Abstract
Hepatocellular carcinoma (HCC) ranks as the most prevalent of primary liver cancers and stands as the third leading cause of cancer-related deaths. Early-stage HCC can be effectively managed with available treatment modalities ranging from invasive techniques, such as liver resection and thermoablation, to systemic therapies primarily employing tyrosine kinase inhibitors. Unfortunately, these interventions take a significant toll on the body, either through physical trauma or the adverse effects of pharmacotherapy. Consequently, there is an understandable drive to develop novel HCC therapies. Adipose-derived stem cells (ADSCs) are a promising therapeutic tool. Their facile extraction process, coupled with the distinctive immunomodulatory capabilities of their secretome, make them an intriguing subject for investigation in both oncology and regenerative medicine. The factors they produce are both enzymes affecting the extracellular matrix (specifically, metalloproteinases and their inhibitors) as well as cytokines and growth factors affecting cell proliferation and invasiveness. So far, the interactions observed with various cancer cell types have not led to clear conclusions. The evidence shows both inhibitory and stimulatory effects on tumor growth. Notably, these effects appear to be dependent on the tumor type, prompting speculation regarding their potential inhibitory impact on HCC. This review briefly synthesizes findings from preclinical and clinical studies examining the effects of ADSCs on cancers, with a specific focus on HCC, and emphasizes the need for further research.
Keywords: hepatocellular carcinoma, adipose-derived stem cells, conditioned medium, anti-cancer therapy, cell-based therapy
1. Introduction
Liver cancers are a major challenge for public health, as they rank third in mortality and sixth in incidence of all malignant neoplasms. Both incidence and mortality are expected to rise by over 50% by the year 2040. Among liver cancers, the most common subtype is hepatocellular carcinoma (HCC), which accounts for 80% of cases [1]. HCC is usually preceded by liver damage caused by alcohol consumption, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis, and hepatitis B or C [2].
The treatment of hepatocellular carcinoma depends on the stage of the disease at the time of diagnosis and preserved liver function. For early and intermediate HCC, treatment options usually include liver resection, orthotopic liver transplantation, or the thermal ablation of the tumor. Advanced HCC requires systemic treatment. HCC is generally resistant to chemotherapy, therefore the first-line treatment in advanced HCC uses tyrosine kinase inhibitors, such as sorafenib [3]. Immunotherapy is emerging as a new potential alternative in the treatment of HCC, mainly in the United States, where it has been accepted as the first-line therapy [4,5]. Radiotherapy is not widely used in HCC, but it may be an alternative in patients who cannot be treated with other therapeutic methods [3,6].
Due to the poor prognosis in advanced hepatocellular carcinoma, new treatment opportunities are being investigated. One such proposal may be stem cell therapy. Cell therapy in cancers is the subject of in vitro and in vivo studies [7] as well as clinical trials [8]. Various types of stem cells are widely used in regenerative medicine, especially in wound healing and diseases involving increased inflammation [9]. Malignant tumors are considered physiologically similar to chronic, non-healing wounds, characterized by the increased production of inflammatory substances, hypoxia, and intense oxidative stress. Moreover, they can also cause actual wounds, leading to ulceration or necrosis, and it is thought that the regenerative potential of stem cells may be useful in treating these diseases and the damage they cause to the body [7]. In patients with non-malignant conditions, such as COVID-19, some mesenchymal stromal/stem cells (MSCs) have been shown to reduce the concentrations of pro-inflammatory cytokines and acute phase proteins such as interleukin 6 and 8 (IL-6 and IL-8), tumor necrosis factor α (TNF-α), C-reactive protein (CRP), and D-dimers, while imaging examinations have shown accelerated regeneration of parenchymal organs [10,11,12].
2. Stem Cells as a Novel Cancer Treatment
Significant scientific evidence suggests the usefulness of various stem cells in the treatment of cancer. Some stem cell therapies, particularly hematopoietic stem cell (HSC) treatment for leukemias and other blood malignancies, are already established as standard practices. HSC transplantation preceded by high-dose chemotherapy is often the gold standard in the treatment of these diseases [13]. Besides hematological malignancies, HSC has demonstrated certain efficacy in the treatment of breast, lung, ovarian, and renal cancers [13,14]. For example, in advanced breast cancer, high-dose chemotherapy followed by HSC provided longer survival in the group of patients with higher-risk cancer (≥10 lymph nodes involved) compared to standard-dose chemotherapy without HSCs; however, this procedure did not provide longer survival in the overall group of patients [8].
Studies on MSCs have shown that these cells exhibit tropism to cancer cells and a propensity to colonize tumors [15], and preclinical studies have demonstrated their anti-proliferative and pro-apoptotic effects on cancer cells. The exact mechanisms responsible for these effects have yet to be elucidated, and unfortunately, only a few clinical studies are being conducted on the potential use of MSCs in the treatment of solid tumors. One phase I study (NCT02530047) concerning ovarian cancer involved genetically modified MSCs secreting interferon β (IFNβ). The ongoing phase I/II trial NCT02068794 also focuses on ovarian cancer but includes oncolytic measles virus (MV-NIS)-infected adipose-derived stem cells (ADSCs). MV-NIS is a modified strain of the measles virus that has oncolytic effects and stimulates the T-cell response against tumor cells [16]. Another ongoing trial is investigating the effect of umbilical cord-derived MSCs modified to express the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on lung adenocarcinoma (NCT03298763) [17]. The one completed phase 1 trial (NCT01844661) examined the effects of autologous bone marrow-derived MSCs (BM-MSCs) infected with the oncolytic adenovirus ICOVIR5 on various metastatic and refractory tumors in children and adults. These cells were shown to allow large amounts of the virus to be safely stored in the body, and some patients demonstrated disease stabilization in response to the treatment with BM-MSCs [18].
3. ADSC Characteristics
ADSCs are a population of adult multipotent stem cells of mesodermal origin [19], which can be easily extracted from adipose tissue in large amounts [20]. ADSCs are spindle-shaped [21] and express markers typical for mesenchymal stem cells, such as CD44, CD73, CD90, and CD105 [22,23]. Unlike other mesenchymal stem cells, ADSCs at early passages express CD34, a surface marker whose expression decreases in further cultures [20,22]. ADSCs are capable of self-renewal [19] and differentiation towards cells representing all three germ layers [22]. ADSCs are obtained mainly by liposuction (lipoaspiration) [24,25] but also from tissues excised during plastic surgeries, such as abdominoplasty or dermolipectomy [21,23]. The excision of intact tissue provides a higher yield of ADSCs [21] or other adipose tissue cells [26]. ADSCs are usually extracted from a tissue sample via enzymatic digestion with collagenases [21,23], which can also be supported by mechanical sorting [21].
Recent studies have developed a concept in which ADSCs may differ within their subpopulations based on their anatomical origin. For instance, ADSCs from abdominal and gluteofemoral adipose tissue were found to show significant differences in RNA expression, which may play a role in cell metabolism [27]. RNA expression differs not only between ADSCs of different origins but also in other cell populations, such as immune cells derived from visceral or subcutaneous adipose tissue [28]. Similar results for ADSC subpopulations were obtained by Nahmgoong et al., who investigated the differentiation potential, metabolic features, and interactions with the microenvironment of ADSCs derived from visceral epididymal adipose tissue and subcutaneous inguinal adipose tissue [29]. A study on ADSC spheroids distinguished three subpopulations of subcutaneous fat: superficial, deep, and the superficial retinacula cutis [30].
The regenerative properties of various ADSC subtypes make them a recognized field of research in trauma regeneration. Particularly great potential lies in the use of autologous grafts of subcutaneous ADSCs or their exosomes in promoting scar-free wound healing and fractured bone fusion, with a strong focus on the treatment of complications such as aseptic non-unions [31,32]. Tendinopathies and tendon injuries are also areas where ADSCs appear to show significant effectiveness. ADSCs derived from the buttocks, ADSCs from visceral fat, periumbilical abdominal ADSCs, and ADSCs from thigh adipose tissue have all been used in clinical trials for these indications [33]. The clinical application of ADSCs is not limited to the treatment of injuries. In the setting of chronic diseases, they have shown efficacy in the management of many complications of diabetes, including skin ulcers and ophthalmic disorders involving subcutaneous ADSCs and eyelid ADSCs, respectively [34,35].
Furthermore, a large body of evidence, including a recent randomized, double-blind study by Tak et al., indicates the efficacy of ADSC-derived constituent extracts in the treatment of androgenetic alopecia [36]. The results from preclinical studies suggest that ADSCs modified for overexpressing miR-188-3p may be helpful in the treatment of Parkinson’s disease [37]. ADSCs grafts, along with autologous fat grafting and visceral stromal fraction administration, appear to be efficient in systemic sclerosis treatment, suggesting the broader applicability of ADSCs in rheumatology [38]. Abdominal ADSC-derived nanovesicles have been shown to affect nucleus pulposus cells in patients with intervertebral disc degeneration, reducing the severity of inflammation and painful symptoms [39].
The anti-inflammatory properties of ADSCs make them an attractive therapeutic agent [40]. It has been postulated that by regulating immune function, mainly by reducing lymphocytic infiltration in transplanted organs, ADSCs may alleviate the course of graft versus host disease [41], which is a serious side effect of organ transplantation. In clinical settings, the autologous transplantation of human ADSCs is immunologically safe and has positive effects on, for example, wound healing [42] and spinal cord injury [43]. Another alternative may be an allogeneic transplant or the use of the ADSC secretome [44]. Due to their multipotent properties, when cultured with small molecules and cell factors, human ADSCs have the ability to differentiate into cells of mesenchymal, ectodermal (neurons and Schwann cells) [45], and endodermal origin, including functional hepatocyte-like cells [46,47]. The effect of differentiation towards hepatocyte-like cells was improved with the use of laminin-containing scaffolds [47]. ADSCs alleviated liver damage in numerous in vivo studies in animal models of liver fibrosis [48,49,50,51], non-alcoholic fatty liver disease (NAFLD) [52], ischemia-reperfusion injury [53], and liver transplantation [54]. The wound-healing properties of ADSCs may significantly increase the regenerative potential of the liver after surgery, ablation, or embolization.
The regulatory and immunomodulatory properties of ADSCs are mostly due to the factors they secrete into the extracellular matrix (ECM). The ADSC secretome consists of three main components: membrane proteins (including major histocompatibility complex antigens), soluble agents in the form of cytokines and growth factors, and nucleic acid fragments [55,56]. These substances are released into the environment immediately or via exosomes and are able to affect inflammation directly by reversing the effects of pro-inflammatory cytokines or indirectly by regulating the activity of specific populations of immune and other cells.
Notable substances produced by ADSCs and other MSCs include hepatocyte growth factor (HGF), which enhances regeneration, especially of the parenchymal organs, such as the liver and lungs, vascular endothelial growth factor (VEGF), which promotes angiogenesis [55,57,58,59], and transforming growth factor β (TGFβ), which stimulates tissue regeneration and regulates T-cell activity [60,61]. The immunosuppressive components produced by ADSCs include prostaglandin E2 (PGE2) and indoleamine 2,3-dioxygenase (IDO) [62,63,64]. The components identified in the ADSC secretome are the tissue inhibitors of metalloproteinases TIMP1 and TIMP2 [55], which reduce NK cell activity and are involved in immunosuppression of tumorigenic processes [65]. Interestingly, some of these factors, such as HGF, have also been described as promoting tumor growth [66,67]. In turn, one contribution of ADSCs to cell migration is through their impact on the ICAM-1/RANTES pathway [68].
The ADSC secretome contains significant amounts of various types of collagen, including type I, III, VI, and XII collagen chains [69]. Indirectly, ADSCs increase collagen content in the ECM of cartilage tissue by secreting insulin-like growth factor 1 (IGF-1) and TGFβ, which stimulate chondrocytes to produce type II collagen, proteoglycans, and aggrecans [24]. The secretome of ADSCs also contains other components of the ECM that consolidate its structure and are essential for regenerating damage, including fibronectin, vimentin, laminin, and fibrillin [69].
ADSCs produce various substances that affect the ECM, but it seems that the ECM can also simultaneously affect these stem cells: the enzymes present in the matrix and their inhibitors have been shown to influence the direction of MSC differentiation towards adipocytes, osteoblasts, or chondroblasts [70]. In a study of pancreatic cancer in mice, mucins produced by cancer cells induced the proliferation and migration of ADSCs [71]. An additional factor affecting ADSCs is obesity, which influences the cell metabolism of ADSCs in mammary fat [72]. Similar relationships have been observed in epicardial adipose tissue ADSCs in diabetes, and diabetic ADSCs play a role in cardiovascular disease development [73]. The exact nature of the interaction between ADSC subpopulations and the ECM is still not comprehensively understood and promises significant discoveries in the future.
4. Advantages and Disadvantages of the Therapeutic Use of ADSCs in Cancer Therapy
ADSCs possess several advantages over MSCs isolated from other sources. Their superior safety profile, easy and safe extraction, and exceptional immunomodulatory properties distinguish themselves among stem cell populations. ADSCs are much easier to obtain than some other types of MSCs: adipose tissue, collected through liposuction, is a rich source of stem cells, and the procedure itself is much less traumatic than, for example, bone marrow extraction [74]. Some other features of ADSCs should also be considered, such as their outstanding ability to stimulate the proliferation and differentiation of target cells as well as their ability to stimulate senescent MSCs in surrounding tissue [19].
It has been concluded in some studies that ADSCs represent a high safety profile in cancer treatment because they inhibit tumor growth. However, this effect was mainly shown in in vitro or in vivo xenograft models, and some researchers have questioned the safety of ADSC use in cancer patients [75]. The anti-tumorigenic effect of ADSCs was proven in different types of cancer, such as thyroid [76], bladder [77], prostate [78,79], ovarian [80], colon [81], and gastric cancers [82], as well as in hepatocellular carcinoma [83,84,85,86]. Another study found no harmful effects of ADSCs when cultured in vitro with HSC-3 squamous cell carcinoma cells, which was in contrast to the bone marrow-derived MSCs (BM-MSCs) also examined in that study, which seemed to promote the invasion of cancer cells and increase their aggressiveness [87].
The first clinical trials on the use of ADSCs in anti-cancer therapy have already begun and are described in more detail in Table 1.
Table 1.
The use of ADSCs in clinical trials for various types of cancer, accessed at clinicaltrials.gov on 24 April 2024.
| Study Number | Cancer Type | ADSC Source | ADSC Quantity | Administration | Results | Ref. |
|---|---|---|---|---|---|---|
| NCT 05789394 |
Glioblastoma multiforme | Allogeneic transplant | 5 × 106 −2 × 107 |
Intratumoral | Ongoing study | [88] |
| NCT 04087889 |
Pancreatic cancer | Allogeneic transplant from a first-degree relative | 2 × 108 | Intravenous | Ongoing study | Unpublished |
| NCT 02068794 |
Ovarian cancer | Not specified | Not specified | Intraperitoneal | Ongoing study | Unpublished |
The anti-cancer effects of ADSCs include actions on cancer cells and the tumor microenvironment involving the ECM, blood vessels, fibroblasts, endothelial cells, immune system cells, and other cells. Most genes that play a role in communication between ADSCs and fibroblasts or endothelial cells are related to the ECM and vascularization (genes encoding TIMPs; metalloproteinases (MMPs); collagens; members of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), VEGF, and fibroblast growth factor (FGF) families [89]). Intracellular adhesion molecule 1 (ICAM-1), a cytokine produced by ADSCs, increases the expression of the RANTES factor (also known as chemokine ligand 5, CCL5), which in turn increases the expression of metalloproteinases MMP2 and MMP9. These enzymes, through the proper degradation of the ECM, allow cells to migrate and grow and therefore facilitate healing processes [68]. ADSCs also have the ability to stimulate other human cells to express genes for MMPs. In a study by Tilotta et al., the ADSC secretome increased the gene expression of MMP13 and ADAMTS5 in human nucleus pulposus cells after IL-1β stimulation [90].
The effect of ADSCs on matrix organization may be of particular interest in cancers where ADSCs are a natural component of the tumor environment, such as breast [91,92], pancreatic [71], or colorectal cancers [93]. The ECM, as an environment for the cells suspended in it, also plays an important role in tissue regeneration processes, including effective wound healing [94]. The tumor ECM may become a target for future anti-cancer therapies [95], potentially also in hepatocellular carcinoma [96].
In contrast to these data, there are some doubts arising from observations that the ADSC secretome may both inhibit and support cancer cell growth (Table 2).
Table 2.
Selected substances produced by ADSCs identified as having either stimulatory (↑) or suppressive (↓) effects on cancer cell growth.
The potential stimulatory effects of ADSCs on cancer cell growth have been demonstrated in several studies [98,99,104,106], especially in ovarian [103,107,108,109] and breast cancers [100,101,102,110,111,112], with some suggestions that this effect of ADSCs may result from their influence on the microenvironment [113]. Moreover, ADSCs co-cultured with melanoma cells from different lines (G-361, SK-Mel-5, MeWo, and A2058) increased the migration capacity of malignant cells, which was correlated with the overexpression of genes such as CXCL12, PTGS2, IL-6, IL-8, and, most notably, HGF, and increased the expression of CCL2, IL-6, IL-8, VEGF, MMP-2, and the EMM-PRIN proteins [66]. Visceral ADSCs obtained from colorectal cancer patients promoted cancer cell proliferation in in vitro and in vivo xenograft models [93]. Hypoxia-induced ADSCs produced interleukin 10 (IL-10), which promoted the growth of Burkitt’s lymphoma cells [114].
The suggestions of differences in the metabolic profile and interactions with the microenvironment of certain ADSC subpopulations may shed light on the problem of ADSC interactions with cancer cells, especially breast cancer, as adipose tissue grafts are used in breast reconstruction after mastectomy [115,116]. Furthermore, ADSCs derived from mammary tissue may constitute a subpopulation of cells underrepresented in breast cancer studies [117], and this population may have pro-cancerous properties [72,117]. It has been found that mammary tissue ADSCs from patients with breast cancer derived from locations close to and distant from the tumor site show disparities in RNA expression and differentiation potential [72].
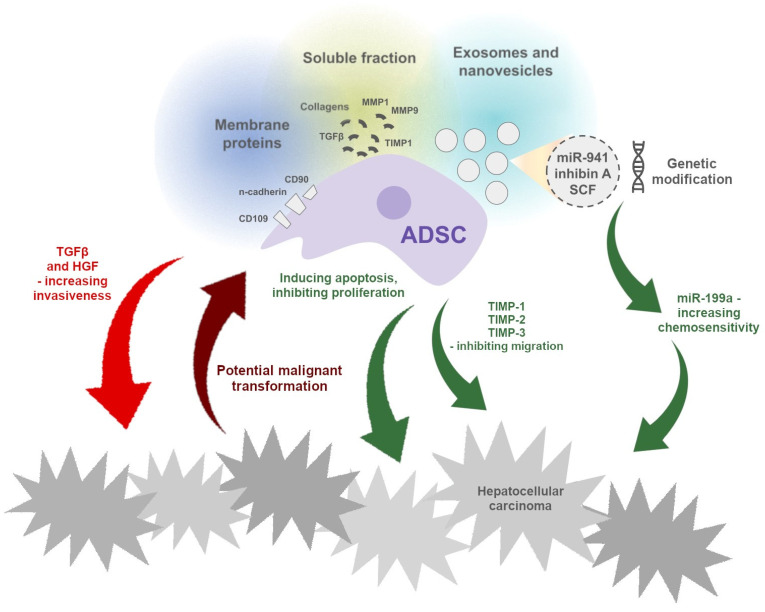
To summarize, the effect of ADSCs, a possible anti-cancer agent, on the growth of malignant tumors is not entirely clear, and studies have yielded conflicting results (Figure 1). It is likely that the outcome depends on the patient and the type of tumor.
Figure 1.
Indicators of the potential mechanisms of the pro- and anti-cancer effects of ADSCs, as well as the possible impact of cancer cells on ADSCs, promoting their neoplastic transformation. Unspecified factors secreted by ADSCs cause apoptosis and the inhibition of cancer cell proliferation in co-cultures in vitro. On the other hand, cancer cells can, through an unknown mechanism, cause the malignant transformation of ADSCs, manifested by the expression of a cancerous phenotype.
4.1. The Effect of ADSCs on Hepatocellular Carcinoma Cells
Some preclinical studies have suggested the potential use of human ADSCs or ADSC-derived conditioned media in the treatment of hepatocellular carcinoma due to their anti-proliferative effects on multiple hepatocellular carcinoma cell lines, such as HepG2 [83,84], Huh7, SMMC7721, Bel7402 [83], and PLC-PRF-5 cells [84]. ADSCs also inhibited HCC cell migration in HepG2 and PLC-PRF-5 cell cultures. Migration inhibition may have been related to the overexpression of a tissue inhibitor of metalloproteinases [84]. Human ADSCs pretreated with curcumin reduced the proliferation, migration, and invasion of HepG2 hepatocellular carcinoma cells and induced their apoptosis [85]. A similar effect was described for rat ADSC-derived conditioned media and the HepG2 cell line [118] or human ADSC-derived conditioned media and the SMMC7721 cell line (via Akt signaling) [83]. Contrary to these findings, a conditioned medium from canine ADSCs enhanced the proliferation and invasion of the canine HCC cell line [119]. The effects of ADSCs on hepatocellular carcinoma cells are summarized in Table 3.
Table 3.
Summary of the experimental studies related to the harmful and beneficial effects of ADSCs on hepatocellular carcinoma cell lines.
| Effect | Method | Cell Line | Ref. |
|---|---|---|---|
| Inhibition of proliferation | Co-culture with ADSCs | HepG2 | [84,85] |
| PLC-PRF-5 | [84] | ||
| Culture with ADSC-derived conditioned medium | HepG2 | [83,84,118] | |
| Huh7 | [83] | ||
| SMMC7721 | [83] | ||
| Bel7402 | [83] | ||
| Induction of apoptosis | Co-culture with ADSCs | HepG2 | [84,85] |
| PLC-PRF-5 | [84] | ||
| Culture with ADSC-derived | SMMC7721 | [83] | |
| conditioned medium | HepG2 | [84,118] | |
| PLC-PRF-5 | [84] | ||
| Inhibition of migration | Co-culture with ADSCs | HepG2 | [84,85] |
| PLC-PRF-5 | [84] | ||
| Culture with ADSC-derived conditioned medium | HepG2 | [84,118] | |
| Reduction of invasiveness | Co-culture with ADSCs | HepG2 | [84] |
| Culture with ADSC-derived conditioned medium | HepG2 | [84,85,118] | |
| Promotion of proliferation and migration | Culture with ADSC-derived conditioned medium | AZACH | [119] |
On the other hand, several studies have described a potential reverse effect of hepatocellular carcinoma cells on ADSCs, namely the risk of malignant transformation of stem cells. The co-culturing of the Huh-7 hepatocellular carcinoma cell line and ADSCs resulted in the acquisition of carcinogenic features in ADSCs, such as higher gene expression of pluripotency markers (OCT-4 and NANOG), proliferation markers (KRAS and CDK6), and adhesion markers (EPCAM and CD44), as well as the downregulation of differentiation markers [120]. Moreover, co-culture with Huh-7 improved the proliferation and migration capacity of ADSCs and induced the expression of miRNA related to the oncogenic miR-17-5p and the suppressor miR-615-5p moieties [120]. In addition, Huh-7.5 hepatocellular carcinoma cells co-cultured with ADSCs supported their differentiation toward the hepatic cell line [121].
4.2. ADSCs as a Potential Complementary Treatment in Standard Therapies
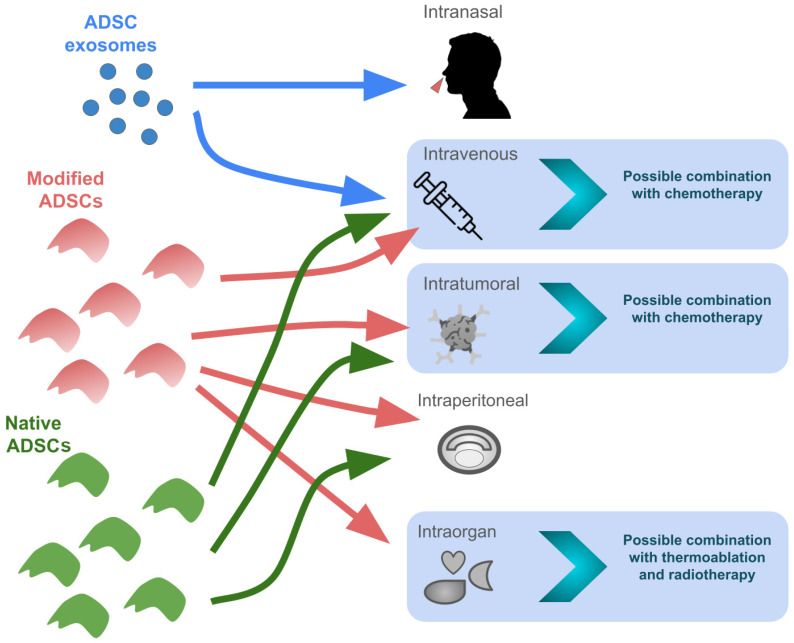
The ability of ADSCs to migrate towards cancer cells has created the opportunity to use ADSCs and ADSC-derived extracellular vesicles as drug carriers (Figure 2). Some studies have confirmed this concept in vitro and in vivo [122,123]. Human ADSCs transfected with the TRAIL migrated toward hepatocellular carcinoma cells, induced apoptosis, and inhibited the proliferation of HCC cells in vitro, as well as reduced the tumor weight and metastatic abilities in an in vivo mouse model [124].
Figure 2.
Potential ways of administrating native and modified ADSCs and ADSC exosomes independently or in combination therapies. The intranasal administration of ADSC-derived exosomes has been proven to be effective in the treatment of ischemic nerve damage in mice [125], but their effectiveness in the treatment of cancer has not yet been confirmed.
It has been shown that ADSCs and their extracellular vesicles tend to accumulate in damaged livers. Human ADSC-derived nanovesicles tended to accumulate more often in fibrotic livers compared to normal livers [50]. This effect might be due to the increased activity of hepatic macrophages in damaged livers [50]. Murine ADSC-derived extracellular vesicles were able to incorporate into cells and reduced HepG2 cell growth [126]. In the same study, extracellular vesicles derived from ADSCs were able to encapsulate astaxanthin, suggesting their possible use as drug carriers [126], which, in addition to the ability to migrate toward a damaged liver, may improve the drug distribution profile. A similar effect was described in breast cancer cells. ADSCs were able to carry and release paclitaxel, a standard chemotherapy drug in breast cancer, thus inhibiting breast cancer cell proliferation in vitro, whereas in the case of in vivo xenografts, paclitaxel-loaded ADSCs inhibited tumor growth [127].
ADSCs may also increase the sensitivity to commonly used HCC drugs. Human ADSCs transfected with miRNA plasmids were able to deliver miRNA into hepatocellular carcinoma cells, which increased their sensitivity to 5-fluorouracil (a chemotherapy agent) and sorafenib [128], a drug classically used in HCC, in vitro. Moreover, these plasmids, when used with sorafenib, demonstrated anti-cancer effects in a mouse model of hepatocellular carcinoma [128]. A similar effect of ADSC exosomes was proven for doxorubicin, where exosomes derived from ADSCs transfected with miR-199a increased the sensitivity of PLC/PRF/5 hepatocellular carcinoma cells for chemotherapy, which resulted in the inhibition of proliferation and the induction of apoptosis [129]. In a study on anti-tumor chemotherapy with doxorubicin, ADSCs were shown to display a similar half-maximal inhibitory concentration (IC50) for doxorubicin between serial passages, suggesting their potential role in further in vitro studies on anti-cancer drugs as a stable control [130].
ADSCs could improve other types of HCC therapies, such as thermal ablation or radiotherapy. Porcine ADSCs transfected with iron oxide-coated gold nanoparticles were shown to accumulate in damaged mouse livers and were able to mediate laser irradiation, resulting in the thermal ablation of HepG2 hepatocellular carcinoma cells in vitro [131]. When combined with radiotherapy, human ADSCs enhanced its anti-cancer effect. The results showed reduced viability, proliferation, sphere formation, and colony formation in liver cancer cells in vitro, as well as impaired migration, wound healing, and invasion of hepatocellular carcinoma cells [86]. The effect was consistent with in vivo experiments in a mouse model, where the combination of radiotherapy and ADSCs resulted in reduced tumor volume and weight, lower proliferation index, and a higher percentage of apoptotic cancer cells [86].
5. Conclusions
ADSCs have a wide variety of potential applications, including drug delivery, chemosensitization, liver regeneration after surgery, thermal ablation, and radiotherapy. However, most studies have referred to in vitro interactions between ADSCs and human cancer cells or in vivo animal models. The studies have usually focused on cell-to-cell interactions between ADSCs and certain human HCC cell lines. The potential of such an HCC model is limited since a cell line is usually derived from a single patient and is homogeneous and therefore does not represent cancer heterogeneity. Moreover, recent studies have suggested that one of the most commonly used cell lines, HepG2, may not represent hepatocellular carcinoma but another liver malignancy: hepatoblastoma [132]. Most studies on the effects of ADSCs in cancer models do not consider immunomodulation and interactions with the extracellular matrix of the tumor, which may be important complementary effects to the usually reported cell-to-cell interactions between ADSCs and cancer cells. There are no clinical studies testing the use of ADSCs in hepatocellular carcinoma or describing their role in other cancers. In conclusion, comprehensive studies, including in vitro and in vivo experimental models, as well as clinical trials, are essential to prove the concept of the therapeutic potential of ADSCs in HCC in the near future. However, regardless of the uncertainties described here, the use of stem cells in hepatocellular carcinoma may still become a promising addition to standard therapies due to their regenerative potential, positive effect on extracellular matrix remodeling, and anti-apoptotic effect on HCC cells.
Author Contributions
Conceptualization, writing—original draft preparation, A.G., A.M. and P.C.; funding acquisition, A.G.; writing—review and editing, supervision, P.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and will be made available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the Polish Ministry of Science and Education grant ‘Pearls of Science’ PN/01/0126/2022 and the Medical University of Silesia in Katowice (Poland), grant BNW-2-017/N/3/O.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rumgay H., Ferlay J., De Martel C., Georges D., Ibrahim A.S., Zheng R., Wei W., Lemmens V.E.P.P., Soerjomataram I. Global, Regional and National Burden of Primary Liver Cancer by Subtype. Eur. J. Cancer. 2022;161:108–118. doi: 10.1016/j.ejca.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Ganesan P., Kulik L.M. Hepatocellular Carcinoma. Clin. Liver Dis. 2023;27:85–102. doi: 10.1016/j.cld.2022.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Vogel A., Cervantes A., Chau I., Daniele B., Llovet J.M., Meyer T., Nault J.-C., Neumann U., Ricke J., Sangro B., et al. Hepatocellular Carcinoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018;29:iv238–iv255. doi: 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- 4.Sperandio R.C., Pestana R.C., Miyamura B.V., Kaseb A.O. Hepatocellular Carcinoma Immunotherapy. Annu. Rev. Med. 2022;73:267–278. doi: 10.1146/annurev-med-042220-021121. [DOI] [PubMed] [Google Scholar]
- 5.Sangro B., Sarobe P., Hervás-Stubbs S., Melero I. Advances in Immunotherapy for Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021;18:525–543. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y., Feng M. Radiotherapy for Hepatocellular Carcinoma. Semin. Radiat. Oncol. 2018;28:277–287. doi: 10.1016/j.semradonc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Chu D.-T., Nguyen T.T., Tien N.L.B., Tran D.-K., Jeong J.-H., Anh P.G., Thanh V.V., Truong D.T., Dinh T.C. Recent Progress of Stem Cell Therapy in Cancer Treatment: Molecular Mechanisms and Potential Applications. Cells. 2020;9:563. doi: 10.3390/cells9030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steenbruggen T.G., Steggink L.C., Seynaeve C.M., Van Der Hoeven J.J.M., Hooning M.J., Jager A., Konings I.R., Kroep J.R., Smit W.M., Tjan-Heijnen V.C.G., et al. High-Dose Chemotherapy with Hematopoietic Stem Cell Transplant in Patients with High-Risk Breast Cancer and 4 or More Involved Axillary Lymph Nodes: 20-Year Follow-up of a Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:528. doi: 10.1001/jamaoncol.2019.6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng N., Chen H., Wu Y., Liu Z. Adipose Stem Cell-Based Treatments for Wound Healing. Front. Cell Dev. Biol. 2022;9:821652. doi: 10.3389/fcell.2021.821652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi L., Huang H., Lu X., Yan X., Jiang X., Xu R., Wang S., Zhang C., Yuan X., Xu Z., et al. Effect of Human Umbilical Cord-Derived Mesenchymal Stem Cells on Lung Damage in Severe COVID-19 Patients: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial. Signal Transduct. Target. Ther. 2021;6:58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang B., Chen J., Li T., Wu H., Yang W., Li Y., Li J., Yu C., Nie F., Ma Z., et al. Clinical Remission of a Critically Ill COVID-19 Patient Treated by Human Umbilical Cord Mesenchymal Stem Cells: A Case Report. Medicine. 2020;99:e21429. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snowden J.A., Sánchez-Ortega I., Corbacioglu S., Basak G.W., Chabannon C., De La Camara R., Dolstra H., Duarte R.F., Glass B., Greco R., et al. Indications for Haematopoietic Cell Transplantation for Haematological Diseases, Solid Tumours and Immune Disorders: Current Practice in Europe, 2022. Bone Marrow Transplant. 2022;57:1217–1239. doi: 10.1038/s41409-022-01691-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawsawi Y.M., Al-Zahrani F., Mavromatis C., Baghdadi M.A., Saggu S., Oyouni A.A.A. Stem Cell Applications for Treatment of Cancer and Autoimmune Diseases: Its Promises, Obstacles, and Future Perspectives. Technol. Cancer Res. Treat. 2018;17:153303381880691. doi: 10.1177/1533033818806910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng X.-B., He X.-W., Zhang L.-J., Qin H.-B., Lin X.-T., Liu X.-H., Zhou C., Liu H.-S., Hu T., Cheng H.-C., et al. Bone Marrow-Derived CXCR4-Overexpressing MSCs Display Increased Homing to Intestine and Ameliorate Colitis-Associated Tumorigenesis in Mice. Gastroenterol. Rep. 2019;7:127–138. doi: 10.1093/gastro/goy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundstrom K. Therapeutic Applications for Oncolytic Self-Replicating RNA Viruses. Int. J. Mol. Sci. 2022;23:15622. doi: 10.3390/ijms232415622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies A., Sage B., Kolluri K., Alrifai D., Graham R., Weil B., Rego R., Bain O., Patrick P., Champion K., et al. TACTICAL: A Phase I/II Trial to Assess the Safety and Efficacy of MSCTRAIL in the Treatment of Metastatic Lung Adenocarcinoma. J. Clin. Oncol. 2019;37:TPS9116. doi: 10.1200/JCO.2019.37.15_suppl.TPS9116. [DOI] [Google Scholar]
- 18.Ruano D., López-Martín J.A., Moreno L., Lassaletta Á., Bautista F., Andión M., Hernández C., González-Murillo Á., Melen G., Alemany R., et al. First-in-Human, First-in-Child Trial of Autologous MSCs Carrying the Oncolytic Virus Icovir-5 in Patients with Advanced Tumors. Mol. Ther. 2020;28:1033–1042. doi: 10.1016/j.ymthe.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sowa Y., Mazda O., Tsuge I., Inafuku N., Kishida T., Morimoto N. Roles of Adipose-Derived Stem Cells in Cell-Based Therapy: Current Status and Future Scope—A Narrative Review. Dig. Med. Res. 2022;5:57. doi: 10.21037/dmr-22-32. [DOI] [Google Scholar]
- 20.Strioga M., Viswanathan S., Darinskas A., Slaby O., Michalek J. Same or Not the Same? Comparison of Adipose Tissue-Derived Versus Bone Marrow-Derived Mesenchymal Stem and Stromal Cells. Stem Cells Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 21.Alstrup T., Eijken M., Brunbjerg M.E., Hammer-Hansen N., Møller B.K., Damsgaard T.E. Measured Levels of Human Adipose Tissue–Derived Stem Cells in Adipose Tissue Is Strongly Dependent on Harvesting Method and Stem Cell Isolation Technique. Plast. Reconstr. Surg. 2020;145:142–150. doi: 10.1097/PRS.0000000000006404. [DOI] [PubMed] [Google Scholar]
- 22.Huang S.-J., Fu R.-H., Shyu W.-C., Liu S.-P., Jong G.-P., Chiu Y.-W., Wu H.-S., Tsou Y.-A., Cheng C.-W., Lin S.-Z. Adipose-Derived Stem Cells: Isolation, Characterization, and Differentiation Potential. Cell Transplant. 2013;22:701–709. doi: 10.3727/096368912X655127. [DOI] [PubMed] [Google Scholar]
- 23.Silva A.C., Percegona L.S., França A.L., Dos Santos T.M., Perini C.C., González P., Rebelatto C.L.K., Câmara N.O.S., Aita C.A.M. Expression of Pancreatic Endocrine Markers by Mesenchymal Stem Cells From Human Adipose Tissue. Transplant. Proc. 2012;44:2495–2496. doi: 10.1016/j.transproceed.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 24.Lee S., Chae D.-S., Song B.-W., Lim S., Kim S.W., Kim I.-K., Hwang K.-C. ADSC-Based Cell Therapies for Musculoskeletal Disorders: A Review of Recent Clinical Trials. Int. J. Mol. Sci. 2021;22:10586. doi: 10.3390/ijms221910586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu M., Heydarkhan-Hagvall S., Hedrick M., Benhaim P., Zuk P. Manual Isolation of Adipose-Derived Stem Cells from Human Lipoaspirates. J. Vis. Exp. 2013;79:50585. doi: 10.3791/50585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eto H., Suga H., Matsumoto D., Inoue K., Aoi N., Kato H., Araki J., Yoshimura K. Characterization of Structure and Cellular Components of Aspirated and Excised Adipose Tissue. Plast. Reconstr. Surg. 2009;124:1087–1097. doi: 10.1097/PRS.0b013e3181b5a3f1. [DOI] [PubMed] [Google Scholar]
- 27.Divoux A., Whytock K.L., Halasz L., Hopf M.E., Sparks L.M., Osborne T.F., Smith S.R. Distinct Subpopulations of Human Subcutaneous Adipose Tissue Precursor Cells Revealed by Single-Cell RNA Sequencing. Am. J. Physiol. Cell Physiol. 2024;326:C1248–C1261. doi: 10.1152/ajpcell.00726.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vijay J., Gauthier M.-F., Biswell R.L., Louiselle D.A., Johnston J.J., Cheung W.A., Belden B., Pramatarova A., Biertho L., Gibson M., et al. Single-Cell Analysis of Human Adipose Tissue Identifies Depot- and Disease-Specific Cell Types. Nat. Metab. 2019;2:97–109. doi: 10.1038/s42255-019-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nahmgoong H., Jeon Y.G., Park E.S., Choi Y.H., Han S.M., Park J., Ji Y., Sohn J.H., Han J.S., Kim Y.Y., et al. Distinct Properties of Adipose Stem Cell Subpopulations Determine Fat Depot-Specific Characteristics. Cell Metab. 2022;34:458–472.e6. doi: 10.1016/j.cmet.2021.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Côrtes I., Alves G., Claudio-Da-Silva C., Baptista L.S. Mimicking Lipolytic, Adipogenic, and Secretory Capacities of Human Subcutaneous Adipose Tissue by Spheroids from Distinct Subpopulations of Adipose Stromal/Stem Cells. Front. Cell Dev. Biol. 2023;11:1219218. doi: 10.3389/fcell.2023.1219218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smakaj A., De Mauro D., Rovere G., Pietramala S., Maccauro G., Parolini O., Lattanzi W., Liuzza F. Clinical Application of Adipose Derived Stem Cells for the Treatment of Aseptic Non-Unions: Current Stage and Future Perspectives—Systematic Review. Int. J. Mol. Sci. 2022;23:3057. doi: 10.3390/ijms23063057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C., Wei S., Xu Q., Sun Y., Ning X., Wang Z. Application of ADSCs and Their Exosomes in Scar Prevention. Stem Cell Rev. Rep. 2022;18:952–967. doi: 10.1007/s12015-021-10252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senesi L., De Francesco F., Marchesini A., Pangrazi P.P., Bertolini M., Riccio V., Riccio M. Efficacy of Adipose-Derived Mesenchymal Stem Cells and Stromal Vascular Fraction Alone and Combined to Biomaterials in Tendinopathy or Tendon Injury: Systematic Review of Current Concepts. Medicina. 2023;59:273. doi: 10.3390/medicina59020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musa M., Zeppieri M., Enaholo E.S., Salati C., Parodi P.C. Adipose Stem Cells in Modern-Day Ophthalmology. Clin. Pract. 2023;13:230–245. doi: 10.3390/clinpract13010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang T., Liu S., Wu Z., Li Q., Ren S., Chen J., Xu X., Wang C., Lu C., Yang X., et al. ADSC-exo@MMP-PEG Smart Hydrogel Promotes Diabetic Wound Healing by Optimizing Cellular Functions and Relieving Oxidative Stress. Mater. Today Bio. 2022;16:100365. doi: 10.1016/j.mtbio.2022.100365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tak Y.J., Lee S.Y., Cho A.R., Kim Y.S. A Randomized, Double-Blind, Vehicle-Controlled Clinical Study of Hair Regeneration Using Adipose-Derived Stem Cell Constituent Extract in Androgenetic Alopecia. Stem Cells Transl. Med. 2020;9:839–849. doi: 10.1002/sctm.19-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q., Wang Z., Xing H., Wang Y., Guo Y. Exosomes Derived from miR-188-3p-Modified Adipose-Derived Mesenchymal Stem Cells Protect Parkinson’s Disease. Mol. Ther. Nucleic Acids. 2021;23:1334–1344. doi: 10.1016/j.omtn.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao Y., Kan H., Ma X., Zhang Y., Huang J., Long X. Autologous Fat or Adipose-Derived Stem Cell Grafting in Systemic Sclerosis Treatment: A Systematic Review and Meta-Analysis. Clin. Exp. Rheumatol. 2023;41:1659–1669. doi: 10.55563/clinexprheumatol/ycy3k7. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z., Zhang L., Yang J., Huang J., Cai J., Zhang S., Feng X., Wang Q. Influence of Extracellular Nanovesicles Derived from Adipose-derived Stem Cells on Nucleus Pulposus Cell from Patients with Intervertebral Disc Degeneration. Exp. Ther. Med. 2021;22:1431. doi: 10.3892/etm.2021.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres-Torrillas M., Rubio M., Damia E., Cuervo B., Del Romero A., Peláez P., Chicharro D., Miguel L., Sopena J. Adipose-Derived Mesenchymal Stem Cells: A Promising Tool in the Treatment of Musculoskeletal Diseases. Int. J. Mol. Sci. 2019;20:3105. doi: 10.3390/ijms20123105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang M., Duan X., Pang N., Wang H., Yuan H., Zhang R., Cui L. Adipose Tissue-derived Stem Cells Modulate Immune Function In Vivo and Promote Long-term Hematopoiesis In Vitro Using the aGVHD Model. Exp. Ther. Med. 2020;19:1725–1732. doi: 10.3892/etm.2020.8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dung T.N., Han V.D., Tien G.N., Lam H.Q. Autologous Adipose-Derived Stem Cells (ADSCs) Transplantation in the Management of Chronic Wounds. Wound Pract. Res. 2021;29:41–48. doi: 10.33235/wpr.29.1.41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tien N.L.B., Hoa N.D., Thanh V.V., Van Thach N., Ngoc V.T.N., Dinh T.C., Phuong T.N.T., Toi P.L., Chu D.T. Autologous Transplantation of Adipose-Derived Stem Cells to Treat Acute Spinal Cord Injury: Evaluation of Clinical Signs, Mental Signs, and Quality of Life. Open Access Maced. J. Med. Sci. 2019;7:4399–4405. doi: 10.3889/oamjms.2019.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ceccarelli S., Pontecorvi P., Anastasiadou E., Napoli C., Marchese C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front. Cell Dev. Biol. 2020;8:236. doi: 10.3389/fcell.2020.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naderi N., Combellack E.J., Griffin M., Sedaghati T., Javed M., Findlay M.W., Wallace C.G., Mosahebi A., Butler P.E., Seifalian A.M., et al. The Regenerative Role of Adipose-derived Stem Cells (ADSC) in Plastic and Reconstructive Surgery. Int. Wound J. 2017;14:112–124. doi: 10.1111/iwj.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu Y., Deng J., Jiang Q., Wang Y., Zhang Y., Yao Y., Cheng F., Chen X., Xu F., Huang M., et al. Rapid Generation of Functional Hepatocyte-like Cells from Human Adipose-Derived Stem Cells. Stem Cell Res. Ther. 2016;7:105. doi: 10.1186/s13287-016-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohammadpour A., Arjmand S., Lotfi A.S., Tavana H., Kabir-Salmani M. Promoting Hepatogenic Differentiation of Human Mesenchymal Stem Cells Using a Novel Laminin-Containing Gelatin Cryogel Scaffold. Biochem. Biophys. Res. Commun. 2018;507:15–21. doi: 10.1016/j.bbrc.2018.10.121. [DOI] [PubMed] [Google Scholar]
- 48.Qu Y., Zhang Q., Cai X., Li F., Ma Z., Xu M., Lu L. Exosomes Derived from miR-181-5p-modified Adipose-derived Mesenchymal Stem Cells Prevent Liver Fibrosis via Autophagy Activation. J. Cell. Mol. Med. 2017;21:2491–2502. doi: 10.1111/jcmm.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu B., Feng J., Guo J., Wang J., Xiu G., Xu J., Ning K., Ling B., Fu Q., Xu J. ADSCs-Derived Exosomes Ameliorate Hepatic Fibrosis by Suppressing Stellate Cell Activation and Remodeling Hepatocellular Glutamine Synthetase-Mediated Glutamine and Ammonia Homeostasis. Stem Cell Res. Ther. 2022;13:494. doi: 10.1186/s13287-022-03049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han H.S., Lee H., You D., Nguyen V.Q., Song D.-G., Oh B.H., Shin S., Choi J.S., Kim J.D., Pan C.-H., et al. Human Adipose Stem Cell-Derived Extracellular Nanovesicles for Treatment of Chronic Liver Fibrosis. J. Control. Release. 2020;320:328–336. doi: 10.1016/j.jconrel.2020.01.042. [DOI] [PubMed] [Google Scholar]
- 51.Lai Y.-J., Sung Y.-T., Lai Y.-A., Chen L.-N., Chen T.-S., Chien C.-T. L-Theanine-Treated Adipose-Derived Mesenchymal Stem Cells Alleviate the Cytotoxicity Induced by N-Nitrosodiethylamine in Liver. Tissue Eng. Regen. Med. 2022;19:1207–1221. doi: 10.1007/s13770-022-00472-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niu Q., Wang T., Wang Z., Wang F., Huang D., Sun H., Liu H. Adipose-Derived Mesenchymal Stem Cell-Secreted Extracellular Vesicles Alleviate Non-Alcoholic Fatty Liver Disease via Delivering miR-223-3p. Adipocyte. 2022;11:572–587. doi: 10.1080/21623945.2022.2098583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma Y., Jiao Z., Liu X., Zhang Q., Piao C., Xu J., Wang H. Protective Effect of Adipose-Derived Stromal Cell-Secretome Attenuate Autophagy Induced by Liver Ischemia–Reperfusion and Partial Hepatectomy. Stem Cell Res. Ther. 2022;13:427. doi: 10.1186/s13287-022-03109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao W., Zhang L., Zhang Y., Sun C., Chen X., Wang Y. Adipose-Derived Mesenchymal Stem Cells Promote Liver Regeneration and Suppress Rejection in Small-for-Size Liver Allograft. Transpl. Immunol. 2017;45:1–7. doi: 10.1016/j.trim.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Kalinina N., Kharlampieva D., Loguinova M., Butenko I., Pobeguts O., Efimenko A., Ageeva L., Sharonov G., Ischenko D., Alekseev D., et al. Characterization of Secretomes Provides Evidence for Adipose-Derived Mesenchymal Stromal Cells Subtypes. Stem Cell Res. Ther. 2015;6:221. doi: 10.1186/s13287-015-0209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitchell R., Mellows B., Sheard J., Antonioli M., Kretz O., Chambers D., Zeuner M.-T., Tomkins J.E., Denecke B., Musante L., et al. Secretome of Adipose-Derived Mesenchymal Stem Cells Promotes Skeletal Muscle Regeneration through Synergistic Action of Extracellular Vesicle Cargo and Soluble Proteins. Stem Cell Res. Ther. 2019;10:116. doi: 10.1186/s13287-019-1213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakagami H., Morishita R., Maeda K., Kikuchi Y., Ogihara T., Kaneda Y. Adipose Tissue-Derived Stromal Cells as a Novel Option for Regenerative Cell Therapy. J. Atheroscler. Thromb. 2006;13:77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 58.Dzhoyashvili N.A., Efimenko A.Y., Kochegura T.N., Kalinina N.I., Koptelova N.V., Sukhareva O.Y., Shestakova M.V., Akchurin R.S., Tkachuk V.A., Parfyonova Y.V. Disturbed Angiogenic Activity of Adipose-Derived Stromal Cells Obtained from Patients with Coronary Artery Disease and Diabetes Mellitus Type 2. J. Transl. Med. 2014;12:337. doi: 10.1186/s12967-014-0337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miceli V., Bulati M., Iannolo G., Zito G., Gallo A., Conaldi P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021;22:763. doi: 10.3390/ijms22020763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen W. TGF-β Regulation of T Cells. Annu. Rev. Immunol. 2023;41:483–512. doi: 10.1146/annurev-immunol-101921-045939. [DOI] [PubMed] [Google Scholar]
- 61.De Araújo Farias V., Carrillo-Gálvez A.B., Martín F., Anderson P. TGF-β and Mesenchymal Stromal Cells in Regenerative Medicine, Autoimmunity and Cancer. Cytokine Growth Factor Rev. 2018;43:25–37. doi: 10.1016/j.cytogfr.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Najar M., Raicevic G., Boufker H.I., Kazan H.F., Bruyn C.D., Meuleman N., Bron D., Toungouz M., Lagneaux L. Mesenchymal Stromal Cells Use PGE2 to Modulate Activation and Proliferation of Lymphocyte Subsets: Combined Comparison of Adipose Tissue, Wharton’s Jelly and Bone Marrow Sources. Cell. Immunol. 2010;264:171–179. doi: 10.1016/j.cellimm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 63.Barpour N., Ghorbani M., Baradaran B., Jodari-Mohammadpour Z., Nejati-Koshki K., Abdollahpour-Alitappeh M., Dabbaghi R., Gharibi T. Development of an Injectable Chitosan-Based Hydrogel Containing Nano-Hydroxy-Apatite and Alendronate for MSC-Based Therapy. Int. J. Biol. Macromol. 2024;261:129737. doi: 10.1016/j.ijbiomac.2024.129737. [DOI] [PubMed] [Google Scholar]
- 64.Heidari F., Razmkhah M., Razban V., Erfani N. Effects of Indoleamine 2,3-dioxygenase (IDO) Silencing on Immunomodulatory Function and Cancer-promoting Characteristic of Adipose-derived Mesenchymal Stem Cells (ASCs) Cell Biol. Int. 2021;45:2544–2556. doi: 10.1002/cbin.11698. [DOI] [PubMed] [Google Scholar]
- 65.Albini A., Gallazzi M., Palano M.T., Carlini V., Ricotta R., Bruno A., Stetler-Stevenson W.G., Noonan D.M. TIMP1 and TIMP2 Downregulate TGFβ Induced Decidual-like Phenotype in Natural Killer Cells. Cancers. 2021;13:4955. doi: 10.3390/cancers13194955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Preisner F., Leimer U., Sandmann S., Zoernig I., Germann G., Koellensperger E. Impact of Human Adipose Tissue-Derived Stem Cells on Malignant Melanoma Cells in An In Vitro Co-Culture Model. Stem Cell Rev. Rep. 2018;14:125–140. doi: 10.1007/s12015-017-9772-y. [DOI] [PubMed] [Google Scholar]
- 67.Zambelli A., Biamonti G., Amato A. HGF/c-Met Signalling in the Tumor Microenvironment. In: Birbrair A., editor. Tumor Microenvironment. Volume 1270. Springer International Publishing; Cham, Switzerland: 2021. pp. 31–44. Advances in Experimental Medicine and Biology. [DOI] [PubMed] [Google Scholar]
- 68.Hermann M., Peddi A., Gerhards A., Schmid R., Schmitz D., Arkudas A., Weisbach V., Horch R.E., Kengelbach-Weigand A. Secretome of Adipose-Derived Stem Cells Cultured in Platelet Lysate Improves Migration and Viability of Keratinocytes. Int. J. Mol. Sci. 2023;24:3522. doi: 10.3390/ijms24043522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.An Y.-H., Kim D.H., Lee E.J., Lee D., Park M.J., Ko J., Kim D.W., Koh J., Hong H.S., Son Y., et al. High-Efficient Production of Adipose-Derived Stem Cell (ADSC) Secretome through Maturation Process and Its Non-Scarring Wound Healing Applications. Front. Bioeng. Biotechnol. 2021;9:681501. doi: 10.3389/fbioe.2021.681501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Assis-Ribas T., Forni M.F., Winnischofer S.M.B., Sogayar M.C., Trombetta-Lima M. Extracellular Matrix Dynamics during Mesenchymal Stem Cells Differentiation. Dev. Biol. 2018;437:63–74. doi: 10.1016/j.ydbio.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Ganguly K., Cox J.L., Ghersi D., Grandgenett P.M., Hollingsworth M.A., Jain M., Kumar S., Batra S.K. Mucin 5AC–Mediated CD44/ITGB1 Clustering Mobilizes Adipose-Derived Mesenchymal Stem Cells to Modulate Pancreatic Cancer Stromal Heterogeneity. Gastroenterology. 2022;162:2032–2046.e12. doi: 10.1053/j.gastro.2022.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ritter A., Kreis N.-N., Roth S., Friemel A., Safdar B.K., Hoock S.C., Wildner J.M., Allert R., Louwen F., Solbach C., et al. Cancer-Educated Mammary Adipose Tissue-Derived Stromal/Stem Cells in Obesity and Breast Cancer: Spatial Regulation and Function. J. Exp. Clin. Cancer Res. 2023;42:35. doi: 10.1186/s13046-022-02592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meechem M., Jadli A.S., Patel V.B. Uncovering the Link Between Diabetes and Cardiovascular Disease: Insights from Adipose-Derived Stem Cells. Can. J. Physiol. Pharmacol. 2024;102:229–241. doi: 10.1139/cjpp-2023-0282. [DOI] [PubMed] [Google Scholar]
- 74.Harasymiak-Krzyżanowska I., Niedojadło A., Karwat J., Kotuła L., Gil-Kulik P., Sawiuk M., Kocki J. Adipose Tissue-Derived Stem Cells Show Considerable Promise for Regenerative Medicine Applications. Cell. Mol. Biol. Lett. 2013;18:479–493. doi: 10.2478/s11658-013-0101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Halloran N., Courtney D., Kerin M.J., Lowery A.J. Adipose-Derived Stem Cells in Novel Approaches to Breast Reconstruction: Their Suitability for Tissue Engineering and Oncological Safety. Breast Cancer. 2017;11:117822341772677. doi: 10.1177/1178223417726777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rezaei-Tazangi F., Samadi A., Azandeh S., Khoshnood S., Mahmoudvand S. Secretome of Adipose Derived Stem Cells Induced Apoptosis in Anaplastic Thyroid Carcinoma C-643 Cells. Immunopathol. Persa. 2021;8:e20. doi: 10.34172/ipp.2022.20. [DOI] [Google Scholar]
- 77.Wang T., Yu X., Lin J., Qin C., Bai T., Xu T., Wang L., Liu X., Li S. Adipose-Derived Stem Cells Inhibited the Proliferation of Bladder Tumor Cells by S Phase Arrest and Wnt/β-Catenin Pathway. Cell. Reprogramming. 2019;21:331–338. doi: 10.1089/cell.2019.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim J.H., Oh E., Han Y.S., Lee S.H., Song Y.S. Enhanced Inhibition of Tumor Growth Using TRAIL-Overexpressing Adipose-Derived Stem Cells in Combination with the Chemotherapeutic Agent CPT-11 in Castration-Resistant Prostate Cancer. Prostate Int. 2021;9:31–41. doi: 10.1016/j.prnil.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahara K., Ii M., Inamoto T., Komura K., Ibuki N., Minami K., Uehara H., Hirano H., Nomi H., Kiyama S., et al. Adipose-Derived Stromal Cells Inhibit Prostate Cancer Cell Proliferation Inducing Apoptosis. Biochem. Biophys. Res. Commun. 2014;446:1102–1107. doi: 10.1016/j.bbrc.2014.03.080. [DOI] [PubMed] [Google Scholar]
- 80.Suzuki H., Yokoi A., Uno K., Yoshida K., Kitagawa M., Asano-Inami E., Matsuo S., Nagao Y., Suzuki K., Nakamura K., et al. Small Extracellular Vesicles from Adipose-Derived Stem Cells Suppress Cell Proliferation by Delivering the Let-7 Family of microRNAs in Ovarian Cancer. Biochem. Biophys. Res. Commun. 2023;680:211–219. doi: 10.1016/j.bbrc.2023.09.022. [DOI] [PubMed] [Google Scholar]
- 81.Khodayar M.J., Rezaei Tazangi F., Samimi A., Alidadi H. Adipose-Derived Mesenchymal Stem Cells Secretome Induces Apoptosis in Colon Carcinoma HT-29 Cells. Jentashapir J. Cell. Mol. Biol. 2023;13:e133934. doi: 10.5812/jjcmb-133934. [DOI] [Google Scholar]
- 82.Zhao J., Zhang Z., Cui Q., Zhao L., Hu Y., Zhao S. Human Adipose-Derived Mesenchymal Stem Cells Inhibit Proliferation and Induce Apoptosis of Human Gastric Cancer HGC-27 Cells. 3 Biotech. 2020;10:129. doi: 10.1007/s13205-020-2090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao W., Ren G., Zhang L., Zhang Z., Liu J., Kuang P., Yin Z., Wang X. Efficacy of Mesenchymal Stem Cells Derived from Human Adipose Tissue in Inhibition of Hepatocellular Carcinoma Cells In Vitro. Cancer Biother. Radiopharm. 2012;27:606–613. doi: 10.1089/cbr.2011.1150. [DOI] [PubMed] [Google Scholar]
- 84.Serhal R., Saliba N., Hilal G., Moussa M., Hassan G., Atat O.E., Alaaeddine N. Effect of Adipose-Derived Mesenchymal Stem Cells on Hepatocellular Carcinoma: In Vitro Inhibition of Carcinogenesis. World J. Gastroenterol. 2019;25:567–583. doi: 10.3748/wjg.v25.i5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghufran H., Azam M., Mehmood A., Ashfaq R., Baig M.T., Malik K., Shahid A.A., Riazuddin S. Tumoricidal Effects of Unprimed and Curcumin-Primed Adipose-Derived Stem Cells on Human Hepatoma HepG2 Cells under Oxidative Conditions. Tissue Cell. 2022;79:101968. doi: 10.1016/j.tice.2022.101968. [DOI] [PubMed] [Google Scholar]
- 86.Wu L., Tang Q., Yin X., Yan D., Tang M., Xin J., Pan Q., Ma C., Yan S. The Therapeutic Potential of Adipose Tissue-Derived Mesenchymal Stem Cells to Enhance Radiotherapy Effects on Hepatocellular Carcinoma. Front. Cell Dev. Biol. 2019;7:267. doi: 10.3389/fcell.2019.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sinha S., Narjus-Sterba M., Tuomainen K., Kaur S., Seppänen-Kaijansinkko R., Salo T., Mannerström B., Al-Samadi A. Adipose-Derived Mesenchymal Stem Cells Do Not Affect the Invasion and Migration Potential of Oral Squamous Carcinoma Cells. Int. J. Mol. Sci. 2020;21:6455. doi: 10.3390/ijms21186455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramos-Fresnedo A., Al-Kharboosh R., Twohy E.L., Basil A.N., Szymkiewicz E.C., Zubair A.C., Trifiletti D.M., Durand N., Dickson D.W., Middlebrooks E.H., et al. Phase 1, Dose Escalation, Nonrandomized, Open-Label, Clinical Trial Evaluating the Safety and Preliminary Efficacy of Allogenic Adipose-Derived Mesenchymal Stem Cells for Recurrent Glioblastoma: A Clinical Trial Protocol. Neurosurg. Pract. 2024;4:e00062. [PMC free article] [PubMed] [Google Scholar]
- 89.Frommer M.L., Langridge B.J., Awad L., Jasionowska S., Denton C.P., Abraham D.J., Abu-Hanna J., Butler P.E.M. Single-Cell Analysis of ADSC Interactions with Fibroblasts and Endothelial Cells in Scleroderma Skin. Cells. 2023;12:1784. doi: 10.3390/cells12131784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tilotta V., Vadalà G., Ambrosio L., Cicione C., Di Giacomo G., Russo F., Papalia R., Denaro V. Mesenchymal Stem Cell-Derived Secretome Enhances Nucleus Pulposus Cell Metabolism and Modulates Extracellular Matrix Gene Expression In Vitro. Front. Bioeng. Biotechnol. 2023;11:1152207. doi: 10.3389/fbioe.2023.1152207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmid R., Wolf K., Robering J.W., Strauß S., Strissel P.L., Strick R., Rübner M., Fasching P.A., Horch R.E., Kremer A.E., et al. ADSCs and Adipocytes Are the Main Producers in the Autotaxin–Lysophosphatidic Acid Axis of Breast Cancer and Healthy Mammary Tissue In Vitro. BMC Cancer. 2018;18:1273. doi: 10.1186/s12885-018-5166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ambrosio M.R., Mosca G., Migliaccio T., Liguoro D., Nele G., Schonauer F., D’Andrea F., Liotti F., Prevete N., Melillo R.M., et al. Glucose Enhances Pro-Tumorigenic Functions of Mammary Adipose-Derived Mesenchymal Stromal/Stem Cells on Breast Cancer Cell Lines. Cancers. 2022;14:5421. doi: 10.3390/cancers14215421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Di Franco S., Bianca P., Sardina D.S., Turdo A., Gaggianesi M., Veschi V., Nicotra A., Mangiapane L.R., Lo Iacono M., Pillitteri I., et al. Adipose Stem Cell Niche Reprograms the Colorectal Cancer Stem Cell Metastatic Machinery. Nat. Commun. 2021;12:5006. doi: 10.1038/s41467-021-25333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin P., Zhang G., Li H. The Role of Extracellular Matrix in Wound Healing. Dermatol. Surg. 2023;49:S41–S48. doi: 10.1097/DSS.0000000000003779. [DOI] [PubMed] [Google Scholar]
- 95.Huang J., Zhang L., Wan D., Zhou L., Zheng S., Lin S., Qiao Y. Extracellular Matrix and Its Therapeutic Potential for Cancer Treatment. Signal Transduct. Target. Ther. 2021;6:153. doi: 10.1038/s41392-021-00544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roy A.M., Iyer R., Chakraborty S. The Extracellular Matrix in Hepatocellular Carcinoma: Mechanisms and Therapeutic Vulnerability. Cell Rep. Med. 2023;4:101170. doi: 10.1016/j.xcrm.2023.101170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chu Y., Wang Y., Peng W., Xu L., Liu M., Li J., Hu X., Li Y., Zuo J., Ye Y. STAT3 Activation by IL-6 from Adipose-Derived Stem Cells Promotes Endometrial Carcinoma Proliferation and Metastasis. Biochem. Biophys. Res. Commun. 2018;500:626–631. doi: 10.1016/j.bbrc.2018.04.121. [DOI] [PubMed] [Google Scholar]
- 98.Lu J.-H., Wei H.-J., Peng B.-Y., Chou H.-H., Chen W.-H., Liu H.-Y., Deng W.-P. Adipose-Derived Stem Cells Enhance Cancer Stem Cell Property and Tumor Formation Capacity in Lewis Lung Carcinoma Cells through an Interleukin-6 Paracrine Circuit. Stem Cells Dev. 2016;25:1833–1842. doi: 10.1089/scd.2016.0163. [DOI] [PubMed] [Google Scholar]
- 99.Wei H.-J., Zeng R., Lu J.-H., Lai W.-F.T., Chen W.-H., Liu H.-Y., Chang Y.-T., Deng W.-P. Adipose-Derived Stem Cells Promote Tumor Initiation and Accelerate Tumor Growth by Interleukin-6 Production. Oncotarget. 2015;6:7713–7726. doi: 10.18632/oncotarget.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goto H., Shimono Y., Funakoshi Y., Imamura Y., Toyoda M., Kiyota N., Kono S., Takao S., Mukohara T., Minami H. Adipose-Derived Stem Cells Enhance Human Breast Cancer Growth and Cancer Stem Cell-like Properties through Adipsin. Oncogene. 2019;38:767–779. doi: 10.1038/s41388-018-0477-8. [DOI] [PubMed] [Google Scholar]
- 101.Li W., Qian C., Ma F., Liu M., Sun X., Liu X., Liu C., Chen Z., Ma W., Liu J., et al. MAPK/ERK-CBP-RFPL-3 Mediates Adipose-Derived Stem Cell-Induced Tumor Growth in Breast Cancer Cells by Activating Telomerase Reverse Transcriptase Expression. Stem Cells Int. 2022;2022:8540535. doi: 10.1155/2022/8540535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu H., Li W., Luo S., Yuan J., Hao L. Adipose Derived Stem Cells Promote Tumor Metastasis in Breast Cancer Cells by Stem Cell Factor Inhibition of miR20b. Cell. Signal. 2019;62:109350. doi: 10.1016/j.cellsig.2019.109350. [DOI] [PubMed] [Google Scholar]
- 103.Liu X., Zhao G., Huo X., Wang Y., Tigyi G., Zhu B.-M., Yue J., Zhang W. Adipose-Derived Stem Cells Facilitate Ovarian Tumor Growth and Metastasis by Promoting Epithelial to Mesenchymal Transition through Activating the TGF-β Pathway. Front. Oncol. 2021;11:756011. doi: 10.3389/fonc.2021.756011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liang Z., Liu H., Zhang Y., Xiong L., Zeng Z., He X., Wang F., Wu X., Lan P. Cyr61 from Adipose-derived Stem Cells Promotes Colorectal Cancer Metastasis and Vasculogenic Mimicry Formation via Integrin αVβ5. Mol. Oncol. 2021;15:3447–3467. doi: 10.1002/1878-0261.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mohd Ali N., Yeap S.K., Ho W.Y., Boo L., Ky H., Satharasinghe D.A., Tan S.W., Cheong S.K., Huang H.D., Lan K.C., et al. Adipose MSCs Suppress MCF7 and MDA-MB-231 Breast Cancer Metastasis and EMT Pathways Leading to Dormancy via Exosomal-miRNAs Following Co-Culture Interaction. Pharmaceuticals. 2020;14:8. doi: 10.3390/ph14010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Airuddin S.S., Halim A.S., Wan Sulaiman W.A., Kadir R., Nasir N.A.M. Adipose-Derived Stem Cell: “Treat or Trick”. Biomedicines. 2021;9:1624. doi: 10.3390/biomedicines9111624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chu Y., Zhu C., Wang Q., Liu M., Wan W., Zhou J., Han R., Yang J., Luo W., Liu C., et al. Adipose-derived Mesenchymal Stem Cells Induced PAX8 Promotes Ovarian Cancer Cell Growth by Stabilizing TAZ Protein. J. Cell. Mol. Med. 2021;25:4434–4443. doi: 10.1111/jcmm.16511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chu Y., You M., Zhang J., Gao G., Han R., Luo W., Liu T., Zuo J., Wang F. Adipose-Derived Mesenchymal Stem Cells Enhance Ovarian Cancer Growth and Metastasis by Increasing Thymosin Beta 4X-Linked Expression. Stem Cells Int. 2019;2019:9037197. doi: 10.1155/2019/9037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chu Y., Tang H., Guo Y., Guo J., Huang B., Fang F., Cai J., Wang Z. Adipose-Derived Mesenchymal Stem Cells Promote Cell Proliferation and Invasion of Epithelial Ovarian Cancer. Exp. Cell Res. 2015;337:16–27. doi: 10.1016/j.yexcr.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 110.Adelipour M., Allameh A., Sheikhi A., Ranjbaran M., Naghashpour M., Nazeri Z., Mojiri-Foroshani H., Golabi S. Role of the Mesenchymal Stem Cells Derived from Adipose Tissue in Changing the Rate of Breast Cancer Cell Proliferation and Autophagy, In Vitro and In Vivo. Iran. J. Basic Med. Sci. 2021;24:98–107. doi: 10.22038/ijbms.2020.51461.11678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu S., Wang Y., Yuan Z., Wang S., Du H., Liu X., Wang Q., Zhu X. Human Adipose-derived Mesenchymal Stem Cells Promote Breast Cancer MCF7 Cell Epithelial-mesenchymal Transition by Cross Interacting with the TGF-β/Smad and PI3K/AKT Signaling Pathways. Mol. Med. Rep. 2018;19:177–186. doi: 10.3892/mmr.2018.9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.De Miranda M.C., Ferreira A.D.F., De Melo M.I.A., Kunrath-Lima M., Goes A.M.D., Rodrigues M.A., Gomes D.A., Faria J.A.Q.A. Adipose-Derived Stem/Stromal Cell Secretome Modulates Breast Cancer Cell Proliferation and Differentiation State towards Aggressiveness. Biochimie. 2021;191:69–77. doi: 10.1016/j.biochi.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 113.Eterno V., Zambelli A., Pavesi L., Villani L., Zanini V., Petrolo G., Manera S., Tuscano A., Amato A. Adipose-Derived Mesenchymal Stem Cells (ASCs) May Favour Breast Cancer Recurrence via HGF/c-Met Signaling. Oncotarget. 2014;5:613–633. doi: 10.18632/oncotarget.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu L., Wang X., Wang J., Liu D., Wang Y., Huang Z., Tan H. Hypoxia-Induced Secretion of IL-10 from Adipose-Derived Mesenchymal Stem Cell Promotes Growth and Cancer Stem Cell Properties of Burkitt Lymphoma. Tumor Biol. 2016;37:7835–7842. doi: 10.1007/s13277-015-4664-8. [DOI] [PubMed] [Google Scholar]
- 115.Fang J., Chen F., Liu D., Gu F., Wang Y. Adipose Tissue-Derived Stem Cells in Breast Reconstruction: A Brief Review on Biology and Translation. Stem Cell Res. Ther. 2021;12:8. doi: 10.1186/s13287-020-01955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Valente D.S., Ely P.B., Kieling L., Konzen A.T., Steffen L.P., Lazzaretti G.S., Zanella R.K. Breast Fat Grafting and Cancer: A Systematic Review of the Science behind Enhancements and Concerns. Transl. Breast Cancer Res. 2024;5:14. doi: 10.21037/tbcr-23-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao M., Sachs P.C., Wang X., Dumur C.I., Idowu M.O., Robila V., Francis M.P., Ware J., Beckman M., Rizki A., et al. Mesenchymal Stem Cells in Mammary Adipose Tissue Stimulate Progression of Breast Cancer Resembling the Basal-Type. Cancer Biol. Ther. 2012;13:782–792. doi: 10.4161/cbt.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xie H., Liao N., Lan F., Cai Z., Liu X., Liu J. 3D-Cultured Adipose Tissue-Derived Stem Cells Inhibit Liver Cancer Cell Migration and Invasion through Suppressing Epithelial-Mesenchymal Transition. Int. J. Mol. Med. 2017;41:1385–1396. doi: 10.3892/ijmm.2017.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Teshima T., Matsumoto H., Koyama H. Soluble Factors from Adipose Tissue-Derived Mesenchymal Stem Cells Promote Canine Hepatocellular Carcinoma Cell Proliferation and Invasion. PLoS ONE. 2018;13:e0191539. doi: 10.1371/journal.pone.0191539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salah R.A., Nasr M.A., El-Derby A.M., Abd Elkodous M., Mohamed R.H., El-Ekiaby N., Osama A., Elshenawy S.E., Hamad M.H.M., Magdeldin S., et al. Hepatocellular Carcinoma Cell Line-Microenvironment Induced Cancer-Associated Phenotype, Genotype and Functionality in Mesenchymal Stem Cells. Life Sci. 2022;288:120168. doi: 10.1016/j.lfs.2021.120168. [DOI] [PubMed] [Google Scholar]
- 121.Wang Y., Lee J.-H., Shirahama H., Seo J., Glenn J.S., Cho N.-J. Extracellular Matrix Functionalization and Huh-7.5 Cell Coculture Promote the Hepatic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells in a 3D ICC Hydrogel Scaffold. ACS Biomater. Sci. Eng. 2016;2:2255–2265. doi: 10.1021/acsbiomaterials.6b00487. [DOI] [PubMed] [Google Scholar]
- 122.Liu T., Li T., Zheng Y., Xu X., Sun R., Zhan S., Guo X., Zhao Z., Zhu W., Feng B., et al. Evaluating Adipose-derived Stem Cell Exosomes as miRNA Drug Delivery Systems for the Treatment of Bladder Cancer. Cancer Med. 2022;11:3687–3699. doi: 10.1002/cam4.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Borghese C., Casagrande N., Corona G., Aldinucci D. Adipose-Derived Stem Cells Primed with Paclitaxel Inhibit Ovarian Cancer Spheroid Growth and Overcome Paclitaxel Resistance. Pharmaceutics. 2020;12:401. doi: 10.3390/pharmaceutics12050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu Z., Li S., Ma T., Zeng J., Zhou X., Li H., Tang M., Liu X., Li F., Jiang B., et al. Secreted TRAIL Gene-modified Adipose-derived Stem Cells Exhibited Potent Tumor-suppressive Effect in Hepatocellular Carcinoma Cells. Immun. Inflamm. Dis. 2021;9:144–156. doi: 10.1002/iid3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y., Niu H., Li L., Han J., Liu Z., Chu M., Sha X., Zhao J. Anti-CHAC1 Exosomes for Nose-to-Brain Delivery of miR-760-3p in Cerebral Ischemia/Reperfusion Injury Mice Inhibiting Neuron Ferroptosis. J. Nanobiotechnol. 2023;21:109. doi: 10.1186/s12951-023-01862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang X., Han C., Du B., Nan D., Zhang W., He G. Isolation and Identification of Adipose Stem Cell Exosomes and the Study of Its Potential as Drug Delivery Carrier In Vitro. Appl. Biochem. Biotechnol. 2022;194:2594–2603. doi: 10.1007/s12010-022-03835-6. [DOI] [PubMed] [Google Scholar]
- 127.Scioli M.G., Artuso S., D’Angelo C., Porru M., D’Amico F., Bielli A., Gentile P., Cervelli V., Leonetti C., Orlandi A. Adipose-Derived Stem Cell-Mediated Paclitaxel Delivery Inhibits Breast Cancer Growth. PLoS ONE. 2018;13:e0203426. doi: 10.1371/journal.pone.0203426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lou G., Song X., Yang F., Wu S., Wang J., Chen Z., Liu Y. Exosomes Derived from miR-122-Modified Adipose Tissue-Derived MSCs Increase Chemosensitivity of Hepatocellular Carcinoma. J. Hematol. Oncol. 2015;8:122. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lou G., Chen L., Xia C., Wang W., Qi J., Li A., Zhao L., Chen Z., Zheng M., Liu Y. MiR-199a-Modified Exosomes from Adipose Tissue-Derived Mesenchymal Stem Cells Improve Hepatocellular Carcinoma Chemosensitivity through mTOR Pathway. J. Exp. Clin. Cancer Res. 2020;39:4. doi: 10.1186/s13046-019-1512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pham P.V., Nguyen S.T., Phan N.L.-C., Do N.M., Vo P.H. Adipose-Derived Stem Cells Can Replace Fibroblasts as Cell Control for Anti-Tumor Screening Assay. OncoTargets Ther. 2020;13:6417–6423. doi: 10.2147/OTT.S259114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao J., Vykoukal J., Abdelsalam M., Recio-Boiles A., Huang Q., Qiao Y., Singhana B., Wallace M., Avritscher R., Melancon M.P. Stem Cell-Mediated Delivery of SPIO-Loaded Gold Nanoparticles for the Theranosis of Liver Injury and Hepatocellular Carcinoma. Nanotechnology. 2014;25:405101. doi: 10.1088/0957-4484/25/40/405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arzumanian V.A., Kiseleva O.I., Poverennaya E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021;22:13135. doi: 10.3390/ijms222313135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the article and will be made available upon reasonable request.