Abstract
The ability to deliver genes as therapeutics requires an understanding of the vector pharmacokinetics similar to that required for conventional drugs. A first question is the half-life of the vector in the bloodstream. Retroviral vectors produced in certain human cell lines differ from vectors produced in nonhuman cell lines in being substantially resistant to inactivation in vitro by human serum complement (F. L. Cosset, Y. Takeuchi, J. L. Battini, R. A. Weiss, and M. K. Collins, J. Virol. 69:7430–7436, 1995). Thus, use of human packaging cell lines (PCL) may produce vectors with longer half-lives, resulting in more-efficacious in vivo gene therapy. However, survival of human PCL-produced vectors in vivo following systemic administration has not been explored. In this investigation, the half-lives of retroviral vectors packaged by either canine D17 or human HT1080 PCL were measured in the bloodstreams of macaques and chimpanzees. Human PCL-produced vectors exhibited significantly higher concentrations of circulating biologically active vector at the earliest time points measured (>1,000-fold in chimpanzees), as well as substantially extended half-lives, compared to canine PCL-produced vectors. In addition, the circulation half-life of human PCL-produced vector was longer in chimpanzees than in macaques. This was consistent with in vitro findings which demonstrated that primate serum inactivation of vector produced from human PCL increased with increasing phylogenetic distance from humans. These results establish that in vivo retroviral vector half-life correlates with in vitro resistance to complement. Furthermore, these findings should influence the choice of animal models used to evaluate retroviral-vector-based therapies.
Early studies reported that serum from primates, but not that from a variety of lower mammals or birds, rapidly inactivates a wide variety of retroviruses, including Moloney murine leukemia virus (MLV). The retroviruses tested in these studies were produced from nonhuman cell lines, and the inactivation occurred by a complement-dependent mechanism (8, 13, 40, 46, 47). High-titer MLV retroviral vectors produced in canine (D17 cell) packaging cell lines (PCL) can be delivered effectively in small mammals and can lead to in vivo expression of the transgene at levels expected to be therapeutic in humans (15, 20, 21, 41). However, small-mammal models are unlike humans in that their complement systems do not show significant inactivation of retroviral vectors. One obstacle to the extension of such studies to the clinic is the potential for human and nonhuman primate complement-mediated inactivation of these vectors, which may lead to greatly reduced in vivo half-lives. This concern may be most relevant for direct in vivo administration to compartments in humans where complement is abundant, such as the circulation. In fact, a previous investigation demonstrated that murine cell-produced retrovirus had an immeasurably short half-life (<1 min) after intravenous administration in macaques (9).
Historically, retroviral vectors have been predominantly made in murine or other nonhuman PCL. We and others have described certain human PCL which produce vectors resistant to inactivation by human serum complement in vitro (10, 19, 31, 32). While human PCL-produced vectors were designed to facilitate direct in vivo gene delivery in nonhuman primates and humans, their circulation half-lives in vivo are not known. Assays in vitro may not reflect the kinetics and overall effects of complement on vector stability in vivo.
PCL derived from the human HT1080 and 293 cell lines were developed in our laboratory to exploit several potential advantages over PCL derived from nonhuman sources (1, 19), the most relevant of which is the production of retroviral vectors more resistant to inactivation by human complement than those packaged in cells of nonprimate origin. One proposed mechanism is the absence of Galα(1-3)Gal (alpha-Gal) glycosylation epitopes (33, 34, 43). These epitopes are abundant in other species such as the mouse but are not present on human cells or other Old World primate cells because these cells lack alpha1-3 galactosyl transferase activity (11, 12, 16, 22). Therefore, human PCL produce retroviral vectors lacking these terminal glycosidic alpha-Gal epitopes. Retroviral vectors without this epitope would be resistant to complement inactivation mediated by the anti-alpha-Gal antibodies present in all Old World primate sera (12). Additional resistance mechanisms may also involve positive factors, such as incorporation of natural human cell membrane species-specific complement control proteins into the vector membranes, as has been shown with human immunodeficiency virus (HIV) and other viruses (28, 36, 37).
It is not clear, a priori, to what extent the resistance of vectors to complement inactivation in vitro correlates with in vivo stability. Complement activity is likely to be more robust in vivo than in vitro, and complement protein deposition may facilitate clearance of viral vectors by additional mechanisms in vivo. Even if direct inactivation of retroviral vectors by complement were blocked, complement decoration could promote clearance by the reticuloendothelial system, as has been suggested for liposome clearance (2, 6, 23, 25). We reasoned that vector clearance by complement might be substantially faster than that by other clearance mechanisms. This would be reflected in measurable differences in half-life in serum between human PCL-produced vectors and retroviral vectors produced in other PCL.
The primary goals of the investigation reported here were (i) to assess whether vectors that are resistant to complement in vitro have significantly enhanced stability in vivo, (ii) to assess the appropriateness of different species of primates for preclinical testing of retroviral vectors, and (iii) to measure the kinetics of vector clearance from the circulation following intravenous injection. Replication-competent retrovirus-free preparations of beta-galactosidase (β-Gal)-encoding retroviral vectors from producer cell lines were used to determine relative sensitivities to inactivation by human and primate sera in vitro and to assess the half-lives in the primate circulatory system in vivo.
Equivalent titers of either human- or canine-cell-packaged vector were injected, and the in vivo stability of biologically active vector from serum recovered from the circulation at various times postinjection was measured. Improved survival conferred by packaging in a human cell line would be evident as significant differences in concentrations of infectious particles in serum over time. These experiments were carried out with nonhuman primates, which inactivate the vector via the complement pathway, as a model for vector stability in humans.
In this investigation, the extent of in vitro inactivation of vector produced from human cells in chimpanzee, baboon, and macaque sera corresponded with evolutionary distance from humans. Results of in vivo serum half-life experiments performed with chimpanzees and macaques demonstrated that the relative serum stability in vivo correlated with the in vitro stability for vector produced in canine and human PCL. These data suggest significant species specificity of complement resistance among nonhuman Old World primate model systems. These results underline the importance of combining human PCL-produced retroviral vector with appropriate animal models for the development of systemic human gene therapies.
(Some in vitro assay portions of this work were presented at the Keystone Gene Therapy meeting in March 1995, and some other portions were presented at the Cold Spring Harbor Gene Therapy meeting in September 1998.)
MATERIALS AND METHODS
Cells.
All PCL were amphotropic or xenotropic “split-genome” PCL (26) with cytomegalovirus promoter-driven separate gag-pol and env expression constructs, and specific helper expression plasmids and PCL construction as described previously (1, 17, 19, 24). Table 1 lists the PCL, which were based on the canine D17 osteosarcoma cell line (ATCC CRL 8468), the human 293 embryonic kidney cell line (ATCC CRL1573), or the human HT1080 fibrosarcoma cell line (ATCC CCL 121). Cells were maintained in Dulbecco modified Eagle medium (DMEM; Gibco-BRL) containing 10% fetal bovine serum (FBS).
TABLE 1.
Packaging cell type, envelope type, and vector combinations tested
| PCL and vector | Cell type | Envelope | Gene | Selection marker |
|---|---|---|---|---|
| DA β-Gal | D-17 (canine)a | Amphotropic MLVd | β-Gal | Neor |
| DX β-Gal | D-17 (canine) | Xenotropic MLVe | β-Gal | Neor |
| DX Neor del β-Gal | D-17 (canine) | Xenotropic MLV | β-Gal | None |
| 2A β-Gal | 293 (human)b | Amphotropic MLV | β-Gal | Neor |
| 2X β-Gal | 293 (human) | Xenotropic MLV | β-Gal | Neor |
| HA Neor del β-Gal | HT1080 (human)c | Amphotropic MLV | β-Gal | None |
Osteosarcoma.
Embryonic kidney.
Fibrosarcoma.
4070A envelope.
NZB 9-1 envelope.
Vectors.
N2 β-Gal is an N2-based retroviral reporter vector expressing β-Gal from the long terminal repeat and neomycin phosphotransferase (neo gene) from an internal simian virus 40 promoter (17). N2 neo del β-Gal lacks the neo gene. “Crude” vector preparations refer to producer cell growth medium supernatant clarified by 0.45-μm-pore-size filtration. Purified vector preparations were concentrated and purified as previously described (17, 20). Vector preparations and vector-producing cell lines were shown to be free of replication-competent retrovirus by hygromycin marker rescue assay of vector or of supernatant from Mus dunni producer cell cocultivations as described previously (17, 27). Either crude or purified preparations of the same vector showed similar in vitro inactivation by serum.
Titer assays.
Samples from in vitro or in vivo assays were titered on HT1080 target cells on six-well plates in the presence of 8 μg of Polybrene (Sigma)/ml. Blue-colony-forming unit (BCFU) titers were determined following 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining by standard methods (5).
Human and nonhuman primate sera and plasma.
Serum samples were collected from nine healthy human volunteers, five chimpanzees (Laboratory for Experimental Medicine and Surgery in Primates, New York University Medical Center, Tuxedo, N.Y.), six baboons, and six macaques (Coulston Foundation, White Sands Research Center, Alamogordo, N. Mex.). Serum samples were aliquoted and frozen at −70°C. Heat inactivation was carried out by incubation at 56°C for 1 h. All human sera were tested for total classical complement activity by using a kit (Sanofi Diagnostics, Kallistad, Minn.), and only serum preparations with activity in the normal range after one freeze-thaw cycle (≥70 100% hemolytic complement [CH100] units) were used.
In vitro serum inactivation assays.
Assays were conducted in 90% serum. Twenty to 100 μl of retroviral vector was mixed in a 1:9 ratio (vol/vol) with either native serum, heat-inactivated serum, or a control consisting of heat-inactivated FBS (undiluted or a 10% solution in DMEM). Equal input titers of each vector type were used in an experiment. Approximately 105 to 106 BCFU of vector/ml was mixed with serum as described above and incubated at 37°C for 30 min, unless otherwise noted.
Experimental animals.
Normal male rhesus macaques and HIV-positive chimpanzees of both genders were obtained by and maintained at the Southwest Foundation for Biomedical Research, San Antonio, Tex. All animals were treated according to U.S. Department of Agriculture regulations.
In vivo primate assays.
Retroviral vectors expressing β-Gal and packaged in human HA (HT1080-derived) or canine DA (D17-derived) PCL were used for intravenous injection via the cephalic vein (macaques) or the antecubital vein (chimpanzees). Injection volumes were 3 ml (macaques) or 5 ml (chimpanzees), followed by a flush with an equal volume of saline. Vector administration and serum sampling were performed at the Southwest Foundation for Biomedical Research. Blood samples were drawn at 5, 10, 20, 30, 45, 60, 90, and 120 min postinjection. Serum was prepared and frozen at −70°C. Animals were observed for 2 days to ensure that no acute toxic effects occurred.
Rhesus macaques.
Males A and B received 3 × 109 BCFU of canine DA-packaged β-Gal retroviral vector (2.8 × 108 to 3.0 × 108 BCFU/kg of body weight). Males C and D received 3 × 109 BCFU of human HA-packaged β-Gal retroviral vector (2.6 × 108 to 3.7 × 108 BCFU/kg).
Chimpanzees.
Females A and B were treated with 5 × 109 BCFU of canine DA-packaged β-Gal retroviral vector (0.9 × 108 to 1.1 × 108 BCFU/kg). Chimpanzees C (female) and D (male) received 5 × 109 BCFU of human HA-packaged β-Gal retroviral vector (0.7 × 108 to 0.9 × 108 BCFU/kg).
RESULTS
Serum inactivation in vitro depends on vector titer.
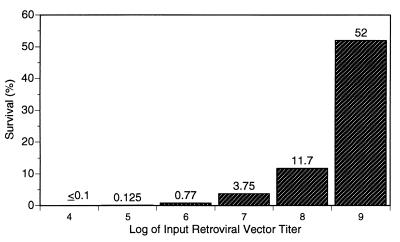
A variety of preparations of β-Gal-expressing retroviral vectors were tested to assess their relative stabilities in serum. These vectors were produced from stably transduced human- or canine-cell-derived PCL of either amphotropic or xenotropic envelope specificity, as listed in Table 1. Preliminary experiments suggested that all canine cell-produced β-Gal retroviral vectors were rapidly inactivated by heat-labile human serum activity, but the magnitude of the inactivation increased when lower titers of the vector were mixed with a fixed volume of serum. This suggested that (i) titers of different vector stocks should be matched and in an appropriate range in order to best quantify relative serum sensitivity and (ii) inactivation in vivo by complement might be overcome with sufficiently high titers. To better define these issues, in vitro inactivation of retroviral vector in 90% normal human serum over a wide range of input titers was quantitatively measured. A high-titer canine-cell-derived β-Gal vector was serially diluted, and the percentage of survival after treatment with 90% complement-active human serum was determined as a function of input titer over a range of 5 orders of magnitude. The results shown in Fig. 1 indicate that the percentage of survival increased dramatically with increasing titer over the range tested, with greater than 50% survival at the highest titer tested. Similar results were obtained with serum from another donor.
FIG. 1.
DX β-Gal retroviral vector survival following in vitro inactivation by human serum as a function of initial titer. A purified, concentrated stock of vector at 109 BCFU/ml was used either undiluted or after serial 10-fold dilutions. Vector dilutions were incubated with normal human serum or FBS under standard in vitro assay conditions. Percent survival is defined as the BCFU titer remaining after normal serum treatment relative to the BCFU titer for the FBS-treated controls at each input titer. For all in vitro serum inactivation experiments, heat-inactivated serum controls for each vector and serum showed no significant effect on titer.
Inhibition of vector inactivation appeared to be vector dependent and to be independent of other serum- or cell-derived components (which might copurify with vector), based on control experiments where dilute vector was spiked with serial dilutions of equivalently purified and concentrated conditioned medium from D17 cells, with little effect on vector sensitivity to serum (data not shown). Thus, inactivation by serum in vitro was dependent on vector concentration, and it appears that inactivation by complement can be largely overcome with sufficiently high titers of retroviral vector. To compare the relative sensitivities to complement of various retroviral vectors quantitatively, subsequent in vitro experiments used vector titers well below the saturation range, 104 to 105 BCFU/ml, in 90% serum. This was intended to approximate in vivo conditions, such as those for intravenous injection, more realistically.
Retroviral vectors from human 293 cell-based amphotropic or xenotropic packaging cells are equally resistant to inactivation by 90% human serum.
Our unpublished results, as well as findings published in reports from several groups, demonstrate that retroviral vector packaged in one of several human cell types, including HT1080 and 293 cells, is substantially resistant to complement inactivation by normal human serum, as judged by in vitro assays with human serum diluted most often to the 20-to-50% range (10, 31, 32). The envelope proteins present on the vector have also been reported to affect relative complement sensitivity (44). In order to assess whether the nature of the envelope protein or the species of origin of the PCL used to generate the vector had greater influence on sensitivity to inactivation by complement and in order to measure the degree of inactivation, studies were conducted in the presence of stringent 90% serum conditions. Figure 2 shows the data from a panel of four β-Gal vectors, with either amphotropic or xenotropic envelopes, generated from producer cells of either human 293 or canine D17 origin. Each was tested against sera from three individual human donors. Inactivation by serum was not detected when retroviral vector of either envelope tropism was packaged by human 293-derived producer cells, as the average level of 2X vector survival was 111% and 2A vector survival averaged 126%. In contrast, the same retroviral vectors were quite sensitive to inactivation by serum when they were generated by PCL with a canine D17-derived cell background. Somewhat stronger inactivation may occur in vectors expressing the xenotropic envelope than in those expressing the amphotropic envelope, with an average DX vector survival of ∼0.4% versus a DA vector survival of 7.3%. In all cases heat-inactivated serum controls produced no significant change in titer, showing that all significant inactivation depended on a heat-labile mechanism, a hallmark of complement (data not shown). This is in general agreement with previous reports of experiments testing the sensitivity of 293 cell-produced vector with an amphotropic envelope to human sera in vitro (31, 32) and extends the results to xenotropic-enveloped vector.
FIG. 2.
In vitro survival of human serum-treated vector from various PCL. Amphotropic or xenotropic retroviral vector packaged by human 293- or canine D17-derived PCL was incubated with serum as described in Materials and Methods. Vector titers were standardized at approximately 104 BCFU/ml, and all vectors were tested against a panel of three human sera. Each vector was tested at least twice, with a representative result shown here. Percent survival is defined relative to FBS-treated controls as for Fig. 1.
Human cell-produced retroviral vector has species-dependent levels of resistance to primate sera.
Since nonhuman primate models may be important for preclinical development of in vivo retroviral vector transduction protocols, the above studies were extended to the inactivation abilities of nonhuman primate sera. The same panel of four β-Gal retroviral vectors shown in Fig. 2 was tested in multiple experiments against sera from chimpanzees, baboons, or macaques. For each primate species, sera from two or more individuals were tested, with similar results. One typical set of such experiments is shown in Fig. 3. Inactivation by human serum is included for the purpose of comparison. Amphotropic and xenotropic retroviral vectors produced from human PCL show little sensitivity to human serum (average survival after 30 min, 72%) and only partial sensitivity to inactivation by chimpanzee serum (44% survival). Greater sensitivity to baboon (11.5% survival) and macaque (0.8% survival) sera was observed. The degree of sensitivity of human PCL-packaged vector to sera from the different species increased with increasing phylogenetic distance from humans. In contrast, both retroviral vectors produced from the canine D17 background showed high sensitivity to inactivation by all four sera, confirming that all had potent ability to inactivate MLV-based vectors. In each case, heat-inactivated serum produced no significant change in titer (data not shown). These results show that the mechanism by which packaging in these human PCL confers vector resistance to inactivation by serum is dependent on the species origin of the serum within the tested group of Old World primates.
FIG. 3.
Survival in human, chimpanzee, baboon, and macaque sera of β-Gal vectors from human and canine PCL. Survival of amphotropic or xenotropic retroviral vector packaged by human 293 or canine D17 PCL was determined as in the experiment for which results are shown in Fig. 2. Percent survival was defined as for Fig. 1. The full panel of vectors was tested against at least two individual donor sera from each species with comparable results.
Human PCL-produced retroviral vector has a substantially increased half-life in the circulatory systems of intravenously injected chimpanzees.
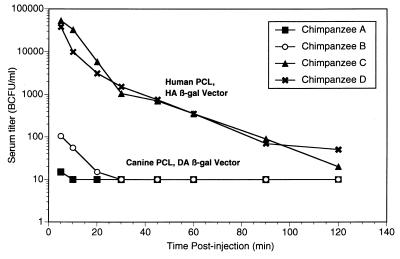
The in vitro tests suggested that chimpanzee serum may be most equivalent to human serum in terms of its inability to inactivate vector made in human PCL. To directly measure the in vivo stability of biologically active vector produced from a nonhuman versus a human PCL, an infectivity assay was performed on vector recovered from the chimpanzee circulation following intravenous injection. To maximize the initial concentrations of injected β-Gal vector in serum and thus the sensitivity of titer assays, higher-titer vectors available from a human PCL were required. Accordingly, in vivo studies used β-Gal-expressing vector packaged in a human HA (HT1080-derived) PCL which gives significantly higher titers (unpublished data) than the 293 PCL-produced vector used in the in vitro experiments for which results are shown in Fig. 2 and 3. We had previously observed that 293 and HT1080 PCL produce vectors equivalently resistant to human serum inactivation in vitro (data not shown). Either human- or canine-PCL-produced β-Gal vector was injected intravenously; equal titers of the two vectors were used. Serial serum samples were frozen, and aliquots were later titered in a single experiment to minimize interassay titer variability.
The results are plotted in Fig. 4. In chimpanzees given human-cell-produced HA/β-Gal, a level of ∼50,000 BCFU/ml was observed at 5 min postinjection. This level dropped 1,000-fold to ∼50 BCFU/ml at 120 min. By contrast, in chimpanzees given canine cell-produced DA/β-Gal, only 100 or fewer BCFU/ml of blood was detected at 5 min. This had decayed to undetectable levels (≤ 10 BCFU/ml) by 30 min. The clearance rate of the human HA PCL vector was initially rapid and then exhibited apparently steady first-order kinetics, with a half-life of ∼15 to 20 min in chimpanzees, while the half-life of the canine DA PCL vector was immeasurably short in chimpanzees.
FIG. 4.
In vivo half-lives of intravenously injected canine- and human-PCL-produced retroviral vectors in chimpanzees. Equal titers (∼5 × 109 BCFU) of DA- or HA-produced β-Gal vector were injected intravenously into chimpanzees, two animals per group. Animals A and B received canine PCL (DA) vector, and animals C and D received human PCL (HA) vector. At the times indicated, blood was drawn, and sera were prepared and frozen. The biological titer in serum samples was determined by a standard BCFU assay on tissue culture HT1080 cells. Chimpanzees A and B had very low or undetectable titers (shown here as 10 BCFU/ml).
Human PCL-produced retroviral vector has an increased half-life in the circulatory systems of intravenously injected macaques.
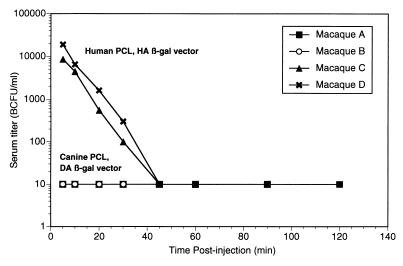
The data in Fig. 3 suggest that serum from the macaque may be more vigorous than human or chimpanzee serum in its ability to inactivate vector made in human PCL. Thus it was of interest to determine if the greater degree of inactivation in macaque serum reflected the sensitivity of the vector in vivo. Since the in vitro results (Fig. 3) suggested that more inactivation of vector might occur in macaque serum than in chimpanzee serum, the macaques were given a threefold-higher input dose of vectors (∼3 × 108 BCFU/kg, compared to 1 × 108 BCFU/kg for chimpanzees). As before, either human HA or canine DA PCL-produced β-Gal vectors, used at equivalent titers, was injected intravenously. Serial serum samples were frozen, and aliquots were later titered together to minimize interassay titer variability.
The results are plotted in Fig. 5. In macaques given human HA/β-Gal, ∼10,000 BCFU/ml of blood was found at 5 min postinjection, but by contrast, no vector activity was detected by 5 min in rhesus macaques given canine DA/β-Gal. The clearance rate of the human HA PCL vector after 5 min postinjection exhibited apparently steady first-order kinetics with a half-life of ∼5 min in macaques, while the half-life of the canine DA PCL vector was immeasurably brief. The human HA vector became undetectable by 45 min postinjection in macaques, much faster than in chimpanzees, despite a threefold-higher dose on a weight basis. The initial concentrations of human HA/β-Gal in the sera of macaques, ∼10,000 BCFU/ml at 5 min postinjection, are also about five times lower than those in the sera of chimpanzees, despite the higher dosing. Lower resistance to the macaque serum complement is one plausible explanation for this difference and for the shorter half-life in macaques.
FIG. 5.
In vivo half-lives of intravenously injected canine- and human PCL-produced retroviral vectors in macaques. Equal titers (∼3 × 109 BCFU) of DA- or HA-produced β-Gal vector were injected intravenously into macaques. There were two animals per group. Animals A and B received canine PCL (DA) vector; animals C and D received human PCL (HA) vector. At the times indicated, blood was drawn, and sera were prepared and frozen. The biological titer in serum samples was determined by a standard BCFU assay on tissue culture HT1080 cells. Macaques A and B had undetectable titers (shown here as 10 BCFU/ml).
DISCUSSION
While retroviral-vector gene therapy continues to be a promising mode of therapeutic intervention for various clinical states, the activity of these vectors in vivo remains poorly understood. To allow broad clinical applications, the function of retroviral vectors following direct administration will need to be more clearly defined. Among these issues are the role of host complement in inactivating vector following intravenous administration and the rate of vector clearance from the circulation. This study addresses these issues in nonhuman primates.
Recent reports have suggested that the resistance of human PCL-produced vectors to complement may be due primarily to the absence of alpha-Gal glycosidic epitopes (33, 34, 43). This would predict that human-PCL-produced vectors would be similarly resistant to the sera of all Old World primates or great apes, including humans. In contrast, we observed differential sensitivity to complement inactivation correlating with increasing evolutionary distance from humans (sensitivity to human serum is lowest, followed, in ascending order, by sensitivity to chimpanzee, baboon, and macaque sera). Resistance to inactivation in the sera of various Old World primates or great apes, tested in vitro here, is surprisingly species specific, involving a mechanism(s) that remains to be elucidated.
Following bolus intravenous injection into chimpanzees, concentrations of human-PCL-produced infectious vector in serum were about 1,000-fold higher initially than those of canine-PCL-produced vector and persisted at detectable levels for more than 2 h. This compared to 30 min or less for injections of equivalent titers of canine-PCL-produced vector. The concentration time course of the human-PCL-produced vector appears to have an initial rapid-distribution phase, followed by first-order kinetics of clearance with a half-life of about 15 to 20 min in chimpanzees. To our knowledge, this study is the first report of a circulation half-life of any intravenously injected viral vector. It is also the first evidence of an extended in vivo half-life of a retroviral vector produced from a human PCL.
Although the highest level of active vector in the bloodstream was observed with a combination of human PCL-produced vectors in chimpanzees, the concentration at the initial time point can represent only a small portion of the total injected active vector. With human-PCL-produced vectors in chimpanzees, initial concentrations of ∼50,000 BCFU/ml of serum were detected at 5 min postinjection. Assuming that chimpanzees have a blood volume of approximately 100 ml/kg, the total amount of vector theoretically available equals total input/blood volume, or 106 BCFU/ml. Thus the observed concentration of 50,000 BCFU/ml at 5 min represents around 5% of this amount. Several possible explanations for this initial rapid decrease in circulating vector concentration include inactivation, aggregation, trapping in locations such as the lung, spleen, and kidney, and binding to serum proteins or receptors. Even if a significant portion of the vector is rapidly bound to the normal amphotropic receptor, it is still most likely susceptible to complement inactivation until entry into target cells. Many such issues concerning both rapid and long-term biolocalization will need to be addressed in future studies with retroviral and other viral vectors.
The observed primate species dependence may provide insights into the mechanisms of complement resistance of retroviral vectors. The mechanism of inactivation by primate serum was initially reported to be an antibody-independent complement lysis mechanism which involves direct binding of C1q to the retroviral envelope (3, 4, 8, 13, 40, 46, 47). More-recent findings have suggested and strengthened evidence for a major, perhaps predominant role for an antibody-dependent mechanism of inactivation by complement, which is directed by antibody to specific terminal alpha-Gal glycosylation epitopes. In this model, retroviruses or vectors made in human cells do not display alpha-Gal terminal glycosidic epitopes because humans and Old World primates lack alpha-galactosyl transferase enzyme activity. Retroviral vectors lacking these epitopes thus lack sensitivity to the abundant anti-alpha-Gal antibody present in Old World primate or great ape serum, which can direct complement inactivation and lysis (12, 16, 22, 33, 34, 43).
However, a number of results suggest that other mechanisms must also play a role in vector inactivation. For example, significant levels of antibody-independent inactivation have often been described (7, 8), and various human cells, all purportedly alpha-Gal negative, can produce vectors with different levels of resistance to complement (31, 35, 44). It has also been observed that the viral envelope protein as well as the host cell can influence relative complement sensitivity in MLV (44) and that the sensitivities of panels of other enveloped viruses to complement show a complex pattern of virus-specific alpha-Gal dependence, differing greatly among different enveloped viruses (42, 45). Together these results suggest a role for other factors in resistance to serum complement. One such factor may be incorporation into the retroviral membrane of natural complement control proteins, such as decay-accelerating factor, from the human cell membranes, as has been reported for HIV (28, 36, 37). Because these complement control proteins exhibit stringent species dependence (29), such a mechanism would be consistent with the effect of the species origin of serum on complement sensitivity as described here.
It has been shown that high titers of canine-PCL-produced (DA) retroviral vector can induce cytotoxic T-lymphocyte responses to vector-coded antigens following intramuscular administration in macaques (17) and chimpanzees (38) despite the presumed sensitivity of the vector to complement. The in vitro results presented here showing high-titer saturation of serum inactivation provide one plausible mechanism by which this could occur. The delivery of sufficiently high-titer vector to a restricted site with a limited local complement supply may allow efficacy with a complement-sensitive retroviral vector. Conversely, for intravenous administration, the results suggest that much higher-titer doses than those used in the primate studies reported here would be needed to saturate complement in the circulation. Cornetta et al., using macaque serum in vitro over a more-limited titer range also reported a similar saturation effect (9).
These results with regard to vector clearance kinetics set the stage for more-detailed pharmacokinetic analysis of intravenously delivered retroviral vectors, a subject largely unexplored for most viral gene therapy vectors to date. One exception was a recent herpesvirus vector study using a novel radiolabeling and imaging methods, which examined physical particle biodistribution over time in mice (39). Localization at several minutes post-intravenous injection was predominantly in the bloodstream, lung, liver, and spleen, as determined by scintigraphic rough imaging, and predominantly in the liver and spleen at 12 h, as determined by imaging and by gamma-counting of tissue samples. Circulation half-lives were not examined. The infectious particle half-life in the present investigation measures clinically active vector rather than the physical particle half-life. Both types of measurement will be needed to best describe the pharmacokinetics of gene delivery vectors, illustrating some of the complexity of this aspect of gene therapy.
In summary, the results presented here demonstrate that in vitro complement resistance assays correlate well with in vivo half-life measurements and that the in vivo advantage of human PCL-produced retroviral vectors appears highly significant. It is likely that these results obtained with MLV-based retroviral vectors will also be relevant to other retroviral vectors, including lentiviral vectors such as those based on HIV and feline immunodeficiency virus (14, 18, 30). Whether these results pertain to other enveloped viruses and viral vectors can also be tested. The results also show that preclinical models need to be carefully evaluated for their relevance to the clinical situation. For example, macaques may be of limited utility in pharmacokinetic or other modeling of the intravenous administration of human PCL-produced retroviral vectors. Chimpanzees would be more suitable, although it would be difficult to perform any large-scale experiments with such a cumbersome model. Small mammals such as rabbits, whose serum does not appear to inactivate retroviral vectors from human or nonhuman PCL (10a), may be well suited for initial pharmacokinetic and biodistribution studies. From a broader perspective, the results presented here give confidence that in vitro screening of animal sera for inactivation of a specific retroviral vector preparation may give a reliable estimate of in vivo performance.
ACKNOWLEDGMENTS
We thank the many scientists formerly and presently at Chiron Technologies who contributed to the making of vector and cell reagents or to planning that made this work possible, in particular James Respess, Sunil Chada, and Michelle Burrascano. We thank many colleagues at Chiron for their help with critical reading and suggestions. Finally, we thank Nelle Cronen and Judy Dryden for excellent assistance with figures, graphics, and references.
REFERENCES
- 1.Barber, J. R., D. J. Jolly, J. G. Respess, and S. M. W. Chang. January 1997. Retroviral packaging cell lines. U.S. patent 5,591,624.
- 2.Barron L G, Meyer K B, Szoka F C., Jr Effects of complement depletion on the pharmacokinetics and gene delivery mediated by cationic lipid-DNA complexes. Hum Gene Ther. 1998;9:315–323. doi: 10.1089/hum.1998.9.3-315. [DOI] [PubMed] [Google Scholar]
- 3.Bartholomew R M, Esser A F. Differences in activation of human and guinea pig complement by retroviruses. J Immunol. 1978;121:1748–1751. [PubMed] [Google Scholar]
- 4.Bartholomew R M, Esser A F, Muller Eberhard H J. Lysis of oncornaviruses by human serum. Isolation of the viral complement (C1) receptor and identification as p15E. Virology. 1978;152:844–853. doi: 10.1084/jem.147.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cepko C. Transduction of genes using retroviral vectors. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates; 1992. pp. 9.10.1–9.10.7. [Google Scholar]
- 6.Chonn A, Cullis P R. Recent advances in liposomal drug-delivery systems. Curr Opin Biotechnol. 1995;6:698–708. doi: 10.1016/0958-1669(95)80115-4. [DOI] [PubMed] [Google Scholar]
- 7.Cooper N R. Complement evasion strategies of microorganisms. Immunol Today. 1991;12:327–331. doi: 10.1016/0167-5699(91)90010-Q. [DOI] [PubMed] [Google Scholar]
- 8.Cooper N R, Jensen F C, Welsh R M, Jr, Oldstone M B. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. J Exp Med. 1976;144:970–984. doi: 10.1084/jem.144.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornetta K, Moen R C, Culver K, Morgan R A, McLachlin J R, Sturm S, Selegue J, London W, Blaese R M, Anderson W F. Amphotropic murine leukemia retrovirus is not an acute pathogen for primates. Hum Gene Ther. 1990;1:15–30. doi: 10.1089/hum.1990.1.1-15. [DOI] [PubMed] [Google Scholar]
- 10.Cosset F L, Takeuchi Y, Battini J L, Weiss R A, Collins M K. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.DePolo, N. J. Unpublished data.
- 11.Galili U, Clark M R, Shohet S B, Buehler J, Macher B A. Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1----3Gal epitope in primates. Proc Natl Acad Sci USA. 1987;84:1369–1373. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galili U, Shohet S B, Kobrin E, Stults C L, Macher B A. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- 13.Gallagher R E, Schrecker A W, Walter C A, Gallo R C. Oncornavirus lytic activity in the serum of gibbon apes. J Natl Cancer Inst. 1978;60:677–682. doi: 10.1093/jnci/60.3.677. [DOI] [PubMed] [Google Scholar]
- 14.Gasmi M, Glynn J, Jin M-J, Jolly D J, Yee J K, Chen S-T. Requirements for efficient production and transduction of HIV-1-based vectors. J Virol. 1999;73:1828–1834. doi: 10.1128/jvi.73.3.1828-1834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greengard J S, Bodner M, McCormick J, Edwards W R, Sensintaffar J L, Sheridan P L, Nichols T C, Read M, Brinkhous K M, Jolly D J, Chang S M W. Keystone Symposium: Molecular and Cellular Biology of Gene Therapy. 1998. In vivo delivery with retroviral vectors: factor VIII gene therapy, abstr. O27; p. 41. [Google Scholar]
- 16.Hamadeh R M, Jarvis G A, Galili U, Mandrell R E, Zhou P, Griffiss J M. Human natural anti-Gal IgG regulates alternative complement pathway activation on bacterial surfaces. J Clin Investig. 1992;89:1223–1235. doi: 10.1172/JCI115706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irwin M J, Laube L S, Lee V, Austin M, Chada S, Anderson C G, Townsend K, Jolly D J, Warner J F. Direct injection of a recombinant retroviral vector induces human immunodeficiency virus-specific immune responses in mice and nonhuman primates. J Virol. 1994;68:5036–5044. doi: 10.1128/jvi.68.8.5036-5044.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston J C, Gasmi M, Lim L E, Elder J H, Yee J-K, Jolly D J, Campbell K P, Davidson B L, Sauter S L. Minimal requirements for efficient transduction of dividing and nondividing cells by feline immunodeficiency virus vectors. J Virol. 1999;73:4991–5000. doi: 10.1128/jvi.73.6.4991-5000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolly D. Viral vector systems for gene therapy. Cancer Gene Ther. 1994;1:51–64. [PubMed] [Google Scholar]
- 20.Karavodin L M, Robbins J, Chong K, Hsu D, Ibanez C, Mento S, Jolly D, Fong T C. Generation of a systemic antitumor response with regional intratumoral injections of interferon gamma retroviral vector. Hum Gene Ther. 1998;9:2231–2241. doi: 10.1089/hum.1998.9.15-2231. [DOI] [PubMed] [Google Scholar]
- 21.Kay M A, Rothenberg S, Landen C N, Bellinger D A, Leland F, Toman C, Finegold M, Thompson A R, Read M S, Brinkhous K M. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993;262:117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- 22.Koren E, Kujundzic M, Koscec M, Neethling F A, Richards S V, Ye Y, Zuhdi N, Cooper D K. Cytotoxic effects of human preformed anti-Gal IgG and complement on cultured pig cells. Transplant Proc. 1994;26:1336–1339. [PubMed] [Google Scholar]
- 23.Lasic D D, Papahadjopoulos D. Liposomes revisited. Science. 1995;267:1275–1276. doi: 10.1126/science.7871422. [DOI] [PubMed] [Google Scholar]
- 24.Laube L S, Burrascano M, Dejesus C E, Howard B D, Johnson M A, Lee W T, Lynn A E, Peters G, Ronlov G S, Townsend K S. Cytotoxic T lymphocyte and antibody responses generated in rhesus monkeys immunized with retroviral vector-transduced fibroblasts expressing human immunodeficiency virus type-1 IIIB ENV/REV proteins. Hum Gene Ther. 1994;5:853–862. doi: 10.1089/hum.1994.5.7-853. [DOI] [PubMed] [Google Scholar]
- 25.Mahato R I, Anwer K, Tagliaferri F, Meaney C, Leonard P, Wadhwa M S, Logan M, French M, Rolland A. Biodistribution and gene expression of lipid/plasmid complexes after systemic administration. Hum Gene Ther. 1998;9:2083–2099. doi: 10.1089/hum.1998.9.14-2083. [DOI] [PubMed] [Google Scholar]
- 26.Miller A D. Retrovirus packaging cells. Hum Gene Ther. 1990;1:5–14. doi: 10.1089/hum.1990.1.1-5. [DOI] [PubMed] [Google Scholar]
- 27.Miller A D, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montefiori D C, Cornell R J, Zhou J Y, Zhou J T, Hirsch V M, Johnson P R. Complement control proteins, CD46, CD55, and CD59, as common surface constituents of human and simian immunodeficiency viruses and possible targets for vaccine protection. Virology. 1994;205:82–92. doi: 10.1006/viro.1994.1622. [DOI] [PubMed] [Google Scholar]
- 29.Morgan B P, Meri S. Membrane proteins that protect against complement lysis. Semin Immunopathol. 1994;15:397–416. doi: 10.1007/BF01837366. [DOI] [PubMed] [Google Scholar]
- 30.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 31.Pensiero M N, Wysocki C A, Nader K, Kikuchi G E. Development of amphotropic murine retrovirus vectors resistant to inactivation by human serum. Hum Gene Ther. 1996;7:1095–1101. doi: 10.1089/hum.1996.7.9-1095. [DOI] [PubMed] [Google Scholar]
- 32.Rigg R J, Chen J, Dando J S, Forestell S P, Plavec I, Bohnlein E. A novel human amphotropic packaging cell line: high titer, complement resistance, and improved safety. Virology. 1996;218:290–295. doi: 10.1006/viro.1996.0194. [DOI] [PubMed] [Google Scholar]
- 33.Rother R P, Fodor W L, Springhorn J P, Birks C W, Setter E, Sandrin M S, Squinto S P, Rollins S A. A novel mechanism of retrovirus inactivation in human serum mediated by anti-alpha-galactosyl natural antibody. J Exp Med. 1995;182:1345–1355. doi: 10.1084/jem.182.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rother R P, Squinto S P. The alpha-galactosyl epitope: a sugar coating that makes viruses and cells unpalatable. Cell. 1996;86:185–188. doi: 10.1016/s0092-8674(00)80090-2. [DOI] [PubMed] [Google Scholar]
- 35.Russell D W, Berger M S, Miller A D. The effects of human serum and cerebrospinal fluid on retroviral vectors and packaging cell lines. Hum Gene Ther. 1995;6:635–641. doi: 10.1089/hum.1995.6.5-635. [DOI] [PubMed] [Google Scholar]
- 36.Saifuddin M, Hedayati T, Atkinson J P, Holguin M H, Parker C J, Spear G T. Human immunodeficiency virus type 1 incorporates both glycosyl phosphatidylinositol-anchored CD55 and CD59 and integral membrane CD46 at levels that protect from complement-mediated destruction. J Gen Virol. 1997;78:1907–1911. doi: 10.1099/0022-1317-78-8-1907. [DOI] [PubMed] [Google Scholar]
- 37.Saifuddin M, Parker C J, Peeples M E, Gorny M K, Zolla-Pazner S, Ghassemi M, Rooney I A, Atkinson J P, Spear G T. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J Exp Med. 1995;182:501–509. doi: 10.1084/jem.182.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sallberg M, Hughes J, Javadian A, Ronlov G, Hultgren C, Townsend K, Anderson C G, O’Dea J, Alfonso J, Eason R, Murthy K K, Jolly D J, Chang S M, Mento S J, Milich D, Lee W T. Genetic immunization of chimpanzees chronically infected with the hepatitis B virus, using a recombinant retroviral vector encoding the hepatitis B virus core antigen. Hum Gene Ther. 1998;9:1719–1729. doi: 10.1089/hum.1998.9.12-1719. [DOI] [PubMed] [Google Scholar]
- 39.Schellingerhout D, Bogdanov A, Jr, Marecos E, Spear M, Breakefield X, Weissleder R. Mapping the in vivo distribution of herpes simplex virions. Hum Gene Ther. 1998;9:1543–1549. doi: 10.1089/hum.1998.9.11-1543. [DOI] [PubMed] [Google Scholar]
- 40.Sherwin S A, Benveniste R E, Todaro G J. Complement-mediated lysis of type-C virus: effect of primate and human sera on various retroviruses. Int J Cancer. 1978;21:6–11. doi: 10.1002/ijc.2910210103. [DOI] [PubMed] [Google Scholar]
- 41.Smiley W R, Laubert B, Howard B D, Ibanez C, Fong T C, Summers W S, Burrows F J. Establishment of parameters for optimal transduction efficiency and antitumor effects with purified high-titer HSV-TK retroviral vector in established solid tumors. Hum Gene Ther. 1997;8:965–977. doi: 10.1089/hum.1997.8.8-965. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi Y, Liong S-H, Bieniasz P D, Jäger U, Porter C D, Friedmann T, McClure M O, Weiss R A. Sensitization of rhabdo-, lenti-, and spumaviruses to human serum by galactosyl(α1–3)galactosylation. J Virol. 1997;71:6174–6178. doi: 10.1128/jvi.71.8.6174-6178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeuchi Y, Porter C D, Strahan K M, Preece A F, Gustafsson K, Cosset F L, Weiss R A, Collins M K. Sensitization of cells and retroviruses to human serum by (alpha 1-3) galactosyltransferase. Nature. 1996;379:85–88. doi: 10.1038/379085a0. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi Y, Cosset F-L C, Lachmann P J, Okada H, Weiss R A, Collins M K L. Type C retrovirus inactivation by human complement is determined by both the viral genome and the producer cell. J Virol. 1994;68:8001–8007. doi: 10.1128/jvi.68.12.8001-8007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welsh R M, O’Donnell C L, Reed D J, Rother R P. Evaluation of the Galα1-3Gal epitope as a host modification factor eliciting natural humoral immunity to enveloped viruses. J Virol. 1998;72:4650–4656. doi: 10.1128/jvi.72.6.4650-4656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welsh R M, Jr, Cooper N R, Jensen F C, Oldstone M B. Human serum lyses RNA tumour viruses. Nature. 1975;257:612–614. doi: 10.1038/257612a0. [DOI] [PubMed] [Google Scholar]
- 47.Welsh R M, Jr, Jensen F C, Cooper N R, Oldstone M B. Inactivation of lysis of oncornaviruses by human serum. Virology. 1974;74:432–440. doi: 10.1016/0042-6822(76)90349-4. [DOI] [PubMed] [Google Scholar]