Abstract
Background
Research on the sequencing of brain radiotherapy and targeted chemotherapy after brain metastasis (BM) in HER2-positive breast cancer patients is limited and inconclusive. This study investigated the efficacy of sequential delivery of radiotherapy and targeted therapy in patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer with BM.
Methods
Fifty-seven patients were categorized into two groups: the targeted-radiotherapy group (receiving 2–8 cycles of anti-HER2-targeted therapy followed by radiotherapy after BM) and the radiotherapy-targeted group (undergoing radiotherapy first, followed by regular anti-HER2-targeted therapy). The study endpoints were intracranial progression-free survival (iPFS) and overall survival. Factors associated with intracranial progression and mortality were assessed by univariate and multivariate Cox proportional hazards analysis.
Results
Patients in the radiotherapy-targeted group had better iPFS (P < 0.001), while there was no significant difference in overall survival between the two groups (P = 0.145). Multivariate Cox analysis showed that different sequential treatment groups were independent prognostic factors for iPFS. In patients with a modified breast graded prognostic assessment score of 3.5–4.0, the median survival time was 26 months in the radiotherapy-targeted group and 22 months in the targeted-radiotherapy group (P = 0.019).
Conclusion
Overall, radiotherapy followed by targeted therapy may improve survival in HER2-positive breast cancer patients with BM, particularly in those with a modified breast graded prognostic assessment score of 3.5–4.0.
Keywords: HER2, breast cancer, brain metastasis, craniocerebral radiotherapy, HER2-targeted drugs
Introduction
In recent years, breast cancer not only has surpassed lung cancer as the most commonly diagnosed cancer worldwide but also remains the leading cause of cancer-related death among women.1 Approximately 10–30% of breast cancer patients develop brain metastasis (BM),2,3 with 20–25% showing over-expression of the human epidermal growth factor receptor 2 (HER2) gene.4 HER2 positivity is associated with aggressive behavior, which leads to increased invasion and proliferation of breast cancer cells.5 During the course of the disease, up to 50% of patients with advanced HER2-positive breast cancer develop BM,6,7 leading to a poor prognosis.8,9 Moreover, about 50% of advanced HER2-positive breast cancer patients succumb to central nervous system progression.10 In addition, there is a high consistency of HER2 expression between the primary tumor and its corresponding BM.11
Over the years, several therapies have been developed for targeting HER2-positive breast cancer. For example, trastuzumab, a monoclonal antibody known as Herceptin, has been specifically designed to target HER2 expression. Trastuzumab can significantly prolong overall survival (OS) in patients with HER2-positive breast cancer.10,12–14 Soffietti et al15 pointed out that HER2-targeted drugs (especially trastuzumab) could reduce the risk of BM in patients with HER2-positive breast cancer and improve the prognosis of patients with BM.
Besides HER2-targeted drugs, treatment options for breast cancer patients with BM include surgery, whole-brain radiotherapy (WBRT), and stereotactic radiosurgery (SRS).15–17 Although WBRT is able to significantly improve the control of intracranial tumors after SRS treatment, it can also negatively impact cognitive function.18,19 Brown et al18 compared SRS and WBRT after resection of BM in a randomized controlled trial. They discovered that the cognitive decline of patients in the WBRT group was more frequent than that in the SRS group, but there was no statistical difference in OS time between the two groups. Therefore, surgery is preferable to radiotherapy alone if pathological findings need to be determined.
Stemmler et al20 stated that the concentration ratio of trastuzumab in serum to cerebrospinal fluid changed from 420:1 before radiotherapy to 76:1 after radiotherapy, indicating a significant increase in the concentration of trastuzumab in cerebrospinal fluid.21 Some other studies have also suggested that targeted therapy after radiotherapy can increase trastuzumab in the cerebrospinal fluid. For example, Dijkers et al21 claimed that a small amount of trastuzumab could cross the blood-brain barrier (BBB) in HER2-positive breast cancer patients with BM and enter the site of BM.
Patients with BM have a median OS ranging from 2 to 25.3 months.22 Given the wide range in median OS time for patients with BM, the prognostic score is crucial for these patients.23 Studies reported that the breast graded prognostic assessment (breast-GPA) score and the modified breast-GPA score could be used to evaluate the survival time of BM patients.24,25 Among 10,000 clinical trials investigating breast cancer treatment, less than 1% have included patients with BM.26,27 The sequencing studies of brain radiotherapy and targeted chemotherapy after BM in HER2-positive breast cancer patients are rare and inconclusive.
This study examined the long-term treatment efficacy of anti-HER2-targeted drugs and craniocerebral radiotherapy for HER2-positive breast cancer patients with BM. In addition, a modified breast-GPA was applied to assess the difference in the prognosis of patients.
Methods
Patients
This study enrolled 506 breast cancer patients diagnosed with BM via computed tomography (CT) and magnetic resonance imaging (MRI) and admitted to Guangxi Medical University Cancer Hospital from January 2010 to September 2019. Inclusion criteria were shown as follows: (1) age > 18 years old; (2) immunohistochemistry 3+ (>10% of tumor cells showed uniform and strong staining of the membrane) or positive fluorescence in situ hybridization (FISH) confirmed positive for HER2 gene amplification; (3) all patients underwent radiotherapy combined with targeted therapy. Exclusion criteria were listed as follows: (1) male breast cancer patients; (2) patients with incomplete clinical and pathological data; (3) patients undergoing craniocerebral tumor surgery; (4) patients who failed to return to the hospital for re-examination or gave up continuing treatment; (5) patients with meningeal metastasis.
Fifty-seven HER2-positive breast cancer patients treated with radiotherapy combined with targeted therapy were finally included. The cut-off date was 2020.12.31. OS was calculated from the date of BM diagnosis to the cut-off date of follow-up or the date of death; intracranial progression-free survival (iPFS) was calculated from the initial diagnosis of BM to the progression of the original intracranial tumor or the emergence of a new intracranial BM. iPFS was used to measure intracranial lesion control.
Grouping
Patients were divided into two groups, namely, the targeted-radiotherapy group (n = 20) and the radiotherapy-targeted group (n = 37).
Patients in the targeted-radiotherapy group underwent 2–8 cycles of anti-HER2-targeted therapy, followed by periodic CT or MRI reviews following the development of BM. Radiotherapy was initiated after a clinical evaluation of intracranial progression. Upon completion of radiotherapy, the radiotherapist recommended whether to continue anti-HER2-targeted therapy based on the patient’s condition.
Patients in the radiotherapy-targeted group (n = 37) were administered radiotherapy after the onset of BM, followed by routine anti-HER2-targeted therapy.
Basic Information
Basic information included time from the first diagnosis to the occurrence of BM, histological type, the location of BM, clinical stage, receptor status (estrogen receptor, progesterone receptor, HER2 status), Ki-67 levels, molecular subtype, the first treatment of a local tumor (surgery, breast/chest radiotherapy, targeted therapy, chemotherapy, endocrine therapy), other metastatic sites (lung, liver, bone), number of BM, Karnofsky Performance Status (KPS) at the diagnosis of BM, age at BM, treatment after BM (chemotherapy, endocrine therapy), time of intracranial progression, and survival time after BM.
Radiotherapy
Patients receiving brain radiotherapy were treated with linear accelerator 6MV-X-ray. A total of 36 patients received WBRT with specific doses of 30 Gy/10F (n = 34) and 40 Gy/20 F (n = 2). Six patients received local dose addition after complete WBRT: whole-brain: 30 Gy/10 f, the local push of 9–24 Gy/3–8 f (3 Gy/f) or 12–20 Gy/6–10 f (2 Gy/f) for visible craniocerebral tumor. Fifteen patients received whole-brain synchronous and dose-adding radiotherapy, BM: 52.2–54 Gy/18 F, whole-brain: 37.8–39.6 Gy/18 F (n = 13), BM: 50–54 Gy/ 20 F, whole-brain: 36–40 Gy/20 F (n = 2). During radiotherapy, mannitol, dexamethasone injection, and glycerin fructose were combined or used alone to reduce intracranial pressure and cerebral edema.
Targeted Drug Therapy
Fifty-seven patients with BM received trastuzumab at an initial load dose of 8 mg/kg, followed by 6 mg/kg every 3 weeks. A total of 41 patients received chemotherapeutic therapy after BM: capecitabine (n = 6), pemetrexed (n = 4), pemetrexed plus carboplatin (n = 1), paclitaxel liposome (n = 2), paclitaxel liposome plus carboplatin (n = 4), docetaxel (n = 10), docetaxel plus carboplatin (n = 5), doxorubicin liposome (n = 2), raltitrexed plus vinorelbine (n = 3), and temozolomide (n = 4). In addition, six patients were treated with endocrine therapy after BM: tamoxifen plus goserelin (n = 3), exemestane (n = 1), toremifene (n = 1), and letrozole (n = 1).
Statistical Analysis
SPSS 25.0 software package was used for statistical analysis. Characteristics of patients in the two groups were compared descriptively. χ2 test or Fisher’s test was used for categorical variables. Kaplan-Meier analysis was used to assess OS and iPFS, whereas Log rank testing was used to estimate differences between the groups. Besides, factors associated with intracranial progression and risk of death were assessed through univariate and multivariate Cox proportional hazards analysis. Combined with literature data, the covariates of multivariate analysis were determined by univariate Cox proportional risk model analysis. A P value < 0.05 was considered statistically significant.
Results
Basic Information
A total of fifty-seven patients with pathologically confirmed HER2-positive tumors and BM were included in this study, achieving a 100% follow-up rate. The flow chart of included patients was shown in Figure 1. Before BM, 41 cases underwent primary surgical treatment, 52 cases received chemotherapy, 21 cases were given endocrine therapy, 41 cases received targeted therapy, and 16 cases underwent breast/chest radiotherapy. There were 23 patients with stage IV at first diagnosis.
Figure 1.
Flow chart of patients included in the study.
At the time of BM diagnosis, the median age was 49 years (27–69 years); the median age of BM diagnosis in the targeted-radiotherapy and radiotherapy-targeted groups was 48 years (30–69 years) and 50 years (27–65 years), respectively. The median KPS score was 90 (range: 60–100). Forty-two patients had KPS scores ranging from 90 to 100, and 15 patients had scores ranging from 60 to 80. The median time from the first diagnosis to BM was 29 months (0–112 months). Besides, there are 50 cases with invasive ductal carcinoma, five cases with lobular carcinoma, and two cases suffering from ductal carcinoma in situ with microinvasion. The number of BM was 1–3 in 29 cases and > 3 in 28 cases. There were 26 cases of Luminal B (HER2+) type and 31 cases of HER2 over-expression type. BM primarily concentrated in multiple intracranial locations (9 cases), the left cerebellar hemisphere (6 cases), the right cerebellar hemisphere (5 cases), bilateral cerebellar hemispheres (4 cases), both cerebral hemispheres and cerebellar hemispheres (6 cases), and the right frontal lobe (4 cases). Other locations included the brain parenchyma, temporal lobe, and occipital lobe.
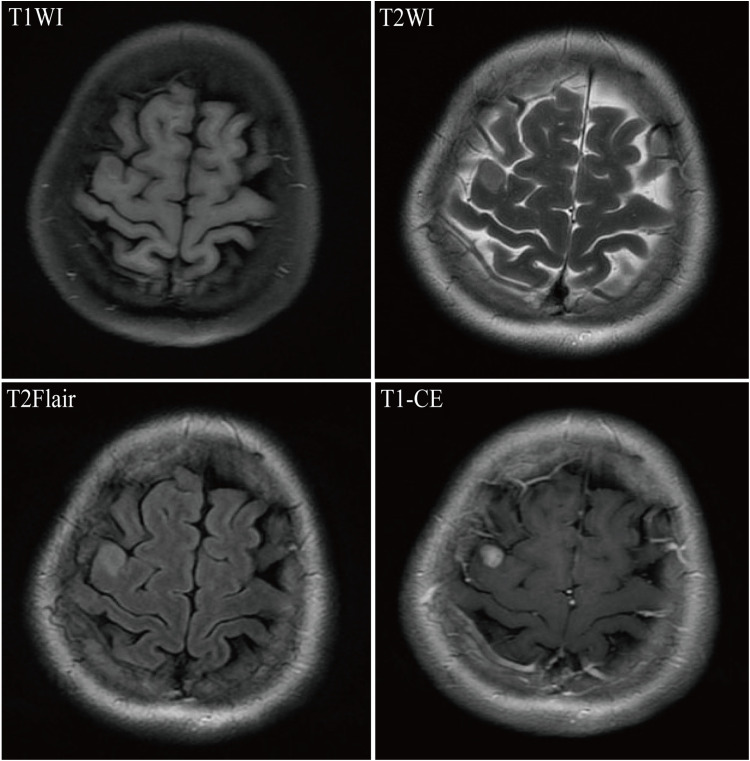
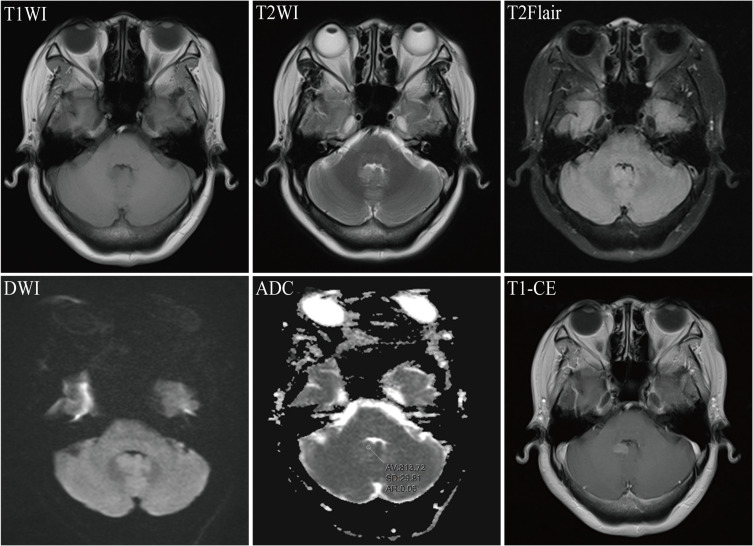
After BM, all patients received craniocerebral radiotherapy and targeted therapy, including endocrine therapy in six cases and chemotherapy in 41 cases. Other metastatic sites mainly included liver, lung, and bone, of which 38 cases had liver metastasis, 32 cases had lung metastasis, and 41 cases had bone metastasis. Figures 2 and 3 showed the MRI images of a patient in the targeted-radiotherapy group and the radiotherapy-targeted group, respectively, after the occurrence of BM. In addition, 23 cases (40.3%) presented with metastases at three different sites concurrently. Among 20 patients in the targeted-radiotherapy group who received targeted therapy after the occurrence of BM, only one patient developed extracranial (liver) progression in addition to intracranial progression after the completion of 8 cycles of trastuzumab re-examination. Furthermore, three breast cancer patients with BM exhibited a marked increase in intracranial pressure or reduced muscle strength in one limb due to significant intracranial space occupation before treatment. All three had multiple BMs, with two cases in the targeted-radiotherapy group and one in the radiotherapy-targeted group.
Figure 2.
Magnetic Resonance Imaging (MRI) for breast cancer combined with brain metastasis in a 45-year-old female patient. T1WI revealed an irregular hypointense nodule in the right precentral gyrus; T2WI showed a slightly hyperintense signal; T2Flair demonstrated a hyperintense signal; and T1-CE displayed significant enhancement of the lesion.
Figure 3.
Magnetic Resonance Imaging (MRI) for breast cancer combined with brain metastasis in a 50-year-old female patient. T1WI revealed an irregular hypointense nodule in the cerebellar vermis; T2WI and T2Flair showed slightly hyperintense signals; diffusion-weighted imaging (DWI) presented an isointense signal; the mean apparent diffusion coefficient (ADC) value of the lesions was 0.813 x 10(-3) mm²/s; and T1-CE demonstrated significant enhancement of the lesion.
Comparison of Basic Data Between Two Groups
The modified GPA score of breast cancer was applied to analyze the prognosis, the specific score calculations were listed in the Appendix 1. The maximum age at the time of BM was set as 50 years old, and the number of BM was set as 1–3, >3. There was no difference in age at BM, molecular subtype, KPS at the diagnosis of BM, Ki-67 levels, treatment of primary breast disease (primary surgical treatment, breast/chest radiotherapy, targeted therapy, chemotherapy, endocrine therapy), and treatment after BM endocrine therapy between the two groups (all P > 0.05). However, the number of BM and the treatment after BM chemical therapy were different (P < 0.05) (Table 1).
Table 1.
Characteristics of Patients in the Radiotherapy-Targeted and the Targeted-Radiotherapy Groups
| Characteristics | Classification | Radiotherapy- Targeted Group (n) | Targeted- Radiotherapy Group (n) | χ2 | P-value |
|---|---|---|---|---|---|
| Patients (n) | 37 | 20 | |||
| Age at BM (year) | ≤ 50 | 22 | 11 | 0.106 | 0.745 |
| > 50 | 15 | 9 | |||
| Number of BM | 1–3 | 15 | 14 | 4.508 | 0.034 |
| > 3 | 22 | 6 | |||
| KPS | 90–100 | 26 | 16 | 0.634 | 0.426 |
| 60–80 | 11 | 4 | |||
| Molecular subtype (n) | Luminal B (HER2+) | 16 | 10 | 0.239 | 0.625 |
| HER2 overexpression | 21 | 10 | |||
| Ki-67 | < 14% | 4 | 2 | Fisher’s exact test | 1.000 |
| ≥ 14% | 33 | 18 | |||
| Treatment of Primary breast disease | |||||
| Primary surgical treatment | Yes | 28 | 13 | 0.733 | 0.392 |
| No | 9 | 7 | |||
| Chemotherapy | Yes | 35 | 17 | Fisher’s exact test | 0.332 |
| No | 2 | 3 | |||
| Endocrine therapy | Yes | 14 | 7 | 0.045 | 0.832 |
| No | 23 | 13 | |||
| Breast/chest radiotherapy | Yes | 12 | 4 | 0.994 | 0.319 |
| No | 25 | 16 | |||
| Targeted therapy | Yes | 28 | 13 | 0.733 | 0.392 |
| No | 9 | 7 | |||
| Treatment after BM | |||||
| Chemotherapy | Yes | 30 | 11 | 4.374 | 0.036 |
| No | 7 | 9 | |||
| Endocrine therapy | Yes | 4 | 2 | Fisher’s exact test | 1.000 |
| No | 33 | 18 | |||
Note: Bold values indicate statistically significant differences.
Abbreviations: BM, brain metastasis; KPS, Karnofsky performance status; HER2, human epidermal growth factor receptor 2.
Comparison of Intracranial Progression-Free Survival Times
The Evaluation of Intracranial Conditions for the First Time
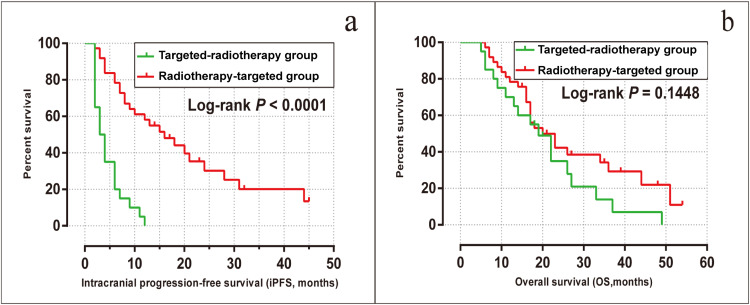
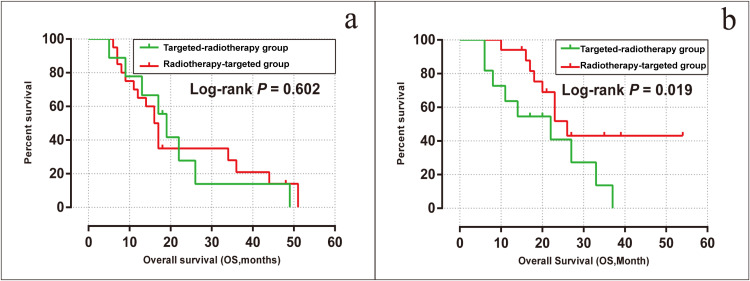
The median iPFS was 3 months [95% confidence interval (CI), 1.53–4.46] and 16 months (95% CI, 8.78–23.21) in the targeted-radiotherapy and radiotherapy-targeted groups, respectively (log-rank P < 0.001). Moreover, the intracranial progression rate was 20 (100%) in the targeted-radiotherapy group and 25 (67.6%) in the radiotherapy-targeted group, respectively (Figure 4a).
Figure 4.
Kaplan-Meier analysis comparing intracranial progression-free survival (a) and overall survival (b) in the radiotherapy-targeted group and the targeted-radiotherapy group.
The Evaluation of Intracranial Conditions for the Second Time
After radiotherapy combined with targeted therapy, the overall objective response rate of the two groups was 24.6%, and the total disease control rate of the two groups was 40.4%. The overall objective response rate of the targeted-radiotherapy group and radiotherapy-targeted group was 15% and 29.7%, respectively (Fisher’s test P = 0.336). The total disease control rate was 50% and 35.1%, respectively (P = 0.275).
The Result of Univariate and Multivariate Cox Analysis
The univariate Cox analysis was conducted to evaluate the factors associated with iPFS, and the evaluation results were detailed in Table 2. The analysis results revealed that the treatment groups, differentiated by varying time sequences, were a significant factor affecting iPFS. Subsequently, a multivariate Cox analysis was performed to adjust for potential confounders and to better understand the independent effects of these variables. Notably, the treatment groups defined by different time sequences also showed a highly significant association with iPFS (P < 0.001), as shown in Table 3.
Table 2.
Univariable Analyses of Covariables Associated with Intracranial Progression-Free Survival
| Factors | HR | 95% CI | P-value |
|---|---|---|---|
| Group | |||
| Targeted-radiotherapy vs Radiotherapy-targeted | 0.178 | 0.088–0.360 | < 0.001 |
| Age at BM (year) | |||
| ≤ 50 vs > 50 | 0.665 | 0.363–1.220 | 0.188 |
| KPS | |||
| ≥ 90 vs < 90 | 0.929 | 0.475–1.815 | 0.829 |
| Number of BM | |||
| 1–3 vs > 3 | 1.144 | 0.635–2.061 | 0.654 |
| Time from the first diagnosis to BM (m) | |||
| ≥ 29 vs < 29 | 0.857 | 0.466–1.576 | 0.62 |
| Molecular subtype (n) | |||
| Luminal B (HER2+) vs HER2 overexpression | 1.130 | 0.615–2.077 | 0.693 |
| Treatment of Primary breast disease (Targeted therapy) | |||
| Yes vs No | 1.117 | 0.589–2.119 | 0.734 |
| Primary surgical treatment | |||
| Yes vs No | 1.808 | 0.932–3.507 | 0.08 |
| Ki-67 | |||
| ≥ 14 vs < 14 | 1.515 | 0.631–3.639 | 0.353 |
| Treatment after BM (Chemotherapy) | |||
| Yes vs No | 0.577 | 0.226–1.471 | 0.249 |
Abbreviations: HR, hazard ratio; CI, confidence interval; iPFS, intracranial progression-free survival; BM, brain metastasis; KPS, Karnofsky performance status; HER2, human epidermal growth factor receptor 2.
Table 3.
Multivariable Analyses of Covariables Associated with Intracranial Progression-Free Survival
| Factors | HR | 95% CI | P-value |
|---|---|---|---|
| Group | |||
| Targeted-radiotherapy vs Radiotherapy-targeted | 0.160 | 0.073–0.352 | < 0.001 |
| Time from the first diagnosis to BM (m) | |||
| ≥ 29 vs < 29 | 0.961 | 0.495–1.866 | 0.906 |
| Number of BM | |||
| 1–3 vs > 3 | 1.805 | 0.949–3.433 | 0.072 |
| Molecular subtype (n) | |||
| Luminal B (HER2+) vs HER2 overexpression | 0.812 | 0.428–1.539 | 0.523 |
| Treatment of Primary breast disease | |||
| Primary surgical treatment | |||
| Yes vs No | 0.808 | 0.394–0.656 | 0.560 |
| Treatment after BM Chemotherapy | |||
| Yes vs No | 0.872 | 0.320–2.375 | 0.789 |
Abbreviations: HR, hazard ratio; CI, confidence interval; iPFS, intracranial progression-free survival; BM, brain metastasis; HER2, human epidermal growth factor receptor 2.
Comparison of Overall Survival Time
The median OS of all patients included in this study was 20 months (95% CI, 14.0–26.0). The median OS was 19 months (95% CI, 13.4–24.6) for patients in the targeted-radiotherapy group and 20 months (95% CI, 12.8–27.2) for patients in the radiotherapy-targeted group, with no significant difference observed between the two groups (log-rank P = 0.145, Figure 4b). The 1-year OS rates were 70.0% for the targeted-radiotherapy group and 81.1% for the radiotherapy-targeted group; the 3-year OS rates were 10.0% and 18.9%, respectively. By the end of the follow-up period, there were 43 deaths in total, with 17 deaths (85%) in the targeted-radiotherapy group and 26 deaths (68.9%) in the radiotherapy-targeted group. Of them, 19% (n = 8) died solely due to intracranial progression, including three cases from the targeted-radiotherapy group and five cases from the radiotherapy-targeted group.
The univariate Cox proportional hazard models were utilized for the analysis of prognostic factors affecting OS, as detailed in Table 4. Further analysis of multivariable Cox proportional hazard models showed that the time from the first diagnosis to BM (P = 0.037) and the number of BMs (P = 0.037) were significant factors; these factors were found to be higher in the radiotherapy-targeted group than in the targeted-radiotherapy group (Table 5).
Table 4.
Univariable Analyses of Covariables Associated with Overall Survival
| Factors | HR | 95% CI | P-value |
|---|---|---|---|
| Group | |||
| Targeted-radiotherapy vs Radiotherapy-targeted | 0.636 | 0.341–1.187 | 0.156 |
| Age at BM (y) | |||
| ≤50 vs > 50 | 0.910 | 0.487–-1.697 | 0.766 |
| KPS | |||
| ≥ 90 vs < 90 | 1.038 | 0.533–2.022 | 0.913 |
| Number of BM | |||
| 1–3 vs > 3 | 1.502 | 0.807–2.795 | 0.199 |
| Time from the first diagnosis to BM (m) | |||
| ≥ 29 vs < 29 | 0.585 | 0.314–1.091 | 0.092 |
| Molecular subtype (n) | |||
| Luminal B (HER2+) vs HER2 overexpression | 1.141 | 0.611–2.132 | 0.678 |
| Treatment of Primary breast disease (Targeted therapy) | |||
| Yes vs No | 0.758 | 0.372–1.548 | 0.447 |
| Primary surgical treatment | |||
| Yes vs No | 1.881 | 0.982–3.605 | 0.057 |
| Ki-67 | |||
| ≥ 14 vs < 14 | 0.905 | 0.375–2.179 | 0.823 |
| Treatment after BM | |||
| Endocrine therapy | |||
| Yes vs No | 0.580 | 0.221–1.523 | 0.269 |
| Chemotherapy | |||
| Yes vs No | 0.566 | 0.199–1.611 | 0.286 |
Abbreviations: HR, hazard ratio; CI, confidence interval; OS, overall survival; BM, brain metastasis; KPS, Karnofsky performance status; HER2, human epidermal growth factor receptor 2.
Table 5.
Multivariable Analyses of Covariables Associated with Overall Survival
| Factors | HR | 95% CI | P-value |
|---|---|---|---|
| Group | |||
| Targeted-radiotherapy vs Radiotherapy-targeted | 0.690 | 0.344–1.384 | 0.296 |
| Number of BM | |||
| 1–3 vs > 3 | 2.038 | 1.045–3.976 | 0.037 |
| Time from the first diagnosis to BM (m) | |||
| ≥ 29 vs < 29 | 0.508 | 0.268–0.960 | 0.037 |
| Treatment of Primary breast disease (Primary surgical treatment) | |||
| Yes vs No | 1.847 | 0.925–3.688 | 0.082 |
| Treatment after BM (Chemotherapy) | |||
| Yes vs No | 0.646 | 0.216–1.931 | 0.434 |
Abbreviations: HR, hazard ratio; CI, confidence interval; OS, overall survival; BM, brain metastasis.
Stratified Analysis Based on Modified Breast Graded Prognostic Assessment
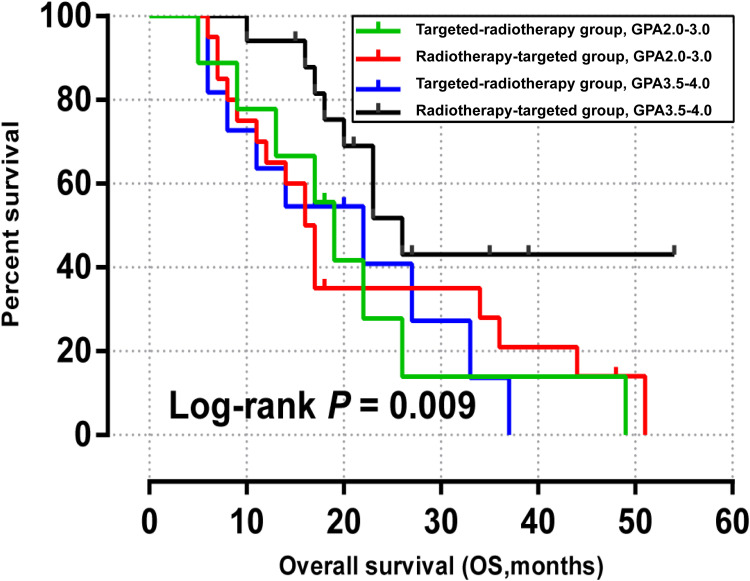
Modified breast-GPA was used in this study. In the modified breast-GPA score, HER2 over-expression type and Luminal B (HER2+) type accounted for 1 and 1.5 points, respectively (the specific score calculation was shown in the Appendix 1). Therefore, the modified breast-GPA score of all patients in this retrospective study was 2.0–4.0. Combined with the data collected, the included patients were further divided into subgroup 2.0–3.0 and subgroup 3.5–4.0 for modified breast-GPA.
In the two groups with modified breast-GPA 2.0–3.0, the median OS of the radiotherapy-targeted group and targeted-radiotherapy group was 16 months and 19 months, respectively (P = 0.602, Figure 5a). In the modified breast-GPA 3.5–4.0 subgroup, the median OS of radiotherapy-targeted group and targeted-radiotherapy group was 26 months and 22 months, respectively (P = 0.019, Figure 5b). All patients were divided into 4 groups according to modified breast-GPA score for Kaplan-Meier analysis (P = 0.009, Figure 6).
Figure 5.
Kaplan-Meier overall survival curves for modified breast graded prognostic assessment 2.0–3.0 group (a) and 3.5–4.0 group (b) in the radiotherapy-group and the targeted-radiotherapy group.
Figure 6.
Kaplan-Meier overall survival curves for modified breast graded prognostic assessment scores in the radiotherapy-targeted group and the targeted-radiotherapy group.
Acute Toxic Reactions
In the radiotherapy-targeted group, there was one case of grade III myelosuppression and one case of apathy caused by high intracranial pressure delaying radiotherapy; the treatment was successfully completed following active management. Other patients did not have grade II or higher myelosuppression or gastrointestinal reactions.
Discussion
Evidence from clinical studies suggests that systemic therapy with anti-HER2-targeted agents can increase the median OS in patients with HER2-positive breast cancer and BM.10,12–14 For example, trastuzumab can significantly improve OS and iPFS of HER2-positive breast cancer patients.28,29 However, whether trastuzumab prolongs OS by controlling extracranial and intracranial diseases remains unclear.30 In this study, intracranial progression was seen in 20 patients who received targeted drug therapy after BM but not radiotherapy; in addition, one patient developed extracranial progression. Hence, we concluded that trastuzumab might have better efficacy in controlling extracranial diseases than intracranial diseases.
Trastuzumab is a monoclonal antibody with a molecular weight of 145,000 Daltons, which theoretically cannot pass the BBB under normal conditions.31 However, studies have shown that the integrity of the BBB is compromised in patients with BM, allowing some trastuzumab to cross. For example, Dijkers et al32 found that the concentration of zirconium-89 (89Zr)-trastuzumab in BM was 18 times higher than in normal brain tissue, suggesting altered BBB integrity due to the presence of tumor blood vessels lacking normal BBB function. In this research, radiotherapy followed by targeted therapy significantly improved iPFS in HER2-positive breast cancer patients with BM. These data are consistent with findings from Kim et al.33 Such effect was particularly evident in patients receiving radiotherapy at the time of progression, underscoring the critical role of timely radiotherapy. This improvement is likely due to the increased intracranial concentration of trastuzumab following radiotherapy, which further damages the BBB, enhancing drug delivery to intracranial lesions.34 Without radiotherapy, the concentration of anti-HER2-targeted drugs in intracranial lesions may be insufficient for effective disease control. Interestingly, our study observed intracranial progression in all patients treated with targeted drugs alone, further confirming the benefit of adding radiotherapy.
Additionally, the multivariate analysis revealed that the sequential treatment was an independent prognostic factor for intracranial progression in HER2-positive breast cancer patients with BM. Although both sequential treatment groups showed significant improvements in one-year and three-year OS rates compared to previous studies using WBRT,35 the median OS between the groups did not significantly differ. Hence, the order of therapy administration may not greatly impact OS. However, the potential influence of trastuzumab following radiotherapy could explain the improved intracranial disease control in the targeted-radiotherapy group, despite a higher mortality rate. These findings highlight the importance of radiotherapy in managing BM and suggest that further disruption of the BBB through radiotherapy can enhance the effectiveness of anti-HER2-targeted therapies. Nonetheless, the relatively short follow-up period and small sample size are limitations that may affect the generalizability of our results, underscoring the need for further prospective studies.
The multivariate Cox regression analysis results showed that interval time from the first breast cancer diagnosis to the occurrence of BM and the number of BM were independent prognostic factors in HER2-positive breast cancer patients with BM, contrary to the data previously published by Ou et al.36 The difference may lay in different grouping criteria; in our study, the interval time from the first diagnosis of breast cancer to the occurrence of BM was divided by 29 months (median), while in Ou’s study36 was divided by 12 months. In addition, the number of BM has been included as a scoring criterion for breast cancer prognosis with BM.24
The stratified analysis using the modified breast-GPA demonstrated a clear correlation between higher GPA scores and improved median OS, consistent with previously reported trends.37 Our findings indicated that the sequence of radiotherapy and targeted therapy administration could significantly influence outcomes in patients with high GPA scores. Specifically, the radiotherapy-targeted group exhibited a more favorable prognosis than the targeted-radiotherapy group. Adverse reactions were generally manageable, with only a few instances of more severe complications, underscoring the overall tolerability of the treatment protocols. These results emphasized the importance of treatment sequencing and provided valuable insights for optimizing therapeutic strategies for HER2-positive breast cancer patients with BM.
There also certain limitations in this study. Firstly, this was a retrospective study with inherent flaws in the experimental design. The data collected spans from 2010 to 2019, predating the most recent international guidelines which recommend upfront systemic therapy alone in carefully selected, asymptomatic patients, and in cases of advanced disease. This temporal difference may account for discrepancies between our study design and current practices. Moreover, retrospective studies have limited the use of second- or third-line targeted therapies (eg, trastuzumab emtanzine, lapatinib, tucatinib), which may affect treatment outcomes. Secondly, being a single-center retrospective study, there is an inherent risk of selection bias, which limits the generalizability of the findings to wider populations. Thirdly, this study had a relatively small sample size, which may affect the statistical power and robustness of the conclusions. Also, the limited number of patients makes it challenging to detect smaller differences between treatment groups and may increase the variability of the results. Furthermore, radiological evaluation included both CT and MRI due to the retrospective nature and varied departmental management, potentially impacting intracranial assessments, introducing confounding factor. Additionally, the follow-up period was relatively short, which may not capture the long-term outcomes and potential late effects of the treatments. Consequently, further studies need to strive to include a larger and more diverse patient population, standardize treatment protocols, and ensure longer follow-up periods. Based on these, a further validation can be performed to analyze the efficacy and safety of sequential radiotherapy and targeted therapy in HER2-positive breast cancer patients with BM.
Conclusion
To sum up, our study demonstrated that radiotherapy followed by targeted therapy significantly improved iPFS in HER2-positive breast cancer patients with BM. The data suggested that the sequence of treatments, specifically administering radiotherapy before anti-HER2 therapy, plays a pivotal role in disease management. However, additional research is required to further elucidate the impact of anti-HER2 therapy on intracranial disease control and OS.
Acknowledgments
The authors are thankful to Guangxi Medical University Cancer Hospital for supplying the patients used in this study. The authors would also like to thank Prof. Xinbin Pan, Liuting Yang, and Zhongguo Liang from Guangxi Medical University Cancer Hospital for modifying the manuscript. We are also grateful to all editors and reviewers.
Funding Statement
This study was supported by the Key Research and Development Program of Guangxi (Grant No. GuikeAB23026001).
Data Sharing Statement
All data generated and analyzed during this study are included in this published article.
Ethics Statement
This study was approved by the Ethics Committee of Guangxi Medical University Cancer Hospital (LW2022152), and all study participants provided informed written consent. The guidelines outlined in the Declaration of Helsinki were followed.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest for this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52(12):2349–2354. doi: [DOI] [PubMed] [Google Scholar]
- 3.Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Goncalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32(11):4655–4662. [PubMed] [Google Scholar]
- 4.Yardley DA, Kaufman PA, Huang W, et al. Quantitative measurement of HER2 expression in breast cancers: comparison with ‘real-world’ routine HER2 testing in a multicenter collaborative biomarker study and correlation with overall survival. Breast Cancer Res. 2015;17(1):41. doi: 10.1186/s13058-015-0543-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol. 2006;17(6):935–944. doi: 10.1093/annonc/mdl064 [DOI] [PubMed] [Google Scholar]
- 6.Cortes J, Dieras V, Ro J, et al. Afatinib alone or Afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): a randomised, open-label, multicentre, Phase 2 trial. Lancet Oncol. 2015;16(16):1700–1710. doi: 10.1016/S1470-2045(15)00373-3 [DOI] [PubMed] [Google Scholar]
- 7.Ramakrishna N, Temin S, Chandarlapaty S, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: american Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(19):2100–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin C, Wu J, Ding S, et al. Subdivision of M1 Stage for de novo metastatic breast cancer to better predict prognosis and response to primary tumor surgery. J Natl Compr Canc Netw. 2019;17(12):1521–1528. doi: 10.6004/jnccn.2019.7332 [DOI] [PubMed] [Google Scholar]
- 9.Xiao W, Li X, Yang A, et al. Analysis of prognostic factors affecting the brain metastases free survival and survival after brain metastases in breast cancer. Front Oncol. 2020;10:431. doi: 10.3389/fonc.2020.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. doi: 10.1002/cncr.11436 [DOI] [PubMed] [Google Scholar]
- 11.Shen Q, Sahin AA, Hess KR, et al. Breast cancer with brain metastases: clinicopathologic features, survival, and paired biomarker analysis. Oncologist. 2015;20(5):466–473. doi: 10.1634/theoncologist.2014-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eichler AF, Kuter I, Ryan P, Schapira L, Younger J, Henson JW. Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer. 2008;112(11):2359–2367. doi: 10.1002/cncr.23468 [DOI] [PubMed] [Google Scholar]
- 13.Church DN, Modgil R, Guglani S, et al. Extended survival in women with brain metastases from HER2 overexpressing breast cancer. Am J Clin Oncol. 2008;31(3):250–254. doi: 10.1097/COC.0b013e31815a43c4 [DOI] [PubMed] [Google Scholar]
- 14.Kirsch DG, Ledezma CJ, Mathews CS, et al. Survival after brain metastases from breast cancer in the trastuzumab era. J Clin Oncol. 2005;23(9):2114–2116. doi: 10.1200/JCO.2005.05.249 [DOI] [PubMed] [Google Scholar]
- 15.Soffietti R, Ahluwalia M, Lin N, Ruda R. Management of brain metastases according to molecular subtypes. Nat Rev Neurol. 2020;16(10):557–574. doi: 10.1038/s41582-020-0391-x [DOI] [PubMed] [Google Scholar]
- 16.Nabors LB, Portnow J, Ahluwalia M, et al. Central nervous system cancers, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(11):1537–1570. doi: 10.6004/jnccn.2020.0052 [DOI] [PubMed] [Google Scholar]
- 17.Venur VA, Leone JP. Targeted therapies for brain metastases from breast cancer. Int J Mol Sci. 2016;17(9):1543. doi: 10.3390/ijms17091543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, Phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. doi: 10.1016/S1470-2045(17)30441-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiotherapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. doi: 10.1001/jama.2016.9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007;18(1):23–28. doi: 10.1097/01.cad.0000236313.50833.ee [DOI] [PubMed] [Google Scholar]
- 21.Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87(5):586–592. doi: 10.1038/clpt.2010.12 [DOI] [PubMed] [Google Scholar]
- 22.Leone JP, Leone BA. Breast cancer brain metastases: the last frontier. Exp Hematol Oncol. 2015;4(1):33. doi: 10.1186/s40164-015-0028-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venur VA, Ahluwalia MS. Prognostic scores for brain metastasis patients: use in clinical practice and trial design. Chin Clin Oncol. 2015;4(2):18. doi: 10.3978/j.issn.2304-3865.2015.06.01 [DOI] [PubMed] [Google Scholar]
- 24.Subbiah IM, Lei X, Weinberg JS, et al. Validation and development of a modified breast graded prognostic assessment as a tool for survival in patients with breast cancer and brain metastases. J Clin Oncol. 2015;33(20):2239–2245. doi: 10.1200/JCO.2014.58.8517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fares J, Kanojia D, Cordero A, et al. Current state of clinical trials in breast cancer brain metastases. Neurooncol Pract. 2019;6(5):392–401. doi: 10.1093/nop/npz003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fares J, Kanojia D, Rashidi A, Ulasov I, Lesniak MS. Landscape of combination therapy trials in breast cancer brain metastasis. Int J Cancer. 2020;147(7):1939–1952. doi: 10.1002/ijc.32937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–3752. doi: 10.1200/JCO.2014.55.5730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–131. doi: 10.1056/NEJMoa1703643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gori S, Puglisi F, Moroso S, et al. The HERBA study: a retrospective multi-institutional Italian study on patients with brain metastases from HER2-positive breast cancer. Clin Breast Cancer. 2019;19(4):e501–e510. doi: 10.1016/j.clbc.2019.05.006 [DOI] [PubMed] [Google Scholar]
- 31.Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol. 2000;18(11):2349–2351. doi: 10.1200/JCO.2000.18.11.2349 [DOI] [PubMed] [Google Scholar]
- 32.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002;3(1):53–57. doi: 10.1016/S1470-2045(01)00622-2 [DOI] [PubMed] [Google Scholar]
- 33.Kim JS, Kim K, Jung W, et al. New brain metastases after whole-brain radiotherapy of initial brain metastases in breast cancer patients: the significance of molecular subtypes (KROG 16-12). Breast Cancer Res Treat. 2021;186(2):453–462. doi: 10.1007/s10549-020-06043-0 [DOI] [PubMed] [Google Scholar]
- 34.Yau T, Swanton C, Chua S, et al. Incidence, pattern and timing of brain metastases among patients with advanced breast cancer treated with trastuzumab. Acta Oncol. 2006;45(2):196–201. doi: 10.1080/02841860500486630 [DOI] [PubMed] [Google Scholar]
- 35.Mahmoud-Ahmed AS, Suh JH, Lee SY, Crownover RL, Barnett GH. Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int J Radiat Oncol Biol Phys. 2002;54(3):810–817. doi: 10.1016/S0360-3016(02)02967-X [DOI] [PubMed] [Google Scholar]
- 36.Ou D, Cao L, Xu C, Kirova Y, Chen JY. Upfront brain radiotherapy may improve survival for unfavorable prognostic breast cancer brain metastasis patients with Breast-GPA 0-2.0. Breast J. 2019;25(6):1134–1142. doi: 10.1111/tbj.13426 [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, Chen J, Yu X, et al. Systemic treatment after whole-brain radiotherapy may improve survival in RPA class II/III breast cancer patients with brain metastasis. J Neurooncol. 2013;114(2):181–189. doi: 10.1007/s11060-013-1169-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study are included in this published article.