Abstract
Vocal cord dysfunction (VCD) is often misdiagnosed as asthma or complicates coexisting asthma. This study aimed to identify distinguishing clinical characteristics in patients with VCD, asthma, and coexisting VCD and asthma. We conducted a retrospective analysis of demographic and clinical data from 292 patients with VCD, asthma, coexisting VCD and asthma, and control subjects from an outpatient university asthma/allergy clinic. Concomitant asthma was present in 32.6% of VCD subjects. Overall, 42.4% of all VCD subjects were previously misdiagnosed as having asthma for an average of 9.0 years. Upper airway symptoms were more prevalent in the VCD population and nocturnal apnea was more prevalent in comorbid VCD and asthma compared with either condition alone. Irritable bowel syndrome and chronic pain were identified as new comorbidities associated with VCD. VCD subjects who had been misdiagnosed with asthma had significantly more health care and asthma medication use compared to VCD subjects who had not mimicked asthma. There was no difference in asthma severity between those with and without VCD. Comorbid VCD and asthma led to an increase in long-acting β-agonist use only, but no difference in health care usage, compared with asthma alone. These findings suggest that the main morbidity associated with VCD may not lie in its inherent disease process, but instead in its ability to mimic asthma.
Vocal cord dysfunction (VCD) is defined by abnormal adduction of the vocal cords, primarily during inspiration. The exact prevalence of VCD in the general population is unknown, but it has a higher incidence among ▪women.1,2 Symptoms of VCD include dyspnea (especially on inspiration), cough, wheezing, dysphonia, and throat tightness. Although symptoms may be absent at rest, they can often be exacerbated by specific irritants.3–5
The differential diagnosis for VCD includes upper airway anatomic defects, neurologic problems involving the vagus or recurrent laryngeal nerves, laryngeal edema, and uncontrolled asthma.3–5 Although the exact cause is unknown, it has been hypothesized that the laryngeal hyperresponsiveness observed in VCD may be secondary to inflammation and/or irritation of the vocal cords. The potential triggers include gastroesophageal reflux disease (GERD), rhinitis with postnasal drip, viral upper respiratory tract infections, cold and/or dry air, and chemical or occupational irritants.3–5 Additionally, various psychosocial factors have been found to be more prevalent in VCD patients.6,7
Proposed guidelines for the diagnosis of VCD include appropriate clinical history, evidence of abnormal vocal cord motion on laryngoscopy, and pulmonary function test criteria.8 Spirometry can be normal without bronchodilator response or show flattening of the inspiratory flow volume loop.9,10 Additionally, bronchoprovocation testing should be negative for asthma although it may trigger symptoms of abnormal vocal cord movement.11,12 Laryngoscopic exam can be normal in between attacks if the appropriate trigger is absent. Conversely, laryngoscopic exam can be abnormal in the absence of symptoms.13 Recently, dynamic volume computed tomography scan was shown to be effective in identifying VCD.14 The management of VCD includes treatment directed at underlying comorbid conditions in addition to speech and behavioral therapy.3
Recent evidence suggests that asthma prevalence is currently around 8% in the American population.15 Several studies have attempted to examine the presence of VCD in patients diagnosed with asthma and found wide-ranging estimates, with VCD present in 1.6–75% of asthmatic patients.1,2,16–18 Other studies have evaluated the presence of asthma in patients with underlying VCD and have found a 29–66% prevalence of asthma in this population.19,20 Most of the studies have not used objective criteria for making the diagnosis of asthma and instead have relied on clinical history, which can make interpretation difficult when attempting to distinguish the two disorders.
In this study, we aimed to identify subjects with VCD alone or VCD and asthma. We then retrospectively compared a large cohort of adult patients with coexistent VCD and asthma, VCD alone, and asthma alone. We hypothesized that there would be distinguishing features, including demographics, symptoms, medication usage, comorbidities, and health care usage, which would help differentiate these groups of patients. Furthermore, we hypothesized that some subjects who presented with an a priori diagnosis of asthma would, in fact, only have VCD and would also have distinguishing features from the remainder of the VCD population. To date, this is the largest analysis examining the relationship of VCD and asthma.
METHODS
Study Design
We conducted a retrospective analysis of patients seen in an outpatient university asthma-allergy center from July 2004 through January 2010 by a single provider. The VCD group was selected by identifying all subjects with the ICD-9 code 478.5 (vocal cord disease not elsewhere classified) seen during this time frame (n = 259). Only subjects who met criteria for VCD diagnosis were included in analysis. The diagnosis of VCD was based on the following diagnostic criteria: a consistent clinical history (prolonged symptoms, recurrent or intermittent episodes, and reproducible inciting factors), symptoms (including dyspnea, upper airway stridor or wheezing, throat tightness, chest tightness, cough, or dysphonia), and positive findings on laryngoscopy.8 Laryngoscopy was performed in all subjects by a single provider during which abnormal vocal cord motion or vocal cord collapse was noted. If no abnormal motion or collapse of the vocal cords was detected, but the subject related a history of irritants such as strong scents, odors, or exercise eliciting their symptoms, a laryngoscopy was repeated with provocation (exercise challenge or exposure to a strong perfume) and vocal cords were observed for abnormal motion and/or collapse. If there was no laryngoscopic findings of VCD, these subjects were excluded from analysis (n = 127). The remaining subjects with confirmed VCD (n = 132) were then evaluated for the presence of asthma based on ICD-9 code 493 and diagnostic criteria for asthma. The diagnosis of asthma was made using currently accepted criteria, including a consistent history, evidence of obstruction and bronchodilator response on spirometry, and/or positive bronchoprovocation challenge testing with methacholine, consistent with the 2007 National Asthma Education and Prevention Program Guidelines.21 Given the concern for false positive methacholine challenge tests in subjects with VCD, we imposed strict criteria for positive bronchoprovocation, which included a disproportionate decrease in forced expiratory volume in 1 s (FEV1) with concomitant decrease in FEV1/forced vital capacity ratio, a concave expiratory flow volume loop, and absence of flattening of the inspiratory flow volume loop. There were 43 subjects who had coexistent asthma and VCD and 89 who had VCD only based on these diagnostic criteria. For our asthma alone group, 100 computer-generated randomly selected asthma patients (ICD-9 code of 493) were evaluated objectively for presence of asthma based on aforementioned criteria. Fifty-nine subjects met criteria for asthma diagnosis. The controls in our study were computer-generated randomly selected patients who did not have a diagnosis of any respiratory disease (including asthma) or vocal cord disease (n = 101). They were seen in our clinic and by the same provider over the same time period as the identified subjects with VCD and/or asthma. Demographic and clinical data were obtained on all subjects. Comorbidities were extracted from the patient's reported medical history. No attempt was made to prove or disprove any of the diagnosis examined, except for asthma and VCD, as described previously. In all asthmatic patients severity was determined.22 This study was approved by the Institutional Review Board at our facility.
Statistical Analysis
Statistical analysis was performed using JMP software (SAS Institute, Cary, NC). Pearson χ-square tests compared categorical variables. For normally distributed data, analysis of variance was used, and for nonparametric numerical demographic data the Kruskal-Wallis test was used to compare medians. A value of p < .05 was considered statistically significant. Ninety-five percent confidence intervals were also recorded.
RESULTS
Study Population
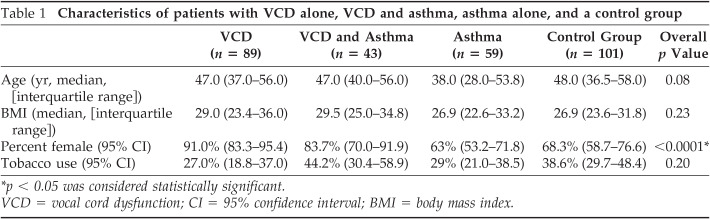
The total study population (n = 292) was divided into four groups: (1) subjects with VCD alone (n = 89), (2) asthma alone (n = 59), (3) coexistent asthma and VCD (n = 43), and (4) controls without either diagnosis (n = 101). The control population without asthma or VCD was used strictly for comparing demographics and comorbidities and to allow comparisons of our VCD population with results of previous studies. The control group was not included in the remainder of analyses comparing those with VCD alone, asthma alone, or coexistent VCD and asthma. There was no difference in age, body mass index, or history of tobacco use among groups (Table 1). There were significantly more ▪women in the VCD population, consistent with previous studies.3 There was no difference in asthma severity between those with asthma alone and those with VCD and asthma. Those with asthma alone consisted of 53.5, 25.8, and 20.7% of mild, moderate, and severe asthmatic patients, respectively, and those with VCD and asthma consisted of 50.5, 26.7, and 22.8%, respectively (p = .77). There was no difference in preor postbronchodilator FEV1% predicted values among asthmatic patients alone compared with those with coexistent VCD and asthma (p > .05).
Table 1.
Characteristics Of patients with VCD alone, VCD and asthma, asthma alone, and a control group
| VCD (n = 89) | VCD and Asthma (n = 43) | Asthma (n = 59) | Control Group (n = 101) | Overall p Value | |
|---|---|---|---|---|---|
| Age (yr, median, [interquartile range]) | 47.0 (37.0–56.0) | 47.0 (40.0–56.0) | 38.0 (28.0–53.8) | 48.0 (36.5–58.0) | 0.08 |
| BMI (median, [interquartile range]) | 29.0 (23.4–36.0) | 29.5 (25.0–34.8) | 26.9 (22.6–33.2) | 26.9 (23.6–31.8) | 0.23 |
| Percent female (95% CI) | 91.0% (83.3–95.4) | 83.7% (70.0–91.9) | 63% (53.2–71.8) | 68.3% (58.7–76.6) | <0.0001* |
| Tobacco use (95% CI) | 27.0% (18.8–37.0) | 44.2% (30.4–58.9) | 29% (21.0–38.5) | 38.6% (29.7–48.4) | 0.20 |
p < .05 was considered statistically significant.
VCD = vocal cord dysfunction; CI = 95% confidence interval; BMI = body mass index.
Relationship between VCD and Asthma
Comorbidities.
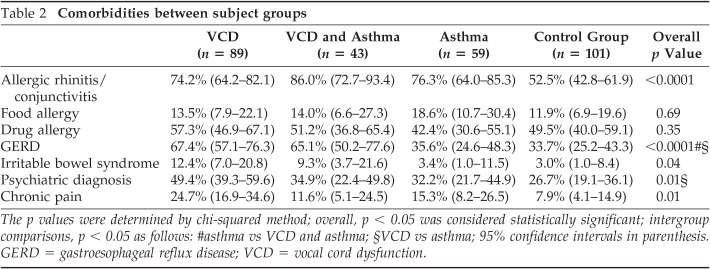
We evaluated all 132 VCD subjects for the presence of coexistent asthma and found that 32.6% (n = 43) of VCD subjects also had asthma, and 67.4% (n = 89) had VCD alone. Among the groups, there were significant differences in reported medical history, including allergic rhinitis/conjunctivitis, GERD, irritable bowel syndrome, chronic pain, and a psychiatric diagnosis. Intergroup comparisons revealed that GERD, psychiatric diagnoses, irritable bowel syndrome, and chronic pain were increased in those with VCD compared with controls, although only GERD and psychiatric diagnoses remained statistically significant when those with VCD alone were compared with those with asthma (Table 2 and data not shown). In this study a diagnosis of chronic pain was given if subjects reported a history of chronic musculoskeletal pain of any type, fibromyalgia, or chronic headaches.
Table 2.
Comorbidities Between subject groups
| VCD (n = 89) | VCD and Asthma (n = 43) | Asthma (n = 59) | Control Group (n = 101) | Overall p Value | |
|---|---|---|---|---|---|
| Allergic rhinitis/conjunctivitis | 74.2% (64.2–82.1) | 86.0% (72.7–93.4) | 76.3% (64.0–85.3) | 52.5% (42.8–61.9) | <0.0001 |
| Food allergy | 13.5% (7.9–22.1) | 14.0% (6.6–27.3) | 18.6% (10.7–30.4) | 11.9% (6.9–19.6) | 0.69 |
| Drug allergy | 57.3% (46.9–67.1) | 51.2% (36.8–65.4) | 42.4% (30.6–55.1) | 49.5% (40.0–59.1) | 0.35 |
| GERD | 67.4% (57.1–76.3) | 65.1% (50.2–77.6) | 35.6% (24.6–48.3) | 33.7% (25.2–43.3) | <0.0001#§ |
| Irritable bowel syndrome | 12.4% (7.0–20.8) | 9.3% (3.7–21.6) | 3.4% (1.0–11.5) | 3.0% (1.0–8.4) | 0.04 |
| Psychiatric diagnosis | 49.4% (39.3–59.6) | 34.9% (22.4–49.8) | 32.2% (21.7–44.9) | 26.7% (19.1–36.1) | 0.01§ |
| Chronic pain | 24.7% (16.9–34.6) | 11.6% (5.1–24.5) | 15.3% (8.2–26.5) | 7.9% (4.1–14.9) | 0.01 |
The p values were determined by χ-squared method; overall, p < .05 was considered statistically significant; intergroup comparisons, p < .05 as follows: #asthma vs VCD and asthma; §VCD vs asthma; 95% confidence intervals in parenthesis.
GERD = gastroesophageal reflux disease; VCD = vocal cord dysfunction.
Symptoms and Triggers.
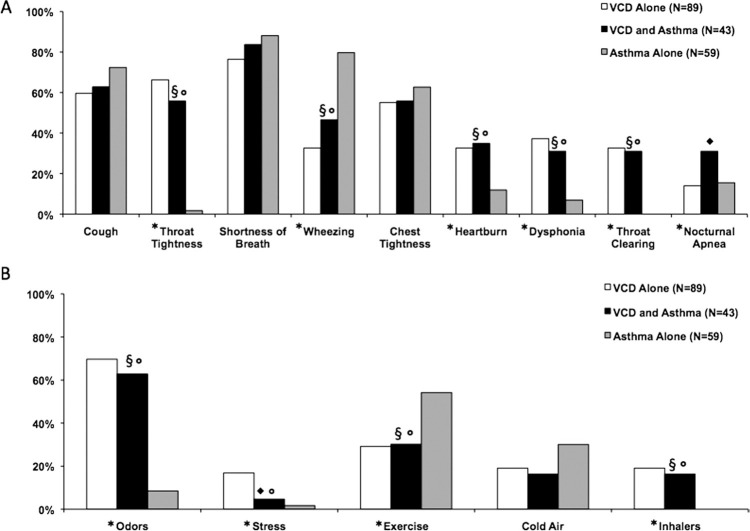
Overall, there were significant differences in throat tightness (p < .0001), wheezing (p < .0001), heartburn (p < .008), dysphonia (p = .0002), throat clearing (p < .0001), and nocturnal apnea (p = .05) between the groups, and no differences were observed for shortness of breath, chest tightness, or cough (Fig. 1). Intergroup comparisons were also made and significant differences are indicated in Fig. 1 Wheezing tended to be more prevalent in those with asthma, as one might expect. Symptoms of heartburn, dysphonia, throat clearing, and throat tightness were more common in those with VCD with or without asthma compared with those with asthma alone. Nocturnal apnea was more common in patients with coexistent VCD and asthma compared with those with VCD or asthma alone. Triggers of odors, stress, and inhaler use were more common in those with VCD with or without asthma than asthma alone, and exercise was a more common trigger in those with asthma alone.
Fig. 1.
Reported (A) symptoms and (B) symptom triggers among subject groups. *Overall, p < .05;◆p < .05 for VCD versus VCD and asthma; §p < .05 for asthma versus VCD and asthma;◦p < .05 for VCD versus asthma.
Medication Use at Initial Visit.
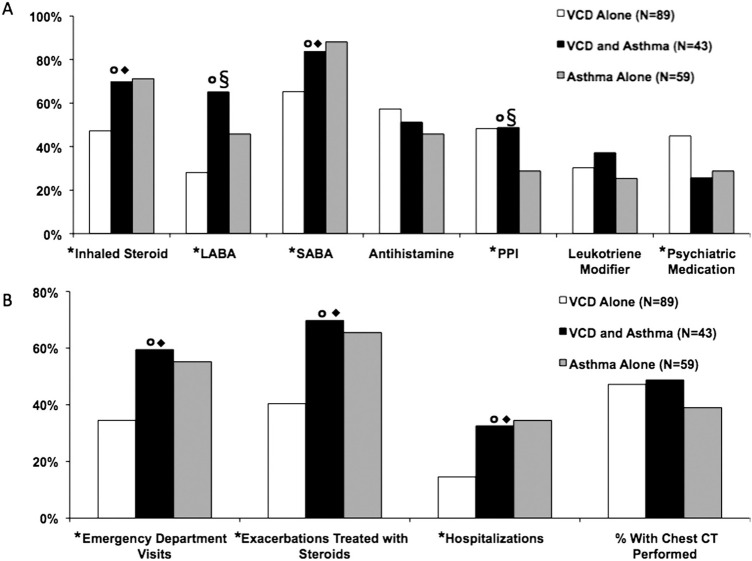
Overall, there were significant differences in inhaled corticosteroid (p = .004), long-acting β-agonists (LABA; p = .0002), and short-acting β-agonists (SABA; p = .002), proton pump inhibitors (p = .04), and psychiatric medication usage (p = .04) between the groups. There was no difference in antihistamine or leukotriene modifier use. VCD subjects with or without asthma were using more proton pump inhibitors compared with asthma alone (Fig. 2 A). Intergroup comparisons revealed that asthmatic patients with or without VCD were generally prescribed an SABA and inhaled corticosteroid more often. LABA use was increased in those with coexistent VCD and asthma compared with those with VCD or asthma alone. Interestingly, those with VCD alone were more likely to be prescribed psychiatric medications compared with those with coexistent VCD and asthma or asthma alone.
Fig. 2.
Comparison of (A) medication usage and (B) health care use among subject groups. *Overall, p < .05;p < .05 for VCD versus VCD and asthma; §p < .05 for asthma versus VCD and asthma;◆p < .05 for VCD versus asthma.
Health Care Usage.
Overall, there were significant differences in health care use among the three groups (VCD alone, VCD and asthma, and asthma alone), including emergency department (ED) visits (p = .01), hospitalizations (p = .01), and exacerbations treated with systemic steroids (p = .002; Fig. 2 B). When comparing VCD alone with coexistent VCD and asthma or comparing VCD alone with asthmatic patients alone, these differences persisted. These intergroup comparisons suggest that this difference is caused by the presence of asthma, regardless of the presence of VCD. Importantly, there was no difference in health care use between those with coexistent VCD and asthma, compared with those with asthma alone.
Asthma Misdiagnosis.
Overall, 42.4% (56/132) of all VCD subjects had been previously misdiagnosed with asthma. Those who had been misdiagnosed had carried an asthma diagnosis for an average of 9.0 years. Within the 132 VCD subjects, we performed a subanalysis of the 56 VCD-only subjects (previously misdiagnosed as having asthma) with 76 VCD-only subjects (not having ever been given a diagnosis of asthma). There were no differences between the groups in terms of basic demographics and comorbidities. However, the VCD subjects who had been misdiagnosed with asthma had significantly more health care use, including asthma medication use (inhaled steroids, LABAs, SABAs, and leukotriene modifiers), ED visits, and "exacerbations" treated with steroids (all, p < .05). Moreover, there was also a strong trend toward increased hospitalizations in the misdiagnosed group as well (p = .07).
DISCUSSION
The relationship between VCD and asthma remains unclear. It was first recognized that VCD could mimic asthma in 1983, and given its symptom overlap, it can remain misdiagnosed as asthma for long periods of time.23 It has since been suggested that both disorders can coexist, although there are varying opinions regarding their exact relationship. Glottic narrowing (particularly during expiration) may be a physiologic compensatory mechanism to bronchial obstruction.24 Others have suggested that laryngeal dysfunction may be intrinsic to asthma.17 Alternatively, VCD and asthma may be isolated disorders that can coexist.25 Given the paucity of available literature, we used objective data to diagnose both VCD and asthma to better characterize the relationship.6,15 This is the largest retrospective study to date examining the relationship between VCD and asthma and is the first to use objective evidence to diagnose asthma using spirometry or methacholine challenge rather than relying on patient history. It has been shown that methacholine challenge can induce VCD, resulting in a false positive test.11,12 In an effort to eliminate this confounding possibility, we used strict criteria for a positive methacholine challenge. Exhaled nitric oxide may also be beneficial in diagnosing asthma in a subset of patients, but these data were not routinely available in this study. Overall, using strict diagnostic criteria, we believe we have identified a population with coexistent VCD and asthma.
In our study, 32.6% of subjects with VCD had concomitant asthma. Given the high prevalence of asthma in the VCD population, one could suggest that screening all patients with VCD for asthma is warranted. The present study examined the relationship between asthma and VCD in more detail and compared subjects with comorbid VCD and asthma, VCD alone, and asthma alone. Two prior studies evaluated these relationships in 95 and 94 subjects, respectively.16,19
Similar to previous studies, our VCD patients (with or without asthma) were more likely to be ▪women and have comorbid GERD or a psychiatric diagnosis.19,20,26,27 Moreover, we observed an association of VCD with irritable bowel syndrome and chronic pain, which has not been previously described. Although Yelken et al reported increased allergy in coexistent asthma and VCD compared with asthmatic patients alone, our study found no difference among the groups, likely reflecting the presence of atopy in the asthmatic population and a known association of allergic rhinitis with VCD.16
Health care use is an important measure in asthma management. ▪Newman et al reported more ED visits in those with VCD alone compared with coexistent VCD and asthma and more hospitalizations in asthmatic patients alone compared with those with coexistent VCD and asthma.19 They also found that VCD patients with coexistent asthma had a trend toward increased medication usage. In our study, those with coexistent VCD and asthma had similar health care usage compared with those with asthma alone, with the exception of increased LABA use. Notably, those with coexistent VCD and asthma had more ED visits, hospitalizations, and exacerbations treated with steroids compared with those with VCD alone, but such findings were not observed when compared with asthmatic patients.
In the study by ▪Newman et al, 44% had VCD alone and were previously misdiagnosed with asthma. This was consistent with our findings that 42.4% of VCD subjects were misdiagnosed with asthma for an average of 9.0 years, slightly higher than the 4.8 years previously published.19 Furthermore, our subanalysis of the VCD population revealed that those with a prior misdiagnosis of asthma had significantly more health care and medication use, which has not been previously reported.
Prior studies suggest that when compared with coexistent VCD and asthma, patients with VCD experience more stridor, and patients with asthma report more chest pain, sputum production, and choking sensation.19 However, our study found that VCD patients with or without asthma compared with asthma alone had significantly more throat tightness, dysphonia, throat clearing, and reflux. Also, we found more wheezing in asthmatic patients with or without VCD compared with VCD alone. Our findings are similar to those of Yelken et al, who found that dysphonia and choking were more common in those with coexistent VCD and asthma compared with asthma alone, and wheezing was more common in asthmatic patients.16
Interestingly, we found nocturnal apnea to be more common in patients with coexistent VCD and asthma than in those with either condition alone, which is a novel observation. Parsons et al previously reported a trend toward an increased prevalence of sleep apnea in patients with coexistent VCD and asthma compared with asthmatic patients, but did not compare sleep apnea in coexistent VCD and asthma versus VCD alone.1 In our study, there was no difference in sleep apnea across groups (data not shown). Exercise was also a common VCD trigger, which has been previously reported.8 However, exercise did not help differentiate VCD patients from asthmatic patients because it was an even more common symptom trigger in those with asthma alone. It is likely that a combination of specific upper airway symptoms along with exercise is needed to distinguish asthma from VCD.
We found that those with VCD with or without asthma compared with asthma alone were more likely to report odors, stress, or inhaler use as triggers for their symptoms, and there was no difference in these triggers between patients with VCD compared with patients with coexistent VCD and asthma. These results suggest that in patients with a confirmed diagnosis of asthma, VCD might be considered a comorbidity if any of the aforementioned symptoms or triggers are present.
Our study has several limitations, including the retrospective nature, and possible selection bias in a tertiary referral center, because our control population is likely overrepresented by subjects with allergic conditions. Although our subjects, based on comparison of four groups, did not differ in age, body mass index, and tobacco use, we did not make a specific attempt to match subjects by any criteria other than the one provider evaluated all subjects, in one location, and over the same time frame. Although we implemented strict criteria for positive methacholine challenge, false positive results are still possible. A single provider performed all laryngoscopies in this study, which should eliminate interobserver differences in the diagnosis of abnormal vocal cord motion or collapse. Laryngoscopies were not performed in the subjects with asthma alone and it therefore remains possible that some of these subjects had coexistent VCD, although their clinical history and available testing did not suggest that was the case. In addition, previous studies have suggested that two phenotypes of VCD may exist, one that is exercise induced and one that is spontaneous.28 Given the general lack of consensus and literature regarding the relationship of VCD and asthma, we chose to focus instead on establishing a clear relationship between the two disorders. Once this relationship is more generally accepted it would be interesting to examine specific phenotypes of VCD associated with asthma. For similar reasons, we did not examine different patterns of upper airway obstruction that have been described, including paradoxical vocal cord movement with adduction on inspiration, paradoxical movement with closure on expiration, and adduction of vocal cords during both inspiration and expiration.
In summary, the results of this study suggest that although those with coexistent VCD and asthma had some increased medication use, there were no differences in key aspects of health care usage, including ED visits, hospitalizations, or exacerbations treated with steroids. Therefore, in subjects with true coexistent VCD and asthma, VCD itself did not confer any increased asthma risk. However, there were key symptoms and triggers that could help distinguish the disorders, which may lead to a more accurate and timely diagnosis. Interestingly, the subpopulation of VCD subjects who had been misdiagnosed with asthma did, in fact, show increased medication and health care use, underscoring the need for adherence to strict criteria when diagnosing either disorder. These findings suggest that the main morbidity associated with VCD may not lie in its inherent disease process, but instead in its ability to mimic asthma, leading to misdiagnosis. Our study represents the largest analysis to date of patients with VCD and asthma. Additional prospective studies are needed to confirm and extend these findings.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1.Parsons JP, Benninger C, Hawley MP, et al. Vocal cord dysfunction: Beyond severe asthma. Respir Med. 2010; 104:504–509. [DOI] [PubMed] [Google Scholar]
- 2.Bisaccioni C, Aun MV, Cajuela E, et al. Comorbidities in severe asthma: Frequency of rhinitis, nasal polyposis, gastroesophageal reflux disease, vocal cord dysfunction and bronchiectasis. Clinics. 2009; 64:769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gimenez LM, Zafra H. Vocal cord dysfunction: An update. Ann Allergy Asthma Immunol. 2011; 106:267–274. [DOI] [PubMed] [Google Scholar]
- 4.Balkissoon R, Kenn K. Asthma: Vocal cord dysfunction (VCD) and other dysfunctional breathing disorders. Semin Respir Crit Care Med. 2012; 33:595–605. [DOI] [PubMed] [Google Scholar]
- 5.Al-Alwan A, Kaminsky D. Vocal cord dysfunction in athletes: Clinical presentation and review of the literature. Phys Sportsmed. 2012; 40:22–27. [DOI] [PubMed] [Google Scholar]
- 6.Freedman MR, Rosenberg SJ, Schmaling KB. Childhood sexual abuse in patients with paradoxical vocal cord dysfunction. J Nerv Ment Dis. 1991; 179:295–298. [DOI] [PubMed] [Google Scholar]
- 7.Husein OF, Husein TN, Gardner R, et al. Formal psychological testing in patients with paradoxical vocal fold dysfunction. Laryngoscope. 2008; 118:740–747. [DOI] [PubMed] [Google Scholar]
- 8.Morris MJ, Christopher KL. Diagnostic criteria for the classification of vocal cord dysfunction. Chest. 2010; 138:1213–1223. [DOI] [PubMed] [Google Scholar]
- 9.Watson MA, King CS, Holley AB, et al. Clinical and lungfunction variables associated with vocal cord dysfunction. Respir Care. 2009; 54:467–473. [PubMed] [Google Scholar]
- 10.Vlahakis NE, Patel AM, Maragos NE, et al. Diagnosis of vocal cord dysfunction: The utility of spirometry and plethysmography. Chest. 2002; 122:2246–2249. [DOI] [PubMed] [Google Scholar]
- 11.Guss J, Mirza N. Methacholine challenge testing in the diagnosis of paradoxical vocal fold motion. Laryngoscope. 2006; 116:1558–1561. [DOI] [PubMed] [Google Scholar]
- 12.Perkins PJ, Morris MJ. Vocal cord dysfunction induced by methacholine challenge testing. Chest. 2002; 122:1988–1993. [DOI] [PubMed] [Google Scholar]
- 13.Treole K, Trudeau MD, Forrest LA. Endoscopic and stroboscopic description of adults with paradoxical vocal fold dysfunction. J Voice. 1999; 13:143–152. [DOI] [PubMed] [Google Scholar]
- 14.Holmes PW, Lau KK, Crossett M, et al. Diagnosis of vocal cord dysfunction in asthma with high resolution dynamic volume computerized tomography of the larynx. Respirology. 2009; 14:1106–1113. [DOI] [PubMed] [Google Scholar]
- 15.Meltzer EO, Blaiss MS, Nathan RA, et al. Asthma burden in the United States: Results of the 2009 Asthma Insight and Management survey. Allergy Asthma Proc. 2012; 33:36–46. [DOI] [PubMed] [Google Scholar]
- 16.Yelken K, Yilmaz A, Guven M, et al. Paradoxical vocal fold motion dysfunction in asthma patients. Respirology. 2009; 14:729–733. [DOI] [PubMed] [Google Scholar]
- 17.Low K, Lau KK, Holmes P, et al. Abnormal vocal cord function in difficult-to-treat asthma. Am J Respir Crit Care Med. 2011; 184:50–56. [DOI] [PubMed] [Google Scholar]
- 18.Jain S, Bandi V, Officer T, et al. Role of vocal cord function and dysfunction in patients presenting with symptoms of acute asthma exacerbation. J Asthma. 2006; 43:207–212. [DOI] [PubMed] [Google Scholar]
- 19.Newman KB, Mason UG, Schmaling KB. Clinical features of vocal cord dysfunction. Crit Care Med. 1995; 152:1382–1386. [DOI] [PubMed] [Google Scholar]
- 20.Gurevich-Uvena J, Parker JM, Fitzpatrick TM, et al. Medical comorbidities for paradoxical vocal fold motion (vocal cord dysfunction) in the military population. J Voice. 2010; 24:728–731. [DOI] [PubMed] [Google Scholar]
- 21.Busse WW, Boushe HA, Camargo CA, et al. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma summary report. J Allergy Clin Immunol. 2007; 120:S94–S138. 2007. [DOI] [PubMed] [Google Scholar]
- 22.Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: Asthma control and exacerbations: Standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009; 180:59–99. [DOI] [PubMed] [Google Scholar]
- 23.Christopher KL, Wood RP, Ecker R, et al. Vocal-cord dysfunction presenting as asthma. N Engl J Med. 1983; 308:1566–1570. [DOI] [PubMed] [Google Scholar]
- 24.Collett PW, Brancatisano T, Engel LA. Changes in the glottic aperture during bronchial asthma. Am Rev Respir Dis. 1983; 128:719–723. [DOI] [PubMed] [Google Scholar]
- 25.Elshami AA, Tino G. Coexistent asthma and functional upper airway obstruction: Case reports and review of the literature. Chest. 1996; 110:1358–1361. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim WH, Gheriani HA, Almohamed AA, et al. Paradoxical vocal cord motion disorder: Past, present and future. Postgrad Med J. 2007; 83:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell DM, Karanfilov BI, Beechler KB, et al. Paradoxical vocal cord dysfunction in juveniles. Arch Otolaryngol Head Neck Surg. 2000; 126:29–34. [DOI] [PubMed] [Google Scholar]
- 28.Doshi DR, Weinberger MM. Long-term outcome of vocal cord dysfunction. Ann Allergy Asthma Immunol. 2006; 96:794–799. [DOI] [PubMed] [Google Scholar]