Abstract
The layered orthorhombic quaternary tellurides EuRECuTe3 (RE = Ho, Tm, Sc) with Cmcm symmetry were first synthesized. Single crystals of the compounds up to 500 μm in size were obtained by the halide-flux method at 1120 K from elements taken in a ratio of Eu/RE/Cu/Te = 1:1:1:3. In the series of compounds, the changes in lattice parameters were in the ranges a = 4.3129(3)–4.2341(3) Å, b = 14.3150(9)–14.1562(9) Å, c = 11.2312(7)–10.8698(7) Å, V = 693.40(8)–651.52(7) Å3. In the structures, the cations Eu2+, RE3+ (RE = Ho, Tm, Sc), and Cu+ occupied independent crystallographic positions. The structures were built with distorted copper tetrahedra forming infinite chains [CuTe4]7− and octahedra [RETe6]9− forming two-dimensional layers along the a-axis. These coordination polyhedra formed parallel two-dimensional layers . Between the layers, along the a-axis, chains of europium trigonal prisms [EuTe6]10− were located. Regularities in the variation of structural parameters and the degree of distortion of coordination polyhedra depending on the ionic radius of the rare-earth metal in the compounds EuRECuCh3 (RE = Ho, Er, Tm, Lu, Sc; Ch = S, Se, Te) were established. It is shown that with a decrease in the ionic radius ri(RE3+) in the compounds EuRECuTe3, the unit-cell volume, bond length d(RE–Te), distortion degree [CuTe4]7−, and crystallographic compression of layers [RECuTe3]2− decreased. The distortion degree of tetrahedral polyhedra [CuCh4]7−, as well as the structural parameters in europium rare-earth copper tellurides EuRECuTe3, were higher than in isostructural quaternary chalcogenides. Ab initio calculations of the crystalline structure, phonon spectrum, and elastic properties of compounds EuRECuTe3 (RE = Ho, Tm, and Sc) ere conducted. The types and wave numbers of fundamental modes were determined, and the involvement of ions in IR and Raman modes was assessed. The calculated data of the crystal structure correlated well with the experimental results.
Keywords: quaternary europium copper tellurides, syntheses, crystal structure, DFT calculations, rare-earth metals
1. Introduction
Studying four-component tellurides opens up new perspectives for creating materials with unique properties and potential applications in various fields, including electronics, optics, and energy [1,2,3,4,5,6]. Understanding the influence of structural features on the properties of chalcogenide materials allows for the modification of the band gap width, electrical, and optical properties of the materials [7,8,9,10,11,12,13,14,15]. The presence of a large variety of coordination polyhedra and different ways of connecting them in tellurides creates conditions for the formation of layered or tunnel structures [6,9]. Layered tellurides of the space group Cmcm demonstrate promising efficiency in solar energy conversion and are considered as prospective absorbers in solar cell structures, as well as thermoelectric materials [1,2,3,4]. It is assumed that in compounds of the space group Cmcm, such as MRECuTe3 (M = d-element) with the structure type of KZrCuS3, there is a covalently bonded sublattice [RECuTe3]2−, leading to improved electrical transport properties, and the M2+ cations induce low lattice thermal conductivity [1,2]. Tellurides of the space group Cmcm contain non-centrosymmetric tetrahedral coordination polyhedra [16], allowing them to be used in nonlinear optical devices as photon media [17,18]. In the EuRECuCh3 structures, a tetrahedral structural motif is clearly visible in the form of one-dimensional chains of distorted [CuCh3]5− [18,19,20,21,22,23,24,25,26] tetrahedra. Compounds BaRECuCh3 (Ch = chalcogen) are used as hole transport materials in solar cells, significantly increasing the efficiency of the solar cell (up to 45%) compared to a cell without this chalcogenide [1,27,28]. It was previously established that replacing the alkaline earth M2+ ions in the quaternary chalcogenides MRECuCh3 with the Eu2+ cation makes it possible to narrow the band gap of the compound due to the presence of the 4f–5d transition in the Eu2+ ion [8]. Also, an increase in the chalcogen radius in the series ri(S2−) > ri(Se2−) > ri(Te2−) reduces the value of the band gap [7]. Thus, it is expected that the synthesis of EuRECuTe3 compounds will make it possible in the future to obtain semiconductor materials with the bandgap value required for photovoltaic materials and use them as a photoanode in multilayer solar cells, improving charge separation as well as reducing the recombination of electrons and holes at the interface of the photoanode and electrolyte.
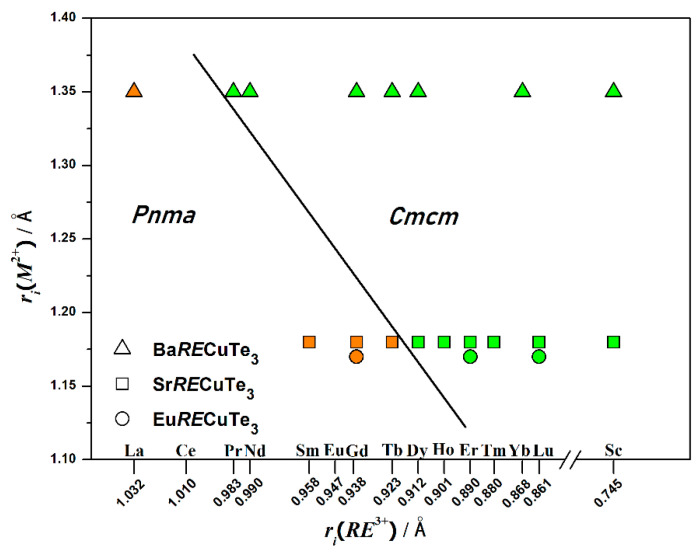
Experimental and theoretical studies of the sulfide series of europium compounds EuRECuS3 were carried out for quite a long time from 1986 to 2021 [8,22,23,24,29], and similar studies of the selenide series EuRECuSe3 were carried out from 2022 to 2024 [18,25,26]. Research has shown that the compounds are p-type semiconductors, low-temperature ferro- and ferrimagnets, with a negative magnetization effect, high-temperature stability in an inert atmosphere, and polymorphism [8,16,18,22,23,24,25,26,29]. A decrease in incongruent melting temperatures and band gaps with changes in chalcogen has been established [8,24,26]. From the EuRECuTe3 series of europium tellurides, only three have been obtained to date: EuGdCuTe3 [20], EuErCuTe3 [19], and EuLuCuTe3 [20] (Figure 1). However, quaternary tellurides with alkaline earth elements BaRECuTe3 (RE = Pr–Yb, Sc) [1,16,17,30,31] and SrRECuTe3 (RE = Dy–Lu, Sc) [1,12,16] are described in detail in the literature. The smaller the ionic radius of the M2+ cation in the compounds MRECuTe3, the fewer compounds will crystallize in the space group Cmcm [12]. The Eu2+ cation has a smaller ionic radius than the Sr2+ and Ba2+ cations [32]. It can be assumed that compounds EuRECuTe3 (RE = Ho–Lu and Sc) will crystallize in the space group Cmcm.
Figure 1.
Structure field diagram of MRECuTe3 tellurides with M = Ba [1,16,17,30,31], Sr [1,12,16], Eu [19,20]. Description: color background corresponds to a defined structure type (space group Pnma—orange (Eu2CuS3); space group Cmcm—green (KZrCuS3)). The black line delimits the regions of existence of the space groups Pnma and Cmcm.
For compounds EuTmCuTe3 and EuScCuTe3, the structure type of NaCuTiS3 has been predicted [2], crystallizing in the space group Pnma [33]. According to DFT calculations using the PBE functional in this space group, the band gap widths of these compounds are 0.59 and 0.40 eV, respectively [2]. The structure of the orthorhombic crystal NaCuTiS3 is characterized by distorted copper tetrahedra and titanium octahedra forming two-dimensional layers [CuTiS3]− along the c-axis [33]. Single-capped trigonal prisms of sodium are located between the layers. However, previously obtained scandium and thulium quaternary chalcogenides MRECuCh3 (M = Eu [8,18,22,24,25], Sr [8,12,34,35,36], Ba [1,37,38,39]; RE = Sc, Tm; Ch = S, Se, Te) crystallize in the space group Cmcm with the KZrCuS3-type structure. It is expected that compounds EuScCuTe3 and EuTmCuTe3 will crystallize in the same structure type and space group as compounds with isostructural composition [22,24,25,35,36,37,38,39].
There are no literature data on attempts to synthesize and establish the crystal structure of tellurides EuRECuTe3 (RE = Ho, Tm, Yb, Sc). In this work, we first describe the single-crystal synthesis of EuRECuTe3 (RE = Ho, Tm, and Sc), investigating their crystal structure both experimentally and using theoretical methods.
2. Experimental
2.1. Materials
Ho (99.9%), Tm (99.9%), Yb (99.9%), Sc (99.9%), Eu (99.99%), Te (99.9%), Ar (99.99%), C2H2 (99.9%), and CsI (99.9%) were purchased from ChemPur (Karlsruhe, Germany). Cu (99.999%) was obtained from Aldrich (Milwaukee, WI, USA).
2.2. Synthesis
Single crystals of EuRECuTe3 (RE = Ho, Tm, Sc) were synthesized from elements taken in a ratio of Cu/Eu/RE/Te = 1:1:1:3 with the addition of cesium iodide as a flux. Due to the rapid oxidizability of rare-earth metals by components of the air (oxygen, carbon dioxide, water vapor) at room temperature, the starting components were weighed in an inert atmosphere using a glovebox. A layer of amorphous carbon obtained by pyrolysis of acetylene was pre-applied to the inner walls of the silica ampoules. After filling the ampoules with the starting components, they were tightly sealed with a quick-release seal, removed from the glove box, connected to a vacuum pump, evacuated to a residual pressure of 2 × 10−3 mbar, sealed, and heated in a muffle furnace. The temperature in the furnace was raised from room temperature to 1120 K over 30 h with subsequent isothermal holding for 96 h. Cooling was carried out to 570 K over 140 h and to 300 K over 3 h. Residual flux from the sample was removed with demineralized water. The products were black needle-like crystals of EuRECuTe3 (RE = Ho, Tm, Sc) up to 500 µm in size (Figure 2). The obtained crystals were suitable for single crystal X-ray diffraction analysis. Unfortunately, it was not possible to obtain high-quality powder diffraction patterns, as copper compounds strongly absorb molybdenum radiation. The yield of tellurides was 10–15%, which did not allow for the experimental determination of the optical properties of the samples. Unfortunately, despite numerous attempts, we were unable to obtain the compound EuYbCuTe3 using the flux method. The samples after synthesis contained single crystals of Cu0.37YbTe2, EuTe, and YbTe. The phase of EuYbCuTe3 was not found in the samples, possibly due to the weak oxidizing ability of tellurium interfering with the Yb0–3e−→Yb3+ redox reaction.
Figure 2.
Photograph of EuHoCuTe3 (left) and EuScCuTe3 (right) crystals placed in a capillary for X-ray diffraction analysis.
2.3. X-ray Diffraction Analysis
The intensities from a single crystal of the EuRECuTe3 (RE = Ho, Tm, Sc) were collected at 293(2) K using a SMART APEX II single-crystal diffractometer (Bruker AXS, Billerica, MA, USA) equipped with a CCD-detector, graphite monochromator, and Mo-Kα radiation source. The parameters of the unit cell were determined and refined for a set of 11,880 reflections. The parameters of the unit cell correspond to the orthorhombic crystal system. The space group Cmcm was determined from the statistical analysis of all intensities. Absorption corrections were applied using the SADABS program. The crystal structure was solved by direct methods using the SHELXS program and refined in an anisotropic approximation using the SHELXL program [40]. Structural investigations for the presence of missing symmetry elements were conducted using the PLATON program [41]. The crystallographic data are deposited in Cambridge Crystallographic Data Centre. The data can be downloaded from the site www.ccdc.cam.ac.uk/data_request/cif (accessed on 25 February 2024).
2.4. DFT Calculations
Calculations were performed at the theoretical level DFT by using hybrid functional B3LYP [42] that take into account non-local exchange at Hartree–Fock formalism. This approach is suitable for to compounds with ionic and covalent chemical bonds. We used CRYSTAL17 code [43]. The program is designed for modeling periodic compounds within the framework of the MO-LCAO approach. Stuttgart pseudopotentials ECPnMWB for Eu2+ and RE3+ cations were used [44]. Here, n is the number of electrons that replaces this pseudopotential. For Eu2+, n was equal Z–10, and for RE3+, n was equal Z–11 (Z is atomic number). For outer shells 5s25p6 of rare-earth metal ions, which were involved in chemical bonds, attached basis sets of TZVP type were used [44]. For scandium and copper, we used all-electron basis sets «Sc_pob_TZVP_2012» and «Cu_86-4111(41D)G_doll_2000», available on the website of program CRYSTAL [43]. For tellurium, we used an all-electron basis set [45] also. Gaussian-type orbitals with exponents lower than 0.1 were cancelled from the basis sets. Self-consistent field was calculated with tolerance 10−9 a.u. Monkhorst-Pack grid used was 8 × 8 × 8 k-points.
Optimization of the crystal structure was performed at first. After that, elastic constants and phonon spectrum were calculated for optimized crystal structure.
3. Results
3.1. Crystal Structures of the EuRECuTe3 (RE = Ho, Tm, Sc)
In the series of europium tellurides EuRECuTe3, only three orthorhombic compounds EuRECuTe3 (RE = Gd [20], Er [19], Lu [20]) have been previously obtained using the halide flux method. The compound EuGdCuTe3 [20] crystallizes in the space group Pnma in the structure type of Eu2CuS3, while the compounds EuErCuTe3 [19] and EuLuCuTe3 [20] crystallize in the space group Cmcm in the structure type of KZrCuS3. Single-crystal X-ray diffraction analysis revealed that the compounds EuRECuTe3 (RE = Ho, Tm, Sc) are isostructural to EuRECuTe3 (RE = Er [19], Lu [20]). Crystallographic data and data collection conditions are presented in Table 1.
Table 1.
Main parameters of processing and refinement of the EuRECuTe3 (RE = Ho, Tm, Sc) samples.
| EuHoCuTe3 | EuTmCuTe3 | EuScCuTe3 | |
|---|---|---|---|
| Molecular weight (g/mol) | 765.56 | 767.23 | 643.26 |
| Space group | Cmcm (no. 63) | ||
| Structure type | KZrCuS3 | ||
| Z | 4 | ||
| a (Å) | 4.3129(3) | 4.3054(3) | 4.2341(3) |
| b (Å) | 14.3150(9) | 14.3017(9) | 14.1562(9) |
| c (Å) | 11.2312(7) | 11.1683(7) | 10.8698(7) |
| V (Å3) | 693.40(8) | 687.68(8) | 651.52(7) |
| ρcal (g/cm3) | 7.311 | 7.410 | 6.558 |
| μ (mm−1) | 35.512 | 37.203 | 26.781 |
| Reflections measured | 6492 | 6639 | 6286 |
| Reflections independent | 481 | 478 | 451 |
| Reflections with Fo > 4σ(Fo) | 0.030 | 0.021 | 0.031 |
| θmax (°) | 27.44 | 27.48 | 27.53 |
| h, k, l limits | −5 ≤ h ≤ 5, −18 ≤ k ≤ 18, −14 ≤ l ≤ 14 | ||
| Rint, Rσ | 0.109, 0.047 | 0.069, 0.024 | 0.065, 0.026 |
| Refinement results | |||
| Number of refinement parameters | 24 | ||
| R1 with Fo > 4σ(Fo) | 0.030 | 0.021 | 0.024 |
| wR 2 | 0.062 | 0.047 | 0.052 |
| Goof | 1.084 | 1.117 | 1.056 |
| ∆ρmax (e/Å3) | 1.857 | 1.431 | 1.503 |
| ∆ρmin (e/Å3) | −1.783 | −1.362 | −1.467 |
| Extinction coefficient, ε | 0.00104(9) | 0.00141(8) | 0.0029(2) |
| CSD number | 2261646 | 2261648 | 2354996 |
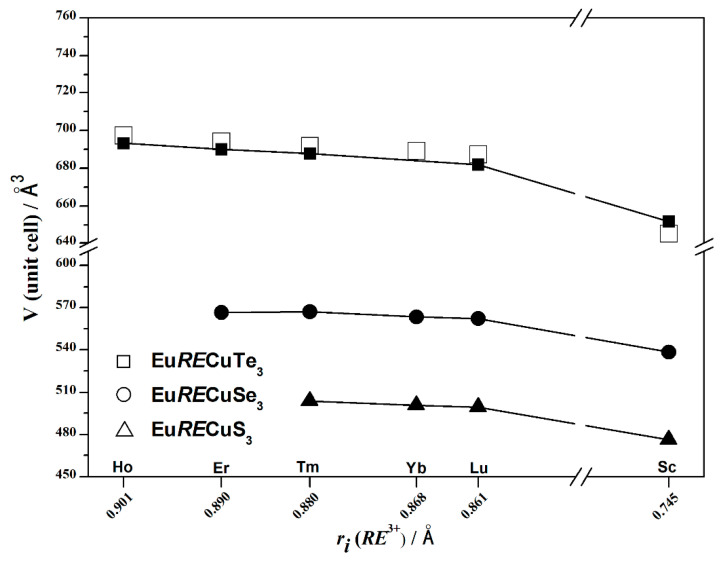
The atomic coordinates, thermal displacement parameters, bond lengths, and valence angles are presented in Tables S1–S3 of the Supplementary Material. The lattice parameters obtained from DFT calculations of EuRECuTe3 (RE = Ho, Tm, Lu, Sc; Table S4) were in good agreement with the experimentally determined values (Table 1). The compound EuYbCuTe3 was unable to be obtained using the halide flux method, but since Yb3+ lies between Tm3+ and Lu3+, whose quaternary tellurides crystallize in the structure type of KZrCuS3, if EuYbCuTe3 is experimentally obtained by another method, it will also crystallize in this structure type. Based on this assumption, DFT calculations of the crystal structure, phonon spectrum, and elastic properties of EuYbCuTe3 were carried out in this study. The calculated values of the electronic parameters and volume were in line with the general trend of their variations (Figure 3 and Figure 4).
Figure 3.
Relationship between the experimental (shaded shapes) and calculated (unshaded shapes) values of the unit-cell volume as a function of the ionic radius of the rare-earth metal cation in the series of the compounds EuRECuTe3 (RE = Ho [this work], Er [19], Tm [this work], Yb [this work], Lu [20], Sc [this work]), EuRECuSe3 (RE = Er [26], Tm–Lu [25], Sc [18]), and EuRECuS3 (RE = Tm–Lu [22], Sc [8]).
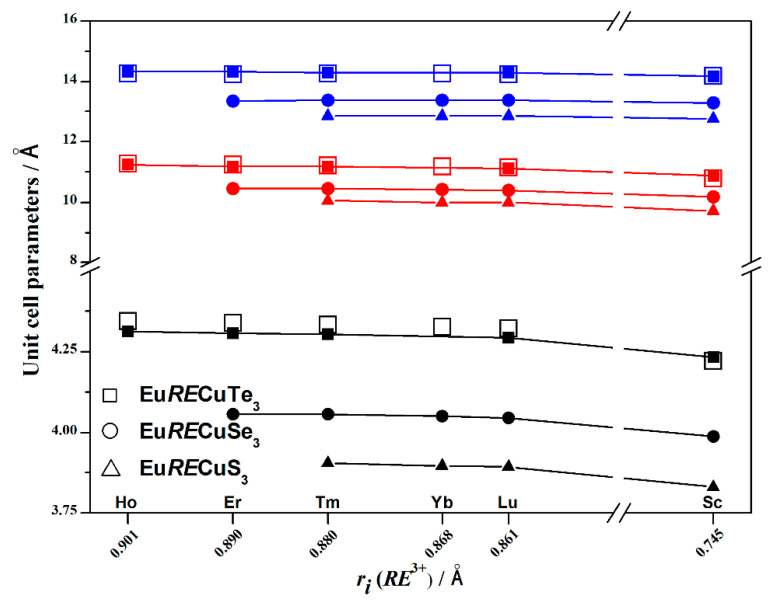
Figure 4.
Relationship between the unit-cell parameters (a: black, b: blue, c: red) and the ionic radius of the rare-earth metal cation in the series of compounds EuRECuTe3 (RE = Ho [this work], Er [19], Tm [this work], Yb [this work], Lu [20], Sc [this work]), EuRECuSe3 (RE = Er [26], Tm–Lu [25], Sc [18]), and EuRECuS3 (RE = Tm–Lu [22], Sc [8]).
In the series of quaternary tellurides EuRECuTe3, where RE = Ho–Lu and Sc, the unit-cell volume decreases from 693.40 to 651.52 Å3 (Table 1, Figure 3), and the unit-cell parameters (a = 4.3129–4.2341 Å, b = 14.3150–14.1562 Å, c = 11.2312–10.8698 Å) decreased, which is consistent with the change in structural parameters as the ionic radius of RE3+ decreases in the series of EuRECuCh3 chalcogenides (Ch = S [22,24], Se [25]), crystallizing in the space group Cmcm (Figure 3 and Figure 4). When changing the chalcogenide in the series Te2− → Se2− → S2−, the anion radii decrease by 10% and 7% (ri(Te2−) = 2.21 Å, ri(Se2−) = 1.98 Å, ri(S2−) = 1.84 Å [32]), respectively. As a result, the unit-cell volumes changed by approximately 18% and 12% (Figure 3), and the unit-cell parameters changed by 6% and 4%, respectively (Figure 4).
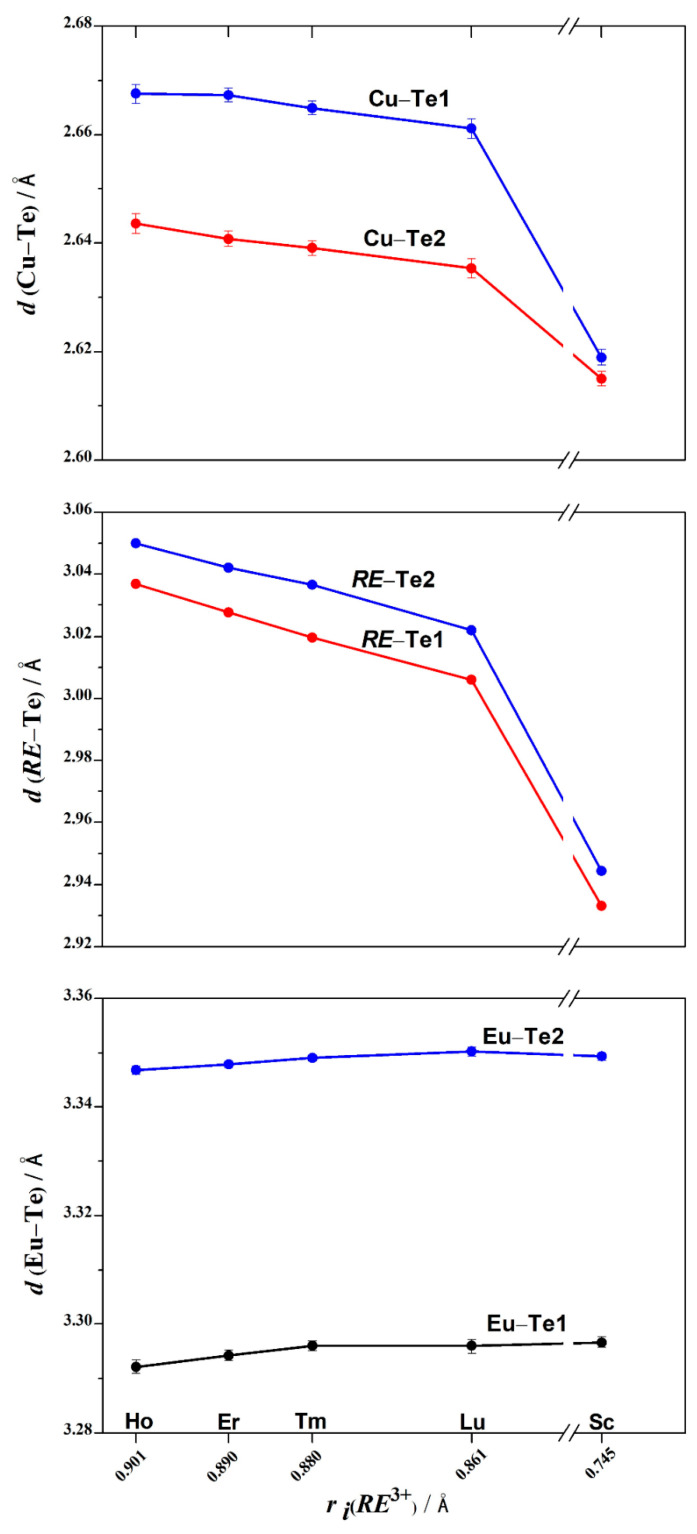
In the structures of EuRECuTe3 (RE = Ho, Tm, Sc), four distances d(Cu–Te) are shorter than the theoretical value of 2.81 Å [32] by 5–7%, while the fifth and sixth distances (4.293–4.086 Å) are longer by 45–52% (Figure S1). The typical coordination polyhedron of copper in this structure is a tetrahedron (Figure 5 and Figure S1). The tetrahedra [CuTe4]7− are linked by shared anions (Te1)2− along the a-axis (Figure 5). As the rare-earth metal cation radius decreased in the quaternary tellurides, a decrease in the ionicity of the Cu–Te bond was observed in the tetrahedra, resulting in the bond lengths d(Cu–Te) changing from 2.644 to 2.615 Å and from 2.668 to 2.620 Å (Table S3, Figure 6).
Figure 5.
Projection of the crystal structure of the EuRECuTe3 representatives in the space group Cmcm.
Figure 6.
The distance d(M–Te) in the structures of EuRECuTe3 compounds with M = Eu, Cu, RE (= Ho [this work], Er [19], Tm [this work], Lu [20], and Sc [this work]).
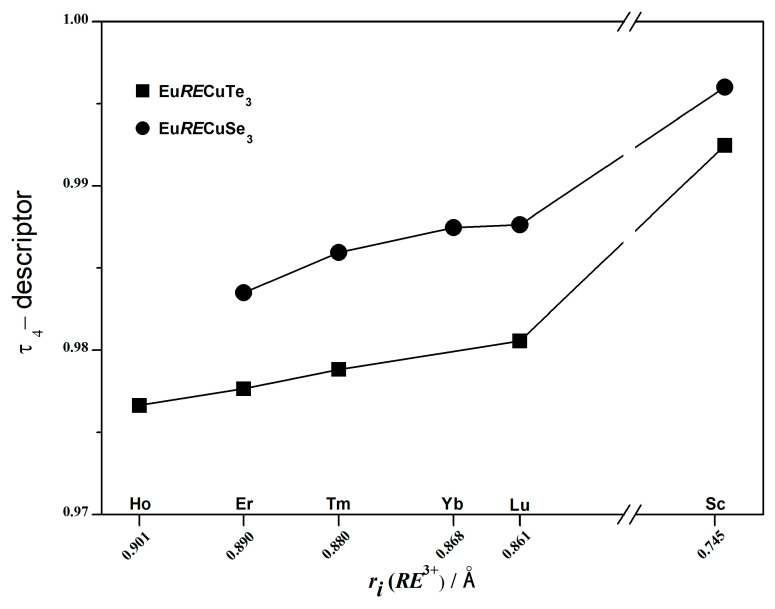
The values of the valence angles ∠(Te–Cu–Te), ranging from 104.4 to 111.0° (Table S3), deviate from the ideal tetrahedral angle by 1–4%. As the compound changed from EuHoCuTe3 to EuScCuTe3, this deviation decreases. The reduction in the distortion of [CuTe4]7− in the series of compounds EuRECuTe3 (RE = Ho–Lu, Sc) is confirmed by the calculation of the τ4 descriptor [46], which increases in this series from 0.977 to 0.992 (Figure 7). Since the τ4 descriptor values for an ideal tetrahedron, trigonal pyramid, square pyramid, and ideal square are 1.00, 0.85, 0.64–0.07, and 0.00, respectively [46], in the structures of EuRECuTe3, a distortion of the copper coordination polyhedron from an ideal tetrahedron to a trigonal pyramid is observed at 2.3–0.8%. The scandium telluride possesses an almost ideal tetrahedral coordination environment. The degree of distortion of the tetrahedral [CuCh4]7− polyhedra (Ch = Se, Te) in the tellurides EuRECuTe3 was found to be higher than in the selenides EuRECuSe3 (Figure 7).
Figure 7.
Calculated values of the τ4 descriptor for the polyhedra [CuCh4]7− (Ch = Se, Te) in the structures of the compounds EuRECuTe3 (RE = Ho [this work], Er [19], Tm [this work], Yb [this work], Lu [20], Sc [this work]) and EuRECuSe3 (RE = Er [26], Tm–Lu [25], Sc [18]) in the space group Cmcm.
In the structures of the EuRECuTe3 compounds in the space group Cmcm, the distances d(RE–Te) (Table S3) deviate from theoretical values (2.96 Å (EuScCuTe3)–3.11 Å (EuHoCuTe3) [32]) by 1–2%. The coordination polyhedra of RE3+ in the structures of EuRECuTe3 (RE = Ho, Tm, Sc) are octahedra, which show distortions. The valence angles ∠(Te–RE–Te), ranging from 86.3 to 93.7° (Table S3), deviate from the ideal octahedral angle by 4%. The octahedral units [RETe6]9− are connected to each other through (Te1)2− anions along the c-axis and through (Te2)2− anions along the a-axis (Figure 5). The coordination polyhedra [RETe6]9− and [CuTe4]7− share common anions (Se1)2− and (Se2)2− and form two-dimensional layers in the ac-plane. In the octahedron, as the radius of RE3+ decreases, there is a reduction in the bond lengths d(RE–Te) from 3.0368 to 2.9334 Å and from 3.0501 to 2.9449 Å (Table S3, Figure 6), leading to a crystal-chemical compression of the two-dimensional layers [RECuTe3]2−.
The anions (Te2)2− and (Te1)2− form trigonal prisms [EuTe6]10− around the Eu2+ cations (Figure S1) connected to each other along the a-axis. The length of the four bonds d(Eu–Te2) in the structures range from 3.3468 to 3.3491 Å, while the lengths of the other two bonds d(Eu–Te1) are between 3.292 and 3.2961 Å (Figure 6, Table S3). Six distances d(Eu–Te) deviate from the theoretical value of 3.38 Å [32] by 2.5–2.6%, while the seventh and eighth distances (3.877–3.706 Å) are longer by 9.6–14.7% (Figure S1). The sums of valence efforts for the compounds EuRECuTe3 (RE = Ho–Lu and Sc) taking coordination into account are Eu (1.63–1.65), RE (2.66–3.09), and Cu (1.33–1.57) (Table S5).
The crystal structure of compounds EuRECuTe3 (RE = Ho–Lu, Sc) is formed by parallel two-dimensional layers in the ac-plane, consisting of octahedra and tetrahedra, which are separated by one-dimensional chains of trigonal prisms (Figure 5).
3.2. Band Structure
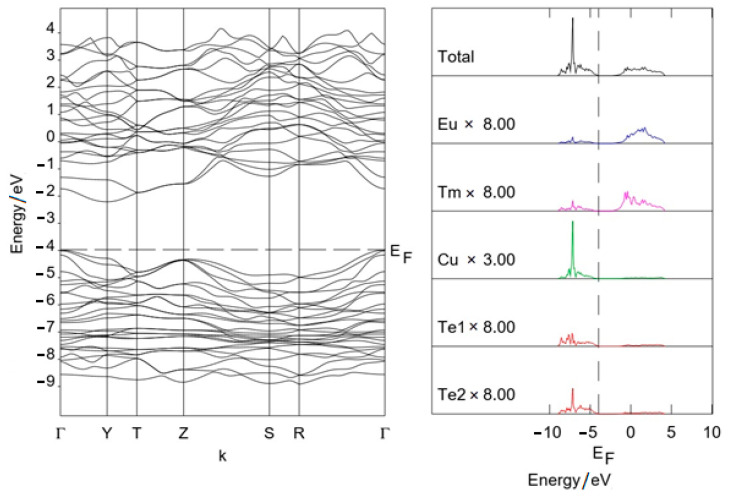
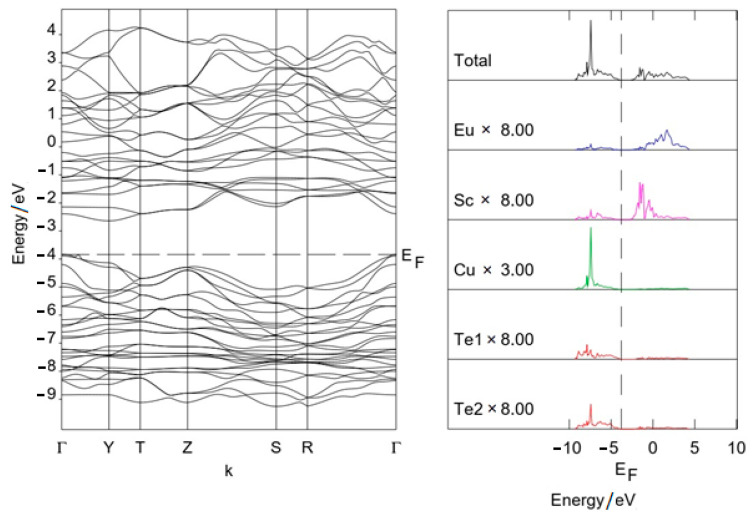
The path in the Brillouin zone of the space group Cmcm is Г(0,0,0), Y(1/2,1/2,0), T(1/2,1/2,1/2), Z(0,0,1/2), S (0,1/2,0), R(0,1/2,1/2). The band structure (Figure 8 and Figure 9) does not include the 4f states of europium and RE3+, since they are replaced by pseudopotentials. As can be seen from the figures, copper and tellurium orbitals provide the main contribution to states near the top of the VB. Orbitals of RE3+ (Sc3+) and europium provide the main contribution to the bottom of the CB. Calculations predicted for EuRECuTe3 (RE = Ho, Tm, Yb, Lu) the indirect band gap Г–Y. The value of the band gap (1.7–1.8 eV) was a HOMO–LUMO estimation (Table 2). This value is close to experimental data for selenides (EuErCuSe3 1.79 eV [26]). For EuScCuTe3, the calculation predicted a smaller gap, equal to 1.2 eV.
Figure 8.
Band structure of EuTmCuTe3.
Figure 9.
Band structure of EuScCuTe3.
Table 2.
Calculated band gaps of the tellurides EuRECuTe3 (RE = Ho–Lu and Sc) in eV.
| Crystal | Indirect (Г–Y) | Crystal | Indirect (Г–Y) | Crystal | Indirect (Г–Y) |
|---|---|---|---|---|---|
| EuHoCuTe3 | 1.72 | EuYbCuTe3 | 1.79 | EuLuCuTe3 | 1.81 |
| EuTmCuTe3 | 1.77 | EuErCuTe3 | 1.75 [19] | EuScCuTe3 | 1.19 |
3.3. Elastic Constants and Elastic Modulus
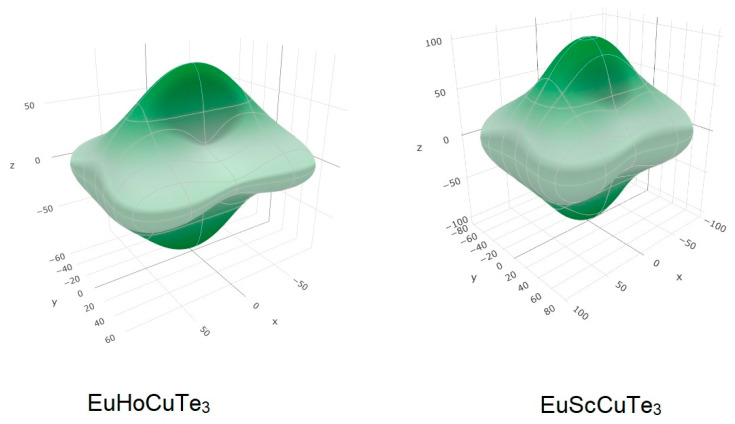
The elastic constants and elastic modulus of the compound EuRECuTe3 (RE = Ho, Tm, Yb, Lu, Sc) are presented in Table 3. The table presents the bulk module (B), shear module (G), Young’s module (Y), and Poisson ratio. These are values for a polycrystal and were calculated by averaging the schemes of Voigt, Reuss, and Hill. The Voigt scheme assumes the uniformity of local strains. The Reuss scheme assumes the uniformity of local stresses. The Voigt scheme provides the upper bound, whereas the Reuss scheme provides the lower bound of value. The approximation of Hill is the average of Voigt and Reuss estimations [47]. The Voigt and Reuss estimates were found to be very different (Table 3), which indicates anisotropy of the elastic properties. The dependence of Young’s modulus on direction also illustrates the strong anisotropy of elastic properties (Figure 10).
| (1) |
Table 3.
Calculated elastic constants and modulus, as well as Vickers hardness (GPa) of the EuRECuTe3 series (RE = Ho, Tm, Yb, Lu, and Sc).
| RE | C11 | C12 | C13 | C22 | C23 | C33 | C44 | C55 | C66 | Averaging Scheme | B | G | Y | Poisson Ratio | HV | AU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ho | 121 | 52 | 40 | 94 | 47 | 113 | 9 | 31 | 45 | Voigt | 67 | 30 | 77 | 0.308 | 3.0 | 1.94 |
| Reuss | 67 | 21 | 58 | 0.356 | ||||||||||||
| Hill | 67 | 25 | 68 | 0.332 | ||||||||||||
| Tm | 121 | 52 | 40 | 96 | 48 | 114 | 11 | 31 | 45 | Voigt | 68 | 30 | 79 | 0.306 | 3.3 | 1.40 |
| Reuss | 68 | 24 | 64 | 0.343 | ||||||||||||
| Hill | 68 | 27 | 71 | 0.324 | ||||||||||||
| Yb | 122 | 52 | 40 | 96 | 47 | 114 | 11 | 31 | 45 | Voigt | 68 | 30 | 79 | 0.305 | 3.4 | 1.37 |
| Reuss | 68 | 24 | 64 | 0.342 | ||||||||||||
| Hill | 68 | 27 | 72 | 0.323 | ||||||||||||
| Lu | 122 | 52 | 40 | 97 | 48 | 115 | 12 | 31 | 45 | Voigt | 68 | 31 | 80 | 0.305 | 3.4 | 1.27 |
| Reuss | 68 | 24 | 65 | 0.339 | ||||||||||||
| Hill | 68 | 27 | 73 | 0.322 | ||||||||||||
| Sc | 127 | 59 | 42 | 103 | 50 | 127 | 18 | 33 | 45 | Voigt | 73 | 33 | 86 | 0.304 | 4.0 | 0.61 |
| Reuss | 73 | 29 | 78 | 0.322 | ||||||||||||
| Hill | 73 | 31 | 82 | 0.313 |
Figure 10.
Young’s modulus (GPa). Dependence of the direction in the crystals EuHoCuTe3 and EuScCuTe3.
We also calculated the universal elastic anisotropy index (1). The closer to zero this index is, the lower the anisotropy of elastic properties [48]. By the lanthanoid pressure, at the row EuRECuTe3 (RE = Ho–Lu), anisotropy decreases (Table 3).
| (2) |
The empirical Formula (2) was used to calculate hardness. According to [49], the formula is based on correlations between Vickers hardness (HV) and the ratio of shear and bulk moduli. The parameters of the formula were determined from reproducing the hardness of more than forty compounds with ionic and covalent bonds [49]. The shear (G) and bulk (B) modulus in (2) is according to the Hill estimate.
3.4. Raman, IR, and Phonon Spectra
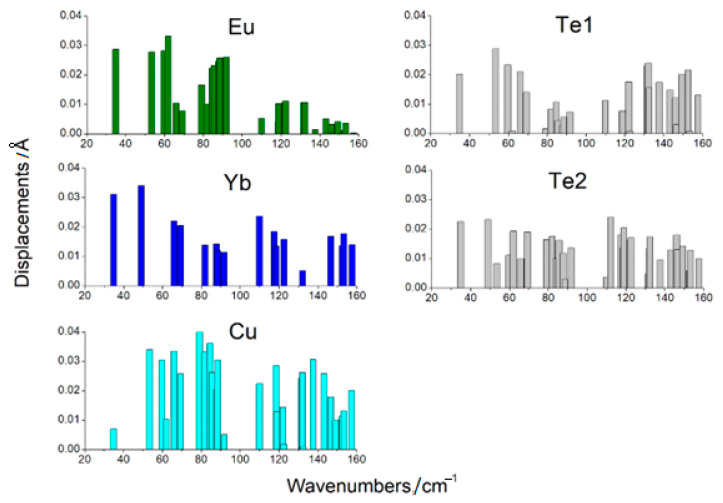
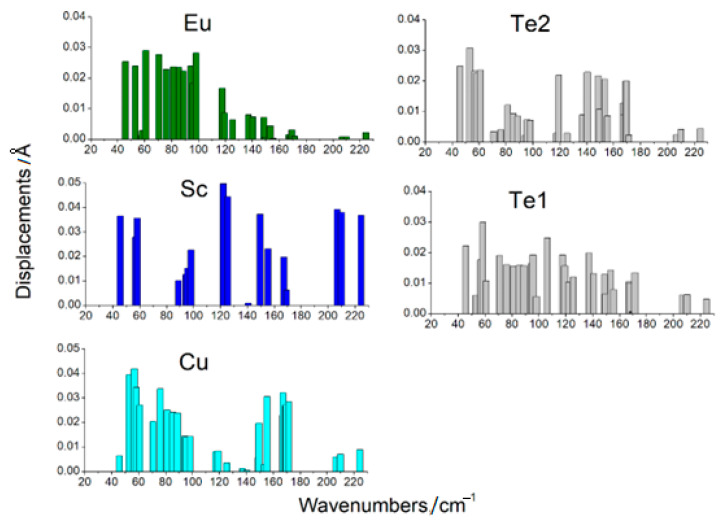
From the DFT calculation, the wavenumbers and types of modes were determined (Table 4 and Table 5). The displacement vectors were obtained from the calculations also. This made it possible to evaluate the participation of each ion in a particular mode. The values of ion displacements characterized their participation in the mode (Figure 11 and Figure 12). According to calculations, the phonon spectrum of the crystals EuRECuTe3 (RE = Ho, Tm, Yb, Lu) at the gamma point lay in the frequency range up to 160 cm−1 (Table 4, Figure 11). In this frequency range, not only were light copper ions involved, but also telluride anions and RE3+ cations (Figure 11 and Figure 12). The phonon spectrum of the crystals EuScCuTe3 at the gamma point lay in the frequency range up to 230 cm−1 (Figure 12, Table 5). This corresponded to the fact that the mass of scandium is less than that of the other RE3+ cations. IR, Raman, and «silent» modes for all crystals are presented in Tables S6–S8.
Table 4.
Phonons at the gamma point of EuYbCuTe3.
| Frequency, cm−1 | Type | IR | Raman | Involved Ions 1 | ||
|---|---|---|---|---|---|---|
| Active/Inactive | Intensity IR (km·mol−1) | Active/Inactive | Intensity Raman (Arbitrary Units) | |||
| 35 | B1u | A | 8.5 | I | Eu S, Yb S, Cu W, Te1 S, Te2 S | |
| 49 | Au | I | 0 | I | Yb S, Te2 S | |
| 53 | B1g | I | 0 | A | 384 | Eu S, Cu S, Te1 S, Te2 W |
| 60 | Ag | I | 0 | A | 576 | Eu S, Cu S, Te1 S, Te2 |
| 62 | B2g | I | 0 | A | 330 | Eu S, Cu W, Te2 |
| 66 | B2u | A | 10.5 | I | Eu, Yb S, Cu S, Te1 S, Te2 W | |
| 69 | B1u | A | 10.3 | I | Eu W, Yb S, Cu S, Te1, Te2 | |
| 79 | B2g | I | 0 | A | 346 | Eu, Cu S, Te2 |
| 82 | B3u | A | 39.1 | I | Eu, Yb, Cu S, Te1 W, Te2 | |
| 85 | B1g | I | 0 | A | 218 | Eu S, Cu S, Te1, Te2 |
| 86 | Ag | I | 0 | A | 138 | Eu S, Cu S, Te2 |
| 87.87 | B1u | A | 88.2 | I | Eu S, Yb, Cu S, Te2 | |
| 88.48 | B2u | A | 0.4 | I | Eu S, Yb, Cu S, Te1 W | |
| 92 | B3u | A | 128.3 | I | Eu S, Yb, Cu W, Te1 W, Te2 | |
| 110 | B3u | A | 13.5 | I | EuW, YbS, CuS, Te1 | |
| 112 | B3g | I | 0 | A | 133 | Te2 S |
| 117 | Au | I | 0 | I | Yb, Te2 | |
| 118.56 | B1u | A | 390.4 | I | Yb, Cu S, Te1 W, Te2 | |
| 118.83 | B1g | I | 0 | A | 37 | Eu, Cu, Te1 W, Te2 S |
| 122 | B2g | I | 0 | A | 0.00 | Eu, Cu, Te1, Te2 |
| 123 | B2u | A | 529.2 | I | Eu, Yb, Te2 | |
| 131.52 | B1g | I | 0 | A | 43 | Eu, Cu S, Te1 S |
| 132.12 | B3u | A | 78.8 | I | Eu W, Yb W, Cu W, Te1 S, Te2 | |
| 132.17 | B2u | A | 83.2 | I | Eu W, Cu S, Te1 S | |
| 132.34 | Ag | I | 0 | A | 93 | Eu, Te1, Te2 |
| 138 | Ag | I | 0 | A | 62 | Cu S, Te1, Te2 W |
| 143 | B2g | I | 0 | A | 174 | Eu W, Cu S, Te1, Te2 |
| 146 | Ag | I | 0 | A | 1000 | Cu, Te1, Te2 |
| 147 | B1u | A | 0.4 | I | Yb, Cu, Te2 | |
| 149 | B2g | I | 0 | A | 25 | Cu, Te1 S, Te2 |
| 153 | B1u | A | 111.2 | I | Yb, Cu, Te1 S, Te2 W | |
| 153 | B3u | A | 170.4 | I | Yb, Cu, Te2 | |
| 158 | B3u | A | 10.2 | I | Yb, Cu S, Te1, Te2 W | |
1 Superscripts “S” and “W” denote strong and weak ion displacements in the mode, respectively.
Table 5.
Phonons at the gamma point of EuScCuTe3.
| Frequency, cm−1 | Type | IR | Raman | Involved Ions 1 | ||
|---|---|---|---|---|---|---|
| Active/Inactive | Intensity IR (km·mol−1) |
Active/Inactive | Intensity Raman (Arbitrary Units) | |||
| 46 | B1u | A | 0 | I | Eu S, Sc S, Cu W, Te1 S, Te2 S | |
| 53 | B1g | I | 0 | A | 0.00 | Eu S, Cu S, Te1 S, Te2 W |
| 57 | B2u | A | 13.9 | I | Sc S, Cu S, Te1 S, Te2 | |
| 58.23 | B1u | A | 20.3 | I | Sc S, Cu S, Te1 S, Te2 S | |
| 58.30 | Au | I | 0 | I | Sc S, Te2 S | |
| 61 | Ag | I | 0 | A | 0.00 | Eu S, Cu S, Te1 S, Te2 |
| 71 | B2g | I | 0 | A | 896.00 | Eu S, Cu S, Te2 |
| 76 | B2g | I | 0 | A | 0.31 | Eu S, Cu S, Te2 |
| 81 | B1g | I | 0 | A | 0.00 | Eu S, Cu S, Te1, Te2 |
| 86 | Ag | I | 0 | A | 0.00 | Eu S, Cu S, Te1 W, Te2 |
| 89 | B3u | A | 142.4 | I | Eu S, Sc, Cu S, Te1 W, Te2 | |
| 94 | B1u | A | 192.2 | I | Eu S, Sc, Cu, Te2 | |
| 96 | B3u | A | 122.4 | I | Eu, Sc, Cu, Te1 W, Te2 | |
| 98 | B2u | A | 0.01 | I | Eu S, Sc S, Cu, Te1 W, Te2 W | |
| 106 | B3g | I | 0 | A | 0.00 | Te2 S |
| 118 | B1g | I | 0 | A | 0.00 | Eu, Cu W, Te2 |
| 119 | B2g | I | 0 | A | 89.31 | Eu W, Cu W, Te1 S, Te2 |
| 122 | Au | I | 0 | I | Sc S, Te2 | |
| 125 | B2u | A | 1388.7 | I | Eu W, Sc S, Te2 | |
| 137 | Ag | I | 0 | A | 0.00 | Eu W, Te1 W, Te2 |
| 141 | B3u | A | 30.2 | I | Eu W, Te1 S, Te2 | |
| 149 | B2g | I | 0 | A | 41.83 | Eu W, Cu W, Te1 S, Te2 |
| 150 | B1u | A | 1108.3 | I | Sc S, Cu, Te1, Te2 W | |
| 153 | Ag | I | 0 | A | 0.00 | Te1 S, Te2 |
| 155 | B3u | A | 5.3 | I | Sc S, Cu S, Te1 W, Te2 W | |
| 167.18 | B1u | A | 96.4 | I | Sc, Cu S, Te1, Te2 | |
| 167.26 | Ag | I | 0 | A | 0.00 | Cu S, Te1 W, Te2 |
| 169 | B2u | A | 131.6 | I | Sc W, Cu S, Te1 | |
| 170 | B1g | I | 0 | A | 0.00 | Cu S, Te1 |
| 172 | B2g | I | 0 | A | 1000.00 | Cu S, Te2 |
| 207 | B1u | A | 0.5 | I | Sc S, Cu W, Te2 W | |
| 210 | B3u | A | 33.6 | I | Sc S, Cu W, Te2 W | |
| 225 | B3u | A | 276.5 | I | Sc S, Cu W | |
1 Superscripts “S” and “W” denote strong and weak ion displacements in the mode, respectively.
Figure 11.
The values of ion displacements at phonon modes in EuYbCuTe3 (space group: Cmcm).
Figure 12.
The values of ion displacements at phonon modes in EuScCuTe3 (space group: Cmcm).
A strong mixing of vibrations of structural units in crystals EuRECuTe3 can be noted. In all compounds, the europium ions participated in the frequency range up to ≈95 cm−1. Calculations predicted a gap in the phonon spectrum in the region ≈ 92–110 cm−1 for EuRECuTe3 (Figure 11). Note that a similar gap was practically absent in the crystal EuScCuTe3 (Figure 12).
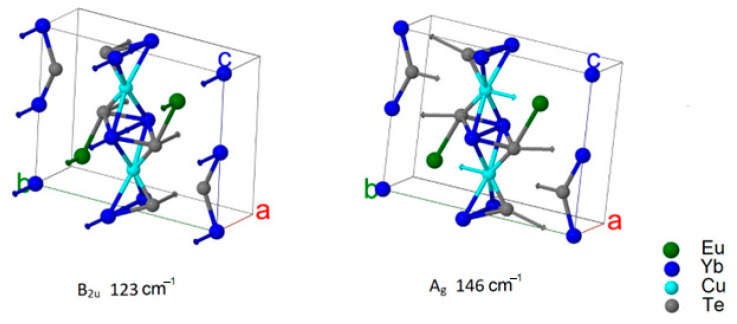
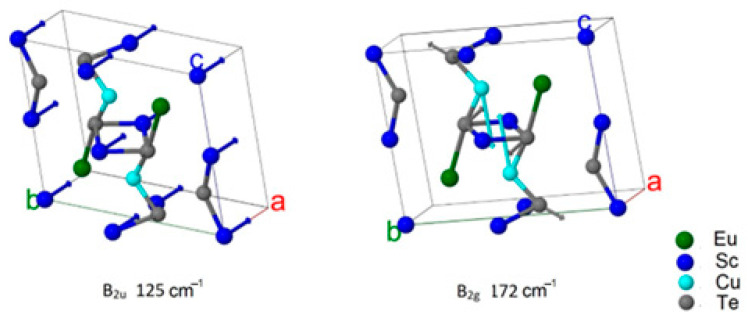
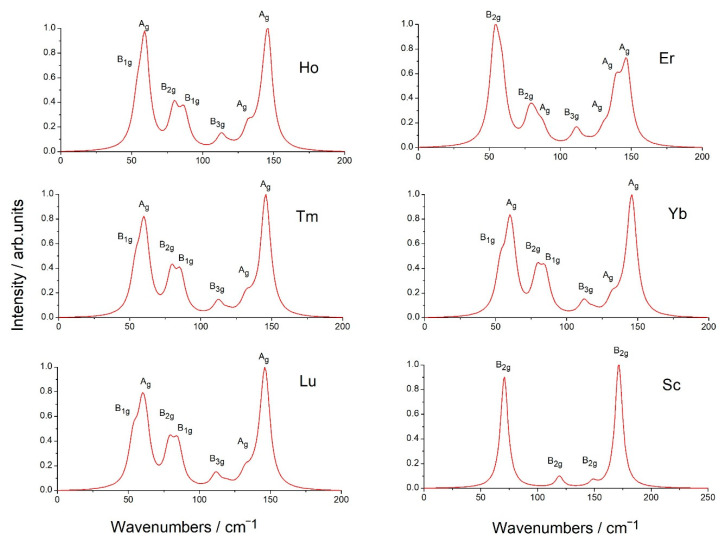
Calculations predicted that in crystals EuRECuTe3 (RE = Ho, Tm, Yb, Lu), the most intense Raman mode has a frequency of about 146 cm−1 (A1g), and the most intense infrared mode has a frequency of about 123 cm−1 (B2u). At the crystal EuScCuTe3, the most intense Raman mode has a frequency of about 172 cm−1 (B2g), and the most intense infrared modes has a frequency of about 125 cm−1 (B2u). These modes are illustrated in Figure 13 and Figure 14. The calculated Raman spectrum for all crystals is shown in Figure 15. The results of calculating the phonon spectrum can be useful for interpreting IR and Raman spectra of rare-earth metal tellurides EuRECuTe3. The IR and Raman spectra of EuRECuTe3 (RE = Ho, Tm, Lu) obtained from calculations are similar to the spectra of EuErCuTe3 [19]. It can be assumed that the experimental spectra of EuRECuTe3 (RE = Ho, Tm, Lu) will be similar to the previously presented experimental spectrum of EuErCuTe3 [19].
Figure 13.
Ion displacements in IR and Raman modes with maximum intensity at EuYbCuTe3.
Figure 14.
Ion displacements in IR and Raman modes with maximum intensity at EuScCuTe3.
Figure 15.
The simulated Raman spectra. The calculation was carried out for the exciting laser wavelength λ = 532 nm and T = 300 K. When modelling the Raman spectra based on the calculated wavenumbers and intensities, the functions pseudo-Voigt with dumping factor 8 cm−1 were used.
The largest ion displacement was 0.040 Å (Cu). In the case when the displacement was greater than or equal to 0.02 Å, the displacement is indicated by “S”. If the displacement did not exceed 0.01 Å, then the displacement is indicated by “W”. If the value of displacement was less than 0.005 Å, then the ion is not mentioned in the column “participants”.
The largest ion displacement is 0.05 Å (Sc). In the case when the displacement was greater than or equal to 0.02 Å, the displacement is indicated by “S”. If the displacement did not exceed 0.01 Å, then the displacement is indicated by “W”. If the value of displacement was less than 0.005 Å, then the ion is not mentioned in the column “participants”.
4. Conclusions
Single crystals of layered heterometallic tellurides EuRECuTe3 (RE = Ho, Tm, and Sc) were synthesized for the first time. The orthorhombic compounds crystallized in the space group Cmcm. With a decrease in the parameters and volume of the unit cell, the bond length d(RE–Te) occurred as the ionic radius of the rare-earth metal cation decreases (RE3+ = Ho3+, Er3+, Tm3+, Yb3+, Lu3+, Sc3+), leading to a crystal-chemical compression of the two-dimensional layers in the crystal structures of the EuRECuTe3 compounds. The patterns of changes in structural parameters were compared with the isostructural chalcogenides EuRECuCh3 (Ch = S, Se, Te) in the space group Cmcm. In the series of chalcogenides, the degree of distortion of the copper coordination polyhedra was found to be highest in the tellurides. Within the framework of the DFT approach, by using the hybrid functional, which takes into account non-local HF exchange, the crystal structure and IR, Raman, and “silent” modes were studied. Elastic tensor as well as elastic moduli and hardness were calculated. The theoretical calculations allow for the assignment of vibrational modes as well as revealing the involved ions that participated in these modes.
Supplementary Materials
The following Supporting Information can be downloaded at: https://www.mdpi.com/article/10.3390/ma17143378/s1, Figure S1. Coordination polyhedra of [CuTe4]7− (left), [EuTe6]10− (right) in EuRECuTe3 (RE = Ho (this work), Er [19], Tm (this work), Lu [20], Sc (this work) from top to bottom). Table S1. Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters of the EuRECuTe3 (RE = Ho, Tm, Sc) samples. Table S2. Atomic displacement parameters (Å2) of the EuRECuTe3 (RE = Ho, Tm, Sc) samples. Table S3. Bond lengths (d /Å) and angles (∠ /°) in the crystal structures of the EuRECuTe3 representatives with RE = Ho, Tm and Sc. Table S4. Unit-cell parameters obtained by DFT methods. Table S5. Bond-valence calculation data for the Eu, RE and Cu cations in the EuRECuTe3 structure. Table S6. Calculated IR modes of the EuRECuTe3 representatives with RE = Ho, Tm, Yb, Lu and Sc. Table S7. Calculated Raman modes of the EuRECuTe3 representatives with RE = Ho, Tm, Yb, Lu and Sc. Table S8. Calculated “silent” modes of the EuRECuTe3 representatives with RE = Ho, Tm, Yb, Lu and Sc.
Author Contributions
Conceptualization, A.V.R. and T.S.; software, A.V.R. and M.V.G.; validation, A.V.R.; formal analysis, M.V.G. (synthesis), V.A.C. (DFT calculations) and R.J.C.L. (XRD); data curation, V.A.C., M.V.G., A.V.R. and T.S.; writing—original draft preparation, A.V.R., T.S., V.A.C. and M.V.G.; writing—review and editing, A.V.R., M.V.G., V.A.C. and T.S.; visualization, A.V.R. and M.V.G.; project administration, A.V.R.; funding acquisition, M.V.G. and A.V.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by grant support Russian Science Foundation, grant number 24-23-00416 (https://rscf.ru/project/24-23-00416/ (accessed on 25 April 2024)).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ishtiyak M., Jana S., Karthikeyan R., Mamindla R., Tripathy B., Malladi S.K., Niranjan M., Prakash J. Syntheses of five new layered quaternary chalcogenides SrScCuSe3, SrScCuTe3, BaScCuSe3, BaScCuTe3, and BaScAgTe3: Crystal structures, thermoelectric properties, and electronic structures. Inorg. Chem. Front. 2021;8:4086–4101. doi: 10.1039/D1QI00717C. [DOI] [Google Scholar]
- 2.Pal K., Xia Y., Shen J., He J., Luo Y., Kanatzidis M.G., Wolverton C. Accelerated discovery of a large family of quaternary chalcogenides with very low lattice thermal conductivity. Comput. Mater. 2021;7:82. doi: 10.1038/s41524-021-00549-x. [DOI] [Google Scholar]
- 3.Pal K., Xia Y., He J., Wolverton C. High thermoelectric performance in BaAgYTe3 via low lattice thermal conductivity induced by bonding heterogeneity. Phys. Rev. Mater. 2019;3:085402. doi: 10.1103/PhysRevMaterials.3.085402. [DOI] [Google Scholar]
- 4.Pal K., Hua X., Xia Y., Wolverton C. Unraveling the structure-valence-property relationships in AMM′Q3 chalcogenides with promising thermoelectric performance. ACS Appl. Energy Mater. 2019;3:2110–2119. doi: 10.1021/acsaem.9b02139. [DOI] [Google Scholar]
- 5.Babo J.-M., Choi E.S., Albrecht-Schmitt T.E. Synthesis, structure, magnetism, and optical properties of Cs2Cu3DyTe4. Inorg. Chem. 2012;51:11730–11735. doi: 10.1021/ic301649p. [DOI] [PubMed] [Google Scholar]
- 6.Meng C.-Y., Chen H., Wan P., Chen L. Syntheses, Structures, and Magnetic and Thermoelectric Properties of Double-Tunnel Tellurides: AxRE2Cu6–xTe6 (A = K–Cs; RE = La–Nd) Chem. Mater. 2011;23:4910–4919. doi: 10.1021/cm201574a. [DOI] [Google Scholar]
- 7.Pavlyuk M.D. Ph.D. Thesis. A.V. Shubnikov Institute of Crystallography RAS; Moscow, Russia: Aug 25, 2020. Detector Crystals Based on CdTe and Cd1–xZnxTe for Direct Detection of X-ray and Gamma Quanta. [Google Scholar]
- 8.Ruseikina A.V., Molokeev M.S., Chernyshev V.A., Aleksandrovsky A.S., Krylov A.S., Krylova S.N., Velikanov D.A., Grigoriev M.V., Maximov N.G., Shestakov N.P., et al. Synthesis, structure, and properties of EuScCuS3 and SrScCuS3. J. Solid State Chem. 2021;296:121926. doi: 10.1016/j.jssc.2020.121926. [DOI] [Google Scholar]
- 9.Ishtiyak M., Jana S., Panigrahi G., Srivastava A.K., Narayanswamy S., Bhattacharjee P.P., Niranjan M.K., Prakash J. Syntheses, crystal structures, optical, and theoretical study of two ternary chalcogenides CsSc5Te8 and Cs0.6(1)Ti6Se8 with tunnel structures. Solid State Sci. 2021;114:106577. doi: 10.1016/j.solidstatesciences.2021.106577. [DOI] [Google Scholar]
- 10.Chen L., Corbett J.D. Synthesis, Structure, and Bonding of Sc6MTe2 (M = Ag, Cu, Cd): Heterometal-Induced Polymerization of Metal Chains in Sc2Te. Inorg. Chem. 2002;41:2146–2150. doi: 10.1021/ic0112544. [DOI] [PubMed] [Google Scholar]
- 11.Ishtiyak M., Panigrahi G., Jana S., Prakash J., Mesbah A., Malliakas C.D., Lebègue S., Ibers J.A. Modulated Linear Tellurium Chains in Ba3ScTe5: Synthesis, Crystal Structure, Optical and Resistivity Studies, and Electronic Structure. Inorg. Chem. 2020;59:2434–2442. doi: 10.1021/acs.inorgchem.9b03319. [DOI] [PubMed] [Google Scholar]
- 12.Ruseikina A.V., Grigoriev M.V., Molokeev M.S., Garmonov A.A., Elyshev A.V., Locke R.J.C., Schleid Th. Synthesis, Crystal Structure and Properties of the New Laminar Quaternary Tellurides SrLnCuTe3 (Ln = Sm, Gd–Tm and Lu) Crystals. 2023;13:291. doi: 10.3390/cryst13020291. [DOI] [Google Scholar]
- 13.Babo J.-M., Albrecht-Schmitt T.E. Ce2AgYb5/3Se6, La2CuErTe5, and Ce2CuTmTe5: Three new quaternary interlanthanide chalcogenides. J. Solid State Chem. 2013;197:414–419. doi: 10.1016/j.jssc.2012.08.024. [DOI] [Google Scholar]
- 14.Huang F.Q., Ibers J.A. Syntheses, Structures, and Theoretical Study of LaCuSTe and SmCuSTe. Inorg. Chem. 1999;38:5978–5983. doi: 10.1021/ic990835e. [DOI] [PubMed] [Google Scholar]
- 15.Babo J.M., Schleid Th. CsCu2Sc3Te6 and CsCuY2Te4: Two new quaternary cesium copper rare-earth metal tellurides. Solid State Sci. 2010;12:238–245. doi: 10.1016/j.solidstatesciences.2009.11.001. [DOI] [Google Scholar]
- 16.Koscielski L.A., Ibers J.A. The structural chemistry of quaternary chalcogenides of the type AMM’Q3. Z. Anorg. Allg. Chem. 2012;638:2585–2593. doi: 10.1002/zaac.201200301. [DOI] [Google Scholar]
- 17.Huang F.Q., Choe W., Lee S., Chu J.S. Syntheses and Crystal Structures of Three Copper Tellurides: BaDyCuTe3, K1.5Dy2Cu2.5Te5, and Acentric K0.5Ba0.5DyCu1.5Te3. Chem. Mater. 1998;10:1320–1326. doi: 10.1021/cm970700b. [DOI] [Google Scholar]
- 18.Grigoriev M.V., Ruseikina A.V., Chernyshev V.A., Oreshonkov A.S., Garmonov A.A., Molokeev M.S., Locke R.J.C., Elyshev A.V., Schleid T. Single Crystals of EuScCuSe3: Synthesis, Experimental and DFT Investigations. Materials. 2023;16:1555. doi: 10.3390/ma16041555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruseikina A.V., Grigoriev M.V., Locke R.J.C., Chernyshev V.A., Garmonov A.A., Schleid Th. Synthesis, Crystal Structure, and Optical and Magnetic Properties of the New Quaternary Erbium Telluride EuErCuTe3: Experiment and Calculation. Materials. 2024;17:2284. doi: 10.3390/ma17102284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruseikina A.V., Grigoriev M.V., Garmonov A.A., Molokeev M.S., Schleid Th., Safin D.A. Synthesis, structures and magnetic properties of the Eu-based quaternary tellurides EuGdCuTe3 and EuLuCuTe3. Cryst. Eng. Comm. 2023;25:1716–1722. doi: 10.1039/D2CE01578A. [DOI] [Google Scholar]
- 21.Ruseikina A.V., Andreev O.V. Regularities of Change in the Structural Parameters of EuLnCuS3 (Ln = La–Nd, Sm, Gd, Ho) Russ. J. Inorg. Chem. 2017;62:160–167. doi: 10.1134/S0036023617020140. [DOI] [Google Scholar]
- 22.Wakeshima M., Furuuchi F., Hinatsu Y. Crystal structures and magnetic properties of novel rare-earth copper sulfides, EuRCuS3 (R = Y, Gd–Lu) J. Phys. Condens. Matter. 2004;16:5503–5518. doi: 10.1088/0953-8984/16/30/012. [DOI] [Google Scholar]
- 23.Furuuchi F., Wakeshima M., Hinatsu Y. Magneticproperties and 151Eu Mo¨ssbauer effects of mixed valence europium copper sulfide, Eu2CuS3. J. Solid State Chem. 2004;177:3853–3858. doi: 10.1016/j.jssc.2004.04.034. [DOI] [Google Scholar]
- 24.Ruseikina A.V., Chernyshev V.A., Velikanov D.A., Aleksandrovsky A.S., Shestakov N.P., Molokeev M.S., Grigoriev M.V., Andreev O.V., Garmonov A.A., Matigorov A.V., et al. Regularities of the property changes in the compounds EuLnCuS3 (Ln = La–Lu) J. Alloys Compd. 2021;874:159968. doi: 10.1016/j.jallcom.2021.159968. [DOI] [Google Scholar]
- 25.Grigoriev M.V., Solovyov L.A., Ruseikina A.V., Aleksandrovsky A.S., Chernyshev V.A., Velikanov D.A., Garmonov A.A., Molokeev M.S., Oreshonkov A.S., Shestakov N.P., et al. Quaternary Selenides EuLnCuSe3: Synthesis, Structures, Properties and In Silico Studies. Int. J. Mol. Sci. 2022;23:1503. doi: 10.3390/ijms23031503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andreev O.V., Atuchin V.V., Aleksandrovsky A.S., Denisenko Y.G., Zakharov B.A., Tyutyunnik A.P., Habibullayev N.N., Velikanov D.A., Ulybin D.A., Shpindyuk D.D. Synthesis, structure, and properties of EuLnCuSe3 (Ln = Nd, Sm, Gd, Er) Crystals. 2022;12:17. doi: 10.3390/cryst12010017. [DOI] [Google Scholar]
- 27.Shahid O., Ray A.K., Yadav S., Deepa M., Niranjan M.K., Prakash J. Structure-property relationships and DFT studies of three quaternary chalcogenides: BaCeCuSe3, BaCeAgS3, and BaCeAgSe3. Mater. Res. Bull. 2023;168:112469. doi: 10.1016/j.materresbull.2023.112469. [DOI] [Google Scholar]
- 28.Shahid O., Yadav S., Maity D., Deepa M., Niranjan M.K., Prakash J. Synthesis, crystal structure, DFT, and photovoltaic studies of BaCeCuS3. New J. Chem. 2023;47:5378–5389. doi: 10.1039/D2NJ06301H. [DOI] [Google Scholar]
- 29.Murashko Y.A., Ruseikina A.V., Kislitsyn A.A., Andreev O.V. Optical and Thermal Properties of the EuLnCuS3 (Ln = La, Pr, Sm, Gd) Compounds. Inorg. Mater. 2015;51:1213–1218. doi: 10.1134/S0020168515120079. [DOI] [Google Scholar]
- 30.Yang Y., Ibers J.A. Synthesis and characterization of a series of quaternary chalcogenides BaLnMQ3 (Ln = Rare Earth, M = Coinage Metal, Q = Se or Te) J. Solid State Chem. 1999;147:366–371. doi: 10.1006/jssc.1999.8359. [DOI] [Google Scholar]
- 31.Prakash J., Mesbah A., Beard J.C., Ibers J.A. Syntheses and crystal structures of BaAgTbS3, BaCuGdTe3, BaCuTbTe3, BaAgTbTe3, and CsAgUTe3. Z. Anorg. Allg. Chem. 2015;641:1253–1257. doi: 10.1002/zaac.201500027. [DOI] [Google Scholar]
- 32.Shannon R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1976;A32:751–767. doi: 10.1107/S0567739476001551. [DOI] [Google Scholar]
- 33.Mansuetto M.F., Keane P.M., Ibers J.A. Synthesis and Structures of the New Group IV Chalcogenides NaCuTiS3 and NaCuZrQ3 (Q = S, Se, Te) J. Solid State Chem. 1993;105:580–587. doi: 10.1006/jssc.1993.1251. [DOI] [Google Scholar]
- 34.Eberle M.A., Schleid Th. Expanding the SrCuRES3 Series with the Rare-Earth Metals Scandium and Yttrium. Z. Kristallogr. 2016;S36:82. [Google Scholar]
- 35.Eberle M.A. Ph.D. Thesis. University of Stuttgart; Stuttgart, Germany: 2016. Darstellung und Charakterisierung Quaternärer Seltenerdmetall-Verbindungen in Kombination mit Kupfer und Schwefel. [Google Scholar]
- 36.Ruseikina A.V., Grigoriev M.V., Solovyov L.A., Chernyshev V.A., Aleksandrovsky A.S., Krylov A.S., Krylova S.N., Shestakov N.P., Velikanov D.A., Garmonov A.A., et al. A Challenge toward Novel Quaternary Sulfides SrLnCuS3 (Ln = La, Nd, Tm): Unraveling Synthetic Pathways, Structures and Properties. Int. J. Mol. Sci. 2022;23:12438. doi: 10.3390/ijms232012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azarapin N.O. Ph.D. Thesis. University of Tyumen; Tyumen, Russia: Oct 4, 2022. Synthesis, Structure and Properties of Compounds BaRECuS3 (RE = Rare-Earth Element) [Google Scholar]
- 38.Wu P., Christuk A.E., Ibers J.A. New Quaternary Chalcogenides BaLnMQ3 (Ln = Rare Earth or Sc; M = Cu, Ag; Q = S, Se). II. Structure and Property Variation vs. Rare-Earth Element. J. Solid State Chem. 1994;110:337–344. doi: 10.1006/jssc.1994.1177. [DOI] [Google Scholar]
- 39.Ruseikina A.V., Pinigina A.N., Grigoriev M.V., Safin D.A. Elucidating a Series of the Quaternary Selenides BaRECuSe3 with a Rich Library of Optical Properties. Cryst. Growth Des. 2024;24:2485–2492. doi: 10.1021/acs.cgd.3c01479. [DOI] [Google Scholar]
- 40.Sheldrick G.M. A short history of SHELX. Acta Crystallogr. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 41.PLATON . A Multipurpose Crystallographic Tool. Utrecht University; Utrecht, The Netherlands: 2008. [Google Scholar]
- 42.Becke A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993;98:5648–5652. doi: 10.1063/1.464913. [DOI] [Google Scholar]
- 43.Crystal. [(accessed on 10 April 2024)]. Available online: http://www.crystal.unito.it/index.php.
- 44.Energy-Consistent Pseudopotentials of the Stuttgart/Cologne Group. [(accessed on 10 April 2024)]. Available online: http://www.tc.uni-koeln.de/PP/clickpse.en.html.
- 45.Towler M. CRYSTAL Resourses Page. [(accessed on 10 April 2024)]. Available online: https://vallico.net/mike_towler/crystal.html.
- 46.Yang L., Powell D.R., Houser R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007;9:955–964. doi: 10.1039/B617136B. [DOI] [PubMed] [Google Scholar]
- 47.Korabelnikov D.V., Zhuravlev Y.N. Ab initio investigations of the elastic properties of chlorates and perchlorates. Phys. Solid State. 2016;58:1166–1171. doi: 10.1134/S1063783416060251. [DOI] [Google Scholar]
- 48.Ranganathan S.I., Ostoja-Starzewski M. Universal Elastic Anisotropy Index. Phys. Rev. Let. 2008;101:055504. doi: 10.1103/PhysRevLett.101.055504. [DOI] [PubMed] [Google Scholar]
- 49.Tian Y., Xu B., Zhao Z. Microscopic theory of hardness and design of novel superhard crystals. Int. J. Refract. Met. Hard Mater. 2012;33:93–106. doi: 10.1016/j.ijrmhm.2012.02.021. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.