Abstract
Introduction:
Seasonal malaria chemoprevention (SMC) with Sulfadoxine pyrimethamine plus amodiaquine (SP + AQ) consist of a monthly administration of therapeutic dose to children under five years of age during the high risk of malaria in area where malaria is highly seasonal. According to SMC recommendation, both non-infected and asymptomatic Plasmodium falciparum infected children will receive similar treatment. The gap in our knowledge is how the effect of asymptomatic infection on the efficacy of SMC in preventing clinical malaria over a four-week period. Thus, this study aimed to assess the risk of clinical malaria and its association with children’s infection status when SMC treatment is given.
Methodology:
The study was carried out in the Koulikoro health district in Mali and concerned children under 10 years of age. A total of 726 and 1452 children were randomly selected and followed over the SMC campaign in the years 2019 and 2020 respectively. Prevalence of asymptomatic P. falciparum infection was determined each round by microscopy before SMC drugs intake. Children were passively followed over a four-week period to determine incidence of clinical malaria. R-Studio software was used for analysis. The risk of clinical malaria by infection status was estimated using a logistic regression. A Kaplan-Meier curve was used to determine the survival time between infected and uninfected children. The Pearson Chi-square test was used to compare proportions with the significant level at p< 0.05.
Results:
The average prevalence of asymptomatic infection was 11.0% both years, and it was higher among children aged 5 to 9 years old in 2019 (p<0.001) and 2020 (p=0.016). The risk of clinical malaria was significantly higher among asymptomatic infected children 2019: (RR=3.05, CI [2.04–4.72]) and 2020 (RR=1.43, CI [1.04–1.97]) transmission seasons. Likewise, the time of the first malaria occurrence was statistically lower among infected children regardless the year (p<0.001 in 2019 and p=0.01 in 2020).
Conclusion:
Results show a high risk of clinical malaria in asymptomatic infected children during SMC delivery. Screening for P. falciparum infection before the SMC treatment could significantly enhance the impact of the strategy on malaria morbidity in endemic areas.
Background
Seasonal malaria chemoprevention (SMC) has been recommended by the World Health Organization (WHO) for malaria prevention in children under five years in Sub-Saharan African countries where the transmission is highly seasonal since 2012. It involves the regular administration of antimalarial drugs on a monthly basis for a duration of three days, namely during periods characterized by elevated malaria transmission rates, which typically occur over a span of three to five months annually[1, 2]. Progressively, SMC has been deployed in specific regions of Mali as pilot studies from 2012 to 2015, then the strategy becomes in 2016 a countrywide malaria prevention tools for children less than five years. Several studies have shown that SMC significantly reduces burden by reducing malaria-related morbidity, mortality and malaria anemia [1–5]. Despite the proven effectiveness of the strategy, malaria remains the most common and deadly disease in Mali with 3,204,275 confirmed cases and fatality rate at 1.4%0 in 2022 according to the National Malaria Control Report (NMCP) [6]. Asymptomatic carriage of parasites consists in the absence of clinical manifestations despite the presence of parasites in the blood. These individuals are very important for transmission because they constitute a reservoir of parasites [7–11].
As per WHO recommendations, only symptomatic children must be tested for malaria before the intake of SP + AQ while malaria RDT test is not required in the absence of symptom. Thus, in malaria endemic area, a significant proportion of asymptomatic infection may receive SMC without knowing their infection status. Since the implementation of SMC as community intervention in malaria endemic region of sub-Saharan Africa, fewer studies have assessed the possible impact of asymptomatic infection on the success of the strategy to protect against clinical malaria among eligible children. that SMC could have on the success of taking drugs in asymptomatic infected children. A risk assessment of the incidence of clinical malaria with respect to infection status at the time treatment is given could help explain the occurrence of the disease among children within four weeks after receiving SMC treatment. This study conducted during the SMC campaign will try to estimate the risk of presenting with malaria symptoms plus a positive RDT within four weeks after completing SMC treatment among eligible children living in Koulikoro health district of Mali. Result could inform on how giving SMC only to non-infected children could increase the likelihood of not having clinical malaria among treated population in endemic area.
Methods
Study sites.
The study was carried out in the health district of Koulikoro located in the tropical zone of Mali, at 60 kilometers from the capital Bamako, Within the district there is different ecological patterns leading to different length of the malaria transmission season (from 4 to 5 months a year). A total of nine (9) villages, both having a community health center and representing both ecological patterns. Sirakorola, Chola, Monzombala were in the dry area with a short transmission season (3 months), Doumba, Sinzani, Koula with a transmission season over four months, Gouni, Kenenkou and Kamani, located along the river; with a transmission season lasting for five months (Fig. 1).
Figure 1.
Study site
Study population.
The target population was those eligible for SMC (children aged 3 months to less than 10 years). After a census enumeration of each village, the total population size of the 9 villages was approximately 27,867 with 6,326 and 6,638 children eligible for SMC in 2019 and 2020 respectively.
Sampling
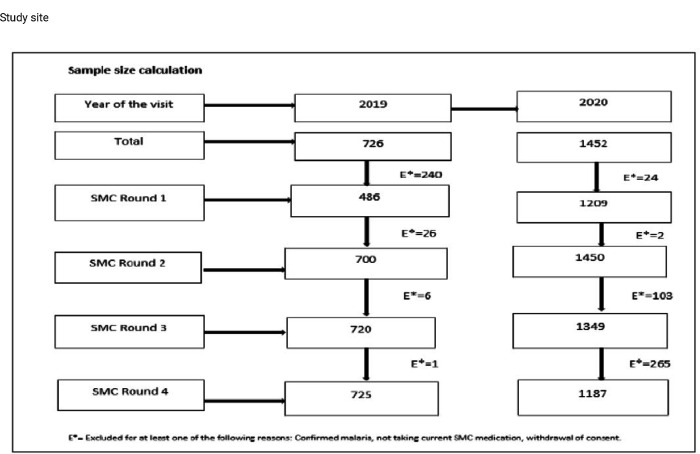
Prior to the SMC season, within all villages, parents, or guardians of children under 10 years of age eligible for SMC were asked for voluntary consent and only upon completing a signed consent form, children were enrolled and given a unique identifying number for the study. A sub sample of 726 and 1452 children enrolled respectively in 2019 and 2020 was then chosen randomly per village to be tested for asymptomatic P. falciparum infection by microscopy before the SMC drug administration. The Fig. 2 (Fig. 2) shows the sample size estimation per year and per month.
Figure 2.
Sample size calculation
The sample size was 726 children in 2019 and for more statistical power, this size was double in year 2020 for a total of 1452 children. Monthly sample size was calculated based on the seasonal variation of asymptomatic malaria prevalence in the study area.
Data collection and collection tools
Before each SMC delivery, parasite assessment was done for each participant and the follow up consisted of a passive case detection of clinical malaria at the community health center. Each year, the study was conducted from July (first SMC round) to November (a month after the last SMC round) Sociodemographic, clinical symptoms as well as malaria RDT test and smear were done at each visit. Electronic data capture was used to collect data through the Redcap platform and synchronized daily.
Statistical analysis
Redcap data were exported as an Excel file for further analysis in the statistical program R version 4.2. To compare percentages, we used the Chi-squared test, and to analyze risk across groups for infection, we utilized logistic regression. Statistical significance was assumed when the p-value was less than 0.05. The Kaplan Meier method was then used to estimate how long it would be before the first clinical malaria episode occurred after SMC therapy.
Results
Descriptive of study population.
On average, children under five years old accounted for 52.9% (1394/2631), while those aged 5–9 years old were 47.1% (1237/2631) in 2019. These proportions were 48.0% (2494/5195) and 52.0% (2701/5195) for children aged 3–59 months and 5–9 years respectively in 2020 (Table 1).
Table 1.
Socio-demographic characteristics
| Characteristic | 2019 | 2020 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| July = 486 | August, n = 700 | September, n = 720 | October, n = 725 | Overall, N = 2,631 | July, n = 1.209 | August, n = 1.450 | September, n = 1.349 | October, n = 1.187 | Overall, N = 5,195 | |
| n (%) | n (%) | n (%) | n (%) | n(%) | n (%) | n (%) | n(%) | n (%) | n (%) | |
| GENDER | ||||||||||
| Male | 253 (52.1) | 371 (53.0) | 396 (55.0) | 377 (52.0) | 1,394 (52.9) | 677 (55.9) | 754 (52.0) | 728 (53.9) | 617 (51.9) | 2,753 (52.9) |
| Female | 233 (47.9) | 329 (47.0) | 324 (45.0) | 348 (48.0) | 1,237 (47.1) | 532 (44.1) | 696 (48.0) | 621 (46.1) | 570 (48.1) | 2,442 (47.1) |
| Age | ||||||||||
| Under 5 y.o | 252 (51.85) | 399 (57.0) | 382 (53.0) | 377 (52.0) | 1,394 (52.9) | 556 (45.90) | 696(48.0) | 661 (48.9) | 605(50.9) | 2,494(48.0) |
| 5–9 y.o | 234 (48.15) | 301 (43.0) | 338 (47.0) | 348 (48.0) | 1,237 (47.1) | 653 (54.1) | 754 (52.0) | 688 (51.1) | 582 (49.1) | 2,701 (52.0) |
The prevalence of Plasmodium falciparum asymptomatic infection by month, age group and year.
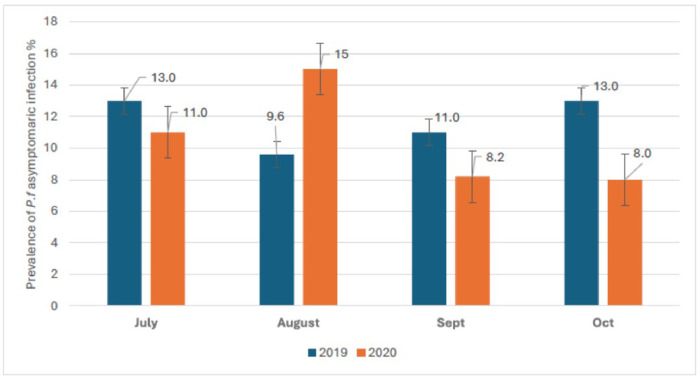
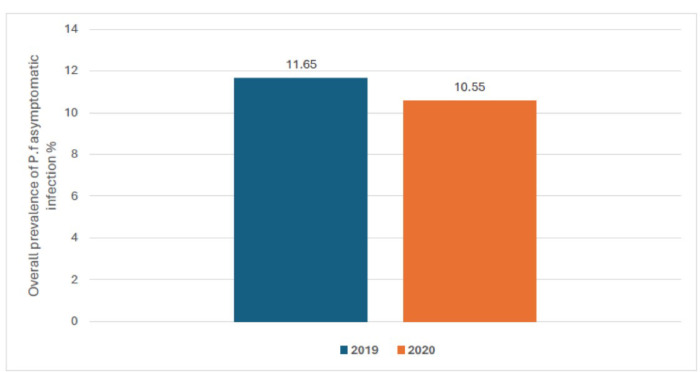
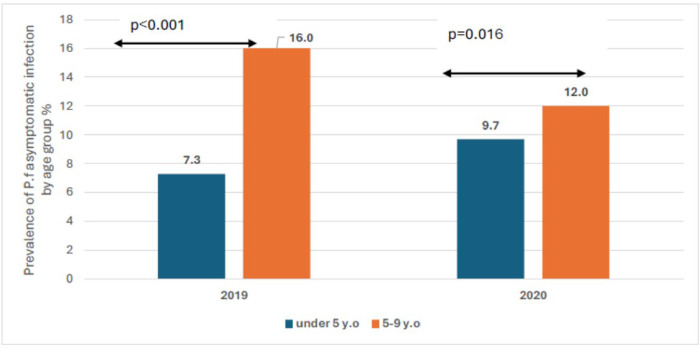
The prevalence of Plasmodium falciparum asymptomatic infection was respectively 13.0%, 9.6%, 11.0% and 13.0% in July, august, September and October of 2019. It was 11.0%, 15.0%, 8.2%, and 8.0% respectively in July, august, September and October of 2020 (Fig. 3). The overall prevalence of malaria infection during the season was 11.65% in 2019 and 10.55% in 2020 (Fig. 4). Regardless of the year, the age-specific prevalence shows significantly high prevalence of asymptomatic infection among older children aged 5 to 9 years old compared to children under five years old. We observed respectively 7.3% vs. 16.0% in year 2019 (p < 0.001) and 9.7% vs. 12.0% in year 2020 (p = 0.016) (Fig. 5).
Figure 3.
Prevalence of asymptomatic P. falciparumcarriage by SMC round by year.
Figure 4.
overall prevalence of asymptomatic P. falciparum carriage by year
Figure 5.
overall age specific prevalence of asymptomatic P. falciparum carriage per year
The overall incidence of clinical malaria according to asymptomatic Plasmodium falciparum carriage prior to SMC medications.
The average cumulative incidence of clinical malaria was 23.58% among asymptomatic children compared to 7.47% among healthy children in 2019 (RR = 3.16, 95% CI [2.49–40]; p < 0.001). In 2020, the cumulative clinical incidence of malaria was 3.60% among asymptomatic children and 5.19% among healthy children (RR = 1.45; 95% CI [1.12–2.16]; p = 0.047) (Table 2).
Table 2.
The overall incidence of clinical malaria according to asymptomatic Plasmodium falciparum carriage prior to SMC medications.
| Year of visit | P. falciparum Infection status before SMC by microscopy | Cumulative malaria Incidence per 100 during SMC season | RR 95% [IC] | P |
|---|---|---|---|---|
| 2019 | Negative | 7.47 | <0.001 | |
| Positive | 23.58 | 3.16[2.49–40] | ||
| 2020 | Negative | 3.60 | 0.047 | |
| Positive | 5.19 | 1.45[1.12–2.16] | ||
| List of fi gures | ||||
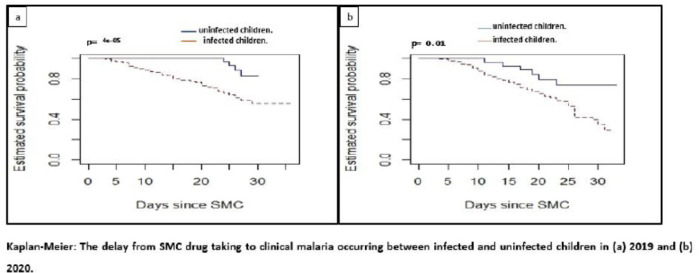
The likelihood of becoming clinically symptomatic over time by asymptomatic statute before SMC.
Survival curves between the two groups based on infection status prior to the first SMC treatment are presented in Figs. 6 and 7. Approximately 50.0% (2019) and 75.0% (2020) of infected children show up at the clinic within four weeks with clinical malaria after receiving SMC treatment. In both years, children who were smear negative had a higher survival probability compared to those who were smear positive. Children who have taken the SMC and are infected are more likely to have clinical malaria than those who were not infected when they took the SMC (p < 0.001 in 2019 and p = 0.01 in 2020).
Figure 6.
Kaplan-Meier: The delay from SMC drug taking to clinical malaria occurring between infected and uninfected children in (a) 2019 and (b) 2020.
Discussions
Methodological approaches
SMC remains an important intervention by reducing malaria-related morbidity and mortality among target population in seasonal transmission areas. Asymptomatic P. falciparum carriage play important role in the maintain of malaria transmission and thus, could slow down the progress toward elimination [11–16]. As SMC strategy did not recommend a test-to-treat approach for all eligible children, those with no malaria like symptom at the administration of SMC may receive SP + AQ while carrying the parasite sometimes at low density in their blood. Our study explored the risk of clinical malaria after SMC treatment with respect to infection status among children less than 10 years old during two malaria transmission seasons.
The prevalence of asymptomatic malaria parasitemia
Our data shows that while SMC is widely implemented with significantly high coverage rates reported in countries like Mali, a non-negligible proportion of children receiving SMC treatment (about 11.0%) are already carrying P. falciparum without any symptoms. Studies have shown that in malaria-endemic countries, there is a high prevalence of asymptomatic carriage of P. falciparum [9, 17–21] and that this parasitemia remains detectable in some children despite the administration of SP plus AQ during SMC [22].
Prevalence was statistically higher among older children compared to those less than 5 years old as reported elsewhere and defined as an age-shift in malaria prevalence and incidence in sub–Saharan Africa with older children being more at risk. Similar observations were made in Gamby by Ahmad and al. [20] and in Mali by Tran TM. and al [23], Toure M. and al.[24] and Coulibaly D. and al. [25].
Clinical malaria incidence after taking seasonal malaria chemoprevention drugs and asymptomatic malaria parasitemia carriage before
Asymptomatic infection prior to SMC delivery was significantly associated with high risk of clinical malaria over the next four weeks as shown here. While SP + AQ remain effective to prevent malaria in Mali, they are not recommended as first or second line by the National Malaria Control Program (NMCP). Thus, one can state that this combination by failing to clear asymptomatic infection reservoir, will not prevent from developing clinical malaria within the next four weeks. The survival analysis shows a short time between SMC treatment and symptoms appearance among children infected while receiving SP + AQ compared to those not infected. It is known that one of the principles of SMC is to maintain an optimal concentration of SP and AQ over four weeks leading to prevent any new infection during this period [2, 26], however the efficacy of SP + AQ as a therapy in most endemic countries for Plasmodium falciparum malaria has not been well studied since the implementation of SMC, as is the case with its widely recognized effectiveness as a chemoprevention agent [27–29]. Furthermore, malaria parasite sensitivity to SMC drugs as well as the parasite load in blood at the time of SMC treatment could impact the drugs metabolite and the pharmacokinetics leading to short protection[22]. Parasite pressure on SMC drugs is also known to reduce its concentration on plasma of asymptomatic infected children which can lead to a reduce protection against clinical malaria by reducing the strength of prophylaxis inducing by par SMC drugs[22, 30, 31].
Conclusion
Asymptomatic infection remains significant among SMC eligible children in malaria endemic area and risk of clinical malaria is significantly high among infected children. Mass drug administration of antimalarial treatment aiming to clear parasite reservoir before the first round of SMC campaign in this area could significantly reduce the disease burden during the transmission season.
Acknowledgements
We thank the ICEMR program team, the community of Dangassa, and Mali’s NMCP. We would also like to thank the Fogarty International Center of the National Institutes of Health of the United States for the support given to Dr Daouda Sanogo under Grant D43TW008652.
Funding
This study was supported by the National Institutes of Health Cooperative Agreements 3U19AI129387-03S1 for the International Center of Excellence for Malaria Research (ICEMR).
Abbreviations
- SMC
Seasonal Malaria Chemoprevention
- SP+AQ
Sulfadoxine-Pyrimethamine+ Amodiaquin
- RDT
Rapid Diagnostic Test
- CI
Confidence Interval
- P. falciparum
Plasmodium falciparum
- WHO
World Health Organization
- RR
Relative Risk
- NMCP
National Malaria Control Program
Footnotes
Conflict of interest
The authors declare that they have no competing interests.
Declarations
Ethical aspects and consent to participate.
The Ethic Committee of the University of Sciences, techniques, and Technologies of Bamako (USTTB) granted ethical approval for this study (N°2021/108/CE/FMPOS). All parents or guardians of individuals provided written informed permission. Furthermore, prior to the recruitment in the research, written consent was acquired from the children’s parents.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Daouda Sanogo, University of Sciences, Techniques and Technologies of Bamako.
Mahamoudou Toure, University of Sciences, Techniques and Technologies of Bamako.
Moussa Keita, University of Sciences, Techniques and Technologies of Bamako.
Fousseyni Kane, University of Sciences, Techniques and Technologies of Bamako.
Soumba Keita, University of Sciences, Techniques and Technologies of Bamako.
Ibrahim Sanogo, University of Sciences, Techniques and Technologies of Bamako.
Sory Ibrahim Diawara, University of Sciences, Techniques and Technologies of Bamako.
Hamady Coulibaly, University of Sciences, Techniques and Technologies of Bamako.
Sidibé M’Baye Thiam, University of Sciences, Techniques and Technologies of Bamako.
Mahamadou Diakite, University of Sciences, Techniques and Technologies of Bamako.
Nafomon Sogoba, University of Sciences, Techniques and Technologies of Bamako.
Seydou Doumbia, University of Sciences, Techniques and Technologies of Bamako.
References
- 1.World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organisation; 2020. Licence CC-BY-NC-SA 3 0 IGO [Internet]. [cited 2021 Jun 10]. Available from: https://cdn.who.int/media/docs/default-source/malaria/world-malaria-reports/world-malaria-report-2020-briefing-kit-fre.pdf?sfvrsn=69c55393_9 [Google Scholar]
- 2.World Health Organization. World Health Organization. (2012). WHO policy recommendation: seasonal malaria chemoprevention (SMC) for plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub-region in Africa. World Health Organization. [Internet]. World Health Organization; 2012. Report No.: WHO/HTM/GMP/2012.02. Available from: https://apps.who.int/iris/handle/10665/337978 [Google Scholar]
- 3.Konaté D, Diawara SI, Touré M, Diakité SAS, Guindo A, Traoré K, et al. Effect of routine seasonal malaria chemoprevention on malaria trends in children under 5 years in Dangassa, Mali. Malar J. 2020;19:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Druetz T, Corneau-Tremblay N, Millogo T, Kouanda S, Ly A, Bicaba A, et al. Impact Evaluation of Seasonal Malaria Chemoprevention under Routine Program Implementation: A Quasi-Experimental Study in Burkina Faso. Am J Trop Med Hyg. 2018;98:524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diawara F, Steinhardt LC, Mahamar A, Traore T, Kone DT, Diawara H, et al. Measuring the impact of seasonal malaria chemoprevention as part of routine malaria control in Kita, Mali. Malar J. 2017;16:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Programme National de Lutte contre le Paludisme du Mali. Rapport Annuel 2022 sur le Paludisme au Mali. [Internet]. Programme National de Lutte contre le Paludisme; Available from: https://pnlp.ml/ [Google Scholar]
- 7.Rasamoel P, Jambou R, Ralamboranto L, Raharimalala L, Roux J. Portage asymptomatique et accès palustre: un équilibre complexe. 2021;
- 8.Frimpong A, Amponsah J, Adjokatseh AS, Agyemang D, Bentum-Ennin L, Ofori EA, et al. Asymptomatic Malaria Infection Is Maintained by a Balanced Pro- and Anti-inflammatory Response. Front Microbiol. 2020;11:559255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontecha G, Maradiaga A, García J, Mejía-Torres R, Escober L, Matamoros J, et al. Asymptomatic Malaria Infections in an Endemic City of Honduras. Human Parasitic Diseases. 2016;37. [Google Scholar]
- 10.Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12:833–40. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad A, Mohammed NI, Joof F, Affara M, Jawara M, Abubakar I, et al. Asymptomatic Plasmodium falciparum carriage and clinical disease: a 5-year community-based longitudinal study in The Gambia. Malar J. 2023;22:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12:833–40. [DOI] [PubMed] [Google Scholar]
- 13.Babiker HA, Gadalla AAH, Ranford-Cartwright LC. The role of asymptomatic P. falciparum parasitaemia in the evolution of antimalarial drug resistance in areas of seasonal transmission. Drug Resist Updat. 2013;16:1–9. [DOI] [PubMed] [Google Scholar]
- 14.Chen I, Clarke SE, Gosling R, Hamainza B, Killeen G, Magill A, et al. “Asymptomatic” Malaria: A Chronic and Debilitating Infection That Should Be Treated. PLoS Med. 2016;13:e1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caroline A, Hannah F, Richard T-L, Safiatou D, Nf L, C A, et al. Increased circulation time of Plasmodium falciparum underlies persistent asymptomatic infection in the dry season. Nature medicine [Internet]. 2020. [cited 2021 Aug 12];26. Available from: https://pubmed.ncbi.nlm.nih.gov/33106664/ [DOI] [PubMed] [Google Scholar]
- 16.Heinemann M, Phillips R, Vinnemeier C, Rolling C, Tannich E, Rolling T. High prevalence of asymptomatic malaria infections in adults, Ashanti Region, Ghana, 2018. Malaria Journal. 2020;19:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mabunda S, Aponte JJ, Tiago A, Alonso P. A country-wide malaria survey in Mozambique. II. Malaria attributable proportion of fever and establishment of malaria case definition in children across different epidemiological settings. Malaria Journal. 2009;8:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vafa M, Troye-Blomberg M, Anchang J, Garcia A, Migot-Nabias F. Multiplicity of Plasmodium falciparum infection in asymptomatic children in Senegal: relation to transmission, age and erythrocyte variants. Malaria Journal. 2008;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akindeh NM, Ngum LN, Niba PTN, Ali IM, Ayem OLO, Chedjou JPK, et al. Assessing Asymptomatic Malaria Carriage of Plasmodium falciparum and Non-falciparum Species in Children Resident in Nkolbisson, Yaoundé, Cameroon. Children (Basel). 2021;8:960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad A, Mohammed NI, Joof F, Affara M, Jawara M, Abubakar I, et al. Asymptomatic Plasmodium falciparum carriage and clinical disease: a 5-year community-based longitudinal study in The Gambia. Malar J. 2023;22:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayuma P, Wang C, Liheluka E, Baraka V, Madebe R, Minja T, et al. Prevalence of asymptomatic malaria, submicroscopic parasitaemia and anaemia in Korogwe District, north-eastern Tanzania. Malaria Journal. 2021;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somé FA, Bazié T, Ehrlich HY, Goodwin J, Lehane A, Neya C, et al. Investigating selected host and parasite factors potentially impacting upon seasonal malaria chemoprevention in Bama, Burkina Faso. Malaria Journal. 2020;19:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran TM, Li S, Doumbo S, Doumtabe D, Huang C-Y, Dia S, et al. An Intensive Longitudinal Cohort Study of Malian Children and Adults Reveals No Evidence of Acquired Immunity to Plasmodium falciparum Infection. Clin Infect Dis. 2013;57:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Touré M, Sanogo D, Dembele S, Diawara SI, Oppfeldt K, Schiøler KL, et al. Seasonality and shift in age-specific malaria prevalence and incidence in Binko and Carrière villages close to the lake in Selingué, Mali | Malaria Journal | Full Text. Malar J [Internet]. [cited 2023 Sep 7]; Available from: 10.1186/s12936-016-1251-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coulibaly D, Guindo B, Niangaly A, Maiga F, Konate S, Kodio A, et al. A Decline and Age Shift in Malaria Incidence in Rural Mali following Implementation of Seasonal Malaria Chemoprevention and Indoor Residual Spraying. The American Journal of Tropical Medicine and Hygiene. 2021;104:1342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Seasonal malaria chemoprevention with sulfadoxine–pyrimethamine plus amodiaquine in children: a field guide [Internet]. Chimioprévention du paludisme saisonnier par administration de sulfadoxine-pyriméthamine et d’amodiaquine aux enfants : guide de terrain. Geneva: World Health Organization; 2013. [cited 2022 Jun 10]. Available from: https://apps.who.int/iris/handle/10665/85726 [Google Scholar]
- 27.Zongo I, Dorsey G, Rouamba N, Tinto H, Dokomajilar C, Guiguemde RT, et al. Artemether-lumefantrine versus amodiaquine plus sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Burkina Faso: a randomised non-inferiority trial. The Lancet. 2007;369:491–8. [DOI] [PubMed] [Google Scholar]
- 28.Zongo I, Dorsey G, Rouamba N, Dokomajilar C, Lankoande M, Ouedraogo J-B, et al. AMODIAQUINE, SULFADOXINE-PYRIMETHAMINE, AND COMBINATION THERAPY FOR UNCOMPLICATED FALCIPARUM MALARIA: A RANDOMIZED CONTROLLED TRIAL FROM BURKINA FASO. The American Journal of Tropical Medicine and Hygiene. 2005;73:826–32. [PubMed] [Google Scholar]
- 29.Somé AF, Séré YY, Dokomajilar C, Zongo I, Rouamba N, Greenhouse B, et al. Selection of Known Plasmodium falciparum Resistance-Mediating Polymorphisms by Artemether-Lumefantrine and Amodiaquine-Sulfadoxine-Pyrimethamine but Not Dihydroartemisinin-Piperaquine in Burkina Faso. Antimicrobial Agents and Chemotherapy. 2010;54:1949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chotsiri P, White NJ, Tarning J. Pharmacokinetic considerations in seasonal malaria chemoprevention. Trends in Parasitology. 2022;38:673–82. [DOI] [PubMed] [Google Scholar]
- 31.White NJ. Does antimalarial mass drug administration increase or decrease the risk of resistance? The Lancet Infectious Diseases. 2017;17:e15–20. [DOI] [PubMed] [Google Scholar]