Abstract
Sheep pulmonary adenomatosis (SPA) is a contagious and experimentally transmissible lung cancer of sheep resembling human bronchiolo-alveolar carcinoma. A type D retrovirus, known as jaagsiekte sheep retrovirus (JSRV), has been associated with the etiology of SPA, but its exact role in the induction of the tumor has not been clear due to the lack of (i) a tissue culture system for the propagation of JSRV and (ii) an infectious JSRV molecular clone. To investigate the role of JSRV in the etiology of SPA, we isolated a full-length JSRV proviral clone, pJSRV21, from a tumor genomic DNA library derived from a natural case of SPA. pJSRV21 was completely sequenced and showed open reading frames in agreement with those deduced for the original South African strain of JSRV. In vivo transfection of three newborn lambs by intratracheal inoculation with pJSRV21 DNA complexed with cationic lipids showed that pJSRV21 is an infectious molecular clone. Viral DNA was detected in the peripheral blood mononuclear cells (PBMCs) of the transfected animals by a highly sensitive JSRV-U3 heminested PCR at various time points ranging from 2 weeks to 6 months posttransfection. In addition, proviral DNA was detected in the PBMCs, lungs, and mediastinal lymph nodes of two lambs sacrificed 9 months posttransfection, but no macroscopic or histological SPA lesion was induced. We prepared JSRV particles by transient transfection of 293T cells with a JSRV construct (pCMV2JS21) in which the upstream U3 was replaced with the cytomegalovirus early promoter. Four newborn lambs were inoculated with JSRV21 particles produced in this manner, and two of them showed the classical signs of SPA 4 months postinfection. The resulting tumors were positive for JSRV DNA and protein. Thus, JSRV21 is an infectious and pathogenic molecular clone and is necessary and sufficient to induce sheep pulmonary adenomatosis.
Animal models of retrovirus-induced tumors have provided many insights into the mechanisms governing cell transformation (44). Sheep pulmonary adenomatosis (SPA), also known as ovine pulmonary carcinoma, is a bronchiolo-alveolar carcinoma that is present in widely distributed agricultural populations (13, 29). SPA strongly resembles human bronchiolo-alveolar carcinoma (BAC); both tumors have the same clinical, macroscopic, histopathologic, and ultrastructural features (18, 31). BAC has many pathological and epidemiological characteristics that distinguish it from other types of human lung cancer, including adenocarcinoma (2, 4, 5). The incidence of BAC is rising, and it now represents up to a quarter of primary lung cancers in the United States (3). Most notably, lung cancer is the main cause of death from cancer in both men and women (21, 46), but very few animal models are available. The common characteristics between human BAC and SPA suggest that SPA could be a unique experimental model and could offer novel insights into pulmonary carcinogenesis.
SPA also is a significant veterinary problem in countries such as the United Kingdom, South Africa, and Spain. The cumulative lifetime risk for developing SPA approaches 25% in high-risk flocks in these countries (37).
Previous experiments provided evidence for the presence of a retrovirus (jaagsiekte sheep retrovirus [JSRV]) in the tumors and lung secretions of SPA-affected sheep (12, 24, 33, 39). An important development was the deduction of the complete nucleotide sequence of a South African strain of JSRV (JSRV-SA) (47, 48); this was accomplished by piecing together cDNA clones and reverse transcriptase PCR (RT-PCR) products from a cDNA library constructed from virus isolated by isopycnic centrifugation from the lung fluid of an SPA-affected animal. The nucleotide sequence indicated that JSRV is most closely related to type D and type B retroviruses, which was consistent with previous serologic data (20, 39).
The availability of the JSRV-SA sequence allowed the generation of highly specific immunologic and molecular probes; these probes made it possible to determine that exogenous JSRV sequences are present in the tumor tissues of SPA-affected (or experimentally infected) sheep but not in unaffected animals (1, 27). Normal sheep have 15 to 20 copies of JSRV-related endogenous retroviruses (12, 14, 47), some of which are transcriptionally active (27). The tumor cells from the lungs of SPA-affected sheep are the main sites of JSRV replication (28), but viral DNA and RNA also can be detected in various lymphoid tissues (30), where the virus appears to infect a wide variety of lymphoid cell (16).
Despite the recent data strongly suggesting that JSRV is the cause of SPA, a reconstructed JSRV-SA provirus failed to reproduce SPA in sheep (32a). Therefore, it has been unclear if JSRV is alone sufficient to induce lung cancer in sheep, if it is a helper virus for an unidentified acutely transforming retrovirus, or if it simply is a passenger that replicates preferentially in SPA tumor cells. To explore the role of JSRV in the etiology of SPA, we molecularly cloned a JSRV provirus from a sheep with a natural case of SPA and assessed the infectivity and pathogenicity of this clone in vivo. The results established that JSRV is necessary and sufficient for induction of SPA.
MATERIALS AND METHODS
Molecular cloning.
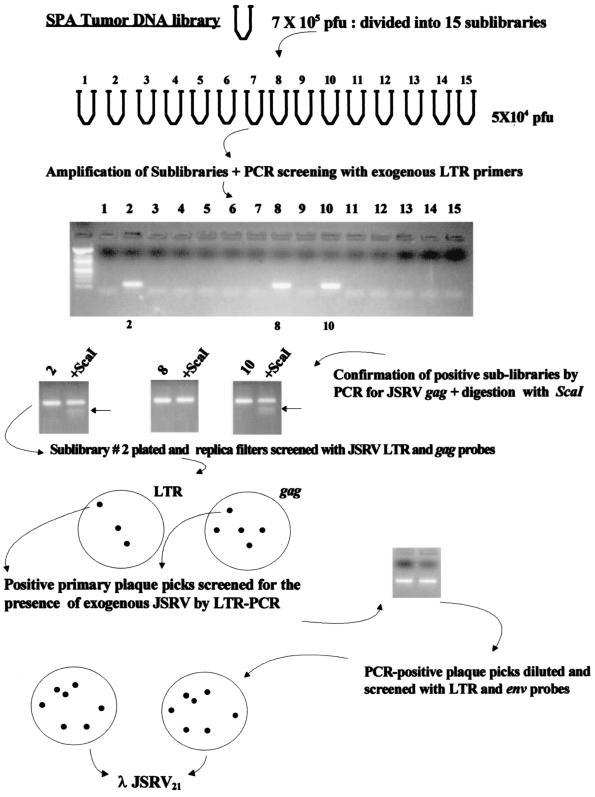
Molecular cloning of JSRV proviral DNA was carried out by established procedures (36). The strategy is shown in Fig. 1. High-molecular-weight genomic DNA was isolated from a lung tumor collected from an adult sheep with naturally acquired SPA. The genomic DNA was digested to completion with XbaI (an enzyme believed not to cut within JSRV DNA on the basis of the previously published cDNA sequence [47]), ligated to XbaI-digested λ Dash II phage vector (Stratagene), and packaged into phage particles by using Gigapack III gold-packaging extracts (Stratagene). The resulting phage library (750,000 PFU) was divided into 15 sublibraries, and each sublibrary was independently amplified. DNA was extracted from an aliquot of each sublibrary and subjected to PCR for JSRV provirus by using a JSRV U3-specific heminested PCR (U3 hn-PCR) to discriminate between exogenous JSRV and endogenous JSRV-related proviruses (30). Of the 15 sublibraries, 3 were positive for exogenous JSRV sequences. The positive sublibraries also were tested for the presence of exogenous JSRV by PCR amplification of a portion of the gag region followed by digestion of the PCR product with ScaI (27). The gag ScaI site is a molecular marker for exogenous JSRV (27). Sublibrary 2 was then plated onto bacterial agar plates and subjected to hybridization of plaque lifts with two 32P-labelled probes on replica filters: a JSRV long terminal repeat (LTR)-specific probe and a gag-specific probe. Under the hybridization conditions used, these probes hybridized with both endogenous and exogenous JSRV sequences. Primary plaques positive for both LTR and gag probes were picked, and DNA was extracted from a portion and screened for the presence of exogenous JSRV sequences by U3 hn-PCR. Exogenous JSRV-positive primary plaque picks were further purified by dilution and plating for isolated plaques on bacterial lawns, followed by hybridization with both LTR and env probes. A recombinant phage carrying a seemingly full-length JSRV provirus was subcloned into pBluescript (Stratagene) to give pJSRV21. Both strands of pJSRV21 were completely sequenced with an automated liquid fluorescence sequencer in the University of California Irvine Biotechnology Resource Facility.
FIG. 1.
Cloning of JSRV21. The strategy used for the isolation of λJSRV21 is shown.
Plasmid pCMV2JS21 was generated by replacing the U3 region in the upstream LTR of pJSRV21 with the human cytomegalovirus (CMV) immediate-early promoter by established procedures (36). The CMV promoter was obtained by PCR amplification from the pCDNA3 plasmid (Invitrogen) with primers CMVNotIf (AAAGGGTTGCGGCCGCCGATGTACGGGCCAGATATAC) and CMV-r2 (CAGAGAGCTCTGCTTATATAGACCTCCCAC) and the Pfu Turbo polymerase (Stratagene) under PCR conditions as suggested by the manufacturer. The resulting PCR product was cut with NotI and then ligated to an amplified portion of JSRV21 spanning position −13 in the U3 to +618 in gag. This portion of JSRV21, which includes R, U5, and the beginning of gag, was amplified by PCR with primers JS21-R (GCATTGTAATAAAGCAGAGTATCAGCC) and JS21663-r (GGAACCAAGGGCAAACTTCCTCAATAAATGAA) and the Pfu Turbo polymerase as above. The ligation reaction mixture was reamplified by PCR with primers CMVNotIf and JS21663-r, and the resulting product was digested with NotI and PacI and inserted into NotI-PacI-digested JSRV21 to give pCMV2JS21.
In vitro transfections.
293T cells were grown in Dulbecco minimum essential medium supplemented with 10% fetal bovine serum in 10-cm tissue culture dishes at 37°C under 5% CO2. The cells were transfected with 45 μg of pCMV2JS21 DNA by using the CalPhos mammalian transfection kit (Clonetech) as recommended by the manufacturer. Medium was replaced after 12 to 16 h with 5 ml of fresh medium. The medium was then harvested at 24, 48, and 72 h after the first medium change. The medium was filtered through a 0.45-μm-pore-size filter, and virus was pelleted by ultracentrifugation through a double layer of glycerol (25 and 50%, vol/vol) at 100,000 × g for 1 h at 4°C. The viral pellet was resuspended in TNE buffer (100 mM NaCl, 10 mM Tris, 1 mM EDTA) at a 300-fold-higher concentration with respect to the initial supernatant and stored at −140°C until further use.
Western blotting.
Western blotting was performed on 15-μl aliquots of concentrated JSRV21 particles obtained from 293T cells transiently transfected with pCMV2JS21. A rabbit antiserum to JSRV major capsid protein (CA) (28) was used essentially as described previously (28, 39), except that an enhanced chemiluminescence detection system (Supersignal; Pierce) was used as recommended by the manufacturer. Concentrated lung fluid collected from an animal with a natural case of SPA was prepared as described previously (28, 39) and used as a positive control.
Analysis of JSRV21 buoyant density.
Approximately 700 μl of concentrated JSRV21 particles was analyzed by isopycnic centrifugation on a linear 25 to 60% (wt/wt) continuous sucrose gradient in an SW41 rotor (Beckman) at 25,000 rpm for 16 h at 4°C. Fractions of approximately 450 μl were collected, and their density was determined by refractometry. Consecutive fractions were pooled two at a time and diluted with 10 ml of TNE buffer, and virus was recovered by centrifugation in an SW41 rotor at 35,000 rpm for 1 h at 4°C. Viral pellets were resuspended in 20 μl of TNE buffer and used in a conventional exogenous RT assay with poly(rA)-oligo(dT) essentially as previously described (49). For analysis of viral cores, 700 μl of concentrated JSRV was treated with a final concentration of 0.1% (vol/vol) Triton X-100 for 8 min at room temperature prior to density gradient analysis.
In vivo DNA transfections.
All animal experiments were performed in the high-security unit and the containment facilities (BL3 level) of the Moredun Research Institute (Penicuik, Scotland). All the lambs used in this study were obtained from a maedi-visna virus-free flock raised at the Moredun Research Institute. Three newborn lambs were inoculated intratracheally with pJSRV21 DNA complexed with a cationic lipid (GL-67) formulated with the neutral colipid DOPE in a molar ratio of 1:2. GL-67–DOPE was a gift from S. Cheng (Genzyme) and was prepared as already described (22). For each transfected animal, a total of 1 mg of pJSRV21 DNA was complexed with GL-67–DOPE at the suggested molar ratio and diluted to a final volume of 5 ml with distilled water. Five animals kept in the high-security unit were used as uninoculated controls.
Peripheral blood mononuclear cells (PBMCs) were collected from transfected or control lambs at various times postinoculation (Table 1) and stored at −70°C. Two inoculated lambs and two uninoculated controls were killed 38 weeks postinoculation, and samples of lungs, mediastinal lymph nodes, spleens, and kidneys were collected. The tissues were split into two portions: the first portion was snap-frozen in liquid nitrogen and stored at −70°C for subsequent isolation of nucleic acids; the remainder was fixed in 10% neutral buffered formalin, processed routinely in an automatic tissue processor, embedded in paraffin wax, and sectioned into 4- to 6-μm-thick slices. Genomic DNA was prepared as already described (30).
TABLE 1.
JSRV U3 hn-PCR on samples from in vivo-transfected lambs
| Lamb | Presence of JSRV provirusa:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Antemortemb
|
Postmortemc in:
|
|||||||
| 6 wk | 12 wk | 22 wk | PBMC | Lungs | MLNd | Spleen | Kidneys | |
| 71 | 4/5 | 1/5 | 1/5 | NDe | ND | ND | ND | ND |
| 73 | 4/5 | 1/5 | 0/5 | 0/5 | 5/5 | 0/5 | 0/5 | 0/5 |
| 74 | 5/5 | 4/5 | 1/5 | 1/5 | 0/5 | 1/5 | 0/5 | 0/5 |
Number of positive reactions/total number of replicate reactions on 500 ng of DNA.
Weeks postinfection. PBMC were used for these experiments.
Animals were sacrificed for postmortem analysis at 38 weeks postinfection.
MLN, mediastinal lymph nodes.
ND, not done.
PCR analysis.
The presence of JSRV proviral DNA in PBMCs and tissues collected from the in vivo-transfected animals and from uninoculated control animals was investigated by the use of a JSRV U3 hn-PCR as already described (30), except that for each sample, five 500-ng replicates of DNA were used (2.5 μg in total) and the samples were considered positive when one or more PCR replicate was positive. In each PCR assay, 5 to 10 500-ng replicates of calf thymus DNA were subjected to JSRV-specific U3 hn-PCR as additional negative controls.
In vivo infections.
Four newborn lambs were inoculated intratracheally with 1 ml each of concentrated supernatant collected from 293T cells transiently transfected with pCMV2JS21. The inoculum was diluted in phosphate-buffered saline (5 ml [final volume]) immediately before use. Two lambs were inoculated with phosphate-buffered saline alone and were kept as uninoculated controls. All the lambs were killed 4 months postinoculation, and tissues were treated as for the in vivo-transfected animals.
Assays for exogenous JSRV provirus in lungs and lung tumors of inoculated and control animals included exogenous virus-specific PCR in the U3 region of the LTR (30) and testing for an exogenous virus-specific ScaI site in an LTR-gag hn-PCR product (27) as described previously.
Histologic examination and immunohistochemistry.
Lung sections (4 to 6 μm thick) were stained with hematoxylin and eosin and examined by light microscopy for tumor lesions. Sections were also examined for the presence of JSRV major capsid protein by immunohistochemistry as described previously (28), except that an antigen retrieval step was included by microwaving the sections at 800 W twice for 7 min. SPA tumor tissue was used as a positive control.
Nucleotide sequence accession number.
The nucleotide sequence of JSRV21 has been deposited in GenBank under accession no. AF105220.
RESULTS
Isolation of JSRV21.
As described in detail in Materials and Methods, we cloned an integrated copy of JSRV provirus from DNA isolated from an animal with a spontaneous case of SPA. A lambda phage library from a lung tumor of an animal with SPA was screened by combining classical plaque hybridizations involving JSRV DNA probes with sib selection procedures. The sib selection involved PCR amplifications that could distinguish exogenous JSRV from multicopy endogenous JSRV-related sequences present in the sheep genome. This combination of techniques was important because the available JSRV hybridization probes cross-hybridized with the endogenous JSRV-related sequences. The cloning strategy resulted in the isolation of a full-length exogenous JSRV proviral clone (λJSRV21). The insert from this clone was subcloned into a plasmid to give pJSRV21.
Sequence analysis showed that JSRV21 possesses the hallmarks of integrated retroviral proviruses (9, 17, 19, 23, 40), such as the presence of a CA-TG dinucleotide pair present at the termini of the upstream and downstream viral LTRs, the loss of 2 nucleotides (nt) from the termini of the LTRs during integration, and an apparent duplication of 6 nt of cellular flanking sequences (TGTGTC) at the integration site. The flanking cellular sequences in the JSRV21 clone were 393 and 1,006 bp long and did not align with known cellular sequences (including proto-oncogenes).
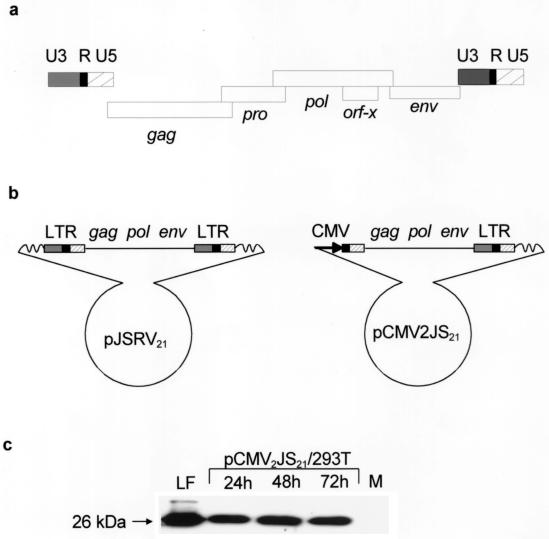
JSRV21 provirus is 7,834 bp long, and the viral genome (R to R) is 7,455 nt. JSRV21 shows the characteristic genomic organization of type D and type B retroviruses, with pro in a different open reading frame from pol (Fig. 2a). These results were consistent with the genomic organization of JSRV deduced by York et al. (47) for JSRV-SA (the only other complete JSRV sequence published). JSRV21 showed high homology (93%) to JSRV-SA. The homology was 90% in the LTRs (89% in U3), 91% in gag, 96% in pol, and 91% in env. JSRV21 is 7 bp shorter than JSRV-SA and in particular has a 5-bp deletion in U3 with respect to JSRV-SA. One difference between the coding regions of JSRV-SA and JSRV21 was in the pro region: the pro open reading frame in JSRV21 starts 53 nt downstream from the putative pro start in JSRV-SA; in particular, there are two stop codons in JSRV21 at positions 1919 and 1931 that are not present in JSRV-SA. Thus, for JSRV21, the −1 translational frameshift that presumably occurs during synthesis of the gag-pro-pol polyprotein precursor must occur downstream of the stop codon at nt 1932. It is interesting that the gag protein sequences for these two viruses have 100% identity in the region shown, so that the differences in the pro sequences did not affect the overlapping gag gene product. The orf-x open reading frame first identified in JSRV-SA was conserved in pJSRV21, suggesting that it plays a functional role.
FIG. 2.
JSRV21-based plasmid constructs and in vitro synthesis of viral particles. (a) Schematic representation of the genomic organization of the JSRV21 provirus; standard retroviral notation is used. The proviral genome is typical of type B and type D retroviruses, with pro in a different open reading frame from pol. Note the presence of an accessory open reading frame (orf-x) overlapping pol. (b) pJSRV21 and pCMV2JS21 plasmid constructs. In pCMV2JS21, the U3 region of the proximal LTR was replaced by the human CMV promoter. (c) Western blot of 300-fold-concentrated supernatant from 293T cells transiently transfected with pCMV2JS21 and collected 24, 48, and 72 h posttransfection. The filters were probed with a rabbit polyclonal antiserum against the major capsid protein (CA) of JSRV (28). Lung secretions collected from an SPA-affected animal and concentrated in the same way as the 293T supernatant were used as a positive control (LF). Concentrated supernatant from mock-transfected 293T cells was used as a negative control (M). The 26-kDa CA protein is indicated.
The presence of the ScaI site at position 1726 in gag and the nucleotide sequence of the U3 indicated that JSRV21 has the molecular markers of an exogenous JSRV and that it was not an endogenous JSRV-related provirus (1, 27).
In vivo DNA transfection of pJSRV21.
To test the infectivity of the JSRV21 provirus, we used in vivo DNA transfection in sheep, since no in vitro culture system for the propagation of the virus was available. Three newborn black-face lambs were inoculated intratracheally with pJSRV21 DNA complexed with a cationic lipid (GL-67–DOPE) that favors transfection of lung cells (22). PBMCs were collected at various times postinoculation (2 to 22 weeks), and the presence of JSRV provirus and transcripts was assessed by U3 hn-PCR (30) (Table 1). All three lambs showed detectable JSRV sequences at various times postinoculation. The levels of JSRV DNA in PBMCs from inoculated lambs were low, as judged by the fraction of replicate PCR products that scored positive. However, they were similar to the levels of JSRV DNA detected in PBMCs from lambs experimentally inoculated with concentrated SPA lung fluid (16). These results indicated that pJSRV21 contained an infectious JSRV provirus. On the other hand, when two inoculated animals were sacrificed at 9 months postinfection, no SPA lesions were observed in the lungs by macroscopic or histologic examinations. JSRV antigens were not detected by immunohistochemistry for JSRV CA antigen in the lungs of the inoculated animals; only the highly sensitive U3 hn-PCR allowed the detection of JSRV provirus in the lungs of one inoculated animal and in the mediastinal lymph nodes and PBMCs of another (Table 1). As controls, PBMCs from five uninoculated control animals were tested by U3 hn-PCR, and they were always negative (none of five replicates were positive for each animal). In addition, two uninoculated animals were sacrificed and lung, mediastinal lymph node, spleen, and kidney samples were tested by U3 hn-PCR; they were also negative. In each PCR assay, 5 to 10 500-ng replicates of calf thymus DNA were subjected to JSRV-specific U3 hn-PCR as additional negative controls, and they were always negative.
Synthesis of JSRV particles in vitro.
Since pJSRV21 contained an infectious provirus, we focused on it as a source of infectious virus for further experiments. Because no in vitro culture systems were available for JSRV, we attempted to recover virus particles by direct transfection of a derivative of pJSRV21 containing a simian virus 40 origin of replication into highly transfectable human 293T cells. This did not result in the production of detectable virus in the culture supernatants (data not shown). It seemed possible that the JSRV LTR was not active in 293T cells, and so we replaced the U3 region of the upstream LTR in pJSRV21 with the human CMV immediate-early promoter, which is highly active in these cells (Fig. 2b). The CMV promoter was positioned so that the resulting RNA transcript would be very similar to wild-type JSRV RNA. When the resulting plasmid (pCMV2JS21) was transfected into 293T cells, substantial amounts of JSRV21 virus were released into the supernatant. Western blot analysis for JSRV CA protein indicated that the amount of virions produced from transfected 293T cells was comparable to that present in lung fluid from SPA-affected sheep (Fig. 2c). Moreover, the fact that the supernatants from transfected 293T cells showed CA protein of the mature (cleaved) size strongly suggested that normal virion morphogenesis and polyprotein cleavage (presumably mediated by functional JSRV protease) took place. Enzymatically active RT could also be detected in the 293T cell supernatants by standard exogenous assays.
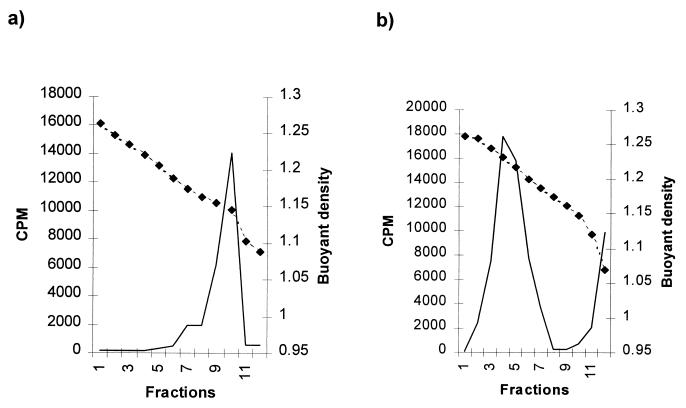
Supernatants from pCMV2JS21-transfected 293T cells were analyzed by isopycnic centrifugation in sucrose density gradients. Supernatants from transfected cells contained RT activity that could be measured by an exogenous RT assay with poly(rA)-oligo(dT) as the template primer. RT assays across the sucrose gradient indicated a peak of RT activity with a buoyant density of approximately 1.15 g/ml (Fig. 3a). This was consistent with the buoyant density of retroviruses in general (45), although it was slightly lower than that reported for JSRV (1.16 to 1.18 g/ml) when the virus was isolated directly from the lung secretions of SPA-affected animals (15, 28, 39, 48). Treatment of the 293T supernatants with 0.1% Triton X-100 prior to centrifugation shifted the RT peak to 1.218 to 1.238 g/ml, consistent with the release of viral cores (Fig. 3b) and suggesting that complete viral particles had been synthesized. Supernatants from mock-transfected 293T cells showed no RT activity (data not shown).
FIG. 3.
Buoyant-density analysis of JSRV21. (a) Intact JSRV21 particles prepared by transient transfection of 293T cells with pCMV2JS21 were analyzed by isopycnic centrifugation in a 25 to 60% (wt/wt) sucrose gradient. Adjacent fractions were pooled, and exogenous RT activity was determined (solid lines). The densities of each fraction are shown in grams per milliliter (dashed lines). (b) JSRV21 was treated with 0.1% Triton X-100 and analyzed as in panel a.
Experimental induction of SPA.
Four newborn black-face lambs were inoculated intratracheally with concentrated JSRV21 stocks obtained from transfected 293T cells. At 4 months postinoculation, one of the animals showed clinical signs of respiratory distress suggestive of SPA. All four animals were sacrificed at this time.
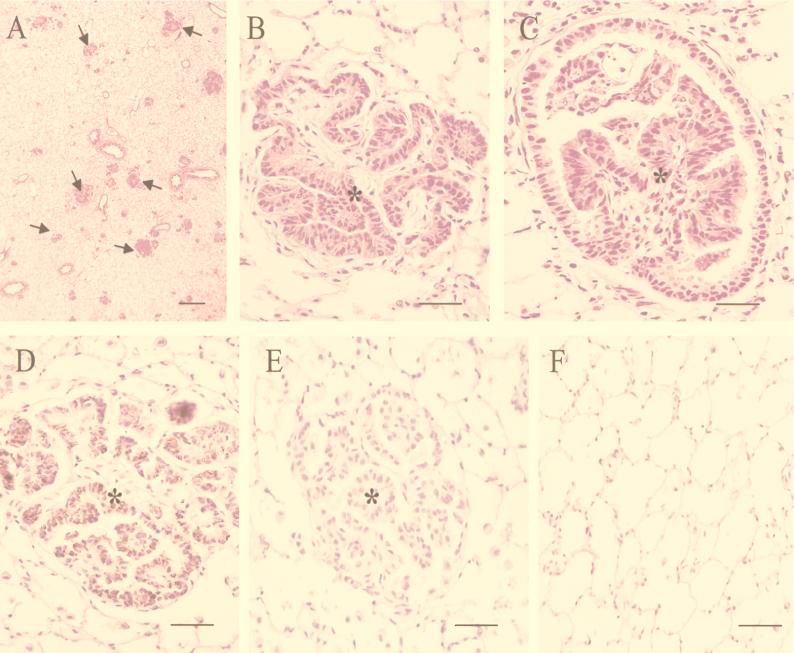
At necropsy, the lungs of the clinically affected lamb showed gross pathological changes typical of SPA. They were both considerably enlarged and heavier than normal due to extensive lesions in the dependent areas of the cranial, medial, and caudal lobes. The lesions had a reddish translucent appearance and were well demarcated from the unaffected dorsal areas of the lungs, although some isolated small foci were scattered throughout the lungs. At the margins of the lesions, small reddish white nodules, approximately 3 to 5 mm in diameter, were observed. Transverse sections of the affected areas were clearly consolidated, and a moderate amount of clear, foamy fluid exuded from the cut surface and airways, as seen in naturally occurring and experimentally transmitted SPA. A few small foci with similar features also were observed in the caudal lobes of a second lamb. Histologic examination revealed the presence of multifocal neoplastic foci in both of these animals (Fig. 4A). Lesions comprised many small intra-alveolar (Fig. 4B) and bronchiolar (Fig. 4C) papilliform proliferations of cuboidal or prismatic epithelial cells. Some of these neoplastic nodules had an interstitial myxoid or fibrotic appearance. Alveoli adjacent to tumor nodules contained a small number of alveolar macrophages. The above lesions were consistent with previously described features of SPA. To test if the tumors expressed JSRV protein, immunohistochemistry with an antiserum raised against JSRV CA protein was carried out (Fig. 4D and E). The tumor cells showed readily detectable staining for JSRV CA protein (reddish brown stain), while the surrounding normal tissue was negative for viral protein. As expected, two uninoculated control lambs showed no signs of disease, and at necropsy their lungs showed no signs of macroscopic or histologic SPA lesions, as well as no immunoreactive material (Fig. 4F).
FIG. 4.
Induction of SPA in JSRV21-infected lambs. (A to C) Lung tumor tissues from JSRV21-infected lambs were fixed in neutral 10% formalin, embedded in paraffin, and sectioned by routine procedures. Hematoxylin and eosin-stained lung tumor sections are shown. (A) Low-magnification micrograph (magnification, ×23; bar, 380 μm) showing many neoplastic foci in the microscopic field (some are indicated by arrows). (B) High-magnification micrograph (magnification, ×372; bar, 40 μm) of a neoplastic nodule with a clear papillary pattern (∗). Myxoid tissue containing cells with elongated or round nuclei is present in the interstitium of the neoplastic tissue. (C) Papillary proliferation (∗) occluding the lumen of a bronchiolus. Magnification, ×372; bar, 40 μm. (D to E) Immunohistochemistry for JSRV CA antigen developed with an avidin-biotin peroxidase complex kit (ABC; Vector Laboratories). Carazzi’s hematoxylin alone was used as counterstain. Magnification, ×372; bar, 40 μm. (D) A neoplastic focus (∗) is shown where most of the neoplastic cells have a brownish cytoplasmatic stain indicative of JSRV CA antigen after staining with a rabbit anti-CA antiserum as primary antibody. No staining is present in the cells infiltrating the tumor or in adjacent normal cells. (E) Rabbit preimmune serum was used as the primary antibody in lung tumor sections in parallel to those in panel D; no brown staining was visible in the tumor nodule (∗). (F) A lung section from an uninoculated lamb was tested for JSRV CA antigen under conditions similar to those in panel D; there were no antigen-positive cells.
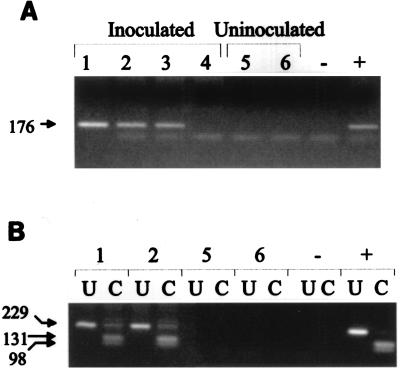
To further test if the tumors in the JSRV21-inoculated lambs resulted from exogenous viral infection, we tested tumor tissue DNA from the infected animals for the presence of exogenous JSRV21 DNA sequences. PCR was carried out with primers from the U3 region of the LTR that are specific for exogenous JSRV (30) (Fig. 5A). Lung tissues from both of the infected lambs with SPA lesions and from one of the infected animals that did not show SPA were positive for exogenous JSRV LTR sequences. As expected, no exogenous JSRV sequences were amplified from lung DNA from the uninoculated controls. To further confirm the presence of exogenous JSRV sequences in tumor DNA, we tested for an exogenous JSRV-specific ScaI site present in the gag region by performing hn-PCR that favors amplification of exogenous JSRV sequences on tissue DNAs followed by digestion of the PCR product with ScaI (27). As shown in Fig. 5B, DNA from the tumor tissues of both lambs that developed SPA showed evidence of exogenous JSRV, as indicated by the appearance of more rapidly migrating ScaI cleavage products in the gel. These results indicated that both tumors induced by JSRV21 infection contained exogenous JSRV sequences from the LTR and gag regions. This further supported the conclusion that JSRV21 induced the SPA lesions in the infected animals.
FIG. 5.
Exogenous viral DNA in JSRV21-induced tumors. (A) Lung DNA (500 ng) from four lambs (lanes 1 to 4) inoculated with JSRV21 was tested for exogenous virus sequences by PCR amplification with primers from the U3 region of the LTR that are specific for exogenous JSRV (30). Three of four inoculated lambs showed evidence of exogenous JSRV21 DNA in lung tissue, including the two with SPA tumors (lanes 1 and 2). As expected, no PCR product was evident in amplifications of lung DNA from the uninoculated control lambs (lanes 5 and 6). Other controls included distilled water in the PCR amplification (lane −) and pJSRV21 plasmid DNA in the amplification (lane +). (B) The presence of the exogenous virus-specific ScaI restriction site in gag was tested in the tumor DNAs of lambs 1 and 2. Lung tumor or normal lung DNA (500 ng) was subjected to LTR-gag hn-PCR followed by digestion with ScaI as described previously (27). The products were then analyzed by agarose gel electrophoresis. U, uncut PCR product; C, PCR product cut with ScaI. Both lambs 1 and 2 showed the ScaI restriction site specific for the exogenous JSRV sequences; lambs 5 and 6 showed no PCR products. PCR controls included distilled water (lanes −) and pJSRV21 plasmid DNA (lanes +). Molecular weight markers are indicated on the side of both panels (in base pairs).
DISCUSSION
These experiments show conclusively for the first time that JSRV is necessary and sufficient to induce SPA. This was accomplished by molecular cloning of an infectious JSRV provirus from an animal with a spontaneous SPA case, recovering infectious virus from the clone by transient transfection of pCMV2JS21 plasmid into 293T cells, and infecting lambs with this virus. The resulting tumors (which developed in the same time frame as for experimentally induced SPA) were histologically identical to those of spontaneous and experimentally induced SPA, and the tumor cells expressed JSRV CA antigen. Moreover, the tumor tissue showed evidence of infection by the exogenous JSRV21 by two criteria: exogenous JSRV-specific PCR in the LTR, and the presence of an exogenous JSRV-specific ScaI site in the gag region. Thus, it can be concluded that JSRV is the etiologic agent of SPA.
Future studies on the oncogenesis and pathogenesis of SPA will greatly benefit from the availability of the infectious and pathogenic JSRV21 molecular clone. It is now possible for the first time to produce JSRV infectious particles in vitro without relying on material collected from naturally or experimentally SPA-affected sheep. The manipulation of the JSRV21 genome will allow us to investigate the oncogenic determinants of this virus and will facilitate studies of the mechanisms of carcinogenesis. Many studies of the virus that were previously limited by difficulty in obtaining viral preparations in sufficient quantity and purity are now feasible.
It should be emphasized that the infectious JSRV21 was obtained by transient transfection of pCMV2JS21 plasmid DNA into human 293T cells. While the virus obtained by this technique is infectious and should be identical to that obtained from infected ovine tumor tissues (since the RNA transcribed in the transfected 293T cells is identical to genomic JSRV21 RNA), there was an additional advantage. It has been shown that uninfected sheep cells carry several copies of highly related endogenous JSRV DNA but that human DNA does not contain cross-hybridizing sequences (14, 47). Thus, the potential for recombination between the exogenous JSRV21 genome and ovine endogenous JSRV-related viruses during generation of JSRV21 virus stocks was eliminated. Moreover, the fact that the virus was obtained by a transient transfection further minimized the likelihood of any low-level genetic interaction between the JSRV21 genome and potential distantly related (nonhybridizing) endogenous viruses of humans.
Animal retroviruses have provided great insights into steps in oncogenesis for both animal and human cancers (34, 44). However, with the notable exception of murine mammary tumor virus (26, 35, 41), most oncogenic retroviruses typically induce tumors of the hematopoietic system. JSRV is unique among retroviruses in transforming lung epithelial cells (type II pneumocytes and Clara cells). The strong resemblance of human BAC and ovine SPA (18, 31) suggests that studies of JSRV oncogenesis may also provide new insights into the development of human BAC. SPA is a naturally occurring disease of an outbred animal species and therefore may be a particularly useful animal model for the human disease.
Interestingly, two other JSRV-related retroviruses of small ruminants, enzootic nasal tumor virus of sheep and goats (6–8, 43), are associated with tumors of the ethmoid turbinates that arise from secretory epithelial cells. Thus, small-ruminant type D retroviruses could offer novel insight into oncogenic mechanisms in secretory epithelial cells.
Other oncogenic retroviruses exert their pathogenic effects by carrying transforming genes (oncogenes) or by insertionally activating cellular proto-oncogenes (34). It is noteworthy that JSRV induces lung cancer in sheep quite rapidly: 4 months in these experiments and as quickly as 3 to 4 weeks in previous experiments with uncloned virus (38, 42). Moreover, the pattern of the tumor cells was more consistent with multifocal disease (Fig. 4A). By analogy to other retrovirus complexes that induce disease rapidly, it initially seemed possible that a defective acute transforming retrovirus carrying a viral oncogene was the cause of the SPA tumors. However, the cloned JSRV21 can induce disease within the same time frame as field isolates. Thus, the oncogenic potential for SPA is contained within the JSRV21 sequences, even though no obvious oncogenes with homology to cellular proto-oncogenes are present (but see below). On the other hand, insertional activation of proto-oncogenes is typically associated with multiple rounds of infection, high viral loads, and long incubation periods. Previous results suggest that JSRV oncogenesis may not fit this paradigm either. In animals with spontaneous or experimentally induced SPA, the only cells in which JSRV protein can be detected are the tumor cells themselves. In particular, in these animals, normal lung epithelial cells (the targets for transformation) do not show detectable viral antigen. Also, in experimentally infected animals, viral DNA in circulating blood cells can be detected only by extremely sensitive nested PCR techniques (16), and there is no evidence for viral expression. Thus, it will be extremely interesting to determine the mechanism of oncogenesis for JSRV.
As described in Results, the JSRV genome carries an alternate open reading frame (orf-x) overlapping pol. This reading frame shows no homologies to any other known gene (viral or cellular), and its function remains to be determined. The fact that orf-x is conserved as an open reading frame for both the South African and British isolates of JSRV (JSRV-SA and JSRV21) strongly suggests that it plays a role in viral replication, oncogenesis, or both. We are currently addressing this by site-directed mutagenesis of orf-x in JSRV21.
Another area of interest is the strict association of JSRV expression with cells of the lungs (28). In vivo, JSRV infects several cell types (16, 28, 30), but viral antigens can be detected in great abundance only in the epithelial tumor cells of the lungs. Perhaps the JSRV LTR contains enhancers specific for the cells in which the tumor originates (type II pneumocytes, Clara cells, and/or a common precursor). This possibility is currently being tested. Once the molecular basis of the association between epithelial respiratory cells and JSRV infection and expression is understood, it might be possible to design new retroviral vectors that are specifically expressed in these cells. This would be particular useful given the difficulty in transducing airway epithelial cells by many viral and nonviral vectors (10, 11, 25, 32).
ACKNOWLEDGMENTS
We thank S. Cheng (Genzyme) for providing the cationic lipid GL-67, L. Gonzales for performing postmortem examinations, C. Lee and P. Dewar for providing technical assistance, and M. Graham for providing animal care.
M.P. is a recipient of a Wellcome Prize Travelling Research Fellowship. Additional funding was provided by SOAFD (to J.M.S.) and by the Comisión Interministerial de Ciencia y Tecnología (AGF96-0535-C02-01, to M.H.). Support from the UCI Cancer Research Institute and the Chao Family Comprehensive Cancer Center is acknowledged.
REFERENCES
- 1.Bai J, Zhu R-Y, Stedman K, Cousens C, Carlson J, Sharp J M, DeMartini J C. Unique long terminal repeat U3 sequences distinguish exogenous jaagsiekte sheep retroviruses associated with ovine pulmonary carcinoma from endogenous loci in the sheep genome. J Virol. 1996;70:3159–3168. doi: 10.1128/jvi.70.5.3159-3168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkley J E, Green M R. Bronchioloalveolar carcinoma. J Clin Oncol. 1996;14:2377–2386. doi: 10.1200/JCO.1996.14.8.2377. [DOI] [PubMed] [Google Scholar]
- 3.Barsky S H, Cameron R, Osann K E, Tomita D, Holmes E C. Rising incidence of bronchioloalveolar lung carcinoma and its unique clinicopathologic features. Cancer. 1994;73:1163–1170. doi: 10.1002/1097-0142(19940215)73:4<1163::aid-cncr2820730407>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Carney D N, De Leij L. Lung cancer biology. Semin Oncol. 1988;15:199–214. [PubMed] [Google Scholar]
- 5.Clayton F. The spectrum and significance of bronchioloalveolar carcinomas. Pathol Annu. 1988;23:361–394. [PubMed] [Google Scholar]
- 6.Cousens C, Minguijon E, Garcia M, Ferrer L M, Dalziel R G, Palmarini M, De las Heras M, Sharp J M. PCR-based detection and partial characterization of a retrovirus associated with contagious intranasal tumors of sheep and goats. J Virol. 1996;70:7580–7583. doi: 10.1128/jvi.70.11.7580-7583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De las Heras M, Sharp J M, Ferrer L M, Garcia de Jalon J A, Cebrian L M. Evidence for a type D-like retrovirus in enzootic nasal tumour of sheep. Vet Rec. 1993;132:441. doi: 10.1136/vr.132.17.441. [DOI] [PubMed] [Google Scholar]
- 8.De las Heras M, Sharp J M, Garcia de Jalon J A, Dewar P. Enzootic nasal tumour of goats: demonstration of a type D-related retrovirus in nasal fluids and tumours. J Gen Virol. 1991;72:2533–2535. doi: 10.1099/0022-1317-72-10-2533. [DOI] [PubMed] [Google Scholar]
- 9.Dhar R, McClements W L, Enquist L W, Vande Woude G F. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci USA. 1980;77:3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman M J, Lee P S, Yang J S, Wilson J M. Lentiviral vectors for gene therapy of cystic fibrosis. Hum Gene Ther. 1997;8:2261–2268. doi: 10.1089/hum.1997.8.18-2261. [DOI] [PubMed] [Google Scholar]
- 11.Grubb B R, Pickles R J, Ye H, Yankaskas J R, Vick R N, Engelhardt J F, Wilson J M, Johnson L G, Boucher R C. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature. 1994;371:802–806. doi: 10.1038/371802a0. [DOI] [PubMed] [Google Scholar]
- 12.Hecht S J, Carlson J O, DeMartini J C. Analysis of a type D retroviral capsid gene expressed in ovine pulmonary carcinoma and present in both affected and unaffected sheep genomes. Virology. 1994;202:480–484. doi: 10.1006/viro.1994.1366. [DOI] [PubMed] [Google Scholar]
- 13.Hecht S J, Sharp J M, Demartini J C. Retroviral aetiopathogenesis of ovine pulmonary carcinoma: a critical appraisal. Br Vet J. 1996;152:395–409. doi: 10.1016/s0007-1935(96)80034-0. [DOI] [PubMed] [Google Scholar]
- 14.Hecht S J, Stedman K E, Carlson J O, DeMartini J C. Distribution of endogenous type B and type D sheep retrovirus sequences in ungulates and other mammals. Proc Natl Acad Sci USA. 1996;93:3297–3302. doi: 10.1073/pnas.93.8.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herring A J, Sharp J M, Scott F M, Angus K W. Further evidence for a retrovirus as the aetiological agent of sheep pulmonary adenomatosis (jaagsiekte) Vet Microbiol. 1983;8:237–249. doi: 10.1016/0378-1135(83)90076-7. [DOI] [PubMed] [Google Scholar]
- 16.Holland M J, Palmarini M, Garcia-Goti M, Gonzalez L, McKendrick I, De las Heras M, Sharp J M. Jaagsiekte retrovirus is widely distributed in both T and B lymphocytes and in mononuclear phagocytes of sheep with naturally and experimentally acquired pulmonary adenomatosis. J Virol. 1999;73:4004–4008. doi: 10.1128/jvi.73.5.4004-4008.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes S H, Mutschler A, Bishop J M, Varmus H E. A Rous sarcoma virus provirus is flanked by short direct repeats of a cellular DNA sequence present in only one copy prior to integration. Proc Natl Acad Sci USA. 1981;78:4299–4303. doi: 10.1073/pnas.78.7.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ives J C, Buffler P A, Greenberg S D. Environmental associations and histopathologic patterns of carcinoma of the lung: the challenge and dilemma in epidemiologic studies. Am Rev Respir Dis. 1983;128:195–209. doi: 10.1164/arrd.1983.128.1.195. [DOI] [PubMed] [Google Scholar]
- 19.Ju G, Skalka A M. Nucleotide sequence analysis of the long terminal repeat (LTR) of avian retroviruses: structural similarities with transposable elements. Cell. 1980;22:379–386. doi: 10.1016/0092-8674(80)90348-7. [DOI] [PubMed] [Google Scholar]
- 20.Kajikawa O, Dahlberg J E, Rosadio R H, De Martini J C. Detection and quantitation of a type D retrovirus gag protein in ovine pulmonary carcinoma (sheep pulmonary adenomatosis) by means of a competition radioimmunoassay. Vet Microbiol. 1990;25:17–28. doi: 10.1016/0378-1135(90)90089-e. [DOI] [PubMed] [Google Scholar]
- 21.Landis S H, Murray T, Bolden S, Wingo P A. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- 22.Lee E R, Marshall J, Siegel C S, Jiang C, Yew N S, Nichols M R, Nietupski J B, Ziegler R J, Lane M B, Wang K X, Wan N C, Scheule R K, Harris D J, Smith A E, Cheng S H. Detailed analysis of structures and formulations of cationic lipids for efficient gene transfer to the lung. Hum Gene Ther. 1996;7:1701–1717. doi: 10.1089/hum.1996.7.14-1701. [DOI] [PubMed] [Google Scholar]
- 23.Majors J E, Varmus H E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981;289:253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- 24.Martin W B, Scott F M, Sharp J M, Angus K W, Norval M. Experimental production of sheep pulmonary adenomatosis (jaagsiekte) Nature. 1976;264:183–185. doi: 10.1038/264183a0. [DOI] [PubMed] [Google Scholar]
- 25.Matsui T, Ozaki S, Watanabe Y. On the formation and reactivity of compound I of the His-64 myoglobin mutants. J Biol Chem. 1997;272:32735–32738. doi: 10.1074/jbc.272.52.32735. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzawa A. Biology of mouse mammary tumour virus (MMTV) Cancer Lett. 1994;90:3–11. doi: 10.1016/0304-3835(94)03671-5. [DOI] [PubMed] [Google Scholar]
- 27.Palmarini M, Cousens C, Dalziel R G, Bai J, Stedman K, DeMartini J C, Sharp J M. The exogenous form of jaagsiekte retrovirus is specifically associated with a contagious lung cancer of sheep. J Virol. 1996;70:1618–1623. doi: 10.1128/jvi.70.3.1618-1623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmarini M, Dewar P, De las Heras M, Inglis N F, Dalziel R G, Sharp J M. Epithelial tumour cells in the lungs of sheep with pulmonary adenomatosis are major sites of replication for jaagsiekte retrovirus. J Gen Virol. 1995;76:2731–2737. doi: 10.1099/0022-1317-76-11-2731. [DOI] [PubMed] [Google Scholar]
- 29.Palmarini M, Fan H, Sharp J M. Sheep pulmonary adenomatosis: a unique model of retrovirus-associated lung cancer. Trends Microbiol. 1997;5:478–483. doi: 10.1016/S0966-842X(97)01162-1. [DOI] [PubMed] [Google Scholar]
- 30.Palmarini M, Holland M J, Cousens C, Dalziel R G, Sharp J M. Jaagsiekte retrovirus establishes a disseminated infection of the lymphoid tissues of sheep affected by pulmonary adenomatosis. J Gen Virol. 1996;77:2991–2998. doi: 10.1099/0022-1317-77-12-2991. [DOI] [PubMed] [Google Scholar]
- 31.Perk K, Hod I. Sheep lung carcinoma: an endemic analogue of a sporadic human neoplasm. JNCI. 1982;69:747–749. [PubMed] [Google Scholar]
- 32.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Querat, G. Personal communication.
- 33.Rosadio R H, Lairmore M D, Russell H I, DeMartini J C. Retrovirus-associated ovine pulmonary carcinoma (sheep pulmonary adenomatosis) and lymphoid interstitial pneumonia. I. Lesion development and age susceptibility. Vet Pathol. 1988;25:475–483. doi: 10.1177/030098588802500611. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg N, Jolicoeur P. Retroviral pathogenesis. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 475–585. [PubMed] [Google Scholar]
- 35.Ross S R, Dzuris J L, Golovkina T V, Clemmons W C, van den Hoogen B. Mouse mammary tumor virus (MMTV), a retrovirus that exploits the immune system. Genetics of susceptibility to MMTV infection. Medicina. 1997;57:34–42. [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Sharp J M, Angus K. Sheep pulmonary adenomatosis: studies on its etiology. In: Petursson G, Hoff-Jogensen R, editors. Maedi-Visna and related diseases. Boston, Mass: Kluwer Academic Publishers; 1990. pp. 177–185. [Google Scholar]
- 38.Sharp J M, Angus K W, Gray E W, Scott F M. Rapid transmission of sheep pulmonary adenomatosis (jaagsiekte) in young lambs. Brief report. Arch Virol. 1983;78:89–95. doi: 10.1007/BF01310861. [DOI] [PubMed] [Google Scholar]
- 39.Sharp J M, Herring A J. Sheep pulmonary adenomatosis: demonstration of a protein which cross-reacts with the major core proteins of Mason-Pfizer monkey virus and mouse mammary tumour virus. J Gen Virol. 1983;64:2323–2327. doi: 10.1099/0022-1317-64-10-2323. [DOI] [PubMed] [Google Scholar]
- 40.Van Beveren C, Goddard J G, Berns A, Verma I M. Structure of Moloney murine leukemia viral DNA: nucleotide sequence of the 5′ long terminal repeat and adjacent cellular sequences. Proc Natl Acad Sci USA. 1980;77:3307–3311. doi: 10.1073/pnas.77.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Leeuwen F, Nusse R. Oncogene activation and oncogene cooperation in MMTV-induced mouse mammary cancer. Cancer Biol. 1995;6:127–133. doi: 10.1006/scbi.1995.0018. [DOI] [PubMed] [Google Scholar]
- 42.Verwoerd D W, Williamson A L, De Villiers E M. Aetiology of jaagsiekte: transmission by means of subcellular fractions and evidence for the involvement of a retrovirus. Onderstepoort J Vet Res. 1980;47:275–280. [PubMed] [Google Scholar]
- 43.Vitellozzi G, Mughetti L, Palmarini M, Mandara M T, Mechelli L, Sharp J M, Manocchio I. Enzootic intranasal tumour of goats in Italy. Zentbl Vetmed Reihe B. 1993;40:459–468. doi: 10.1111/j.1439-0450.1993.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 44.Vogt P K. Historical introduction to the general properties of retroviruses. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 1–25. [PubMed] [Google Scholar]
- 45.Vogt V M. Retroviral virions and genomes. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 27–69. [PubMed] [Google Scholar]
- 46.Wingo P A, Ries L A, Rosenberg H M, Miller D S, Edwards B K. Cancer incidence and mortality, 1973–1995: a report card for the U.S. Cancer. 1998;82:1197–1207. doi: 10.1002/(sici)1097-0142(19980315)82:6<1197::aid-cncr26>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 47.York D F, Vigne R, Verwoerd D W, Querat G. Nucleotide sequence of the jaagsiekte retrovirus, an exogenous and endogenous type D and B retrovirus of sheep and goats. J Virol. 1992;66:4930–4939. doi: 10.1128/jvi.66.8.4930-4939.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.York D F, Vigne R, Verwoerd D W, Querat G. Isolation, identification, and partial cDNA cloning of genomic RNA of jaagsiekte retrovirus, the etiological agent of sheep pulmonary adenomatosis. J Virol. 1991;65:5061–5067. doi: 10.1128/jvi.65.9.5061-5067.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.York D F, Williamson A, Barnard B J, Verwoerd D W. Some characteristics of a retrovirus isolated from transformed bovine cells. Virology. 1989;171:394–400. doi: 10.1016/0042-6822(89)90607-7. [DOI] [PubMed] [Google Scholar]