Abstract
Calcific aortic stenosis (AS) is a major cause of morbidity and mortality in high-income countries. AS presents sex-specific features impacting pathophysiology, outcomes, and management strategies. In women, AS often manifests with a high valvular fibrotic burden, small valvular annuli, concentric left ventricular (LV) remodeling/hypertrophy, and, frequently, supernormal LV ejection fraction coupled with diastolic dysfunction. Paradoxical low-flow low-gradient AS epitomizes these traits, posing significant challenges post-aortic valve replacement due to limited positive remodeling and significant risk of patient–prosthesis mismatch. Conversely, men present more commonly with LV dilatation and dysfunction, indicating the phenotype of classical low-flow low-gradient AS, i.e., with decreased LV ejection fraction. However, these distinctions have not been fully incorporated into guidelines for AS management. The only treatment for AS is aortic valve replacement; women are frequently referred late, leading to increased heart damage caused by AS. Therefore, it is important to reassess surgical planning and timing to minimize irreversible cardiac damage in women. The integrity and the consideration of sex differences in the management of AS is critical. Further research, including sufficient representation of women, is needed to investigate these differences and to develop individualized, sex-specific management strategies.
Keywords: aortic stenosis, sex differences, diagnosis, complications, outcomes, aortic valve replacement, patient–prosthesis mismatch
1. Introduction
Calcific aortic valve stenosis (AS) is the most common valvular heart disease requiring intervention in high-income countries [1] and the AS global burden is expected to grow in the next years due to the progressive aging of the population [2]. As medical research progresses, it is becoming increasingly clear that men and women do not share the same anatomic features, but they also show differences in the manifestation and progression of AS. These disparities raise important questions about diagnostic approaches, management, and sex-specific clinical management. Both the actual European [3] and American Guidelines [4] report class I indication for aortic valve replacement (AVR) for severe symptomatic AS or severe asymptomatic AS, if this latter is accompanied by left-ventricular (LV) dysfunction or coexists with an indication for other concomitant cardiac surgery. In asymptomatic severe AS there is a growing body of evidence regarding risk stratification through additional markers such as pro N-Type Brain Natriuretic Peptide dosing [5], the detection of very severe AS (peak aortic jet velocity (Vpeak) > 5 m/s or mean gradient > 60 mmHg) [6], and exercise stress testing [7], aiming to identify patients who could potentially benefit from early AVR (class II indications). In the context of asymptomatic AS, speckle tracking echocardiography with both LV [8,9] and left-atrial strain assessment have been revealed as risk stratification tools [10]. The research focus now extends to optimal management in both severe and non-severe AS, exploring evidence for early AVR in other specific scenarios [11]. A recent study from Généreux P. et al. highlights that the risk of mortality increases continuously with the AS severity degree, starting from mild and moderate forms [12], according to the findings of Strange et al. [13], suggesting the currently unmet optimized management of non-severe AS. The trial PROGRESS (NCT04889872) is ongoing, aiming to specifically evaluate the safety and effectiveness of transcatheter aortic valve replacement (TAVR) also in moderate AS. The consideration of pseudo-severe AS, a specific phenotype characterized by low flow, LV dysfunction and severely reduced aortic valvular area despite non-severe confirmation at second-line imaging, is important. While not a primary indication for AVR, a recent multicentric retrospective study by Ludwig S. et al. revealed that TAVR is a significant predictor of improved long-term outcomes among patients with pseudo-severe AS [14]. This emphasizes the necessity for further research also in this particular setting. All these described scenarios are focusing on researching the best early aortic intervention timing, but this aspect is facing some other big challenges. Recent findings underscore that women with severe symptomatic AS are often referred later for aortic valve intervention, leading to increased mortality and cardiovascular events [15]. This unfavorable condition is amplified by the evidence that more advanced extracardiac valvular damage [16], higher surgical risk scores [17], and concomitant low-flow statuses are observed in women undergoing AVR [18], so future research is warranted to equally optimize AS management in both sexes. The aim of this review is to focus specifically on the female-related AS context from a pathophysiological and clinical point of view, also encompassing the management of AS and considerations of possible future perspectives.
2. Aortic Valve Anatomy
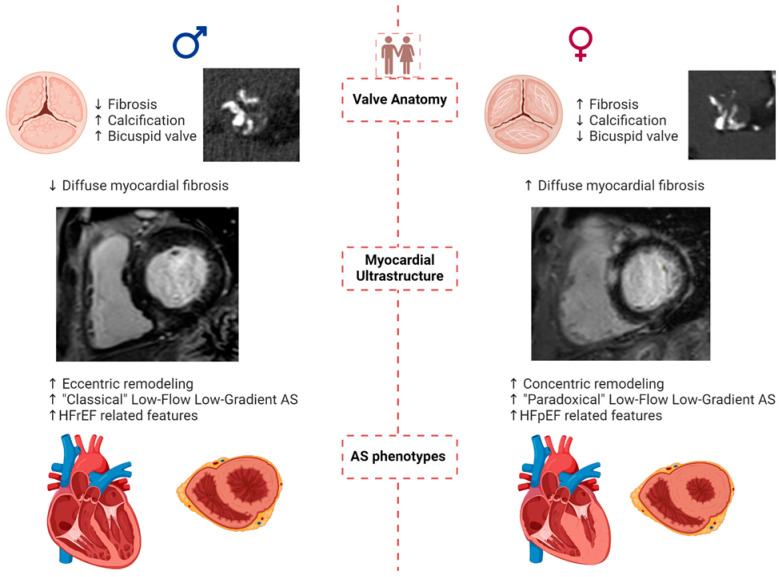
Women present a smaller aortic annulus than men and a shorter distance between the annulus and the coronary ostia, corresponding in part to their smaller body surface area [19]. As a matter of fact, even with similar body surface area, women have smaller hearts [20]. The incidence of AS is higher in males than in females for younger patients due to a higher prevalence of congenital bicuspid aortic valve (BAV), which has a male-to-female ratio of 3:1 (Figure 1) [21,22,23]. Based on systematic echocardiographic screening, the prevalence of BAV is estimated to be 0.6% to 0.8% in men and 0.2% in women [24,25]. Currently, no difference has been found in the distribution of phenotypic types of bicuspid valve morphology between the two sexes [26], but data remain insufficient regarding this topic.
Figure 1.
Pathophysiology and presentation of aortic stenosis in women and men. Legend: AS: aortic stenosis; HFrEF: Heart Failure with reduced Ejection Fraction; HFpEF: Heart Failure with preserved Ejection Fraction.
After the age of 75 years, the incidence of AS is reversed and slightly higher in women [27].
3. Calcification and Fibrosis of the Aortic Valve According to Sex
Valvular lesions in AS were, for a long time, considered to be similar between sexes, and male sex was identified as a predictive factor for occurrence and hemodynamic progression of AS. However, previous research has underrepresented the female sex, leading to a lack of conclusive results [21]. Several recent studies have examined the sex-related AS differences, including hemodynamic severity and aortic valve calcification (AVC), and valve remodeling.
AVC, which is the primary lesion of calcific AS, could be assessed by computed tomography (CT) and correlates with hemodynamic severity measured by echocardiography. AVC is a powerful determinant of the severity of AS and a major risk factor for AS progression and adverse outcomes [28,29,30]. For a similar hemodynamic AS severity, women have less aortic valvular calcification and higher levels of valvular fibrosis (Figure 1) with more dense connective tissue [31,32,33,34,35]. Consequently, specific calcification score thresholds of 1200 or 1300 Agatston units for women and 2000 Agatston units for men have been established to identify severe AS [3,4,28,36].
The impact of sex on the mechanism of AVC remains poorly understood. In the pathophysiology of AVC, inflammation, lipoprotein profile, and matrix remodeling are the main factors involved in the calcification process [37]. Additionally, gene expression and hormonal status, particularly testosterone, has been implicated in vascular smooth muscle culture calcification and AS progression in animal models [38,39,40,41,42,43]. These differences could potentially explain the variation and underlying mechanisms that may differentiate AVC burden between sexes. Mechanisms underlying sex differences in fibrosis include different gene expression profiles and phenotypes [41]. Valvular fibrosis in women may be attributed to enhanced activation of the myofibroblasts pathway in the interstitial cells of the aortic valve associated with genes that escape X-chromosome inactivation [44].
Due to various limitations and underrepresentation of women in studies, it remains challenging to draw definitive conclusions regarding potential differences in hemodynamic or anatomic progression of AVC between men and women. In the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) study, the annual hemodynamic progression of mild to moderate AS was found to be similar between men and women [45]. Similar results were observed by Tastet et al. in a study with a similar patient profile; however, the correlation slope between mean gradient progression and AVC was more pronounced in women than in men [30]. The COFRASA-GENERAC study demonstrated that female sex was an independent predictor of both hemodynamic and anatomic progression [46]. These results underscore a distinct progression profile between men and women in AS. However, increased female participation is necessary to deepen our understanding of the impact of sex on AS progression. This will also help to guide clinical practice in terms of follow-up timing and diagnosis.
4. LV Remodeling, Comorbidities, and AS Presentation According to Sex
AS has, for a long time, been described as a pathological condition which involves both the valve and the LV as a consequence of remodeling against chronic increased afterload [47,48]. Geometric patterns of LV include a normal pattern, concentric remodeling, concentric hypertrophy, and eccentric hypertrophy [49,50]. LV concentric remodeling with concomitant smaller cardiac chambers and lower LV mass is more prevalent in women [19,51]. Concentric hypertrophy was associated with worse clinical outcomes in women (Figure 1) [51]. Men have a higher prevalence of both concentric and eccentric hypertrophy across the spectrum of AS severity [52,53]. Some pivotal echocardiographic studies have shown that women present greater LV thickness than men both in severe [54,55] and non-severe [47,56] AS. In addition, women develop a restrictive LV pattern and are more likely than men to develop heart failure in response to cardiac overload [57].
CMR findings on myocardial fibrosis and sex differences are discordant. Dobson LE et al. found no sex difference in late gadolinium enhancement (LGE), indicating long-standing fibrosis [58], whereas Treibel TA et al. observed more LGE in men [53]. More recently, Tastet et al. [16] reported, in patients with a similar amount of LGE between sexes, a higher extracellular volume in women, suggesting diffuse fibrosis [59,60]. The discordance in findings may be attributed to differences in patient’s baseline characteristics across studies. Tastet et al.’s cohort [16] included a wider range of AS severity, different from other research that has been mainly focused on severe symptomatic AS. Further sub-analyses of this study provided compelling insights regarding the development of fibrosis, demonstrating that women exhibit fibrosis from earlier AS stages, a process driven by the renin–angiotensin–aldosterone system [61,62]. LV fibrosis and concentric remodeling are prominent features of paradoxical low-flow (PLF) AS, characterized by reduced cardiac output despite preserved ejection fraction. Due to the low-flow state, low-gradient (<40 mmHg) and small AVA (<1 cm2) are often concomitant [63]. This phenotype is highly prevalent in hypertensive women (Figure 1) and may be a reason for underdiagnosis and underestimation of AS severity in women [18,64]. PLF is associated with negative outcomes [64,65,66]. On the other hand, men are more frequently affected by coronary artery disease and reduced LV ejection faction (LVEF), i.e., classical low flow (CLF), which is also associated with low-gradient/small AVA, and worse outcomes [21]. The prevalence of PLF AS (up to 20%) is higher than the prevalence of CLF (up to 10%) AS [67].
Scarsini R et al. found that patients with PLF low-gradient AS often exhibit severe microcirculatory dysfunction and reduced peak atrial longitudinal strain [68], with the latter being a potential indicator of LV fibrosis [69] and yielding significant prognostic information [70] in AS. Guzzetti et al. also recently described a PLF high-gradient AS phenotype, associated with a worse prognosis than PLF low-gradient AS and related to sex-specific stroke volume index thresholds linked to a better risk stratification [71]. Further mechanisms combined with the increased afterload imposed by AS have been described as responsible for the concentric remodeling tendency observed in women.
Hypertension could play an important role [15] not only in sustaining concentric LV remodeling [72] and diastolic heart failure with preserved ejection fraction [73] but also yielding challenges in echocardiographic evaluation, potentially leading to a normal-flow low gradient, despite reduced aortic valve area presentation, which can be misleading and result in underestimation of AS [74]. There is concordance in the literature regarding the increased prevalence of diastolic dysfunction in women with AS in comparison to men [16,75], regardless of AS severity [76].
Dayan et al. [77] identified that PLF low-gradient AS shares similar features with Heart Failure with preserved Ejection Fraction (HFpEF), such as diastolic dysfunction and concentric remodeling, with altered ventricular–arterial coupling being a crucial link between the two conditions [78,79]. The H2FPEF score, validated for identifying likely HFpEF [80] also correlates with increased prevalence of PLF AS [81] and is associated with poorer exercise capacity and adverse hemodynamics in moderate to severe AS [82].
However, a recent position paper categorizes valvular heart disease as a “HFpEF mimic” rather than true HFpEF, naming it “HF attributed to valvular heart disease” to distinguish it from HFpEF [83]. De Biase N et al. recently identified overlaps in demographic–clinical characteristics, functional capacity impairment, and epicardial adipose tissue accumulation patterns between PLF AS and HFpEF [84]. These aspects, as reported by the authors in the conclusion [84], reinforce the hypothesized concept of PLF AS as a specific HFpEF sub-phenotype and warrant further investigation.
Conversely from the more widespread tendency of concentric remodeling, diastolic impairment, and PLF AS evolution observed in women, the progression towards LV dilation and heart failure with reduced ejection fraction (HFrEF) are features linked mainly to long-standing AS in men [85]. Men represent approximately 70–75% of patients with CLF AS [86,87], regardless of the severity degree. Coronary artery diseases, more commonly observed in men, could concurrently enhance the evolution towards LV dilatation and dysfunction in AS [88].
5. Multimaging in AS Evaluation
Clinical examination and non-invasive imaging assessment are essential in the diagnostic process of AS, not only focusing on valve structure and function but also enabling extra-valvular cardiac damage detection, facilitating the decision-making process in routine clinical practice.
5.1. Transthoracic Echocardiography (TTE)
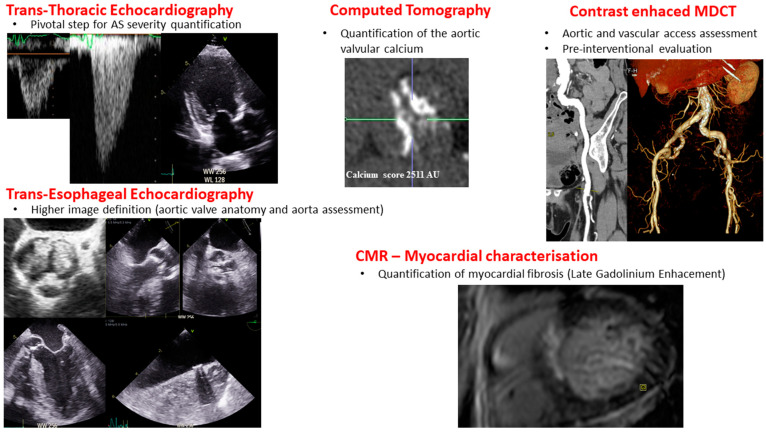
Two-dimensional TTE is the primary imaging modality for the assessment, diagnosis, and severity grading of AS, and it enables evaluation of AV morphology—as shown in Figure 2 [89,90,91,92,93]. Vpeak, mean transvalvular gradient, and AVA are the main echocardiographic parameters used to determine the severity of AS. AVA can be indexed to account for size differences, especially in small patients. Other parameters could be assessed in case of uncertainty or for risk stratification, such as the Doppler velocity index, valvulo-arterial impedance, stroke volume index, etc. TTE is also used to assess both LV structure and function. Calculation of LV mass from ventricular diameter measurements is used to assess the hypertrophic response in AS [89].
Figure 2.
Multi-imaging modalities in aortic stenosis diagnosis. Legend: AS: aortic stenosis, CMR: Cardiac Magnetic Resonance, MDCT: Multi-Detector Computed Tomography.
Women typically have smaller LV dimensions than men, resulting in a smaller LV mass [94,95,96,97,98,99]. Global longitudinal strain (GLS) is an important parameter in the evaluation of LV contractile function. Several studies have demonstrated the prognostic value of this imaging biomarker in predicting cardiovascular events, morbidity, and mortality in patients with AS, especially when the LVEF is preserved [89,100,101,102,103,104,105]. Heterogeneity in GLS cut-offs is present in the literature, and it is also partially dependent on the software utilized for speckle-tracking analysis. Nevertheless, a GLS threshold between −18% and −16% finds agreement by most studies [106,107,108]. The adoption of GLS could be useful in clinical practice for the risk stratification of patients with asymptomatic severe AS with preserved LV ejection fraction, not strictly presenting a Class I indication for AVR [8,106].
GLS and amyloidosis identification in the setting of AS is controversial since the presence of the so-called “apical sparing” pattern is also present in advanced remodeled LV [109]. A multi-imaging approach associated with careful clinical evaluation is key to resolve this issue [110]. Indeed, amyloidosis often presents often with low-gradient, small AVA and lower aortic valve calcium burden than expected, as well as granular myocardial texture, severe diastolic dysfunction, and specific clinical manifestations (e.g., renal disease, neuropathy, disturbance of cardiac conduction).
Although TTE remains the primary tool for diagnosing AS and monitoring its progression, it has certain limitations. Other advanced imaging modalities play an important complementary role, allowing us to examine a patient’s cardiac health with enhanced precision (Table 1 and Table 2).
5.2. Dobutamine Stress Echocardiography (DSE)
DSE as a second-line imaging method is indicated in patients with low-flow, low-gradient AS with depressed LV ejection fraction to establish the AS severity. Reduced LVEF with AS is more common in men. Although DSE is safe in patients with preserved ejection fraction, its accuracy in patients with mildly reduced or normal LVEF is lower than in patients with severely reduced LVEF [111]. Moreover, the use of mean gradient > 40 mmHg and AVA < 1 cm2 at any stage of DSE to confirm severe AS has been shown to be inconclusive or inaccurate in many patients, as many patients do not reach normal flow during DSE [112]. Hence, the measurement of aortic valve calcium score (AVC) should be preferred, especially in case of PLF LG.
5.3. Computed Tomography (CT)
CT is a valuable imaging modality for anatomical assessment, allowing precise measurement of AVC that is a truly flow-independent marker of AS severity—as shown in Figure 2 [36,90,113]. AVC score and volume are measured on a non-contrast ECG gated CT scan [36,90,114]. Calcium scoring measurements are highly reproducible markers of LV decompensation that demonstrate excellent agreement with echocardiographic measurements and serve as a robust predictor of future clinical events, outperforming echocardiography in all patient groups, even those with discordant classification, CLF, and/or PLF [29,90,114,115,116]. Sex-specific thresholds (i.e., 1200 or 1300 Agatston units for women and 2000 Agatston units for men) have been validated in several international cohorts and are used to predict AS progression and adverse clinical events [36,90,117]. In order to take into account aortic valve size, AVC could be indexed to the cross-sectional area of the aortic annulus, i.e., the AVC density, which is mostly useful in patients with small or large annulus, such as patients with bicuspid valve [28,36,118].
Contrast-enhanced CT can enhance the anatomical evaluation of AS severity and provides several advantages compared to non-contrast examination [90,119]. In a post hoc analysis by Cartlidge et al., contrast-enhanced CT assessment of calcified and non-calcified volumes in the aortic valve showed correlations with the severity of AS [119]. Further studies are needed to develop a reliable methodology and establish severity thresholds to assist in clinical decision-making.
Finally, contrast-enhanced CT is also the cornerstone of the peripheral vascular evaluation pre-procedural strategy (Figure 2), which is especially useful in women where iliofemoral dissections and perforations are more common than men [120], as sex differences also exist in terms of the access difficulty for a transcatheter approach.
5.4. Positron Emission Tomography (PET Scan)
Although CT can identify anatomical lesions in AS, it does not provide information about the calcification process. PET scans can be used to measure the activity of the calcification process [121]. Radiotracers are injected intravenously and localize in areas where the pathological process of interest is active. Valvular calcification activity in AS is evaluated using 18F-fluoride [90]. Dweck et al. demonstrated that 91% of patients with AS had increased 18F-NaF uptake, with a progressive rise in tracer activity correlating with AS severity [122]. In addition, longitudinal studies have shown that baseline 18 F-NaF uptake is a predictor of AS progression and AVR [123,124]. The use of 18F-NaF PET may be beneficial for bioprosthesis evaluation and early detection of degeneration. In observational studies, 18F-NaF uptake has been demonstrated as an independent and early predictor of bioprosthesis degeneration, outperforming other factors such as echocardiographic findings [125,126]. While PET scans enable early detection of AVC, their clinical utility in AS is limited. Indeed, CT provides similar prognostic information at a reduced cost and with lower radiation exposure [90].
5.5. Cardiac Magnetic Resonance (CMR)
CMR is currently adopted to evaluate myocardial-related features (Figure 2), particularly through late gadolinium enhancement and extracellular volume mapping assessment, providing structural information and myocardial tissue characterization, as discussed in the LV remodeling paragraph [90].
6. Symptoms and Clinical Profile in AS: Sex Differences
Women are more likely to present nuanced symptoms compared to men, often complaining of less specific symptoms such as shortness of breath and dizziness. This trend may be explained by a higher prevalence of microvascular dysfunction, a higher frequency of concomitant tricuspid/mitral valve disease, a smaller LV cavity, and a lower LV mass associated with diastolic dysfunction. Additionally, women have a shorter duration of exercise and a lower anaerobic threshold [15,97,127,128]. On the other hand, men are more likely to suffer from angina, which may be due to the higher incidence of coronary heart disease in men [19]. In studies of severe AS, while men had atherosclerotic comorbidities, particularly coronary artery disease, women were older, more often frail, had a higher Society of Thoracic Surgeon (STS) score, and a higher prevalence of hypertension and chronic obstructive pulmonary disease [19]. Moreover, women are more likely than men to develop symptoms during follow-up despite similar initial severity of AS [97].
Finally, the delay to diagnosis and treatment in women could also be associated with gender bias, as symptoms and AS severity in women are often undermined by physicians. Indeed, delay to intervention has been shown in women with similar AS severity and symptoms than men [15]. However, sex-and gender-specific studies in AS are needed to confirm this point.
7. AVR Referral: Surgical AVR, TAVR, and Ross Procedure
7.1. Surgical AVR (SAVR)
The onset of symptoms, which indicates LV decompensation and is associated with a poor prognosis in severe AS, is a trigger for AVR according to the guidelines [3,4]. Differences between sexes in the timing and manifestation of symptoms can significantly influence therapeutic decisions, long-term health preservation, and the risk of irreversible cardiac damage associated with delayed referral for AVR, peri/postoperative complications, mortality, and long-term outcomes. Consequently, it is crucial not to base therapeutic decisions solely on symptoms. Women are referred for AVR later than men, despite having more symptoms. Male patients are more likely to have Class I indications for AVR based on echocardiographic parameters [129,130]. The tendency for men to be referred earlier for AVR is in part related to a higher frequency of concomitant procedures such as coronary artery bypass grafting or aorta repair/replacement. Discordance of echocardiographic parameters is a second explanation for delayed referral of female patients for AVR [15]. Another important reason for this lack of intervention is the conviction that symptoms are unrelated to AS, but unoperated patients are at higher risk of mortality [131].
Women exhibit higher rates of in-hospital mortality compared to men [132]. In the Society of Thoracic Surgeons’ national database, focusing on patients undergoing isolated SAVR, female patients had an increased risk of mortality, stroke, and postoperative hospitalization compared to male patients [133]. Another study showed that overall survival was worse in women than in men; however, after adjusting for preoperative risk factors, there was no significant difference in overall survival between the two sexes [134]. Fuchs et al. presented contrasting results, demonstrating that operative and long-term mortality did not increase in women after SAVR. Furthermore, women experienced better outcomes [135]. This discrepancy in outcomes may be attributed to pre-procedural comorbidities and cardiac damage, as well as the PLF AS [15,51,71,132].
7.2. TAVR
Women are more likely to undergo TAVR [17,19]. Overall, women generally have fewer cardiovascular risk factors and less calcium burden, with lower rates of prior myocardial infarction, revascularization, prior stroke, and peripheral vascular disease. Additionally, they often have better LVEF at presentation [19,21]. Several studies suggest that TAVR offers significant benefits to women. In the CoreValve US High-Risk Pivotal Trial, women treated with TAVR had lower one-year all-cause mortality compared to those undergoing SAVR (12.7% vs. 21.8%) [136]. In the majority of TAVR studies, women had comparable or favorable results to men after one or two years [137,138,139,140,141,142,143,144,145,146,147,148]. Women have a lower risk of developing paravalvular regurgitation, an important prognostic factor after TAVR [149,150], than men [151,152]. Less AVC and a smaller annular size in women may explain this difference in results [153]. In contrast to men, women had a higher incidence of major bleeding and in-hospital vascular complications after TAVR (Figure 3), as well as device-related complications, stroke, and conversion to conventional SAVR [133,140,141,154,155], but with a lower rate of re-hospitalization [156]. The increased incidence of vascular complications and major bleeding in women undergoing TAVR may be due to their advanced age and the relatively smaller dimensions of the vessels, annulus, and LV ejection pathway [19,157]. The most recent study regarding the non-inferiority of TAVR in low-risk, symptomatic severe AS—the PARTNER trial—presents results after 5 years of follow up [158]. There is a tendency of more adverse outcomes after SAVR in women shown by different studies [159,160,161,162], potentially representing pivotal clinical issues in favor of TAVR in women with severe AS requiring intervention. Furthermore, a specific multicenter register (Women’s International Transcatheter Aortic Valve Implantation [WIN-TAVR] Registry) including only woman with intermediate to high risk undergoing TAVR [163] reported a 1-year VARC-2 efficacy endpoint of 16.5% with low incidence of 1-year mortality and stroke rate of 13.9%. A subsequent recent sub-analysis highlights that the VARC-2 endpoint was higher and mostly sustained by patients with concurrent frailty/prefrailty criteria [164]. Nevertheless, there is not yet available evidence from randomized studies regarding the superiority of TAVR compared to SAVR in women with symptomatic AS, independently from surgical risk. An ongoing randomized multicentric controlled trial has been specifically designed in order to clarify this aspect (Randomized researcH in womEn all comers wIth Aortic stenosis [RHEIA] trial) [165].
Figure 3.
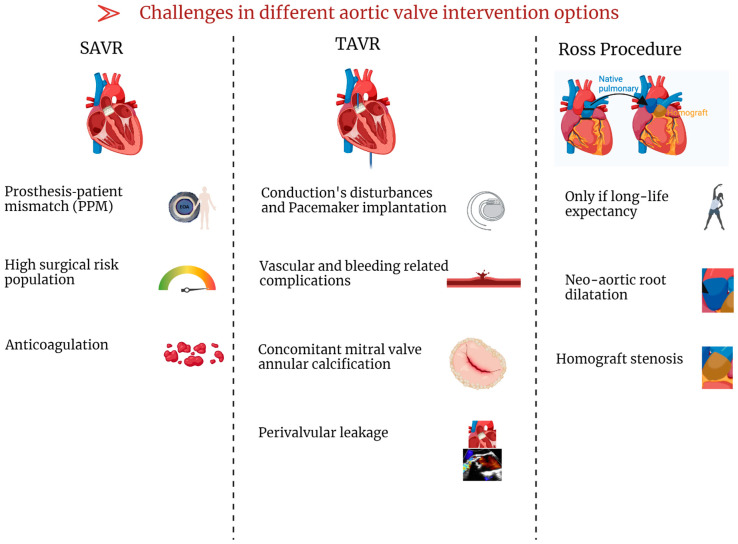
Challenges in the different aortic valve intervention options. Legend: SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement.
7.3. Ross Procedure
The Ross procedure appears to be beneficial and particularly appropriate for young women [166], especially those considering pregnancy [167]. This is mainly due to (1) the advantage of avoiding the need for anticoagulation, which can be a challenge in managing pregnancy for both maternal and fetal health [168] and (2) the longer valve durability of the autograft (from Ross procedure) compared to bioprostheses [169]. Indeed, several studies have reported various cardiovascular and obstetric complications in pregnant women with mechanical valve replacement, and thus bioprostheses are mainly implanted in young women planning pregnancy. However, studies have shown no adverse associations between pregnancy and the Ross procedure in pregnant women [170,171,172,173]. It is important to highlight that, despite the generally favorable tolerance of pregnancy in these women, there may be a risk of neo-aortic dilatation (Figure 3), especially in those who have had multiple pregnancies [173,174]. As women have unique characteristics, a special approach is required, particularly with regard to pregnancy planning given the potential risks to maternal and fetal health. Individual counseling prior to conception in young women with bicuspid aortic valve is essential to assess risks and discuss options. Close follow-up during pregnancy is essential to evaluate disease progression and make any necessary treatment adjustments while minimizing risks to both the mother and the fetus. In addition, postpartum and long-term follow-up are required to assess and prevent long-term complications.
Specific indications for the three types of interventional treatment for AS are shown in Table 3.
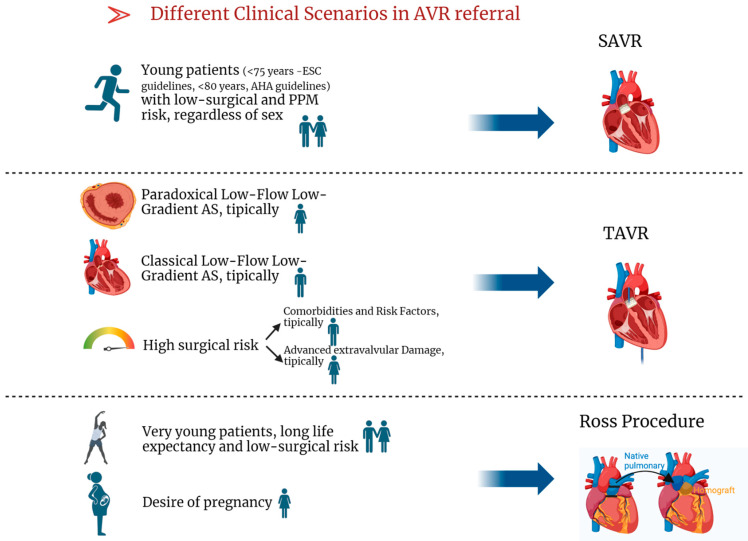
Figure 4 aims to summarize common scenarios of severe AS encountered in clinical practice referred for AVR and their proposed best treatment option.
Figure 4.
Different Clinical Scenarios in AVR referral. Legend: AS: aortic stenosis, AVR: aortic valve replacement, SAVR: surgical aortic valve replacement, TAVR: transcatheter aortic valve replacement.
8. Current Challenges in AVR
The planning of aortic valve intervention has some important aspects: Prosthesis–patient mismatch (PPM) occurrence is more common after SAVR, while conduction disturbance and vascular/bleeding complications are mostly observed in TAVR. Also, the presence of severe mitral annular calcification represents an important field of research because evidence in the literature regarding its prognostic role after TAVR is discordant.
8.1. Prosthesis–Patient Mismatch (PPM)
PPM after AVR occurs when the effective orifice area (EOA) is too small for the patient’s cardiovascular requirements [175,176]. Severe PPM after SAVR occurs in 11% of patients, mostly in women (57%) [177,178,179,180]. Recently, the definition of PPM has been updated, lowering the threshold for patients with a body mass index (BMI) above 30 kg/m2 (VARC 3 Definition) [181,182], which decreases the prevalence of severe PPM to 3% [183]. Severe PPM is less common after TAVR than SAVR, regardless of the definition [184,185,186].
In clinical practice, a small aortic annulus (less than 400 mm2) in patients with severe AS represents a significant challenge [187]. This condition is identified as a risk factor for PPM following AVR [188,189], and it predominantly affects elderly women with a small LV cavity and concentric remodeling [187,190]. Choosing SAVR over TAVR in such cases may potentially increase the risk of PPM (Figure 3), leading to poorer long-term clinical outcomes [191]. The VIVA trial, enrolling 151 patients, showed no difference in medium-term outcomes between SAVR and TAVR in the context of severe AS with small annulus [192]. However, in women with PLF AS, TAVR may improve outcomes compared to SAVR [193]. The RHEIA multicentric randomized trial aims to further elucidate specifically whether TAVR is a better option than SAVR in women with small annulus, regardless of the patients’ baseline clinical surgical risk [165]. The minimization risk of PPM after AVR is important especially in patients with CLF [194,195] or PLF AS [196], which are specific phenotypes where PPM contributes significantly to worse outcomes [196,197].
Severe PPM should be avoided in all patients and in certain “vulnerable subsets” even moderate PPM should be avoided, such as those with CLF AS, PLF AS, severe LV hypertrophy, and mitral regurgitation [191]. The occurrence of significant PPM could be predicted before (or during) the procedure, using published EOA values for the type and size of the prosthesis that is planned to be implanted according to the aortic annulus size measured by CT or echocardiography [198] and indexed to the body surface area. For “vulnerable patients” at risk of severe or moderate PPM, options to avoid PPM include selecting an alternative prosthesis (with better hemodynamics), aortic root enlargement, or consideration of TAVR, which presents lower PPM prevalence [191].
8.2. Pacemaker Implantation
While permanent pacemaker implantation was common in women after TAVR in the WIN-TAVR registry [199], a metanalysis involving 46 studies and encompassing more than 70,000 patients with TAVR highlighted that pacemaker implantation incidences occur less frequently in women, suggesting that conduction disturbances such as TAVR-related complications are more common in men [200]. This issue could be partially explained by the lower calcium burden detected in women with AS, especially in the non-coronary cusp [201]. The anatomical proximity of both the atrium-ventricular node and left Tawara branch to the non-coronary cusps, susceptible to compression during valve implantation, may explain this complication after TAVR. The aortic calcium burden is additionally linked to the development of paravalvular leak after TAVR. Women are more likely to present paravalvular aortic leak than men, a feature linked to adverse long-term outcomes [151,152,153].
Finally, other important anatomic determinants of pacemaker implantation after TAVR are very severe reduced aortic valve area (below 0.75 cm2) [202], differences between implantation depth and membranous septum length above 3 mm [203], greater aortic annulus diameter, and the presence of mitral annular calcification [204].
8.3. Frailty and Bleeding Risk
A particular phenotype of AS where TAVR in women demonstrates poorer outcomes than in men is represented by low-flow, low-gradient AS [205]. This trend was also observed in patients who were very elderly and frail [206], as well as in women reporting coexistent pulmonary hypertension [207]. Additionally, some studies report that women are often a technically challenging subset of patients for TAVR, also exhibiting more vascular complications and bleeding in the post-operative period (Figure 3) [208,209].
8.4. Mitral Annular Calcification
The presence of severe mitral annular calcification (MAC) has been previously reported to be an independent predictor of long-term negative outcomes after TAVR. Severe MAC is mostly observed in women, and after adjustment for other clinical parameters it was associated with increased all-cause cardiovascular mortality and conduction disturbance after TAVR [210]. However, if MAC is an independent predictor of prognosis regardless of concomitant mitral valve disease (regurgitation or stenosis), its impact remains a matter of debate since the available evidence reports discordant findings [211,212,213].
9. Conclusions and Future Perspectives
Sex-related differences are encountered in AS, ranging from pathophysiology, encompassing extra-valvular damage, and the diagnostic process, up to intervention when needed [21,209]. The conclusions of this review can be summarized as follows:
-
(1)
The calcific burden in AS is less represented in women, with more concomitant widespread fibrotic patterns sustained by different biological pathways.
-
(2)
HFpEF and PLF AS, especially in woman, present common features, warranting further studies aiming to elucidate if PLF AS could be included in the HFpEF spectrum.
-
(3)
Referrals for aortic valve intervention tend to be later in women, leading to an augmented extra-valvular damage-related burden.
-
(4)
SAVR carries a higher risk of severe PPM compared to TAVR, especially in women, PLF AS, and concomitant small aortic anulus. TAVR in those scenarios could be preferred; ongoing studies are focusing on this strategy in women with small annulus.
-
(5)
TAVR is facing some challenges in women, especially considering the bleeding risk, frailty and higher prevalence of mitral annular calcification.
-
(6)
The Ross procedure performed in highly specialized centers is a valid option in patient with low surgical risk and without underlying comorbidities.
Further studies are required to deepen our knowledge of sex-specific AVC and structural heart changes, along with the creation of tailored antithrombotic treatments designed to lower the heightened risk of bleeding in women after TAVR [148]. Moreover, to enhance patient outcomes in AS, it is essential to establish sex-specific thresholds for early and accurate diagnosis in women [209]. It is also important to examine disparities in patient referrals to guarantee equal opportunities for both sexes with severe AS to access the best possible care [214]. Through these measures, we can devise individualized treatment plans for patients with severe AS accounting for sex differences, leading to better health outcomes.
Table 1.
Advantages and limitations of the established imaging modalities used to assess AS.
| Indication/Diagnostic | Advantages | Limits | |
|---|---|---|---|
| TTE [18,67,89,90,91,92,93,215,216,217,218,219] |
|
|
|
| DSE [89,220,221,222] |
|
|
|
| Exercise Stress Echocardiography [3,4,223,224] |
|
|
|
| CT [31,113,114,225] |
|
|
|
| TEE [3,4,226] |
|
|
|
| CMR [3,4,226,227] |
|
|
|
AS = aortic stenosis; AV = aortic valve; AVA = aortic valve area; DSE = dobutamine stress echocardiography; CLF: classical low flow; CMR = Cardiac Magnetic Resonance; CT = computed tomography; LV = left ventricle; LVEF = left ventricle ejection fraction; TAVR = transcatheter aortic valve replacement; TEE = transesophageal echocardiography; TTE = transthoracic echocardiography; Vpeak = peak aortic jet velocity.
Table 2.
New emerging imaging modalities for the management of AS.
| Indication/Diagnostic | Advantages | Limits | |
|---|---|---|---|
| Contrast-enhanced CT [90,119,228] |
|
|
|
| PET scan [229,230] |
|
|
|
| CT angiography [231,232] |
|
|
|
CT = computed tomography; GLS = global longitudinal strain; TAVR = transcatheter aortic valve replacement; PET = positron emission tomography.
Table 3.
Interventional treatment of AS.
| SAVR [3,4] | TAVR [3,4] | ROSS [3,4,233,234,235,236,237,238,239] | |
|---|---|---|---|
| Indications |
|
|
|
AS = aortic stenosis; AVR = aortic valve replacement; BNP = Brain Natriuretic Peptide; SAVR = surgical aortic valve replacement; TAVR = transcatheter aortic valve replacement; PPM = prosthesis–patient mismatch.
Abbreviations
| AS | aortic stenosis |
| AVC | aortic valve calcification |
| AVR | aortic valve replacement |
| BAV | bicuspid aortic valve |
| CLF | classical low flow |
| CMR | Cardiac Magnetic Resonance |
| CT | computed tomography |
| EOA | effective orifice area |
| EOAi | indexed effective orifice area |
| HFpEF | Heart Failure with preserved Ejection Fraction |
| HFrEF | Heart Failure with reduced Ejection Fraction |
| LGE | late gadolinium enhancement |
| LV | left ventricle |
| LVEF | left ventricular ejection fraction |
| PET scan | positron emission tomography scan |
| PLF | paradoxical low flow |
| SAVR | surgical aortic valve replacement |
| TAVR | transcatheter aortic valve replacement |
| TTE | transthoracic echocardiography |
| TEE | transesophageal echocardiography |
| Vpeak | peak aortic jet velocity |
Conflicts of Interest
P.S. and K.A. has nothing to declare. M.A.C. has received funding from Edwards Lifesciences for CT core laboratory and research grants from Edwards Lifesciences, Medtronic, and Pi-Cardia, with no direct compensation.
Funding Statement
This research did not receive direct funding, but Dr. Clavel holds the Canada research Chair on Women’s Cardiac Valvular Health funded by the Canadian Institutes of Health Research (CIHR)—grand number: 950-232973. P.S. holds a grant from the University of Verona (Italy).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.d’Arcy J.L., Coffey S., Loudon M.A., Kennedy A., Pearson-Stuttard J., Birks J., Frangou E., Farmer A.J., Mant D., Wilson J., et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: The OxVALVE Population Cohort Study. Eur. Heart J. 2016;37:3515–3522. doi: 10.1093/eurheartj/ehw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benfari G., Essayagh B., Michelena H.I., Ye Z., Inojosa J.M., Ribichini F.L., Crestanello J., Messika-Zeitoun D., Prendergast B., Wong B.F., et al. Severe aortic stenosis: Secular trends of incidence and outcomes. Eur. Heart J. 2024;45:1877–1886. doi: 10.1093/eurheartj/ehad887. [DOI] [PubMed] [Google Scholar]
- 3.Vahanian A., Beyersdorf F., Praz F., Milojevic M., Baldus S., Bauersachs J., Capodanno D., Conradi L., De Bonis M., De Paulis R., et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur. Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 4.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., 3rd, Gentile F., Jneid H., Krieger E.V., Mack M., McLeod C., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J. Am. Coll. Cardiol. 2021;77:450–500. doi: 10.1016/j.jacc.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 5.Nakatsuma K., Taniguchi T., Morimoto T., Shiomi H., Ando K., Kanamori N., Murata K., Kitai T., Kawase Y., Izumi C., et al. B-type natriuretic peptide in patients with asymptomatic severe aortic stenosis. Heart. 2019;105:384–390. doi: 10.1136/heartjnl-2018-313746. [DOI] [PubMed] [Google Scholar]
- 6.Lancellotti P., Magne J., Dulgheru R., Clavel M.A., Donal E., Vannan M.A., Chambers J., Rosenhek R., Habib G., Lloyd G., et al. Outcomes of patients with asymptomatic aortic stenosis followed up in heart valve clinics. JAMA Cardiol. 2018;3:1060–1068. doi: 10.1001/jamacardio.2018.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancellotti P., Lebois F., Simon M., Tombeux C., Chauvel C., Pierard L.A. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation. 2005;112:I377–I382. doi: 10.1161/CIRCULATIONAHA.104.523274. [DOI] [PubMed] [Google Scholar]
- 8.Tastet L., Tribouilloy C., Maréchaux S., Vollema E.M., Delgado V., Salaun E., Shen M., Capoulade R., Clavel M.A., Arsenault M., et al. Staging cardiac damage in patients with asymptomatic aortic valve stenosis. J. Am. Coll. Cardiol. 2019;74:550–563. doi: 10.1016/j.jacc.2019.04.065. [DOI] [PubMed] [Google Scholar]
- 9.Magne J., Cosyns B., Popescu B.A., Carstensen H.G., Dahl J., Desai M.Y., Kearney L., Lancellotti P., Marwick T.H., Sato K., et al. Distribution and prognostic significance of left ventricular global longitudinal strain in asymptomatic significant aortic stenosis: An individual participant data meta-analysis. JACC Cardiovasc. Imaging. 2019;12:84–92. doi: 10.1016/j.jcmg.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Galli E., Fournet M., Chabanne C., Lelong B., Leguerrier A., Flecher E., Mabo P., Donal E. Prognostic value of left atrial reservoir function in patients with severe aortic stenosis: A 2D speckle-tracking echocardiographic study. Eur. Heart J. Cardiovasc. Imaging. 2016;17:533–541. doi: 10.1093/ehjci/jev230. [DOI] [PubMed] [Google Scholar]
- 11.Stassen J., Ewe S.H., Pio S.M., Pibarot P., Redfors B., Leipsic J., Genereux P., Van Mieghem N.M., Kuneman J.H., Makkar R., et al. Managing Patients with Moderate Aortic Stenosis. JACC Cardiovasc. Imaging. 2023;16:837–855. doi: 10.1016/j.jcmg.2022.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Généreux P., Sharma R.P., Cubeddu R.J., Aaron L., Abdelfattah O.M., Koulogiannis K.P., Marcoff L., Naguib M., Kapadia S.R., Makkar R.R., et al. The mortality burden of untreated aortic stenosis. J. Am. Coll. Cardiol. 2023;82:2101–2109. doi: 10.1016/j.jacc.2023.09.796. [DOI] [PubMed] [Google Scholar]
- 13.Strange G., Stewart S., Celermajer D., Prior D., Scalia G.M., Marwick T., Ilton M., Joseph M., Codde J., Playford D. Poor long-term survival in patients with moderate aortic stenosis. J. Am. Coll. Cardiol. 2019;74:1851–1863. doi: 10.1016/j.jacc.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig S., Schofer N., Abdel-Wahab M., Urena M., Jean G., Renker M., Hamm C.W., Thiele H., Iung B., Ooms J.F., et al. Transcatheter aortic valve replacement in patients with reduced ejection fraction and nonsevere aortic stenosis. Circ. Cardiovasc. Interv. 2023;16:e012768. doi: 10.1161/CIRCINTERVENTIONS.122.012768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bienjonetti-Boudreau D., Fleury M.A., Voisine M., Paquin A., Chouinard I., Tailleur M., Duval R., Magnan P.O., Beaudoin J., Salaun E., et al. Impact of sex on the management and outcome of aortic stenosis patients. Eur. Heart J. 2021;42:2683–2691. doi: 10.1093/eurheartj/ehab242. [DOI] [PubMed] [Google Scholar]
- 16.Tastet L., Kwiecinski J., Pibarot P., Capoulade R., Everett R.J., Newby D.E., Shen M., Guzzetti E., Arsenault M., Bédard E., et al. Sex-related differences in the extent of myocardial fibrosis in patients with aortic valve stenosis. JACC Cardiovasc. Imaging. 2020;13:699–711. doi: 10.1016/j.jcmg.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Chandrasekhar J., Dangas G., Yu J., Vemulapalli S., Suchindran S., Vora A.N., Baber U., Mehran R., STS/ACC TVT Registry Sex-based differences in outcomes with transcatheter aortic valve therapy: TVT registry from 2011 to 2014. J. Am. Coll. Cardiol. 2016;68:2733–2743. doi: 10.1016/j.jacc.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 18.Hachicha Z., Dumesnil J.G., Bogaty P., Pibarot P. Paradoxical low flow, low gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 19.Shan Y., Pellikka P.A. Aortic stenosis in women. Heart. 2020;106:970–976. doi: 10.1136/heartjnl-2019-315407. [DOI] [PubMed] [Google Scholar]
- 20.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Cote N., Clavel M.A. Sex differences in the pathophysiology, diagnosis, and management of aortic stenosis. Cardiol. Clin. 2020;38:129–138. doi: 10.1016/j.ccl.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Andell P., Li X., Martinsson A., Andersson C., Stagmo M., Zöller B., Sundquist K., Smith J.G. Epidemiology of valvular heart disease in a swedish nationwide hospital-based register study. Heart. 2017;103:1696–1703. doi: 10.1136/heartjnl-2016-310894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coffey S., Roberts-Thomson R., Brown A., Carapetis J., Chen M., Enriquez-Sarano M., Zühlke L., Prendergast B.D. Global epidemiology of valvular heart disease. Nat. Rev. Cardiol. 2021;18:853–864. doi: 10.1038/s41569-021-00570-z. [DOI] [PubMed] [Google Scholar]
- 24.Tutar E., Ekici F., Atalay S., Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am. Heart J. 2005;150:513–515. doi: 10.1016/j.ahj.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 25.Nistri S., Basso C., Marzari C., Mormino P., Thiene G. Frequency of bicuspid aortic valve in young male conscripts by echocardiogram. Am. J. Cardiol. 2005;96:718–721. doi: 10.1016/j.amjcard.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 26.Granath C., Mohamed S.A., Olsson C., Grattan M., Mertens L., Franco-Cereceda A., Bjorck H.M. Valve disease and aortopathy associations of bicuspid aortic valve phenotypes differ between men and women. Open Heart. 2021;8:e001857. doi: 10.1136/openhrt-2021-001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nkomo V.T., Gardin J.M., Skelton T.N., Gottdiener J.S., Scott C.G., Enriquez-Sarano M. Burden of valvular heart diseases: A population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 28.Clavel M.A., Pibarot P., Messika-Zeitoun D., Capoulade R., Malouf J., Aggarval S., Araoz P.A., Michelena H.I., Cueff C., Larose É., et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: Results of an international registry study. J. Am. Coll. Cardiol. 2014;64:1202–1213. doi: 10.1016/j.jacc.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pawade T., Clavel M.A., Tribouilloy C., Dreyfus J., Mathieu T., Tastet L., Renard C., Gun M., Jenkins W.S.A., Macron L., et al. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ. Cardiovasc. Imaging. 2018;11:e007146. doi: 10.1161/CIRCIMAGING.117.007146. [DOI] [PubMed] [Google Scholar]
- 30.Tastet L., Enriquez-Sarano M., Capoulade R., Malouf J., Araoz P.A., Shen M., Michelena H.I., Larose É., Arsenault M., Bédard E., et al. Impact of aortic valve calcification and sex on hemodynamic progression and clinical outcomes in AS. J. Am. Coll. Cardiol. 2017;69:2096–2098. doi: 10.1016/j.jacc.2017.02.037. [DOI] [PubMed] [Google Scholar]
- 31.Simard L., Côté N., Dagenais F., Mathieu P., Couture C., Trahan S., Bossé Y., Mohammadi S., Pagé S., Joubert P., et al. Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis: Is valvular fibrosis the explanation? Circ. Res. 2017;120:681–691. doi: 10.1161/CIRCRESAHA.116.309306. [DOI] [PubMed] [Google Scholar]
- 32.Voisine M., Hervault M., Shen M., Boilard A.J., Filion B., Rosa M., Bossé Y., Mathieu P., Côté N., Clavel M.A. Age, sex, and valve phenotype differences in fibro-calcific remodeling of calcified aortic valve. J. Am. Heart Assoc. 2020;9:e015610. doi: 10.1161/JAHA.119.015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thaden J.J., Nkomo V.T., Suri R.M., Maleszewski J.J., Soderberg D.J., Clavel M.A., Pislaru S.V., Malouf J.F., Foley T.A., Oh J.K., et al. Sex-related differences in calcific aortic stenosis: Correlating clinical and echocardiographic characteristics and computed tomography aortic valve calcium score to excised aortic valve weight. Eur. Heart J. 2016;37:693–699. doi: 10.1093/eurheartj/ehv560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linde L., Carter-Storch R., Christensen N.L., Ovrehus K.A., Diederichsen A.C.P., Laursen K., Jensen P.S., Rasmussen L.M., Moller J.E., Dahl J.S. Sex differences in aortic valve calcification in severe aortic valve stenosis: Association between computer tomography assessed calcification and valvular calcium concentrations. Eur. Heart J. Cardiovasc. Imaging. 2021;22:581–588. doi: 10.1093/ehjci/jeaa096. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal S.R., Clavel M.A., Messika-Zeitoun D., Cueff C., Malouf J., Araoz P.A., Mankad R., Michelena H., Vahanian A., Enriquez-Sarano M. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ. Cardiovasc. Imaging. 2013;6:40–47. doi: 10.1161/CIRCIMAGING.112.980052. [DOI] [PubMed] [Google Scholar]
- 36.Clavel M.A., Messika-Zeitoun D., Pibarot P., Aggarwal S., Malouf J., Araoz P., Michelena H., Cueff C., Larose É., Capoulade R., et al. The complex nature of discordant severe calcified aortic valve disease grading: New insights from combined Doppler-echocardiographic and computed tomographic study. J. Am. Coll. Cardiol. 2013;62:2329–2338. doi: 10.1016/j.jacc.2013.08.1621. [DOI] [PubMed] [Google Scholar]
- 37.Lindman B.R., Clavel M.A., Mathieu P., Iung B., Lancellotti P., Otto C.M., Pibarot P. Calcific aortic stenosis. Nat. Rev. Dis. Primers. 2016;2:16006. doi: 10.1038/nrdp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Husseini D., Boulanger M.C., Fournier D., Mahmut A., Bossé Y., Pibarot P., Mathieu P. High expression of the pi-transporter SLC20A1/Pit1 in calcific aortic valve disease promotes mineralization through regulation of akt-1. PLoS ONE. 2013;8:e53393. doi: 10.1371/journal.pone.0053393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parra-Izquierdo I., Castanos-Mollor I., Lopez J., Gomez C., San Roman J.A., Sanchez Crespo M., Garcia-Rodriguez C. Calcification Induced by Type I Interferon in Human Aortic Valve Interstitial Cells Is Larger in Males and Blunted by a Janus Kinase Inhibitor. Arterioscler. Thromb. Vasc. Biol. 2018;38:2148–2159. doi: 10.1161/ATVBAHA.118.311504. [DOI] [PubMed] [Google Scholar]
- 40.El Husseini D., Boulanger M.C., Mahmut A., Bouchareb R., Laflamme M.H., Fournier D., Pibarot P., Bossé Y., Mathieu P. P2Y2 receptor represses IL-6 expression by valve interstitial cells through Akt: Implication for calcific aortic valve disease. J. Mol. Cell Cardiol. 2014;72:146–156. doi: 10.1016/j.yjmcc.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 41.McCoy C.M., Nicholas D.Q., Masters K.S. Sex-related differences in gene expression by porcine aortic valvular interstitial cells. PLoS ONE. 2012;7:e39980. doi: 10.1371/journal.pone.0039980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarajlic P., Plunde O., Franco-Cereceda A., Bäck M. Artificial intelligence models reveal sex-specific gene expression in aortic valve calcification. JACC Basic. Transl. Sci. 2021;6:403–412. doi: 10.1016/j.jacbts.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleury M.A., Annabi M.S., Voisine M., Hervault M., Boilard A.J., Shen M., Marette A., Côté N., Clavel M.A. Impact of sex and sex hormones on pathophysiology and progression of aortic stenosis in a murine model. Physiol. Rep. 2022;10:e15433. doi: 10.14814/phy2.15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aguado B.A., Walker C.J., Grim J.C., Schroeder M.E., Batan D., Vogt B.J., Rodriguez A.G., Schwisow J.A., Moulton K.S., Weiss R.M., et al. Genes that escape X chromosome inactivation modulate sex differences in valve myofibroblasts. Circulation. 2022;145:513–530. doi: 10.1161/CIRCULATIONAHA.121.054108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cramariuc D., Rogge B.P., Lønnebakken M.T., Boman K., Bahlmann E., Gohlke-Bärwolf C., Chambers J.B., Pedersen T.R., Gerdts E. Sex differences in cardiovascular outcome during progression of aortic valve stenosis. Heart. 2015;101:209–214. doi: 10.1136/heartjnl-2014-306078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen V., Mathieu T., Melissopoulou M., Cimadevilla C., Codogno I., Huart V., Duval X., Vahanian A., Messika-Zeitoun D. Sex differences in the progression of aortic stenosis and prognostic implication: The COFRASA-GENERAC study. JACC Cardiovasc. Imaging. 2016;9:499–501. doi: 10.1016/j.jcmg.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Stassen J., Pio S.M., Ewe S.H., Amanullah M.R., Hirasawa K., Butcher S.C., Singh G.K., Sin K.Y.K., Ding Z.P., Chew N.W.S., et al. Sex-Related Differences in Medically Treated Moderate Aortic Stenosis. Struct. Heart. 2022;6:100042. doi: 10.1016/j.shj.2022.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ito S., Miranda W.R., Nkomo V.T., Connolly H.M., Pislaru S.V., Greason K.L., Pellikka P.A., Lewis B.R., Oh J.K. Reduced left ventricular ejection fraction in patients with aortic stenosis. J. Am. Coll. Cardiol. 2018;71:1313–1321. doi: 10.1016/j.jacc.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 49.Grossman W., Jones D., McLaurin L.P. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Investig. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerdts E. Left ventricular structure in different types of chronic pressure overload. Eur. Heart J. 2008;10:E23–E30. doi: 10.1093/eurheartj/sun015. [DOI] [Google Scholar]
- 51.Capoulade R., Clavel M.A., Le Ven F., Dahou A., Thébault C., Tastet L., Shen M., Arsenault M., Bédard É., Beaudoin J., et al. Impact of left ventricular remodelling patterns on outcomes in patients with aortic stenosis. Eur. Heart J. Cardiovasc. Imaging. 2017;18:1378–1387. doi: 10.1093/ehjci/jew288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cramariuc D., Rieck A.E., Staal E.M., Wachtell K., Eriksen E., Rossebo A.B., Gerdts E. Factors influencing left ventricular structure and stress-corrected systolic function in men and women with asymptomatic aortic valve stenosis (a SEAS Substudy) Am. J. Cardiol. 2008;101:510–515. doi: 10.1016/j.amjcard.2007.09.100. [DOI] [PubMed] [Google Scholar]
- 53.Treibel T.A., Kozor R., Fontana M., Torlasco C., Reant P., Badiani S., Espinoza M., Yap J., Diez J., Hughes A.D., et al. Sex dimorphism in the myocardial response to aortic stenosis. JACC Cardiovasc. Imaging. 2018;11:962–973. doi: 10.1016/j.jcmg.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Douglas P.S., Otto C.M., Mickel M.C., Labovitz A., Reid C.L., Davis K.B. Gender differences in left ventricle geometry and function in patients undergoing balloon dilatation of the aortic valve for isolated aortic stenosis. NHLBI Balloon Valvuloplasty Registry. Br. Heart J. 1995;73:548–554. doi: 10.1136/hrt.73.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrov G., Regitz-Zagrosek V., Lehmkuhl E., Krabatsch T., Dunkel A., Dandel M., Dworatzek E., Mahmoodzadeh S., Schubert C., Becher E., et al. Regression of myocardial hypertrophy after aortic valve replacement: Faster in women? Circulation. 2010;122:S23–S28. doi: 10.1161/CIRCULATIONAHA.109.927764. [DOI] [PubMed] [Google Scholar]
- 56.Lee J.M., Park S.J., Lee S.P., Park E., Chang S.A., Kim H.K., Lee W., Kim Y.J., Lee S.C., Park S.W., et al. Gender difference in ventricular response to aortic stenosis: Insight from cardiovascular magnetic resonance. PLoS ONE. 2015;10:e0121684. doi: 10.1371/journal.pone.0121684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Douglas P.S., Katz S.E., Weinberg E.O., Chen M.H., Bishop S.P., Lorell B.H. Hypertrophy remodeling: Gender differences in the early response to left ventrcular pressure overload. J. Am. Coll. Cardiol. 1998;32:1118–1125. doi: 10.1016/S0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 58.Dobson L.E., Fairbairn T.A., Musa T.A., Uddin A., Mundie C.A., Swoboda P.P., Ripley D.P., McDiarmid A.K., Erhayiem B., Garg P., et al. Sex-related differences in left ventricular remodeling in severe aortic stenosis and reverse remodeling after aortic valve replacement: A cardiovascular magnetic resonance study. Am. Heart J. 2016;175:101–111. doi: 10.1016/j.ahj.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Bull S., White S.K., Piechnik S.K., Flett A.S., Ferreira V.M., Loudon M., Francis J.M., Karamitsos T.D., Prendergast B.D., Robson M.D., et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–937. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azevedo C.F., Nigri M., Higuchi M.L., Pomerantzeff P.M., Spina G.S., Sampaio R.O., Tarasoutchi F., Grinberg M., Rochitte C.E. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J. Am. Coll. Cardiol. 2010;56:278–287. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 61.Keller K.M., Howlett S.E. Sex differences in the biology and pathology of the aging heart. Can. J. Cardiol. 2016;32:1065–1073. doi: 10.1016/j.cjca.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 62.Greiten L.E., Holditch S.J., Arunachalam S.P., Miller V.M. Should there be sex-specific criteria for the diagnosis and treatment of heart failure? J. Cardiovasc. Transl. Res. 2014;7:139–155. doi: 10.1007/s12265-013-9514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clavel M.A., Magne J., Pibarot P. Low-gradient aortic stenosis. Eur. Heart J. 2016;37:2645–2657. doi: 10.1093/eurheartj/ehw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clavel M.A., Dumesnil J.G., Capoulade R., Mathieu P., Sénéchal M., Pibarot P. Outcome of patients with aortic stenosis, small valve area and low-flow, low-gradient despite preserved left ventricular ejection fraction. J. Am. Coll. Cardiol. 2012;60:1259–1267. doi: 10.1016/j.jacc.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 65.Eleid M.F., Michelena H.I., Nkomo V.T., Nishimura R.A., Malouf J.F., Scott C.G., Pellikka P.A. Causes of death and predictors of survival after aortic valve replacement in low flow vs. normal flow severe aortic stenosis with preserved ejection fraction. Eur. Heart J. Cardiovasc. Imaging. 2015;16:1270–1275. doi: 10.1093/ehjci/jev091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clavel M.A., Berthelot-Richer M., Le Ven F., Capoulade R., Dahou A., Dumesnil J.G., Mathieu P., Pibarot P. Impact of classic and paradoxical low flow on survival after aortic valve replacement for severe aortic stenosis. J. Am. Coll. Cardiol. 2015;65:645–653. doi: 10.1016/j.jacc.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 67.Clavel M.A., Burwash I.G., Pibarot P. Cardiac imaging for assessing low-gradient severe aortic stenosis. JACC Cardiovasc. Imaging. 2017;10:185–202. doi: 10.1016/j.jcmg.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Scarsini R., Pighi M., Mainardi A., Portolan L., Springhetti P., Mammone C., Della Mora F., Fanti D., Tavella D., Gottin L., et al. Proof of concept study on coronary microvascular function in low flow low gradient aortic stenosis. Heart. 2023;109:785–793. doi: 10.1136/heartjnl-2022-321907. [DOI] [PubMed] [Google Scholar]
- 69.Benfari G., Noni M., Onorati F., Cerrito L.F., Pernigo M., Vinco G., Cameli M., Mandoli G.E., Borio G., Geremia G., et al. Effects of aortic valve replacement on left ventricular diastolic function in patients with aortic valve stenosis. Am. J. Cardiol. 2019;124:409–415. doi: 10.1016/j.amjcard.2019.04.046. [DOI] [PubMed] [Google Scholar]
- 70.Springhetti P., Tomaselli M., Benfari G., Milazzo S., Ciceri L., Penso M., Pilan M., Clement A., Rota A., Del Sole P.A., et al. Peak atrial longitudinal strain and risk stratification in moderate and severe aortic stenosis. Eur. Heart J. Cardiovasc. Imaging. 2024;25:947–957. doi: 10.1093/ehjci/jeae040. [DOI] [PubMed] [Google Scholar]
- 71.Guzzetti E., Poulin A., Annabi M.S., Zhang B., Kalavrouziotis D., Couture C., Dagenais F., Pibarot P., Clavel M.A. Transvalvular flow, sex, and survival after valve replacement surgery in patients with severe aortic stenosis. J. Am. Coll. Cardiol. 2020;75:1897–1909. doi: 10.1016/j.jacc.2020.02.065. [DOI] [PubMed] [Google Scholar]
- 72.Basile C., Fucile I., Lembo M., Manzi M.V., Ilardi F., Franzone A., Mancusi C. Arterial hypertension in aortic valve stenosis: A critical update. J. Clin. Med. 2021;10:5553. doi: 10.3390/jcm10235553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goliasch G., Lang I.M. Impact of sex on the management and outcome of aortic stenosis patients: A female aortic valve stenosis paradox, and a call for personalized treatments? Eur. Heart J. 2021;42:2692–2694. doi: 10.1093/eurheartj/ehab331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Côté N., Simard L., Zenses A.S., Tastet L., Shen M., Clisson M., Clavel M.A. Impact of vascular hemodynamics on aortic stenosis evaluation: New insights into the pathophysiology of normal flow-small aortic valve area-low gradient pattern. J. Am. Heart Assoc. 2017;6:e006276. doi: 10.1161/JAHA.117.006276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ito S., Miranda W.R., Nkomo V.T., Lewis B.R., Oh J.K. Sex differences in LV remodeling and hemodynamics in aortic stenosis: Sex-specific criteria for severe stenosis? JACC Cardiovasc. Imaging. 2022;15:1175–1189. doi: 10.1016/j.jcmg.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 76.Hariri E.H., El Halabi J., Kassis N., Al Hammoud M.M., Badwan O.Z., Layoun H., Kassab J., Al Shuab W., Bansal A., Farwati M., et al. Sex differences in the progression and long-term outcomes of native mild to moderate aortic stenosis. JACC Cardiovasc. Imaging. 2024;17:1–12. doi: 10.1016/j.jcmg.2023.06.006. [DOI] [PubMed] [Google Scholar]
- 77.Dayan V., Vignolo G., Magne J., Clavel M.A., Mohty D., Pibarot P. Outcome and impact of aortic valve replacement in patients with preserved LV ejection fraction and low gradient aortic stenosis: A meta-analysis. J. Am. Coll. Cardiol. 2015;66:2594–2603. doi: 10.1016/j.jacc.2015.09.076. [DOI] [PubMed] [Google Scholar]
- 78.Lam C.S., Roger V.L., Rodeheffer R.J., Bursi F., Borlaug B.A., Ommen S.R., Kass D.A., Redfield M.M. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chin C.W.L., Ding Z.P., Lam C.S.P., Ling L.H. Paradoxical low-gradient aortic stenosis: The HFpEF of aortic stenosis. J. Am. Coll. Cardiol. 2016;67:2447–2448. doi: 10.1016/j.jacc.2016.02.070. [DOI] [PubMed] [Google Scholar]
- 80.Reddy Y.N.V., Carter R.E., Obokata M., Redfield M.M., Borlaug B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ludwig S., Pellegrini C., Gossling A., Rheude T., Voigtländer L., Bhadra O.D., Linder M., Kalbacher D., Koell B., Waldschmidt L., et al. Prognostic value of the H(2) FPEF score in patients undergoing transcatheter aortic valve implantation. ESC Heart Fail. 2021;8:461–470. doi: 10.1002/ehf2.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoedemakers S., Verwerft J., Reddy Y.N.V., Delvaux R., Stroobants S., Jogani S., Claessen G., Droogmans S., Cosyns B., Borlaug B.A., et al. Cardiac dysfunction rather than aortic valve stenosis severity drives exercise intolerance and adverse haemodynamics. Eur. Heart J. Cardiovasc. Imaging. 2024;25:302–312. doi: 10.1093/ehjci/jead276. [DOI] [PubMed] [Google Scholar]
- 83.Kittleson M.M., Panjrath G.S., Amancherla K., Davis L.L., Deswal A., Dixon D.L., Januzzi J.L., Jr., Yancy C.W. 2023 ACC Expert Consensus Decision Pathway on management of heart failure with preserved ejection fraction: A report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2023;81:1835–1878. doi: 10.1016/j.jacc.2023.03.393. [DOI] [PubMed] [Google Scholar]
- 84.De Biase N., Mazzola M., Del Punta L., Di Fiore V., De Carlo M., Giannini C., Costa G., Paneni F., Mengozzi A., Nesti L., et al. Haemodynamic and metabolic phenotyping of patients with aortic stenosis and preserved ejection fraction: A specific phenotype of heart failure with preserved ejection fraction? Eur. J. Heart Fail. 2023;25:1947–1958. doi: 10.1002/ejhf.3018. [DOI] [PubMed] [Google Scholar]
- 85.Carroll J.D., Carroll E.P., Feldman T., Ward D.M., Lang R.M., McGaughey D., Karp R.B. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86:1099–1107. doi: 10.1161/01.CIR.86.4.1099. [DOI] [PubMed] [Google Scholar]
- 86.Maes F., Lerakis S., Barbosa Ribeiro H., Gilard M., Cavalcante J.L., Makkar R., Herrmann H.C., Windecker S., Enriquez-Sarano M., Cheema A.N., et al. Outcomes from transcatheter aortic valve replacement in patients with low-flow, low-gradient aortic stenosis and left ventricular ejection fraction less than 30%: A substudy from the TOPAS-TAVI registry. JAMA Cardiol. 2019;4:64–70. doi: 10.1001/jamacardio.2018.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kolte D., Bhardwaj B., Lu M., Alu M.C., Passeri J.J., Inglessis I., Vlahakes G.J., Garcia S., Cohen D.J., Lindman B.R., et al. Association between early left ventricular ejection fraction improvement after transcatheter aortic valve replacement and 5-year clinical outcomes. JAMA Cardiol. 2022;7:934–944. doi: 10.1001/jamacardio.2022.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Williams M., Kodali S.K., Hahn R.T., Humphries K.H., Nkomo V., Cohen D.J., Douglas P.S., Mack M., McAndrew T.C., Svensson L., et al. Sex-related differences in outcomes following transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis: Insights from the PARTNER Trial. J. Am. Coll. Cardiol. 2014;63:1522–1528. doi: 10.1016/j.jacc.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 89.Baumgartner H., Hung J., Bermejo J., Chambers J.B., Edvardsen T., Goldstein S., Lancellotti P., LeFevre M., Miller F., Jr., Otto C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017;30:372–392. doi: 10.1016/j.echo.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 90.Dweck M.R., Loganath K., Bing R., Treibel T.A., McCann G.P., Newby D.E., Leipsic J., Fraccaro C., Paolisso P., Cosyns B., et al. Multi-modality imaging in aortic stenosis: An EACVI clinical consensus document. Eur. Heart J. Cardiovasc. Imaging. 2023;24:1430–1443. doi: 10.1093/ehjci/jead153. [DOI] [PubMed] [Google Scholar]
- 91.Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., Guyton R.A., O’Gara P.T., Ruiz C.E., Skubas N.J., Sorajja P., et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary. J. Am. Coll. Cardiol. 2014;63:2438–2488. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 92.Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., 3rd, Fleisher L.A., Jneid H., Mack M.J., McLeod C.J., O’Gara P.T., et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J. Am. Coll. Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 93.Michelena H.I., Della Corte A., Evangelista A., Maleszewski J.J., Edwards W.D., Roman M.J., Devereux R.B., Fernández B., Asch F.M., Barker A.J., et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and Its aortopathy, for clinical, surgical, interventional and research purposes. Ann. Thorac. Surg. 2021;112:e203–e235. doi: 10.1016/j.athoracsur.2020.08.119. [DOI] [PubMed] [Google Scholar]
- 94.Stangl V., Baldenhofer G., Knebel F., Zhang K., Sanad W., Spethmann S., Grubitzsch H., Sander M., Wernecke K.D., Baumann G., et al. Impact of gender on three-month outcome and left ventricular remodeling after transfemoral transcatheter aortic valve implantation. Am. J. Cardiol. 2012;110:884–890. doi: 10.1016/j.amjcard.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 95.Lee L., Cotella J.I., Miyoshi T., Addetia K., Schreckenberg M., Hitschrich N., Blankenhagen M., Amuthan V., Citro R., Daimon M., et al. Normal Values of Left Ventricular Mass by Two-Dimensional and Three-Dimensional Echocardiography: Results from the World Alliance Societies of Echocardiography Normal Values Study. J. Am. Soc. Echocardiogr. 2023;36:533–542.e1. doi: 10.1016/j.echo.2022.12.016. [DOI] [PubMed] [Google Scholar]
- 96.Ngiam J.N., Chew N., Tan Y.Q.B., Sim H.W., Sia C.H., Low T.T., Kong W.K.F., Tay E.L., Kang G.S., Yeo T.C., et al. An Asian Perspective on Gender Differences in Clinical Outcomes and Echocardiographic Profiles of Patients with Medically Managed Severe Aortic Stenosis. Heart Lung Circ. 2021;30:115–120. doi: 10.1016/j.hlc.2019.06.725. [DOI] [PubMed] [Google Scholar]
- 97.Singh A., Chan D.C.S., Greenwood J.P., Dawson D.K., Sonecki P., Hogrefe K., Kelly D.J., Dhakshinamurthy V., Lang C.C., Khoo J.P., et al. Symptom onset in aortic stenosis: Relation to sex differences in left ventricular remodeling. JACC Cardiovasc. Imaging. 2019;12:96–105. doi: 10.1016/j.jcmg.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 98.Kuneman J.H., Singh G.K., Milhorini Pio S., Hirasawa K., Hautemann D., van der Kley F., Ajmone Marsan N., Knuuti J., Delgado V., Bax J.J. Sex differences in left ventricular remodelling in patients with severe aortic valve stenosis. Eur. Heart J. Cardiovasc. Imaging. 2022;23:781–789. doi: 10.1093/ehjci/jeab174. [DOI] [PubMed] [Google Scholar]
- 99.Ninomiya R., Orii M., Fujiwara J., Yoshizawa M., Nakajima Y., Ishikawa Y., Kumagai A., Fusazaki T., Tashiro A., Kin H., et al. Sex-Related Differences in Cardiac Remodeling and Reverse Remodeling after Transcatheter Aortic Valve Implantation in Patients with Severe Aortic Stenosis in a Japanese Population. Int. Heart J. 2020;61:961–969. doi: 10.1536/ihj.20-154. [DOI] [PubMed] [Google Scholar]
- 100.Agoston-Coldea L., Bheecarry K., Petra C., Strambu L., Ober C., Revnic R., Lupu S., Mocan T., Fodor D. The value of global longitudinal strain and galectin-3 for predicting cardiovascular events in patients with severe aortic stenosis. Med. Ultrason. 2018;20:205. doi: 10.11152/mu-1456. [DOI] [PubMed] [Google Scholar]
- 101.Arbucci R., Lowenstein Haber D.M., Rousse M.G., Saad A.K., Martinez Golleti L., Gastaldello N., Amor M., Caniggia C., Merlo P., Zambrana G., et al. Long Term Prognostic Value of Contractile Reserve Assessed by Global Longitudinal Strain in Patients with Asymptomatic Severe Aortic Stenosis. J. Clin. Med. 2022;11:689. doi: 10.3390/jcm11030689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stassen J., Pio S.M., Ewe S.H., Singh G.K., Hirasawa K., Butcher S.C., Cohen D.J., Genereux P., Leon M.B., Marsan N.A., et al. Left Ventricular Global Longitudinal Strain in Patients with Moderate Aortic Stenosis. J. Am. Soc. Echocardiogr. 2022;35:791–800.e4. doi: 10.1016/j.echo.2022.03.008. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y., Zhang M., Chen H., Li H. Prognostic Value of Global Longitudinal Strain in Asymptomatic Aortic Stenosis: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022;9:778027. doi: 10.3389/fcvm.2022.778027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gutierrez-Ortiz E., Olmos C., Carrion-Sanchez I., Jimenez-Quevedo P., Nombela-Franco L., Parraga R., Gil-Abizanda S., Mahia P., Luaces M., de Agustin J.A., et al. Redefining cardiac damage staging in aortic stenosis: The value of GLS and RVAc. Eur. Heart J. Cardiovasc. Imaging. 2023;24:1608–1617. doi: 10.1093/ehjci/jead140. [DOI] [PubMed] [Google Scholar]
- 105.Delgado V., Tops L.F., van Bommel R.J., van derr Kley F., Marsan N.A., Klautz R.J., Versteegh M.I., Holman E.R., Schalij M.J., Bax J.J. Strain analysis in patients with severe aortic stenosis and preserved left ventricular ejection fraction undergoing surgical valve replacement. Eur. Heart J. 2009;30:3037–3047. doi: 10.1093/eurheartj/ehp351. [DOI] [PubMed] [Google Scholar]
- 106.Vollema E.M., Sugimoto T., Shen M., Tastet L., Ng A.C.T., Abou R., Marsan N.A., Mertens B., Dulgheru R., Lancellotti P., et al. Association of left ventricular global longitudinal strain with asymptomatic severe aortic stenosis natural course and prognostic value. JAMA Cardiol. 2018;3:839–847. doi: 10.1001/jamacardio.2018.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nagata Y., Takeuchi M., Wu V.C., Izumo M., Suzuki K., Sato K., Seo Y., Akashi Y.J., Aonuma K., Otsuji Y. Prognostic value of LV deformation parameters using 2D and 3D speckle-tracking echocardiography in asymptomatic patients with severe aortic stenosis and preserved LV ejection fraction. JACC Cardiovasc. Imaging. 2015;8:235–245. doi: 10.1016/j.jcmg.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 108.Carstensen H.G., Larsen L.H., Hassager C., Kofoed K.F., Jensen J.S., Mogelvang R. Basal longitudinal strain predicts future aortic valve replacement in asymptomatic patients with aortic stenosis. Eur. Heart J. Cardiovasc. Imaging. 2016;17:283–292. doi: 10.1093/ehjci/jev143. [DOI] [PubMed] [Google Scholar]
- 109.Abecasis J., Gomes Pinto D., Ramos S., Masci P.G., Cardim N., Gil V., Félix A. Left ventricular remodeling in degenerative aortic valve stenosis. Curr. Probl. Cardiol. 2021;46:100801. doi: 10.1016/j.cpcardiol.2021.100801. [DOI] [PubMed] [Google Scholar]
- 110.Ternacle J., Krapf L., Mohty D., Magne J., Nguyen A., Galat A., Gallet R., Teiger E., Cote N., Clavel M.A., et al. Aortic stenosis and cardiac amyloidosis: JACC review topic of the week. J. Am. Coll. Cardiol. 2019;74:2638–2651. doi: 10.1016/j.jacc.2019.09.056. [DOI] [PubMed] [Google Scholar]
- 111.Mogensen N.S.B., Ali M., Carter-Storch R., Annabi M.-S., Grenier-Delaney J., Møller J.E., Øvrehus K.A., Pellikka P.A., Pibarot P., Clavel M.-A., et al. Dobutamine Stress Echocardiography in Low-Gradient Aortic Stenosis. J. Am. Soc. Echocardiogr. 2024. in press . [DOI] [PubMed]
- 112.Annabi M.S., Touboul E., Dahou A., Burwash I.A., Bergler-Klein J., Enriquez-Sarano M., Orwat S., Baumgartner H., Mascherbauer J., Mundigler G., et al. Dobutamine stress echocardiography for management of low-flow, low-gradient aortic stenosis. J. Am. Coll. Cardiol. 2018;71:475–485. doi: 10.1016/j.jacc.2017.11.052. [DOI] [PubMed] [Google Scholar]
- 113.Dweck M.R., Chin C., Newby D.E. Small valve area with low gradient aortic stenosis: Beware the hard hearted. J. Am. Coll. Cardiol. 2013;62:2339–2340. doi: 10.1016/j.jacc.2013.08.1620. [DOI] [PubMed] [Google Scholar]
- 114.Pawade T., Sheth T., Guzzetti E., Dweck M.R., Clavel M.A. Why and how to measure aortic valve calcification in patients with aortic stenosis. JACC Cardiovasc. Imaging. 2019;12:1835–1848. doi: 10.1016/j.jcmg.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 115.Khurrami L., Moller J.E., Lindholt J.S., Dahl J.S., Fredgart M.H., Obel L.M., Steffensen F.H., Urbonaviciene G., Lambrechtsen J., Diederichsen A.C.P. Aortic valve calcification among elderly males from the general population, associated echocardiographic findings, and clinical implications. Eur. Heart J. Cardiovasc. Imaging. 2022;23:177–184. doi: 10.1093/ehjci/jeab182. [DOI] [PubMed] [Google Scholar]
- 116.Mogensen N.S.B., Sanchez Dahl J., Ali M., Annabi M.-S., Haujir A., Powers A., Carter-Storch R., Grenier-Delaney J., Møller J.E., Øvrehus K.A., et al. Usefulness of aortic valve calcification in patients with low flow aortic stenosis. Medrxiv Prepr. Serv. Health Sci. 2024. preprint . [DOI]
- 117.Shen M., Guzzetti E., Oh J.K., Singh G.K., Pawade T., Tastet L., Clavel M.A., Delgado V., Bax J.J., Dweck M.R., et al. Computed tomography aortic valve calcium scoring in patients with bicuspid aortic valve stenosis. Struct. Heart. 2022;6:100027. doi: 10.1016/j.shj.2022.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Powers A., Ali M., Lavoie N., Haujir A., Mogensen N.S.B., Ludwig S., Øvrehus K.A., Tastet L., Rhéaume C., Schofer N., et al. Aortic valve calcification density measured by MDCT in the assessment of aortic stenosis severity. Circ. Cardiovasc. Imaging. 2024;17:e016267. doi: 10.1161/CIRCIMAGING.123.016267. [DOI] [PubMed] [Google Scholar]
- 119.Cartlidge T.R., Bing R., Kwiecinski J., Guzzetti E., Pawade T.A., Doris M.K., Adamson P.D., Massera D., Lembo M., Peeters F., et al. Contrast-enhanced computed tomography assessment of aortic stenosis. Heart. 2021;107:1905–1911. doi: 10.1136/heartjnl-2020-318556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gaglia M.A., Lipinski M.J., Torguson R., Gai J., Ben-Dor I., Bernardo N.L., Satler L.F., Pichard A.D., Waksman R. Comparison in Men Versus Women of Co-morbidities, Complications, and Outcomes after Transcatheter Aortic Valve Implantation for Severe Aortic Stenosis. Am. J. Cardiol. 2016;118:1692–1697. doi: 10.1016/j.amjcard.2016.08.049. [DOI] [PubMed] [Google Scholar]
- 121.Czernin J., Satyamurthy N., Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J. Nucl. Med. 2010;51:1826–1829. doi: 10.2967/jnumed.110.077933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dweck M.R., Boon N.A., Newby D.E. Calcific aortic stenosis: A disease of the valve and the myocardium. J. Am. Coll. Cardiol. 2012;60:1854–1863. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 123.Dweck M.R., Jenkins W.S., Vesey A.T., Pringle M.A., Chin C.W., Malley T.S., Cowie W.J., Tsampasian V., Richardson H., Fletcher A., et al. 18F-NaF uptake Is a marker of active calcification and disease progression in patients with aortic stenosis. Circ. Cardiovasc. Imaging. 2014;7:371–378. doi: 10.1161/CIRCIMAGING.113.001508. [DOI] [PubMed] [Google Scholar]
- 124.Jenkins W.S., Vesey A.T., Shah A.S., Pawade T.A., Chin C.W., White A.C., Fletcher A., Cartlidge T.R., Mitchell A.G., Pringle M.A., et al. Valvular (18)F-fluoride and (18)F-fluorodeoxyglucose uptake predict disease progression and clinical outcome in patients with aortic stenosis. J. Am. Coll. Cardiol. 2015;66:1200–1201. doi: 10.1016/j.jacc.2015.06.1325. [DOI] [PubMed] [Google Scholar]
- 125.Cartlidge T.R.G., Doris M.K., Sellers S.L., Pawade T.A., White A.C., Pessotto R., Kwiecinski J., Fletcher A., Alcaide C., Lucatelli C., et al. Detection and prediction of bioprosthetic aortic valve degeneration. J. Am. Coll. Cardiol. 2019;73:1107–1119. doi: 10.1016/j.jacc.2018.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kwiecinski J., Tzolos E., Cartlidge T.R.G., Fletcher A., Doris M.K., Bing R., Tarkin J.M., Seidman M.A., Gulsin G.S., Cruden N.L., et al. Native aortic valve disease progression and bioprosthetic valve degeneration in patients with transcatheter aortic valve implantation. Circulation. 2021;144:1396–1408. doi: 10.1161/CIRCULATIONAHA.121.056891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Larsson S.C., Wolk A., Hakansson N., Back M. Overall and abdominal obesity and incident aortic valve stenosis: Two prospective cohort studies. Eur. Heart J. 2017;38:2192–2197. doi: 10.1093/eurheartj/ehx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Legget M.E., Kuusisto J., Healy N.L., Fujioka M., Schwaegler R.G., Otto C.M. Gender differences in left ventricular function at rest and wih exercis in asymptomatic aortic stenosis. Am. Heart J. 1996;131:94–100. doi: 10.1016/S0002-8703(96)90056-3. [DOI] [PubMed] [Google Scholar]
- 129.Tribouilloy C., Bohbot Y., Rusinaru D., Belkhir K., Diouf M., Altes A., Delpierre Q., Serbout S., Kubala M., Levy F., et al. Excess mortality and undertreatment of women with severe aortic stenosis. J. Am. Heart Assoc. 2021;10:e018816. doi: 10.1161/JAHA.120.018816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hartzell M., Malhotra R., Yared K., Rosenfield H.R., Walker J.D., Wood M.J. Effect of gender on treatment and outcomes in severe aortic stenosis. Am. J. Cardiol. 2011;107:1681–1686. doi: 10.1016/j.amjcard.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Freed B.H., Sugeng L., Furlong K., Mor-Avi V., Raman J., Jeevanandam V., Lang R.M. Reasons for nonadherence to guidelines for aortic valve replacement in patients with severe aortic stenosis and potential solutions. Am. J. Cardiol. 2010;105:1339–1342. doi: 10.1016/j.amjcard.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 132.Chaker Z., Badhwar V., Alqahtani F., Aljohani S., Zack C.J., Holmes D.R., Rihal C.S., Alkhouli M. Sex differences in the utilization and outcomes of surgical aortic valve replacement for severe aortic stenosis. J. Am. Heart Assoc. 2017;6:e006370. doi: 10.1161/JAHA.117.006370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brown J.M., O’Brien S.M., Wu C., Sikora J.A., Griffith B.P., Gammie J.S. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: Changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J. Thorac. Cardiovasc. Surg. 2009;137:82–90. doi: 10.1016/j.jtcvs.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 134.Ter Woorst J.F., Hoff A.H.T., van Straten A.H.M., Houterman S., Soliman-Hamad M.A. Impact of Sex on the Outcome of Isolated Aortic Valve Replacement and the Role of Different Preoperative Profiles. J. Cardiothorac. Vasc. Anesth. 2019;33:1237–1243. doi: 10.1053/j.jvca.2018.08.196. [DOI] [PubMed] [Google Scholar]
- 135.Fuchs C., Mascherbauer J., Rosenhek R., Pernicka E., Klaar U., Scholten C., Heger M., Wollenek G., Czerny M., Maurer G., et al. Gender differences in clinical presentation and surgical outcome of aortic stenosis. Heart. 2010;96:539–545. doi: 10.1136/hrt.2009.186650. [DOI] [PubMed] [Google Scholar]
- 136.Skelding K.A., Yakubov S.J., Kleiman N.S., Reardon M.J., Adams D.H., Huang J., Forrest J.K., Popma J.J. Transcatheter aortic valve replacement versus surgery in women at high risk for surgical aortic valve replacement (from the CoreValve US high risk pivotal trial) Am. J. Cardiol. 2016;118:560–566. doi: 10.1016/j.amjcard.2016.05.051. [DOI] [PubMed] [Google Scholar]
- 137.Sannino A., Szerlip M., Harrington K., Schiattarella G.G., Grayburn P.A. Comparison of Baseline Characteristics and Outcomes in Men Versus Women with Aortic Stenosis Undergoing Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2018;121:844–849. doi: 10.1016/j.amjcard.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 138.Hayashida K., Morice M.C., Chevalier B., Hovasse T., Romano M., Garot P., Farge A., Donzeau-Gouge P., Bouvier E., Cormier B., et al. Sex-related differences in clinical presentation and outcome of transcatheter aortic valve implantation for severe aortic stenosis. J. Am. Coll. Cardiol. 2012;59:566–571. doi: 10.1016/j.jacc.2011.10.877. [DOI] [PubMed] [Google Scholar]
- 139.Saad M., Nairooz R., Pothineni N.V.K., Almomani A., Kovelamudi S., Sardar P., Katz M., Abdel-Wahab M., Bangalore S., Kleiman N.S., et al. Long-term outcomes with transcatheter aortic valve replacement in women compared with men: Evidence from a meta-analysis. JACC Cardiovasc. Interv. 2017;11:24–35. doi: 10.1016/j.jcin.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 140.Kodali S., Williams M.R., Doshi D., Hahn R.T., Humphries K.H., Nkomo V.T., Cohen D.J., Douglas P.S., Mack M., Xu K., et al. Sex-specific differences at presentation and outcomes among patients undergoing transcatheter aortic valve replacement: A cohort study. Ann. Intern. Med. 2016;164:377–384. doi: 10.7326/M15-0121. [DOI] [PubMed] [Google Scholar]
- 141.Humphries K.H., Toggweiler S., Rodés-Cabau J., Nombela-Franco L., Dumont É., Wood D.A., Willson A.B., Binder R.K., Freeman M., Lee M.K., et al. Sex differences in mortality after transcatheter aortic valve replacement for severe aortic stenosis. J. Am. Coll. Cardiol. 2012;60:882–886. doi: 10.1016/j.jacc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 142.Biere L., Launay M., Pinaud F., Hamel J.F., Eltchaninoff H., Iung B., Laskar M., Leguerrier A., Gilard M., Furber A. Influence of sex on mortality and perioperative outcomes in patients undergoing TAVR: Insights from the FRANCE 2 registry. J. Am. Coll. Cardiol. 2015;65:755–757. doi: 10.1016/j.jacc.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 143.Czarnecki A., Qiu F., Koh M., Prasad T.J., Cantor W.J., Cheema A.N., Chu M.W.A., Feindel C., Fremes S.E., Kingsbury K., et al. Clinical outcomes after trans-catheter aortic valve replacement in men and women in Ontario, Canada. Catheter. Cardiovasc. Interv. 2017;90:486–494. doi: 10.1002/ccd.26906. [DOI] [PubMed] [Google Scholar]
- 144.Katz M., Carlos Bacelar Nunes Filho A., Caixeta A., Antonio Carvalho L., Sarmento-Leite R., Alves Lemos Neto P., Eduardo Koenig Sao Thiago L., Dias Dourado Oliveira A., Antonio Marino M., Tadeu Tumelero R., et al. Gender-related differences on short- and long-term outcomes of patients undergoing transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2017;89:429–436. doi: 10.1002/ccd.26658. [DOI] [PubMed] [Google Scholar]
- 145.Szerlip M., Gualano S., Holper E., Squiers J.J., White J.M., Doshi D., Williams M.R., Hahn R.T., Webb J.G., Svensson L.G., et al. Sex-specific outcomes of transcatheter aortic valve replacement with the SAPIEN 3 valve: Insights from the PARTNER II S3 high-risk and intermediate-risk cohorts. JACC Cardiovasc. Interv. 2018;11:13–20. doi: 10.1016/j.jcin.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 146.Zhao Z.G., Liao Y.B., Peng Y., Chai H., Liu W., Li Q., Ren X., Wang X.Q., Luo X.L., Zhang C., et al. Sex-related differences in outcomes after transcatheter aortic valve implantation: A systematic review and meta-analysis. Circ. Cardiovasc. Interv. 2013;6:543–551. doi: 10.1161/CIRCINTERVENTIONS.113.000529. [DOI] [PubMed] [Google Scholar]
- 147.Stangl V., Baldenhofer G., Laule M., Baumann G., Stangl K. Influence of sex on outcome following transcatheter aortic valve implantation (TAVI): Systematic review and meta-analysis. J. Interv. Cardiol. 2014;27:531–539. doi: 10.1111/joic.12150. [DOI] [PubMed] [Google Scholar]
- 148.Avvedimento M., Nuche J., Farjat-Pasos J.I., Rodés-Cabau J. Bleeding events after transcatheter aortic valve replacement: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2023;81:684–702. doi: 10.1016/j.jacc.2022.11.050. [DOI] [PubMed] [Google Scholar]
- 149.Kodali S.K., Williams M.R., Smith C.R., Svensson L.G., Webb J.G., Makkar R.R., Fontana G.P., Dewey T.M., Thourani V.H., Pichard A.D., et al. Two-Year outcomes after transcatheter or surgical aortic-valve replacement. N. Engl. J. Med. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 150.Adams D.H., Popma J.J., Reardon M.J., Yakubov S.J., Coselli J.S., Deeb G.M., Gleason T.G., Buchbinder M., Hermiller J., Jr., Kleiman N.S., et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 151.Abdel-Wahab M., Zahn R., Horack M., Gerckens U., Schuler G., Sievert H., Eggebrecht H., Senges J., Richardt G. Aortic regurgitation after transcatheter aortic valve implantation: Incidence and early outcome. Results from the German transcatheter aortic valve interventions registry. Heart. 2011;97:899–906. doi: 10.1136/hrt.2010.217158. [DOI] [PubMed] [Google Scholar]
- 152.Unbehaun A., Pasic M., Dreysse S., Drews T., Kukucka M., Mladenow A., Ivanitskaja-Kuhn E., Hetzer R., Buz S. Transapical aortic valve implantation: Incidence and predictors of paravalvular leakage and transvalvular regurgitation in a series of 358 patients. J. Am. Coll. Cardiol. 2012;59:211–221. doi: 10.1016/j.jacc.2011.10.857. [DOI] [PubMed] [Google Scholar]
- 153.Ferrante G., Pagnotta P., Petronio A.S., Bedogni F., Brambilla N., Fiorina C., Giannini C., Mennuni M., De Marco F., Klugmann S., et al. Sex differences in postprocedural aortic regurgitation and mid-term mortality after transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2014;84:264–271. doi: 10.1002/ccd.25377. [DOI] [PubMed] [Google Scholar]
- 154.Conrotto F., D’Ascenzo F., Presbitero P., Humphries K.H., Webb J.G., O’Connor S.A., Morice M.C., Lefevre T., Grasso C., Sbarra P., et al. Effect of gender after transcatheter aortic valve implantation: A meta-analysis. Ann. Thorac. Surg. 2015;99:809–816. doi: 10.1016/j.athoracsur.2014.09.089. [DOI] [PubMed] [Google Scholar]
- 155.O’Connor S.A., Morice M.C., Gilard M., Leon M.B., Webb J.G., Dvir D., Rodes-Cabau J., Tamburino C., Capodanno D., D’Ascenzo F., et al. Revisiting Sex Equality with Transcatheter Aortic Valve Replacement Outcomes: A Collaborative, Patient-Level Meta-Analysis of 11,310 Patients. J. Am. Coll. Cardiol. 2015;66:221–228. doi: 10.1016/j.jacc.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 156.Van Mieghem N.M., Reardon M.J., Yakubov S.J., Heiser J., Merhi W., Windecker S., Makkar R.R., Cheng W., Robbins M., Fail P., et al. Clinical outcomes of TAVI or SAVR in men and women with aortic stenosis at intermediate operative risk: A post hoc analysis of the randomised SURTAVI trial. EuroIntervention. 2020;16:833–841. doi: 10.4244/EIJ-D-20-00303. [DOI] [PubMed] [Google Scholar]
- 157.Ahmed B., Lischke S., Holterman L.A., Straight F., Dauerman H.L. Angiographic predictors of vascular complications among women undergoing cardiac catheterization and intervention. J. Invasive Cardiol. 2010;22:512–516. [PubMed] [Google Scholar]
- 158.Mack M.J., Leon M.B., Thourani V.H., Pibarot P., Hahn R.T., Genereux P., Kodali S.K., Kapadia S.R., Cohen D.J., Pocock S.J., et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N. Engl. J. Med. 2023;389:1949–1960. doi: 10.1056/NEJMoa2307447. [DOI] [PubMed] [Google Scholar]
- 159.Duncan A.I., Lin J., Koch C.G., Gillinov A.M., Xu M., Starr N.J. The impact of gender on in-hospital mortality and morbidity after isolated aortic valve replacement. Anesth. Analg. 2006;103:800–808. doi: 10.1213/01.ane.0000231890.95212.12. [DOI] [PubMed] [Google Scholar]
- 160.Elhmidi Y., Piazza N., Mazzitelli D., Wottke M., Lange R., Bleiziffer S. Sex-related differences in 2197 patients undergoing isolated surgical aortic valve replacement. J. Card. Surg. 2014;29:772–778. doi: 10.1111/jocs.12442. [DOI] [PubMed] [Google Scholar]
- 161.López-de-Andrés A., Méndez-Bailón M., Perez-Farinos N., Hernández-Barrera V., de Miguel-Díez J., Muñoz-Rivas N., Jiménez-García R. Gender differences in incidence and in-hospital outcomes of surgical aortic valve replacement in Spain, 2001–2015. Eur. J. Public Health. 2019;29:674–680. doi: 10.1093/eurpub/ckz019. [DOI] [PubMed] [Google Scholar]
- 162.Onorati F., D’Errigo P., Barbanti M., Rosato S., Covello R.D., Maraschini A., Ranucci M., Santoro G., Tamburino C., Grossi C., et al. Different impact of sex on baseline characteristics and major periprocedural outcomes of transcatheter and surgical aortic valve interventions: Results of the multicenter Italian OBSERVANT Registry. J. Thorac. Cardiovasc. Surg. 2014;147:1529–1539. doi: 10.1016/j.jtcvs.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 163.Chieffo A., Petronio A.S., Mehilli J., Chandrasekhar J., Sartori S., Lefevre T., Presbitero P., Capranzano P., Tchetche D., Iadanza A., et al. 1-Year Clinical Outcomes in Women after Transcatheter Aortic Valve Replacement: Results From the First WIN-TAVI Registry. JACC Cardiovasc. Interv. 2018;11:1–12. doi: 10.1016/j.jcin.2017.09.034. [DOI] [PubMed] [Google Scholar]
- 164.Petrovic M., Spirito A., Sartori S., Vogel B., Tchetche D., Petronio A.S., Mehilli J., Lefevre T., Presbitero P., Capranzano P., et al. Prognostic impact of prefrailty and frailty in women undergoing TAVR: Insights from the WIN-TAVI registry. Can. J. Cardiol. 2024;40:457–467. doi: 10.1016/j.cjca.2023.10.024. [DOI] [PubMed] [Google Scholar]
- 165.Eltchaninoff H., Bonaros N., Prendergast B., Nietlispach F., Vasa-Nicotera M., Chieffo A., Pibarot P., Bramlage P., Sykorova L., Kurucova J., et al. Rationale and design of a prospective, randomized, controlled, multicenter study to evaluate the safety and efficacy of transcatheter heart valve replacement in female patients with severe symptomatic aortic stenosis requiring aortic valve intervention (Randomized researcH in womEn all comers wIth Aortic stenosis [RHEIA] trial) Am. Heart J. 2020;228:27–35. doi: 10.1016/j.ahj.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 166.David T.E., Woo A., Armstrong S., Maganti M. When is the Ross operation a good option to treat aortic valve disease? J. Thorac. Cardiovasc. Surg. 2010;139:68–73; discussion 73–65. doi: 10.1016/j.jtcvs.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 167.Pieper P.G., Balci A., Van Dijk A.P. Pregnancy in women with prosthetic heart valves. Neth. Heart J. 2008;16:406–411. doi: 10.1007/BF03086187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McLintock C. Anticoagulant therapy in pregnant women with mechanical prosthetic heart valves: No easy option. Thromb. Res. 2011;127((Suppl. S3)):S56–S60. doi: 10.1016/S0049-3848(11)70016-0. [DOI] [PubMed] [Google Scholar]
- 169.Simard L., Perron J., Shen M., Tastet L., Mohammadi S., Clisson M., Poulin A., Clavel M.A. Vascular burden impact on echocardiographic valvular graft degeneration following a Ross procedure in young adults. J. Am. Coll. Cardiol. 2017;70:1099–1101. doi: 10.1016/j.jacc.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 170.Bouhout I., Poirier N., Mazine A., Dore A., Mercier L.A., Leduc L., El-Hamamsy I. Cardiac, obstetric, and fetal outcomes during pregnancy after biological or mechanical aortic valve replacement. Can. J. Cardiol. 2014;30:801–807. doi: 10.1016/j.cjca.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 171.Basude S., Trinder J., Caputo M., Curtis S.L. Pregnancy outcome and follow-up cardiac outcome in women with aortic valve replacement. Obstet. Med. 2014;7:29–33. doi: 10.1177/1753495X13514382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Heuvelman H.J., Arabkhani B., Cornette J.M., Pieper P.G., Bogers A.J., Takkenberg J.J., Roos-Hesselink J.W. Pregnancy outcomes in women with aortic valve substitutes. Am. J. Cardiol. 2013;111:382–387. doi: 10.1016/j.amjcard.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 173.Carvajal H.G., Lindley K.J., Shah T., Brar A.K., Barger P.M., Billadello J.J., Eghtesady P. Impact of pregnancy on autograft dilatation and aortic valve function following the Ross procedure. Congenit. Heart Dis. 2018;13:217–221. doi: 10.1111/chd.12554. [DOI] [PubMed] [Google Scholar]
- 174.Thompson S.E., Prabhakar C.R.K., Creasey T., Stoll V.M., Gurney L., Green J., Fox C., Morris R.K., Thompson P.J., Thorne S.A., et al. Pregnancy outcomes in women following the Ross procedure. Int. J. Cardiol. 2023;371:135–139. doi: 10.1016/j.ijcard.2022.09.069. [DOI] [PubMed] [Google Scholar]
- 175.Rahimtoola S.H. The problem of valve prosthesis-patient mismatch. Circulation. 1978;58:20–24. doi: 10.1161/01.CIR.58.1.20. [DOI] [PubMed] [Google Scholar]
- 176.Pibarot P., Dumesnil J.G. Prosthesis-patient mismatch: Definition, clinical impact, and prevention. Heart. 2006;92:1022–1029. doi: 10.1136/hrt.2005.067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fallon J.M., DeSimone J.P., Brennan J.M., O’Brien S., Thibault D.P., DiScipio A.W., Pibarot P., Jacobs J.P., Malenka D.J. The incidence and consequence of prosthesis-patient mismatch after surgical aortic valve replacement. Ann. Thorac. Surg. 2018;106:14–22. doi: 10.1016/j.athoracsur.2018.01.090. [DOI] [PubMed] [Google Scholar]
- 178.Dahlbacka S., Laakso T., Kinnunen E.M., Moriyama N., Laine M., Virtanen M., Maaranen P., Ahvenvaara T., Tauriainen T., Husso A., et al. Patient-prosthesis mismatch worsens long-term survival: Insights from the FinnValve registry. Ann. Thorac. Surg. 2021;111:1284–1290. doi: 10.1016/j.athoracsur.2020.06.026. [DOI] [PubMed] [Google Scholar]
- 179.Blais C., Dumesnil J.G., Baillot R., Simard S., Doyle D., Pibarot P. Impact of valve prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation. 2003;108:983–988. doi: 10.1161/01.CIR.0000085167.67105.32. [DOI] [PubMed] [Google Scholar]
- 180.Ternacle J., Abbas A.E., Pibarot P. Prosthesis-patient mismatch after transcatheter aortic valve replacement: Has it become obsolete ? JACC Cardiovasc. Interv. 2021;14:977–980. doi: 10.1016/j.jcin.2021.03.039. [DOI] [PubMed] [Google Scholar]
- 181.Généreux P., Piazza N., Alu M.C., Nazif T., Hahn R.T., Pibarot P., Bax J.J., Leipsic J.A., Blanke P., Blackstone E.H., et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021;42:1825–1857. doi: 10.1016/j.jacc.2021.02.038. [DOI] [PubMed] [Google Scholar]
- 182.Kappetein A.P., Head S.J., Généreux P., Piazza N., Van Mieghem N.M., Blackstone E.H., Brott T.G., Cohen D.J., Cutlip D.E., van Es G.A., et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. Eur. J. Cardiothorac. Surg. 2012;42:S45–S60. doi: 10.1093/ejcts/ezs533. [DOI] [PubMed] [Google Scholar]
- 183.Thourani V.H., Abbas A.E., Ternacle J., Hahn R.T., Makkar R., Kodali S.K., George I., Kapadia S., Svensson L.G., Szeto W.Y., et al. Patient-prosthesis mismatch after surgical aortic valve replacement: Analysis of the PARTNER trials. Ann. Thorac. Surg. 2024;117:1164–1171. doi: 10.1016/j.athoracsur.2024.01.023. [DOI] [PubMed] [Google Scholar]
- 184.Levesque T., Eltchaninoff H., Chabannes R., Barbe T., Dosseh O., Tron C., Bettinger N., Bouhzam N., Hemery T., Le Pessec G., et al. Impact of prosthesis-patient mismatch after transcatheter aortic valve replacement. Can. J. Cardiol. 2024;40:113–122. doi: 10.1016/j.cjca.2023.09.012. [DOI] [PubMed] [Google Scholar]
- 185.Pibarot P., Weissman N.J., Stewart W.J., Hahn R.T., Lindman B.R., McAndrew T., Kodali S.K., Mack M.J., Thourani V.H., Miller D.C., et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis- A PARTNER trial cohort A analysis. J. Am. Coll. Cardiol. 2014;64:1323–1334. doi: 10.1016/j.jacc.2014.06.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Herrmann H.C., Daneshvar S.A., Fonarow G.C., Stebbins A., Vemulapalli S., Desai N.D., Malenka D.J., Thourani V.H., Rymer J., Kosinski A.S. Prosthesis-patient mismatch in patients undergoing transcatheter aortic valve replacement: From the STS/ACC TVT registry. J. Am. Coll. Cardiol. 2018;72:2701–2711. doi: 10.1016/j.jacc.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 187.Freitas-Ferraz A.B., Tirado-Conte G., Dagenais F., Ruel M., Al-Atassi T., Dumont E., Mohammadi S., Bernier M., Pibarot P., Rodés-Cabau J. Aortic stenosis and small aortic annulus. Circulation. 2019;139:2685–2702. doi: 10.1161/CIRCULATIONAHA.118.038408. [DOI] [PubMed] [Google Scholar]
- 188.Pibarot P., Dumesnil J.G. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J. Am. Coll. Cardiol. 2000;36:1131–1141. doi: 10.1016/S0735-1097(00)00859-7. [DOI] [PubMed] [Google Scholar]
- 189.Elmahdy W., Osman M., Farag M., Shoaib A., Saad H., Sullivan K., Krishnan U., Nashef S., Berman M. Prosthesis-patient mismatch increases early and late mortality in low risk aortic valve replacement. Semin. Thorac. Cardiovasc. Surg. 2021;33:23–30. doi: 10.1053/j.semtcvs.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 190.Mihos C.G., Klassen S.L., Yucel E. Sex-specific considerations in women with aortic stenosis and outcomes after transcatheter aortic valve replacement. Curr. Treat. Options Cardiovasc. Med. 2018;20:52. doi: 10.1007/s11936-018-0651-x. [DOI] [PubMed] [Google Scholar]
- 191.Pibarot P., Magne J., Leipsic J., Côté N., Blanke P., Thourani V.H., Hahn R. Imaging for predicting and assessing prosthesis-patient mismatch after aortic valve replacement. JACC Cardiovasc. Imaging. 2019;12:149–162. doi: 10.1016/j.jcmg.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 192.Rodes-Cabau J., Ribeiro H.B., Mohammadi S., Serra V., Al-Atassi T., Iniguez A., Vilalta V., Nombela-Franco L., Saez de Ibarra Sanchez J.I., Auffret V., et al. Transcatheter or Surgical Aortic Valve Replacement in Patients with Severe Aortic Stenosis and Small Aortic Annulus: A Randomized Clinical Trial. Circulation. 2024;149:644–655. doi: 10.1161/CIRCULATIONAHA.123.067326. [DOI] [PubMed] [Google Scholar]
- 193.Carter-Storch R., Hahn R.T., Abbas A.E., Daubert M.A., Douglas P.S., Elmariah S., Zhao Y., Mack M.J., Leon M.B., Pibarot P. Effect of Sex and Flow Status on Outcomes after Surgical or Transcatheter Aortic Valve Replacement. JACC Adv. 2024;3:100853. doi: 10.1016/j.jacadv.2024.100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ruel M., Al-Faleh H., Kulik A., Chan K., Mesana T.G., Burwash I.G. Prosthesis-patient mismatch after aortic valve replacement predominantly affects patients with pre-existing left ventricular dysfunction: Effect on survival, freedom from heart failure, and left ventricular mass regression. J. Thorac. Cardiovasc. Surg. 2006;131:1036–1044. doi: 10.1016/j.jtcvs.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 195.Mohty D., Dumesnil J.G., Echahidi N., Mathieu P., Dagenais F., Voisine P., Pibarot P. Impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: Influence of age, obesity, and left ventricular dysfunction. J. Am. Coll. Cardiol. 2009;53:39–47. doi: 10.1016/j.jacc.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 196.Mohty D., Boulogne C., Magne J., Pibarot P., Echahidi N., Cornu E., Dumesnil J., Laskar M., Virot P., Aboyans V. Prevalence and long-term outcome of aortic prosthesis-patient mismatch in patients with paradoxical low-flow severe aortic stenosis. Circulation. 2014;130:S25–S31. doi: 10.1161/CIRCULATIONAHA.113.007819. [DOI] [PubMed] [Google Scholar]
- 197.Annabi M.S., Clisson M., Clavel M.A., Pibarot P. Workup and management of patients with paradoxical low-flow, low-gradient aortic stenosis. Curr. Treat. Options Cardiovasc. Med. 2018;20:49. doi: 10.1007/s11936-018-0642-y. [DOI] [PubMed] [Google Scholar]
- 198.Zoghbi W.A., Jone P.N., Chamsi-Pasha M.A., Chen T., Collins K.A., Desai M.Y., Grayburn P., Groves D.W., Hahn R.T., Little S.H., et al. Guidelines for the evaluation of prosthetic valve function with cardiovascular imaging: A report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2024;37:2–63. doi: 10.1016/j.echo.2023.10.004. [DOI] [PubMed] [Google Scholar]
- 199.Panoulas V.F., Chandrasekhar J., Busi G., Ruparelia N., Zhang Z., Mehilli J., Sartori S., Lefevre T., Presbitero P., Capranzano P., et al. Prevalence, predictors, and outcomes of patient prosthesis mismatch in women undergoing TAVI for severe aortic stenosis: Insights from the WIN-TAVI registry. Catheter. Cardiovasc. Interv. 2021;97:516–526. doi: 10.1002/ccd.29227. [DOI] [PubMed] [Google Scholar]
- 200.Ravaux J.M., Di Mauro M., Vernooy K., Van’t Hof A.W., Veenstra L., Kats S., Maessen J.G., Lorusso R. Do Women Require Less Permanent Pacemaker after Transcatheter Aortic Valve Implantation? A Meta-Analysis and Meta-Regression. J. Am. Heart Assoc. 2021;10:e019429. doi: 10.1161/JAHA.120.019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jung S., Ammon F., Smolka S., Moshage M., Marwan M., Achenbach S. Calcification of aortic valve cusps as independent predictor for permanent pacemaker requirement after transcatheter aortic valve implantation. Eur. Heart J. 2023;44:ehad655.025. doi: 10.1093/eurheartj/ehad655.025. [DOI] [Google Scholar]
- 202.Fadahunsi O.O., Olowoyeye A., Ukaigwe A., Li Z., Vora A.N., Vemulapalli S., Elgin E., Donato A.J. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: Analysis from the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT registry. J. Am. Coll. Cardiol. Intv. 2016;9:2189–2199. doi: 10.1016/j.jcin.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 203.Chang S., Jiang Z., Liu X., Tang Y., Bai M., Xu J., Wang H., Chen Y., Li C., Chen Y., et al. Permanent pacemaker reduction using temporary-permanent pacemaker as a 1-month bridge after transcatheter aortic valve replacement: A prospective, multicentre, single-arm, observational study. eClinicalMedicine. 2024;72:102603. doi: 10.1016/j.eclinm.2024.102603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sammour Y., Krishnaswamy A., Kumar A., Puri R., Tarakji K.G., Bazarbashi N., Harb S., Griffin B., Svensson L., Wazni O., et al. Incidence, Predictors, and Implications of Permanent Pacemaker Requirement after Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2021;14:115–134. doi: 10.1016/j.jcin.2020.09.063. [DOI] [PubMed] [Google Scholar]
- 205.Bartko P.E., Clavel M.A., Annabi M.S., Dahou A., Ristl R., Goliasch G., Baumgartner H., Hengstenberg C., Cavalcante J.L., Burwash I., et al. Sex-related differences in low-gradient, low-ejection fraction aortic stenosis: Results from the multicenter TOPAS study. JACC Cardiovasc. Imaging. 2019;12:203–205. doi: 10.1016/j.jcmg.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 206.Pighi M., Piazza N., Martucci G., Lachapelle K., Perrault L.P., Asgar A.W., Lauck S., Webb J.G., Popma J.J., Kim D.H., et al. Sex-specific determinants of outcomes after transcatheter aortic valve replacement. Circ. Cardiovasc. Qual. Outcomes. 2019;12:e005363. doi: 10.1161/CIRCOUTCOMES.118.005363. [DOI] [PubMed] [Google Scholar]
- 207.Zhou C., Xia Z., Chen B., Song Y., Lian Z. Gender differences in age-stratified early outcomes in patients with transcatheter aortic valve implantation. Am. J. Cardiol. 2023;187:100–109. doi: 10.1016/j.amjcard.2022.10.038. [DOI] [PubMed] [Google Scholar]
- 208.Avvedimento M., Real C., Nuche J., Farjat-Pasos J., Galhardo A., Trinh K.H., Robichaud M., Delarochellière R., Paradis J.M., Poulin A., et al. Incidence, predictors, and prognostic impact of bleeding events after TAVR according to VARC-3 criteria. JACC Cardiovasc. Interv. 2023;16:2262–2274. doi: 10.1016/j.jcin.2023.07.005. [DOI] [PubMed] [Google Scholar]
- 209.Stehli J., Zaman S., Stähli B.E. Sex discrepancies in pathophysiology, presentation, treatment, and outcomes of severe aortic stenosis. Front. Cardiovasc. Med. 2023;10:1256970. doi: 10.3389/fcvm.2023.1256970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Abramowitz Y., Kazuno Y., Chakravarty T., Kawamori H., Maeno Y., Anderson D., Allison Z., Mangat G., Cheng W., Gopal A., et al. Concomitant mitral annular calcification and severe aortic stenosis: Prevalence, characteristics and outcome following transcatheter aortic valve replacement. Eur. Heart J. 2017;38:1194–1203. doi: 10.1093/eurheartj/ehw594. [DOI] [PubMed] [Google Scholar]
- 211.Ahmad S., Yousaf A., Ghumman G.M., Dvalishvili M., Ahsan M.J., Dilibe A., Reis H.L., Qavi A.H., Szerlip M., Goldsweig A.M. Outcomes of transcatheter aortic valve replacement in patients with mitral annular calcification and concomitant mitral valve dysfunction: A systematic review and meta-analysis. Cardiovasc. Revasc Med. 2023;61:99–109. doi: 10.1016/j.carrev.2023.10.010. [DOI] [PubMed] [Google Scholar]
- 212.Okuno T., Asami M., Khan F., Praz F., Heg D., Lanz J., Kassar M., Khalique O.K., Gräni C., Brugger N., et al. Does isolated mitral annular calcification in the absence of mitral valve disease affect clinical outcomes after transcatheter aortic valve replacement? Eur. Heart J. Cardiovasc. Imaging. 2020;21:522–532. doi: 10.1093/ehjci/jez208. [DOI] [PubMed] [Google Scholar]
- 213.Moin A., Lak H.M., Zafar M., Tariq R., Shaikh F.H., Mussa M., Bansal A., Shekhar S., Harb S., Unai S., et al. A systematic review and meta-analysis of prevalence, characteristics, and impact of mitral annular calcification on outcomes after transcatheter aortic valve implantation. Am. J. Cardiol. 2023;201:123–130. doi: 10.1016/j.amjcard.2023.05.069. [DOI] [PubMed] [Google Scholar]
- 214.Messika-Zeitoun D., Baumgartner H., Burwash I.G., Vahanian A., Bax J., Pibarot P., Chan V., Leon M., Enriquez-Sarano M., Mesana T., et al. Unmet needs in valvular heart disease. Eur. Heart J. 2023;44:1862–1873. doi: 10.1093/eurheartj/ehad121. [DOI] [PubMed] [Google Scholar]
- 215.Khalili A., Drummond J., Ramjattan N., Zeltser R., Makaryus A.N. Diagnostic and treatment utility of echocardiography in the management of the cardiac patient. World J. Cardiol. 2020;12:262–268. doi: 10.4330/wjc.v12.i6.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hahn R.T., Pibarot P. Accurate measurement of left ventricular outflow tract diameter: Comment on the updated recommendations for the echocardiographic assessment of aortic valve stenosis. J. Am. Soc. Echocardiogr. 2017;30:1038–1041. doi: 10.1016/j.echo.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 217.Altes A., Thellier N., Rusinaru D., Marsou W., Bohbot Y., Chadha G., Leman B., Paquet P., Ennezat P.V., Tribouilloy C., et al. Dimensionless index in patients with low-gradient severe aortic stenosis and preserved ejection fraction. Circ. Cardiovasc. Imaging. 2020;13:e010925. doi: 10.1161/CIRCIMAGING.120.010925. [DOI] [PubMed] [Google Scholar]
- 218.Minners J., Allgeier M., Gohlke-Baerwolf C., Kienzle R.P., Neumann F.J., Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur. Heart J. 2008;29:1043–1048. doi: 10.1093/eurheartj/ehm543. [DOI] [PubMed] [Google Scholar]
- 219.Berthelot-Richer M., Pibarot P., Capoulade R., Dumesnil J.G., Dahou A., Thébault C., Le Ven F., Clavel M.A. Discordant grading of aortic stenosis severity: Echocardiographic predictors of survival benefit associated with aortic valve replacement. JACC Cardiovasc. Imaging. 2016;9:797–805. doi: 10.1016/j.jcmg.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 220.Monin J.L., Quere J.P., Monchi M., Petit H., Baleynaud S., Chauvel C., Pop C., Ohlmann P., Lelguen C., Dehant P., et al. Low-gradient aortic stenosis: Operative risk stratification and predictors for long-term outcome: A multicenter study using dobutamine stress hemodynamics. Circulation. 2003;108:319–324. doi: 10.1161/01.CIR.0000079171.43055.46. [DOI] [PubMed] [Google Scholar]
- 221.Reis G., Motta M.S., Barbosa M.M., Estives W.A., Souza S.F., Bocchi E.A. Dobutamine stress echocardiography for noninvasive assessment and risk stratification of patients with rheumatic mitral stenosis. J. Am. Coll. Cardiol. 2004;43:393–401. doi: 10.1016/j.jacc.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 222.Monin J.L., Monchi M., Gest V., Duval-Moulin A.M., Dubois-Rande J.L., Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: Risk stratification by low-dose dobutamine echocardiography. J. Am. Coll. Cardiol. 2001;37:2101–2107. doi: 10.1016/S0735-1097(01)01339-0. [DOI] [PubMed] [Google Scholar]
- 223.Lancellotti P., Dulgheru R., Go Y.Y., Sugimoto T., Marchetta S., Oury C., Garbi M. Stress echocardiography in patients with native valvular heart disease. Heart. 2018;104:807–813. doi: 10.1136/heartjnl-2017-311682. [DOI] [PubMed] [Google Scholar]
- 224.Roger V.L., Pellikka P.A., Oh J.K., Miller F.A., Seward J.B., Tajik A.J. Stress echocardiography. Part I. Exercise echocardiography: Techniques, implementation, clinical applications, and correlations. Mayo Clin. Proc. 1995;70:5–15. doi: 10.1016/S0025-6196(11)64659-4. [DOI] [PubMed] [Google Scholar]
- 225.Geers J., Bing R. Computed tomographic imaging of patients with native and prosthetic aortic valve stenosis. Heart. 2023;109:1327–1337. doi: 10.1136/heartjnl-2022-321660. [DOI] [PubMed] [Google Scholar]
- 226.Bohbot Y., Renard C., Manrique A., Levy F., Marechaux S., Gerber B.L., Tribouilloy C. Usefulness of Cardiac Magnetic Resonance Imaging in Aortic Stenosis. Circ. Cardiovasc. Imaging. 2020;13:e010356. doi: 10.1161/CIRCIMAGING.119.010356. [DOI] [PubMed] [Google Scholar]
- 227.Garcia J., Marrufo O.R., Rodriguez A.O., Larose É., Pibarot P., Kadem L. Cardiovascular magnetic resonance evaluation of aortic stenosis severity using single plane measurement of effective orifice area. J. Cardiovasc. Magn. Reson. 2012;14:23. doi: 10.1186/1532-429X-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Scully P.R., Patel K.P., Saberwal B., Klotz E., Augusto J.B., Thornton G.D., Hughes R.K., Manisty C., Lloyd G., Newton J.D., et al. Identifying cardiac amyloid in aortic stenosis: ECV quantification by CT in TAVR patients. JACC Cardiovasc. Imaging. 2020;13:2177–2189. doi: 10.1016/j.jcmg.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Maddahi J., Packard R.R. Cardiac PET perfusion tracers: Current status and future directions. Semin. Nucl. Med. 2014;44:333–343. doi: 10.1053/j.semnuclmed.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nayfeh M., Ahmed A.I., Saad J.M., Alahdab F., Al-Mallah M. The role of cardiac PET in diagnosis and prognosis of ischemic heart disease: Optimal modality across different patient populations. Curr. Atheroscler. Rep. 2023;25:351–357. doi: 10.1007/s11883-023-01107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kim C., Hong S.J., Shin D.H., Kim J.S., Kim B.K., Ko Y.G., Choi D., Jang Y., Hong M.K. Limitations of coronary computed tomographic angiography for delineating the lumen and vessel contours of coronary arteries in patients with stable angina. Eur. Heart J. Cardiovasc. Imaging. 2015;16:1358–1365. doi: 10.1093/ehjci/jev100. [DOI] [PubMed] [Google Scholar]
- 232.Chang S.M., Bhatti S., Nabi F. Coronary computed tomography angiography. Curr. Opin. Cardiol. 2011;26:392–402. doi: 10.1097/HCO.0b013e32834938c6. [DOI] [PubMed] [Google Scholar]
- 233.Starnes V.A., Bowdish M.E., Cohen R.G., Baker C.J., Elsayed R.S. The Ross procedure utilizing the pulmonary autograft inclusion technique in adults. JTCVS Tech. 2021;10:372–376. doi: 10.1016/j.xjtc.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Beltran-Sanchez H., Subramanian S.V. Period and cohort-specific trends in life expectancy at different ages: Analysis of survival in high-income countries. SSM Popul. Health. 2019;8:100422. doi: 10.1016/j.ssmph.2019.100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chauvette V., Bouhout I., Tarabzoni M., Wong D., Bozinovski J., Chu M.W.A., El-Hamamsy I., Canadian Ross R. The Ross procedure in patients older than 50: A sensible proposition? J. Thorac. Cardiovasc. Surg. 2022;164:835–844.e5. doi: 10.1016/j.jtcvs.2020.09.121. [DOI] [PubMed] [Google Scholar]
- 236.Mazine A., El-Hamamsy I., Verma S., Peterson M.D., Bonow R.O., Yacoub M.H., David T.E., Bhatt D.L. Ross procedure in adults for cardiologists and cardiac surgeons: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018;72:2761–2777. doi: 10.1016/j.jacc.2018.08.2200. [DOI] [PubMed] [Google Scholar]
- 237.Oeser C., Uyanik-Uenal K., Kocher A., Laufer G., Andreas M. The Ross procedure in adult patients: A single-centre analysis of long-term results up to 28 years. Eur. J. Cardiothorac. Surg. 2022;62:ezac379. doi: 10.1093/ejcts/ezac379. [DOI] [PubMed] [Google Scholar]
- 238.Kouchoukos N.T., Davila-Roman V.G., Spray T.L., Murphy S.F., Perrillo J.B. Replacement of the aortic root with a pulmonary autograft in children and young adults with aortic valve disease. N. Engl. J. Med. 1994;330:1–6. doi: 10.1056/NEJM199401063300101. [DOI] [PubMed] [Google Scholar]
- 239.Reece T.B., Welke K.F., O’Brien S., Grau-Sepulveda M.V., Grover F.L., Gammie J.S. Rethinking the Ross procedure in adults. Ann. Thorac. Surg. 2014;97:175–181. doi: 10.1016/j.athoracsur.2013.07.036. [DOI] [PubMed] [Google Scholar]