy cell types where they are unable to replicate [105,119,147]. Its therapeutic use began at the end of the 19th century, with some controversy due to the poor clinical outcomes observed in early cancer patient trials. However, it was not until the end of the 20th century that oncolytic virotherapy was revived, due to the development of molecular biology and the introduction of genetic modification techniques, which not only allowed viruses with greater affinity for tumour cells to be isolated, but also modified or assembled to enhance their anti-tumour properties [119,148,149,150].
Most viruses used in oncolytic virotherapy, whether single- or double-stranded DNA or RNA, are usually based on human pathogens such as adenovirus (Ad), herpes simplex virus (HSV), measles virus (MV), coxsackievirus, vaccinia virus or reovirus, among others. However, viruses from other animal species, such as Newcastle virus, vesicular stomatitis virus or retroviruses, can also be used, and more than 10 viral families with their serotypes and subgroups have been studied as anti-tumour therapeutic agents [149,150,151,152]. Their mechanisms of action are dual, based on: (1) the selective destruction of tumour cells after infection (direct cellular oncolysis); and (2) the stimulation of the patient systemic anti-tumour immunity induced by the release of new viral particles generated by the lysis of tumour cells, which create a more pro-inflammatory tumour microenvironment that favours the immune attack and limits the evasion capacity of tumour cells (indirect cellular oncolysis) [105,119,147,148,151,153].
Unfortunately, these anti-cancer effects associated with oncolytic virus may be reduced when administered systemically intravenously, resulting in more occasional and transient responses than when administered locally (intratumorally) [154]. The main factors associated with this low anti-tumour efficacy after intravenous administration are (1) the ability of the patient immune system to recognize and neutralize the oncolytic viruses before they can reach the tumour and exert their therapeutic effects; (2) the presence of an inadequate tropism, whereby these oncolytic viruses are directed to tissues where they can be trapped or retained, such as the liver or spleen; (3) the presence of factors associated with the tumour development; and (4) the occurrence of factors associated with the tumour microenvironment that prevent their pre-penetration and intratumoural diffusion, including the release of chemokines and cytokines such as IL-10 and transforming growth factor β (TGF-β), among others [105,119,147,148,150,154].
Therefore, in recent years, new strategies have been developed to overcome these limitations: (1) increase of the tumour tropism (selection of entry receptors that are highly expressed by tumours); (2) improve their safety (restriction of viral replication to tumour cells only); (3) increase their therapeutic efficacy by inserting therapeutic transgenes that co-express cytokines and other molecules with anti-tumour activity; and (4) improve their biodistribution in the tumour environment [149].
Among the strategies to improve the biodistribution of oncolytic virus to tumour cells, different cell types (lymphocytes, myeloid cells, mesenchymal stem/stromal cells, etc.) as carriers of these oncolytic viruses to the tumour environment have been tested [150,152]. Of all these, MSCs have attracted most interest as oncolytic carriers due to (1) their easy capacity to be infected by oncolytic viruses, which allows them to replicate and produce new virions, remaining viable long enough to reach the tumour microenvironment; (2) their capacity for selective migration, achieving a more direct transport towards the tumour microenvironment (tumour tropism), where a large amount of pro-inflammatory cytokines and chemokines are released, favouring the chemoattraction of these MSCs carriers; and (3) their immunomodulatory properties, which prevent the recognition of these viruses by the patient innate and adaptive immune system [105,119,150,151,152,154,155,156].
The main preclinical studies evaluating the use of human MSCs as oncolytic virus vectors are summarized in Table 2.
Table 2.
Preclinical studies using MSCs as carrier for oncolytic viruses.
| Family | Natural Host | Type of OV | Host Cell | Target Cancer | Route | References |
|---|---|---|---|---|---|---|
| Adenoviridae | Human | Ad-ICOVIR5 | AT-MSCs | Lung adenocarcinoma | IP | [157] |
| AT-MSCs | Osteosarcoma | IP | [158] | |||
| AT-MSCs | Lung adenocarcinoma | IT | [159] | |||
| Ad-ICOVIR15-Ad.IC9 | BM-MSCs | Lung cancer | IV | [160] | ||
| Ad-ICOVIR15 | Men-MSCs, BM-MSCs |
Lung and pancreatic adenocarcinoma, melanoma |
IP | [161] | ||
| Men-MSCs + PBMNCs | Lung adenocarcinoma, epidermoid carcinoma, pharynx squamous cell carcinoma | IP | [162] | |||
| Ad-ICOVIR15, Ad-ICOVIR15-cBITE |
Men-MSCs | Lung adenocarcinoma and epidermoid carcinoma | IP | [163] | ||
| Ad-ICOVIR15, Ad-ICOVIR17 |
AT-MSCs | Glioblastoma multiforme | IT, IV | [164] | ||
| Ad-ICOCAV17 | AT-MSCs | Osteosarcoma and brain tumours | IV | [165] | ||
| Spontaneous lung carcinoma | IV | [166] | ||||
| Ad-hOC-E1 | BM-MSCs | Renal carcinoma | IP | [167] | ||
| Ad-RLX-PCDP | BM-MSCs | Pancreatic cancer | IV | [168] | ||
| Ad-Ad5-HexPos3 | BM-MSCs, AT-MSCs |
Head and neck squamous cells carcinoma | IV, IP | [169] | ||
| Ad-Ad5-Ki67/IL-15 | Source of MSCs non-specified |
Glioblastoma multiforme | IT | [170] | ||
| Ad-5, Ad-3, Ad-5.Pk7-Delta24 | BM-MSCs, AT-MSCs |
Lung and breast tumours | IV | [171] | ||
| Ad-AFPp-E1A, Ad-AFPp-E1A-122 | WJ-MSCs | Hepatocellular carcinoma | IV | [172] | ||
| Ad5/3-Δ19K-Luc-GFP, Ad5/3-TRAIL-GFP, Ad5/3- FCU1-GFP/5-FC |
BM-MSCs | Pancreatic cancer | - | [173] | ||
| Ad-Ad5/3 Ad-Ad5/3RGD-Luc |
BM-MSCs | Ovarian carcinoma | IP | [174] | ||
| Ad-Ad5/3-TRAIL | BM-MSCs | Pancreatic ductal adenocarcinoma | IV | [175] | ||
| Ad-hTERTp-IL24 | WJ-MSCs | Hepatocellular carcinoma | IV | [176] | ||
| Ad-5-E3, Ad-WNTi | BM-MSCs | Hepatocellular carcinoma | IV | [177,178] | ||
| Ad-CRAd-EGFP | BM-MSCs | Colon cancer | IV, IP | [17] | ||
| Ad-CRAd, Ad-bic | MSCs-E1 (Gene Ad E1A/E1B) |
Prostate cancer | IT | [179] | ||
| Ad-CRAd, Ad5/3.CXCR4 |
BM-MSCs | Lung metastases of breast carcinoma | IV | [180] | ||
| Ad-CRAdNTR (PS1217H6) |
BM-MSCs | Colorectal cancer | IV | [181] | ||
| Ad-CRAd5/F11 | Men-MSCs | Colorectal cancer | IV, IP, IT | [182] | ||
| AD-5/3-kBF5HRE-E1Awt | BM-MSCs | Melanoma, breast tumour | IO | [183] | ||
| Ad-WT, Ad-RGD, Ad-5/3, Ad-CRAd | Source of MSCs non-specified |
Gliomas (Glioblastoma multiforme) |
IC | [184] | ||
| Ad-WT, Ad-5-CRAd-S-pk7 |
BM-MSCs | Breast cancer | IT | [185] | ||
| Ad-rAd.DCN Ad-rAd.Null |
WJ-MSCs | Breast cancer lung metastatic | IV | [186] | ||
| Ad5-Delta-24-RGD | Source of MSCs non-specified |
Ovarian and breast cancer | IV | [187] | ||
| Ad-Delta-24-RGD | BM-MSCs | Gliomas | IC, IA | [188,189,190] | ||
| Ad5/35-Tet-on-E1b Pro-D24-ES-IL-24 | UCB-MSCs | Gliomas | IV | [191] | ||
| Ad-YSCH-01 | DP-MSCs | Glossopharyngeal, bladder and breast squamous cancer | IV, IP, IT | [192] | ||
| Herpesviridae | Human | Herpes Simplex Virus (HSV) |
Source of MSCs non-specified |
Melanoma brain metastatic |
IA | [193] |
| HSV-R-LM249 | BM-MSCs, AM-MSCs, AT-MSCs, DP-MSCs |
Lung and brain metastases |
IV | [194] | ||
| Poxviridae | Human and Bovine | Vaccinia virus (CAL1) |
AT-MSCs | Colon cancer | IT | [195] |
| Vaccinia virus (Copenhagen, Wyeth and LIVP strains) |
AT-MSCs | Canine soft tissue sarcoma | IV | [196] | ||
| Vaccinia virus (WT1/ACAM2000 and L14 strains) |
AT-MSCs | Melanoma, lung carcinoma, myelogenous leukaemia | - | [197] | ||
| Rabbit | MYXV-IL15 | BM-MSCs | Pulmonary melanoma | IV | [198] | |
| MYXV-TNFSF14 | AT-MSCs | Pancreatic adenocarcinoma | IP | [199,200] | ||
| MYXV | AT-MSCs | Glioblastoma multiforme | IC | [201] | ||
| Paramyxoviridae | Human | MV | AT-MSC | Ovarian cancer | IP | [202,203] |
| BM-MSC | Hepatocellular carcinoma | IV | [204] | |||
| Lymphoblastic leukaemia | IV | [205] | ||||
| Birds | NDV (LaSota strain) |
BM-MSC + Lactobacillus casei extract |
Colorectal cancer | - | [206] | |
| BM-MSC | Human papillomavirus associated malignancy | [207] | ||||
| NDV (MTH-68/H) |
BM-MSCs, AT-MSCs, WJ-MSCs |
Glioblastoma multiforme | - | [208] | ||
| Reoviridae | Mammalians | Reovirus (T3D strain) |
AT-MSC | Lung cancer | IV | [209] |
| AT-MSC | Glioblastoma multiforme | - | [210] | |||
| AT-MSC | Colorectal cancer | IT | [211] | |||
| AT-MSC | Lung cancer | - | [212] | |||
| WJ-MSC | Acute myeloid leukaemia | IV | [213] |
Abbreviations: OV (oncolytic virus), Ad (adenovirus), MV (measles virus), MYXV (myxoma virus), NDV (Newcastle disease virus), RLX (relaxin), PCDP (biodegradable polymer), WNTi (Wnt-inhibiting decoy receptor), MSCs (mesenchymal stem/stromal cells), BM (bone marrow), AM (amniotic membrane), AT (adipose tissue), DP (dental pulp), WJ (Wharton’s jelly), Men (menstrual blood), UCB (umbilical cord blood), BITE (bispecific T cell engager), IT (intratumoural), IV (intravenous), IA (intra-arterial), IP (intraperitoneal), IC (intracranial), IO (intraocular), WT (wild-type), PBMNCs (peripheral blood mononuclear cells), DCN (decorin).
2.3.3. Suicide Genes
Gene-directed enzyme prodrug therapy, also known as suicide gene therapy, is a promising alternative of cancer treatment that goes far beyond the limits of conventional chemotherapy. This innovative therapy modality involves delivering a gene construct into the therapeutic cells encoding an enzyme that is capable of converting a non-toxic prodrug into a cytotoxic metabolite subsequently within the tumour environment, thereby concentrating the cytotoxic effect onto the malignant cells while minimizing the effect on healthy cells [214].
The most common example of this approach is the combination of the thymidine kinase (TK) gene from herpes simplex virus (HSV) with ganciclovir (GCV) as a prodrug. TK catalyses the phosphorylation of deoxythymidine and a wide range of nucleotide analogues, including GCV, which is then further phosphorylated by cellular kinases to its active and cytotoxic triphosphate form, GCV-TP [215]. Then, GCV-TP is incorporated into DNA during replication, ultimately leading to premature chain termination, and eventually inducing apoptotic cell death [216]. Although GCV-TP is not able to diffuse passively to adjacent cells, the gap junctions established between MSCs encoding the HSV-TK gene and the surrounding tumour cells allow the entry of GCV-TP into the tumour cells, hence causing cell apoptosis. This mechanism of action has been termed “bystander effect”. Additionally, the bystander effect can be augmented by cells that do not express the TK gene through the phagocytosis of apoptotic vesicles that contain GCV-TP. For example, MSCs expressing the HSV-TK gene in combination with GCV have provided promising results in the treatment of a variety of tumours, including colorectal cancer, melanoma, glioblastoma and breast cancer [217,218,219,220].
Another approach of suicide gene therapy is based on the use of the Escherichia coli cytosine deaminase (CD) gene in combination with the prodrug 5-fluorocytosine (5-FC). In this setting, therapeutic MSCs are bioengineered to express the CD enzyme, which converts 5-FC into 5-fluorouracil (5-FU), a potent cytotoxic metabolite for the neighbouring tumour cells [221,222]. Other examples include the combinations of the carboxylesterase gene with irinotecan, and the cytochrome P450 gene with cyclophosphamide. These combined treatments have been successfully tested in preclinical animal models of osteosarcoma, melanoma, and brain, breast and colorectal tumours [223,224,225,226,227].
The use of MSCs as delivery vectors for the suicide genes has shown remarkable progress in the targeted transfer of suicide genes. MSCs, known for their tumour-homing capabilities, can be genetically engineered to express the suicide gene, thereby directly delivering the gene to the tumour site. This strategy increases the specificity and efficacy of the therapy and reduces the risk of systemic toxicity. Moreover, some studies have explored the combination of suicide gene therapy with other therapeutic strategies to enhance its efficacy. For example, combining HSV-TK/GCV with immune checkpoint inhibitors, radiation or other targeted therapies (e.g., histone deacetylase inhibitors and valproic acid) can synergize to produce more robust anti-tumour responses, potentially overcoming resistance mechanisms and improving treatment outcomes [228,229,230].
Preclinical cancer models using MSCs as therapeutic vehicles of suicide genes are summarized in Table 3.
Table 3.
Preclinical anti-tumour models using suicide genes expressing MSCs.
| Suicide Gene/Prodrug | Tumour Preclinical Model | References |
|---|---|---|
| Thymidine kinase + ganciclovir |
Melanoma lung metastasis | [217] |
| Breast cancer Glioblastoma multiforme Colon cancer Malignant melanoma Intracranial glioma |
[218] [219,231] [220] [228] [229] |
|
|
E. coli cytosine deaminase + 5-Fluorocytosine |
Glioblastoma multiforme Osteosarcoma |
[221] [222] |
| Colon cancer | [225] | |
| Carboxylesterase + irinotecan |
Glioma | [226] |
| Cytochrome P450 + cyclophosphamide |
Colorectal and breast cancer | [227] |
3. Clinical Trials
Among the changing scenarios in cancer therapy, MSCs have emerged as a promising tool, not only because of their regenerative capabilities, but also because of their abovementioned potential utility as delivery vehicles for anticancer agents. The use of MSCs with oncolytic viruses, genetically modified and engineered cells, and other therapeutic modalities is opening new avenues in the fight against various malignancies, as evidenced by completed and ongoing Phase I and II clinical trials (Table 4).
Table 4.
Human MSC-based clinical trials targeting solid tumours.
| Clinical Trial ID | Target Cancer | Therapeutic MSCs | Status | Location |
|---|---|---|---|---|
| NCT01844661 | Solid metastatic and refractory tumours | Autologous MSC-ICOVIR-5 (CELYVIR) |
Completed | Spain |
| NCT04758533 | Diffuse intrinsic pontine glioma and medulloblastoma |
Allogeneic MSC-ICOVIR-5 (AloCELYVIR) |
Recruiting | Spain |
| NCT03896568 | Recurrent glioblastoma, gliosarcoma and astrocytoma |
Allogeneic MSC-DNX-2401 | Recruiting | United States |
| NCT02068794 | Recurrent ovarian, primary peritoneal or fallopian tube cancer | MSC-MV-NIS | Recruiting | United States |
| NCT02008539 | Advanced gastrointestinal adenocarcinoma |
Autologous MSC-HSV-TK (MSC_apceth_101) + GCV | Completed | Germany |
| NCT03298763 | Metastatic lung adenocarcinoma | MSC-TRAIL | Recruiting | United Kingdom |
| NCT02530047 | Ovarian cancer | MSC-IFN-β | Completed | United States |
| NCT02079324 | Head and neck cancer | MSC-IL-12 (GX-051) | Unknown status | South Korea |
| NCT03608631 | Metastatic pancreatic ductal adenocarcinoma with KrasG12D mutation |
MSC-EV-siRNA KrasG12D (iExosomes) |
Active, Not recruiting | United States |
Initial clinical attempts to fight different types of tumours combined MSCs with oncolytic viruses. For example, a Phase I/II clinical trial studied the systemic administration of autologous hBM-MSCs infected with the oncolytic adenovirus ICOVIR-5 (CELYVIR) for the treatment of paediatric and adult patients with solid metastatic and refractory tumours (NCT01844661). The authors found that CELYVIR had a very low systemic toxicity and a great safety profile and beneficial anti-tumour effects [103,232]. The same strategy but using allogeneic MSCs infected with ICOVIR-5 (AloCELYVIR), is being investigated alone or in combination with radiotherapy, to assess its safety, tolerability and preliminary efficacy for the treatment of diffuse intrinsic pontine glioma and medulloblastoma (NCT04758533). Another clinical study, identified as NCT03896568, which is still recruiting patients, involves the intra-arterial administration of allogeneic hBM-MSCs loaded with the oncolytic virus DNX-2401, also known as Delta-24-RGD. This treatment is being investigated in patients with recurrent glioblastoma, gliosarcoma and astrocytoma, while the Phase I/II clinical trial NCT02068794 is still evaluating the therapeutic efficacy of intraperitoneal administration of MSCs infected with oncolytic measles virus encoding thyroidal sodium iodide symporter (MV-NIS) in the treatment of ovarian, primary peritoneal or fallopian tube cancer.
Subsequent studies have broadened the scope of bioengineered MSC applications. For example, the phase I/II TREAT-ME1 clinical trial evaluated the safety and efficacy of autologous MSCs delivering the gene HSV-TK (MSC_apceth_101) in combination with GCV, showing acceptable safety, tolerability and some signs of effectiveness in reducing tumour growth and metastases in advanced gastrointestinal adenocarcinoma (NCT02008539) [233].
The ability of MSCs to release therapeutic drugs or bioactive molecules into the tumour microenvironment has also been explored in several clinical trials. Thus, the TACTICAL trial is evaluating the combination of allogeneic MSCs overexpressing the TRAIL gene and chemotherapy in patients with metastatic lung adenocarcinoma to determine tolerability and efficacy (NCT03298763), while IFN-β or IL-12-producing MSCs are being evaluated for ovarian cancer or head and neck cancer treatment, respectively (NCT02530047 and NCT02079324).
Furthermore, the clinical use of MSC-EVs for cancer treatment is currently one of the future challenges for cell-free therapies. An example of this approach is an active Phase I clinical trial to evaluate the therapeutic effects of MSC-EVs loaded with small interfering RNA (siRNA) against KrasG12D (iExosomes) in metastatic pancreatic ductal adenocarcinoma patients with KrasG12D mutation (NCT03608631). MSC-EVs are considered as a promising platform through which to further enhance the anti-cancer effects of traditional therapies, and of course more translational studies will be conducted in the foreseeable future to further reveal their therapeutic potential.
4. Challenges and Future Prospects
Mesenchymal stem/stromal cells are widely used to treat various inflammatory and degenerative diseases due to their ability to engraft and repair damaged tissues, differentiate into different cell types and secrete a variety of soluble mediators with pleiotropic effects. However, the use of MSCs in the treatment of cancer must be approached with caution to minimize their potential to support tumour growth while maximizing their anti-tumour effects. Developing effective strategies involve genetically modifying MSCs or extracellular vesicles to enhance their anti-cancer effects or to deliver therapeutic agents to cancer cells as “Trojan horses”. Future understanding of the complex interactions between MSCs, the immune system and the tumour environment is essential for these advances. While challenging, these strategies aimed at enhancing MSC homing and engraftment in tumours following systemic administration, hold promise for fully unlocking the therapeutic potential of MSCs in precise and tailored anti-tumour therapies. Continued preclinical and clinical research is essential to develop safe and effective MSC-based cancer therapies that will ultimately improve the survival and quality of life of patients suffering from a wide range of malignancies.
Author Contributions
Literature search, preparation of tables and writing, J.I.G.-C. and D.G.-B., with supervision from A.G.Z. and J.M.M. Critical feedback, review, and editing, D.G.-B., A.G.Z. and J.M.M. All authors contributed to the article. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by Instituto de Salud Carlos III (ISCIII) through the Spanish Network of Advanced Therapies (Terav), RICORS subprogram, projects RD21/0017/0010 (to A.G.Z.) and RD21/0017/0001 (to J.M.M.), co-funded by ERDF-Next Generation EU “Plan de Recuperación, Transformación y Resiliencia”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein A.J., Chailakhjan R.K., Lalykina K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 4.Mattar P., Bieback K. Comparing the Immunomodulatory Properties of Bone Marrow, Adipose Tissue, and Birth-Associated Tissue Mesenchymal Stromal Cells. Front. Immunol. 2015;6:560. doi: 10.3389/fimmu.2015.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puissant B., Barreau C., Bourin P., Clavel C., Corre J., Bousquet C., Taureau C., Cousin B., Abbal M., Laharrague P., et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Lozano F.J., Bueno C., Insausti C.L., Meseguer L., Ramirez M.C., Blanquer M., Marin N., Martinez S., Moraleda J.M. Mesenchymal stem cells derived from dental tissues. Int. Endod. J. 2011;44:800–806. doi: 10.1111/j.1365-2591.2011.01877.x. [DOI] [PubMed] [Google Scholar]
- 7.Stappenbeck T.S., Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 8.Yañez R., Oviedo A., Aldea M., Bueren J.A., Lamana M.L. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp. Cell Res. 2010;316:3109–3123. doi: 10.1016/j.yexcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Salgado A.J., Reis R.L., Sousa N.J., Gimble J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 10.Tian R., Su S., Yu Y., Liang S., Ma C., Jiao Y., Xing W., Tian Z., Jiang T., Wang J. Revolutionizing osteoarthritis treatment: How mesenchymal stem cells hold the key. Biomed. Pharmacother. 2024;173:116458. doi: 10.1016/j.biopha.2024.116458. [DOI] [PubMed] [Google Scholar]
- 11.Le Blanc K., Rasmusson I., Sundberg B., Gotherstrom C., Hassan M., Uzunel M., Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 12.Barrere-Lemaire S., Vincent A., Jorgensen C., Piot C., Nargeot J., Djouad F. Mesenchymal stromal cells for improvement of cardiac function following acute myocardial infarction: A matter of timing. Physiol. Rev. 2024;104:659–725. doi: 10.1152/physrev.00009.2023. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y., Sun Z., Han Q., Liao L., Wang J., Bian C., Li J., Yan X., Liu Y., Shao C., et al. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia. 2009;23:925–933. doi: 10.1038/leu.2008.384. [DOI] [PubMed] [Google Scholar]
- 14.Qiao L., Xu Z., Zhao T., Zhao Z., Shi M., Zhao R.C., Ye L., Zhang X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500–507. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 15.Otsu K., Das S., Houser S.D., Quadri S.K., Bhattacharya S., Bhattacharya J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113:4197–4205. doi: 10.1182/blood-2008-09-176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francois S., Usunier B., Forgue-Lafitte M.E., L’Homme B., Benderitter M., Douay L., Gorin N.C., Larsen A.K., Chapel A. Mesenchymal Stem Cell Administration Attenuates Colon Cancer Progression by Modulating the Immune Component within the Colorectal Tumor Microenvironment. Stem Cells Transl. Med. 2019;8:285–300. doi: 10.1002/sctm.18-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y.F., Chen M.J., Wu M.H., Hung S.C. The use of hypoxic cultured mesenchymal stem cell for oncolytic virus therapy. Cancer Gene Ther. 2013;20:308–316. doi: 10.1038/cgt.2013.22. [DOI] [PubMed] [Google Scholar]
- 18.Beckermann B.M., Kallifatidis G., Groth A., Frommhold D., Apel A., Mattern J., Salnikov A.V., Moldenhauer G., Wagner W., Diehlmann A., et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br. J. Cancer. 2008;99:622–631. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poggi A., Varesano S., Zocchi M.R. How to Hit Mesenchymal Stromal Cells and Make the Tumor Microenvironment Immunostimulant Rather Than Immunosuppressive. Front. Immunol. 2018;9:262. doi: 10.3389/fimmu.2018.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karnoub A.E., Dash A.B., Vo A.P., Sullivan A., Brooks M.W., Bell G.W., Richardson A.L., Polyak K., Tubo R., Weinberg R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 21.Luo J., Ok Lee S., Liang L., Huang C.K., Li L., Wen S., Chang C. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene. 2014;33:2768–2778. doi: 10.1038/onc.2013.233. [DOI] [PubMed] [Google Scholar]
- 22.Slama Y., Ah-Pine F., Khettab M., Arcambal A., Begue M., Dutheil F., Gasque P. The Dual Role of Mesenchymal Stem Cells in Cancer Pathophysiology: Pro-Tumorigenic Effects versus Therapeutic Potential. Int. J. Mol. Sci. 2023;24:13511. doi: 10.3390/ijms241713511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papait A., Stefani F.R., Cargnoni A., Magatti M., Parolini O., Silini A.R. The Multifaceted Roles of MSCs in the Tumor Microenvironment: Interactions With Immune Cells and Exploitation for Therapy. Front. Cell Dev. Biol. 2020;8:447. doi: 10.3389/fcell.2020.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridge S.M., Sullivan F.J., Glynn S.A. Mesenchymal stem cells: Key players in cancer progression. Mol. Cancer. 2017;16:31. doi: 10.1186/s12943-017-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidd S., Spaeth E., Dembinski J.L., Dietrich M., Watson K., Klopp A., Battula V.L., Weil M., Andreeff M., Marini F.C. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quante M., Tu S.P., Tomita H., Gonda T., Wang S.S., Takashi S., Baik G.H., Shibata W., Diprete B., Betz K.S., et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taheri M., Tehrani H.A., Dehghani S., Alibolandi M., Arefian E., Ramezani M. Nanotechnology and bioengineering approaches to improve the potency of mesenchymal stem cell as an off-the-shelf versatile tumor delivery vehicle. Med. Res. Rev. 2024;44:1596–1661. doi: 10.1002/med.22023. [DOI] [PubMed] [Google Scholar]
- 28.Taeb S., Rostamzadeh D., Amini S.M., Rahmati M., Golshekan M., Abedinzadeh M., Ahmadi E., Neha S., Najafi M. Revolutionizing Cancer Treatment: Harnessing the Power of Mesenchymal Stem Cells for Precise Targeted Therapy in the Tumor Microenvironment. Curr. Top. Med. Chem. 2024 doi: 10.2174/0115680266299112240514103048. [DOI] [PubMed] [Google Scholar]
- 29.Han Y., Yang J., Fang J., Zhou Y., Candi E., Wang J., Hua D., Shao C., Shi Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022;7:92. doi: 10.1038/s41392-022-00932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X., Song E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Reviews. Drug Discov. 2019;18:99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 31.Ma F., Chen D., Chen F., Chi Y., Han Z., Feng X., Li X., Han Z. Human Umbilical Cord Mesenchymal Stem Cells Promote Breast Cancer Metastasis by Interleukin-8- and Interleukin-6-Dependent Induction of CD44(+)/CD24(−) Cells. Cell Transplant. 2015;24:2585–2599. doi: 10.3727/096368915X687462. [DOI] [PubMed] [Google Scholar]
- 32.Xie C., Yang Z., Suo Y., Chen Q., Wei D., Weng X., Gu Z., Wei X. Systemically Infused Mesenchymal Stem Cells Show Different Homing Profiles in Healthy and Tumor Mouse Models. Stem Cells Transl. Med. 2017;6:1120–1131. doi: 10.1002/sctm.16-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith C.L., Chaichana K.L., Lee Y.M., Lin B., Stanko K.M., O’Donnell T., Gupta S., Shah S.R., Wang J., Wijesekera O., et al. Pre-exposure of human adipose mesenchymal stem cells to soluble factors enhances their homing to brain cancer. Stem Cells Transl. Med. 2015;4:239–251. doi: 10.5966/sctm.2014-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rattigan Y., Hsu J.M., Mishra P.J., Glod J., Banerjee D. Interleukin 6 mediated recruitment of mesenchymal stem cells to the hypoxic tumor milieu. Exp. Cell Res. 2010;316:3417–3424. doi: 10.1016/j.yexcr.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Ringe J., Strassburg S., Neumann K., Endres M., Notter M., Burmester G.R., Kaps C., Sittinger M. Towards in situ tissue repair: Human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J. Cell. Biochem. 2007;101:135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 36.Schar M.O., Diaz-Romero J., Kohl S., Zumstein M.A., Nesic D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin. Orthop. Relat. Res. 2015;473:1635–1643. doi: 10.1007/s11999-015-4192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalimuthu S., Oh J.M., Gangadaran P., Zhu L., Lee H.W., Rajendran R.L., Baek S.H., Jeon Y.H., Jeong S.Y., Lee S.W., et al. In Vivo Tracking of Chemokine Receptor CXCR4-Engineered Mesenchymal Stem Cell Migration by Optical Molecular Imaging. Stem Cells Int. 2017;2017:8085637. doi: 10.1155/2017/8085637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kidd S., Spaeth E., Watson K., Burks J., Lu H., Klopp A., Andreeff M., Marini F.C. Origins of the tumor microenvironment: Quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS ONE. 2012;7:e30563. doi: 10.1371/journal.pone.0030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Housman G., Byler S., Heerboth S., Lapinska K., Longacre M., Snyder N., Sarkar S. Drug resistance in cancer: An overview. Cancers. 2014;6:1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christodoulou I., Goulielmaki M., Devetzi M., Panagiotidis M., Koliakos G., Zoumpourlis V. Mesenchymal stem cells in preclinical cancer cytotherapy: A systematic review. Stem Cell Res. Ther. 2018;9:336. doi: 10.1186/s13287-018-1078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraitchman D.L., Tatsumi M., Gilson W.D., Ishimori T., Kedziorek D., Walczak P., Segars W.P., Chen H.H., Fritzges D., Izbudak I., et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbash I.M., Chouraqui P., Baron J., Feinberg M.S., Etzion S., Tessone A., Miller L., Guetta E., Zipori D., Kedes L.H., et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 43.Kean T.J., Lin P., Caplan A.I., Dennis J.E. MSCs: Delivery Routes and Engraftment, Cell-Targeting Strategies, and Immune Modulation. Stem Cells Int. 2013;2013:732742. doi: 10.1155/2013/732742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Bernal D., Garcia-Arranz M., Yanez R.M., Hervas-Salcedo R., Cortes A., Fernandez-Garcia M., Hernando-Rodriguez M., Quintana-Bustamante O., Bueren J.A., Garcia-Olmo D., et al. The Current Status of Mesenchymal Stromal Cells: Controversies, Unresolved Issues and Some Promising Solutions to Improve Their Therapeutic Efficacy. Front. Cell Dev. Biol. 2021;9:650664. doi: 10.3389/fcell.2021.650664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai X., Xi J., Bi Y., Zhao X., Bing W., Meng X., Liu Y., Zhu Z., Song G. TNF-alpha promotes survival and migration of MSCs under oxidative stress via NF-kappaB pathway to attenuate intimal hyperplasia in vein grafts. J. Cell. Mol. Med. 2017;21:2077–2091. doi: 10.1111/jcmm.13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazennec G., Lam P.Y. Recent discoveries concerning the tumor—Mesenchymal stem cell interactions. Biochim. Biophys. Acta. 2016;1866:290–299. doi: 10.1016/j.bbcan.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Li M., Zeng L., Liu S., Dangelmajer S., Kahlert U.D., Huang H., Han Y., Chi X., Zhu M., Lei T. Transforming Growth Factor-beta Promotes Homing and Therapeutic Efficacy of Human Mesenchymal Stem Cells to Glioblastoma. J. Neuropathol. Exp. Neurol. 2019;78:315–325. doi: 10.1093/jnen/nlz016. [DOI] [PubMed] [Google Scholar]
- 48.Magne B., Dedier M., Nivet M., Coulomb B., Banzet S., Lataillade J.J., Trouillas M. IL-1beta-Primed Mesenchymal Stromal Cells Improve Epidermal Substitute Engraftment and Wound Healing via Matrix Metalloproteinases and Transforming Growth Factor-beta1. J. Investig. Dermatol. 2020;140:688–698. doi: 10.1016/j.jid.2019.07.721. [DOI] [PubMed] [Google Scholar]
- 49.Tsai L.K., Wang Z., Munasinghe J., Leng Y., Leeds P., Chuang D.M. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke. 2011;42:2932–2939. doi: 10.1161/STROKEAHA.110.612788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Q., Chen L., You Y., Zou C., Zhang Y., Liu Q., Cheng F. Erythropoietin combined with granulocyte colony-stimulating factor enhances MMP-2 expression in mesenchymal stem cells and promotes cell migration. Mol. Med. Rep. 2011;4:31–36. doi: 10.3892/mmr.2010.387. [DOI] [PubMed] [Google Scholar]
- 51.Najafi R., Sharifi A.M. Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin-diabetic rats. Expert Opin. Biol. Ther. 2013;13:959–972. doi: 10.1517/14712598.2013.782390. [DOI] [PubMed] [Google Scholar]
- 52.Zheng X.B., He X.W., Zhang L.J., Qin H.B., Lin X.T., Liu X.H., Zhou C., Liu H.S., Hu T., Cheng H.C., et al. Bone marrow-derived CXCR4-overexpressing MSCs display increased homing to intestine and ameliorate colitis-associated tumorigenesis in mice. Gastroenterol. Rep. 2019;7:127–138. doi: 10.1093/gastro/goy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shao Y., Zhou F., He D., Zhang L., Shen J. Overexpression of CXCR7 promotes mesenchymal stem cells to repair phosgene-induced acute lung injury in rats. Biomed. Pharmacother. 2019;109:1233–1239. doi: 10.1016/j.biopha.2018.10.108. [DOI] [PubMed] [Google Scholar]
- 54.Kumar S., Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007;21:3917–3927. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]
- 55.Levy O., Zhao W., Mortensen L.J., Leblanc S., Tsang K., Fu M., Phillips J.A., Sagar V., Anandakumaran P., Ngai J., et al. mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood. 2013;122:e23–e32. doi: 10.1182/blood-2013-04-495119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hervas-Salcedo R., Fernandez-Garcia M., Hernando-Rodriguez M., Suarez-Cabrera C., Bueren J.A., Yanez R.M. Improved efficacy of mesenchymal stromal cells stably expressing CXCR4 and IL-10 in a xenogeneic graft versus host disease mouse model. Front. Immunol. 2023;14:1062086. doi: 10.3389/fimmu.2023.1062086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sackstein R., Merzaban J.S., Cain D.W., Dagia N.M., Spencer J.A., Lin C.P., Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Bernal D., Blanquer M., Martinez C.M., Garcia-Guillen A.I., Garcia-Hernandez A.M., Carmen Alguero M., Yanez R., Lamana M.L., Moraleda J.M., Sackstein R. Enforced mesenchymal stem cell tissue colonization counteracts immunopathology. NPJ Regen. Med. 2022;7:61. doi: 10.1038/s41536-022-00258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Bernal D., Garcia-Arranz M., Garcia-Guillen A.I., Garcia-Hernandez A.M., Blanquer M., Garcia-Olmo D., Sackstein R., Moraleda J.M., Zapata A.G. Exofucosylation of Adipose Mesenchymal Stromal Cells Alters Their Secretome Profile. Front. Cell Dev. Biol. 2020;8:584074. doi: 10.3389/fcell.2020.584074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarkar D., Vemula P.K., Teo G.S., Spelke D., Karnik R., Wee L.Y., Karp J.M. Chemical engineering of mesenchymal stem cells to induce a cell rolling response. Bioconjugate Chem. 2008;19:2105–2109. doi: 10.1021/bc800345q. [DOI] [PubMed] [Google Scholar]
- 61.Cheng H., Byrska-Bishop M., Zhang C.T., Kastrup C.J., Hwang N.S., Tai A.K., Lee W.W., Xu X., Nahrendorf M., Langer R., et al. Stem cell membrane engineering for cell rolling using peptide conjugation and tuning of cell-selectin interaction kinetics. Biomaterials. 2012;33:5004–5012. doi: 10.1016/j.biomaterials.2012.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lo C.Y., Antonopoulos A., Dell A., Haslam S.M., Lee T., Neelamegham S. The use of surface immobilization of P-selectin glycoprotein ligand-1 on mesenchymal stem cells to facilitate selectin mediated cell tethering and rolling. Biomaterials. 2013;34:8213–8222. doi: 10.1016/j.biomaterials.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Won Y.W., Patel A.N., Bull D.A. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials. 2014;35:5627–5635. doi: 10.1016/j.biomaterials.2014.03.070. [DOI] [PubMed] [Google Scholar]
- 64.Abels E.R., Breakefield X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bui T.M., Mascarenhas L.A., Sumagin R. Extracellular vesicles regulate immune responses and cellular function in intestinal inflammation and repair. Tissue Barriers. 2018;6:e1431038. doi: 10.1080/21688370.2018.1431038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mendt M., Rezvani K., Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54:789–792. doi: 10.1038/s41409-019-0616-z. [DOI] [PubMed] [Google Scholar]
- 67.Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A., Coscia C., Iessi E., Logozzi M., Molinari A., et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu W., Huang L., Li Y., Zhang X., Gu J., Yan Y., Xu X., Wang M., Qian H., Xu W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315:28–37. doi: 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Weng Z., Zhang B., Wu C., Yu F., Han B., Li B., Li L. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J. Hematol. Oncol. 2021;14:136. doi: 10.1186/s13045-021-01141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong L., Pu Y., Zhang L., Qi Q., Xu L., Li W., Wei C., Wang X., Zhou S., Zhu J., et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 2018;9:218. doi: 10.1038/s41419-018-0323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ren W., Hou J., Yang C., Wang H., Wu S., Wu Y., Zhao X., Lu C. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J. Exp. Clin. Cancer Res. CR. 2019;38:62. doi: 10.1186/s13046-019-1027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Casson J., Davies O.G., Smith C.A., Dalby M.J., Berry C.C. Mesenchymal stem cell-derived extracellular vesicles may promote breast cancer cell dormancy. J. Tissue Eng. 2018;9:2041731418810093. doi: 10.1177/2041731418810093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ji R., Zhang B., Zhang X., Xue J., Yuan X., Yan Y., Wang M., Zhu W., Qian H., Xu W. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle. 2015;14:2473–2483. doi: 10.1080/15384101.2015.1005530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ono M., Kosaka N., Tominaga N., Yoshioka Y., Takeshita F., Takahashi R.U., Yoshida M., Tsuda H., Tamura K., Ochiya T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 2014;7:ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 75.Wu S., Ju G.Q., Du T., Zhu Y.J., Liu G.H. Microvesicles derived from human umbilical cord Wharton’s jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS ONE. 2013;8:e61366. doi: 10.1371/journal.pone.0061366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang H., Wang J., Ren T., Huang Y., Liang X., Yu Y., Wang W., Niu J., Guo W. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020;490:54–65. doi: 10.1016/j.canlet.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 77.Pakravan K., Babashah S., Sadeghizadeh M., Mowla S.J., Mossahebi-Mohammadi M., Ataei F., Dana N., Javan M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1alpha/VEGF signaling axis in breast cancer cells. Cell. Oncol. 2017;40:457–470. doi: 10.1007/s13402-017-0335-7. [DOI] [PubMed] [Google Scholar]
- 78.Herrmann I.K., Wood M.J.A., Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 79.Walker S., Busatto S., Pham A., Tian M., Suh A., Carson K., Quintero A., Lafrence M., Malik H., Santana M.X., et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9:8001–8017. doi: 10.7150/thno.37097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haney M.J., Klyachko N.L., Harrison E.B., Zhao Y., Kabanov A.V., Batrakova E.V. TPP1 Delivery to Lysosomes with Extracellular Vesicles and their Enhanced Brain Distribution in the Animal Model of Batten Disease. Adv. Healthc. Mater. 2019;8:e1801271. doi: 10.1002/adhm.201801271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuhrmann G., Serio A., Mazo M., Nair R., Stevens M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release Off. J. Control. Release Soc. 2015;205:35–44. doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 82.Cho N.J., Hwang L.Y., Solandt J.J.R., Frank C.W. Comparison of Extruded and Sonicated Vesicles for Planar Bilayer Self-Assembly. Materials. 2013;6:3294–3308. doi: 10.3390/ma6083294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cocce V., Farronato D., Brini A.T., Masia C., Gianni A.B., Piovani G., Sisto F., Alessandri G., Angiero F., Pessina A. Drug Loaded Gingival Mesenchymal Stromal Cells (GinPa-MSCs) Inhibit In Vitro Proliferation of Oral Squamous Cell Carcinoma. Sci. Rep. 2017;7:9376. doi: 10.1038/s41598-017-09175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abas B.I., Demirbolat G.M., Cevik O. Wharton jelly-derived mesenchymal stem cell exosomes induce apoptosis and suppress EMT signaling in cervical cancer cells as an effective drug carrier system of paclitaxel. PLoS ONE. 2022;17:e0274607. doi: 10.1371/journal.pone.0274607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamilton G., Teufelsbauer M. Adipose-derived stromal/stem cells and extracellular vesicles for cancer therapy. Expert Opin. Biol. Ther. 2022;22:67–78. doi: 10.1080/14712598.2021.1954156. [DOI] [PubMed] [Google Scholar]
- 86.Song Y., Song Q., Hu D., Sun B., Gao M., Liang X., Qu B., Suo L., Yin Z., Wang L. The potential applications of artificially modified exosomes derived from mesenchymal stem cells in tumor therapy. Front. Oncol. 2023;13:1299384. doi: 10.3389/fonc.2023.1299384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Munoz J.L., Bliss S.A., Greco S.J., Ramkissoon S.H., Ligon K.L., Rameshwar P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol. Ther. Nucleic Acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katakowski M., Buller B., Zheng X., Lu Y., Rogers T., Osobamiro O., Shu W., Jiang F., Chopp M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee H.K., Finniss S., Cazacu S., Bucris E., Ziv-Av A., Xiang C., Bobbitt K., Rempel S.A., Hasselbach L., Mikkelsen T., et al. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget. 2013;4:346–361. doi: 10.18632/oncotarget.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lou G., Song X., Yang F., Wu S., Wang J., Chen Z., Liu Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015;8:122. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lang F.M., Hossain A., Gumin J., Momin E.N., Shimizu Y., Ledbetter D., Shahar T., Yamashita S., Parker Kerrigan B., Fueyo J., et al. Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro-oncology. 2018;20:380–390. doi: 10.1093/neuonc/nox152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roccaro A.M., Sacco A., Maiso P., Azab A.K., Tai Y.T., Reagan M., Azab F., Flores L.M., Campigotto F., Weller E., et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J. Clin. Investig. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yuan Z., Kolluri K.K., Gowers K.H., Janes S.M. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J. Extracell. Vesicles. 2017;6:1265291. doi: 10.1080/20013078.2017.1265291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ye Z., Zhang T., He W., Jin H., Liu C., Yang Z., Ren J. Methotrexate-Loaded Extracellular Vesicles Functionalized with Therapeutic and Targeted Peptides for the Treatment of Glioblastoma Multiforme. ACS Appl. Mater. Interfaces. 2018;10:12341–12350. doi: 10.1021/acsami.7b18135. [DOI] [PubMed] [Google Scholar]
- 95.Bagheri E., Abnous K., Farzad S.A., Taghdisi S.M., Ramezani M., Alibolandi M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 2020;261:118369. doi: 10.1016/j.lfs.2020.118369. [DOI] [PubMed] [Google Scholar]
- 96.Dembinski J.L., Wilson S.M., Spaeth E.L., Studeny M., Zompetta C., Samudio I., Roby K., Andreeff M., Marini F.C. Tumor stroma engraftment of gene-modified mesenchymal stem cells as anti-tumor therapy against ovarian cancer. Cytotherapy. 2013;15:20–32. doi: 10.1016/j.jcyt.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zischek C., Niess H., Ischenko I., Conrad C., Huss R., Jauch K.W., Nelson P.J., Bruns C. Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Ann. Surg. 2009;250:747–753. doi: 10.1097/SLA.0b013e3181bd62d0. [DOI] [PubMed] [Google Scholar]
- 98.Shams F., Pourjabbar B., Hashemi N., Farahmandian N., Golchin A., Nuoroozi G., Rahimpour A. Current progress in engineered and nano-engineered mesenchymal stem cells for cancer: From mechanisms to therapy. Biomed. Pharmacother. 2023;167:115505. doi: 10.1016/j.biopha.2023.115505. [DOI] [PubMed] [Google Scholar]
- 99.Bonomi A., Steimberg N., Benetti A., Berenzi A., Alessandri G., Pascucci L., Boniotti J., Cocce V., Sordi V., Pessina A., et al. Paclitaxel-releasing mesenchymal stromal cells inhibit the growth of multiple myeloma cells in a dynamic 3D culture system. Hematol. Oncol. 2017;35:693–702. doi: 10.1002/hon.2306. [DOI] [PubMed] [Google Scholar]
- 100.Pessina A., Bonomi A., Cocce V., Invernici G., Navone S., Cavicchini L., Sisto F., Ferrari M., Vigano L., Locatelli A., et al. Mesenchymal stromal cells primed with paclitaxel provide a new approach for cancer therapy. PLoS ONE. 2011;6:e28321. doi: 10.1371/journal.pone.0028321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Babajani A., Soltani P., Jamshidi E., Farjoo M.H., Niknejad H. Recent Advances on Drug-Loaded Mesenchymal Stem Cells With Anti-neoplastic Agents for Targeted Treatment of Cancer. Front. Bioeng. Biotechnol. 2020;8:748. doi: 10.3389/fbioe.2020.00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taeb S., Rostamzadeh D., Mafi S., Mofatteh M., Zarrabi A., Hushmandi K., Safari A., Khodamoradi E., Najafi M. Update on Mesenchymal Stem Cells: A Crucial Player in Cancer Immunotherapy. Curr. Mol. Med. 2024;24:98–113. doi: 10.2174/1566524023666221226143814. [DOI] [PubMed] [Google Scholar]
- 103.Garcia-Castro J., Alemany R., Cascallo M., Martinez-Quintanilla J., Arriero Mdel M., Lassaletta A., Madero L., Ramirez M. Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: An exploratory study. Cancer Gene Ther. 2010;17:476–483. doi: 10.1038/cgt.2010.4. [DOI] [PubMed] [Google Scholar]
- 104.Hmadcha A., Martin-Montalvo A., Gauthier B.R., Soria B., Capilla-Gonzalez V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020;8:43. doi: 10.3389/fbioe.2020.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lan T., Luo M., Wei X. Mesenchymal stem/stromal cells in cancer therapy. J. Hematol. Oncol. 2021;14:195. doi: 10.1186/s13045-021-01208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao Y., Tang S., Guo J., Alahdal M., Cao S., Yang Z., Zhang F., Shen Y., Sun M., Mo R., et al. Targeted delivery of doxorubicin by nano-loaded mesenchymal stem cells for lung melanoma metastases therapy. Sci. Rep. 2017;7:44758. doi: 10.1038/srep44758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petrella F., Cocce V., Masia C., Milani M., Sale E.O., Alessandri G., Parati E., Sisto F., Pentimalli F., Brini A.T., et al. Paclitaxel-releasing mesenchymal stromal cells inhibit in vitro proliferation of human mesothelioma cells. Biomed. Pharmacother. 2017;87:755–758. doi: 10.1016/j.biopha.2017.01.118. [DOI] [PubMed] [Google Scholar]
- 108.Clavreul A., Pourbaghi-Masouleh M., Roger E., Lautram N., Montero-Menei C.N., Menei P. Human mesenchymal stromal cells as cellular drug-delivery vectors for glioblastoma therapy: A good deal? J. Exp. Clin. Cancer Res. CR. 2017;36:135. doi: 10.1186/s13046-017-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rimoldi I., Cocce V., Facchetti G., Alessandri G., Brini A.T., Sisto F., Parati E., Cavicchini L., Lucchini G., Petrella F., et al. Uptake-release by MSCs of a cationic platinum(II) complex active in vitro on human malignant cancer cell lines. Biomed. Pharmacother. 2018;108:111–118. doi: 10.1016/j.biopha.2018.09.040. [DOI] [PubMed] [Google Scholar]
- 110.Pacioni S., D’Alessandris Q.G., Giannetti S., Morgante L., Cocce V., Bonomi A., Buccarelli M., Pascucci L., Alessandri G., Pessina A., et al. Human mesenchymal stromal cells inhibit tumor growth in orthotopic glioblastoma xenografts. Stem Cell Res. Ther. 2017;8:53. doi: 10.1186/s13287-017-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brini A.T., Cocce V., Ferreira L.M., Giannasi C., Cossellu G., Gianni A.B., Angiero F., Bonomi A., Pascucci L., Falchetti M.L., et al. Cell-mediated drug delivery by gingival interdental papilla mesenchymal stromal cells (GinPa-MSCs) loaded with paclitaxel. Expert Opin. Drug Deliv. 2016;13:789–798. doi: 10.1517/17425247.2016.1167037. [DOI] [PubMed] [Google Scholar]
- 112.Hung S.W., Marrache S., Cummins S., Bhutia Y.D., Mody H., Hooks S.B., Dhar S., Govindarajan R. Defective hCNT1 transport contributes to gemcitabine chemoresistance in ovarian cancer subtypes: Overcoming transport defects using a nanoparticle approach. Cancer Lett. 2015;359:233–240. doi: 10.1016/j.canlet.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bosco D.B., Kenworthy R., Zorio D.A., Sang Q.X. Human mesenchymal stem cells are resistant to Paclitaxel by adopting a non-proliferative fibroblastic state. PLoS ONE. 2015;10:e0128511. doi: 10.1371/journal.pone.0128511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang X., Chen H., Zeng X., Guo W., Jin Y., Wang S., Tian R., Han Y., Guo L., Han J., et al. Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm. Sin. B. 2019;9:167–176. doi: 10.1016/j.apsb.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kalimuthu S., Gangadaran P., Rajendran R.L., Zhu L., Oh J.M., Lee H.W., Gopal A., Baek S.H., Jeong S.Y., Lee S.W., et al. A New Approach for Loading Anticancer Drugs Into Mesenchymal Stem Cell-Derived Exosome Mimetics for Cancer Therapy. Front. Pharmacol. 2018;9:1116. doi: 10.3389/fphar.2018.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vizoso F.J., Eiro N., Cid S., Schneider J., Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017;18:1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bonomi A., Sordi V., Dugnani E., Ceserani V., Dossena M., Cocce V., Cavicchini L., Ciusani E., Bondiolotti G., Piovani G., et al. Gemcitabine-releasing mesenchymal stromal cells inhibit in vitro proliferation of human pancreatic carcinoma cells. Cytotherapy. 2015;17:1687–1695. doi: 10.1016/j.jcyt.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 118.Pessina A., Cocce V., Pascucci L., Bonomi A., Cavicchini L., Sisto F., Ferrari M., Ciusani E., Crovace A., Falchetti M.L., et al. Mesenchymal stromal cells primed with Paclitaxel attract and kill leukaemia cells, inhibit angiogenesis and improve survival of leukaemia-bearing mice. Br. J. Haematol. 2013;160:766–778. doi: 10.1111/bjh.12196. [DOI] [PubMed] [Google Scholar]
- 119.Golinelli G., Mastrolia I., Aramini B., Masciale V., Pinelli M., Pacchioni L., Casari G., Dall’Ora M., Soares M.B.P., Damasceno P.K.F., et al. Arming Mesenchymal Stromal/Stem Cells Against Cancer: Has the Time Come? Front. Pharmacol. 2020;11:529921. doi: 10.3389/fphar.2020.529921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Studeny M., Marini F.C., Champlin R.E., Zompetta C., Fidler I.J., Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 121.Mueller L.P., Luetzkendorf J., Widder M., Nerger K., Caysa H., Mueller T. TRAIL-transduced multipotent mesenchymal stromal cells (TRAIL-MSC) overcome TRAIL resistance in selected CRC cell lines in vitro and in vivo. Cancer Gene Ther. 2011;18:229–239. doi: 10.1038/cgt.2010.68. [DOI] [PubMed] [Google Scholar]
- 122.Nakamura K., Ito Y., Kawano Y., Kurozumi K., Kobune M., Tsuda H., Bizen A., Honmou O., Niitsu Y., Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 123.Chen X., Lin X., Zhao J., Shi W., Zhang H., Wang Y., Kan B., Du L., Wang B., Wei Y., et al. A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Mol. Ther. J. Am. Soc. Gene Ther. 2008;16:749–756. doi: 10.1038/mt.2008.3. [DOI] [PubMed] [Google Scholar]
- 124.Ryu C.H., Park S.H., Park S.A., Kim S.M., Lim J.Y., Jeong C.H., Yoon W.S., Oh W.I., Sung Y.C., Jeun S.S. Gene therapy of intracranial glioma using interleukin 12-secreting human umbilical cord blood-derived mesenchymal stem cells. Hum. Gene Ther. 2011;22:733–743. doi: 10.1089/hum.2010.187. [DOI] [PubMed] [Google Scholar]
- 125.Jing W., Chen Y., Lu L., Hu X., Shao C., Zhang Y., Zhou X., Zhou Y., Wu L., Liu R., et al. Human umbilical cord blood-derived mesenchymal stem cells producing IL15 eradicate established pancreatic tumor in syngeneic mice. Mol. Cancer Ther. 2014;13:2127–2137. doi: 10.1158/1535-7163.MCT-14-0175. [DOI] [PubMed] [Google Scholar]
- 126.Sun Y.P., Zhang B.L., Duan J.W., Wu H.H., Wang B.Q., Yu Z.P., Yang W.J., Shan Y.F., Zhou M.T., Zhang Q.Y. Effect of NK4 transduction in bone marrow-derived mesenchymal stem cells on biological characteristics of pancreatic cancer cells. Int. J. Mol. Sci. 2014;15:3729–3745. doi: 10.3390/ijms15033729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang X.J., Lu J.T., Tu H.J., Huang K.M., Fu R., Cao G., Huang M., Cheng L.H., Dai L.J., Zhang L. TRAIL-engineered bone marrow-derived mesenchymal stem cells: TRAIL expression and cytotoxic effects on C6 glioma cells. Anticancer Res. 2014;34:729–734. [PubMed] [Google Scholar]
- 128.Ren C., Kumar S., Chanda D., Chen J., Mountz J.D., Ponnazhagan S. Therapeutic potential of mesenchymal stem cells producing interferon-alpha in a mouse melanoma lung metastasis model. Stem Cells. 2008;26:2332–2338. doi: 10.1634/stemcells.2008-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li X., Lu Y., Huang W., Xu H., Chen X., Geng Q., Fan H., Tan Y., Xue G., Jiang X. In vitro effect of adenovirus-mediated human Gamma Interferon gene transfer into human mesenchymal stem cells for chronic myelogenous leukemia. Hematol. Oncol. 2006;24:151–158. doi: 10.1002/hon.779. [DOI] [PubMed] [Google Scholar]
- 130.Zhao C., Pu Y., Zhang H., Hu X., Zhang R., He S., Zhao Q., Mu B. IL10-modified Human Mesenchymal Stem Cells inhibit Pancreatic Cancer growth through Angiogenesis Inhibition. J. Cancer. 2020;11:5345–5352. doi: 10.7150/jca.38062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim N., Nam Y.S., Im K.I., Lim J.Y., Lee E.S., Jeon Y.W., Cho S.G. IL-21-Expressing Mesenchymal Stem Cells Prevent Lethal B-Cell Lymphoma Through Efficient Delivery of IL-21, Which Redirects the Immune System to Target the Tumor. Stem Cells Dev. 2015;24:2808–2821. doi: 10.1089/scd.2015.0103. [DOI] [PubMed] [Google Scholar]
- 132.Takayama Y., Kusamori K., Nishikawa M. Mesenchymal stem/stromal cells as next-generation drug delivery vehicles for cancer therapeutics. Expert Opin. Drug Deliv. 2021;18:1627–1642. doi: 10.1080/17425247.2021.1960309. [DOI] [PubMed] [Google Scholar]
- 133.Kim S.M., Woo J.S., Jeong C.H., Ryu C.H., Jang J.D., Jeun S.S. Potential application of temozolomide in mesenchymal stem cell-based TRAIL gene therapy against malignant glioma. Stem Cells Transl. Med. 2014;3:172–182. doi: 10.5966/sctm.2013-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yan C., Li S., Li Z., Peng H., Yuan X., Jiang L., Zhang Y., Fan D., Hu X., Yang M., et al. Human umbilical cord mesenchymal stem cells as vehicles of CD20-specific TRAIL fusion protein delivery: A double-target therapy against non-Hodgkin’s lymphoma. Mol. Pharm. 2013;10:142–151. doi: 10.1021/mp300261e. [DOI] [PubMed] [Google Scholar]
- 135.Yang P.M., Hsieh Y.Y., Du J.L., Yen S.C., Hung C.F. Sequential Interferon beta-Cisplatin Treatment Enhances the Surface Exposure of Calreticulin in Cancer Cells via an Interferon Regulatory Factor 1-Dependent Manner. Biomolecules. 2020;10:643. doi: 10.3390/biom10040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang X., Zhang X., Fu M.L., Weichselbaum R.R., Gajewski T.F., Guo Y., Fu Y.X. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu G., Guo Y., Seng Z., Cui G., Qu J. Bone marrow-derived mesenchymal stem cells co-expressing interleukin-18 and interferon-beta exhibit potent antitumor effect against intracranial glioma in rats. Oncol. Rep. 2015;34:1915–1922. doi: 10.3892/or.2015.4174. [DOI] [PubMed] [Google Scholar]
- 138.Mirlekar B., Pylayeva-Gupta Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers. 2021;13:167. doi: 10.3390/cancers13020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gao P., Ding Q., Wu Z., Jiang H., Fang Z. Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Lett. 2010;290:157–166. doi: 10.1016/j.canlet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 140.Chen D., Tang P., Liu L., Wang F., Xing H., Sun L., Jiang Z. Bone marrow-derived mesenchymal stem cells promote cell proliferation of multiple myeloma through inhibiting T cell immune responses via PD-1/PD-L1 pathway. Cell Cycle. 2018;17:858–867. doi: 10.1080/15384101.2018.1442624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Musso A., Catellani S., Canevali P., Tavella S., Vene R., Boero S., Pierri I., Gobbi M., Kunkl A., Ravetti J.L., et al. Aminobisphosphonates prevent the inhibitory effects exerted by lymph node stromal cells on anti-tumor Vdelta 2 T lymphocytes in non-Hodgkin lymphomas. Haematologica. 2014;99:131–139. doi: 10.3324/haematol.2013.097311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kanehira M., Xin H., Hoshino K., Maemondo M., Mizuguchi H., Hayakawa T., Matsumoto K., Nakamura T., Nukiwa T., Saijo Y. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 2007;14:894–903. doi: 10.1038/sj.cgt.7701079. [DOI] [PubMed] [Google Scholar]
- 143.Choi S.H., Tamura K., Khajuria R.K., Bhere D., Nesterenko I., Lawler J., Shah K. Antiangiogenic variant of TSP-1 targets tumor cells in glioblastomas. Mol. Ther. J. Am. Soc. Gene Ther. 2015;23:235–243. doi: 10.1038/mt.2014.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mangraviti A., Tzeng S.Y., Gullotti D., Kozielski K.L., Kim J.E., Seng M., Abbadi S., Schiapparelli P., Sarabia-Estrada R., Vescovi A., et al. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials. 2016;100:53–66. doi: 10.1016/j.biomaterials.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guo X.R., Yang Z.S., Tang X.J., Zou D.D., Gui H., Wang X.L., Ma S.N., Yuan Y.H., Fang J., Wang B., et al. The application of mRNA-based gene transfer in mesenchymal stem cell-mediated cytotoxicity of glioma cells. Oncotarget. 2016;7:55529–55542. doi: 10.18632/oncotarget.10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guo X.R., Hu Q.Y., Yuan Y.H., Tang X.J., Yang Z.S., Zou D.D., Bian L.J., Dai L.J., Li D.S. PTEN-mRNA engineered mesenchymal stem cell-mediated cytotoxic effects on U251 glioma cells. Oncol. Lett. 2016;11:2733–2740. doi: 10.3892/ol.2016.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Asija S., Chatterjee A., Goda J.S., Yadav S., Chekuri G., Purwar R. Oncolytic immunovirotherapy for high-grade gliomas: A novel and an evolving therapeutic option. Front. Immunol. 2023;14:1118246. doi: 10.3389/fimmu.2023.1118246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zheng M., Huang J., Tong A., Yang H. Oncolytic Viruses for Cancer Therapy: Barriers and Recent Advances. Mol. Ther. Oncolytics. 2019;15:234–247. doi: 10.1016/j.omto.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hemminki O., Dos Santos J.M., Hemminki A. Oncolytic viruses for cancer immunotherapy. J. Hematol. Oncol. 2020;13:84. doi: 10.1186/s13045-020-00922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moreno R. Mesenchymal stem cells and oncolytic viruses: Joining forces against cancer. J. Immunother. Cancer. 2021;9:e001684. doi: 10.1136/jitc-2020-001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kaufman H.L., Kohlhapp F.J., Zloza A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Reale A., Calistri A., Altomonte J. Giving Oncolytic Viruses a Free Ride: Carrier Cells for Oncolytic Virotherapy. Pharmaceutics. 2021;13:2192. doi: 10.3390/pharmaceutics13122192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hadrys A., Sochanik A., McFadden G., Jazowiecka-Rakus J. Mesenchymal stem cells as carriers for systemic delivery of oncolytic viruses. Eur. J. Pharmacol. 2020;874:172991. doi: 10.1016/j.ejphar.2020.172991. [DOI] [PubMed] [Google Scholar]
- 154.Ruano D., Lopez-Martin J.A., Moreno L., Lassaletta A., Bautista F., Andion M., Hernandez C., Gonzalez-Murillo A., Melen G., Alemany R., et al. First-in-Human, First-in-Child Trial of Autologous MSCs Carrying the Oncolytic Virus Icovir-5 in Patients with Advanced Tumors. Mol. Ther. J. Am. Soc. Gene Ther. 2020;28:1033–1042. doi: 10.1016/j.ymthe.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chulpanova D.S., Kitaeva K.V., Tazetdinova L.G., James V., Rizvanov A.A., Solovyeva V.V. Application of Mesenchymal Stem Cells for Therapeutic Agent Delivery in Anti-tumor Treatment. Front. Pharmacol. 2018;9:259. doi: 10.3389/fphar.2018.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liang W., Chen X., Zhang S., Fang J., Chen M., Xu Y. Mesenchymal stem cells as a double-edged sword in tumor growth: Focusing on MSC-derived cytokines. Cell Mol. Biol. Lett. 2021;26:3. doi: 10.1186/s11658-020-00246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morales-Molina A., Gambera S., Cejalvo T., Moreno R., Rodriguez-Milla M.A., Perise-Barrios A.J., Garcia-Castro J. Antitumor virotherapy using syngeneic or allogeneic mesenchymal stem cell carriers induces systemic immune response and intratumoral leukocyte infiltration in mice. Cancer Immunol. Immunother. CII. 2018;67:1589–1602. doi: 10.1007/s00262-018-2220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Morales-Molina A., Gambera S., Leo A., Garcia-Castro J. Combination immunotherapy using G-CSF and oncolytic virotherapy reduces tumor growth in osteosarcoma. J. Immunother. Cancer. 2021;9:e001703. doi: 10.1136/jitc-2020-001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rincon E., Cejalvo T., Kanojia D., Alfranca A., Rodriguez-Milla M.A., Gil Hoyos R.A., Han Y., Zhang L., Alemany R., Lesniak M.S., et al. Mesenchymal stem cell carriers enhance antitumor efficacy of oncolytic adenoviruses in an immunocompetent mouse model. Oncotarget. 2017;8:45415–45431. doi: 10.18632/oncotarget.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hoyos V., Del Bufalo F., Yagyu S., Ando M., Dotti G., Suzuki M., Bouchier-Hayes L., Alemany R., Brenner M.K. Mesenchymal Stromal Cells for Linked Delivery of Oncolytic and Apoptotic Adenoviruses to Non-small-cell Lung Cancers. Mol. Ther. J. Am. Soc. Gene Ther. 2015;23:1497–1506. doi: 10.1038/mt.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moreno R., Rojas L.A., Villellas F.V., Soriano V.C., Garcia-Castro J., Fajardo C.A., Alemany R. Human Menstrual Blood-Derived Mesenchymal Stem Cells as Potential Cell Carriers for Oncolytic Adenovirus. Stem Cells Int. 2017;2017:3615729. doi: 10.1155/2017/3615729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moreno R., Fajardo C.A., Farrera-Sal M., Perise-Barrios A.J., Morales-Molina A., Al-Zaher A.A., Garcia-Castro J., Alemany R. Enhanced Antitumor Efficacy of Oncolytic Adenovirus-loaded Menstrual Blood-derived Mesenchymal Stem Cells in Combination with Peripheral Blood Mononuclear Cells. Mol. Cancer Ther. 2019;18:127–138. doi: 10.1158/1535-7163.MCT-18-0431. [DOI] [PubMed] [Google Scholar]
- 163.Barlabe P., Sostoa J., Fajardo C.A., Alemany R., Moreno R. Enhanced antitumor efficacy of an oncolytic adenovirus armed with an EGFR-targeted BiTE using menstrual blood-derived mesenchymal stem cells as carriers. Cancer Gene Ther. 2020;27:383–388. doi: 10.1038/s41417-019-0110-1. [DOI] [PubMed] [Google Scholar]
- 164.Martinez-Quintanilla J., He D., Wakimoto H., Alemany R., Shah K. Encapsulated stem cells loaded with hyaluronidase-expressing oncolytic virus for brain tumor therapy. Mol. Ther. J. Am. Soc. Gene Ther. 2015;23:108–118. doi: 10.1038/mt.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cejalvo T., Perise-Barrios A.J., Del Portillo I., Laborda E., Rodriguez-Milla M.A., Cubillo I., Vazquez F., Sardon D., Ramirez M., Alemany R., et al. Remission of Spontaneous Canine Tumors after Systemic Cellular Viroimmunotherapy. Cancer Res. 2018;78:4891–4901. doi: 10.1158/0008-5472.CAN-17-3754. [DOI] [PubMed] [Google Scholar]
- 166.Delgado-Bonet P., Tomeo-Martin B.D., Ortiz-Diez G., Perise-Barrios A.J. Tumor-Homing of Mesenchymal Stem Cells Infected with Oncolytic Virus in a Canine Patient. Vet. Sci. 2022;9:285. doi: 10.3390/vetsci9060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hsiao W.C., Sung S.Y., Liao C.H., Wu H.C., Hsieh C.L. Vitamin D3-inducible mesenchymal stem cell-based delivery of conditionally replicating adenoviruses effectively targets renal cell carcinoma and inhibits tumor growth. Mol. Pharm. 2012;9:1396–1408. doi: 10.1021/mp200649g. [DOI] [PubMed] [Google Scholar]
- 168.Na Y., Nam J.P., Hong J., Oh E., Shin H.C., Kim H.S., Kim S.W., Yun C.O. Systemic administration of human mesenchymal stromal cells infected with polymer-coated oncolytic adenovirus induces efficient pancreatic tumor homing and infiltration. J. Control. Release Off. J. Control. Release Soc. 2019;305:75–88. doi: 10.1016/j.jconrel.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nilson R., Krutzke L., Wienen F., Rojewski M., Zeplin P.H., Funk W., Schrezenmeier H., Kochanek S., Kritzinger A. Evaluation of Human Mesenchymal Stromal Cells as Carriers for the Delivery of Oncolytic HAdV-5 to Head and Neck Squamous Cell Carcinomas. Viruses. 2023;15:218. doi: 10.3390/v15010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang P., Zhang J., Zhang Q., Liu F. Mesenchymal stem cells loaded with Ad5-Ki67/IL-15 enhance oncolytic adenovirotherapy in experimental glioblastoma. Biomed. Pharmacother. 2023;157:114035. doi: 10.1016/j.biopha.2022.114035. [DOI] [PubMed] [Google Scholar]
- 171.Hakkarainen T., Sarkioja M., Lehenkari P., Miettinen S., Ylikomi T., Suuronen R., Desmond R.A., Kanerva A., Hemminki A. Human mesenchymal stem cells lack tumor tropism but enhance the antitumor activity of oncolytic adenoviruses in orthotopic lung and breast tumors. Hum. Gene Ther. 2007;18:627–641. doi: 10.1089/hum.2007.034. [DOI] [PubMed] [Google Scholar]
- 172.Yuan X., Zhang Q., Li Z., Zhang X., Bao S., Fan D., Ru Y., Dong S., Zhang Y., Zhang Y., et al. Mesenchymal stem cells deliver and release conditionally replicative adenovirus depending on hepatic differentiation to eliminate hepatocellular carcinoma cells specifically. Cancer Lett. 2016;381:85–95. doi: 10.1016/j.canlet.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 173.Hammer K., Kazcorowski A., Liu L., Behr M., Schemmer P., Herr I., Nettelbeck D.M. Engineered adenoviruses combine enhanced oncolysis with improved virus production by mesenchymal stromal carrier cells. Int. J. Cancer. 2015;137:978–990. doi: 10.1002/ijc.29442. [DOI] [PubMed] [Google Scholar]
- 174.Komarova S., Kawakami Y., Stoff-Khalili M.A., Curiel D.T., Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol. Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 175.Kaczorowski A., Hammer K., Liu L., Villhauer S., Nwaeburu C., Fan P., Zhao Z., Gladkich J., Gross W., Nettelbeck D.M., et al. Delivery of improved oncolytic adenoviruses by mesenchymal stromal cells for elimination of tumorigenic pancreatic cancer cells. Oncotarget. 2016;7:9046–9059. doi: 10.18632/oncotarget.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li Z., Ye Z., Zhang X., Zhang Q., Fan D., Zhang Y., Luo H.R., Yuan X., Li Z., Xiong D. E1A-engineered human umbilical cord mesenchymal stem cells as carriers and amplifiers for adenovirus suppress hepatocarcinoma in mice. Oncotarget. 2016;7:51815–51828. doi: 10.18632/oncotarget.10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yoon A.R., Hong J., Li Y., Shin H.C., Lee H., Kim H.S., Yun C.O. Mesenchymal Stem Cell-Mediated Delivery of an Oncolytic Adenovirus Enhances Antitumor Efficacy in Hepatocellular Carcinoma. Cancer Res. 2019;79:4503–4514. doi: 10.1158/0008-5472.CAN-18-3900. [DOI] [PubMed] [Google Scholar]
- 178.Mahasa K.J., de Pillis L., Ouifki R., Eladdadi A., Maini P., Yoon A.R., Yun C.O. Mesenchymal stem cells used as carrier cells of oncolytic adenovirus results in enhanced oncolytic virotherapy. Sci. Rep. 2020;10:425. doi: 10.1038/s41598-019-57240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Muhammad T., Sakhawat A., Khan A.A., Ma L., Gjerset R.A., Huang Y. Mesenchymal stem cell-mediated delivery of therapeutic adenoviral vectors to prostate cancer. Stem Cell Res. Ther. 2019;10:190. doi: 10.1186/s13287-019-1268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stoff-Khalili M.A., Rivera A.A., Mathis J.M., Banerjee N.S., Moon A.S., Hess A., Rocconi R.P., Numnum T.M., Everts M., Chow L.T., et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res. Treat. 2007;105:157–167. doi: 10.1007/s10549-006-9449-8. [DOI] [PubMed] [Google Scholar]
- 181.Ho C.T., Wu M.H., Chen M.J., Lin S.P., Yen Y.T., Hung S.C. Combination of Mesenchymal Stem Cell-Delivered Oncolytic Virus with Prodrug Activation Increases Efficacy and Safety of Colorectal Cancer Therapy. Biomedicines. 2021;9:548. doi: 10.3390/biomedicines9050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Guo Y., Zhang Z., Xu X., Xu Z., Wang S., Huang D., Li Y., Mou X., Liu F., Xiang C. Menstrual Blood-Derived Stem Cells as Delivery Vehicles for Oncolytic Adenovirus Virotherapy for Colorectal Cancer. Stem Cells Dev. 2019;28:882–896. doi: 10.1089/scd.2018.0222. [DOI] [PubMed] [Google Scholar]
- 183.Bolontrade M.F., Sganga L., Piaggio E., Viale D.L., Sorrentino M.A., Robinson A., Sevlever G., Garcia M.G., Mazzolini G., Podhajcer O.L. A specific subpopulation of mesenchymal stromal cell carriers overrides melanoma resistance to an oncolytic adenovirus. Stem Cells Dev. 2012;21:2689–2702. doi: 10.1089/scd.2011.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sonabend A.M., Ulasov I.V., Tyler M.A., Rivera A.A., Mathis J.M., Lesniak M.S. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26:831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 185.Ahmed A.U., Rolle C.E., Tyler M.A., Han Y., Sengupta S., Wainwright D.A., Balyasnikova I.V., Ulasov I.V., Lesniak M.S. Bone marrow mesenchymal stem cells loaded with an oncolytic adenovirus suppress the anti-adenoviral immune response in the cotton rat model. Mol. Ther. J. Am. Soc. Gene Ther. 2010;18:1846–1856. doi: 10.1038/mt.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang Y., Liu C., Wang T., Kong F., Zhang H., Yi J., Dong X., Duan H., Tao N., Yang Y., et al. Therapeutic effects of mesenchymal stem cells loaded with oncolytic adenovirus carrying decorin on a breast cancer lung metastatic mouse model. Mol. Ther. Oncolytics. 2022;24:486–496. doi: 10.1016/j.omto.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dembinski J.L., Spaeth E.L., Fueyo J., Gomez-Manzano C., Studeny M., Andreeff M., Marini F.C. Reduction of nontarget infection and systemic toxicity by targeted delivery of conditionally replicating viruses transported in mesenchymal stem cells. Cancer Gene Ther. 2010;17:289–297. doi: 10.1038/cgt.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yong R.L., Shinojima N., Fueyo J., Gumin J., Vecil G.G., Marini F.C., Bogler O., Andreeff M., Lang F.F. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69:8932–8940. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shinojima N., Hossain A., Takezaki T., Fueyo J., Gumin J., Gao F., Nwajei F., Marini F.C., Andreeff M., Kuratsu J., et al. TGF-beta mediates homing of bone marrow-derived human mesenchymal stem cells to glioma stem cells. Cancer Res. 2013;73:2333–2344. doi: 10.1158/0008-5472.CAN-12-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shimizu Y., Gumin J., Gao F., Hossain A., Shpall E.J., Kondo A., Parker Kerrigan B.C., Yang J., Ledbetter D., Fueyo J., et al. Characterization of patient-derived bone marrow human mesenchymal stem cells as oncolytic virus carriers for the treatment of glioblastoma. J. Neurosurg. 2022;136:757–767. doi: 10.3171/2021.3.JNS203045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang J., Chen H., Chen C., Liu H., He Y., Zhao J., Yang P., Mao Q., Xia H. Systemic administration of mesenchymal stem cells loaded with a novel oncolytic adenovirus carrying IL-24/endostatin enhances glioma therapy. Cancer Lett. 2021;509:26–38. doi: 10.1016/j.canlet.2021.03.027. [DOI] [PubMed] [Google Scholar]
- 192.He X., Yao W., Zhu J.D., Jin X., Liu X.Y., Zhang K.J., Zhao S.L. Potent antitumor efficacy of human dental pulp stem cells armed with YSCH-01 oncolytic adenovirus. J. Transl. Med. 2023;21:688. doi: 10.1186/s12967-023-04539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Du W., Seah I., Bougazzoul O., Choi G., Meeth K., Bosenberg M.W., Wakimoto H., Fisher D., Shah K. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proc. Natl. Acad. Sci. USA. 2017;114:E6157–E6165. doi: 10.1073/pnas.1700363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Leoni V., Gatta V., Palladini A., Nicoletti G., Ranieri D., Dall’Ora M., Grosso V., Rossi M., Alviano F., Bonsi L., et al. Systemic delivery of HER2-retargeted oncolytic-HSV by mesenchymal stromal cells protects from lung and brain metastases. Oncotarget. 2015;6:34774–34787. doi: 10.18632/oncotarget.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nguyen D.H., Herrmann T., Hartl B., Draganov D., Minev I., Neuharth F., Gomez A., Alamillo A., Schneider L.E., Kleinholz D., et al. Development of Allogeneic Stem Cell-Based Platform for Delivery and Potentiation of Oncolytic Virotherapy. Cancers. 2022;14:6136. doi: 10.3390/cancers14246136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Petrov I., Gentschev I., Vyalkova A., Elashry M.I., Klymiuk M.C., Arnhold S., Szalay A.A. Canine Adipose-Derived Mesenchymal Stem Cells (cAdMSCs) as a “Trojan Horse” in Vaccinia Virus Mediated Oncolytic Therapy against Canine Soft Tissue Sarcomas. Viruses. 2020;12:750. doi: 10.3390/v12070750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Draganov D.D., Santidrian A.F., Minev I., Nguyen D., Kilinc M.O., Petrov I., Vyalkova A., Lander E., Berman M., Minev B., et al. Delivery of oncolytic vaccinia virus by matched allogeneic stem cells overcomes critical innate and adaptive immune barriers. J. Transl. Med. 2019;17:100. doi: 10.1186/s12967-019-1829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jazowiecka-Rakus J., Sochanik A., Rusin A., Hadrys A., Fidyk W., Villa N., Rahman M.M., Chmielik E., Franco L.S., McFadden G. Myxoma Virus-Loaded Mesenchymal Stem Cells in Experimental Oncolytic Therapy of Murine Pulmonary Melanoma. Mol. Ther. Oncolytics. 2020;18:335–350. doi: 10.1016/j.omto.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jazowiecka-Rakus J., Hadrys A., Rahman M.M., McFadden G., Fidyk W., Chmielik E., Pazdzior M., Grajek M., Kozik V., Sochanik A. Myxoma Virus Expressing LIGHT (TNFSF14) Pre-Loaded into Adipose-Derived Mesenchymal Stem Cells Is Effective Treatment for Murine Pancreatic Adenocarcinoma. Cancers. 2021;13:1394. doi: 10.3390/cancers13061394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jazowiecka-Rakus J., Sochanik A., Hadrys A., Fidyk W., Chmielik E., Rahman M.M., McFadden G. Combination of LIGHT (TNFSF14)-Armed Myxoma Virus Pre-Loaded into ADSCs and Gemcitabine in the Treatment of Experimental Orthotopic Murine Pancreatic Adenocarcinoma. Cancers. 2022;14:2022. doi: 10.3390/cancers14082022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Josiah D.T., Zhu D., Dreher F., Olson J., McFadden G., Caldas H. Adipose-derived stem cells as therapeutic delivery vehicles of an oncolytic virus for glioblastoma. Mol. Ther. J. Am. Soc. Gene Ther. 2010;18:377–385. doi: 10.1038/mt.2009.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mader E.K., Maeyama Y., Lin Y., Butler G.W., Russell H.M., Galanis E., Russell S.J., Dietz A.B., Peng K.W. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009;15:7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mader E.K., Butler G., Dowdy S.C., Mariani A., Knutson K.L., Federspiel M.J., Russell S.J., Galanis E., Dietz A.B., Peng K.W. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J. Transl. Med. 2013;11:20. doi: 10.1186/1479-5876-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ong H.T., Federspiel M.J., Guo C.M., Ooi L.L., Russell S.J., Peng K.W., Hui K.M. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J. Hepatol. 2013;59:999–1006. doi: 10.1016/j.jhep.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Castleton A., Dey A., Beaton B., Patel B., Aucher A., Davis D.M., Fielding A.K. Human mesenchymal stromal cells deliver systemic oncolytic measles virus to treat acute lymphoblastic leukemia in the presence of humoral immunity. Blood. 2014;123:1327–1335. doi: 10.1182/blood-2013-09-528851. [DOI] [PubMed] [Google Scholar]
- 206.Ghorbani Alvanegh A., Mirzaei Nodooshan M., Dorostkar R., Ranjbar R., Jalali Kondori B., Shahriary A., Parastouei K., Vazifedust S., Afrasiab E., Esmaeili Gouvarchinghaleh H. Antiproliferative effects of mesenchymal stem cells carrying Newcastle disease virus and Lactobacillus Casei extract on CT26 Cell line: Synergistic effects in cancer therapy. Infect. Agents Cancer. 2023;18:46. doi: 10.1186/s13027-023-00521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Keshavarz M., Ebrahimzadeh M.S., Miri S.M., Dianat-Moghadam H., Ghorbanhosseini S.S., Mohebbi S.R., Keyvani H., Ghaemi A. Oncolytic Newcastle disease virus delivered by Mesenchymal stem cells-engineered system enhances the therapeutic effects altering tumor microenvironment. Virol. J. 2020;17:64. doi: 10.1186/s12985-020-01326-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kazimirsky G., Jiang W., Slavin S., Ziv-Av A., Brodie C. Mesenchymal stem cells enhance the oncolytic effect of Newcastle disease virus in glioma cells and glioma stem cells via the secretion of TRAIL. Stem Cell Res. Ther. 2016;7:149. doi: 10.1186/s13287-016-0414-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Seyed-Khorrami S.M., Soleimanjahi H., Soudi S., Habibian A. MSCs loaded with oncolytic reovirus: Migration and in vivo virus delivery potential for evaluating anti-cancer effect in tumor-bearing C57BL/6 mice. Cancer Cell Int. 2021;21:244. doi: 10.1186/s12935-021-01848-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Babaei A., Bannazadeh Baghi H., Nezhadi A., Jamalpoor Z. In Vitro Anti-cancer Activity of Adipose-Derived Mesenchymal Stem Cells Increased after Infection with Oncolytic Reovirus. Adv. Pharm. Bull. 2021;11:361–370. doi: 10.34172/apb.2021.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Babaei A., Soleimanjahi H., Soleimani M., Arefian E. Mesenchymal stem cells loaded with oncolytic reovirus enhances antitumor activity in mice models of colorectal cancer. Biochem. Pharmacol. 2021;190:114644. doi: 10.1016/j.bcp.2021.114644. [DOI] [PubMed] [Google Scholar]
- 212.Banijamali R.S., Soleimanjahi H., Soudi S., Karimi H. Mesenchymal stem cells support delivery and boost the efficacy of oncolytic reoviruses in TC-1 tumor cells. J. Cell. Biochem. 2021;122:1360–1375. doi: 10.1002/jcb.29955. [DOI] [PubMed] [Google Scholar]
- 213.Wang X., Zhao X., He Z. Mesenchymal stem cell carriers enhance anti-tumor efficacy of oncolytic virotherapy. Oncol. Lett. 2021;21:238. doi: 10.3892/ol.2021.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zarogoulidis P., Darwiche K., Sakkas A., Yarmus L., Huang H., Li Q., Freitag L., Zarogoulidis K., Malecki M. Suicide Gene Therapy for Cancer—Current Strategies. J. Genet. Syndr. Gene Ther. 2013;4:16849. doi: 10.4172/2157-7412.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Moolten F.L. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: Paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. [PubMed] [Google Scholar]
- 216.Fillat C., Carrio M., Cascante A., Sangro B. Suicide gene therapy mediated by the Herpes Simplex virus thymidine kinase gene/Ganciclovir system: Fifteen years of application. Curr. Gene Ther. 2003;3:13–26. doi: 10.2174/1566523033347426. [DOI] [PubMed] [Google Scholar]
- 217.Zhang T.Y., Huang B., Wu H.B., Wu J.H., Li L.M., Li Y.X., Hu Y.L., Han M., Shen Y.Q., Tabata Y., et al. Synergistic effects of co-administration of suicide gene expressing mesenchymal stem cells and prodrug-encapsulated liposome on aggressive lung melanoma metastases in mice. J. Control. Release Off. J. Control. Release Soc. 2015;209:260–271. doi: 10.1016/j.jconrel.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 218.Leng L., Wang Y., He N., Wang D., Zhao Q., Feng G., Su W., Xu Y., Han Z., Kong D., et al. Molecular imaging for assessment of mesenchymal stem cells mediated breast cancer therapy. Biomaterials. 2014;35:5162–5170. doi: 10.1016/j.biomaterials.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Alieva M., Bago J.R., Aguilar E., Soler-Botija C., Vila O.F., Molet J., Gambhir S.S., Rubio N., Blanco J. Glioblastoma therapy with cytotoxic mesenchymal stromal cells optimized by bioluminescence imaging of tumor and therapeutic cell response. PLoS ONE. 2012;7:e35148. doi: 10.1371/journal.pone.0035148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yang J., Lv K., Sun J., Guan J. Anti-tumor effects of engineered mesenchymal stem cells in colon cancer model. Cancer Manag. Res. 2019;11:8443–8450. doi: 10.2147/CMAR.S209880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chang D.Y., Jung J.H., Kim A.A., Marasini S., Lee Y.J., Paek S.H., Kim S.S., Suh-Kim H. Combined effects of mesenchymal stem cells carrying cytosine deaminase gene with 5-fluorocytosine and temozolomide in orthotopic glioma model. Am. J. Cancer Res. 2020;10:1429–1441. [PMC free article] [PubMed] [Google Scholar]
- 222.NguyenThai Q.A., Sharma N., Luong D.H., Sodhi S.S., Kim J.H., Kim N., Oh S.J., Jeong D.K. Targeted inhibition of osteosarcoma tumor growth by bone marrow-derived mesenchymal stem cells expressing cytosine deaminase/5-fluorocytosine in tumor-bearing mice. J. Gene Med. 2015;17:87–99. doi: 10.1002/jgm.2826. [DOI] [PubMed] [Google Scholar]
- 223.Nowakowski A., Drela K., Rozycka J., Janowski M., Lukomska B. Engineered Mesenchymal Stem Cells as an Anti-Cancer Trojan Horse. Stem Cells Dev. 2016;25:1513–1531. doi: 10.1089/scd.2016.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Abrate A., Buono R., Canu T., Esposito A., Del Maschio A., Luciano R., Bettiga A., Colciago G., Guazzoni G., Benigni F., et al. Mesenchymal stem cells expressing therapeutic genes induce autochthonous prostate tumour regression. Eur. J. Cancer. 2014;50:2478–2488. doi: 10.1016/j.ejca.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 225.Kim M.G., Lee Y.J., Jung J.H., Chang D.Y., Bashyal N., Suh-Kim H., Kim S.S. Enhanced antitumor efficacy of mesenchymal stem cells expressing cytosine deaminase and 5-fluorocytosine combined with alpha-galactosylceramide in a colon cancer model. Am. J. Cancer Res. 2023;13:2439–2451. [PMC free article] [PubMed] [Google Scholar]
- 226.Choi S.A., Lee J.Y., Wang K.C., Phi J.H., Song S.H., Song J., Kim S.K. Human adipose tissue-derived mesenchymal stem cells: Characteristics and therapeutic potential as cellular vehicles for prodrug gene therapy against brainstem gliomas. Eur. J. Cancer. 2012;48:129–137. doi: 10.1016/j.ejca.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 227.Amara I., Pramil E., Senamaud-Beaufort C., Devillers A., Macedo R., Lescaille G., Seguin J., Tartour E., Lemoine F.M., Beaune P., et al. Engineered mesenchymal stem cells as vectors in a suicide gene therapy against preclinical murine models for solid tumors. J. Control. Release Off. J. Control. Release Soc. 2016;239:82–91. doi: 10.1016/j.jconrel.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 228.Yamamoto S., Yamano T., Tanaka M., Hoon D.S., Takao S., Morishita R., Aikou T., Kaneda Y. A novel combination of suicide gene therapy and histone deacetylase inhibitor for treatment of malignant melanoma. Cancer Gene Ther. 2003;10:179–186. doi: 10.1038/sj.cgt.7700551. [DOI] [PubMed] [Google Scholar]
- 229.Ryu C.H., Park K.Y., Kim S.M., Jeong C.H., Woo J.S., Hou Y., Jeun S.S. Valproic acid enhances anti-tumor effect of mesenchymal stem cell mediated HSV-TK gene therapy in intracranial glioma. Biochem. Biophys. Res. Commun. 2012;421:585–590. doi: 10.1016/j.bbrc.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 230.Sun K., Xu Y., Zhang L., Niravath P., Darcourt J., Patel T., Teh B.S., Farach A.M., Guerrero C., Mathur S., et al. A Phase 2 Trial of Enhancing Immune Checkpoint Blockade by Stereotactic Radiation and In Situ Virus Gene Therapy in Metastatic Triple-Negative Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022;28:4392–4401. doi: 10.1158/1078-0432.CCR-22-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Duhrsen L., Hartfuss S., Hirsch D., Geiger S., Maire C.L., Sedlacik J., Guenther C., Westphal M., Lamszus K., Hermann F.G., et al. Preclinical analysis of human mesenchymal stem cells: Tumor tropism and therapeutic efficiency of local HSV-TK suicide gene therapy in glioblastoma. Oncotarget. 2019;10:6049–6061. doi: 10.18632/oncotarget.27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Melen G.J., Franco-Luzon L., Ruano D., Gonzalez-Murillo A., Alfranca A., Casco F., Lassaletta A., Alonso M., Madero L., Alemany R., et al. Influence of carrier cells on the clinical outcome of children with neuroblastoma treated with high dose of oncolytic adenovirus delivered in mesenchymal stem cells. Cancer Lett. 2016;371:161–170. doi: 10.1016/j.canlet.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 233.Von Einem J.C., Peter S., Gunther C., Volk H.D., Grutz G., Salat C., Stoetzer O., Nelson P.J., Michl M., Modest D.P., et al. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells—TREAT-ME-1—A phase I, first in human, first in class trial. Oncotarget. 2017;8:80156–80166. doi: 10.18632/oncotarget.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]