Abstract
We have recently reported that the in vitro inhibition of human immunodeficiency virus type 1 (HIV-1) reverse transcription by inhibitors of reverse transcriptase (RT) occurred most efficiently when the expected DNA products of RT reactions were long (Quan et al., Nucleic Acids Res. 26:5692–5698, 1998). Here, we have used a quantitative PCR to analyze HIV-1 reverse transcription within acutely infected cells treated with RT inhibitors. We found that levels of minus-strand strong-stop DNA [(−)ssDNA] formed in acutely infected MT2 cells were only slightly reduced if cells were infected with viruses that had been generated in the presence of either azidothymidine or nevirapine (5 μM) and maintained in the presence of this drug throughout the viral adsorption period and thereafter. Control experiments in which virus inoculation of cells was performed at 4°C, followed directly by cell extraction, showed that less than 1% of total (−)ssDNA within acutely infected cells was attributable to its presence within adsorbed virions. In contrast, synthesis of intermediate-length reverse-transcribed DNA products decreased gradually as viral DNA strand elongation took place in the presence of either of these inhibitors. This establishes that nucleoside and nonnucleoside RT inhibitors can exert similar temporal impacts in regard to inhibition of viral DNA synthesis. Generation of full-length viral DNA, as expected, was almost completely blocked in the presence of these antiviral drugs. These results provide insight into the fact that high concentrations of drugs are often needed to yield inhibitory effects in cell-free RT assays performed with short templates, whereas relatively low drug concentrations are often strongly inhibitory in cellular systems.
Several reports have shown that the nucleoside analog chain-terminating drug azidothymidine (AZT) can efficiently block the reverse transcriptase (RT)-mediated formation of full-length human immunodeficiency virus (HIV) viral DNA but not of minus-strand strong-stop DNA [(−)ssDNA] during acute infection (3, 13, 24, 25). However, the reasons for this discrepancy remain unclear, in spite of the fact that the inhibitory potential of RT inhibitors is dependent on the length of the template being copied and the number of sites at which inhibitors might exert their effect (15, 23).
We have previously used cell-free reactions to study the relationship between the inhibitory effects of the active form of AZT, i.e., AZT 5′-triphosphate, and the lengths of products generated in cell-free assays performed with recombinant RT. We have also studied the nonnucleoside RT inhibitor nevirapine in this regard. The results showed that the inhibitory effects of these molecules were directly related to the expected lengths of the RT products generated (19). Similar findings were obtained with endogenous RT reactions performed with purified virus particles (18), consistent with theoretical predictions (8). However, it is not known whether intracellular reverse transcription follows the same pattern, and the failure of AZT to affect the intracellular synthesis of (−)ssDNA is complicated by the fact that HIV type 1 (HIV-1) can initiate reverse transcription prior to infection and can carry (−)ssDNA product into target cells (14, 22, 26–28). Conceivably, in fact, such DNA might contribute to the efficiency of infection (26–28). We now report on the intracellular effects of both AZT and nevirapine in the synthesis of specific RT products.
The RT products that have been specifically monitored include (−)ssDNA, intermediate-length viral DNA, and full-length viral DNA and were detected by quantitative PCR. Our results show that more than 99% of the (−)ssDNA made during acute infection of MT2 cells was formed after infection and was not carried into cells by infecting virions. However, (−)ssDNA was made to the same extent in acutely infected cells that had been exposed to virus particles derived either from untreated, chronically infected H9 cells or from H9 cells that had been continuously treated with either AZT or nevirapine. However, the number of intermediate viral DNA products made in acutely infected cells treated with these drugs diminished gradually as a function of DNA strand elongation, and the synthesis of full-length viral DNA product was almost completely blocked by these RT inhibitors, as previously demonstrated (3, 13, 24, 25).
MATERIALS AND METHODS
Generation of infectious virus.
MT2 and H9 cells were routinely maintained in RPMI 1640 medium (GIBCO Laboratories, Mississauga, Canada) supplemented with 10% fetal bovine serum (Flow Laboratories, Toronto, Canada), 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. HIV-1 wild-type virus stocks were prepared by transfection of 2 μg of proviral DNA (HxB2D) into 5 × 105 MT2 cells with Lipofectin according to the manufacturer’s recommendations (GIBCO BRL, Montreal, Canada).
To minimize the amount of virion-carried DNA as well as to obtain enough HIV for study, all viruses used in subsequent infection experiments were harvested from chronically infected H9 cells in the absence or presence of RT inhibitors and were purified, whenever appropriate, to eliminate the inhibitor. Recombinant wild-type viruses were harvested from H9 cells that were chronically infected with the progeny of the MT2 cell transfections. The IIIb strain of HIV-1 (HIV-IIIb) was similarly harvested from chronically infected H9 cells (a gift of R. C. Gallo, Bethesda, Md.).
Chronically infected H9 cells were pelleted, and equal numbers of cells were then incubated in the absence or presence of either 5 μM AZT or 5 μM nevirapine. After 10 h, the cells were again pelleted and resuspended in fresh drug-free medium or medium containing 5 μM AZT or 5 μM nevirapine; in the case of drug-containing culture fluids, the intent was to limit the formation of any (−)ssDNA in viral particles generated from cells that contained integrated viral DNA. Viral particles in culture fluids were harvested after a further 16 h of incubation. Viruses were ultracentrifuged (at 80,000 × g for 1 h at 4°C) to eliminate RT inhibitors in culture fluids and to limit any influence of such molecules during a subsequent round of infection, although, in some cases, fresh drug was included in these subsequent rounds both during the adsorption period and thereafter. Pelleted viruses were resuspended in fresh drug-free medium, evaluated for p24 CA content (Abbott Laboratories, North Chicago, Ill.) and RT activity, and kept frozen at −70°C until further study.
Wild-type (HxB2D) viruses harvested from H9 cells grown in the absence of drug or in the presence of either AZT or nevirapine were termed H9-wt, H9-wt-AZT, and H9-wt-Nev, respectively. The corresponding IIIb viruses were termed H9-IIIb, H9-IIIb-AZT, and H9-IIIb-Nev, respectively.
De novo infection.
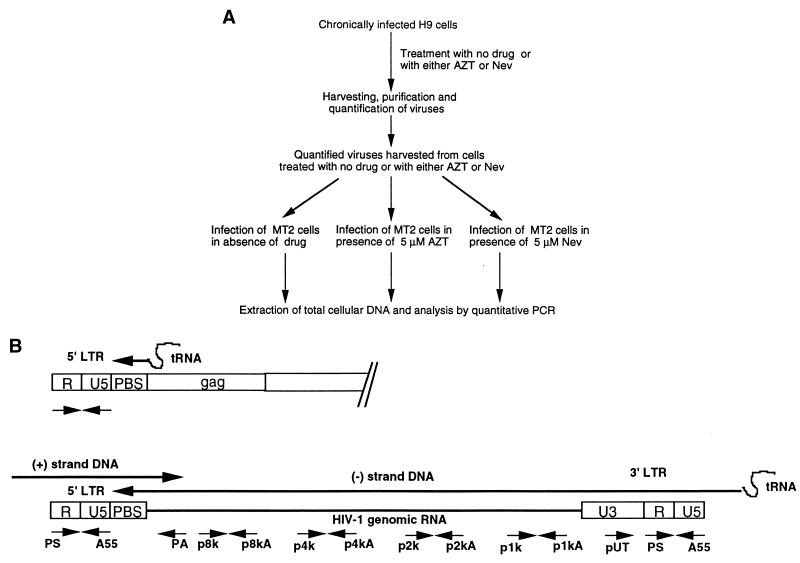
A total of 106 MT2 cells were infected with HxB2D or IIIb viruses (equivalent to 10 ng of p24), derived from either drug-treated or untreated H9 cells, as described above. Following a 2-h adsorption at 37°C, the cells were pelleted at 4°C and were resuspended in fresh medium containing either no drug, 5 μM AZT, or 5 μM nevirapine. Prior to infection, the cells had either been left untreated or treated with 5 μM AZT or 5 μM nevirapine for 3 h so as to ensure that the drugs would act against HIV-1 reverse transcription from the time of viral adsorption. The viruses used for infection were diluted into drug-free medium or medium supplemented with 5 μM AZT or nevirapine, depending on the individual experiment. Figure 1A summarizes the process of infection and analysis.
FIG. 1.
(A) Schema of experimental procedures used; (B) schematic representation of DNA oligonucleotide primer pairs used to amplify regions of the HIV-1 genome by quantitative PCR. The direction of a sense or antisense primer is indicated by an arrow pointing to the 3′ or 5′ terminus. LTR, long terminal repeat.
MT2 cells, exposed to virus for periods between 2 and 12 h, were pelleted, washed once with medium, and resuspended in lysis buffer containing 0.5% sodium dodecyl sulfate and 1 mg of protease K/ml for 6 h (12, 16), and extracted DNA was analyzed by quantitative PCR with primer pairs able to detect both full-length and partially elongated viral DNA products (HIV genomic sites amplified by these primer pairs are listed in Table 1 and diagrammed in Fig. 1B). For example, (−)ssDNA and full-length DNA were detected by primer pairs PS–A55 and PS–PA, respectively (12, 13, 24, 25). The shortest products after the first template switch (≈300 bases) were amplified by primer pair pUT–A55. Template-switched DNA strands that were elongated to lengths of about 1, 2, 4, and 8 kb were amplified by primer pairs p1k–p1kA, p2k–p2kA, p4k–p4kA, and p8k–p8kA, respectively.
TABLE 1.
Primer pairs used in quantitative PCR
| Primer no. | Name | Length (nt) | Sequence (5′→3′) | Location |
|---|---|---|---|---|
| 1 | PS | 22 | AGACCAGATCTGAGCCTGGGAG | 15–36 |
| 2 | A55 | 24 | CTGCTAGAGATTTTCCACACTGAC | 182–159 |
| 3 | PA | 20 | CCCATCGATCTAATTCTCCC | 382–363 |
| 4 | pUT | 20 | GCTGACATCGAGCTTGCTAC | 8990–9009 |
| 5 | p1k | 21 | AAAGTAGTGTGGTTGGATGGC | 8393–8413 |
| 6 | p1kA | 20 | CTCCTCTTGTGCTTCTAGCC | 8562–8543 |
| 7 | p2k | 22 | AACCATTAGGAGTAGCACCCAC | 7276–7297 |
| 8 | p2kA | 22 | CTGCTGCTGCACTATACACGAC | 7457–7436 |
| 9 | p4k | 22 | ACTTATGGGGATACTTGGGCAG | 5283–5304 |
| 10 | p4kA | 22 | GTACAAGCAGTTTTAGGCTGAC | 5478–5457 |
| 11 | p8k | 22 | GAATTCTGTTGACTCAGATTGG | 2092–2113 |
| 12 | p8kA | 23 | GAAATTTTCCCTTCCTCTTCCAT | 2282–2250 |
RESULTS
Virus production by chronically infected H9 cells treated with AZT or nevirapine.
H9 cells that were chronically infected with recombinant HxB2 virus were left untreated or treated with either 5 μM AZT or 5 μM nevirapine for 5 h to minimize the extent of synthesis of (−)ssDNA in harvested viral particles. The cells were pelleted and resuspended in drug-containing or drug-free medium. The results (Table 2) show that after 16 h, levels of both viral p24 CA and RT activities were similar in the drug-treated and control cultures. This shows, as expected, that these drugs, both of which affect RT preintegrationally, had not affected the production of viral proteins.
TABLE 2.
Production of HIV-1 by chronically infected H9 cells treated with AZT or nevirapine
| Viral strain | Treatment | Production (mean ± SD) of:
|
|
|---|---|---|---|
| p24 (ng/ml) | RT (105 cpm/ml) | ||
| HxB2D | 154 ± 12 | 45.6 ± 5.2 | |
| AZT (5 μM) | 119 ± 11 | 42.4 ± 3.8 | |
| Nevirapine (5 μM) | 129 ± 10 | 52.3 ± 4.4 | |
| IIIb | 334 ± 27 | 144.6 ± 9.6 | |
| AZT (5 μM) | 325 ± 21 | 143 ± 11.7 | |
| Nevirapine (5 μM) | 329 ± 18 | 165.4 ± 10.3 | |
Inhibition of synthesis of (−)ssDNA and full-length viral DNA in acutely infected cells grown in the presence of RT inhibitors.
A number of reports have shown that AZT treatment of acutely infected cells had little effect on the formation of (−)ssDNA while blocking the production of full-length viral DNA (3, 13, 24, 25). To further address this issue, we have performed a more-extensive series of experiments that are better controlled than those previously carried out. We adopted two strategies to control the influence of virion-carried DNA that may interfere with subsequent PCR analysis. First, the viruses used were harvested from cells that had been subjected to treatment with RT inhibitors. For this purpose, we used viruses derived from chronically infected H9 cells that had themselves been grown in the presence of drug (i.e., 5 μM AZT or nevirapine). The inhibitors were subsequently removed from virions by the purification step. We then used these viruses to acutely infect MT2 cells in the presence of the same compounds; drugs were present during the pretreatment period, during the adsorption period, and for various times thereafter, following which DNA was extracted from the cells as described above. As an additional control, viral adsorption was carried out for 2 h at 4°C in order to allow viral attachment but not internalization and initiation of reverse transcription, and DNA was extracted immediately thereafter (6).
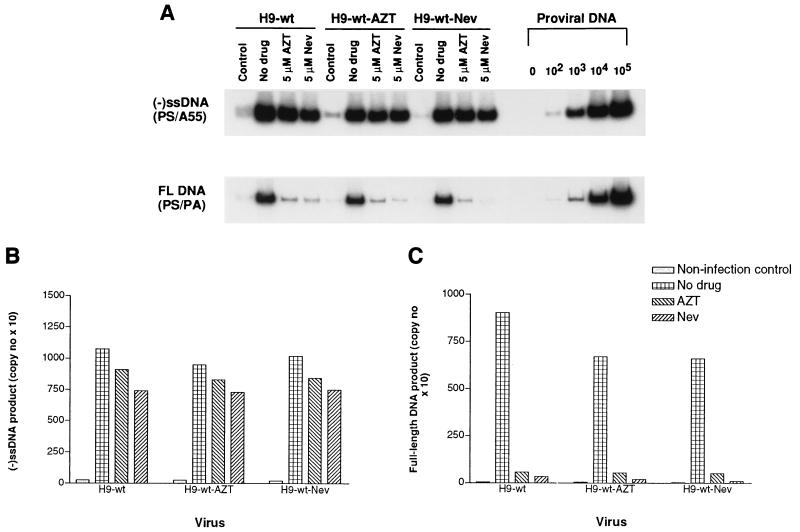
Figure 2A shows that levels of (−)ssDNA were similar whether MT2 cells had been infected with the HxB2D clone of HIV derived from untreated H9 cells or with that from H9 cells that had been treated with either 5 μM AZT or 5 μM nevirapine, and that treatment of the MT2 target population with either AZT or nevirapine (5 μM) had no significant effect on the levels of (−)ssDNA that were generated. The control lanes show the much lower levels of (−)ssDNA that were present within virus particles that had attached to MT2 cells at 4°C. These results are presented in graphic form on the basis of molecular imaging analysis in Fig. 2B.
FIG. 2.
(A) PCR analysis of inhibition of synthesis of (−)ssDNA and full-length viral DNA (FL DNA) by AZT or nevirapine (Nev) in experiments in which MT2 cells were acutely infected either with wild-type HIV-1 or with viruses grown in chronically infected H9 cells that had been treated with these drugs as described in Materials and Methods. PCR products were electrophoresed on 4% acrylamide gels under nondenaturing conditions. Control lanes, results with MT2 cells that had been coincubated with virus for 2 h at 4°C. Copy numbers of proviral DNA are shown on the right side of the gel. (B and C) Quantification of (−)ssDNA (B) and full-length DNA (C) by molecular imaging under conditions of no drug treatment or exposure to either 5 μM AZT or 5 μM Nevirapine.
Figure 2A also shows the levels of full-length viral DNA that were synthesized in acutely infected MT2 cells in the absence of the two drugs studied. In this case, it is apparent that both AZT and nevirapine (5 μM) had caused significant impairment in the completion of viral DNA synthesis and that nevirapine played a more significant role in this regard than AZT (Fig. 2C).
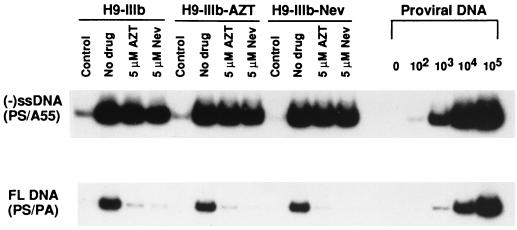
Figure 3 reports similar findings with regard to the IIIb strain of HIV-1. Again, it is apparent that both pretreatment of chronically infected virus-producing H9 cells and pretreatment of acutely infected MT2 cells had little effect on the synthesis of (−)ssDNA in target cells. In contrast, both drugs impacted significantly on the generation of full-length viral DNA.
FIG. 3.
Inhibition of synthesis of (−)ssDNA and of full-length (FL) DNA in MT2 cells acutely infected with IIIb virus in the presence of 5 μM AZT or nevirapine (Nev). Details are identical to those for Fig. 2. Control lanes, results obtained with MT2 cells coincubated with virus for 2 h at 4°C. Copy numbers of proviral DNA are shown on the right.
Time course inhibition of synthesis of (−)ssDNA and full-length viral DNA by antiviral drugs.
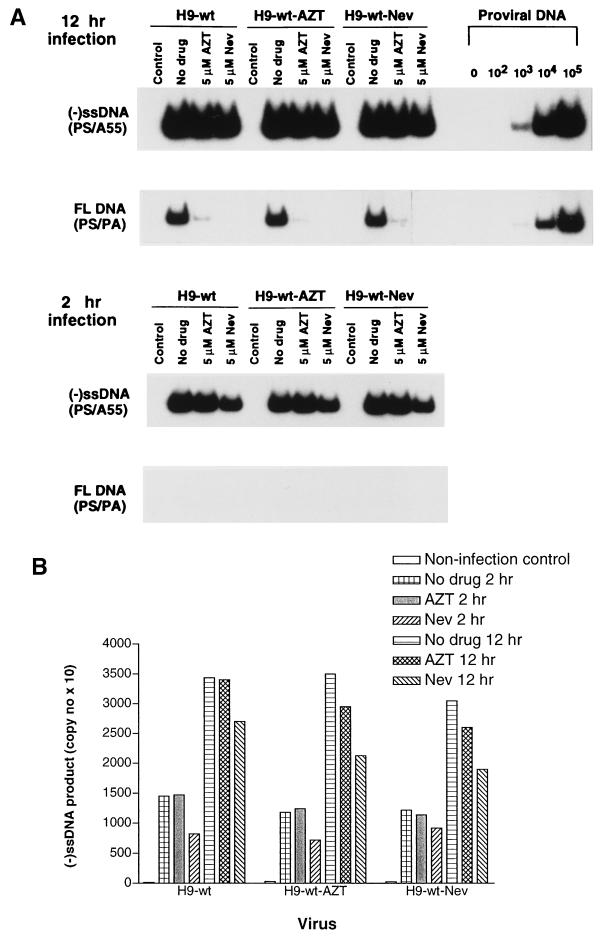
We next performed time course studies to determine whether any increased synthesis of either (−)ss viral DNA or full-length viral DNA occurred during infection. The results of time course experiments show that the extent of synthesis of (−)ssDNA increased more than twofold between 2 and 12 h after infection and that the synthesis of this material was not significantly affected by the RT inhibitors. The production of full-length viral DNA increased even more significantly over 12 h in the absence of drug; however, both AZT and nevirapine (5 μM) inhibited this process (Fig. 4A). These results also show that the synthesis of (−)ssDNA had largely been completed after 2 h, when the synthesis of full-length viral DNA had barely started. This finding is in agreement with other reports that HIV-1 requires more than 10 h to complete reverse transcription within cells (11).
FIG. 4.
Time course of inhibition of synthesis of (−)ssDNA and of full-length (FL) viral DNA by 5 μM AZT or nevirapine (Nev) in acutely infected MT2 cells. (A) PCR results were obtained on the basis of gel electrophoresis as in Fig. 2, except that infection proceeded for either 2 or 12 h. Control lanes, results obtained with MT2 cells coincubated with virus for 2 h at 4°C. (B) Analysis of results of synthesis of (−)ssDNA by molecular imaging.
Quantitation of the (−)ssDNA results on the basis of molecular imaging is provided in Fig. 4B.
Effects of RT inhibitors on intermediate-length reverse-transcribed DNA products.
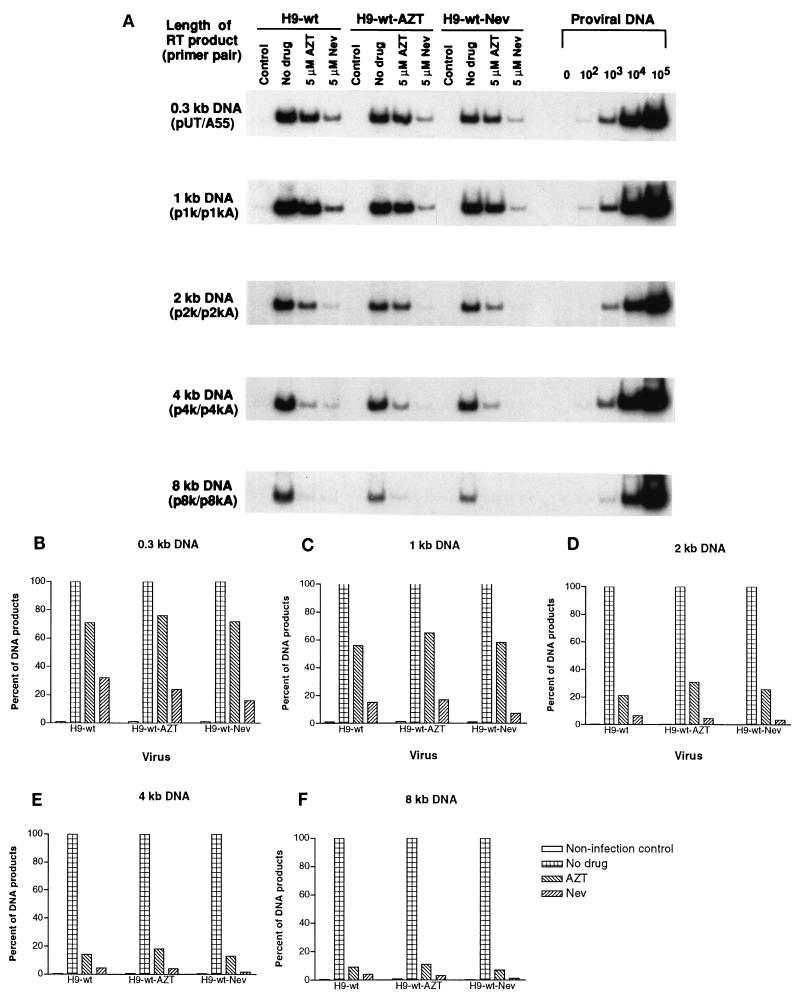
We next used PCR to detect intermediate RT products generated in acutely infected MT2 cells by probing DNA that had been extracted at 10 h after infection. Figure 5A shows that synthesis of these intermediate products decreased gradually as viral DNA strands became further elongated in the presence of either 5 μM AZT or 5 μM nevirapine. This tendency toward reduction in the amount of product is displayed in Fig. 5B through F. When products grew larger than 4 kb, the extent of drug-mediated inhibition was similar to that observed for the production of full-length viral DNA, with nevirapine exerting an effect even stronger than that of AZT.
FIG. 5.
Inhibition of synthesis of intermediate-sized HIV-1 wild-type DNA in acutely infected MT2 cells treated with 5 μM AZT or nevirapine (Nev). (A) PCR products were electrophoresed as described in the legend to Fig. 2 and analyzed with primer pairs specifically designed to amplify intermediate DNA products within the HIV-1 genome. Control lanes, results obtained with MT2 cells coincubated with virus for 2 h at 4°C. The shortest DNA products after the first template switch were amplified by the primer pair pUT–AA55. Template-switched DNA strands that were elongated to approximately 1, 2, 4, and 8 kb were analyzed with primer pairs p1k–p1kA, p2k–p2kA, p4k–p4kA, and p8k–p8kA, respectively. (B through F) Analysis of synthesis of intermediate-length viral DNA by molecular imaging.
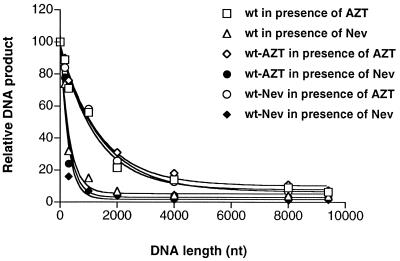
The relationship between the reduction in the amount of the DNA product and the length of the DNA product in the presence of RT inhibitor was further analyzed by using the following equation, which fits the results to second-order dynamics (8, 19): y = 100 · e−kx (where y represents the total amount of the product, x represents the product length, and k is a constant). Our results are consistent with those predicted by this equation, as shown in the normalized curves of Fig. 6, which presents a graphic analysis of these data on the basis of molecular imaging analysis. No significant effects on the extent of viral DNA synthesis were detected when viruses from drug-treated chronically infected H9 cells were used to infect MT2 cells in the presence or absence of the same RT inhibitors, suggesting that virion-carried DNA did not play a significant role in our assays.
FIG. 6.
DNA production versus anticipated DNA length. Results were obtained from Fig. 2 and 5 by dividing the copy number of viral DNA produced in the presence of RT inhibitors by that produced in the absence of drugs. MT2 cells were acutely infected with viruses harvested from H9 cells. Curve annealing was achieved by use of a Graphpad Prism 2.0 program (Graphpad Software Inc., San Diego, Calif.). For curve fitting of the synthesis of DNA by wild-type (wt) virus in the presence of either AZT or nevirapine (Nev), the equations obtained were yAZT = 100 · e−0.000789x + 7.95 and yNev = 100 · e−0.0031x + 5.40.
DISCUSSION
Reverse transcription reactions have mostly been studied in vitro in regard to short template strands. It is known that standard enzyme values, such as Kis and 50% inhibitory concentrations (IC50s), are dependent on experimental conditions such as template length and substrate and inhibitor concentrations (2, 5, 8, 15, 18, 19, 23).
The relationship between DNA elongation and the inhibitory effects of anti-RT compounds has been intensively studied in cell-free reactions (1, 9, 10, 15, 17–21). We have now investigated the specific production of DNA products of varying lengths in acutely infected cells grown in the presence of either AZT or nevirapine. The results show that both drugs caused diminutions in the amounts of DNA products in infected cells and that this occurred gradually as a function of DNA chain elongation. Furthermore, nevirapine appeared to be more active in each case than AZT.
Since RT reactions are processive events, the influence of RT inhibitors should be amplified during successive cycles of DNA polymerization. Factors that may impact only slightly on RT activity in regard to work performed with short templates might have more-dramatic effects on the synthesis of full-length products generated from long templates. We have previously reported a diminution in the synthesis of RT products alongside the elongation of DNA chains in cell-free experiments that were shown to follow the equation described above (19). Our data presented here imply that a second-order dynamic process can also be used to describe reverse transcription under intracellular conditions (Fig. 6).
Table 3 highlights the relationship between the production of DNA and DNA chain elongation; it contains values deduced from the equation given above by using a variety of constants. These predicted values show the influence of the length of DNA chain elongation on total DNA synthesis. In the case of k = 0.1 in Table 3, about 90% of products will have elongated to a length of 0.2 kb (i.e., ∼200 nucleotides [nt]), while only 35% will have elongated to 2 kb and fewer than 2% to 8 kb, values similar to the results of our experiments (see the normalized curves in Fig. 6). Our data also provide an explanation for the fact that higher drug concentrations are often required to significantly impede viral DNA synthesis in cell-free RT reactions than in cellular systems.
TABLE 3.
Predicted relative amount of DNA versus length of elongated DNA strand
| DNA strand length (× 200 nt) | Relative amt of DNAa where:
|
|||||
|---|---|---|---|---|---|---|
| k = 0.2 | k = 0.1 | k = 0.05 | k = 0.03 | k = 0.02 | k = 0.01 | |
| 0 | 100 | 100 | 100 | 100 | 100 | 100 |
| 1 | 81.5 | 90 | 95 | 97 | 98 | 99 |
| 5 | 36.8 | 60 | 75 | 85 | 90.5 | 95 |
| 10 | 13.6 | 35 | 60 | 73 | 82 | 90 |
| 20 | 1.85 | 12 | 36 | 54.4 | 67 | 82 |
| 40 | 0.04 | 1.5 | 13 | 29 | 44.5 | 67 |
| 50 | 0.0044 | 0.53 | 7 | 21 | 37 | 60 |
The predicted values (y) were obtained from the equation y = 100 · e−kx (0 ≤ k ≤1), where x is the anticipated DNA strand length.
The fact that AZT can block formation of full-length DNA but not (−)ssDNA can be explained by differences in patterns of DNA chain elongation. Theoretically, nevirapine should affect every cycle of RT polymerization while AZT triphosphate should affect the incorporation of dTMP only, i.e., about one-fourth of the total nucleotides in a DNA strand. In addition, nevirapine as a nonnucleoside RT inhibitor is already in its active form when added to cells and needs only to penetrate into the cytoplasm to be effective. In contrast, AZT must both enter the target cell and be metabolized to its triphosphate. Obvious considerations are the levels and function of cellular enzymes involved in these phosphorylation steps, performed by thymidine kinase and thymidylate kinase (7).
We have also confirmed that the vast majority of HIV-1 (−)ssDNA is synthesized in infected cells and not in virions, even though the process of reverse transcription begins before viral entry (14, 22, 26–28). In our studies, viruses grown in chronically infected H9 cells in the presence or absence of drugs were able to initiate infection with near-equal efficiency, regardless whether the target cells were treated with antiviral compounds or not. An extensive series of control studies in which viruses were allowed to adsorb to MT2 cells at 4°C, followed by extraction of DNA, revealed that the proportion of total (−)ssDNA found in H9 cell-derived virions was low compared to that produced intracellularly, although it should be acknowledged that cell types other than H9, when infected by HIV-1, may conceivably produce greater amounts of (−)ssDNA. These data also provide further verification that virion-carried (−)ssDNA is probably unimportant in regard to de novo infection of actively dividing cells (4) but may be important in regard to nondividing cells (26). In this context, it should be noted that the use of 5 μM AZT or 5 μM nevirapine in chronically infected cells, i.e., drug concentrations 500- to 1,000-fold higher than the IC50s of these drugs for HIV replication, was intended to minimize the amount of (−)ssDNA in virions. Indeed, Fig. 2 and 3 (see the cold-incubation control lanes) demonstrate that some (−)ssDNA was indeed produced within virus particles in this circumstance.
The fact that our data clearly document a relationship between the observed inhibitory effects of both AZT and nevirapine and the length of target template makes it unlikely that these drugs have any direct effect on RT through mechanisms other than direct inhibition of viral DNA chain elongation. Otherwise, we would likely have observed a stepwise inhibition of DNA synthesis as a function of increased product length, rather than the smooth curves depicted in Fig. 6. This figure also documents that the production of (−)ssDNA falls on the same curves as those predicted by the equation shown above. The shortest (−)ssDNA fragment analyzed in Fig. 6 corresponds to about 200 bp. In acutely infected cells exposed to antiviral drugs, the high levels of this product are a direct consequence of their short length.
REFERENCES
- 1.Arts E J, Li X, Gu Z, Kleiman L, Parniak M, Wainberg M A. Comparison of deoxyoligonucleotide and tRNALys-3 as primers in an endogenous human immunodeficiency virus-1 in vitro reverse transcription/template-switching reaction. J Biol Chem. 1994;269:14672–14680. [PubMed] [Google Scholar]
- 2.Arts E J, Marois J-P, Gu Z, Le Grice S F J, Wainberg M A. Effects of 3′-deoxynucleoside 5′-triphosphate concentrations on chain termination by nucleoside analogs during human immunodeficiency virus type 1 reverse transcription of minus-strand strong-stop DNA. J Virol. 1996;70:712–720. doi: 10.1128/jvi.70.2.712-720.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arts E J, Wainberg M A. Preferential incorporation of nucleoside analogs after template switching during human immunodeficiency virus reverse transcription. Antimicrob Agents Chemother. 1994;38:1008–1016. doi: 10.1128/aac.38.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arts E J, Mak J, Kleiman L, Wainberg M A. DNA found in human immunodeficiency virus type 1 particles may not be required for infectivity. J Gen Virol. 1994;75:1605–1613. doi: 10.1099/0022-1317-75-7-1605. [DOI] [PubMed] [Google Scholar]
- 5.Back N K T, Nijhuis M, Keulen W, Boucher C A B, Oude Essink B B, van Kuilenburg A B P, van Gennip A H, Berkhout B. Reduced replication of 3TC-resistant HIV-1 variants in primary cells due to a processivity defect of the reverse transcriptase enzyme. EMBO J. 1996;15:4040–4049. [PMC free article] [PubMed] [Google Scholar]
- 6.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 7.Gao W Y, Shirasaka T, Johns D G, Broder S, Mitsuya H. Differential phosphorylation of azidothymidine, dideoxycytidine and dideoxyinosine in resting and activated peripheral blood mononuclear cells. J Clin Investig. 1993;91:2326–2333. doi: 10.1172/JCI116463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goody R S, Muller B, Restle T. Factors contributing to the inhibition of HIV reverse transcriptase by chain-terminating nucleotides in vitro and in vivo. FEBS Lett. 1991;291:1–5. doi: 10.1016/0014-5793(91)81089-q. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh J, Zinnen S, Modrich P. Kinetic mechanism of the DNA-dependent DNA polymerase activity of human immunodeficiency virus reverse transcriptase. J Biol Chem. 1993;268:24607–24613. [PubMed] [Google Scholar]
- 10.Kati W M, Johnson K A, Jerva L F, Anderson K S. Mechanism and fidelity of HIV reverse transcriptase. J Biol Chem. 1992;267:25988–25997. [PubMed] [Google Scholar]
- 11.Kim S, Byrn R, Groopman J, Baltimore D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol. 1989;63:3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Mak J, Arts E J, Gu Z, Kleiman L, Wainberg M A, Parniak M A. Effects of alteration of primer binding site sequences in human immunodeficiency virus type 1 replication. J Virol. 1994;68:6198–6206. doi: 10.1128/jvi.68.10.6198-6206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Liang C, Quan Y, Chandok R, Laughrea M, Parniak M A, Kleiman L, Wainberg M A. Identification of sequences downstream of the primer binding site that are important for efficient replication of human immunodeficiency virus type 1. J Virol. 1997;71:6003–6010. doi: 10.1128/jvi.71.8.6003-6010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lori F, Veronese F, De Viro A L, Lusso P, Reitz M S, Gallo R C. Viral DNA carried by human immunodeficiency virus type 1 virions. J Virol. 1992;66:5067–5074. doi: 10.1128/jvi.66.8.5067-5074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Q F, Bathurst I C, Barr P J, Kenyon G L. The observed inhibitory potency of 3′-azido-3′-deoxythymidine 5′-triphosphate for HIV-1 reverse transcriptase depends on the length of the poly(rA) region of the template. Biochemistry. 1992;31:1375–1379. doi: 10.1021/bi00120a013. [DOI] [PubMed] [Google Scholar]
- 16.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 17.Patel P H, Jacobo-Molina A, Ding J, Taillo C, Clark A D, Jr, Raag R, Nanni R G, Hughes S H, Arnold E. Insights into DNA polymerization mechanisms from structure and function analysis of HIV-1 reverse transcriptase. Biochemistry. 1995;34:5351–5356. doi: 10.1021/bi00016a006. [DOI] [PubMed] [Google Scholar]
- 18.Quan Y, Gu Z, Li X, Liang C, Parniak M A, Wainberg M A. Endogenous reverse transcriptase assays reveal synergy between combinations of the M184V and the other drug resistance-conferring mutations in interactions with nucleoside analog triphosphates. J Mol Biol. 1998;277:237–247. doi: 10.1006/jmbi.1997.1592. [DOI] [PubMed] [Google Scholar]
- 19.Quan Y, Liang C, Inouye P, Wainberg M A. Enhanced impairment of chain elongation by inhibitors of HIV reverse transcriptase in cell-free reactions yielding longer DNA products. Nucleic Acids Res. 1998;26:5692–5698. doi: 10.1093/nar/26.24.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reardon J E. Human immunodeficiency virus reverse transcriptase: steady-state and pre-steady-state kinetics of nucleotide incorporation. Biochemistry. 1992;31:4473–4479. doi: 10.1021/bi00133a013. [DOI] [PubMed] [Google Scholar]
- 21.Rittinger K, Divta G, Goody R S. Human immunodeficiency virus reverse transcriptase substrate-induced conformational changes and the mechanism of inhibition by nonnucleoside inhibitors. Proc Natl Acad Sci USA. 1995;92:8046–8049. doi: 10.1073/pnas.92.17.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trono D. Partial reverse transcripts in virions from human immunodeficiency and murine leukemia viruses. J Virol. 1992;66:4893–4900. doi: 10.1128/jvi.66.8.4893-4900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villahermosa M L, Martinez-Irujo J J, Cabodevilla F, Santiago E. Synergistic inhibition of HIV-1 reverse transcriptase by combinations of chain-terminating nucleosides. Biochemistry. 1997;36:13223–13231. doi: 10.1021/bi970852k. [DOI] [PubMed] [Google Scholar]
- 24.Zack J A, Arrigo S J, Weitman S R, Go A S, Haislip A M, Chen I S Y. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 25.Zack J A, Haislip A M, Krogstad P, Chen I S Y. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Dornadula G, Pomerantz R. Endogenous reverse transcription of human immunodeficiency virus type 1 in physiological microenvironments: an important stage for viral infection of nondividing cells. J Virol. 1996;70:2809–2824. doi: 10.1128/jvi.70.5.2809-2824.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Zhang Y, Spicer T, Henrard D, Poiesz B. Reverse transcription take place within extracellular HIV-1 virions: potential biological significance. AIDS Res Hum Retroviruses. 1993;9:1287–1296. doi: 10.1089/aid.1993.9.1287. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Zhang Y, Spicer T, Henrard D, Poiesz B. Nascent human immunodeficiency virus type 1 reverse transcription occurs within an enveloped particle. J Virol. 1995;69:3675–3682. doi: 10.1128/jvi.69.6.3675-3682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]