Abstract
Background: It has been shown that obesity and a higher body mass index (BMI) are associated with a higher recurrence rate of atrial fibrillation (AF) after successful catheter ablation (CA). The same has been proven for the left atrial volume index (LAVI). It has also been shown that there is a correlation between LAVI and BMI. However, whether the LAVI’s prognostic impact on AF recurrence is BMI-independent remains unclear. Methods: We prospectively included 62 patients with paroxysmal AF who were referred to our institution for CA. All patients underwent radiofrequency CA with standard pulmonary veins isolation. Transthoracic 2-D echocardiography was performed one day after CA to obtain standard measures of cardiac function and morphology. Recurrence was defined as documented AF within 6 months of the follow-up period. Patients were also instructed to visit our outpatient clinic earlier in case of symptoms suggesting AF recurrence. Results: We observed AF recurrence in 27% of patients after 6 months. The mean BMI in our cohort was 29.65 ± 5.08 kg/cm2 and the mean LAVI was 38.04 ± 11.38 mL/m2. We further divided patients into two groups according to BMI. Even though the LAVI was similar in both groups, we found it to be a significant predictor of AF recurrence only in obese patients (BMI ≥ 30) and not in the non-obese group (BMI < 30). There was also no significant difference in AF recurrence between both cohorts. The significance of the LAVI as an AF recurrence predictor in the obesity group was also confirmed in a multivariate model. Conclusions: According to our results, the LAVI tends to be a significant predictor of AF recurrence after successful catheter ablation in obese patients, but not in normal-weight or overweight patients. This would suggest different mechanisms of AF in non-obese patients in comparison to obese patients. Further studies are needed in this regard.
Keywords: atrial fibrillation, catheter ablation, left atrial volume index, body mass index
1. Introduction
Atrial fibrillation (AF) is a chronic degenerative disorder of pandemic proportions. It was stated that it correlates with increasing age, as well as with common cardiovascular risk factors such hypertension, diabetes, hyperlipidaemia, and obesity [1]. Studies have been conducted to further enlighten the role of body mass index (BMI), body surface area (BSA), and height and weight in the pathophysiology of atrial remodelling and the subsequent incidence and prevalence of AF [2,3]. Regarding the left atrial remodelling process, computed tomography (CT) studies showed that excessive peri-atrial and pericardial adipose tissue could play a significant role in the loss of atrial architecture and left atrial dilatation [4,5]. However, this could be the main pathophysiologic process in obese patients but not in those with normal or near-normal weight. It has also been shown that obesity and a higher BMI are associated with a higher recurrence rate of atrial fibrillation (AF) after successful catheter ablation (CA) [6]. The same has been proven for the left atrial volume index (LAVI) [7]. It was also proven that there is a correlation between the LAVI and BMI [8]. However, whether LAVI’s prognostic impact on AF recurrence is BMI-independent remains unclear. The aim of this study was to compare correlations between the LAVI and AF recurrence after a successful CA in obese and non-obese patients and to further stratify whether the LAVI’s prognostic impact is the same across the whole BMI spectrum.
2. Methods
We prospectively included 62 patients with paroxysmal AF. All patients underwent radiofrequency CA with pulmonary vein isolation (PVI) as the primary target of the procedure. Sex, age, body height, body weight, body mass index (BMI), and type of antiarrhythmic therapy (AAT) were obtained before the procedure.
Prior to ablation, transoesophageal echocardiography was performed in all patients to rule out the presence of thrombus in the LA. The ablation procedure was performed under conscious sedation. Under fluoroscopic guidance, a transseptal puncture was performed. A detailed bipolar voltage map of the left atrium (LA) was then constructed using 20-polar catheter (Pentaray; Biosense-Webster, Irvine, CA, USA). An automated 3D mapping system (Carto, Biosense Webster, Irvine, CA, USA) was used in all patients. We used standard respiratory gating to minimise respiratory movement bias. Prior to ablation, contact force calibration was performed. Endocardial contact was ensured mainly by local electrogram and contact force measurements. Ablation was performed with a 3.5 mm irrigated-tipped catheter (SmartTouch Thermocool, Biosense Webster, Irvine, CA, USA). PVI was achieved with wide antral circumferential ablation. The isolation of the ablated region was then confirmed with entrance and exit block pacing manoeuvres. Direct current cardioversion to restore sinus rhythm was also performed after the successful procedure if patients were still in AF. Mapping and CA were performed by a single operator.
Standard transthoracic two-dimensional echocardiography was performed one day after CA to obtain a standard recording of cardiac function and morphology (Vivid E95, General Electric Vingmed, Milwaukee, WI, USA). Standard two-dimensional and Doppler measurements were obtained according to the current recommendations [9].
2.1. Follow-Up
All patients had a 12-lead ECG (25 mm/s, 10 mm/mV) recorded at their follow-up visit 6 months after CA to evaluate their basic rhythm. Patients were also instructed to visit an outpatient clinic earlier in case of symptoms suggesting AF recurrence. We excluded any observed AF during the blanking period: within 3 months after the procedure. In patients where AF was documented with Holter monitoring, this observation was also regarded as AF recurrence. The endpoint of our study was to estimate the AF recurrence rate diagnosed by ECG. Recurrence was defined as documented AF within the first 6 months of the follow-up period.
2.2. Statistical Analysis
Obtained data were analysed using SPSS version 26 (SPSS Inc., Chicago, IL, USA). We used Student’s t-test for evaluating differences between continuous variables and the chi-square test for the analysis of categorical variables. Continuous data are given as mean ± standard deviation (SD) and categorical variables are expressed as absolute values and percentages. A simple logistic regression method was used to assess the association between the LAVI and AF recurrence. The significance of other potential confounders was adjusted via multivariate logistic regression and the enter method.
3. Results
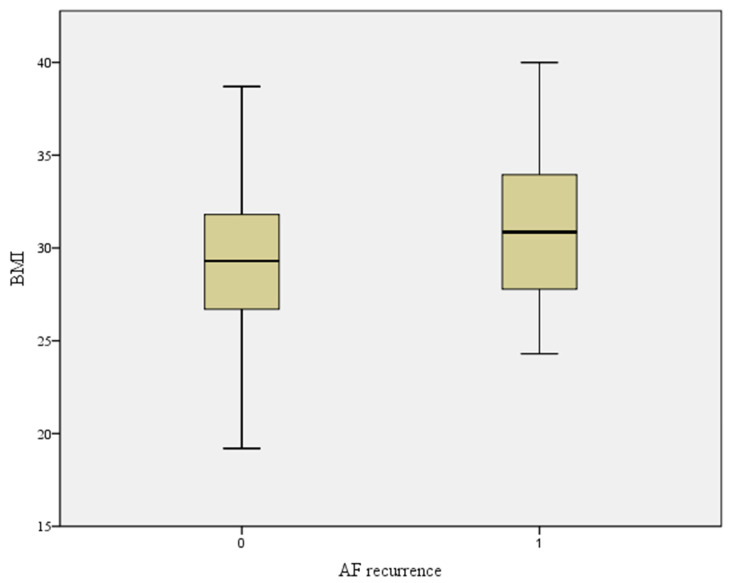
The patients’ data are displayed in Table 1. We observed AF recurrence in 27% of patients after 6 months. The mean BMI in our cohort was 29.65 ± 5.08 kg/cm2 and the mean LAVI was 38.04 ± 11.38 mL/m2. BMI was also elevated in AF recurrence patients, but not to the point of statistical significance (Figure 1). We further divided patients into two groups according to BMI (≥30 and <30). We found no statistically significant differences in age, sex, left ventricular ejection fraction, and comorbidities between both groups (Table 2). The LAVI was similar in both groups. There was a slightly higher percentage of AF recurrences in the obese group; however, the difference was not statistically significant (Table 2). We found the LAVI to be a significant predictor of AF recurrence in all patients. When we further divided patients according to BMI, we found the LAVI to be significantly associated with the AF recurrence rate only in obese patients (BMI ≥ 30) and not in normal-weight or overweight patients (BMI < 30) (Table 3). The significance of the LAVI as an AF recurrence predictor according to BMI was also confirmed in a multivariate model in both groups (adjusted for the confounding effects of hypertension, diabetes, left ventricular ejection fraction, and antiarrhythmic therapy) (Table 3).
Table 1.
Patient characteristics.
| Parameter | Mean and Standard Deviation |
|---|---|
| Age (years) | 61.52 ± 9.87 |
| Body height (cm) | 175.00 ± 9.43 |
| Body weight (kg) | 92.13 ± 15.58 |
| LAVI (mL/m2) | 38.04 ± 11.38 |
| BMI (kg/m2) | 29.65 ± 5.08 |
Legend (Table 1): LAVI—left atrial volume index; BMI—body mass index.
Figure 1.
BMI according to AF recurrence. Legend (Figure 1): BMI—body mass index, AF—atrial fibrillation; p—0.1.
Table 2.
Comparison of patients stratified according to BMI.
| BMI ≥ 30 kg/cm2 | BMI < 30 kg/cm2 | p Value | |
|---|---|---|---|
| Number of patients | 27 | 35 | |
| Age (years) | 61.3 ± 11.5 | 61.7 ± 7.3 | 0.9 |
| Sex (female) | 11(40.7%) | 6(17.1%) | 0.06 |
| LAVI (mL/m2) | 38.2 ± 13.2 | 37.8 ± 8.8 | 0.9 |
| AF recurrence after 6 months (%) | 9(33.3%) | 8(22.9%) | 0.4 |
| Antiarrhythmic treatment after CA | 26(89.7%) | 32(84.2%) | 0.7 |
| Left ventricular ejection fraction (%) | 58.7 ± 2.5 | 59.3 ± 2.8 | 0.4 |
| Hypertension | 18(66.6%) | 19(54.3%) | 0.5 |
| Diabetes | 1(4.0%) | 2(5.7%) | 1.0 |
Legend (Table 2): LAVI—left atrial volume index; AF—atrial fibrillation; BMI—body mass index; CA—catheter ablation.
Table 3.
LAVI as a predictor of AF recurrence after catheter ablation in both groups.
| Number of Patients | Odds Ratio for AF Recurrence | Confidence Interval | p Value | |
|---|---|---|---|---|
| LAVI (BMI < 30 kg/cm2)—unadjusted | 35 | 1.08 | 0.87–1.17 | 0.1 |
| LAVI (BMI < 30 kg/cm2)—adjusted * | 35 | 1.07 | 0.97–1.17 | 0.16 |
| LAVI (BMI ≥ 30 kg/cm2)—unadjusted | 27 | 1.29 | 1.07–1.54 | 0.007 |
| LAVI (BMI ≥ 30 kg/cm2)—adjusted * | 27 | 1.35 | 1.02–1.78 | 0.03 |
Legend (Table 3): LAVI—left atrial volume index; AF—atrial fibrillation; BMI—body mass index; * Adjustment for confounding effects of hypertension, diabetes, left ventricular ejection fraction, beta blockers, and antiarrhythmic therapy.
4. Discussion
Previous studies have demonstrated an association between left atrial (LA) size and the incidence of new-onset AF, as well as AF recurrence, after CA [3,6,7]. However, it is still controversial whether this is due to the enlargement of the LA per se or a consequence of the accompanying risk factors, such as obesity and its metabolic derangements. In a recent study, a CT-derived cut-off value for the LAVI of 51.99 mL/m2 was proposed as a prognostic marker for AF recurrence after CA, while the impact of BMI and other measures of obesity was not studied [3]. In our study, the LAVI was a predictor of AF recurrence only in obese patients, while no association of the LAVI with AF recurrence was demonstrated in normal-weight and overweight patients.
Obesity was proven to be an independent risk factor for the incidence of AF, with a 10–30% higher risk of AF for every 5 kg/m2 increase in BMI [10,11,12]. According to the reported estimations, it already accounts for almost one-fifth of AF cases [13,14]. Interestingly, Pranata et al. demonstrated a non-linear relationship of BMI with AF recurrence after CA, with a steeper curve in those with a BMI >30–35 [6]. Besides hemodynamic stress due to persistent volume overload, obesity was found to increase proinflammatory cytokines, induce insulin resistance, alter metabolic pathways, and induce gene expression profiles associated with cardiac hypertrophy, which result in subsequent electrophysiological, mechanical, and structural LA remodelling [12,15,16,17,18]. Obesity is also commonly associated with other risk factors for LA remodelling and AF, such as arterial hypertension, type 2 diabetes mellitus, and obstructive sleep apnea [19,20]. In obese patients, compared to non-obese patients, a lower global LA longitudinal strain, revealing LA mechanical dysfunction, was also demonstrated [21].
Besides BMI, additional clinical measures, such as waist circumference and waist-to-hip ratio, have been suggested to define regional body fat distribution more precisely and assess visceral fat. A correlation of the BMI and waist-to-hip ratio with visceral adiposity was reported, though it was influenced by gender and race [22,23,24,25]. Extensive evidence has demonstrated the more unfavourable metabolic and cardiovascular effects of visceral compared to subcutaneous fat, mediated by its endocrine proinflammatory and immunological mechanisms [26]. Most studies have evaluated the extra-thoracic part of visceral fat, especially its intra-abdominal distribution, and most of them have confirmed its association with an adverse metabolic phenotype and an enhanced cardiovascular risk [27,28]. In recent years, epicardial adipose tissue (EAT) has been increasingly advocated for as a critical part of visceral fat compartment, associated with LA size and function [5,22,29]. EAT promotes AF by direct fat infiltration of the underlying atrial myocardium, increased oxidative stress, local autonomic dysfunction, accelerated interstitial atrial fibrosis, and subsequent conduction slowing and heterogeneity [28,30,31,32]. EAT and associated inflammatory cytokines were linked with the incidence, severity, and recurrence of AF [33,34].
As noted in a study by van Rosendael et al., the incidence of paroxysmal AF was the highest in patients with a large amount of EAT, while increased LA size predicted AF persistency [5]. Similarly, a recently published meta-analysis confirmed the predictive role of EAT for AF recurrence after CA [35]. Based on these results, an increased EAT in normal-sized LAs might reflect an early LA disease, followed by LA enlargement and the transition from paroxysmal to persistent/permanent AF. Adding our findings to the recent data, one can expect the most frequent AF recurrences in obese patients with a large amount of EAT and subsequent LA enlargement, which warrants further confirmation.
According to our results, LA enlargement unrelated to obesity might have a lower propensity for AF recurrence and the mechanisms behind this are crucial. In non-obese patients, LA enlargement is possibly merely a consequence of prolonged hemodynamic stress due to heterogeneous conditions with LA pressure or volume overload, which can also be aggravated by atrial stand-still during AF episodes. On the other hand, genetic and innervational factors promoting LA enlargement and AF have also been identified [36,37,38]. However, LA enlargement in obese patients seems to carry a higher propensity for AF compared to in non-obese patients, which highlights the additional proarrhythmogenic impact of local and systemic inflammatory conditions mediated by obesity. It is possible that an enlarged left atrium in obese patients reflects different and more aggravating pathophysiological circumstances and processes than in non-obese patients. Due to the inflammatory and direct mechanical impacts of adipose tissue and the additional deranged atrial position in obese patients, it is plausible that there are vast microscopic and cellular changes taking place. Even though the LAVI is similarly enlarged, as in non-obese patients, their atrial micro-architectonics is probably significantly damaged, thus leading to a higher percentage of destructed atrial tissue and hence a higher probability of arrhythmic foci outside of the pulmonary veins and respective antral regions. Further studies that could show more specific differences in atrial tissue with respect to adipose tissue and BMI are needed to prove this hypothesis.
Our results are hypothesis-generating and should be interpreted in the context of certain limitations. In our patients, visceral fat was not specifically evaluated, and our results are based only on BMI. However, BMI is the most frequently used anthropometric measure in clinical routine and its results are easily applied in everyday clinical practise. Since BMI was found to correlate with the amount of visceral fat to a certain degree, we believe that adding more specific measures of visceral fat would not significantly change our results [22]. Among other potential limitations we must mention the lack of data regarding specific antiarrhythmic therapy after the procedure; however, all patients were treated following the current guidelines and there were no major differences in the percentage of prescribed therapy between both groups. We also did not measure the time between the ablation procedure and the registered AF, which would give us further insight in AF recurrence and possible correlations with BMI; however, we postulated that a 3-month window between the blanking period and final observation time would be too short for any meaningful conclusions. Although the number of included patients was limited, all interventional procedures were performed by the same skilled electrophysiologist and echocardiographic examinations were performed by the same experienced echocardiographer on the same ultrasound machine. Thereby, we completely avoided potential interobserver procedural and measurement variability issues. Finally, we also did not perform a 24 h Holter routinely in all patients; however, due to the known low detection value of this method and short observation window, we concluded that symptom-driven diagnostical procedures (Holter or ECG) and clinical presentation with a 12-lead ECG would suffice for the registration of follow-up data [1].
5. Conclusions
According to our results, the LAVI tends to be a significant predictor of AF recurrence after CA in obese patients, but not in normal-weight or overweight patients. This would suggest different mechanisms of AF in patients with normal weight or slightly over-weighted patients in comparison to obese patients. Further studies are needed in this regard.
Author Contributions
Conceptualization, F.H.N.; Methodology, J.A.; Investigation, F.H.N., J.A., I.B. and D.S.; Resources, I.B.; Writing—original draft, F.H.N.; Writing—review & editing, D.S.; Funding acquisition, I.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was made in accordance with the declaration of Helsinki and approved by the Committee of Medical Ethics in University Clinical Center Maribor (approval code is UKC-MB-KME-36/24, Approval date 4 July 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Authors have the access to all research data.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.-A., Dilaveris P.E., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 2.Labarbera M.A., Atta-Fosu T., Feeny A.K., Firouznia M., Mchale M., Cantlay C., Roach T., Axtell A., Schoenhagen P., Barnard J., et al. New Radiomic Markers of Pulmonary Vein Morphology Associated with Post-Ablation Recurrence of Atrial Fibrillation. IEEE J. Transl. Eng. Health Med. 2022;10:1800209. doi: 10.1109/JTEHM.2021.3134160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maier J., Blessberger H., Nahler A., Hrncic D., Fellner A., Reiter C., Hönig S., Schmit P., Fellner F., Lambert T., et al. Cardiac Computed Tomography-Derived Left Atrial Volume Index as a Predictor of Long-Term Success of Cryo-Ablation in Patients with Atrial Fibrillation. Am. J. Cardiol. 2021;140:69–77. doi: 10.1016/j.amjcard.2020.10.061. [DOI] [PubMed] [Google Scholar]
- 4.van Rosendael A.R., Smit J.M., El’mahdiui M., van Rosendael P.J., Leung M., Delgado V., Bax J.J. Association between left atrial epicardial fat, left atrial volume, and the severity of atrial fibrillation. Europace. 2022;24:1223–1228. doi: 10.1093/europace/euac031. [DOI] [PubMed] [Google Scholar]
- 5.van Rosendael A.R., Dimitriu-Leen A.C., van Rosendael P.J., Leung M., Smit J.M., Saraste A., Knuuti J., van der Geest R.J., van der Arend B.W., van Zwet E.W., et al. Association between Posterior Left Atrial Adipose Tissue Mass and Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2017;10:e004614. doi: 10.1161/CIRCEP.116.004614. [DOI] [PubMed] [Google Scholar]
- 6.Pranata R., Henrina J., Yonas E., Putra I.C.S., Cahyadi I., Lim M.A., Munawar D.A., Munawar M. BMI and atrial fibrillation recurrence post catheter ablation: A dose-response meta-analysis. Eur. J. Clin. Investig. 2021;51:e13499. doi: 10.1111/eci.13499. [DOI] [PubMed] [Google Scholar]
- 7.Njoku A., Kannabhiran M., Arora R., Reddy P., Gopinathannair R., Lakkireddy D., Dominic P. Left atrial volume predicts atrial fi-brillation recurrence after radiofrequency ablation: A meta-analysis. Europace. 2018;20:33–42. doi: 10.1093/europace/eux013. [DOI] [PubMed] [Google Scholar]
- 8.Chien S.C., Chandramouli C., Lo C.I., Lin C.F., Sung K.T., Huang W.H., Lai Y.-H., Yun C.-H., Su C.-H., Yeh H.-I., et al. Associations of obesity and malnutrition with cardiac remodeling and cardiovascular outcomes in Asian adults: A cohort study. PLoS Med. 2021;18:e1003661. doi: 10.1371/journal.pmed.1003661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., et al. Recommendations for cardiac chamber quantifi-cation by echocardiography in adults: An update from the American Society of Echocardiography and the European Associ-ation of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Wong C.X., Sullivan T., Sun M.T., Mahajan R., Pathak R.K., Middeldorp M., Twomey D., Ganesan A.N., Rangnekar G., Roberts-Thomson K.C., et al. Obesity and the Risk of Incident, Post-Operative, and Post-Ablation Atrial Fibrillation: A Meta-Analysis of 626,603 Individuals in 51 Studies. JACC Clin. Electrophysiol. 2015;1:139–152. doi: 10.1016/j.jacep.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Wang T.J., Parise H., Levy D., D’Agostino R.B., Sr., Wolf P.A., Vasan R.S., Benjamin E.J. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 12.Wanahita N., Messerli F.H., Bangalore S., Gami A.S., Somers V.K., Steinberg J.S. Atrial fibrillation and obesity—Results of a meta-analysis. Am. Heart J. 2008;155:310–315. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Huxley R.R., Lopez F.L., Folsom A.R., Agarwal S.K., Loehr L.R., Soliman E.Z., Maclehose R., Konety S., Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: The Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnabel R.B., Yin X., Gona P., Larson M.G., Beiser A.S., McManus D.D., Newton-Cheh C., Lubitz S.A., Magnani J.W., Ellinor P.T., et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahajan R., Lau D.H., Brooks A.G., Shipp N.J., Manavis J., Wood J.P., Finnie J.W., Samuel C.S., Royce S.G., Twomey D.J., et al. Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity. J. Am. Coll. Cardiol. 2015;66:1–11. doi: 10.1016/j.jacc.2015.04.058. [DOI] [PubMed] [Google Scholar]
- 16.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontana L., Eagon J.C., Trujillo M.E., Scherer P.E., Klein S. Visceral fat adipokine secretion is associated with systemic inflam-mation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 18.Newman M.S., Nguyen T., Watson M.J., Hull R.W., Yu H.-G. Transcriptome profiling reveals novel BMI- and sex-specific gene expression signatures for human cardiac hypertrophy. Physiol. Genom. 2017;49:355–367. doi: 10.1152/physiolgenomics.00122.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson P.W.F., D’Agostino R.B., Sullivan L., Parise H., Kannel W.B. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch. Intern. Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 20.Gami A.S., Hodge D.O., Herges R.M., Olson E.J., Nykodym J., Kara T., Somers V.K. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 21.Cichoń M., Wieczorek J., Wybraniec M., Woźniak-Skowerska I., Hoffmann A., Nowak S., Szydło K., Wnuk-Wojnar A., Mizia-Stec K. Left atrial function in obese and non-obese patients undergoing percutaneous pulmonary vein isolation. Heart Vessel. 2019;34:343–351. doi: 10.1007/s00380-018-1243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabkin S.W. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: A systematic review and meta-analysis. Metab. Syndr. Relat. Disord. 2014;12:31–42. doi: 10.1089/met.2013.0107. [DOI] [PubMed] [Google Scholar]
- 23.Iacobellis G., Ribaudo M.C., Assael F., Vecci E., Tiberti C., Zappaterreno A., Di Mario U., Leonetti F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J. Clin. Endocrinol. Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 24.Hatem S.N., Sanders P. Epicardial adipose tissue and atrial fibrillation. Cardiovasc. Res. 2014;102:205–213. doi: 10.1093/cvr/cvu045. [DOI] [PubMed] [Google Scholar]
- 25.Camhi S.M., Bray G.A., Bouchard C., Greenway F.L., Johnson W.D., Newton R.L., Ravussin E., Ryan D.H., Smith S.R., Katzmarzyk P.T. The Relationship of Waist Circumference and BMI to Visceral, Subcutaneous, and Total Body Fat: Sex and Race Differences. Obesity. 2011;19:402–408. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox C.S., Massaro J.M., Hoffmann U., Pou K.M., Maurovich-Horvat P., Liu C.Y., Vasan R.S., Murabito J.M., Meigs J.B., Cupples L.A., et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 27.Shen W., Wang Z., Punyanita M., Lei J., Sinav A., Kral J.G., Imielinska C., Ross R., Heymsfield S.B. Adipose tissue quantification by imaging methods: A proposed classification. Obes. Res. 2003;11:5–16. doi: 10.1038/oby.2003.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salgado-Somoza A., Teijeira-Fernández E., Fernández A.L., González-Juanatey J.R., Eiras S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H202–H209. doi: 10.1152/ajpheart.00120.2010. [DOI] [PubMed] [Google Scholar]
- 29.Mahabadi A.A., Berg M.H., Lehmann N., Kälsch H., Bauer M., Kara K., Dragano N., Moebus S., Jöckel K.-H., Erbel R., et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: The Heinz Nixdorf Recall Study. J. Am. Coll. Cardiol. 2013;61:1388–1395. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 30.Venteclef N., Guglielmi V., Balse E., Gaborit B., Cotillard A., Atassi F., Amour J., Leprince P., Dutour A., Clément K., et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J. 2015;36:795–805. doi: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- 31.Wong C.X., Ganesan A.N., Selvanayagam J.B. Epicardial fat and atrial fibrillation: Current evidence, potential mechanisms, clinical implications, and future directions. Eur. Heart J. 2017;38:1294–1302. doi: 10.1093/eurheartj/ehw045. [DOI] [PubMed] [Google Scholar]
- 32.Haemers P., Hamdi H., Guedj K., Suffee N., Farahmand P., Popovic N., Claus P., LePrince P., Nicoletti A., Jalife J., et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 2017;38:53–61. doi: 10.1093/eurheartj/ehv625. [DOI] [PubMed] [Google Scholar]
- 33.Aviles R.J., Martin D.O., Apperson-Hansen C., Houghtaling P.L., Rautaharju P., Kronmal R.A., Tracy R.P., Van Wagoner D.R., Psaty B.M., Lauer M.S., et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 34.Malouf J.F., Kanagala R., Al Atawi F.O., Rosales A.G., Davison D.E., Murali N.S., Tsang T.S.M., Chandrasekaran K., Ammash N.M., Friedman P.A., et al. High sensitivity C-reactive protein: A novel predictor for recurrence of atrial fibrillation after successful cardioversion. J. Am. Coll. Cardiol. 2005;46:1284–1287. doi: 10.1016/j.jacc.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 35.Chen J., Mei Z., Yang Y., Dai C., Wang Y., Zeng R., Liu Q. Epicardial adipose tissue is associated with higher recurrence risk after catheter ablation in atrial fibrillation patients: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2022;22:264. doi: 10.1186/s12872-022-02703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ezeani M., Prabhu S. PI3K(p110α) as a determinant and gene therapy for atrial enlargement in atrial fibrillation. Mol. Cell. Biochem. 2023;478:471–490. doi: 10.1007/s11010-022-04526-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L., Di Tullio M.R., Beecham A., Slifer S., Rundek T., Homma S., Blanton S.H., Sacco R.L. A comprehensive genetic study on left atrium size in caribbean hispanics identifies potential candidate genes in 17p10. Circ. Cardiovasc. Genet. 2010;3:386–392. doi: 10.1161/CIRCGENETICS.110.938381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Călburean P., Osorio T.G., Sorgente A., Almorad A., Pannone L., Monaco C., Miraglia V., Al Housari M., Mojica J., Bala G., et al. High vagal tone predicts pulmonary vein reconnection after cryoballoon ablation for paroxysmal atrial fibrillation. Pacing Clin. Electrophysiol. 2021;44:2075–2083. doi: 10.1111/pace.14408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Authors have the access to all research data.