Abstract
Objectives
There is an active debate regarding whether metformin use improves survival in people with ovarian cancer. We examined this issue using methods designed to avoid immortal time bias—as bias that occurs when participants in a study cannot experience the outcome for a certain portion of the study time.
Methods
We used time-dependent analyses to study the association between metformin use for all 4,951 patients diagnosed with ovarian cancer in 1997 through 2018 in the province of British Columbia, Canada. Cox proportional hazards models were run to estimate the association between metformin and survival in the full cohort of ovarian cancer patients and among a cohort restricted to patients with diabetes.
Results
Metformin use was associated with a 17 % better ovarian cancer survival in the full cohort (adjusted hazard ratio (aHR) = 0.83 (95 %CI 0.67, 1.02)), and a 16 % better ovarian cancer survival for serous cancers patient's cohort (aHR = 0.84 (95 %CI 0.66, 1.07)), although both were not significant. However, a statistically significant protective effect was observed when restricting to the diabetic cohort (aHR = 0.71 (95 %CI 0.54–0.91)), which was also seen among serous cancers (aHR = 0.73 (95 %CI 0.54–0.98)).
Conclusion
Metformin use was associated with improved ovarian cancer survival. The lack of statistical significance in the full cohort may reflect that diabetes is associated with reduced cancer survival, and thus diabetes itself may offset the benefit of metformin when examining the full cohort. Future research should examine metformin use among non-diabetic ovarian cancer patients.
Keywords: Metformin, Ovarian cancer, Immortal time bias, Survival
Introduction
Ovarian cancer is the fifth most common cause of cancer deaths among females in developed countries. There is still no effective screening method for ovarian cancer [1], and most people are diagnosed in late stages with 5-year survival rates of 15–30 %.[2,3].
Metformin, a member of the biguanide class of drugs, is widely used as first-line therapy for type 2 diabetes to normalize hyperglycemia [4]. Epidemiologic studies have reported that metformin use is associated with a reduced risk of cancer in diabetic patients, including a reduced incidence of epithelial ovarian cancer [[5], [6], [7]]. There has been some suggestion that use of metformin after diagnosis might also improve survival in people with ovarian cancer [[8], [9], [10], [11], [12]]. Indirect mechanisms include suppression of hepatic gluconeogenesis, resulting in increased insulin sensitivity and a reduction in circulating glucose and insulin levels, which may lead to decreased growth factor stimulation of tumor cells [13]. Direct effects include inhibiting respiratory complex 1 in the mitochondria, interfering with oxidative phosphorylation resulting in decreased adenosine triphosphate production and increased energetic stress [14]. In tumor cells that are unable to cope with metformin-induced energetic stress, energetic crisis and ultimately cell death may occur in response to metformin treatment [14].
However, the effect of metformin use on survival in ovarian cancer patients is inconsistent, as several other studies have reported no association [[15], [16], [17], [18]]. There is also the possibility that patient or study characteristics have influenced previous findings, including whether the control group included diabetic or non-diabetic patients, as well as timing and duration of the metformin use. The inconsistencies may be due to methodologic differences. Many previous studies of metformin use and ovarian cancer survival were prone to suffering from immortal time bias, wherein immortal time (a period of follow-up during which the outcome cannot occur due to the exposure definition) biases the study toward finding a survival effect of the medication. These designs introduce time between cohort entry and the first prescription (or the number of prescriptions that define cohort entry) as ‘immortal time’ for the exposed in that they require that the exposed survived long enough to receive the treatment [19]. A 2020 systematic review concluded that only two previous studies of metformin use and ovarian cancer survival were likely free of immortal time bias [19]. One of the included studies examined ovarian cancer specific mortality and the other examined all-cause mortality, and both suggested no survival benefit associated with metformin use [17,18]. Thus, carefully designed studies that account for how these patient and study characteristics might influence results are still needed to understand whether there is a role for metformin in increasing ovarian cancer survival.
Methods
We conducted a population-based retrospective cohort study of all patients who were diagnosed with ovarian cancer at age 30 or older in the Canadian province of British Columbia (population of 5 million) between January 1st, 1997 and December 31st, 2018, with follow-up through to December 31st, 2020. With approval of all data stewards, we work with Population Data BC to access the BC Cancer Registry, which we used to identify all ovarian cancer patients. In BC, cancer is a reportable disease and all cases are entered into the provincial BC Cancer Registry. The registry sources include hematology and pathology reports, death certificates, hospital reports, and cancer treatments. The data available includes details about the type of cancer diagnosed and the date of diagnosis. These data were linked with vital statistics death data, as well as the Discharge Abstract Database (DAD), which contains information on all hospital stays and day surgeries in the province and the medical services plan file (MSP), which includes data on all physician visits in an outpatient settings. Data on metformin and other medication use was obtained from linking with the BC PharmaNet, into which every medication dispensed in an outpatient setting in the province of British Columbia must be entered, by law. Thus, it is a complete capture of medications dispensed throughout the province. You can find further information regarding these data sets by visiting the Population Data BC’s project webpage at: https://my.popdata.bc.ca/project_listings/21-105/collection_approval_dates.
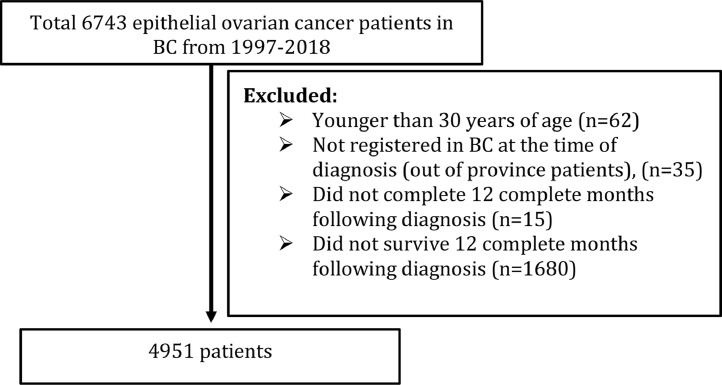
Ovarian cancer patients were identified in the BC Cancer Registry using the International Classification of Diseases for Oncology ICD10 CM codes for ovarian cancer, fallopian tube cancer or peritoneal cancer, not otherwise specified (C56; C57.0; C48.2, respectively). We included all epithelial ovarian cancers diagnosed between 1997 and 2018 and excluded all borderline tumors. We excluded women who did not survive for 12 complete months following diagnosis, as medication exposure is unlikely to make a difference to survival in very advanced and aggressive cancers. We also excluded patients if they were not registered in BC at the time of diagnosis and did not complete 12 months follow up after diagnosis. This information was checked using registry data which contains information about all people who were registered in BC's Health Insurance system. We set the cohort entry date as 12 months after ovarian cancer diagnosis. We identified the diabetic ovarian cancer patients using diagnostic codes indicating the presence of the disease in either the hospital (DAD) or the physician data (MSP) (ICD9 code 250.x and ICD 10 code E10.x (type 1 diabetes) and E11.x (type 2 diabetes) and E14.x (unspecified diabetes mellitus).
Ethics approval was obtained from the University of British Columbia's Behavioral Research Ethics Board. All inferences, opinions and conclusions are those of the authors and do not reflect the opinions or policies of the Data Stewards. Access to data provided by the Data Stewards is subject to approval but can be requested for research projects through the Data Stewards or their designated service providers.
Assessment of medication use
Medication dispensations were classified according to their Anatomical Therapeutic Chemical code. Metformin monotherapy has ATC code A10BA02, and metformin combinations have codes (A10BD03, A10BD07, A10BD10, A10BD11, A10BD13, and A10BD15). We grouped all patients using metformin monotherapy or a metformin combination as the metformin exposed group. We also identified patients using other diabetes medications: sulfonylureas (ATC 4 A10BB), thiazolidinediones (ATC4 A10BG) and insulin monotherapy (ATC3 A10A). Post-diagnosis exposure to metformin was defined as at least two prescriptions for metformin or a metformin combination on different dates after cohort entry date. We modeled this as a time dependent variable. Their exposure was lagged by 6 months as it is biologically implausible that short duration exposure would meaningfully impact ovarian cancer survival. Patients were considered unexposed until 6 months after the date of their second prescription (lag period) and exposed thereafter, allowing them to move from a period of non-exposure to a period of exposure which was done to remove immortal time bias. Ovarian cancer patients entered the cohort one year after the date they were diagnosed with ovarian cancer, which imposed the 12-month survival restriction.
We modeled use of metformin dichotomously (ever use vs. never use) and examined cumulative duration of use post-diagnosis. Cumulative duration of use was defined as the total months of exposure to the medication of interest, which was calculated by adding the total duration of all the metformin prescriptions that were filled between the date of their first exposure (6 months after second prescription) to the end of follow-up or their date of death. We categorized this time-dependent variable as: less than 12 months, 12–24 months, 24–36 months and greater than or equal to 36 months of use. We also conducted a user-type analysis where we grouped patients according to whether they were new users of metformin post-diagnosis with ovarian cancer (they had not filled a single metformin for the 12 months period prior to their ovarian cancer diagnosis but met our criteria as a user post-diagnosis), whether they were continuing users of metformin (they filled ≥2 prescriptions in the 12 months before diagnosis and continued to fill prescriptions after their diagnosis), and whether they were pre-diagnosis users of metformin who stopped after their diagnosis with ovarian cancer (they filled ≥2 prescriptions for metformin in the 12 months before diagnosis and did not fill any in the 1 year after their diagnosis). User-type was classified into 3 categories and all groups were compared with never users.
Outcome of interest: We examined the relationship between medication use and all-cause mortality, as well as ovarian cancer-specific mortality. We considered anyone with an underlying cause of death code for any gynecologic cancer (C48.2, C51-C58), as well as site unspecified malignant neoplasm and neoplasms of multiple independent primary sites (C80, C97) to have died from ovarian cancer. The date of death was classified as the date of their event. Individuals were censored as of the earlier of the date they left the province, or the end of study follow-up on December 31st, 2020.
Statistical analysis
We examined both ovarian cancer specific mortality and all-cause mortality. We ran time-dependent Cox proportional hazards models to estimate hazard ratio and 95 % confidence intervals, and censored patients at the time of last follow-up or at the end of study follow-up on December 31st, 2020. We ran these models for all epithelial ovarian cancer, and for all serous ovarian cancers. We lacked reliable data on grade for a significant proportion of our study period, so high-grade and low-grade serous were combined; however, most of these cancers are high-grade serous ovarian cancers. We did not have enough metformin users to run the models for endometrioid, clear cell or mucinous ovarian cancers.
All models were adjusted for categorical variables such as age at diagnosis (30–59,60–69,70–79,80+), diagnosis year with categories as 1997–2002, 2003–2008, 2009–2014, 2015–2018, income quintiles at diagnosis, debulking surgery after diagnosis (yes/no), previous non-gynecologic cancer (yes/no), and separate dichotomous (yes/no) variables for comorbidities in their health record in the 5 years before the date of ovarian cancer diagnosis. The comorbidities included diabetes, cerebrovascular disease, peripheral vascular disease, renal disease, and cardiopulmonary disease (yes/no). We also adjusted for use of menopausal hormone therapy (ATC codes: G03A, G03C, G03D and L02AA, LO2AB), statins (ATC codes: C10AA, C10BA, C10BX, A10BH51, A10BH52) and β-blockers (ATC codes: C07) and use of insulin or any other diabetes medication (ATC codes outlined above) in the 6 months before ovarian cancer diagnosis. These other medications were included as they been previously associated with ovarian cancer survival analyses [[20], [21], [22]], and may be confounded with metformin use, particularly the cardiovascular medications which are commonly prescribed to diabetic patients [23]. All variables that were adjusted in the model were time-fixed covariates. The only time dependent variable in the model was exposure to metformin. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient characteristics
There were 4,951 people diagnosed with an epithelial ovarian cancer and included in the analysis. Of these, 711 (14.4 %) had a diagnostic code indicating they were diabetic (either type 1, type 2 or unspecified) in their health records in the 5 years before their ovarian cancer diagnosis. Of these, 236 (4.8 %) were metformin users in the 12 months before their diagnosis. Nearly all metformin users were diabetic (98.7 %). There were 166 (3.3 %) patients who were lost to follow up after 1 year (Fig. 1).
Fig. 1.
Flow diagram of final study population.
The mean age at diagnosis of the entire cohort was 62.2(SD 12.5) and the median years of follow-up were 3.1 (IQR 1.3 to 6.9). Follow up time is low because 70 % of ovarian cancer patients die within 5 years. During the follow-up period, 2984 people died; 2628 (88 %) of them from ovarian cancer. Metformin users were diagnosed at an older average age than non-users (66.1 vs. 61.9) and were more likely to have other comorbidities including cardiopulmonary disease, cardiovascular disease, peripheral vascular disease, renal disease, and cerebrovascular disease (Table 1). They were more likely have serous ovarian cancers (66.1 % vs. 58.7 %), and to have filled a prescription for beta-blockers, statins, and other diabetes medications in the 6 months before diagnosis with their ovarian cancer. Metformin users were also more likely to be diagnosed in the later half of the study period (2009–2018). Within the diabetic cohort (Table 2), users and non-users were similar in terms of many of the characteristics examined, but metformin users were more likely to have filled a prescription for beta-blockers, statins, insulin, and other diabetes medications than non-users.
Table 1.
Baseline characteristics of the entire cohort stratified by metformin use.
| Entire cohort (n=4951) | Pre-diagnostic metformin use (12 months pre-diagnosis) |
||
|---|---|---|---|
| Metformin user (n = 239) |
Non-user (n = 4712) |
||
|
Age at diagnosis (years) Mean (SD) |
62.2 (12.5) (Range: 30.1–99.2) Median 62.2 (IQR: 52.9–71.4) |
66.1 (10.9) (Range: 38.7–91.8) Median 67.2 (IQR: 58.6–73.6) |
61.9 (12.6) (Range: 30.1–99.2) Median 61.8 (IQR: 52.7–71.1) |
| Age at diagnosis (years), n(%) | |||
| 30–59 | 2054 (41.5) | 62 (25.9) | 1992 (42.3) |
| 60–69 | 1227 (24.8) | 74 (31.0) | 1153 (24.5) |
| 70–79 | 931 (18.8) | 63 (26.4) | 868 (18.4) |
| 80+ | 739 (14.9) | 40 (16.7) | 699 (14.8) |
| Year of diagnosis, n(%) | |||
| 1997–2002 | 1152 (23.3) | 28 (11.7) | 1124 (23.8) |
| 2003–2008 | 1191 (24.1) | 52 (21.8) | 1139 (24.2) |
| 2009–2014 | 1524 (30.8) | 92 (38.5) | 1432 (30.4) |
| 2015–2018 | 1084 (21.9) | 67 (28.0) | 1017 (21.6) |
| Income quintile, n(%) | |||
| Q1 | 902 (18.5) | 58 (24.5) | 844 (18.2) |
| Q 2 | 948 (19.5) | 43 (18.1) | 905 (19.5) |
| Q 3 | 961 (19.7) | 48 (20.2) | 913 (19.7) |
| Q 4 | 1019 (20.9) | 50 (21.1) | 969 (20.9) |
| Q 5 | 1037 (21.3) | ∼40 (16.0) | ∼1000 (21.6) |
| Missing | 84 | <5 ** | ∼80 |
| Histotype | |||
| Serous | 2923 (59.0) | 158 (66.1) | 2765 (58.7) |
| Endometrioid | 640 (12.9) | 26 (10.9) | 614 (13.0) |
| Mucinous | 248 (5.0) | 9 (3.8) | 239 (5.1) |
| Clear cell | 392 (7.9) | 10 (4.2) | 382 (8.1) |
| Carcinoma, NOS/Mixed | 748 (15.1) | 36 (15.1) | 712 (15.1) |
|
Median follow up, years (Interquartile range) |
3.1 (1.3 to 6.9) | 2.7 (1.3 to 5.3) | 3.1 (1.3 to 7.0) |
| Debulking surgery | 3723 (75.2) | 173 (72.4) | 3550 (75.3) |
| Previous cancer | 654 (13.2) | 32 (13.4) | 622 (13.2) |
|
Comorbidities (5 years before diagnosis) |
|||
| Diabetes history | 711 (14.4) | 236 (98.7) | 475 (10.1) |
| Cardiopulmonary disease | 971 (19.6) | 62 (25.9) | 909 (19.3) |
| Cardiovascular disease* | 633 (12.8) | 43 (17.9) | 590 (12.5) |
| Peripheral vascular disease | 205 (4.1) | 14 (5.9) | 191 (4.0) |
| Renal disease | 231 (4.7) | 31 (12.9) | 200 (4.2) |
| Cerebrovascular disease | 221 (4.5) | 13 (5.4) | 208 (4.4) |
|
Medication history (6 months before diagnosis) |
|||
| Hormone replacement therapy | 677 (13.7) | 23 (9.6) | 654 (13.8) |
| β-blockers | 538 (10.9) | 58 (24.3) | 480 (10.2) |
| Statins | 690 (13.9) | 127 (53.1) | 563 (11.9) |
| Insulin | 72 (1.5) | 36 (15.1) | 36 (0.8) |
| Other diabetes medicine§ | 77 (1.6) | 57 (23.8) | 20 (0.4) |
congenital heart failure or rheumatic heart disease or myocardial infarction
all medications excluding metformin (ATC code A10B) and insulin (ATC code A10A)
**Privacy agreements with data stewards include not publishing cell sizes with values <5
Table 2.
Baseline characteristics of DIABETIC PATIENTS stratified by metformin use.
| Entire diabetic cohort (n=711) | Pre-diagnostic metformin use (12 months pre-diagnosis) |
||
|---|---|---|---|
| Metformin user (n=236) |
Non-user (n=475) |
||
|
Age at diagnosis (years) Mean (SD) |
66.3 (11.4) (Range: 32.2–97.6) Median 66.9 (IQR: 58.5-74.4) |
66.0 (10.8) (Range: 38.7–91.8) Median 67.1 (IQR: 58.6-73.6) |
66.4 (11.7) (Range: 32.2–97.6) Median 66.6 (IQR: 58.4-74.9) |
| 30–59 | 189 (26.6) | 62 (26.3) | 127 (26.7) |
| 60–69 | 199 (27.9) | 73 (30.9) | 126 (26.5) |
| 70–79 | 184 (25.9) | 63 (26.7) | 121 (25.5) |
| 80+ | 139 (19.5) | 38 (16.1) | 101 (21.3) |
| Year of diagnosis | |||
| 1997–2002 | 111 (15.6) | 27 (11.4) | 84 (17.7) |
| 2003–2008 | 162 (22.8) | 52 (22.0) | 110 (23.2) |
| 2009–2014 | 254 (35.7) | 92 (38.9) | 162 (34.1) |
| 2015–2018 | 184 (25.9) | 66 (27.5) | 119 (25.0) |
| Income quintile | |||
| Q1 | 143 (20.3) | 57 (24.4) | 86 (18.3) |
| Q 2 | 155 (21.9) | 42 (17.9) | 113 (23.9) |
| Q 3 | 138 (19.6) | 47 (20.1) | 91 (19.3) |
| Q 4 | 134 (19.0) | 50 (21.4) | 84 (17.8) |
| Q 5 | 135 (19.1) | ∼40 (16.2) | ∼95 (20.6) |
| Missing | 6 | <5⁎⁎ | <5⁎⁎ |
| Histotype | |||
| Serous | 453 (63.7) | 156 (66.1) | 297 (62.5) |
| Endometrioid | 69 (9.7) | 26 (11.0) | 43 (9.0) |
| Mucinous | 22 (3.1) | 9 (3.8) | 13 (2.7) |
| Clear cell | 40 (5.6) | 10 (4.2) | 30 (6.3) |
| Carcinoma, NOS/Mixed | 127 (17.9) | 35 (14.8) | 92 (19.4) |
|
Median follow up, years (Interquartile range) |
2.7 (1.2 to 5.6) | 2.8 (1.3 to 5.3) | 2.6 (1.1 to 5.7) |
| Surgery after diagnosis | 510 (71.7) | 171 (72.5) | 339 (71.4) |
| Previous cancer | 110 (15.5) | 32 (13.6) | 78 (16.4) |
|
Comorbidities (5 years before diagnosis) |
|||
| Cardiopulmonary disease | 171 (24.0) | 60 (25.4) | 111 (23.4) |
| Cardiovascular disease* | 131 (18.4) | 43 (18.2) | 88 (18.5) |
| Peripheral vascular disease | 42 (5.9) | 14 (5.9) | 28 (5.9) |
| Renal disease | 76 (10.7) | 31 (13.1) | 45 (9.5) |
| Cerebrovascular disease | 45 (6.3) | 12 (5.1) | 33 (6.9) |
|
Medication history (6 months before diagnosis) |
|||
| Hormone replacement therapy | 72 (10.1) | 22 (9.3) | 50 (10.5) |
| β-blockers | 134 (18.8) | 58 (24.6) | 76 (16.0) |
| Statins | 262 (36.8) | 125 (52.9) | 137 (28.8) |
| Insulin | 72 (10.1) | 36 (15.2) | 36 (7.6) |
| Other diabetes medicine§ | 75 (10.5) | 56 (23.7) | 19 (4.0) |
congenital heart failure or rheumatic heart disease or myocardial infarction
all medications excluding metformin (ATC code A10B) and insulin (ATC code A10A)
Privacy agreements with data stewards include not publishing cell sizes with values <5
Postdiagnosis use of metformin and survival
Results from the time variant Cox proportional hazards regression models for the association between postdiagnosis use of metformin and both ovarian cancer specific and all-cause mortality are presented in Table 3. Crude hazard ratios for any metformin use was 0.95 (95 %CI 0.79–1.14) for ovarian cancer specific mortality and 1.02 (95 % CI 0.86–1.20) for all-cause mortality. After adjusting for covariates, the adjusted hazard ratio of an ovarian cancer death was 0.83 (95 %CI 0.67, 1.02). The adjusted hazard ratio for a death due to any cause was 0.88 (95 % CI 0.73, 1.06). There was no clear trend with respect to cumulative duration of use, for either ovarian cancer specific mortality or all-cause mortality, and no statistically significant results by user-type (whether the person used metformin pre-diagnosis, continued use after diagnosis, or began use post diagnosis). The results for the analysis that was restricted to serous ovarian cancers also revealed no statistically significant results (Supplemental Table S1).
Table 3.
Post-diagnosis Metformin prescription dispensation and ovarian cancer specific and all-cause mortality.
| Ovarian cancer specific mortality |
All-cause mortality |
||||||
|---|---|---|---|---|---|---|---|
| Person- years | Mortality events (n=2628) |
Crude HR (95 % CI) | Adjusted HR* (95 % CI) | Mortality events (n=2984) |
Crude HR (95 % CI) | Adjusted HR* (95 % CI) | |
| Metformin user | |||||||
| Metformin user | 1567.76 | 118 | 0.95 (0.79–1.14) | 0.83 (0.67–1.02) | 150 | 1.02 (0.86–1.20) | 0.88 (0.73–1.06) |
| Non-user | 23,619.28 | 2510 | 1 | 1 | 2834 | 1 | 1 |
| Cumulative duration of metformin use, months | |||||||
| <12 | 461.77 | 57 | 0.99 (0.76-1.29) | 0.88 (0.66-1.17) | 65 | 1.03 (0.80-1.32) | 0.91 (0.70-1.18) |
| 12-24 | 299.01 | 28 | 0.89 (0.61-1.29) | 0.76 (0.52-1.13) | 37 | 1.05 (0.75-1. 45) | 0.88 (0.63-1.24) |
| 24-36 | 215.93 | 16 | 0.99 (0.60-1.63) | 0.83 (0.50-1.38) | 18 | 0.94 (0.59-1.51) | 0.77 (0.48-1.24) |
| >36 | 591.05 | 17 | 0.87 (0.53-1.41) | 0.76 (0.46-1.26) | 30 | 1.01 (0.70-1.46) | 0.89 (0.61-1.29) |
| Metformin User type | |||||||
| New use post diagnosis | 866.46 | 47 | 0.91 (0.68-1.22) | 0.90 (0.67-1.22) | 62 | 0.95 (0.74-1.23) | 0.94 (0.72-1.22) |
| Continuing use from pre-diagnosis | 701.30 | 71 | 0.97 (0.77-1.24) | 0.81 (0.61-1.06) | 88 | 1.08 (0.87-1.33) | 0.86 (0.67-1.11) |
| Use only pre-diagnosis | 244.24 | 62 | 1.47 (1.14-1.90) | 1.16 (0.87-1.55) | 68 | 1.50 (1.18-1.92) | 1.15 (0.87-1.51) |
| Never use | 23,375.04 | 2448 | 1 | 1 | 2766 | 1 | 1 |
*Models adjusted for age at diagnosis, diagnosis year, income, surgery after diagnosis, previous cancer, diabetes history, cardiovascular disease, cerebrovascular disease, peripheral vascular disease, renal disease, cardiopulmonary disease, hormone replacement therapy, statin, β-blockers, insulin, other diabetic medications
When restricted to the cohort of diabetic patients, crude hazard ratios for any use of metformin were 0.65 (95 %CI 0.51–0.84) for ovarian cancer specific mortality and 0.74 (95 %CI 0.59–0.92) for all-cause mortality (Table 4). After adjusting for all covariates, the adjusted hazard ratios were 0.71 (95 %CI 0.54–0.91) for ovarian cancer specific mortality and 0.81 (95 %CI 0.64–1.02) for all-cause mortality. The crude hazard ratios for new use post diagnosis and continuing use from pre-diagnosis were 0.56 (95 % CI 0.35-0.88) and 0.70 (95 %CI 0.54–0.93), respectively for ovarian cancer specific mortality. However, after adjusting the confidence intervals included 1 aHR=0.64 (95 %CI 0.40–1.02) for new use post diagnosis and aHR=0.76 (95 %CI 0.56–1.01) for continuing use from pre-diagnosis. For all-cause mortality, only crude hazard ratios for continuing use from pre-diagnosis was significant 0.77 (95 % CI: 0.60–0.98). The adjusted hazard ratios were protective for ovarian cancer specific mortality (aHR=0.73 (95 %CI 0.54–0.98)) and all-cause mortality (aHR=0.74 (95 %CI 0.56–0.98)) when restricting to serous ovarian cancers (Supplemental Table S2), but all cumulative duration and user type analyses were not statistically significant.
Table 4.
Post-diagnostic metformin prescription dispensation and risk of ovarian cancer specific and all-cause mortality in a diabetic ovarian cancer patients.
| Ovarian cancer specific mortality |
All-cause mortality |
||||||
|---|---|---|---|---|---|---|---|
| Person- years | Mortality events (n=412) |
Crude HR (95 % CI) | Adjusted HR* (95 % CI) | Mortality events (n=477) |
Crude HR (95 % CI) | Adjusted HR* (95 % CI) | |
| Diabetic medication users | |||||||
| Metformin users | 1073.66 | 91 | 0.65 (0.51-0.84) | 0.71 (0.54-0.91) | 121 | 0.74 (0.59-0.92) | 0.81 (0.64-1.02) |
| Non-users | 1921.99 | 321 | 1 | 1 | 356 | 1 | 1 |
| Cumulative duration of metformin use, months | |||||||
| <12 | 294.75 | 43 | 0.69 (0.50-0.97) | 0.74 (0.53-1.05) | 50 | 0.75 (0.55-1.03) | 0.82 (0.59-1.13) |
| 12-24 | 214.06 | 24 | 0.71 (0.46-1.11) | 0.74 (0.47-1.16) | 33 | 0.87 (0.59-1.28) | 0.89 (0.60-1.33) |
| 24-36 | 154.90 | 13 | 0.67 (0.37-1.21) | 0.70 (0.38-1.27) | 14 | 0.60 (0.34-1.06) | 0.64 (0.36-1.13) |
| ≥36 | 409.95 | 11 | 0.44 (0.22-0.84) | 0.52 (0.27-1.02) | 24 | 0.66 (0.41-1.06) | 0.81 (0.50-1.30) |
| Metformin User type | |||||||
| New use post diagnosis | 373.86 | 21 | 0.56 (0.35-0.88) | 0.64 (0.40-1.02) | 34 | 0.73 (0.51-1.06) | 0.88 (0.60-1.28) |
| Continuing use from pre-diagnosis | 699.80 | 70 | 0.70 (0.54-0.93) | 0.76 (0.56-1.01) | 87 | 0.77 (0.60-0.98) | 0.82 (0.63-1.07) |
| Use only pre-diagnosis | 240.68 | 60 | 1.25 (0.93-1.70) | 1.23 (0.90-1.68) | 66 | 1.33 (0.99-1.77) | 1.28 (0.95-1.72) |
| Never use | 1681.31 | 261 | 1 | 1 | 290 | 1 | 1 |
*Models adjusted for age at diagnosis, diagnosis year, income, surgery after diagnosis, previous cancer, cardiovascular disease, cerebrovascular disease, Peripheral vascular disease, renal disease, Cardiopulmonary disease, hormone replacement therapy, statin, β-blockers, insulin, other diabetic medications
Discussion
In this large, population-based study including nearly 5000 ovarian cancer patients, and over 700 diabetic ovarian cancer patients, we found an ∼30 % reduction in ovarian cancer mortality and all-cause mortality among diabetic patients using metformin compared to diabetic patients who did not use metformin. When comparing patients using metformin to all other patients not using metformin, we found a reduction in both ovarian cancer and all-cause mortality that did not reach statistical significance.
Our finding of no significant mortality benefit for metformin users when comparing with non-diabetic non-users is consistent with a meta-analysis that reported that when correcting for immortal time bias, there was no survival benefit for metformin users [19]. We addressed immortal time bias by using a time-dependent variable to categorize exposure to metformin, allowing people to move from a period of non-exposure to exposure and thus eliminating immortal time bias. Our finding of a significant mortality benefit within diabetic patients is also inconsistent with a recent meta-analysis that reported finding consistent results comparing metformin users with nondiabetic non-users and studies comparing metformin users with diabetic non-users [24]. While that meta-analysis reported that metformin use was associated with reduced ovarian cancer mortality of approximately 30 % in both groups, we only found that reduced mortality in the diabetic group.
We also found no statistically significant relationships by cumulative duration of use of metformin, or by user-type in our adjusted models. However, in the unadjusted models in the diabetic cohort, new use post-diagnosis was associated with the largest reduction in ovarian cancer specific mortality. This was no longer significant following adjustment. While the hazard ratio was in the direction of improved survival, the confidence interval widened to include 1. While this could suggest confounding by prognosis, where the diabetic patients who receive a favourable ovarian cancer prognosis are more likely to be prescribed metformin, there was also a significant reduction in mortality with those continuing use from pre-diagnosis in the unadjusted models. The adjusted hazard ratio for continuing users also remained protective but lost statistical significance following adjustment for covariates. Thus, we cannot rule out the possibility of residual confounding by ovarian cancer prognosis in this analysis, and it is possible that the diabetic patients who seem most likely to survive their ovarian cancer are the same patients who are started on metformin or are encouraged to continue using their metformin.
The fact that the protective effect of metformin reached statistical significance only within the diabetic cohort may be related to the consistent finding that diabetes is associated with reduced survival after ovarian cancer [25,26]. While metformin appears to be associated with increased survival among non-diabetics, the cohort used in this study included less than 5 non-diabetic metformin users (exact number cannot be disclosed due to privacy restrictions). Thus, when examining the role of metformin in the entire cohort, the comparison was between diabetic metformin users and non-diabetic non-users. It is possible that the reduced survival inherent to having comorbid diabetes may have offset some of the protection afforded by the metformin. Thus, future research should examine the role of metformin by examining whether it is protective among non-diabetic users. This would likely require a prospective study, and preferably a randomized controlled trial to also eliminate the confounding by ovarian cancer prognosis.
Some strengths of our study include the large size of 4951 ovarian cancer patients, the population-based nature of the study, and the long follow-up. The most important limitations are those that cannot be overcome when using observational study designs. Firstly, as mentioned above, we have no information on metformin use among people without diabetes, and thus our estimates of the effect of the medication on survival cannot be separated from the effects of diabetes on survival. We also suffer from limitations common to studies using administrative datasets, including possible misclassification of exposure, given that our prescription drug database provides information on prescriptions that were dispensed at a pharmacy, which does not mean those prescriptions were used completely and as prescribed. However, the use of two filled prescriptions to classify a person as a user would have limited this misclassification, as people who do not use or completely use a medication generally do not refill that prescription. We are also limited in our ability to classify ovarian cancers by histotype, as we are relying on ICD-O morphology codes, which can be vague. These codes also vary in precision over time, as ovarian cancer pathology improved during our study period. We were also limited by our small number of metformin users, which made is impossible to conduct histotype-specific analyses for any histotype other than serous ovarian cancer. Given that metformin is generally used to treat type 2 diabetes, we may have ended up with selection bias in the diabetic cohort, wherein more type 1 diabetics ended up in the non-user group. However, type 1 diabetics generally use insulin, and there were only 36 (7.6 %) of people using insulin in the non-user group and 36 (15.2 %) using insulin the metformin suggesting this is unlikely to be a significant source of bias. Finally, access to richer data on ovarian cancer prognosis would have helped us address what we hypothesize is confounding by prognosis in our diabetic cohort.
Conclusion
This study found evidence of improved survival following a diagnosis of ovarian cancer for people using metformin, particularly when comparing diabetic metformin users to diabetic non-users. While we cannot rule out bias, particularly due to ovarian cancer prognosis, future research should examine whether metformin use among non-diabetic patients might improve ovarian cancer survival. The best study design for this question is a randomized controlled trial, as it will also eliminate the confounding by prognosis that is impossible to completely address using observational research designs.
CRediT authorship contribution statement
Paramdeep Kaur: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Andrew Berchuck: Methodology, Writing – review & editing. Anne Chase: Methodology, Writing – review & editing. Bronwyn Grout: Methodology, Writing – review & editing. Cindy McKinnon Deurloo: Methodology, Writing – review & editing. Leigh C. Pearce: Conceptualization, Methodology, Writing – review & editing. Malcolm C. Pike: Methodology, Writing – review & editing. Jean Richardson: Methodology, Writing – review & editing. Kathryn L. Terry: Methodology, Writing – review & editing. Penelope M. Webb: Methodology, Writing – review & editing. Gillian E. Hanley: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Paramdeep Kaur: This work is supported by United States Department of Defence Ovarian Cancer Research Program. Malcolm Pike is the PI of the grant supporting this work. Payments were made to Dr. Pike and subawards were included to UBC, my organization.
Penelope M. Webb: Speaker's fee (Dec 2021) paid by AstraZeneca. Gillian E. Hanley: This work is supported by United States Department of Defence Ovarian Cancer Research Program. Malcolm Pike is the PI of the grant supporting this work. Payments were made to Dr. Pike and subawards were included to UBC, my organization. I also received grant from Canadian Institutes of Health Research related to ovarian cancer.
Acknowledgments
This study was supported by the Department of Defense. PMW is supported by an Investigator Grant from the National Health and Medical Research Council of Australia (APP1173346). MCP was supported in part through the NIH/NCI Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center. The funding sources played no role in study design, collection of data, interpretation of data, writing of the report or decision to submit the article for publication.
References
- 1.Menon U., Gentry-Maharaj A., Burnell M., et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK collaborative trial of ovarian cancer screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397(10290):2182–2193. doi: 10.1016/S0140-6736(21)00731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surveillance epidemiology and end results program. Ovary cancer survival statistics. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed November 25, 2023.
- 3.Tone A.A., Salvador S., Finlayson S.J., et al. The role of the fallopian tube in ovarian cancer. Clin. Adv. Hematol. Oncol. 2012;10(5):296–306. [PubMed] [Google Scholar]
- 4.Quinn B.J., Kitagawa H., Memmott R.M., Gills J.J., Dennis PA. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol. Metab. 2013;24(9):469–480. doi: 10.1016/j.tem.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Tseng CH. Metformin reduces ovarian cancer risk in Taiwanese women with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2015;31(6):619–626. doi: 10.1002/dmrr.2649. [DOI] [PubMed] [Google Scholar]
- 6.Mekuria A.N., Ayele Y., Tola A., Mishore KM. Monotherapy with metformin versus sulfonylureas and risk of cancer in type 2 diabetic patients: a systematic review and meta-analysis. J. Diabetes Res. 2019;2019 doi: 10.1155/2019/7676909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen Q., Zhao Z., Wen J., et al. The association between metformin therapy and risk of gynecological cancer in patients: two meta-analyses. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019;237:33–41. doi: 10.1016/j.ejogrb.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Currie C.J., Poole C.D., Jenkins-Jones S., Gale E.A.M., Johnson J.A., Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes impact of metformin on survival. Diabetes Care. 2012;35(2):299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero I.L., McCormick A., McEwen K.A., et al. Relationship of Type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet. Gynecol. 2012;119(1):61–67. doi: 10.1097/AOG.0b013e3182393ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar S., Meuter A., Thapa P., et al. Metformin intake is associated with better survival in ovarian cancer A Case-Control Study. Cancer. 2013;119(3):555–562. doi: 10.1002/cncr.27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S.B., Lei K.J., Liu J.P., Jia YM. Continuous use of metformin can improve survival in type 2 diabetic patients with ovarian cancer: a retrospective study. Medicine. 2017;96(29):e7605. doi: 10.1097/MD.0000000000007605. (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez R., Gockley A.A., Melamed A., et al. Multivariable analysis of association of beta-blocker use and survival in advanced ovarian cancer. Gynecol. Oncol. 2020;157(3):700–705. doi: 10.1016/j.ygyno.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Kirpichnikov D., McFarlane S.I., Sowers JR. Metformin: an update. Ann. Intern. Med. 2002;137(1):25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 14.Pollak M.N. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2(9):778–790. doi: 10.1158/2159-8290.cd-12-0263. [DOI] [PubMed] [Google Scholar]
- 15.Shah M.M., Erickson B.K., Matin T., et al. Diabetes mellitus and ovarian cancer: more complex than just increasing risk. Gynecol. Oncol. 2014;135(2):273–277. doi: 10.1016/j.ygyno.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bar D., Lavie O., Stein N., Feferkorn I., Shai A. The effect of metabolic comorbidities and commonly used drugs on the prognosis of patients with ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;207:227–231. doi: 10.1016/j.ejogrb.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Garcia C., Yao A., Camacho F., Balkrishnan R., Cantrell LA. A SEER-Medicare analysis of the impact of metformin on overall survival in ovarian cancer. Gynecol. Oncol. 2017;146(2):346–350. doi: 10.1016/j.ygyno.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Urpilainen E., Marttila M., Hautakoski A., et al. Prognosis of ovarian cancer in women with type 2 diabetes using metformin and other forms of antidiabetic medication or statins: a retrospective cohort study. BMC Cancer. 2018;18(1):767. doi: 10.1186/s12885-018-4676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suissa S. Immortal time bias in pharmaco-epidemiology. Am. J. Epidemiol. 2008;167(4):492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 20.Majidi A., Na R., Dixon-Suen S., Jordan S.J., Webb PM. Common medications and survival in women with ovarian cancer: a systematic review and meta-analysis. Gynecol. Oncol. 2020;157(3):678–685. doi: 10.1016/j.ygyno.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 21.Hanley G.E., Kaur P., Berchuck A., et al. Cardiovascular medications and survival in people with ovarian cancer: a population-based cohort study from British Columbia, Canada. Gynecol. Oncol. 2021;162(2):461–468. doi: 10.1016/j.ygyno.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brieger K.K., Peterson S., Lee A.W., et al. Menopausal hormone therapy prior to the diagnosis of ovarian cancer is associated with improved survival. Gynecol. Oncol. 2020;158(3):702–709. doi: 10.1016/j.ygyno.2020.06.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnett D.K., Khera A., Blumenthal RS. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: part 1, lifestyle and behavioral factors. JAMA Cardiol. 2019;4(10):1043–1044. doi: 10.1001/jamacardio.2019.2604. [DOI] [PubMed] [Google Scholar]
- 24.Guo M., Shang X., Guo D. Metformin use and mortality in women with ovarian cancer: an updated meta-analysis. Int. J. Clin. Pract. 2022;2022 doi: 10.1155/2022/9592969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barone B.B., Yeh H.C., Snyder C.F., et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300(23):2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipscombe L.L., Goodwin P.J., Zinman B., McLaughlin J.R., Hux J.E. The impact of diabetes on survival following breast cancer. Breast Cancer Res. Treat. 2008;109(2):389–395. doi: 10.1007/s10549-007-9654-0. [DOI] [PubMed] [Google Scholar]