Abstract
Endocrine disrupting contaminants, in combination with other environmental variables, are associated with altered reproductive health. Assisted reproductive technology (ART) procedures offer valuable opportunities to explore the connections between environmental contaminants in the ovarian microenvironment and measures of fertility, including impaired responsiveness to gonadotropins. Here, we investigate an emerging class of environmental contaminants, the perfluorinated alkyl acids (PFAAs), to determine whether ovarian contaminant levels are associated with measures of ovarian responsiveness and fertility outcomes in a South Carolina population of women undergoing ART. Levels of PFAAs in plasma and follicular fluid samples collected from women undergoing ovarian stimulation were analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS). Six PFAAs were detected in both plasma and follicular fluid. PFAA concentrations in plasma correlate strongly to those detected in ovary and, with the exception of one compound, remain stable throughout ovarian stimulation. The concentration of PFHxS in follicular fluid inversely relates to baseline follicle counts. While no significant relationships were detected between ovarian response measures and PFAA concentrations, we identified a negative relationship between follicular fluid PFDA and PFuNA and blastocyst conversion rates. Our assessments indicate that plasma levels of PFAAs serve as a sound proxy of those in the ovarian compartment and that follicular fluid levels of specific PFAA compounds are inversely related to important clinical measures of reproductive health including baseline follicle count and post-fertilization success.
Keywords: PFAAs, EDCs, follicular fluid, ART
1. INTRODUCTION
Current estimates of human fecundity and fertility in the United States indicate that 10.9% (7.3 million) of married women between ages 15–44 display impaired fecundity and 6% (1.5 million) are classified as infertile [1, 2]. While it is difficult to evaluate whether these numbers have increased over recent decades, it is clear that the number of individuals seeking assisted reproductive technology (ART) therapy is on the rise [3]. Various reasons have been postulated to explain this trend including delayed age of first pregnancy, improved access to fertility clinics, and increased reporting of fertility problems. Additionally, exposure to environmental contaminants can significantly influence reproductive health, and exposures have been suggested as a likely contributor [4–7]. Here, we investigate an emerging class of environmental contaminants, the perfluorinated alkyl acids (PFAAs), to determine whether ovarian contaminant levels are associated with measures of overall ovarian health in a population of women undergoing fertility treatment.
The ovarian follicle represents the functional unit of the ovary, and follicular development is tightly regulated by both intra- and extra-ovarian factors [8]. Whereas pre-antral stages can occur independent of gonadotropin receptor activation and are characterized by growth and differentiation of the oocyte, the subsequent antral phases of follicular development are dependent upon pituitary derived gonadotropins (follicle stimulating hormone and luteinizing hormone, FSH and LH, respectively) and are characterized by differentiation and enlargement of the follicle [8]. Thus, the ability of the ovary to properly respond to gonadotropin stimulation is a fundamental aspect of its function. Current ART takes advantage of the regulatory mechanisms that control follicle growth to promote the development of multiple follicles. In this setting, controlled ovarian stimulation is conducted by pharmacologically silencing the hypothalamus-pituitary-ovary axis and subsequent stimulation with exogenous FSH, allowing for careful control and monitoring of the developing follicle pool. When optimal follicle count and size is achieved, ovulation is triggered and measures to promote fertilization (e.g., intrauterine insemination, harvesting of follicles subsequent in vitro fertilization) are performed [9]. Ovarian responsiveness to FSH treatment is not a standardized parameter across different fertility practices, but typically incorporates a combination of more common measures including peak 17β-estradiol (E2), peak follicle count, total FSH administered, and total oocytes retrieved. The etiology of variation in responsiveness to FSH is likely rooted in a complex of influences including age, body mass index (BMI), ovarian reserve, as well as genetic factors. There is, however, also concern that exposures to environmental contaminants may also impair the mechanisms that regulate ovarian follicle development [10–12]. The treatment protocol and samples collected as part of standard ART procedures offer valuable opportunities to explore the connections between environmental contaminants in the ovarian microenvironment potentially providing insights into the underlying influences of impaired responsiveness to gonadotropins.
Here we focus on PFAAs, which exhibit surfactant properties that make them desirable components of various products including adhesives, water-repellant surfaces, lubricants, and aqueous film-forming foams finding use in packaging, as stain repellants on fabrics and as firefighting foams. Structurally, PFAAs consist of one or more carbon atoms where all of the hydrogen atoms have been replaced by fluorine atoms and an acid functional group [13]. The carbon-fluorine bond is the strongest known bond in organic chemistry hence PFAAs are stable and long-lived in the environment. PFAAs have also been shown to bioaccumulate and biomagnify in the ecosystem [14, 15]. In humans, PFAAs are poorly eliminated and exhibit half-lives up to 5 years [16]. Primary exposure routes include inhalation of air particles contaminated with PFAAs originating from numerous consumer products including non-stick cookware and water-resistant consumables as well as ingestion through food and water [17]. Some epidemiological data exists associating PFAAs and health outcomes including recent studies demonstrating an association between circulating PFAA levels and kidney dysfunction (increased uric acid, reduced glomerular filtration), prostate cancer risk, lipid metabolism, and sperm quality [18–21].
Both epidemiological and in vitro studies suggest that PFAA compounds might influence ovarian cell signaling and measures of overall reproductive health [22]. In human populations, elevated concentrations of perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) are associated with moderate to severe endometriosis as well as polycystic ovarian syndrome [23, 24]. Approaches that incorporate transient transfection assays have linked PFAA exposure to activation of mouse and human peroxisome proliferator activated receptor alpha (PPARα) [25, 26]. PPARs are ligand dependent nuclear receptors with roles in many physiological processes including inflammation, energy homeostasis, glucose metabolism and cellular proliferation and differentiation. The observed interaction between PFAAs and receptors of the PPAR family is intriguing given the documented role of PPAR receptor activation in the regulation of folliculogenesis and steroidogenesis and offers rationale for inclusion of the PFAA survey conducted here [27, 28]. To our knowledge, only two studies have directly assessed the relationship between PFAAs and ovarian health, specifically responsiveness to gonadotropin stimulation through fertility treatment. The first did not present details regarding individual PFAA concentrations [29]. A more recent study involved assessments in a population of Belgium women seeking ART and focused on examining post-oocyte retrieval outcomes such as fertilization and cleavage rates and identified a direct relationship between PFAA concentrations and embryo quality [30].
The development of the ovarian follicle is a tightly regulated process dependent on a critical balance of hormones and growth factors. Given the observations linking PFAAs to disrupted ovarian signaling, we set out to address four primary questions (1) Which PFAAs are measurable in the ovarian follicular fluid?, (2) Are plasma PFAA levels predictive of levels measured in follicular fluid?, (3) Do the concentrations of PFAAs fluctuate over the course of ovarian stimulation with FSH?, and (4) Is there relationship between measures of ovarian responsiveness or fertilization success and PFAA body burden? The results will provide information regarding abundance and distribution of these compounds in a population of women seeking ART. Also important is that the analysis framework described here can be applied to study the influence of other compounds of interest on ovarian function and responsiveness in the setting of ART.
2. METHODS
2.1. Patients
This study was approved by the Medical University of South Carolina institutional review board (IRB# Pro00015729). Informed consent was obtained from all subjects before inclusion in the study. A total of 50 subjects undergoing in vitro fertilization at the Coastal Fertility Center in Mount Pleasant, South Carolina, USA, were enrolled between May 2013 and August 2014. Of the enrolled subjects, we collected follicular fluid from 36 subjects. No exclusions were applied. All subjects underwent pelvic ultrasound examination to determine baseline antral follicle counts. The initial dosage of gonadotropin was determined based on baseline antral follicle counts, baseline FSH levels, and patient age. Ultrasound tracking and hormone measures were carried out at appropriate intervals and human chorionic gonadotropin (hCG) was administered to trigger final oocyte maturation. Ultrasound guided oocyte retrieval was carried out approximately 36 hours post hCG trigger and all visible follicles larger than 10 mm in diameter were aspirated.
2.2. Sampling Protocol
Blood and follicular fluid were collected from enrolled subjects. A baseline blood and urine sample was collected prior to the gonadotropin stimulation phase. Additional blood samples were collected throughout the stimulation phase. At the time of oocyte retrieval, ovarian follicular fluid was collected from follicles >10mm in diameter. The total volume of follicular fluid from each patient was pooled and collected directly into glass containers, transferred to 50 mL conical centrifuge tubes (Thermo Fisher Scientific, Grand Island, NY, USA) and immediately processed by centrifugation at 600 RCF for 10 min to pellet associated follicular cells. The cleared supernatant was removed and stored in glass vials at −40 °C until further analysis. Whole blood was centrifuged at 1500 RCF for 20 min at room temperature and the cleared plasma supernatant was stored at −40 °C until further analysis.
2.3. Calculating ovarian responsiveness index and measures of fertilization success
For each patient, data regarding patient age, body mass index (BMI), basal and peak E2 levels (pg/mL), amount of gonadotropin applied, number of oocytes retrieved (>10 mm), and other related fertility measures were collected. From this information, we calculated three measures of ovarian responsiveness: ΔE2 (pg/mL), Δ antral follicle account and oocytes at retrieval. The ΔE2 was calculated by determining the proportional increase of E2 over the course of FSH stimulation. The Δ antral follicle count was calculated by determining proportional change in antral follicle count over the course of FSH stimulation (as determined by ultrasound examination). The oocytes at retrieval measure reflects the total number of oocytes retrieved. Additionally, fertilization rate, blastocyst conversion rate, and pregnancy outcomes were collected for each subject and incorporated into the analysis. Fertilization rate was determined 17–20 hours post insemination by calculating the number of zygotes with two pronuclei (2pn) / total number of mature oocytes retrieved. Blastocyst development was defined as embryos with blastocoel development and expansion of at least grade 2 using the Gardner scale on day 5 or 6 of development [31]. Blastocyst conversion is defined as the number of blastocysts generated by day 6 of development / the number of embryos cultured past day 3 of development. Whether the in vitro fertilization procedure resulted in the birth of one or more babies was recorded and categorized as pregnancy outcome.
2.4. Measurement of perfluorinated alkyl acids
The National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 1958 organic contaminants in fortified human serum was employed as a control material during PFAA analysis. The SRM was reconstituted with deionized water based on the certificate of analysis (www.nist.gov/srm/).
Calibration solutions were comprised of two solutions produced by the NIST Reference Materials (RMs): RM 8446 Perfluorinated Carboxylic Acids and Perfluorooctane Sulfonamide in Methanol and RM 8447 Perfluorinated Sulfonic Acids in Methanol. Together, the solution contained 15 PFAAs as follows: perfluorobutyric acid (PFBA), perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), PFOA, perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnA), perfluorododecanoic acid (PFDoA), perfluorotridecanoic acid (PFTriA), perfluorotetradecanoic acid (PFTA), perfluorobutanesulfonic acid (PFBS), perfluorohexanesulfonic acid (PFHxS), PFOS, and perfluorooctanesulfonamide (PFOSA). Internal standards (IS) were purchased from Cambridge Isotope Laboratories (Andover, MA), RTI International (Research Triangle Park, NC), and Wellington Laboratories (Guelph, Ontario) to generate an internal standard (IS) mixture containing eleven isotopically labeled PFAAs, and they were as follows: [13C4]PFBA, [13C2]PFHxA, [13C8]PFOA, [13C9]PFNA, [13C9]PFDA, [13C2]PFUnA, [13C2]PFDoA, [18O2]PFBS, [18O2]PFHxS, [13C4]PFOS, and [18O2]PFOSA.
The isolation and purification methods are outlined in detail by Reiner and colleagues [32]. Purified extracts from 1 mL of follicular fluid were analyzed for PFAAs by liquid chromatography tandem mass spectrometry (LC-MS/MS) using an Agilent 1100 LC system (HPLC; Santa Clara, CA) coupled to an Applied Biosystems API 4000 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA) with electrospray ionization in negative mode. A total of 5 μL of resulting sample extract was injected onto an Agilent Zorbax Eclipse Plus C18 analytical column (2.1 mm x 150 mm x 5 μm). The following solvent gradient flow was employed: 50% 20 mmol/L ammonium acetate in methanol, 50% 20 mmol/L ammonium acetate in water (at a flow rate of 250 μL/min). By 20 min, the methanol increased to 75% methanol and held for 5 min, followed by an increase to 95% methanol by 35 min with a subsequent return to 50% methanol at 40 min. Two multiple reaction monitoring (MRM) transitions for each PFAA were monitored to ensure no interferences with PFAA measurement, one MRM was employed for quantitation and the other one was used for confirmation [32]. All plasma and follicular fluid samples were processed alongside quality control material NIST SRM 1958, a follicular fluid quality control sample, and blanks to determine the accuracy and precision of the method. The PFAA concentrations of SRM 1958 processed during our extraction met established values reported on the Certificate of Analysis (CoA). Repeated measurements of the follicular fluid quality control material showed good agreement (RSD < 15 %). The PFAAs were considered to be above the limit of detection (LOD) if the mass of an analyte in the sample was greater than the mean plus three standard deviations of all blanks.
2.5. Statistics
All statistical analyses were performed with SPSS Statistics version 22 (IBM, Armonk, NY, USA) and GraphPad Prism version 6.01 (GraphPad Software, La Jolla, CA, USA). PFAA concentrations were log transformed prior to analysis. Measures were analyzed using a ROUT test to identify outliers, Q=0.5. For each individual PFAA, a T-test was used to detect differences between plasma and follicular fluid concentrations. To determine whether plasma PFAA concentration related to follicular fluid PFAA concentration, Pearson correlation analyses were used. For assessments aimed at determining if concentrations of PFAAs changed over the course of ovarian stimulation, a repeated measures ANOVA was utilized. To identify relationships among ovarian response and contaminant burden, a partial correlation analysis was conducted with a correction for age built into the model. A correction for BMI was not applied in this context because unlike age, a direct association between BMI and ovarian responsiveness was not detected. For statistical analysis of PFAA measurement data, PFAAs measured below the LOD were set equal to half the LOD prior to running the statistical tests [33]. Statistical significance was set at p<0.05 for all tests.
3. RESULTS
3.1. Description of Subjects
Of the 50 subjects enrolled, we collected both baseline and stimulatory phase plasma samples, along with follicular fluid samples, for 26 individual women participating in the standard in vitro fertilization treatment protocol. Baseline and stimulatory phase samples were collected (i.e. plasma samples) from an additional 8 patients but corresponding follicular fluid samples at time of egg retrieval were not collected. These samples were used when addressing questions regarding fluctuations of PFAAs over the course of ovarian stimulation and ovarian responsiveness. Descriptive data including average age (33.7 ± 4.5 years) and average BMI (24.8 ± 4.2 kg/m2) were determined for the study cohort and accounted for in subsequent statistical analyses.
3.2. PFAA burden in ovarian follicular fluid and plasma
Of the panel of fifteen PFAAs measured, six were detected above the LOD in all patient follicular fluid samples, with the exception of one patient that had PFDA and PFUnA concentrations below the LOD ( PFDA >0.3 ng/g, PFUnA > 0.2). In order of abundance, these include: PFOS, PFOA, PFNA, PFHxS, PFDA, and PFUnA (Figure 1A). Correlation analysis revealed a high degree of association among the individual PFAA levels, indicating that exposures to the compounds are linked either by source or perhaps by behavior (Table 1).
Figure 1.
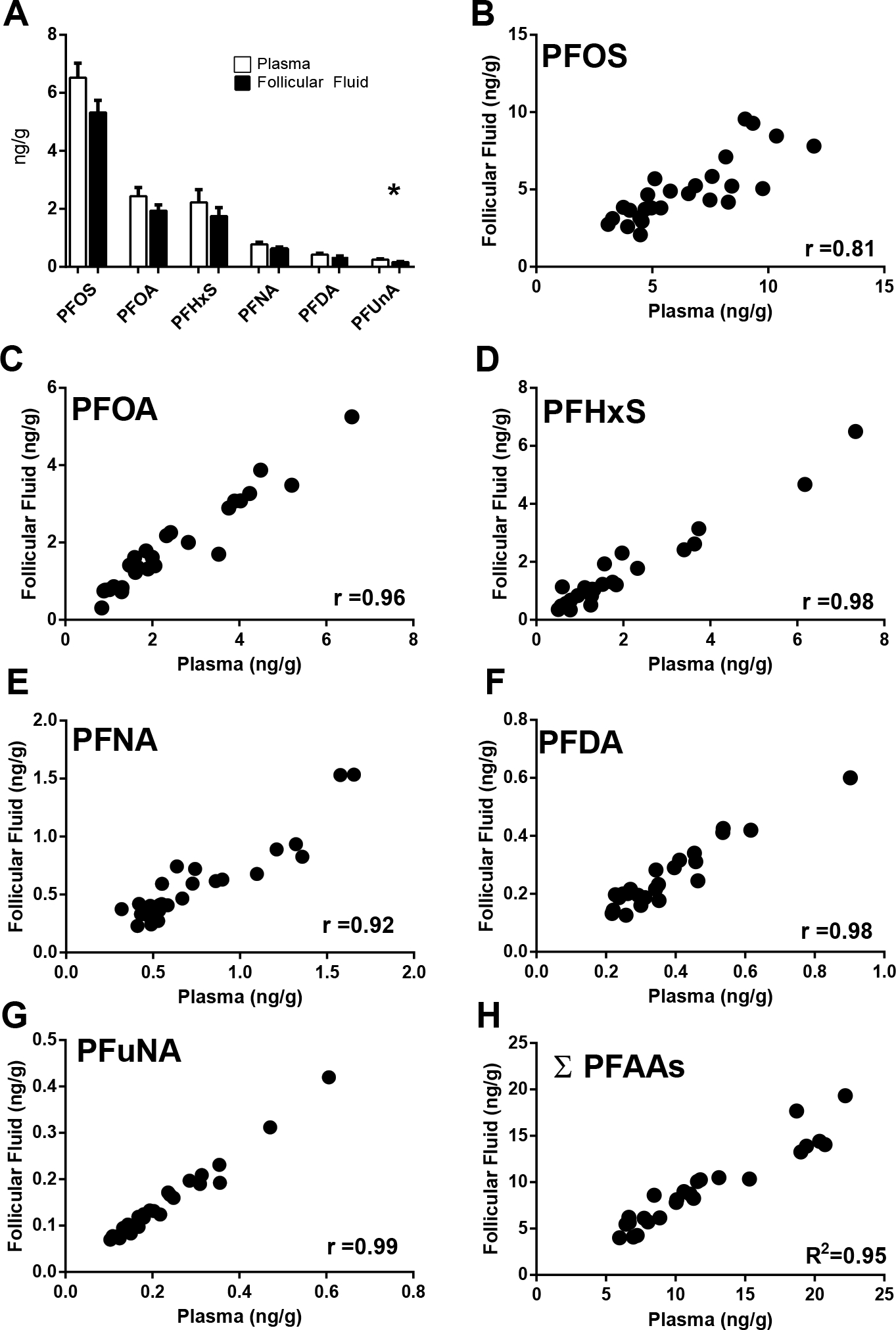
Measurable PFAA compounds in plasma and follicular fluid. Mean concentrations (A) and tissue level correlations (B-H) for each individual compound are displayed. The asterisk (*) in panel A denotes a statistically significant difference was determined by T-test (p<0.05). The Pearson correlation coefficients are displayed in panels B-H, p<0.05. Error bars displayed in panel A reflect SEM.
Table 1.
Intra-PFAA correlations values (r) in plasma and follicular fluid.
| Follicular Fluid | lnPFOA | lnPFNA | lnPFDA | lnPFUnA | lnPFHxS | lnPFOS |
|---|---|---|---|---|---|---|
|
| ||||||
| PFOA | - | 0.741 ** | 0.648 ** | 0.326 | 0.363 * | 0.524 ** |
| PFNA | - | 0.850 ** | 0.438 * | 0.411 * | 0.770 ** | |
| PFDA | - | 0.586 ** | 0.276 | 0.714 ** | ||
| PFUnA | - | 0.336 | 0.383 * | |||
| PFHxS | - | 0.451 * | ||||
| PFOS | - | |||||
|
| ||||||
| Plasma | lnPFOA | lnPFNA | lnPFDA | lnPFUnA | lnPFHxS | lnPFOS |
|
| ||||||
| PFOA | - | 0.814 ** | 0.617 ** | 0.289 | 0.173 | 0.540 ** |
| PFNA | - | 0.809 ** | 0522 ** | 0.421 * | 0613 ** | |
| PFDA | - | 0.699 ** | 0.324 | 0.506 ** | ||
| PFUnA | - | 0.571 ** | 0.363 | |||
| PFHxS | - | 0534 ** | ||||
| PFOS | - | |||||
significant at the 0.05 level (2-tailed).
significant at the 0.01 level (2-tailed).
Overall, follicular fluid levels of PFAAs are similar to those detected in plasma. Of the six compounds detected, only PFUnA concentrations differed between the two matrices with plasma levels being approximately 1.5 times higher than those detected in follicular fluid (unpaired T-Test, p=0.01) (Figure 1, Table 2).
Table 2.
Detected concentrations (ng/g) of individual and total PFAAs in plasma and follicular fluid (FF).
| Plasma (ng/g; n=26) | FF (ng/g; n=31) | Plasma/ FF (n=26) | p value | |
|---|---|---|---|---|
|
| ||||
| PFOS | 6.52 ± 0.50 | 5.33 ± 0.42 | 1.22 | 0.07 |
| PFOA | 2.44 ± 0.30 | 1.94 ± 0.20 | 1.26 | 0.16 |
| PFHxS | 2.23 ± 0.44 | 1.75 ± 0.30 | 1.27 | 0.36 |
| PFNA | 0.78 ± 0.08 | 0.63 ± 0.06 | 1.24 | 0.12 |
| PFDA | 0.42 ± 0.05 | 0.33 ± 0.04 | 1.27 | 0.19 |
| PFUnA | 0.25 ± 0.03 | 0.17 ± 0.02 | 1.47 | 0.01* |
|
| ||||
| ΣPFAAs | 12.6 ± 1.1 | 10.1 ± 0.8 | 1.25 | 0.06 |
3.3. PFAA concentrations in plasma are highly correlated to those detected in follicular fluid
For total and individual PFAA compounds, significant positive correlations between plasma and follicular fluid concentrations are detected (Figure 1, B–H). However, the strength of these relationships varies among the individual compounds (range r=0.81 – 0.99).
3.4. PFAA plasma concentrations remain stable over the course of ovarian stimulation
To evaluate whether the average plasma levels of PFAA compounds vary significantly over the course of ovarian stimulation, we measured compounds in plasma samples collected from women prior to stimulation with FSH and at three subsequent time points during the stimulation protocol, reflecting an average of 17 days total (min. 12 days, max. 26 days). Results indicate that levels generally remain unchanged (Figure 2, Supplementary Table 1), with the exception of PFOA, which decreased over the course of stimulation (repeated measures ANOVA, p=0.001, F=7.4).
Figure 2.
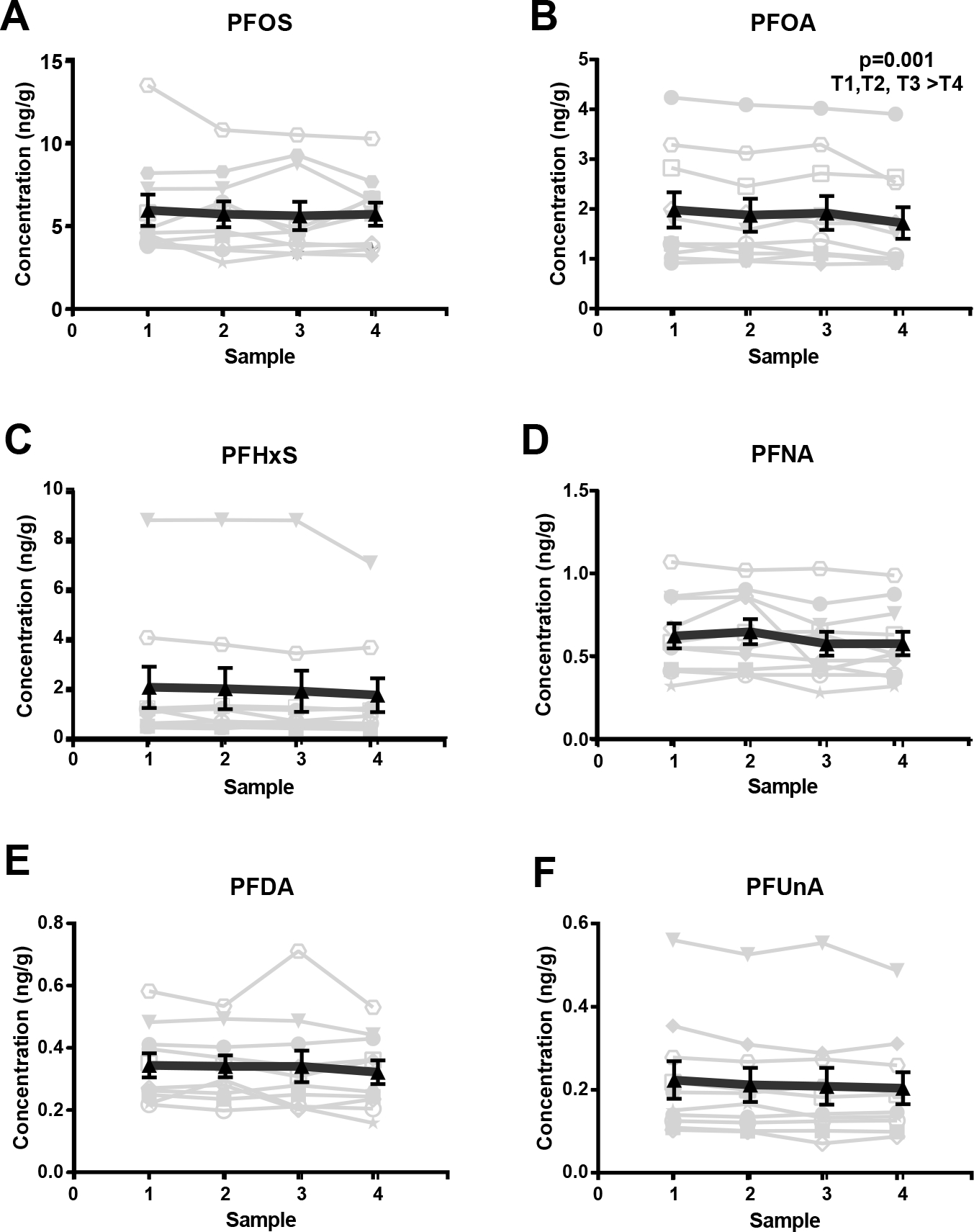
Plasma PFAA concentrations (ng/g) measured over the study time period: (A) PFOS, (B) PFOA, (C) PFHxS, (D) PFNA, (E) PFDA, and (F) PFUnA. Plots depict the detectable levels of individual plasma PFAAs collected from women (n=10) prior to and throughout the course of ovarian stimulation. Individual (light grey) and average (n=10, black) values are displayed. Error bars reflect S.E.M.
3.5. Connections between ovarian responsiveness and plasma PFAAs
To assess the potential influence of PFAAs on ovarian health, we first considered whether detected plasma and follicular fluid PFAAs relate to baseline follicle counts, E2 levels, and patient age (Figure 3, Table 3). A non-significant relationship was determined for total PFAA burden and baseline measures of E2 and follicle counts (Figure 3). However, the follicular fluid levels of one particular PFAA, PFHxS, negatively correlate to baseline follicle counts (Pearson correlation, r= −0.43, Table 3). We did not detect an association between patient age and PFAA body burden (Table 3).
Figure 3.
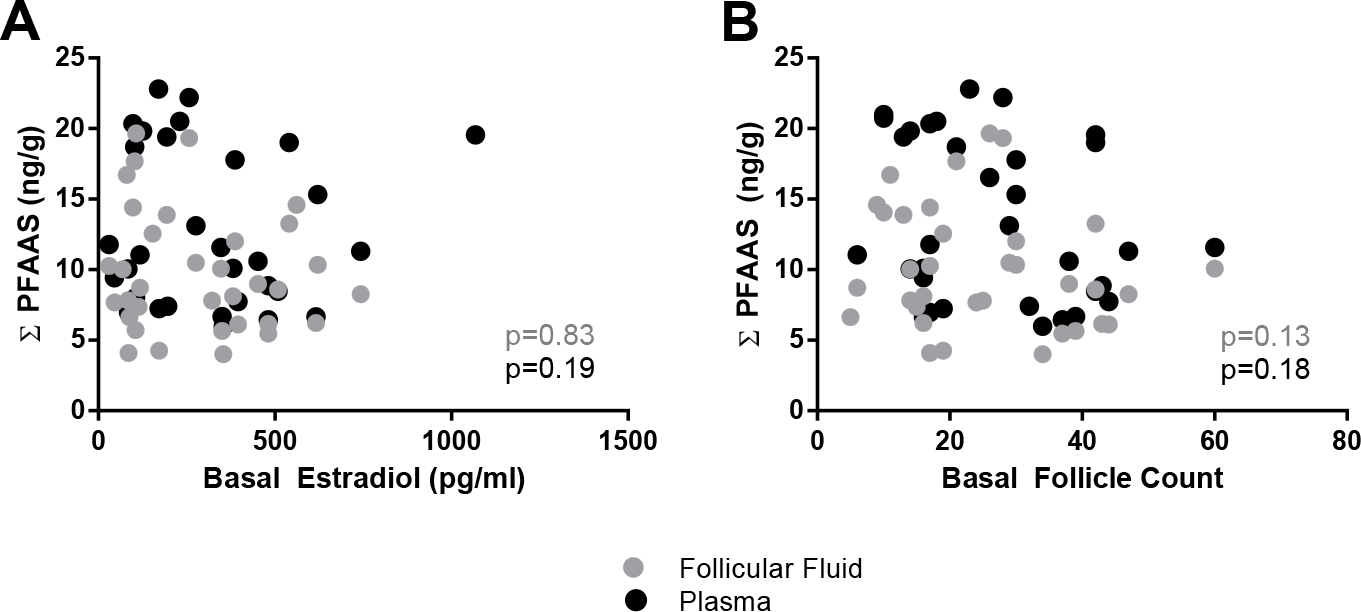
PFAA concentrations (ng/g) related to basal E2 and follicle count measurements. Plasma (black circles) and follicular fluid (gray circles) are depicted along with significance values (p) obtained from partial correlation analysis.
Table 3.
Correlation values (r) between PFAA levels (ng/g) and clinical measurements
| Follicular Fluid | lnPFHxS | lnPFOA | lnPFOS | lnPFNA | lnPFDA | lnPFUnA | lnΣPFAAs |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Estradiol (pg/ml) | −0.34 | −0.11 | 0.07 | 0.05 | 0.08 | 0.18 | −0.04 |
| Follicle Count | −0.43 | −0.25 | −0.16 | −0.23 | −0.25 | −0.29 | −0.29 |
| Δ E2 | −0.09 | 0.02 | −0.19 | −0.20 | −0.17 | −0.36 | −0.18 |
| Δ Follicle # | 0.19 | 0.07 | 0.09 | 0.15 | 0.30 | 0.40 | 0.14 |
| Ooocytes Retrieved | −0.23 | −0.16 | −0.07 | −0.18 | −0.13 | −0.09 | −0.17 |
| Fertilization (%) | −0.07 | 0.04 | 0.15 | −0.12 | −0.06 | −0.18 | 0.06 |
| Blast Conversion | 0.00 | −0.24 | −0.13 | −0.33 | −0.52 | −0.60 | −0.23 |
| Age | −0.08 | −0.04 | −0.25 | −0.20 | −0.24 | −0.37 | −0.18 |
|
| |||||||
|
| |||||||
| Plasma | lnPFHxS | lnPFOA | lnPFOS | lnPFNA | lnPFDA | lnPFUnA | lnΣPFAAs |
|
| |||||||
| Estradiol (pg/ml) | −0.18 | 0.07 | −0.47 | −0.16 | −0.25 | −0.35 | −0.27 |
| Follicle Count | −0.32 | −0.06 | −0.38 | −0.20 | −0.22 | −0.27 | −0.27 |
| Δ E2 | −0.18 | −0.07 | −0.06 | −0.13 | 0.07 | −0.27 | −0.18 |
| Δ Follicle # | 0.06 | 0.22 | −0.08 | 0.14 | 0.18 | 0.25 | 0.06 |
| Ooocytes Retrieved | −0.29 | −0.13 | −0.13 | −0.24 | −0.08 | −0.15 | −0.22 |
| Fertilization (%) | 0.01 | 0.20 | −0.05 | 0.19 | 0.14 | 0.03 | 0.08 |
| Blast Conversion | −0.10 | −0.17 | −0.11 | −0.21 | −0.29 | −0.54 | −0.23 |
| Age | −0.27 | −0.21 | −0.15 | −0.12 | −0.07 | −0.29 | −0.22 |
Bolded values are significant at the 0.05 level (2-tailed).
Subsequently, we assessed whether PFAAs relate to ovarian response to exogenous gonadotropins. Three different, but related, measures of ovarian responsiveness were considered: (1) ΔE2, (2) Δ antral follicle count, and (3) oocytes retrieved. The range of responsiveness observed in our sample set is displayed in Figure 4. The oocytes retrieved measure of responsiveness negatively correlates with age (Pearson correlation, r= −0.47, p = 0.007). To control for this relationship, an age correction was applied when examining the link between PFAA burden and ovarian responsiveness. The analysis did not reveal any significant relationships linking ovarian responsiveness to total or individual PFAA concentrations in plasma or follicular fluid (Figure 4, Table 3).
Figure 4.
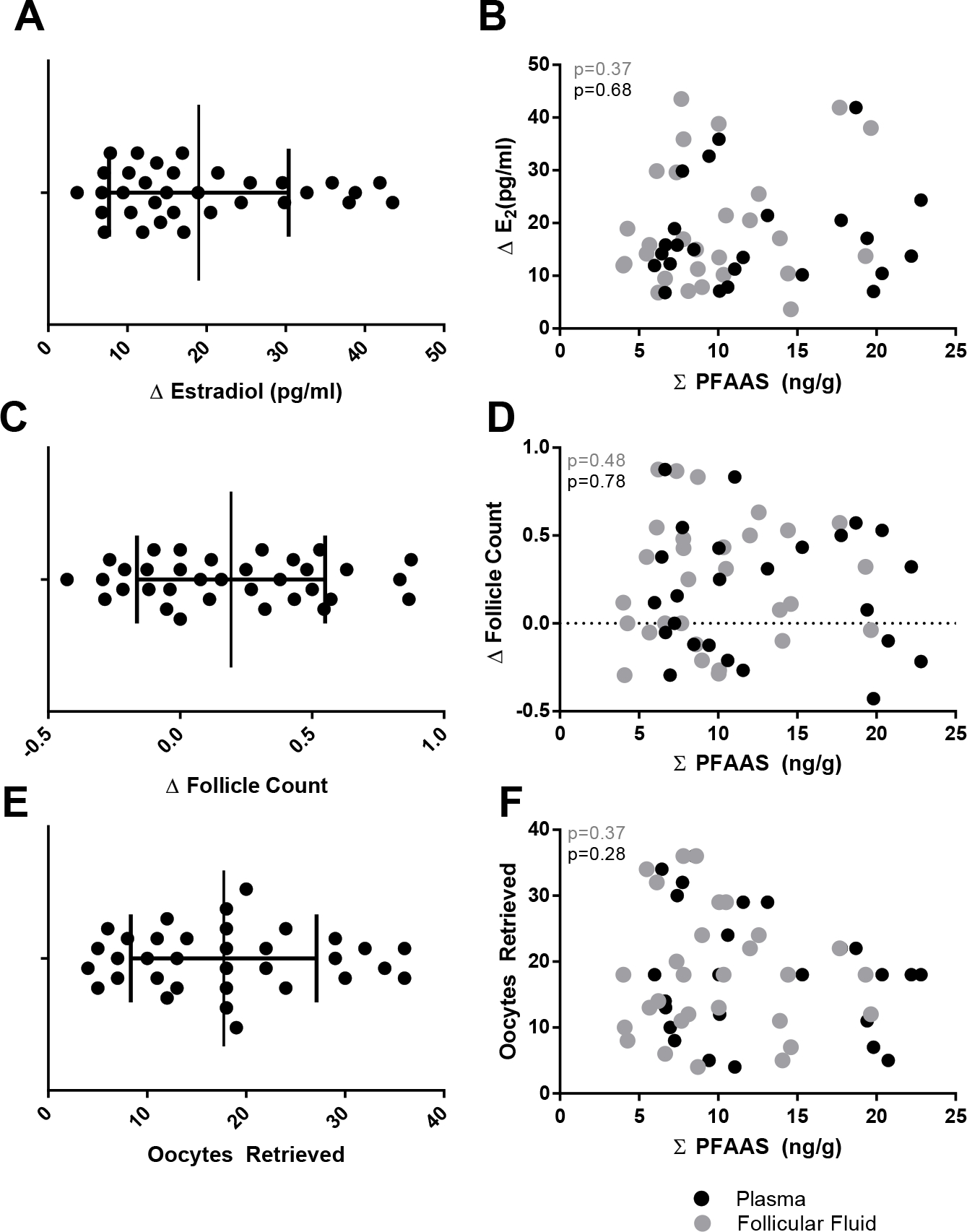
Ovarian response measures related to total PFAA concentrations (ng/g). The range of observed ovarian responsiveness for three different response measures is displayed in panels A, C, and E. The relationship between ovarian responsiveness and total PFAA concentrations (ng/g) are depicted in panels B, D, and F. Plasma (black circles) and follicular fluid (gray circles) are depicted along with significance values (p) obtained from partial correlation analysis.
3.6. Fertility outcome measures and PFAAs
When considering the relationship between PFAA body burden and fertilization measures we found that both PFDA and PFuNA levels in follicular fluid are inversely related to blastocyst conversion rate (r=−0.52 and r=−0.60, respectively, Table 3). A relationship was not detected between PFAA levels and fertilization rates or pregnancy outcomes (Table 3).
4. DISCUSSION
Previously, we demonstrated that wildlife exposed to environmental endocrine disrupting contaminants display reduced ovarian responsiveness upon exogenous stimulation with gonadotropins [34]. In the context of human fertility, women also display a wide range of ovarian responsiveness (Figures 4A, C and E). We hypothesized that certain contaminants present in the ovarian microenvironment influence the ability of the ovary to properly respond to stimulatory signals, such as gonadotropins. This study represents one of the first efforts to quantify multiple PFAAs in the plasma and ovarian follicular fluid of women and subsequently connect those levels to measures of ovarian health in response to gonadotropin stimulation. In addition, it examines the relationship between PFAAs and fertilization outcomes and establishes a valuable framework for future investigations into the environmental determinants of ovarian sensitivity to gonadotropin stimulation.
Six PFAAs were quantified in plasma and ovarian follicular fluid samples, providing evidence that these persistent environmental compounds accumulate in the ovary at levels similar to those detected in the plasma. In previous assessments aimed at quantifying different classes of environmental contaminants (mostly organochlorines, OCs), a two-fold or greater difference was reported between the two tissues, with levels being greater in the plasma relative to the ovary [4]. This stands in contrast to our findings and those reported previously for PFAA compounds, which were found to be approximately equal in both compartments. A likely explanation for this lies in the distinct chemical properties that characterize the different contaminant groups. While OC pollutants are lipophilic and stored in adipose tissue, PFFAs display lipophobic properties and are frequently detected in human plasma samples [13]. PFAAs circulate bound to carrier proteins, which unlike lipid bound OCs, and easily transported across the blood follicle barrier [35, 36]. Therefore, it is likely that the observed near equal abundance of PFAAs in circulation and in follicular fluid may be due to the chemical properties that facilitate transport of these compounds into the follicle from the local bloodstream.
The two most abundant PFAAs detected in our samples (both plasma and follicular fluid) were PFOS and PFOA, which is similar to recent NHANES reports examining PFAA burden in human samples [37]. Our results show that circulating PFAAs are highly predictive of PFAA burden in the ovarian follicular fluid, indicating that plasma sampling can serve as an appropriate proxy for ovarian exposure levels. The time course results indicate that PFAAs remain fairly stable over the course of ovarian stimulation (Figure 2). This finding further supports the use of plasma measurements of PFAAs as an indicator of exposures in the ovarian compartment. If a high degree of variation had been observed, then predictions linking plasma PFAA levels to ovarian health would be challenging. We did note a decrease in the measurable levels of one PFAA compound, PFOA, over the stimulatory course. Due to the long half-life of PFAAs, it is doubtful that this observation is linked to changes in lifestyle (exposure) during the period of ovarian stimulation, but instead might reflect physiological changes associated with a change in circulating hormones (i.e. increased E2). Hormonal fluctuations associated with the menstrual cycle have been linked to observed differences in the pharmacokinetics and pharmacodymamics of certain pharmaceuticals [38, 39]. In addition, PFAA dosing studies in rodents have revealed sex differences in clearance rates. Relative to female rats, males display a reduced excretion rate of PFOA. Treatment of castrated male rats with E2 results in an increased excretion of PFOA, similar to that observed in females [40].Therefore, it seems plausible that the changes associated with exogenous FSH treatment may directly impact clearance of circulating PFAAs and contribute to the reduction observed in PFOA over the course of ovarian stimulation.
We tested the hypothesis that ovarian health is correlated with PFAA body burden in women seeking ART. We found that baseline follicle count is inversely related to plasma PFHxS concentrations, flagging this particular PFAA as a potential compound of interest in the context of ovarian pathology. The identified relationship between PFHxS and baseline fertility measures is intriguing given the documented ability of PFAAs to bind PPARs in vitro and recognized roles for PPAR signaling in proper ovarian follicle maturation and ovulation [26]. Gene expression analysis in rodents reveals that fluctuations in ovarian PPAR transcription characterize different stages of follicle development. Transcript abundance of PPARγ, for example, declines dramatically as follicles transition into the late antral stage and prepare for ovulation through activation of human chorionic gonadotropin (HCG) signaling cascades [28]. Therefore, it seems plausible that persistent activation of ovarian PPARs, through binding to PFAA compounds, could impede follicle maturation and responsiveness to ovulation triggers contributing to impaired fertility. Support linking environmental contaminant exposure to impaired ovarian responsiveness via PPAR mediated signaling comes from in vitro studies demonstrating the ability of monoethylhexyl phthalate (MEHP) to activate PPARs and subsequently lead to reduced aromatase mRNA and protein along with a corresponding reduction in E2 synthesis, an action that could ultimately lead to impaired follicle maturation and ovulation [41]. Given the correlative nature and small sample size of these analyses, a causal relationship is certainly not demonstrated with our current study design. Future studies employing larger samples sizes are still needed to test the robustness of the relationship between PFAA exposure and ovarian responsiveness to FSH. Further, experimental approaches capable of establishing causality will be required to determine the potential molecular mechanisms by which PFAAs might influence ovarian health.
In addition to ovarian responsiveness, we also assessed whether PFAA body burden relates to ART outcome measures and found that increased follicular fluid PFOA and PFuNA levels are associated with lower rates of blastocyst conversion, but not fertilization or pregnancy outcomes. In human IVF, there is a trend toward blastocyst stage transfer and cryopreservation [42]. Culturing embryos to the blastocyst stage allows for the in vitro selection of embryos with the highest implantation potential through morphokinetic assessment [43]. Blastocyst conversion is thus an important measure of IVF cycle quality. Interestingly, a previous report observed a positive relationship between PFAA levels and fertilization rates as well as higher top quality embryo rates [30]. However, this study incorporated a principle component analysis in which potential negative impacts of organochlorine endocrine disrupting contaminants (e.g., p,p’-DDE) on these endpoints were corrected for [44]. The current analyses did not measure or include corrections for this class of contaminants, and thus, observed negative correlations between blastocyst conversion rate and PFOA and PFuNA levels could be confounded by effects of co-occurring compounds to which individuals are exposed.
The current study as well as the Petro, et al., 2014 report are based on relatively small sample sizes and future studies examining the relationship of these compounds to clinical measures of fertility more broadly across the population are warranted. Further, mechanistic studies, aimed at identifying causal roles of PFAAs in affecting early embryonic development, are needed to more fully understand the impact of these exposures on reproduction.
In summary, the experimental design and analysis approach presented here provides a framework for investigating the relationships between ovarian contaminant burden and function. Our assessments indicate that plasma levels of PFAAs are fairly stable and highly predictive of those present in the ovarian compartment follicular fluid. While future studies incorporating an increased number of patient samples as well as unbiased, untargeted analytical approaches to profile the suite of contaminants that accumulate in the follicular fluid will certainly provide insight and targets for future investigations, additional mechanistic studies are also needed to better understand the downstream effects of PFAA exposure in the ovary. An in vitro system incorporating ovarian granulosa cells cultured in the presence or absence of PFAAs, and stimulated with FSH could be used to identify potential genomic or transcriptional level PFAA responsive targets.
Supplementary Material
Supplemental Table 1. PFFA concentrations over the course of stimulation with gonadotropins. For each subject, the detected levels (ng/g) of PFAA compounds prior to (Sample 1) and during the course of gonadotropin stimulation (Samples 2–4) are displayed. Because the actual time between sample collections varied for each subject, the sample collection day is also displayed (Baseline sample = day 1) representing a sampling range of 12–26 days.
Acknowledgements:
We would like to dedicate this manuscript to our co-author, Lou Guillette. This work was supported through South Carolina Center for Economic Excellence funds to LJG. We would like to acknowledge the staff at Coastal Fertility Specialist for aiding sample collections. The authors declare they have no actual or potential competing financial interests.
Footnotes
Disclaimer
Certain commercial equipment or instruments are identified in the paper to specify adequately the experimental procedures. Such identification does not imply recommendations or endorsement by NIST; nor does it imply that the equipment or instruments are the best available for the purpose
REFERENCES:
- 1.Chandra A, Copen CE, Stephen EH: Infertility and impaired fecundity in the United States, 1982–2010: data from the National Survey of Family Growth. National health statistics reports 2013(67):1–18, 11 p following 19. [PubMed] [Google Scholar]
- 2.Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J: Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital and health statistics Series 23, Data from the National Survey of Family Growth 2005(25):1–160. [PubMed] [Google Scholar]
- 3.Boivin J, Bunting L, Collins JA, Nygren KG: International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Human reproduction (Oxford, England) 2007, 22(6):1506–1512. [DOI] [PubMed] [Google Scholar]
- 4.Younglai EV, Foster WG, Hughes EG, Trim K, Jarrell JF: Levels of environmental contaminants in human follicular fluid, serum, and seminal plasma of couples undergoing in vitro fertilization. Archives of environmental contamination and toxicology 2002, 43(1):121–126. [DOI] [PubMed] [Google Scholar]
- 5.Bloom MS, Parsons PJ, Steuerwald A, Schisterman EF, Browne RW, Kim K, Coccaro GA, Conti GC, Narayan N, Fujimoto VY: Toxic trace metals and human oocytes during in vitro fertilization (IVF). Reproductive Toxicology 2010, 29(3):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernanke J, Kohler HR: The impact of environmental chemicals on wildlife vertebrates. Reviews of environmental contamination and toxicology 2009, 198:1–47. [DOI] [PubMed] [Google Scholar]
- 7.Herbst AL, Bern HA (eds.): Developmental effects of diethylstilbestrol (DES) in pregnancy. New York: Thieme-Stratton; 1981. [Google Scholar]
- 8.Edson MA, Nagaraja AK, Matzuk MM: The mammalian ovary from genesis to revelation. Endocrine reviews 2009, 30(6):624–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang DZ, Yang W, Li Y, He Z: Progress in understanding human ovarian folliculogenesis and its implications in assisted reproduction. Journal of assisted reproduction and genetics 2013, 30(2):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillette LJ, Moore BC: Environmental contaminants, fertility, and multioocytic follicles: a lesson from wildlife? Seminars in reproductive medicine 2006, 24(3):134–141. [DOI] [PubMed] [Google Scholar]
- 11.Borgeest C, Symonds D, Mayer LP, Hoyer PB, Flaws JA: Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian epithelium in the mouse. Toxicological sciences : an official journal of the Society of Toxicology 2002, 68(2):473–478. [DOI] [PubMed] [Google Scholar]
- 12.Gupta RK, Meachum S, Hernandez-Ochoa I, Peretz J, Yao HH, Flaws JA: Methoxychlor inhibits growth of antral follicles by altering cell cycle regulators. Toxicology and applied pharmacology 2009, 240(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ: Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr Enviro Assess Manage 2011, 7(4):513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houde M, De Silva AO, Muir DCG, Letcher RJ: Monitoring of Perfluorinated Compounds in Aquatic Biota: An Updated Review. Environ Sci Technol 2011, 45(19):7962–7973. [DOI] [PubMed] [Google Scholar]
- 15.Rotander A, Kärrman A, van Bavel B, Polder A, Rigét F, Auðunsson GA, Víkingsson G, Gabrielsen GW, Bloch D, Dam M: Increasing levels of long-chain perfluorocarboxylic acids (PFCAs) in Arctic and North Atlantic marine mammals, 1984–2009. Chemosphere 2012, 86(3):278–285. [DOI] [PubMed] [Google Scholar]
- 16.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR: Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environmental health perspectives 2007, 115(9):1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Hollander W, de Voogt P, De Coen W, Bervoets L: Perfluorinated substances in human food and other sources of human exposure. Reviews of environmental contamination and toxicology 2010, 208:179–215. [DOI] [PubMed] [Google Scholar]
- 18.Hardell E, Karrman A, van Bavel B, Bao J, Carlberg M, Hardell L: Case-control study on perfluorinated alkyl acids (PFAAs) and the risk of prostate cancer. Environment international 2014, 63:35–39. [DOI] [PubMed] [Google Scholar]
- 19.Kataria A, Trachtman H, Malaga-Dieguez L, Trasande L: Association between perfluoroalkyl acids and kidney function in a cross-sectional study of adolescents. Environmental health : a global access science source 2015, 14:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher T, Galloway TS, Melzer D, Holcroft P, Cipelli R, Pilling LC, Mondal D, Luster M, Harries LW: Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environment international 2013, 57–58:2–10. [DOI] [PubMed] [Google Scholar]
- 21.Vested A, Ramlau-Hansen CH, Olsen SF, Bonde JP, Kristensen SL, Halldorsson TI, Becher G, Haug LS, Ernst EH, Toft G: Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environmental health perspectives 2013, 121(4):453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford NM, Fenton SE, Strynar M, Hines EP, Pritchard DA, Steiner AZ: Effects of perfluorinated chemicals on thyroid function, markers of ovarian reserve, and natural fertility. Reproductive toxicology (Elmsford, NY) 2017, 69:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vagi SJ, Azziz-Baumgartner E, Sjodin A, Calafat AM, Dumesic D, Gonzalez L, Kato K, Silva MJ, Ye X, Azziz R: Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol A in polycystic ovary syndrome: a case-control study. BMC endocrine disorders 2014, 14:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis GM, Peterson CM, Chen Z, Hediger ML, Croughan MS, Sundaram R, Stanford JB, Fujimoto VY, Varner MW, Giudice LC et al. : Perfluorochemicals and endometriosis: the ENDO study. Epidemiology (Cambridge, Mass) 2012, 23(6):799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takacs ML, Abbott BD: Activation of Mouse and Human Peroxisome Proliferator–Activated Receptors (α, β/δ, γ) by Perfluorooctanoic Acid and Perfluorooctane Sulfonate. Toxicological Sciences 2007, 95(1):108–117. [DOI] [PubMed] [Google Scholar]
- 26.Wolf CJ, Schmid JE, Lau C, Abbott BD: Activation of mouse and human peroxisome proliferator-activated receptor-alpha (PPARalpha) by perfluoroalkyl acids (PFAAs): further investigation of C4-C12 compounds. Reproductive toxicology (Elmsford, NY) 2012, 33(4):546–551. [DOI] [PubMed] [Google Scholar]
- 27.Velez LM, Abruzzese GA, Motta AB: The biology of the peroxisome proliferator-activated receptor system in the female reproductive tract. Current pharmaceutical design 2013, 19(25):4641–4646. [DOI] [PubMed] [Google Scholar]
- 28.Froment P, Gizard F, Defever D, Staels B, Dupont J, Monget P: Peroxisome proliferator-activated receptors in reproductive tissues: from gametogenesis to parturition. The Journal of endocrinology 2006, 189(2):199–209. [DOI] [PubMed] [Google Scholar]
- 29.Governini L, Orvieto R, Guerranti C, Gambera L, De Leo V, Piomboni P: The impact of environmental exposure to perfluorinated compounds on oocyte fertilization capacity. Journal of Assisted Reproduction and Genetics 2011, 28(5):415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petro EML, D’Hollander W, Covaci A, Bervoets L, Fransen E, De Neubourg D, De Pauw I, Leroy JLMR, Jorssen EPA, Bols PEJ: Perfluoroalkyl acid contamination of follicular fluid and its consequence for in vitro oocyte developmental competence. Science of The Total Environment 2014, <HT>496</HT>:282–288. [DOI] [PubMed] [Google Scholar]
- 31.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB: Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 2000, 73(6):1155–1158. [DOI] [PubMed] [Google Scholar]
- 32.Reiner JL, Phinney KW, Keller JM: Determination of perfluorinated compounds in human plasma and serum Standard Reference Materials using independent analytical methods. Analytical and Bioanalytical Chemistry 2011, 401(9):2899. [DOI] [PubMed] [Google Scholar]
- 33.Keller JM, McClellan-Green PD, Kucklick JR, Keil DE, Peden-Adams MM: Effects of organochlorine contaminants on loggerhead sea turtle immunity: comparison of a correlative field study and in vitro exposure experiments. Environmental health perspectives 2006, 114(1):70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore BC, Roark AM, Kohno S, Hamlin HJ, Guillette LJ Jr.: Gene-environment interactions: the potential role of contaminants in somatic growth and the development of the reproductive system of the American alligator. Molecular and cellular endocrinology 2012, 354(1–2):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP: Binding of perfluorinated fatty acids to serum proteins. Environmental toxicology and chemistry 2003, 22(11):2639–2649. [DOI] [PubMed] [Google Scholar]
- 36.Schweigert FJ, Gericke B, Wolfram W, Kaisers U, Dudenhausen JW: Peptide and protein profiles in serum and follicular fluid of women undergoing IVF. Human Reproduction 2006, 21(11):2960–2968. [DOI] [PubMed] [Google Scholar]
- 37.Jain RB: Estimation of the total concentration of perfluoroalkyl acids (PFAA) in human serum: Data from NHANES 2005–2012. Chemosphere 2015, 134:387–394. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell SC, Smith RL, Waring RH: The menstrual cycle and drug metabolism. Current drug metabolism 2009, 10(5):499–507. [DOI] [PubMed] [Google Scholar]
- 39.Kashuba AD, Nafziger AN: Physiological changes during the menstrual cycle and their effects on the pharmacokinetics and pharmacodynamics of drugs. Clinical pharmacokinetics 1998, 34(3):203–218. [DOI] [PubMed] [Google Scholar]
- 40.Ylinen M, Hanhijärvi H, Jaakonaho J, Peura P: Stimulation by Oestradiol of the Urinary Excretion of Perfluorooctanoic Acid in the Male rat. Pharmacology & Toxicology 1989, 65(4):274–277. [DOI] [PubMed] [Google Scholar]
- 41.Lovekamp-Swan T, Davis BJ: Mechanisms of phthalate ester toxicity in the female reproductive system. Environmental health perspectives 2003, 111(2):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D: Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. The Cochrane Library; 2016. [DOI] [PubMed] [Google Scholar]
- 43.Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarselli F, Spinella F, Fiorentino F, Varricchio MT, Greco E: Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod 2016. [DOI] [PubMed] [Google Scholar]
- 44.Petro EM, Leroy JL, Covaci A, Fransen E, De Neubourg D, Dirtu AC, De Pauw I, Bols PE: Endocrine-disrupting chemicals in human follicular fluid impair in vitro oocyte developmental competence. Hum Reprod 2012, 27(4):1025–1033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. PFFA concentrations over the course of stimulation with gonadotropins. For each subject, the detected levels (ng/g) of PFAA compounds prior to (Sample 1) and during the course of gonadotropin stimulation (Samples 2–4) are displayed. Because the actual time between sample collections varied for each subject, the sample collection day is also displayed (Baseline sample = day 1) representing a sampling range of 12–26 days.


