Abstract
Caffeic acid (CA) is a polyphenol belonging to the phenylpropanoid family, commonly found in plants and vegetables. It was first identified by Hlasiwetz in 1867 as a breakdown product of caffetannic acid. CA is biosynthesized from the amino acids tyrosine or phenylalanine through specific enzyme-catalyzed reactions. Extensive research since its discovery has revealed various health benefits associated with CA, including its antioxidant, anti-inflammatory, and anticancer properties. These effects are attributed to its ability to modulate several pathways, such as inhibiting NFkB, STAT3, and ERK1/2, thereby reducing inflammatory responses, and activating the Nrf2/ARE pathway to enhance antioxidant cell defenses. The consumption of CA has been linked to a reduced risk of certain cancers, mitigation of chemotherapy and radiotherapy-induced toxicity, and reversal of resistance to first-line chemotherapeutic agents. This suggests that CA could serve as a useful adjunct in cancer treatment. Studies have shown CA to be generally safe, with few adverse effects (such as back pain and headaches) reported. This review collates the latest information from Google Scholar, PubMed, the Phenol-Explorer database, and ClinicalTrials.gov, incorporating a total of 154 articles, to underscore the potential of CA in cancer prevention and overcoming chemoresistance.
Keywords: caffeic acid, vegetal sources, metabolism, adjuvant cancer treatment
1. Introduction
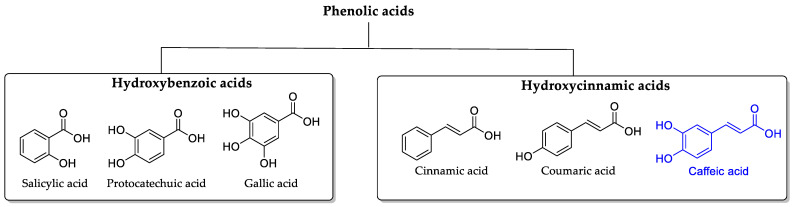
Caffeic acid (CA) was first identified by Hlasiwetz in 1867 during the hydrolysis of caffetannic acid using caustic potash [1]. CA is a naturally occurring polyphenol found widely in the plant kingdom, particularly in beverages like coffee and yerba mate, which are consumed worldwide. Polyphenols are characterized by a benzene structure functionalized with one or more hydroxyl groups. Presently, there are over 8000 known polyphenolic compounds, categorized into various families, Figure 1, including phenolic acids (such as hydroxybenzoic acids and hydroxycinnamic acids), flavonoids (encompassing flavones, flavonols, flavonones, isoflavones, flavononols, anthocyanins, flavan-3-ols, and chalcones), tannins (both hydrolysable and non-hydrolysable (condensed) tannins), stilbenes, lignans, quinones (benzoquinone, naphthoquinone, and anthraquinones), and coumarins (simple coumarins, furanocoumarins, pyranocoumarins, benzocoumarins, coumestans, and biscoumarins) [2,3,4,5,6,7,8,9,10].
Figure 1.
Classification of phenolic acids. Chemical structure of principal hydroxybenzoic and hydroxycinnamic acids, including caffeic acid (CA) in blue (figure created in ChemDraw Ultra 12.0 software).
The properties of CA stem from its distinctive chemical structure. The presence of phenolic hydroxyl groups in the catechol moiety, coupled with a double bond in the carbon chain, imparts both antioxidant and pro-oxidant characteristics to CA. Within cells, CA serves as a primary antioxidant by counteracting harmful reactive oxygen species (ROS) that pose threats to deoxyribonucleic acid (DNA), proteins, and lipids, thus mitigating the risk of carcinogenesis. Moreover, CA exhibits secondary antioxidant activity by stimulating the intracellular nuclear erythroid 2-related factor 2/antioxidant response element (Nrf2/ARE) pathway. The Nrf2 regulates the expression of phase II antioxidant enzymes, including glutathione S-transferase (GST), heme oxygenase 1 (HO-1), and NADPH Quinone Dehydrogenase 1 (NQO1), which play pivotal roles in preserving cellular redox homeostasis [11,12]. Research has shown that CA exhibits hepatoprotective properties in HepG2 cells, shielding them from oxidative stress triggered by tert-butyl hydroperoxide (t-BHP). This suggests that the activation of Nrf2 and a rise in levels of HO-1 and glutamate-cysteine ligase (GCL) within the cell nuclei occur in response [13]. Furthermore, CA has been shown to mitigate cell and tissue damage in the brains of rats exposed to neurotoxic compounds such as quinolinic acid (QUIN, 100 μM), ferrous sulfate (FeSO4, 25 μM), and 6-hydroxydopamine (6-OHDA, 100 μM). At a concentration of 100 μM, CA reduced oxidative damage and improved brain function in rats. Similar findings were observed in the worm model C. elegans, where CA directly activated the Nrf2/ARE pathway [14]. Additionally, CA has been found to down-regulate inflammatory interleukins, specifically interleukin-6 (IL-6) and interleukin-1β (IL-1β), as well as nuclear factor kappa B (NF-κB) in the inflammatory cascade and inhibit signal transducer and activator of transcription 3 (STAT3) and signal-regulated kinase 1/2 (ERK1/2) actions in vitro [15,16,17]. However, an antioxidant agent such as CA can become a pro-oxidant due to its ability to chelate metals such as copper (Cu), inducing lipid peroxidation and causing DNA damage through oxidation or the formation of covalent adducts with DNA [18].
These actions play a vital role in both cancer prevention and treatment. Furthermore, evidence indicates that caffeic acid might enhance the susceptibility of cancer cells to certain drugs frequently employed in chemotherapy, implying its potential as a promising compound for combating chemoresistance. This review aims to underscore the phytochemical characteristics of CA and its direct involvement in cellular pathways linked to cancer development and advancement, in contrast to other reviews that demonstrate the general activity of CA. Here, we provide a strong basis for the use of CA as an adjuvant in cancer chemotherapy and radiotherapy.
2. Methodology
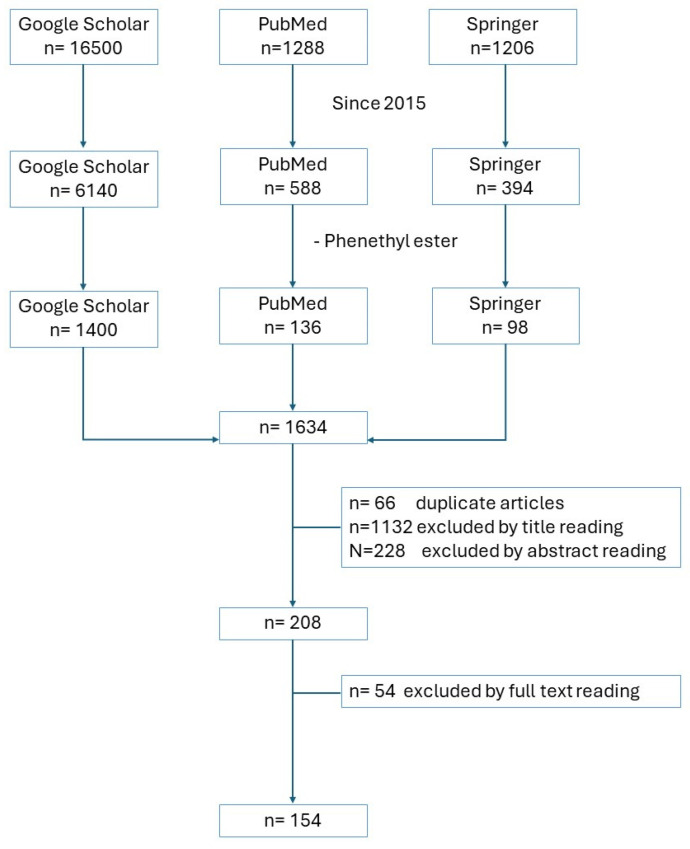
The literature review was conducted across multiple databases, including Google Scholar, PubMed, and Springer (Figure 2). Initially, a search was conducted using keywords such as caffeic acid, chemoresistance, adjuvant, biosynthesis, and metabolism. Subsequently, additional terms like inflammation and molecular targets were combined with caffeic acid for a more comprehensive search. The literature exploration involved examining the bibliographies of selected publications featuring original research to compile this review article.
Figure 2.
Flowchart with exclusion criteria and number of items found.
3. Biosynthesis of Caffeic Acid
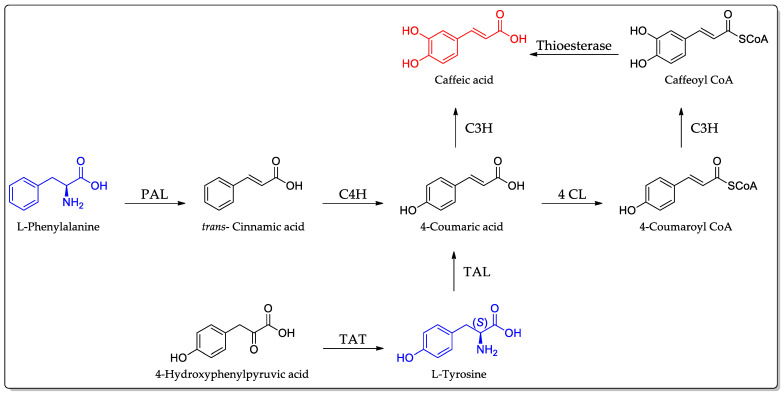
CA is synthesized via the phenylpropanoid pathway, which originates from the amino acids phenylalanine or tyrosine. Tyrosine undergoes a two-step conversion process to produce p-coumaric acid, initially catalyzed by the enzyme tyrosine ammonia-lyase (TAL). Subsequently, p-coumaric acid is transformed into CA by 4-coumarate 3-hydroxylase (C3H), which introduces a hydroxyl group at position 3. Another route involves the conversion of p-coumaric acid to coumaroyl-CoA by 4-coumarate (i.e., CoA ligase (4CL)), followed by m-hydroxylation by C3H to yield caffeoyl-CoA. The hydrolysis of caffeoyl-CoA by CoA thioesters results in the production of caffeic acid and CoA [19]. The enzymes involved in this biosynthetic pathway have been elucidated through knockout mutations and RNAi-mediated studies in Arabidopsis plants, facilitating the identification and functional characterization of key genes and enzymes [20,21]. Figure 3 shows a schematic representation of the biosynthetic pathway.
Figure 3.
Biosynthetic pathways for the formation of CA (shown in red) from its precursor amino acids (in blue). The involved enzymes are abbreviated: PAL = phenylalanine ammonia-lyase; C4H = cinnamate 4-hydroxylase; 4CL = 4-coumaric acid CoA ligase; TAT = tyrosine aminotransferase; and C3H = p-coumarate 3-hydroxylase.
4. Natural Sources with High Content of CA
CA is abundantly present in a wide range of plant-derived foods, including fruits, vegetables, and certain beverages. The concentration of CA within a particular plant species varies depending on factors such as the plant part (e.g., fruits, leaves, stems, and roots), degree of ripeness, processing techniques, and storage conditions. Natural sources rich in CA include coffee beans, especially when freshly roasted, which can contain significant amounts of CA depending on the coffee type and brewing method [22,23]. Fruits such as apples, pears, cherries, and grapes are known to contain CA [24,25], while vegetables like bell peppers, broccoli, carrots, and spinach also contribute to CA intake [26,27]. Herbs and spices like thyme, oregano, sage, rosemary, and basil are recognized as rich sources of CA [28,29]. Additionally, whole grains like wheat, oats, and rice, particularly their bran and outer layers, contain this polyphenol [30,31]. Recent research has revealed the presence of CA in certain mushrooms, including Agaricus bisporus, Coprinus atramentarius, Morchella elata, and Laetiporus sulphureus [32]. Furthermore, the traditional consumption of artichoke (Cynara scolymus) has been identified as a significant source of CA, particularly in its leaves [33,34]. The Phenol-Explorer database (accessed on 12 March 2024) (http://phenol-explorer.eu/) offers comprehensive information on polyphenol levels in various foods, comprising over 35,000 data points encompassing 500 distinct polyphenols across more than 400 food items. Table 1 provides an overview of the major natural sources of CA.
Table 1.
Assessing caffeic acid levels in various plants, including both edible and medicinal plants and plants consumed as is, as used culinary ingredients, or as infusions.
| Edible Vegetables or Fruits | Culinary Plants or Herbal Infusion | ||||
|---|---|---|---|---|---|
| Scientific Name/ Common Name |
Caffeic Acid mg/kg |
References | Scientific Name/ Common Name |
Caffeic Acid mg/kg |
References |
|
Allium sativum (Garlic) |
50 | [35] |
Andrographis paniculata (Green chiretta) |
450 | [35] |
|
Apium graveolens (Celery) |
880–1120 | [35] |
Anethum graveolens (Dill) |
1840 | [35] |
| Averrhoa carambola (Carambola) | 90 | [35] |
Artemisia dracunculus (Tarragon) |
620 | [35] |
|
Brassica juncea (Brown mustard) |
830 | [35] |
Artemisia pallens (Davana) |
110 | [35] |
|
Capsicum annuum (Red Pepper) |
250–850 | [35] |
Artemisia vulgaris (Mugwort) |
25.4 | [28] |
|
Carica papaya (Papaya) |
5080 | [35] |
Camellia sinensis (Tea plant) |
510 | [35] |
|
Coriandrum sativum (Coriander) |
240 | [35] |
Centella asiatica (Indian pennywort) |
860 | [35] |
|
Eryngium foetidum (Cilantro) |
1600 | [35] |
Chromolaena odorata (Devil weed) |
6210 | [35] |
|
Daucus carota (Carrot) |
850 | [35] |
Ilex paraguariensis (Yerba mate) |
63.5 | [36,37] |
| Helianthus annuus (Sunflower) | 30–1100 | [35] |
Clerodendrum indicum (Bharangi) |
110 | [35] |
|
Ipomoea aquatica (Water spinach) |
1130 | [35] |
Clerodendrum
thomsoniae |
770 | [35] |
|
Ipomoea batatas (Sweet Potato) |
125 | [38] |
Coffea canephora (Coffea) |
12,330 | [35] |
|
Lactuca sativa (Lettuce) |
2580 | [35] |
Ginkgo biloba (Ginkgo) |
398 | [39] |
|
Morus alba (Mulberry) |
250 | [35] |
Cymbopogon citratus (Lemon grass) |
730 | [35] |
|
Morinda citrifolia (Noni) |
100 | [35] |
Echinacea purpurea (Purple coneflower) |
115.9 | [28] |
|
Persea americana (Avocado) |
80 | [35] |
Euphorbia hirta (Asthma plant) |
210 | [35] |
|
Persicaria odorata (Vietnamese coriander) |
910 | [35] |
Eucommia ulmoides (Hardy rubber tree) |
190 | [35] |
|
Spinacia oleracea (Spinach) |
2.4–5.3 * 370 ** |
[29] [35] |
Gnaphalium polycaulon (Western cudweed) |
2360 | [35] |
|
Psidium guajava (Guava) |
220 | [35] |
Hibiscus sabdariffa (Roselle) |
3510 | [35] |
|
Punica granatum (Pomegranate) |
3050–3630 | [35] |
Hyptis suaveolens (Bamburral) |
1110 | [35] |
|
Raphanus sativus (Radish) |
330 | [35] |
Leonotis nepetifolia (Klip dagga) |
4180 | [35] |
|
Petroselinum crispum (Parsley) |
480 | [35] |
Leonurus sibiricus (Motherwort) |
120 | [35] |
| Phyllanthus emblica (Gooseberry) | 290 | [35] |
Salvia officinalis (Sage) |
1660 ** 74.2 * |
[35] [39] |
|
Physalis angulata (Poppers) |
120 | [35] |
Perilla frutescens (Purple mint) |
1890 870 |
[35] [40] |
|
Physalis peruviana (Goldenberry) |
860 | [35] |
Melissa officinalis (Lemon balm) |
26.8 1740 |
[35] [28] |
|
Satureja hortensis (Summer savory) |
248 | [41] |
Mentha arvensis (Pudina) |
1080 | [35] |
|
Solanum melongena (Eggplant) |
3.8 | [42] | Mentha cordifolia | 1000 | [35] |
| Vaccinium myrtillus (Blueberry) | 59.66 | [43] |
Mentha piperita (Peppermint) |
57.6 | [28] |
|
Moringa oleifera (Moringa) |
40–300 | [35] |
Rosmarinus officinalis (Rosemary) |
1460 ** 43.6 ** |
[35] [28] |
|
Thymus vulgaris (Thyme) |
117 * 1550 ** 69.4 ** |
[39] [35] [28] |
Origanum majorana (Marjoram) |
104 * 67 ** |
[39] [28] |
| Ocimum basilicum (Basil) | 16.6–41.1 * 54.4 ** 3110 ** |
[29] [28] [35] |
Origanum vulgare (Oregano) |
4100 ** 140 ** 41.4 ** |
[35] [41] [28] |
* mg/kg of fresh weight. ** mg/kg of dried plant.
5. Absorption, Distribution, and Metabolism of CA
The potential therapeutic use of CA relies heavily on its pharmacokinetic behavior, which encompasses its stability within the digestive system, including exposure to acidic pH, bile, and metabolic enzymes, as well as its absorption in the intestines, distribution throughout the body, and subsequent metabolic processes leading to excretion. Studies have demonstrated that CA can traverse the blood–brain barrier (BBB) and reach concentrations of 0.02 µM in the cerebrospinal fluid, thereby exerting beneficial effects on the brain [44]. To investigate the pharmacokinetics of CA, experiments were conducted in rats using radioactive-labeled CA [3-14C]. Rats were orally administered 1.52 mg of labeled CA [3-14C] (140 × 106 dpm) via gavage, and various samples, including organs, tissues, plasma, urine, and feces, were collected over a duration of up to 72 h. These samples were then analyzed using high-performance liquid chromatography (HPLC) coupled with online radioactivity detection and tandem mass spectrometry, allowing for the determination of residual radioactivity in the samples. The results of these analyses are summarized in Table 2.
Table 2.
Distribution of radioactivity (%) in the gastrointestinal tract of rats after ingestion of [3-14C] CA by baggage.
| Sample | 1 h | 3 h | 6 h | 12 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|---|---|---|
| Stomach | 62 | 7.1 | 0.2 | 0.4 | 0.1 | 0.2 | 0.1 |
| Duodenum | 7.9 | 8.1 | 0.2 | 0.3 | 0.1 | 0.1 | <0.1 |
| Jejunum/ileum | 9.1 | 16 | 1.1 | 1.4 | 1.1 | 0.7 | <0.1 |
| Cecum | <0.1 | 7.4 | 6.6 | 3.9 | 1.9 | 0.3 | <0.1 |
| Colon | <0.1 | 7.4 | 6.6 | 3.9 | 1.9 | 0.3 | <0.1 |
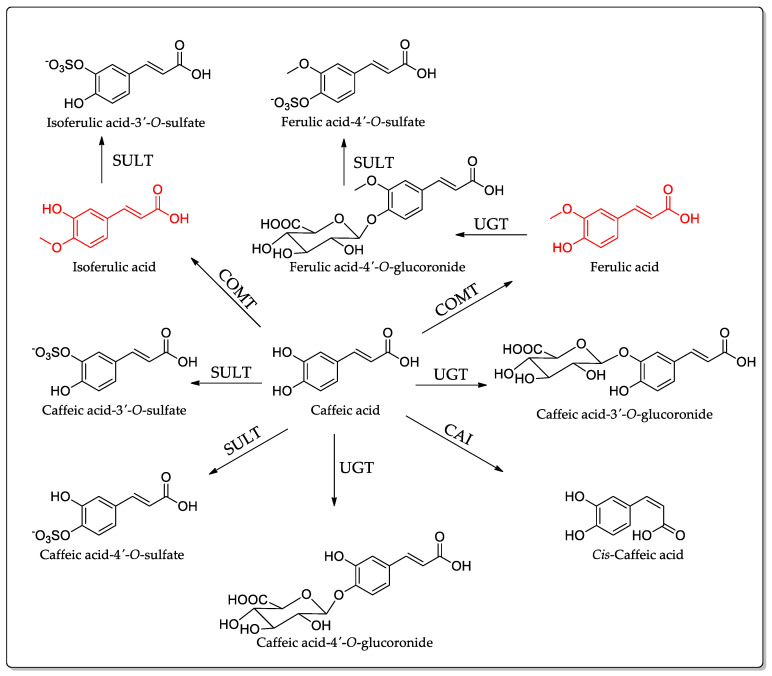
One hour after ingestion, approximately 80% of the radioactivity remained in the gastrointestinal (GI) tract, while the remaining 20% had already entered the bloodstream and kidneys. This indicates that the absorption of CA starts in the stomach and is subsequently excreted, primarily through urine, in the form of nine identified radioactive compounds, including trans-caffeic acid, cis-caffeic acid, caffeic acid 3′-O-sulfate, caffeic acid-4′-O-sulfate, caffeic acid 3′-O-glucuronide, caffeic acid 4′-O-glucuronide, isoferulic acid-3′-O-sulfate, ferulic acid-4′-O-sulfate, and ferulic acid-4′-O-glucuronide (refer to structures in Figure 4), along with four unidentified metabolites. By the 72-h mark, there was minimal to no accumulation of radioactivity in various body tissues, including the kidney, brain, testes, lung, heart, muscle, liver, spleen, and red blood cells. Only a small quantity of an unidentified 14C-labeled metabolite was excreted in feces. The overall recovery of radioactivity was approximately 80% [45].
Figure 4.
Metabolism of caffeic acid by rats. The involved enzymes are abbreviated: CAI = caffeic acid isomerase; COMT = catechol-O-methyltransferase; SULT = sulfotransferase; and UGT = uridine-5′-diphosphate-glucuronosyltransferase [45].
Kishida and Matsumoto conducted a study on the metabolism of CA in male Wistar rats that were administered a dosage of 40 mg/kg. They found that at 48 h after administration, approximately 61.6% of CA was excreted, with the excretion primarily consisting of glucuronidated and/or sulfated CA-conjugates. To analyze these conjugates and quantify the amount of free CA, specific enzymes were utilized to release CA from the conjugates. Subsequently, CA levels in the urine were measured using HPLC-DAD [46].
6. Signaling Pathways Affected in Cancer Progression
Despite continuing advances in treatments, cancer remains a major health challenge, with cardiovascular diseases and cancers being the most common causes of death worldwide [47]. Understanding the detailed mechanisms of carcinogenesis, which are triggered by prolonged exposure to various risk factors, is crucial for preventing cancer development and progression [48]. Hanahan and Weinberg have summarized several cellular processes that contribute to the emergence of neoplasms and their malignant progression, including self-sufficiency in growth signals, insensitivity to growth-inhibitory signals, evasion of apoptosis, unlimited replicative potential, tissue invasion and metastasis, and sustained angiogenesis [49].
Although cancer is a multifactorial disease, a common outcome of exposure to risk factors is inflammation and uncontrolled production of ROS. ROS and RNS are a group of radical and non-radical molecules produced by cellular metabolism or induced by external sources. Excessive ROS formation can damage macromolecules such as DNA, proteins, and lipids, leading to genomic instability and altered cell growth [50]. ROS can influence cell cycle progression by affecting the activity of proteins like cyclin-dependent kinase inhibitor p21 or the serine/threonine mutant ataxia telangiectasia protein kinase (ATM). ATM is crucial for DNA repair and impacts cell signaling pathways that are important for proliferation and apoptosis, such as the Akt or p53 pathway [48]. Normally, intracellular ROS accumulation due to carcinogen exposure is mitigated by antioxidant enzymes like catalase (CAT), superoxide dismutase (SOD), or the glutathione (GSH) system. However, when ROS levels exceed the cell’s antioxidant capacity, extensive oxidative damage to cellular components occurs, increasing the likelihood of mutations in oncogenes or tumor suppressor genes and leading to the formation of precancerous lesions. Consequently, ROS are attributed to a tumor-promoting role during carcinogenesis as they can inactivate or activate proteins involved in cancer-related signaling pathways.
ROS, chronic infections, and inflammation all activate the NF-κB pathway, promoting the dimerization of the inhibitor of κB kinase (IKK) gamma (IKKγ), also known as the nuclear factor κB (NF-κB) essential modulator (NEMO). This component of the IKK complex is crucial for the canonical activation of the NF-κB pathway [51,52,53]. The dimerization of IKKγ/NEMO triggers the phosphorylation of inhibitory NF-κB proteins (IκBs), leading to their degradation by the proteasome. This process induces the phosphorylation of p50/p65 dimers via the activation of protein kinase-A (PKAc), facilitating the translocation of NF-κB to the nucleus, where it regulates the transcription of genes associated with survival, cell proliferation, proinflammatory cytokines, and ROS-related genes [54,55]. The activation of the NF-κB signaling pathway is linked to various cellular processes, including inflammation and immune response. The disruption of this pathway has been associated with inflammatory diseases and the development and progression of cancer. In cancer, specifically, NF-κB activation is linked to apoptosis resistance as it negatively regulates anti-apoptotic proteins B-cell leukemia/lymphoma 2 (Bcl-2) and B-cell lymphoma-extra large (Bcl-XL). It is also linked to increased malignancy by modulating the transcription of genes related to cell proliferation of cyclin D1 (CCND1) and cellular Myc (c-Myc). It also influences the expression of genes involved in angiogenesis, such as vascular endothelial growth factor (VEGF), proinflammatory cytokines related to carcinogenesis, such as IL-1β and IL-6, genes involved in detoxification and drug resistance, and antioxidant enzymes such as SOD, CAT, thioredoxin (TRX), HO-1, and glutathione peroxidase (GPX1) [54].
Another pathway activated by ROS is the Nrf2 pathway. Nrf2 is a key regulator of cellular redox homeostasis, controlling the expression of various antioxidant and cytoprotective genes involved in drug detoxification, cell proliferation, metabolism, autophagy, apoptosis, proteasome function, DNA repair, and antioxidant response, collectively known as phase II genes. Under normal conditions, Nrf2 associates with the Kelch-like Echassociated protein 1 (Keap1), part of the Cullin 3 (CUL3)-based ubiquitin ligase complex that regulates Nrf2 stability, and promotes its degradation via the ubiquitin/proteasome pathway. However, in the presence of electrophilic molecules or oxidative stress, cysteine residues in Keap1 are modified, leading to its inactivation and reducing its binding to Nrf2 or CUL3, thereby preventing Nrf2 degradation. Stabilized Nrf2 translocates to the nucleus, where it dimerizes with other leucine zipper proteins of the v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog (Maf) family to activate AREs on phase II genes. Additionally, Nrf2 degradation can be mediated by β-transducin repeat-containing protein (β-TrCP) in the nucleus, which binds to phosphorylated Nrf2 and Cullin 1 (CUL1), inducing Nrf2 ubiquitination. Another mechanism involves Keap1 degradation mediated by phosphorylated p62/Sequestosome-1 (p62/SQSTM1) [54,56]. Strong cellular antioxidant, drug-detoxifying, and cytoprotective activities have been linked to cancer malignancy, with evidence showing that cancer cells often exhibit aberrant Nrf2 activation. Various mechanisms have been identified, including somatic mutations in Nrf2, Keap1, or CUL3 genes, epigenetic silencing of Keap1, accumulation of Keap1-interacting proteins like p62/SQSTM1 or p21, and cysteine modification by oncometabolites such as fumarate, which affect Keap1 activity [54]. Nrf2 activation has been associated with resistance to chemotherapy and radiotherapy and poor prognosis in cancers such as head and neck, lung, ovarian, and breast cancer [57,58,59,60,61,62,63]. Importantly, several natural and chemical compounds are being tested to inhibit Nrf2 signaling in cancer cells with high Nrf2 activity, while Nrf2 inducers are being explored to protect normal cells from carcinogens [64].
Oxygen deprivation in tumors significantly influences cancer development and metastasis. Hypoxia-inducible transcription factors (HIFs) are crucial in promoting the survival, proliferation, adaptability, and motility of malignant cells. Additionally, hypoxia elevates Programmed death-ligand 1 (PD-L1) expression, also known as cluster of differentiation 274 (CD274) or B7 homolog 1 (B7-H1), in both malignant and immunoregulatory cells [65]. ROS also play a role by oxidizing prolyl hydroxylase domain (PHDs) proteins, essential for the binding of von Hippel–Lindau tumor suppressor protein (p-VHL) to HIF-1α, leading to its ubiquitination and degradation in the proteasome. HIF-1 operates as a heterodimeric complex composed of an alpha and a beta subunit. HIF-1β is constitutively expressed, while HIF-1α accumulation is induced under hypoxic conditions. In normoxia, PHDs hydroxylate two prolyl residues of HIF-1α, marking it for ubiquitination and subsequent von Hippel–Lindau (VHL) complex-mediated degradation [54]. Conversely, during hypoxia, HIF-1α accumulates, and the HIF-1 complex activates the transcription of genes containing hypoxia response elements (HREs) in the cell nucleus. HIF-1 signaling has been extensively studied in various cancers, including pancreatic, gastric, and prostate cancers, due to its regulation of genes involved in angiogenesis, metabolism, glucose transport, and cell migration [66,67,68,69,70].
In neoplastic cells, the ErbB family of proteins and their signaling pathways are frequently altered. This family consists of four receptor tyrosine kinases related to the epidermal growth factor receptor (EGFR); the name of the family is derived from the viral oncogene homologous to erythroblastic leukemia viral oncogene. In humans, this family includes Human epidermal growth factor receptor 1 or Her 1 (EGFR, ErbB1), Her2 (ErbB2), Her3 (ErbB3), and Her4 (ErbB4) [71]. ErbB signaling influences cell proliferation, migration, differentiation, apoptosis, and motility via the Phosphoinositide 3-kinase/serine threonine-protein kinases (PI3K/Akt), the Janus kinase/signal transducer and activator of transcription (JAK/STAT), and Mitogen-activated protein kinase (MAPK) pathways. Aberrant signaling of ErbB family members is critical in tumorigenesis and immune evasion in various malignancies. Excessive ErbB signaling is linked to the development of numerous solid tumors, and direct alterations in these receptor-dependent pathways are associated with cancer progression [72].
The PI3K/AKT pathway is pivotal for cell survival, proliferation, and protein biosynthesis. Phosphoinositide 3-kinase (PI3K) is a family of lipid kinase enzymes that phosphorylate the 3’-OH group of phosphatidylinositols (PtdIns) in the plasma membrane (Class I) or intracellular membranes for vesicular trafficking regulation (Classes II and III). These proteins are biologically significant as their activation influences cellular processes such as growth, survival, metabolism, inflammation, motility, proliferation, and therapy resistance [73]. Class I PI3K and its downstream effector AKT/PKB are activated by extracellular growth factors like epidermal growth factor (EGF), platelet-derived growth factor (PDGF), or insulin, triggering a phosphorylation cascade that culminates in the activation of the mammalian target of rapamycin (mTOR) kinase and the Ras/MAPK pathway. Ras proteins, from the acronym Rat sarcoma virus, are located in the plasma membrane of cells and act as molecular switches that send signals to activate cell growth. The Ras family is a protein superfamily of small GTPases. Members of the superfamily are divided into families and subfamilies based on their structure, sequence, and function. The five main families are Ras, Ras homologous (Rho), Ras-related nuclear protein (Ran), Ras-related in brain (Rab), and ADP-ribosylation factor (Arf) GTPases. The activation of the PI3K/AKT pathway is widely associated with carcinogenesis and is frequently observed in various human cancers [74,75,76,77]. High levels of ROS can oxidize and inactivate the phosphatase and tensin homolog (PTEN), promoting PI3K/AKT pathway activation [54]. PTEN acts as a phosphatase to dephosphorylate PtdIns, counteracting PI3Ks by specifically catalyzing the dephosphorylation of the 3phosphate of the inositol ring at phosphatidylinositol 3,4,5-trisphosphate (PIP3) to produce phosphatidylinositol 3,4-bisphosphate (PIP2). This dephosphorylation is crucial because it inhibits the Akt signaling pathway; thus, sustained PTEN inactivation leads to excessive PI3K/AKT pathway activation [78]. The PTEN phosphatase is encoded by the PTEN tumor suppressor gene, and mutations or deletions of this gene contribute to constitutive PI3K/AKT signaling, facilitating the development of cancers such as glioblastoma, lung cancer, breast cancer, and prostate cancer [79,80,81,82,83]. Clinical trials are currently investigating PI3K/AKT pathway inhibition for treating pancreatic, lung, ovarian, breast, melanoma, and hematological malignancies [54].
Mitogen-activated protein kinases (MAPKs) are a group of enzymes that phosphorylate various cellular proteins, including transcription factors, nuclear proteins, membrane transporters, cytoskeletal elements, and other kinases, upon activation. This phosphorylation regulates crucial cellular processes, such as proliferation, migration, differentiation, senescence, and cell death. MAPKs are activated by extracellular signals like growth factors (EGF, PDGF, insulin), cytokines, and intracellular stressors. Key MAPK subfamilies, such as ERK1/2, Jun N-terminal kinase (JNK), and p38, respond to different stimuli. The Ras/Raf/MEK/ERK1/2 pathway is notably relevant in cancer, often altered by excessive growth factors, hormonal signaling, or oncogenic mutations, leading to abnormal mitogenic signaling, increased proliferation, and apoptosis resistance. Raf is an acronym for Rapidly Accelerated Fibrosarcoma and is the best characterized Ras effector and a member of a family of serine/threonine kinases. Mutations in Ras family genes, found in about 30% of human cancers, activate proteins like PI3K, Rac, and Rho, which influence cytoskeletal dynamics and cell invasiveness. Rac-GTPase represents a subfamily of the Rho family. Specific small molecule inhibitors targeting the Ras/MAPK pathway are under clinical trials, including those for the KRAS-G12C mutation, with promising clinical outcomes anticipated. The Ras/MAPK pathway activation is intricately regulated and closely linked to ROS production. ROS can directly activate MAPKs without external stimuli like EGF, and antioxidant inhibition has been shown to prevent this activation in various experimental setups. Ras-mediated transformation involves NADPH oxidase 1 (NOX1) activation via the Ras-Rac1-NOX1 pathway. Key redox sensors in the MAPK cascade include TRX and apoptosis signal-regulating kinase 1 (ASK1). TRX inhibits ASK1 when reduced, but ROS-induced oxidation releases ASK1, activating JNK and p38 signaling, which affects cell proliferation, differentiation, apoptosis, inflammation, and stress response. Additionally, ROS can activate ERK signaling, potentially through the upstream activation of the EGF receptor (EGFR). This process involves NOX-mediated H2O2 generation and SHP-2 phosphatase inactivation, sustaining EGFR activation and promoting cell signaling via the Ras-Raf-MEK-ERK pathway, impacting cell proliferation and differentiation. MAPK activation is also linked to mitochondrial changes, including increased mitochondrial ROS production. Mutations in Ras or ERK2 activation can induce mitochondrial fission and increase mitochondrial mass, affecting ATP production and contributing to cancerous traits in cells with Ras mutations [54].
The intracellular JAK/STAT pathway and modification of histone marks on nucleosomes regulate the expression of proinflammatory mediators, playing a crucial role in carcinogenesis [84]. The activation of the JAK/STAT pathway occurs in response to various hormones (prolactin, growth hormone, leptin, and erythropoietin), cytokines, and growth factors through their respective receptors. This signaling pathway regulates cell proliferation, differentiation, survival, motility, and apoptosis in different tissues [85]. STATs are latent cytoplasmic transcription factors activated by phosphorylation through Janus kinase (JAK). The JAK family includes JAK1-3 and tyrosine kinase 2 (TYK2), which interact non-covalently with membrane receptor domains or intracellular portions of growth factor receptors. Upon cytokine binding, JAKs phosphorylate tyrosine residues to transmit signals from membrane-bound receptors. Phosphorylated STAT molecules then dimerize and translocate to the nucleus, initiating gene transcription that regulates cell cycle progression, apoptosis initiation, and occurrence. These processes are vital for cellular homeostasis, and disruptions in this pathway contribute to cancer progression, inflammatory diseases, and autoimmune disorders. STAT family proteins are implicated in creating and sustaining a procarcinogenic inflammatory microenvironment, initiating malignant transformation, and promoting cancer progression. Inflammation plays a critical role in tumor initiation and progression, both as an initiator of oncogenic transformations and as a promoter through genetic and epigenetic alterations that foster an inflammatory microenvironment conducive to tumor growth. Additionally, immune responses and inflammatory mediators mediated by STAT3, STAT5, and STAT6 suppress antitumor immunity, further contributing to carcinogenesis. Given the JAK-STAT pathway’s role in regulating cell cycle and apoptosis effector molecules, it is evident that this pathway promotes carcinogenesis by enhancing proliferation and modulating programmed cell death [84].
In human cancer, the TP53 tumor suppressor gene, situated on chromosome 17, is the most frequently mutated. It encodes p53, a transcription factor binding to specific DNA sequences within the genome, activating adjacent genes and those controlled by enhancers with p53 binding sites. Additionally, p53 can repress the transcription of numerous genes, typically through indirect mechanisms [86]. The primary biological role of p53 is well-understood: it induces cell cycle arrest, senescence, or apoptosis in response to DNA damage, thereby preventing the accumulation of oncogenic mutations, earning it the nickname “guardian of the genome”. Moreover, p53 participates in other cellular processes like autophagy, metabolism, and cellular plasticity [87]. It enhances glutamine catabolism, supports antioxidant activity, reduces lipid synthesis, promotes fatty acid oxidation, and stimulates gluconeogenesis. Under normal conditions in unstressed cells, p53 levels remain low due to continuous proteasomal degradation mediated by the Mouse double minute 2 homolog (MDM2), an E3 ubiquitin ligase, and the principal inhibitor of p53, along with COP1 and p53-induced RING-H2 protein (Pirh2). Additionally, transformed mouse 3T3 cell double minute 4 (MDM4), formally named MDMX, restricts p53’s biochemical activity as a transcription factor, serving as a physiological inhibitor [86]. At basal levels, p53 maintains the transcription of several antioxidant genes (SESN1/2, GPX1, and AIF). However, during stress conditions, p53 accumulates in the cytoplasm and induces the expression of NQO1 and proline oxidase (POX), enzymes that generate ROS, alongside Bcl2-associated X, apoptosis regulator (Bax), and p53-upregulated modulator of apoptosis (PUMA), which promote mitochondrial uncoupling and ROS production, culminating in oxidative stress and apoptosis. Bax and Bcl-2 homologous antagonist/killer (Bak) are members of the Bcl-2 family and core regulators of the intrinsic pathway of apoptosis. Upon apoptotic stimuli, they are activated and oligomerized at the mitochondrial outer membrane (MOM) to mediate its permeabilization, which is considered a key step in apoptosis. PUMA is a proapoptotic homolog domain-3-only member of the Bcl-2 protein family, which has been demonstrated to be critical for some cells’ apoptosis induced by ER stress. A crucial target of p53 involved in ROS metabolism and regulation is the TP-53-induced regulator of apoptosis and glycolysis (TIGAR), which inhibits glycolysis and enhances glucose flux into the pentose phosphate pathway, a major source of NADPH utilized in reducing GSH. Conversely, p53 mutations in cancer cells impair mitochondrial respiration and elevate glycolysis [54].
In summary, multiple pathways and factors contribute to the emergence and progression of various cancers. Investigating the dysregulation of intracellular pathways in cancer and exploring molecules capable of restoring these pathways are crucial endeavors.
7. Anti-Inflammatory Activity of Caffeic Acid against Cancer
As mentioned in the previous section, chronic inflammation has been linked to the initiation and progression of cancer [88,89,90]. ROS and RNS, generated during inflammation, can inflict DNA damage, potentially leading to mutations that contribute to the maintenance of tumors [91,92]. Chronic inflammation often triggers cellular proliferation as part of tissue repair mechanisms, inadvertently creating conditions favorable for the expansion of cells with damaged DNA or mutations, thus heightening cancer risk. Moreover, inflammation has been implicated in cancer progression by promoting the production of inflammatory cytokines that support cell proliferation, inhibit apoptosis, stimulate tumor angiogenesis and vascularization, and facilitate tumor invasion and metastasis.
NF-κB is a crucial protein complex responsible for controlling DNA transcription, cytokine synthesis, and cell viability. The dysregulation of NF-κB is associated with immune response irregularities and cancer advancement, making it a significant factor in tumor biology. It influences pathways like JNK/p38 MAPK and positively regulates c-Myc, thus playing a role in various reported neoplasms. Consequently, the inhibition of NF-κB holds promise as a target for cancer therapy development [93]. In this context, CA has shown the capability to regulate this pathway, thereby disrupting cancer cell proliferation, viability, and invasion in endothelial cells, triggered by lipopolysaccharide (LPS), as well as in mammary epithelial cells [94,95]. Consequently, CA modulation of cytokines may affect the production and function of various inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) [96] and interleukins IL-1β, IL-6, and IL-8 [97,98,99,100].
CA has been discovered to impede Cyclooxygenase-2 (COX-2), an enzyme pivotal in the inflammatory cascade. Elevated COX-2 expression is evident during prolonged inflammation, where it can foster the onset and progression of chronic ailments, such as cancer. Studies suggest that CA, at concentrations ranging from 50 to 10 µM, effectively suppresses COX-2 and its byproduct, prostaglandin E2 (PGE2), in human colon myofibroblast cells (CCD-18Co) [99]. Consequently, CA also hampers the enzyme Nitric Oxide Synthase (iNOS), curtailing nitric oxide (NO) production, which may aid in inhibiting tumor angiogenesis [101].
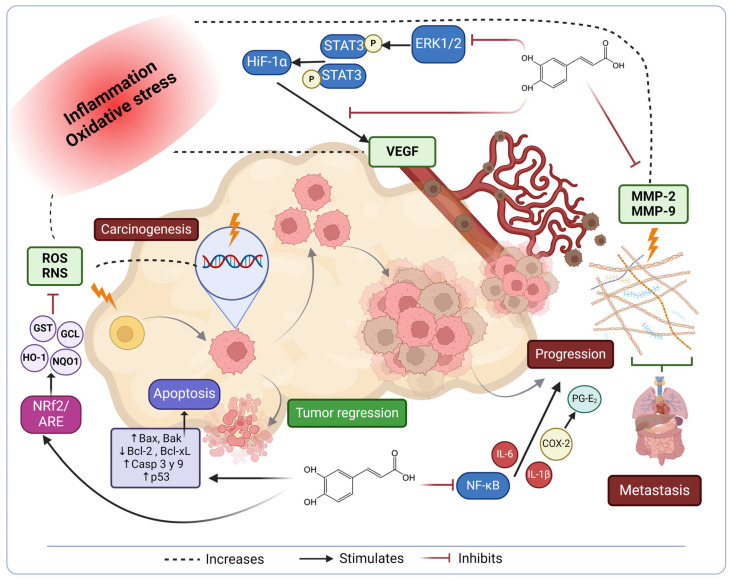
Inflammation induces the secretion of molecules like VEGF, which stimulates the development of new blood vessels that are crucial for supplying oxygen and nutrients to tumors. The activity of VEGF is amplified by HIF-1α and STAT3. In a Caki-I carcinoma animal model, CA was observed to suppress STAT3 phosphorylation, thus diminishing its function and consequently impeding HIF-1α, resulting in reduced tumor vascularization and VEGF gene expression [16]. In retinal endothelial cells, CA demonstrated potent anti-angiogenic effects, mitigating vaso-proliferative retinopathies associated with ROS-induced VEGF [102]. In hepatocellular carcinoma cells (HCC), CA curbed VEGF and vascularization induced by CoCl2, accomplishing this through the stabilization of STAT3/JNK1/HIF-1α [103]. Guo et al. discovered that CA serves as a potent inhibitor of prolyl hydroxylase-2 (PHD2), an enzyme responsible for HIF hydroxylation and degradation. In neuronal cells such as PC12 and SH-SY5Y, CA attenuated cell apoptosis induced by hypoxia. Furthermore, in mice, CA administration decreased injury markers such as lactate dehydrogenase, malondialdehyde, and lactic acid [104].
Another process associated with inflammation in cancer is the production of enzymes such as metalloproteinases (MMPs), which play a role in breaking down the extracellular matrix of cells. MMP-2 and MMP-9 can degrade these barriers, facilitating the invasion of cancer cells into nearby tissues and their spread to distant areas. Through computational studies, CA has been identified as an inhibitor of MMP-9 and dipeptidyl peptidase-4 (DPP-4) enzymes, with respective IC50 values of 158.19 and 88.99 µM [105]. Chung et al. explored the enzymatic inhibitory effects of CA extracted from Euonymus alatus and its synthetic derivative caffeic acid phenethyl ester (CAPE) in animal experiments using rats. The animals were administered CA and CAPE at doses of 5 mg/kg subcutaneously or 20 mg/kg orally. Both compounds exhibited the inhibition of MMP-2 and -9, with no effect on MMP-1, -3, and -7. Moreover, in xenograft models, CA and CAPE hindered the growth of HepG2 tumors and liver metastasis by suppressing MMP-9 activity and decreasing NF-κB expression [106]. These multifaceted mechanisms orchestrated by CA in cancer progression are depicted in Figure 5.
Figure 5.
Antioxidant and anti-inflammatory mechanisms modulated by CA in cancer progression.
8. Caffeic Acid Properties against Cancer
Apoptosis, a regulated cell death mechanism, serves to eliminate damaged or unnecessary cells. CA has demonstrated the ability to trigger apoptosis in cancer cells by influencing the mitochondrial pathway. This is accomplished by the inhibition of the Bcl-2 family of proteins, leading to an increase in pro-apoptotic members (such as Bax and Bak) and a decrease in anti-apoptotic ones (such as Bcl-2 and Bcl-xL) [107]. This action can result in the release of cytochrome c from the mitochondria into the cytosol, initiating the activation of caspases—protease enzymes crucial for programmed cell death [108]. Specifically, CA at a concentration of 10 µg/mL can activate both initiator caspases (like caspase-9) and executioner caspases (such as caspase-3 and caspase-7), leading to the breakdown of cellular components necessary for apoptosis (Figure 4) [109]. Furthermore, CA has been found to induce the expression of p53, a tumor suppressor protein pivotal in preventing cancer development [110]. The activation of p53 can result in cell cycle arrest and apoptosis.
In general, it has been shown that CA is a potent modulator of apoptosis and autophagy in cancer cells, thus affecting their proliferation and survival, including in carcinoma cells in the head and neck [111] and MCF-7 [112], multiple myeloma (MM.1R, RPMI8226, and U266) [113], leukemia (K562) [114], and osteosarcoma cells (MG-63) [115]. In human melanoma SK-Mel-28 cells, the administration of CA (0–200 μM) induces apoptosis and cell cycle arrest by increasing the expression profile of caspase 1 and caspase 3 [116]. Moreover, CA (200–800 μM) has been shown to promote Ca2+ accumulation in cells in a concentration-dependent manner, an effect that is closely related to apoptosis. CA releases Ca2+ from the endoplasmic reticulum by inducing protein phospholipase C and then induces apoptosis in SCM1 gastric cancer cells [117]. In vivo, the effect of novel caffeic acids conjugated with silver nanoparticles with and without gamma radiation exposure has been found to be effective against experimentally induced Ehrlich tumors, resulting in growth inhibition in solid tumor cells, whose underlying mechanism involves an apoptotic effect [118].
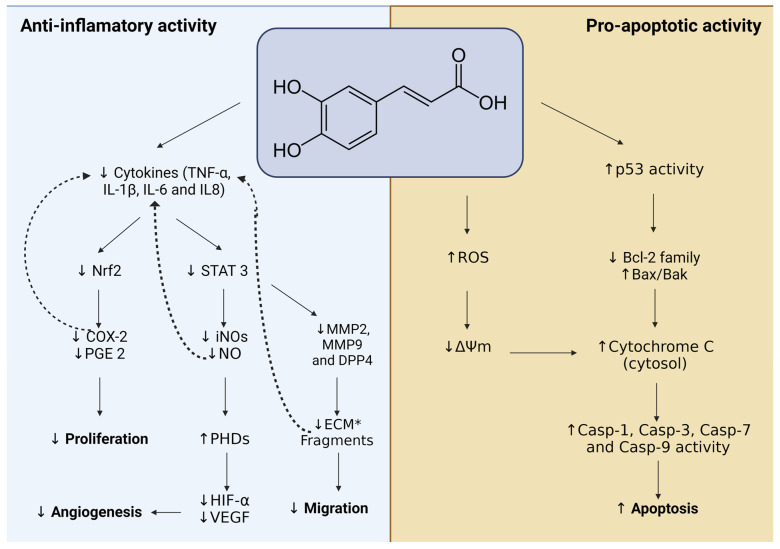
The impact of CA on mitochondria has been explored, particularly in breast cancer cells like MCF-7 and MDA-MB-468 cell lines. Treatment with CA at a concentration of 20 μM disrupts mitochondrial function, which leads to several effects: increased Caspase-9 activity, elevated levels of ROS, and a decrease in membrane potential (Δψm) [119]. Additionally, it has been suggested that CA may specifically influence protein kinase C delta (PKCδ), a protein that is involved in apoptosis and affects mitochondria by reducing ΔΨm. Consequently, CA promotes apoptosis in MG-63 osteosarcoma cells by inducing the translocation of PKCδ to mitochondria and reducing ΔΨm, resulting in mitochondrial membrane potential (MMP) alterations [115]. Additionally, an antioxidant derived from CA, known as AntiOxCIN6, has been developed. This compound is formed by linking the antioxidant core to the lipophilic TPP+ via a 10-carbon aliphatic chain. Interestingly, AntiOxCIN6 has demonstrated the potential to indirectly induce apoptosis. Despite increasing the antioxidant defense system, this complex is inefficient in eliminating ROS. Consequently, it affects mitochondrial function by impairing its ATP production capacity. Although this impairment does not directly affect cell viability, it sensitizes A549 adenocarcinoma cells to apoptotic death induced by cisplatin [120]. A summary of both the anti-inflammatory and proapoptotic anticancer activity of CA can be seen in the flowchart in Figure 6. Here, it is observed that CA affects signaling pathways that result in cell death, decreased proliferation, and migration of cancer cells, as well as a decrease in angiogenesis processes.
Figure 6.
Flowchart summarizing the reported anti-inflammatory and proapoptotic activity of CA in cancer cells. * ECM: extracellular matrix.
Regarding the modulation of CA in oncogenic signaling pathways in cancer, it has been reported that the administration of CA (100 μM) alone or in combination with metformin (10 mM) is efficient in stimulating the AMPK signaling pathway, which acts by preventing de novo synthesis of unsaturated fatty acids, consequently reducing cancer cell survival [121]. AMP-activated protein kinase (AMPK) is a vital enzyme in regulating cellular metabolism and maintaining energy balance. It also plays a significant role in tumor progression by influencing the expression of genes associated with invasion, metastasis, and angiogenesis. Consequently, activators of AMPK hold promise for exerting antitumor effects by augmenting sensitivity to chemotherapy and radiotherapy while mitigating metastasis [122]. CA has also been found to disrupt the PI3K/Akt signaling pathway, both in laboratory studies and in living organisms. This interference shows promise in inhibiting the proliferation and self-renewal ability of cancer stem cells (CSCs). Such effects have been investigated in CSC populations derived from the human colon adenocarcinoma cell line HCT116 [121,123]. In melanoma, TGFβ is one of the key signaling pathways in progression because, in some circumstances, it can provide the ideal microenvironment for tumor development. In this sense, a study showed that CA (1 mmol/L), together with a moderately potent SMF (0.7 T), decreased the expression of TGFβ, which could support anticancer therapy [124]. Conversely, tissue transglutaminase type 2 (TG2) is an enzyme involved in apoptosis, wherein its activity, among other roles, stimulates caspase activation, a process often induced by oxidative stress. In this context, a study conducted by Feriotto et al. revealed that CA effectively stimulates the activation pathways of TG2. This was shown by the antiproliferative impact observed in the K562 cell line (chronic myeloid leukemia), which correlated with TG2 activity. Such activation of TG2 contributed to the elevation of ROS levels, consequently leading to cell death [114].
In hepatocellular carcinoma (HCC), CA inhibits the activity of GRP75 (75 KDa Glucose-Regulated Protein) induced by low doses of the carcinogen Benzo(a)pyrene (B[a]P) through both transcriptional and post-transcriptional modifications. This inhibition is closely linked to CA’s role as an inhibitor of NF-κB, which serves as an upstream transcriptional regulator of GRP75. Additionally, CA induces the cleavage of the GRP75–p53 complex, leading to the nuclear translocation and activation of p53. This promotes the induction or maintenance of anti-apoptotic capacity and multidrug resistance (MDR) characteristics in HCC. Given its pivotal role, GRP75 is implicated in the onset and progression of cancer [125].
9. Caffeic Acid as an Adjuvant for Chemotherapy
Drug resistance represents a significant obstacle to the success of chemotherapy in treating many types of human tumors, and the extent of its effect varies depending on factors such as the cancer type, the specific drug used, and patient-related factors [126]. One mechanism through which cancer cells develop resistance to chemotherapy involves the overexpression of drug efflux pumps, such as P-glycoprotein (P-gp), which diminishes the intracellular concentration of chemotherapeutic drugs. CA has demonstrated the ability to regulate the activity of these pumps, potentially mitigating drug efflux and enhancing the retention of chemotherapeutic agents within cells, thus improving the effectiveness of cancer treatments. Research conducted by Teng et al. investigated the impact of CA on P-gp both in vitro and in silico. Their findings revealed that the combination of CA with chemotherapeutic agents like doxorubicin reduced the activity of P-gp, as indicated by lower IC50 values in resistant cancer cells. Additionally, CA inhibited the efflux of rhodamine 123. In silico analysis suggested that CA binds to P-gp through polar interactions with specific residues, including Glu74 and Tyr117 [127]. A search and compilation of studies where CA was tested as a coadjuvant for the treatment of various types of cancer are presented below, divided into in vitro trials, in vivo trials, and clinical trials.
9.1. In Vitro and In Vivo Trials
CA exhibits adjuvant effects, enhancing the apoptotic response when combined with cisplatin in various cancer cell lines such as Lacks’ cervical cancer cells (HeLa), cervical cancer (CaSki), non-small cell lung cancer cell (A549), and hepatoblastoma cell line (HEPG2) [128,129]. Additionally, Sirota et al. demonstrated that the combined treatment with cisplatin (5 µM) and CA (10 µM) restored the chemo-sensitizing effect against cisplatin-resistant ovarian endometrioid adenocarcinoma cells (A2780). This combined treatment resulted in a reduction of cell viability comparable to that achieved in sensitive cells treated solely with cisplatin at the same concentration. However, it was noted that pre-incubation with CA before cisplatin treatment could induce resistance by activating Nrf2. This highlights the importance of cautious examination when using CA as an adjuvant to cisplatin [130]. On another note, the cocrystallization of CA with 5-fluorouracil (5-FU) has been found to enhance the physicochemical properties of 5-FU, such as solubility and tissue permeability. This synergistic interaction leads to an improved anticancer activity of 5-FU, as indicated by a combination index (CI) of less than 1 [131]. Table 3 summarizes the results of in vitro and in vivo co-treatments using CA against cancer cells.
In a recent study examining the impact of caffeic acid on lung cancer cells, it was observed that CA effectively inhibits proliferation, migration, and apoptosis by targeting the TMEM16A protein, which is a calcium-activated chloride channel. The inhibitory concentration (IC50) of CA for TMEM16A was determined to be 29.47 ± 3.19 μM. Further animal studies corroborated these findings, demonstrating a significant reduction in tumor size. Notably, when combined with 5.4 mg/kg caffeic acid and hydroxydaunorubicin (DOX) at a dosage of 4.1 mg/kg, the treatment resulted in an 85.6% reduction in tumor size and minimized adverse effects. In conclusion, the combined therapy of caffeic acid and DOX proved more effective in inhibiting lung cancer cell growth compared to either drug administered alone, even at higher doses [132].
In addition to the re-sensitization of cancer cells, a protective effect of CA towards other organs has also been seen during the use of chemotherapeutics. For example, the effect of CA encapsulated in a nanoemulsion on the reduction of nephrotoxicity towards non-cancerous cells of the HEK 293 renal line was evaluated, showing an improvement in cell viability in renal cells from 33% to more than 95% during treatment with cisplatin [129]. This finding and similar results are also summarized in Table 3.
Table 3.
Synergetic effect of CA in cancer cells.
| Aim | Cancer Type: Model | Treatment Conditions | Finding | Reference |
|---|---|---|---|---|
|
To assess the efficacy of cisplatin + CA treatment in human cervical cancer |
• In vitro: human cervical cancer cell lines: HeLa, SiHa, CaSki (HPV-positive), and C33A (HPV- negative) cells. |
CA (300 µM) and cisplatin (11 µM) for 24 h |
The combination of cisplatin and CA significantly inhibited cell growth of HeLa and CaSki cell lines, with a combination index < 1, indicating a synergistic effect. The combination significantly increased the expression of caspases 3, 7, and 9, demonstrating apoptosis. | [128] |
| To assess the efficacy of the combined treatment with cisplatin + CA against ovarian carcinoma |
• In vitro: ovarian carcinoma cells A2780 and ovarian carcinoma- resistant A2780cisR cells. |
CA (10 µM) and cisplatin (5 µM) for 24 h |
The combined therapy restores the sensitivity of resistant cells to cisplatin, achieving a similar level of cell viability as that observed in sensitive cells (60% viability). When the cisplatin/caffeic acid ratio was increased to 1:10 (5:50 µM), the caspase activity rose significantly by 4.3-fold. |
[130] |
| To evaluate the effects of metformin (Met) and CA on metastatic human cervical cancer |
• In vitro: metastatic human cervical HTB-34 cell line. |
CA (100 µM) and Met (10 mM) for 24 h |
CA (100 µM) and Met (10 mM) activated AMPK. CA increased oxidative stress, affecting bioenergetics pathways and making HTB-34 cells more sensitive to Met. CA and Met suppressed HTB-34 cells by different mechanisms. |
[133] |
|
To determine the efficacy and underlying mechanisms of CA in combination with paclitaxel for the treatment in human non-small cell lung carcinoma (NSCLC) |
• In vitro: human non-small cell lung carcinoma H1299 cells. • In vivo: mouse xenograft model by subcutaneous injections of H1299 cells. |
In vitro: 100 μM CA + 10 μM of paclitaxel for 24 h In vivo: 20 mg/kg CA and 10 mg/kg paclitaxelad ministered concomitantly for three weeks. |
In vitro, combination treatment decreased the proliferation of NSCLC H1299 cells by the MAPK pathway. CA induced sub-G1 cell cycle arrest in H1299 cells. In vivo, the combined treatment with CA and paclitaxel exerted a more effective suppressive effect on tumor growth in H1299 xenografts without causing significant adverse effects. |
[134] |
|
To evaluate the synergistic antitumor activity and the physicochemical and pharmacokinetic properties of caffeic acid/5-FU-cocrystal in vitro and in vivo. |
• In vitro: human colon cancer HCT-116, breast cancer MDA-MB-231, and lung cancer A549 cell lines • In vivo: Sprague Dawley rats |
In vitro: HCT-116; MDA-MB-231 (15.19 μM); and A549 (11.57 μM) of caffeic acid/5-FU cocrystal for 48 h. In vivo: oral dose of 50 mg kg−1. |
In vitro: Cocrystallization of CA + 5-FU optimized the physicochemical properties of 5-FU and exerted a synergistic antitumor effect (CI < 1), thus enhancing the anticancer activity of 5-FU. In vivo: The aqueous solubility and permeability of 5-FU in the cocrystal increased by 1.92 and 4.22-fold, respectively, compared to the original drug 5-FU. |
[131] |
|
To evaluate the effects of CA and imatinib (IM) and their synergistic effects on chronic myeloid leukemia model |
• In vitro: human myelogenous leukemia cell line K562 and (IM)-resistant cells. |
Synergistic effects of CA (up to 38 µM) and IM (up to 0.15 µM) on K562 cells. | CA induced apoptosis in IM-resistant cells and reduced their proliferation. Combination treatment with CA and IM showed synergistic effects, increasing the antiproliferative activity. | [114] |
|
To assess the activity of Pancreatic Ductal Adenocarcinoma (PDAC) by treatment with CA, gemcitabine (Gem), and doxorubicin (DOX) |
• In vitro: Human epithelioid carcinoma attached cell lines Panc-1 and Mia-PaCa-2. Both have increased potential of migration and invasion, as well as Gem resistance |
Cytotoxic analysis of CA was measured at 24 and 48 h in combination with Gem and DOX. |
CA showed cytotoxic concentrations (IC50) of 37.37 µM and 15.06 µM against Panc-1 and Mia-PaCa-2, respectively. Cotreatment with a combination of CA and Gem or DOX did not show synergic activity; in contrast, it showed antagonism, suggesting that CA could display interactions with Gem or DOX. |
[135] |
| To study the effect of CA and DOX on lung cancer |
• In vitro: mouse pulmonary system adenocarcinoma LA795 cell line • In vivo: Balb/c mice and Sprague Dawley (SD) rats |
In vitro: Not specified In vivo: CA (5.4 mg/kg bw) + DOX (4.1 mg/kg bw) |
In vitro: CA inhibited TMEM16A with an IC50 of 29.47 ± 3.19 μM. CA regulated the proliferation, migration, and apoptosis of lung cancer cells targeting TMEM16A (binding sites: D439, E448, and R753). CA regulated the growth of lung cancer through the MAPK pathway. CA + DOX inhibited lung cancer cell growth more than a double dose of either one. In vivo: CA + DOX achieved a tumor suppression rate of 85.6% and compensated for side effects. |
[132] |
| To evaluate the effects of tocotrienols and CA encapsulated in a nanoemulsion with cisplatin on lung and liver cancer |
• In vivo: human lung cancer cell A549 and liver HEP G2 cancer cells. |
Not specified |
TRF, CA, and CIS synergistically enhanced late-phase apoptosis and improved cell cycle arrest in the G0/G1 phase. ROS generation was enhanced using TRF:CA:CIS by 16.9% and 30.2% for A549 and HEP G2, respectively. |
[129] |
|
To evaluate the oxidative stress induced by multi-walled carbon nanotube (MWCNT) treatment on islets and β-cells. |
• In vivo: islets and β-cells |
CA significantly reduced ROS production after MWCNT treatment and increased insulin secretion together with the enzymes SOD, GSH-Px, CAT, and GSH, but it decreased the level of MDA. |
[136] | |
|
To evaluate the effect of CA encapsulated in a nanoemulsion on the reduction of nephrotoxicity effects |
• In vitro: non-cancer cells of the HEK 293 kidney line |
CA (0.08–1.75 μM) + CIS 0.03 μM | Improved cell viability in kidney cells from 33% to over 95%. | [129] |
|
To evaluate delivery systems with CA for the treatment of breast cancer, loaded on oxidant carbon nanotube (OCNT) and/or chitosan (CS). |
• In vitro: human breast cancer MDA-MB-231 cell line |
CA (100 µg/mL); oxidant carbon nanotube (OCNT)/CA (80 µg/mL); and chitosan (CS)/OCNT/CA (30 µg/mL) |
The delivery system based on CS/OCNT/CA showed a higher cytotoxic effect on MDA-MB-231 compared to OCNT/CA and CA alone through apoptosis. | [137] |
9.2. Clinical Trials
To assess the efficacy of CA in cancer therapy, we searched the clinical trial database clinicaltrials.gov using the keyword “caffeic acid”. One trial with the code NCT02050334, titled “CC100: Safety and Tolerability of Single Doses”, involved 18 participants aged 18 to 65. They were administered CA at a maximum concentration of 24 mg/kg. The findings indicated that CA did not lead to severe adverse effects; the main reported adverse effects were back pain and headache. However, the trial outcomes have not been published.
The effectiveness and safety of CA were assessed in 103 patients with primary immune thrombocytopenia (ITP), with a median age of 48 years. They received an oral regimen of CA tablets at a dose of 300 mg three times per day for 12 weeks. The results demonstrated that CA treatment was effective, with minimal adverse effects, including mild nausea in one case and elevated liver enzymes in another [138]. Furthermore, a meta-analysis involving 2533 patients indicated that CA is statistically effective in treating ITP, leading to an increase in platelet counts (standardized mean difference [SMD] = 1.50, 95% CI [1.09, 1.91], p < 0.00001), with few adverse effects (relative risk ratio [RR] = 1.24, 95% CI [1.17, 1.31], p < 0.00001) [139]. However, other clinical trials, such as NCT04648917 (GASC1 Inhibitor Caffeic Acid for Squamous Esophageal Cell Cancer [ESCC]), NCT03070262 (The Efficacy and Safety of Caffeic Acid for Esophageal Cancer), and NCT02351622 (Caffeic Acid Tablets as a Second-line Therapy for ITP), have not published their results yet.
10. Radioprotective Potential of Caffeic Acid in Chemotherapy
Radiotherapy, a potent cytotoxic treatment, is widely utilized for localized solid tumors, either alone or in conjunction with chemotherapy [140,141]. However, despite its efficacy, it also damages normal cells due to the minimal distinction between cancerous and healthy cells, particularly affecting skin cells, leading to adverse effects such as dermatological conditions and potential cancer development [142]. Notably, nearly 95% of patients undergoing radiotherapy experience symptoms like pain, redness, or ulcers [143]; for instance, pelvic cancer treatment often results in damage to testicular tissue or neuropathy [144,145]. Advanced techniques like intensity-modulated radiotherapy aim to mitigate these toxicities [141]. Nonetheless, akin to chemotherapy, radiotherapy poses significant side effects that can substantially impact the quality of life of patients and treatment adherence [146,147]. Table 4 outlines the protective effects of CA in conjunction with radiotherapy.
Table 4.
Protective activity of CA in radiotherapy treatment.
| Aim | Model | Treatment Conditions | Finding | Reference |
|---|---|---|---|---|
|
To investigate the radioprotective potential of CA against γ radiation-induced cellular changes |
In vitro: human peripheral blood lymphocytes |
CA 66 µM for 30 min before γ radiation (1, 2, 3 y, and 4 Gy) |
Pre-treatment with CA before γ radiation treatment showed significant cell protection (around 80–85%). Overall, CA protects lymphocytes by decreasing (p < 0.01) DNA damage in micronucleus frequencies (MNs) by comet assay, decreasing the level of lipid peroxidation index by TBARS, and improving the antioxidant activity by increasing GSH, SOD, CAT, and GPx levels. |
[148] |
|
To investigate the protective role of CA in human epidermal keratinocytes and carcinogenesis induced by cancer treatments with ionizing radiation (γ or X-rays) |
In vitro: human epidermal keratinocyte line HaCaT cells |
0.1 µg/mL of CA for 24 h prior to γ radiation at 4 Gy (1 Gy/min) for 10 min. | Pre-treatment with CA increased the cell survival significantly (p < 0.05) by about 40% at 8 Gy level and reduced ROS production by 38% (p < 0.05), which was induced radiation. CA pre-treatment considerably reduced the number of foci of DNA strand breaks at each time point compared to the control. |
[149] |
| To assess the activity of zinc oxide–caffeic acid nanoparticles (ZnO-CA NPs) against cancer cell lines and evaluated on Ehrlich carcinoma treated with γ radiation. |
In vitro: human breast cancer MCF-7 cell line and human liver cancer cell line HepG2 In vivo: Ehrlich carcinoma bearing mice (EC mice) |
In vitro: Not specified. In vivo: Animals were treated with γ radiation at a dose rate of 0.45 Gy/min in a treatment of 2 Gy/week for 3 successive doses. Animals were injected IP with ZnO-CA NPs (5 mg/100 g) in different experiments. |
In vitro: ZnO-CA NPs showed antiproliferative activity against cancer cell lines. The IC50 values of ZnO-CA NPs were 9.22 and 11.53 µg/mL for MCF7 and HepG2, respectively. In vivo: ZnO-CA NPs increased the antitumor activity in mice treated with γ radiation. The LD50 for ZnO-CA NPs was determined in 50.0 mg/100 g bw. The tumor weight decreased from 56.1% (ZnO-CA NPs) to 71.9% in the combination treatment with γ radiation after 4 weeks compared to untreated solid EC tumor. |
[150] |
11. Conclusions
Further research is necessary to comprehensively ascertain the viability of CA as an anticancer treatment. However, preliminary clinical evidence suggests promising benefits. Studies conducted on cells and animals indicate that CA enhances the efficacy of chemotherapy and radiotherapy, potentially mitigating their adverse effects and improving patient outcomes with minimal side effects. This study has some limitations, including a lack of evidence for certain cancer types and omissions in many studies regarding whether the models are drug-resistant or simply aim to potentiate the action in non-resistant cancer cells. Additionally, in vivo testing is limited by small sample sizes, which may restrict the generalizability of the results. Cancer heterogeneity presents another significant limitation. Within a single tumor, cancer cells can vary significantly in differentiation, proliferation rate, metastatic capacity, and therapy susceptibility. This diversity can lead to subpopulations of cells responding differently to specific treatments [151]. Consequently, in vitro models with a single cell type or minimal heterogeneity make it difficult to predict how a tumor with cells in varying degrees of neoplasia will behave. This variability implies that the adjuvant action of CA may differ considerably from patient to patient, even within the same cancer type. While a study may focus on a specific type, the results may not apply to other cases of the same cancer due to biological and molecular differences.
Another interesting property to continue studying is the dual capacity of CA to act as an antioxidant during carcinogenesis and as a pro-oxidant against cancer cells, promoting their apoptosis or sensitizing them to chemotherapeutic drugs. Future challenges include exploring different administration methods for CA as an adjuvant in therapies to treat different neoplasms. Some authors mention that CA’s chemical instability and limited bioavailability restrict its therapeutic potential in vivo. Currently, new formulations are being developed for CA administration in different pathologies, such as transferrin (Tf)-modified nanoparticles (NPs) loaded with CA for Alzheimer’s disease (AD) [152], topical cream with nanostructured lipid carriers (NLCs) loaded with CA for anti-inflammatory action [153], and poloxamer 407 designed to improve the stability of caffeic acid in the stomach [154]. Nonetheless, the utilization of CA in cancer therapy requires more extensive investigation through clinical trials.
Author Contributions
Manuscript writing, N.C., C.V., L.O. and V.B.; manuscript editing, J.R.C.-P., I.G.-C., V.-A.N.-N. and C.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The authors are thankful for the financial support from ANID FONDECYT 1220831 to C.P.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kohn M. Heinrich Hlasiwetz (1825–1875) J. Chem. Educ. 1945;22:55. doi: 10.1021/ed022p55. [DOI] [Google Scholar]
- 2.Kiokias S., Proestos C., Oreopoulou V. Phenolic Acids of Plant Origin-A Review on Their Antioxidant Activity In Vitro (O/W Emulsion Systems) Along with Their in Vivo Health Biochemical Properties. Foods. 2020;9:534. doi: 10.3390/foods9040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dias M.C., Pinto D., Silva A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules. 2021;26:5377. doi: 10.3390/molecules26175377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzanova M., Atanasov V., Yaneva Z., Ivanova D., Dinev T. Selectivity of current extraction techniques for flavonoids from plant materials. Processes. 2020;8:1222. doi: 10.3390/pr8101222. [DOI] [Google Scholar]
- 5.Bule M., Khan F., Nisar M.F., Niaz K., Nabavi S., Saeedi M., Sanches Silva A. Recent Advances in Natural Products Analysis. Elsevier; Amsterdam, The Netherlands: 2020. Tannins (hydrolysable tannins, condensed tannins, phlorotannins, flavono-ellagitannins) pp. 132–146. [Google Scholar]
- 6.Su X., Zhou D., Li N. Bioactive stilbenes from plants. Stud. Nat. Prod. Chem. 2022;73:265–403. [Google Scholar]
- 7.Wang L.X., Wang H.L., Huang J., Chu T.Z., Peng C., Zhang H., Chen H.L., Xiong Y.A., Tan Y.Z. Review of lignans from 2019 to 2021: Newly reported compounds, diverse activities, structure-activity relationships and clinical applications. Phytochemistry. 2022;202:113326. doi: 10.1016/j.phytochem.2022.113326. [DOI] [PubMed] [Google Scholar]
- 8.Dulo B., Phan K., Githaiga J., Raes K., De Meester S. Natural quinone dyes: A review on structure, extraction techniques, analysis and application potential. Waste Biomass Valorization. 2021;12:6339–6374. doi: 10.1007/s12649-021-01443-9. [DOI] [Google Scholar]
- 9.Gouda M.A., Hussein B.H.M., El-Demerdash A., Ibrahim M.E., Salem M.A., Helal M.H., Hamama W.S. A review: Synthesis and medicinal importance of coumarins and their analogues (Part II) Curr. Bioact. Compd. 2020;16:993–1008. doi: 10.2174/1573407215666191111120604. [DOI] [Google Scholar]
- 10.Lončar M., Jakovljević M., Šubarić D., Pavlić M., Buzjak Služek V., Cindrić I., Molnar M. Coumarins in Food and Methods of Their Determination. Foods. 2020;9:645. doi: 10.3390/foods9050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saha S., Buttari B., Panieri E., Profumo E., Saso L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules. 2020;25:5474. doi: 10.3390/molecules25225474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S.Y., Pyo M.C., Nam M.H., Lee K.W. ERK/Nrf2 pathway activation by caffeic acid in HepG2 cells alleviates its hepatocellular damage caused by t-butylhydroperoxide-induced oxidative stress. BMC Complement. Altern. Med. 2019;19:139. doi: 10.1186/s12906-019-2551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colonnello A., Aguilera-Portillo G., Rubio-López L.C., Robles-Bañuelos B., Rangel-López E., Cortez-Núñez S., Evaristo-Priego Y., Silva-Palacios A., Galván-Arzate S., García-Contreras R., et al. Comparing the Neuroprotective Effects of Caffeic Acid in Rat Cortical Slices and Caenorhabditis elegans: Involvement of Nrf2 and SKN-1 Signaling Pathways. Neurotox. Res. 2020;37:326–337. doi: 10.1007/s12640-019-00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerriero E., Sorice A., Capone F., Costantini S., Palladino P., D’Ischia M., Castello G. Effects of lipoic acid, caffeic acid and a synthesized lipoyl-caffeic conjugate on human hepatoma cell lines. Molecules. 2011;16:6365–6377. doi: 10.3390/molecules16086365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung J.E., Kim H.S., Lee C.S., Park D.H., Kim Y.N., Lee M.J., Lee J.W., Park J.W., Kim M.S., Ye S.K., et al. Caffeic acid and its synthetic derivative CADPE suppress tumor angiogenesis by blocking STAT3-mediated VEGF expression in human renal carcinoma cells. Carcinogenesis. 2007;28:1780–1787. doi: 10.1093/carcin/bgm130. [DOI] [PubMed] [Google Scholar]
- 17.Yang G., Fu Y., Malakhova M., Kurinov I., Zhu F., Yao K., Li H., Chen H., Li W., Lim D.Y., et al. Caffeic acid directly targets ERK1/2 to attenuate solar UV-induced skin carcinogenesis. Cancer Prev. Res. 2014;7:1056–1566. doi: 10.1158/1940-6207.CAPR-14-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espíndola K.M.M., Ferreira R.G., Narvaez L.E.M., Silva Rosario A.C.R., Da Silva A.H.M., Silva A.G.B., Vieira A.P.O., Monteiro M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019;9:541. doi: 10.3389/fonc.2019.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez-Ahumada Mdel C., Timmermann B.N., Gang D.R. Biosynthesis of curcuminoids and gingerols in turmeric (Curcuma longa) and ginger (Zingiber officinale): Identification of curcuminoid synthase and hydroxycinnamoyl-CoA thioesterases. Phytochemistry. 2006;67:2017–2029. doi: 10.1016/j.phytochem.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Fraser C.M., Chapple C. The phenylpropanoid pathway in Arabidopsis. Arab. Book. 2011;9:e0152. doi: 10.1199/tab.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogt T. Phenylpropanoid biosynthesis. Mol. Plant. 2010;3:2–20. doi: 10.1093/mp/ssp106. [DOI] [PubMed] [Google Scholar]
- 22.Fujioka K., Shibamoto T. Quantitation of volatiles and nonvolatile acids in an extract from coffee beverages: Correlation with antioxidant activity. J. Agric. Food Chem. 2006;54:6054–6058. doi: 10.1021/jf060460x. [DOI] [PubMed] [Google Scholar]
- 23.Mattila P., Kumpulainen J. Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J. Agric. Food Chem. 2002;50:3660–3667. doi: 10.1021/jf020028p. [DOI] [PubMed] [Google Scholar]
- 24.Al-Farsi M., Alasalvar C., Morris A., Baron M., Shahidi F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005;53:7592–7599. doi: 10.1021/jf050579q. [DOI] [PubMed] [Google Scholar]
- 25.Nakatani N., Kayano S., Kikuzaki H., Sumino K., Katagiri K., Mitani T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.) J. Agric. Food Chem. 2000;48:5512–5516. doi: 10.1021/jf000422s. [DOI] [PubMed] [Google Scholar]
- 26.Bianco A., Buiarelli F., Cartoni G., Coccioli F., Jasionowska R., Margherita P. Analysis by liquid chromatography-tandem mass spectrometry of biophenolic compounds in olives and vegetation waters, Part I. J. Sep. Sci. 2003;26:409–416. doi: 10.1002/jssc.200390053. [DOI] [Google Scholar]
- 27.Rossetto M., Lante A., Vanzani P., Spettoli P., Scarpa M., Rigo A. Red chicories as potent scavengers of highly reactive radicals: A study on their phenolic composition and peroxyl radical trapping capacity and efficiency. J. Agric. Food Chem. 2005;53:8169–8175. doi: 10.1021/jf051116n. [DOI] [PubMed] [Google Scholar]
- 28.Lee J. Caffeic acid derivatives in dried Lamiaceae and Echinacea purpurea products. J. Funct. Foods. 2010;2:158–162. doi: 10.1016/j.jff.2010.02.003. [DOI] [Google Scholar]
- 29.Meinhart A.D., Damin F.M., Caldeirão L., de Jesus Filho M., da Silva L.C., da Silva Constant L., Teixeira Filho J., Wagner R., Teixeira Godoy H. Study of new sources of six chlorogenic acids and caffeic acid. J. Food Compos. Anal. 2019;82:103244. doi: 10.1016/j.jfca.2019.103244. [DOI] [Google Scholar]
- 30.Zielinski H., Kozlowska H., Lewczuk B. Bioactive compounds in the cereal grains before and after hydrothermal processing. Innov. Food Sci. Emerg. Technol. 2001;2:159–169. doi: 10.1016/S1466-8564(01)00040-6. [DOI] [Google Scholar]
- 31.Weidner S., Amarowicz R., Karamać M., Dąbrowski G. Phenolic acids in caryopses of two cultivars of wheat, rye and triticale that display different resistance to pre-harvest sprouting. Eur. Food Res. Technol. 1999;210:109–113. doi: 10.1007/s002170050544. [DOI] [Google Scholar]
- 32.Çayan F., Deveci E., Tel-Çayan G., Duru M.E. Identification and quantification of phenolic acid compounds of twenty-six mushrooms by HPLC–DAD. J. Food Meas. Charact. 2020;14:1690–1698. doi: 10.1007/s11694-020-00417-0. [DOI] [Google Scholar]
- 33.Pérez-García F., Adzet T., Cañigueral S. Activity of artichoke leaf extract on reactive oxygen species in human leukocytes. Free Radic. Res. 2000;33:661–665. doi: 10.1080/10715760000301171. [DOI] [PubMed] [Google Scholar]
- 34.Llorach R., Espín J.C., Tomás-Barberán F.A., Ferreres F. Artichoke (Cynara scolymus L.) byproducts as a potential source of health-promoting antioxidant phenolics. J. Agric. Food Chem. 2002;50:3458–3464. doi: 10.1021/jf0200570. [DOI] [PubMed] [Google Scholar]
- 35.Chaowuttikul C., Palanuvej C., Ruangrungsi N. Quantification of chlorogenic acid, rosmarinic acid, and caffeic acid contents in selected Thai medicinal plants using RP-HPLC-DAD. Braz. J. Pharm. Sci. 2020;56:e17547. doi: 10.1590/s2175-97902019000317547. [DOI] [Google Scholar]
- 36.Vieira M.A., Maraschin M., Pagliosa C.M., Podestá R., de Simas K.N., Rockenbach I.I., Amboni R.D., Amante E.R. Phenolic acids and methylxanthines composition and antioxidant properties of mate (Ilex paraguariensis) residue. J Food Sci. 2010;75:C280–C285. doi: 10.1111/j.1750-3841.2010.01548.x. [DOI] [PubMed] [Google Scholar]
- 37.Berté K.A., Beux M.R., Spada P.K., Salvador M., Hoffmann-Ribani R. Chemical composition and antioxidant activity of yerba-mate (Ilex paraguariensis A.St.-Hil., Aquifoliaceae) extract as obtained by spray drying. J. Agric. Food Chem. 2011;59:5523–5527. doi: 10.1021/jf2008343. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki K., Oki T., Kobayashi T., Kai Y., Okuno S. Single-laboratory validation for the determination of caffeic acid and seven caffeoylquinic acids in sweet potato leaves. Biosci. Biotechnol. Biochem. 2014;78:2073–2080. doi: 10.1080/09168451.2014.942253. [DOI] [PubMed] [Google Scholar]
- 39.Zheng W., Wang S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 40.Lee H.-S., Lee H.-A., Hong C.-O., Yang S.-Y., Hong S.-Y., Park S.-Y., Lee H.-J., Lee K.-W. Quantification of caffeic acid and rosmarinic acid and antioxidant activities of hot-water extracts from leaves of Perilla frutescens. Korean J. Food Sci. Technol. 2009;41:302–306. [Google Scholar]
- 41.Exarchou V., Troganis A., Gerothanassis I.P., Tsimidou M., Boskou D. Identification and quantification of caffeic and rosmarinic acid in complex plant extracts by the use of variable-temperature two-dimensional nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2001;49:2–8. doi: 10.1021/jf990928e. [DOI] [PubMed] [Google Scholar]
- 42.Kowalski R., Kowalska G. Phenolic acid contents in fruits of aubergine (Solanum melongena L.) Pol. J. Food Nutr. Sci. 2005;14:37–41. [Google Scholar]
- 43.Meinhart A.D., Damin F.M., Caldeirão L., de Jesus Filho M., da Silva L.C., da Silva Constant L., Filho J.T., Wagner R., Godoy H.T. Chlorogenic and caffeic acids in 64 fruits consumed in Brazil. Food Chem. 2019;286:51–63. doi: 10.1016/j.foodchem.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Grabska-Kobylecka I., Kaczmarek-Bak J., Figlus M., Prymont-Przyminska A., Zwolinska A., Sarniak A., Wlodarczyk A., Glabinski A., Nowak D. The Presence of Caffeic Acid in Cerebrospinal Fluid: Evidence That Dietary Polyphenols Can Cross the Blood-Brain Barrier in Humans. Nutrients. 2020;12:1531. doi: 10.3390/nu12051531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Omar M.H., Mullen W., Stalmach A., Auger C., Rouanet J.M., Teissedre P.L., Caldwell S.T., Hartley R.C., Crozier A. Absorption, disposition, metabolism, and excretion of [3-(14)C]caffeic acid in rats. J. Agric. Food Chem. 2012;60:5205–5214. doi: 10.1021/jf3001185. [DOI] [PubMed] [Google Scholar]
- 46.Kishida K., Matsumoto H. Urinary excretion rate and bioavailability of chlorogenic acid, caffeic acid, p-coumaric acid, and ferulic acid in non-fasted rats maintained under physiological conditions. Heliyon. 2019;5:e02708. doi: 10.1016/j.heliyon.2019.e02708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization WHO MORTALITY DATABASE. Interactive Platform Visualizing Mortality Data. Noncommunicable Diseases. [(accessed on 5 April 2024)]. Available online: https://platform.who.int/mortality/themes/theme-details/mdb/noncommunicable-diseases.
- 48.Helfinger V., Schröder K. Redox control in cancer development and progression. Mol. Asp. Med. 2018;63:88–98. doi: 10.1016/j.mam.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 50.Gào X., Schöttker B. Reduction-oxidation pathways involved in cancer development: A systematic review of literature reviews. Oncotarget. 2017;8:51888–51906. doi: 10.18632/oncotarget.17128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shifera A.S. The zinc finger domain of IKKγ (NEMO) protein in health and disease. J. Cell. Mol. Med. 2010;14:2404–2414. doi: 10.1111/j.1582-4934.2010.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barczewski A.H., Ragusa M.J., Mierke D.F., Pellegrini M. The IKK-binding domain of NEMO is an irregular coiled coil with a dynamic binding interface. Sci. Rep. 2019;9:2950. doi: 10.1038/s41598-019-39588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 54.Sarmiento-Salinas F.L., Perez-Gonzalez A., Acosta-Casique A., Ix-Ballote A., Diaz A., Treviño S., Rosas-Murrieta N.H., Millán-Perez-Peña L., Maycotte P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021;284:119942. doi: 10.1016/j.lfs.2021.119942. [DOI] [PubMed] [Google Scholar]
- 55.Giridharan S., Srinivasan M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018;11:407–419. doi: 10.2147/JIR.S140188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitamura H., Motohashi H. NRF2 addiction in cancer cells. Cancer Sci. 2018;109:900–911. doi: 10.1111/cas.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin D., Kim E.H., Lee J., Roh J.L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic. Biol. Med. 2018;129:454–462. doi: 10.1016/j.freeradbiomed.2018.10.426. [DOI] [PubMed] [Google Scholar]
- 58.Best S.A., Sutherland K.D. “Keaping” a lid on lung cancer: The Keap1-Nrf2 pathway. Cell Cycle. 2018;17:1696–1707. doi: 10.1080/15384101.2018.1496756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Konstantinopoulos P.A., Spentzos D., Fountzilas E., Francoeur N., Sanisetty S., Grammatikos A.P., Hecht J.L., Cannistra S.A. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. 2011;71:5081–5089. doi: 10.1158/0008-5472.CAN-10-4668. [DOI] [PubMed] [Google Scholar]
- 60.da Costa D.R.C. NRF2 and Keap1 Genetic Polymorphisms in Breast Cancer. 2022. [(accessed on 10 June 2024)]. Available online: https://ubibliorum.ubi.pt/bitstream/10400.6/12896/1/9344_19837.pdf.
- 61.Sánchez-Ortega M., Carrera A.C., Garrido A. Role of NRF2 in lung cancer. Cells. 2021;10:1879. doi: 10.3390/cells10081879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tossetta G., Fantone S., Montanari E., Marzioni D., Goteri G. Role of NRF2 in Ovarian Cancer. Antioxidants. 2022;11:663. doi: 10.3390/antiox11040663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alves F.M., dos Santos Jaques H., Orrutéa J.F.G., de Oliveira Silva A.G., Machado M.G., Smaniotto L.L., Federige A.C.L., Colleto M.I.O., de Souza J.A.O., Rech D., et al. Changes in systemic oxidative stress correlate to chemoresistance and poor prognosis features in women with breast cancer. Rev. Senol. Patol. Mamar. 2024;37:100598. doi: 10.1016/j.senol.2024.100598. [DOI] [Google Scholar]
- 64.Taguchi K., Yamamoto M. The KEAP1-NRF2 System in Cancer. Front. Oncol. 2017;7:85. doi: 10.3389/fonc.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shurin M.R., Umansky V. Cross-talk between HIF and PD-1/PD-L1 pathways in carcinogenesis and therapy. J. Clin. Investig. 2022;132:e159473. doi: 10.1172/JCI159473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin X., Dai L., Ma Y., Wang J., Liu Z. Implications of HIF-1α in the tumorigenesis and progression of pancreatic cancer. Cancer Cell Int. 2020;20:273. doi: 10.1186/s12935-020-01370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ucaryilmaz Metin C., Ozcan G. The HIF-1α as a potent inducer of the hallmarks in gastric cancer. Cancers. 2022;14:2711. doi: 10.3390/cancers14112711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li M., Li G., Yang X., Yin W., Lv G., Wang S. HIF in Gastric Cancer: Regulation and Therapeutic Target. Molecules. 2022;27:4893. doi: 10.3390/molecules27154893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohamed O.A.A., Tesen H.S., Hany M., Sherif A., Abdelwahab M.M., Elnaggar M.H. The role of hypoxia on prostate cancer progression and metastasis. Mol. Biol. Rep. 2023;50:3873–3884. doi: 10.1007/s11033-023-08251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elzakra N., Kim Y. HIF-1α Metabolic Pathways in Human Cancer. Adv. Exp. Med. Biol. 2021;1280:243–260. doi: 10.1007/978-3-030-51652-9_17. [DOI] [PubMed] [Google Scholar]
- 71.Green K.J., Niessen C.M., Rübsam M., Perez White B.E., Broussard J.A. The Desmosome-Keratin Scaffold Integrates ErbB Family and Mechanical Signaling to Polarize Epidermal Structure and Function. Front. Cell Dev. Biol. 2022;10:903696. doi: 10.3389/fcell.2022.903696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumagai S., Koyama S., Nishikawa H. Antitumour immunity regulated by aberrant ERBB family signalling. Nat. Rev. Cancer. 2021;21:181–197. doi: 10.1038/s41568-020-00322-0. [DOI] [PubMed] [Google Scholar]
- 73.Chu E.C., Tarnawski A.S. PTEN regulatory functions in tumor suppression and cell biology. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2004;10:Ra235–Ra241. [PubMed] [Google Scholar]
- 74.Choudhury A.D. PTEN-PI3K pathway alterations in advanced prostate cancer and clinical implications. Prostate. 2022;82((Suppl. S1)):S60–S72. doi: 10.1002/pros.24372. [DOI] [PubMed] [Google Scholar]
- 75.Tortorella E., Giantulli S., Sciarra A., Silvestri I. AR and PI3K/AKT in Prostate Cancer: A Tale of Two Interconnected Pathways. Int. J. Mol. Sci. 2023;24:2046. doi: 10.3390/ijms24032046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan A.C. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC) Thorac. Cancer. 2020;11:511–518. doi: 10.1111/1759-7714.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu K., Wu Y., He P., Fan Y., Zhong X., Zheng H., Luo T. PI3K/AKT/mTOR-Targeted Therapy for Breast Cancer. Cells. 2022;11:2508. doi: 10.3390/cells11162508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.NCBI PTEN Phosphatase and Tensin Homolog [Homo sapiens (Human)] [(accessed on 10 June 2024)]; Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=ShowDetailView&TermToSearch=5728.
- 79.Xiao J., Hu C.P., He B.X., Chen X., Lu X.X., Xie M.X., Li W., He S.Y., You S.J., Chen Q. PTEN expression is a prognostic marker for patients with non-small cell lung cancer: A systematic review and meta-analysis of the literature. Oncotarget. 2016;7:57832–57840. doi: 10.18632/oncotarget.11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elwy F., Assem M.M., Hassan N.H.A., Helwa R. PTEN mutations prevalence in HER2-positive breast cancer patients. Rev. Senol. Patol. Mamar. 2023;36:100410. doi: 10.1016/j.senol.2022.02.004. [DOI] [Google Scholar]
- 81.Patnam S., Samal R., Koyyada R., Joshi P., Singh A.D., Nagalla B., Soma M.R., Sannareddy R.R., Ippili K., Raju S. Exosomal PTEN as a predictive marker of aggressive gliomas. Neurol. India. 2022;70:215–222. doi: 10.4103/0028-3886.338731. [DOI] [PubMed] [Google Scholar]
- 82.Benitez J.A., Finlay D., Castanza A., Parisian A.D., Ma J., Longobardi C., Campos A., Vadla R., Izurieta A., Scerra G., et al. PTEN deficiency leads to proteasome addiction: A novel vulnerability in glioblastoma. Neuro-Oncology. 2021;23:1072–1086. doi: 10.1093/neuonc/noab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nóbrega M., Cilião H.L., Souza M.F., Souza M.R., Serpeloni J.M., Fuganti P.E., Cólus I.M.S. Association of polymorphisms of PTEN, AKT1, PI3K, AR, and AMACR genes in patients with prostate cancer. Genet. Mol. Biol. 2020;43:e20180329. doi: 10.1590/1678-4685-gmb-2018-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li W., Zhuang Y., Shao S.J., Trivedi P., Zheng B., Huang G.L., He Z., Zhang X. Essential contribution of the JAK/STAT pathway to carcinogenesis, lytic infection of herpesviruses and pathogenesis of COVID-19 (Review) Mol. Med. Rep. 2024;29:39. doi: 10.3892/mmr.2024.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smirnova O.V., Ostroukhova T.Y., Bogorad R.L. JAK-STAT pathway in carcinogenesis: Is it relevant to cholangiocarcinoma progression? World J. Gastroenterol. 2007;13:6478–6491. doi: 10.3748/wjg.v13.i48.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hassin O., Oren M. Drugging p53 in cancer: One protein, many targets. Nat. Rev. Drug Discov. 2023;22:127–144. doi: 10.1038/s41573-022-00571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marei H.E., Althani A., Afifi N., Hasan A., Caceci T., Pozzoli G., Morrione A., Giordano A., Cenciarelli C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021;21:703. doi: 10.1186/s12935-021-02396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang T., Ma C., Zhang Z., Zhang H., Hu H. NF-κB signaling in inflammation and cancer. MedComm. 2021;2:618–653. doi: 10.1002/mco2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hibino S., Kawazoe T., Kasahara H., Itoh S., Ishimoto T., Sakata-Yanagimoto M., Taniguchi K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021;22:5421. doi: 10.3390/ijms22115421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Piotrowski I., Kulcenty K., Suchorska W. Interplay between inflammation and cancer. Rep. Pract. Oncol. Radiother. 2020;25:422–427. doi: 10.1016/j.rpor.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kruk J., Aboul-Enein H.Y. Reactive Oxygen and Nitrogen Species in Carcinogenesis: Implications of Oxidative Stress on the Progression and Development of Several Cancer Types. Mini-Rev. Med. Chem. 2017;17:904–919. doi: 10.2174/1389557517666170228115324. [DOI] [PubMed] [Google Scholar]
- 92.Moldogazieva N.T., Lutsenko S.V., Terentiev A.A. Reactive Oxygen and Nitrogen Species-Induced Protein Modifications: Implication in Carcinogenesis and Anticancer Therapy. Cancer Res. 2018;78:6040–6047. doi: 10.1158/0008-5472.CAN-18-0980. [DOI] [PubMed] [Google Scholar]
- 93.Lambrou G.I., Hatziagapiou K., Vlahopoulos S. Inflammation and tissue homeostasis: The NF-κB system in physiology and malignant progression. Mol. Biol. Rep. 2020;47:4047–4063. doi: 10.1007/s11033-020-05410-w. [DOI] [PubMed] [Google Scholar]
- 94.Kim S.R., Jung Y.R., Kim D.H., An H.J., Kim M.K., Kim N.D., Chung H.Y. Caffeic acid regulates LPS-induced NF-κB activation through NIK/IKK and c-Src/ERK signaling pathways in endothelial cells. Arch. Pharmacal. Res. 2014;37:539–547. doi: 10.1007/s12272-013-0211-6. [DOI] [PubMed] [Google Scholar]
- 95.Liu M., Fang G., Yin S., Zhao X., Zhang C., Li J., Liu Z. Caffeic Acid Prevented LPS-Induced Injury of Primary Bovine Mammary Epithelial Cells through Inhibiting NF-κB and MAPK Activation. Mediat. Inflamm. 2019;2019:1897820. doi: 10.1155/2019/1897820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sandra F., Ketherin K. Caffeic acid inhibits RANKL and TNF-α-induced phosphorylation of p38 mitogen-activated protein kinase in RAW-D cells. Indones. Biomed. J. 2018;10:140–143. doi: 10.18585/inabj.v10i2.437. [DOI] [Google Scholar]
- 97.Zhang M., Zhou J., Wang L., Li B., Guo J., Guan X., Han Q., Zhang H. Caffeic acid reduces cutaneous tumor necrosis factor alpha (TNF-α), IL-6 and IL-1β levels and ameliorates skin edema in acute and chronic model of cutaneous inflammation in mice. Biol. Pharm. Bull. 2014;37:347–354. doi: 10.1248/bpb.b13-00459. [DOI] [PubMed] [Google Scholar]
- 98.Wang L., Lu M., Yi M., Chen L., Shen J., Li Z., Li L., Yang Y., Zhang J., Li Y. Caffeic acid attenuates the autocrine IL-6 in hepatocellular carcinoma via the epigenetic silencing of the NF-κB-IL-6-STAT-3 feedback loop. RSC Adv. 2015;5:52952–52957. doi: 10.1039/C5RA05878C. [DOI] [Google Scholar]
- 99.Zielińska D., Zieliński H., Laparra-Llopis J.M., Szawara-Nowak D., Honke J., Giménez-Bastida J.A. Caffeic Acid Modulates Processes Associated with Intestinal Inflammation. Nutrients. 2021;13:554. doi: 10.3390/nu13020554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang Y.K., Tseng K.F., Tsai P.H., Wang J.S., Lee C.Y., Shen M.Y. IL-8 as a Potential Therapeutic Target for Periodontitis and Its Inhibition by Caffeic Acid Phenethyl Ester In Vitro. Int. J. Mol. Sci. 2021;22:3641. doi: 10.3390/ijms22073641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alam M., Ahmed S., Elasbali A.M., Adnan M., Alam S., Hassan M.I., Pasupuleti V.R. Therapeutic implications of caffeic acid in cancer and neurological diseases. Front. Oncol. 2022;12:860508. doi: 10.3389/fonc.2022.860508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim J.H., Lee B.J., Kim J.H., Yu Y.S., Kim K.W. Anti-angiogenic effect of caffeic acid on retinal neovascularization. Vasc. Pharmacol. 2009;51:262–267. doi: 10.1016/j.vph.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 103.Gu W., Yang Y., Zhang C., Zhang Y., Chen L., Shen J., Li G., Li Z., Li L., Li Y., et al. Caffeic acid attenuates the angiogenic function of hepatocellular carcinoma cells via reduction in JNK-1-mediated HIF-1α stabilization in hypoxia. RSC Adv. 2016;6:82774–82782. doi: 10.1039/C6RA07703J. [DOI] [Google Scholar]
- 104.Guo Z., Yang Y., Li L., Zhao Q., Li Y., Liu Z., Hao L., Guo B., Diao A. The novel prolyl hydroxylase-2 inhibitor caffeic acid upregulates hypoxia inducible factor and protects against hypoxia. Eur. J. Pharmacol. 2022;934:175307. doi: 10.1016/j.ejphar.2022.175307. [DOI] [PubMed] [Google Scholar]
- 105.Istyastono E.P., Yuniarti N., Prasasty V.D., Mungkasi S., Waskitha S.S.W., Yanuar M.R.S., Riswanto F.D.O. Caffeic Acid in Spent Coffee Grounds as a Dual Inhibitor for MMP-9 and DPP-4 Enzymes. Molecules. 2023;28:7182. doi: 10.3390/molecules28207182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chung T.W., Moon S.K., Chang Y.C., Ko J.H., Lee Y.C., Cho G., Kim S.H., Kim J.G., Kim C.H. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: Complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004;18:1670–1681. doi: 10.1096/fj.04-2126com. [DOI] [PubMed] [Google Scholar]
- 107.Chang W.C., Hsieh C.H., Hsiao M.W., Lin W.C., Hung Y.C., Ye J.C. Caffeic acid induces apoptosis in human cervical cancer cells through the mitochondrial pathway. Taiwan. J. Obstet. Gynecol. 2010;49:419–424. doi: 10.1016/S1028-4559(10)60092-7. [DOI] [PubMed] [Google Scholar]
- 108.Sandra F., Rizal M.I., Wahid A.H.A., Andajana M., Celinna M. Caffeic acid induces intrinsic apoptotic pathway in mg-63 osteosarcoma cells through bid truncation and cytochrome c release. Indones. Biomed. J. 2022;14:323–328. doi: 10.18585/inabj.v14i3.2032. [DOI] [Google Scholar]
- 109.Sandra F., Hudono K.F., Putri A.A., Putri C.A.P. Caspase inhibitor diminishes caffeic acid-induced apoptosis in osteosarcoma cells. Indones. Biomed. J. 2017;9:160–164. doi: 10.18585/inabj.v9i3.334. [DOI] [Google Scholar]
- 110.Rezaei-Seresht H., Cheshomi H., Falanji F., Movahedi-Motlagh F., Hashemian M., Mireskandari E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: An in silico and in vitro study. Avicenna J. Phytomed. 2019;9:574–586. doi: 10.22038/AJP.2019.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dziedzic A., Kubina R., Kabała-Dzik A., Tanasiewicz M. Induction of Cell Cycle Arrest and Apoptotic Response of Head and Neck Squamous Carcinoma Cells (Detroit 562) by Caffeic Acid and Caffeic Acid Phenethyl Ester Derivative. Evid. Based Complement. Altern. Med. 2017;2017:6793456. doi: 10.1155/2017/6793456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kabała-Dzik A., Rzepecka-Stojko A., Kubina R., Wojtyczka R.D., Buszman E., Stojko J. Caffeic Acid Versus Caffeic Acid Phenethyl Ester in the Treatment of Breast Cancer MCF-7 Cells: Migration Rate Inhibition. Integr. Cancer Ther. 2018;17:1247–1259. doi: 10.1177/1534735418801521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun L., Ren J., Feng X., Li S., Wang Y., Jiang Y., Zheng C. Caffeic Acid Markedly Induced Apoptosis of Human Multiple Myeloma Cells through the Caspase-dependent Pathway. Pharmacogn. Mag. 2023;19:720–726. doi: 10.1177/09731296231170926. [DOI] [Google Scholar]
- 114.Feriotto G., Tagliati F., Giriolo R., Casciano F., Tabolacci C., Beninati S., Khan M.T.H., Mischiati C. Caffeic Acid Enhances the Anti-Leukemic Effect of Imatinib on Chronic Myeloid Leukemia Cells and Triggers Apoptosis in Cells Sensitive and Resistant to Imatinib. Int. J. Mol. Sci. 2021;22:1644. doi: 10.3390/ijms22041644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sandra F., Rizal M.I., Aliwarga C.C., Hadimartana J.C., Celinna M. Caffeic Acid Induces Apoptosis in MG-63 Osteosarcoma Cells via Protein Kinase C Delta (PKCδ) Translocation and Mitochondrial Membrane Potential Reduction. Indones. Biomed. J. 2022;14:358–364. doi: 10.18585/inabj.v14i4.2089. [DOI] [Google Scholar]
- 116.Pelinson L.P., Assmann C.E., Palma T.V., da Cruz I.B.M., Pillat M.M., Mânica A., Stefanello N., Weis G.C.C., de Oliveira Alves A., de Andrade C.M., et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019;46:2085–2092. doi: 10.1007/s11033-019-04658-1. [DOI] [PubMed] [Google Scholar]
- 117.Chang H.T., Chen I.L., Chou C.T., Liang W.Z., Kuo D.H., Shieh P., Jan C.R. Effect of caffeic acid on Ca(2+) homeostasis and apoptosis in SCM1 human gastric cancer cells. Arch. Toxicol. 2013;87:2141–2150. doi: 10.1007/s00204-013-1075-8. [DOI] [PubMed] [Google Scholar]
- 118.Abdelwahab T.S., Abdelhamed R.E., Ali E.N., Mansour N.A., Abdalla M.S. Evaluation of Silver Nanoparticles Caffeic Acid Complex Compound as New Potential Therapeutic Agent against Cancer Incidence in Mice. Asian Pac. J. Cancer Prev. APJCP. 2021;22:3189–3201. doi: 10.31557/APJCP.2021.22.10.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robati A.K., Shahsavari Z., Vaezi M.A., Safizadeh B., Shirian F.I., Tavakoli-Yaraki M. Caffeic acid stimulates breast cancer death through Reactive oxygen species (ROS) formation, Caspase activation and mitochondrial membrane potential depletion. Acta Biochim. Iran. 2024;1:176–182. doi: 10.18502/abi.v1i4.14719. [DOI] [Google Scholar]
- 120.Amorim R., Magalhães C.C., Benfeito S., Cagide F., Tavares L.C., Santos K., Sardão V.A., Datta S., Cortopassi G.A., Baldeiras I., et al. Mitochondria dysfunction induced by decyl-TPP mitochondriotropic antioxidant based on caffeic acid AntiOxCIN(6) sensitizes cisplatin lung anticancer therapy due to a remodeling of energy metabolism. Biochem. Pharmacol. 2024;219:115953. doi: 10.1016/j.bcp.2023.115953. [DOI] [PubMed] [Google Scholar]
- 121.Mirzaei S., Gholami M.H., Zabolian A., Saleki H., Farahani M.V., Hamzehlou S., Far F.B., Sharifzadeh S.O., Samarghandian S., Khan H., et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol. Res. 2021;171:105759. doi: 10.1016/j.phrs.2021.105759. [DOI] [PubMed] [Google Scholar]
- 122.Cao W., Li J., Hao Q., Vadgama J.V., Wu Y. AMP-activated protein kinase: A potential therapeutic target for triple-negative breast cancer. Breast Cancer Res. BCR. 2019;21:29. doi: 10.1186/s13058-019-1107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park S.R., Kim S.R., Hong I.S., Lee H.Y. A Novel Therapeutic Approach for Colorectal Cancer Stem Cells: Blocking the PI3K/Akt Signaling Axis With Caffeic Acid. Front. Cell Dev. Biol. 2020;8:585987. doi: 10.3389/fcell.2020.585987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kimsa-Dudek M., Synowiec-Wojtarowicz A., Krawczyk A. Phenolic acids and a static magnetic field change the expression of transforming growth factor β isoforms in amelanotic melanoma cells. Mol. Biol. Rep. 2023;50:4207–4216. doi: 10.1007/s11033-023-08336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang Y., Jin M., Meng Y., Dai Y., Chen S., Zhou Y., Li Y., Tang L. Involvement and targeted intervention of benzo(a)pyrene-regulated apoptosis related proteome modification and muti-drug resistance in hepatocellular carcinoma. Cell Death Dis. 2023;14:265. doi: 10.1038/s41419-023-05771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gatti L., Zunino F. Overview of tumor cell chemoresistance mechanisms. Methods Mol. Med. 2005;111:127–148. doi: 10.1385/1-59259-889-7:127. [DOI] [PubMed] [Google Scholar]
- 127.Teng Y.N., Wang C.C.N., Liao W.C., Lan Y.H., Hung C.C. Caffeic Acid Attenuates Multi-Drug Resistance in Cancer Cells by Inhibiting Efflux Function of Human P-glycoprotein. Molecules. 2020;25:247. doi: 10.3390/molecules25020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koraneekit A., Limpaiboon T., Sangka A., Boonsiri P., Daduang S., Daduang J. Synergistic effects of cisplatin-caffeic acid induces apoptosis in human cervical cancer cells via the mitochondrial pathways. Oncol. Lett. 2018;15:7397–7402. doi: 10.3892/ol.2018.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raviadaran R., Ng M.H., Chandran D., Ooi K.K., Manickam S. Stable W/O/W multiple nanoemulsion encapsulating natural tocotrienols and caffeic acid with cisplatin synergistically treated cancer cell lines (A549 and HEP G2) and reduced toxicity on normal cell line (HEK 293) Mater. Sci. Eng. C Mater. Biol. Appl. 2021;121:111808. doi: 10.1016/j.msec.2020.111808. [DOI] [PubMed] [Google Scholar]
- 130.Sirota R., Gibson D., Kohen R. The timing of caffeic acid treatment with cisplatin determines sensitization or resistance of ovarian carcinoma cell lines. Redox Biol. 2017;11:170–175. doi: 10.1016/j.redox.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu Y.-M., Wang L.-Y., Bu F.-Z., Wang L.-L., Li Y.-T., Wang C., Wu Z.-Y. The supramolecular self-assembly of 5-fluorouracil and caffeic acid through cocrystallization strategy opens up a new way for the development of synergistic antitumor pharmaceutical cocrystal. CrystEngComm. 2020;22:7992–8006. doi: 10.1039/D0CE01297A. [DOI] [Google Scholar]
- 132.Bai X., Li S., Liu X., An H., Kang X., Guo S. Caffeic Acid, an Active Ingredient in Coffee, Combines with DOX for Multitarget Combination Therapy of Lung Cancer. J. Agric. Food Chem. 2022;70:8326–8337. doi: 10.1021/acs.jafc.2c03009. [DOI] [PubMed] [Google Scholar]
- 133.Tyszka-Czochara M., Konieczny P., Majka M. Caffeic Acid Expands Anti-Tumor Effect of Metformin in Human Metastatic Cervical Carcinoma HTB-34 Cells: Implications of AMPK Activation and Impairment of Fatty Acids De Novo Biosynthesis. Int. J. Mol. Sci. 2017;18:462. doi: 10.3390/ijms18020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Min J., Shen H., Xi W., Wang Q., Yin L., Zhang Y., Yu Y., Yang Q., Wang Z.N. Synergistic Anticancer Activity of Combined Use of Caffeic Acid with Paclitaxel Enhances Apoptosis of Non-Small-Cell Lung Cancer H1299 Cells in Vivo and in Vitro. Cell. Physiol. Biochem. 2018;48:1433–1442. doi: 10.1159/000492253. [DOI] [PubMed] [Google Scholar]
- 135.Gupta S., Tak H., Rathore K., Banavath H.N., Tejavath K.K. Caffeic acid, a dietary polyphenol pre-sensitizes PDAC to chemotherapeutic drug. J. Biomol. Struct. Dyn. 2023. ahead of print . [DOI] [PubMed]
- 136.Ahangarpour A., Alboghobeish S., Oroojan A.A., Dehghani M.A. Caffeic acid protects mice pancreatic islets from oxidative stress induced by multi-walled carbon nanotubes (MWCNTs) Vet. Res. Forum. 2021;12:77–85. doi: 10.30466/vrf.2019.94666.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rahimi H., Mohammadgholi A., Koosha M. Cytotoxic Effect of Caffeic Acid Loaded in Chitosan-Grafted Carbon Nanotubes on MDA-MB-231 Breast Cancer Cell Lines. Iran. J. Sci. 2023;47:631–640. doi: 10.1007/s40995-023-01465-z. [DOI] [Google Scholar]
- 138.Qin P., Wei Y., Hou M., Zhao C., Shen Z. A multicenter clinical trial of caffeic acid tablet in treatment of 103 primary immune thrombocytopenia patients. Zhonghua Xue Ye Xue Za Zhi. 2015;36:103–106. doi: 10.3760/cma.j.issn.0253-2727.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yu H., Chen R., Zhou Z., Liu R., Wen J. Efficacy and safety of caffeic acid tablets in the treatment of thrombocytopenia: A systematic review and meta-analysis. Medicine. 2023;102:e35353. doi: 10.1097/MD.0000000000035353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schaue D., McBride W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015;12:527–540. doi: 10.1038/nrclinonc.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.De Ruysscher D., Niedermann G., Burnet N.G., Siva S., Lee A.W.M., Hegi-Johnson F. Radiotherapy toxicity. Nat. Rev. Dis. Primers. 2019;5:13. doi: 10.1038/s41572-019-0064-5. [DOI] [PubMed] [Google Scholar]
- 142.Hall E.J. Radiation, the two-edged sword: Cancer risks at high and low doses. Cancer J. 2000;6:343–350. [PubMed] [Google Scholar]
- 143.Borrelli M.R., Shen A.H., Lee G.K., Momeni A., Longaker M.T., Wan D.C. Radiation-Induced Skin Fibrosis: Pathogenesis, Current Treatment Options, and Emerging Therapeutics. Ann. Plast. Surg. 2019;83((4S Suppl. 1)):S59–S64. doi: 10.1097/SAP.0000000000002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rakici S.Y., Guzel A.I., Tumkaya L., Sevim Nalkiran H., Mercantepe T. Pelvic radiation-induced testicular damage: An experimental study at 1 Gray. Syst. Biol. Reprod. Med. 2020;66:89–98. doi: 10.1080/19396368.2019.1679909. [DOI] [PubMed] [Google Scholar]
- 145.Delanian S., Lefaix J.L., Pradat P.F. Radiation-induced neuropathy in cancer survivors. Radiother. Oncol. 2012;105:273–282. doi: 10.1016/j.radonc.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 146.Kuderer N.M., Desai A., Lustberg M.B., Lyman G.H. Mitigating acute chemotherapy-associated adverse events in patients with cancer. Nat. Rev. Clin. Oncol. 2022;19:681–697. doi: 10.1038/s41571-022-00685-3. [DOI] [PubMed] [Google Scholar]
- 147.van den Boogaard W.M.C., Komninos D.S.J., Vermeij W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers. 2022;14:627. doi: 10.3390/cancers14030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Devipriya N., Sudheer A.R., Menon V.P. Caffeic acid protects human peripheral blood lymphocytes against gamma radiation-induced cellular damage. J. Biochem. Mol. Toxicol. 2008;22:175–186. doi: 10.1002/jbt.20228. [DOI] [PubMed] [Google Scholar]
- 149.Lukmanul Hakkim F., Miura M., Matsuda N., Alharassi A.S., Guillemin G., Yamauchi M., Arivazhagan G., Song H. An in vitro evidence for caffeic acid, rosmarinic acid and trans cinnamic acid as a skin protectant against γ-radiation. Int. J. Low Radiat. 2014;9:305–316. doi: 10.1504/IJLR.2014.063414. [DOI] [Google Scholar]
- 150.Sayed H.M., Said M.M., Morcos N.Y.S., El Gawish M.A., Ismail A.F.M. Antitumor and Radiosensitizing Effects of Zinc Oxide-Caffeic Acid Nanoparticles against Solid Ehrlich Carcinoma in Female Mice. Integr. Cancer Ther. 2021;20:15347354211021920. doi: 10.1177/15347354211021920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hausser J., Alon U. Tumour heterogeneity and the evolutionary trade-offs of cancer. Nat. Rev. Cancer. 2020;20:247–257. doi: 10.1038/s41568-020-0241-6. [DOI] [PubMed] [Google Scholar]
- 152.Andrade S., Pereira M.C., Loureiro J.A. Caffeic acid loaded into engineered lipid nanoparticles for Alzheimer’s disease therapy. Colloids Surf. B Biointerfaces. 2023;225:113270. doi: 10.1016/j.colsurfb.2023.113270. [DOI] [PubMed] [Google Scholar]
- 153.Kamath P.P., Rajeevan R., Maity S., Nayak Y., Narayan R., Mehta C.H., Velagacherla V., Konuri A., Nayak U.Y. Development of nanostructured lipid carriers loaded caffeic acid topical cream for prevention of inflammation in wistar rat model. J. Appl. Pharm. Sci. 2023;13:064–075. doi: 10.7324/JAPS.2023.130106-1. [DOI] [Google Scholar]
- 154.Stanciauskaite M., Poskute M., Kurapkiene V., Marksa M., Jakstas V., Ivanauskas L., Kersiene M., Leskauskaite D., Ramanauskiene K. Optimization of Delivery and Bioavailability of Encapsulated Caffeic Acid. Foods. 2023;12:1993. doi: 10.3390/foods12101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.