Abstract
Typical herpes simplex virus (HSV) capsids contain seven proteins that form a T=16 icosahedron of 1,250-Å diameter. Infection of cells with recombinant baculoviruses expressing two of these proteins, VP5 (which forms the pentons and hexons in typical HSV capsids) and VP19C (a component of the triplexes that connect adjacent capsomeres), results in the formation of spherical particles of 880-Å diameter. Electron cryomicroscopy and computer reconstruction revealed that these particles possess a T=7 icosahedral symmetry, having 12 pentons and 60 hexons. Among the characteristic structural features of the particle are the skewed appearance of the hexons and the presence of intercapsomeric mass densities connecting the middle domain of one hexon subunit to the lower domain of a subunit in the adjacent hexon. We interpret these connecting masses as being formed by VP19C. Comparison of the connecting masses with the triplexes, which occupy equivalent positions in the T=16 capsid, reveals the probable locations of the single VP19C and two VP23 molecules that make up the triplex. Their arrangement suggests that the two triplex proteins have different roles in controlling intercapsomeric interactions and capsid stability. The nature of these particles and of other aberrant forms made in the absence of scaffold demonstrates the conformational adaptability of the capsid proteins and illustrates how VP23 and the scaffolding protein modulate the nature of the VP5-VP19C network to ensure assembly of the functional T=16 capsid.
The herpes simplex virus-1 (HSV-1) virion consists of four distinct compartments: glycoprotein-containing envelope, tegument, capsid, and DNA core. The T=16 icosahedral capsid shell is 160 Å thick and has a diameter of 1,250 Å. Historically, capsids have been considered to exist in three forms, B-capsids (containing scaffolding proteins), C-capsids (containing viral DNA), and A-capsids (empty). B-capsids contain seven structural proteins (24). Four of these constitute the capsid shell: VP5 and VP26 make up the capsomeres, while VP19C and VP23 together form the triplexes, which fit between, and link together, adjacent capsomeres (19, 43). The three remaining proteins (VP21, VP24, and VP22a) are involved in formation and processing of the scaffold.
Functional scaffolding proteins are essential for HSV-1 capsid assembly and maturation, and considerable effort has gone into determining their properties and roles. They are expressed from a pair of overlapping genes, UL26 and UL26.5, in which the open reading frame of UL26 is an in-frame N-terminal extension of the UL26.5 open reading frame (12). The larger gene encodes a protease, which cleaves itself internally to give the proteins VP24 and VP21. It also cleaves both itself and the product of the smaller gene (the abundant scaffolding protein pre-VP22a) at a second site near its C terminus (12, 23). This maturational cleavage removes a 25-amino-acid sequence that is known to interact with the major capsid protein, VP5 (8). The interaction is essential for correct capsid shell formation, and in circumstances where it cannot take place, the outer-shell proteins self-assemble into aberrant structures (9, 14, 30). The maturational cleavage is also an essential step, and if it is inhibited the scaffolding proteins are retained within the capsid, preventing packaging of the viral genome (6, 22).
Although the internal capsid scaffold is not icosahedrally ordered (33, 42), it interacts with the icosahedral shell in a regular manner (42) and clearly has a role in controlling the symmetry of the particle. If the three essential shell proteins (VP5, VP19C, and VP23) are expressed in the absence of functional scaffolding proteins, they form structures that have the appearance of partial and incomplete shells in negatively stained samples (2, 29, 31). These structures have recognizable capsomeres that are organized into hexagonal networks. This suggests that the scaffold is important for determining the curvature and ultimate closure of the capsid shell rather than in controlling the precise interactions of its subunits. A different type of particle is made when only VP5 and VP19C are present. In this case, densely staining spherical particles are formed (25, 31), which are markedly smaller than the intact capsid. These VP5-VP19C particles also contain recognizable capsomeres organized in a hexagonal pattern, but, in contrast to the VP5-VP19C-VP23 shells, many of them appear to be closed, intact spheres. The formation of VP5-VP19C particles demonstrates that VP19C alone is sufficient to link capsomeres together and that it does not require the other triplex protein, VP23. However, the differences between the shell structures formed in the presence and absence of VP23 indicate that VP23 does have a role in modulating the nature of the interaction between VP5 and VP19C and/or between VP5 subunits of adjacent capsomeres.
Triplexes are heterotrimers, formed by two copies of one protein (VP23; 34 kDa) and a single copy of an unrelated protein (VP19C; 53 kDa) (19). It has recently been shown that functional triplexes can assemble in isolation from the other capsid proteins (28). This demonstrates that heterotrimer formation is an intrinsic property of the component proteins. In addition, purified VP23 is known to be in a partially folded “molten globule” state (10), suggesting that its final conformation is influenced by its local environment and interactions.
In this paper, we report the three-dimensional (3-D) structure of recombinant VP5-VP19C particles by electron cryomicroscopy and computer image processing. Structural comparisons between VP5-VP19C particles and typical capsids have allowed us to localize VP19C and VP23 in the triplex and to suggest possible ways in which the scaffolding and triplex proteins influence capsid morphogenesis.
MATERIALS AND METHODS
Capsid preparation.
The VP26− capsids were purified from Sf21 cells coinfected with five different recombinant baculoviruses expressing VP5, VP19C, VP23, VP22a, and the HSV-1 protease (41). VP5-VP19C particles were purified from cells infected with recombinant baculoviruses expressing only VP5 and VP19C as described previously (25).
Electron cryomicroscopy.
Purified VP26− particles were embedded in vitreous ice suspended across the holes in holey carbon grids. Electron cryomicroscopy observation was done by spot scan illumination following established procedures (43). The ice-embedded VP5-VP19C particles were imaged with flood beam rather than spot scan illumination. Microscope alignment, specimen assessment, and focusing were performed with a Gatan (Pleasanton, Calif.) 1,024- by 1,024-pixel slow-scan charge-coupled device camera (27). All micrographs were recorded at a magnification of ×30,000 on Kodak SO163 film in a JEOL4000 electron cryomicroscope operating at 400 kV with a LaB6 filament under minimal dose conditions (∼7 electrons/Å2).
Image digitization and preprocessing.
Forty selected micrographs were digitized on a SCAI scanner (Carl Zeiss, Englewood, Colo.) at a step size of 4.67 Å/pixel. A total of 1,300 particle images (240 by 240 pixels) were selected automatically (26). The image quality was assessed quantitatively with EMAN software (12a) by evaluating the contrast transfer function rings visualized in the incoherently averaged Fourier transforms of particle images. We used 700 particles from these micrographs with the first zeros of their contrast transfer functions between 1/20 and 1/24 Å−1 (40) for further analysis.
Most of the subsequent computational steps were performed with IMAGIC-5 software (37) on a Silicon Graphics, Inc. Onyx2 supercomputer with 24 parallel processors. The major steps, as detailed below, include noise reduction, particle symmetry determination, generation of a low-resolution model from a small number of particle images, and iterative refinement of the center and orientation parameters of the particles by using the projection images computed from this model. In some of the computationally intensive steps, such as particle alignment and orientation determination, we used the strategy of distributed computing in order to reduce the actual computational time.
Data compression and noise reduction.
The raw images of individual VP5-VP19C recombinant particles are noisy and relatively large (880 Å). In order to enhance the signal-to-noise ratio and reduce the time needed for an initial reconstruction, we employed the wavelet transformation method (13, 15, 16). We used the first component, called the “approximation” from the first level of wavelet decomposition. The approximation is obtained after the application of a low-pass filter (11) and subsampling of each particle image by a factor of 2 for each dimension to reduce the size of the images to 120 by 120 pixels. In order to get a higher signal-to-noise ratio, we grouped the reduced images with similar orientations into classes as employed in single-particle reconstruction methods (4, 5, 35, 36).
Evaluation of particle symmetry.
Determining the particle symmetry is an important step in the 3-D-reconstruction procedure because it allows one to simplify the particle orientation search and to impose symmetry averaging. In order to determine the symmetry of the VP5-VP19C particle, we analyzed the real-space self-common lines of class-averaged images with different point group symmetry assumptions. We computed the Self-Sincorr (37) functions for each class-averaged image and searched for the Euler angles of the particle by finding the smallest standard deviation between the peaks of assumed point group symmetries. This evaluation indicated that the selected particles had icosahedral symmetry, and thus, in all subsequent data-processing steps, icosahedral symmetry was assumed.
Low-resolution model.
During the initial low-resolution reconstruction step, we used the class-averaged images obtained after wavelet transformation. The angular reconstitution method (34, 38) was used to assign Euler angles to each class average. Then a 3-D reconstruction was calculated by using the exact-filter back-projection algorithm (7). The orientation of an individual particle was initially assigned to that of the class average to which it belonged. The particle orientations were then iteratively refined by using projections computed from the preliminary reconstruction. The resolution of the model obtained from the wavelet-filtered images was ∼37 Å.
Final reconstructions.
The low-resolution model of the VP5-VP19C particles did not make use of all the information inherent in our raw image data. Therefore, we reconstructed its final map from the original VP5-VP19C particle images without wavelet compression and class averaging. First, the low-resolution model was scaled up to the same dimensions as the original image and projections were computed from this model to refine the Euler angles for each of the original particle images by the angular-reconstitution technique. Consequently, an improved 3-D reconstruction was computed and used for further angle and center refinement. This reconstruction and refinement procedure was iterated for several rounds until no further significant improvement was obtained.
The reconstruction procedure for the VP26− particles has been published elsewhere (41). Although the VP26− particle was reconstructed previously at 19-Å resolution, in the present study we truncated the data at about 26 Å in order to carry out proper structural comparison with the VP5-VP19C particle. The final reconstruction was computed from 305 particles.
The effective resolution of the final structures was evaluated based on the Fourier shell correlation coefficient between independent reconstructions being larger than 0.5 (7). Based on this criterion, both the VP5-VP19C and VP26− reconstructions have an effective resolution of 26 Å.
Comparison of connecting densities.
Structure extraction and graphic rendering were carried out by using the Explorer software package (NAG, Downers Grove, Ill.) with custom-designed modules (3). The VP19C density was computationally isolated from the VP5-VP19C particle by assuming that its interface with VP5 was a plane forming a tangent with the surface of the hexon. The triplex was extracted from the 3-D reconstruction of another recombinant capsid that contains all the capsid proteins except VP26 (the VP26− capsid) (41) by the same procedure.
The two unique VP19C connections from the VP5-VP19C particle were aligned and averaged, as were four of the triplexes (Tb, Tc, Td, and Te) from the VP26− capsid. To carry out the alignment, we first assigned a value of zero to all densities below the selected contour level and then scaled the densities of the maps to have the same means and standard deviations. The alignment procedure performs an exhaustive comparison of the maps for all possible Euler angles at a step size of 0.2°. The best alignment is the one that gives the highest correlation between the two maps. The two maps were compared voxel by voxel. Their differences were considered to be significant when the density was at or above the selected threshold in a voxel in one map but not in the corresponding voxel in the other map.
RESULTS
3D structure of VP5-VP19C particles.
The protein composition of VP5-VP19C particles (Fig. 1A) and their appearance in thin-section and negative-stain electron microscopy have been described previously (25). Figure 1B shows a 400-kV electron microscopic image of ice-embedded VP5-VP19C particles. The intact particles appear spherical but have different sizes. Particles of an apparently uniform size class (∼880 Å), which constitute over 50% of the population, were chosen for computer analysis and reconstruction. At the start of the data analysis, we had to determine if the particle had any symmetry. Therefore, we examined different possible point group symmetries, including dihedral, icosahedral, cubic, and tetragonal, as described in Materials and Methods. This analysis concluded that the particle is icosahedral, and thus icosahedral symmetry was assumed in our subsequent data processing.
FIG. 1.
(A) Sodium dodecyl sulfate gel of purified wild-type B-capsids (left) and recombinant VP5-VP19C particles (right). In the right lane, two bands are evident, with molecular masses of ∼150 and 53 kDa, corresponding to VP5 and VP19C, respectively. (B and C) 400-kV electron microscopic images of ice-embedded VP5-VP19C particles (B) and VP26− particles (C). One VP5-VP19C particle is enclosed by a dotted circle. The defocus values are 2.9 (B) and 2.6 (C) μm.
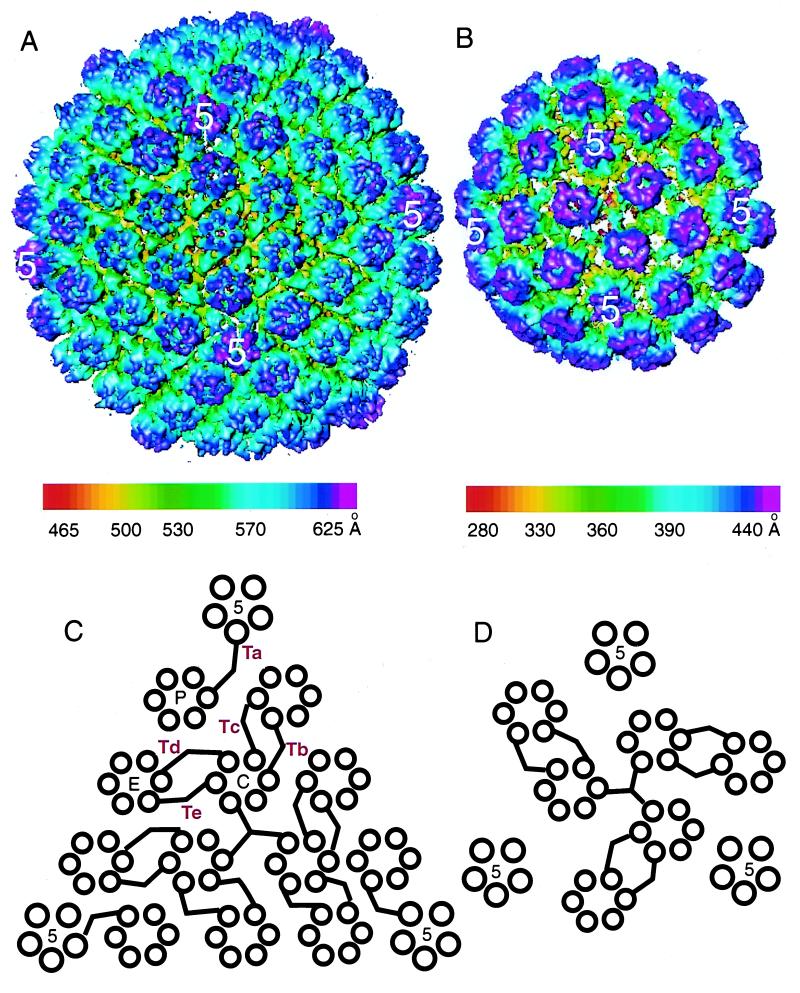
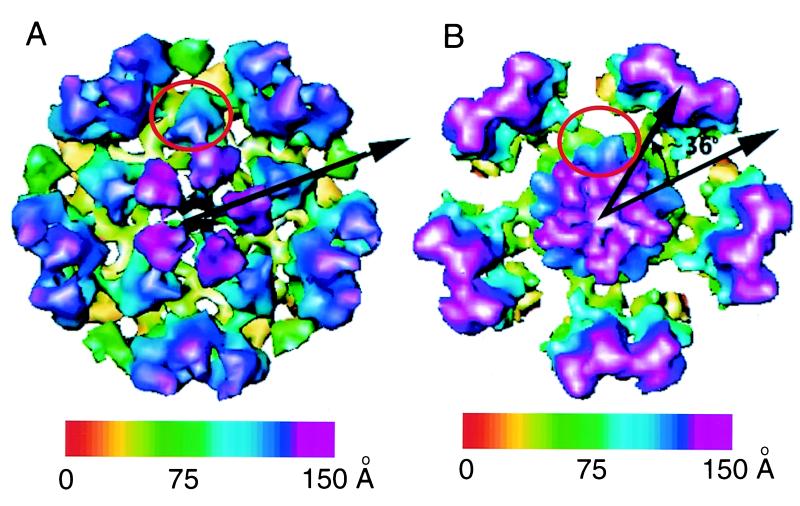
The VP5-VP19C particle was reconstructed to 26-Å resolution. It forms a T=7 icosahedral lattice which has an external diameter of ∼880 Å and an internal diameter of ∼580 Å, consisting of 60 hexons and 12 pentons (Fig. 2B). An asymmetric unit contains one penton subunit, one hexon, and 1 1/3 copies of a connecting density joining neighboring hexons. The size of 700 Å previously estimated from negatively stained images (25) was smaller, presumably due to specimen shrinkage caused by negative stain. For comparison, we also obtained a 26-Å-resolution reconstruction of recombinant VP26− capsids (Fig. 1C and 2A) (41). VP26− capsids were chosen because, like the VP5-VP19C particles, they lack VP26, which is normally present on the tips of the hexons in wild-type capsids. Apart from this difference, the VP26− capsid structure is identical to that of wild-type capsids, forming a T=16 icosahedral lattice with an external diameter of ∼1,250 Å and an internal diameter of ∼985 Å. The VP26− capsid shell is made up of 150 hexons, 12 pentons, and 320 triplexes containing 960 copies of VP5, 320 copies of VP19C, and 640 copies of VP23 per capsid (39). An asymmetric unit consists of one penton subunit, 2 1/2 hexons, and 5 1/3 triplexes (41). Figure 2C and D shows schematics illustrating the connection patterns between capsomeres in both types of particle.
FIG. 2.
Three-dimensional reconstructions of the recombinant VP26− capsid (A) and VP5-VP19C particle (B). (A) The VP26− capsid forms a T=16 icosahedron with a diameter of 1,250 Å. (B) The VP5-VP19C particle forms a T=7 icosahedron with a diameter of 880 Å. Both maps are viewed along an icosahedral twofold axis and color coded according to the particle radius, as indicated by the color bars. All isosurfaces are displayed with a contour level of 2 standard deviations from the mean. The arrangement of capsomeres and intercapsomeric connections in one triangular face are illustrated for the VP26− capsid (C) and the VP5-VP19C particle (D), respectively. The pentons (5) are labeled, as are single P, E, and C hexons and Ta to Tf triplexes in panel C.
The VP26− capsid has a polyhedral shape due to flattening of the icosahedral faces, which results in the pentons being further from the center of the particle than the hexons. By contrast, the VP5-VP19C particles are uniformly spherical. The shell thicknesses of both types of particle are similar (about ∼160 Å), and the intercapsomeric distances are the same, with the distance between two neighboring hexons being ∼77 Å in either case. The dimensions of the hexons in the VP5-VP19C particle correspond closely to those in the VP26− capsid, with an average diameter of ∼145 Å and a height of ∼150 Å. There is a central channel of ∼18-Å diameter. However, despite these similarities, the two maps reveal not only differences in the overall sizes and gross morphologies of the particles but also conformational differences in their structural subunits.
Comparison of hexons.
In order to illustrate differences affecting the hexons of the VP26− and VP5-VP19C particles, we computationally extracted a C hexon from the VP26− capsid and a hexon from the VP5-VP19C capsid, together with portions of the surrounding capsomeres. Figure 3A and B shows outside views of corresponding portions from the VP26− capsid and the VP5-VP19C particle, respectively. Figure 3E and F shows the equivalent inside views. In both views, the maps appear very different. For example, the individual VP5 subunits in the VP5-VP19C particle are much less well resolved than those in the VP26− capsid, making it harder to see the sixfold nature of the hexon. This is particularly obvious in the views of the inner surface (compare Fig. 3E and F), where the floor density of the VP5-VP19C particle is irregular and discontinuous between adjacent subunits. In addition, the hexon subunits in the VP26− capsid are arranged as a regular hexagon while those in the VP5-VP19C particle appear skewed and deviate from sixfold symmetry. This is more readily seen in the contour plots of a 5-Å-thick slice from the upper regions of the hexons (Fig. 3C and D).
FIG. 3.
Comparison of hexons in VP26− capsids and VP5-VP19C particles. The outer (A and B) and inner (E and F) views of the hexon environment in VP26− capsids and VP5-VP19C particles, respectively, are shown. The triangles denote local threefold positions that are occupied by connecting densities. That between the central hexon and the hexons labeled 1 and 6 denotes the strict icosahedral threefold position. The yellow circles in panels A and B highlight the connecting masses between two adjacent hexons. The black circles highlight regions where the capsid floor is present in the VP26− capsid (E) but absent in the VP5-VP19C particle (F). (C and D) Contour plots of 5-Å-thick slices taken at 25 Å below the uppermost part of each type of hexon, which reveal the contrast between the sixfold symmetry in the VP26− hexon (C) and the skewed nature of the subunits in the VP5-VP19C hexon (D). The densities are color coded according to the capsid radius, as indicated by the color bars.
Intercapsomeric connections.
The pattern of intercapsomeric connections in the VP5-VP19C particle, shown in Fig. 2D, exhibits similarities with the simplified representation of the pattern of triplex connections in the VP26− capsid (Fig. 2C), with pairs of connections between adjacent hexons. However, the network of connections is less extensive and, notably, there are no connections to the pentons. Examination of the hexon environment (Fig. 3) reveals that, although mass density is present at the strict, and some of the local, threefold positions in the VP5-VP19C particle (Fig. 3B), it does not have the characteristic appearance of triplexes (Fig. 3A). Since the VP5-VP19C particles do not contain the second triplex protein, VP23, we interpret these connecting masses as each consisting of a single molecule of VP19C. Though density is clearly present at the icosahedral threefold positions in the center of each triangular face, its shape cannot be ascertained due to the imposition of symmetry in the reconstruction step.
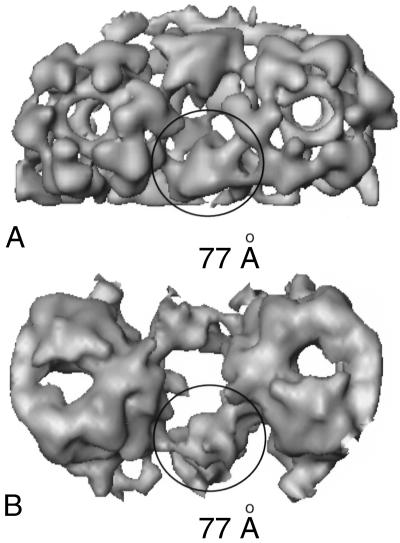
To determine the degree of similarity between the triplex and the VP19C connections, we computationally extracted two adjacent hexons and their connecting masses from equivalent parts of the VP26− and VP5-VP19C maps (Fig. 4). The triplex in the VP26− particle (Fig. 4A) and the VP19C connection in the VP5-VP19C particle (Fig. 4B) are highlighted. The triplex and VP19C connections fit equally well between the hexons, each spanning a gap of ∼77 Å. The upper surfaces of triplexes typically appear as tilted triangles with elevated “head” and lowered “tail” domains (43). In the VP26− capsid, the head of each triplex connects to the middle domain of a VP5 subunit in one hexon and the tail to the lower domain of a subunit in the adjacent hexon (Fig. 4A). In the VP5-VP19C particle, the general appearance of the VP19C connections is similar, when viewed from above, and they form contacts on the capsomeres with equivalent locations and spacing (Fig. 4B).
FIG. 4.
Spatial arrangement of hexons in VP26− capsids and VP5-VP19C particles. Two adjacent hexons with connecting masses, computationally extracted from the VP26− (A) and VP5-VP19C (B) particles, are shown in similar orientations. The circles highlight the positions of a triplex in the VP26− particle and a VP19C connection in the VP5-VP19C particle. The diameter of the circle (∼77 Å) is the distance spanned by VP19C and the triplex.
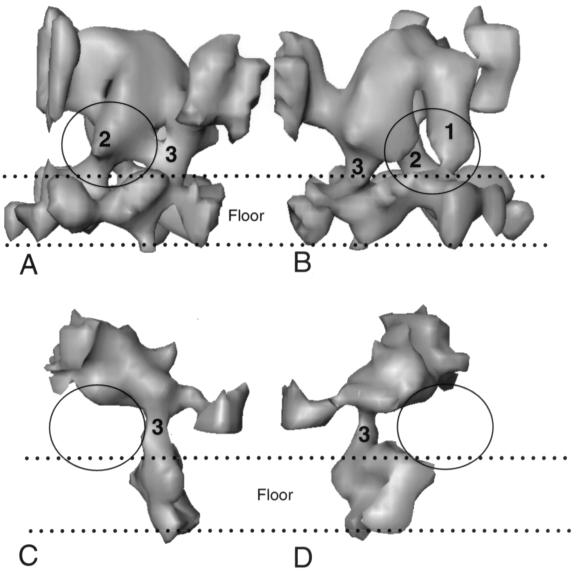
In addition to linking the middle and lower domains of adjacent hexons, both triplexes and VP19C connections also make contact with regions in the floors of their respective particles. Figure 5A and B show views from either side of a Tc triplex, illustrating the contacts it makes with the floor of the VP26− capsid. Two contacts are present under the head of the triplex and a third is near the tail. These correspond to the two legs and tail previously described in the A-capsid (43). In the VP5-VP19C particle the floor is discontinuous and poorly formed and only a single contact between the VP19C connection and the floor domain is detected (Fig. 5C and D). The similar locations of the floor contacts made by VP19C and the triplex tail suggest that both are formed by VP19C. In contrast, the two legs below the head of the triplex are absent from VP5-VP19C structure.
FIG. 5.
Floor connections of triplexes and VP19C. (A and B) Views from opposite sides of an isolated Tc triplex from the VP26− capsid together with the underlying floor density; the space between the two dotted lines represents the floor. (C and D) Similar orientations of an isolated VP19C connection and its associated floor from the VP5-VP19C particle. The two legs (1 and 2) and the tail (3) that connect the triplex to the floor are indicated in panels A and B. In panels C and D, the single contact between the VP19C connection and the floor density that corresponds to the tail connection is indicated (3). The two legs in the triplex (A and B) and the equivalent positions below the VP19C connection (C and D) are circled.
Locations of the triplex proteins.
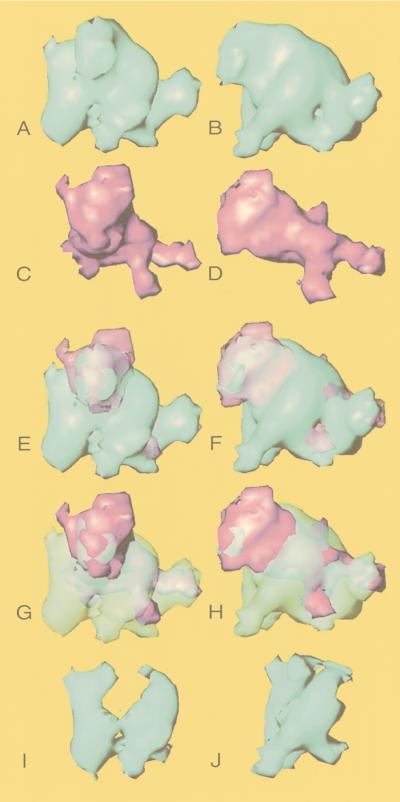
In an attempt to identify the locations of VP19C (53 kDa) and VP23 (34 kDa) in the triplex, we compared an averaged triplex and an averaged VP19C connection (Fig. 6). To calculate the averages, we used the four structurally similar triplexes, Tb, Tc, Td, and Te, and the two icosahedrally unrelated VP19C connections. In each case we carried out a 3-D alignment of the isolated densities before averaging. Figure 6A to D shows the averaged triplex and the averaged VP19C connection viewed from head on and from one side. Figure 6E to H shows the two structures superimposed. The presence of density from the VP19C connection, extending beyond the apparent molecular boundaries of the triplex, implies that the conformation of VP19C is not perfectly conserved between the VP26− and VP5-VP19C particles. However, the major portion of the upper part of the triplex and the regions which make contact with the hexons are clearly formed by VP19C. By contrast, most of the lower part of the triplex has no counterpart in the VP19C connection. Presumably, this part is largely contributed by VP23.
FIG. 6.
Localization of VP19C and VP23. A computationally averaged (see Materials and Methods) triplex (blue) and an averaged VP19C connection (magenta) are shown from a front (A and C) and a side (B and D) view. The two structures are shown superimposed in the same orientations in panels E to H. For each pair of images, either the VP19C (E and F) or the triplex (G and H) density is shown in semitransparency to allow the position of the other structure to be seen. The densities thought to represent the two molecules of VP23 (I and J) were computationally isolated from the difference map between the triplex and the VP19C connection.
Based on the above analyses, we have attempted to assign boundaries to the two copies of VP23. The isolated region shown in Fig. 6I and J corresponds to the two legs extending below the body of the triplex. These two legs resemble each other structurally and are connected at the top to the body of the triplex and at the bottom to the floor domain of VP5. On the basis of their apparent relatedness and their absence from the VP19C connection, we consider these legs to represent the two copies of VP23. Their combined mass of ∼47 kDa is less than that expected for two molecules of VP23 (∼68 kDa), but this probably reflects the difficulty of defining their upper boundaries in the rather monolithic mass of the triplex. The additional, unresolved VP23 material presumably contributes to the excess density present in neighboring regions of the triplex.
Comparison of pentons.
We computationally extracted the pentons and part of the surrounding capsid shell from the 3-D structures of the VP26− capsid and the VP5-VP19C particle (Fig. 7). Both types of penton have similar diameters (∼145 Å), although the VP26− penton has a central channel which is not present in the VP5-VP19C penton. The alignment of the VP5 subunits with respect to the adjacent hexons appears to have changed such that the VP5-VP19C penton seems to be rotated by about 36° relative to the VP26− penton. Each of the penton subunits in the VP26− particle (Fig. 7A) is connected to a triplex, Ta. By contrast, there are no densities linking the hexons and pentons in the VP5-VP19C particle which might correspond to the VP19C connections between neighboring hexons seen in Fig. 3B, and the only visible connections between the pentons and hexons are the limited contacts formed at the floor of the shell. Although the boundaries of a single VP5 subunit in the VP5-VP19C particle are not easily discerned, there are obvious differences between the penton subunits in the two capsid types. Some of these may be due to conformational changes that particularly affect the upper portions of VP5. However, a striking difference is due to the presence of five prominent extra masses around the middle of the VP5-VP19C penton (Fig. 7B).
FIG. 7.
Comparison of pentons in VP26− capsids and VP5-VP19C particles. Outer views of the penton environment in VP26− capsids (A) and VP5-VP19C particles (B) are shown. The red circle highlights the position of a triplex in panel A. An additional mass present on the side of the penton in the VP5-VP19C particle that may represent a molecule of VP19C is circled in panel B. The arrow in panel A indicates the alignment of a single VP5 subunit with respect to the neighboring hexons. The pair of arrows in panel B indicates the apparent rotation of subunit density in the VP5-VP19C penton relative to the VP26− penton. The maps are color coded according to the particle radius, as indicated by the color bars.
DISCUSSION
Conformational variation in penton and hexon.
Apart from the obvious gross differences between the appearance of VP26− capsids and VP5-VP19C particles, there are subtle variations in the appearance of their pentons and hexons. The alteration to the penton can be partly explained by changes in the conformation of the subunits; however, as can be seen in Fig. 7, the lack of connecting masses equivalent to Ta triplexes also causes obvious differences. The reason why there are no connections between the penton and hexons may be related to the apparent rotation of the penton in the VP5-VP19C particles, since this would move the VP19C binding sites on the penton and hexon out of alignment. Changes in either the separation or orientation of the binding sites could prevent VP19C from establishing connections at both ends. It is notable, therefore, that a prominent additional mass is present at the midpoint of each VP5-VP19C penton subunit (Fig. 7B). Although the precise boundaries of this mass are uncertain, it corresponds to at least ∼37 kDa. The midpoint of each penton VP5 is the region to which the Ta triplex would normally bind, and the extra mass may, therefore, represent a “collapsed” VP19C molecule which has bound at only one end.
Although the VP5-VP19C hexons are of similar overall size and shape to those in the VP26− capsid, they differ notably in two respects. They are skewed, with the subunits arranged in a distorted oval rather than a regular hexagon, and their connections to each other in the floor of the particle are limited and discontinuous. Interestingly, these features resemble those in in vitro-assembled procapsids (33). The procapsid is the first complete product of capsid assembly (18), and therefore, the organization of its subunits presumably reflects the manner in which they initially interact. The structures seen in polyhedral capsids are the products of a subsequent maturational step that involves extensive conformational adjustment of the proteins. The VP5-VP19C particle represents an unnatural assembly product and consequently may not provide a suitable environment in which the subsequent rearrangements of the subunits can take place. Therefore, the presence of procapsid-like features in the VP5-VP19C particles could indicate that the component proteins are locked in immature, or partially rearranged, configurations. A similar situation occurs in phage P22, where, in the smaller particles that are formed in the absence of scaffold, the hexons resemble those in procapsids rather than those in mature phage heads (32).
Capsid size and curvature.
The size of an icosahedral capsid is determined by the dimensions, separation, and arrangement of its component subunits. As described above, in VP5-VP19C particles the sizes of individual subunits are similar to those in normal capsids but the VP5-VP19C particle is markedly smaller (880 instead of 1,250 Å) and has a different arrangement of capsomeres (T=7 instead of T=16). Such behavior is not unique to herpesviruses and is also found in a number of bacteriophages. An example that shows considerable similarity to HSV is bacteriophage P22 (32), in which the capsid shell protein can also assemble into smaller-than-normal particles. Thus, in the absence of the P22 scaffolding protein, two different types of structure can form. Large particles, which are essentially identical to the normal T=7 P22 procapsid head, and more abundant, smaller, T=4 particles.
In P22, therefore, although the scaffolding protein can influence the outcome of the assembly process, it does not direct the basic pattern of subunit interactions which results in icosahedron formation. This property appears to be inherent in the capsid shell proteins themselves. The icosahedral nature of the VP5-VP19C particles suggests that a similar situation applies to HSV. Interestingly, in both HSV and P22, the major shell proteins seem to have a preference for forming particles of smaller radius than normal capsids. Presumably, the scaffolding proteins prevent this by imposing a larger radius of curvature on the growing capsid shell (32). Although in vitro studies have shown that the HSV shell and scaffold normally coassemble, the scaffolding protein can self-assemble, in the absence of shell proteins, into spherical particles of approximately 600-Å diameter (17, 21). Since this is slightly too large to be enclosed by an 880-Å shell with an internal diameter of ∼580 Å, the presence of a core of this size would preclude formation of such small particles. It is clear that the potential interactions between shell proteins are sufficiently diverse to accommodate the switch to the alternative T=16 arrangement. Thus, one function of the scaffold may be to enforce a size constraint on the range of possible interactions among the shell proteins and hence direct the formation of the desired T=16 icosahedron.
Structural roles of VP19C and VP23.
It is clear that VP19C forms intercapsomeric interactions with VP5 in both the VP26− and VP5-VP19C particles. The comparison shown in Fig. 4, demonstrates that VP19C alone determines the basic dimensions of the triplex and forms its connections with the capsomeres. Thus the VP5-VP19C interaction appears to be the key to forming an icosahedral particle. However, VP23 is able to influence the formation of complexes between VP5 and VP19C. Thus, if VP5, VP19C, and VP23 are expressed together, neither the 880-Å particle nor the normal capsid is formed and only incomplete shells and spiral forms are seen (2, 29, 31).
Both in vitro-assembled HSV procapsids and VP5-VP19C particles are basically spherical. In contrast, the mature HSV capsid is polyhedral, with flattened triangular faces joined at distinct vertices. The transformation (angularization) from spherical to polyhedral form is associated with changes to the shell structure involving reorganization of the subunits in both pentons and hexons and formation of a continuous capsid floor. The mechanism of this angularization is unknown, although it occurs spontaneously on extended incubation of procapsids. Interestingly, in the polyhedral capsid the two molecules of VP23 appear to connect the body of the triplex to the capsid floor (Fig. 5). Since the capsid floor is made up predominantly of domains of VP5 molecules that extend outwards from surrounding capsomeres (7a, 42), this strongly suggests that VP23 interacts directly with VP5 (Fig. 5A and B). Although no such interaction has been detected in either fluorescence (20) or yeast two-hybrid (1) assays, Rixon et al. (25) suggested that it might form in the context of the capsid. Our proposed model for the triplex structure supports this suggestion, since the organization of the capsid floor is markedly different in mature capsids and procapsids (33). It seems likely, therefore, that the conformation of the site on the VP5 molecule which binds VP23 is substantially altered in polyhedral capsids compared to procapsids or the isolated protein. Indeed, binding of VP23 to VP5 may help to shape the arrangement of the VP5 floor domains, thereby ensuring that they adopt the correct conformation and stabilizing their interactions. The absence of the interaction between VP5 and VP23 could, therefore, be one of the reasons why the floor of the VP5-VP19C particle is poorly formed.
A plausible scenario for capsid assembly can be envisaged in which the capsid shell components initially come together in a T=16 icosahedral network dictated by a combination of their own intrinsic properties and the curvature imposed by the scaffolding proteins. During assembly, the triplexes interact with neighboring capsomere subunits through their VP19C backbones, holding them in place until the procapsid shell is complete. The floor domains of the VP5 subunits then come together to form the capsid floor, and in the process, the spatial relationship among capsomeres alters, resulting in angularization. Formation of the floor is accompanied by binding of VP23 to VP5, which fixes and stabilizes the structure. Whatever the exact sequence of events, the result of this process would be that the unstable and possibly inexact contacts between protein subunits seen in procapsids are transformed into the robust network of precise contacts seen in mature capsids.
As our knowledge of their structures increases, it becomes increasingly apparent that an HSV capsid protein or subunit cannot be considered as having a single defined conformation but rather as encompassing a range of possible forms which can alter to suit the requirements of its local environment and morphogenic status (10, 33, 43). It appears from the studies described here, that the interaction between VP5 and VP19C is central to the formation of an icosahedral shell. However, by influencing the conformations of these proteins and the nature of their interactions at a local level, VP23 and the scaffolding proteins together help to define the curvature of the shell and the size of the particle and thus ensure the formation of normal capsids.
ACKNOWLEDGMENTS
This work was supported by NIH (AI38469 and RR02250), NSF (BIR-9413229), and the Human Frontier Science Program (RG-537/96).
We thank Joyce Mitchell for expert technical assistance and Jing He and Amy McGough for helpful discussions.
REFERENCES
- 1.Desai P, Person S. Molecular interactions between the HSV-1 capsid proteins as measured by the yeast two-hybrid system. Virology. 1996;220:516–521. doi: 10.1006/viro.1996.0341. [DOI] [PubMed] [Google Scholar]
- 2.Desai P, Watkins S C, Person S. The size and symmetry of B-capsids of herpes simplex virus type 1 are determined by the gene products of the UL26 open reading frame. J Virol. 1994;68:5365–5374. doi: 10.1128/jvi.68.9.5365-5374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dougherty M, Chiu W. Using animation to enhance 3d visualization: a strategy for a production environment. Microsc Microanal. 1998;4(Suppl. 2):452–453. [Google Scholar]
- 4.Frank J. Classification of macromolecular assemblies studied as “single particles.”. Quart Rev Biophys. 1990;23:281–329. doi: 10.1017/s0033583500005564. [DOI] [PubMed] [Google Scholar]
- 5.Frank J, Verschoor A, Boublik M, van Heel M. Multivariate statistical analysis of ribosome electron micrographs. L and R lateral views of the 40S subunit from HeLa cells. J Mol Biol. 1982;161:107–137. doi: 10.1016/0022-2836(82)90281-9. [DOI] [PubMed] [Google Scholar]
- 6.Gao M, Matusick-Kumar L, Hurlburt W, DiTusa S F, Newcomb W W, Brown J C, McCann III P J, Deckman I, Colonno R J. The protease of herpes simplex virus type 1 is essential for functional capsid formation and viral growth. J Virol. 1994;68:3702–3712. doi: 10.1128/jvi.68.6.3702-3712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harauz G, van Heel M. Exact filters for general geometry three dimensional reconstruction. Optik. 1986;73:146–156. [Google Scholar]
- 7a.He, J. Personal communication.
- 8.Hong Z, Beaudet-Miller M, Durkin J, Zhang R, Kwong A D. Identification of a minimal hydrophobic domain in the herpes simplex virus 1 scaffolding protein which is required for interaction with the major capsid protein. J Virol. 1996;70:533–540. doi: 10.1128/jvi.70.1.533-540.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennard J, Rixon F J, McDougall I M, Tatman J D, Preston V G. The 25 amino acid residues at the carboxy terminus of the herpes simplex virus 1 UL26.5 protein are required for the formation of the capsid shell around the scaffold. J Gen Virol. 1995;76:1611–1621. doi: 10.1099/0022-1317-76-7-1611. [DOI] [PubMed] [Google Scholar]
- 10.Kirkitadze M D, Barlow P N, Price N C, Kelly S M, Boutell C J, Rixon F J, McClelland D A. The herpes simplex virus triplex protein, VP23, exists as a molten globule. J Virol. 1998;72:10066–10072. doi: 10.1128/jvi.72.12.10066-10072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laws K I. Textured image segmentation. USCIPI report 940. Los Angeles: University of South California; 1980. [Google Scholar]
- 12.Liu F, Roizman B. The herpes simplex virus 1 gene encoding a protease also contains within its coding domain the gene encoding the more abundant substrate. J Virol. 1991;65:5149–5156. doi: 10.1128/jvi.65.10.5149-5156.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Ludtke, S., and P. Baldwin. September 1998, posting date. [Online.] EMAN, version 1.0. http://ncmi.bioch.bcm.tmc.edu. [January 1999, last date accessed.]
- 13.Mallat S. Multi-frequency channel decompositions of images and wavelet models. IEEE Trans Acoust Speech Signal Process. 1989;37:2091–2110. [Google Scholar]
- 14.Matusick-Kumar L, Newcomb W W, Brown J C, McCann III P J, Hurlburt W, Weinheimer S P, Gao M. The C-terminal 25 amino acids of the protease and its substrate ICP35 of herpes simplex virus type 1 are involved in the formation of sealed capsids. J Virol. 1995;69:4347–4356. doi: 10.1128/jvi.69.7.4347-4356.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer Y. Wavelets and operators. Cambridge, United Kingdom: Cambridge University Press; 1993. [Google Scholar]
- 16.Morlet J, Arens G, Fourgeau E, Giard D. Wave propagation and sampling theory. Geophysics. 1982;47:222–236. [Google Scholar]
- 17.Newcomb W W, Brown J C. Structure of the herpes simplex virus capsid: effects of extraction with guanidine hydrochloride and partial reconstitution of extracted capsids. J Virol. 1991;65:613–620. doi: 10.1128/jvi.65.2.613-620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newcomb W W, Homa F L, Thomsen D R, Booy F P, Trus B L, Steven A C, Spencer J V, Brown J C. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid formation. J Mol Biol. 1996;263:432–446. doi: 10.1006/jmbi.1996.0587. [DOI] [PubMed] [Google Scholar]
- 19.Newcomb W W, Trus B L, Booy F P, Steven A C, Wall J S, Brown J C. Structure of the herpes simplex virus capsid: molecular composition of the pentons and the triplexes. J Mol Biol. 1993;232:499–511. doi: 10.1006/jmbi.1993.1406. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson P, Addison C, Cross A M, Kennard J, Preston V G, Rixon F J. Localization of the herpes simplex virus type 1 major capsid protein VP5 to the cell nucleus requires the abundant scaffolding protein VP22a. J Gen Virol. 1994;75:1091–1099. doi: 10.1099/0022-1317-75-5-1091. [DOI] [PubMed] [Google Scholar]
- 21.Preston V G, Al-Kobaisi M F, McDougall I M, Rixon F J. The herpes simplex virus gene UL26 proteinase in the presence of the UL26.5 gene product promotes the formation of scaffold-like structures. J Gen Virol. 1994;75:2355–2366. doi: 10.1099/0022-1317-75-9-2355. [DOI] [PubMed] [Google Scholar]
- 22.Preston V G, Coates J A V, Rixon F J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983;45:1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preston V G, Rixon F J, McDougall I M, McGregor M, Al-Kobaisi M F. Processing of the herpes simplex virus assembly protein ICP35 near its carboxy terminal end requires the product of the whole UL26 reading frame. Virology. 1992;186:87–98. doi: 10.1016/0042-6822(92)90063-u. [DOI] [PubMed] [Google Scholar]
- 24.Rixon F J. Structure and assembly of herpesviruses. Semin Virol. 1993;4:135–144. [Google Scholar]
- 25.Rixon F J, Addison C, McGregor A, Macnab S J, Nicholson P, Preston V G, Tatman J D. Multiple interactions control the intracellular localization of the herpes simplex virus type 1 capsid proteins. J Gen Virol. 1996;77:2251–2260. doi: 10.1099/0022-1317-77-9-2251. [DOI] [PubMed] [Google Scholar]
- 26.Saad A, Chiu W, Thuman-Commike P. IEEE Signal Processing Society International Conference on Image Processing. 1998. Multi-resolution approach for automatic selection of spherical particles; pp. 8647–8651. [Google Scholar]
- 27.Sherman M B, Brink J, Chiu W. Performance of a slow-scan CCD camera for macromolecular imaging in a 400 keV electron cryomicroscope. Micron. 1996;27:129–139. doi: 10.1016/0968-4328(96)00018-2. [DOI] [PubMed] [Google Scholar]
- 28.Spencer J V, Newcomb W W, Thomsen D R, Homa F L, Brown J C. Assembly of the herpes simplex virus capsid: preformed triplexes bind to the nascent capsid. J Virol. 1998;72:3944–3951. doi: 10.1128/jvi.72.5.3944-3951.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatman J D, Preston V G, Nicholson P, Elliott R M, Rixon F J. Assembly of herpes simplex virus type 1 capsids using a panel of recombinant baculoviruses. J Gen Virol. 1994;75:1101–1113. doi: 10.1099/0022-1317-75-5-1101. [DOI] [PubMed] [Google Scholar]
- 30.Thomsen D R, Newcomb W W, Brown J C, Homa F L. Assembly of the herpes simplex virus capsid: requirement for the carboxyl-terminal 25 amino acids of the proteins encoded by the UL26 and UL26.5 genes. J Virol. 1995;69:3690–3703. doi: 10.1128/jvi.69.6.3690-3703.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomsen D R, Roof L L, Homa F L. Assembly of herpes simplex virus (HSV) intermediate capsids in insect cells infected with recombinant baculoviruses expressing HSV capsid proteins. J Virol. 1994;68:2442–2457. doi: 10.1128/jvi.68.4.2442-2457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thuman-Commike P A, Greene B, Malinski J A, King J, Chiu W. Role of the scaffolding protein in P22 procapsid size determination suggested by T=4 and T=7 procapsid structures. Biophys J. 1998;74:559–568. doi: 10.1016/S0006-3495(98)77814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trus B L, Booy F P, Newcomb W W, Brown J C, Homa F L, Thomsen D R, Steven A C. The herpes simplex virus procapsid: structure, conformational changes upon maturation, and roles of the triplex proteins V19c and VP23 in assembly. J Mol Biol. 1996;263:447–462. doi: 10.1016/s0022-2836(96)80018-0. [DOI] [PubMed] [Google Scholar]
- 34.van Heel M. Angular reconstitution: a posteriori assignment of projection directions for 3D reconstruction. Ultramicroscopy. 1987;21:111–124. doi: 10.1016/0304-3991(87)90078-7. [DOI] [PubMed] [Google Scholar]
- 35.van Heel M. Classification of very large electron microscopical image data sets. Optik. 1989;82:114–126. [Google Scholar]
- 36.van Heel M, Frank J. Use of multivariate statistics in analysing the images of biological macromolecules. Ultramicroscopy. 1981;6:187–194. doi: 10.1016/0304-3991(81)90059-0. [DOI] [PubMed] [Google Scholar]
- 37.van Heel M, Harauz G, Orlova E V, Schmidt R, Schatz M. A new generation of the IMAGIC image processing system. J Struct Biol. 1996;116:17–24. doi: 10.1006/jsbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- 38.van Heel M, Winkler H, Orlova E V, Schatz M. Structural analysis of ice-embedded single particles. Scanning Microsc Suppl. 1992;6:23–42. [Google Scholar]
- 39.Zhou Z H, Chiu W, Haskell K, Spears H J, Jakana J, Rixon F J, Scott L R. Refinement of herpesvirus B-capsid structure on parallel supercomputers. Biophys J. 1998;74:576–588. doi: 10.1016/S0006-3495(98)77816-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Z H, Hardt S, Wang B, Sherman M B, Jakana J, Chiu W. CTF determination of images of ice-embedded single particles using a graphics interface. J Struct Biol. 1996;116:216–222. doi: 10.1006/jsbi.1996.0033. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Z H, He J, Jakana J, Tatman J, Rixon F, Chiu W. Assembly of VP26 in HSV-1 inferred from structures of wild-type and recombinant capsids. Nat Struct Biol. 1995;2:1026–1030. doi: 10.1038/nsb1195-1026. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Z H, Macnab S J, Jakana J, Scott L R, Chiu W, Rixon F J. Identification of the sites of interaction between the scaffold and outer shell in HSV-1 capsids by difference electron imaging. Proc Natl Acad Sci USA. 1998;95:2778–2783. doi: 10.1073/pnas.95.6.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Z H, Prasad B V V, Jakana J, Rixon F, Chiu W. Protein subunit structures in the herpes simplex virus A-capsid determined from 400 kV spot-scan electron cryomicroscopy. J Mol Biol. 1994;242:458–469. doi: 10.1006/jmbi.1994.1594. [DOI] [PubMed] [Google Scholar]