Abstract
Background/Objectives: Although oxytocin administration is recommended for delayed labor progress, there is no consensus over the preferred optimal dose of oxytocin. We aimed to perform a meta-analysis of pregnancy outcomes comparing high-dose versus low-dose oxytocin regimens for augmentation of delayed labor. Methods: PubMed, Embase, and Cochrane databases were systematically searched for studies comparing high-dose with low-dose oxytocin for labor augmentation from inception up to May 2023. The outcomes assessed were cesarean rate, instrumental delivery rate, postpartum hemorrhage, neonatal death, and uterine tachysystole. Subgroup analysis was performed with randomized controlled trials (RCTs) and propensity-matched studies. Statistical analysis was performed using Rstudio. Heterogeneity was assessed with I2 statistics, and a random-risk effect was used if I2 > 50%. Results: Twenty-one studies met inclusion criteria, and eighteen were RCTs. A total of 14.834 patients were included, of whom 7.921 (53.3%) received high-dose and 6.913 (46.6%) received low-dose oxytocin during labor augmentation. No statistical differences were found in cesarean delivery, neonatal mortality, postpartum hemorrhage and vaginal instrumentation rate. However, uterine tachysystole incidence was significantly higher with high-dose oxytocin (95% Cl, 1.30–1.94, p = 0.3; 0.6; I2 = 9%). Conclusions: Labor augmentation with a low-dose oxytocin regimen is effective as with a high-dose regimen, but with significantly less uterine tachysystole events, which can lead to intrauterine and neonatal complications. Our findings suggest that a low-dose regimen may be safe and effective for labor augmentation in medical practice.
Keywords: high-dose, low-dose, oxytocin, labor augmentation
1. Introduction
Prolonged labor is a frequent obstetric complication associated with maternal and perinatal morbidity and mortality [1]. Various conditions can contribute to its development, including abnormal fetal presentation, cephalopelvic disproportion, inadequate uterine contractions, and alterations in maternal soft tissue [2]. Currently, prolonged labor represents one of the main indications for cesarean section, a widely used surgical procedure. In this context, the search for less invasive interventions becomes crucial to reduce unnecessary cesarean rates [3]. Amniotomy and oxytocin are traditional methods used to stimulate uterine contractions in cases of dysfunctional labor [4].
Oxytocin, a hormone produced in the hypothalamus, acts in the hormonal cascade of labor, promoting an increase in the frequency, duration, and intensity of uterine contractions after the spontaneous onset of labor [5]. The stimulation of this hormone occurs directly on the smooth muscle and is physiologically increased during labor and immediately after childbirth, at which time the oxytocin receptors in the myometrium are increased [6,7].
Since the 1960s, synthetic oxytocin, commonly known as pitocin, has been widely used for labor induction and management. This medication plays a crucial role in obstetric care by aiming to promote efficient uterine contractions, favorable cervical modification, and the progression of labor towards vaginal birth [8]. By mimicking the natural hormone oxytocin, pitocin stimulates the uterus, helping to initiate and regulate contractions. This can be particularly beneficial in cases where labor needs to be induced for medical reasons, such as when a pregnancy extends beyond the due date, or if there are concerns about the health of the mother or baby [8]. The use of synthetic oxytocin can also help manage labor that is progressing too slowly. In such scenarios, it can enhance the frequency and intensity of contractions, reducing the likelihood of complications and the need for cesarean delivery [9]. Pitocin is administered intravenously, allowing healthcare providers to control the dosage precisely and adjust it according to the laboring woman’s needs and responses. Despite its widespread use and benefits, the administration of synthetic oxytocin requires careful monitoring to avoid potential risks, such as uterine hyperstimulation, which can lead to fetal distress or other complications [9].
However, oxytocin administration may be associated with adverse maternal effects, such as headache, nausea, vomiting, bradycardia, tachycardia, and cardiac arrhythmias [9]. Additionally, it can induce fetal distress, potentially culminating in hypoxia, fetal or neonatal death, and hyponatremia in the newborn [10]. A recent study demonstrated an association between oxytocin administered during labor and an increased risk of postpartum hemorrhage [11].
The optimal dose of oxytocin for stimulating contractions in cases of dysfunctional labor remains a subject of debate [12]. High doses may shorten the duration of labor, but at the cost of potentially dangerous side effects. These include uterine hypertonicity (excessive muscle tone), uterine rupture, and fetal hypoxia [12]. Conversely, low doses, while seemingly safer, might not be effective in managing prolonged labor. This dilemma underlines a critical question: can we find a middle ground between achieving efficient labor progression and ensuring the safety of both mother and baby? This ongoing debate highlights the need for further research to determine the most effective and safe oxytocin dosing strategies for labor augmentation. By pinpointing the “sweet spot” between efficacy and safety, we can strive for optimal birth outcomes for both mothers and newborns.
This systematic review and meta-analysis endeavor to delve deeper into this debate. We will meticulously analyze recently published randomized controlled trials (RCTs) to assess the safety and efficacy of high- versus low-dose oxytocin protocols for labor augmentation. Our investigation will encompass not only the success rates of vaginal delivery but also delve into crucial maternal and neonatal indicators of well-being. By critically appraising the current evidence, this meta-analysis aspires to illuminate the optimal path for oxytocin dosing in labor augmentation, ultimately promoting a smoother and safer birthing experience for mothers and their newborns.
2. Materials and Methods
2.1. Protocol and Registration
This meta-analysis was performed according to the guidelines of the declaration Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (Tables S1 and S2) and the recommendations of the Cochrane Collaboration [13]. This review was registered on the Prospective International Registry of Systematic Reviews—PROSPERO (https://www.crd.york.ac.uk/, accessed on 27 January 2024) as number CRD42024521779.
2.2. Eligibility Criteria
Eligibility criteria: (1) randomized controlled trials (RCTs); (2) those comparing high-dose with low-dose oxytocin for labor augmentation; (3) involving patients aged 18 years or older who were pregnant women in spontaneous labor needing oxytocin augmentation due to delayed or slow progress of labor; (4) clinical outcomes of interest. Excluded were studies with overlapping populations, those employing medications in combination with others, non-randomized clinical trials, case reports, reviews, opinion pieces, technical reports, guidelines, animal studies, and in vitro experiments.
2.3. Search Strategy
We conducted a systematic search of published studies on PubMed, Cochrane and Embase. Additionally, our search extended to abstracts, articles, and scientific presentations. The database searches were carried out in May 2023. For each selected database, both Medical Subject Headings (MeSH) terms and input terms were adapted according to specific syntax rules, utilizing Boolean connectors (OR, AND). To ensure the inclusion of additional studies, we evaluated the references of the included articles and systematic reviews of the literature. Furthermore, an alert was set up in each database for notifications regarding the publication of studies relevant to our search criteria.
The search strategy was executed by two authors (F.A.K. and F.C.A.M.). In pursuit of including further studies, we assessed the references and abstracts of the included articles.
Studies identified in the databases and references from articles were integrated into the reference management software (Rayyan version 1.1)8. Duplicates were removed through both automatic and manual screening. Titles and abstracts of the identified articles were independently reviewed by two authors (F.A.K. and F.C.A.M.), who also independently extracted data according to predefined search criteria and quality assessment protocols. In cases of discrepancy between reviewers, a third reviewer made the final decision on inclusion.
2.4. Data Extraction and Risk of Bias Assessment
To summarize the main findings, three authors (V.M.S., F.A.K., and M.E.C.S.) independently collated data extracted from the included articles, including authors and year, study design, characteristics of the patient sample (size, age, clinical and pathological data, and study group), assessment method, and conclusions regarding cesarean rate, instrumental delivery rate, postpartum hemorrhage, neonatal death, and uterine tachysystole.
We employed the Cochrane Collaboration tool for assessing the risk of bias in randomized trials (RoB 2) for the quality assessment of individual randomized controlled trials (RCTs) [12]. For each trial, a risk of bias score was assigned, indicating whether it was at a high, low, or unclear risk of bias across five domains: randomization process, deviations from intended interventions, missing outcomes, measurement of outcomes, and selection of reported results. The risk of bias was independently analyzed by two researchers (F.C.A.M. and F.A.K.). The discrepancy between reviewers was resolved in agreement by the two reviewers for the final decision. We present in Figure S1 a summary of the risks of bias for the studies selected for this meta-analysis.
2.5. Endpoints and Definitions
The primary outcomes of interest were: (1) cesarean rate, (2) instrumental delivery rate, (3) postpartum hemorrhage, (4) neonatal death, and (5) uterine tachysystole.
2.6. Statistical Analysis
Researchers investigated the strength of associations between interventions and outcomes using odds ratios (ORs) and reported a range of certainty with 95% confidence intervals (CIs). To identify inconsistencies across studies (heterogeneity), Cochran’s Q-test and I2 statistic were employed. Studies with p-values below 0.10 and I2 exceeding 25% were considered to have significant variation [14]. The Sidik–Jonkman method was used to estimate the variation between studies (tau2). The DerSimonian and Laird random-effects models were implemented for all endpoints. Statistical analyses were performed using R software, version 4.4.1.
3. Results
3.1. Study Selection and Baseline Characteristics
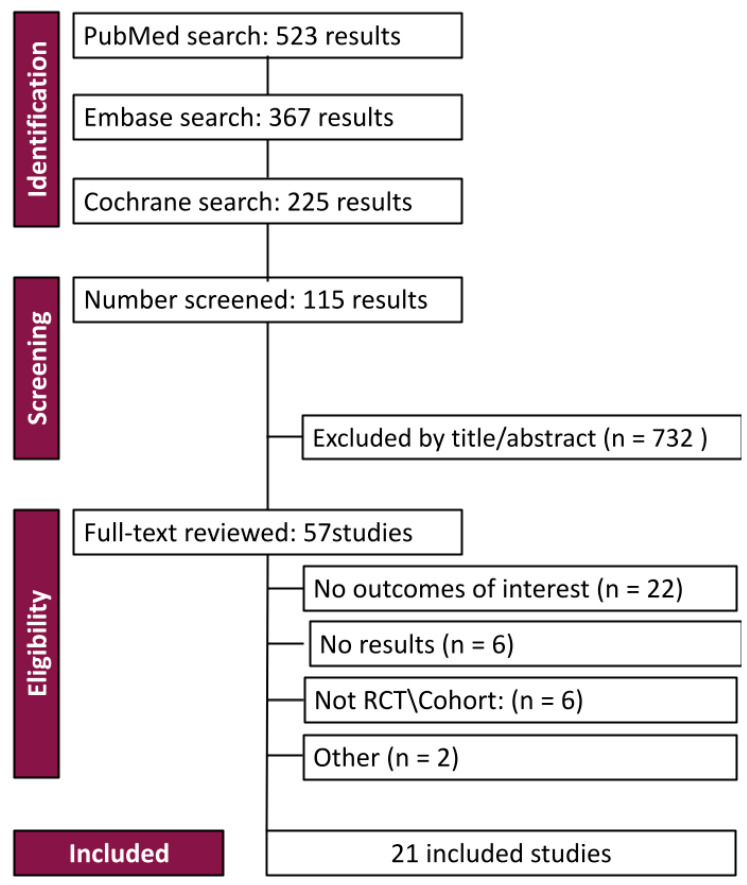
In the initial literature search, 1115 results were identified. After removing duplicates and ineligible studies, twenty-one studies remained, of which eighteen were RCTs, as seen in Figure 1.
Figure 1.
PRISMA flow diagram of study screening and selection.
After full review according to prespecified criteria, a total of 14.834 patients were evaluated through comparison of 7.921 (53.39%) women who received high doses of oxytocin with 6.913 patients (46.6%) who received low doses for labor augmentation. The mean age was reported as 26.47 years for the high-dose group and 27.61 years for the low-dose group. Moreover, the mean gestational age presented a minimal difference of 0.12 weeks between the groups. Nulliparous women constituted the majority of the participants, with a count of 12,207, while the initial oxytocin dosage averaged at 5.05 mU/min for the intervention group and 1.82 mU/min for the control group. Study characteristics are shown in Table 1 [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. We received no notifications in any of the databases that had set up alerts. No studies were included after the review.
Table 1.
Design and characteristics of studies included in the meta-analysis.
| Study, Year | Design | Intervention | Control | Gestational Age | Age (Mean) | Nullipara | Cervical Dilatation |
|---|---|---|---|---|---|---|---|
| Bidgood, 1987 [15] | RCT | high dose (19) | low dose (21) | >34 weeks | NI | NI | <0.5 cm/h |
| Frigoletto, 1995 [16] | RCT | high dose (678) | low dose (585) | ≥36 weeks | NI | I: 678 (100%) C:585 (100%) |
I: 3.3 ± 2.0 C: 3.6 ± 2.1 |
| Hourvitz, 1996 [17] | RCT | high dose (83) | low dose (65) | I: 41.0 ± 51.5 C: 40.7 ± 1.5 |
I: 28.4 ± 5.0 C: 28.7 ± 5.4 |
I: 47 (56.1%) C:34 (51.9%) |
I: 1.8 ± 0.9 C: 1.7 ± 0.9 |
| Jamal, 2004 [18] | RCT | high dose (100) | low dose (100) | I: 39.1 ± 1.3 C: 38.0 ± 1.3 |
I: 25.4 ± 4.9 C: 26.3 ± 5.1 |
I: 50 (50%) C:41 (41%) |
I: 3.5 ± 0.7 C: 3.7 ± 0.7 |
| Kenyon, 2013 [19] | RCT | high dose (47) | low dose (47) | ≥37 weeks | NI | I: 47 (100%) C:47 (100%) |
>4 cm |
| Liu, 2018 [20] | RCT | high dose (324) | low dose (162) | I: 278 ± 4 days C: 281 ± 5 days |
I: 25.53 ± 2.46 C: 25.46 ± 1.95 |
I: 138 (85%) C:142 (88%) |
>28 mm |
| Majoko, 2001 [21] | RCT | high dose (125) | low dose (133) | I: 39.2 ± 2.1 C: 39.7 ± 2.1 |
I: 20.2 ± 3.3 C: 20.4 ± 3.5 |
I: 125 (100%) C:133 (100%) |
I: 6.0 ± 1.6 C: 6.3 ± 1.7 |
| Manjula, 2015 [22] | RCT | high dose (100) | low dose (100) | I: 38.2 ± 1.07 C: 38.0 ± 1.11 |
I: 26.0 ± 3.4 C: 26.2 ± 3.7 |
I: 65 (65%) C:63 (63%) |
≥3 cm |
| Merril, 1999 [23] | double-blind RCT | high dose (249) | low dose (242) | I: 39.1 ± 0.1 C: 38.9 ± 0.1 |
I: 25.4 ± 0.4 C: 25.4 ± 0.4 |
I: 135 (54.2%) C:115 (47.5%) |
I: 4.7 ± 0.1 C: 5.0 ± 0.1 |
| Muller, 1992 [24] | RCT | high dose (70) | low dose (68) | I: 40.5 ± 1.6 C: 40.6 ± 1.5 |
I: 22.7 ± 5.7 C: 22.3 ± 5.3 |
I: 46 (65.7%) C:45 (66.2%) |
I: 1.7 ± 1.1 C:1.8 ± 1.1 |
| NCT, 2022 [25] | RCT | high dose (10) | low dose (10) | 38.7 ± 1.85 | 26.2 ± 5.26 | NI | NI |
| Neerukonda, 2018 [26] | RCT | high dose (200) | low dose (200) | I: 38.9 ± 0.88 C: 39.0 ± 0.86 |
I: 24.1 ± 3.5 C: 24.4 ± 3.2 |
I: 98 (49%) C:98 (49%) |
NI |
| Padmaja, 2022 [27] | RCT | high dose (90) | low dose (90) | I: 38.6 ± 2.5 C: 38.1 ± 1.2 |
I: 25.0 ± 4.0 C: 24.7 ± 4.1 |
I: 90 (100%) C:90 (100%) |
≥3 cm |
| Prichard, 2019 [28] | retrospective | high dose (2674) | low dose (2211) | I: 39.9 ± 1.30 C: 39.7 ± 1.30 |
I: 30.3 ± 4.7 C: 30.7 ± 4.6 |
I: 2674 (100%) C:2211 (100%) |
I: 645 (29.2%) C: 1016 (38%) |
| Selin, 2019 [29] | RCT | high dose (647) | low dose (648) | I: 29.9 ± 4.8 C: 29.9 ± 4.6 |
I: 29.0 ± 4.8 C: 29.0 ± 4.6 |
I: 647 (100%) C:648 (100%) |
I: 3.47 ± 1.55 C: 3.50 ± 1.60 |
| Satin, 1992 [30] | RCT | high dose (1537) | low dose (1252) | I: 39.0 0 ± 0.1 C: 39.0 0 ± 0.1 |
I: 22.7 0 ± 0.2 C: 22.9 ± 0.2 |
I: 938 (61%) C:751 (60%) |
≥3 cm I: 48% C:47% |
| Son, 2020 [31] | double-blind RCT | high dose (502) | standard dose (501) | I: 39.1 ± 0.8 C: 39.1 ± 0.7 |
I: 31.5 ± 4.4 C: 31.7 ± 4.3 |
I: 502 (100%) C:501 (100%) |
≥3 cm |
| Tesemma, 2020 [32] | cross-sectional | high dose (108) | low dose (108) | 39.4 weeks | 26 years | I: 56 (51.9%) C:32 (29.6%) |
≥4 cm |
| Toaff, 1978 [33] | prospective | pharmacological dose (134) | physiological dose (144) | I: 39.51 ± 0.95 C: 43.62 ± 4.27 |
I: 24- 1 ± 2.5 C: 23.2 ± 2.3 |
I: 64 (47.74%) C:62 (43.06%) |
NI |
| Wei, 2022 [34] | double-blind RCT | high dose (70) | low dose (70) | ≥37 weeks | I: 27.67 ± 4.86 C: 29.73 ± 5.54 |
I: 21 (30%) C:22 (31.43%) |
NI |
| Xenakis, 1995 [35] | RCT | high dose (154) | low dose (156) | I: 40.2 ± 1.6 C: 39.9 ± 2.0 |
I: 24.4 ± 5.9 C: 24.2 ± 6.9 |
I: 72 (46.75%) C:94 (60.26%) |
I: 5.4 cm C: 6.2 cm |
I: intervention; C: control; NI: not informed. Values in mean ± SD or n (%).
3.2. Cesarean
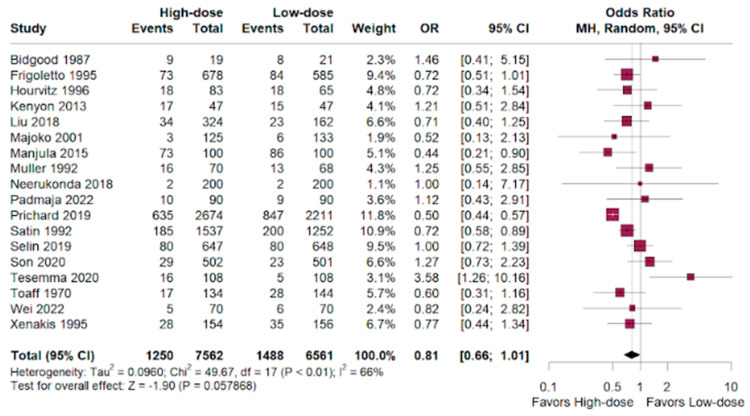
Cesarean section had a favorable outcome for the high-dosage group in patients randomized compared to the low-dose outcomes. (OR 0.81; 95% CI 0.66–1.01; p < 0.01; I2 = 66%; Figure 2).
Figure 2.
Cesarean outcomes of women who underwent labor augmentation using oxytocin, comparing high-dose versus low-dose treatments [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
3.3. Hemorrhage
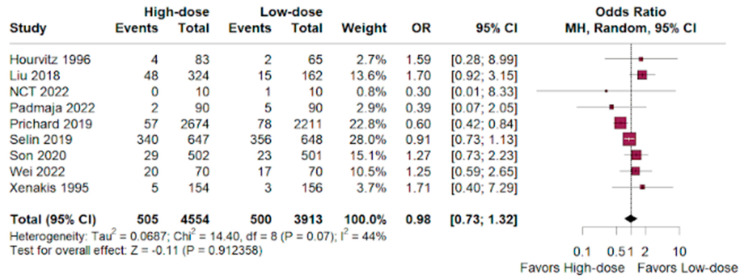
The hemorrhage odds ratio was nearly identical between both groups, with a slight lean towards the high-dose group versus low-dosage group. (OR 0.98; 95% CI 0.73–1.32; p < 0.01; I2 = 44%; Figure 3).
Figure 3.
Hemorrhage outcomes of women who underwent labor augmentation using oxytocin, comparing high-dose versus low-dose treatments [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
3.4. Neonatal Death
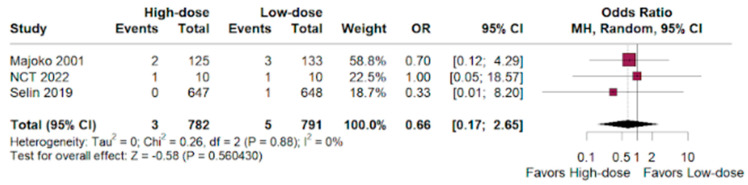
The odds ratio indicated prevalence for the high-dose group over the low-dose group in women receiving labor augmentation with oxytocin. (OR 0.66; 95% CI 0.17–2.65; p < 0.01; I2 = 0%; Figure 4).
Figure 4.
Neonatal deaths by newborns of women who underwent labor augmentation using oxytocin, comparing high-dose versus low-dose treatments [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
3.5. NICU
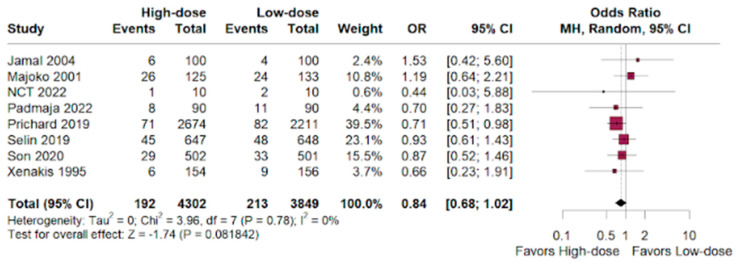
Among NICU admissions, there was a slight benefit for the high-dose group versus the low-dose group in women who underwent labor augmentation. (OR 0.84; 95% CI 0.68–1.02; p < 0.01; I2 = 0%; Figure 5).
Figure 5.
NICU admissions by newborns of women who underwent labor augmentation using oxytocin, comparing high-dose versus low-dose treatments [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
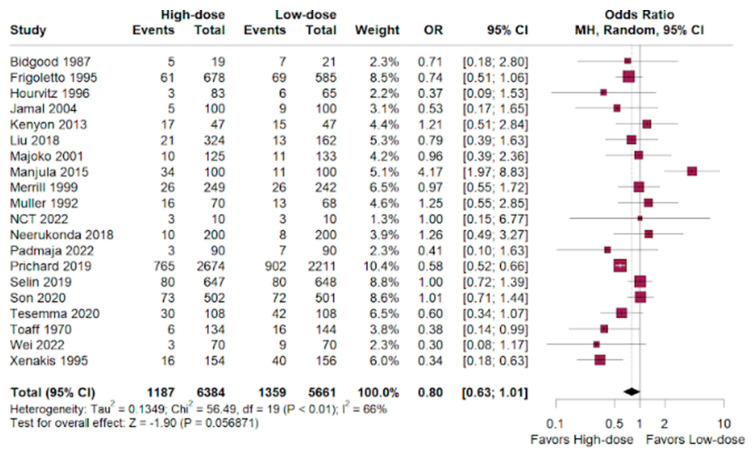
3.6. Instrumental Vaginal Delivery
The OR was slightly favorable for the high-dosage women over the low-dosage women, who were administered oxytocin for labor augmentation. (OR 0.80; 95% CI 0.63–1.01; p < 0.01; I2 = 66%; Figure 6).
Figure 6.
Instrumental vaginal delivery performed in women who underwent labor augmentation using oxytocin, comparing high-dose versus low-dose treatments [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
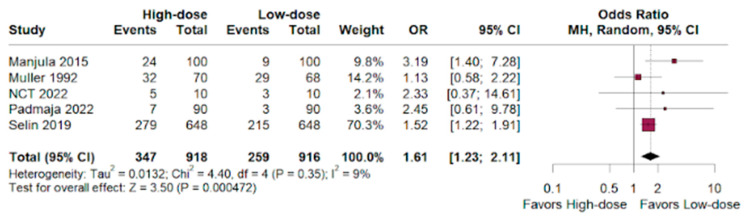
3.7. Uterine Tachysystole
The odds ratio for uterine tachysystoles showed a benefit for the low-dose group versus the high-dose group of women who were given oxytocin for labor augmentation (HR 1.61; 95% CI 1.23–2.11; p < 0.01; I2 = 9%; Figure 7).
Figure 7.
Uterine tachysystole in deliveries of women who underwent labor augmentation using oxytocin, comparing high-dose versus low-dose treatments [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35].
4. Discussion
This systematic review and meta-analysis of randomized controlled trials investigated the use of high-dose versus low-dose oxytocin for labor augmentation, analyzing data from 14.834 patients across 21 studies. While the primary focus of this work was on the cesarean delivery rate, the analysis also considered other relevant outcomes such as hemorrhage, neonatal death, NICU admission, instrumental vaginal delivery, and uterine tachysystole.
As previous studies had divergent conclusions [36,37], this analysis found unsubstantial differences in the cesarean delivery rate between the high-dose and low-dose oxytocin groups (OR 0.81; 95% CI 0.66–1.01; p < 0.01). Hemorrhage rates were similar between groups, with a slight non-significant tendency towards the high-dose group (OR 0.98; 95% CI 0.73–1.32; p < 0.01). High-dose oxytocin showed a potential advantage in terms of neonatal deaths, with a lower odds ratio compared to low-dose oxytocin (OR 0.66; 95% CI 0.17–2.65; p < 0.01), but the confidence interval was wide and the only eight events demonstrate that these benefits are statistically insignificant and hinder the validation of the results.
A insignificant benefit was observed for high-dose oxytocin for NICU admissions (OR 0.84; 95% CI 0.68–1.02; p < 0.01), and we observed an inconclusive advantage for the high-dose group compared to the low-dose group in achieving instrumental vaginal delivery (OR 0.80; 95% CI 0.63–1.01; p < 0.01). The high-dose group had a significantly higher risk of uterine tachysystole compared to the low-dose group (HR 1.61; 95% CI 1.23–2.11; p < 0.01).
Thus, our meta-analysis suggests that low-dose oxytocin might be a viable alternative to high-dose for labor augmentation. While high-dose oxytocin may offer a potential benefit in terms of a shorter duration of labor, it comes with a significantly increased risk of uterine tachysystole, which can diminish fetal oxygenation by reducing placenta blood flow [38]. Other results such as NICU admissions, cesarean delivery rates, and postpartum hemorrhages (blood loss of more than 500 mL on both vaginal and cesarean deliveries) [39] showed statistically insignificance, and considering the lack of significant differences in delivery outcomes and potential adverse events, low-dose oxytocin appears to be a safer and equally effective choice [40].
This meta-analysis has some limitations. First, the heterogeneity of some outcomes may reflect the high variability in the characteristics of the patients included in this study. Second, some characteristics such as race, location, previous diseases, and BMI were not available, which may have limited the generalizability of our results to different populations. However, these limitations did not prevent robust conclusions from being drawn, and low-dose oxytocin appears to be as effective as high-dose oxytocin for labor augmentation in terms of cesarean and instrumental delivery; it is also potentially safer due to a lower risk of uterine tachysystole.
Future studies are needed to confirm our results and further explore neonatal outcomes and women’s experiences; we must also assess the influence of parity and BMI on treatment outcomes. Additionally, it is crucial to investigate the long-term effects of administering low-dose oxytocin on both mothers and infants to ensure its safety and efficacy over extended periods. Furthermore, a deeper examination of the psychological and emotional impacts on women undergoing this treatment could provide a more comprehensive understanding of their overall well-being.
Moreover, research should aim to identify any potential variations in treatment outcomes across different demographic groups, considering factors such as age, ethnicity, and socioeconomic status. These studies could help in tailoring treatment protocols to better suit diverse populations. It would also be beneficial to conduct large-scale, multi-center trials to enhance the generalizability of the findings and to solidify the evidence base supporting the use of low-dose oxytocin.
In parallel, qualitative studies focusing on women’s subjective experiences and satisfaction with low-dose oxytocin treatment could offer valuable insights into patient-centered care approaches. Understanding the preferences and expectations of women could inform the development of guidelines that align more closely with their needs and improve overall treatment experiences.
However, this paper provides valuable insights into low-dose oxytocin as a potentially safer and comparably effective alternative to high-dose oxytocin. The findings suggest that low-dose oxytocin may reduce the risk of adverse effects associated with higher doses, while still achieving desirable clinical outcomes. This represents a significant step forward in optimizing labor induction practices and enhancing maternal and neonatal health.
5. Conclusions
This meta-analysis of randomized controlled trials provides convincing evidence that low-dose oxytocin can be as effective as high-dose oxytocin for labor augmentation. In addition, it has proven to be safer for use in medical practice, being effective without the increased risk of uterine tachysystoles observed with the high-dose regimen, which can lead to complications even if it extends the duration of labor. Moreover, to address the limitations of heterogeneity in outcomes and high variability in patient features, further research is needed to explore the optimal dosage of oxytocin that considers individual patient’s characteristics, risk factors, neonatal outcomes, and women’s experiences. Ultimately, the choice of the ideal oxytocin dosage should be individualized through a personalized approach, considering the potential benefits and risks in each case.
Acknowledgments
We acknowledge Universidade Federal do Pará (UFPA).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm14070724/s1. Table S1: PRISMA 2020 Checklist; Table S2: PRISMA 2020 for Abstract Checklist; Figure S1: Methodological quality summary using RoB2. A Overall risk of bias.
Author Contributions
Conceptualization: F.C.A.d.M., M.G.H.S.J.L. and L.D.M.; methodology: F.C.A.d.M. and M.G.H.S.J.L.; resources: F.C.A.d.M., V.M., F.A.K. and M.G.H.S.J.L.; data curation: M.G.H.S.J.L. and F.A.K.; writing—original draft preparation: F.C.A.d.M., F.A.K. and R.M.R.B.; writing—review and editing, R.M.R.B.; visualization: F.C.A.d.M.; supervision: F.C.A.d.M. and R.M.R.B.; project administration: F.C.A.d.M.; funding acquisition: F.C.A.d.M. and R.M.R.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Pró-Reitoria de Pesquisa e Pós-Graduação da UFPA (PROPESP).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pospiech K., Czajkowski K. Amniotic fluid lactate level as a diagnostic tool for prolonged labour. J. Mother Child. 2020;24:3–7. doi: 10.34763/jmotherandchild.20202403.2027.d-20-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matta P., Turner J., Flatley C., Kumar S. Prolonged second stage of labour increases maternal morbidity but not neonatal morbidity. Aust. N. Z. J. Obstet. Gynaecol. 2019;59:555–560. doi: 10.1111/ajo.12935. [DOI] [PubMed] [Google Scholar]
- 3.Miseljic N., Ibrahimovic S. Health Implications of Increased Cesarean Section Rates. Mater. Sociomed. 2020;32:123–126. doi: 10.5455/msm.2020.32.123-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upawi S.N., Ahmad M.F., Abu M.A., Ahmad S. Amniotomy and early oxytocin infusion vs amniotomy and delayed oxytocin infusion for labour augmentation amongst nulliparous women at term: A randomised controlled trial. Midwifery. 2022;105:103238. doi: 10.1016/j.midw.2021.103238. [DOI] [PubMed] [Google Scholar]
- 5.Iovino M., Messana T., Tortora A., Giusti C., Lisco G., Giagulli V.A., Guastamacchia E., De Pergola G., Triggiani V. Oxytocin Signaling Pathway: From Cell Biology to Clinical Implications. Endocr. Metab. Immune Disord. Drug Targets. 2021;21:91–110. doi: 10.2174/1871530320666200520093730. [DOI] [PubMed] [Google Scholar]
- 6.Hermesch A.C., Kernberg A.S., Layoun V.R., Caughey A.B. Oxytocin: Physiology, Pharmacology, and Clinical Application for Labor Management. Am. J. Obstet. Gynecol. 2023;230:S729–S739. doi: 10.1016/j.ajog.2023.06.041. [DOI] [PubMed] [Google Scholar]
- 7.Ferraz Barbosa B., de Moraes F.C.A., Araujo Alves da Silva B., Bordignon Barbosa C., Pereira da Silva I., da Silva E.R., Barros J.C.M., Rebouças L.W.C., Dos Santos N.P.C., Fernandes M.R. The Use of Honey for Cicatrization and Pain Control of Obstetric Wounds: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2024;16:185. doi: 10.3390/nu16020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson N., Ellis J., Page K., Dunn Amore A., Phillippi J. Review of Evidence-Based Methods for Successful Labor Induction. J. Midwifery Womens Health. 2021;66:459–469. doi: 10.1111/jmwh.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burguet A., Rousseau A. Oxytocin Administration during Spontaneous Labor: Guidelines for Clinical Practice. Chapter 6: Fetal, Neonatal and Pediatric Risks and Adverse Effects of Using Oxytocin Augmentation during Spontaneous Labor. J. Gynecol. Obstet. Hum. Reprod. 2017;46:523–530. doi: 10.1016/j.jogoh.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Hinshaw K., Simpson S., Cummings S., Hildreth A., Thornton J. A Randomised Controlled Trial of Early versus Delayed Oxytocin Augmentation to Treat Primary Dysfunctional Labour in Nulliparous Women. BJOG Int. J. Obstet. Gynaecol. 2008;115:1289–1295; discussion 1295–1296. doi: 10.1111/j.1471-0528.2008.01819.x. [DOI] [PubMed] [Google Scholar]
- 11.Bernitz S., Betran A.P., Gunnes N., Zhang J., Blix E., Øian P., Eggebøs T.M., Dalbye R. Association of oxytocin augmentation and duration of labour with postpartum haemorrhage: A cohort study of nulliparous women. Midwifery. 2023;123:103705. doi: 10.1016/j.midw.2023.103705. [DOI] [PubMed] [Google Scholar]
- 12.Mori R., Tokumasu H., Pledge D., Kenyon S. High Dose versus Low Dose Oxytocin for Augmentation of Delayed Labour. Cochrane Database Syst. Rev. 2011:CD007201. doi: 10.1002/14651858.CD007201.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bidgood K.A., Steer P.J. A Randomized Control Study of Oxytocin Augmentation of Labour. 1. Obstetric Outcome. Br. J. Obstet. Gynaecol. 1987;94:512–517. doi: 10.1111/j.1471-0528.1987.tb03142.x. [DOI] [PubMed] [Google Scholar]
- 16.Frigoletto F.D., Lieberman E., Lang J.M., Cohen A., Barss V., Ringer S., Datta S. A Clinical Trial of Active Management of Labor. N. Engl. J. Med. 1995;333:745–750. doi: 10.1056/NEJM199509213331201. [DOI] [PubMed] [Google Scholar]
- 17.Hourvitz A., Alcalay M., Korach J., Lusky A., Barkai G., Seidman D.S. A Prospective Study of High- versus Low-Dose Oxytocin for Induction of Labor. Acta Obstet. Gynecol. Scand. 1996;75:636–641. doi: 10.3109/00016349609054688. [DOI] [PubMed] [Google Scholar]
- 18.Jamal A., Kalantari R. High and Low Dose Oxytocin in Augmentation of Labor. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2004;87:6–8. doi: 10.1016/j.ijgo.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Kenyon S., Armstrong N., Johnston T., Walkinshaw S., Petrou S., Howman A., Cheed V., Markham C., McNicol S., Willars J., et al. Standard- or High-Dose Oxytocin for Nulliparous Women with Confirmed Delay in Labour: Quantitative and Qualitative Results from a Pilot Randomised Controlled Trial. BJOG Int. J. Obstet. Gynaecol. 2013;120:1403–1412. doi: 10.1111/1471-0528.12331. [DOI] [PubMed] [Google Scholar]
- 20.Liu J., Yi Y., Weiwei X. Effects of Increased Frequency, High Dose, and Pulsatile Oxytocin Regimens on Abnormal Labor Delivery. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018;24:2063–2071. doi: 10.12659/MSM.906728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majoko F. Effectiveness and Safety of High Dose Oxytocin for Augmentation of Labour in Nulliparous Women. Cent. Afr. J. Med. 2001;47:247–250. doi: 10.4314/cajm.v47i11.8624. [DOI] [PubMed] [Google Scholar]
- 22.Manjula B.G., Bagga R., Kalra J., Dutta S. Labour Induction with an Intermediate-Dose Oxytocin Regimen Has Advantages over a High-Dose Regimen. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2015;35:362–367. doi: 10.3109/01443615.2014.968103. [DOI] [PubMed] [Google Scholar]
- 23.Merrill D.C., Zlatnik F.J. Randomized, Double-Masked Comparison of Oxytocin Dosage in Induction and Augmentation of Labor. Obstet. Gynecol. 1999;94:455–463. doi: 10.1016/s0029-7844(99)00338-5. [DOI] [PubMed] [Google Scholar]
- 24.Muller P.R., Stubbs T.M., Laurent S.L. A Prospective Randomized Clinical Trial Comparing Two Oxytocin Induction Protocols. Am. J. Obstet. Gynecol. 1992;167:373–380; discussion 380–381. doi: 10.1016/S0002-9378(11)91415-X. [DOI] [PubMed] [Google Scholar]
- 25.Northwestern University Comparison of Low-Dose and High-Dose Oxytocin Regimens for Labor Augmentation (No. NCT02487797) [(accessed on 16 March 2024)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT02487797.
- 26.Neerukonda M.N., More S., Himabindu N. High Dose Oxytocin Versus Low Dose Oxytocin for Augmentation of Labor: A Prospective, Comparative, Randomized Study. Int. J. Med. Res. Health Sci. 2018;7:6–10. [Google Scholar]
- 27.Padmaja J. The Comparative Study Between High Dose Versus Low Dose Oxytocin for Augmentation of Labour Concerning Maternal and Fetal Outcome. [(accessed on 16 March 2024)]. Available online: https://ejmcm.com/issue-content/the-comparative-study-between-high-dose-versus-low-dose-oxytocin-for-augmentation-of-labour-concerning-maternal-and-fetal-outcome-5543.
- 28.Prichard N., Lindquist A., Hiscock R., Ruff S., Tong S., Brownfoot F.C. High-dose compared with low-dose oxytocin for induction of labour of nulliparous women at term. J. Matern. Fetal Neonatal Med. 2019;32:362–368. doi: 10.1080/14767058.2017.1378338. [DOI] [PubMed] [Google Scholar]
- 29.Selin L., Wennerholm U.-B., Jonsson M., Dencker A., Wallin G., Wiberg-Itzel E., Almström E., Petzold M., Berg M. High-Dose versus Low-Dose of Oxytocin for Labour Augmentation: A Randomised Controlled Trial. Women Birth J. Aust. Coll. Midwives. 2019;32:356–363. doi: 10.1016/j.wombi.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Satin A.J., Leveno K.J., Sherman M.L., Brewster D.S., Cunningham F.G. High- versus Low-Dose Oxytocin for Labor Stimulation. Obstet. Gynecol. 1992;80:111–116. [PubMed] [Google Scholar]
- 31.Son M., Roy A., Stetson B.T., Grady N.T., Vanecko M.C., Bond N., Swanson K., Grobman W.A., Miller E.S., Peaceman A.M. High-Dose Compared with Standard-Dose Oxytocin Regimens to Augment Labor in Nulliparous Women: A Randomized Controlled Trial. Obstet. Gynecol. 2021;137:991–998. doi: 10.1097/AOG.0000000000004399. [DOI] [PubMed] [Google Scholar]
- 32.Tesemma M.G., Sori D.A., Gemeda D.H. High Dose and Low Dose Oxytocin Regimens as Determinants of Successful Labor Induction: A Multicenter Comparative Study. BMC Pregnancy Childbirth. 2020;20:232. doi: 10.1186/s12884-020-02938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toaff M.E., Hezroni J., Toaff R. Induction of Labour by Pharmacological and Physiological Doses of Intravenous Oxytocin. Br. J. Obstet. Gynaecol. 1978;85:101–108. doi: 10.1111/j.1471-0528.1978.tb10461.x. [DOI] [PubMed] [Google Scholar]
- 34.Wei R.M., Bounthavong M., Hill M.G. High- vs Low-Dose Oxytocin in Lean and Obese Women: A Double-Blinded Randomized Controlled Trial. Am. J. Obstet. Gynecol. MFM. 2022;4:100627. doi: 10.1016/j.ajogmf.2022.100627. [DOI] [PubMed] [Google Scholar]
- 35.Xenakis E.M., Langer O., Piper J.M., Conway D., Berkus M.D. Low-Dose versus High-Dose Oxytocin Augmentation of Labor--a Randomized Trial. Am. J. Obstet. Gynecol. 1995;173:1874–1878. doi: 10.1016/0002-9378(95)90444-1. [DOI] [PubMed] [Google Scholar]
- 36.Boie S., Glavind J., Uldbjerg N., Steer P.J., Bor P. CONDISOX trial group Continued versus Discontinued Oxytocin Stimulation in the Active Phase of Labour (CONDISOX): Double Blind Randomised Controlled Trial. BMJ. 2021;373:n716. doi: 10.1136/bmj.n716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aboshama R.A., Abdelhakim A.M., Shareef M.A., AlAmodi A.A., Sunoqrot M., Alborno N.M., Gadelkarim M., Abbas A.M., Bakry M.S. High dose vs. low dose oxytocin for labor augmentation: A systematic review and meta-analysis of randomized controlled trials. J. Perinat. Med. 2020;49:178–190. doi: 10.1515/jpm-2020-0042. [DOI] [PubMed] [Google Scholar]
- 38.Leathersich S.J., Vogel J.P., Tran T.S., Hofmeyr G.J. Acute tocolysis for uterine tachysystole or suspected fetal distress. Cochrane Database Syst. Rev. 2018;7:CD009770. doi: 10.1002/14651858.CD009770.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofer S., Blaha J., Collins P.W., Ducloy-Bouthors A.S., Guasch E., Labate F., Lança F., Nyfløt L.T., Steiner K., Van de Velde M. Haemostatic support in postpartum haemorrhage: A review of the literature and expert opinion. Eur. J. Anaesthesiol. 2023;40:29–38. doi: 10.1097/EJA.0000000000001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavez M.P., Pasqualotto E., Ferreira R.O.M., Hohl A., de Moraes F.C.A., Schmidt P.H.S., Rodrigues A.L.S.d.O., de Sa J.R. Fezolinetant for the Treatment of Vasomotor Symptoms Associated with Menopause: A Meta-Analysis. Climacteric. 2024;27:245–254. doi: 10.1080/13697137.2024.2334083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article.