Abstract
Nitrogen heterocycles have drawn considerable attention because of their structurally novel and significant biological activities. Marine-derived fungi, especially the Aspergillus species, possess unique metabolic pathways to produce secondary metabolites with novel structures and potent biological activities. This review prioritizes the structural diversity and biological activities of nitrogen heterocycles that are produced by marine-derived Aspergillus species from January 2019 to January 2024, and their relevant biological activities. A total of 306 new nitrogen heterocycles, including seven major categories—indole alkaloids, diketopiperazine alkaloids, quinazoline alkaloids, isoquinoline alkaloids pyrrolidine alkaloids, cyclopeptide alkaloids, and other heterocyclic alkaloids—are presented in this review. Among these nitrogen heterocycles, 52 compounds had novel skeleton structures. Remarkably, 103 compounds showed various biological activities, such as cytotoxic, antimicrobial, anti-inflammatory, antifungal, anti-virus, and enzyme-inhibitory activities, and 21 compounds showed potent activities. This paper will guide further investigations into the structural diversity and biological activities of nitrogen heterocycles derived from the Aspergillus species and their potential contributions to the future development of new natural drug products in the medicinal and agricultural fields.
Keywords: Aspergillus sp., marine, secondary metabolite, nitrogen heterocycles, biological activity
1. Introduction
The marine ecosystem has attracted the attention of natural product chemists due to the prospects of biologically active marine natural products. Marine natural products have attracted much attention from both natural product chemists and pharmacologists due to their novel structure and potential bioactivities [1,2,3,4]. One of the most interesting classes of marine natural products is nitrogen heterocycles. Marine-derived nitrogen-containing secondary metabolites exhibit a variety of biological activities, such as cytotoxic, antifungal, antibacterial, anti-inflammatory, and enzyme-inhibitory activities [1,5,6].
Marine-derived fungi, especially marine-derived Aspergillus species, are the richest sources of these basic nitrogen-containing secondary metabolites, which can produce a large number of structurally unique heterocyclic alkaloids [5,6,7]. In the present review, we have updated the diversity and biological activities of nitrogen heterocycles that are produced by marine-derived Aspergillus species. Herein, a total of 306 new compounds (including 13 pairs of racemates) reported from the beginning of January 2019 to January 2024 are included, and 115 references are cited in this review. We only report the previously undescribed compounds isolated during this period, while the compounds reported before this period and mentioned in the references used in this review are not included. The relevant biological and pharmacological activities of some potential alkaloids are also highlighted, which will benefit future drug development and innovation.
2. Structural and Biological Activity Studies
Based on the literature search, 306 previously undescribed nitrogen heterocycles (1–293, including 13 pairs of racemates) were obtained from marine-derived Aspergillus species from 2019 to 2024 (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14 and Figure 15). These heterocyclic alkaloids could be classified into seven major categories—indole alkaloids (1–43), diketopiperazine alkaloids (44–149), cyclopeptide alkaloids (150–181), quinazoline alkaloids (182–218), isoquinoline alkaloids (219–235), pyrrolidine alkaloids (236–256), and other heterocyclic alkaloids (257–293)—based on their structural patterns. The racemates were purified by chiral HPLC analysis. The structures and the absolute configurations of the new compounds and novel skeleton compounds were elucidated by a detailed spectroscopic analysis of NMR and MS data, time-dependent density functional theory (TDDFT)/ECD calculations, DP4+ probability predictions, as well as single-crystal X-ray diffraction. The absolute configurations of the amino acid residues of the peptides were determined by Marfey’s method.
Figure 1.
Indole alkaloids produced by marine-derived Aspergillus species (1–18).
Figure 2.
Indole alkaloids produced by marine-derived Aspergillus species (19–43).
Figure 3.
Diketopiperazine alkaloids produced by marine-derived Aspergillus species (44–54).
Figure 4.
Diketopiperazine alkaloids produced by marine-derived Aspergillus species (55–72).
Figure 5.
Diketopiperazine alkaloids produced by marine-derived Aspergillus species (73–93).
Figure 6.
Diketopiperazine alkaloids produced by marine-derived Aspergillus species (94–120).
Figure 7.
Diketopiperazine alkaloids produced by marine-derived Aspergillus species (121–149).
Figure 8.
Cyclopeptide alkaloids produced by marine-derived Aspergillus species (150–161).
Figure 9.
Cyclopeptide alkaloids produced by marine-derived Aspergillus species (172–181).
Figure 10.
Quinazoline alkaloids produced by marine-derived Aspergillus species (182–218).
Figure 11.
Isoquinoline alkaloids produced by marine-derived Aspergillus species (219–235).
Figure 12.
Pyrrolidine alkaloids produced by marine-derived Aspergillus species (236–256).
Figure 13.
Other heterocyclic alkaloids produced by marine-derived Aspergillus species (257–268).
Figure 14.
Other heterocyclic alkaloids produced by marine-derived Aspergillus species (269–278).
Figure 15.
Other heterocyclic alkaloids produced by marine-derived Aspergillus species (279–293).
2.1. Indole Alkaloids
Indole alkaloids are a class of alkaloids containing an indole moiety. The indole moiety, presented in a wide range of marine natural products, showed various bioactivities, which may be one of the most important components of the heterocycles in the discovery of new drugs [7,8,9]. A total of 44 new indole alkaloids (1–43, including 1 pair of racemates) were discovered from marine-derived Aspergillus species, including 11 compounds with novel skeleton structures (Figure 1 and Figure 2). Remarkably, 14 of them showed cytotoxic, pro-angiogenic, protein tyrosine phosphatase 1B-inhibitory, antibacterial, antifungal, and antivirus activities. Intriguingly, many of these indole alkaloids possess unique structural features as well as interesting biological and pharmacological activities.
One new paraherquamide, aculeaquamide A (1), was isolated from the marine-derived fungus Aspergillus aculeatinus WHF0198. Compound 1 showed cytotoxicity against Bel-7402 with an IC50 value of 3.3 μM [10]. A new indole alkaloid, asterriquinone F (2), was isolated from the brown alga-derived fungus Aspergillus terreus LM.1.5 [11]. A new tryptoquivaline analog, asperdiazapinone G (3), was isolated from the mangrove soil-derived fungus Aspergillus sp. WHUF03110 [12]. Two new prenylated indole alkaloid homodimers, di-6-hydroxydeoxybrevianamide E (4) and dinotoamide J (5), were obtained from the seawater-derived fungus Aspergillus austroafricanus Y32-2. Compound 5 exhibited pro-angiogenic activity in a PTK787-induced vascular injury zebrafish model at the concentration of 70 µg/mL [13]. Six new indole-diterpenoids, penerpene O (6), penerpenes Q–R (7–8) and penerpenes T–V (9–11), were isolated from the marine soft coral-derived fungus Aspergillus sp. ZF-104. Compound 8 bears a rare indolin-2-one unit in its structure, and 9 contains a reconstructed novel skeleton in which the indole ring and the terpenoid substructure were cleaved before being reconnected through the nitrogen atom. Compounds 6 and 10 showed protein tyrosine phosphatase 1B (PTP1B)-inhibitory activities, with IC50 values of 17.7 ± 0.7 and 28.1 ± 2.2 μM, respectively, comparable to that of the positive control NaVO3 (IC50, 33.6 ± 0.92 μM) [14]. One new indole glucoside, 6-methoxyindole-3-carboxylic acid O-β-D-glucopyranosyl ester (12), was isolated from sea cucumber-derived fungus Aspergillus fumigatus M580 [15]. Six new alkaloids, sclerotiamides C−H (13–18), were isolated from gorgonian-derived fungus Aspergillus sclerotiorum LZDX-33-4. Compounds 13 and 14 were notoamide-type alkaloids with the incorporation of a unique 2,2-diaminopropane unit, 15 and 16 were unprecedented notoamide hybrids with a new coumarin unit. Compound 18 represented a new highly oxidized notoamide scaffold. The whole genome was sequenced and analyzed using antiSMASH, in order to deduce the biogenetic pathway of the unprecedented notoamides. Compound 13 showed significant inhibitory activity against HeLa, A549, HepG2, and SMMC7721 cell lines with IC50 values of 1.7 ± 0.1, 1.6 ± 0.1, 1.8 ± 0.1, and 1.5 ± 0.1 μM, respectively. Compound 16 showed significant inhibitory activity against HeLa, A549, HepG2, and SMMC7721 cell lines with IC50 values of 7.9± 0.2, 7.8 ± 0.1, 8.1 ± 0.2, and 6.7 ± 0.2 μM, respectively. The preliminary study of mechanism indicated that 13 induced apoptosis in HeLa cells by arresting the cell cycle, activating ROS production, and regulating apoptosis-related proteins in the MAPK signaling pathway. The significant anti-HeLa effect of 13 suggested it to be a potential lead compound for further development as an anti-cervical tumor agent [16] (Figure 1).
Three new indole alkaloids, fumindolines, A–C (19–21), were obtained from the marine seawater-derived fungus Aspergillus fumigatus H22 [17]. Three new β-carboline alkaloids, aspercarbolines A–C (22–24), were isolated from the fungus Aspergillus sp. XBB-4 was isolated from the inner tissue of geoduck Panopea abbreviate, which was collected from the South China Sea. Compound 24 exhibited significant cytotoxic activity against human nasopharyngeal carcinoma cell lines (CNE1, CNE2, HONE1, and SUNE1) and human hepatocellular carcinomacell lines (hepG2 and QGY7701), with IC50 values of 16.29, 20.58, 20.11, 45.31, 50.85, and 28.97 μM, respectively [18]. Two new indole alkaloids, flavonoids A (25) and B (26), were isolated from the marine deep-sea-derived fungus Aspergillus flavipes DS720. Compound 25 showed high and broad-spectrum cytotoxic activities against HeLa, 5637, CAL-62, PATU8988T, A-375, and A-673 cell lines, with inhibition rates of (96.94 ± 0.62)%, (99.49 ± 0.50)%, (96.16 ± 1.34)%, (90.83 ± 3.31)%, (99.32 ± 0.11)%, and (90.01 ± 5.81)%, respectively, at the concentration of 20 µM, indicating that 25 may possess certain potential for the development of lead compounds in the future [19]. A pair of inseparable mixtures of secofumitremorgins A (27a) and B (27b), which differed in the configuration of the nitrogen atom, were isolated and identified from the deep-sea sediment-derived fungus Aspergillus fumigatus SD-406. Their structures were determined through a detailed spectroscopic analysis of NMR and MS data, a chiral HPLC analysis of the acidic hydrolysate, an X-ray crystallographic analysis, a J-based configuration analysis, and quantum chemical calculations of ECD, OR, and NMR (with DP4+ probability analysis). Compounds 27, 27a, and 27b exhibited activity against aquatic pathogenic bacteria Vibrio alginolyticus and Edwardsiella tarda and the plant pathogenic fungi Fusarium graminearum Schw, with minimum inhibitory concentration (MIC) values of 32, 64, and 4 µg/mL, respectively [20]. Aspergillipeptides H–I (28–29) were isolated from the marine gorgonian-associated fungus Aspergillus sp. SCSIO 41501 [21]. Four new indole diterpenoids, ascandinines A–D (30–33), were isolated from an Antarctic sponge-derived fungus Aspergillus candidus HDN15-152. Compound 30 possesses an unprecedented 2-oxabicyclo [2.2.2]octan-3-ol motif embedded in a pentacyclic ring system, while 31–33 represent a rare type of indole diterpenoid featuring the 6/5/5/6/6/6/6-fused ring system. Compound 32 displayed anti-influenza virus A (H1N1) activity with an IC50 value of 26 μM, while compound 33 showed cytotoxicity against HL-60 cells with an IC50 value of 7.8 μM [22]. Two new β-carboline alkaloids, aspergillspins A–B (34–35), were isolated from the marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501 [23]. Three new bisindolylquinones, asterriquinones I–K (36–38), and three new bis-indolylbenzenoids asterriquinols G–I (39–41), were isolated from the sponge-derived fungus Aspergillus sp. SCSIO 41018. Asterriquinone I (41) represented the first reported bis-indolylquinone possessing a chlorine atom. Compound 36 exhibited cytotoxic activities against K562, BEL-7042, and SGC-7901 cell lines, with IC50 values of 17.9 ± 0.62, 25.8 ± 0.16, and 29.2 ± 0.32, respectively. Compound 37 displayed cytotoxic activities against K562, BEL-7042, and SGC-7901 cell lines, were IC50 values of 8.5 ± 0.17, 11.1 ± 0.22, and 18.7 ±0.45 μM, respectively. Compound 38 showed cytotoxic activities against K562, BEL-7042, and SGC-7901 cell lines, with IC50 values of 13.0 ± 0.36, 14.9 ± 0.55, and 26.2 ± 0.13 μM, respectively [24]. Two new isomeric modified tripeptides, aspergillamides C and D (42 and 43), were obtained from the marine sponge Callyspongia sp.-derived fungus Aspergillus terreus SCSIO 41008 [25] (Figure 2).
2.2. Diketopiperazine Alkaloids
Diketopiperazine alkaloids have proven to be the most abundant heterocyclic alkaloids up to now, usually possessing diverse scaffolds and rich biological activities. Diketopiperazine alkaloids are widely distributed in filamentous fungi, especially in the genera Aspergillus and Penicillium of the phylum Ascomycota or sac fungi [26,27]. A total of 115 new diketopiperazine alkaloids (44–149, including 9 pairs of racemates) were discovered from marine-derived Aspergillus species (Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7), 18 of them with novel skeleton structures. Thirty-seven of them showed anti-virus, cytotoxic, inhibit thioredoxin reductase, antibacterial, antifungal, anti-inflammatory, α-glucosidase-inhibitory, and angiotensin-converting enzyme-inhibitory activities.
Six new diketopiperazines, (±)-7,8-epoxy-brevianamide Q ((±)−44), (±)-8-hydroxy-brevianamide R ((±)−45), and (±)-8-epihydroxy-brevianamide R ((±)−46), were isolated from a marine sediment-derived fungus Aspergillus versicolor MF180151. Compound 44 is the first sample of brevianamides with an epoxy moiety. The racemates of 44–46 were isolated by chiral HPLC analysis, and their structures were clarified using the calculated ECD, and DP4+ probability methods. [28]. A pair of new spirocyclic alkaloids, (-)-5-isopentenyl-cryptoechinuline D (47a) and (+)-5-isopentenyl-cryptoechinuline D (47b), were obtained from a marine moss-derived fungus Aspergillus ruber TX-M4-1. Compound 47a was shown to inhibit thioredoxin reductase (TrxR) activity with an IC50 value of 6.2 μmol/L [29]. Two pairs of new dimeric diketopiperazine alkaloids, (±)-dibrevianamides Q1 ((±)-48) and Q2 ((±)-49), were obtained from a marine-derived fungus Aspergillus sp. ZA-01. Compounds (+)-48 and (−)-49 were clarified using the calculated ECD and DP4+ probability methods. Compounds 48 and 49 displayed anti-H1N1 virus activity, with IC50 values of 12.6 and 19.5 μM, respectively. Compound (+)-48 showed significant activity against Mycobacterium tuberculosis (MIC, 10.2 μg/mL) [30]. A new diketopiperazine alkaloid, versicolamide C (50), was isolated from a soft coral-derived fungus Aspergillus sp. SCSIO 41036 [31]. Two new thiodiketopiperazines, emestrins L (51) and M (52), were obtained from the sea hare Aplysia pulmonica-derived fungus Aspergillus terreus RA2905 [32]. Two pairs of diketopiperazine alkaloids, (±)-brevianamide Z [(±)-53] and (±)-brevianamide Z1 [(±)-54], were isolated from the sea mud-derived fungus Aspergillus versicolor. Their absolute configurations were assigned based on extensive spectroscopic analysis and calculated using the NMR, ECD, ORD, and DP4+ methods [33] (Figure 3).
Ten new diketopiperazine alkaloids, pyranamides A–D (55–58), secopyranamide C (59), and protuboxepins F–J (60–64), were isolated from the marine sponge-derived fungus Aspergillus versicolor SCSIO 41016. Compounds 55–58 were obtained as rare 1-oxa-8,10-diazaspiro [5.5]undecane containing diketopiperazine alkaloids, while 59 was obtained as a tetrahydropyran-ring cleavage derivative. Plausible biosynthetic pathways of 55–64 were postulated [34]. Two new diketopiperazine alkaloids, sclerotioloids A (65) and C (66), were obtained from the sponge-derived fungus Aspergillus sclerotiorum ST0501 [35]. One new oxepin-containing diketopiperazinetype alkaloid, oxepinamide L (67), was isolated from the culture of the marine coral-derived fungus Aspergillus puniceus [36]. Three new diketopiperazine alkaloids, asperindopiperazines A–C (68–70), were isolated from the Mariana-Trench-associated fungus Aspergillus sp. SY2601 [37]. One new diketopiperazine alkaloid, 12β,13β-hydroxy-asperfumigatin (71), was isolated from the marine-derived fungus Aspergillus fumigatus H22 [17]. Two new diketopiperazine alkaloids, (+)- and (−)-brevianamide X ((±)-72), were isolated from the seaweed Enteromorpha prolifera-derived fungus Aspergillus versicolor OUCMDZ-2738. Their structures were elucidated based on spectroscopic analysis, specific rotation analysis, ECD, and X-ray crystallographic analysis. Compound 72 was further resolved into the corresponding optically pure enantiomers and its absolute configurations were determined for the first time. A plausible biosynthetic relationship of 72, through a sequence of oxidative transformations and hydrolysis or methanolysis, was illustrated [38] (Figure 4).
A new asymmetric diketopiperazine dimer, asperflocin (73), was isolated from the sponge-associated fungus Aspergillus versicolor 16F-11. Compound 73 showed moderate cytotoxic activity against A375 cell lines, with an IC50 value of 10.29 ± 2.37 μM [39]. Four new indole diketopiperazine alkaloids, aspechinulins A-D (74–77), were isolated from the deep-sea sediment-derived fungus Aspergillus sp. FS445. Compounds 74–76 represented the first examples of indole diketopiperazine derivatives constructing a C5 unit at 11-NH through an imide linkage. Compound 76 exhibited potential inhibitory activities against NO production with the same IC50 value of the positive control aminoguanidine (IC50, 23.7 μM) [40]. Two new diketopiperazines, 5-prenylcryptoechinulin A (78) and 9-epi-didehydroechinulin (79), were obtained from the deep-sea-derived fungus Aspergillus chevalieri MCCC M23426. Compound 78 displayed selective antibacterial activities against Staphylococcus aureus CICC 10384, with an inhibition rate of over 90%, at the concentration of 250 µM [41]. One new piperazinedione, asperdione A (80), was isolated from the Aspergillus sp. XBB-4, which was isolated from the inner tissue of geoduck Panopea abbreviate, which was collected from the South China Sea. Compound 80 exhibited significant cytotoxic activity against human nasopharyngeal carcinoma cell lines (CNE1, CNE2, HONE1 and SUNE1) and human hepatocellular carcinomacell lines (hepG2 and QGY7701), with IC50 values of 22.00, 18.93, 21.61, 16.93, 12.33, and 10.72, respectively [18]. Two new diketopiperazine alkaloids, (+)19-epi-sclerotiamide (81) and (–)19-epi-sclerotiamide (82), were isolated from a soft coral-associated epiphytic fungus Aspergillus versicolor CGF 9-1-2 [42]. Three new emestrin-type thiodiketopiperazines, didethio-11α-methylthioemestrin (83), 7′-epi-didethio-11α-methylthioemestrin (84), and 2″-desmethyl-MPC1001F (85), were isolated and identified from the deep-sea cold seep sediment-derived fungus Aspergillus nidulans SD-531. Compound 85 exhibited significant antimicrobial activity against E. tarda, V. alginolyticus, P. aeruginosa, and V. parahaemolyticus, with MIC values of 0.5, 1.0, 16.0, and 16.0 μg/mL, respectively. Compound 85 showed cytotoxic activity against the tumor cell line Huh 7.5, with an IC50 value of 8.0 μΜ [43]. Six new prenylated indole diketopiperazine alkaloids, asperthrins A–D (88–91), asperthrin E (86), and asperthrin F (87), were isolated from the marine-derived fungus Aspergillus sp. YJ191021. Compound 88 exhibited moderate antifungal and antibacterial activities against V. anguillarum, Xanthomonas oryzae pv. Oryzicola, and Rhizoctonia solani, with MIC values of 8, 12.5, and 25 µg/mL, respectively. Furthermore, 88 displayed notable anti-inflammatory activity, with an IC50 value of 1.46 ± 0.21 µM, in Propionibacterium acnes-induced human monocyte cell line (THP-1) [44]. Two new dimeric diketopiperazines, stereoisomers (92–93), were isolated from the culture broth of the marine-derived fungus Aspergillus sp. Z3, which was found in the gut of marine isopod Ligia exotica [45] (Figure 5).
Three new diketopiperazine alkaloids, 3-hydroxyprotuboxepin K (94), 3,15-dehydroprotuboxepin K (95), and versiamide A (96), were isolated from the marine red algal Rhodomela confervoides-derived fungus Aspergillus creber EN-602. Compound 96 represented the first example of a naturally occurring quinazolinone alkaloid with a diketopiperazine ring derived from phenylalanine (Phe) and leucine (Leu). Compound 94 exhibited inhibitory activity against the angiotensin-converting enzyme (ACE) with an IC50 value of 22.4 μM. Compound 95 exhibited antimicrobial activity against E. tarda, E. coli and M. luteus, P. aeruginosa, and V. harveyi, with MIC values of 64, 8 and 16, 32 and 64 μg/mL, respectively. Compound 96 exhibited antimicrobial activity against Aeromonas hydrophila, E. coli, M. luteus, and P. aeruginosa, with MIC values of 64, 16, 64, and 64 μg/mL, respectively [46]. Three new diketopiperazine derivatives, Waikikiamides A–C (97–99), were isolated from a Hawaiian marine-derived fungus, Aspergillus sp. FM242. Compound 99 featured the first unique heterodimer of two notoamide analogs with an N-O-C bridge. The hybrids of dipeptidylpyruvate and polyketides (97–98) had quite rare secondary metabolites, and the dipeptidylpyruvate heterodimer (99) with an N-O-C bridge is unprecedented. The discovery of 97–99 expanded the chemical diversity of the known dipeptidylpyruvate scaffolds and provided intriguing templates for synthetic and biosynthetic chemists. The Waikikiamide biosynthetic gene cluster derived from the complete sequencing and bioinformatic mining of the Aspergillus sp. FM 242 genome. The gene clusters mined from the sequenced genome support their putative biosynthetic pathways. Compound 97 showed antiproliferative activity against cell lines (HT1080, PC3, Jurkat, A2780S), with IC50 values of 0.519, 1.855, 0.62, and 0.78 μM, respectively. Compound 99 exhibited antiproliferative activity against cell lines (HT1080, PC3, Jurkat, A2780S), with IC50 values of 1.135, 1.805, 1.79, and 1.127 μM, respectively. Furthermore, compound 97, a dipeptidylpyruvate-polyketide hybrid with a nitrogenated methoxy group, showed the most antiproliferative activity, and could potentially attract widespread attention from cancer biologists, biochemists, medicinal chemists, and pharmacologists focusing on new anticancer drug discovery and development [47]. Four new indolyl diketopiperazines, aspamides A–D (100–103), and two new diketopiperazines, aspamides F–G (104–105), were isolated from the solid culture of Aspergillus versicolor, which is an endophyte with the sea crab (Chiromantes haematocheir). All compounds were selected for virtual screening on the 3CL hydrolase (Mpro) of SARS-CoV-2, which was exploited as a potential drug target to fight COVID-19. The docking scores of compounds 100, 101, 104, and 105 were top among all screened molecules (docking scores: −5.389; −4.772; −5.146; −4.962; −5.158), and the score of ritonavir (a potent in vitro inhibitor of human immunodeficiency virus type 1 protease) was −7.039, suggesting that these compounds may be helpful in fighting COVID-19, although further studies are required [48]. Ten prenylated notoamide-type alkaloids, sclerotiamides I-R (106–115), were isolated from marine gorgonian-derived fungus Aspergillus sclerotiorum LZDX-33-4. Compound 107 possessed inhibitory effects against LDH and IL-1β expression in BV-2 cells. Further investigation revealed that 107 significantly inhibited NLRP3 inflammasome activation and blocked NLRP3 inflammasome-induced pyroptosis via the amelioration of mitochondria damage. Compound 108 exhibited potent inhibition against pathogenic S. aureus ATCC 29213, MRSA T144, and E. faecalis ATCC 29212, with MIC values of 4.0, 4.0, and 16.0 μM, respectively [49]. Three new indole diketopiperazine alkaloids, 11-methylneoechinulin E (116) and variecolorin M (117), and (+)-variecolorin G (118), were isolated from a soft coral-associated epiphytic fungus Aspergillus sp. EGF 15-0-3. The enantiomeric mixtures and (+)-variecolorin G (118), with a ratio of 1:2, were separated using chiral HPLC separation, and the absolute configurations of 118 were determined by experimental and quantum-chemical ECD investigations and single-crystal X-ray diffraction analysis [50]. Two new quinazolinone diketopiperazine alkaloids, versicomide E (119) and cottoquinazoline H (120), were isolated from deep-sea coral Hemicorallium cf. Imperiale-derived fungus Aspergillus versicolor AS-212. Compound 120 exhibited inhibitory effects against Vibrio harveyi and V. parahaemolyticus with MIC values of 18.1 and 9.0 µM, respectively [51] (Figure 6).
Four new oxepine-containing pyrazinopyrimidine alkaloids, versicoxepines A–D (121–124), were isolated from Aspergillus versicolor AS-212, a fungus isolated from the deep-sea coral Hemicorallium cf. imperiale. Compounds 122 and 123 represented the first example of a new oxepine-containing pyrazinopyrimidine alkaloid whose cyclic dipeptide moiety is composed of the same type of amino acid (Val or Ile) [52]. Two new dipeptides, asperopiperazines A and B (125 and 126), were isolated from the tunicate-derived Aspergillus sp. DY001. Compound 125 displayed antimicrobial activity against E. coli and S. aureus with the same MIC value of 8 μM. Compound 126 displayed antimicrobial effects against E. coli and S. aureus, with MIC values of 4 and 8 μM, respectively. Compound 125 displayed growth-inhibitory effects towards HCT 116 and MDA-MB-231 cells, with IC50 values of 15.1 ± 0.1 and 24.3 ± 0.2 μM, respectively. Compound 126 displayed growth-inhibitory effects towards HCT 116 and MDA-MB-231 cells, with IC50 values of 16.2 ± 0.1 and 26.3 ± 0.3 μM, respectively [53]. Six new diketopiperazine alkaloids, aspergiamides A–F (127–132), were isolated from the mangrove Sonneratia apetala endophytic fungus Aspergillus sp. 16-5c. Compound 127 exhibited significant α-glucosidase-inhibitory effects, with an IC50 value of 18.2 µM. Compound 129 exhibited moderate α-glucosidase inhibition with an IC50 value of 83.9 µM [54]. Five new antibacterial indole diketopiperazine alkaloids, 24,25-dihydroxyvariecolorin G (133), 25-hydroxyrubrumazine B (134), 22-chloro-25-hydroxyrubrumazine B (135), 25-hydroxyvariecolorin F (136), and 27-epi-aspechinulin D (137), were isolated from a fungal strain of deep-sea cold seep-derived Aspergillus chevalieri CS-122. Compound 134, with hydroxyl groups at C-22 and C-23, exhibited broad-spectrum antibacterial activity against five tested bacterial strains (V. harveyi, E. tarda, A. hydrophila, E. coli, and M. luteus), with MIC values of 32, 16, 32, 16, and 32 µg/mL, respectively. Compound 137, with hydroxyl groups at C-27 and C-28, exhibited broad-spectrum antibacterial activity against five tested bacterial strains (V. harveyi, E. tarda, A. hydrophila, E. coli, and M. luteus). Compound 136 showed moderate activity against the human pathogen E. coli and the aquatic bacterium V. harveyi, with the same MIC value of 32 µg/mL,. Compound 133 displayed significant inhibitory effects against E. coli, with an MIC value of 4 µg/mL, while compound 135 displayed noticeable inhibitory effects against V. harveyi, with an MIC value of 8 µg/mL [55]. Three new tripeptide derivatives, asterripeptides A–C (138–140), were isolated from mangrove Kandelia candel-derived fungus Aspergillus terreus LM.5.2. Compounds 138–140 contain a rare fungi cinnamic acid residue [56]. Two new compounds, 19S,20-epoxy-18-oxotryprostatin A (141) and 20-hydroxy-18-oxotryprostatin A (142), were isolated from the marine sediment-derived fungus Aspergillus fumigatus MF071. A genomic data analysis revealed the putative biosynthetic gene clusters ftm for fumitremorgins, pso for pseurotins, fga for fumigaclavines, and hel for helvolinic acid [57]. One new piperazinedione derivative, nigerpiperazine A (143), was isolated from the mangrove plant Ceriops tagal fungus Aspergillus niger JX-5. Compound 143 showed inhibitory activities against Helicoverpa armigera Hubner, with the IC50 value of 200 μg/mL [58]. Four new diketopiperazine-type alkaloids, oxepinamides H–K (144–147), were isolated from the culture broth extracts of the deep-sea-derived fungus Aspergillus puniceus SCSIO z021. Compounds 144–146 showed significant transcriptional activation on LXRα, with EC50 values of 15, 15, and 16 μM, respectively [59]. Two new diketopiperazine alkaloids, chevalinulins A (148) and B (149), containing an unprecedented spiro-[bicyclo [2.2.2]octane-diketopiperazine] skeleton, were isolated from the deep-sea cold-seep-derived fungus Aspergillus chevalieri CS-122. Their structures were determined by single-crystal X-ray diffraction, specific rotation (SR), and NMR calculations. The putative biosynthesis of 148 and 149 might start with the production of cyclo-L-Trp-L-Ala catalyzed by a nonribosomal peptide synthetase (NRPS) or by a cyclodipeptide synthase (CDPS), followed by monoprenylation catalyzed by a prenyltransferase to form preechuinulin. Compounds 148 and 149 both remarkably increased the number of intersegmental blood vessels (ISVs) in model zebrafish at the concentrations of 40 and 80 μg/mL, indicating that 148 and 149 exhibited significant proangiogenic activity. The above proangiogenic activities of 148 and 149 provide additional potential leads in the treatment of a series of diseases related to insufficient angiogenesis, such as ischemic heart disease, coronary artery disease, and stroke [60] (Figure 7).
2.3. Cyclopeptide Alkaloids
Cyclopeptides are macrocyclic compounds, the ring system of which consists of a hydroxystyrylamine moiety, an amino acid, and a β-hydroxy amino acid, and which is substituted with one or two additional units. A large number of natural cyclopeptides have been reported to be highly effective against different cancer cells, some of which are renowned for their clinical uses [61,62]. A total of 32 new cyclopeptide alkaloids were discovered from marine-derived Aspergillus species; among them, 12 compounds have novel skeleton structures (Figure 8, Figure 9 and Figure 10). Eleven of them showed cytotoxic, antibacterial activities, antifungal activities, anti-inflammatory, and pancreatic lipase-inhibitory activities.
A novel cyclic tripeptide, sclerotiotide M (150), was isolated from the culture of a marine-derived fungus, Aspergillus ochraceopetaliformis DSW-2 [63]. Four new peptides, JG002CPA (151), JG002CPB (152), FJ120DPA (153), and FJ120DPB (154), were isolated from the culture broths of the marine-derived fungi Aspergillus allahabadii JG002 and A. ochraceopetaliformis FJ120. Compound 152 exhibited the strongest inhibitory activity (IC50, 53.1 μM), better than the positive controls berberine chloride (IC50, 104.3 μM) and curcumin (IC50, 47.8 μM), and 152 also showed weak inhibition (IC50, 104.3 μM) of isocitrate lyase (ICL) derived from C. albicans [64]. Five new pentadepsipeptides, aspertides A–E (155–159), were isolated from the co-culture of mangrove Sonneratia paracaseolaris-derived fungus Aspergillus tamarii MA-21 and Aspergillus insuetus SD-512. Compounds 156–159 possessed uncommon amino acid residues, such as 3-hydroxyproline, 2,3-dihydroxyproline, or pipecolinic acid. Compounds 158 and 159 exhibited antibacterial activities against E. tarda, V. alginolyticus, V. anguillarum, V. vulniffcus, and S. aureus, with MIC values of 8–32 μg/mL, respectively. Compound 158 showed antibacterial activity against E. tarda, V. alginolyticus, V. anguillarum, and V. vulniffcus, with MIC values of 8, 16, 32, and 8 μg/mL, respectively. Compound 159 exhibited an inhibitory effect against E. tarda and S. aureus, with MIC values of 16 and 8 μg/mL, respectively [65]. Two new cyclohexadepsipeptides, japonamides A (160) and B (161), were isolated from the marine sponge-derived fungus Aspergillus japonicus MCCC 3A00261 based on molecular networking. Compounds 160 and 161 revealed synergistic antifungal activity against Candida albicans, with the same MIC value of 3.125 µM and FICI value of 0.03. The absence of toxicity to mammalian cells indicated the safety of the drug, which has important implications for the research and development of drugs. The above results suggest that the combination of 160 or 161 with antifungal drugs may be an effective anti-C. albicans regimen. Additionally, the results opened up a new method for cyclic peptides as synergistic antifungal active molecules in combination with fluconazole, ketoconazole, and rapamycin against resistant C. albicans. The underlying synergistic mechanism requires further exploration [66] (Figure 8).
Seven new cyclopentapeptides, pseudoviridinutans A–G (162–168), were isolated from the marine sediment-derived fungus Aspergillus pseudoviridinutans TW58-5 based on molecular networking. Compounds 162–168, with a rare amino acid moiety, O,β-dimethyltyrosine, were observed for the first time from a marine-derived fungus. Compound 167, displayed obvious inhibitory effects on NO production, stimulated by LPS at 20 μM, with no obvious cytotoxicity. Further, the dose study suggested that 167 dose-dependently inhibited NO production stimulated by LPS and compound 167 inhibited LPS-stimulated NO production in cultured murine macrophage RAW264.7 cells by reducing the expression of iNOS and NLRP3 [67]. Three new cyclohexapeptides, petrosamides A–C (169–171), were isolated from the marine sponge Petrosia sp.-derived fungus Aspergillus sp. 151304. Compounds 169–171 displayed significant and dose-dependent pancreatic lipase (PL)-inhibitory activities, with IC50 values of 7.6 ± 1.5, 1.8 ± 0.3, and 0.5 ± 0.1 μM, respectively. Further inhibition kinetics analyses showed that 171 inhibited PL in a noncompetitive manner, while molecular dynamics simulation revealed that it could bind to PL at the entrance of the catalytic pocket [68]. A new cyclic pentapeptide, cotteslosin C (172), was isolated from a co-culture of the sponge-associated fungus Aspergillus versicolor with Bacillus subtilis [69]. A new cyclic tetrapeptide, asperhiratide (173), was identified, obtained from the soft coral-derived fungus Aspergillus hiratsukae SCSIO 5Bn1003 [70]. One new cyclopeptide, versicolide A (174), was isolated from the seafloor-derived Aspergillus versicolor PS108-62 [71]. Three undescribed cyclic lipopeptides, maribasins C–E (175–177), were isolated from the marine gorgonian-associated fungus Aspergillus sp. SCSIO 41501. Compound 175 showed significant antifungal activity against five phytopathogenic fungal strains (Fusarium oxysporum, Curvularia australiensis, Pyricularia oryzae, Alternaria solani, and Colletotrichum gloeosporioiles), with MIC values of 25, 6.25, 50, 25, and 12.5 μg/disc, respectively. Compound 176 showed significant antifungal activity against F. oxysporum, C. australiensis, P. oryzae, A. solani, and C. gloeosporioiles, with MIC values of 12.5, 3.12, 12.5, 25, and 12.5 μg/disc, respectively. Compound 177 showed significant antifungal activity against F. oxysporum, C. australiensis, P. oryzae, A. solani, and C. gloeosporioiles, with MIC values of 50, 12.5, 50, 25, and 25 μg/disc, respectively. The structure–bioactivity relationship exhibited that the β-amino fatty acid chain could significantly affect the antifungal activity of this type of cyclic lipopeptides [21]. Three new aspochracin-type cyclic tripeptides, sclerotiotides M–O (178–180), were obtained from the fungus Aspergillus insulicola HDN151418, which was isolated from an unidentified Antarctica sponge. Compound 178 showed broad antimicrobial activity against a panel of pathogenic strains, including B. cereus, Proteus species, Mycobacterium phlei, B. subtilis, V. parahemolyticus, E. tarda, MRCNS, and MRSA, with MIC values of 3.13, 3.13, 3.13, 6.25, 3.13, 1.56, 12.5, and 25.0 µM, respectively. Compound 179 showed broad antimicrobial activity against B. cereus, P. species, M. phlei, B. subtilis, V. parahemolyticus, E. tarda, MRCNS, and MRSA, with MIC values of 6.25, 6.25, 12.5, 12.5, 6.25, 1.56, 25.0, and 25.0 µM, respectively [72]. A new N-methyl cyclic pentapeptide, caletasin (181), was isolated from the marine-facultative Aspergillus sp. MEXU 27854 from Caleta Bay, Mexico [73] (Figure 9).
2.4. Quinazoline Alkaloids
Quinazoline is a nitrogen-containing heterocyclic compound illustrated by a double-ring structure that contains a benzene ring system fused to pyrimidine at two adjacent carbon atoms, and has several pharmacological activities [74]. A total of 39 new quinoline alkaloids (182–218, including 2 pairs of racemates) were discovered from the marine-derived Aspergillus species. Among them, three compounds were found to have novel skeleton structures and fifteen compounds showed cytotoxic and antibacterial activities (Figure 10).
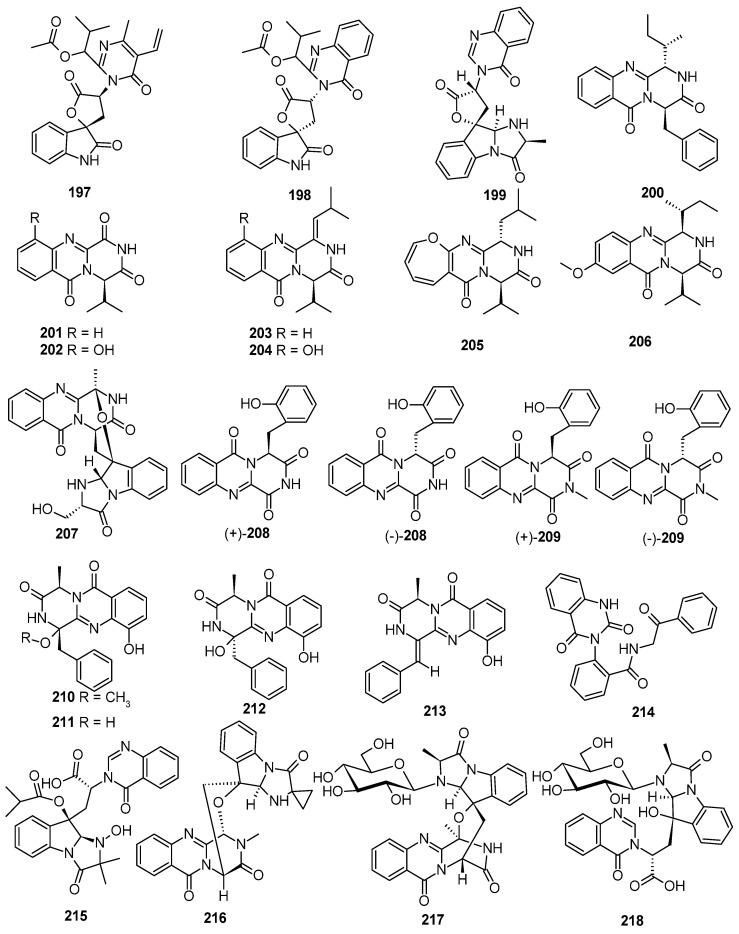
One new quinazoline alkaloid, chaetominine A (182), was isolated from the strain Aspergillus fumigatus MF029, which was obtained from a marine sponge, Hymeniacidon perleve, collected from the Bohai Sea, China [75]. Three new quinazoline-containing indole alkaloids, aspertoryadins H–J (183–185), were isolated from the marine-derived fungus Aspergillus sp. HNMF114 [76]. Two new quinazoline alkaloids, chaetominines A (186) and B (187), were isolated from marine sponge Callyspongia sp.-derived fungus Aspergillus versicolour SCSIO XWS04 F52. Compounds 186 and 187 showed cytotoxic activity against leukemia K562 and colon cancer cells SW1116, with IC50 ranging from 7.5 to 12.5 μM, and also exhibited significant protection against H1N1 virus-induced cytopathogenicity in MDCK cells, with IC50 values of 15.5 and 24.5 μM (oseltamivir as a positive control, IC50 3.19 μM), respectively [77]. Three new 4-quinazolinone alkaloids, puniceloids E–G (188–190), were isolated from the culture of the marine-derived fungus Aspergillus puniceus FAHY0085. Compounds 189 and 190 showed the significant transcriptional activation of liver X receptor α with the same EC50 value of 1.7 μM, [36]. One new quinazolinone alkaloid, 2-(4-hydroxybenzyl)-4-(3-acetyl)quinazolin-one (191), was isolated from the seawater-derived fungus Aspergillus sydowii SW9. Compound 191 exhibited selective inhibitory activities against the human pathogenic bacteria E. coli, S.aureus, S. epidermidis, and S. pneumoniae, with MIC values of 16, 8.0, 4.0, and 16 µg/mL, respectively [78]. Seven new quinazoline-containing indole alkaloids, aspertoryadins A–G (192–198), were isolated from the Sanguinolaria chinensis-derived fungus Aspergillus sp. HNMF114. Compound 192 had a rarely aminosulfonyl group in its structure. Compounds 197 and 198 exhibited quorum-sensing-inhibitory activity against Chromobacterium violaceum CV026, with the same MIC values of 32 μg/well, respectively [79]. One new quinazoline alkaloid, 2-epi-tryptoquivaline F (199), was isolated from the marine-derived fungus Aspergillus fumigatus H22 [17]. A new quinazoline alkaloid, protuboxepin K (200), was isolated from the culture broth of the marine-derived fungal strain Aspergillus sp. BFM-0085, collected from a sediment sample of Tokyo Bay. Compound 200 inhibited bone morphogenetic protein (BMP)-induced alkaline phosphatase activity with an IC50 value of 4.7 μM in mutant BMP receptor-carrying C2C12 (R206H) cells [80]. Five new quinazolinone alkaloids, felicarnezolines A–E (201–205), were isolated from the co-culture of marine-derived fungi Amphichorda sp. KMM 4639 and Aspergillus carneus KMM 4638. Compounds 203–205 showed cytotoxic activity against human breast cancer MCF-7 cells with IC50 values of 92.5 ± 3.1, 68.7 ± 1.6, and 86.3 ± 2.3 μM, respectively. Compound 204 protected rat cardiomyocytes H9c2 and human neuroblastoma SH-SY5Y cells against CoCl2-induced damage, with the IC50 value of 72.9 ± 2.8 μM, showing a good protective effect in hypoxiamimic conditions via antioxidant pathways. Compound 205 showed cytotoxic effects against human prostate cancer PC-3 cells, with an IC50 value of 83.8 ± 5.5 μM [81]. One new quinazoline, (−)-isoversicomide A (206), was isolated from the fungus Aspergillus versicolor PS108-62 [71]. One new quinazoline alkaloid, 29-hydroxyfumiquinazoline C (207), was isolated and identified from the deep-sea sediment-derived fungus Aspergillus fumigatus SD-406 [20]. Two new alkaloid racemates, (±)-17-hydroxybrevianamide N (208) and (±)-N1-methyl-17-hydroxybrevianamide N (209), featuring a rare O-hydroxyphenylalanine residue and an imide subunit, were isolated from a soft-coral Sinularia sp.-derived fungus Aspergillus sp. CHNSCLM-0151. Simultaneously, their structures, including their absolute configurations, were elucidated on the basis of comprehensive spectroscopic analysis, ECD calculations, and X-ray diffraction data. Interestingly, the basic solution promotes the racemization of (+)-208 and (−)-208, whereas acidic solution suppresses the transformation [82]. Four new 4-quinazolinone alkaloids, puniceloids A–D (210–213), were isolated from the culture broth extracts of the deep-sea sediment-derived fungus Aspergillus puniceus SCSIO z021. Compounds 210–213 showed significant transcriptional activation on LXRα, with EC50 values of 5.1, 5.3, 1.7, and 1.7 μM, respectively [59]. One new quinazolinone alkaloid, novobenzomalvin D (214), was isolated from Aspergillus terreus SCAU011, a fungus from the rhizosphere sediment of a mangrove plant Rhizophora stylosa. Compound 214 exhibited better COX-2-inhibitory activity (inhibition rate of 91.1%) than the positive control celecoxib (56.7%) at 20 nM [83]. A new alkaloid, tryptoquivaline Y (215), was purified from a Hawaiian beach soil-derived fungus Aspergillus felis FM324 [84]. A new fumiquinazoline-type alkaloid, 2-methyl-versiquinazoline C (216), was isolated from Aspergillus flavipes PJ03-11 [85]. Two new glucosidated indole-containing quinazoline alkaloids, fumigatosides G (217) and H (218), were isolated from mangrove-derived fungus Aspergillus fumigatus SAl12 [86] (Figure 10).
2.5. Isoquinoline Alkaloids
Isoquinoline alkaloids, a large class of natural products, are mostly found in plants and also found in the extracts of bacterial and fungal cultures. They possess a broad range of biological activities, including antimicrobial, antitumor, antileukemic, and anti-inflammatory properties [87]. A total of 17 new isoquinoline alkaloids were discovered from marine-derived Aspergillus species (Figure 11), and 5 of them showed cytotoxic activities, antibacterial inhibitory activity, and inhibitory activity against protein tyrosine phosphatase CD45.
Sixteen new isoquinoline alkaloids, puniceusines A–N (221–234), puniceusine O (219), and (±)-puniceusine P (220), were isolated from the extracts of a deep-sea sediment-derived fungus, Aspergillus puniceus SCSIO z021. Compounds 223 and 224 showed selective inhibitory activity against protein tyrosine phosphatase CD45 with IC50 values of 8.4 and 5.6 µM, respectively, and 224 also showed moderate cytotoxicity towards human lung adenocarcinoma cell line H1975 with an IC50 value of 11.0 µM. Compound 234 showed medium antibacterial activity towards S. aureus, methicillin-resistant S. aureus (MRSA), and E. coli, with MIC values of 100 µg/mL, and compound 224 could inhibit the growth of E. coli with an MIC value of 100 µg/mL. The structure–activity relationship of 221–234 revealed that the substituents at C-7 of the isoquinoline nucleus could greatly affect their bioactivity [88,89]. One new alkaloid, 2-(quinoline-8-carboxamido)benzoic acid (235), was isolated from the deep-sea sediment-derived fungus Aspergillus sp. SCSIO06786 [90] (Figure 11).
2.6. Pyrrolidine Alkaloids
Pyrrolidine alkaloids have been shown to possess several important biological activities, including antioxidant, anti-inflammatory, antibacterial, antifungal, antiparasitic and anthelmintic, anticancer, anti-hyperglycemic, organ-protective, and neuropharmacological activities [91]. A total of 22 new pyrrolidine alkaloids (236–256, including 1 pair of racemates) were discovered from marine-derived Aspergillus species. Three compounds have novel skeleton structures (Figure 12) and nine of them showed cytotoxic activities, antibacterial activities, antifungal activities, and anti-inflammatory activities.
Two rare tetracyclic skeleton alkaloids, perinadines B and C (236 and 237), the first reported derivatives of a rare type of tetracyclic alkaloid, perinadine A, were isolated as mixtures of epimers from the marine sponge Haliclona sp.-derived fungus Aspergillus sp. LS116, driven by molecular networking. Compounds 236 and 237 showed moderate antibacterial activity against B. subtilis with MIC values of 32 and 64 μg/mL, respectively [92]. One new pyrrolidine alkaloid, ochraceopetalin (238), a mixed-biogenetic salt compound, with a sulfonated diphenylether–aminol–amino acid ester guanidinium salt of an unprecedented structural class, was isolated from a marine sediment-derived fungus Aspergillus ochraceopetaliformis FJ120. Compound 238 exhibited significant cytotoxicity against K562 and A549 cells, with IC50 values of 9.5 and 6.8 µM, respectively [93]. Three new tricyclic cyclopiazonic acid-related alkaloids, asperorydines N–P (239–241), were isolated and characterized from the fungus Aspergillus flavus SCSIO F025, derived from the deep-sea sediments of South China Sea [94]. One new pyrrolidine alkaloid, variotin B (242), was isolated from the deep-sea shrimp fungus Aspergillus unguis IV17-109. Compound 242 showed moderate anti-inflammatory activity, with an IC50 value of 20.0 µM [95]. One new pyrrolidine alkaloid, (E)-6-hydroxy-5-(1-propenyl)-1,2-dihydropyrano [3,2-b]pyrrole-3,7-dione (243), was obtained from the rhizosphere soil of mangrove Bruguiera gymnorrhiza (L.)-derived fungus Aspergillus sp. DM94 [96]. Two new spiro-heterocyclic γ-lactam derivatives, cephalimysins M (244) and N (245), were isolated from the fermentation cultures of the marine sediment-derived fungus Aspergillus fumigatus CUGBMF170049 [97]. Five new azaspirene derivatives, azaspirenes A–E (246–250), were isolated from the marine mud-derived endophytic fungus Aspergillus micronesiensis NF666, and a plausible biosynthetic pathway of azaspirenes was proposed [98]. One new pyrrolidine alkaloid, 10R-15-methylpseurotin A (251), was isolated from the deep-sea sediment-derived fungus Aspergillus fumigatus SD-406. Compound 251 exhibited moderate activity against the plant-pathogenic fungi F. graminearum Schw., with an MIC value of 16 µg/mL [20]. A new alkaloid, pseurotin I (252), was purified from a fungal strain Aspergillus felis FM324, which was isolated from a Hawaiian beach soil sample. Compound 252 inhibited NF-κB with an IC50 value of 30.9 μM, respectively [84]. Two pyrrolinone-fused benzoazepine alkaloids, (±)-asperazepanones A (253) and B (254), were isolated from the coral-derived Aspergillus candidus fungus. Compound 254 showed obviously inhibitory activity against nitric oxide (NO) production, with an inhibition rate of 43 ± 4% at the concentration of 1 μM. The levels of TNF-α (p < 0.0001) and IL-6 (p < 0.01) significantly decreased, with 40 ± 2% and 77 ± 7% inhibition rates, respectively, at the concentration of 0.1 μM [99]. A new oxygenated tricyclic cyclopiazonic acid alkaloid, asperorydine Q (255), was isolated from the coral-associated Aspergillus flavus GXIMD 02503. Compound 255 exhibited moderate inhibitory activity against NF-κB activation, with an IC50 value of 14.1 ± 1.5 μM [100]. A new alkaloid, pyranonigrin L (256), was isolated from mangrove endophytic fungus Aspergillus fumigatus SAS10 [101] (Figure 12).
2.7. Other Heterocyclic Alkaloids
A total of 37 new other heterocyclic alkaloids were discovered from the marine-derived Aspergillus species (Figure 13, Figure 14 and Figure 15); 11 compounds have novel skeleton structures and 12 of them showed cytotoxic activities, antibacterial activities, antifungal activities, α-glucosidase-inhibitory activities, PTP1B inhibitory activities, and so on.
Six new benzoic acid-containing alkaloids, asperalin A–F (257–262), were isolated and identified from a seagrass Enhalus acoroides-derived Aspergillus alabamensis SYSU-6778. Compounds 259 and 260 showed strong activity against S. aureus, S. iniae, and S. parauberis, with MIC values of 10.1, 5.0, and 10.1 μM, respectively. Compound 261, an N-alkylated product of 260, exhibited the strongest inhibitory effects against S. iniae, with an MIC value of 2.2 μM. Compound 262, as a new natural bactericide, showed moderate to potent inhibitory activity against Gram-negative bacterium E. ictalurid and four Gram-positive bacteria (S. Iniae, S. aureus, S. parauberis and Bacillus subtilis), with MIC values of 10.9, 43.6, 21.8, 87.3, and 21.8 μM, respectively [102]. A new aflaquinolone, 22-epi-aflaquinolone B (263), was isolated from a co-culture of the sponge-associated fungus Aspergillus versicolor with B. subtilis [69]. Three new quinolone alkaloids, aspergillspins C–E (264–266), were isolated from the marine gorgonian Melitodes squamata-derived fungus Aspergillus sp. SCSIO 41501 [23]. Two new alkaloids, citriquinolinones A (267) and B (268), were isolated from the deep-sea whale Mesoplodon densirostris-derived fungus Aspergillus versicolor 170217. Compounds 267 and 268, featuring a unique isoquinolinone-embedded citrinin scaffold, represented the first examples of citrinin-isoquinolinone hybrids [103] (Figure 13).
A new benzodiazepine alkaloid, circumdatin M (269), with a rare pyrimidone-4-pyrone moiety, was isolated from a Hawaiian marine fungus Aspergillus sp. FM242. The structure and absolute configuration of 269 was determined through an analysis of HRMS and NMR spectroscopic data, DP4+ NMR calculations, and CD calculations [104]. Two new nucleoside derivatives, kipukasins M (270) and N (271), were obtained from the sea mud-derived fungus Aspergillus versicolor TJ-LHQ-AV507. Interestingly, intramolecular transesterification occurred in 270 and 271, which existed as a pair of inseparable regioisomers [105]. One new pteridine alkaloid, asperpteridinate A (272), was isolated from the seawater sample-derived fungus Aspergillus austroafricanus Y32-2 [13]. A new alkaloid, pyripyropene U (273), was obtained from the marine sponge-derived fungus Aspergillus sp. SCSIO41420 [106]. A new alkaloid, aspernigrin E (274), was isolated from mangrove endophytic fungus Aspergillus fumigatus SAS10 [101]. One new azaphthalide derivative, (S)-3-hydroxy-2,7-dimethylfuro [3,4-b]pyridin-5(7H)-one (275), was isolated from the coral-derived fungus Aspergillus sp. SCSIO41405 [107]. A new alkaloid, asperalumazine A (276), was isolated and identified from a seagrass-derived Aspergillus alabamensis SYSU-6778. Compound 276 represented the first example of a lumazine derivative directly coupled to a benzoic acid moiety via a hydroxymethyl group [102]. Three new polypropionate derivatives, fiscpropionates D–E (277–278), representing the first examples of polypropionate derivatives containing a 3-hydroxypiperidin-2-one as part of an imide linkage, were obtained from the deep-sea-derived fungus Aspergillus fischeri FS452. Compound 277 exhibited significant inhibitory activities against Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB), with an IC50 value of 11 μM [108] (Figure 14).
One new compound, rhizoaspergillin A (279), was isolated from the mangrove Rhizophora mucronata endophytic fungus Aspergillus sp. A1E3. From the perspective of biosynthesis, 279 could originate from the combined assembly of three building blocks, viz., orsellinic acid, β-D-ribofuranose, and L-glutamine. It is an unprecedented alkaloid-N-oxide involving the biosynthetic pathways of polyketides, pentose, and amino acids [109]. One new acremolin-type alkaloid, acremolin D (280), was isolated from the deep-sea-derived fungus Aspergillus sydowii MCCC 3A00324. Compound 280 showed inhibitory effects against Hela-S3 and K562 cell lines, with inhibition rates of 30.6% and 25.1%, respectively, at the concentration of 20 μM [110]. A new oxidized phomaligol derivative, phomaligol H (281), was isolated from the culture of the fungus Aspergillus flavus BB1; the fungus was isolated from the marine shellfish Meretrix meretrix, collected on Hailing Island. Compound 281 demonstrated cytotoxic activity against the A549 cell line, with an IC50 value of 65.53 μM [111]. Four undescribed pyrazinopyrimidine-type alkaloids, including three natural products, pyrasplorines A–C (282–284), and an artifact deg-pyrasplorine B (285), as well as a biogenetically related versicoloid A (286), were discovered from the extract of a mangrove Thespesia populnea-derived fungus Apergillus verisicolor HDN11-84. Compound 282 had a unique spiral-type skeleton (composed of a cyclopentenone ring with a pyrazino [1,2-a] pyrimidine core), which is unprecedented in pyrazinopyrimidine-type alkaloids [112]. A new compound, penilumamide K (287), was obtained from the deep-sea-derived fungus Aspergillus sp. SCSIO 41029. Compound 287 represented the first lumazine peptide reported from deep-sea-derived fungus. Compound 287 had significant potency against α-glucosidase, with an IC50 value of 18.61 μΜ [113]. One new compound (6-benzyl-1-isopentyl-4-oxo-1,4-dihydropyridin-3-yl)-carboxamide (288) was obtained from a mangrove Bruguiera gymnorrhiza (L.)-derived fungus strain of Aspergillus sp. DM94 [96]. Three unusual chlorinated metabolites, flavipesides A–C (289–291), were isolated from the marine sponge-derived fungus Aspergillus flavipes 164013. Their structures were determined by spectroscopic data analysis, and absolute configurations were assigned through single-crystal X-ray diffraction with ECD spectral analysis. Compounds 289–291 represented a new structural family of PKS-NRPS hybrid metabolites with an uncommon assembly of structural scaffolds and functional groups. Each of them was composed of a chlorinated, methylated, and hydroxylated xanthone and an aminoethyl-modified pyrazol, as well as a methylated dipeptide. Compound 289–291 exhibited potent inhibitory activity against PL, with IC50 values of 0.23 ± 0.03, 0.07 ± 0.01, and 0.14 ± 0.02 μM, respectively. They were 6–21 times more potent than the positive control kaempferol with an IC50 value of 1.50 ± 0.21 μM. A preliminary structure–activity relationship analysis revealed that the inhibitory activity potency of 289–291 was influenced by the variations in chlorination positions on the xanthone substructure: 2-chloridated > 4-chloridated > 2,4-dichloridated. These isolates were discovered to be a new family of PL inhibitors with therapeutic potential to prevent hyperlipidemia and obesity [114]. Two new nucleoside derivatives, kipukasins K (292) and L (293), were obtained from the gorgonian Dichotella gemmacea-derived fungus Aspergillus versicolor XS-20090066-based molecular networking; the fungus was induced by chemical epigenetic manipulation with a combination of 100 µM SAHA and 100 µM 5-Aza. Chemical epigenetic manipulation should be a feasible and effective strategy to trigger the production of bioactive secondary metabolites from marine-derived fungi [115] (Figure 15).
3. Conclusions
In this review, the sources, structural diversity, and biological activity of secondary metabolites from marine-derived Aspergillus species are summarized, covering the period between January 2019 and January 2024. A total of 306 new nitrogen heterocycles were obtained from the genus Aspergillus. Remarkably, among them, 52 compounds had novel skeleton structures. One hundred and three compounds, along with their biological activities, producing strains, and habitats, are summarized in Table 1. The structure type and the bioactivity distribution of the new nitrogen heterocycles isolated from Aspergillus pp. are also shown in Figure 16.
Table 1.
The producing strains, habitats, Genbank accession number, and bioactivities of secondary metabolites from marine-derived Aspergillus species.
| Compounds | Producing Strains | Habitats | Genbank Accession Number | Bioactivities | Refs. |
|---|---|---|---|---|---|
| Aculeaquamide A (1) | A. aculeatinus WHF0198 | Deep-sea sediment, South China Sea |
– | IC50 (cytotoxicity) 3.3 μM | [10] |
| Asterriquinone F (2) | A. terreus LM.1.5 | Leaves of an unidentified mangrove tree, Vietnam, South China Sea |
MN788658.1 | – | [11] |
| Asperdiazapinone G (3) | Aspergillus sp. WHUF03110 | Mangrove soil, Yalong Bay, at Sanya, Hainan, China |
MZ661122 | – | [12] |
| Di-6-hydroxydeoxybrevianamide E (4) | A. austroafricanus Y32-2 | Seawater, Indian Ocean | MK267449 | – | [13] |
| Dinotoamide J (5) | A. austroafricanus Y32-2 | Seawater, Indian Ocean | MK267449 | Pro-angiogenic activity | [13] |
| at concentration of 70 µg/mL | |||||
| Penerpene O (6) | Aspergillus sp. ZF-104 | Marine soft coral, Haikou Bay, Hainan province, China |
OM320573 | IC50 (PTP1B-inhibitory activities) 17.7 ± 0.7 μM | [14] |
| Penerpenes Q-R (7–8) | Aspergillus sp. ZF-104 | Marine soft coral, Haikou Bay, Hainan province, China |
OM320573 | – | [14] |
| Penerpenes T (9) | Aspergillus sp. ZF-104 | Marine soft coral, Haikou Bay, Hainan province, China |
OM320573 | – | [14] |
| Penerpene U (10) | Aspergillus sp. ZF-104 | Marine soft coral, Haikou Bay, Hainan province, China |
OM320573 | IC50 (PTP1B-inhibitory activities) | [14] |
| 28.1 ± 2.2 μM | |||||
| Penerpene V (11) | Aspergillus sp. ZF-104 | Marine soft coral, Haikou Bay, Hainan province, China |
OM320573 | – | [14] |
| O-β-D-glucopyranosyl ester (12) | A. fumigatus M580 | Sea cucumber, Co To-Thanh Lan island, Vietnam |
MW015802 | – | [15] |
| Sclerotiamide C (13) | A. sclerotiorum LZDX-33-4 | Marine gorgonian, South China Sea |
OK012383.1 | IC50 (cytotoxicity) 1.5–1.8 μM | [16] |
| Sclerotiamides D-E (14–15) | A. sclerotiorum LZDX-33-4 | Marine gorgonian, South China Sea |
OK012383.1 | – | [16] |
| Sclerotiamide F (16) | A. sclerotiorum LZDX-33-4 | Marine gorgonian, South China Sea |
OK012383.1 | IC50 (cytotoxicity) 0.05–0.07 μM | [16] |
| Sclerotiamides G-H (17–18) | A. sclerotiorum LZDX-33-4 | Marine gorgonian, South China Sea |
OK012383.1 | – | [16] |
| Fumindoline A–C (19–21) | A. fumigatus H22 | Seawater, Western Pacific | NRRL 163 s | – | [17] |
| Aspercarbolines A–B (22–23) | Aspergillus sp. XBB-4 | Inner tissue of geoduck Panopea abbreviate, South China Sea | MK863524 | – | [18] |
| Aspercarboline C (24) | Aspergillus. sp. XBB-4 | Inner tissue of geoduck Panopea abbreviate, South China Sea | MK863524 | IC50 (cytotoxicity) 16.29–50.85 μM | [18] |
| Flavonoid A (25) | A. flavipes DS720 | Deep seawater, Mariana Trench |
ON340751 | Inhibition rates of (cytotoxicity) (90.83 ± 3.31)%–(99.49 ± 0.50)%, at the concentration of 20 µM | [19] |
| Flavonoid B (26) | A. flavipes DS720 | Deep seawater, the Mariana Trench |
ON340751 | – | [19] |
| Secofumitremorgins A and B (27a and 27b) | A. fumigatus SD-406 | Deep-sea sediment, the East China Sea |
MT635279 | MIC (antimicrobial) 4–64 µg/mL | [20] |
| Aspergillipeptides H–I (28–29) | Aspergillus sp. SCSIO 41501 | Marine gorgonian Melitodes squamata Nutting, the South China Sea, Sanya, Hainan |
JN851015 | – | [21] |
| Ascandinines A–B (30–31) | A. candidus HDN15-152 | Sponge, Pulitzer bay, Antarctica | MH430037 | – | [22] |
| Ascandinine C (32) | A. candidus HDN15-152 | Sponge, Pulitzer bay, Antarctica | MH430037 | IC50 (anti-influenza virus) 26 μM | [22] |
| Ascandinine D (33) | A. candidus HDN15-152 | Sponge, Pulitzer bay, Antarctica | MH430037 | IC50 (cytotoxicity) 7.8 μM | [22] |
| Aspergillspins A-B (34–35) | Aspergillus sp. SCSIO 41501 | Marine gorgonian Melitodes squamata Nutting, the South China Sea, Sanya, Hainan |
JN851015 | – | [23] |
| Asterriquinone I (36) | Aspergillus sp. SCSIO 41018 | Sponge, Xuwen, Guangdong Province |
MH109740.1 | IC50 (cytotoxicity) | [24] |
| 17.9 ± 0.62–29.2 ± 0.32 μM | |||||
| Asterriquinone J (37) | Aspergillus sp. SCSIO 41018 | Sponge, Xuwen, Guangdong Province |
MH109740.1 | IC50 (cytotoxicity) | [24] |
| 8.5 ± 0.17–18.7 ± 0.45 μM | |||||
| Asterriquinone K (38) | Aspergillus sp. SCSIO 41018 | Sponge, Xuwen, Guangdong Province |
MH109740.1 | IC50 (cytotoxicity) | [24] |
| 13.0 ± 0.36–26.2 ± 0.13 μM | |||||
| Asterriquinols G–I (39–41) | Aspergillus sp. SCSIO 41018 | Sponge, Xuwen, Guangdong Province |
MH109740.1 | – | [24] |
| Aspergillamides C-D (42–43) | A. terreus SCSIO 41008 | Marine sponge Callyspongia sp., Xuwen County, Guangdong Province, China |
MF536093 | – | [25] |
| (±)-7,8-epoxy-brevianamide Q ((±)-44) | A. versicolor MF180151 | Marine sediment, Bohai Sea, China. |
MK680178 | – | [28] |
| (±)-8-hydroxy-brevianamide R ((±)-45) | A. versicolor MF180151 | Marine sediment, Bohai Sea, China. |
MK680178 | – | [28] |
| (±)-8-epihydroxy-brevianamide R ((±)-46) | A. versicolor MF180151 | Marine sediment, Bohai Sea, China. |
MK680178 | – | [28] |
| (-)-5-isopentenyl-cryptoechinuline D (47a) | A. ruber TX-M4-1 | Marine moss, Weizhou Island | OL989330 | IC50 (inhibits TrxR activity) | [29] |
| 6.2 μM | |||||
| (+)-5-isopentenyl-cryptoechinuline D (47b) | A. ruber TX-M4-1 | Marine moss, Weizhou Island | OL989330 | – | [29] |
| (±)-dibrevianamide Q1 ((±)-48) | Aspergillus sp. ZA-01 | Marine sediment, Bohai Sea | – | IC50 (anti-H1N1 virus activity, (+)-44) 12.6 μM; MIC (anti-bacterium) 10.2 μg/mL |
[30] |
| (±)-dibrevianamide Q2 ((±)-49) | Aspergillus sp. ZA-01 | Marine sediment, Bohai Sea | – | IC50 (anti-H1N1 virus activity, (−)-45) 19.5 μM | [30] |
| Versicolamide C (50) | Aspergillus sp. SCSIO 41036 | Soft coral, Beihai, Guangxi, China |
OM441922 | – | [31] |
| Emestrins L-M (51–52) | A. terreus RA2905 | Sea hare Aplysia pulmonica, Weizhou, South China Sea |
MK611650 | – | [32] |
| (±)-brevianamide Z ((±)-53) | A. versicolor HBU-7 | Sea mud, Bohai, China | KY814754 | – | [33] |
| (±)-brevianamide Z1 ((±)-54) | A. versicolor HBU-7 | Sea mud, Bohai, China | KY814754 | – | [33] |
| Pyranamides A-D (55–58) | A. versicolor SCSIO 41016 | Marine sponge Callyspongia sp., Xuwen County, Guangdong Province, China |
MH244341 | – | [34] |
| Secopyranamide C (59) | A. versicolor SCSIO 41016 | Marine sponge Callyspongia sp., Xuwen County, Guangdong Province, China |
MH244341 | – | [34] |
| Protuboxepins F-J (60–64) | A. versicolor SCSIO 41016 | Marine sponge Callyspongia sp., Xuwen County, Guangdong Province, China |
MH244341 | – | [34] |
| Sclerotioloid A (65) | A. sclerotiorum ST0501 | Sponge, Guangdong, China | MT534582 | – | [35] |
| Sclerotioloid C (66) | A. sclerotiorum ST0501 | Sponge, Guangdong, China | MT534582 | – | [35] |
| Oxepinamide L (67) | A. puniceus FAHY0085 | Marine coral, South China Sea | OQ825098 | – | [36] |
| Asperindopiperazines A–C (68–70) | Aspergillus sp. SY2601 | Mariana-Trench sediments | OR646740 | – | [37] |
| 12β,13β-hydroxy-asperfumigatin (71) | A. fumigatus H22 | Seawater, Western Pacific | NRRL 163 s | – | [17] |
| (+)- and (-)-brevianamide X ((±)-72) | A. versicolor OUCMDZ-2738 |
Enteromorpha prolifera, Shilaoren beach, Qingdao, China |
MH150818 | – | [38] |
| Asperflocin (73) | A. versicolor 16F-11 | Sponge, Yongxing Island, South China Sea, China |
KM605199 | IC50 (cytotoxicity) 10.29 ± 2.37 μM | [39] |
| Aspechinulins A-B (74–75) | Aspergillus sp. FS445 | Deep-Sea sediment, Indian Ocean |
MW386823 | – | [40] |
| Aspechinulin C (76) | Aspergillus sp. FS445 | Deep-Sea sediment, Indian Ocean |
MW386823 | IC50 (inhibition against NO production) 23.7 μM | [40] |
| Aspechinulin D (77) | Aspergillus sp. FS445 | Deep-Sea sediment, Indian Ocean |
MW386823 | – | [40] |
| 5-prenylcryptoechinulin A (78) | A. chevalieri MCCC M23426 | Deep-Sea | NR_135340 | Inhibition rate (antibacterial) of over 90% at the concentration of 250 µM | [41] |
| 9-epi-didehydroechinulin (79) | A. chevalieri MCCC M23426 | Deep-Sea | NR_135340 | – | [41] |
| Asperdione A (80) | Aspergillus sp. XBB-4 | Inner tissue of geoduck Panopea abbreviate, South China Sea | MK863524 | IC50 (cytotoxicity) 10.72–22.00 μM | [18] |
| (+) and (-)19-epi-sclerotiamide | A. versicolor CGF 9-1-2 | Soft coral, South China Sea | MG827180.1 | – | [42] |
| (81 and 82) | |||||
| Didethio-11α-methylthioemestrin (83) | A. nidulans SD-531 | Deep-Sea cold-seep sediment, South China Sea | MN901610.1 | – | [43] |
| 7′-epi-didethio-11α-methylthioemestrin (84) | A. nidulans SD-531 | Deep-Sea cold-seep sediment, South China Sea | MN901610.1 | IC50 (antimicrobial) 0.5–16.0 μM | [43] |
| 2′′-desmethyl-MPC1001F (85) | A. nidulans SD-531 | Deep-Sea cold-seep sediment, South China Sea | MN901610.1 | IC50 (cytotoxicity) 8.0 μM | [43] |
| Asperthrins E–F (86–87) | Aspergillus sp. YJ191021 | Soil, Zhoushan, Zhejiang, China | – | – | [44] |
| Asperthrin A (88) | Aspergillus sp. YJ191021 | Soil, Zhoushan, Zhejiang, China | – | MIC (antimicrobial) 8–25 µg/mL; IC50 (anti-inflammatory) 1.46 ± 0.21 µM | [44] |
| Asperthrins B–D (89–91) | Aspergillus sp. YJ191021 | Soil, Zhoushan, Zhejiang, China | – | – | [44] |
| Stereoisomers (92–93) | Aspergillus sp. Z3 | Marine isopod Ligia exotica, Dinghai, Zhoushan, Zhejiang, China |
– | – | [45] |
| 3-hydroxyprotuboxepin K (94) | A. creber EN-602 | Red algal Rhodomela
confervoides, Qingdao, China |
MW186501 | IC50 (enzyme-inhibitory activity) 22.4 μM | [46] |
| 3,15-dehydroprotuboxepin K (95) | A. creber EN-602 | Red algal Rhodomela
confervoides, Qingdao, China |
MW186501 | MIC (antimicrobial) 8–64 µM | [46] |
| Versiamide A (96) | A. creber EN-602 | Red algal Rhodomela
confervoides, Qingdao, China |
MW186501 | MIC (antimicrobial) 16–64 µM; | [46] |
| Waikikiamide A (97) | Aspergillus sp. FM242 | soil, Waikiki beach of Oahu, Honolulu, Hawaii | MH879469 | IC50 (cytotoxicity) 0.591–1.855 μM | [47] |
| Waikikiamide B (98) | Aspergillus sp. FM242 | soil, Waikiki beach of Oahu, Honolulu, Hawaii | MH879469 | – | [47] |
| Waikikiamide C (99) | Aspergillus sp. FM242 | soil, Waikiki beach of Oahu, Honolulu, Hawaii | MH879469 | IC50 (cytotoxicity) 1.127–1.805 μM | [47] |
| Aspamides A-D (100–103) | A. versicolor DY180635 | Sea crab (Chiromantes haematocheir), Zhoushan, Zhejiang, China | MT361076 | – | [48] |
| Aspamides F-G (104–105) | A. versicolor DY180635 | Sea crab (Chiromantes haematocheir), Zhoushan, Zhejiang, China | MT361076 | – | [48] |
| Sclerotiamide I (106) | A. sclerotiorum LZDX-33-4 | Marine gorgonian, South China Sea |
OK012383.1 | – | [49] |
| Sclerotiamide J (107) | A. sclerotiorum LZDX-33-4 | Marine gorgonian, South China Sea |
OK012383.1 | Inhibited NLRP3 inflammasome activation |
[49] |
| Sclerotiamide K (108) | A. sclerotiorum LZDX-33-4 | Marine gorgonian, South China Sea |
OK012383.1 | MIC (antimicrobial) 4–16 µM | [49] |
| Sclerotiamides L–R (109–115) | A. sclerotiorum LZDX-33-4 | Marine gorgonian, South China Sea |
OK012383.1 | – | [49] |
| 11-methylneoechinulin E (116) | Aspergillus sp. EGF 15-0-3 | Soft coral, South China Sea | FJ941865.1 | – | [50] |
| Variecolorin M (117) | Aspergillus sp. EGF 15-0-3 | Soft coral, South China Sea | FJ941865.1 | – | [50] |
| (+)-variecolorin G (118) | Aspergillus sp. EGF 15-0-3 | Soft coral, South China Sea | FJ941865.1 | – | [50] |
| Versicomide E (119) | A. versicolor AS-212 | Deep-sea coral Hemicorallium cf. imperiale, Magellan Seamounts | OP009765.1 | – | [51] |
| Cottoquinazoline H (120) | A. versicolor AS-212 | Deep-sea coral Hemicorallium cf. imperiale, Magellan Seamounts | OP009765.1 | MIC (antimicrobial) 9.0–18.1 µM | [51] |
| Versicoxepines A–D (121–124) | A. versicolor AS-212 | Deep-sea coral Hemicorallium cf. imperiale, Magellan Seamounts | OP009765.1 | – | [52] |
| Asperopiperazine A (125) | Aspergillus sp. DY001 | Sea tunicate Didemnum sp., Jizan, Saudi Red Sea coast |
MN818770 | MIC (antimicrobial) 8 µM; IC50 (cytotoxicity) 15.1 ± 0.1–24.3 ± 0.2 μM | [53] |
| Asperopiperazine B (126) | Aspergillus sp. DY001 | Sea tunicate Didemnum sp., Jizan, Saudi Red Sea coast |
MN818770 | MIC (antimicrobial) 4–8 µM; IC50 (cytotoxicity) 16.2 ± 0.1–26.3 ± 0.3 μM | [53] |
| Aspergiamide A (127) | Aspergillus sp. 16-5c | Leaves of S. apetala, a mangrove, Hainan Island, China | JX993829 | IC50 (α-glucosidase-inhibitory) 18.2 Μm |
[54] |
| Aspergiamide B (128) | Aspergillus sp. 16-5c | Leaves of S. apetala, a mangrove, Hainan Island, China | JX993829 | – | [54] |
| Aspergiamide C (129) | Aspergillus sp. 16-5c | Leaves of S. apetala, a mangrove, Hainan Island, China | JX993829 | IC50 (α-glucosidase-inhibitory) 83.9 μM |
[54] |
| Aspergiamides D-F (130–132) | Aspergillus sp. 16-5c | Leaves of S. apetala, a mangrove, Hainan Island, China | JX993829 | – | [54] |
| 24,25-dihydroxyvariecolorin G (133) | A. chevalieri CS-122 | Deep-sea cold-seep sediment, South China Sea |
OM304365.1 | MIC (anti-E. coli) 4 µg/mL | [55] |
| 25-hydroxyrubrumazine B (134) | A. chevalieri CS-122 | Deep-sea cold-seep sediment, South China Sea |
OM304365.1 | MIC (antibacterial) 16–32 µg/mL | [55] |
| 22-chloro-25-hydroxyrubrumazine B (135) | A. chevalieri CS-122 | Deep-sea cold-seep sediment, South China Sea |
OM304365.1 | MIC (anti-V. harveyi) 8 µg/mL | [55] |
| 25-hydroxyvariecolorin F (136) | A. chevalieri CS-122 | Deep-sea cold-seep sediment, South China Sea |
OM304365.1 | MIC (antimicrobial) 32 µg/mL | [55] |
| 27-epi-aspechinulin D (137) | A. chevalieri CS-122 | Deep-sea cold-seep sediment, South China Sea |
OM304365.1 | Potent broad-spectrum antibacterial activity |
[55] |
| Asterripeptides A–C (138–140) | A. terreus LM.5.2 | Mangrove tree leaves Kandelia candelcoast, Hoa province, Vietnam, South China Sea |
MN788658.1 | – | [56] |
| 19S,20-epoxy-18-oxotryprostatin A (141) | A. fumigatus MF071 | Marine sediment, Bohai Sea, China |
MN700176 | – | [57] |
| 20-hydroxy-18-oxotryprostatin A (142) | A. fumigatus MF071 | Marine sediment, Bohai Sea, China |
MN700176 | – | [57] |
| Nigerpiperazine A (143) | A. niger JX-5 | Mangrove plant Ceriops tagal, Dongzhaigang, Hainan, China | MK234873 | IC50 (insecticidal activity) 200 μg/mL | [58] |
| Oxepinamides H–J (144–146) | A. puniceus SCSIO z021 | Deep-sea sediment, Okinawa Trough |
KX258801 | EC50 (transcriptional activation on LXRα) 15–16 μM | [59] |
| Oxepinamide K (147) | A. puniceus SCSIO z021 | Deep-sea sediment, Okinawa Trough |
KX258801 | – | [59] |
| Chevalinulins A (148) | A. chevalieri CS-122 | Deep-sea cold seep, South China Sea |
OM304365 | Proangiogenic activity at the concentrations of 40 μg/mL | [60] |
| Chevalinulins B (149) | A. chevalieri CS-122 | Deep-sea cold seep, South China Sea |
OM304365 | Proangiogenic activity at the concentration of 80 μg/mL | [60] |
| Sclerotiotide M (150) | A. ochraceopetaliformis | Seawater, Dongshan Island, Fujian, China |
MW047023 | – | [63] |
| DSW-2 | |||||
| JG002CPA (151) | A. allahabadii JG002 | Underwater sediment, the coast of Jeju-do, Korea |
MK424488 | – | [64] |
| JG002CPB (152) | A. allahabadii JG002 | Underwater sediment, the coast of Jeju-do, Korea |
MK424488 | IC50 (antimicrobial) 47.8–104.3 μM | [64] |
| FJ120DPA (153) | A. ochraceopetaliformis FJ120 | Underwater sediment, the coast of Jeju-do, Korea |
KF384187 | – | [64] |
| FJ120DPB (154) | A. ochraceopetaliformis FJ120 | Underwater sediment, the coast of Jeju-do, Korea |
KF384187 | – | [64] |
| Aspertides A–C (155–157) | co-culture of A. tamarii MA-21 and A. insuetus SD-512 | Mangrove plant S. paracaseolaris, Wenchang, Hainan, China and deep-sea sediment, South China Sea | HQ891663 and MN696202 | – | [65] |
| Aspertide D (158) | co-culture of A. tamarii MA-21 and A. insuetus SD-512 | Mangrove plant S. paracaseolaris, Wenchang, Hainan, China and deep-sea sediment, South China Sea | HQ891663 and MN696202 | MIC (antibacterial) 8–32 µg/mL | [65] |
| Aspertide E (159) | co-culture of A. tamarii MA-21 and A. insuetus SD-512 | Mangrove plant S. paracaseolaris, Wenchang, Hainan, China and deep-sea sediment, South China Sea | HQ891663 and MN696202 | MIC (antibacterial) 8–16 µg/mL | [65] |
| Japonamides A–B (160–161) | A. japonicus MCCC 3A00261 | Marine sponge, Arctic 6700-4 sea area |
HM573340 | – | [66] |
| Pseudoviridinutans A–E (162–166) | A. pseudoviridinutans TW58-5 | Marine sediment, Kueishantao, Taiwan |
OQ405296 | – | [67] |
| Pseudoviridinutan F (167) | A. pseudoviridinutans TW58-5 | Marine sediment, Kueishantao, Taiwan |
OQ405296 | Inhibited LPS and stimulated NO production | [67] |
| Pseudoviridinutan G (168) | A. pseudoviridinutans TW58-5 | Marine sediment, Kueishantao, Taiwan |
OQ405296 | – | [67] |
| Petrosamides A-C (169–171) | Aspergillus sp. 151304 | Marine sponge Petrosia sp., Yongxing Island, China |
SUB7318612 | IC50 (PL-inhibitory activities) 0.5 ± 0.1–7.6 ± 1.5 μM |
[68] |
| Cotteslosin C (172) | co-culture of A. versicolor 8.1.3a and B. subtilis 168 trpC2 | Sponge and laboratory strain | KY174984 | – | [69] |
| Asperhiratide (173) |
A. hiratsukae SCSIO 5Bn1003 |
Soft coral, South China Sea | KY806121.1 | – | [70] |
| Versicolide A (174) | A. versicolor PS108-62 | Marine sediment, Arctic Ocean | OP807024 | – | [71] |
| Maribasin C (175) | Aspergillus sp. SCSIO 41501 | Marine gorgonian Melitodes squamata Nutting, the South China Sea, Sanya, Hainan |
JN851015 | MIC (antifungal) 6.25–50 µg/disc | [21] |
| Maribasin D (176) | Aspergillus sp. SCSIO 41501 | Marine gorgonian Melitodes squamata Nutting, the South China Sea, Sanya, Hainan |
JN851015 | MIC (antifungal) 3.12–25 µg/disc | [21] |
| Maribasin E (177) | Aspergillus sp. SCSIO 41501 | Marine gorgonian Melitodes squamata Nutting, the South China Sea, Sanya, Hainan |
JN851015 | MIC (antifungal) 12.5–50 µg/disc | [21] |
| Sclerotiotide M (178) | A. insulicola HDN151418 | Sponge, Prydz Bay, Antarctica | MT898544 | MIC (antimicrobial) 1.56–12.5 µM | [72] |
| Sclerotiotide N (179) | A. insulicola HDN151418 | Sponge, Prydz Bay, Antarctica | MT898544 | MIC (antimicrobial) 1.56–25.0 µM | [72] |
| Sclerotiotide O (180) | A. insulicola HDN151418 | Sponge, Prydz Bay, Antarctica | MT898544 | – | [72] |
| Caletasin (181) | Aspergillus sp. MEXU 27854 | Mairne sand, Caleta Bay, Mexico | KY406733 | – | [73] |
| Chaetominine A (182) | A. fumigatus MF029 | Marine sponge Hymeniacidon perleve, Bohai Sea, China | MH974808 | – | [75] |
| Aspertoryadins H-J (183–185) | Aspergillus sp. HNMF114 |
Sanguinolaria chinensi, Haikou Bay |
MK732953 | – | [76] |
| Chaetominines A (186) and B (187) |
A. versicolour SCSIO XWS04 F52 |
Marine sponge Callyspongia sp., Xuwen County, Guangdong Province, China | MN788648 | IC50 (cytotoxicity) 7.5–24.5 µM | [77] |
| Puniceloid E (188) | A. puniceus FAHY0085 | Marine coral, South China Sea | OQ825098 | – | [36] |
| Puniceloid F (189) | A. puniceus FAHY0085 | Marine coral, South China Sea | OQ825098 | EC50 (transcriptional activation on LXRα) 2 µM | [36] |
| Puniceloid G (190) | A. puniceus FAHY0085 | Marine coral, South China Sea | OQ825098 | – | [36] |
| 2-(4-hydroxybenzyl)-4-(3-acetyl) | A. sydowii SW9 | Seawater, Yangma Island, Yantai, China |
MN696205 | MIC (antibacterial) 8–16 µg/mL | [78] |
| quinazolin-one (191) | |||||
| Aspertoryadins A–E (192–196) | Aspergillus sp. HNMF114 |
Sanguinolaria chinensi, Haikou Bay |
MK732953 | – | [79] |
| Aspertoryadin F (197) | Aspergillus sp. HNMF114 |
Sanguinolaria chinensi, Haikou Bay |
MK732953 | MIC (antifungal) 32 µg/well | [79] |
| Aspertoryadin G (198) | Aspergillus sp. HNMF114 |
Sanguinolaria chinensi, Haikou Bay |
MK732953 | MIC (antifungal) 32 µg/well | [79] |
| 2-epi-tryptoquivaline F (199) | A. fumigatus H22 | Seawater, Western Pacific | NRRL 163 s | – | [17] |
| Protuboxepin K (200) | Aspergillus sp. BFM-0085 | Sediment, Tokyo Bay, Tokyo, Japan |
– | IC50 (cytotoxicity) 4.7 µM | [80] |
| Felicarnezolines A–B (201–202) | co-culture of Amphichorda sp. KMM 4639 and A. carneus KMM 4638 | Van Phong Bay, the South China Sea, Vietnam, and Brown alga Laminaria sachalinensis, Kunashir Island |
OQ344667 | – | [81] |
| Felicarnezoline C (203) | co-culture of Amphichorda sp. KMM 4639 and A. carneus KMM 4638 | Van Phong Bay, the South China Sea, Vietnam, and Brown alga Laminaria sachalinensis, Kunashir Island |
OQ344667 | IC50 (cytotoxicity) 92.5 ± 3.1 µM |
[81] |
| Felicarnezoline D (204) | co-culture of Amphichorda sp. KMM 4639 and A. carneus KMM 4638 | Van Phong Bay, the South China Sea, Vietnam, and Brown alga Laminaria sachalinensis, Kunashir Island |
OQ344667 | IC50 (cytotoxicity) 68.7 ± 1.6–72.9 ± 2.8 µM |
[81] |
| Felicarnezoline E (205) | co-culture of Amphichorda sp. KMM 4639 and A. carneus KMM 4638 | Van Phong Bay, the South China Sea, Vietnam, and Brown alga Laminaria sachalinensis, Kunashir Island |
OQ344667 | IC50 (cytotoxicity) 83.8 ± 5.5–86.3 ± 2.3 µM |
[81] |
| (-)-isoversicomide A (206) | A. versicolor PS108-62 | Marine sediment, Arctic Ocean | OP807024 | – | [71] |
| 29-hydroxyfumiquinazoline C (207) | A. fumigatus SD-406 | Deep-sea sediment, the East China Sea |
MT635279 | – | [20] |
| (±)-17-hydroxybrevianamide N (208) |
Aspergillus sp. CHNSCLM-0151 |
Soft coral, South China Sea | KY235298 | – | [82] |
| (±)-N1-methyl-17-hydroxybrevianamide N (209) |
Aspergillus sp. CHNSCLM-0151 |
Soft coral, South China Sea | KY235298 | – | [82] |
| Puniceloids A–D (210–213) | A. puniceus SCSIO z021 | Deep-sea sediment, Okinawa Trough |
KX258801 | EC50 (transcriptional activation on LXRα) 1.7–5.3 µM | [59] |
| Novobenzomalvin D (214) | A. terreus SCAU011 | Mangrove plant Rhizophora
stylosa, Techeng Isle, China |
KY827341 | COX-2 inhibition rate of 91.1% at 20 nM |
[83] |
| Tryptoquivaline Y (215) | A. felis FM324 | Hawaiian beach soil | MZ227547 | – | [84] |
| 2-methyl-versiquinazoline C (216) | A. flavipes PJ03-11 | Wetland mud, Panjin Red Beach National Nature Reserve, Liaoning Province, China |
KT809365 | – | [85] |
| Fumigatosides G-H (217–218) | A. fumigatus SAl12 | Leaves of mangrove plant Sonneratia apetala Buch.-Ham., Dongzhaigang National Nature Reserve, south China’s Hainan Province |
– | – | [86] |
| Puniceusine O (219) | A. puniceus SCSIO z021 | Deep-sea sediment, Okinawa Trough |
KX258801 | – | [88] |
| (±)-puniceusine P (220) | A. puniceus SCSIO z021 | Deep-sea sediment, Okinawa Trough |
KX258801 | – | [88] |
| Puniceusines A-B (221–222) | A. puniceus SCSIO z021 | Deep-sea sediment, Okinawa Trough |
KX258801 | – | [89] |
| Puniceusine C (223) | A. puniceus SCSIO z021 | Deep-sea sediment, Okinawa Trough |
KX258801 | IC50 (CD45-inhibitory activities) 8.4 µM | [89] |
| Puniceusine D (224) | A. puniceus SCSIO z021 | Deep-sea sediment, Okinawa Trough |
KX258801 | IC50 (CD45-inhibitory activities) 5.6 μM; IC50 (cytotoxicity) 11.0 µM; MIC (anti-E. coli) 100 µg/mL | [89] |
| Puniceusines E-M (225–233) | A. puniceus SCSIO z021 | Deep-sea sediment, Okinawa Trough |
KX258801 | – | [89] |
| Puniceusine N (234) | A. puniceus SCSIO z021 | Deep-sea sediment, Okinawa Trough |
KX258801 | MIC (antibacterial) 100 µg/mL | [89] |
| 2-(quinoline-8-carboxamido) | Aspergillus sp. SCSIO06786 | Deep-sea sediment, Indian Ocean |
MN203718 | – | [90] |
| benzoic acid (235) | |||||
| perinadine B-C (236–237) | Aspergillus sp. LS116 | Marine sponge Haliclona sp., Lingshui, Hainan, China | FJ864703 | MIC (antibacterial) 32–64 µg/mL | [92] |
| Ochraceopetalin (238) | A. ochraceopetaliformis FJ120 | Marine sediment, Jeju-do, Korea | KF384187 | IC50 (cytotoxicity) 6.8–9.5 µM | [93] |
| Asperorydines N-P (239–241) | A. flavus SCSIO F025 | Deep-sea sediment, the central South China Sea |
KF682370 | – | [94] |
| Variotin B (242) | A. unguis IV17-109 | Deep-sea shrimp, Indian Ocean | OL700797 | IC50 (anti-inflammatory) 20.0 µM | [95] |
| (E)-6-hydroxy-5-(1-propenyl)-1,2-dihydropyrano [3,2-b]pyrrole-3,7-dione (243) | Aspergillus sp. DM94 | Rhizosphere soil of mangrove Bruguiera gymnorrhiza (L.) Poir | M20191003 | – | [96] |
| Cephalimysins M–N (244–245) | A. fumigatus CUGBMF170049 | Marine sediment, Bohai Sea, China |
MK453215 | – | [97] |
| Azaspirenes A–E (246–250) | A. micronesiensis NF666 | Marine mud, South China Sea | – | – | [98] |
| 10R-15-methylpseurotin A (251) | A. fumigatus SD-406 | Deep-sea sediment, the East China Sea |
MT635279 | MIC (antifungal) 16 µM | [20] |
| Pseurotin I (252) | A. felis FM324 | Hawaiian beach soil | MZ227547 | IC50 (NF-κB-inhibitory activities) 30.9 µM |
[84] |
| (±)-asperazepanone A (253) | A. candidus CHNSCLM-0393 | Nansha Islands coral reef, South China |
MF681708 | – | [99] |
| (+)-asperazepanone B (254) | A. candidus CHNSCLM-0393 | Nansha Islands coral reef, South China |
MF681708 | Against nitric oxide (NO) production with an inhibition rate of 43 ± 4% at the concentration of 1 μM | [99] |
| Asperorydine Q (255) | A. flavus GXIMD 02503 | Coral Porites lutea, Guangxi Zhuang Autonomous Region, China |
MT510157 and MT510158 | IC50 (NF-κB-inhibitory activities) 14.1 ± 1.5 µM |
[100] |
| pyranonigrin L (256) | A. fumigatus SAS10 | Mangrove, Dongzhai Harbor, Hainan Province, China |
– | – | [101] |
| Asperalins A–B (257–258) | A. alabamensis SYSU-6778 | Seagrass E. acoroides, Dongzhai Port, Hainan Island, China | ON845600 | – | [102] |
| Asperalins C–D (259–260) | A. alabamensis SYSU-6778 | Seagrass E. acoroides, Dongzhai Port, Hainan Island, China | ON845600 | MIC (antimicrobial) 5–10.1 µM | [102] |
| Asperalin E (261) | A. alabamensis SYSU-6778 | Seagrass E. acoroides, Dongzhai Port, Hainan Island, China | ON845600 | MIC (antimicrobial) 2.2 µM | [102] |
| Asperalin F (262) | A. alabamensis SYSU-6778 | Seagrass E. acoroides, Dongzhai Port, Hainan Island, China | ON845600 | MIC (antimicrobial) 10.9–87.3 µM | [102] |
| 22-epi-aflaquinolone B (263) | co-culture of A. versicolor 8.1.3a and B. subtilis 168 trpC2 | Sponge and laboratory strain | KY174984 | – | [69] |
| Aspergillspins C–E (264–266) | Aspergillus sp. SCSIO 41501 | Marine gorgonian Melitodes squamata Nutting, the South China Sea, Sanya, Hainan |
JN851015 | – | [23] |
| Citriquinolinones A–B (267–268) | A. versicolor 170217 | Deep-sea whale Mesoplodon densirostris, Ningde, East China Sea | SUB13826338 | – | [103] |
| Circumdatin M (269) | Aspergillus sp. FM242 | Soil, Waikiki beach of Oahu, Honolulu, Hawaii | MH879469 | – | [104] |
| Kipukasins M (270) and N (271) | A. versicolor TJ-LHQ-AV507 | Sea mud, South China Sea | 2081031 | – | [105] |
| Asperpteridinate A (272) | A. austroafricanus Y32-2 | Seawater, Indian Ocean | MK267449 | – | [13] |
| Pyripyropene U (273) | Aspergillus sp. SCSIO41420 | Marine sponge, Weizhou Island, Guangxi, China |
NR_ OP363213 | – | [106] |
| Aspernigrin E (274) | A. fumigatus SAS10 | Mangrove, Dongzhai Harbor, Hainan Province, China |
– | – | [101] |
| (S)-3-hydroxy-2,7-dimethylfuro [3,4-b]pyridin-5(7H)-one (275) | Aspergillus sp. SCSIO41405 | Coral, Luhuitou waters, Sanya Bay, South China Sea |
– | – | [107] |
| Asperalumazine A (276) | A. alabamensis SYSU-6778 | Seagrass E. acoroides, Dongzhai Port, Hainan Island, China | ON845600 | – | [102] |
| Fiscpropionate D (277) | A. fischeri FS452 | Deep-sea sludge, Indian Ocean | KF294264 | IC50 (MptpB-inhibitory activities) 11 μM | [108] |
| Fiscpropionate E (278) | A. fischeri FS452 | Deep-sea sludge, Indian Ocean | KF294264 | – | [108] |
| Rhizoaspergillin A (279) | Aspergillus sp. A1E3 | Mangrove Rhizophora mucronata, Trang Province | – | – | [109] |
| Acremolin D (280) | A. sydowii MCCC 3A00324 | Deep-sea sediment, South Atlantic Ocean |
MN918102 | Inhibition rate (cytotoxicity) of 25.1–30.6% at the concentration of 20 µM | [110] |
| Phomaligol H (281) | A. flavus BB1 | Marine shellfish Meretrix meretrix, Hailing Island, Yangjiang, China | MT584825 | IC50 (cytotoxicity) 65.53 µM | [111] |
| Pyrasplorines A–C (282–284) | A. verisicolor HDN11-84 |
Thespesia populnea, Guangxi Province, China |
KU950433 | – | [112] |
| Deg-pyrasplorine B (285) | A. verisicolor HDN11-84 |
Thespesia populnea, Guangxi Province, China |
KU950433 | – | [112] |
| Versicoloid A (286) | A. verisicolor HDN11-84 |
Thespesia populnea, Guangxi Province, China |
KU950433 | – | [112] |
| Penilumamide K (287) | Aspergillus sp. SCSIO 41029 | Deep-sea sediment, South China Sea | MH591418.1 | IC50 (α-glucosidase inhibitory) 18.61 μM | [113] |
| (6-benzyl-1-isopentyl-4-oxo-1,4-dihydropyridin-3-yl)-carboxamide (288) | Aspergillus sp. DM94 | Rhizosphere soil of mangrove Bruguiera gymnorrhiza (L.) Poir | M20191003 | – | [96] |
| Flavipesides A–C (289–291) | A. flavipes 164013 | Cyanobacterium Lyngbya majuscula, South China Sea | – | IC50 (PL inhibitory activities) 0.07 ± 0.01–0.23 ± 0.03 μM | [114] |
| kipukasins K–L (292–293) | A. versicolor XS-20090066 | Gorgonian Dichotella gemmacea, Xisha Islands coral reef, South China Sea | MN880095 | – | [115] |
Figure 16.
Structural diversity (left) and bioactivities (right) of new nitrogen heterocycles isolated from Aspergillus pp. that were discovered from January 2019 to January 2024.
The chemical structures of the 306 new secondary metabolites from Aspergillus species can mainly be classified into six types, including 44 indole alkaloids (including 1 pair of racemates), 115 diketopiperazine alkaloids (including 9 pairs of racemates), 32 cyclopeptide alkaloids, 39 quinazoline alkaloids (including 2 pairs of racemates), 17 isoquinoline alkaloids, 22 pyrrolidine alkaloids (including 1 pair of racemates), and 37 other heterocyclic alkaloids. However, among these 306 new compounds, diketopiperazine alkaloids accounted for 37.58%, while indole alkaloids, cyclopeptide alkaloids, quinazoline alkaloids, isoquinoline alkaloids, pyrrolidine alkaloids, and other heterocyclic alkaloids accounted for 14.38%, 10.46%, 12.74%, and 5.56%, 7.19%, and 12.09%, respectively (Figure 16). Due to the high salt levels of the marine environment, some nitrogen-containing secondary metabolites from marine-derived Aspergillus species contain halogen atoms, such as compounds 33, 37 and 259–262.
Moreover, it is worth noting that nearly 33.66% (103 compounds) showed broad-spectrum biological activities, including antimicrobial activities (30 compounds), cytotoxic activities (25 compounds), enzyme-inhibitory activities (14 compounds), antifungal activities (6 compounds), anti-inflammatory activities (6 compounds), and other activities (22 compounds). Notably, cytotoxic (24.27%), antimicrobial (29.13%), and other activities (21.36%) represent the top three bioactivities (Figure 16). It is important to highlight that 21 compounds exhibit potent activities. For example a, new indole alkaloid, sclerotiamide C (13), showed significant inhibitory activity against HeLa, A549, HepG2, and SMMC7721 cell lines, with IC50 values of 1.7 ± 0.1, 1.6 ± 0.1, 1.8 ± 0.1, and 1.5 ± 0.1 μM, respectively, and 13 could induce apoptosis in HeLa cells by arresting the cell cycle, activating ROS production, and regulating apoptosis-related proteins in the MAPK signaling pathway, suggesting that 13 is a potential lead for further development as an anti-cervical tumor agent. A new diketopiperazine alkaloid, waikikiamide A (97), showed antiproliferative activity against cell lines (HT1080, PC3, Jurkat, A2780S) with IC50 values of 0.519, 1.855, 0.62, and 0.78 μM, respectively. New peptides, JG002CPB (152), exhibited the strongest inhibitory activity (IC50 = 53.1 μM), better than that of the positive controls berberine chloride (IC50 = 104.3 μM). One new quinazolinone alkaloid, novobenzomalvin D (214), exhibited better COX-2-inhibitory activity (an inhibition rate of 91.1%) than the positive control celecoxib (56.7%) at 20 nM.
Additionally, the samples were obtained from various environments: 25.82% from sediment, 19.61% from coral, 14.38% from sponge, 8.17% from mangrove, 6.54% from sea mud, 3.92% from seawater, and 21.57% from shellfish, seagrass, and other marine resources. Most significantly, 25.82% originated from marine sediment samples (Figure 17).
Figure 17.
The proportion of Aspergillus species from different marine sources.
In summary, Aspergillus species have been proven to be an important source of novel structures and diverse secondary metabolites with a broad range of biological activities, revealing their great untapped potential in medicinal and agrochemical applications. However, for most of the bioactive secondary metabolites, the lack of deep pharmacological mechanisms limited their applications. We should focus on the pharmacological mechanisms, pharmacokinetics, medicinal chemistry, biosynthesis, etc., to promote the development of innovative drugs in future studies.
Author Contributions
C.Z. and G.H. conceived and revised this article; J.S. prepared and wrote the original draft; M.Y., Z.X. and W.C. conducted the literature analysis; S.C., Y.Q. and Y.W. reviewed and edited this article. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no competing financial interests.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Nos. 32160108 and 2217702), the Key Research and Development Program of Hainan Province (No. ZDYF2024SHFZ116 and ZDYF2021SHFZ270), the Team Innovation Center for Academicians of Hainan Province, the Specific Research Fund for the Innovation Center of Hainan Province Academicians (No. YSPTZX202309), and the Key Science and Technology Program of Hainan Province (No. ZDKJ202008).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Carroll A.R., Copp B.R., Grkovic T., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2024;41:162–207. doi: 10.1039/D3NP00061C. [DOI] [PubMed] [Google Scholar]
- 2.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 3.Xu W.F., Wu N.N., Wu Y.W., Qi Y.X., Wei M.Y., Pineda L.M., Ng M.G., Spadafora C., Zheng J.Y., Lu L., et al. Structure modification, antialgal, antiplasmodial, and toxic evaluations of a series of new marine-derived 14-membered resorcylic acid lactone derivatives. Mar. Life Sci. Technol. 2022;4:88–97. doi: 10.1007/s42995-021-00103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rida P.C., LiVecche D., Ogden A., Zhou J., Aneja R. The noscapine chronicle: A pharmaco-historic biography of the opiate alkaloid family and its clinical applications. Med. Res. Rev. 2015;35:107–1096. doi: 10.1002/med.21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han Y.Q., Zhang Q., Xu W.F., Hai Y., Chao R., Wang C.F., Hou X.M., Wei M.Y., Gu Y.C., Wang C.Y., et al. Targeted isolation of antitubercular cycloheptapeptides and an unusual pyrroloindoline-containing new analog, asperpyrroindotide A, using LC-MS/MS-based molecular networking. Mar. Life Sci. Technol. 2023;5:85–93. doi: 10.1007/s42995-022-00157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hai Y., Wei M.Y., Wang C.Y., Gu Y.C., Shao C.L. The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987–2020) Mar. Life Sci. Technol. 2021;3:488–518. doi: 10.1007/s42995-021-00101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu K., Yuan X.L., Li C., Li X.D. Recent discovery of heterocyclic alkaloids from marine-derived Aspergillus Species. Mar. Drugs. 2020;18:54. doi: 10.3390/md18010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wibowo J.T., Ahmadi P., Rahmawati S.I., Bayu A., Putra M.Y., Kijjoa A. Marine-Derived indole alkaloids and their biological and pharmacological Activities. Mar. Drugs. 2022;20:3. doi: 10.3390/md20010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh P.T., Singh M.Q. Recent progress in biological activities of indole and indole alkaloids. Mini-Rev. Med. Chem. 2018;18:9–25. doi: 10.2174/1389557517666170807123201. [DOI] [PubMed] [Google Scholar]
- 10.Wu J., Wang F., He L.M., Zhou S.Y., Wang S.B., Jia J., Hong K., Cai Y.S. Aculeaquamide A, cytotoxic paraherquamide from the marine fungus Aspergillus aculeatinus WHUF0198. Nat. Prod. Res. 2021;36:4382–4387. doi: 10.1080/14786419.2021.1998047. [DOI] [PubMed] [Google Scholar]
- 11.Girich E.V., Yurchenko A.N., Smetanina O.F., Trinh P.T.H., Ngoc N.T.D., Pivkin M.V., Popov R.S., Pislyagin E.A., Menchinskaya E.S., Chingizova E.A., et al. Neuroprotective metabolites from vietnamese marine derived fungi of Aspergillus and Penicillium genera. Mar. Drugs. 2020;18:608. doi: 10.3390/md18120608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv H., Wang K., Xue Y., Chen J., Su H., Zhang J., Wu Y., Jia J., Bi H., Wang H., et al. Three new metabolites from the marine-derived Fungus Aspergillus sp. WHUF03110. Nat. Pro. Commun. 2021;16:10936–10940. doi: 10.1177/1934578X211055009. [DOI] [Google Scholar]
- 13.Li P., Zhang M., Li H., Wang R., Hou H., Li X., Liu K., Chen H. New prenylated indole homodimeric and pteridine alkaloids from the marine-derived fungus Aspergillus austroafricanus Y32-2. Mar. Drugs. 2021;19:98. doi: 10.3390/md19020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F., Yang L., Xie Q.Y., Guo J.C., Ma Q.Y., Dai L.T., Zhou L.M., Dai H.F., Kong F.D., Luo D.Q., et al. Diverse indole-diterpenoids with protein tyrosine phosphatase 1B inhibitory activities from the marine coral-derived fungus Aspergillus sp. ZF-104. Phytochemistry. 2023;216:113888. doi: 10.1016/j.phytochem.2023.113888. [DOI] [PubMed] [Google Scholar]
- 15.Tuan C.D., Hung N.V., Minh L.T.H., Lien H.T.H., Chae J.W., Yun H.Y., Kim Y.H., Cuong P.V., Huong D.T.M. A new indole glucoside and other constituents from the Sea cucumber-derived Aspergillus fumigatus M580 and their biological activities. Rec. Nat. Prod. 2022;16:633–638. doi: 10.25135/rnp.310.2110.2248. [DOI] [Google Scholar]
- 16.Guo X., Meng Q.Y., Liu J., Wu J.S., Jia H.L., Liu D., Gu Y.C., Liu J.R., Huang J., Fan A.L., et al. Sclerotiamides C-H notoamides from a marine gorgonian-derived fungus with cytotoxic activities. J. Nat. Prod. 2022;85:1067–1078. doi: 10.1021/acs.jnatprod.1c01194. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R., Wang H., Chen B., Dai H., Sun J., Han J., Liu H. Discovery of anti-MRSA secondary metabolites from a marine-derived fungus Aspergillus fumigatus. Mar. Drugs. 2022;20:302. doi: 10.3390/md20050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu Y., Guo Q., Ran Y.Q., Lan W.J., Lam C.K., Feng G.K., Deng R., Zhu X.F., Li H.J., Chen L.P. Cytotoxic alkaloids from the marine shellfish-associated fungus Aspergillus sp. XBB-4 induced by an amino acid-directed strategy. RSC Adv. 2020;10:4243–4250. doi: 10.1039/C9RA10306F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu A., Xu X.N., Zhang M., Li C.L., Liu L., Fu D.Y. Cytotoxic indole alkaloids and polyketides produced by a marine-derived fungus Aspergillus flavipes DS720. Front. Microbiol. 2022;13:959754. doi: 10.3389/fmicb.2022.959754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan L.H., Li X.M., Chi L.P., Li X., Wang B.G. Six new antimicrobial metabolites from the deep-sea sediment-derived fungus Aspergillus fumigatus SD-406. Mar. Drugs. 2022;20:4. doi: 10.3390/md20010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao F.H., Liang X., Cheng X., Dong J.D., Qi S.H. Antifungal peptides from the marine gorgonian-associated fungus Aspergillus sp. SCSIO41501. Phytochemistry. 2021;192:112967. doi: 10.1016/j.phytochem.2021.112967. [DOI] [PubMed] [Google Scholar]
- 22.Zhou G.L., Sun C.X., Hou X.W., Che Q., Zhang G.J., Gu Q.Q., Liu C.G., Zhu T.J., Li D.H. Ascandinines A–D, indole diterpenoids, from the sponge-derived fungus Aspergillus candidus HDN15-152. J. Org. Chem. 2021;86:2431–2436. doi: 10.1021/acs.joc.0c02575. [DOI] [PubMed] [Google Scholar]
- 23.Ma X., Liang X., Huang Z.H., Qi S.H. New alkaloids and isocoumarins from the marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Nat. Prod. Res. 2019;34:1992–2000. doi: 10.1080/14786419.2019.1569660. [DOI] [PubMed] [Google Scholar]
- 24.Guo C., Wang P., Lin X.P., Salendra L., Kong F.D., Liao S.R., Yang B., Zhou X.F., Wang J.F., Liu Y.H. Phloroglucinol heterodimers and bis-indolyl alkaloids from the sponge-derived fungus Aspergillus sp. SCSIO 41018. Org. Chem. Front. 2019;6:3053. doi: 10.1039/C9QO00351G. [DOI] [Google Scholar]
- 25.Luo X.W., Lin Y., Lu Y.J., Zhou X.F., Liu Y.H. Peptides and polyketides isolated from the marine sponge-derived fungus Aspergillus terreus SCSIO 41008. Chin. J. Nat. Med. 2019;17:0149–0154. doi: 10.1016/S1875-5364(19)30017-2. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y.M., Liang X.A., Kong Y., Jia B. Structural diversity and biological activities of indole diketopiperazine alkaloids from fungi. J. Agric. Food Chem. 2016;64:6659–6671. doi: 10.1021/acs.jafc.6b01772. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y.H., Du H.F., Liu Y.F., Cao F., Luo D.Q., Wang C.Y. Novel anti-inflammatory diketopiperazine alkaloids from the marine-derived fungus Penicillium brasilianum. Appl. Microbiol. Biotechnol. 2024;108:194. doi: 10.1007/s00253-024-13026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J.S., Li Z., Gao J.Y., He H.T., Dai H.Q., Xia X.K., Liu C.H., Zhang L.X., Song F.H. New diketopiperazines from a marine-derived fungus strain Aspergillus versicolor MF180151. Mar. Drugs. 2019;17:262. doi: 10.3390/md17050262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C., Wang Y.F., Sun J., Wang S.Y., Du W.S., Zhou L.M., Kong F.D. A pair of new spirocyclic alkaloid enantiomers with TrxR inhibitory activities were isolated from marine-derived Aspergillus ruber TX-M4-1. J. Ocean Univ. China. 2023;22:1677–1682. doi: 10.1007/s11802-023-5607-4. [DOI] [Google Scholar]
- 30.Zhao Y.J., Li L., Zhang Y.H., Yang Y.Y., Li L.F., Yang K., Liu Y.F., Cao F. (±)-Dibrevianamides Q1 and Q2, the key precursors of asperginulin A from a marine-derived fungus. Appl. Microbiol. Biotechnol. 2023;107:6459–6467. doi: 10.1007/s00253-023-12739-2. [DOI] [PubMed] [Google Scholar]
- 31.Long J.Y., Pang X.Y., Lin X.P., Liao S.R., Zhou X.F., Wang J.F., Yang B., Liu Y.H. Asperbenzophenone A and versicolamide C, new fungal metabolites from the soft coral derived Aspergillus sp. SCSIO 41036. Chem. Biodivers. 2022;19:e202100925. doi: 10.1002/cbdv.202100925. [DOI] [PubMed] [Google Scholar]
- 32.Wu J.S., Shi X.H., Yao G.S., Shao C.L., Fu X.M., Zhang X.L., Guan H.S., Wang C.Y. New thiodiketopiperazine and 3,4-dihydroisocoumarin derivatives from the marine-derived fungus Aspergillus terreus. Mar. Drugs. 2020;18:132. doi: 10.3390/md18030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L., Chang Q.H., Zhang S.S., Yang K., Chen F.L., Zhu H.J., Cao F., Liu Y.F. (±)-Brevianamides Z and Z1, new diketopiperazine alkaloids from the marine-derived fungus Aspergillus versicolor. J. Mol. Struct. 2022;1261:132904. doi: 10.1016/j.molstruc.2022.132904. [DOI] [Google Scholar]
- 34.Lu X.W., Chen C.M., Tao H.M., Lin X.P., Yang B., Zhou X.F., Liu Y.H. Structurally diverse diketopiperazine alkaloids from the marine-derived fungus Aspergillus versicolor SCSIO 41016. Org. Chem. Front. 2019;6:736–740. doi: 10.1039/C8QO01147H. [DOI] [Google Scholar]
- 35.Mao J.Q., Zheng Y.Y., Wang C.Y., Liu Y., Yao G.S. Sclerotioloids A–C: Three new alkaloids from the marine-derived fungus Aspergillus sclerotiorum ST0501. Mar. Drugs. 2023;21:219. doi: 10.3390/md21040219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong Q., Guo M.M., Yang J., Wei X., Liao L., Xin X.J., Zhang D., An F.L. Four previously undescribed diketopiperazines from marine fungus Aspergillus puniceus FAHY0085 and their effects on liver X receptor α. Phytochemistry. 2023;214:113816. doi: 10.1016/j.phytochem.2023.113816. [DOI] [PubMed] [Google Scholar]
- 37.Sun C., Ha Y., Liu X., Wang N., Lian X.Y., Zhang Z. Isolation and structure elucidation of new metabolites from the mariana-trench-associated fungus Aspergillus sp. SY2601. Molecules. 2024;29:459. doi: 10.3390/molecules29020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W., Wang L.P., Wang B., Xu Y.C., Zhu G.L., Lan M.M., Zhu W.M., Sun K.L. Diketopiperazine and diphenylether derivatives from Marine algae-derived Aspergillus versicolor OUCMDZ-2738 by epigenetic activation. Mar. Drugs. 2019;17:6. doi: 10.3390/md17010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu B.B., Gui Y.H., Liu L., Su Z.Y., Jiao W.H., Li L., Sun F., Wang S.P., Yang F., Lin H.W. A new asymmetric diketopiperazine dimer from the sponge-associated fungus Aspergillus versicolor 16F-11. Magn. Reson. Chem. 2019;57:49–54. doi: 10.1002/mrc.4780. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z.M., Chen Y.C., Li S.N., Hu C.Y., Liu H.X., Zhang W.M. Indole diketopiperazine alkaloids from the deep-sea-derived fungus Aspergillus sp. FS445. Nat. Prod. Res. 2021;36:5213–5221. doi: 10.1080/14786419.2021.1925271. [DOI] [PubMed] [Google Scholar]
- 41.Lv D., Xia J., Guan X., Lai Q., Zhang B., Lin J., Shao Z., Luo S., Zhangsun D., Qin J.J., et al. Indole diketopiperazine alkaloids isolated from the marine-derived fungus Aspergillus chevalieri MCCC M23426. Front Microbiol. 2022;13:950857. doi: 10.3389/fmicb.2022.950857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu J.S., He Y.P., Zhou F.G., Wu P.P., Chen L.Y., Ni C., Zhang Z.K., Xiao X.J., An L.K., He X.X., et al. New indole diketopiperazine alkaloids from soft coral-associated epiphytic fungus Aspergillus versicolor CGF 9-1-2. Chem. Biodivers. 2023;20:e202300301. doi: 10.1002/cbdv.202300301. [DOI] [PubMed] [Google Scholar]
- 43.Lv F.V., Mándi A., Li X.M., Chi L.P., Li X., Wang B.G., Kurtán T., Meng L.H. Emestrin-type thiodiketopiperazines from Aspergillus nidulans SD-531, a fungus obtained from the deep-sea sediment of cold seep in the south china sea. Deep-Sea Res. PT I. 2023;195:104004. doi: 10.1016/j.dsr.2023.104004. [DOI] [Google Scholar]
- 44.Yang J., Gong L.Z., Guo M.M., Jiang Y., Ding Y., Wang Z.J., Xin X.J., An F.L. Bioactive indole diketopiperazine alkaloids from the marine endophytic fungus Aspergillus sp. YJ191021. Mar. Drugs. 2021;19:157. doi: 10.3390/md19030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X.Y., Xu J.Z., Wang P.M., Ding W.J. Novel indole diketopiperazine stereoisomers from a marine-derived fungus Aspergillus sp. Mycology. 2022;14:1–10. doi: 10.1080/21501203.2022.2069173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H.L., Yang S.Q., Li X.M., Li X., Wang B.G. Structurally diverse alkaloids produced by Aspergillus creber EN-602, an endophytic fungus obtained from the marine red alga Rhodomela confervoides. Bioorg. Chem. 2021;110:104822. doi: 10.1016/j.bioorg.2021.104822. [DOI] [PubMed] [Google Scholar]
- 47.Wang F., Sarotti A.M., Jiang G., Huguet-Tapia J.C., Zheng S.L., Wu X., Li C., Ding Y., Cao S. Waikikiamides A-C: Complex diketopiperazine dimer and diketopiperazine-polyketide hybrids from a Hawaiian marine fungal strain Aspergillus sp. FM242. Org. Lett. 2020;22:4408–4412. doi: 10.1021/acs.orglett.0c01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding Y., Zhu X.J., Hao L.L., Zhao M.Y., Hua Q., An F.L. Bioactive indolyl diketopiperazines from the marine derived endophytic Aspergillus versicolor DY180635. Mar. Drugs. 2020;18:338. doi: 10.3390/md18070338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng Q.Y., Guo X., Wu J.S., Liu D., Gu Y.C., Huang J., Fan A.L., Lin W.H. Prenylated notoamide-type alkaloids isolated from the fungus Aspergillus sclerotiorum and their inhibition of NLRP3 inflammasome activation and antibacterial activities. Phytochemistry. 2022;203:113424. doi: 10.1016/j.phytochem.2022.113424. [DOI] [PubMed] [Google Scholar]
- 50.Wei X., Feng C., Wang S.Y., Zhang D.M., Li X.H., Zhang C.X. New indole diketopiperazine alkaloids from soft coral-associated epiphytic fungus Aspergillus sp. EGF 15-0-3. Chem. Biodivers. 2020;17:e2000106. doi: 10.1002/cbdv.202000106. [DOI] [PubMed] [Google Scholar]
- 51.Dong Y.L., Li X.M., Shi X.S., Wang Y.R., Wang B.G., Meng L.H. Diketopiperazine alkaloids and bisabolene sesquiterpenoids from Aspergillus versicolor AS-212, an endozoic fungus associated with deep-sea coral of Magellan Seamounts. Mar. Drugs. 2023;21:293. doi: 10.3390/md21050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong Y.L., Li X.M., Wang Y.R., Shi X.S., Wang B.G., Meng L.H. Oxepine-containing pyrazinopyrimidine alkaloids and quinolinone derivatives produced by Aspergillus versicolor AS-212, a deep-sea-derived endozoic fungus. Fitoterapia. 2023;168:105559. doi: 10.1016/j.fitote.2023.105559. [DOI] [PubMed] [Google Scholar]
- 53.Youssef D.T.A., Shaala L.A., Genta-Jouve G. Asperopiperazines A and B: Antimicrobial and cytotoxic dipeptides from a tunicate-derived fungus Aspergillus sp. DY001. Mar. Drugs. 2022;20:451. doi: 10.3390/md20070451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye G.T., Huang C.Y., Li J.L., Chen T., Tang J., Liu W.B., Long Y.H. Isolation, structural characterization and antidiabetic activity of new diketopiperazine alkaloids from mangrove endophytic fungus Aspergillus sp. 16-5c. Mar. Drugs. 2021;19:402. doi: 10.3390/md19070402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan L.H., Du F.Y., Li X.M., Yang S.Q., Wang B.G., Li X. Antibacterial indole diketopiperazine alkaloids from the deep-sea cold seep-derived fungus Aspergillus chevalieri. Mar. Drugs. 2023;21:195. doi: 10.3390/md21030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Girich E.V., Rasin A.B., Popov R.S., Yurchenko E.A., Chingizova E.A., Trinh P.T.H., Ngoc N.T.D., Pivkin M.V., Zhuravleva O.I., Yurchenko A.N. New tripeptide derivatives asperripeptides A-C from Vietnamese mangrove-derived fungus Aspergillus terreus LM.5.2. Mar. Drugs. 2022;20:77. doi: 10.3390/md20010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han J., Liu M., Jenkins I.D., Liu X., Zhang L., Quinn R.J., Feng Y. Genome-inspired chemical exploration of marine fungus Aspergillus fumigatus MF071. Mar. Drugs. 2020;18:352. doi: 10.3390/md18070352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bai M., Wang Y., Liu T., Lian Y.X., Bai Q.Q., Song X.P., Han C.R., Zheng C.J., Chen G.Y. One new piperazinedione isolated from a mangrove-derived fungus Aspergillus niger JX-5. Nat. Prod. Res. 2020;36:2277–2283. doi: 10.1080/14786419.2020.1828407. [DOI] [PubMed] [Google Scholar]
- 59.Liang X., Zhang X.L., Lu X.H., Zheng Z.H., Ma X., Qi S.H. Diketopiperazine-Type alkaloids from a deep-Sea-derived Aspergillus puniceus fungus and their effects on liver X receptor α. J. Nat. Prod. 2019;82:1558–1564. doi: 10.1021/acs.jnatprod.9b00055. [DOI] [PubMed] [Google Scholar]
- 60.Yan L.H., Li P.H., Li X.M., Yang S.Q., Liu K.C., Wang B.G., Li X. Chevalinulins A and B, proangiogenic alkaloids with a spiro[bicyclo [2.2.2]octane-diketopiperazine] skeleton from deep-sea cold-seep-derived fungus Aspergillus chevalieri CS-122. Org. Lett. 2022;24:2684–2688. doi: 10.1021/acs.orglett.2c00781. [DOI] [PubMed] [Google Scholar]
- 61.Tuenter E., Exarchou V., Apers S., Pieters L. Cyclopeptide alkaloids. Phytochem. Rev. 2017;16:623–637. doi: 10.1007/s11101-016-9484-y. [DOI] [Google Scholar]
- 62.Zhang J.N., Xia Y.X., Zhang H.J. Natural cyclopeptides as anticancer agents in the Last 20 Years. Int. J. Mol. Sci. 2021;22:3973. doi: 10.3390/ijms22083973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liao Y.Y., Hong X., Yang J., Qin J.J., Zhang B.B., Lin J.H., Shao Z.Z., Wang W.Y. Structure elucidation of a novel cyclic tripeptide from the marine-derived fungus Aspergillus ochraceopetaliformis DSW-2. Nat. Prod. Res. 2021;36:3572–3578. doi: 10.1080/14786419.2020.1869969. [DOI] [PubMed] [Google Scholar]
- 64.Hwang J.Y., Lee J.H., Park S.C., Lee J., Oh D.C., Oh K.B., Shin J. New peptides from the marine-derived fungi Aspergillus allahabadii and Aspergillus ochraceopetaliformis. Mar. Drugs. 2019;17:488. doi: 10.3390/md17090488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chi L.P., Liu D., Li X.M., Wan Y., Wang B.G., Li X. Aspertides A-E: Antimicrobial pentadepsipeptides with a unique p-methoxycinnamoyl amide group from the marine isolates Aspergillus tamarii MA-21 and Aspergillus insuetus SD-512. J. Agric. Food. Chem. 2023;71:13316–13324. doi: 10.1021/acs.jafc.3c02610. [DOI] [PubMed] [Google Scholar]
- 66.Wang H.F., Zhang R., Ma B., Wang W.Z., Yu C., Han J.J., Zhu L.J., Zhang X., Dai H.Q., Liu H.W., et al. Japonamides A and B, two new cyclohexadepsipeptides from the marine-sponge-derived fungus Aspergillus japonicus and their synergistic antifungal activities. J. Fungi. 2022;8:1058. doi: 10.3390/jof8101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding W.J., Tian D.M., Chen M., Xia Z.X., Tang X.Y., Zhang S.H., Wei J.H., Li X.N., Yao X.S., Wu B., et al. Molecular networking-guided isolation of cyclopentapeptides from the hydrothermal vent sediment derived fungus Aspergillus pseudoviridinutans TW58-5 and their anti-inflammatory effects. J. Nat. Prod. 2023;86:1919–1930. doi: 10.1021/acs.jnatprod.3c00287. [DOI] [PubMed] [Google Scholar]
- 68.Tang W.Z., Liu J.T., Hu Q., He R.J., Guan X.Q., Ge G.B., Han H., Yang F., Lin H.W. Pancreatic lipase inhibitory cyclohexapeptides from the marine sponge-derived fungus Aspergillus sp. 151304. J. Nat. Prod. 2020;83:2287–2293. doi: 10.1021/acs.jnatprod.0c00549. [DOI] [PubMed] [Google Scholar]
- 69.Abdel-Wahab N.M., Scharf S., Özkaya F.C., Kurtán T., Mándi A., Fouad M.A., Kamel M.S., Müller W.E.G., Kalscheuer R., Lin W., et al. Induction of secondary metabolites from the marine-derived fungus Aspergillus versicolor through co-cultivation with Bacillus subtilis. Planta Med. 2019;85:503–512. doi: 10.1055/a-0835-2332. [DOI] [PubMed] [Google Scholar]
- 70.Zeng Q., Chen Y.C., Wang J.F., Shi X.F., Che Y.H., Chen X.Y., Zhong W.M., Zhang W.M., Wei X.Y., Wang F.Z., et al. Diverse secondary metabolites from the coral-derived fungus Aspergillus hiratsukae SCSIO 5Bn1003. Mar. Drugs. 2022;20:150. doi: 10.3390/md20020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Magot F., Van Soen G., Buedenbender L., Li F., Soltwedel T., Grauso L., Mangoni A., Blümel M., Tasdemir D. Bioactivity and metabolome mining of deep-sea sediment-derived microorganisms reveal new hybrid PKS-NRPS macrolactone from Aspergillus versicolor PS108-62. Mar. Drugs. 2023;21:95. doi: 10.3390/md21020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun C.X., Zhang Z.P., Ren Z.L., Yu L., Zhou H., Han Y.X., Shah M., Che Q., Zhang G.J., Li D.H., et al. Antibacterial cyclic tripeptides from antarctica-sponge-derived fungus Aspergillus insulicola HDN151418. Mar. Drugs. 2020;18:532. doi: 10.3390/md18110532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aparicio-Cuevas M.A., González M.D.C., Raja H., Rivero-Cruz I., Kurina S.J., Burdette J.E., Oberlies N.H., Figueroa M. Metabolites from the marine-facultative Aspergillus sp. MEXU 27854 and Gymnoascus hyalinosporus MEXU 29901 from Caleta Bay, Mexico. Tetrahedron Lett. 2019;60:1649–1652. doi: 10.1016/j.tetlet.2019.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alagarsamy V., Chitra K., Saravanan G., Raja Solomon V., Sulthana M.T., Narendhar B. An overview of quinazolines: Pharmacological significance and recent developments. Eur. J. Med. Chem. 2018;151:628–685. doi: 10.1016/j.ejmech.2018.03.076. [DOI] [PubMed] [Google Scholar]
- 75.Song Z.J., Liu Y., Gao J.Y., Hu J.S., He H.T., Dai S.W., Wang L.Q., Dai H.Q., Zhang L.X., Song F.H. Antitubercular metabolites from the marine-derived fungus strain Aspergillus fumigatus MF029. Nat. Prod. Res. 2019;35:2647–2654. doi: 10.1080/14786419.2019.1660331. [DOI] [PubMed] [Google Scholar]
- 76.Liu S.S., Yang L., Kong F.D., Zhao J.H., Yao L., Yuchi Z.G., Ma Q.Y., Xie Q.Y., Zhou L.M., Guo M.F., et al. Three new quinazoline-containing indole alkaloids from the marine-derived fungus Aspergillus sp. HNMF114. Front. Microbiol. 2021;12:680879. doi: 10.3389/fmicb.2021.680879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fredimoses M., Ai W., Lin X.P., Zhou X.F., Liao S.R., Pan L., Liu Y.H. Two new Aspera chaetominines A and B, and a new derivative of terrein, isolated from marine sponge associated fungus Aspergillus versicolour SCSIO XWS04 F52. Nat. Prod. Res. 2023;7:1–13. doi: 10.1080/14786419.2023.2275744. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y.J., Zhang J.L., Li C., Mu X.G., Liu X.L., Wang L., Zhao Y.C., Zhang P., Li X.D., Zhang X.X. Antimicrobial secondary metabolites from the seawater-derived fungus Aspergillus sydowii SW9. Molecules. 2019;24:4596. doi: 10.3390/molecules24244596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kong F.D., Zhang S.L., Zhou S.Q., Ma Q.Y., Xie Q.Y., Chen J.P., Li J.H., Zhou L.M., Yuan J.Z., Hu Z., et al. Quinazoline-Containing indole alkaloids from the marine-derived fungus Aspergillus sp. HNMF114. J. Nat. Prod. 2019;82:3456–3463. doi: 10.1021/acs.jnatprod.9b00845. [DOI] [PubMed] [Google Scholar]
- 80.Ohte S., Shiokawa T., Koyama N., Katagiri T., Imada C., Tomoda H. A new diketopiperazine-like inhibitor of bone morphogenetic protein-induced osteoblastic differentiation produced by marine-derived Aspergillus sp. BFM-0085. J. Antibiot. 2020;73:554–558. doi: 10.1038/s41429-020-0316-3. [DOI] [PubMed] [Google Scholar]
- 81.Belousova E.B., Zhuravleva O.I., Yurchenko E.A., Oleynikova G.K., Antonov A.S., Kirichuk N.N., Chausova V.E., Khudyakova Y.V., Menshov A.S., Popov R.S., et al. New anti-hypoxic metabolites from co-culture of marine-derived fungi Aspergillus carneus KMM 4638 and Amphichorda sp. KMM 4639. Biomolecules. 2023;13:741. doi: 10.3390/biom13050741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu W.F., Chao R., Hai Y., Guo Y.Y., Wei M.Y., Wang C.Y., Shao C.L. 17-Hydroxybrevianamide N and its N1-methyl derivative, quinazolinones from a soft-coral-derived Aspergillus sp. Fungus: 13S enantiomers as the true natural products. J. Nat. Prod. 2021;84:1353–1358. doi: 10.1021/acs.jnatprod.1c00098. [DOI] [PubMed] [Google Scholar]
- 83.Bao J., Li X.X., Zhu K.K., He F., Wang Y.Y., Yu J.H., Zhang X.Y., Zhang H. Bioactive aromatic butenolides from a mangrove sediment originated fungal species, Aspergillus terreus SCAU011. Fitoterapia. 2021;150:104856. doi: 10.1016/j.fitote.2021.104856. [DOI] [PubMed] [Google Scholar]
- 84.Wang C., Sarotti A.M., Zaman K.A.U., Wu X.H., Cao S.G. New alkaloids from a Hawaiian fungal strain Aspergillus felis FM324. Front. Chem. 2021;9:724617. doi: 10.3389/fchem.2021.724617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Si Y.Y., Tang M.X., Lin S., Chen G., Hua H.M., Bai J., Wang Y.B., Wang H.F., Pei Y.H. 2-Methyl-versiquinazoline C, a new fumiquinazoline-type alkaloid from the fungus Aspergillus flavipes PJ03-11. J. Asian Nat. Prod. Res. 2019;21:528–534. doi: 10.1080/10286020.2018.1463999. [DOI] [PubMed] [Google Scholar]
- 86.Guo S.L., Zhou H.M., Huang X., Peng S.Y., Li J.Y., Ding B., Tao Y.W., Huang H.B. New glucosidated indole-quinazoline alkaloids from mangrove endophytic fungus Aspergillus fumigatus SAl12. Nat. Prod. Res. 2023:1–6. doi: 10.1080/14786419.2023.2209822. [DOI] [PubMed] [Google Scholar]
- 87.Shuai H., Myronovskyi M., Rosenkränzer B., Paulus C., Nadmid S., Stierhof M., Kolling D., Luzhetskyy A. Novel biosynthetic route to the isoquinoline scaffold. ACS Chem. Biol. 2022;17:598–608. doi: 10.1021/acschembio.1c00869. [DOI] [PubMed] [Google Scholar]
- 88.Liu C.M., Liang X., Yao F.H., Qi S.H. Five new aromatic polyketides and isoquinoline alkaloids from the deep-sea-derived fungus Aspergillus puniceus SCSIO z021. Tetrahedron. 2022;126:133067. doi: 10.1016/j.tet.2022.133067. [DOI] [Google Scholar]
- 89.Liu C.M., Yao F.H., Lu X.H., Zhang X.X., Luo L.X., Liang X., Qi S.H. Isoquinoline alkaloids as protein tyrosine phosphatase inhibitors from a deep-sea-derived fungus Aspergillus puniceus. Mar. Drugs. 2022;20:78. doi: 10.3390/md20010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pang X.Y., Lin X.P., Zhou X.F., Yang B., Tian X.P., Wang J.F., Xu S.H., Liu Y.H. New quinoline alkaloid and bisabolane-type sesquiterpenoid derivatives from the deep-sea-derived fungus Aspergillus sp. SCSIO06786. Fitoterapia. 2020;140:104406. doi: 10.1016/j.fitote.2019.104406. [DOI] [PubMed] [Google Scholar]
- 91.Islam M.T., Mubarak M.S. Pyrrolidine alkaloids and their promises in pharmacotherapy. Adv. Tradit. Med. 2020;20:13–22. doi: 10.1007/s13596-019-00419-4. [DOI] [Google Scholar]
- 92.Liu Y., Ding L.J., Shi Y.T., Yan X.J., Wu B., He S. Molecular networking-driven discovery of antibacterial perinadines, new tetracyclic alkaloids from the marine sponge-derived fungus Aspergillus sp. ACS Omega. 2022;7:9909–9916. doi: 10.1021/acsomega.2c00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park S.C., Lee J.H., Hwang J.Y., Kwon O.S., Liao L., Oh D.C., Oh K.B., Shin J. Ochraceopetalin, a mixed-biogenetic salt of polyketide and amino acid origins from a marine-derived Aspergillus ochraceopetaliformis fungus. Mar. Drugs. 2021;19:413. doi: 10.3390/md19080413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiang Y., Zeng Q., Mai Z.M., Chen Y.C., Shi X.F., Chen X.Y., Zhong W.M., Wei X.Y., Zhang W.M., Zhang S., et al. Asperorydines N-P, three new cyclopiazonic acid alkaloids from the marine-derived fungus Aspergillus flavus SCSIO F025. Fitoterapia. 2021;150:104839. doi: 10.1016/j.fitote.2021.104839. [DOI] [PubMed] [Google Scholar]
- 95.Anh C.V., Yoon Y.D., Kang J.S., Lee H.S., Heo C.S., Shin H.J. Nitrogen-Containing secondary metabolites from a deep-sea fungus Aspergillus unguis and their anti-inflammatory activity. Mar. Drugs. 2022;20:217. doi: 10.3390/md20030217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gou X., Jia J., Xue Y., Ding W., Dong Z., Tian D., Chen M., Bi H., Hong K., Tang J. New pyrones and their analogs from the marine mangrove-derived Aspergillus sp. DM94 with antibacterial activity against Helicobacter pylori. Appl. Microbiol. Biotechnol. 2020;104:7971–7978. doi: 10.1007/s00253-020-10792-9. [DOI] [PubMed] [Google Scholar]
- 97.Xu X., Han J., Wang Y., Lin R., Yang H., Li J., Wei S., Polyak S.W., Song F. Two new spiro-heterocyclic γ-lactams from A marine-derived Aspergillus fumigatus strain CUGBMF170049. Mar. Drugs. 2019;17:289. doi: 10.3390/md17050289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiao F.W., Xing Y.N., Liu T.Y. New azaspirene derivatives from marine-derived fungus Aspergillus micronesiensis NF666. Tetrahedron Lett. 2023;123:154566. doi: 10.1016/j.tetlet.2023.154566. [DOI] [Google Scholar]
- 99.Xu L., Guo F.W., Zhang X.Q., Zhou T.Y., Wang C.J., Wei M.Y., Gu Y.C., Wang C.Y., Shao C.L. Discovery, total syntheses and potent anti-inflammatory activity of pyrrolinone-fused benzoazepine alkaloids asperazepanones A and B from Aspergillus candidus. Commun. Chem. 2022;5:80. doi: 10.1038/s42004-022-00696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang J.M., Li Z.C., Zhang Y.T., Chen C.M., Chen W.H., Gao C.H., Liu Y.H., Tan Y.H., Luo X.W. A new α-cyclopiazonic acid alkaloid identified from the Weizhou Island coral-derived fungus Aspergillus flavus GXIMD 02503. J. Ocean Univ. China. 2022;21:1307–1312. doi: 10.1007/s11802-022-4959-5. [DOI] [Google Scholar]
- 101.Chen Z.H., Liu H.S., Ding B., Guo S.Y., Huang H.B., Tao Y.W. Two new alkaloids from the endophytic fungus Aspergillus fumigatus SAS10 isolated from the mangrove tree Sonneratia apetala. Nat. Prod. Res. 2023:1–6. doi: 10.1080/14786419.2023.2272289. [DOI] [PubMed] [Google Scholar]
- 102.Hu Z.B., Zhu Y.J., Chen J.J., Chen J., Li C.Y., Gao Z.Z., Li J., Liu L. Discovery of novel bactericides from Aspergillus alabamensis and their antibacterial activity against fish pathogens. J. Agric. Food Chem. 2023;71:4298–4305. doi: 10.1021/acs.jafc.2c09141. [DOI] [PubMed] [Google Scholar]
- 103.Lin S.H., Yan Q.X., Zhang Y., Wu T.Z., Zou Z.B., Liu Q.M., Jiang J.Y., Xie M.M., Xu L., Hao Y.J., et al. Citriquinolinones A and B: Rare isoquinolinone-embedded citrinin analogues and related metabolites from the deep-sea-derived Aspergillus versicolor 170217. Mar. Drugs. 2023;21:504. doi: 10.3390/md21100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang F.Q., Hu Z.Q., Li C.S., Wu X.H., Cao S.G. Circumdatin M, a new benzodiazepine alkaloid with a unique pyrimidone-4-pyrone moiety from a Hawaiian marine fungus Aspergillus sp. FM242. Tetrahedron Lett. 2019;60:1724–1726. doi: 10.1016/j.tetlet.2019.05.061. [DOI] [Google Scholar]
- 105.Li H.Q., Guo J.R., Zhang R.G., Wang J.P., Hu Z.X., Zhang Y.H. Two new nucleoside derivatives isolated from the marine-derived Aspergillus versicolor and their intramolecular transesterification. Nat. Prod. Res. 2020;36:3346–3352. doi: 10.1080/14786419.2020.1858409. [DOI] [PubMed] [Google Scholar]
- 106.Chen Z., She J.L., Liu J., Peng B., Yuan Z.M., Zhou X.F. Pyripyropene U, a new alkaloid from the sponge-derived fungus Aspergillus sp. SCSIO41420. Nat. Prod. Res. 2023:1–6. doi: 10.1080/14786419.2023.2284866. [DOI] [PubMed] [Google Scholar]
- 107.Peng Q.Y., Cai J., Long J.Y., Yang B., Lin X.P., Wang J.F., Xiao J., Liu Y.H., Zhou X.F. New azaphthalide and phthalide derivatives from the marine coral-derived fungus Aspergillus sp. SCSIO41405. Phytochem. Lett. 2021;43:94–97. doi: 10.1016/j.phytol.2021.03.019. [DOI] [Google Scholar]
- 108.Liu Z.M., Wang Q.L., Li S.N., Cui H., Sun Z.H., Chen D.N., Lu Y.J., Liu H.X., Zhang W.M. Polypropionate derivatives with Mycobacterium tuberculosis protein tyrosine phosphatase B inhibitory activities from the deep-sea-derived fungus Aspergillus fischeri FS452. J. Nat. Prod. 2019;82:3440–3449. doi: 10.1021/acs.jnatprod.9b00834. [DOI] [PubMed] [Google Scholar]
- 109.Wu B.B., Xu C.L., Chen J.J., Chen G.Y. Rhizoaspergillin A and rhizoaspergillinol A, including a unique orsellinic acid-ribose-pyridazinone-N-oxide hybrid, from the mangrove endophytic fungus Aspergillus sp. A1E3. Mar. Drugs. 2023;21:598. doi: 10.3390/md21110598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Niu S.W., Chen Z.M., Pei S.X., Shao Z.Z., Zhang G.Y., Hong B.H. Acremolin D, a new acremolin alkaloid from the deep-sea sediment derived Aspergillus sydowii fungus. Nat. Prod. Res. 2021;36:4936–4942. doi: 10.1080/14786419.2021.1913587. [DOI] [PubMed] [Google Scholar]
- 111.Liu X.J., Li H.J., Ma W.Z., Zhang F.M., Xu M.Y., Mahmud T., Lan W.J. Phomaligols F-I, polyoxygenated cyclohexenone derivatives from marine-derived fungus Aspergillus flavus BB1. Bioorg. Chem. 2021;115:105269. doi: 10.1016/j.bioorg.2021.105269. [DOI] [PubMed] [Google Scholar]
- 112.Li F., Sun C.X., Che Q., Zhu T.J., Gu Q.Q., Guan H.S., Zhang G.J., Li D.H. Pyrazinopyrimidine alkaloids from a mangrove-derived fungus Aspergillus versicolor HDN11-84. Phytochemistry. 2021;188:112817. doi: 10.1016/j.phytochem.2021.112817. [DOI] [PubMed] [Google Scholar]
- 113.Chen W.H., Chen C.M., Long J.Y., Lan S.J., Lin X.P., Liao S.R., Yang B., Zhou X.F., Wang J.F., Liu Y.H. Bioactive secondary metabolites from the deep-sea derived fungus Aspergillus sp. SCSIO 41029. J. Antibiot. 2021;74:156–159. doi: 10.1038/s41429-020-00378-y. [DOI] [PubMed] [Google Scholar]
- 114.Jiao W.H., Xu Q.H., Ge G.B., Shang R.Y., Zhu H.R., Liu H.Y., Cui J., Sun F., Lin H.W. Flavipesides A-C, PKS-NRPS hybrids as pancreatic lipase inhibitors from a marine sponge symbiotic fungus Aspergillus flavipes 164013. Org. Lett. 2020;22:1825–1829. doi: 10.1021/acs.orglett.0c00150. [DOI] [PubMed] [Google Scholar]
- 115.Wu J.S., Yao G.S., Shi X.H., Rehman S.U., Xu Y., Fu X.M., Zhang X.L., Liu Y., Wang C.Y. Epigenetic agents trigger the production of bioactive nucleoside derivatives and bisabolane sesquiterpenes from the marine-derived fungus Aspergillus versicolor. Front. Microbiol. 2020;11:85. doi: 10.3389/fmicb.2020.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]