Abstract
Therapeutic suppression of human immunodeficiency virus type 1 (HIV-1) replication may help elucidate interactions between the host cellular immune responses and HIV-1 infection. We performed a detailed longitudinal evaluation of two subjects before and after the start of highly active antiretroviral therapy (HAART). Both subjects had evidence of in vivo-activated and memory cytotoxic T-lymphocyte precursor (CTLp) activity against multiple HIV-1 gene products. After the start of therapy, both subjects had declines in the levels of in vivo-activated HIV-1-specific CTLs and had immediate increases in circulating HIV-1-specific CTL memory cells. With continued therapy, and continued suppression of viral load, levels of memory CTLps declined. HLA A*0201 peptide tetramer staining demonstrated that declining levels of in vivo-activated CTL activity were associated with a decrease in the expression of the CD38+ activation marker. Transient increases in viral load during continued therapy were associated with increases in the levels of virus-specific CTLps in both individuals. The results were confirmed by measuring CTL responses to discrete optimal epitopes. These studies illustrate the dynamic equilibrium between the host immune response and levels of viral antigen burden and suggest that efforts to augment HIV-1-specific immune responses in subjects on HAART may decrease the incidence of virologic relapse.
Human immunodeficiency virus type 1 (HIV-1) infection is associated with an extremely vigorous virus-specific cytotoxic T-lymphocyte (CTL) response that declines with disease progression (3, 17). Several studies have found evidence for high levels of CTL precursors (CTLp) in subjects with control of HIV-1 replication, suggesting that CTLs may control viremia (11, 17, 28). However, the presence of high levels of CTLs has also been documented in some subjects with high viral loads, suggesting that levels of HIV-specific CTLs may be driven by high levels of HIV-1 replication (7). These observations are not necessarily mutually exclusive. Although the level of HIV-specific CTLs in an individual may be dependent on some degree of viral antigen persistence, it is likely that subjects able to generate more-vigorous CTL responses with smaller antigenic burdens may have more effective immune-mediated control of viral replication. Recent studies would support this interpretation. A study of the CTL response directed against a dominant HLA-A2-restricted Gag epitope demonstrated that the number of CTLs directed against this epitope, as measured with HLA-A2–peptide tetramers, negatively correlates with levels of plasma viremia. This study also demonstrated that the numbers of CTLs declined after initiation of highly active antiretroviral therapy (HAART) (22).
If the viral set-point is the result of an equilibration between immune responses directed against the virus and the rate of viral replication, the level of immune responses would be expected to decline with drug-induced viral suppression. We performed detailed longitudinal studies of two subjects with evidence of in vivo-activated and memory HIV-specific CTL activity and followed the evolution of these immune responses before and after HAART to evaluate the effect that therapeutic suppression of HIV-1 replication had on the level of immune activation in these individuals. Both subjects responded to HAART with sharp declines in plasma HIV-1 RNA levels. Functional assays of HIV-specific CTL activity measured with recombinant vaccinia viruses and epitopic peptides showed that direct CTL lysis declined to undetectable levels in both subjects shortly after initiation of HAART. In contrast, the levels of memory CTLs initially increased after HAART was started and then steadily declined. The results of tetramer staining in these individuals corroborated the results obtained with direct CTL assays and precursor frequency analysis and demonstrate the dynamic relationship between plasma viremia and virus-specific CTL responses.
MATERIALS AND METHODS
Subjects.
Subject 115i is an individual with well-characterized virus-specific CTL responses against multiple viral epitopes (13, 14, 16). This subject has been infected at least since 1987 and was started on zidovudine, lamivudine, and indinavir in 1996. At the time of initiation of therapy, subject 115i had a CD4 T-cell count of 269. Subject 221l has been infected since before 1985 and at the time of initiation of therapy in 1996 had a CD4 T-cell count of 457. This subject’s drug regimen consisted of stavudine, lamivudine, and indinavir. Both subjects gave written informed consent.
Viral load measurements.
HIV-1 plasma RNA levels were quantitated by reverse transcriptase (RT) PCR with the UltraDirect assay (Roche Molecular Systems, Branchburg, N.J.) according to the manufacturer’s instructions.
Synthetic peptides.
The nine amino acid peptides p17/77–85 and gp41/584–592 were synthesized as COOH-terminal acids on a Synergy 432A peptide synthesizer (Applied Biosystems, Foster City, Calif.). The amino acid numbering is according to the HXB2 sequence.
Recombinant vaccinia viruses.
Recombinant vaccinia viruses constructed from the HIV-1 IIIb isolate included those expressing Gag (vAbT141) (18), RT (vCF21) (30), and Envelope (vPE11) (26). The recombinant vaccinia virus expressing the Nef protein was constructed from the HIV-1 SF2 isolate and was the generous gift of T. Yilma. The NYCBH strain of vaccinia virus or vaccinia virus expressing the Escherichia coli β-galactosidase protein (4) was used as a control.
In vivo-activated CTL assays.
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll separation and directly incubated with chromium-labeled target cells infected with recombinant vaccinia virus expressing Lac, Gag, RT, Env, or Nef or sensitized with epitopic peptides. Assays were performed at designated effector-to-target cell (E/T) ratios in a 6-h cytotoxicity release assay as previously described (16).
CTLp frequency assays.
CTLp assays were performed as previously described (10, 15). PBMC were cultured at 250 to 16,000 per well in 24 replicate wells of a 96-well microtiter plate. To each well was added 2.5 × 104 gamma-irradiated (30 Gy) PBMC from an HIV-1-seronegative donor and the monoclonal antibody 12F6. Ten to 14 days later, the wells were split and assayed for cytotoxicity on 51Cr-labeled autologous B-LCL infected with vaccinia virus expressing HIV-1 gene products. The percent lysis for each well was determined from the formula 100 × [(experimental release − spontaneous release)/(maximum release − spontaneous release)]. A well was scored as positive in this assay if the lysis was >8%. Using 8% lysis rather than 3 standard deviations was more stringent (3 standard deviations above background on average represented 5% lysis) and gave more consistent results. In subject 115i background levels of CTLp were <20/106 PBMC in 20 of 21 assays and 74/106 PBMC in 1 assay. In subject 221l, background CTLp frequencies were <50/106 PBMC in 17 of 18 assays and 73/106 PBMC in 1 assay. Background CTLp frequencies were subtracted from frequencies against HIV-1 gene products. Based on 95% confidence intervals, 50 CTLp/106 PBMC above background was a positive result. Activated cell frequency was estimated by the maximum likelihood method (3, 6). Spontaneous release was <30% for all reported assays.
Major histocompatibility complex-tetramer synthesis.
Peptide-major histocompatibility complex tetrameric complexes were synthesized as previously described (1). Purified HLA heavy chain and β2m were synthesized by means of a prokaryotic expression system (pETR+D). The heavy chain was modified by deletion of the transmembrane cytosolic tail and COOH-terminal addition of a sequence containing the BirA enzymatic biotinylation site. Heavy chain, β2m, and peptide were refolded by dilution. The 45-kDa refolded product was isolated by fast protein liquid chromatography and then biotinylated by BirA in the presence of biotin (Sigma)–adenosine 5′-triphosphate (Sigma). Streptavidin-phycoerythrin conjugate (Sigma) was added in a 1:4 molar ratio, and the tetrameric product was concentrated to 1 mg/ml. Cryopreserved PBMC were simultaneously stained with HLA class I-peptide tetramers as described by Ogg et al. (22), anti-CD8, and anti-CD38.
RESULTS
Effect of antiretroviral therapy on viral burden and CD4 cell numbers.
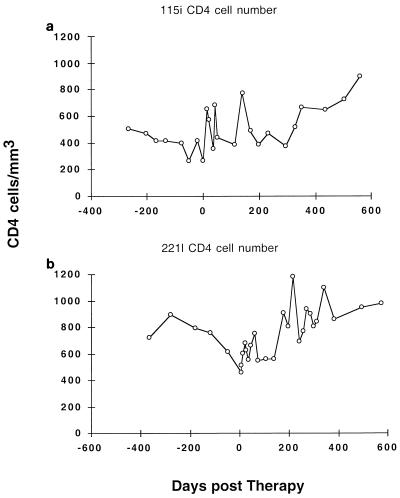
Antiretroviral therapy was first initiated in subject 115i at a viral burden of 35,518 copies/ml and a CD4 cell number of 269/mm3. This was followed by a rapid decline in the plasma HIV-1 RNA level after the initiation of therapy, with a half-life (t1/2) of 8.5 days, and by day 71 of therapy the viral burden was <50 copies/ml of plasma (Fig. 1). Viral-load measurements were not available frequently enough to delineate separate phases of decay; thus, this value represents the summation of what is likely a multiphasic process (12, 25, 31). The viral burden remained undetectable at this threshold through day 231 of therapy. Subsequently, there were small increases in plasma RNA levels on days 260, 323, and 504 with corresponding values of 522, 99, and 58 copies/ml, respectively, and these increases could not be attributed to lapses in adherence to the prescribed antiretroviral regimen. There was a corresponding gradual increase in CD4 cell numbers on therapy to nearly 900 CD4 cells/mm3 on day 560 of therapy (Fig. 2).
FIG. 1.
Longitudinal plasma HIV-1 RNA levels in subjects 115i (a) and 221l (b). All assays were performed on plasma with the Amplicor assay (Roche).
FIG. 2.
Longitudinal CD4 cell numbers in subjects 115i (a) and 221l (b).
Subject 221l had a substantially higher viral burden of 586,000 copies/ml of plasma at the initiation of therapy and a CD4 cell number of 457 cells/mm3. Despite the higher initial level of plasma HIV-1 RNA, this subject had a similarly good response to HAART, with a 3-log10 unit reduction in viral load to a nadir of 76 copies/ml at day 105 (t1/2, 9.6 days) (Fig. 1). However, this subject maintained a persistently detectable viral load with the UltraDirect assay until day 574 after therapy, when it was undetectable at <50 copies/ml. After the initial nadir of 76 copies/ml was reached at day 105, there was a small increase in viral load to 2,462 copies/ml on day 298 of therapy. The CD4 cell count of this subject steadily increased to values in the 800 to 1,100 range (Fig. 2).
Effect of antiretroviral therapy on direct CTL lysis.
Prior to the initiation of therapy, both subjects consistently demonstrated evidence of in vivo-activated HIV-specific CTL activity, as shown with fresh PBMC and target cells infected with vaccinia–HIV-1 recombinants (Fig. 3 and 4). Subject 115i demonstrated fluctuating responses to Gag, Env, and RT (Fig. 3). Subject 221l demonstrated direct activity against Gag-expressing target cells on multiple occasions prior to the initiation of therapy; however, this subject did not have consistent recognition of target cells expressing Env, RT, or Nef (Fig. 4). CTL responses were then monitored with initiation of potent antiretroviral therapy with fresh PBMC in direct assays and in precursor frequency assays.
FIG. 3.
Longitudinal in vivo-activated CTL lysis of vaccinia virus-expressed HIV-1 proteins or peptides representing optimal HLA class I-restricted CTL epitopes by subject 115i. Freshly isolated PBMC were incubated with vaccinia virus-infected autologous B-LCL or peptide-pulsed B-LCL at an E/T ratio of 100:1. Background lysis against NYCBH vaccinia virus-infected target cells was <12% at all time points except on day 20 after the start of therapy, when it was 24%. In each case background lysis was subtracted. Background lysis against unpulsed B-LCL targets was always <5%. The targets used were Vac-Gag (a), Vac-RT (b), Vac-Env (c), Vac-Nef (d), SLYNTVATL (HLA A2 restricted) (e), and ERYLKDQQL (HLA B14 restricted) (f).
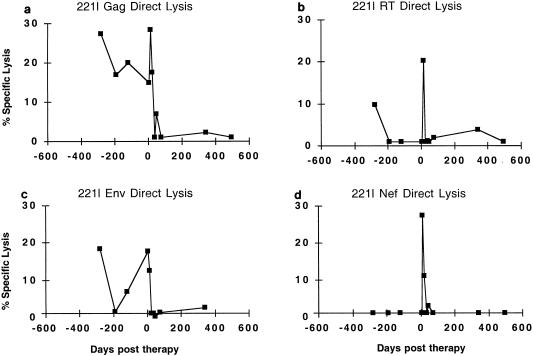
FIG. 4.
Longitudinal direct CTL lysis of vaccinia virus-expressed HIV-1 proteins by subject 221l. The mean background lysis against NYCBH-infected target cells was 19% (range 2.3 to 42%) and was subtracted. The targets used were Vac-Gag (a), Vac-RT (b), Vac-Env (c), and Vac-Nef (d).
In subject 115i direct Gag-specific CTL activity remained detectable throughout the period of decline in viral load to undetectable levels over the first 71 days of therapy. Despite some initial fluctuations in the levels of direct lysis through this period, from day 71 through day 260 there was a steady decline in direct Gag-specific lysis. By day 260 direct Gag and RT CTL activities were undetectable (<10% above background lysis at an E/T ratio of 100:1), and on day 260 there was a small breakthrough in viremia (viral load, 522 copies/ml); however, the next time that direct CTL activity was measured (day 294), Gag-specific lysis was just detectable at 11.3%. This value remained elevated at 11.4% above background lysis on day 323 then subsequently declined to undetectable levels through day 514 of therapy. Direct CTL lysis against RT and Env similarly showed transient elevations that declined to background levels over this same period (Fig. 3a to c).
The levels of in vivo-activated CTL activity in subject 221l were lower than those found in subject 115i, but CTL activity against Gag was detectable in each assay prior to the initiation of therapy. This patient’s viral burden was also much higher than that of subject 115i and took much longer to become undetectable once therapy was initiated. Although a nadir of 76 copies/ml was reached by day 105, it took until day 574 of therapy to reach <50 copies/ml. By day 36 after therapy, direct CTL activity against Gag was no longer detectable, and it was not detected again after the start of therapy, even in the face of a small peak of viremia to 2,462 copies/ml on day 298 of therapy (despite no documented lapses in drug adherence). Direct responses to RT and Env waned prior to therapy, and responses to Nef were always negative. On day 13 after initiation of therapy, in vivo-activated CTL activity against Gag, RT, and Nef targets was the highest ever observed. However, the CTL activity quickly decayed. On the next assay (day 22 of therapy) Nef direct lysis had declined to 11% above background and no lysis against RT or Env was present; by day 36 of therapy, no in vivo-activated CTLs were detectable (Fig. 4).
In summary, both subjects showed evidence of in vivo-activated HIV-specific CTLs prior to the initiation of HAART, with higher levels in the person with the lower viral load (115i). Although in vivo-activated CTL decreased after initiation of HAART, a small rebound in viremia in subject 115i was associated with a return of in vivo-activated CTL, but a resurgence of lytic activity was not evident in subject 221l despite a higher rebound in viremia. These data indicate that control of viremia with HAART is associated with a decline in in vivo-activated CTL activity and that subjects may have different viral thresholds associated with the presence of this immune response.
Effect of antiretroviral therapy on epitope-specific direct CTL responses.
In subject 115i we had previously characterized CTL responses to a dominant epitope in the envelope protein. Thus, the effects of HAART on epitope-specific lysis were measured as well. Similar to the data from target cells expressing recombinant HIV-1 antigens, epitope-specific CTL assays showed that after therapy was initiated, in vivo-activated CTL lysis declined. A direct CTL response against an A2-restricted Gag epitope in p17 that is recognized by the majority of HLA-A2-positive individuals (2, 9) declined after the initiation of therapy, and by day 112 of therapy it was negative (<10% at an E/T ratio of 100:1). Activity against an envelope epitope previously shown to be immunodominant in this individual (15, 16) remained detectable throughout day 200, well beyond the period after the viral load had declined to <50 copies/ml. As observed for the response against vaccinia virus-infected target cells, this response also rose again after a transient rise in viral load at day 294, when direct activity was present at 11% above background (Fig. 3e and f).
Effect of antiretroviral therapy on CTLp frequency.
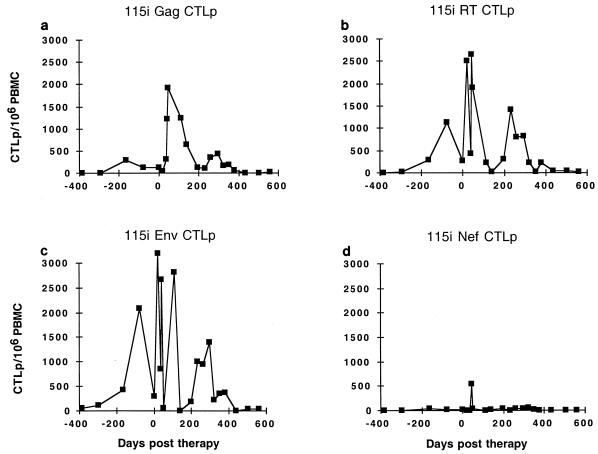
The antiviral CTL response consists of not only in vivo-activated effector cells but also memory CTLs directed against viral antigens. Prior to therapy, both subjects had detectable CTLp against several HIV-1 antigens. In both subjects the levels of memory CTLs increased shortly after the initiation of therapy, at a time when in vivo-activated CTLs were declining. In subject 115i Gag-specific CTLp frequency rose over the first 46 days of therapy to a peak of 1,921/106 PBMC, a full log10 higher than ever previously recorded in this person over 10 years of follow-up (Fig. 5a and data not shown). During this time, direct CTL activity fluctuated, reaching a peak by day 46 of therapy, but then steadily declined (Fig. 3). CTLp declined to a nadir of 105/106 PBMC by day 231 of therapy (Fig. 5a). Viral burden from day 71 through day 231 was undetectable at <50 copies/ml. A small subsequent peak of viremia on day 260 to 522 copies/ml was associated with a corresponding peak in circulating Gag CTLp. For the remainder of the study (days 261 to 504) viremia was generally <50 copies/ml and CTLp were not detectable in our assay (sensitivity, 50 CTLp/106 PBMC).
FIG. 5.
Longitudinal CTLp frequency in subject 115i. The targets used were Vac-Gag (a), Vac-RT (b), Vac-Env (c), and Vac-Nef (d).
The patterns of CTLp frequencies against RT, Env, and Nef mirrored those directed against Gag for subject 115i. On day 139 of therapy, CTLp against RT, Env, and Nef were at their nadirs of 21, 1, and 10/106 PBMC, respectively. With the increase in viral load to 522 copies/ml (day 260), increases in RT and Env CTLp can be seen that were even greater than those directed against Gag (Fig. 5b to d), consistent with the relative levels prior to the initiation of therapy.
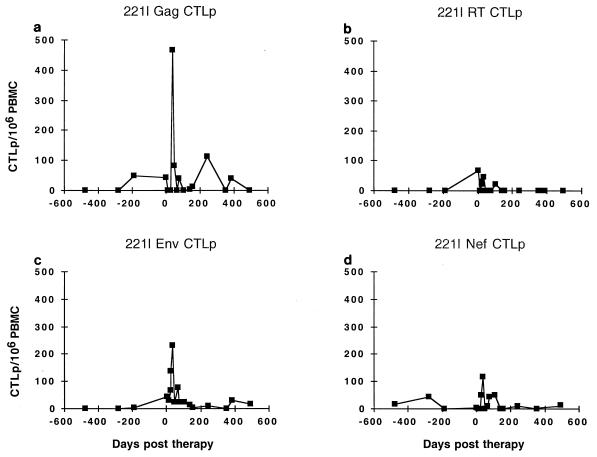
Although CTLp frequencies in subject 221l were lower than those of 115i at the start of therapy, the pattern of responses after therapy showed a similar rise and then decline. Gag CTLp frequency rose to a peak of 466/106 PBMC on day 36 after the start of therapy (Fig. 6a). Although CTLp to Env, RT, and Nef also appeared to rise after the start of therapy, Env activity showed the most consistent increase, to a peak of 232/106 on day 36 of therapy (Fig. 6b to d). Gag CTLp reached another small peak of 113/106 on day 243 of therapy, which coincided with a late rise in viremia to 2,462 on day 298 of therapy. Subsequently, the viral load declined to the 100- to 400-copy/ml range, and Gag CTLp likewise fell to levels just at the limit of detection.
FIG. 6.
Longitudinal CTLp frequency in subject 221l. The targets used were Vac-Gag (a), Vac-RT (b), Vac-Env (c), and Vac-Nef (d).
Together, the data on these two subjects show that CTLp frequencies increase after the initiation of HAART and then decrease with sustained suppression of viral replication. Small subsequent increases in viral burden while on therapy can be associated with detectable increases in the CTLp frequency, suggesting that the immune responses directed against the virus are maintained by continued exposure to viral antigen.
Effect of HAART on SL9-specific CTL tetramer staining.
Recent studies have described a negative correlation between levels of HIV-1 viremia and the number of CD8+ cells that stain with tetramer-peptide complexes (22). The tetramers used in the present study were specific for CTL clones that recognize an HLA-A2-restricted epitope in p17 (SLYNTVATL) that is recognized by the majority of HLA-A2-positive subjects (2, 9). The ability of PBMC from subject 115i to lyse B-LCL pulsed with this peptide is demonstrated in Fig. 3e. Subject 221l never demonstrated direct lysis of peptide-pulsed B-LCL above 10% at an E/T ratio of 100:1 (data not shown). Cryopreserved PBMC from each subject were simultaneously stained with tetramer, anti-CD8+, and anti-CD38 antibodies. The CD38 molecule has been shown to be an activation marker that is up-regulated in HIV-infected subjects with high levels of CTL activity (8); therefore, the effect of HAART on the activation state of tetramer-positive (tet+) cells could be determined.
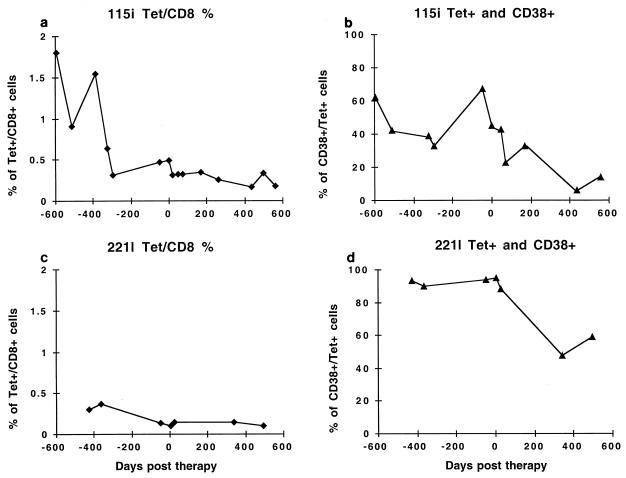
Subject 115i had a gradual decline in the number of tet+ CD8+ cells over the 600 days prior to the initiation of therapy, at the same time that the viral load was gradually increasing. Over this same period the proportion of CD38+ cells remained relatively constant at 40 to 60%, indicating a high percentage of activated cells (Fig. 7). At these times direct CTL activity against the SL9 epitope was also present. After the initiation of therapy the number of tet+ CD8+ cells continued to decrease at a low rate (t1/2, 392 days); however, in contrast to the pretherapy values, the percentage of tet+ CD38+ cells dramatically decreased to 11 to 12%, with a shorter t1/2 of 155 days. This decay in the percentage of activation marker expressing tet+ CD8+ cells was closer to the decay rate of direct SLYNTVATL-pulsed 115i B-LCL (54 days) (Fig. 7).
FIG. 7.
Longitudinal flow cytometric visualization of A*0201-restricted, SLYNTVATL-specific CTL in subjects 115i and 221l. PBMC were stained with HLA-A*0201/SLYNTVATL tetramers and monoclonal antibodies specific for CD8 and CD38. The limit of detection was 0.02% of CD8+ T cells, and the lowest value obtained in either subject was 0.1%. (a) Percentage of tet+ CD8+ T cells in subject 115i. (b) Percentage of CD38+ tet+ cells in subject 115i. (c) Percentage of tet+ CD8+ T cells in subject 221l. (d) Percentage of CD38+ tet+ cells in subject 221l.
Subject 221l had a lower initial frequency of tet+ CD8+ cells prior to the initiation of therapy (highest pretherapy value, 0.37%, compared to 1.8% for subject 115i). The frequency of these cells similarly declined over the 400 days prior to the start of therapy. There was no further decline in the already low frequency of these cells posttherapy. Prior to and just after the start of therapy, the percentage of these cells expressing the activation marker CD38 was >90%, and it declined to <60% after 340 days of therapy. The higher percentage of cells expressing CD38 suggests ongoing antigenic stimulation, as is consistent with both the persistent low-level plasma viremia and persistent detection of tet+ CD8+ cells.
Thus, the direct quantitation of these CD8+ antigen-specific cells indicates a gradual decrease in their frequency as the viral load increased prior to therapy. In both subjects a decrease in the activation of these cells, reflected by down-regulation of the CD38+ marker, was observed with continued suppression of viral replication.
DISCUSSION
In this study we have examined the effect of HAART on both in vivo-activated and memory CTLs. We find that suppression of viral replication results in a decrease in in vivo-activated CTLs that precedes the decline in memory CTLs. Remarkably, CTLp in both individuals studied increased shortly after the initiation of therapy. This increase in activity was polyclonal, as evidenced by assays of CTL activity with target cells infected with vaccinia virus constructs expressing HIV-1 viral gene products or sensitized with epitopic peptides. Changes in the quantity and activation state of CTLs were further verified by direct flow cytometric visualization of CTLs with HLA-peptide tetramers directed against an HLA A*0201-restricted epitope in p17.
This study has further defined the interaction between the virus-specific CTL response and viral burden. Few studies have evaluated HIV-specific CTL responses longitudinally after the initiation of HAART. These previous studies did not include multiple evaluations of CTL activity with direct CTL assays of HIV-1 gene products and specific viral epitopes and memory CTL assays prior to and after the initiation of therapy (5, 23). We had the benefit of studying two individuals followed for almost 2 years before and after the initiation of HAART. Both individuals had excellent virologic responses to therapy and sustained increases in CD4 cell numbers. Likewise, in both subjects direct CTL activity declined shortly after the initiation of HAART. Interestingly, CTLp frequencies against multiple HIV-1 antigens increased initially even as direct CTL activity declined. Both subjects showed marked decreases in the expression of the CD38 activation marker on the tet+ CD8+ cells after the initiation of HAART. These changes in the quantity and quality of immune reactivity in response to an abrupt decrease in plasma viremia highlight the dependence of immune activation on exposure to antigen.
The magnitude of CTL responses in these HLA A*0201-positive subjects prior to the initiation of HAART, as measured by tetramer analysis, is consistent with what had been observed in an earlier study (22). Ogg et al. found in a cross-sectional study that the level of HIV-1 plasma viremia was negatively correlated with the percentage of CD8+ cells that stained with HLA A*0201 peptide tetramers. They also found that the frequency of tetramer-positive CTLs declined after the initiation of therapy. A more recent study has calculated that the “decay” t1/2 of tetramer-positive cells was on average 45 days after the initiation of HAART (23). We were able to prospectively follow our subjects with direct CTL assays performed with freshly isolated PBMC as well as with tetramer staining. Tetramer-positive cells were detectable in each subject and, consistent with prior results, were higher in the subject with the lower pretreatment viral load. In both subjects the frequency of these cells declined as the viral burden was increasing prior to therapy (with a t1/2 of 335 days in subject 115i and 247 days in subject 221l). This emphasizes the inverse relation between the frequency of virus-specific CTLs and viral burden. The low frequency of tet+ CD8+ cells in subject 221l is consistent with the low level of direct lysis against the p17/77–85 epitope (data not shown). However, other CTL responses were present, as evidenced by direct lysis of vaccinia virus-Gag-infected target cells. In subject 115i there was a continued decline in the number of these cells after therapy was started. Decay in tet+ CD8+ cells was not observed in subject 221l after the start of therapy, probably due to a lack of sustained suppression of viral load in this individual. Both subjects showed marked decreases in the expression of the CD38 activation marker on the tet+ CD8+ cells after the initiation of HAART, which is consistent with the observed decline of direct lytic activity after the initiation of HAART. Only subject 115i had significant direct CTL effector responses to the SLYNTVATL peptide; however, the decay rate of direct effector activity was closer to that of the CD38 activation marker than it was to that of the absolute frequency of tet+ CD8+ cells.
These studies extend previous studies by showing the relationship between HAART and functional CTLp and direct CTL activities. The standard measurement of memory CTLp has been through limiting-dilution assays that require several rounds of in vitro replication before expansion of a single memory cell sufficient to detect lysis in a chromium release assay occurs. It has been hypothesized that activated effector cells that mediate lysis in direct killing assays are not able to expand in precursor frequency assays (20). In both subjects studied here, levels of memory CTLs as measured by a standard CTLp frequency assay arose to their highest levels after HAART was initiated, and these rises occurred as the level of direct CTL activity declined. These results are consistent with the generalized finding that shortly after the initiation of HAART levels of memory CD8 cells rise, followed by a slow decline to baseline levels (19). A possible explanation for the concurrent drop in levels of CTL effector activity and increase in the number of CTLp after HAART is a reversion of activated CTL effector cells to a resting-cell phenotype (27, 29). The decline in the percentage of CD38+ tet+ cells as the number of CTLp rises would also support this hypothesis. Alternatively, these results may arise from a perturbation of steady-state production of activated CTL from CTLp. Another possible explanation is that CTLp redistribute from lymph node sites when the antigen load is decreased.
With continued therapy, and continued declines in the antigenic burden, levels of memory CTLs also declined. This phenomenon has also been described by Dalod et al. (5). These investigators used a bulk-stimulation technique (21) to generate CTL responses and used lytic unit calculations to determine the magnitude of the responses. In that study, subjects with good control of viremia (6 of 13) to undetectable levels showed a decrease in HIV-specific CTL activity. It has also been documented that in general, overall levels of memory CD8+ T cells decline after 12 weeks of HAART (24), consistent with the HIV-1-specific CTLp results shown here.
We also found that small increases in viremia, once an initial nadir of plasma HIV-1 RNA viremia was reached, were accompanied by proportional increases in CTLp in each subject. Subject 115i had a much higher elevation of the number of CTLp (a peak of 439 CTLp/million) in response to a very small increase in viral load (to 522 copies/ml on day 260), while subject 221l had a much smaller peak of 113 CTLp/million in response to a viral-load peak of 2,462 copies/ml. The higher-magnitude immune response to a lower antigen burden may be one explanation for the more effective control of viral replication in this subject. It is interesting to note that subject 115i had the higher pretreatment CTL response and lower pretreatment viral load. The subsequent decline in viral burden in each subject was possibly driven by the increase in detectable CTLp. Although this is an attractive explanation, transient unreported nonadherence to the HAART regimen or a subclinical illness that transiently elevated the viral burden cannot be ruled out.
This study has several implications for vaccine development and immunity-based therapy protocols. Prior studies have shown that higher frequencies of HIV-1-specific CTLs are associated with lower viral burdens in chronically infected subjects (22). This study demonstrates a dynamic equilibrium between these effector cells and the viral burden, with rises in CTLs in response to increases in viremia after therapy. The brisk response to a small increase in viral load suggests that the immune system maintains the ability to rapidly respond, even as CTLp fall to low levels. Since the nature of the immune system is to respond to an invading pathogen, it is not surprising that in the presence of HAART, and a subsequent decline in the antigenic burden, levels of virus-specific CTLs fall precipitously. If these effector cells are playing a protective role, then efforts to increase their numbers through therapeutic vaccination in subjects on HAART may decrease the rate of virologic relapse in these subjects by driving the levels of viral replication below the threshold required for drug resistance mutations to accumulate.
ACKNOWLEDGMENTS
This work was supported by NIH grants R01 AI39966, R01 AI40873, R01 AI28568, and CA12464.
We thank M. Gately and Hoffman-LaRoche for the generous gift of interleukin-2.
REFERENCES
- 1.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Brander C, Hartman K E, Trocha A K, Jones N G, Johnson R P, Korber B, Wentworth P, Buchbinder S P, Wolinsky S, Walker B D, Kalams S A. Lack of strong immune selection pressure by the immunodominant, HLA-A*0201-restricted cytotoxic T lymphocyte response in chronic human immunodeficiency virus-1 infection. J Clin Investig. 1998;101:2559–2566. doi: 10.1172/JCI2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalod M, Harzic M, Pellegrin I, Dumon B, Hoen B, Sereni D, Deschemin J C, Levy J P, Venet A, Gomard E. Evolution of cytotoxic T lymphocyte responses to human immunodeficiency virus type 1 in patients with symptomatic primary infection receiving antiretroviral triple therapy. J Infect Dis. 1998;178:61–69. doi: 10.1086/515587. [DOI] [PubMed] [Google Scholar]
- 6.de St. Groth F. The evaluation of limiting dilution assays. J Immunol Methods. 1982;49:R11–R23. doi: 10.1016/0022-1759(82)90269-1. [DOI] [PubMed] [Google Scholar]
- 7.Ferbas J, Kaplan A H, Hausner M A, Matud J L, Liu Z, Panicali D L, Nerng-Ho H, Detels R, Giorgi J V. Virus burden in long-term survivors of human immunodeficiency virus (HIV) infection is a determinant of anti-HIV CD8+ lymphocyte activity. J Infect Dis. 1995;172:329–339. doi: 10.1093/infdis/172.2.329. [DOI] [PubMed] [Google Scholar]
- 8.Giorgi J V, Ho H N, Hirji K, Chou C C, Hultin L E, O’Rourke S, Park L, Margolick J B, Ferbas J, Phair J P. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion: development of HLA-DR+ CD38− CD8+ cells is associated with subsequent stable CD4+ cell levels. The Multicenter AIDS Cohort Study Group. J Infect Dis. 1994;170:775–781. doi: 10.1093/infdis/170.4.775. [DOI] [PubMed] [Google Scholar]
- 9.Goulder P J, Sewell A K, Lalloo D G, Price D A, Whelan J A, Evans J, Taylor G P, Luzzi G, Giangrande P, Phillips R E, McMichael A J. Patterns of immunodominance in HIV-1-specific cytotoxic T lymphocyte responses in two human histocompatibility leukocyte antigens (HLA)-identical siblings with HLA-A*0201 are influenced by epitope mutation. J Exp Med. 1997;185:1423–1433. doi: 10.1084/jem.185.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrer T, Harrer E, Kalams S A, Barbosa P, Trocha A, Johnson R P, Elbeik T, Feinberg M B, Buchbinder S P, Walker B D. Cytotoxic T lymphocytes in asymptomatic long-term nonprogressing HIV-1 infection. Breadth and specificity of the response and relation to in vivo viral quasispecies in a person with prolonged infection and low viral load. J Immunol. 1996;156:2616–2623. [PubMed] [Google Scholar]
- 11.Harrer T, Harrer E, Kalams S A, Elbeik T, Staprans S I, Feinberg M B, Cao Y, Ho D D, Yilma T, Caliendo A M, Johnson R P, Buchbinder S P, Walker B D. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:585–592. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 12.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 13.Johnson R P, Trocha A, Buchanan T M, Walker B D. Identification of overlapping HLA class I-restricted cytotoxic T cell epitopes in a conserved region of the human immunodeficiency virus type 1 envelope glycoprotein: definition of minimum epitopes and analysis of the effects of sequence variation. J Exp Med. 1992;175:961–971. doi: 10.1084/jem.175.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson R P, Trocha A, Yang L, Mazzara G P, Panicali D L, Buchanan T M, Walker B D. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J Immunol. 1991;147:1512–1521. [PubMed] [Google Scholar]
- 15.Kalams S A, Johnson R P, Dynan M J, Hartman K E, Harrer T, Harrer E, Trocha A K, Blattner W A, Buchbinder S P, Walker B D. T cell receptor usage and fine specificity of human immunodeficiency virus 1-specific cytotoxic T lymphocyte clones: analysis of quasispecies recognition reveals a dominant response directed against a minor in vivo variant. J Exp Med. 1996;183:1669–1679. doi: 10.1084/jem.183.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalams S A, Johnson R P, Trocha A K, Dynan M J, Ngo H S, D’Aquila R T, Kurnick J T, Walker B D. Longitudinal analysis of T cell receptor (TCR) gene usage by human immunodeficiency virus 1 envelope-specific cytotoxic T lymphocyte clones reveals a limited TCR repertoire. J Exp Med. 1994;179:1261–1271. doi: 10.1084/jem.179.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koup R A, Pikora C A, Luzuriaga K, Brettler D B, Day E S, Mazzara G P, Sullivan J L. Limiting dilution analysis of cytotoxic T lymphocytes to human immunodeficiency virus gag antigens in infected persons: in vitro quantitation of effector cell populations with p17 and p24 specificities. J Exp Med. 1991;174:1593–1600. doi: 10.1084/jem.174.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lederman M M, Connick E, Landay A, Kuritzkes D R, Spritzler J, St. Clair M, Kotzin B L, Fox L, Chiozzi M H, Leonard J M, Rousseau F, Wade M, Roe J D, Martinez A, Kessler H. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis. 1998;178:70–79. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 20.McMichael A J, O’Callaghan C A. A new look at T cells. J Exp Med. 1998;187:1367–1371. doi: 10.1084/jem.187.9.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nixon D F, Townsend A R, Elvin J G, Rizza C R, Gallwey J, McMichael A J. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988;336:484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- 22.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 23.Ogg G S, Jin X, Bonhoeffer S, Moss P, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Hurley A, Markowitz M, Ho D D, McMichael A J, Nixon D F. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pakker N G, Notermans D W, de Boer R J, Roos M T, de Wolf F, Hill A, Leonard J M, Danner S A, Miedema F, Schellekens P T. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 25.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 26.Polydefkis M, Koenig S, Flexner C, Obah E, Gebo K, Chakrabarti S, Earl P L, Moss B, Siliciano R F. Anchor sequence-dependent endogenous processing of human immunodeficiency virus 1 envelope glycoprotein gp160 for CD4+ T cell recognition. J Exp Med. 1990;171:875–887. doi: 10.1084/jem.171.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razvi E S, Welsh R M, McFarland H I. In vivo state of antiviral CTL precursors. Characterization of a cycling cell population containing CTL precursors in immune mice. J Immunol. 1995;154:620–632. [PubMed] [Google Scholar]
- 28.Rinaldo C, Huang X L, Fan Z F, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selin L K, Welsh R M. Cytolytically active memory CTL present in lymphocytic choriomeningitis virus-immune mice after clearance of virus infection. J Immunol. 1997;158:5366–5373. [PubMed] [Google Scholar]
- 30.Walker B D, Flexner C, Paradis T J, Fuller T C, Hirsch M S, Schooley R T, Moss B. HIV-1 reverse transcriptase is a target for cytotoxic T lymphocytes in infected individuals. Science. 1988;240:64–66. doi: 10.1126/science.2451288. [DOI] [PubMed] [Google Scholar]
- 31.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]