Abstract
Cucumber mosaic virus (CMV) has been divided into two subgroups based on serological data, peptide mapping of the coat protein, nucleic acid hybridization, and nucleotide sequence similarity. Analyses of a number of recently isolated strains suggest a further division of the subgroup I strains. Alignment of the 5′ nontranslated regions of RNA 3 for 26 strains of CMV suggests the division of CMV into subgroups IA, IB, and II and suggests that rearrangements, deletions, and insertions in this region may have been the precursors of the subsequent radiation of each subgroup. Phylogeny analyses of CMV using the coat protein open reading frame of 53 strains strongly support the further division of subgroup I into IA and IB. In addition, strains within each subgroup radiate from a single point of origin, indicating that they have evolved from a single common ancestor for each subgroup.
Cucumber mosaic virus (CMV), genus Cucumovirus, family Bromoviridae, is a positive-sense RNA plant virus with a tripartite genome (for a review see reference 12). RNAs 1 and 2 encode the nonstructural proteins involved in viral replication. RNA 3 encodes a movement protein and the coat protein (CP), which is translated from a subgenomic mRNA, RNA 4. A fifth open reading frame (ORF), the 2b ORF, is also encoded on RNA 2. It has been implicated in virus movement and symptom severity (3–5). CMV has an extremely broad host range (approximately 1,000 species), and numerous strains of CMV have been described. Serological data, peptide mapping of the CP, and nucleic acid hybridization divided CMV strains into two subgroups, designated I and II. Sequence analysis of a representative strain from each subgroup verified the designations (12). Over the past several years a large number of additional strains have been described and partially sequenced, and recent analysis of the CP genes of several subgroup I strains (2) suggests that they can be further divided into two groups.
While divergence and speciation of RNA viruses probably occurs rapidly and frequently, little is known about the mechanisms of these events (14). Clearly, rapid mutation rates can play a role (6, 7), but other events like reassortment in viruses with divided genomes and RNA-RNA recombination are probably also important driving forces in RNA virus evolution (9, 15). Previously it was shown that phylogenetic estimates for the Cucumovirus genus were different when the ORFs from different RNAs were used for analysis, suggesting that reassortment of the three genomic segments may have played an important role in the speciation of this genus (16). A naturally occurring reassortant between CMV and Peanut stunt virus (PSV), genus Cucumovirus, supported this theory (16). Although speculation about the role of RNA recombination in the speciation of RNA viruses has been common, the capacity for rapid change by mutation in RNA virus genomes has made the footprints of previous recombination events difficult to uncover. In one study comparing two members of the genus Bromovirus, family Bromoviridae, however, evidence for past recombination events in the 5′ nontranslated regions (NTRs) of RNAs 3 was noted (1). Among the Bromoviridae, the 5′ NTRs of RNAs 1 and 2 are conserved, both within and between viruses of the same species. The 5′ NTRs of RNAs 3 are less similar to the 5′ NTRs of RNAs 1 and 2 of the same virus but are conserved between viruses.
In this study, the 5′ NTRs of RNA 3 for 26 strains of CMV were aligned. The NTRs fall into three groups, one corresponding to subgroup II strains and two corresponding to subgroup I strains, suggesting further division of the subgroup I strains into IA and IB.
To confirm the subgrouping, a phylogenetic analysis was done on CMV strains. Most of the sequence data available for CMV are for the CP gene. A limited number of strains have had the entire RNA 3 analyzed, and still fewer have had the complete sequence of RNAs 1 and 2 determined. All previous taxonomic considerations for CMV have been based on the CP. Here we used the sequences of the CP genes of 53 strains of CMV and of the CP gene from the ER strain of PSV (11), another Cucumovirus species, to estimate the phylogenetic relationships of CMV. The analysis clearly supports the same subdivision of subgroup I strains into IA and IB groupings as seen in the 5′ NTR analysis.
MATERIALS AND METHODS
Source of sequence data.
Sequence data for RNA 3 and the CP were obtained from GenBank (accession numbers are shown in Table 1). The cloning of LS-CMV has been described elsewhere (17). The cDNA clone of RNA 3 was sequenced by standard dideoxy sequencing techniques using Sequenase (U.S. Biochemical). K-CMV RNA 3 was cloned by using a cDNA cloning kit (Amersham) and a primer specific to the 3′ end of subgroup I CMV strains. The 5′ 642 nucleotides of the K-CMV RNA 3 cDNA clone (pK302) were derived by reverse transcription-PCR using avian myeloblastosis virus reverse transcriptase, 30 thermocycles with Taq polymerase, primer NheI (5′CACGCTAGCTGTGGTACCGG3′), and 5′-terminal primer (BamHI site; T7 RNA polymerase promoter-GTAATCTTACCACTG). Plasmid pK302 was sequenced by using a Taq DyeDeoxy Terminator cycle sequencing kit (Perkin-Elmer/Applied Biosystems, Foster City, Calif.). The products of the reaction were separated electrophoretically, and the data were processed by an ABI model 373A automated DNA sequencer (Perkin-Elmer/Applied Biosystems). DNA sequences were assembled and edited with the PC/Gene program ASSEMGEL (IntelliGenetics, Mountain View, Calif.).
TABLE 1.
Strain abbreviations and GenBank accession numbers
| Abbreviation | Accession no. | Strain | Country of origina |
|---|---|---|---|
| Fny | D10538 | Fny-CMV | United States (NY) |
| Sny-CMV | U66094 | Sny-CMV | United States (NY) |
| M | D10539 | M-CMV | United Kingdom |
| E5 | D42080 | E5-CMV | Japan |
| Y | D12499 | Y-CMV | Japan |
| Leg | D16405 | Legume CMV | Japan |
| O | D00385 | O-CMV | Japan |
| CS | D28489 | Cs-CMV | Japan |
| D8 | AB004781 | D8-CMV | Japan |
| Km | AB004780 | Km-CMV | [Japan] |
| N | D28486 | N-CMV | Japan |
| Pepo2 | D28488 | Pepo strain 2 | Japan |
| Ft | D28487 | Ft-CMV | Japan |
| Kor | L36251 | Korean CMV | Korea |
| NT9 | D28780 | NT9-CMV | Taiwan |
| SD | AB008777 | SD-CMV | [China] |
| As | AF013291 | As-CMV | Korea |
| M48 | D49496 | M48-CMV | [Taiwan] |
| C | D00462 | C-CMV | United States (NY) |
| Ny | U22821 | Ny-CMV | [Australia] |
| I17F | X16386 | I17F-CMV | France |
| Ban | U43888 | Banana-CMV | [Israel] |
| C7-2 | D42079 | C7-2-CMV | Japan |
| Musa | U32859 | Musa-CMV | [Columbia] |
| Plant | U32858 | Plantain-CMV | [Columbia] |
| Ix | U20219 | Ixora CMV | Philippines |
| ChCu | X65017 | Chinese cucumber CMV | China |
| P6 | D10545 | Price’s 6 CMV | United Kingdom |
| Hi | U31219 | Hawaii CMV | United States (HI) |
| FC | D10544 | Fulton’s C CMV | United States (AR) |
| Pepo | D43800 | Pepo CMV | Japan |
| PR50 | M98501 | PuertoRico 50 CMV | United States (PR) |
| ABI | L36525 | ABI-CMV | Korea |
| P1 | AJ006988 | P1 CMV | [China] |
| PR36 | M98500 | PuertoRico 36 CMV | United States (PR) |
| PR1 | M98499 | PuertoRico 1 CMV | United States (PR) |
| RB | AJ006990 | RB-CMV | [China] |
| PhyM | X89652 | Physalis CMV | [India] |
| Oahu | U31220 | Oahu-CMV | United States (HI) |
| Kin | Z12818 | Kin-CMV | Scotland |
| M2 | AB006813 | M2-CMV | [Japan] |
| Q | M21464 | Q-CMV | Australia |
| Trk7 | L15336 | Trk-7 CMV | Hungary |
| K | AF127977 | K-CMV | China |
| LS | AF127976 | LS-CMV | United States (NY) |
| S | AF063610 | S-CMV | South Africa |
| WL | D00463 | Wl-CMV | United States (NY) |
| SN | U22822 | Sn-CMV | [Australia] |
| DRKD | U10922 | Drkd-CMV | United States (AR) |
| SP104 | U10924 | Spinach 104 CMV | United States (AR) |
| SP103 | U10923 | Spinach 103 CMV | United States (AR) |
| Wem | L40953 | Wem CMV | Unknown |
Individual states or territories in the United States are shown in parentheses in standard postal code abbreviation; countries shown in brackets are locations of the researchers reporting the sequence, where no description of the strain origin is given.
5′ NTR and phylogenetic analyses.
Nucleotide sequences of the 5′ NTR or of the CP gene were initially aligned with the Pileup program of the Wisconsin Package, version 9.0 (Genetics Computer Group, Madison, Wis.). Alignments were edited with the SEQUENCE editing and analysis program, version 3.0.4, from Gary Olsen. Phylogenetic analyses were performed with PAUP 4.0b1 (Smithsonian Institution). Characters were either unordered or assigned user-defined weights, and gaps were treated as a fifth character state. All analyses were tested by at least 100 bootstrap replicates for confidence levels, and branches with less than 70% bootstrap support were collapsed. The larger data set was analyzed by the heuristic algorithm in the parsimony setting. Smaller data sets were also analyzed by the branch-and-bound algorithm. In addition, all data sets were analyzed in the maximum likelihood setting, using a fast branch swapping search. Character state changes were calculated by using MacClade 3.0 (developed by D. Maddison and W. Maddison; obtained from Sinauer Associates, Inc.).
Nucleotide sequence accession numbers.
The sequences of LS-CMV RNA 3 and K-CMV RNA 3 were deposited in GenBank with accession no. AF127976 and AF127977, respectively.
RESULTS
Analysis of the 5′ NTR of RNA 3.
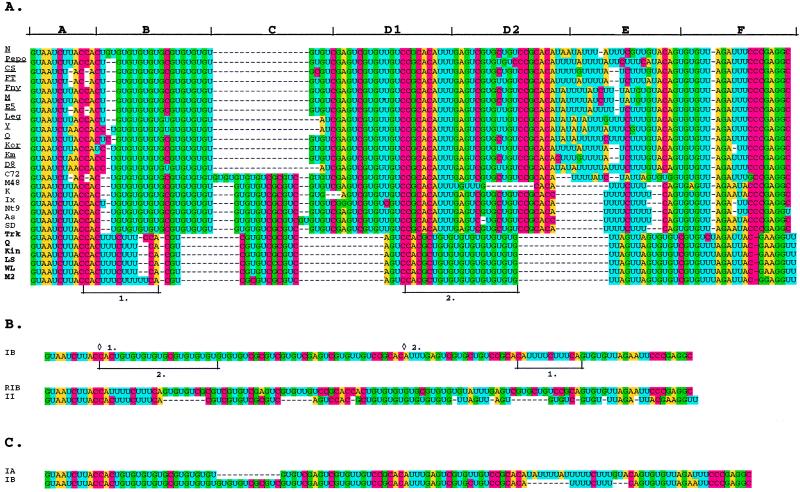
A set of 26 strains of CMV that have sequence data available for the entire RNA 3 were used for the 5′ NTR analysis. When these were aligned with PileUp, the alignment was very poor, even when the gap penalties were adjusted. The use of color-highlighted fonts for the A, C, G, and U nucleotides assisted in constructing a better alignment and revealed several interesting features of these sequences (Fig. 1A). The subgroup II 5′ NTR sequences are nearly identical, with only strains Trk7 and M2 showing minor differences compared with the other four strains. However, the subgroup I strains are more divergent and can be clearly divided into two further subgroups (Fig. 1). A number of motifs can be identified in the 5′ NTRs. Boxes A and F, at the 5′ and 3′ termini, respectively, of the NTR, are highly conserved among all strains. Box D is a tandem repeat in all of the subgroup I sequences but is not found in subgroup II (Fig. 1A).
FIG. 1.
Nucleotide sequence rearrangements in the 5′ NTR of CMV. (A) Alignment of the 5′ NTRs of 26 strains. Bases are shown with colored highlights. Boxes A and F are conserved in all strains. Boxes B, C, and E are variable. Boxes D1 and D2 are direct repeats conserved in all subgroup I strains. Motifs that have apparently been rearranged are indicated as 1. and 2. (B) Alignments and potential rearrangements in the consensus sequences of the 5′ NTRs of subgroup IB and II. The top portion shows the consensus sequence for subgroup IB. 1. and 2. indicate the two motifs that are rearranged to form the sequence in the lower portion (RIB, rearranged IB) by deletion and insertion at the respective diamonds. The rearranged sequence is shown in the bottom portion, aligned with the consensus subgroup II sequence. (C) Nucleotide sequence alignment with potential deletion events indicated, of the subgroup IA and IB consensus sequences.
A more striking observation concerns the differences in the 5′ NTRs of these RNAs. Boxes B, C, and E contain regions where there is considerable subgroup-specific variation. Boxes B and E contain motifs in subgroup I strains that appear in another position in subgroup II strains. This rearrangement is most obvious when we compare the second subgroup I set, designated IB, and the subgroup II strains (1. and 2. in Fig. 1A and B). If two rearrangement events are invoked in the subgroup IB consensus sequence, the resulting sequence is very similar to that for the subgroup II 5′ NTRs (Fig. 1B, RIB) requiring only several deletions of short regions of nucleotides to generate the extant sequence. An alignment of the consensus sequence for subgroups IA and IB shows two deletions, one in the IA sequence and the other in the IB sequence (Fig. 1C), suggesting that these subgroups could have arisen from a progenitor CMV by separate deletion events.
Phylogenetic analyses.
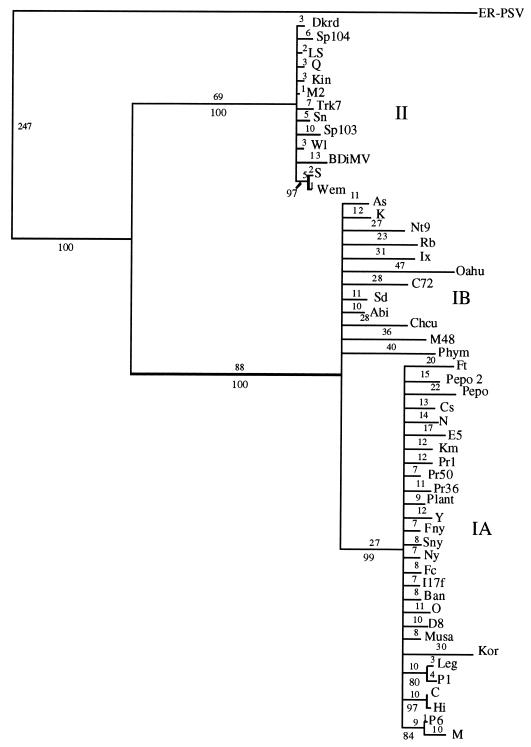
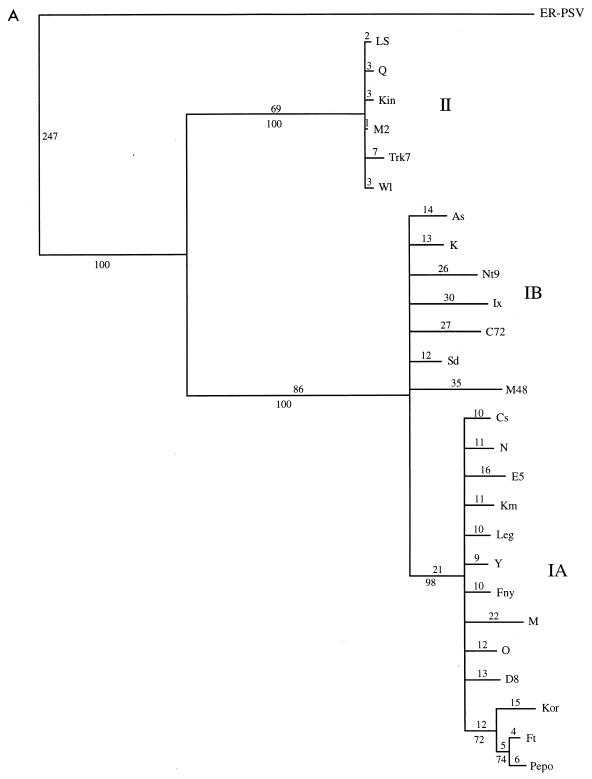
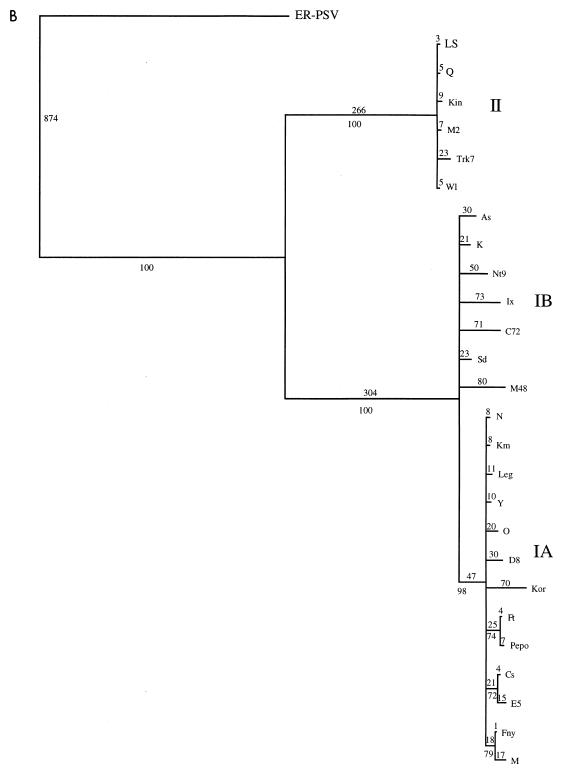
The initial data set containing 53 CMV taxa was analyzed by both a heuristic bootstrap search using the maximum parsimony setting and a fast branch swapping search using maximum likelihood. The CP gene of the ER strain of PSV was used as an outgroup. The topologies of both trees were essentially identical, with three major clades coinciding with strains from CMV subgroups IA, IB, and II (Fig. 2 and data not shown). The large size of the data set precluded a more detailed analysis, but a subset of the entire group, using the 26 strains from the 5′ NTR study, was analyzed in more detail, using a branch-and-bound search (Fig. 3A). The topologies of the two trees were essentially identical, with very little internal branching within the groups. To analyze the within-group relationships, each group was subjected to a more rigorous analysis with 1,000 bootstrap replications of a branch-and-bound search. For these analyses, two members of the most closely related group were used as an outgroup: LS- and Q-CMV for subgroup I; K- and Nt9-CMV for subgroup IA; Fny- and O-CMV for subgroup IB; and Fny- and K-CMV for subgroup II. The results from maximum parsimony and maximum likelihood analyses gave nearly identical results. In all cases, the groups have identical patterns of relationship to the complete analysis, and the CMV strains appear to have a “star phylogeny” pattern of evolution (Fig. 2 and 3).
FIG. 2.
Phylogenetic estimation of 53 strains of CMV based on the CP ORF, derived from 100 bootstrap replicates, using the heuristic search method of PAUP 4.0b1. All branches with less than 70% bootstrap support were collapsed. Numbers above the lines indicate branch lengths determined from unordered character state changes (i.e., number of changes); numbers below the lines indicate bootstrap values from the corresponding cladogram.
FIG. 3.
Detailed analysis of a subset of 26 strains of CMV. (A) Phylogram showing branch lengths when characters are unordered. Numbers represent actual number of nucleotide changes. Bootstrap values from the corresponding cladogram are shown below the branch points. (B) Phylogram generated by using a step matrix of character state changes as described in the text. Branch lengths are proportional to the cost of the nucleotide changes. Numbers represent total relative cost. Bootstrap values from the corresponding cladogram are shown below the branch points.
The default settings for standard phylogeny estimations are for unordered character states; that is, each type of nucleotide change is given an equal probability of occurring. However, in comparisons of viral sequences, certain types of changes are far more common than other types, and these biases can be different for different viruses (reviewed in reference 13). Transitions between A and G or between C and U are much more common than transversions between A and U. Transversions between C and A or G are extremely rare. Hence the character state changes are probably ordered, such that while a U-to-C change may be a single step that could be given a relative value of 1, an A-to-C change would have a higher cost, and the change may occur more readily by two steps: A to U (value greater than 1) and U to C (value of 1), for example. The PAUP program allows the user to supply a step matrix of character state changes to account for ordered change.
All phylogeny analyses are, at best, estimations. It is not possible to be certain that the relationships indicated are completely accurate. To determine the most likely ordering of character state changes for CMV CP evolution, we estimated the frequency of various changes by constructing four separate trees with one member of each subgroup (ER-PSV as the outgroup in all cases, and different members of CMV subgroups IA, IB, and II as ingroups). By using simple trees with only one member of each subgroup the confidence in each tree is very high, and the resulting information about the character state changes is most likely accurate. The frequency of each type of change was calculated from these simple trees by using MacClade 3.0. The character state changes were very similar for each tree, and Table 2 shows the average frequency of each type of change occurring at all homologous sites. These numbers were used to generate a step matrix of character state changes, such that a greater cost is assigned to changes that are very rare or may require more than one step. The phylogeny estimation was repeated for both the individual groups and the subset of sequences, using the new step matrix. The relationships of the strains were not changed, and each subgroup still exhibited a star phylogeny. However, the branches lengths were increased in a nonproportional manner, suggesting that the very rare changes may be more common in some lineages than in others (compare Fig. 3A and B).
TABLE 2.
Summary of frequency of specific nucleotide changes in CMV CP gene evolution
| Nucleotide | Frequency changes
|
|||
|---|---|---|---|---|
| A | C | G | U | |
| A | 0 | 4 | 1 | |
| C | 1 | 0 | 6 | |
| G | 6 | 0 | 0 | |
| U | 1 | 8 | 1 | |
DISCUSSION
Mechanisms of RNA-RNA recombination in plant viruses have been studied in the bromoviruses and in Turnip crinkle virus (reviewed in reference 15). These mechanisms can involve either heterologous or homologous recombination. Heterologous recombination events require a short region of complementarity between two templates, which can then form a short heteroduplex. The polymerase switches from one template to the other rather than unwinding the heteroduplex region. Homologous recombination uses a copy-choice mechanism, whereby the replication complex falls off one template, the nascent chain anneals with another template, and the replication proceeds on the new template. The apparent rearrangements in the 5′ NTRs of RNA 3 must have occurred via RNA-RNA recombination events. There is no clear evidence, however, for the regions either of complementarity or of similarity that are required for these mechanisms, in the extant sequences of the CMV RNAs 3 5′ NTRs, and hence the mechanism of recombination that gave rise to the 5′ NTRs of the various CMV subgroup RNAs 3 is not apparent. The progenitor virus could have contained sequences that have since been lost in all of the extant strains, or the recombination events could have involved novel mechanisms of recombination.
One of the subgroup I CMV strains used in this analysis, C72-CMV, appears to be an intermediate between the IA and IB strains by the 5′ NTR analysis, with the 5′ portion (box C) more similar to IB and the 3′ portion (box E) more similar to IA. However, the phylogenetic analysis clearly places the C72 CP into the subgroup IB viruses. C72 could contain a more ancestral form of the 5′ NTR, or it could be the product of a more recent recombination event between subgroups IA and IB.
Although sequence data are available for RNAs 1 and 2 of only a limited number of these strains, such rearrangements were not apparent in the other 5′ NTRs, nor was their any evidence of rearrangements in the 3′ NTRs or the intergenic region of RNAs 3 (data not shown). It is possible that the rearrangements of the RNA 3 5′ NTRs were the initial events that gave rise to the various subgroups, which later accumulated differences in their various ORFs. Estimations of relatedness based on the CP genes of these strains have previously relied only on sequence similarity (distance methods), and hence it was not possible to determine if the subgroup II strains are closer to the IA or the IB strains. The phylogeny estimations shown here indicate that the subgroup II strains are the most closely related to the ancestral state, with the IB subgroup falling out as a sister clade to them. This suggests that the 5′ NTR shown in Fig. 1B (RIB) may be the closest to the ancestral 5′ NTR and that deletions gave rise to subgroup II, whereas rearrangements gave rise to subgroup IB. Subgroup IA falls as a sister clade to the IB strains, indicating that it arose from IB and is the result of the most recent subspeciation event.
The evolutionary patterns indicated by the phylogeny estimations of CMV using CP sequences are consistent with a few events in which CMV passed through a narrow bottleneck and was disseminated from that point to many parts of the world. The first radiation, of subgroup II strains, is worldwide. From those strains, a second radiation gave rise to the subgroup IB strains. This distribution may have been more limited, as all subgroup IB strains described so far originated in Asia. From the subgroup IB strains there occurred a third radiation event that gave rise to the subgroup IA strains, and again there was a worldwide distribution. These types of radiation patterns, or star phylogenies, have been noted in recently emerged viruses such as Simian and Human immunodeficiency viruses, and rapid radiation may be a common outcome of emergence (10). This would suggest that CMV may also have emerged relatively recently. However, without a time line for virus evolution, it is very difficult to speculate about when these events occurred.
An alternate hypothesis that could also be consistent with the data is that the three subgroups of CMV represent the collection of variants around three fitness peaks. This would indicate that, rather than being a recently emerged virus, CMV has simply reached three stable equilibria instead of one. Were this the case, however, one might expect the three subgroups to fall out as sister clades, rather than successive subclades. In addition, our recent studies on mutation frequencies in CMV (to be published elsewhere) suggest that it is a rapidly evolving virus.
The phylograms show another interesting feature of CMV evolution. The branch lengths of the subgroup II strains are quite short in comparison to those for the subgroup I strains, and the subgroup IB strains show the most divergence from each other. This suggests that the subgroup I strains are evolving more rapidly, although it is not possible to put any timeline on this evolution, and hence rates are not directly comparable. These differences may be reflective of the broader host range and higher incidence of subgroup I strains compared with subgroup II strains.
The enormous amount of variation seen in RNA virus genomes has allowed them to be very successful at infecting new hosts and evading the host’s defense responses. Clearly variation is an advantage, and viruses are believed to exist at the threshold of catastrophe (8). The evolution of RNA viruses is undoubtedly a series of complex events that involves all possible mechanisms to introduce variation, including mutation, reassortment, and recombination. Any or all of these events may play a pivotal role in virus speciation.
ACKNOWLEDGMENTS
We thank Peter Nagy for helpful discussions on RNA recombination, and we thank Stan Flasinski, Joachim deMiranda, Richard Nelson, William Schneider, and Peter Palukaitis for careful reading of the manuscript.
This work was supported by the S. R. Noble Foundation.
REFERENCES
- 1.Allison R F, Janda M, Ahlquist P. Sequence of cowpea chlorotic mottle virus RNAs 2 and 3 and evidence of a recombination event during bromovirus evolution. Virology. 1989;172:321–330. doi: 10.1016/0042-6822(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 2.Chaumpluk P, Sasaki Y, Nakajima N, Nagano H, Nakamura I, Suziki K, Mise K, Inouye N, Okuno T, Furusawa I. Six new subgroup I members of Japanese cucumber mosaic virus as determined by nucleotide sequence analysis of RNA3’s cDNAs. Ann Phytopathol Soc Jpn. 1996;62:40–44. [Google Scholar]
- 3.Ding S-W, Anderson B J, Haase H R, Symons R H. New overlapping gene encoded by the cucumber mosaic virus genome. Virology. 1994;198:593–601. doi: 10.1006/viro.1994.1071. [DOI] [PubMed] [Google Scholar]
- 4.Ding S-W, Li W X, Symons R H. A novel naturally occurring hybrid gene encoded by a plant RNA virus facilitates long distance virus movement. EMBO J. 1995;14:5762–5772. doi: 10.1002/j.1460-2075.1995.tb00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding S-W, Shi B-J, Li W-X, Symons R H. An interspecies hybrid RNA virus is significantly more virulent than either parental virus. Proc Natl Acad Sci USA. 1996;93:7470–7474. doi: 10.1073/pnas.93.15.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domingo E, Holland J J. High error rates, population equilibrium, and evolution of RNA replication systems. In: Domingo E, Holland J J, Ahlquist P, editors. Variability of RNA genomes. 1st ed. III. Boca Raton, Fla: CRC Press; 1988. pp. 3–36. [Google Scholar]
- 7.Domingo E, Holland J J. Mutation rates and rapid evolution of RNA viruses. In: Morse S S, editor. The evolutionary biology of viruses. New York, N.Y: Raven Press, Ltd.; 1994. pp. 161–184. [Google Scholar]
- 8.Holland J J, Domingo E, delaTorre J C, Steinhauer D A. Mutation frequencies at defined single codon sites in vesicular stomatitis virus and poliovirus can be increased only slightly by chemical mutagenesis. J Virol. 1990;64:3960–3962. doi: 10.1128/jvi.64.8.3960-3962.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai M M C. Genetic recombination in RNA viruses. Curr Top Microbiol Immunol. 1992;176:21–32. doi: 10.1007/978-3-642-77011-1_2. [DOI] [PubMed] [Google Scholar]
- 10.Myers G, MacInnes K, Myers L. Phylogenetic moments in the AIDS epidemic. In: Morse S S, editor. Emerging viruses. New York, N.Y: Oxford University Press; 1993. pp. 120–137. [Google Scholar]
- 11.Naidu R A, Collins G B, Ghabrial S A. Nucleotide sequence analysis of a cDNA clone encoding the coat protein gene of peanut stunt virus. Plant Mol Biol. 1991;17:175–177. doi: 10.1007/BF00036826. [DOI] [PubMed] [Google Scholar]
- 12.Palukaitis P, Roossinck M J, Dietzgen R G, Francki R I B. Cucumber mosaic virus. Adv Virus Res. 1992;41:281–348. doi: 10.1016/s0065-3527(08)60039-1. [DOI] [PubMed] [Google Scholar]
- 13.Ramírez B-C, Barbier P, Séron K, Haenni A-L, Bernardi F. Molecular mechanisms of point mutations in RNA viruses. In: Gibbs A J, Calisher C H, García-Arenal F, editors. Molecular basis of virus evolution. Cambridge, England: Cambridge University Press; 1995. pp. 105–118. [Google Scholar]
- 14.Roossinck M J. Mechanisms of plant virus evolution. Annu Rev Phytopathol. 1997;35:191–209. doi: 10.1146/annurev.phyto.35.1.191. [DOI] [PubMed] [Google Scholar]
- 15.Simon A E, Bujarski J J. RNA-RNA recombination and evolution in virus-infected plants. Annu Rev Phytopathol. 1994;32:337–362. [Google Scholar]
- 16.White P S, Morales F J, Roossinck M J. Interspecific reassortment in the evolution of a cucumovirus. Virology. 1995;207:334–337. doi: 10.1006/viro.1995.1088. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Hanada K, Palukaitis P. Mapping local and systemic symptom determinants of cucumber mosaic cucumovirus in tobacco. J Gen Virol. 1994;75:3185–3191. doi: 10.1099/0022-1317-75-11-3185. [DOI] [PubMed] [Google Scholar]