Highlights
-
•
The study presents a novel microbial utilization approach to sewer management.
-
•
This approach involves n-DAMO and microbial sulfide oxidation processes.
-
•
Both processes can occur simultaneously by dosing nitrite into the sewer system.
-
•
This approach reduces dissolved methane by 58 % and sulfide by over 90 %.
Keywords: Sewer system, n-DAMO, Sulfide oxidation, Microbial utilization, Nitrite dosing, Integrated urban water management
Abstract
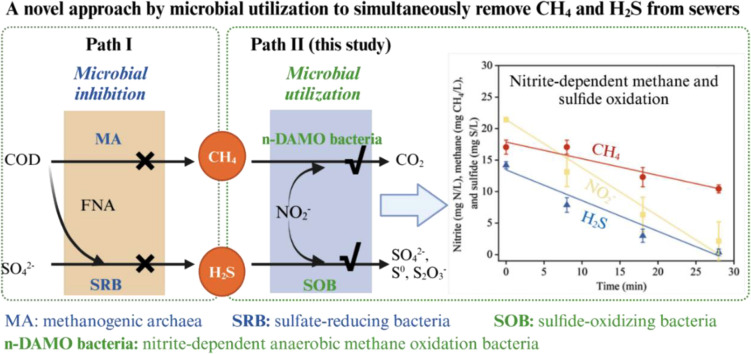
Chemicals are commonly dosed in sewer systems to reduce the emission of hydrogen sulfide (H2S) and methane (CH4), incurring high costs and environmental concerns. Nitrite dosing is a promising approach as nitrite can be produced from urine wastewater, which is a feasible integrated water management strategy. However, nitrite dosing usually requires strict conditions, e.g., relatively high nitrite concentration (e.g., ∼200 mg N/L) and acidic environment, to inhibit microorganisms. In contrast to “microbial inhibition”, this study proposes “microbial utilization” concept, i.e., utilizing nitrite as a substrate for H2S and CH4 consumption in sewer. In a laboratory-scale sewer reactor, nitrite at a relatively low concentrations of 25–48 mg N/L was continuously dosed. Two nitrite-dependent microbial utilization processes, i.e., nitrite-dependent anaerobic methane oxidation (n-DAMO) and microbial sulfide oxidation, successfully occurred in conjunction with nitrite reduction. The occurrence of both processes achieved a 58 % reduction in dissolved methane and over 90 % sulfide removal in the sewer reactor, with microbial activities measured as 15.6 mg CH4/(L·h) and 29.4 mg S/(L·h), respectively. High copy numbers of n-DAMO bacteria and sulfide-oxidizing bacteria (SOB) were detected in both sewer biofilms and sediments. Mechanism analysis confirmed that the dosed nitrite at a relatively low level did not cause the inhibition of sulfidogenic process due to the downward migration of activity zones in sewer sediments. Therefore, the proposed “microbial utilization” concept offers a new alternative for simultaneous removal of sulfide and methane in sewers.
Graphical abstract
1. Introduction
Hydrogen sulfide (H2S) and methane (CH4) are two significant contaminants in global sewer networks. The H2S formation poses multiple threats including corrosion of concrete infrastructure, sewer odors, and health hazards for sewage treatment workers (Pikaar et al., 2014; Zuo et al., 2019). CH4 is a potent greenhouse gas (GHG), with a global warming potential approximately 28 times that of CO2 when measured over a 100-year period (Soued et al., 2022). Sewer systems are significant sources of CH4 production, with a high rate of 1–2 g CH4/(m2·d), leading to a liquid-phase CH4 concentration of up to 20–25 mg/L in real rising main sewers (Guisasola et al., 2008; Liu et al., 2015a). To address both H2S and CH4 emissions in sewers, a well-known approach is to reduce the source production of sulfide and methane by suppressing the activities of SRB and MA in sewer biofilms and sediments (Zhang et al., 2023). This suppression can be primarily achieved by the dosing of various chemicals, such as free nitrous acid (FNA), free ammonia (FA) and ferrate (Jiang et al., 2011; Yan et al., 2023; Zuo et al., 2023b). However, microbial inhibition typically requires the exposure of sewer microbial communities to chemicals with high concentration for several hours, presenting a challenge in maintaining such harsh conditions in real sewers. Several factors, including chemical degradation and dilution along sewer pipes, tend to result in the relatively low efficiency of these approaches. For instance, previous reports have demonstrated that 0.2–0.3 mg HNO2—N/L (e.g., ∼200 mg NO2−-N/L at pH=6) of FNA achieved 80 % inactivation of SRB in a lab-scale biofilm reactor (Jiang et al., 2011), but even 13.3 mg HNO2—N/L of FNA was insufficient for sustained SRB inactivation in a real pressure sewer (Despot et al., 2021).
In-sewer dosing of nitrite has emerged as a practical approach because nitrite can be derived from partial nitridation (NH3+1.5O2→NO2−+H2O) of high-strength wastewater (e.g., source-separated urine) (Mackey et al., 2016; Zuo et al., 2023a). Zheng et al. (2017) reported that using nitrite produced from urine would lower the operational costs by approximately 2/3 and GHG emissions by 1/3, compared with using commodity chemicals. Although nitrite is widely recognized as toxic to sewer microbes and causes microbial inhibition (Jiang et al., 2010; Mohanakrishnan et al., 2008), studies indicated that nitrite could also serve as an electron acceptor for sulfide removal ((1), (2)), facilitated by sulfide oxidizing bacteria (SOB) like Thiobacillus (Mahmood et al., 2007; Yang et al., 2005). This microbial utilization mechanism inspired us to seek any potential microbial oxidation of methane by dosed nitrite, contradictory to the previous microbial inhibition mechanism. Searching in the literature, a novel microbial process of nitrite-dependent anaerobic methane oxidation (n-DAMO) was discovered (Raghoebarsing et al., 2006). This process was demonstrated to have an important role in mitigating methane in many natural environments like marine, wetland, and deep lakes (Deutzmann et al., 2014; Haroon et al., 2013; Hu et al., 2014). The bacteria responsible for n-DAMO belong to the genus “Candidates’ Methylomirabilis” of the NC10 phylum, which can utilize methane as an electron donor and nitrite as an electron acceptor (Eq. (3)) (Ettwig et al., 2010). Consequently, we hypothesize that these bacteria, if present in sewers, are likely to act as a sink of methane, as do in natural environments. Over past years, various n-DAMO technologies have been developed to remove dissolved methane and nitrogen in wastewater treatment (An et al., 2024; Fan et al., 2023; Liu et al., 2020; van Kessel et al., 2018; Wang et al., 2023). Zuo et al. (2024) found that methane emission was reduced by half due to the growth of n-DAMO bacteria in sewers, pointing to the potential of utilizing microbes to enhance sewer management. However, a detailed approach to leveraging nitrite for both sulfide oxidation and DAMO processes in sewers is currently lacking, and the efficiencies of these processes remain unknown.
| (1) |
| (2) |
| (3) |
Therefore, this research aims to study in-sewer nitrite dosing for simultaneous sulfide and methane control via "microbial utilization" in addition to conventional "microbial inhibition". This microbial utilization process is expected to involve SOB and n-DAMO bacteria as key microbes which co-utilize nitrite as an electron acceptor to oxidize sulfide and methane simultaneously. To test this hypothesis, a lab-scale sewer reactor was operated for over 240 days with continuous nitrite dosing, during which sulfide and methane removal performance was systematically monitored. A comprehensive experimental approach involving cycle studies, activity batch tests and microbial analysis was employed to dissect the underlying mechanism behind the reactor's performance. Outcomes from this study suggest that nitrite-dependent microbial utilization concept hold promise as a useful method for sewer management.
2. Results and discussion
2.1. Simultaneous removal of methane and sulfide through nitrite dosing
A long-term experiment was conducted in the lab-scale sewer reactor receiving real wastewater that contained high concentrations of sulfide and methane, produced from another identical lab-scale sewer reactor without any chemical dosing (control reactor). Fig. 1A shows the long-term performance of methane removal by nitrite dosing, as observed from the methane concentrations in the influent and effluent at varying nitrite dosages. In the initial 120 days at 33 mg N/L nitrite dosage (excluding days 35–45), the average methane concentration in the effluent was only 8.5 ± 2.9 mg CH4/L. Compared with the influent level (18.5 ± 3.5 mg CH4/L), the methane removal ratio reached around 53 %, demonstrating the stable occurrence of n-DAMO process through nitrite dosing. Mass balance analysis, based on the average removed amount of methane (i.e., approx.10 mg CH4/L) and the stoichiometry of n-DAMO reaction (Eq. (3)), revealed that about 70 % of nitrite was consumed through the n-DAMO process, suggesting a significant contribution of n-DAMO in nitrite reduction. Simultaneously, the sulfide concentration in the effluent was also significantly reduced to approx. 8.1 ± 6.6 mg S/L during the first 120 days (excluding days 35–45) (Fig. 1B). An average sulfide removal ratio of ∼71% was obtained, higher than the methane removal ratio (Fig. 1A). The simultaneous removal of methane and sulfide in the nitrite-dosed sewer reactor confirmed the reliability of the proposed microbial utilization approach.
Fig. 1.
Long-term removal performance of methane (A) and sulfide (B) in the sewer reactor receiving nitrite dosing and is summarized in box figures, respectively (C and D).
During the period of day 35–45, reducing the nitrite concentration to 25 mg N/L resulted in a rapid recovery in effluent methane and sulfide concentrations (15 ± 2.3 mg CH4/L and 16 ± 6 mg S/L). The relatively low ratio of 36 ± 9 % (methane) and 34 ± 24 % (sulfide) indicated that their removal relied on nitrite dosing concentration (Fig. 1C). As such, a subsequent increase in nitrite concentration to 44 mg N/L was conducted after day 120 to improve methane and sulfide removal performance. The results showed that the overall methane removal performance was slightly enhanced, with an average removal ratio of 58 % (Fig. 1C). However, it was observed that the influent dissolved methane concentration was unstable during day 165–182, which was only 7.4 ± 1.2 mg CH4/L (Fig. 1A), due to the fluctuations of COD in raw sewage (Figure S1). Within this period, the effluent methane concentration only dropped to 3.6 ± 0.4 mg CH4/L (Fig. 1A). The low methane removal amount suggests that methane removal also relies on methane concentration, in addition to nitrite dosing amount. After day 182, the influent dissolved methane concentration was stable in the range of 15–20 mg CH4/L. The methane removal ratio at the steady state was 67 ± 4 %, which was significantly higher than that in the period with nitrite dosage of 33 mg N/L (p < 0.05).
In contrast, increasing the nitrite concentration to 48 mg N/L could effectively enhance sulfide removal (Fig. 1D). After day 150, the sulfide concentration in the effluent was only 1.2 ± 1.5 mg S/L, with a high removal ratio of over 90 % (Fig. 1D). Within this period, sulfide control was relatively stable, compared to the dynamic fluctuations in the effluent sulfide concentration before day 120 (Fig. 1B).
2.2. Evidence of nitrite-dependent microbial methane and sulfide oxidation
Copy numbers of n-DAMO bacteria and SOB. The abundance of n-DAMO bacteria and SOB was assessed using qPCR. n-DAMO bacteria were quantified by targeting both n-DAMO bacterial 16S rRNA genes and the functional gene pomA (Fig. 2A), while SOB bacteria were quantified using the functional gene soxB (Fig. 2B). The copy number of n-DAMO bacterial16S rRNA genes in sewer biofilm and sediment in the nitrite-dosed sewer reactor was found to be 1.4 (± 0.2) × 106 copies/g sludge and 0.9 (± 0.2) × 106 copies/g sludge, representing a substantial increase compared to those observed in the control reactor (0.1 (± 0.003) × 106 copies/g sludge and 0.1 (± 0.02) × 106 copies/g sludge) (Fig. 2A). They were in line with the increased abundance of the pmoA gene, collectively supporting the prevalence of n-DAMO bacteria in sewer biofilms and sediment in the nitrite-dosed sewer reactor (Fig. 2A). In addition, the abundance of the soxB gene in the nitrite-dosed sewer reactor was notably high, i.e. 329 (± 72) × 106 copies/g sludge (biofilm) and 62 (± 13) × 106 copies/g sludge (sediment), indicating the dominance of SOB under nitrite-dosed sewer conditions.
Fig. 2.
The abundance (copy number) of n-DAMO bacteria related 16S RNA gene (A) and function gene pmoA, and SOB related function gene soxB (B) in the biofilm and sediment sludge in the nitrite-dosed sewer reactor and control reactor, respectively.
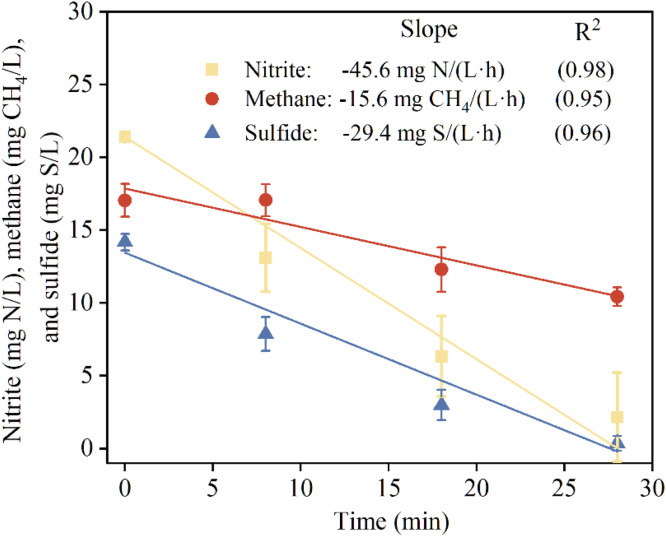
Nitrite-dependent methane and sulfide oxidation activity. Independent batch tests were carried out in triplicate to show the simultaneous oxidation of methane and sulfide coupled with nitrite reduction in the nitrite-dosed sewer reactor (Fig. 3). Following the dose of nitrite with an initial concentration of 22 mg N/L, sulfide and methane rapidly oxidized at an average rate of 29.4 mg S/(L·h) and 15.6 mg CH4/(L·h), respectively, while nitrite also dropped at a comparable rate of 45.6 mg N/(L·h). The simultaneous decline in these three indicators suggests that n-DAMO and sulfide oxidation occur concurrently. According to Eq. (3), the nitrite reduction rate by n-DAMO bacteria was determined to be 36 mg N/(L·h), approx. 78 % of the observed total nitrite reduction rate. This ratio was close to the average contribution (∼70 %) in the long-term result. In comparison, the same nitrite dosing test was conducted in the control reactor, but no methane and sulfide decline were observed (Figure S2), suggesting that both sulfide and methane oxidation are driven microbially rather than chemically. To repeat the phenomenon of diminished methane removal observed between days 165–182 with a low influent methane concentration (Fig. 1A), an additional batch test was undertaken in the nitrite-dosed reactor with the initial methane concentration hovered around 6 mg CH4/L. As expected, the fluctuations in methane levels were negligible (Figure S3), again suggesting that n-DAMO activity may be limited at low methane concentrations.
Fig. 3.
Nitrite-dependent simultaneous methane and sulfide oxidation activities in the sewer reactor with nitrite dosing. The lines present the linear fitting, with the slope presenting the rates. The error bars are the results of triplicate tests.
2.3. Cycling study results
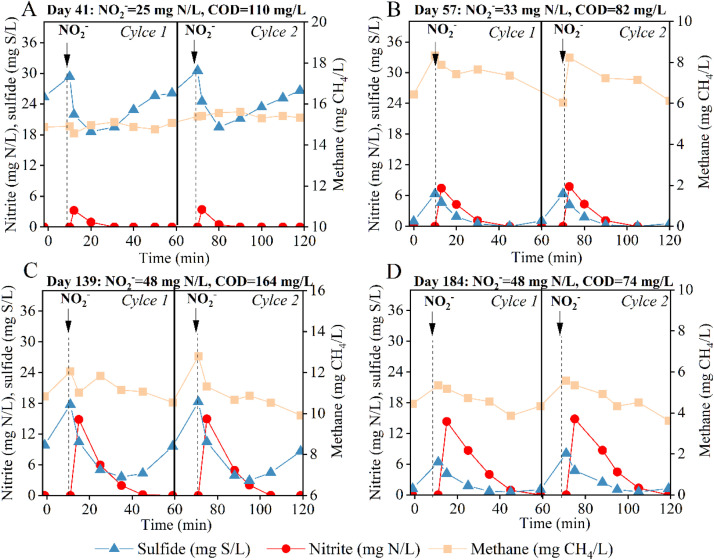
To understand the dynamic fluctuation in sulfide and methane removal by nitrite dosing, a series of periodic cycle tests were conducted (days 41, 57, 139, and 184), as illustrated in Fig. 4. On day 41, with 10 mL nitrite (25 mg N/L) dosed into the reactor, the initial nitrite concentration reached around 4 mg N/L due to dilution (Fig. 4A). Following that, the nitrite concentration appeared to decrease, concomitant with the decrease in sulfide concentration. This concurrent decline indicates the occurrence of nitrite-dependent sulfide oxidation. However, after the depletion of nitrite after 20–30 min, the sulfide concentration showed a remarkable recovery and reached around 25 mg S/L at the end of the cycle — a high level observed before nitrite dosing. This suggests that the present nitrite dosage did not effectively inhibit SRB activity. Overall, the amount of removed sulfide via nitrite-dependent sulfide oxidation was offset by the production of sulfide once nitrite was depleted, leading to the worse sulfide removal performance during days 35–45 (Fig. 1B).
Fig. 4.
Dynamic variations in the nitrite, sulfide, and methane concentrations during two typical cycles in the nitrite-dosed sewer reactor: day 41 (A), day 57 (B), day 139 (C) and day 184 (D).
In comparison, at 33 mg N/L of nitrite dosage on day 57, a low sulfide concentration was evident in a cycle (Fig. 4B). After nitrite dosing, sulfide rapidly declined to zero, and was not re-produced after nitrite was depleted, which is probably due to the absence of available COD as the substrate. To further explore the key effect of COD on sulfide production rebound, Fig. 4C and 4D depict the cycle results with higher nitrite concentrations (48 mg N/L) on days 139 and 184, respectively. On day 139, a substantial recovery in sulfide production (Fig. 4C), like that in Fig. 4A, also occurred after the nitrite depletion, again suggesting the high tolerance of SRB activity to nitrite dosing. On day 184, there is no recovery in sulfide concentration (Fig. 4D), resembling the scenario of COD insufficiency in Fig. 4B. These results indicate that SRB were not inhibited even at the highest nitrite dosage of 48 mg N/L. Once nitrite was depleted, whether sulfide could be re-produced by SRB was dependent on the availability of COD.
Additionally, the variations in methane concentration were measured in all cycles. Methane concentrations exhibited a clear decline under three conditions (Figs. 4B-D), except for the neglected variation under 25 mg N/L nitrite dosing (Fig. 4A). This characteristic aligns with long-term methane removal performance (Fig. 1A and 1C). Notably, there is no recovery in methane production after the depletion of nitrite in all cycles, indicating a low methane production activity.
2.4. The inhibitory effect of nitrite on sulfidogenic and methanogenic processes
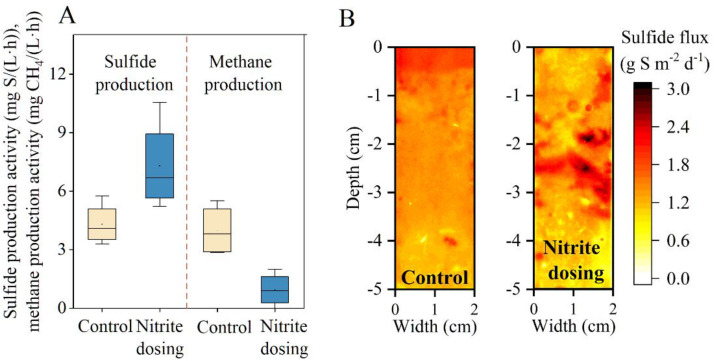
To ascertain the impact of nitrite dosing on methane and sulfide production activity, batch tests were conducted, as illustrated in Fig. 5A. The control lab-scale sewer reactor without any chemical dosing displayed a similar microbial activity between sulfide production and (4 mg S/(L·h)) and methane production (3.8 mg CH4/(L·h)). In contrast, the experimental reactor, subjected to long-term nitrite dosing, exhibited significant changes.
Fig. 5.
Sulfide and methane production activities in the control reactor without chemical dosing and the experiment reactor with nitrite dosing (A), and the 2D profiles of sulfide flux in the sediments of both reactors (B). The depth of 0 cm represents the water-sediment interface.
Methane production activity dropped below 0.5 mg CH4/(L·h), indicating effective inactivation of MA by nitrite dosing, consistent with previous studies reporting the sensitivity of MA to chemicals such as FA, FNA, and ferrate (Jiang et al., 2011; Yan et al., 2023; Zuo et al., 2023b). The low methane production activity reasonably explained no recovery of methane production after nitrite was depleted (Fig. 4).
In contrast, the long-term addition of nitrite enhanced rather than inhibited sulfide production activity (Fig. 5A). However, previous studies demonstrated that nitrite is toxic to most microbes including SRB (Mohanakrishnan et al., 2008). To delve into the enhanced mechanism of nitrite dosing on sulfidogenic activity, the novel diffusive gradient in thin films (DGT) technique was applied to measure microscale sulfidogenic activity within the sediment. Previous studies have shown that the DGT technique enables the direct identification of the hotspot layer of sulfide generation along the sediment depth (Zuo et al., 2020). Fig. 5B shows that the control reactor exhibited varying sulfide generation flux within the sediment, with the active zone located within the surface layer (0–0.5 cm) in the control reactor, with a sulfide production flux of 1.87 ± 0.04 g S m−2 d−1, similar with previous reports. In contrast, under the long-term pressure of nitrite dosing, sulfide production flux with the surface layer dropped to 0.8 ± 0.01 g S m−2 d−1. Instead, the sulfide generation hotspot shifted to the deeper layer (2–3 cm), providing the potential protection of sulfidogenic process against nitrite exposure (Fig. 5B). The downward migration of activity zones in sewer sediments has been reported in previous studies whose dosed various chemicals like nitrate and iron salts (Cao et al., 2019; Liu et al., 2015b).
2.5. Microbial utilization vs. microbial inhibition: implication for urban integrated water management
In sewer management, sulfide-induced pipe corrosion and odor are persistent challenges, prompting decades of focused attention and mitigation measures globally. As the imperative for achieving net-zero carbon emissions gains prominence, a corresponding commitment to reducing methane emissions in the sewer sector emerges. This study demonstrates a novel microbial utilization approach which is capable of removing sulfide and methane simultanously (Path II) (Fig. 6A). The long-term experimental result showed a 58 % removal of methane and a 91 % removal of sulfide at a practical nitrite dosing concentration (48 mg N/L), demonstrating the reliability of the proposed concept for sewer management.
Fig. 6.
A conceptual diagram involoving two paths for simulaneous sulfide and methane removal from sewers (A), and a novel urban wastewater management concept integrating urine collection and nitritation based on two paths (B).
Recent studies mainly focused on microbial inhibition strategies (Path I) to suppress source production of sulfide and methane within sewer biofilms or sediments (Fig. 6A). However, challenges remain. Microbial inhibition strategies require the identification of sulfide and methane production hotspots, which is difficult in actual sewer networks (Zuo et al., 2021). In addition, microbial inhibition usually relies on a several-hour exposure to chemicals with high concentrations, e.g., 200 mg N/L of nitrite at pH 6 for 8–24 h. This is difficult because the added chemicals can quickly degrade and dilute along the pipeline. SRB and MA are often protected within sewer biofilms and sediments, which may also result in the added chemicals having a weaker inactivation effect. As evidenced in Fig. 5B, nitrite dosing simulated a high sulfide production activity in the deep layer of sediments. A crucial reason for the limited application of microbial utilization (path II) was the lack of methane oxidation process in sewers. Until recently, we discovered the growth of n-DAMO bacteria in sewer systems, pointing the possibility of developing next-generation biotechnologies in sewer management (Zuo et al., 2024). Herein this study demonstrated the feasibility of leveraging the n-DAMO bacteria and SOB for methane and sulfide removal in sewer systems. Besides, the competition between sulfide oxidation and methane oxidation driven by nitrite reduction is comparable, but with methane consumption, n-DAMO activity will be significantly limited, whereas sulfide oxidation activity will remain high (Fig. 1). This difference warrants further studies.
While nitrite-dependent microbial utilization approach is attractive, the practical application may be limited to the associated high cost due to continuous nitrite inputs. The search for a sustainable nitrite source becomes imperative. Recent interest in the recycling of urine for sewer management presents a noteworthy advancement. Studies demonstrated that urine can serve as a stable and cost-effective source of nitrite through microbial nitritation, thereby delivering huge economic and environmental benefits (Mackey et al., 2016; Zheng et al., 2017). Based on the findings of this study, we can further propose a new urban integrated wastewater management concept, i.e., urine nitritation for sewer sulfide and methane oxidation (Fig. 6B). In earlier studies (Chen et al., 2013; Zheng et al., 2017; Zuo et al., 2023b), urine has been introduced into sewer systems for sulfide control, either by inhibition or oxidation mechnisam, but methane oxidation is missing. Here our proposed nitrite-dependent oxidation approach integrating n-DAMO and sulfide oxidation can simultaneously mtigiate sulfide and methane in sewers, thereby supporting sewer management from a renewed perspective. The added benefit of nitrogen pollutant removal underscores a paradigm shift of sewer system from the conventional “wastewater collection system” to a more advanced “wastewater treatment system”, which encapsulates a forward-thinking vision for the future of urban wastewater management.
It is important to noted that this work remains a proof-of-concept study that requires further understanding and optimzation. For instance, it is estimated that ∼70 % of nitrite was consumed through the n-DAMO process based on chemical analysis of long-term data (Fig. 1). A more accurate mass balance can be achieved using isotope tracing techniques. Besides, the proposed microbial utilization approach was demonstrated in a lab-scale preudo sewer system, highlighting the necessity for further scale-up testing and field trials. Real sewers operate under dynamic anaerobic/aerobic conditions with varying water quality. In our study, the approach demonstrated effectiveness only under anaerobic conditions, making it suitable for anaeobic scenarios in pressure main sewers. However, in gravity sewers, where oxygen is prevalent due to reaeration, the electron acceptor may switch to oxygen instead of nitrite. Additionally, the amount of available COD in wastewater influences the sulfide and methane oxidation effectiveness, as COD serves as the effective electron donor for nitrite. In addition to COD, ammonium may also used as an electron donor for nitrite through anaerobic ammonium oxidation. All these factors warrant systematic investigation. Finally, it is essential to emphasize that the feasibility of microbial utilization (Path II) does not render it a replacement for microbial inhibition (Path I). Path II is deemed suitable for extended sewer pipes with high concentrations of sulfide and methane originating from upstream pipes. Conversely, this control strategy may not be effective in sewer systems where methane and sulfide levels are low. This is a potential reason why previous studies did not discover n-DAMO process in sewer systems with upstream nitrite dosing. Therefore, the selection between the two paths necessitates a thoughtful consideration of real sewer structures and water quality. Moreover, coupling both paths is also possible under specific sewer conditions.
3. Conclusions
This study investigates in-sewer nitrite dosing for simultaneous sulfide and methane control via novel "microbial utilization" path in a simulated sewer reactor. The main findings are as follows:
-
1)
Microbial utilization involves SOB and n-DAMO processes to co-utilize nitrite as an electron acceptor, achieving a 58 % reduction in dissolved methane and over 90 % sulfide removal from influent.
-
2)
The n-DAMO and sulfide oxidation activities were comparable, measured as 15.6 mg CH4/(L·h) and 29.4 mg S/(L·h), respectively, which was supported by high copy numbers of n-DAMO bacteria and SOB found in both sewer biofilms and sediments.
-
3)
Nitrite-dependent microbial utilization strategy serves as a promising alternative for enhancing simultaneous sulfide and methane control in sewer management. Future research should focus on the feasibility of using nitrite produced from urine to drive the microbial utilization pathway.
4. Materials and methods
4.1. Sewer reactor
Nitrite dosing was implemented in a lab-scale sewer reactor to remove sulfide and methane from wastewater. The feeding of the reactor was the real domestic wastewater that contained high concentrations of sulfide and methane, daily produced from another identical lab-scale sewer reactor without any chemical dosing.
The sewer reactor had a volume of 1.8 L (12 cm height and 14 cm inner diameter) and was equipped with a mechanical stirrer (ZT 3IK15RGN—C) operating at 40 rpm. The sewer reactor was covered with a black cloth to simulate the dark environment of sewer systems. The reactor contained mature sewer sediment (about 5 cm deep) and sewer biofilm on the inner walls. The sewer reactor was inoculated with sludge containing n-DAMO bacteria Candidatus Methylomirabilis. The relative abundance of n-DAMO bacteria in the inoculum was about 2 %. The feeding pattern consisted of 20 cycles per day (once per hour, excluding 1:00–5:00am). Each feed pumping event was 10 min, delivering 0.3 L of wastewater into the reactor. The operating temperature was maintained at 20 °C ± 2 °C.
4.2. Long-term nitrite dosing experiments
Nitrite dosing was initiated on day 0. A nitrite stock solution (1 g N/L), prepared with sodium nitrite (NaNO2), was introduced into the reactor after each sewage feeding event (i.e., 20 cycles per day), completing within 1 min via a peristaltic pump (Long, Model: BT100–2 J). The dosed nitrite volume varied, specifically with approximately 10 mL (0–35 days), 8 mL (35–45 days), 10 mL (45–120 days), and 13 mL (120–240 days). These volumes corresponded to 33, 25, 33, and 48 mg of NO2−-N/L in influent wastewater, respectively, which are in the practical range of the nitrite dosage in sewers (Mohanakrishnan et al., 2008).
Throughout the nitrite dosing operation, sulfide and methane concentrations in the influent and effluent of the reactor were measured 3–5 times per week. COD and nitrite concentrations in the influent and effluent were simultaneously monitored. Biofilm and sediment-surface (0–0.5 cm) samples in nitrite-dosed sewer reactor were collected at the end of reactor operation and stored at −80 °C for microbial analysis. In the meantime, the microbial samples from another lab sewer reactor without any chemical dosing were also collected as a control (i.e., control reactor).
Cycle studies were conducted at different periods (days 41, 57, 139 and 184) to gain insights into the dynamic characteristics of sulfide and methane removal by nitrite. In each cycle, 5 mL liquid was collected every 10–20 min to monitor the variations in sulfide, methane, and nitrite concentrations. Each cycle test was conducted in duplicate to prove the reliability of the data.
4.3. Bioactivity assessment
Nitrite-dependent methane and sulfide oxidation activities. To showcase the simultaneous oxidation of methane and sulfide coupling with nitrite reduction in the nitrite-dosed sewer reactor, independent batch tests were conducted in situ at the steady state. Long-term nitrite dosing was halted before batch tests to obtain a high initial concentration of methane and sulfide. Subsequently, 18 mL of nitrite stock solution (1 g N/L) was added, resulting in an initial nitrite concentration of 20–25 mg N/L. Liquid samples were collected every 7 min, and the concentrations of the sulfide, methane, and nitrite in these samples were measured. Batch tests lasted 30 min and were performed in triplicate. Linear regression of concentrations versus time was applied to derive slopes representing nitrite-dependent methane and sulfide oxidation activities. For a control, the same nitrite dosing batch test was also performed in another identical lab-scale sewer reactor without any chemical dosing (control reactor).
Methane and sulfide production activities. To determine the impact of nitrite dosing on methane and sulfide production activities, bioactivity tests were conducted monthly for both the nitrite-dosed sewer reactor and the control reactor. The liquid in sewer reactors was replaced with re-injecting fresh raw wastewater at the beginning of the test, and 4–5 samples were collected over a 2-hour period for measuring dissolved sulfide and methane concentrations. Linear regression of concentrations versus time was applied to obtain slopes representing sulfidogenic and methanogenic activities.
DGT technique. At the end of the operation, a novel technique, namely diffusive gradient in thin films (DGT), was employed to measure two-dimensional, high-resolution sulfidogenic activity in sediments of the nitrite-dosed sewer reactor and the control reactor. The principle of DGT for sulfide measurement in sewer sediment was introduced in previous studies (Zuo et al., 2021, 2020). The DGT was prepared according to the reported study (Ding et al., 2012), with the probe provided by Easysensor Ltd. (www.easysensor.net). To initiate the DGT measurement, the liquid in sewer reactors was replaced with fresh raw wastewater at the beginning of the test. After 10 min, the DGT probe was vertically inserted into the sediment of the reactor. The reactors were hermetically sealed to prevent any H2S loss during the measurement process. The probe was strategically positioned at the sediment-water interface using a ceramic knife. After 40 min, the DGT probes were carefully extracted for subsequent analysis (see more details in Text S1 of Supporting Information (SI)).
4.4. Analytical methods
The concentrations of COD, sulfide, and nitrite were determined using standard methods (Ministry of Environmental Protection, 2006). The sulfate and VFA concentrations were measured using ion chromatography (ICS-2000 Dionex, USA) and gas chromatography (GC-6890 N Agilent, USA), respectively. To measure the dissolved methane concentrations, 2 mL of wastewater was filtered through a 0.45-µm filter membrane using a syringe with a needle into a vacuum tube. After the gas-liquid equilibrium was attained overnight, a gas chromatography instrument (GC-7890A Agilent, USA) equipped with a flame ionization detector was used to determine the methane concentration in the gas phase. The methane concentration in the liquid phase was calculated using the mass balance equation and Henry's law (Guisasola et al., 2008). Additionally, the pH was monitored using a pH probe meter (WTW, pH/Oxi340i, Germany).
Real-time quantitative PCR (qPCR) was used to measure the abundance of the n-DAMO bacteria by quantifying the 16S rRNA genes and pmoA genes of n-DAMO bacteria in the collected samples. Specifically, the 16S rRNA gene of n-DAMO bacteria was determined using the primer sets of qp1F and qp1R, while the pmoA gene of n-DAMO bacteria was quantified using the primer sets of cmo182 and cmo568 (Hu et al., 2014). The detection of both genes made the quantification of n-DAMO bacteria more convincing. More details of qPCR were described in the previous study (Hu et al., 2014).
CRediT authorship contribution statement
Zhiqiang Zuo: Writing – review & editing, Writing – original draft, Supervision, Methodology, Funding acquisition, Conceptualization. Yaxin Xing: Methodology, Investigation. Xi Lu: Validation. Tao Liu: Writing – review & editing. Min Zheng: Writing – review & editing, Supervision. Miao Guo: Writing – review & editing. Yanchen Liu: Writing – review & editing, Supervision, Project administration, Funding acquisition. Xia Huang: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research was supported by the National Key Research and Development Program of China (2022YFC3203200), the National Natural Science Foundation of China (No. 52270091 and No. 52300188). Dr. Min Zheng is the recipient of an Australian Research Council (ARC) Industry Fellowship (IE230100245). Dr. Tao Liu is the recipient of an ARC DECRA Fellowship (DE220101310).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.wroa.2024.100231.
Appendix. Supplementary materials
Data availability
Data will be made available on request
References
- An Z., Chen F., Zheng Y., Zhou J., Liu B., Qi L., Lin Z., Yao C., Wang B., Wang Y. Role of n-DAMO in mitigating methane emissions from intertidal wetlands is regulated by saltmarsh vegetations. Environ. Sci. Technol. 2024;58(2):1152–1163. doi: 10.1021/acs.est.3c07882. [DOI] [PubMed] [Google Scholar]
- Cao J., Zhang L., Hong J., Sun J., Jiang F. Different ferric dosing strategies could result in different control mechanisms of sulfide and methane production in sediments of gravity sewers. Water Res. 2019;164 doi: 10.1016/j.watres.2019.114914. [DOI] [PubMed] [Google Scholar]
- Chen G.H., Qian J., Peng G.L., Liang Z.S., Jiang F. Nitrogen removal capacity of simultaneously autotrophic and heterotrophic denitrification in a sewer receiving nitrified source-separated urine. Water Pract. Techn. 2013;8(1):33–40. [Google Scholar]
- Despot D., Reinhold L., Augustyniak A., Barjenbruch M. Dosing free nitrous acid as an alternative sulphide control technology for pressure sewers in Germany. Water. 2021;13(8):1015. [Google Scholar]
- Deutzmann J.S., Stief P., Brandes J., Schink B. Anaerobic methane oxidation coupled to denitrification is the dominant methane sink in a deep lake. Proceed. Nat. Acad. Sci. 2014;111(51):18273–18278. doi: 10.1073/pnas.1411617111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Sun Q., Xu D., Jia F., He X., Zhang C. High-resolution simultaneous measurements of dissolved reactive phosphorus and dissolved sulfide: the first observation of their simultaneous release in sediments. Environ. Sci. Technol. 2012;46(15):8297–8304. doi: 10.1021/es301134h. [DOI] [PubMed] [Google Scholar]
- Ettwig K.F., Butler M.K., Le Paslier D., Pelletier E., Mangenot S., Kuypers M.M., Schreiber F., Dutilh B.E., Zedelius J., de Beer D. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature. 2010;464(7288):543–548. doi: 10.1038/nature08883. [DOI] [PubMed] [Google Scholar]
- Fan S.Q., Wen W.R., Xie G.J., Lu Y., Nie W.B., Liu B.F., Xing D.F., Ma J., Ren N.Q. Revisiting the engineering roadmap of nitrate/nitrite-dependent anaerobic methane oxidation. Environ. Sci. Technol. 2023;57(50):20975–20991. doi: 10.1021/acs.est.3c02806. [DOI] [PubMed] [Google Scholar]
- Guisasola A., de Haas D., Keller J., Yuan Z. Methane formation in sewer systems. Water Res. 2008;42(6–7):1421–1430. doi: 10.1016/j.watres.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Haroon M.F., Hu S., Shi Y., Imelfort M., Keller J., Hugenholtz P., Yuan Z., Tyson G.W. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature. 2013;500(7464):567–570. doi: 10.1038/nature12375. [DOI] [PubMed] [Google Scholar]
- Hu B.l., Shen L.d., Lian X., Zhu Q., Liu S., Huang Q., He Z.f., Geng S., Cheng D.q., Lou L.p. Evidence for nitrite-dependent anaerobic methane oxidation as a previously overlooked microbial methane sink in wetlands. Proceed. Nat. Acad. Sci. 2014;111(12):4495–4500. doi: 10.1073/pnas.1318393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G., Gutierrez O., Sharma K.R., Yuan Z. Effects of nitrite concentration and exposure time on sulfide and methane production in sewer systems. Water Res. 2010;44(14):4241–4251. doi: 10.1016/j.watres.2010.05.030. [DOI] [PubMed] [Google Scholar]
- Jiang G., Gutierrez O., Yuan Z. The strong biocidal effect of free nitrous acid on anaerobic sewer biofilms. Water Res. 2011;45(12):3735–3743. doi: 10.1016/j.watres.2011.04.026. [DOI] [PubMed] [Google Scholar]
- Liu T., Li J., Khai Lim Z., Chen H., Hu S., Yuan Z., Guo J. Simultaneous removal of dissolved methane and nitrogen from synthetic mainstream anaerobic effluent. Environ. Sci. Technol. 2020;54(12):7629–7638. doi: 10.1021/acs.est.0c00912. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ni B.J., Sharma K.R., Yuan Z. Methane emission from sewers. Sci. Total Environ. 2015;524:40–51. doi: 10.1016/j.scitotenv.2015.04.029. [DOI] [PubMed] [Google Scholar]
- Liu Y., Sharma K.R., Ni B.J., Fan L., Murthy S., Tyson G.Q., Yuan Z. Effects of nitrate dosing on sulfidogenic and methanogenic activities in sewer sediment. Water Res. 2015;74:155–165. doi: 10.1016/j.watres.2015.02.017. [DOI] [PubMed] [Google Scholar]
- Mackey H.R., Morito G.R., Hao T., Chen G.H. Pursuit of urine nitrifying granular sludge for decentralised nitrite production and sewer gas control. Chem. Eng. J. 2016;289:17–27. [Google Scholar]
- Mahmood Q., Zheng P., Cai J., Wu D., Hu B., Li J. Anoxic sulfide biooxidation using nitrite as electron acceptor. J. Hazard. Mater. 2007;147(1–2):249–256. doi: 10.1016/j.jhazmat.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Ministry of Environmental Protection . Monitoring and Analyticalmethods of Water and Wastewater. 4th ed. China environmental science press; Beijing, China: 2006. People's Republic of China. [Google Scholar]
- Mohanakrishnan J., Gutierrez O., Meyer R.L., Yuan Z. Nitrite effectively inhibits sulfide and methane production in a laboratory scale sewer reactor. Water Res. 2008;42(14):3961–3971. doi: 10.1016/j.watres.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Pikaar I., Sharma K.R., Hu S., Gernjak W., Keller J., Yuan Z. Reducing sewer corrosion through integrated urban water management. Science (1979) 2014;345(6198):812–814. doi: 10.1126/science.1251418. [DOI] [PubMed] [Google Scholar]
- Raghoebarsing A.A., Pol A., Van de Pas-Schoonen K.T., Smolders A.J., Ettwig K.F., Rijpstra W.I.C., Schouten S., Damsté J.S.S., Op den Camp H.J., Jetten M.S. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature. 2006;440(7086):918–921. doi: 10.1038/nature04617. [DOI] [PubMed] [Google Scholar]
- Soued C., Harrison J.A., Mercier-Blais S., Prairie Y.T. Reservoir CO2 and CH4 emissions and their climate impact over the period 1900–2060. Nat. Geosci. 2022;15(9):700–705. [Google Scholar]
- van Kessel M.A., Stultiens K., Slegers M.F., Cruz S.G., Jetten M.S., Kartal B., den Camp H.J.O. Current perspectives on the application of N-damo and anammox in wastewater treatment. Curr. Opin. Biotechnol. 2018;50:222–227. doi: 10.1016/j.copbio.2018.01.031. [DOI] [PubMed] [Google Scholar]
- Wang W., Zhang Y., Yin T.M., Zhao L., Xu X.J., Xing D.F., Zhang R.C., Lee D.J., Ren N.Q., Chen C. Prospect of denitrifying anaerobic methane oxidation (DAMO) application on wastewater treatment and biogas recycling utilization. Sci. Total Environ. 2023 doi: 10.1016/j.scitotenv.2023.167142. [DOI] [PubMed] [Google Scholar]
- Yan X., Sun J., Wang Y., Zhang Z., Zhang C., Li W., Xu J., Dai X., Ni B.J. Low-rate ferrate dosing damages the microbial biofilm structure through humic substances destruction and facilitates the sewer biofilm control. Water Res. 2023;235 doi: 10.1016/j.watres.2023.119834. [DOI] [PubMed] [Google Scholar]
- Yang W., Vollertsen J., Hvitved-Jacobsen T. Anoxic sulfide oxidation in wastewater of sewer networks. Water Sci. Techn. 2005;52(3):191–199. [PubMed] [Google Scholar]
- Zhang L., Qiu Y.Y., Sharma K.R., Shi T., Song Y., Sun J., Liang Z., Yuan Z., Jiang F. Hydrogen sulfide control in sewer systems: a critical review of recent progress. Water Res. 2023 doi: 10.1016/j.watres.2023.120046. [DOI] [PubMed] [Google Scholar]
- Zheng M., Zuo Z., Zhang Y., Cui Y., Dong Q., Liu Y., Huang X., Yuan Z. Nitrite production from urine for sulfide control in sewers. Water Res. 2017;122:447–454. doi: 10.1016/j.watres.2017.05.048. [DOI] [PubMed] [Google Scholar]
- Zuo Z., Chang J., Lu Z., Wang M., Lin Y., Zheng M., Zhu D.Z., Yu T., Huang X., Liu Y. Hydrogen sulfide generation and emission in urban sanitary sewer in China: what factor plays the critical role? Environ. Sci. 2019;5(5):839–848. [Google Scholar]
- Zuo Z., Chen Y., Xing Y., Li S., Yang S., Jiang G., Liu T., Zheng M., Huang X., Liu Y. The advantage of a two-stage nitrification method for fertilizer recovery from human urine. Water Res. 2023;235 doi: 10.1016/j.watres.2023.119932. [DOI] [PubMed] [Google Scholar]
- Zuo Z., Ren D., Qiao L., Li H., Huang X., Liu Y. Rapid dynamic quantification of sulfide generation flux in spatially heterogeneous sediments of gravity sewers. Water Res. 2021;203 doi: 10.1016/j.watres.2021.117494. [DOI] [PubMed] [Google Scholar]
- Zuo Z., Sun Z., Zhang Y., Wang M., Ren D., Li S., Yuan Z., Liu Y., Huang X. In situ exploration of the sulfidogenic process at the water-sediment interface in sewers: mechanism and implications. ACS. ES. T. Eng. 2020;1(3):415–423. [Google Scholar]
- Zuo Z., Xing Y., Duan H., Ren D., Zheng M., Liu Y., Huang X. Reducing sulfide and methane production in gravity sewer sediments through urine separation, collection and intermittent dosing. Water Res. 2023;234 doi: 10.1016/j.watres.2023.119820. [DOI] [PubMed] [Google Scholar]
- Zuo Z., Xing Y., Liu T., Zheng M., Lu X., Chen Y., Jiang G., Liang P., Huang X., Liu Y. Methane mitigation via the nitrite-DAMO process induced by nitrate dosing in sewers. Water Res. 2024 doi: 10.1016/j.watres.2024.121701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request