Abstract
Human herpesvirus 8 (HHV8) infects Kaposi’s sarcoma (KS) spindle cells in situ, as well as the lesional endothelial cells considered to be spindle cell precursors. The HHV8 genome contains several oncogenes, suggesting that infection of endothelial and spindle cells could induce cellular transformation and tumorigenesis and promote the formation of KS lesions. To investigate the potential of HHV8 infection of endothelial cells to contribute to the development of KS, we have developed an in vitro model utilizing dermal microvascular endothelial cells that support significant HHV8 infection. In contrast to existing in vitro systems used to study HHV8 pathogenesis, the majority of dermal endothelial cells are infected with HHV8 and the viral genome is maintained indefinitely. Infection is predominantly latent, with a small percentage of cells supporting lytic replication, and latency is responsive to lytic induction stimuli. Infected endothelial cells develop a spindle shape resembling that of KS lesional cells and show characteristics of a transformed phenotype, including loss of contact inhibition and acquisition of anchorage-independent growth. These results describe a relevant model system in which to study virus-host interactions in vitro and demonstrate the ability of HHV8 to induce phenotypic changes in infected endothelial cells that resemble characteristics of KS spindle cells in vivo. Thus, our results are consistent with a direct role for HHV8 in the pathogenesis of KS.
Kaposi’s sarcoma (KS) is a multifocal vascular neoplasm involving the skin, visceral organs, and lymph nodes. KS lesions contain distinctive proliferating spindle cells, activated endothelial cells, fibroblasts, smooth muscle cells, and infiltrating inflammatory cells (38, 42). Infection with the gamma-2 herpesvirus, human herpesvirus 8 (HHV8), also known as KS-associated herpesvirus, is closely associated with the development of these lesions. HHV8 was first identified by representation difference analysis of KS tissue from an AIDS patient (11) and has subsequently been identified in >95% of KS patients with all clinical forms of KS (2, 21, 30, 47). HHV8 sequences have also been identified in primary effusion lymphomas (PEL), a subset of B-cell lymphomas largely confined to AIDS patients (7), and a rare lymphoproliferative disorder known as multicentric Castleman’s disease (50). Seroepidemiologic studies also support a role for HHV8 in the etiology of KS in that HHV8 infection precedes KS development, and there is a consistent correlation between HHV8 seroprevalence and high-risk KS groups (17, 18, 23, 49, 56).
Molecular studies using PCR, in situ hybridization, and immunohistochemical analyses have identified HHV8 in atypical endothelial cells and spindle cells in KS lesions (6, 24, 28, 36, 51, 52). Endothelial cells are generally considered to be the precursors of KS spindle cells (5, 12, 22, 41–45, 48), and HHV8-infected endothelial cells are detected in very early lesions prior to extensive spindle cell formation (24, 51, 52). HHV8 may thus promote the development of KS spindle cells as a direct consequence of endothelial cell infection. In addition, induction of paracrine stimuli that influence uninfected bystander cells may facilitate spindle cell formation (14, 15, 45, 46).
Despite compelling evidence, verification of viral etiology and identification of mechanisms of HHV8 pathogenesis have remained elusive. Clarification of these issues would be greatly assisted by the establishment of in vitro models that accurately reflect HHV8 infection in KS lesional tissue in vivo. The study of latently infected PEL cell lines that support lytic replication following treatment with phorbol esters and sodium butyrate has proved invaluable for virus characterization and development of serological assays (3, 8, 17, 29, 39). However, the in vivo infection status of these cell lines precludes evaluation of the consequences of a de novo viral infection. In addition, HHV8 infection of B-lymphocyte-derived PEL cells may differ in significant aspects from infection of cell lineages that harbor the viral genome in KS lesions.
In vivo, the majority of spindle and endothelial cells in KS lesions maintain a latent HHV8 infection with virus in only a small percentage of cells spontaneously entering the lytic replication cycle (33, 36, 51, 52, 57). Current tissue culture models developed for the study of KS pathogenesis do not adequately reproduce this viral strategy in vitro. Spindle cells isolated from KS lesions generally have a limited life in tissue culture, and even in established KS cell lines, the viral genome is not well maintained (1, 2, 25). In culture systems based on in vitro infection of endothelial, epithelial, and fibroblastoid cells, infection occurs in only low percentages of cells and is generally not well maintained (15, 16, 34, 40). In this report, we describe an endothelial cell-based tissue culture model that reflects the HHV8 life cycle in KS lesions in vivo. This model utilizes immortalized dermal microvascular endothelial cells (DMVEC) that allow the extended maintenance of age- and passage-matched mock-infected controls for evaluation of virus-induced cellular changes. In this model, HHV8 established a robust long-term infection in the majority of DMVEC. Infection was primarily latent with a small percentage of cells spontaneously entering the lytic cycle, and lytic replication could be significantly induced by treatment with chemical agents. Infected DMVEC developed a spindle shape resembling that of KS spindle cells in vivo and displayed elements of a transformed phenotype including loss of contact inhibition and acquisition of anchorage-independent growth. Consequently, HHV8 infection of DMVEC represents an ideal model system in which to study molecular mechanisms of HHV8 infection and KS pathogenesis in vitro.
MATERIALS AND METHODS
Cells.
Primary DMVEC were obtained from Clonetics Corporation (San Diego, Calif.) and maintained in the culture medium recommended by Clonetics. Primary cells were immortalized by infection with the recombinant retrovirus LXSN16 E6E7 derived from the amphotropic retrovirus-packaging cell line PA317 (20, 35). The recombinant virus contains the E6 and E7 genes of human papillomavirus (HPV) type 16 flanked by the long terminal repeats of Moloney murine leukemia virus and a neomycin resistance marker. Briefly, primary DMVEC were infected by exposure to PA317 cell supernatant and cultured in the presence of G418 (200 μg/ml; GIBCO BRL, Gaithersburg, Md.) for selection of resistant clones that were expanded and cryopreserved for subsequent use. Immortalized DMVEC were maintained in Endothelial-SFM medium (GIBCO BRL) supplemented with 10% human AB serum (HS; Sigma, St. Louis, Mo.), 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM glutamine, 25 μg of endothelial cell growth supplement (Becton-Dickinson, Bedford, Mass.) per ml, 40 μg of heparin (Sigma) per ml, and 200 μg of G418 per ml. The BCBL-1 cell line (39) was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (contributed by Michael McGrath and Don Ganem) and cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM glutamine, and 5 × 10−5 M 2-mercaptoethanol.
Virus infections.
BCBL-1 cells were used as a source of HHV8 for DMVEC infections. To generate HHV8-containing supernatants, BCBL cells (106 cells/ml) were exposed to tetradecanoyl phorbol acetate (TPA) (20 ng/ml) for 48 h and cell-free virus inoculum was obtained by filtration through a 0.45-μm-pore-size membrane. Since no protocol for determining the infectious titer of HHV8-containing supernatants currently exists, fresh inocula prepared for different experiments were standardized by ensuring that identical numbers of passage-matched BCBL cells were likewise treated for virus induction. For HHV8 infection, DMVEC were grown to 60 to 80% confluency in 60- or 35-mm-diameter Primaria tissue culture dishes. Heparin was omitted from the culture medium for at least 24 h prior to virus challenge. Virus inoculum was added to monolayers (0.8 ml per 35-mm-diameter dish; 1.5 ml per 60-mm-diameter dish) for 4 h in the presence of Polybrene (2 μg/ml) followed by the addition of equal volumes of heparin-free culture medium for an additional 12 h or overnight. For every experiment, mock infections were performed in parallel with BCBL supernatants exposed to UV light to inactivate HHV8. For inactivation, BCBL supernatants were decanted into six-well trays (2 ml/well) and placed in a Stratalinker UV chamber (Stratagene, La Jolla, Calif.) for two 10-min pulses with tray lids removed. Supernatants were then filtered through 0.45-μm-pore-size membranes and equilibrated in a 7% CO2 incubator for 30 min before use. To remove virus inoculum, cells were rinsed twice in Hanks balanced salt solution and once in culture medium and recultured in culture medium containing heparin. Thereafter, cells were fed every 3 or 4 days and passaged by trypsinization as required or as dictated by the experimental protocol. For infections with virus produced by HHV8-infected DMVEC, supernatants were harvested from uninduced and TPA-treated infected DMVEC, treated as described above, and used to infect naive DMVEC cultures.
DNA PCR.
Infected DMVEC were examined for the presence of HHV8-specific DNA by using primers that amplify a 233-bp fragment of the KS330233 BamHI fragment of open reading frame (ORF) 26 (11, 30). Genomic DNA was prepared from serial dilutions of cells, and PCR was performed on samples equivalent to the amount of DNA from 2 × 103, 4 × 102, 2 × 102, and 4 × 101 HHV8-exposed DMVEC. Samples prepared from 2 × 103 uninduced BCBL-1 cells and mock-infected DMVEC were used as positive and negative controls, respectively. DNA was amplified as follows: 3 min at 94°C (hot start); 35 cycles, with 1 cycle consisting of 1 min at 94°C, 1 min at 58°C, and 1 min at 72°C; and 5 min at 72°C. PCR products were visualized following electrophoresis through agarose-ethidium bromide gels.
RT-PCR.
HHV8 genes ORF 29 and ORF K12 (kaposin) were used as reverse transcriptase PCR (RT-PCR) targets. ORF 29 is spliced and expressed late in the lytic infection cycle following viral DNA replication (40), while ORF K12 is encoded by the latency-associated 0.7-kb transcript T0.7 (57). Total RNA was extracted from DMVEC with a Qiagen RNeasy Kit (Qiagen) according to the manufacturer’s protocol, and cDNA was reverse transcribed by using Superscript RT (Life Technologies, Gaithersburg, Md.) at 200 U/μg of RNA. For each reaction, controls in the absence of RT were included. ORF 29 sequences (300 bp) were amplified with primers flanking the splice donor (ORF 29A [GCACGTAGCCAACTCCGTG]) and acceptor (ORF 29B [GCAGGAAACTCGTGGAGCG]) regions of the gene as previously described (40). ORF K12 sequences (225 bp) were amplified with primers (5′ TCCTCACTCCAATCCCAATGC and 3′ CTTTGGGAGGGCACGCTAGCT) as previously described (31). The cellular hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene was amplified from each sample as a control for cDNA synthesis and yielded consistent amplification products from sample to sample. PCR products were visualized following electrophoresis through agarose-ethidium bromide gels.
IFA for HHV8 proteins.
Immunofluorescence assays (IFA) were performed with a polyclonal antibody raised in rabbit against full-length ORF 73 expressed as a glutathione S-transferase fusion protein in baculovirus and monoclonal antibodies (MAbs) against ORF 59 (MAb 11D1) (9) and ORF K8.1A/B (10). For ORF 73 and ORF 59, HHV8-infected DMVEC monolayers were fixed in 95% ethanol–5% glacial acetic acid, permeabilized with 0.5% Triton X-100, and blocked with 20% normal goat serum (NGS) in phosphate-buffered saline (PBS) for 20 min prior to staining with primary antibodies followed by fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit and anti-mouse secondary antibodies (Tago, Burlingame, Calif.). For ORF K8.1A/B, monolayers were fixed with 2% paraformaldehyde (pH 7.4) in PBS followed by blocking and staining essentially as described above. Antibodies were diluted 1:100 in 1% NGS in PBS and incubated with monolayers for 60 min at 37°C. Staining controls included omission of primary antibody and staining of mock-infected monolayers with MAb 11D1. Stained cells were mounted in SlowFade antifade reagent in 50% glycerol (Molecular Probes, Eugene, Oreg.) and viewed on a Nikon fluorescence microscope.
IFA for cellular proteins.
Uninduced mock- and HHV8-infected DMVEC were stained with MAbs against CD31 (clone JC/70A; DAKO, Carpinteria, Calif.) and VE-cadherin (clone 55-7H1; Pharmingen) followed by a FITC-conjugated goat anti-mouse secondary antibody (TAGO), or a rabbit polyclonal antibody against von Willebrand factor (vWF) (DAKO) followed by a tetramethyl rhodamine isocyanate (TRITC)-conjugated goat anti-rabbit secondary antibody. For CD31 and VE-cadherin staining, cell monolayers were fixed and permeabilized by immersion in 2% paraformaldehyde–0.25% Triton X-100 in PBS and then in 2% paraformaldehyde alone (both immersions for 5 min each at room temperature). For vWF staining, cells were fixed and permeabilized as described above for staining with the HHV8 MAb 11D1. Cells were blocked with 20% NGS and incubated with the relevant antibodies diluted 1:100 in PBS containing 1% NGS for 60 min at 37°C. Cells were mounted and viewed as described above.
Electron microscopy.
To prepare DMVEC for transmission electron microscopy, HHV8-infected and mock-infected monolayers were rinsed twice in cacodylate buffer and scraped into Karnovsky’s fixative. Cell pellets were postfixed in 1% OsO4, dehydrated in a graded series of solutions of ethanol and propylene oxide and embedded in Spurr’s resin for thin sectioning. Thin sections were stained with uranyl acetate and lead citrate and examined on a Philips EM 300 electron microscope.
Morphology changes.
To assess changes in cell morphology in mock- and HHV8-infected DMVEC, cells were examined on a regular basis on an inverted light microscope. Cells were examined for evidence of spindling, virus-induced cytopathic effect, and evidence of transformation reflected by loss of contact inhibition and development of adherent foci in the monolayer.
Soft agar.
Mock- and HHV8-infected DMVEC were trypsinized, resuspended at a concentration of 104 cells/ml in 2× Dulbecco modified Eagle medium additionally supplemented with 5% HS, heparin, and endothelial cell growth supplement in a final amount of 0.36% melted agar and added to an underlay of 0.6% agar in 2× Dulbecco modified Eagle medium with 5% HS. Infected DMVEC cultures used in soft-agar experiments were not exposed to lytic-cycle induction stimuli. Parallel immunostained cultures contained approximately 20% latently infected ORF 73-positive cells and no more than 0.5% of cells expressing the lytic-cycle ORF 59 protein. Cells were fed every 5 days and routinely observed by microscopy for colony formation.
RESULTS
DMVEC are permissive for HHV8 infection.
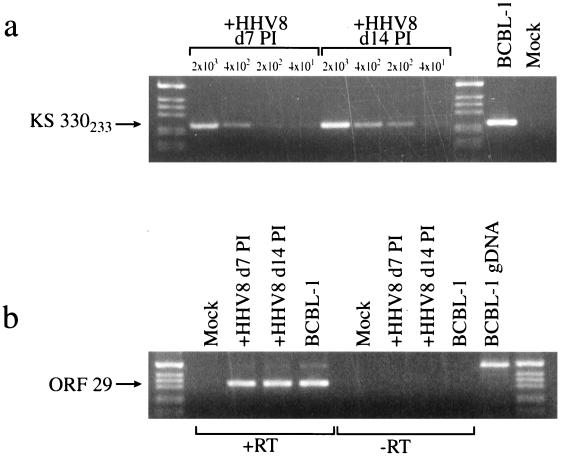
Dermal endothelial cells were obtained from a commercial source and immortalized with the E6 and E7 genes of HPV type 16 (20). DMVEC were infected with HHV8 by exposure to cell-free supernatants derived from phorbol ester-treated BCBL cells. For mock infections, viral inoculum was exposed to UV light to inactivate virus. To initially verify infection, PCR was performed with primers that amplify the KS330233 BamHI region of HHV8 DNA originally associated with KS lesions (11). Figure 1a illustrates product amplified from DNA obtained from as few as 200 HHV8-exposed DMVEC at day 7 postinfection (PI) and at day 14 PI following one tissue culture passage. The intensity of the PCR signal from equivalent cell numbers was enhanced at later times PI. DNA from mock-infected cells was similarly tested but failed to yield detectable amplification products. An RT-PCR assay targeting the ORF 29 gene was used to confirm the presence of de novo viral gene expression in these cells (Fig. 1b). As ORF 29 contains a 4-kb intron, the spliced RT-PCR product is smaller than any product amplified from contaminating input viral DNA or genomic DNA (40). To illustrate this difference, ORF 29 was also amplified from BCBL-1 cell genomic DNA and reverse-transcribed cDNA. HHV8-infected DMVEC expressed transcripts that corresponded to ORF 29 amplified from spliced mRNA, thus indicating de novo viral gene expression in infected cells.
FIG. 1.
DNA and RT-PCR analyses of HHV8-infected DMVEC. (a) DNA PCR amplification of the HHV8-specific KS330233 sequence from serial dilutions of HHV8-exposed DMVEC (+HHV8). DNA was amplified from 2 × 103, 4 × 102, and 2 × 102 and cell equivalents at 7 and 14 days PI (d7 PI and d14 PI, respectively). For positive and negative controls, PCR was performed on genomic DNA prepared from 2 × 103 BCBL-1 cells and mock-infected DMVEC. (b) RT-PCR detection of ORF 29 mRNA using cDNA from 2 × 103 HHV8-exposed DMVEC (+HHV8) at 7 and 14 days PI (d7 PI and d14 PI, respectively) to verify the authenticity of HHV8 infection. cDNA prepared from BCBL-1 cells was used as a positive control. No signal was obtained from mock-infected cells or samples prepared in the absence of RT (−RT). Cellular HPRT was simultaneously amplified from all +RT samples as a control for cDNA synthesis (data not shown). Amplification products from reverse-transcribed BCBL-1 cell cDNA and genomic DNA (BCBL-1 gDNA) demonstrate that the RT-PCR product from spliced mRNA is smaller than the product from genomic DNA. Products amplified from HHV8-infected DMVEC show only the smaller band, indicating lack of contamination from the viral inoculum or replicated virus.
HHV8-infected DMVEC express ORF 73 (LANA).
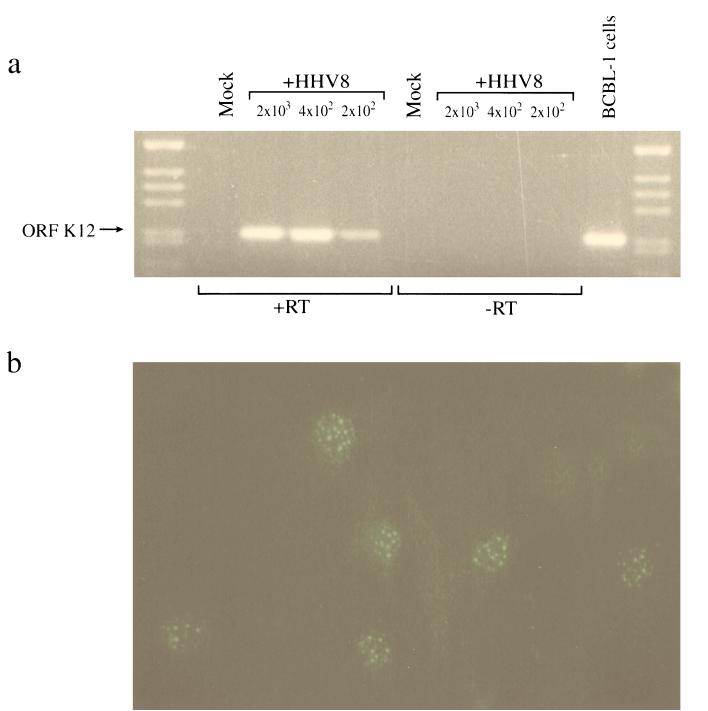
In situ hybridization and immunohistochemical studies illustrate that the majority of endothelial and spindle cells in KS lesions harbor the HHV8 genome in a latent state (36, 51, 52, 57). Such studies demonstrate expression of the latency-associated 0.7-kb transcript (T0.7) which encodes the ORF K12 protein, kaposin, and the latency-associated nuclear antigen (LANA) encoded by ORF 73. To characterize latent infection in DMVEC infected with HHV8 in vitro, we examined expression of ORF K12 by RT-PCR and ORF 73 by IFA. As illustrated in Fig. 2a, ORF K12 transcripts were readily amplified from HHV8-infected DMVEC but not mock-infected DMVEC at day 14 PI. The ability to amplify kaposin from as few as 200 virus-exposed cells indicates the maintenance of latent virus in a significant proportion of cells. IFA using a rabbit polyclonal antiserum against ORF 73 demonstrated typical punctate LANA reactivity in infected DMVEC nuclei. ORF 73 expression was detected as early as 12 h PI but initially never in more than 10% of HHV8-exposed cells. The percentage of ORF 73-positive cells increased with time PI until as many as 80% of cells in a monolayer were latently infected. Figure 2b illustrates ORF 73 expression in approximately 50% of cells at day 7 PI.
FIG. 2.
Expression of latency-associated genes in HHV8-infected DMVEC. (a) RT-PCR detection of HHV8 kaposin (ORF K12) mRNA by RT-PCR from HHV8-infected (+HHV8) but not mock-infected DMVEC. cDNA from uninduced BCBL-1 cells was included as a positive control. Products amplified from cDNA prepared from 2 × 103, 4 × 102, and 2 × 102 and cell equivalents at day 14 PI are shown. No signal was detected in samples prepared in the absence of RT (−RT). Cellular HPRT was simultaneously amplified from all +RT samples as a control for cDNA synthesis (data not shown). (b) Nuclear expression of latent antigen ORF 73 in HHV8-infected DMVEC at day 7 PI visualized with a polyclonal antibody against ORF 73 and a goat anti-rabbit FITC conjugate.
Stimulation of latently infected DMVEC induces lytic replication of HHV8.
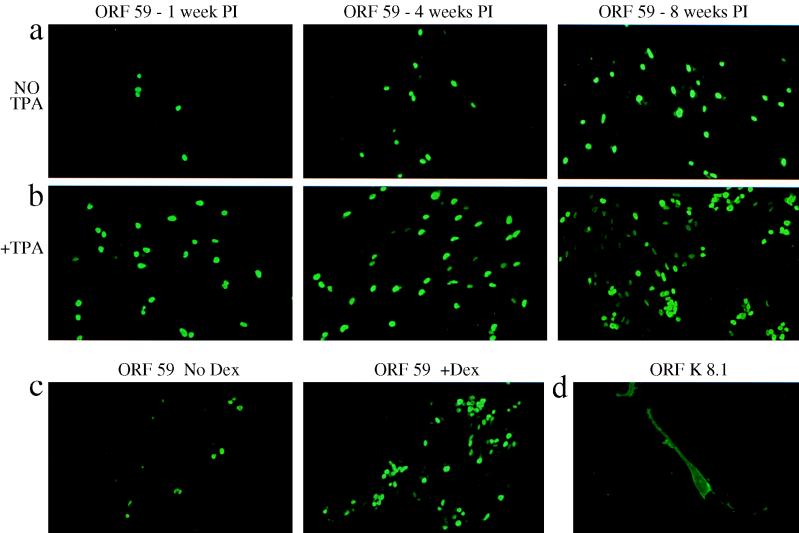
In our previous experiments, the expansion of ORF 73-positive cells and the increased intensity of DNA and RT-PCR signals observed with time PI suggested spread of infection through virus-exposed DMVEC cultures. This spread of infection may reflect the division of latently infected cells or the entry of a percentage of infected cells into the lytic replication cycle with release of viral progeny and reinfection within the culture. Detection of ORF 29 mRNA expression (Fig. 1b) also suggested the capacity for DMVEC to support lytic replication, since in other herpesviruses this gene product functions in DNA packaging during capsid assembly (4). In vivo, only a fraction of spindle cells (2 to 5%) in KS lesions support productive infection as shown by expression of lytic-cycle genes (51, 57) and detection of intranuclear herpesvirus particles (33, 55). To characterize lytic infection in DMVEC in vitro, we evaluated expression of two lytic-cycle-associated proteins encoded by ORF 59 and ORF K8.1A/B in HHV8-infected DMVEC by IFA. ORF 59 encodes a DNA replication accessory protein (9), and ORF K8.1A/B encodes a glycoprotein expressed as a late gene product (10). Nuclear reactivity for ORF 59 was reproducibly detected by 48 h PI and persisted with time and even after multiple tissue culture passages, indicating the maintenance of lytically infected cells within the cell population. The number of cells expressing ORF 59 was initially low (<1%) but increased with time PI, and spontaneous ORF 59 expression was detected in as many as 5% of cells in HHV8-exposed monolayers by 8 weeks PI (Fig. 3a).
FIG. 3.
Expression of lytic-cycle-associated proteins in HHV8-infected DMVEC. (a) Nuclear expression of ORF 59 visualized with MAb 11D1 and a goat anti-mouse FITC conjugate at 1, 4, and 8 weeks PI in HHV8-infected DMVEC cultures that were not induced (No TPA). Cells were subcultured at weekly intervals. The increase in ORF 59-positive cells indicates maintenance of the HHV8 genome and a complete viral replication cycle allowing infection spread. (b) ORF 59 expression in duplicate DMVEC cultures at 1, 4, and 8 weeks PI that had been pretreated with TPA for 48 h (+TPA). (c) ORF 59 expression in HHV8-infected DMVEC at 4 weeks PI without (No Dex) or with (+Dex) pretreatment with dexamethasone for 5 days. Treatment with exogenous induction stimuli (TPA or dexamethasone) increased the percentage of ORF 59-positive cells approximately 10-fold. (d) Expression of glycoprotein ORF K8.1A/B, a late gene product, on the surface of HHV8-infected DMVEC visualized with a MAb against K8.1A/B and a goat anti-mouse FITC conjugate.
In the BCBL-1 cell line in which the majority of cells are latently infected by HHV8, treatment of these cells with chemical stimuli such as TPA or n-butyrate induces lytic replication in up to 40% of cells (10, 26, 49). In order to assess whether latently infected DMVEC were similarly responsive to lytic-cycle induction, DMVEC were treated with TPA, n-butyrate, or the glucocorticoid dexamethasone. A 48-h exposure to TPA (Fig. 3b) or n-butyrate (data not shown) or a 5-day exposure to dexamethasone (Fig. 3c) resulted in a 5- to 10-fold increase in the number of ORF 59-positive cells compared to that in parallel uninduced cultures. However, the percentage of ORF 59-positive cells never exceeded 40% of the induced culture, even when parallel uninduced cultures contained as many as 5% spontaneously ORF 59-positive cells. Thus, similar to PEL cell lines, latently infected DMVEC have the potential to enter the lytic cycle following exposure to the appropriate stimuli, but not every infected cell is responsive to induction. Simultaneous evaluation of ORF 59 and ORF 73 expression demonstrated that, in the absence of exogenous stimuli, lytically infected cells generally represented 0.5 to 5% of the latently infected cell population.
When HHV8-infected DMVEC were evaluated for the presence of the late gene product ORF K8.1A/B following TPA induction, membrane reactivity was readily observed (Fig. 3d), but expression of this glycoprotein was always 5- to 10-fold lower than expression of ORF 59 in parallel cultures. If every ORF 59-positive cell ultimately expresses ORF K8.1A/B, then these results reflect the asynchronous temporal sequence of lytic gene expression within individual cells. Alternately, these observations may indicate that only a fraction of cells expressing early lytic-cycle genes progress to a complete replication cycle.
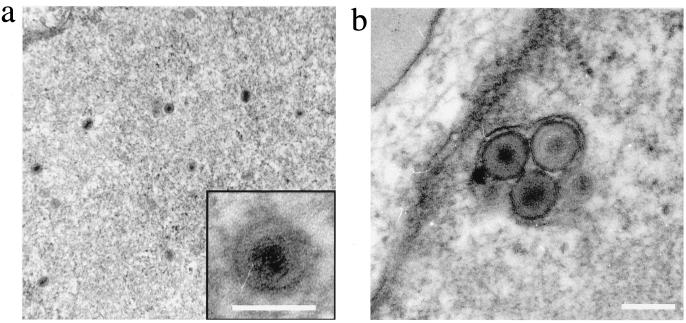
To confirm that DMVEC were permissive for HHV8 replication, transmission electron microscopy was used to detect viral progeny within HHV8-infected DMVEC. Herpesvirus-like structures with diameters of 80 to 100 nm were observed within infected-cell nuclei (Fig. 4a), and particles with diameters of 100 to 120 nm were localized to subcellular compartments that resembled cytoplasmic cisternae (Fig. 4b). Visualization of intracellular virus particles exclusively in HHV8-infected DMVEC confirmed the ability of HHV8 to productively infect these cells.
FIG. 4.
Detection of HHV8 particles in infected DMVEC by electron microscopy. (a) View of a DMVEC nucleus showing herpesvirus-like capsid structures. The insert shows an enlarged view of a single virion (bar = 100 nm). (b) View of a cytoplasmic vesicle containing mature virions sectioned within the nuclear plane (bar = 120 nm).
To determine the ability of infected DMVEC to release infectious virions, cell-free supernatants from infected DMVEC were transferred to uninfected DMVEC and reinfection was evaluated by PCR and IFA. Cells exposed to HHV8-infected DMVEC supernatants expressed HHV8-specific DNA and mRNA transcripts and viral proteins (data not shown), confirming the ability of infected DMVEC to release infectious virus. ORF 73 was expressed in <1% of cells 24 h postexposure to supernatants from uninduced DMVEC, indicating spontaneous production of infectious virus but at a low frequency. In contrast, as many as 10% of cells expressed ORF 73 cells 24 h postexposure to supernatants from TPA-induced DMVEC, reflecting the increased entry of cells into the permissive viral replication cycle. Infection has been successfully transferred over six serial passages to successive naive cultures, suggesting that such transfer could occur indefinitely.
The results presented above indicate that, similar to infection of endothelial and spindle cells in vivo, infection of DMVEC in vitro is primarily latent with only a small fraction of cells expressing lytic-cycle genes and producing infectious viral progeny.
HHV8 infection of DMVEC induces a KS-like cellular phenotype.
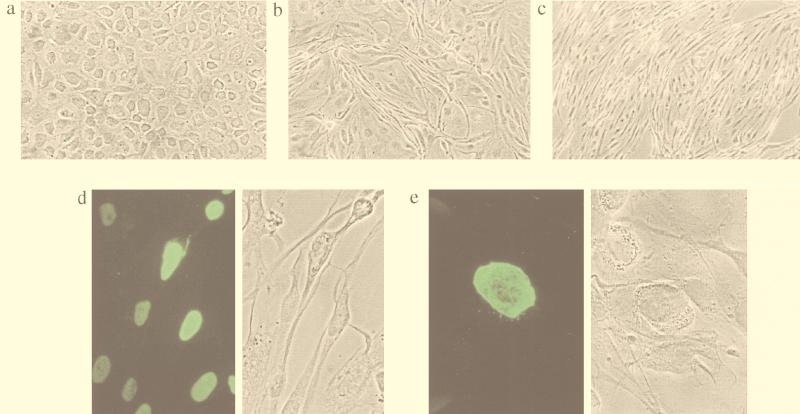
Early KS lesions are characterized by atypical endothelial cells, with spindle cells developing as the disease progresses. The existence of HHV8-infected endothelial cells prior to extensive spindle cell formation strongly supports the hypothesis that endothelial cells are the precursors of spindle cells and that HHV8 infection drives tumorigenesis. Under normal tissue culture conditions, DMVEC display the typical endothelial cobblestone appearance (Fig. 5a). However, following infection with HHV8, cells displaying a spindle shape were observed within DMVEC cultures (Fig. 5b and c). Spindle phenotypes included elongated cells that retained oval cell bodies, elongated cells that were uniformly narrow, and extremely narrow light-refractile cells that displayed scattering. In addition to spindle cells, a percentage of DMVEC exhibiting cell rounding were observed. The extent of phenotypic change within infected-cell monolayers increased with time PI and correlated well with the percentage of HHV8-infected cells. To evaluate the role of direct virus infection on DMVEC morphology, cultures exhibiting foci of phenotypic change were examined by IFA for expression of latent-cycle (ORF 73) and lytic-cycle (ORF 59 and ORF K8.1) HHV8 proteins. Uninfected DMVEC adjacent to infected cells retained cobblestone appearance or were only mildly elongated, implying that while soluble factors produced by infected cells may induce a degree of morphologic change, the marked spindling is a consequence of direct virus infection. While almost all spindle cells were latently infected, as shown by ORF 73 reactivity, not all spindle cells expressed lytic-cycle ORF 59. Expression in spindle cells of a latent gene product but not a gene product essential for viral DNA replication suggested that expression of the latent gene program alone was sufficient to induce morphologic change. However, ORF 59-positive cells demonstrated more-extensive spindling than ORF 73-positive cells and the intensity of ORF 59 expression correlated with the severity of spindling observed. Mildly spindled, ORF 59-positive cells displayed only punctate nuclear reactivity with minimal reactivity throughout the rest of the nucleus, while with enhanced spindling and scattering, the intensity of background nuclear staining increased (Fig. 5d). Cells exhibiting rounding were always strongly ORF 59 positive and importantly, expressed the K8.1A/B glycoprotein (Fig. 5e). The robust expression of this late gene product in rounding cells suggested that cells were rounding as a consequence of a complete viral replication cycle. Electron microscopy studies of KS lesions indicate that a small percentage of infected cells undergo lysis and release of mature virions (33, 55). In DMVEC cultures, cell rounding may represent the stage immediately prior to lysis and release of viral progeny. Every lytically infected cell may progress through stages of spindling and ultimately to rounding and lysis. Alternately, these phenotypes may not represent a predestined sequence but may instead reflect expression of different lytic gene programs.
FIG. 5.
Induction of spindle morphology in DMVEC cultures following HHV8 infection. (a) Phase-contrast microscopy of mock-infected DMVEC, demonstrating the typical cobblestone appearance. (b) Phase-contrast microscopy of HHV8-infected DMVEC at 1 week PI, demonstrating the appearance of cells with a spindle shape within the monolayer. (c) Phase-contrast microscopy of HHV8-infected DMVEC at 4 weeks PI, demonstrating the increase in morphologic change with time PI. (d) Fluorescence (left) and phase-contrast (right) microscopy fields of HHV8-infected DMVEC, demonstrating a correlation between ORF 59 expression and severity of phenotypic change. (e) Fluorescence (left) and phase-contrast (right) microscopy fields, demonstrating strong expression of ORF K8.1A/B in DMVEC exhibiting cell rounding.
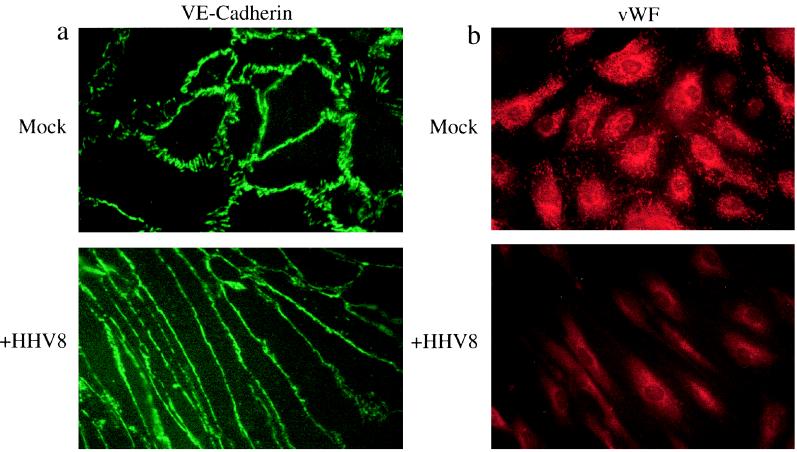
In accordance with an endothelial origin, KS spindle cells express a variety of endothelial cell markers including CD34, VE-cadherin, and CD31 (43, 54). However, spindle cell expression of vWF, a classical marker of endothelial phenotype, is variable or absent (37). In our study, mock-infected DMVEC expressed CD31 (data not shown) and VE-cadherin (Fig. 6a) at cell borders and vWF in Weibel-Palade bodies and perinuclear compartments (Fig. 6b). In HHV8-infected cultures, expression of CD31 (data not shown) and VE-cadherin was maintained at intercellular junctions (Fig. 6c), but vWF was progressively lost from HHV8-infected cultures (Fig. 6d). Expression of ORF 73, but not necessarily ORF 59, in vWF-negative cells indicated that latent infection was sufficient to induce vWF loss (data not shown). These results indicate that following HHV8 infection in vitro, DMVEC develop characteristics such as morphologic change and selective loss of endothelial markers that resemble properties of spindle cells in KS lesions.
FIG. 6.
Expression of endothelial cell phenotypic markers by HHV8-infected DMVEC. (a) IFA demonstrating maintenance of VE-cadherin at cell junctions in spindle-shaped, HHV8-infected DMVEC (+HHV8) and mock-infected, cobblestone DMVEC. CD31 expression was similarly retained following HHV8 infection (data not shown). (b) IFA demonstrating loss of vWF expression in DMVEC cultures following HHV8 infection (+HHV8). In contrast, mock-infected cultures express high levels of vWF in characteristic rod-shaped Weibel-Palade bodies. Cultures were uninduced and were stained at 4 weeks PI when approximately 80% of cells expressed ORF 73 (data not shown).
HHV8 induces growth of DMVEC in soft agar.
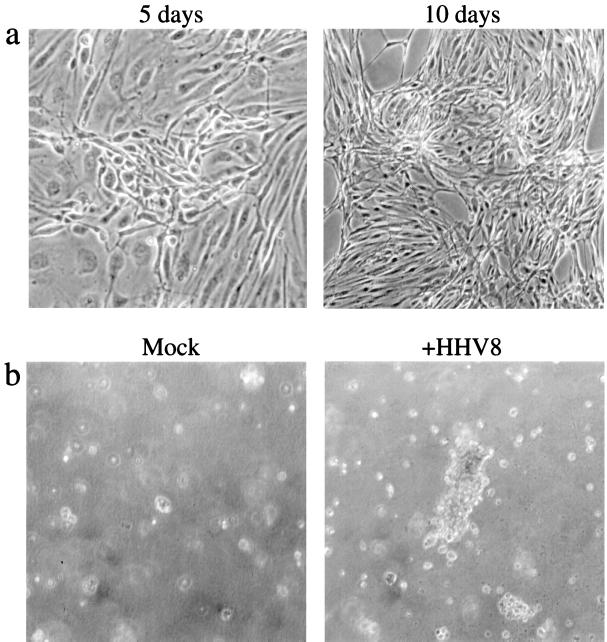
The spindle cell is the distinguishing tumor cell of the KS lesion. A number of HHV8 genes with homology to cellular growth factors and oncogenes have been identified (reviewed in reference 32). These factors and genes include viral interleukin-6 (ORF K2), viral MIP-I (ORF 6) and viral MIP-II (ORF 4), a bcl-2 homolog (ORF 16), an interferon regulatory factor homolog (ORF K9), a viral type D cyclin (ORF 72), and a homolog of a G-protein-coupled receptor (ORF 74). In addition, expression of kaposin from the ORF K12 gene leads to cellular transformation of RAT-3 cells and development of vascular sarcomas in athymic nu/nu mice (31), while the nuclear location and acidic amino acid-rich sequence of ORF 73 suggest that it may function as a regulator of transcription (13). Thus, expression of HHV8 genes may induce cellular transformation. Characteristics of transformed cells in vitro include loss of contact inhibition, as demonstrated by focus formation in monolayer culture, and acquisition of anchorage-independent growth, as demonstrated by the ability to form colonies in soft agar. We thus evaluated HHV8-infected cultures for evidence of a transformed phenotype using these two criteria. Mock-infected cells always maintained a discrete monolayer, even when cultured postconfluency and were unable to grow in soft agar (data not shown). Thus, immortalization by HPV proteins does not confer characteristics of a fully transformed cell. In contrast, when HHV8-infected DMVEC were incubated at postconfluency to allow efficient spread of infection throughout the culture, foci of cells that showed strong expression of HHV8 proteins and displayed loss of contact inhibition were observed (Fig. 7a). In addition, when HHV8-infected DMVEC were plated in soft agar and observed for colony growth, large colonies were observed after 2 weeks (Fig. 7b). In wells where 2 × 103 latently infected cells and no more than 5 × 101 lytically infected cells were seeded, as many as 100 colonies were scored. Since the number of colonies exceeded the number of lytically infected cells seeded, these results suggest that latent infection is sufficient to confer the transformed phenotype.
FIG. 7.
Loss of contact inhibition and growth in soft agar by HHV8-infected DMVEC. (a) Phase-contrast microscopy of an HHV8-infected DMVEC monolayer demonstrating loss of contact inhibition and piling up of cells into foci following culture postconfluency for 5 and 10 days. (b) Phase-contrast microscopy of cultures grown on soft agar 2 weeks after seeding of cells from mock-infected and HHV8-infected (+HHV8) DMVEC. Colonies were formed exclusively by HHV8-infected cells.
DISCUSSION
In this report we describe the extensive, long-term infection of DMVEC with HHV8. This model represents a significant advance over currently available tissue culture systems in which less than 10% of HHV8-challenged cells are infected and infection is not well maintained (15, 16, 34, 40). In KS lesions in vivo, the majority of spindle cells are latently infected with a subpopulation supporting lytic replication (33, 36, 51, 52, 57). Our model accurately represents this viral strategy, as the majority of DMVEC maintained the viral genome in a latent state with only a small number (±5%) of latently infected cells spontaneously entering the lytic cycle. In PEL cell lines, treatment with TPA or n-butyrate induces lytic replication of HHV8 in latently infected cells (10, 26, 49). DMVEC were similarly responsive to these induction stimuli with expression of lytic-cycle proteins in up to 40% of induced cells. Interestingly, the synthetic glucocorticoid dexamethasone was as effective as TPA and n-butyrate for induction of lytic replication in DMVEC. A direct effect of glucocorticoids on the HHV8 replication cycle may contribute to the immunosuppression-related appearance or exacerbation of KS that is documented in patients treated with corticosteroids (19, 53).
The DMVEC used in this study were preimmortalized by HPV E6 and E7 genes to facilitate an extended in vitro life span. While we (data not shown) and others (34) have been able to infect primary DMVEC with HHV8, the number of cells initially infected is low and infection does not spread throughout the culture to yield a high percentage of infected cells. The inability to establish significant productive infection of primary DMVEC may be attributed to their naturally limited life span in culture. Even if infection with HHV8 could confer a growth advantage on primary DMVEC, as described by Flores and colleagues for infection of primary bone marrow endothelial cells (15), the low initial infection rate and limited in vitro passage appear to preclude any growth advantage conferred from the infected cells in the culture. Immortalized DMVEC retain the essential in vitro features of primary cells, but the extended life span facilitates the establishment of infection and also enables the long-term maintenance of age- and passage-matched mock-infected control cells in parallel with HHV8-infected cultures for comparison of HHV8-related phenotypic changes. Following HHV8 infection, DMVEC shape changed from a classical cobblestone to a spindle. In addition, while expression of the endothelial markers CD31 and VE-cadherin was maintained at spindle cell junctions, expression of vWF was lost from infected cells. Importantly, infected DMVEC also acquired features characteristic of a transformed phenotype, including loss of contact inhibition and acquisition of anchorage-independent growth. In these respects, the altered phenotype of DMVEC infected in vitro resembles the phenotype of infected endothelial and spindle cells in vivo and supports the hypothesis that HHV8-infected endothelial cells are the precursors of KS spindle cells.
HHV8 may alter the phenotype of endothelial cells as a direct consequence of infection or as a result of induction of paracrine mechanisms that influence adjacent uninfected cells (14, 15, 45, 46). In our study, mildly spindled uninfected DMVEC were observed in virus-exposed cultures, particularly when culture medium was infrequently changed to allow autologous medium conditioning. Thus, paracrine influences are likely to operate in HHV8-exposed DMVEC cultures, as has been described for HHV8-exposed bone marrow-derived endothelial cell cultures, and similar influences may indeed play an important role in spindle cell formation in vivo. However, the frequent observance of uninfected cobblestone-shaped DMVEC in close proximity to infected spindle-shaped cells strongly suggests a direct influence of HHV8 on the infected cell that cannot be explained by paracrine stimuli alone. Similarly, Flore and colleagues (15) found that direct infection of bone marrow endothelial cells was a prerequisite for HHV8-induced transformation. Viral proteins may induce changes unique to HHV8-infected cells. In addition, cellular proteins may be preferentially upregulated on infected cells, making them more responsive to autocrine and/or paracrine stimuli than adjacent uninfected cells in the identical microenvironment.
In this study, detection in the majority of spindle-shaped DMVEC of latent-cycle, but not lytic-cycle, gene products suggests that latent infection is sufficient to induce phenotypic change. The significance of lytic replication for KS pathogenesis remains to be fully established. Lytic replication would promote expansion of virus infection, but the extent to which lytic gene expression is compatible with cellular proliferation or transformation is unclear. The mechanisms that determine the progression of the HHV8 life cycle from latency to lytic replication in vivo remain to be determined, but presumably there are naturally occurring immunologic stimuli with the capacity to activate lytic replication, as has been shown for chemically induced lytic-cycle induction in PEL lines in vitro (39, 57). Such stimuli likely account for the dynamic nature of the disease in AIDS patients with KS and KS patients undergoing iatrogenic immunosuppression (19, 27, 53). As the HHV8 genome in our DMVEC model is responsive to induction stimuli, this model should assist in the identification of viral and cellular genes that control switching from latent to lytic replication as well as elucidation of immunologic or drug-induced stimuli that could induce or exacerbate this process.
In conclusion, we have developed a tissue culture model for the study of HHV8 and KS which represents a significant advance over current systems for a number of reasons. First, as KS manifests primarily as a skin disease, dermis-derived endothelial cells represent a relevant cell type for evaluation of HHV8 pathogenesis. Second, viral infection accurately reflects the pattern seen in vivo with the majority of cells within the culture being latently infected and a small percentage spontaneously entering the lytic cycle. Third, infected cultures undergo phenotypic changes including spindle cell morphology and characteristics of a transformed phenotype that resemble stages of KS tumorigenesis. We have been able to maintain infected cultures for over a year with multiple tissue culture passages. The ability to routinely passage infected cultures and maintain HHV8 suggests that infected cultures should be indefinitely maintained. Thus, these cells should prove invaluable for the study of the virus life cycle and gene expression patterns inside endothelial cells as well as allowing elucidation of cellular and viral mechanisms involved in tumorigenesis.
ACKNOWLEDGMENTS
We thank Michael Jarvis and Randy Taplitz for helpful discussions on the manuscript and Andrew Townsend for assistance with graphics.
This work was supported in part by a grant from Viral Partners L.L.C. (J.A.N.), Public Health Service grant CA-75911 (B.C.), grant KUMC RI-8906 (B.C.), and a grant from the KUEA-Ernst F. Lied Fund (B.C.).
REFERENCES
- 1.Aluigi M G, Albini A, Carlone S, Repetto L, De Marchi R, Icardi A, Moro M, Noonan D, Benelli R. KSHV sequences in biopsies and cultured spindle cells of epidemic, iatrogenic and Mediterranean forms of Kaposi’s sarcoma. Res Virol. 1996;147:267–275. doi: 10.1016/0923-2516(96)82285-0. [DOI] [PubMed] [Google Scholar]
- 2.Ambroziak J A, Blackbourn D J, Herndier B G, Glougau R G, Gullet J H, McDonald A R, Lennette E T, Levy J A. Herpes-like sequences in HIV-infected and uninfected Kaposi’s sarcoma patients. Science. 1995;268:582–583. doi: 10.1126/science.7725108. [DOI] [PubMed] [Google Scholar]
- 3.Arvanitakis L, Mesri E A, Nador R G, Said J W, Asch A S, Knowles D M, Cesarman E. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;88:2648–2654. [PubMed] [Google Scholar]
- 4.Baines J D, Poon A D, Rovnak J, Roizman B. The herpes simplex virus 1 UL15 gene encodes two proteins and is required for cleavage of genomic viral DNA. J Virol. 1994;68:8118–8124. doi: 10.1128/jvi.68.12.8118-8124.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becksted J H, Woods G S, Fletcher V. Evidence for the origin of Kaposi’s sarcoma from lymphatic endothelium. Am J Pathol. 1985;119:294–300. [PMC free article] [PubMed] [Google Scholar]
- 6.Boshoff C, Schulz T F, Kennedy M M, Graham A K, Fisher C, Thomas A, McGee J O, Weiss R A, O’Leary J J. Kaposi’s sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–1278. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 7.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 8.Cesarman E, Moore P S, Rao P H, Inghirami G, Knowles D M, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 9.Chan S R, Bloomer C, Chandran B. Identification and characterization of human herpesvirus-8 lytic cycle-associated ORF 59 protein and the encoding cDNA by monoclonal antibody. Virology. 1998;240:118–126. doi: 10.1006/viro.1997.8911. [DOI] [PubMed] [Google Scholar]
- 10.Chandran B, Bloomer C, Chan S R, Zhu L, Goldstein E, Horvat R. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology. 1998;249:140–149. doi: 10.1006/viro.1998.9316. [DOI] [PubMed] [Google Scholar]
- 11.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 12.Corbeil J, Evans L A, Vasak E, Cooper D A, Penney R. Culture and properties of cells derived from Kaposi’s sarcoma. J Immunol. 1991;146:2972–2976. [PubMed] [Google Scholar]
- 13.Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. A cluster of latency expressed genes in Kaposi’s sarcoma-associated herpesvirus. J Virol. 1998;72:8309–8315. doi: 10.1128/jvi.72.10.8309-8315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ensoli B, Nakamura S, Salahuddin S Z, Biberfeld P, Larsson L, Beaver B, Wong-Staal F, Gallo R C. AIDS-Kaposi’s sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science. 1989;243:223–226. doi: 10.1126/science.2643161. [DOI] [PubMed] [Google Scholar]
- 15.Flore O, Rafii S, Ely S, O’Leary J J, Hyjek E M, Cesarman E. Transformation of primary human endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Nature. 1998;394:588–592. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- 16.Foreman K E, Friborg J, Jr, Wong W P, Woffendin C, Polverini P J, Nickloff B J, Nabel G J. Propagation of a human herpesvirus from AIDS-associated Kaposi’s sarcoma. N Engl J Med. 1997;336:163–171. doi: 10.1056/NEJM199701163360302. [DOI] [PubMed] [Google Scholar]
- 17.Gao S-J, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, Newton R, Rinaldo C R, Saah A, Phair J, Detels R, Chang Y, Moore P S. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2:925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 18.Gao S-J, Kingsley L, Hoover D R, Spira T J, Rinaldo C R, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore P S. Seroconversion to antibodies against Kaposi’s sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi’s sarcoma. N Engl J Med. 1996;335:233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 19.Gill P S, Loureiro C, Bernstein-Singer M, Rarick M U, Sattler F, Levine A M. Clinical effect of glucocorticoids on Kaposi’s sarcoma related to the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1989;110:937–940. doi: 10.7326/0003-4819-110-11-937. [DOI] [PubMed] [Google Scholar]
- 20.Halbert C L, Demers G W, Galloway D A. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991;65:473–478. doi: 10.1128/jvi.65.1.473-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y Q, Li J J, Kaplan M H, Poiesz B, Katabira E, Zhang W C, Feiner D, Freidman-Kein A E. Human herpesvirus-like nucleic acid in various forms of Kaposi’s sarcoma. Lancet. 1995;345:759–761. doi: 10.1016/s0140-6736(95)90641-x. [DOI] [PubMed] [Google Scholar]
- 22.Jussila L, Valtola R, Partanen T A, Salven P, Heikkila P, Matikainen M T, Renkonen R, Kalpainen A, Detmar M, Tschachler E, Alitalo R, Alitalo K. Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 1998;58:1599–1604. [PubMed] [Google Scholar]
- 23.Kedes D H, Oberskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): Distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2:918–924. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy M M, Cooper K, Howells D D, Picton S, Biddolph S, Lucas S B, McGee J O, O’Leary J J. Identification of HHV8 in early Kaposi’s sarcoma: implications for Kaposi’s sarcoma pathogenesis. Mol Pathol. 1997;51:14–20. doi: 10.1136/mp.51.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebbe C, de Cremoux P, Rybojad M, Costa da Cunha C, Morel P, Calvo F. Kaposi’s sarcoma and new herpesvirus. Lancet. 1995;345:1180. doi: 10.1016/s0140-6736(95)91011-5. [DOI] [PubMed] [Google Scholar]
- 26.Lennette E T, Blackbourne D J, Levy J A. Antibodies to human herpesvirus type 8 in the general population and in Kaposi’s sarcoma patients. Lancet. 1996;348:858–861. doi: 10.1016/S0140-6736(96)03240-0. [DOI] [PubMed] [Google Scholar]
- 27.Levy J A, Ziegler J L. Acquired immunodeficiency syndrome is an opportunistic infection and Kaposi’s sarcoma results from secondary immune stimulation. Lancet. 1983;2:78–81. doi: 10.1016/s0140-6736(83)90062-4. [DOI] [PubMed] [Google Scholar]
- 28.Li J J, Huang Y Q, Cockerell C J, Friedman-Klein A E. Localization of human herpes-like virus type 8 in vascular endothelial cells and perivascular spindle-shaped cells of Kaposi’s sarcoma lesions by in situ hybridization. Am J Pathol. 1996;148:1741–1748. [PMC free article] [PubMed] [Google Scholar]
- 29.Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov V M, Grossberg S, Chang Y. Selective switch between latency and lytic replication of Kaposi’s sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J Virol. 1997;71:314–324. doi: 10.1128/jvi.71.1.314-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore P S, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and without HIV infection. N Engl J Med. 1995;332:1181–1185. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 31.Muralidhar S, Pumfery A M, Hassani M, Sadaie M R, Azumi N, Kishishita M, Brady J N, Doniger J, Medveczky P, Rosenthal L J. Identification of kaposin (open reading frame K12) as a human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus) transforming gene. J Virol. 1998;72:4980–4988. doi: 10.1128/jvi.72.6.4980-4988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neipel F, Albrecht J-C, Fleckenstein B. Cell-homologous genes in the Kaposi’s sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity. J Virol. 1997;71:4187–4192. doi: 10.1128/jvi.71.6.4187-4192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orenstein J M, Alkan S, Blauvelt A, Jeang K T, Weinstein M D, Ganem D, Herndier B. Visualization of human herpesvirus type 8 in Kaposi’s sarcoma by light and transmission electron microscopy. AIDS. 1997;11:F35–F45. doi: 10.1097/00002030-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Panyutich E A, Said J W, Miles S A. Infection of primary dermal microvascular endothelial cells by Kaposi’s sarcoma-associated herpesvirus. AIDS. 1998;12:467–472. doi: 10.1097/00002030-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Prudhomme J G, Sherman I W, Land K M, Moses A V, Stenglein S, Nelson J A. Studies of Plasmodium falciparum cytoadherence using immortalized human brain capillary endothelial cells. Int J Parasitol. 1996;26:647–655. doi: 10.1016/0020-7519(96)00027-6. [DOI] [PubMed] [Google Scholar]
- 36.Rainbow L, Platt G M, Simpson G R, Sarid R, Gao S-J, Stoiber H, Herrington C S, Moore P S, Schulz T F. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J Virol. 1997;71:5915–5921. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rappersberger K, Tschachler E, Zonzits E, Gillitzer R, Hatzakis A, Kaloterakis A, Mann D L, Popow-Kraupp T, Biggar R J, Berger R, Stratigos J, Wolff K, Stingl G. Endemic Kaposi’s sarcoma in human immunodeficiency virus type 1-seronegative persons: demonstration of retrovirus-like particles in cutaneous lesions. J Investig Dermatol. 1990;95:371–381. doi: 10.1111/1523-1747.ep12555450. [DOI] [PubMed] [Google Scholar]
- 38.Regezi J A, MacPhail L A, Daniels T E, Greenspan J S, Dodd C L, Lozada-Nur F, Heinic G S, Chinn H, Silverman S, Hansen L S. Oral Kaposi’s sarcoma: a 10-year retrospective histopathological study. J Oral Pathol Med. 1993;22:292–297. doi: 10.1111/j.1600-0714.1993.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 39.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 40.Renne R, Blackbourn D, Whitby D, Levy J, Ganem D. Limited transmission of Kaposi’s sarcoma-associated herpesvirus in cultured cells. J Virol. 1998;72:5182–5188. doi: 10.1128/jvi.72.6.5182-5188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roth W K, Werner S, Risau W, Remberger K, Hofschneider P H. Cultured, AIDS-related Kaposi’s sarcoma cells express endothelial cell markers and are weakly malignant in vitro. Int J Cancer. 1988;42:767–773. doi: 10.1002/ijc.2910420523. [DOI] [PubMed] [Google Scholar]
- 42.Roth W K, Brandsetter H, Sturzl M. Cellular and molecular features of HIV-associated Kaposi’s sarcoma. AIDS. 1992;6:895–913. doi: 10.1097/00002030-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Russell Jones R, Orchard G, Zelger B, Wilson Jones E. Immunostaining for CD31 and CD34 in Kaposi’s sarcoma. J Clin Pathol. 1995;48:1011–1016. doi: 10.1136/jcp.48.11.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutgers J L, Wieczorek R, Bonetti F, Kaplan K L, Posnett D N, Friedman-Klein A E, Knowles D M. The expression of endothelial cell surface antigens by AIDS-associated Kaposi’s sarcoma. Evidence for a vascular endothelial cell origin. Am J Pathol. 1986;122:493–499. [PMC free article] [PubMed] [Google Scholar]
- 45.Salahuddin S Z, Nakamura S, Biberfeld P, Kaplan M H, Markham P D, Larsson L, Gallo R C. Angiogenic properties of Kaposi’s sarcoma-derived cells after long-term culture in vitro. Science. 1988;242:430–433. doi: 10.1126/science.2459779. [DOI] [PubMed] [Google Scholar]
- 46.Samaniego F, Markham P D, Gendleman R, Watanabe Y, Kao V, Kowalski K, Sonnabend J A, Pintus A, Gallo R C, Ensoli B. Vascular endothelial growth factor and basic fibroblast growth factor present in Kaposi’s sarcoma (KS) are induced by inflammatory cytokines and synergize to promote vascular permeability and KS lesion development. Am J Pathol. 1998;152:1433–1443. [PMC free article] [PubMed] [Google Scholar]
- 47.Schalling M, Ekman M, Kaaya E E, Linde A, Biberfeld P. A role for a new herpes virus (KSHV) in different forms of Kaposi’s sarcoma. Nat Med. 1995;1:707–708. doi: 10.1038/nm0795-707. [DOI] [PubMed] [Google Scholar]
- 48.Scully P A, Steinman H K, Kennedy C, Trueblood K, Frisman D M, Voland J R. AIDS-related Kaposi’s sarcoma displays differential expression of endothelial surface antigens. Am J Pathol. 1988;130:244–251. [PMC free article] [PubMed] [Google Scholar]
- 49.Smith M S, Bloomer C, Horvat R, Goldstein E, Casparian J M, Chandran B. Detection of human herpesvirus 8 DNA in Kaposi’s sarcoma lesions and peripheral blood of human immunodeficiency virus-positive patients and correlation with serologic measurements. J Infect Dis. 1997;176:84–93. doi: 10.1086/514043. [DOI] [PubMed] [Google Scholar]
- 50.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay M-F, Clauvel J-P, Raphael M, Degos L, Sigaux F. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 51.Staskus K A, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson D J, Ganem D, Haase A T. Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sturzl M, Blasig C, Schreier A, Neipel F, Hohenadl C, Cornali E, Ascherl G, Esser S, Brockmeyer N H, Ekman M, Kaaya E E, Tschachler E, Biberfeld P. Expression of HHV-8 latency-associated T0.7 RNA in spindle cells and endothelial cells of AIDS-associated, classical and African Kaposi’s sarcoma. Int J Cancer. 1997;72:68–71. doi: 10.1002/(sici)1097-0215(19970703)72:1<68::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 53.Trattner A, Hodak E, David M, Sandbeck M. The appearance of Kaposi’s sarcoma during corticosteroid therapy. Cancer. 1993;72:1779–1783. doi: 10.1002/1097-0142(19930901)72:5<1779::aid-cncr2820720543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 54.Uccini S, Ruco L P, Monardo F, Stoppacciaro A, Dejana E, La Parola I L, Cerimele D, Baroni C D. Co-expression of endothelial cell and macrophage antigens in Kaposi’s sarcoma cells. J Pathol. 1994;173:23–31. doi: 10.1002/path.1711730105. [DOI] [PubMed] [Google Scholar]
- 55.Walter P R, Philippe E, Nguemby-Mbina C, Chamlian A. Kaposi’s sarcoma: presence of herpes-type virus particles in a tumor specimen. Hum Pathol. 1984;15:1145–1146. doi: 10.1016/s0046-8177(84)80309-3. [DOI] [PubMed] [Google Scholar]
- 56.Whitby D, Howard M R, Tenant-Flowers M, Brink N S, Copas A, Boshoff C, Hatzioannou T, Sugget F E A, Aldam D M, Denton A S, Miller R F, Weller I V D, Weiss R A, Tedder R S, Schulz T F. Detection of Kaposi’s sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet. 1995;346:799–800. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- 57.Zhong W, Wang H, Herndier B, Ganem D. Restricted expression of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi’s sarcoma. Proc Natl Acad Sci USA. 1996;93:6641–6646. doi: 10.1073/pnas.93.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]