Abstract
Background: Psoriasis is an immune-mediated chronic disorder associated with various comorbidities. Even though biologics and small-molecule inhibitors are the mainstay treatment for moderate-to-severe psoriasis, there is no current consensus regarding which agent should be used for a specific type of patient. This paper aims to test the reliability of blood-count-derived inflammatory markers in assessing treatment response to biologics and small-molecule inhibitors in psoriasis. Material and Methods: Bio-naïve adult patients diagnosed with chronic plaque psoriasis fulfilling the inclusion criteria were enrolled. They were divided into study subgroups based on treatment of choice, and blood-count-derived inflammatory markers were analyzed at baseline, three-month, six-month, and at twelve-month visits. Results: A total of 240 patients were included. The highest number of patients underwent treatment with ixekizumab. The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), platelet-to-monocyte ratio (PMR), monocyte-to-lymphocyte ratio (MLR), derived neutrophil-to-lymphocyte ratio (d-NLR), systemic inflammation response index (SIRI), systemic immune inflammation index (SII), and aggregate index of systemic inflammation (AISI) all varied significantly (p < 0.005) between the four visits. The psoriasis area severity index (PASI) score correlated with PLR, d-NLR, and SII, while the psoriasis scalp severity index (PSSI) score correlated with AISI and SIRI. More than half of patients reached the target goal of PASI90 at the six-month visit. A total of 77 patients were super-responders, with the highest number undergoing treatment with ixekizumab. Higher baseline values of d-NLR and SIRI are independent predictors of the super-responder status. Conclusions: Blood-count-derived inflammatory markers can serve as indicators of treatment response to biologics in psoriasis, while d-NLR and SIRI were independent predictors of super-responders in our study.
Keywords: biologics, psoriasis, noninvasive, inflammation, blood count
1. Introduction
Psoriasis is an immune-mediated chronic disorder that significantly impacts patients’ quality of life and is frequently associated with various comorbidities. The most frequent clinical type, chronic plaque psoriasis, is linked to cardiovascular disorders, such as hypertension, myocardial infarction, or atherosclerosis, metabolic syndrome, diabetes mellitus, and depression [1]. Moreover, patients with psoriasis seem to have an increased risk of presenting with associated nonalcoholic fatty liver disease (NAFLD) [2], most likely due to the associated metabolic syndrome and obesity. Also, the hepato-dermal axis was recently described and proposed as being involved in the pathogenesis of this comorbidity [3].
Psoriasis etiopathogenesis involves a complex interplay of genetic, environmental, and immune factors. A continuous interaction between dendritic cells, various subsets of T cells, and keratinocytes leads to an increased inflammatory state in psoriasis, defined by high levels of interleukin (IL)-12, IL-23, IL-17, and tumor necrosis factors (TNF)α [4].
Taking into account lesions’ localization and extension, various treatment options are available for psoriasis. Biologics and small-molecule inhibitors targeting altered immune pathways are currently the gold standard for treating moderate to severe psoriasis. When choosing the optimal treatment, patients’ clinical and biological profiles, including comorbidities, should also be taken into account. A comprehensive, multidimensional approach to patients diagnosed with psoriasis is needed, focusing on skin lesions and all aspects of this disease. A proper choice of treatment would prove useful in addressing not only cutaneous lesions but also associated comorbidities. However, there is no current consensus regarding which agent should be used for a specific type of patient.
In recent years, various blood-count-derived markers have emerged as reliable and easily obtainable markers for systemic inflammation. Apart from being useful in appreciating the evolution and outcome of chronic obstructive pulmonary disorder (COPD) [4], various cancers [5,6,7] and cardiac disorders [8,9] they were also proven to be indicators of activity disease in dermatological disorders. Their utility has been reported so far in bullous disorders [10], hidradenitis suppurativa [11], and atopic dermatitis [12,13]. On the other hand, conflicting data are reported for acne [14,15].
Current evidence of psoriasis focuses mainly on two hematological parameters: the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR). Other parameters, such as the monocyte-to-lymphocyte ratio or the derived neutrophil-to-lymphocyte ratio (d-NLR) [16], have also been investigated. Even fewer papers have analyzed composite markers, such as the systemic immune index (SII), systemic immune response index (SIRI), and aggregate index of systemic inflammation (AISI) [16,17].
Blood-count-derived inflammatory biomarkers have not been extensively studied regarding biological treatment in psoriasis. Data referring to composite markers in this matter are scarce [18], and, to the best of our knowledge, the reliability of AISI and d-NLR has not been assessed so far regarding treatment response to biologics.
2. Materials and Methods
2.1. Study Population
This is a multicentric retrospective study performed on adult patients diagnosed with chronic plaque psoriasis in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Mures Clinical County Hospital (no. 3770/05.04.2023). Bio-naïve patients diagnosed with chronic plaque psoriasis originating from Dermatology Departments of various hospitals (Mures Clinical County Hospital; Cluj Napoca Emergency Clinical County Hospital; Agrippa Ionescu Emergency Clinical Hospital of Bucharest; Sfantul Spiridon Emergency Clinical County Hospital of Iasi, Elias Emergency University Clinical Hospital Bucharest; Emergency Clinical County Hospital of Bistrita Nasaud; Town Hospitals of Odorheiu Secuiesc and Reghin) were enrolled in this study.
Bio-naïve patients undergoing any biological or small-molecule inhibitors treatment were included in this study if complete, appropriate data were available at treatment initiation, at the three-month, six-month, and twelve-month follow-up. Data regarding demographics, comorbidities, disease activity, and laboratory parameters were collected for enrolled patients. Disease activity was assessed using the Psoriasis Area Severity Index (PASI) score, and when special sites were involved, the Nail Psoriasis Area Severity Index (NAPSI) and the Psoriasis Scalp Severity Index (PSSI) were used. Concomitant psoriatic arthritis was also checked for. Pediatric patients, those with incomplete data, and those benefiting from a therapeutical switch at a certain point were excluded from the analysis. Patients’ data were collected using the hospitals’ electronic databases.
2.2. Blood Analysis and Biomarkers
Blood samples were obtained by venipuncture from the upper extremities, more specifically the median cubital vein; when it was not reachable, the cephalic or basilic veins were used. Whole blood venous samples were collected in the morning after an overnight fast for all patients, following each hospital’s drawing blood protocol. Next, the obtained samples were analyzed by spectrophotometry or flow cytometry using an automatic hematology analyzer. Laboratory investigations were performed as per the requirements of our National Psoriasis Protocol, which states that bloodwork should be carried out in psoriatic patients receiving biologics as follows: before the initial visit and three months, six months, and twelve months after starting biologics. Next, a complete clinical and biological assessment of patients would be made every six months as long as the patient receives treatment.
The following blood-count-derived inflammatory markers were calculated and further analyzed for all patients: neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), platelet-to-monocyte ratio (PMR), monocyte-to-lymphocyte ratio (MLR), derived neutrophil-to-lymphocyte ration (d-NLR), systemic inflammation response index (SIRI), systemic immune index (SII), and aggregate index of systemic inflammation (AISI). The equations used are depicted in Table 1.
Table 1.
Biomarkers formulas.
| Marker | Formula |
|---|---|
| NLR | Neutrophil count/lymphocyte count [×103/μL] |
| PLR | Platelet count/lymphocyte count [×103/μL] |
| PMR | Platelet count/monocyte count [×103/μL] |
| MLR | Monocyte count/lymphocyte count [×103/μL] |
| d-NLR | Neutrophil count/(WBC-neutrophil count) [×103/μL] |
| SIRI | (Neutrophil count × monocyte count)/lymphocyte count [×103/μL] |
| SII | (Neutrophil count × platelet count)/lymphocyte count [×103/μL] |
| AISI | (Neutrophil count × monocyte count x platelet count)/lymphocyte count [×103/μL] |
NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PMR, platelet-to-monocyte ratio; MLR, monocyte-to-lymphocyte ratio; d-NLR, derived neutrophil-to-lymphocyte ratio; SIRI, systemic inflammation response index; SII, systemic immune inflammation index; AISI, aggregate index of systemic inflammation.
2.3. Treatment Response Definition
The following treatment target goals were established: PASI 75 at the three-month hallmark, PASI 90 at the six-month follow-up visit, and PASI 100 at the 12-month visit. Patients achieving PASI 100 at the six-month visit were considered super-responders. Based on whether treatment target goals were met or not, patients were afterward divided into study subgroups and further analyzed.
2.4. Study Outcomes
This paper primarily aims to establish whether blood-count-derived inflammatory markers may serve as predictors of treatment response to biologics. Second, we aimed to identify independent prognostic factors for treatment response and to contour the bio-humoral profile of patients with a proper and sustained favorable response to biologics. To the best of our knowledge, this is the first study to evaluate the usefulness of AISI and d-NLR as blood-count-derived inflammatory markers impacted by biologics and small-molecule inhibitors in psoriasis.
2.5. Statistical Analysis
The MedCalc Statistic software for Windows, version 22.023, was used for the statistical analysis. After assessing normality using the Shapiro–Wilk test, categorical variables are expressed as absolute values and percentages. In contrast, continuous variables are depicted using the median or mean values with standard derivations (SD). The Mann–Whitney test for continuous variables or, if referring to categorical variables, the chi-square test was used to evaluate differences between study groups. One-way ANOVA was used to compare data between study groups if the normal distribution was met, and the Kruskal–Wallis test was used for non-normally distributed data. Spearman’s rank correlation coefficient was used to evaluate correlations, while multiple logistic regression was run to identify independent predictors of response to biologics. A p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Patients’ Clinical Profile
A total of 240 out of 289 eligible patients fulfilled all inclusion criteria and were enrolled in this study. The study group included mainly males (n = 143) and had a mean age of 50.76 ± 14.35 years. Male patients had significantly higher BMI than females (p = 0.04), but no differences were noted regarding age (p = 0.68). Forty-five patients were diagnosed with psoriatic arthritis, while 103 presented with cardiovascular diseases, such as hypertension or chronic ischemic cardiomyopathy. The highest number of patients were on ixekizumab (n = 61), followed by risankizumab (n = 44) and secukinumab (n = 41). Sociodemographics and treatment of choice are highlighted in Table 2.
Table 2.
Demographic characteristics and treatment of choice of the patients.
| Parameter | p-Value | |
|---|---|---|
| Age (n = 240) | 50.76 ± 14.35 | |
| Male | 51.30 ± 13.96 | 0.68 |
| Female | 49.96 ± 14.93 | |
| Gender | ||
| Male | 143 | |
| Female | 97 | |
| BMI (n = 240) | 28.89 ± 18.86 | |
| Male | 29.23 ± 17.79 | 0.04 |
| Female | 28.40 ± 20.43 | |
| Psoriatic arthritis (n = 240) | 44 | |
| Male | 31 | |
| Female | 13 | |
| Treatment of choice | ||
| Adalimumab | 22 | |
| Etanercept | 18 | |
| Infliximab | 6 | |
| Certolizumab | 6 | |
| Ixekizumab | 61 | |
| Secukinumab | 41 | |
| Tildrakizumab | 11 | |
| Risankizumab | 44 | |
| Guselkumab | 10 | |
| Ustekinumab | 17 | |
| Apremilast | 4 |
BMI, body mass index.
No statistically significant differences were noted between the four visits (initiation, three-month, six-month, and twelve-month hallmarks) regarding platelet (p = 0.445), neutrophil (p = 0.120), and lymphocyte (p = 0.414) count. Regarding the other analyzed parameters, their values varied in dynamics significantly from visit to visit, as seen in Table 3. Activity scores, PASI, PSSI, and NAPSI varied significantly between visits (p < 0.001).
Table 3.
Laboratory characteristics of the study population across visits.
| Variables | Initial Visit | 3-Month Follow-Up | 6-Month Follow-Up | 12-Month Follow-Up | p-Value |
|---|---|---|---|---|---|
| WBC | 7.12 [6.82–7.32] | 6.87 [6.66–7.04] | 6.81 [6.47–7] | 6.65 [6.48–6.91] | 0.012 |
| Platelets | 247.35 [235.66–256.33] | 242.55 [234–253.66] | 242 [232.33–253.33] | 247 [235.33–251.67] | 0.445 |
| Neutrophils | 4.32 [4.12–4.59] | 4.10 [3.95–4.31] | 3.88 [3.70–4.13] | 3.90 [3.72–4.09- | 0.120 |
| Lymphocytes | 2.02 [1.90–2.12] | 2 [1.93–2.08] | 2.09 [1.99–2.20] | 2.12 [2.10–2.25] | 0.414 |
| Monocytes | 0.48 [0.45–0.50] | 0.50 [0.47–0.52] | 0.48 [0.44–0.51] | 0.50 [0.48–0.53] | 0.016 |
| PLR | 122.54 [115.81–127.92] | 120.28 [112.86–125.72] | 117.72 [109.38–123.36] | 116 [108.81–121.73] | <0.001 |
| NLR | 2.15 [2.01–2.26] | 2.05 [1.84–2.12] | 1.82 [1.73–1.95] | 1.82 [1.70–1.93] | <0.001 |
| d-NLR | 1.57 [1.47–1.65] | 1.42 [1.32–1.48] | 1.39 [1.29–1.47] | 1.38 [1.31–1.48] | <0.001 |
| MLR | 0.24 [0.23–0.26] | 0.25 [0.23–0.27] | 0.23 [0.22–0.25] | 0.24 [0.23–0.25] | <0.001 |
| PMR | 526.32 [491.32–558.69] | 490 [474.01–518.55] | 496.72 [466.67–529.65] | 483.77 [450–533.69] | <0.001 |
| SII | 549.70 [509.36–591.07] | 488.51 [457.35–524.22] | 450.56 [418.62–475.69] | 447.26 [410.83–491.39] | <0.001 |
| SIRI | 1.02 [0.94–1.10] | 0.99 [0.92–1.09] | 0.90 [0.84–0.98] | 0.90 [0.85–0.98] | <0.001 |
| AISI | 254.76 [227.20–278.32] | 242.48 [214.09–279.76] | 214.68 [194.11–238.79] | 241.48 [212.09–277.76] | <0.001 |
| ESR | 12 [10–13] | 9 [8–10] | 9 [8–10.13] | 10 [8–11] | <0.001 |
| Nail involvement (n) | 20 | 14 | 14 | 12 | 0.493 |
| Scalp involvement (n) | 42 | 15 | 15 | 15 | <0.001 |
| Palmoplantar involvement (n) | 18 | 12 | 12 | 10 | 0.428 |
| PASI | 20 [19–20.5] | 1.20 [1–1.8] | 6.6 [5.5–7.5] | 1.56 [1.04–2.09] | <0.001 |
| PSSI | 7.12 [4.78–9.47] | 0.48 [0.19–0.78] | 0.91 [0.32–1.49] | 0.09 [0.015–0.17] | <0.001 |
| NAPSI | 4.97 [2.23–7.70] | 1.49 [0.38–2.61] | 1.08 [0.34–1.84] | 0.45 [0.13–0.77] | <0.001 |
NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PMR, platelet-to-monocyte ratio; MLR, monocyte-to-lymphocyte ratio; d-NLR, derived neutrophil-to-lymphocyte ratio; SIRI, systemic inflammation response index; SII, systemic immune inflammation index; AISI, aggregate index of systemic inflammation; ESR, erythrocyte sedimentation rate; PASI, psoriasis area severity index; PSSI, psoriasis scalp severity index; NAPSI, nail psoriasis severity index.
3.2. Serological Markers Variation and Disease Severity Evolution
Next, we analyzed whether there is any association (Table 4) between disease severity during the analyzed period of time and the aforementioned blood-count-derived inflammatory markers. As the duration of treatment increased, PLR, NLR, d-NLR, SII, and SIRI decreased. Moreover, as the PASI score decreased, so too did the values of WBC (p = 0.006), PLR (p = 0.013), d-NLR (p = 0.008), and ESR (p = 0.045). PSSI values correlated significantly positively with AISI (p = 0.038) and SIRI (p = 0.028). No correlation was noted between the NAPSI score and the analyzed biomarkers.
Table 4.
Correlation between serological markers and disease severity.
| PASI | PSSI | ||||
|---|---|---|---|---|---|
| Marker | rho | p-Value | Marker | rho | p-Value |
| WBC | 0.175 | 0.006 | AISI | 0.198 | 0.038 |
| PLR | 0.159 | 0.013 | SIRI | 0.209 | 0.028 |
| D-NLR | 0.234 | 0.034 | |||
| SII | 0.178 | 0.008 | |||
| ESR | 0.130 | 0.045 | - | ||
PLR, platelet-to-lymphocyte ratio; D-NLR, derived neutrophil-to-lymphocyte ratio; SIRI, systemic inflammation response index; SII, systemic immune inflammation index; AISI, aggregate index of systemic inflammation; ESR, erythrocyte sedimentation rate; PASI, psoriasis area severity index; PSSI, psoriasis scalp severity index.
3.3. Blood-Count-Derived Inflammatory Markers’ Variation Based on the Type of Biologic Used
For patients treated with TNF-α inhibitors, significant variations in blood-count-derived inflammatory markers were noted between the four analyzed periods of time in terms of d-NLR (adalimumab; p = 0.014) and AISI (certolizumab; p = 0.04). If referring to IL-17 inhibitors, notable differences were observed for NLR (both ixekizumab and secukinumab; p < 0.05), SIRI (ixekizumab; p-0.008), MLR (secukinumab; p = 0.008) and AISI (secukinumab; p = 0.008). For the anti-IL-23 agents, risankizumab led to a significant variation in NLR (p = 0.04) during the course of treatment, while guselkumab impacted both NLR (p = 0.02) and SII (p = 0.04). A comprehensive overview of the analyzed parameters variation during the course of treatment with the analyzed agents is depicted in Table 5.
Table 5.
Comparison of blood-count-derived inflammatory markers during treatment course based on the treatment choice.
| Variables * | Initial Visit | 3-Month Follow-Up | 6-Month Follow-Up | 12-Month Follow-Up | p-Value |
|---|---|---|---|---|---|
| Adalimumab | |||||
| PLR | 123.01 [92.79–138.75] | 106.84 [83.49–128.04] | 98.34 [77.34–116.34] | 92.26 [75.92–108.42] | 0.98 |
| NLR | 2.20 [1.85–2.38] | 1.58 [1.34–2.21] | 1.42 [1.20–1.65] | 1.35 [1.17–1.86] | 0.428 |
| d-NLR | 1.49 [1.27–1.65] | 1.12 [0.92–1.28] | 1.11 [0.94–1.29] | 1.08 [0.94–1.22] | 0.014 |
| MLR | 0.24 [0.21–0.29] | 0.21 [0.17–0.33] | 0.22 [0.18–0.35] | 0.24 [0.18–0.29] | 0.054 |
| PMR | 520.89 [357/98–585.73] | 452.80 [274.17–616.10] | 448.23 [410.10–484.45] | 400.79 [295.81–586.02] | 0.060 |
| SII | 314.42 [287.26–435.93] | 371.42 [301.99–458.99] | 372.45 [303.56–456.72] | 314.23 [287.26–425.92] | 0.283 |
| SIRI | 1.15 [0.86–1.45] | 0.81 [0.46–1.21] | 0.81 [0.47–1.22] | 0.79 [0.56–1.21] | 0.149 |
| AISI | 276.50 [181.43–401.89] | 167.91 [112.27–294.04] | 178.23 [145.67–234.56] | 216.95 [118.34–287.56] | 0.15 |
| Etanercept | |||||
| PLR | 142.41 [95.89–182.82] | 105.67 [93.85–155.81] | 115.11 [94.84–147.41] | 124.48 [96.84–156.16] | 0.21 |
| NLR | 2.21 [1.63–3.21] | 1.91 [1.46–2.23] | 1.56 [1.26–2.29] | 1.54 [1.18–2.06] | 0.14 |
| d-NLR | 1.65 [1.38–2.29] | 1.35 [0.99–1.66] | 1.23 [0.99–1.64] | 1.16 [0.93–1.64] | 0.03 |
| MLR | 0.25 [0.22–0.33] | 0.25 [0.21–0.35] | 0.23 [0.17–0.27] | 0.23 [0.17–0.27] | 0.25 |
| PMR | 555.77 [396.00–728.78] | 500.51 [425.16–657.06] | 559.25 [388.18–662.07] | 569.28 [434.54–693.75] | 0.71 |
| SII | 574.33 [332.55–886.15] | 439.47 [306.33–604.07] | 415.99 [324.85–500.69] | 389.38 [259.09–559.77] | 0.04 |
| SIRI | 1.03 [0.72–1.43] | 0.86 [0.65–0.99] | 0.80 [0.54–0.97] | 0.74 [0.50–0.89] | 0.01 |
| AISI | 247.70 [112.15–422.90] | 220.65 [147.27–378.04] | 191.89 [131.84–237.09] | 177.63 [121.47–232.86] | 0.01 |
| Infliximab | |||||
| PLR | 143.59 [98.55–186.66] | 117.95 [104.16–239.14] | 107.60 [84.56–143.34] | 109.26 [96.61–170.79] | 0.41 |
| NLR | 1.27 [1.58–4.35] | 1.81 [1.46–2.91] | 1.71 [0.78–2.48] | 1.88 [1.38–2.20] | 0.45 |
| d-NLR | 1.69 [1.16–3.41] | 1.45 [1.08–2.17] | 1.32 [0.61–2.03] | 1.37 [1.02–1.69] | 0.32 |
| MLR | 0.24 [0.15–0.32] | 0.28 [0.19–0.36] | 0.22 [0.18–0.30] | 0.28 [0.25–0.30] | 0.22 |
| PMR | 580.44 [372.94–1027.58] | 452.30 [387.32–679.04] | 503.87 [328.28–671.34] | 397.04 [356.02–561.60] | 0.12 |
| SII | 629.295 [372.53–1192.20] | 507.645 [294.46–695.64] | 1.01 [0.43–1.11] | 432.81 [358.78–614.63] | 0.20 |
| SIRI | 1.08 [0.70–1.58] | 0.785 [0.64–1.70] | 1.01 [0.431–1.11] | 1.070 [0.87–1.31] | 0.17 |
| AISI | 254.23 [175.91–470.88] | 234.15 [118.12–407.06] | 241.12 [133.90–287.77] | 285.84 [170.91–381.21] | 0.09 |
| Certolizumab | |||||
| PLR | 99.52 [65.05–115.51] | 88.07 [54.37–96.40] | 97.07 [56.71–123.86] | 84.02 [58.47–117.45] | 0.48 |
| NLR | 2.04 [1.587–2.454] | 1.67 [0.94–1.95] | 1.86 [1.03–3.54] | 1.32 [1.02–2.06] | 0.33 |
| d-NLR | 1.68 [1.11–1.79] | 1.36 [0.75–1.53] | 1.38 [0.81–2.15] | 1.05 [0.80–1.59] | 0.88 |
| MLR | 0.23 [0.16–0.30] | 0.17 [0.132–0.30] | 0.22 [0.09–0.51] | 0.22 [0.09–0.26] | 0.23 |
| PMR | 412.01 [309.10–551.90] | 461.54 [194.84–715.28] | 532.50 [168.72–746.38] | 418.58 [237.50–1004.02] | 0.24 |
| SII | 420.83 [309.93–592.62] | 310.84 [140.07–489.79] | 456.93 [216.59–623.88] | 341.13 [155.63–496.81] | 0.07 |
| SIRI | 1.09 [0.79–1.26] | 1.69 [0.41–1.31] | 0.82 [0.48–3.38] | 0.78 [0.37–1.22] | 0.07 |
| AISI | 204.39 [178.58–299.58] | 130.88 [68.59–250.96] | 192.91 [102.45–540.86] | 154.68 [81.20–356.18] | 0.04 |
| Ixekizumab | |||||
| PLR | 124 [108.38–147.07] | 121.435 [112.04–140.54] | 118.15 [101.70–132.99] | 119.09 [107.42–141.70] | 0.03 |
| NLR | 2.14 [1.93–2.38] | 1.89 [1.77–2.28] | 1.97 [1.68–2.27] | 1.93 [1.84–2.43] | 0.04 |
| d-NLR | 1.66 [1.47–1.81] | 1.57 [1.38–1.67] | 1.62 [1.31–1.73] | 1.62 [1.41–1.73] | 0.21 |
| MLR | 0.25 [0.21–0.28] | 0.25 [0.22–0.28] | 0.24 [0.21–0.27] | 0.25 [0.22–0.27] | 0.94 |
| PMR | 522.5 [470.22–607.38] | 506.67 [468.91–576.44] | 496.72 [448.60–542.76] | 503.51 [451.73–597.79] | 0.30 |
| SII | 559.89 [507.75–638.92] | 508.71 [441.64–577.01] | 476 [401.05–525.58] | 512.47 [444.83–581.48] | 0.29 |
| SIRI | 1.08 [0.86–1.27] | 0.95 [0.82–1.04] | 0.89 [0.74–1.08] | 0.98 [0.86–1.25] | 0.29 |
| SIRI | 1.02 [0.86–1.13] | 1.05 [0.88–1.23] | 0.99 [0.82–1.22] | 0.97 [0.74–1.12] | 0.008 |
| AISI | 278.32 [213.44–366.69] | 216.54 [191.36–284.76] | 226.94 [155.31–250.01] | 228.16 [194.74–306.96] | 0.84 |
| Secukinumab | |||||
| PLR | 125.29 [113.65–151.76] | 126.15 [112.78–140.11] | 126.47 [110.40–131.59] | 119 [108.18–141.65] | 0.85 |
| NLR | 2.23 [1.88–2.44] | 2.07 [1.66–2.56] | 1.83 [1.54–2.14] | 1.81 [1.42–2.10] | 0.005 |
| d-NLR | 1.42 [1.36–1.81] | 1.475 [1.15–1.80] | 1.41 [1.25–1.60] | 1.35 [1.11–1.57] | 0.154 |
| MLR | 0.26 [0.21–0.28] | 0.27 [0.23–0.30] | 0.25 [0.21–0.29] | 0.25 [0.21–0.27] | 0.008 |
| PMR | 543.26 [467.35–677.31] | 481 [412.59–554.94] | 461.9 [411.01–493.99] | 460 [416.10–573.17] | 0.23 |
| SII | 520.03 [416.10–645.76] | 501.26 [444.11–580.68] | 439.78 [403.15–524.57] | 412.66 [370.70–507.89] | 0.058 |
| SIRI | 1.08 [0.86–1.27] | 0.95 [0.82–1.04] | 0.89 [0.74–1.08] | 0.98 [0.86–1.25] | 0.29 |
| AISI | 241.12 [206.06–277.64] | 268.665 [213.56–324.45] | 238.55 [186.30–274.01] | 217 [187.28–257.58] | 0.008 |
| Tildrakizumab | |||||
| PLR | 121.68 [103.15–186.73] | 135.79 [98.88–157.40] | 124.35 [82.90–157.45] | 125.52 [112.18–154.51] | 0.23 |
| NLR | 2.24 [1.70–3.18] | 2.3 [1.54–3.46] | 2.42 [1.36–2.86] | 2.18 [1.57–2.95] | 0.86 |
| d-NLR | 1.42 [1.23–2.35] | 1.63 [1.16–2.41] | 1.69 [1.01–2.13] | 1.57 [1.25–2.06] | 0.78 |
| MLR | 0.27 [0.19–0.33] | 0.28 [0.20–0.31] | 0.25 [0.17–0.32] | 0.24 [0.17–0.38] | 0.71 |
| PMR | 474 [440.68–720.67] | 485 [438.03–579.58] | 530.43 [354.84–631.98] | 520 [363.72–785.12] | 0.32 |
| SII | 611.29 [371.10–709.35] | 574.39 [439.87–792.95] | 554.68 [284.14–632.80] | 508.38 [451.50–688.02] | 0.72 |
| SIRI | 0.85 [0.67–1.51] | 1.19 [0.83–1.57] | 1 [0.67–1.47] | 0.91 [0.69–1.61] | 0.88 |
| AISI | 266.63 [158.77–363.59] | 271.6 [163.98–412.84] | 206.97 [153.79–393.27] | 205.03 [160.54–341.37] | 0.99 |
| Risankizumab | |||||
| PLR | 113.17 [103.35–124.92] | 120.62 [101.75–133.97] | 111.915 [99.65–19.96] | 115.17 [102.28–124.96] | 0.16 |
| NLR | 1.9 [1.451–2.23] | 2.185 [1.87–2.42] | 1.735 [1.53–1.89] | 1.75 [1.43–1.99] | 0.04 |
| d-NLR | 1.405 [1.14–1.71] | 1.625 [1.43–1.77] | 1.295 [1.14–1.47] | 1.26 [1.05–1.51] | 0.54 |
| MLR | 0.225 [0.18–0.25] | 0.25 [0.21–0.28] | 0.21 [0.18–0.24] | 0.22 [0.19–0.25] | 0.81 |
| PMR | 545 [491.56–657.59] | 482.8 [427.38–621.04] | 530.78 [459.55–635.14] | 511.76 [425.13–670.27] | 0.62 |
| SII | 464.905 [380.01–556.99] | 501.505 [444.36–605.69] | 415.23 [345.09–510.48] | 435.86 [367.68–544.54] | 0.33 |
| SIRI | 0.935 [0.670–1.01] | 1.145 [0.891–1.23] | 0.79 [0.610–0.98] | 0.88 [0.690–1.02] | 0.20 |
| AISI | 214.425 [153.78–290.81] | 288.22 [211.08–322.75] | 188.23 [155.43–248.86] | 219.33 [159.79–267.39] | 0.15 |
| Guselkumab | |||||
| PLR | 120.225 [100.15–189.98] | 136.48 [92.11–61.93] | 120.615 [80.71–159.89] | 115.155 [80.98–82.98] | 0.80 |
| NLR | 2.18 [1.75–4.20] | 2.11 [1.52–2.80] | 1.75 [1.46–2.09] | 1.95 [1.64–2.28] | 0.02 |
| d-NLR | 1.53 [1.31–2.12] | 1.61 [1.12–1.82] | 1.35 [0.96–1.57] | 1.4 [1.01–1.55] | 0.45 |
| MLR | 0.26 [0.21–0.39] | 0.24 [0.18–0.26] | 0.25 [0.10–0.27] | 0.24 [0.18–0.27] | 0.12 |
| PMR | 468.16 [349.90–828.32] | 551.25 [381.29–832.06] | 589 [356.09–846.16] | 533.61 [401.86–719.85] | 0.53 |
| SII | 717.79 [510.73–1026.12] | 534.54 [390.93–1037.29] | 464.85 [373.21–591.27] | 551.27 [349.65–848.11] | 0.04 |
| SIRI | 1.26 [0.88–2.80] | 1.06 [0.74–1.47] | 0.85 [0.45–1.21] | 1.02 [0.75–1.46] | 0.07 |
| AISI | 393.36 [274.36–715.36] | 291.51 [184.81–470.05] | 251.93 [134.54–295.49] | 276.81 [175.55–499.80] | 0.09 |
| Ustekinumab | |||||
| PLR | 155.11 [104.69–176.66] | 122.69 [98.141–63.520] | 127.41 [98.76–51.63] | 107.69 [83.93–49.17] | 0.08 |
| NLR | 3.03 [1.47–3.61] | 2.4 [1.84–2.87] | 1.95 [1.51–2.73] | 1.93 [1.29–2.19] | 0.08 |
| d-NLR | 2.19 [1.52–2.38] | 1.82 [1.43–2.11] | 1.72 [1.19–2.04] | 1.6 [1.24–1.86] | 0.50 |
| MLR | 0.27 [0.22–0.43] | 0.25 [0.18–0.33] | 0.23 [0.15–0.33] | 0.23 [0.14–0.30] | 0.98 |
| PMR | 512.5 [366.42–763.31] | 490 [415.03–675.81] | 662.96 [335.65–732.27] | 543.48 [416.98–620.79] | 0.43 |
| SII | 668.51 [452.76–997.77] | 524.38 [407.29–708.24] | 530.26 [393.45–695.04] | 440.12 [356.07–572.94] | 0.38 |
| SIRI | 1.15 [0.85–1.94] | 1 [0.60–1.94] | 1.05 [0.51–1.52] | 0.78 [0.68–1.56] | 0.48 |
| AISI | 274.09 [184.63–596.28] | 244.26 [154.35–377.77] | 217.01 [140.54–383.16] | 188.5 [160.67–582.63] | 0.66 |
| Apremilast | |||||
| PLR | 137.60 ± 22.64 | 140.17 ± 29.29 | 133.44 ± 20.92 | 129.52 ± 24.67 | 0.77 |
| NLR | 1.77 ± 0.635 | 1.84 ± 0.57 | 2.12 ± 0.38 | 1.93 ± 0.56 | 0.67 |
| d-NLR | 1.31 ± 0.41 | 1.31 ± 0.23 | 1.60 ± 0.33 | 1.36 ± 0.16 | 0.38 |
| MLR | 0.22 ± 0.09 | 0.27 ± 0.12 | 0.23 ± 0.09 | 0.23 ± 0.10 | 0.91 |
| PMR | 684.32 ± 260.47 | 588.48 ± 243.37 | 624.27 ± 249.77 | 654.06 ± 274.05 | 0.77 |
| SII | 443.36 ± 206.54 | 457.76 ± 206.71 | 552.72 ± 193.12 | 513.89 ± 212.43 | 0.99 |
| SIRI | 0.77 ± 0.47 | 0.96 ± 0.67 | 0.95 ± 0.37 | 0.95 ± 0.59 | 0.43 |
| AISI | 194.02 ± 116.23 | 248.72 ± 201.88 | 252.48 ± 117.71 | 262.15 ± 161.23 | 0.61 |
PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; d-NLR, derived neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; PMR, platelet-to monocyte ratio; SII, systemic immune inflammation index; SIRI, systemic inflammation response index; AISI, aggregate index of systemic inflammation; * is expressed as mean ± SD for normally distributed data (Apremilast) and as median with 95% CI for non-normally distributed data.
3.4. Clinical and Biological Profile of Responders vs. Nonresponders in Dependence of the Biologic Used
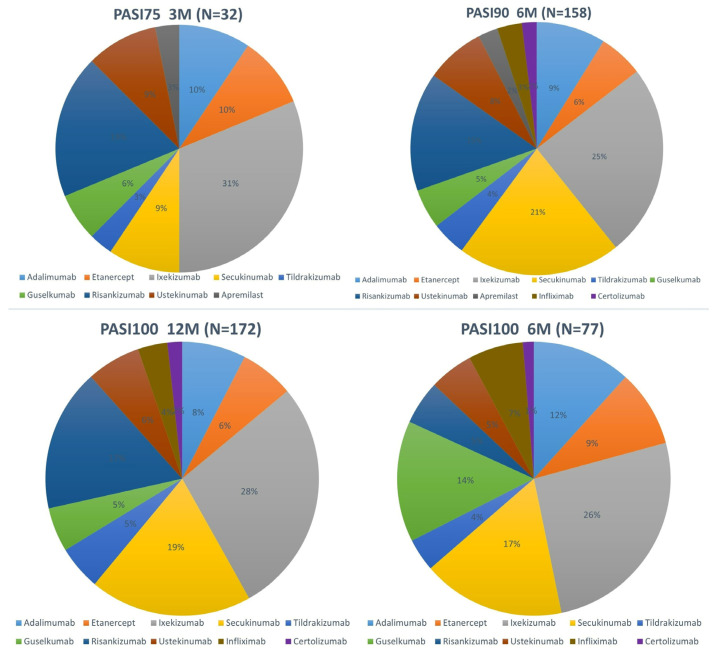
Thirteen percent (n = 32) of patients met the target goal, e.g., PASI75, at the three-month evaluation, while a marked increase (65.83%; n = 158 and 71.66%; n = 172) was noted at the six-month and twelve-month hallmark (as seen in Figure 1). Ixekizumab was the most commonly used treatment for patients achieving treatment target goals at the three-month, six-month, and twelve-month thresholds and was also associated with the highest number of patients being super-responders.
Figure 1.
Percentage of patients reaching target goals based on the treatment of choice.
As depicted in Table 6, blood-count-derived inflammatory markers values did not differ significantly between responders and nonresponders at the three-month and twelve-month hallmark. However, the analyzed markers in both evaluations had lower values in the responders subgroup than in nonresponders. At the 6-month evaluation, for patients meeting the PASI90 target goal, significantly lower values (p < 0.05) were noted for all analyzed markers except NLR and PLR.
Table 6.
Comparison of blood-count-derived inflammatory markers between responders and nonresponders.
| Variable | PLR | NLR | d-NLR | MLR | PMR | SII | SIRI | AISI |
|---|---|---|---|---|---|---|---|---|
| PASI75 3M | ||||||||
| Responder | 108.565 [98.17–120.65] | 1.97 [1.73–2.42] | 1.34 [1.20–1.55] | 0.23 [0.19–0.28] | 493.7 [396.60–575.78] | 506.205 [426.29–576.00] | 1.000 [0.80–1.23] | 250.590 [177.03–372.85] |
| Nonresponder | 122.66 [113.73–129.31] | 2.015 [1.81–2.13] | 1.535 [1.43–1.60] | 0.25 [0.20–0.27] | 491.43 [473.01–525.13] | 488.51 [454.53–520.99] | 0.985 [0.89–1.08] | 241.13 [209.85–283.21] |
| p-value | 0.18 | 0.64 | 0.48 | 0.58 | 0.82 | 0.55 | 0.54 | 0.72 |
| PASI90 6M | ||||||||
| Responder | 108.24 [98.99–115.65] | 1.80 [1.74–2.00] | 1.41 [1.29–1.56] | 0.25 [0.23–0.26] | 480.26 [447.80–518.00] | 472.04 [434.86–513.82] | 0.98 [0.89–1.07] | 158.97 [141.46–191.91] |
| Nonresponder | 121.74 [114.58–127.65] | 1.85 [1.58–1.97] | 1.35 [1.15–1.47] | 0.22 [0.18–0.24] | 522.05 [477.84–591.78] | 404.82 [338.55–450.37] | 0.71 [0.63–0.87] | 244.35 [227.90–269.58] |
| p-value | 0.02 | 0.15 | 0.06 | 0.001 | 0.31 | 0.002 | 0.001 | <0.001 |
| PASI100 12M | ||||||||
| Responder | 115.385 [108.526–122.400] | 1.83 [1.700–1.987] | 1.375 [1.273–1.480] | 0.24 [0.230–0.260] | 494.205 [450.000–534.041] | 442.445 [398.253–499.176] | 0.88 [0.813–0.980] | 217.73 [195.332–242.703] |
| Nonresponder | 119.145 [102.830–129.133] | 1.77 [1.632–1.930] | 1.405 [1.232–1.520] | 0.23 [0.204–0.260] | 478.935 [425.492–593.406] | 453.725 [417.552–539.350] | 0.965 [0.754–1.026] | 227.555 [192.587–264.591] |
| p-value | 0.48 | 0.98 | 0.49 | 0.57 | 0.74 | 0.77 | 0.91 | 0.94 |
PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; d-NLR, derived neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; PMR, platelet-to-monocyte ratio; SII, systemic immune inflammation index; SIRI, systemic inflammation response index; AISI, aggregate index of systemic inflammation.
3.5. Blood-Count-Derived Inflammatory Markers Usefulness in Predicting Super-Responder Status
In a multiple logistic regression model (Table 7), higher baseline values of d-NLR and SIRI and scalp involvement (p < 0.05) proved to be predictive factors of the super-responder status. However, since the 95% CI for PASI, d-NLR, and SIRI cross over 1, these data should be cautiously interpreted.
Table 7.
Predictors of super-responder status.
| Parameter | OR | 95% CI | p-Value |
|---|---|---|---|
| Age | 0.97 | 0.95–0.99 | 0.01 |
| Scalp involvement | 0.29 | 0.11–0.78 | 0.02 |
| PASI | 1.03 | 0.99–1.05 | 0.04 |
| d-NLR | 0.75 | 0.46–1.33 | 0.05 |
| SIRI | 0.20 | 0.02–2.17 | 0.04 |
PASI, psoriasis area severity index; d-NLR, derived neutrophil-to-lymphocyte ratio; SIRI, systemic inflammation response index.
4. Discussion
Blood-count-derived inflammatory markers have been proven useful in estimating and predicting disease severity in psoriasis [16]. Moreover, existing data highlight their usefulness in predicting psoriasis comorbidities [19] and, thus, guiding the clinician in the treatment choice. With respect to biologics, they have been assessed so far in rheumatic disorders, such as ankylosing spondylitis and psoriatic arthritis [20], rheumatoid arthritis [21], and skin diseases like atopic dermatitis [12] and hidradenitis suppurativa [22]. Biologics’ effects on disease progression in psoriasis have been evaluated in a number of studies [23,24,25] but with conflicting results. Until now, no paper has assessed the usefulness of AISI and d-NLR in predicting responses to biologics.
Numerous effective biologics targeting various pathways in psoriasis pathogenesis are currently available. Patients with psoriasis present with a high clinical variation during the course of the disease, which could be translated into high variations in the immunological profile of these patients. As such, different patterns in cytokines expression might be noted from patient to patient and afterward impact how a patient reacts to a specific type of treatment. However, cytokines determination is not currently widely and routinely available, and the need for simple, cost-reliable markers to assess the inflammatory status and treatment response is very high. A personalized approach taking into account predictive biomarkers may aid in obtaining a faster and more complete resolution of skin lesions while improving associated comorbidities.
The highest number of patients included in our study underwent treatment with interleukin inhibitors, specifically IL-17 and IL-23 inhibitors, such as ixekizumab (n = 61), risankizumab (n = 44), and secukinumab (n = 41). In addition to being in the majority, male patients had higher BMI scores than female patients (p = 0.04).
Regarding absolute cell count, WBC and monocyte values decreased linearly and significantly between the four visits (p = 0.012); other cell populations did not differ significantly during the course of treatment.
All blood-count-derived inflammatory markers varied significantly between the four visits (p < 0.001); however, only PLR, NLR, d-NLR, PMR, SII, and SIRI presented with a gradual decrease during the treatment period. On the other hand, the PASI score significantly and positively correlated with PLR (rho = 0.175), d-NLR (rho = 0.234), and SII (rho = 0.178), while PSSI correlated with AISI (rho = 0.198) and SIRI (rho = 0.209). NLR is the marker most extensively studied in relation to psoriasis; it was proved to be higher compared to controls [26] and decreased during the course of treatment with biologics [27,28], as seen in our study as well. In a study by Andersen et al. [25], lower baseline values of NLR between responders and nonresponders to anti-TNF-α agents were noted.
For patients treated with adalimumab, d-NLR significantly varied between the four visits, while in the certolizumab subgroup, the AISI score significantly decreased. In a study published by Albayrak [23], anti-TNF-α agents, such as adalimumab, etanercept, and infliximab, significantly decreased NLR, NMR, and PLR as soon as three months after the start of treatment. Additionally, in the aforementioned study, NLR was correlated with PASI and CRP values, highlighting once more the reliability of this marker in properly assessing the inflammatory status in psoriasis. NLR varied significantly in our study group in patients treated with IL-17 and IL-23 inhibitors. Out of all the parameters that were analyzed, NLR varied significantly the most (in three out of eleven biologics) in our study group. Moreover, high PLR combined with increased serum values of IL-6 and nail involvement was recently proven to be a subclinical indicator of psoriatic arthritis [29].
Apremilast did not show any significant variation in the analyzed parameters in the aforementioned period of time, indicating that small-molecule inhibitors might not significantly modify the inflammatory status. However, it should be kept in mind that our study included only one type of small-molecule inhibitor, namely, apremilast, with a limited number of patients (n = 4), and the results for this choice of treatment should be interpreted cautiously. To the best of our knowledge, no study thus far has analyzed the relationship between apremilast and blood-count-derived inflammatory markers in psoriasis; future ideas might include expanding the spectrum of small-molecule inhibitors to include deucravacitinib as well, and to assess their impact in altering blood-count-derived inflammatory markers in psoriasis.
More than half of patients (65.83%) met their target goals at the 6-month hallmark, while at the 12-month evaluation, 71.66% of them obtained complete clearance of their lesions. Additionally, 32.08% of patients were super-responders, having obtained PASI100 at the 6-month hallmark. Most patients who met target goals underwent treatment with ixekizumab (10, 20, and 48, respectively). Risankizumab and secukinumab closely followed. However, as seen in Figure 1, not only did patients on ixekizumab obtain the fastest response, but the number of patients meeting target goals gradually increased during the follow-up period of time. These data are in accordance with those recently published by Gooderham et al. [30], who identified that patients undergoing treatment with ixekizumab obtained faster skin lesion clearance compared to an IL-23 inhibitor, namely, guselkumab. Moreover, it seems that skin clearance in the head region is achieved faster compared to the trunk, upper, and lower extremities [31,32]. On the other hand, even though risankizumab and secukinumab presented with a slower action in the initial treatment phases, once the target goals were met, they were properly maintained during the follow-up period. Regarding super-responders, the highest number of patients in this group were under treatment with ixekizumab, with slightly fewer patients undergoing treatment with risankizumab and secukinumab. These data suggest that anti-IL-17 and anti-IL-23 agents not only lead to a faster significant clearance of lesions compared to the other classes but have a sustained positive effect, at least, if not more, during a short follow-up period.
No blood-count-derived inflammatory marker varied significantly between patients who achieved clinical response at the three-month hallmark and those who did not. However, it should be kept in mind that at the three-month evaluation, only 32 patients (13.33%) achieved the treatment goal (as defined, PASI 75), while if referring to the treatment target met at 6 months, 65.83% of patients (n = 158) met clinical resolution, e.g., PASI 90. This matter is also reflected by a gradual, significant decrease in inflammatory markers, such as PLR (p = 0.02), MLR (p = 0.001), SII (p = 0.002), SIRI (p = 0.001), and AISI (p < 0.001), between responders and nonresponders.
At the 12-month hallmark, no significant differences between responders and nonresponders were noted between inflammatory markers values. These findings indicate that there is a difference between clinical response and biological response in patients with psoriasis. Treatment should be guided to limit and contain cutaneous lesions and address the possibly associated comorbidities. In this matter, it is once more emphasized the need for a multidisciplinary, comprehensive approach to patients with psoriasis to prevent disease extension, utilizing a careful follow-up of patients during biologics treatment.
Many countries, including ours, mandate a careful follow-up every 6 months for patients undergoing biologics treatment to limit possible adverse reactions. On the other hand, it must be noted that even for patients considered to be nonresponders at the 12-month hallmark (e.g., patients who did not achieve PASI100), all inflammatory markers values were lower compared to baseline values, indicating that, indeed, biologics decrease the overall inflammatory status of psoriatic patients. Future agreements might shed some light onto what should be considered acceptable regarding treatment targets: Should only PASI100 be desired? Or might PASI90 and even PASI75 be reasonable target goals for both prescribing doctors and patients?
A total of 77 patients (32.08%) were super-responders, as defined in Section 2, reaching PASI100 at the 6-month hallmark. Most of them were under treatment with ixekizumab (n = 20). Disease severity quantified by the PASI score (OR = 1.03), scalp involvement (OR = 0.29), advanced age (OR = 0.97), higher baseline values of d-NLR (OR = 0.75), and SIRI (OR = 0.20) predicted super-response to treatment. These data should be interpreted cautiously since for three parameters (PASI, d-NLR, and SIRI), the 95% CI crosses over 1, therefore not providing enough evidence. This situation might also be due to the fact that there were a limited number of super-responders (n = 77). Furthermore, it would be incorrect to interpret a positive odds ratio (OR) with a significant p-value but with a 95% confidence interval (CI) encompassing the null value as evidence of no association between the exposure and outcome [33]; nevertheless, situations like these should be approached with caution. Early response is also useful for the long-term evolution of such patients. Reaching a PASI of 2 or less within the first six months of starting biologics is associated with a reduced risk of flares and a more stable disease course [34].
This is the first paper to assess the usefulness of d-NLR and AISI in predicting treatment response to biologics; our study identified that higher baseline values of d-NLR significantly predicted one patient’s status as a super-responder. On the other hand, AISI significantly decreased during the course of treatment with anti-TNF-α and anti-IL-17 agents, while its values varied between responders and nonresponders at the six-month hallmark.
This study‘s main limitation is its retrospective design. Future ideas might include prospective patient enrolment. As such, disease severity assessment will be associated with lower bias in data collection. Moreover, our study focused solely on chronic plaque psoriasis; it would be interesting to assess various classical immunosuppressive agents’ effects on blood-count-derived inflammatory markers in pustular or erythrodermic psoriasis. A longer follow-up period would help gain additional data regarding psoriasis evolution under biologics, interleukin levels, and neutralizing antibodies determination.
5. Conclusions
In our study group, all blood-count-derived inflammatory markers varied significantly from visit to visit in evaluating response to biologics. Furthermore, PLR, d-NLR, and SII decreased parallelly with the PASI score in our study group. Most super-responder patients underwent treatment with ixekizumab. If referring to d-NLR, a blood-count-derived inflammatory marker tested for the first time in the literature regarding the treatment of psoriasis with biologics in our paper, it proved to be an integrative part of reaching the super-responder status.
Further, larger-scale studies are necessary to better represent the study population. These cost-effective and simple-to-determine markers can be used to assess systemic inflammation in psoriasis, monitor disease course, and predict patients’ response to biologics.
Author Contributions
Conceptualization: S.-H.M., O.S.C., A.B., O.M.T., L.G.-S. and A.C.N.; formal analysis: S.-H.M. and O.M.T.; methodology: S.-H.M., O.M.T., O.S.C. and A.C.N.; resources: A.B., L.G.-S., H.D., I.B., K.S., V.B., D.Ș.-P., I.M., M.A., R.I.I., L.P., A.B.B., M.H., D.L.G., R.A.S. and V.V.; validation: S.-H.M., O.S.C. and A.C.N.; visualization: O.M.T.; writing—original draft: S.-H.M., O.S.C., O.M.T., L.G.-S. and A.C.N.; writing—revision and editing: S.-H.M., O.M.T., R.A.S. and A.C.N.; supervision: S.-H.M., O.S.C. and A.C.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee Mures Clinical County Hospital no. 3770/5 April 2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data presented can be made available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Yamazaki F. Psoriasis: Comorbidities. J. Dermatol. 2021;48:732–740. doi: 10.1111/1346-8138.15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruan Z., Lu T., Chen Y., Yuan M., Yu H., Liu R., Xie X. Association between Psoriasis and Nonalcoholic Fatty Liver Disease among Outpatient US Adults. JAMA Dermatol. 2022;158:745. doi: 10.1001/jamadermatol.2022.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A., Gisondi P., Lonardo A., Targher G. Relationship between Non-Alcoholic Fatty Liver Disease and Psoriasis: A Novel Hepato-Dermal Axis? Int. J. Mol. Sci. 2016;17:217. doi: 10.3390/ijms17020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Man M.-A., Davidescu L., Motoc N.-S., Rajnoveanu R.-M., Bondor C.-I., Pop C.-M., Toma C. Diagnostic Value of the Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) in Various Respiratory Diseases: A Retrospective Analysis. Diagnostics. 2021;12:81. doi: 10.3390/diagnostics12010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misiewicz A., Dymicka-Piekarska V. Fashionable, but What is Their Real Clinical Usefulness? NLR, LMR, and PLR as a Promising Indicator in Colorectal Cancer Prognosis: A Systematic Review. J. Inflamm. Res. 2023;16:69–81. doi: 10.2147/JIR.S391932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maloney S., Pavlakis N., Itchins M., Arena J., Mittal A., Hudson A., Colvin E., Sahni S., Diakos C., Chan D., et al. The Prognostic and Predictive Role of the Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Lymphocyte-to-Monocyte Ratio (LMR) as Biomarkers in Resected Pancreatic Cancer. J. Clin. Med. 2023;12:1989. doi: 10.3390/jcm12051989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovács A.R., Sulina A., Kovács K.S., Lukács L., Török P., Lampé R. Prognostic Significance of Preoperative NLR, MLR, and PLR Values in Predicting the Outcome of Primary Cytoreductive Surgery in Serous Epithelial Ovarian Cancer. Diagnostics. 2023;13:2268. doi: 10.3390/diagnostics13132268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powrózek T., Skwarek-Dziekanowska A., Sobieszek G., Małecka-Massalska T. Correlation between Neutrophil-to-Lymphocyte Ratio, Platelets-to-Lymphocyte Ratio, C-Reactive Protein-to-Albumin Ratio and Clinical Picture of Elderly Chronic Heart Failure Patients. J. Clin. Med. 2024;13:433. doi: 10.3390/jcm13020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamaki S., Nagai Y., Shutta R., Masuda D., Yamashita S., Seo M., Yamada T., Nakagawa A., Yasumura Y., Nakagawa Y., et al. Combination of Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as a Novel Predictor of Cardiac Death in Patients with Acute Decompensated Heart Failure with Preserved Left Ventricular Ejection Fraction: A Multicenter Study. J. Am. Heart Assoc. 2023;12:e026326. doi: 10.1161/JAHA.122.026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun C., Li X., Qian H., Liang G., Xiang R., Zhao C., Li Z., Li S., Jing K., Wang Y., et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are positively correlated with disease activity of bullous pemphigoid. Arch. Dermatol. Res. 2023;315:2383–2391. doi: 10.1007/s00403-023-02639-w. [DOI] [PubMed] [Google Scholar]
- 11.Öksüm Solak E., Baran Ketencioglu B., Cinar S.L., Kartal D., Borlu M. The role of new inflammatory markers in determining disease activation and severity in patients with hidradenitis suppurativa. Int. J. Dermatol. 2023;62:1076–1081. doi: 10.1111/ijd.16744. [DOI] [PubMed] [Google Scholar]
- 12.Zinellu A., Sucato F., Piras V., Addis G.M., Biondi G., Montesu M.A., Mangoni A.A., Carru C., Pirina P., Paliogiannis P., et al. Blood Cells Count Derived Inflammation Indexes as Predictors of Early Treatment Response to Dupilumab in Patients with Moderate-to-Severe Atopic Dermatitis. J. Clin. Med. 2023;12:2104. doi: 10.3390/jcm12062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagino T., Saeki H., Kanda N. Biomarkers and Predictive Factors for Treatment Response to Tumor Necrosis Factor-α Inhibitors in Patients with Psoriasis. J. Clin. Med. 2023;12:974. doi: 10.3390/jcm12030974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen T., Chen Y., Shao X., Chen J., Liu L., Li Y., Pu Y., Chen J. Hematological parameters in patients with acnes. J. Cosmet. Dermatol. 2023;22:2099–2104. doi: 10.1111/jocd.15676. [DOI] [PubMed] [Google Scholar]
- 15.Turan Ç., Metin N. A Novel Inflammatory Marker in the Follow-up of Moderate-to-Severe Acne Vulgaris Administered Isotretinoin: Systemic Immune-Inflammation Index (SII) Curr. Health Sci. J. 2022;48:63–67. doi: 10.12865/CHSJ.48.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiucă O.M., Morariu S.H., Mariean C.R., Tiucă R.A., Nicolescu A.C., Cotoi O.S. Impact of Blood-Count-Derived Inflammatory Markers in Psoriatic Disease Progression. Life. 2024;14:114. doi: 10.3390/life14010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma R., Cui L., Cai J., Yang N., Wang Y., Chen Q., Chen W., Peng C., Qin H., Ding Y., et al. Association between systemic immune inflammation index, systemic inflammation response index and adult psoriasis: Evidence from NHANES. Front. Immunol. 2024;15:1323174. doi: 10.3389/fimmu.2024.1323174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamer F., Edek Y.C., Aksakal A.B. Effect of Treatment with Biologic Agents on the Novel Inflammatory Biomarkers Systemic Immune Inflammation Index and Systemic Inflammation Response Index for Psoriasis. Dermatol. Pract. Concept. 2024;14:e2024065. doi: 10.5826/dpc.1401a65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiucă O.M., Morariu S.H., Mariean C.R., Tiucă R.A., Nicolescu A.C., Cotoi O.S. Predictive Performances of Blood-Count-Derived Inflammatory Markers for Liver Fibrosis Severity in Psoriasis Vulgaris. Int. J. Mol. Sci. 2023;24:16898. doi: 10.3390/ijms242316898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aboud F.M., Galal S., Elwafa M.A.Z.A., Farouk A.M. Impact of biological and non-biological treatment on hematological indices in patients with ankylosing spondylitis and psoriatic arthritis. Egypt. Rheumatol. Rehabil. 2023;50:14. doi: 10.1186/s43166-023-00174-0. [DOI] [Google Scholar]
- 21.Lee H.-N., Kim Y.-K., Kim G.-T., Ahn E., So M.W., Sohn D.H., Lee S.-G. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio as predictors of 12-week treatment response and drug persistence of anti-tumor necrosis factor-α agents in patients with rheumatoid arthritis: A retrospective chart review analysis. Rheumatol. Int. 2019;39:859–868. doi: 10.1007/s00296-019-04276-x. [DOI] [PubMed] [Google Scholar]
- 22.Kearney N., McCourt C., Hambly R., Hughes R., O’Kane D., Kirby B. Association of Biologic Treatment in Hidradenitis Suppurativa with Reduced Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio. JAMA Dermatol. 2023;159:222. doi: 10.1001/jamadermatol.2022.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albayrak H. Neutrophil-to-Lymphocyte Ratio, Neutrophil-to-Monocyte Ratio, Platelet-to-Lymphocyte Ratio, and Systemic Immune-Inflammation Index in Psoriasis Patients: Response to Treatment with Biological Drugs. J. Clin. Med. 2023;12:5452. doi: 10.3390/jcm12175452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An I., Ucmak D., Ozturk M. The effect of biological agent treatment on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, mean platelet volume, and C-reactive protein in psoriasis patients. Postępy Dermatol. Alergol. 2020;37:202–206. doi: 10.5114/ada.2020.94838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen C.S.B., Kvist-Hansen A., Siewertsen M., Enevold C., Hansen P.R., Kaur-Knudsen D., Zachariae C., Nielsen C.H., Loft N., Skov L. Blood Cell Biomarkers of Inflammation and Cytokine Levels as Predictors of Response to Biologics in Patients with Psoriasis. Int. J. Mol. Sci. 2023;24:6111. doi: 10.3390/ijms24076111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paliogiannis P., Satta R., Deligia G., Farina G., Bassu S., Mangoni A.A., Carru C., Zinellu A. Associations between the neutrophil-to-lymphocyte and the platelet-to-lymphocyte ratios and the presence and severity of psoriasis: A systematic review and meta-analysis. Clin. Exp. Med. 2019;19:37–45. doi: 10.1007/s10238-018-0538-x. [DOI] [PubMed] [Google Scholar]
- 27.Aktaş Karabay E., Aksu Çerman A., Demir D., Kıvanç Altunay I. The Effects of Systemic Psoriasis Therapies on the C-Reactive Protein and the Neutrophil-Lymphocyte Ratio. Ann. Dermatol. 2019;31:601. doi: 10.5021/ad.2019.31.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann J., Knoop C., Enk A., Hadaschik E. Detailed Long-term Dynamics of Neutrophil-to-Lymphocyte Ratio under Biologic Treatment Reveals Differential Effects of Tumour Necrosis Factor-alpha and Interleukin 12/23 Antagonists. Acta Derm.-Venereol. 2021;101:271. doi: 10.2340/actadv.v101.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X., Zhao Y., Mu Z., Jia Y., Liu C., Zhang J., Cai L. The Combination of IL-6, PLR and Nail Psoriasis: Screen for the Early Diagnosis of Psoriatic Arthritis. Clin. Cosmet. Investig. Dermatol. 2023;16:1703–1713. doi: 10.2147/CCID.S413853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gooderham M., Vender R., Crowley J., Hong H.C.-H., Feely M., Garrelts A., See K., Konicek B., Green L. Speed and Cumulative Responses According to Body Regions in Patients with Moderate-to-Severe Plaque Psoriasis Treated with Ixekizumab (Interleukin-17A Antagonist) versus Guselkumab (Interleukin-23p19 Inhibitor) Dermatol. Ther. 2024;14:441–451. doi: 10.1007/s13555-023-01075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng P., Hou P., Yang C., Chu C. Variation of body regional responses to Ustekinumab and Secukinumab in psoriasis patients: A real-world retrospective study and literature review. Dermatol. Ther. 2022;35:e15950. doi: 10.1111/dth.15950. [DOI] [PubMed] [Google Scholar]
- 32.Blauvelt A., Muram T.M., See K., Mallinckrodt C.H., Crowley J.J., Van De Kerkhof P. Improvements in psoriasis within different body regions vary over time following treatment with ixekizumab. J. Dermatol. Treat. 2018;29:220–229. doi: 10.1080/09546634.2017.1365114. [DOI] [PubMed] [Google Scholar]
- 33.Szumilas M. Explaining odds ratios. J. Can. Acad. Child Adolesc. Psychiatry. 2010;19:227–229. Erratum in J. Can. Acad. Child Adolesc. Psychiatry 2015, 24, 58. [PMC free article] [PubMed] [Google Scholar]
- 34.Loft N., Egeberg A., Rasmussen M., Bryld L., Nissen C., Dam T., Ajgeiy K., Iversen L., Skov L. Response to Biologics During the First Six Months of Therapy in Biologic-naïve Patients with Psoriasis Predicts Risk of Disease Flares: A Danish Nationwide Study. Acta Derm.-Venereol. 2021;101:adv00357. doi: 10.2340/00015555-3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented can be made available upon request.