Abstract
Administration of adenovirus (Ad) vectors to immunologically naive experimental animals almost invariably results in the induction of systemic anti-Ad neutralizing antibodies. To determine if the human systemic humoral host responses to Ad vectors follow a similar pattern, we evaluated the systemic (serum) anti-Ad serotype 5 (Ad5) neutralizing antibodies in humans after administration of first generation (E1− E3−) Ad5-based gene transfer vectors to different hosts. AdGVCFTR.10 (carrying the normal human cystic fibrosis [CF] transmembrane regulator cDNA) was sprayed (8 × 107 to 2 × 1010 particle units [PU]) repetitively (every 3 months or every 2 weeks) to the airway epithelium of 15 individuals with CF. AdGVCD.10 (carrying the Escherichia coli cytosine deaminase gene) was administered (8 × 108 to 8 × 109 PU; once a week, twice) directly to liver metastasis of five individuals with colon cancer and by the intradermal route (8 × 107 to 8 × 109 PU, single administration) to six healthy individuals. AdGVVEGF121.10 (carrying the human vascular endothelial growth factor 121 cDNA) was administered (4 × 108 to 4 × 109.5 PU, single administration) directly to the myocardium of 11 individuals with ischemic heart disease. Ad vector administration to the airways of individuals with CF evoked no or minimal serum neutralizing antibodies, even with repetitive administration. In contrast, intratumor administration of an Ad vector to individuals with metastatic colon cancer resulted in a robust antibody response, with anti-Ad neutralizing antibody titers of 102 to >104. Healthy individuals responded to single intradermal Ad vector variably, from induction of no neutralizing anti-Ad antibodies to titers of 5 × 103. Likewise, individuals with ischemic heart disease had a variable response to single intramyocardial vector administration, ranging from minimal neutralizing antibody levels to titers of 104. Evaluation of the data from all trials showed no correlation between the peak serum neutralizing anti-Ad response and the dose of Ad vector administered (P > 0.1, all comparisons). In contrast, there was a striking correlation between the peak anti-Ad5 neutralizing antibody levels evoked by vector administration and the level of preexisting anti-Ad5 antibodies (P = 0.0001). Thus, unlike the case for experimental animals, administration of Ad vectors to humans does not invariably evoke a systemic anti-Ad neutralizing antibody response. In humans, the extent of the response is dictated by preexisting antibody titers and modified by route of administration but is not dose dependent. Since the extent of anti-Ad neutralizing antibodies will likely modify the efficacy of administration of Ad vectors, these observations are of fundamental importance in designing human gene therapy trials and in interpreting the efficacy of Ad vector-mediated gene transfer.
Extensive studies in experimental animals have demonstrated the ability of E1− replication-deficient adenovirus (Ad) vectors to transfer and express transgenes in a variety of organs (2, 5, 8, 9, 22, 23, 25, 35, 39, 40, 42, 45, 51, 52, 55, 56, 59, 65, 67, 70, 71, 73–75, 78, 85, 89, 90, 97, 98, 100, 104, 107, 108, 110, 116, 117, 132, 134–138). In experimental animals, the administration of these vectors is almost invariably associated with the development of systemic neutralizing antibodies directed against the Ad vector (11, 25, 27, 31, 35, 44, 47–49, 51–53, 57, 58, 62, 63, 65, 66, 72, 76, 77, 80, 101, 103, 104, 108–110, 114, 118–121, 124, 127, 131, 132, 134–138). The anti-Ad neutralizing antibody response is robust in immunologically naive animals, with generation of a systemic anti-Ad neutralizing humoral response within 2 to 4 weeks, depending on the species. The intensity of systemic anti-Ad humoral immunity in experimental animals is dependent on the dose and on the route of administration of the vector (31, 108, 110, 120, 137).
Based on the ability of Ad vectors to safely mediate transfer and robust expression of transgenes in organs of experimental animals, these vectors are being evaluated in a variety of human gene transfer applications (4). In the context of the observation that administration of Ad vectors by a variety of routes to naive experimental animals rapidly evokes systemic anti-Ad neutralizing antibodies, the present study focuses on several questions regarding the administration of Ad vectors to humans: (i) does the administration of Ad vectors to humans invariably evoke systemic anti-Ad neutralizing antibodies; (ii) does the extent of the neutralizing antibody response depend on the route of administration; (iii) is the systemic anti-Ad humoral response dose dependent; and (iv) does the baseline anti-Ad antibody status of the human recipient modify the humoral response to administration of the vector? To accomplish this, we have evaluated our human experience with Ad vectors administered to the airway epithelium of individuals with cystic fibrosis (CF), metastatic tumors in liver of individuals with colon cancer, the skin of healthy (normal) individuals, and the myocardium of individuals with coronary artery disease. The data demonstrate that humans can mount a systemic anti-Ad neutralizing antibody response following administration of these vectors but that the results are quite different than in experimental animals, with minimal responses in naive humans (i.e., those with no detectable preexisting anti-Ad neutralizing antibodies), different responses dependent on the target organ, dose independence, and a striking relationship to the preexisting systemic anti-Ad neutralizing antibody titer.
MATERIALS AND METHODS
Vectors.
Three different clinical-grade Ad vectors were used: AdGVCD.10, AdGVCFTR.10, and AdGVVEGF121.10 (all produced by GenVec, Inc., Rockville, Md.). All are E1− E3− replication-deficient vectors based on the Ad serotype 5 (Ad5) genome (18, 19, 21, 41). All carry an expression cassette in the E1 position (right to left) containing the cytomegalovirus early-immediate enhancer-promoter, an artificial splice sequence, the transgene, and the simian virus 40 poly(A)-stop signal (18, 19, 21, 41). AdGVCD.10 expresses the Escherichia coli cytosine deaminase gene (18), AdGVCFTR.10 expresses the human CF transmembrane conductance regulator (CFTR) cDNA (19), and AdGVVEGF121.10 expresses the human vascular endothelial growth factor 121 cDNA (21). All Ad vectors were propagated in 293 cells, purified by CsCl density purification, dialyzed, and stored at −70°C (98). All lots fulfilled the in vitro and in vivo safety criteria established by the Food and Drug Administration Bureau of Biologics (FDA BB) for clinical-grade Ad vector preparations (FDA BB-IND 5702, 6442, 6950, and 7381), including the criteria of being free of endotoxin and infectious agents and containing ≤1 replication-competent adenovirus for the total dose to be delivered (18, 19, 21, 41).
Each Ad vector preparation was characterized as to particle units (PU; on the basis of physical particle concentration determined from the absorbance at 260 nm and the extinction coefficient for Ad [9.09 × 10−12 ml × particles−1 cm−1] [82]) and PFU (98). The studies of individuals with CF, normal subjects, and those with metastatic colon cancer were based on dosage in PFU; based on a policy decision by the FDA BB for all future Ad gene transfer studies, the coronary artery disease study was based on dosage in PU. Because anti-Ad neutralizing antibodies are directed against the capsid of the Ad vector and not vector activity, the most relevant dose parameter is PU. In this context, all doses have been converted to PU (Table 1 presents PU and PFU characteristics for each Ad vector lot used). Since the analyses of PU and PFU vary ± 20% (data not shown), for convenience in graphing the data, all PU-to-PFU ratios are rounded off to the nearest 10 U (Table 1).
TABLE 1.
Characteristics of Ad vectors
| Trial | Vector | Lot no. | PU/ml | PFU/ml | PU/PFUa | Doses used for each administration (PU) |
|---|---|---|---|---|---|---|
| Normal skin | AdGVCD.10 | 0061-0002 | 3.7 × 1011 | 1.0 × 1010 | 36 | 8 × 107–8 × 109 |
| CF | AdGVCFTR.10 | 0056-0002 | 2.2 × 1011 | 3.7 × 1010 | 6 | 3 × 107–3 × 108.5 |
| q3mo × 3 (part A) | ||||||
| Individuals 1–8 | ||||||
| AdGVCFTR.10 | 0045-007 | 1.4 × 1012 | 1.6 × 1011 | 9 | 3 × 109–3 × 1010 | |
| Individuals 9–14 | ||||||
| q15d × 4 (part B) | AdGVCFTR.10 | 0045-007 | 1.4 × 1012 | 1.6 × 1011 | 9 | 3 × 109.5 |
| Metastatic colon cancer | AdGVCD.10 | 0061-0002 | 3.7 × 1011 | 1.0 × 1010 | 36 | 8 × 108–8 × 109 |
| Coronary artery disease | AdGVVEGF121.10 | 0061-0003 | 4.0 × 1011 | 1.0 × 1010 | 40 | 4 × 108–4 × 109.5 |
Study design.
All clinical studies were approved by the local Institutional Review Boards and Biosafety Committees, the National Institutes of Health Recombinant DNA Advisory Committee, and the Food and Drug Administration. After informed consent was obtained, all studies were initiated with a baseline evaluation to determine eligibility, baseline parameters for safety evaluation, and serum for baseline anti-Ad neutralizing antibodies. This was followed by the vector administration which differed in each protocol (Table 2). All of the complete protocols are on file and available at the Office of Recombinant DNA Activities, National Institutes of Health, Bethesda, Md. The protocols for the q3mo × 3 (every 3 months, three times) repetitive administration study of the AdGVCFTR.10 vector to individuals with cystic fibrosis and for repetitive administration (q1wk × 2 [once a week, twice]) of the AdGVCD.10 vector colon cancer metastatic to the liver are also available in the literature (18, 19).
TABLE 2.
Study population, doses, intervals, and sites of vector administration
| Group | Vector | Site of administration | Individual no. | Age (yr) | Sexf | Total dose for each administration
|
No. of administrations | Interval between administrations | |
|---|---|---|---|---|---|---|---|---|---|
| PFU | PU | ||||||||
| Normal | AdGVCD.10 | Skin | 1 | 23 | M | 2 × 106 | 8 × 107 | 1 | —c |
| 2 | 25 | M | 2 × 106 | 8 × 107 | 1 | — | |||
| 3 | 42 | M | 2 × 107 | 8 × 108 | 1 | — | |||
| 4 | 40 | M | 2 × 107 | 8 × 108 | 1 | — | |||
| 5 | 46 | M | 2 × 108 | 8 × 109 | 1 | — | |||
| 6 | 29 | M | 2 × 108 | 8 × 109 | 1 | — | |||
| CF | |||||||||
| Part A | AdGVCFTR.10a | Airway epithelium | 1 | 37 | M | 3 × 106 | 3 × 107 | 3 | 3 mo |
| 2 | 24 | M | 3 × 106 | 3 × 107 | 3 | 3 mo | |||
| 3 | 22 | M | 3 × 106.5 | 3 × 107.5 | 1 | — | |||
| 4 | 26 | M | 3 × 106.5 | 3 × 107.5 | 3 | 3 mo | |||
| 5 | 33 | M | 3 × 107 | 3 × 108 | 1 | — | |||
| 6 | 24 | F | 3 × 107 | 3 × 108 | 3 | 3 mo | |||
| 7 | 24 | M | 3 × 107.5 | 3 × 108.5 | 3 | 3 mo | |||
| 8 | 48 | F | 3 × 107.5 | 3 × 108.5 | 1 | — | |||
| 9 | 25 | M | 3 × 108 | 3 × 109 | 3 | 3 mo | |||
| 10 | 20 | M | 3 × 108 | 3 × 109 | 2 | 3 mo | |||
| 11 | 38 | M | 3 × 108.5 | 3 × 109.5 | 3 | 3 mo | |||
| 12 | 42 | M | 3 × 108.5 | 3 × 109.5 | 3 | 3 mo | |||
| 13e | 25 | M | 2 × 109 | 2 × 1010 | 3 | 3 mo | |||
| 14 | 17 | M | 2 × 109 | 2 × 1010 | 3 | 3 mo | |||
| Part B | AdGVCFTR.10 | Airway epithelium | 1b | 25 | M | 3 × 108.5 | 3 × 109.5 | 4 | 15 days |
| 2 | 22 | M | 3 × 108.5 | 3 × 109.5 | 4 | 15 days | |||
| Metastatic colon cancer | AdGVCD.10 | Metastatic liver tumors | 1 | 63 | M | 2 × 107 | 8 × 108 | 2 | 1 wk |
| 2 | 65 | M | 2 × 107 | 8 × 108 | 2 | 1 wk | |||
| 3 | 75 | M | 2 × 107 | 8 × 108 | 2 | 1 wk | |||
| 4 | 75 | M | 2 × 108 | 8 × 109 | 2 | 1 wk | |||
| 5 | 72 | M | 2 × 108 | 8 × 109 | 2 | 1 wk | |||
| Coronary artery disease | AdGVVEGF121.10 | Intramyocardial | 1 | 60 | M | 107 | 4 × 108 | 1 | — |
| 2 | 56 | M | 107 | 4 × 108 | 1 | — | |||
| 3 | 64 | M | 107 | 4 × 108 | 1 | — | |||
| 4 | 66 | M | 107.5 | 4 × 108.5 | 1 | — | |||
| 5 | 60 | M | 107.5 | 4 × 108.5 | 1 | — | |||
| 6 | 66 | M | 107.5 | 4 × 108.5 | 1 | — | |||
| 7 | 65 | F | 108 | 4 × 109 | 1 | — | |||
| 8 | 52 | F | 108 | 4 × 109 | 1 | — | |||
| 9 | 46 | M | 108 | 4 × 109 | 1 | — | |||
| 10 | 45 | M | 108.5 | 4 × 109.5 | 1 | — | |||
| 11 | 51 | F | 108.5 | 4 × 109.5 | 1 | — | |||
Individuals 1 to 8 received lot 0056-0002; individuals 9 to 14 received lot 0045-0007; (Table 1).
Individual 13 in part A was the same as individual 1 in part A; the vector in part B was first administered 4 months after the last vector administration in part A.
—, administered one time only.
Administered two times only.
The first dose given to individual 13 was 3 × 1010 PU; subsequent doses were 2 × 1010 PU.
M, male; F, female.
(i) Normal, intradermal.
The trial seeks to evaluate the host responses to intradermal administration of the first generation AdGVCD.10 vector to normal individuals. Each subject was administered the AdGVCD.10 vector in escalating doses (total dose of 8 × 107 to 8 × 109 PU in log increments, n = 2 per group) in a volume of 100 μl by the intradermal route with a 26-gauge needle to two different sites, each site receiving 50% of the total dose (Table 2). Six individuals, all males 34 ± 4 years of age participated in the study. The subjects were considered to be healthy on the basis of medical history, physical examination, and standard biochemical, radiographic, and pulmonary function tests.
(ii) CF, intrabronchial.
The studies consisted of two parts, A and B. Both evaluated repetitive administration of the AdGVCFTR.10 vector to the airway epithelium of individuals with CF but differed in the interval between doses. All subjects in parts A and B had CF diagnosed by clinical manifestations, genetic analysis, and/or positive sweat chloride test.
In part A, the AdGVCFTR.10 vector was administered by endobronchial spray to individuals with CF q3mo × 3. Part A included seven cohorts of 2 individuals each, for a total of 14 individuals (12 males and 2 females, ages 29 ± 2 years; average forced expiratory volume in 1 s [FEV1] of 53% ± 4% predicted). Each cohort was assigned a dose of the AdGVCFTR.10 vector from 3 × 107 to 2 × 1010 PU (in half-log increments [Table 2]). The AdGVCFTR.10 vector was sprayed through a fiberoptic bronchoscope within a lobar bronchus every 3 months, at days 1, 91, and 181. For example, the two individuals in the 3 × 107-PU dose group received 107 PU of the AdGVCFTR.10 vector at three sites, for a total dose of 3 × 107 PU on day 1, and again at days 91 and 181.
In part B of the protocol, two individuals received the AdGVCFTR.10 vector in similar fashion as in part A, but q2wk × 4, at one dose (3 × 109.5 PU [Table 2]). Part B included two individuals (two males, ages 22 and 25 years). Individuals 1 was also included in part A of the protocol (individual 13; receiving a dose of 2 × 1010 PU 4 months prior to the initiation of part B [Table 2]). The two individuals in part B had FEV1 of 44 and 74% predicted, respectively.
(iii) Colon cancer, administration to liver metastasis.
The trial is a prodrug study to evaluate the toxicity and biologic efficacy following direct administration of an Ad vector coding for the E. coli cytosine deaminase gene (AdGVCD.10) to hepatic metastases of biopsy proven colorectal carcinoma with concomitant oral administration of the prodrug 5-fluorocytosine. The study is based on the knowledge that the cytosine deaminase gene converts the prodrug 5-fluorocytosine to the potent chemotherapeutic agent 5-fluorouracil. Individuals (five males, 70 ± 3 years old) with biopsy-proven diagnosis of colon cancer and two or more liver metastases scheduled for laparotomy (for resection of liver metastasis, hepatic artery catheter placement, and/or other procedures) as part of their regular treatment for colon cancer were divided into three dose groups, each group to receive the AdGVCD.10 vector, from a total dose of 8 × 108 to 8 × 109 PU administered (50% of the total dose) on day 1, and 50% of the total dose again on day 7 (Table 2). On day 1, the AdGVCD.10 vector was administered under computer tomography guidance in four equally divided aliquots to the same liver metastasis, each with a separate entry into the metastatic tumor. Each aliquot was contained in a volume of 100 μl. Seven days later, the AdGVCD.10 vector was administered in an identical fashion in four equally divided aliquots (in 100 μl, under computer tomography guidance) to the same metastasis. Oral 5-fluorocytosine (total of 200 mg/kg of body weight per day in four equally divided doses) was started on day 2 and continued until the day prior to laparotomy. The laparotomy for removal of the metastases was performed on days 11 to 15.
(iv) Coronary artery disease, intramyocardial.
The trial is designed to evaluate myocardial angiogenesis induced by direct intramyocardial administration of the AdGVVEGF121.10 vector to individuals with severe coronary artery disease. The trial is a dose-escalating (4 × 108 to 4 × 109.5 PU) safety study of 11 individuals (eight males and three females; 57 ± 2 years old) divided into four dose groups. All had severe coronary artery disease diagnosed by medical history, electrocardiogram, treadmill stress test, echocardiogram, 99mTc-sestamibi flow studies, and cardiac catheterization with angiography. The AdGVVEGF121.10 vector was administered directly to the myocardium following bypass surgery using a 28-gauge needle at ≤5-mm vertical depth in 10 divided doses of 100 μl at 1.0- to 1.5-cm intervals in a myocardial region in a coronary artery distribution that was not bypassed.
Anti-Ad5 neutralization antibody titers.
Anti-Ad5 neutralizing antibody titers were measured by the ability of serum to prevent infection of A549 cells (CCL 185; American Type Cell Culture Collection, Rockville, Md.) by wild-type Ad5 as previously described (72, 76). Briefly, the A549 cells were cultured in improved Eagle’s minimum essential medium containing 10% fetal bovine serum, 2 mM glutamine, 50 U of penicillin per ml, and 50 μg of streptomycin per ml. Cells were seeded in 96-well plates (Falcon 3072; Becton Dickinson, Lincoln Park, N.J.) at a density of 3 × 104 cells/well in a volume of 200 μl of medium. Serum samples were heat inactivated at 55°C for 40 min, diluted serially, and added to a 96-well plate (Falcon 3077; Becton Dickinson). An amount of Ad5 equivalent to a multiplicity of infection of 1 was added to the serum, and the plates were incubated for 1 h at 37°C. The mixture was subsequently added to the A549 cells, and the cells were incubated until the serum-free control wells exhibited >95% cytopathic effect (typically 6 to 8 days) evaluated by methylene blue staining (EM Diagnostic Systems, Gibbstown, N.J.). The neutralizing antibody titer (per 5 μl of serum) was calculated as the product of the reciprocal of the initial dilution times the reciprocal of the dilution in the last well showing <95% cytopathic effect. All assays were performed in triplicate, and data are presented as the mean of three determinations. Human serum known to have anti-Ad5 neutralizing antibodies was used as a positive control, and human serum known to lack anti-Ad neutralizing antibodies was used as a negative control.
Statistical analysis.
Error estimates are presented as mean ± standard deviation. Comparison of the frequency of undetectable baseline anti-Ad5 neutralizing antibodies among the four groups was made by chi-square test. For comparison of the mean ages among the groups of individuals studied, the data were evaluated by an analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) post-hoc test for unequal sample sizes. To test the difference between age and FEV1 by detectable baseline anti-Ad5 within the CF group, a Welch modified Student two-sample t test for unequal variances was used. Due to the nonnormal distribution of the different parameters evaluated (route, age, dose, and disease state), nonparametric statistical analyses were done to evaluate the difference in anti-Ad neutralizing antibody response for the different routes of vector administration and the anti-Ad neutralizing antibody response in relation to baseline anti-Ad titer and dose of vector administered. For the difference in distribution of systemic anti-Ad5 neutralizing antibody response for the four routes of vector administration, the data were ranked and analyzed by Kruskal-Wallis ANOVA. The relationship between baseline systemic anti-Ad5 neutralizing antibodies and dose of vector administered with the peak systemic anti-Ad5 neutralizing antibody response was measured by the Spearman rank correlation coefficient. Statistical significance was taken for P values of less than 0.05. The statistics analysis was carried out by using the programs S-plus 4.5 for Windows (Mathsoft, Inc., Seattle, Wash.) and SAS (SAS Institute, Cary, N.C.). For individuals without detectable neutralizing antibodies at baseline, fold increase over baseline was determined by using the titer of 9 since 10 is the lowest detectable titer with the assay.
RESULTS
No adverse effects attributable to the vector were observed in any study. The details regarding safety and efficacy (where relevant) of each study will be reported elsewhere.
Preexisting anti-Ad neutralizing antibodies.
Evaluation of the different groups of individuals (normal, CF, metastatic colon cancer, and coronary artery disease) demonstrated a broad range of preexisting anti-Ad neutralizing antibody titers, although the differences among the various groups were not significant (Fig. 1). No detectable serum anti-Ad neutralizing antibodies (titer of <10) were detected in 33% (2 of 6) of normal subjects, 67% (10 of 15) of individuals with CF (all individuals in group A plus one individual in group B; individual 1 in group B previously participated in group A, [Table 2]), 20% (1 of 5) of individuals with metastatic colon cancer, and 27% (3 of 11) of individuals with coronary artery disease. Although a larger proportion of individuals with CF had no detectable pretherapy neutralizing antibodies (>2 times that of any other group), this level did not achieve statistical significance (P = 0.21 [chi-square test] compared to all other groups).
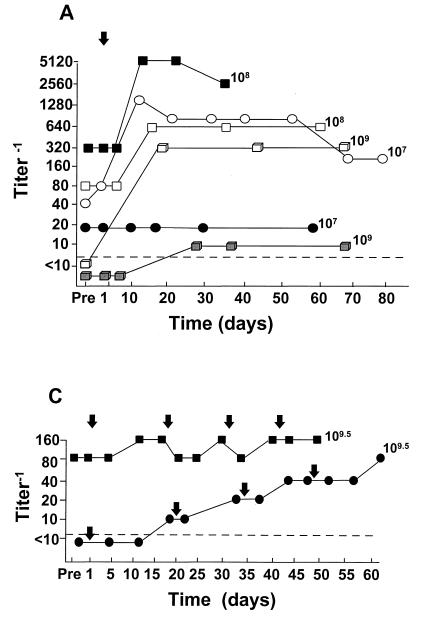
FIG. 1.
Serum anti-Ad5 neutralizing antibody levels as a function of time after administration of E1− Ad gene transfer vectors by various routes to different groups of individuals. Serum levels of anti-Ad5 neutralizing antibodies were quantified pretherapy (Pre) and at various time points as indicated after vector administration. Note that the scale for the titers varies among the panels. Each symbol represents a different individual; each arrow represents a single vector administration. The dose (each administration) of vector (PU) is indicated for each individual. For convenience in plotting the data, only the log dose is given; for the actual doses (e.g., 8 × 107 instead of 107 in panel A), see Table 2. (A) Single intradermal administration (day 1) of the AdGVCD.10 vector to normal individuals; (B) repeat administration (q3m × 3; days 1, 81, and 181) of the AdGVCFTR.10 vector to the airway epithelium of individuals with CF; (C) repeat administration (q15d × 4; days 1, 15, 30, and 45) of the AdGVCFTR.10 vector to the airway epithelium of individuals with CF; (D) repeat administration (q7d × 2; days 1 and 7) of the AdGVCD.10 vector directly to liver metastasis of individuals with colon carcinoma; (E) single direct myocardial administration (day 1) of AdGVVEGF.121.10 to individuals with diffuse coronary artery disease. In all panels, the dashed horizontal line represents the lower limit of detection of the assay.
While the age of the CF group was lower than those of the cancer and coronary artery groups (P < 0.001 [multiple comparison, ANOVA, and Tukey HSD post-hoc test for unequal sample sizes], both comparisons), it was no different from that of the normal group (P = 0.62). Within the CF group, the average age of those with undetectable anti-Ad neutralizing antibodies was no different from that of those with detectable antibodies [30 ± 10 years versus 26 ± 6 years; P = 0.38 [Student’s two-sample t test for unequal variances]), and there was no difference among those in the CF group with nondetectable and detectable antibodies in the average FEV1 (54% ± 17% predicted versus 52% ± 11% predicted, P = 0.79 [Student’s two-sample t test for unequal variances]).
Systemic anti-Ad5 neutralizing antibody responses to vector administration.
Evaluation of systemic anti-Ad5 neutralizing antibodies revealed a variable response depending on the different routes of administration and/or the disorders of the recipients (Fig. 1). The least responses were in individuals with CF after intrabronchial administration of the AdGVCFTR.10 vector (Fig. 1B and C), and the most robust systemic anti-Ad5 neutralizing antibody responses were observed after administration of AdGVCD.10 to liver metastasis of individuals with colon cancer (Fig. 1D). Administration of the Ad vectors by the intradermal and intramyocardial routes resulted in a variable response, with minimal to no neutralizing antibody response in 2 of 6 individuals in the normal trial and 4 of 11 in the coronary artery disease trial (Fig. 1A and E). The data suggest that there is a difference in the distribution of peak anti-Ad5 neutralizing antibody titer according to the route of administration (P = 0.0023 [Kruskal-Wallis test]). In general, for those individuals in whom antibody titers were increased significantly above baseline (in all trials), the peak anti-Ad neutralizing antibody levels were detected by 2 to 4 weeks after vector administration, with levels significantly higher than pre-vector administration levels still present 8 to 12 weeks after Ad vector administration.
(i) Normal (AdGVCD.10, intradermal).
For the six individuals studied, one (8 × 107 PU) had no response, and one (8 × 109 PU) had only a minimal increase in serum systemic anti-Ad5 neutralizing antibody titer from the baseline (Fig. 1A). In contrast, the other four individuals responded to vector administration with significantly increased levels of anti-Ad5 neutralizing antibodies above baseline, with peak levels observed by the second week after the AdGVCD.10 vector was administered. The largest increase was observed in two individuals (8 × 108 and 8 × 109 PU) with 32- and 35-fold increases in the neutralizing titer, respectively.
(ii) CF (AdGVCFTR.10, intrabronchial).
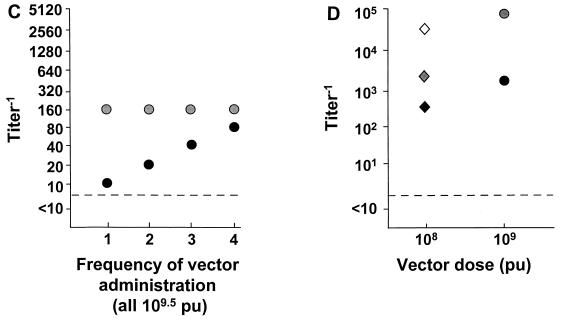
Strikingly, no or minimal systemic anti-Ad5 neutralizing antibodies were observed in individuals with CF, even after repeated lung administration of the AdGVCFTR.10 vector. In part A of the protocol, no significant increase in antibody titers were observed after the first vector administration at any dose (Fig. 1B). Likewise, after the second vector administration, only minimal increases of anti-Ad5 neutralizing antibody titers were observed. The maximum increase (eightfold) was observed in one subject at a dose of 3 × 108.5 PU by the third week after the second vector administration. Following the third vector administration, only minimal (twofold maximum) increases in anti-Ad5 antibody titer were seen in four individuals. In part B of the CF protocol (Fig. 1C), one individual responded with a minimal increase in the anti-Ad5 titer, with successive twofold increase in antibody titers after each lung administration of the AdGVCFTR.10 vector. The individual 2 had no change in anti-Ad antibody titer throughout the study, despite four administrations of the vector 2 weeks apart. Importantly, this same individual had also participated in part A of the CF trial. In part A, he received three lung administrations of AdGVCFTR.10 (2 × 1010 PU q3mo × 3), followed 4 months later, in part B, by four lung administrations (3 × 109.5 PU q15d × 4). Interestingly, even after a total of seven intrabronchial administrations of the AdCFTR.10 vector, the systemic neutralizing antibody titer increased only minimally, with the increase observed only in part A of the trial (Fig. 1B and C).
(iii) Colon carcinoma metastatic to liver (AdGVCD.10).
In striking contrast to intrabronchial administration of an Ad vector, most individuals receiving intratumor (colon carcinoma metastatic to liver) administration of an Ad vector responded with a vigorous systemic anti-Ad5 neutralizing antibody response, with peak antibody levels seen in all individual by the fourth week after vector administration (Fig. 1D). The maximum titers observed (at doses of 8 × 108 and 8 × 109 PU) reached >104. The lowest antibody increase was noted in an individual at a dose of 8 × 108 PU, whose peak level reached 640. In the other two individuals in the study (receiving doses of 8 × 108 and 8 × 109 PU), anti-Ad5 antibody levels increased from <102 at baseline to 103 by the fourth week following vector administration.
(iv) Coronary artery disease (AdGVVEGF.121.10, intramyocardial).
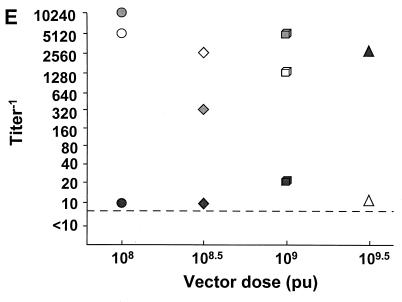
Of the 11 individuals studied, 4 (receiving 4 × 108, 4 × 108.5, 4 × 109, and 4 × 109.5 PU, respectively) had no significant increase in anti-Ad5 neutralizing antibody titer from baseline, and a fifth individual (4 × 108.5 PU) had only a maximum fourfold increase in antibody titer over baseline. The other six individuals had a more vigorous response, with anti-Ad5 antibody titers peaking by the second week after vector administration at 103 to 104. The highest titer was in an individual receiving 4 × 108 PU, with a 512-fold increase in the titer from baseline (Fig. 1E).
Relationship of antibody responses to dose and preexisting antibody titers.
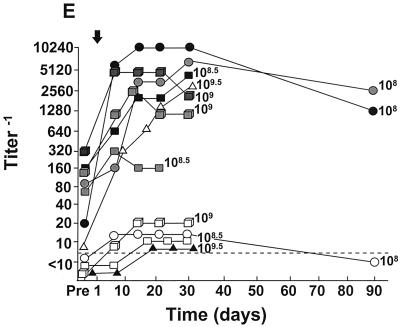
Remarkably, analysis of the peak anti-Ad5 neutralizing antibody responses to administration of the Ad vectors demonstrated no relationship with the dose of vector administered (Fig. 2). Further, the only apparent dependency on frequency of administration was in one individual with CF receiving four administrations of the AdGVCFTR.10 vector (3 × 108.5 PU) (Fig. 2C). Among all other individuals, whether peak antibody responses among different individuals receiving different doses or peak antibody responses among the different individuals receiving repetitive doses were compared, no dose dependency was observed (skin route, correlation coefficient −0.35, P = 0.48; lung route, correlation coefficient −0.39, P = 0.14; intraliver tumor route, correlation coefficient −0.09, P = 0.82; intramyocardial route, correlation coefficient −0.22, P = 0.52 [all Spearman’s rank correlation]). This lack of dose dependency was also true when the analysis was carried out by examining the increase in peak titers (over baseline titers) compared to dose (skin route, correlation coefficient 0.23, P = 0.64; lung route, correlation coefficient 0.23, P = 0.39; intraliver tumor route, correlation coefficient −0.39, P = 0.43; intramyocardial route, correlation coefficient −0.11, P = 0.73 [all Spearman’s rank correlation]). Finally, the lack of dose dependency also held when the individuals with no preexisting anti-Ad neutralizing antibody titers were eliminated from the analysis (not shown; correlation coefficient 0.3, P = 0.13).
FIG. 2.
Peak systemic anti-Ad5 neutralizing antibody levels as a function of dose and frequency of administration. E1− Ad vectors were administered by different routes to different groups of individuals as indicated. Each symbol represents a different individual. For convenience in plotting the data, only the log dose is given; for the actual doses (e.g., 8 × 107− instead of 107 in panel A), see Table 2. (A) Single intradermal administration of the AdGVCD.10 vector to normal individuals; (B) repeat administration (q3mo × 3) of the AdGVCFTR.10 vector to the airway epithelium of individuals with cystic fibrosis; (C) repeat administration (q15d × 4) of the AdGVCFTR.10 vector to the airway epithelium of individuals with CF; (D) repeat administrations (q7d × 2) of the AdGVCD.10 vector directly to liver metastasis of individuals with colon carcinoma; (E) single direct myocardial administration of the AdGVVEGF.121.10 vector to individuals with diffuse coronary artery disease. In all panels, the dashed horizontal line represents the lower limit of detection of the assay. For panels B and C, the timing of the repetitive doses are indicated on the abscissa.
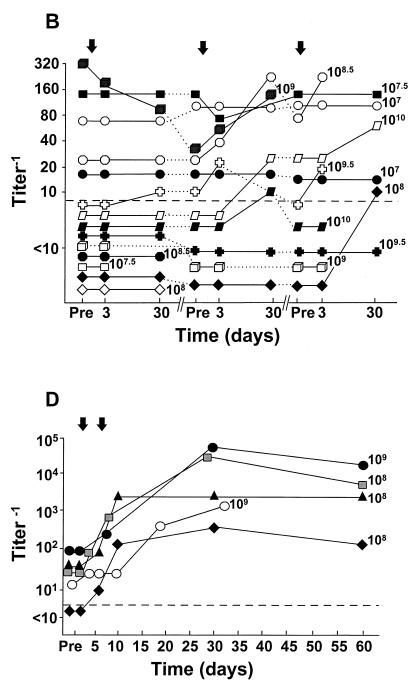
In contrast to the lack of correlation of peak neutralizing antibody titers with dose, there was a remarkable correlation of peak neutralizing antibody titers with the baseline anti-Ad5 neutralizing antibody titers (Fig. 3). This was true for intradermal administration to normal subjects (correlation coefficient 0.84, P = 0.036 [Spearman’s rank correlation] [Fig. 3A]), repetitive (q3mo × 3) airway administration to individuals with CF (correlation coefficient for first administration 0.90, P = 0.0001; correlation coefficient for second administration 0.90, P = 0.0002; correlation coefficient for third administration 0.90, P = 0.0001 [Fig. 3B]), repetitive (q2wk × 4) airway administration to individuals with CF (correlation coefficient 0.91, P = 0.0001 [Fig. 3C]), repetitive administration (q3mo × 3, followed 4 months later by q15d × 4) to the airway epithelium of one individual with CF who participated in parts A and B of the CF trial (correlation coefficient 0.94, P = 0.02 [Fig. 3D]), intratumor administration to individuals with colon cancer metastatic to liver (correlation coefficient 0.98, P = 0.0003 [Fig. 3D]), and intramyocardial administration to individuals with coronary artery disease (correlation coefficient 0.73, P = 0.011 [Fig. 3E] [all analyses by Spearman’s rank correlation]). Finally, analysis of all of the data together demonstrated that despite the disparity in peak titers, there was still a significant correlation of peak neutralizing antibody titers with the baseline titers (correlation coefficient 0.77, P < 0.0001 [Fig. 3F]). This was also true when we analyzed by examining the increase in peak titers (over baseline levels) compared to baseline titers (correlation coefficient 0.34, P = 0.034).
FIG. 3.
Peak systemic anti-Ad5 neutralizing antibody levels as a function of anti-Ad5 neutralizing antibody titer before vector administration. E1− Ad vectors were administered by different routes to different groups of individuals. Each symbol represents a different individual. (A) Single intradermal administration of the AdGVCD.10 vector to normal individuals; (B) repeat administration (q3mo × 3) of the AdGVCFTR.10 vector to the airway epithelium of individuals with CF. Open symbols, first administration; light gray symbols, second administration; dark gray symbols, third administration. (C) Repeat administration (q15d × 4) of the AdGVCFTR.10 vector to the airway epithelium of individuals with CF. Open symbols, first administration; light gray symbols, second administration; dark gray symbols, third administration; black symbols, fourth administration. (D) Repeat administration (q7d × 2) of the AdGVCD.10 vector directly to liver metastasis of individuals with colon carcinoma. (E) Single direct myocardial administration of the AdGVVEGF.121.10 vector to individuals with diffuse coronary artery disease. (F) Data combined from panels A to E. ●, intradermal, normal, from panel A; ▴, airway, CF, from panel B; ▵, airway, CF, from panel C; ■, liver, colon carcinoma, from panel D; □, heart, coronary artery disease, from panel E. In all panels, the dashed horizontal line represents the lower limit of detection of the assay. For convenience in plotting the data, only the log dose is given; for the actual doses (e.g., 8 × 107 instead of 107 in panel A), see Table 2.
DISCUSSION
Neutralizing anti-Ad antibodies, representing a subset of total serum anti-Ad antibodies, function by preventing the virus from binding to cell membrane receptors and/or preventing translocation of the internalized Ad from the endosome into the cytoplasm (13, 128, 129). Based on the experience of administration of Ad gene transfer vectors to experimental animals, the general principle has evolved that the administration of Ad vectors to the immune naive host almost invariably evokes neutralizing antibodies directed against the Ad (11, 25, 27, 31, 35, 44, 47–49, 51–53, 57, 58, 62, 63, 65, 66, 72, 76, 77, 80, 101, 103, 104, 108–110, 114, 118–121, 124, 127, 131, 132, 134–138). However, the present study demonstrates that at the doses used (3 × 107 to 2 × 1010 PU), with the caveat that there is a significant difference in body mass of humans and the experimental animals typically used in gene transfer studies, humans differ from experimental animals in responding to Ad vectors. While humans are capable of increasing systemic anti-Ad neutralizing antibody titers following administration of Ad vectors, the systemic humoral response to Ad vectors in humans is variable, from no response to a vigorous response with neutralizing titers of >104. Whereas naive experimental animals respond with systemic anti-Ad neutralizing antibodies following administration of Ad vectors to all organs and routes evaluated (except for the retina and brain [8, 90]), the present study demonstrates that (i) intrabronchial administration of an Ad vector to individuals with CF results in no or minimal systemic anti-Ad neutralizing antibodies, despite repetitive administration three times over a 6-month period or four times over a 2-month period; (ii) in contrast, at the same or lower doses, administration of Ad vectors to liver tumors (intratumoral) of individuals with metastatic colon cancer, the skin (intradermal) of normal individuals, or the heart (intramyocardial) of individual with coronary artery disease evokes a variable anti-Ad humoral response, from little or no response to striking anti-Ad neutralizing antibody titers of >104; (iii) very differently than for experimental animals (108, 110, 137), the humoral response to Ad vectors in humans at the doses administered in these studies is dose independent; and (iv) whereas immune naive (i.e., no preexisting systemic anti-Ad neutralizing antibodies) experimental animals develop a humoral anti-Ad response within 2 to 4 weeks after Ad vector administration independent of route (11, 25, 27, 31, 35, 44, 47–49, 51–53, 57, 58, 62, 63, 65, 66, 72, 76, 77, 80, 101, 103, 104, 108–110, 114, 118–121, 124, 127, 131, 132, 134–138), the anti-Ad vector response in humans is strikingly dependent on the preexisting anti-Ad neutralizing antibody titer. Together, these observations demonstrate some of the pitfalls of relying on experimental animal studies to predict human host responses to Ad gene transfer vectors and lead to the important conclusion that the design of human Ad gene transfer studies should take into account the preexisting anti-Ad humoral immune status of the potential participants in assessing the safety and efficacy of Ad vectors.
Humoral response to Ad vectors in experimental animals.
Anti-Ad neutralizing antibody responses to administration of Ad vectors to naive animals is dose dependent (108, 110, 137) and is dependent on the route of administration, with the highest Ad neutralization observed after intravenous administration (11, 25, 27, 31, 35, 44, 47–49, 51–53, 57, 58, 62, 63, 65, 66, 72, 76, 77, 80, 101, 103, 104, 108–110, 114, 118–121, 124, 127, 131, 132, 134–138). By most routes, administration of Ad vectors to naive animals is followed by a systemic neutralizing antibody response which peaks within 2 to 3 weeks and decreases by the second or third month after vector administration. No systemic anti-Ad antibody responses are observed following administration to the retina, and a minimal neutralizing antibody response has been reported after administration to the brain (7, 90).
Humoral response to wild-type Ad in humans.
The data regarding humoral responses to wild-type Ad must be viewed with caution in regard to the present study in that Ad gene transfer vectors are E1−, whereas the expression of the E1 genes following wild-type Ad infection likely influences the spectrum and quantity of expression of Ad gene products presented to the immune system for humoral responses. Importantly, all vector preparations in the present study had ≤1 replication-competent Ad per dose administered, and thus the expression of Ad5 E1 genes does not play a role in the anti-Ad neutralizing antibody host responses observed (18, 19, 21, 41). Given this caveat, there are data for normal subjects and individuals with CF regarding anti-Ad systemic neutralizing antibodies following infection with wild-type Ad. Most normal adults (>85%) have systemic anti-Ad antibodies (total antibodies, not necessarily neutralizing) against several of the most frequently encountered Ad serotypes, including subgroup C, serotype 5, the base Ad used for the vectors in the present study (43, 50, 102). Early infection with wild-type Ad in normal humans is followed by a systemic serotype-specific immunoglobulin G (IgG) and IgM anti-Ad antibody response (81). Twenty-seven to 57% of normals are reported to have anti-Ad5 serum neutralizing antibodies (91, 104). Anti-Ad neutralizing antibodies usually develop within 2 weeks of primary infection with wild-type Ad, and if reinfection does not occur, neutralizing antibodies generally decline to undetectable levels over a 2-year period (17, 46). Enteric administration of Ad serotypes 1, 2, 4, 5, 7, and 21 to normal humans also triggers the formation of neutralizing antibodies (26, 84, 105, 106). Based on these studies, an oral vaccine composed of wild-type Ad4 and Ad7 is routinely administered to healthy, military recruits followed by protective systemic neutralizing antibodies (115).
As in normal individuals, the prevalence of systemic anti-Ad5 (total, not specifically neutralizing) antibody titers in individuals with CF are reported to be high, 97% having anti-Ad5 IgG antibodies and 53% having systemic anti-Ad5 IgM antibodies (96). Children with CF have variability in the prevalence of systemic neutralizing antibodies against various Ad serotypes, with neutralizing antibodies against Ad5 in 17% (91). The prevalence of systemic anti-Ad5 neutralizing antibodies in the healthy mothers of the same children was 49%.
Humoral responses to administration of Ad vectors to the respiratory epithelium in humans.
Systemic anti-Ad neutralizing antibody responses to E1− Ad vectors has been evaluated in a variety of clinical trials in which the vector has been administered to the respiratory epithelium. Administration of an Ad5-based vector carrying the normal CFTR cDNA to the nasal epithelium followed by administration to the airway epithelium of four individuals resulted in no change in systemic neutralizing antibody titers (20). Single nasal administration of an Ad5-based vector containing the CFTR cDNA to 12 individuals with CF was followed by no change in anti-Ad neutralizing antibody titer in 11, with only one individual (at the highest dose, 1010 PFU) having a 16-fold increase in the titer from baseline (60). Repeat (four to five times) nasal administration of an Ad2-based vector containing the normal CFTR cDNA to six individuals with CF resulted in minimal systemic neutralizing antibody response, with maximal increases in titer of four- to eightfold above baseline (139). Nasal instillation followed by lung (aerosol) administration of an Ad5-based vector carrying the normal CFTR cDNA to six individuals with CF resulted in no increase in anti-Ad neutralizing antibodies (7). These observations of minimal humoral responses to administration of Ad vectors to the respiratory epithelium in CF is likely not because of a defect in immunity in CF, in that individuals with CF have no evidence of increased susceptibility to respiratory viral infections (91, 93, 125). Consistent with these observations in CF, direct injection of an Ad5-based vector expressing the E. coli β-galactosidase gene to endobronchial tumors in four individuals with lung cancer was followed by a minimal systemic neutralizing antibody response, at most a fourfold increase over baseline (32). Together with the data in the present study, these observations strikingly demonstrate that the anti-Ad neutralizing antibody response elicited after administration of Ad vectors to the human respiratory epithelial surface is minimal compared with other routes of administration, a conclusion that is very different than the experience in experimental animals where the anti-Ad humoral response is invariably robust following Ad vector administration to the respiratory epithelium (53, 72, 76, 120, 121, 134, 137).
Humoral responses to administration of Ad vectors in sites other than the respiratory epithelium in humans.
Other than the present study, there are very few data available relating the administration of E1− Ad vectors to sites in humans other than the respiratory epithelium. Intrapleural administration of an Ad5-based vector expressing the Herpes simplex virus thymidine kinase gene to 21 individuals with localized mesothelioma was followed by a systemic but variable neutralizing antibody response in most individuals (83), and administration of a similar vector to malignant gliomas resulted in the development of systemic neutralizing antibodies (3). Since the Ad capsid is the major factor in inducing anti-Ad antibodies against Ad vectors, it is instructive to review the responses of normal humans to protein components of Ad. Intramuscular and intradermal administration to normal individuals of soluble antigen from Ad1 or Ad2 (both subgroup C) was followed by the development of anti-Ad1 or anti-Ad2 neutralizing antibodies in 90%, with low levels of neutralizing antibodies detected in most individuals after 1 year (6, 54). Intramuscular administration to normal individuals of Ad5 hexon elicited anti-Ad neutralizing antibodies in 80%, and intramuscular administration of Ad5 fiber elicited anti-Ad neutralizing antibodies in 100% (16). All of those developing neutralizing antibodies were protected when challenged with nasal administration of wild-type Ad5, whereas nonimmunized controls developed symptoms of Ad infection (16).
Factors modulating systemic humoral responses to Ad vectors.
An important finding in the present study was the variability of the anti-Ad neutralizing antibody response in the different trials. Differences in the vector per se does not explain the variability of the antibody response, since the three Ad vectors used in the four trials in the present study were all derived from the same Ad5 backbone, and all have identical Ad capsids. Consistent with these observations, when normal individuals with undetectable baseline serum anti-Ad neutralizing antibodies who subsequently had natural wild-type Ad infections (diagnosed by fecal and/or respiratory Ad isolation), a variable proportion developed systemic anti-Ad neutralizing antibodies (29).
The present study was not designed to identify specific Ad capsid epitopes that are recognized by the anti-Ad neutralizing antibodies evoked by the Ad vectors. However, the experience from humans following wild-type Ad infection suggests that the dominant neutralizing antibodies observed against the Ad vectors are against hexon and fiber (16). It has been suggested that Ad vectors also induce neutralizing antibodies against the penton base protein (30).
The data in the present study suggest that the route of administration plays a major role in the systemic anti-Ad neutralizing antibody response, although there are insufficient data to determine whether the underlying disease state modifies the systemic humoral host response to Ad vectors. Administration of the Ad vector to the bronchial epithelium of individuals with CF yielded the lowest antibody response, while direct injection of the Ad vector to colon carcinoma metastases in the liver resulted in the most vigorous antibody response. One factor that may be relevant in regard to minimal responses to bronchial administration of the AdGVCFTR.10 vector in CF is that the airway epithelium of these individuals is covered by high quantities of mucopurulent secretions which could preclude efficient Ad-vector infection of the airway epithelial cells (112, 122).
The strong anti-Ad neutralizing antibody response observed in the metastatic colon cancer trial might be explained by the close proximity of the site of vector administration in the intrahepatic tumors to the liver reticuloendothelial system and/or to the vector gaining access to the systemic circulation. We do not have any direct evidence that the vector reached the circulation following intratumoral administration, but this is a possibility, since administration of the AdGVCD.10 vector was performed with a needle which traveled through normal liver tissue, and the metastatic colon cancer tumors are relatively firm, making it difficult to disperse the vector through the tumor. Another factor that may play a role is the damage to tumor cells induced by the destruction of the tumor by the conversion of 5-fluorocytosine to 5-fluorouracil by the cytosine deaminase expressed by the AdGVCD.10 vector.
While some individuals in the intradermal and intramyocardial trials did not raise the anti-Ad neutralizing antibody titer following administration of the Ad vector, most had a significant systemic anti-Ad neutralizing antibody response, with some individuals in the intramyocardial trial having as vigorous response as some individuals in the colon cancer study. Similar to the intrahepatic tumor administration, it is possible that intradermal or intramyocardial administration introduces small amounts of the vector into the systemic circulation and/or exposes the Ad vector to antigen-presenting cells in the local draining lymph nodes. However, other factors must play a role, since not all individuals in these two trials responded with an increase in the antibody titer.
The most striking finding in the present study was the correlation of the peak systemic anti-Ad5 neutralizing antibody response with the level of baseline anti-Ad5 neutralizing titers. Irrespective of route of administration and underlying disorder, we observed that individuals with higher baseline anti-Ad neutralizing antibody titer mounted a higher neutralizing antibody response after vector administration, consistent with a secondary immune response in those individuals previously exposed to Ad of a similar serotype (29, 92, 94).
Relevance to future gene therapy trials.
There are several important conclusions from these results that have implications for future gene therapy trials. First, unlike in animal studies (53, 72, 76, 120, 134, 138), repeated respiratory epithelial administration of E1− Ad vectors is not followed by a strong systemic anti-Ad neutralizing antibody response, i.e., the generation of anti-Ad systemic neutralizing antibodies does not seem to be a major host immune response after repeated respiratory epithelial administration of Ad vectors. This observation suggests that repeat administration of Ad vectors to the lung may be feasible, at least in regard to the hurdle of systemic humoral immunity against Ad vectors, although local mucosal immunity and other factors (e.g., vector genome and epithelial cell Ad receptors) play a more important role in the efficiency of Ad-mediated gene transfer after repeated lung administration (72, 76, 79, 86–88, 111, 134). A caveat to the minimal anti-Ad neutralizing antibody response observed in the CF trial is that the vector was administered to only a limited region of the airways; i.e., doses higher than the one used in this trial (>2 × 1010 PU) might elicit a different systemic anti-Ad neutralizing antibody response in humans. Second, the extent of the observed humoral immune responses to Ad5-based vectors is related to preexisting systemic anti-Ad5 neutralizing antibodies, likely due to prior exposure to wild-type subgroup C Ad. This observation is important for the design of gene therapy strategies, since (i) high anti-Ad neutralizing antibodies may prevent, or significantly reduce, the efficiency of gene transfer upon administration of a similar serotype-based Ad vector (57, 72, 76, 108, 134) and (ii) in the absence of adenovirus replication, the host immune system still recognizes the Ad capsid proteins and mounts an anti-Ad neutralizing antibody response; i.e., for the design of new “stealth” Ad vectors, simply deleting Ad genes (12, 14, 28, 36, 61, 68, 69) will likely not circumvent the anti-Ad humoral response for in vivo gene therapy applications requiring repeated Ad vector administration.
There are several theoretical strategies that might be used in human gene therapy applications to circumvent the host humoral immune responses to Ad vectors. First, sequential administration of different-serotype Ad vectors to rodents will circumvent systemic and local host immunity elicited by the first vector administration (57, 72, 76). Thus, different-serotype Ad gene transfer vectors, or vectors with capsid components modified at specific capsid sites known to be targets for the anti-Ad neutralizing antibodies, might be used in the context of preexisting, serotype-specific anti-Ad immunity (1, 10, 33, 64, 95, 99, 113, 126). Second, several strategies to suppress humoral immune responses to Ad vectors, including immunotolerization by different routes, cytokine treatment, use of monoclonal antibodies, T-cell depletion strategies, macrophage depletion, thymectomy, and immunosuppressive drugs, have been evaluated in experimental animals (11, 15, 22, 24, 25, 27, 34, 35, 37, 38, 44, 47, 48, 51, 52, 58, 62, 66, 100, 101, 104, 110, 114, 123, 124, 127, 130, 131, 135, 136, 140); many of these strategies have proven successful in blunting humoral immune responses, permitting transgene expression after repetitive administration of Ad vectors (11, 15, 22, 44, 47, 48, 58, 62, 101, 110, 124, 127, 133, 135, 136). These strategies have yet to be evaluated in humans.
ACKNOWLEDGMENTS
We thank our colleagues in the Departments of Medicine, Radiology, and Cardiothoracic Surgery at the Weill Medical College of Cornell University—New York Presbyterian Hospital who helped with these studies.
These studies were supported, in part, by the National Institutes of Health (grants P01 HL51746, R21 CA75153, M01RR00047, and M01RR00102); Will Rogers Memorial Fund, Los Angeles, Calif.; Cystic Fibrosis Foundation, Bethesda, Md.; GenVec, Inc., Rockville, Md.; and the Jeffry and Barbara M. Picower Foundation, Palm Beach, Fla. T.K.R. was also supported by the Thoracic Surgery Research Foundation, Chicago, Ill.
REFERENCES
- 1.Abrahamsen K, Kong H L, Mastrangeli A, Brough D, Lizonova A, Crystal R G, Falck-Pedersen E. Construction of an adenovirus type 7a E1A− vector. J Virol. 1997;71:8946–8951. doi: 10.1128/jvi.71.11.8946-8951.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akli S, Caillaud C, Vigne E, Stratford-Perricaudet L D, Poenaru L, Perricaudet M, Kahn A, Peschanski M R. Transfer of a foreign gene into the brain using adenovirus vectors. Nat Genet. 1993;3:224–228. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- 3.Alavi J B, Judy K, Alavi A, Hackney D, Phillips P, Smith J, Recio A, Wilson J, Eck S. Abstracts of the 1st Annual Meeting of the American Society of Gene Therapy. Thorofare, N.J: American Society of Gene Therapy; 1998. Phase I trial of gene therapy in primary brain tumors; p. 112a. [Google Scholar]
- 4.Anonymous. Human gene marker/therapy clinical protocols. Hum Gene Ther. 1998;9:2805–2852. doi: 10.1089/hum.1998.9.18-2805. [DOI] [PubMed] [Google Scholar]
- 5.Bajocchi G, Feldman S H, Crystal R G, Mastrangeli A. Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vectors. Nat Genet. 1993;3:229–234. doi: 10.1038/ng0393-229. [DOI] [PubMed] [Google Scholar]
- 6.Banks P A, Kasel J A, Huber M, Alford R H, Knight V. Persistence of neutralizing antibody in adult volunteers immunized with adnovirus soluble antigens. Proc Soc Exp Biol Med. 1966;121:240–243. doi: 10.3181/00379727-121-30747. [DOI] [PubMed] [Google Scholar]
- 7.Bellon G, Michel-Calemard L, Thouvenot D, Jagneaux V, Poitevin F, Malcus C, Accart N, Layani M P, Aymard M, Bernon H, Bienvenu J, Courtney M, Doring G, Gilly B, Gilly R, Lamy D, Levrey H, Morel Y, Paulin C, Perraud F, Rodillon L, Sene C, So S, Touraine-Moulin F, Schatz C, Pavirani A. Aerosol administration of a recombinant adenovirus expressing CFTR to cystic fibrosis patients: a phase I clinical trial. Hum Gene Ther. 1997;8:15–25. doi: 10.1089/hum.1997.8.1-15. [DOI] [PubMed] [Google Scholar]
- 8.Bennett J, Pakola S, Zeng Y, Maguire A. Humoral response after administration of E1-deleted adenoviruses: immune privilege of the subretinal space. Hum Gene Ther. 1996;7:1763–1769. doi: 10.1089/hum.1996.7.14-1763. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard K T, Boekelheide K. Adenovirus-mediated gene transfer to rat testis in vivo. Biol Reprod. 1997;56:495–500. doi: 10.1095/biolreprod56.2.495. [DOI] [PubMed] [Google Scholar]
- 10.Bouri K, Feero W, Myerburg M, Wickham T J, Kovesdi I, Hoffman E, Clemens P. Abstracts of the 1st Annual Meeting of the American Society of Gene Therapy. Thorofare, N.J: American Society of Gene Therapy; 1998. Polylysine modification of the fiber protein enhances muscle cell transduction; p. 157a. [Google Scholar]
- 11.Bouvet M, Fang B, Ekmekcioglu S, Ji L, Bucana C D, Hamada K, Grimm E A, Roth J A. Suppression of the immune response to an adenovirus vector and enhancement of intratumoral transgene expression by low-dose etoposide. Gene Ther. 1998;5:189–195. doi: 10.1038/sj.gt.3300564. [DOI] [PubMed] [Google Scholar]
- 12.Brough D E, Lizonova A, Hsu C, Kulesa V A, Kovesdi I. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J Virol. 1996;70:6497–6501. doi: 10.1128/jvi.70.9.6497-6501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown D T, Burlingham B T. Penetration of host cell membranes by adenovirus 2. J Virol. 1973;12:386–396. doi: 10.1128/jvi.12.2.386-396.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H H, Mack L M, Kelly R, Ontell M, Kochanek S, Clemens P R. Persistence in muscle of an adenoviral vector that lacks all viral genes. Proc Natl Acad Sci USA. 1997;94:1645–1650. doi: 10.1073/pnas.94.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cichon G, Strauss M. Transient immunosuppression with 15-deoxyspergualin prolongs reporter gene expression and reduces humoral immune response after adenoviral gene transfer. Gene Ther. 1998;5:85–90. doi: 10.1038/sj.gt.3300555. [DOI] [PubMed] [Google Scholar]
- 16.Couch R B, Kasel J A, Perreira H G, Haase A T, Knight V. Induction of immunity in man by crystalline adenovirus type 5 capsid antigens. Proc Soc Exp Biol Med. 1973;143:905–910. doi: 10.3181/00379727-143-37438. [DOI] [PubMed] [Google Scholar]
- 17.Crawford-Miksza L, Schnurr D P. Seroepidemiology of new AIDS-associated adenoviruses among the San Francisco men’s health study. J Med Virol. 1996;50:230–236. doi: 10.1002/(SICI)1096-9071(199611)50:3<230::AID-JMV4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Crystal R G, Hirschowitz E, Lieberman M, Daly J, Kazam E, Henschke C, Yankelevitz D, Kemeny N, Silverstein R, Ohwada A, Russi T, Mastrangeli A, Sanders A, Cooke J, Harvey B G. Phase I study of direct administration of a replication deficient adenovirus vector containing the E. coli cytosine deaminase gene to metastatic colon carcinoma of the liver in association with the oral administration of the pro-drug 5-fluorocytosine. Hum Gene Ther. 1997;8:985–1001. doi: 10.1089/hum.1997.8.8-985. [DOI] [PubMed] [Google Scholar]
- 19.Crystal R G, Mastrangeli A, Sanders A, Cooke J, King T, Gilbert F, Henschke C, Pascal W, Herena J, Harvey B G, Hirschowitz E, Diaz D, Russi T, Pacheco F, Sikand V, Brion P. Evaluation of repeat administration of a replication deficient, recombinant adenovirus containing the normal cystic fibrosis transmembrane conductance regulator cDNA to the airways of individuals with cystic fibrosis. Hum Gene Ther. 1995;6:667–703. doi: 10.1089/hum.1995.6.5-667. [DOI] [PubMed] [Google Scholar]
- 20.Crystal R G, McElvaney N G, Rosenfeld M A, Chu C S, Mastrangeli A, Hay J G, Brody S L, Jaffe H A, Eissa N T, Danel C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet. 1994;8:42–51. doi: 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- 21.Crystal R G, Rosengart T K, Isom O W, et al. Phase I study of direct administration of a replication deficient adenovirus vector (AdGVVEGF121.10) containing the VEGF121 cDNA to the ischemic myocardium of individuals with life threatening diffuse coronary artery disease. RAC Report 9711221. Bethesda, Md: National Institutes of Health; 1998. [Google Scholar]
- 22.Dai Y, Schwarz E M, Gu D, Zhang W W, Sarvetnick N, Verma I M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson B L, Allen E D, Kozarsky K F, Wilson J M, Roessler B J. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993;3:219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- 24.De Matteo R P, Markmann J F, Kozarsky K F, Barker C F, Raper S E. Prolongation of adenoviral transgene expression in mouse liver by T lymphocyte subset depletion. Gene Ther. 1996;3:4–12. [PubMed] [Google Scholar]
- 25.De Matteo R P, Raper S E, Ahn M, Fisher K J, Burke C, Radu A, Widera G, Claytor B R, Barker C F, Markmann J F. Gene transfer to the thymus. A means of abrogating the immune response to recombinant adenovirus. Ann Surg. 1995;222:229–239. doi: 10.1097/00000658-199509000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edmondson W P, Purcell R H, Gundelfinger B F, Love J W, Ludwig W, Chanock R M. Immunization by selective infection with type 4 adenovirus grown in human diploid tissue culture. II. Specific protective effect against epidemic disease. JAMA. 1966;195:159–165. [PubMed] [Google Scholar]
- 27.Fang B, Eisensmith R C, Wang H, Kay M A, Cross R E, Landen C N, Gordon G, Bellinger D A, Read M S, Hu P C, Brinkhous K M, Woo S L C. Gene therapy for hemophilia B: host immunosuppression prolongs the therapeutic effect of adenovirus-mediated factor IX expression. Hum Gene Ther. 1995;6:1039–1044. doi: 10.1089/hum.1995.6.8-1039. [DOI] [PubMed] [Google Scholar]
- 28.Fisher K J, Choi H, Burda J, Chen S J, Wilson J M. Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology. 1996;217:11–22. doi: 10.1006/viro.1996.0088. [DOI] [PubMed] [Google Scholar]
- 29.Fox J P, Hall C E, Cooney M K. The Seattle virus watch. VII. Observations of adenovirus infections. Am J Epidemiol. 1977;105:362–386. doi: 10.1093/oxfordjournals.aje.a112394. [DOI] [PubMed] [Google Scholar]
- 30.Gahery-Segard H, Farace F, Godfrin D, Gaston J, Lengagne R, Tursz T, Boulanger P, Guillet J G. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J Virol. 1998;72:2388–2397. doi: 10.1128/jvi.72.3.2388-2397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gahery-Segard H, Juillard V, Gaston J, Lengagne R, Pavirani A, Boulanger P, Guillet J G. Humoral immune response to the capsid components of recombinant adenoviruses: routes of immunization modulate virus-induced Ig subclass shifts. Eur J Immunol. 1997;27:653–659. doi: 10.1002/eji.1830270312. [DOI] [PubMed] [Google Scholar]
- 32.Gahery-Segard H, Molinier-Frenkel V, Le Boulaire C, Saulnier P, Opolon P, Lengagne R, Gautier E, Le Cesne A, Zitvogel L, Venet A, Schatz C, Courtney M, Le Chevalier T, Tursz T, Guillet J G, Farace F. Phase I trial of recombinant adenovirus gene transfer in lung cancer. Longitudinal study of the immune responses to transgene and viral products. J Clin Investig. 1997;100:2218–2226. doi: 10.1172/JCI119759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gall J, Kass-Eisler A, Leinwand L, Falck-Pedersen E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J Virol. 1996;70:2116–2123. doi: 10.1128/jvi.70.4.2116-2123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geddes B J, Harding T C, Hughes D S, Byrnes A P, Lightman S L, Conde G, Uney J B. Persistent transgene expression in the hypothalamus following stereotaxic delivery of a recombinant adenovirus: suppression of the immune response with cyclosporin. Endocrinology. 1996;137:5166–5169. doi: 10.1210/endo.137.11.8895393. [DOI] [PubMed] [Google Scholar]
- 35.Gilgenkrantz H, Duboc D, Juillard V, Couton D, Pavirani A, Guillet J G, Briand P, Kahn A. Transient expression of genes transferred in vivo into heart using first-generation adenoviral vectors: role of the immune response. Hum Gene Ther. 1995;6:1265–1274. doi: 10.1089/hum.1995.6.10-1265. [DOI] [PubMed] [Google Scholar]
- 36.Gorziglia M I, Kadan M J, Yei S, Lim J, Lee G M, Luthra R, Trapnell B C. Elimination of both E1 and E2 from adenovirus vectors further improves prospects for in vivo human gene therapy. J Virol. 1996;70:4173–4178. doi: 10.1128/jvi.70.6.4173-4178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guerette B, Moisset P A, Huard C, Tardif F, Gravel C, Tremblay J P. Inflammatory damage following first-generation replication-defective adenovirus controlled by anti-LFA-1. J Leukoc Biol. 1997;61:533–538. doi: 10.1002/jlb.61.4.533. [DOI] [PubMed] [Google Scholar]
- 38.Guerette B, Vilquin J T, Gingras M, Gravel C, Wood K J, Tremblay J P. Prevention of immune reactions triggered by first-generation adenoviral vectors by monoclonal antibodies and CTLA4Ig. Hum Gene Ther. 1996;7:1455–1463. doi: 10.1089/hum.1996.7.12-1455. [DOI] [PubMed] [Google Scholar]
- 39.Guzman R J, Hirschowitz E A, Brody S L, Crystal R G, Epstein S E, Finkel T. In vivo suppression of injury-induced vascular smooth muscle cell accumulation using adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene. Proc Natl Acad Sci USA. 1994;91:10732–10736. doi: 10.1073/pnas.91.22.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guzman R J, Lemarchand P, Crystal R G, Epstein S E, Finkel T. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ Res. 1993;73:1202–1207. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- 41.Harvey B-G, Crystal R G. Immune responses to intradermal administration of an adenovirus type 5 gene transfer vector (AdGVCD.10) in normal individuals. RAC Report 9701-171. Bethesda, Md: National Institutes of Health; 1997. [Google Scholar]
- 42.Huard J, Lochmuller H, Acsadi G, Jani A, Massie B, Karpati G. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995;2:107–115. [PubMed] [Google Scholar]
- 43.Huebner R J, Rowe W P, Ward T G, Parrott R H, Bell J A. Adenoidal-pharyngeal-conjunctival agents. A newly recognized group of common viruses of the respiratory system. N Engl J Med. 1954;251:1077–1086. doi: 10.1056/NEJM195412302512701. [DOI] [PubMed] [Google Scholar]
- 44.Ilan Y, Prakash R, Davidson A, Jona, Droguett G, Horwitz M S, Chowdhury N R, Chowdhury J R. Oral tolerization to adenoviral antigens permits long-term gene expression using recombinant adenoviral vectors. J Clin Investig. 1997;99:1098–1106. doi: 10.1172/JCI119238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaffe H A, Danel C, Longenecker G, Metzger M, Setoguchi Y, Rosenfeld M A, Gant T W, Thorgeirsson S S, Stratford-Perricaudet L D, Perricaudet M, Pavirani A, Lecocq J-P, Crystal R G. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat Genet. 1992;1:372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- 46.Jawetz E, Thygeson P, Hanna L, Nicholas A, Kimura S J. The etiology of epidemic keratoconjunctivitis. Am J Ophthalmol. 1957;43:79–83. doi: 10.1016/0002-9394(57)91483-6. [DOI] [PubMed] [Google Scholar]
- 47.Jooss K, Turka L A, Wilson J M. Blunting of immune responses to adenoviral vectors in mouse liver and lung with CTLA4Ig. Gene Ther. 1998;5:309–319. doi: 10.1038/sj.gt.3300595. [DOI] [PubMed] [Google Scholar]
- 48.Jooss K, Yang Y, Wilson J M. Cyclophosphamide diminishes inflammation and prolongs transgene expression following delivery of adenoviral vectors to mouse liver and lung. Hum Gene Ther. 1996;7:1555–1566. doi: 10.1089/hum.1996.7.13-1555. [DOI] [PubMed] [Google Scholar]
- 49.Juillard V, Villefroy P, Godfrin D, Pavirani A, Venet A, Guillet J G. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur J Immunol. 1995;25:3467–3473. doi: 10.1002/eji.1830251239. [DOI] [PubMed] [Google Scholar]
- 50.Jung D, Wigand R. Epidemiology of group II adenoviruses. Am J Epidemiol. 1967;85:311–319. doi: 10.1093/oxfordjournals.aje.a120694. [DOI] [PubMed] [Google Scholar]
- 51.Kagami H, Atkinson J C, Michalek S M, Handelman B, Yu S, Baum B J, O’Connell B. Repetitive adenovirus administration to the parotid gland: role of immunological barriers and induction of oral tolerance. Hum Gene Ther. 1998;9:305–313. doi: 10.1089/hum.1998.9.3-305. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan J M, Smith A E. Transient immunosuppression with deoxyspergualin improves longevity of transgene expression and ability to readminister adenoviral vector to the mouse lung. Hum Gene Ther. 1997;8:1095–1104. doi: 10.1089/hum.1997.8.9-1095. [DOI] [PubMed] [Google Scholar]
- 53.Kaplan J M, St George J A, Pennington S E, Keyes L D, Johnson R P, Wadsworth S C, Smith A E. Humoral and cellular immune responses of nonhuman primates to long-term repeated lung exposure to Ad2/CFTR2. Gene Ther. 1996;3:117–127. [PubMed] [Google Scholar]
- 54.Kasel J A, Banks P A, Huber M, Alford R H, Knight V. Antigenicity of adenovirus type 4 soluble antigens in man. J Bacteriol. 1966;91:464–464. doi: 10.1128/jb.91.1.464-.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kass-Eisler A, Falck-Pedersen E, Alvira M, Rivera J, Buttrick P M, Wittenberg B A, Cipriani L, Leinwand L A. Quantitative determination of adenovirus-mediated gene delivery to rat cardiac myocytes in vitro and in vivo. Proc Natl Acad Sci USA. 1993;90:11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kass-Eisler A, Falck-Pedersen E, Elfenbein D H, Alvira M, Buttrick P M, Leinwand L A. The impact of developmental stage, route of administration and the immune system on adenovirus-mediated gene transfer. Gene Ther. 1994;1:395–402. [PubMed] [Google Scholar]
- 57.Kass-Eisler A, Leinwand L, Gall J, Bloom B, Falck-Pedersen E. Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 1996;3:154–162. [PubMed] [Google Scholar]
- 58.Kay M A, Holterman A X, Meuse L, Gown A, Ochs H D, Linsley P S, Wilson C B. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat Genet. 1995;11:191–197. doi: 10.1038/ng1095-191. [DOI] [PubMed] [Google Scholar]
- 59.Kay M A, Landen C N, Rothenberg S R, Taylor L A, Leland F, Wiehle S, Fang B, Bellinger D, Finegold M, Thompson A R, Read M, Brinkhous K M, Woo S L. In vivo hepatic gene therapy: complete albeit transient correction of factor IX deficiency in hemophilia B dogs. Proc Natl Acad Sci USA. 1994;91:2353–2357. doi: 10.1073/pnas.91.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knowles M R, Hohneker K W, Zhou Z, Olsen J C, Noah T L, Hu P C, Leigh M W, Engelhardt J F, Edwards L J, Jones K R, Grossman M, Wilson J M, Johnson L G, Boucher R C. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N Engl J Med. 1995;333:823–831. doi: 10.1056/NEJM199509283331302. [DOI] [PubMed] [Google Scholar]
- 61.Kochanek S, Clemens P R, Mitani K, Chen H H, Chan S, Caskey C T. A new adenoviral vector: replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and β-galactosidase. Proc Natl Acad Sci USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kolls J K, Lei D, Odom G, Nelson S, Summer W R, Gerber M A, Shellito J E. Use of transient CD4 lymphocyte depletion to prolong transgene expression of E1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:489–497. doi: 10.1089/hum.1996.7.4-489. [DOI] [PubMed] [Google Scholar]
- 63.Kozarsky K F, McKinley D R, Austin L L, Raper S E, Stratford-Perricaudet L D, Wilson J M. In vivo correction of low density lipoprotein receptor deficiency in the Watanabe heritable hyperlipidemic rabbit with recombinant adenoviruses. J Biol Chem. 1994;269:13695–13702. [PubMed] [Google Scholar]
- 64.Krasnykh V, Dmitriev I, Mikheeva G, Miller C R, Belousova N, Kashentseva E, Wang M-H, Curiel D T. Abstracts of the 1st Annual Meeting of the American Society of Gene Therapy. Thorofare, N.J: American Society of Gene Therapy; 1998. Genetic modification to adenovirus fiber protein as a means to achieve vector targeting; p. 177a. [Google Scholar]
- 65.Kucharczuk J C, Raper S, Elshami A A, Amin K M, Sterman D H, Wheeldon E B, Wilson J M, Litzky L A, Kaiser L R, Albelda S M. Safety of intrapleurally administered recombinant adenovirus carrying herpes simplex thymidine kinase DNA followed by ganciclovir therapy in nonhuman primates. Hum Gene Ther. 1996;7:2225–2233. doi: 10.1089/hum.1996.7.18-2225. [DOI] [PubMed] [Google Scholar]
- 66.Kuzmin A I, Galenko O, Eisensmith R C. Abstracts of the 1st Annual Meeting of the American Society of Gene Therapy. Thorofare, N.J: American Society of Gene Therapy; 1998. Long-term transgene expression and successful readministration of recombinant E1-deleted adenoviral vectors in mice treated with liposome-encapsulated clodronate and MR1 antibody; p. 420. [Google Scholar]
- 67.Lemarchand P, Jones M, Danel C, Yamada I, Mastrangeli A, Crystal R G. In vivo adenovirus-mediated gene transfer to lungs via pulmonary artery. J Appl Physiol. 1994;76:2840–2845. doi: 10.1152/jappl.1994.76.6.2840. [DOI] [PubMed] [Google Scholar]
- 68.Lieber A, He C Y, Kirillova I, Kay M A. Recombinant adenoviruses with large deletions generated by CRE-mediated excision exhibit different biological properties compared with first-generation vectors in vitro and in vivo. J Virol. 1996;70:8944–8960. doi: 10.1128/jvi.70.12.8944-8960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lusky M, Christ M, Rittner K, Dieterle A, Dreyer D, Mourot B, Schultz H, Stoeckel F, Pavirani A, Mehtali M. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2a, or E1/E4 deleted. J Virol. 1998;72:2022–2032. doi: 10.1128/jvi.72.3.2022-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mack C A, Magovern C J, Budenbender K T, Patel S R, Schwarz E A, Zanzonico P, Ferris B, Sanborn T, Isom P, Isom O W, Crystal R G, Rosengart T K. Salvage angiogenesis induced by adenovirus-mediated gene transfer of vascular endothelial growth factor protects against ischemic vascular occlusion. J Vasc Surg. 1998;27:699–709. doi: 10.1016/s0741-5214(98)70236-8. [DOI] [PubMed] [Google Scholar]
- 71.Mack C A, Patel S R, Schwarz E A, Zanzonico P, Hahn R T, Ilercil A, Devereux R B, Goldsmith S J, Christian T F, Sanborn T A, Kovesdi I, Hackett N, Isom O W, Crystal R G, Rosengart T K. Biologic bypass with the use of adenovirus-mediated gene transfer of the complementary deoxyribonucleic acid for vascular endothelial growth factor 121 improves myocardial perfusion and function in the ischemic porcine heart. J Thorac Cardiovasc Surg. 1998;115:168–76. doi: 10.1016/s0022-5223(98)70455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mack C A, Song W R, Carpenter H, Wickham T J, Kovesdi I, Harvey B G, Magovern C J, Isom O W, Rosengart T, Falck-Pedersen E, Hackett N R, Crystal R G, Mastrangeli A. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum Gene Ther. 1997;8:99–109. doi: 10.1089/hum.1997.8.1-99. [DOI] [PubMed] [Google Scholar]
- 73.Magovern C J, Mack C A, Zhang J, Hahn R T, Ko W, Isom O W, Crystal R G, Rosengart T K. Direct in vivo gene transfer to canine myocardium using a replication-deficient adenovirus vector. Ann Thorac Surg. 1996;62:425–433. [PubMed] [Google Scholar]
- 74.Magovern C J, Mack C A, Zhang J, Rosengart T K, Isom O W, Crystal R G. Regional angiogenesis induced in nonischemic tissue by an adenoviral vector expressing vascular endothelial growth factor. Hum Gene Ther. 1997;8:215–227. doi: 10.1089/hum.1997.8.2-215. [DOI] [PubMed] [Google Scholar]
- 75.Mastrangeli A, Danel C, Rosenfeld M A, Stratford-Perricaudet L, Perricaudet M, Pavirani A, Lecocq J P, Crystal R G. Diversity of airway epithelial cell targets for in vivo recombinant adenovirus-mediated gene transfer. J Clin Investig. 1993;91:225–234. doi: 10.1172/JCI116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mastrangeli A, Harvey B G, Yao J, Wolff G, Kovesdi I, Crystal R G, Falck-Pedersen E. “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum Gene Ther. 1996;7:79–87. doi: 10.1089/hum.1996.7.1-79. [DOI] [PubMed] [Google Scholar]
- 77.McClane S J, Chirmule N, Burke C V, Raper S E. Characterization of the immune response after local delivery of recombinant adenovirus in murine pancreas and successful strategies for readministration. Hum Gene Ther. 1997;8:2207–2216. doi: 10.1089/hum.1997.8.18-2207. [DOI] [PubMed] [Google Scholar]
- 78.McClane S J, Hamilton T E, De Matteo R P, Burke C, Raper S E. Effect of adenoviral early genes and the host immune system on in vivo pancreatic gene transfer in the mouse. Pancreas. 1997;15:236–245. doi: 10.1097/00006676-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 79.Mestecky J, Abraham R, Ogra P L. Common mucosal immune system and strategies for the development of vaccines effective at the mucosal surfaces. In: Ogra P L, Mestecky J, Lamm M E, Strober W, McGhee J R, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1994. pp. 357–372. [Google Scholar]
- 80.Michou A I, Santoro L, Christ M, Julliard V, Pavirani A, Mehtali M. Adenovirus-mediated gene transfer: influence of transgene, mouse strain and type of immune response on persistence of transgene expression. Gene Ther. 1997;4:473–482. doi: 10.1038/sj.gt.3300412. [DOI] [PubMed] [Google Scholar]
- 81.Mistchenko A S, Lenzi H L, Thompson F M, Mota E M, Vidaurreta S, Navari C, Grinstein S. Participation of immune complexes in adenovirus infection. Acta Paediatr. 1992;81:983–988. doi: 10.1111/j.1651-2227.1992.tb12159.x. [DOI] [PubMed] [Google Scholar]
- 82.Mittereder N, March K L, Trapnell B C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Molnar-Kimber K L, Sterman D H, Chang M, Kang E H, ElBash M, Lanuti M, Elshami A, Gelfand K, Wilson J M, Kaiser L R, Albelda S M. Impact of preexisting and induced humoral and cellular immune responses in an adenovirus-based gene therapy phase I clinical trial for localized mesothelioma. Hum Gene Ther. 1998;9:2121–2133. doi: 10.1089/hum.1998.9.14-2121. [DOI] [PubMed] [Google Scholar]
- 84.Mufson M A, Webb P A, Kennedy H, Gill V, Chanock R M. Etiology of upper-respiratory-tract illnesses among civilian adults. JAMA. 1966;195:1–7. [PubMed] [Google Scholar]
- 85.Muhlhauser J, Jones M, Yamada I, Cirielli C, Lemarchand P, Gloe T R, Bewig B, Signoretti S, Crystal R G, Capogrossi M C. Safety and efficacy of in vivo gene transfer into the porcine heart with replication-deficient, recombinant adenovirus vectors. Gene Ther. 1996;3:145–153. [PubMed] [Google Scholar]
- 86.Murphy B R. Mucosal immunity to viruses. In: Ogra P L, Mestecky J, Lamm M E, Strober W, McGhee J R, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1994. pp. 333–343. [Google Scholar]
- 87.Murphy B R, Clements M L, Johnson P R, Wright P F. The mucosal and systemic immune responses of children and adults to live and inactivated influenza A virus vaccines. Vaccines. 1996;1996:303–318. [Google Scholar]
- 88.Ogra P L, Karzon D T. Formation and function of poliovirus antibody in different tissues. Prog Med Virol. 1971;13:156–193. [Google Scholar]
- 89.Ohno T, Gordon D, San H, Pompili V J, Imperiale M J, Nabel G J, Nabel E G. Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science. 1994;265:781–784. doi: 10.1126/science.8047883. [DOI] [PubMed] [Google Scholar]
- 90.Parr M J, Wen P Y, Schaub M, Khoury S J, Sayegh M H, Fine H A. Immune parameters affecting adenoviral vector gene therapy in the brain. J Neurovirol. 1998;4:194–203. doi: 10.3109/13550289809114519. [DOI] [PubMed] [Google Scholar]
- 91.Piedra P A, Poveda G A, Ramsey B, McCoy K, Hiatt P W. Incidence and prevalence of neutralizing antibodies to the common adenoviruses in children with cystic fibrosis: implication for gene therapy with adenovirus vectors. Pediatrics. 1998;101:1013–1019. doi: 10.1542/peds.101.6.1013. [DOI] [PubMed] [Google Scholar]
- 92.Poovorawan Y, Theamboonlers A, Sanpavat S, Chumdermpadetsuk S, Safary A, Vandepapeliere P. Long-term antibody persistence after booster vaccination with combined tetravalent diphtheria tetanus, whole-cell bordetella pertussis and hepatitis b vaccine in healthy infants. Ann Trop Paediatr. 1997;17:301–308. doi: 10.1080/02724936.1997.11747902. [DOI] [PubMed] [Google Scholar]
- 93.Ramsey B W, Gore E J, Smith A L, Cooney M K, Redding G J, Foy H. The effect of respiratory viral infections on patients with cystic fibrosis. Am J Dis Child. 1989;143:662–668. doi: 10.1001/archpedi.1989.02150180040017. [DOI] [PubMed] [Google Scholar]
- 94.Rao S P, Rajkumar K, Schiffman G, Desai N, Unger C, Miller S T. Anti-pneumococcal antibody levels three to seven years after first booster immunization in children with sickle cell disease, and after a second booster. J Pediatr. 1995;127:590–592. doi: 10.1016/s0022-3476(95)70119-2. [DOI] [PubMed] [Google Scholar]
- 95.Roelvink P W, Kovesdi I, Bergelson J M, Finberg R W, Wickham T J. Abstracts of the 1st Annual Meeting of the American Society of Gene Therapy. Thorofare, N.J: American Society of Gene Therapy; 1998. Adenovirus retargeting through fiber modification and bifunctional fiber receptor protein (CAR) chemicals; p. 177a. [Google Scholar]
- 96.Rosenecker J, Harms K H, Bertele R M, Pohl-Koppe A, v.Mutius E, Adam D, Nicolai T. Adenovirus infection in cystic fibrosis patients: Implications for the use of adenoviral vectors for gene transfer. Infection. 1996;24:5–8. doi: 10.1007/BF01780642. [DOI] [PubMed] [Google Scholar]
- 97.Rosenfeld M A, Siegfried W, Yoshimura K, Yoneyama K, Fukayama M, Stier L E, Paakko P K, Gilardi P, Stratford-Perricaudet L D, Perricaudet M, Jallat S, Pavirani A, Lecocq J-P, Crystal R G. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- 98.Rosenfeld M A, Yoshimura K, Trapnell B C, Yoneyama K, Rosenthal E R, Dalemans W, Fukayama M, Bargon J, Stier L E, Stratford-Perricaudet L, Perricaudet M, Guggino W B, Pavirani A, Lecocq J-P, Crystal R G. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- 99.Roy S, Shirley P S, McClelland A, Kaleko M. Circumvention of immunity to the adenovirus major coat protein hexon. J Virol. 1998;72:6875–6879. doi: 10.1128/jvi.72.8.6875-6879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sawchuk S J, Boivin G P, Duwel L E, Ball W, Bove K, Trapnell B, Hirsch R. Anti-T cell receptor monoclonal antibody prolongs transgene expression following adenovirus-mediated in vivo gene transfer to mouse synovium. Hum Gene Ther. 1996;7:499–506. doi: 10.1089/hum.1996.7.4-499. [DOI] [PubMed] [Google Scholar]
- 101.Scaria A, St George J A, Gregory R J, Noelle R J, Wadsworth S C, Smith A E, Kaplan J M. Antibody to CD40 ligand inhibits both humoral and cellular immune responses to adenoviral vectors and facilitates repeated administration to mouse airway. Gene Ther. 1997;4:611–617. doi: 10.1038/sj.gt.3300431. [DOI] [PubMed] [Google Scholar]
- 102.Schmitz H, Wigand R, Heinrich W. Worldwide epidemiology of human adenovirus infections. Am J Epidemiol. 1983;117:455–466. doi: 10.1093/oxfordjournals.aje.a113563. [DOI] [PubMed] [Google Scholar]
- 103.Schneider D B, Fly C A, Dichek D A, Geary R L. Adenoviral gene transfer in arteries of hypercholesterolemic nonhuman primates. Hum Gene Ther. 1998;9:815–821. doi: 10.1089/hum.1998.9.6-815. [DOI] [PubMed] [Google Scholar]
- 104.Schulick A H, Vassalli G, Dunn P F, Dong G, Rade J J, Zamarron C, Dichek D A. Established immunity precludes adenovirus-mediated gene transfer in rat carotid arteries. Potential for immunosuppression and vector engineering to overcome barriers of immunity. J Clin Investig. 1997;99:209–219. doi: 10.1172/JCI119149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwartz A R, Togo Y, Hornick R B. Clinical evaluation of live, oral types 1, 2, and 5 adenovirus vaccines. Am Rev Respir Dis. 1974;109:233–239. doi: 10.1164/arrd.1974.109.2.233. [DOI] [PubMed] [Google Scholar]
- 106.Scott R M, Dudding B A, Romano S V, Russell P K. Enteric immunization with live adenovirus type 21 vaccine. II. Systemic and local immune responses following immunization. Infect Immun. 1972;5:300–304. doi: 10.1128/iai.5.3.300-304.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Setoguchi Y, Danel C, Crystal R G. Stimulation of erythropoiesis by in vivo gene therapy: physiologic consequences of transfer of the human erythropoietin gene to experimental animals using an adenovirus vector. Blood. 1994;84:2946–2953. [PubMed] [Google Scholar]
- 108.Setoguchi Y, Jaffe H A, Chu C S, Crystal R G. Intraperitoneal in vivo gene therapy to deliver alpha 1-antitrypsin to the systemic circulation. Am J Respir Cell Mol Biol. 1994;10:369–377. doi: 10.1165/ajrcmb.10.4.8136153. [DOI] [PubMed] [Google Scholar]
- 109.Smith T A, Mehaffey M G, Kayda D B, Saunders J M, Yei S, Trapnell B C, McClelland A, Kaleko M. Adenovirus mediated expression of therapeutic plasma levels of human factor IX in mice. Nat Genet. 1993;5:397–402. doi: 10.1038/ng1293-397. [DOI] [PubMed] [Google Scholar]
- 110.Smith T A, White B D, Gardner J M, Kaleko M, McClelland A. Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Ther. 1996;3:496–502. [PubMed] [Google Scholar]
- 111.Smith T J, Buescher E L, Top F H, Jr, Altemeier W A, McCown J M. Experimental respiratory infection with type 4 adenovirus vaccine in volunteers: clinical and immunological responses. J Infect Dis. 1970;122:239–248. doi: 10.1093/infdis/122.4.239. [DOI] [PubMed] [Google Scholar]
- 112.Stern M, Caplen N J, Browning J E, Griesenbach U, Sorgi F, Huang L, Gruenert D C, Marriot C, Crystal R G, Geddes D M, Alton E W. The effect of mucolytic agents on gene transfer across a CF sputum barrier in vitro. Gene Ther. 1998;5:91–98. doi: 10.1038/sj.gt.3300556. [DOI] [PubMed] [Google Scholar]
- 113.Stevenson S C, Rollence M, Marshall-Neff J, McClelland A. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J Virol. 1997;71:4782–4790. doi: 10.1128/jvi.71.6.4782-4790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sullivan D E, Dash S, Du H, Hiramatsu N, Aydin F, Kolls J, Blanchard J, Baskin G, Gerber M A. Liver-directed gene transfer in non-human primates. Hum Gene Ther. 1997;8:1195–1206. doi: 10.1089/hum.1997.8.10-1195. [DOI] [PubMed] [Google Scholar]
- 115.Takafuji E T, Gaydos J C, Allen R G, Top F H., Jr Simultaneous administration of live, enteric-coated adenovirus types 4, 7, and 21 vaccines: safety and immunogenicity. J Infect Dis. 1979;140:48–53. doi: 10.1093/infdis/140.1.48. [DOI] [PubMed] [Google Scholar]
- 116.Timme T L. Local inflammatory response and vector spread after direct intraprostatic injection of a recombinant adenovirus containing the herpes simplex virus thymidine kinase gene and ganciclovir therapy in mice. Cancer Gene Ther. 1998;5:74–82. [PubMed] [Google Scholar]
- 117.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 118.Ueno H. Adenovirus-mediated gene transfer into injured arteries: quantitative analysis of repeated administration. Gerontology. 1996;42(Suppl. 1):25–36. doi: 10.1159/000213822. [DOI] [PubMed] [Google Scholar]
- 119.Ueno H, Li J J, Tomita H, Yamamoto H, Pan Y, Kanegae Y, Saito I, Takeshita A. Quantitative analysis of repeat adenovirus-mediated gene transfer into injured canine femoral arteries. Arterioscler Thromb Vasc Biol. 1995;15:2246–2253. doi: 10.1161/01.atv.15.12.2246. [DOI] [PubMed] [Google Scholar]
- 120.Van Ginkel F W, Liu C, Simecka J W, Dong J Y, Greenway T, Frizzell R A, Kiyono H, McGhee J R, Pascual D W. Intratracheal gene delivery with adenoviral vector induces elevated systemic IgG and mucosal IgA antibodies to adenovirus and β-galactosidase. Hum Gene Ther. 1995;6:895–903. doi: 10.1089/hum.1995.6.7-895. [DOI] [PubMed] [Google Scholar]
- 121.Van Ginkel F W, McGhee J R, Liu C, Simecka J W, Yamamoto M, Frizzell R A, Sorscher E J, Kiyono H, Pascual D W. Adenoviral gene delivery elicits distinct pulmonary-associated T helper cell responses to the vector and to its transgene. J Immunol. 1997;159:685–693. [PubMed] [Google Scholar]
- 122.Vanheeckeren A, Ferkol T, Tosi M. Effects of bronchopulmonary inflammation induced by Pseudomonas aeruginosa on adenovirus-mediated gene transfer to airway epithelial cells in mice. Gene Ther. 1998;5:345–351. doi: 10.1038/sj.gt.3300593. [DOI] [PubMed] [Google Scholar]
- 123.Vilquin J T, Guerette B, Kinoshita I, Roy B, Goulet M, Gravel C, Roy R, Tremblay J P. FK506 immunosuppression to control the immune reactions triggered by first-generation adenovirus-mediated gene transfer. Hum Gene Ther. 1995;6:1391–1401. doi: 10.1089/hum.1995.6.11-1391. [DOI] [PubMed] [Google Scholar]
- 124.Walter J, You Q, Hagstrom J N, Sands M, High K A. Successful expression of human factor IX following repeat administration of adenoviral vector in mice. Proc Natl Acad Sci USA. 1996;93:3056–3061. doi: 10.1073/pnas.93.7.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang E E, Prober C G, Manson B, Corey M, Levison H. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N Engl J Med. 1984;311:1653–1658. doi: 10.1056/NEJM198412273112602. [DOI] [PubMed] [Google Scholar]
- 126.Wickham T J, Tzeng E, Shears L L2, Roelvink P W, Li Y, Lee G M, Brough D E, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wilson C B, Embree L J, Schowalter D, Albert R, Aruffo A, Hollenbaugh D, Linsley P, Kay M A. Transient inhibition of CD28 and CD40 ligand interactions prolongs adenovirus-mediated transgene expression in the lung and facilitates expression after secondary vector administration. J Virol. 1998;72:7542–7550. doi: 10.1128/jvi.72.9.7542-7550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wohlfart C. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J Virol. 1988;62:2321–2328. doi: 10.1128/jvi.62.7.2321-2328.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wohlfart C E, Svensson U K, Everitt E. Interaction between HeLa cells and adenovirus type 2 virions neutralized by different antisera. J Virol. 1985;56:896–903. doi: 10.1128/jvi.56.3.896-903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wolff G, Worgall S, van Rooijen N, Song W R, Harvey B G, Crystal R G. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J Virol. 1997;71:624–629. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang Y, Greenough K, Wilson J M. Transient immune blockade prevents formation of neutralizing antibody to recombinant adenovirus and allows repeated gene transfer to mouse liver. Gene Ther. 1996;3:412–420. [PubMed] [Google Scholar]
- 132.Yang Y, Haecker S E, Su Q, Wilson J M. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Genet. 1996;5:1703–1712. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- 133.Yang Y, Jooss K U, Su Q, Ertl H C, Wilson J M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- 134.Yang Y, Li Q, Ertl H C, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang Y, Su Q, Grewal I S, Schilz R, Flavell R A, Wilson J M. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J Virol. 1996;70:6370–6377. doi: 10.1128/jvi.70.9.6370-6377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang Y, Trinchieri G, Wilson J M. Recombinant IL-12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung. Nat Med. 1995;1:890–893. doi: 10.1038/nm0995-890. [DOI] [PubMed] [Google Scholar]
- 137.Yei S, Mittereder N, Tang K, O’Sullivan C, Trapnell B C. Adenovirus-mediated gene transfer for cystic fibrosis: quantitative evaluation of repeated in vivo vector administration to the lung. Gene Ther. 1994;1:192–200. [PubMed] [Google Scholar]
- 138.Zabner J, Petersen D M, Puga A P, Graham S M, Couture L A, Keyes L D, Lukason M J, St George J A, Gregory R J, Smith A E, Welsh M J. Safety and efficacy of repetitive adenovirus-mediated transfer of CFTR cDNA to airway epithelia of primates and cotton rats. Nat Genet. 1994;6:75–83. doi: 10.1038/ng0194-75. [DOI] [PubMed] [Google Scholar]
- 139.Zabner J, Ramsey B W, Meeker D P, Aitken M L, Balfour R P, Gibson R L, Launspach J, Moscicki R A, Richards S M, Standaert T A, Williams-Warren J, Wadsworth S C, Smith A E, Welsh M J. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J Clin Investig. 1996;97:1504–1511. doi: 10.1172/JCI118573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zsengeller Z K, Wert S E, Hull W M, Hu X, Yei S, Trapnell B C, Whitsett J A. Persistence of replication-deficient adenovirus-mediated gene transfer in lungs of immune-deficient (nu/nu) mice. Hum Gene Ther. 1995;6:457–467. doi: 10.1089/hum.1995.6.4-457. [DOI] [PubMed] [Google Scholar]