Abstract
Background
Arrhythmogenic cardiomyopathy (ACM) is a genetically determined myocardial atrophy which progressively extends from the epicardium towards the endocardium, resulting in wall thinning. It is one of the leading causes of sudden death in young people. Postmortem studies demonstrate that up to 70–80% of the cases have biventricular involvement. Variable penetrance and expressivity results in a wide phenotypic spectrum, challenging diagnostic accuracy of advanced multimodality imaging tools. Prompt recognition, non-invasive imaging, risk stratification for sudden cardiac death (SCD), and preventive measures are paramount to improve prognosis.
Case summary
Here, we present a 22-year-old Black male who was referred to our electrophysiology clinic with palpitations, remote syncope, and a family history of SCD. Over 3 years, he developed gradually worsening symptomatic palpitations. While physical exam and transthoracic echocardiography were unremarkable, his cardiac magnetic resonance imaging was consistent with biventricular ACM. Genetic testing confirmed ACM, revealing double heterozygosity in DSG2 and PKP2. Given the elevated estimated risk of life-threatening dysrhythmias, a subcutaneous cardiac defibrillator was successfully implanted.
Discussion
Frequently, patients with ACM have more than one mutation in the same gene (compound heterozygosity) or in a second gene (double heterozygosity). Genetic counselling is strongly recommended for family members of the proband. The diagnosis of ACM may be mimicked by other diseases (cardiac sarcoidosis, dilated cardiomyopathy, amyloidosis), thus genetic testing can be useful to determine the presence of the disease. The present report provides an overview of the clinical course, diagnostic criteria, risk stratification, and prognostication for patients with ACM.
Keywords: Arrhythmogenic cardiomyopathy, Sudden cardiac death, Individualized prevention, Genetic testing, CMR, Case report
Graphical Abstract
Graphical Abstract.
Summary of the clinical course of the 22-year-old Black male. *Post hoc review of initial transthoracic echocardiogram by two independent imaging cardiologists revealed mild right ventricular dilatation with normal basal dimensions and subtle apical dyskinesis. PAC, premature atrial contractions; PVC, premature ventricular contractions.
Learning points.
Arrhythmogenic cardiomyopathy (ACM) is a genetically mediated cardiomyopathy involving cell-to-cell interaction with principal presentation of ventricular arrhythmias and sudden cardiac death/arrest.
This genetic arrhythmia syndrome presents with varying severity of the phenotypic manifestation, affecting either right, left, or both ventricles.
Conventionally, ACM follows autosomal dominant pattern of inheritance.
It is genetic architecture challenges precision in prognostication, suggesting benefits from standardized close follow-up and screening.
Early diagnosis and awareness allow for timely surveillance, abstinence from competitive sports, shared decision-making regarding antiarrhythmic drugs, catheter ablation, and implantable cardioverter defibrillator, as indicated.
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC), once thought of as a cardiac disorder of right ventricular (RV) predominance due to fibrofatty infiltration, is more aptly named arrhythmogenic cardiomyopathy (ACM) which incorporates a group of disorders with RV, left ventricular, or biventricular involvement. Arrhythmogenic cardiomyopathy is the genetically mediated dysfunction of the connexome, which is a functional unit within cardiac myocytes, comprising desmosomes, gap junctions, adherens junctions, and various ion channels, which allows for electrical and mechanical coupling during cell-to-cell interaction.1 The dysfunctional connections lead to heart failure and the creation of a nidus for ventricular arrhythmias (VAs). As malignant arrhythmia and sudden cardiac death (SCD) often predominate the initial presentation, early recognition, detection, and appropriate management are imperative.
Case presentation
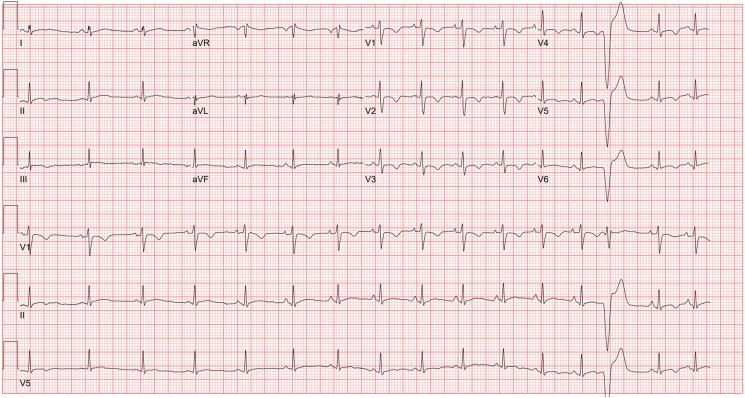
This is a case of a 22-year-old Black male who presented to his primary care physician with a history of intermittent palpitations associated with dizziness for the past 3 years (Graphical Abstract). An isolated episode of syncope at the age of 18 was noted. However, he did not seek medical attention for this episode at the time. Family history was significant for SCD in his paternal grandfather at the age of 47. No autopsy or postmortem genetic testing was performed. No risky behaviours were identified, except for the occasional use of marijuana. Physical examination revealed a physically fit male without any significant findings. The electrocardiogram (ECG) (Figure 1) demonstrated normal sinus rhythm and T-wave inversions in the anterior precordial leads. Also noted were wide premature ventricular contractions with right bundle morphology in V1 with an early precordial transition point.
Figure 1.
Presenting electrocardiogram demonstrates sinus rhythm with sinus arrhythmia. T-wave inversions are seen in the anterior precordial leads as well as occasional premature ventricular contractions.
Further work-up included a 48 h Holter monitor demonstrating predominant sinus rhythm, rare premature atrial and ventricular contractions (<1%), as well as one 6 beat episode of non-sustained ventricular tachycardia (NSVT). Patient-initiated events correlated with sinus rhythm. Transthoracic echocardiogram (TTE) was reported to be unremarkable. Reassurance and surveillance were provided. No significant changes in his symptoms were noted over the following 2 years, until a sudden onset of diaphoresis, flushing, and tachycardia. Self-assessment revealed elevated heart rate at 192 b.p.m., as measured on his smart watch, which was brief and self-resolved. In the Electrophysiology Clinic, the ECG was unchanged from prior (Figure 1). The constellation of a family history of SCD at an early age, personal history of undefined syncope, and T-wave inversions in all anterior precordial leads prompted cardiac MRI.
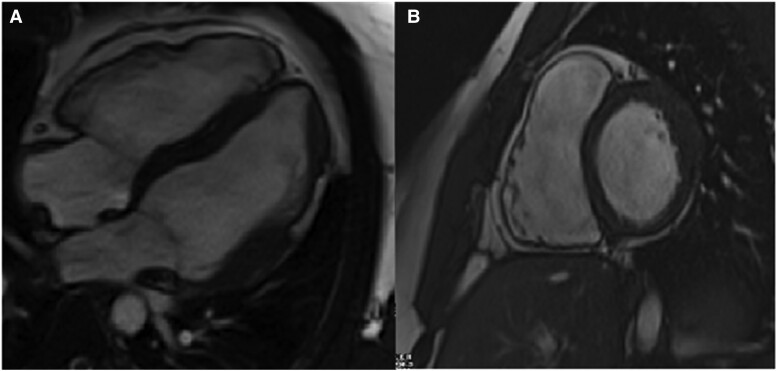
Cardiac MRI (Figure 2; Supplementary material online, Cine Images) was notable for RV enlargement and reduced systolic function, with an estimated ejection fraction (EF) of 37% and regional segments of dyskinesia. The left ventricle assessment demonstrated focal thickening of the mid-anterior, anterolateral, and inferolateral wall segments with associated transmural delayed gadolinium enhancement and hypokinesis. These changes met phenotypic definition of ACM. The patient was started on a beta-blocker. His ACM risk calculator estimated the risk of VAs within 5 years at 20%. A multidisciplinary team discussion and recommendation was made to proceed with an implantable cardiac defibrillator (ICD) for the primary prevention of SCD. The patient opted to obtain genetic testing in addition to the phenotypic characterization.
Figure 2.
Cardiac magnetic resonance four-chamber (A) and short-axis (B) images demonstrating right ventricular enlargement compared with the left ventricle. The right ventricular end-diastolic volume was 260 mL and 135 mL/m2 when indexed to the body surface area. The right ventricular ejection fraction was 37%. Focal thickening of the mid-anterior, anterolateral, and inferolateral wall segments with associated transmural delayed gadolinium enhancement and hypokinesis of the left ventricle. Structural abnormalities are characterized by non-ischaemic late gadolinium enhancement affecting the subepicardial and less often the midmyocardial layers. The origin of the fibrofatty infiltration of the ventricular myocardium is thought to be the second heart field cardiac fibroadipocyte progenitor cells. These cells express desmosome genes and have bimodal potential for differentiation into fibrogenic or adipogenic pathways. Increased accumulation of plakoglobin, also known as junction plakoglobin or gamma-catenin, in the nucleus results in the increase in the adipogenic factors and reduction of the inhibitors of adipogenesis (connective tissue growth factor). Adipogenesis of the heart is also mediated by the downregulation of micro-RNA-184 (miR-184). Cine images are presented in Supplementary material online. The use of diagnostic criteria for arrhythmogenic cardiomyopathy is a two-step process. First, one must determine the number of major and minor criteria for both right and left ventricular involvement by applying the multiparametric approach, covering six categories: (i) morpho-functional abnormalities, (ii) structural myocardial abnormalities, (iii) electrocardiogram repolarization abnormalities, (iv) electrocardiogram depolarization abnormalities, (v) ventricular arrhythmias, and (vi) family history and molecular genetics.
Using next-generation sequencing, there were two well-described pathogenic frameshift variants in (i) the DSG2 gene [NM_001943.5:c.1053_1056dup (p.Ile353fs)] and (ii) the PKP2 gene [NM_001005242.3(PKP2):c.837_838del (p.Val280fs)]. Shared decision-making further resulted in a successful implantation of a subcutaneous ICD. A subcutaneous implantation was prioritized due to the patient’s young age and the absence of bradycardia or atrioventricular block. The patient continues to follow-up closely with our Cardio-Genomics Program. Clinical follow-up through December 2023 demonstrated no adverse cardiovascular events. Rare occasional palpitations with no functional limitations and good tolerance of metoprolol were reported. Serial event monitors and device interrogations showed rare ectopy, occasional accelerated idioventricular rhythm, no treated VAs, or shocks. His recommended lifestyle changes included abstinence from endurance activities and competitive sports.
Discussion
Epidemiology and genetics of arrhythmogenic cardiomyopathy
The reported prevalence of ACM (OMIM: 609040) varies across the globe, with the most cited estimate being 1:5000. Approximately 30% are undiagnosed due to SCD as the initial manifestation of the disease. The first case series of adults with RV dysplasia, dated 1982, was reported in France.2 The first genetic characterization of ACM was described in 2000 in families from Ecuador.3 Global registries reveal no specific racial or geographical predilection. This genetic arrhythmia syndrome presents with varying severity of the phenotypic manifestation, affecting either right, left, or both ventricles, and genetic architecture, which challenges precision in prognostication.
Most familial cases of the disease have an autosomal dominant pattern of inheritance with age-related, reduced penetrance.4 Genetic basis of ACM is localized to the genes encoding the cardiac desmosome (PKP2, DSP, DSC2, DSG2, JUP), as well as extra-desmosomal genes (CTNNA3, PLN, TMEM4, SCN5A, CDH2, DES). Among family members of patients with ACM undergoing genetic testing, many will not have a pathogenic variant identified, reflecting incomplete lifetime penetrance estimated at 30–50%.5 Occasional reports of de novo variants in ACM to date limit systematic assessment of the frequency and characteristics of those cases. Mutation-specific testing is recommended when a genetic diagnosis of ACM is made in the proband to determine the possible risk in first-degree relatives. Frequently, patients with ACM have more than one mutation in the same gene (compound heterozygosity) or in a second gene (double heterozygosity). There is ongoing work on genetic-driven risk stratification as selected common genetic variants are associated with increased arrhythmogenic risk.6
Diagnostic criteria and differential diagnosis
Criteria for diagnosis of ACM were first proposed in 1994 and revised by a Task Force in 2010.7 To incorporate left-sided variants and late-gadolinium enhancement (LGE) findings by MRI, the 2020 Padua criteria have been developed by an international expert consensus.8 Variable expressivity and incomplete penetrance result in a wide differential diagnosis for ACM,9 including cardiac sarcoidosis, dilated cardiomyopathy, athlete’s heart, myocarditis, congenital aneurysm or diverticula, idiopathic outflow tract tachycardia, and Brugada syndrome.
Multimodality diagnostic approach in arrhythmogenic cardiomyopathy
The imaging diagnosis of ACM relies on the demonstration of structural and functional abnormalities resulting from the underlying histological changes.10 The major echocardiographic criteria are associated with 55–75% sensitivity and 95% specificity for diagnosing ACM. Magnetic resonance imaging allows for reproducible volumetric assessment with an overall sensitivity of 100%, specificity 87%, positive predictive value 29%, and negative predictive value of 100%. In patients with ARVC, there is a linear correlation between the reduction of EF and extent of LGE, while in arrhythmogenic left ventricular cardiomyopathy, an inverse correlation has been reported.11 To better understand disease onset and progression in this patient, the initial TTE was independently re-reviewed. Mild RV dilatation with normal basal dimensions and subtle apical dyskinesis were noted, highlighting diagnostic challenges in non-referral centres.
Screening and management
All first-degree relatives and at-risk relatives with the same (likely) pathogenic variant as the proband should undergo ACM screening with a 12-lead ECG, Holter monitoring, and an imaging study. Relatives with possible and borderline ACM should be screened every 5 years and every year, respectively.11 Symptomatic patients and those between 20 and 30 years of age have the highest risk of developing definite ACM.12 Data suggest that genotypic-based classification shows higher precision in predicting the outcome of patients with genetic cardiomyopathies than phenotype-based classification.13
High-intensity exercise increases the penetrance and arrhythmic risk in genotype-positive patients. Competitive or endurance sports are associated with early disease manifestation and increased risk of malignant arrhythmia and SCD.14–16 To date, no significant interaction was found between exercise dose and pathogenic variant carrier status.15 The risk of incident sustained VA appears to increase non-linearly with exercise dose.17 Currently, it is recommended to restrict exercise to 15 MET h/week.17 Further studies exploring mutation burden in those with compound and digenic heterozygosity for desmosomal mutations and the risk of SCD are needed.
Antiarrhythmic therapy is recommended as (i) an adjunct to ICD therapy in patients with frequent appropriate device discharges, (ii) to improve symptoms in patients with frequent premature ventricular beats and/or NSVT, or (iii) as an adjunct to catheter ablation in selected patients with recurrent haemodynamically stable VT.18 Amiodarone in combination with beta-blockers is the most effective with the least pro-arrhythmic risk, even in patients with ventricular dysfunction.18 Beta-blocker use should be considered in all patients with ACM irrespective of the presence of arrhythmias. Risk stratification should be done to determine the risk of SCD and need for ICD. Catheter ablation of VT is recommended for ACM patients with incessant VT or frequent appropriate ICD interventions despite pharmacological therapy. Patients with end-stage heart failure or uncontrollable ventricular tachycardia may require heart transplantation.19
Conclusion
An individualized approach to the selection of testing (MRI, echocardiography, exercise testing, signal-averaged ECG) and counselling on exercising programmes should be provided. Further research is needed for the optimization of genotype- and phenotype-based risk stratification in patients with ACM.
Supplementary Material
Contributor Information
Jeremiah Haines, Division of Cardiovascular Medicine, Department of Medicine, Medical College of Wisconsin, 8701 W Watertown Plank Rd, Milwaukee, WI 53226, USA.
Noelle Garster, Division of Cardiovascular Medicine, Department of Medicine, Medical College of Wisconsin, 8701 W Watertown Plank Rd, Milwaukee, WI 53226, USA.
Divyanshu Mohananey, Division of Cardiovascular Medicine, Department of Medicine, Medical College of Wisconsin, 8701 W Watertown Plank Rd, Milwaukee, WI 53226, USA.
Maya S Safarova, Division of Cardiovascular Medicine, Department of Medicine, Medical College of Wisconsin, 8701 W Watertown Plank Rd, Milwaukee, WI 53226, USA.
Lead author biography

Jeremiah Haines, DO, is a Cardiologist from Milwaukee, WI, USA. Dr Haines received his medical degree from West Virginia School of Osteopathic Medicine. He completed his residency in Internal Medicine at Loyola University Medical Center Maywood, IL, USA, and his fellowship in Cardiovascular Medicine at the Medical College of Wisconsin Milwaukee, WI, USA. He is currently finishing an additional year of advanced training in structural imaging and interventional echocardiography, after which he will be joining Aurora St. Luke’s Medical Center in Milwaukee as multi-modality imaging faculty.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Funding: None declared.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
References
- 1. Krahn AD, Wilde AAM, Calkins H, La Gerche A, Cadrin-Tourigny J, Roberts JD, et al. Arrhythmogenic right ventricular cardiomyopathy. JACC Clin Electrophysiol 2022;8:533–553. [DOI] [PubMed] [Google Scholar]
- 2. Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation 1982;65:384–398. [DOI] [PubMed] [Google Scholar]
- 3. Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, Purkis PE, et al. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet 2000;9:2761–2766. [DOI] [PubMed] [Google Scholar]
- 4. van Lint FHM, Murray B, Tichnell C, Zwart R, Amat N, Deprez RHL, et al. Arrhythmogenic right ventricular cardiomyopathy-associated desmosomal variants are rarely de novo. Circ Genom Precis Med 2019;12:e002467. [DOI] [PubMed] [Google Scholar]
- 5. Fressart V, Duthoit G, Donal E, Probst V, Deharo JC, Chevalier P, et al. Desmosomal gene analysis in arrhythmogenic right ventricular dysplasia/cardiomyopathy: spectrum of mutations and clinical impact in practice. Europace 2010;12:861–868. [DOI] [PubMed] [Google Scholar]
- 6. Protonotarios A, Bariani R, Cappelletto C, Pavlou M, García-García A, Cipriani A, et al. Importance of genotype for risk stratification in arrhythmogenic right ventricular cardiomyopathy using the 2019 ARVC risk calculator. Eur Heart J 2022;43:3053–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corrado D, Zorzi A, Cipriani A, Bauce B, Bariani R, Beffagna G, et al. Evolving diagnostic criteria for arrhythmogenic cardiomyopathy. J Am Heart Assoc 2021;10:e021987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corrado D, Marra MP, Zorzi A, Beffagna G, Cipriani A, Lazzari M, et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol 2020;319:106–114. [DOI] [PubMed] [Google Scholar]
- 9. Molitor N, Duru F. Arrhythmogenic right ventricular cardiomyopathy and differential diagnosis with diseases mimicking its phenotypes. J Clin Med 2022;11:1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malik N, Mukherjee M, Wu KC, Zimmerman SL, Zhan J, Calkins H, et al. Multimodality imaging in arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Imaging 2022;15:e013725. [DOI] [PubMed] [Google Scholar]
- 11. He J, Xu J, Li G, Zhou D, Li S, Zhuang B, et al. Arrhythmogenic left ventricular cardiomyopathy: a clinical and CMR study. Sci Rep 2020;10:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muller SA, Gasperetti A, Bosman LP, Schmidt AF, Baas AF, Amin AS, et al. Individualized family screening for arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol 2023;82:214–225. [DOI] [PubMed] [Google Scholar]
- 13. Paldino A, Dal Ferro M, Stolfo D, Gandin I, Medo K, Graw S, et al. Prognostic prediction of genotype vs phenotype in genetic cardiomyopathies. J Am Coll Cardiol 2022;80:1981–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corrado D, Wichter T, Link MS, Hauer R, Marchlinski F, Anastasakis A, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Eur Heart J 2015;36:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NA III, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. Circulation 2015;132:e273–e280. [DOI] [PubMed] [Google Scholar]
- 16. Saberniak J, Hasselberg NE, Borgquist R, Platonov PG, Sarvari SI, Smith H, et al. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail 2014;16:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bosman LP, Wang W, Lie ØH, van Lint FHM, Rootwelt-Norberg C, Murray B, et al. Integrating exercise into personalized ventricular arrhythmia risk prediction in arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol 2022;15:e010221. [DOI] [PubMed] [Google Scholar]
- 18. Marcus GM, Glidden DV, Polonsky B, Zareba W, Smith LM, Cannom DS, et al. Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: a report from the North American ARVC Registry. J Am Coll Cardiol 2009;54:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tedford RJ, James C, Judge DP, Tichnell C, Murray B, Bhonsale A, et al. Cardiac transplantation in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol 2012;59:289–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.