Digoxin
Use of digoxin for heart failure varies between countries across Europe, with high rates in Germany and low rates in the United Kingdom. It is potentially invaluable in patients with atrial fibrillation and coexistent heart failure, improving control of the ventricular rate and allowing more effective filling of the ventricle. Digoxin is also used in patients with chronic heart failure secondary to left ventricular systolic impairment, in sinus rhythm, who remain symptomatic despite optimal doses of diuretics and angiotensin converting enzyme inhibitors, where it acts as an inotrope.
Digoxin should be considered in patients with sinus rhythm plus (a) continued symptoms of heart failure despite optimal doses of diuretics and angiotensin converting enzyme inhibitors; (b) severe left ventricular systolic dysfunction with cardiac dilatation; or (c) recurrent hospital admissions for heart failure
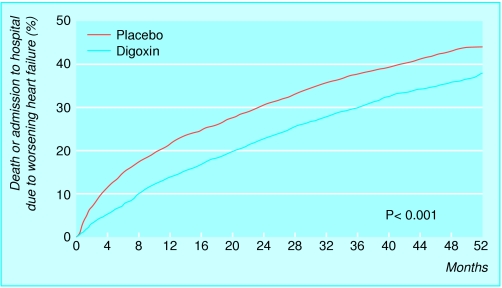
Study of effect of digoxin on mortality and morbidity in patients with heart failure*
Number of participants: 6800
Design: prospective, randomised, double blind, placebo controlled
Participants: left ventricular ejection fraction <45%
Intervention: randomised to digoxin (0.125-0.500 mg) or placebo; follow up at 37 months
Results:
Reduced admissions to hospital owing to heart failure (greater absolute and relative benefits in the patients with resistant symptoms and more severe impairment of left ventricular systolic function)
No effect on overall survival
*The Digitalis Investigation Group's study (see key references box)
Evidence of symptomatic benefit from digoxin in patients with chronic heart failure in sinus rhythm has been reported in several randomised placebo controlled trials and several smaller trials. The RADIANCE and PROVED trials, for example, reported that the withdrawal of digoxin from patients with congestive heart failure who had already been treated with the drug was associated with worsening heart failure and increased hospital readmission rates. The Digitalis Investigation Group's large study found that digoxin was associated with a symptomatic improvement in patients with congestive heart failure, when added to treatment with diuretics and angiotensin converting enzyme inhibitors. Importantly, there were greater absolute and relative benefits in the patients who had resistant symptoms and more severe impairment of left ventricular systolic function. However, although there was a reduction in the combined end points of admission and mortality resulting from heart failure, there was no significant improvement in overall survival. β Blockers were used rarely in the Digitalis Investigation Group's study, and as a result it is not clear whether digoxin is additive to both the β blockers and angiotensin converting enzyme inhibitors in congestive heart failure.
Digoxin: practical aspects
Ensure a maintenance dose of 125-375 μg (0.125-0.375 mg) daily
Give a reduced maintenance dose in elderly people, when renal impairment is present, and when used with drugs that increase digoxin concentrations (amiodarone, verapamil)
Concentrations should be monitored especially in cases of uncertainty about whether therapeutic levels have been achieved (range 6 hours after dose: 1.2-1.9 ng/ml)
Monitor potassium concentrations (avoid hypokalaemia) and renal function
Digoxin toxicity may be associated with: (a) adverse symptoms (for example, nausea, vomiting, headache, confusion, visual symptoms); and (b) arrhythmias (for example, atrioventricular junctional rhythms, atrial tachycardia, atrioventricular block, ventricular tachycardia)
Serious toxicity should be treated by correcting potassium concentrations and with drugs such as β blockers and glycoside binding agents (cholestyramine), and in severe cases specific digoxin antibodies (Digibind)
Source of information: Uretsky et al (J Am Coll Cardiol 1993;22:955) and Packer et al (N Engl J Med 1993;329:1)
Other inotropes
The potential role of inotropic agents other than digoxin in chronic heart failure has been addressed in several studies. Although these drugs seem to improve symptoms in some patients, most have been associated with an increase in mortality.
Inotropic drugs associated with increased mortality in chronic heart failure
| Drug | Class | Inotropic activity |
|---|---|---|
| Xamoterol | β Receptor antagonist | Mild |
| Dobutamine | Dopamine, α, and β receptor antagonist | Strong |
| Ibopamine | Dopamine, α, and β receptor antagonist | Weak |
| Amrinone | Phosphodiesterase inhibitor | Strong |
| Enoximone | Phosphodiesterase inhibitor | Strong |
| Flosequinan | Attenuates inositol triphosphate | Weak |
| Milrinone | Phosphodiesterase inhibitor | Strong |
| Vesnarinone | Phosphodiesterase inhibitor | Mild |
For example, the PRIME II trial (a prospective randomised study) examined ibopamine, a weak inotrope, in patients with chronic heart failure who were already receiving standard treatment. An excess mortality was shown, however, particularly in those with severe symptoms; this was possibly related to an excess of arrhythmias. In addition, a previous trial evaluating intermittent dobutamine infusions in patients with chronic heart failure was stopped prematurely because of excess mortality in the group taking dobutamine. Xamoterol, a β receptor antagonist with mild agonist inotropic effects, also failed to show any positive benefits in patients with heart failure.
Potential mechanisms and benefits of β blockers: improved left ventricular function; reduced sympathetic tone; improved autonomic nervous system balance; up regulation of β adrenergic receptors; reduction in arrhythmias, ischaemia, further infarction, myocardial fibrosis, and apoptosis
In overall terms, no evidence exists at present to support the use of oral catecholamine receptor (or postreceptor pathway) stimulants in the treatment of chronic heart failure. Digoxin remains the only (albeit weak) positive inotrope that is valuable in the management of chronic heart failure.
β Blockers
β Adrenoceptor blockers have traditionally been avoided in patients with heart failure due to their negative inotropic effects. However, there is now considerable clinical evidence to support the use of β blockers in patients with chronic stable heart failure resulting from left ventricular systolic dysfunction. Recent randomised controlled trials in patients with chronic heart failure have reported that combining β blockers with conventional treatment with diuretics and angiotensin converting enzyme inhibitors results in improvements in left ventricular function, symptoms, and survival, as well as a reduction in admissions to hospital.
Randomised, placebo controlled β blocker trials in congestive heart failure
| Study | Treatment | NYHA class* | Outcome |
|---|---|---|---|
| MDC | Metoprolol | II, III | Improved clinical state without effect on survival. Reduction in need for transplantation in patients with dilated cardiomyopathy |
| CIBIS I | Bisoprolol | II, III | Trend (non-significant) towards improved survival |
| ANZ trial | Carvedilol | I, II | Carvedilol superior to placebo for morbidity and mortality |
| Carvedilol (US) | Carvedilol | II, III | Carvedilol superior to placebo for morbidity and mortality |
| CIBIS II | Bisoprolol | III, IV | Bisoprolol superior to placebo for morbidity and mortality |
| MERIT-HF | Metoprolol | II, III | Metoprolol superior to placebo for morbidity and mortality |
Placebo groups in all trials received appropriate conventional treatment (diuretics alone; diuretics plus either digoxin or angiotensin converting enzyme inhibitors; or diuretics plus digoxin and angiotensin converting enzyme inhibitors). Trials still in progress: COMET (carvedilol v metoprolol) and COPERNICUS (carvedilol in severe chronic heart failure).
*Classification of the New York Heart Association (I=no symptoms, II=mild, III=moderate, IV=severe).
Recently, two randomised controlled trials have studied the effects of carvedilol, a β blocker with α blocking and vasodilator properties, in patients with symptomatic heart failure. The US multicentre carvedilol study programme was stopped early because of a highly significant (65%) mortality benefit in patients receiving carvedilol, when compared to placebo, and the Australia/New Zealand heart failure study reported a 41% reduction in the combined primary end point of all cause hospital admission and mortality. Bisoprolol has also been studied, and, although the first cardiac insufficiency bisoprolol study (CIBIS I) reported a trend towards a reduction in mortality and need for cardiac transplantation, there was no conclusive survival benefit. The recent CIBIS II study, however, was stopped prematurely because of the beneficial effects of active treatment on both morbidity and mortality. Metoprolol has also shown similar prognostic advantages in the metoprolol randomised intervention trial in heart failure (MERIT-HF), which was also stopped early. In summary, evidence is now available to support the use of β blockers in chronic heart failure, as the benefits supplement those already obtained from angiotensin converting enzyme inhibitors.
Meta-analysis of effects of β blockers on mortality and admissions to hospital in chronic heart failure
| No of trials (total No of patients) | % receiving placebo | % receiving active treatment | Risk reduction (%) | P value |
|---|---|---|---|---|
| 18 (3023) | 24.6 | 15.8 | 38 | <0.001 |
Summary of the cardiac insufficiency bisoprolol study II (CIBIS II)*
Randomised, double blind, parallel group study
2647 participants (class III-IV (moderate to severe) according to classification of the New York Heart Association)
Bisoprolol, increased in dose to a maximum of 10 mg a day
Trial stopped because of significant mortality benefit in patients treated with bisoprolol:
(a) 32% reduction in all cause mortality
(b) 32% reduction in admissions to hospital for worsening heart failure
(c) 42% reduction in sudden death
*CIBIS II Investigators and Committee (Lancet 1999;353:9-13)
Carvedilol is now licensed in the United Kingdom for use in mild to moderate chronic stable heart failure, although at present its use is still not recommended in patients with severe symptoms (New York Heart Association class IV). This latter group has been underrepresented in the trials to date.
In general, β blockers should be started at very low doses, with the dose being slowly increased, under expert supervision, to the target dose if tolerated. In the short term there may be a deterioration in symptoms, which may improve with alterations in other treatment, particularly diuretics.
Dose and titration of β blockers in large, placebo controlled heart failure trials
| β Blocker | Initial dose (mg) | Weekly titration schedule: total daily dose (mg) | Target total daily dose (mg) | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8–11 | 12–15 | |||||
| Metoprolol (MDC trial) | 5 | 10 | 15 | 20 | 50 | 75 | 100 | 150 | NI | NI | 100–150 | ||
| Carvedilol (US trials) | 3.125 | 6.25 | NI | 12.5 | NI | 25 | NI | 50 | NI | NI | 50 | ||
| Bisoprolol (CIBIS II) | 1.25 | 1.25 | 2.5 | 3.75 | 5 | 5 | 5 | 5 | 7.5 | 10 | 10 | ||
References: Waagstein F et al (Lancet 1993;342:1442-6), Packer M et al (N Engl J Med 1996;334:1349-55), and CIBIS II Investigators and Committee (Lancet 1999;353:9-13).
NI=no increase in dose.
Antithrombotic treatment
In patients with chronic heart failure the incidence of stroke and thromboembolism is significantly higher in the presence of atrial and left ventricular dilatation, particularly in severe left ventricular dysfunction. Nevertheless, there is conflicting evidence of benefit from routine treatment of patients with heart failure who are in sinus rhythm with antithrombotic treatment, although anticoagulation should be considered in the presence of mobile ventricular thrombus, atrial fibrillation, and severe cardiac impairment. Large scale, prospective randomised controlled trials of antithrombotic treatment in heart failure are in progress, such as the WATCH study (a trial of warfarin and antiplatelet therapy); the full results are awaited with interest.
The combination of atrial fibrillation and heart failure (or evidence of left ventricular systolic dysfunction on echocardiography) is associated with a particularly high risk of thromboembolism, which is reduced by long term treatment with warfarin. Aspirin seems to have little effect on the risk of thromboembolism and overall mortality in such patients.
Antiarrhythmic treatment
Chronic heart failure and atrial fibrillation
Restoration and long term maintenance of sinus rhythm is less successful in the presence of severe structural heart disease, particularly when the atrial fibrillation is longstanding. In patients with a deterioration in symptoms that is associated with recent onset atrial fibrillation, treatment with amiodarone increases the long term success rate of cardioversion. Digoxin is otherwise appropriate for controlling ventricular rate in most patients with heart failure and chronic atrial fibrillation, with the addition of amiodarone in resistant cases.
The use of class I antiarrhythmic agents in patients with atrial fibrillation and chronic heart failure substantially increases the risk of mortality
Chronic heart failure and ventricular arrhythmias
Ventricular arrhythmias are a common cause of death in severe heart failure. Precipitating or aggravating factors should thus be addressed, including electrolyte disturbance (for example, hypokalaemia, hypomagnesaemia), digoxin toxicity, drugs causing electrical instability (for example, antiarrhythmic drugs, antidepressants), and continued or recurrent myocardial ischaemia.
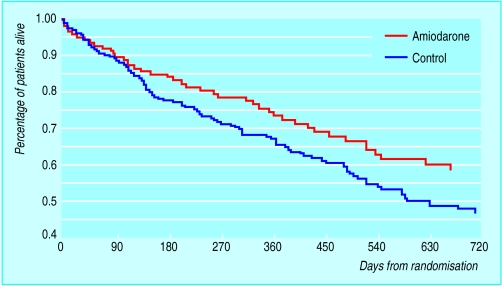
Amiodarone is effective for the symptomatic control of ventricular arrhythmias in chronic heart failure, although most studies have reported that long term antiarrhythmic treatment with amiodarone has a neutral effect on survival. An Argentinian trial (the GESICA study) of empirical amiodarone in patients with chronic heart failure reported, however, that active treatment was associated with a 28% reduction in total mortality, although this trial included a high incidence of patients with non-ischaemic heart failure. In contrast, in the survival trial of antiarrhythmic therapy in congestive heart failure (CHF-STAT), amiodarone did not improve overall survival, although there was a significant (46%) reduction in cardiac death and admission to hospital in the patients with non-ischaemic chronic heart failure.
Summary of drug management in chronic heart failure
| Drug class | Potential therapeutic role |
|---|---|
| Diuretics | Symptomatic improvement of congestion. Spironolactone improves survival in severe (NYHA class IV) heart failure |
| Angiotensin converting enzyme (ACE) inhibitors | Improved symptoms, exercise capacity, and survival in patients with asymptomatic and symptomatic systolic dysfunction |
| Digoxin | Improved symptoms, exercise capacity, and fewer admissions to hospital |
| Angiotensin II receptor antagonists | Treatment of symptomatic heart failure in patients intolerant to ACE inhibitors* |
| Nitrates and hydralazine | Improved survival in symptomatic patients intolerant to ACE inhibitors or angiotensin II receptor antagonists* |
| β Blockers | Improved symptoms and survival in stable patients who are already receiving ACE inhibitors |
| Amiodarone | Prevention of arrhythmias in patients with symptomatic ventricular arrhythmias |
*Recommendations of when these agents might be considered (the use of these agents has not been addressed in randomised trials of patients intolerant to ACE inhibitors).
In general, amiodarone should probably be reserved for patients with chronic heart failure who also have symptomatic ventricular arrhythmias. Interest has also developed in implantable cardioverter defibrillators, which reduce the risk of sudden death in high risk patients with ventricular arrhythmias (MADIT and AVID studies), although the role of these devices in patients with chronic heart failure still remains to be established.
Key references
Australia/New Zealand Heart Failure Research Collaborative Group. Randomized, placebo-controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease. Lancet 1997;349:375-80.
Lip GYH. Intracardiac thrombus formation in cardiac impairment: investigation and the role of anticoagulant therapy. Postgrad Med J 1996;72:731-8.
Massie BM, Fisher SG, Radford M, Deedwania PC, Singh BN, Fletcher RD, et al for the CHF-STAT Investigators. Effect of amiodarone on clinical status and left ventricular function in patients with congestive heart failure. Circulation 1996;93:2128-34.
MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet 1999;353:2001-7.
Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R, et al. Randomised trial of low-dose amiodarone in severe congestive heart failure [GESICA trial]. Lancet 1994;344:493-8.
Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. Effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996;334:1349-55.
Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525-33.
Figure.
Incidence of death or admission to hospital due to worsening heart failure in two groups of patients: those receiving digoxin and those receiving placebo (Digitalis Investigation Group's study—see key references box at end of article)
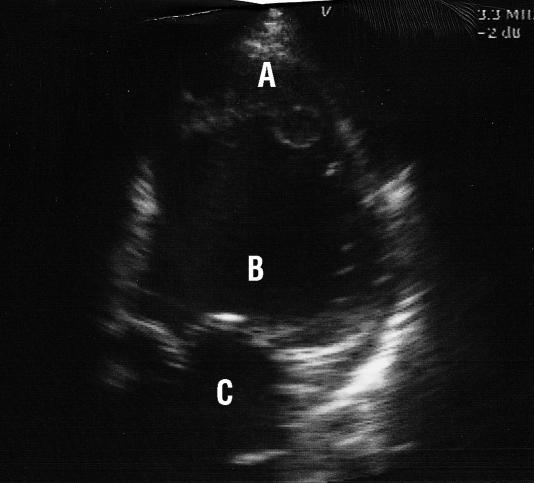
Figure.
Echocardiogram showing thrombus at left ventricular apex in patient with dilated cardiomyopathy (A=thrombus, B=left ventricle, C=left atrium)
Figure.
Survival curves from GESICA trial (see key references box), showing difference between patients taking amiodarone and controls
Acknowledgments
The survival graph is adapted with permission from Doval et al (Lancet 1994;344:493-8). The table of inotropic drugs is adapted with permission from Niebauer et al (Lancet 1997;349:966). The table of results of a meta-analysis of effects of β blockers is adapted with permission from Lechat P et al (Circulation 1998;98:1184-91). The table on doses and titration of β blockers is adapted with permission from Remme WJ (Eur Heart J 1997;18:736-53).
Footnotes
The ABC of heart failure is edited by C R Gibbs, M K Davies, and G Y H Lip. CRG is research fellow and GYHL is consultant cardiologist and reader in medicine in the university department of medicine and the department of cardiology, City Hospital, Birmingham; MKD is consultant cardiologist in the department of cardiology, Selly Oak Hospital, Birmingham. The series will be published as a book in the spring.