Abstract
Human respiratory syncytial virus (RSV) is a major cause of acute respiratory infection. In 2020, RSV was effectively eliminated from the community in New Zealand due to non-pharmaceutical interventions (NPI) used to control the spread of COVID-19. However, in April 2021, following a brief quarantine-free travel agreement with Australia, there was a large-scale nationwide outbreak of RSV that led to reported cases more than five times higher, and hospitalisations more than three times higher, than the typical seasonal pattern. In this study, we generated 1,471 viral genomes of both RSV-A and RSV-B sampled between 2015 and 2022 from across New Zealand. Using a phylodynamics approach, we used these data to better understand RSV transmission patterns in New Zealand prior to 2020, and how RSV became re-established in the community following the relaxation of COVID-19 restrictions. We found that in 2021, there was a large epidemic of RSV in New Zealand that affected a broader age group range compared to the usual pattern of RSV infections. This epidemic was due to an increase in RSV importations, leading to several large genomic clusters of both RSV-A ON1 and RSV-B BA9 genotypes in New Zealand. However, while a number of importations were detected, there was also a major reduction in RSV genetic diversity compared to pre-pandemic seasonal outbreaks. These genomic clusters were temporally associated with the increase of migration in 2021 due to quarantine-free travel from Australia at the time. The closest genetic relatives to the New Zealand RSV genomes, when sampled, were viral genomes sampled in Australia during a large, off-season summer outbreak several months prior, rather than cryptic lineages that were sustained but not detected in New Zealand. These data reveal the impact of NPI used during the COVID-19 pandemic on other respiratory infections and highlight the important insights that can be gained from viral genomes.
Keywords: Respiratory syncytial virus, RSV, genomic epidemiology, phylodynamics, virus evolution, disease outbreak, New Zealand
Introduction
Respiratory syncytial virus (RSV) is a major cause of acute respiratory infection mainly affecting children, the elderly, and those with comorbidities, resulting in a considerable hospitalisation burden among children in particular1,2. This RNA virus is part of the Orthopneumoviruses, a genus of viruses in the Pneumoviradae family that generally cause upper respiratory tract infections in humans and some other vertebrates3. RSV is known to cause severe disease in children, particularly infants4, and in older adults. In 2019, for instance, there were an estimated 33 million RSV-associated acute respiratory infections globally, and over 100,000 deaths in children under five years old5. While serological studies have found that RSV infection is common in infants4, the virus induces poor immunity and thus reinfection can be frequent throughout life6. RSV infection is less severe in adults, typically causing upper respiratory infections with mild to moderate symptoms7. However, in the elderly, RSV can be more severe, and is reported to have morbidity and mortality rates comparable with influenza virus8.
The global circulation of RSV is characterised by a distinct seasonality9–12. In New Zealand, a geographically isolated country in the southern hemisphere with a temperate climate, RSV cases usually increase in autumn (i.e. at the end of April) and peak in mid-winter (late June)13. However, in 2020, the regular circulation of RSV was disrupted due to the introduction of non-pharmaceutical interventions (NPI), the most stringent of which was the closure of international borders, used to curb the spread of SARS-CoV-214. These NPI were effective in eliminating the circulation and reintroduction of SARS-CoV-2 in New Zealand15,16, and consequently appeared to eliminate the introduction and spread of other respiratory viruses too14. During the winter of 2020, reported cases of RSV were reduced by 98% when compared to the average annual cases recorded from 2015 to 201914. Indeed, compared to annual averages, New Zealand also saw reductions in cases of influenza virus (99.9%), metapneumovirus (92%), enterovirus (82%), adenovirus (81%), parainfluenza virus (80%), and rhinovirus (75%)14. A decrease in the circulation of RSV during 2020 was also observed in other countries that used NPI including in the USA17, Australia11, China18, Singapore19, South Africa20, UK, and Ireland21.
In April 2021, following over a year of restricted travel into New Zealand, a brief quarantine-free travel agreement was initiated between New Zealand, Australia, and other countries in the South Pacific22. This relaxation of travel restrictions coincided with an increase in the circulation of respiratory viruses in New Zealand23. Specifically, New Zealand experienced reported RSV cases five times higher, and hospitalisations three times higher, than those typically seen in the pre-pandemic phase24. Australia experienced similar epidemic surges of RSV following the relaxation of COVID-19 restrictions leading to extensive off-season outbreaks and hospitalisations during the summer of 2020 from cryptically circulating lineages11. By August 2021, New Zealand had suspended quarantine free travel and once again closed the border with Australia. New Zealand then entered its second nationwide lockdown in order to control the spread of the highly-transmissible SARS-CoV-2 Delta variant25. This in turn was met with a considerable decline in the number of reported RSV cases26. Beginning early 2022, the New Zealand border became unrestricted, and with the increased migration of people into the country there has been widespread community transmission of respiratory viruses once again, including influenza virus, RSV and the SARS-CoV-2 Omicron variant26,27.
Viral genomic data offers valuable insights into the pattern of viral evolution, and therefore enhances our understanding of outbreak epidemiology by providing a high level of detail and context to transmission patterns. When combined with geographic and population information, these data can reveal pathways of viral spread that can be used to inform effective national and local interventions16,28. Temporal and geographic information can be integrated into phylodynamic analyses to detect the spread of a pathogen in time and space, develop predictions about an outbreak’s trajectory, and identify clusters of similar sequences as an indication of infection sources and superspreading28–30. Herein, we describe the first genome-scale analysis of RSV in New Zealand spanning eight years from 2015 to 2022. This time frame incorporates several significant time points, including the New Zealand border closure in early 2020, nationwide lockdowns during the COVID-19 pandemic, the quarantine-free travel period with Australia and the South Pacific in 2021, and later, the easing of travel restrictions as New Zealand reconnected with the rest of the world in 2022. In this study we investigate the typical patterns and dynamics of RSV transmission in New Zealand prior to 2020, and how RSV became re-established in the community following its elimination.
Materials & Methods
Ethics
The New Zealand Northern A Health and Disability Ethics Committee approved the Southern Hemisphere Influenza Vaccine Effectiveness Research and Surveillance (SHIVERS) study (NTX/11/11/102). Ethics approval was not required for the primary care), laboratory, or hospital-based surveillance platforms as they were conducted in accordance with the Public Health Act 1956.
Sample collection
Viral samples were obtained from several surveillance and research platforms, each serving a different purpose. Community-based samples were obtained from general practitioners (GPs) across New Zealand from patients with influenza-like illness (ILI), for which the surveillance period typically began in late April and ended in late September. Hospital-based samples were obtained from patients with severe acute respiratory infection (SARI) from two major Auckland based hospitals year round. Laboratory-based surveillance samples were referred to the Institute for Environmental Science and Research (ESR) from both community and hospital diagnostic laboratories throughout the year in several major centres: Auckland, Bay of Plenty, Waikato, Hawkes Bay, Wellington, Nelson, Christchurch and Dunedin. Samples were also collected and tested at ESR for the Southern Hemisphere Influenza Vaccine Effectiveness Research and Surveillance (SHIVERS) study. This study included three Wellington-based community cohorts, where samples were obtained from participants that experienced acute respiratory infection (ARI). The surveillance period for these cohorts matched that of the community-based surveillance. In 2021, a fifth iteration of SHIVERS commenced in which samples from diagnostic and hospital laboratories throughout New Zealand referred positive RSV samples to ESR for further testing and analysis. Collection dates of the genomes used in this study were collected from July 2015 up to, and including, October 2022.
Viral RNA extraction and qRT-PCR
Nasopharyngeal samples were received by the Clinical Virology Department at ESR from 2015 to 2022. Viral RNA was extracted using the Thermo Fisher Scientific™ MagMax Viral/Pathogen Nucleic Acid Isolation Kit (A48310) as per manufacturer’s instructions on either the ZiXpress or the Kingfisher Flex automated extraction instruments.An RSV singleplex qRT-PCR was performed on RNA using primer and probes synthesised by biosearch using sequences published by Kim et al31, coupled with the AgPath-ID™ One step RT-PCR buffer and enzyme (4387424). Positive RSV RNA was then genotyped (RSV-A, RSV-B) using primers and probes synthesised by biosearch using sequences published by Hu et al32, coupled with the AgPath-ID™ One step RT-PCR buffer and enzyme (4387424). Samples collected between 2015 and 2021 with a cycle threshold (Ct.) below 25, as well as samples from 2022 with a Ct. below 30, were referred to the genomic sequencing department at ESR.
Generating viral genomes from RSV samples
In brief, sequencing was performed on the Illumina Nextseq platform using the respiratory virus oligo panel v2 (20044311), RNA prep enrichment kit (20040537) and the 500/550 midoutput kit (20024905) and used as per manufacturer’s instructions. Consensus based assembly was performed using a standardised pipeline, based on the Seattle Flu assembly pipeline, and modified for use by ESR, (https://github.com/seattleflu/assembly). Two additional references were used for consensus calling: EPI_ISL_2543807 and EPI_ISL_2543850, available from GISAID33. Consensus viral genomes were generated, and subject to quality testing and those with fewer than 50% ambiguities were selected for further analysis. The genomes generated are available on GISAID under two blocks of consecutive accession numbers (EPI_ISL_16959469 - EPI_ISL_16960152 and EPI_ISL_19206151 - EPI_ISL_19207488).
Genotyping RSV genomes
Reference G glycoprotein gene (G-gene) sequences for each known genotype for both RSV-A and RSV-B were obtained from GenBank (see Supplementary Table 1 for a list of accession numbers and their genotypes). G-gene sequences were first annotated and extracted from the New Zealand genomes using Geneious Prime 2022.1.1 (https://www.geneious.com) and aligned with the reference sequences using MAFFT (v7)34 using the FFT-NS-2 algorithm. A maximum likelihood phylogenetic tree for each of RSV-A and RSV-B was estimated using IQ-TREE (v 1.6.8)35, using the Hasegawa-Kishino-Yano nucleotide substitution model with a gamma distributed rate variation among sites (HKY+Γ)36 (determined to be the best fit model by ModelFinder)37. Using the topology of the reference sequences, the genotypes of the New Zealand RSV G-genes were determined. Genotyping across whole genome sequences was also performed using LABEL38 based on genotypes proposed by Chen et al39 following alignment with MAFFT (v7)34.
Inferring genomic diversity of RSV using phylogenomic analysis
Newly acquired New Zealand RSV genomes were aligned with globally-sourced RSV genomes with MAFFT (v7)34 using the FFT-NS-2 algorithm (see Supplementary Table 2 for global genome accession numbers). These global genomes (3,487 RSV-A and 3,002 RSV-B) were sampled uniformly at random from ~50,000 international sequences on GISAID33 between 2015 and 2022 (as of March 2024). A maximum likelihood phylogenetic tree was estimated using IQ-TREE (v 1.6.8)35, using the HKY+Γ36 nucleotide substitution model. We integrated the in-build Least Squares Dating (LSD2) method to estimate a time-scaled phylogenetic tree using the day of sampling. Phylogenetic branch support was assessed using the ultrafast bootstrap40 approximation. We used TempEst (v 1.5.3)41 to analyse the root-to-tip genetic regression against sampling dates to investigate the temporal signal of RSV-A and RSV-B genomes.
Estimating introductions of RSV using Bayesian phylodynamic analysis
To infer the migration dynamics of RSV into New Zealand over time, we treated geographic location as a discrete trait with two demes where genomes were either sampled as being ‘local’ (i.e. sampled from New Zealand) or ‘global’ (i.e. sampled from the rest of the world). Due to the size of the dataset, local and global RSV-A and RSV-B genomes were randomly split into six subsamples, making sure that near equal numbers of genomes were selected for each deme for each year. For each of RSV-A and RSV-B, each subsample comprised approximately 400 local and 400 global genomes. To correct for geographical biases on GISAID, global genomes were subsampled by iteratively sampling a country and then a genome from that country. Each subsample was aligned using MAFFT (v7)34 and subject to Bayesian phylodynamic analysis using BEAST2 (v2.7)42 with the MASCOT structured coalescent tree prior43. We again used the HKY+Γ36 nucleotide substitution and a strict clock LogNormal(mean=0.001, sigma=0.1) clock rate prior (units: substitutions per site per year). Introduction (i.e. transition from global to local) and export (local to global) events were assumed to be rare occurrences, and introductions were assumed much more common than exports, reflecting prior knowledge about epidemics originating overseas and then coming to New Zealand. The prior on introductions was Exponential(mean=0.1) migrations per year and Exponential(mean=0.001) for exports. The (constant) effective population sizes of the two demes had independent LogNormal(mean=0.2, sigma=0.4) priors. We sampled the posterior distribution using Markov chain Monte Carlo (MCMC) running each analysis for 100 million steps, sampling states every 10,000 steps, and discarding the first 10% as burn-in. Convergence was assessed in Tracer v1.644, ensuring that all analyses had over 200 effective samples for all reported parameters. All 6/6 RSV-A analyses and 2/6 RSV-B analyses were completed to this standard (the failed analyses are not reported here). The number of introductions of RSV-A and RSV-B over time was estimated using the Babel package in BEAST2 and the results were summarised in R (https://ww.R-Project.org/)45.
Results & Discussion
The elimination and reintroduction of RSV during the COVID-19 pandemic
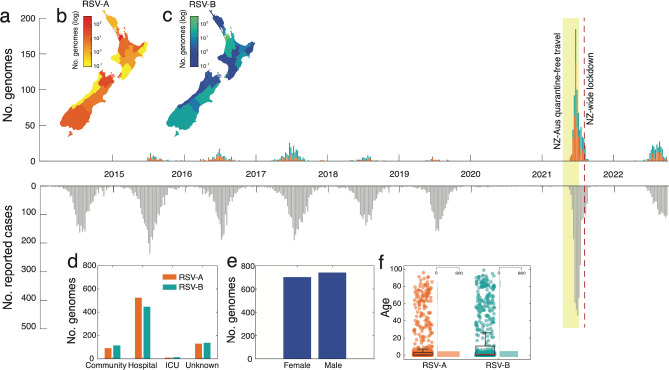
In 2021, New Zealand experienced a large epidemic of RSV, following on from a brief period of quarantine-free travel with Australia and other countries in the South Pacific (Figure 1). To better understand the transmission dynamics of RSV before, during, and after this outbreak, we generated RSV genomes from patients in New Zealand over eight years. In total, 1,471 viral genomes, including 756 RSV-A and 715 RSV-B sequences, were generated from New Zealand between 2015 and 2022. Both RSV-A and RSV-B co-circulated each year in relatively even prevalence. Genomes were generated from the male and female sexes in equal proportions, and samples were mostly collected from young children with an overall median age of 1 (range 0 to 99) (Figure 1). Severity of RSV infections can be inferred by the surveillance platform from which the sample originated. Among samples from a known origin, included in this study, 83% were referred from hospital-based surveillance, including outpatients and ICU, meaning that the vast majority of genomes were most likely generated from severe infections (Figure 1).
Figure 1.
(a) Number of RSV-A (orange) and RSV-B (green) genomes generated over time based on sample collection date. The reflected grey bars below show the number of reported RSV positive cases in New Zealand over the same timeframe46. (b-c) Map of New Zealand, coloured by the number of positive RSV-A and -B cases in each District Health Board. (d) The number of RSV genomes generated from cases reported in the community, hospital, intensive care unit (ICU), and those from unknown sources. (e) The number of RSV genomes generated from female and male patients. (f) The age distribution of patients from which RSV genomes were generated. A box plot indicates the mean (red), lower and upper quartiles (black), and the scatter points show the raw data. An adjacent vertical histogram shows the frequency of patient ages for RSV-A and RSV-B.
Between 2015 and 2019, annual outbreaks of RSV were typically seasonal with cases peaking in late June, coinciding with mid-winter in New Zealand (Figure 1). In 2020, however, reported RSV cases were reduced by 98% and no community transmission of RSV reported as a result of NPI used to control the spread of SARS-CoV-2. Schools and childcare centres were closed during a four-week nationwide lockdown in April 2020, which effectively eliminated SARS-CoV-2 and other respiratory infections from the New Zealand community14, 47. The following year, in April 2021, and for the first time since restrictions were placed on the New Zealand border due to the COVID-19 pandemic, people could travel to and from Australia without having to enter managed quarantine at the border. Within weeks of lifting border restrictions there was an exceptionally large RSV epidemic in New Zealand, with a sharp surge in reported cases of both RSV-A and -B (Figure 1).
The 2021 RSV surge affected a wider age distribution compared to previous years (Supplementary Figure 1). Among people who were sampled in this study, we found a 5% increase in infections among 5–18 year olds and a 15% increase in infections among 19–65 year olds compared to previous years (Supplementary Table 3). This surge cannot be explained by weakened immunity due to COVID-1948, as there were fewer than 3,000 reported COVID-19 cases, (in a population of ~five million) before August 2021. The majority of these COVID-19 cases were associated with returning travellers which had limited community transmission16. Rather it was more likely the result of waned immunity due to prolonged lack of viral exposure, as seen in other countries49–51. However, other factors related to sample collection may have also contributed to the apparent surge. One factor was a change in diagnostic viral testing regimes that were implemented during the COVID-19 pandemic such as the increased use of point of care testing with an expanded respiratory viral diagnostic assay that included RSV, as well as wider testing in the community that included older children and adults. Increased testing not only inflates case counts but can also skew patient age distribution by incorporating less severe cases52. Nevertheless, due to the associated rise in hospitalisations26 and the return to normal levels in 2022, it is likely that the 2021 epidemic reflected a period of reduced exposure to RSV and an increase in the immunologically naïve population.
Quarantine-free travel with Australia was brought to an abrupt end in July 2021 due to the uncontrolled spread of the SARS-CoV-2 Delta Variant of Concern in Australia53. Weeks later, New Zealand entered its second nationwide lockdown due to the incursion of Delta SARS-CoV-2, which was genomically linked to cases in Australia25. The lockdown caused the number of reported RSV cases to dramatically reduce (Figure 1).
Inferring the sources of the 2021 RSV outbreaks using phylodynamics
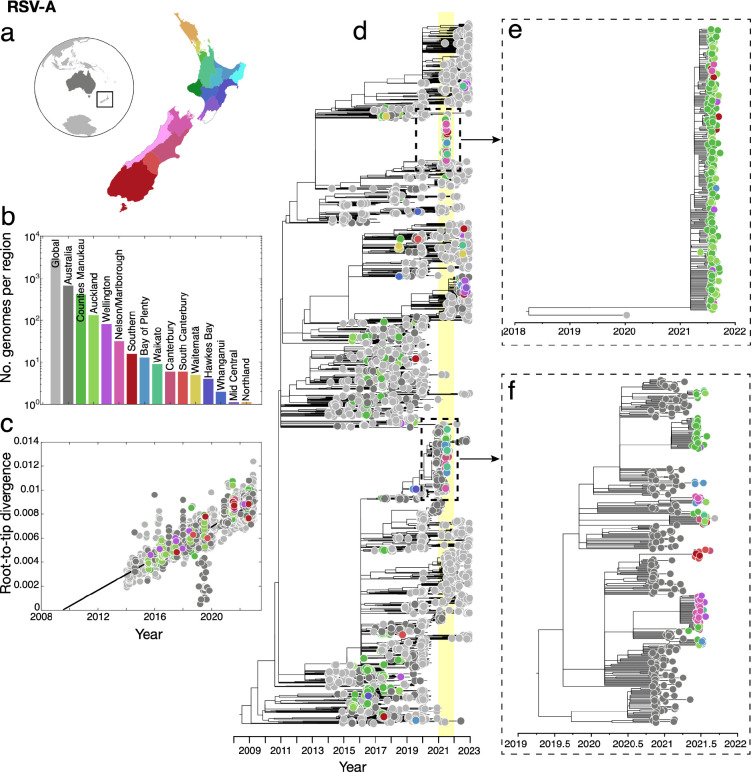
Phylogenomic analysis of RSV-A and RSV-B genomes, together with genomes sampled from Australia and the rest of the world, showed that in the pre-pandemic phase, genomes sampled from New Zealand were widely distributed among globally sampled genomes, falling among a range of genomic lineages. In 2021, however, genomes sampled from New Zealand formed only a small number of tightly grouped clusters. In particular, RSV-A genomes sampled in 2021 fell into only two distinct lineages (Figure 2). One clade of RSV-A genomes, predominantly sampled from Auckland and surrounding districts, formed a monophyletic clade with no close-in-time sampled genomic ancestors, and unrelated to previously circulating New Zealand lineages (Figure 2). Due to the increased testing regimes coupled with managed quarantine at the border for all arrivals besides those from Australia and the South Pacific, this lineage is unlikely to represent undetected transmission in New Zealand. Rather, it is more likely that this lineage is related to unsampled genomes, most probably in Australia.
Figure 2.
(a) Map of New Zealand (where regions are categorised by District Health Board (DHB)), Australia (dark grey), and the rest of the world (light grey). (b) Number of RSV-A genomes sampled per region, where colours correspond to those in (a). (c) Root-to-tip regression analysis of RSV-A genomes versus sampling time. (d) Maximum likelihood time-scaled phylogenetic tree showing 756 RSV-A genomes sampled from New Zealand (coloured circles based on DHB region), 662 RSV-A genomes sampled from Australia (dark grey) and 2,913 RSV-A genomes sampled from the rest of the world (light grey). A yellow vertical bar highlights the year 2021 and a dotted box shows the major New Zealand clades sampled. (e-f) Maximum likelihood time-scaled phylogenetic trees showing the major clades sampled in New Zealand during 2021 and their closest sampled genetic relatives.
A second cluster of New Zealand 2021 genomes were grouped with those sampled from Australia during the months prior (Figure 2). This 2021 New Zealand cluster was due to multiple introductions, forming several monophyletic clades among Australian samples, and included genomes from across a wide geographic region including the North and South Islands of New Zealand. Both the highlighted clusters of RSV-A genomes were determined to be the ON1-like genotype based on their G-gene sequences (genomic clade A.3 based on Chen et al.39), which has become globally dominant in recent years54, 55. This genotype, which evolved from the previous dominant RSV-A genotype NA-1, has a characteristic 72 nucleotide duplication in the G-gene56. All RSV-A genomes sampled in New Zealand conformed to a root-to-tip linear regression reflecting a temporal signal in these data (Pearson correlation coefficient, r = 0.88, p<10−4).
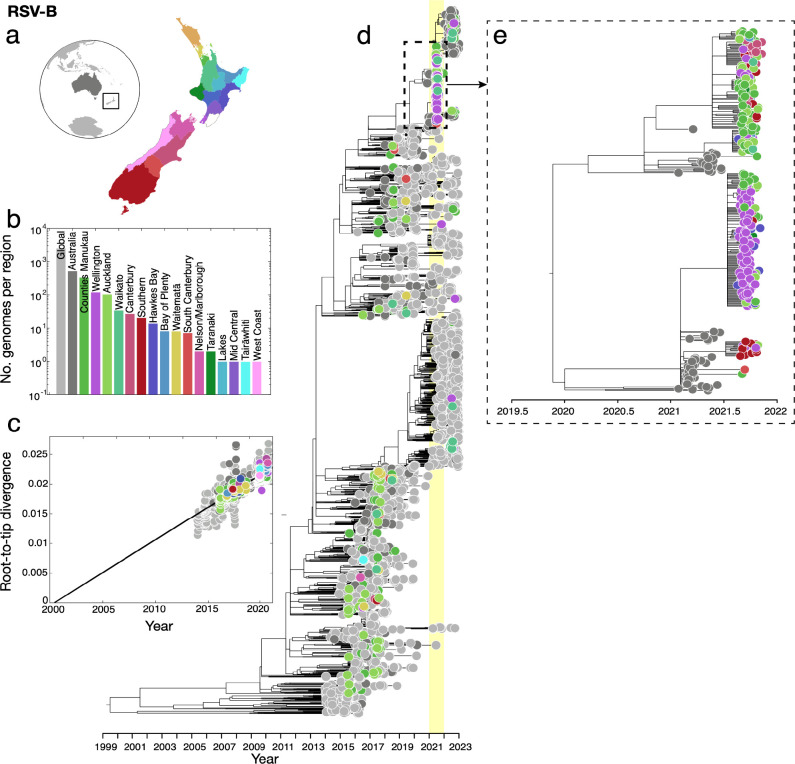
Similarly, RSV-B genomes sampled in 2021 were clustered tightly with those sampled in Australia in the months leading up to the outbreak in New Zealand (Figure 3). These genomes formed several large monophyletic clades among Australian samples and largely clustered according to geographic location in New Zealand. We again found a temporal signal in the New Zealand RSV-B data, illustrated by a root-to-tip linear regression (Pearson correlation coefficient, r = 0.93, p<10−4). These genomes were classified as the BA9 genotype based on their G-gene sequences (or the B.5.8 genomic clade39). BA9 is one of 15 genotypes within the BA lineage reported since its emergence in around 199857. Genotype BA9 was first identified in Japan in 200658 and, like all BA genotypes, is characterised by a 60 nucleotide duplication in the G-gene59. Over the last decade, BA9 has become globally dominant and is now found worldwide54, 56, 60–62. Overall, the RSV-A and RSV-B genomes sampled in 2021 were most likely from new introductions into New Zealand rather than from cryptically circulating lineages that remained undetected in 2020. Indeed, our data support that RSV transmission was eliminated in 2020 and both subtypes were reintroduced in 2021, most likely from Australia following the lifting of border restrictions including quarantine.
Figure 3.
(a) Map of New Zealand (where regions are categorised by District Health Board (DHB)), Australia (dark grey), and the rest of the world (light grey). (b) Number of RSV-B genomes sampled per region, where colours correspond to those in (a). (c) Root-to-tip regression analysis of RSV-B genomes versus sampling time. (d) Maximum likelihood time-scaled phylogenetic tree showing 715 RSV-B genomes sampled from New Zealand (coloured circles based on DHB region), 518 RSV-B genomes sampled from Australia (dark grey) and 2,577 RSV-B genomes sampled from the rest of the world (light grey). A yellow vertical bar highlights the year 2021 and a dotted box shows the major New Zealand clade sampled. (e) Maximum likelihood time-scaled phylogenetic tree showing the major clades sampled in New Zealand during 2021 and their closest sampled genetic relatives.
Examining the spatial transmission dynamics of RSV over time
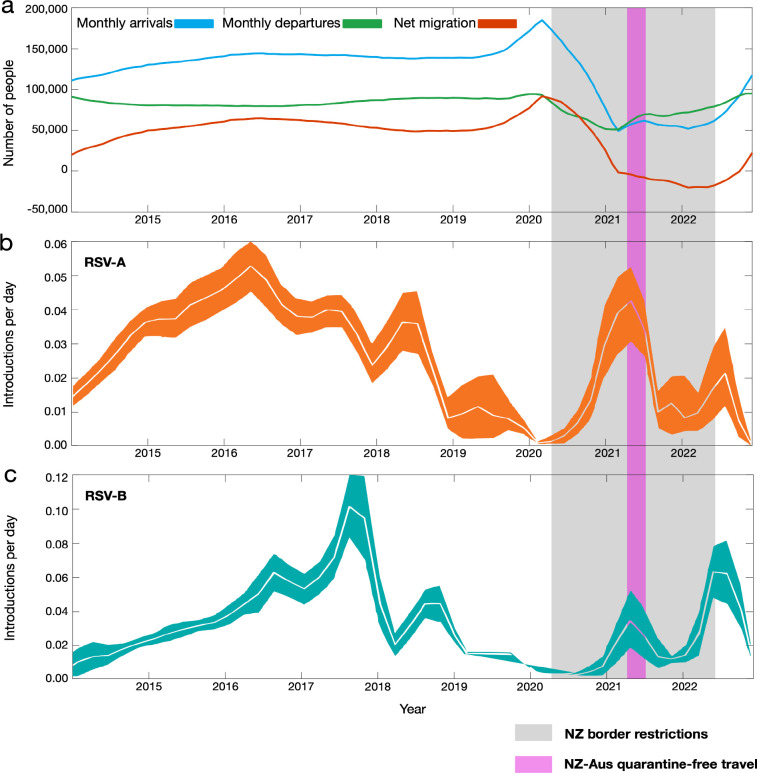
We estimated the spatial transmission dynamics of RSV over time to elucidate how the number of viral introductions changed as a result of NPI, particularly border restrictions, used during the COVID-19 pandemic. While the number of people arriving in New Zealand peaked in early 2020 following an announcement that the border would be closed, a nationwide lockdown meant that any potential new arrivals of RSV did not lead to onward transmission in the community (Figure 4). We inferred the number of introductions of both RSV-A and RSV-B into New Zealand between 2015 and 2022 using genomes sampled from both New Zealand and abroad. Although genomic sequences were too sparse to capture seasonal patterns between 2015 and 2019, introductions of both RSV-A and RSV-B decreased in 2020 resulting in near-zero introductions of either subtype (Figure 4). These results confirm that the number of introductions dramatically increased the following year in 2021, particularly for RSV-A, coinciding with quarantine-free travel to and from Australia. In 2022, RSV-B showed a larger number of introductions, which is consistent with the multiple different lineages that were circulating in 2022. Although both subtypes were introduced many times throughout the eight years (around 80 for RSV-A and 100 for RSV-B), only a small number of these introductions eventuated in large detected outbreaks in 2021 (Figures 2 and 3). This reflects the immunologically naive population of 2021 combined with the highly stochastic nature of disease transmission, which often occurs through superspreader events63,64. In contrast, very few export events were identified. Our analysis showed that RSV-B mutated slightly faster than RSV-A (Table 1), and at a comparable rate with previous studies65. These experiments proved quite robust to genome subsampling, with each replicate yielding similar clock rates and migration counts.
Figure 4.
(a) Number of people arriving into New Zealand (blue), number of people departing New Zealand (green) and the overall net migration of people into New Zealand (red). (b-c) Estimated migration rates of RSV-A and-B into New Zealand over time (mean estimates are shown in white and 95% credible intervals are coloured). A grey shaded area shows the period in which New Zealand underwent border restrictions during the COVID-19 pandemic. A purple shaded area shows the quarantine-free travel period with Australia.
Table 1.
Summary of estimated clock rates and RSV migration events between New Zealand and the rest of the world (BEAST2).
| Rep | Clock rate (subs/site/year) | Number of introductions | Number of exports | |
|---|---|---|---|---|
| RSV-A | 1* | 8.79×10−4 (8.40×10−4, 9.13×10−4) | 85.3 [83, 88] | 2.94 [1, 5] |
| 2 | 8.31×10−4 (7.95×10−4, 8.66×10−4) | 81.9 [80, 84] | 3.93 [3, 5] | |
| 3 | 8.25×10−4 (7.90×10−4, 8.59×10−4) | 89.4 [84, 93] | 2.15 [1, 5] | |
| 4 | 8.68×10−4 (8.29×10−4, 9.04×10−4) | 83.4 [82, 85] | 3.11 [2, 4] | |
| 5 | 8.52×10−4 (8.19×10−4, 8.87×10−4) | 84.6 [82, 87] | 3.71 [2, 6] | |
| 6 | 8.68×10−4 (8.32×10−4, 9.04×10−4) | 80.2 [78, 82] | 4.16 [3, 6] | |
| RSV-B | 1* | 1.04×10−3 (1.00×10−3, 1.09×10−3) | 100 [97, 104] | 0.86 [0, 3] |
| 2 | 1.00×10−3 (9.64×10−4, 1.05×10−3) | 101 [97, 104] | 0.57 [0, 1] |
Each replicate contains a different subsample of genomes. Mean estimates (and 95% credible intervals) are rounded to 3 significant figures. MCMC chains that did not converge (replicates RSV-B 306) are omitted from the table.
These replicates are shown in Figure 4.
Genomic diversity of RSV before and after the COVID-19 pandemic
Despite the border-opening associated increase in both viral introductions and genomic surveillance in New Zealand, the surge of introductions in 2021 had very little genomic diversity compared to the pre-pandemic phase as shown by the limited number of New Zealand genomic clusters in our phylogenetic analysis. Similar genomic bottlenecks have been reported in Europe66 and Australia11, where major lineages of RSV were collapsed following NPI during the COVID-19 pandemic. The resurgence of RSV in 2021 led to very little genomic diversity in circulating lineages, with only two major clades of RSV-A and one clade of RSV-B being reintroduced into New Zealand. Nevertheless, this reduced genetic diversity was seemingly short lived with more widespread lineages reappearing by 2022 when border restrictions were removed. Overall, the extensive NPI used to control SARS-CoV-2 in New Zealand significantly impacted the epidemiology of other respiratory viral infections, including the near-elimination and diminished genetic diversity of typically seasonal viruses.
Supplementary Material
Supplementary Table 1. GenBank accession numbers and genotypes of G-gene RSV reference sequences.
Supplementary Table 3. Age groups of people from which RSV genomes were derived between 2015 and 2022.
Supplementary Table 2. GISAID accession numbers of global genomes.
Supplementary Figure 1. The age distribution of patients from which RSV genomes were generated for each year (RSV-A shown in orange and RSV-B shown in green). A box plot indicates the lower and upper quartiles, and the scatter points show the raw data.
Acknowledgements
We gratefully acknowledge all data contributors, i.e. the Authors and their originating laboratories, responsible for obtaining the specimens, and their submitting laboratories for generating the genetic sequence and metadata and sharing via the GISAID Initiative, on which this research is based.
We would like to thank the participants of the SHIVERS WellKiwi cohorts for their continued enrollment and support in our research. Second, we would like to thank the WellKiwi Clinical and Study teams for SHIVERS research, the organisation and collection of participant samples and metadata used in this study. We would also like to thank the clinical virology and genomic sequencing teams at the Institute of Environmental Science and Research for processing, testing and sequencing all of the samples collected.
We would like to acknowledge staff from all of the diagnostic laboratories and hospital laboratories in New Zealand that take the time and effort to collect, aliquot and refer samples to ESR. A special thanks to Nicolas Zacchi from Pathlab in Bay of Plenty, Radhika Nagappan from Auckland City Hospital, Fifi Tse from Middlemore Hospital, and Samantha Hutton from Awanui Laboratory in Wellington for helping collate retrospective data for the sample analysis. Thank you to former ESR staff who contributed to this study.
Funding
LJ is funded by a University of Otago Doctoral Scholarship. JLG is funded by a New Zealand Royal Society Rutherford Discovery Fellowship (RDF-20-UOO-007). This study was supported by a New Zealand Health Research Council Grant (22/138) awarded to JLG, JdL, DW, SH, LJ, NF and DW. Our grateful thanks to FluLab for funding the SHIVERS-V team to undertake research encompassed by the project called “Influenza in a post-COVID world” which has allowed researchers in Aotearoa New Zealand to collect crucial data on influenza virus and produce local innovations and international impact with a Southern Hemisphere “population laboratory”. The SHIVERS/WellKiwis cohort is funded by the US National Institute of Allergy and Infectious Diseases (NIAID): SHIVERS-III infant funded by the US-NIAID (U01 AI 144616); SHIVERS-IV household funded by the US-NIAID (CEIRR contract: 75N93021C00016).
*. SHIVERS Investigation Team
Clinical Virology Team: Bryden Bird1, Tegan Slater1, Farjana Ahmed1,Mai Anh Sam1, Elaisa Glampe1, Gabriella Davey1
Study and Clinical and data team: Mary Ryan1, Megan Rensburg5, Jort Cueto5, Ernest Caballero5, Joshma John5, Sasha Aizupuru5 & Maritza Marull1, Andrea Bowers1
References
- 1.Prasad N, Newbern EC, Trenholme AA, Thompson MG, Mcarthur C, Wong CA, et al. The health and economic burden of respiratory syncytial virus associated hospitalizations in adults. PLoS One. 2020; 15(6):e0234235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. New England Journal of Medicine. 2005; 352(17):1749–59. [DOI] [PubMed] [Google Scholar]
- 3.Rima B, Collins P, Easton A, et al. ICTV virus taxonomy profile: Pneumoviridae. Journal of General Virology. 2017; 98(12):2912–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clinical Microbiology Reviews. 2017; 30(1):277–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022; 399(10340):2047–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müller-Pebody B, Edmunds WJ, Zambon MC, Gay NJ, Crowcroft NS. Contribution of RSV to bronchiolitis and pneumonia-associated hospitalizations in English children, April 1995–March 1998. Epidemiology and Infection. 2002; 129(1):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clinical Microbiology Reviews. 2000; 13(3):371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haber N. Respiratory syncytial virus infection in elderly adults. Medecine et maladies infectieuses. 2018; 377–382. [DOI] [PubMed] [Google Scholar]
- 9.Robertson M, Eden JS, Levy A, et al. The spatial-temporal dynamics of respiratory syncytial virus infections across the east-west coasts of Australia during 2016–17. Virus Evolution. 2021; 7(2):veab068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Giallonardo F, Kok J, Fernandez M, Carter I, Geoghegan JL, Dwyer D, et al. Evolution of human respiratory syncytial virus (RSV) over multiple seasons in New South Wales, Australia. Viruses 2018; 10(9):476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eden J-S, Sikazwe C, Xie R, Deng Y-M, Sullivan SG, Michie A, et al. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nature Communications. 2022; 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fall A, Dia N, Cisse EHAK, Kiori DE, Sarr FD, Sy S, et al. Epidemiology and molecular characterization of human respiratory syncytial virus in Senegal after four consecutive years of surveillance, 2012–2015. PLoS One. 2016; 11(6):e0157163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad N, Newbern EC, Trenholme AA, Wood T, Thompson MG, Aminisani N, et al. Respiratory syncytial virus hospitalisations among young children: a data linkage study. Epidemiology and Infection. 2019; 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang QS, Wood T, Jelley L, Jennings T, Jefferies S, Daniells K, et al. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nature Communications. 2021; 12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jefferies S, French N, Gilkison C, Graham G, Hope V, Marshall J, et al. COVID-19 in New Zealand and the impact of the national response: a descriptive epidemiological study. The Lancet Public Health. 2020; 5(11):e612–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geoghegan JL, Ren X, Storey M, Hadfield J, Jelley L, Jefferies S, et al. Genomic epidemiology reveals transmission patterns and dynamics of SARS-CoV-2 in Aotearoa New Zealand. Nature Communications. 2020; 11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker RE, Park SW, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proceedings of the National Academy of Sciences USA. 2020; 117(48):30547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu P, Xu M, Cao L, Su L, Lu L, Dong N, et al. Impact of COVID-19 pandemic on the prevalence of respiratory viruses in children with lower respiratory tract infections in China. Virology Journal. 2021; 18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan WY, Thoon KC, Loo LH, Chan KS, Oon LLE, Ramasamy A, et al. Trends in respiratory virus infections during the COVID-19 pandemic in Singapore, 2020. JAMA Network Open. 2021; 4(6):e2115973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tempia S, Walaza S, Bhiman JN, Mcmorrow ML, Moyes J, Mkhencele T, et al. Decline of influenza and respiratory syncytial virus detection in facility-based surveillance during the COVID-19 pandemic, South Africa, January to October 2020. Eurosurveillance. 2021; 26(29). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams TC, Sinha I, Barr IG, Zambon M. Transmission of paediatric respiratory syncytial virus and influenza in the wake of the COVID-19 pandemic. Eurosurveillance. 2021; 26(29). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Department of Prime Minister and Cabinet. Timeline of significant COVID-19 events. Accessed July 2024 (https://www.dpmc.govt.nz/sites/default/files/2023-10/pr-timeline-significant-events-activities.pdf).
- 23.Hatter L, Eathorne A, Hills T, Bruce P, Beasley R. Respiratory syncytial virus: paying the immunity debt with interest. The Lancet Child and Adolescent Health. 2021; 5(12):e44–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant CC, Huang QS, Trenholme A, Taylor S, Wood T. What can we learn from our 2021 respiratory syncytial virus experience? New Zealand Medical Journal. 2021; 134(1540):7–12. [PubMed] [Google Scholar]
- 25.Jelley L, Douglas J, Ren X, Winter D, Mcneill A, Huang S, et al. Genomic epidemiology of Delta SARS-CoV-2 during transition from elimination to suppression in Aotearoa New Zealand. Nature Communications. 2022; 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang QS, Turner N, Wood T, Anglemyer A, Mcintyre P, Aminisani N, et al. Impact of the COVID-19 related border restrictions on influenza and other common respiratory viral infections in New Zealand. Influenza and Other Respiratory Viruses. 2024; 18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douglas J., Winter D., McNeill A., Carr S., Bunce M., French N., et al. Tracing the international arrivals of SARS-CoV-2 Omicron variants after Aotearoa New Zealand reopened its border. Nature Communications, 2022; 13(1), 6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglas J, Geoghegan JL, Hadfield J, Bouckaert R, Storey M, Ren X, et al. Real-time genomics for tracking severe acute respiratory syndrome coronavirus 2 border incursions after virus elimination, New Zealand. Emerging Infectious Diseases. 2021; 27(9):2361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Da Silva Filipe A, Shepherd JG, Williams T, Hughes J, Aranday-Cortes E, Asamaphan P, et al. Genomic epidemiology reveals multiple introductions of SARS-CoV-2 from mainland Europe into Scotland. Nature Microbiology. 2020; 6(1):112–22. [DOI] [PubMed] [Google Scholar]
- 30.Park D, Dudas G, Wohl S, et al. Ebola virus epidemiology, transmission, and evolution during seven months in Sierra Leone. Cell. 2015; 161(7):1516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim C, Ahmed JA, Eidex RB, Nyoka R, Waiboci LW, Erdman D, et al. Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real-time reverse transcription-PCR assays. PLoS One. 2011; 6(6):e21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu A, Colella M, Tam JS, Rappaport R, Cheng S-M. Simultaneous detection, subgrouping, and quantitation of respiratory syncytial virus A and B by real-time PCR. Journal of Clinical Microbiology. 2003; 41(1):149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elbe S, Buckland-Merrett G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Global Challenges. 2017; 1:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013; 30(4):772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen L-T, Schmidt HA, Von Haeseler A, Minh BQ. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution. 2015; 32(1):268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasegawa M, Kishino H, Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution. 1985; 22(2):160–174. [DOI] [PubMed] [Google Scholar]
- 37.Kalyaanamoorthy S, Minh BQ, Wong TKF, Von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods. 2017; 14(6):587–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shepard S.S., Davis C.T., Bahl J., Rivailler P., York I.A., Donis R.O. LABEL: Fast and accurate lineage assignment with assessment of H5N1 and H9N2 influenza A hemagglutinins. PLoS One. 2014; 9, e86921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J., Qiu X., Avadhanula V., Shepard S.S., Kim D., Hixson J., Piedra P.A., Bahl J. Novel and extendable genotyping system for human respiratory syncytial virus based on whole-genome sequence analysis. Influenza and Other Respiratory Viruses. 2022; 16, 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoang D.T., Chernomor O., von Haeseler A., Minh B.Q., and Vinh L.S.. UFBoot2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution. 2018; 35:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rambaut A, Lam TT, Max Carvalho L, Pybus OG. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen). Virus Evolution. 2016; 2(1):vew007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A, et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Computational Biology. 2019; 15(4):e1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Müller N. F., Rasmussen D., Stadler T. MASCOT: Parameter and state inference under the marginal structured coalescent approximation. Bioinformatics. 2018; bty406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarization in Bayesian phylogenetics using tracer 1.7. Systems Biology. 2018; 67(5):901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021; URL: https://www.R-project.org/. [Google Scholar]
- 46.Institute of Environmental Science and Research Limited; 2024. [cited 18 June 2024]. Available from https://www.esr.cri.nz/digital-library
- 47.Cumming J. Going hard and early: Aotearoa New Zealand’s response to Covid-19. Health Economics, Policy and Law. 2022; 17(1):107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Davis PB, Berger N, Kaelber DC, Volkow N, Xu R. Association of COVID-19 with respiratory syncytial virus (RSV) infections in children aged 0–5 years in the USA in 2022: a multicentre retrospective cohort study. Family Medicine and Community Health. 2023; 11(4):e002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Den Hartog G, Van Kasteren PB, Schepp RM, Teirlinck AC, Van Der Klis FRM, Van Binnendijk RS. Decline of RSV-specific antibodies during the COVID-19 pandemic. The Lancet Infectious Diseases. 2023; 23(1):23–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reicherz F, Xu RY, Abu-Raya B, et al. Waning immunity against respiratory syncytial virus during the coronavirus disease 2019 pandemic. Journal of Infectious Diseases. 2022; 226(12):2064–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rios-Guzman E, Simons LM, Dean TJ, Agnes F, Pawlowski A, Alisoltanidehkordi A, et al. Deviations in RSV epidemiological patterns and population structures in the United States following the COVID-19 pandemic. Nature Communications. 2024; 15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petros BA, Milliren CE, Sabeti PC, Ozonoff A. Increased pediatric respiratory syncytial virus case counts following the emergence of SARS-CoV-2 can be attributed to changes in testing. Clinical Infectious Diseases. 2024; 78(6),1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Office of the Minister for COVID-19 Response. Accessed July 2024 (https://covid19.govt.nz/assets/Proactive-Releases/Border/19-Jul-2022/Quarantine-Free-Travel-With-Australia_-Further-Suspension.pdf).
- 54.Umar S, Yang R, Wang X, Liu Y, Ke P, Qin S. Molecular epidemiology and characteristics of respiratory syncytial virus among hospitalized children in Guangzhou, China. Virology Journal. 2023; 20(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eshaghi A, Duvvuri VR, Lai R, Nadarajah JT, Li A, Patel SN, et al. Genetic variability of human respiratory syncytial virus A strains circulating in Ontario: A novel genotype with a 72 nucleotide G gene duplication. PLoS One. 2012; 7(3):e32807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsukagoshi H, Yokoi H, Kobayashi M, Kushibuchi I, Okamoto-Nakagawa R, Yoshida A, et al. Genetic analysis of attachment glycoprotein (G) gene in new genotype ON1 of human respiratory syncytial virus detected in Japan. Microbiology and Immunology. 2013; 57(9):655–9. [DOI] [PubMed] [Google Scholar]
- 57.Muñoz-Escalante JC, Comas-García A, Bernal-Silva S, Noyola DE. Respiratory syncytial virus B sequence analysis reveals a novel early genotype. Scientific Reports. 2021; 11(1):3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song J, Wang H, Shi J, Cui A, Huang Y, Sun L, et al. Emergence of BA9 genotype of human respiratory syncytial virus subgroup B in China from 2006 to 2014. Scientific Reports. 2017; 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trento A, Galiano M, Videla C, et al. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. Journal of General Virology. 2003; 84(11):3115–3120. [DOI] [PubMed] [Google Scholar]
- 60.Tabatabai J, Ihling CM, Rehbein RM, et al. Molecular epidemiology of respiratory syncytial virus in hospitalised children in Heidelberg, Southern Germany, 2014–2017. Infection, Genetics and Evolution. 2022; 98:105209. [DOI] [PubMed] [Google Scholar]
- 61.Razanajatovo Rahombanjanahary NH, Rybkina K, Randriambolamanantsoa TH, Razafimanjato H, Heraud JM. Genetic diversity and molecular epidemiology of respiratory syncytial virus circulated in Antananarivo, Madagascar, from 2011 to 2017: Predominance of ON1 and BA9 genotypes. Journal of Clinical Virology. 2020; 129:104506. [DOI] [PubMed] [Google Scholar]
- 62.Yun K.W., Choi E.H., Lee H.J. Molecular epidemiology of respiratory syncytial virus for 28 consecutive seasons (1990–2018) and genetic variability of the duplication region in the G gene of genotypes ON1 and BA in South Korea. Archives of Virology. 2020; 165, 1069–1077. [DOI] [PubMed] [Google Scholar]
- 63.Stein RA. Super-spreaders in infectious diseases. International Journal of Infectious Diseases. 2011; 15(8):e510–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005; 438(7066):355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pangesti KNA, Abd El Ghany M, Walsh MG, Kesson AM, Hill-Cawthorne GA. Molecular epidemiology of respiratory syncytial virus. Reviews in Medical Virology. 2018; 28(2):e1968. [DOI] [PubMed] [Google Scholar]
- 66.Chow E.J., Uyeki T.M., Chu H.Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nature Reviews Microbiology. 2023; 21, 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. GenBank accession numbers and genotypes of G-gene RSV reference sequences.
Supplementary Table 3. Age groups of people from which RSV genomes were derived between 2015 and 2022.
Supplementary Table 2. GISAID accession numbers of global genomes.
Supplementary Figure 1. The age distribution of patients from which RSV genomes were generated for each year (RSV-A shown in orange and RSV-B shown in green). A box plot indicates the lower and upper quartiles, and the scatter points show the raw data.