Abstract
An adenovirus vector encoding the human Bcl-2 gene (hBcl-2) was derived. In vivo expression of hBcl-2 in murine livers enhanced and prolonged adenovirus-mediated gene expression. Furthermore, in the hBcl-2-treated group a significant reduction in the apoptosis induced by the adenovirus vector was observed. Thus, the cytoprotection of the vector-infected cells with antiapoptotic genes appears promising for successful in vivo gene therapy.
Adenovirus has been widely employed as vector for gene transfer and is currently being evaluated in a number of human clinical gene therapy trials (4, 14, 41a). Despite the utility of this agent, its use has been limited by host-derived immune response. This response results in the elimination of the transduced cell with the consequent attenuation of transgene expression (3, 62, 69, 75, 76). In this regard, vector-infected cells elicit a late host-specific immunological response based on CD8+ cytotoxic T cells against viral and/or transgene products resulting in the elimination of the virus-infected cells (56, 58, 62, 64, 74, 77). In addition, the recombinant adenovirus infection results in the generation of neutralizing antibodies that preclude its readministration (13, 26, 58). The biological basis of these events has been extensively studied, and specific strategies have been developed to mitigate host eradication of vector-infected cells (15, 23, 25, 29, 30, 39, 47, 53).
In addition to this late-phase immunological event, an early innate, inflammatory phase occurs between days 1 and 4 postinfection. This early response is responsible for the elimination of 90% of the vector genome in the liver after intravenous (i.v.) administration. This phase has been characterized by periportal polymorphonuclear leukocyte infiltration; activation of macrophages (Kupffer cells), natural killer (NK) cells, and complement; and proinflammatory cytokine release (5, 17, 37, 58, 70, 71).
An additional factor causing adenovirus vector clearance is apoptosis (programmed cell death) of the transduced cells induced directly by the expression of viral proteins (36, 41, 42). In this regard, apoptosis is controlled through the expression of specific genes. Among these, the Bcl-2 family is the most important (21, 32, 48, 49, 65, 67). The protein encoded by the Bcl-2 gene has been implicated in the prolongation of cell survival by blocking the apoptotic and necrotic processes (16, 34, 52, 55, 61, 72, 80). Whereas Bcl-2 is normally not expressed in hepatocytes, de novo Bcl-2 expression has been observed after bile duct ligation and suggests an adaptive phenomenon to resist apoptosis by toxic bile salts (33).
On these bases, we hypothesized that coexpression of the Bcl-2 gene at the time of systemic administration may have the potential to “cytoprotect” the adenovirus vector-infected cell and might thus allow enhancement and prolongation of the transgene expression. To this end, we derived an adenovirus vector encoding Bcl-2, which blocks cell injury by a variety of stimuli. In the present study, we show that the adenovirus-mediated Bcl-2 delivery can mitigate vector-induced apoptosis within the target parenchyma, with the result of dramatically augmenting transgene expression achieved by an adenovirus vector.
The initial technical step in this endeavor was the construction of a replication-incompetent, recombinant adenovirus vector expressing the human Bcl-2 (hBcl-2) gene without the transmembrane domain. This recombinant was constructed by the two-plasmid homologous recombination method of Graham (20). Plasmid encoding the hBcl-2 open reading frame without the transmembrane domain, generously provided by J. Reed (La Jolla Cancer Research Foundation, La Jolla, Calif.), was used as a source of the hBcl-2 gene. The hBcl-2 gene without the transmembrane domain gene was cloned into the plasmid pcDNA3-Bcl-2, excised with EcoRI and XhoI to release an 0.7-kb fragment containing the hBcl-2 open reading frame, and then subcloned into the EcoRI and XhoI sites of the shuttle plasmid pCA13 (Microbix, Inc.). The resultant plasmid, pCAhBcl-2, sequentially contains 0.5 map units of sequence from the left end of the adenovirus type 5 genome, cytomegalovirus promoter, the hBcl-2 gene, and a unique ClaI site, followed by simian virus 40 poly(A) signal sequences. Restriction endonuclease digestion and direct sequence analysis confirmed the orientation and sequence of the inserted Bcl-2 open reading frame. The resultant plasmid, pCAhBcl-2, was then cotransfected into the adenovirus packaging cell line 293 together with the adenovirus rescue plasmid pJM17 (Microbix, Inc.) by employing DOTAP (BRL, Gaithersburg, Md.). After cotransfection, cells were overlaid with Dulbecco’s modified Eagle’s medium–F-12 (Mediatech/Cellgro, Herndon, Va.) supplemented with 2% heat-inactivated fetal bovine serum (HyClone Laboratories, Logan, Utah) and 0.65% SeaPlaque agarose (Difco FMC Laboratories, Detroit, Mich.). Individual plaques of AdCMVhBcl-2 were picked approximately 10 days posttransfection and carried through three additional steps of plaque purification. The virus was then expanded and purified by equilibrium centrifugation in a CsCl2 gradient. To confirm the identity of AdCMVhBcl-2, its genomic DNA was subjected to restriction enzyme analysis. In addition, PCR utilizing a pair of primers designed to amplify the E1A region of the wild-type adenovirus genome was utilized to test DNA isolated from purified virions of AdCMVhBcl-2. Furthermore, the presence of the expression cassette in the viral genome was confirmed by DNA sequencing. The number of PFU per preparation was determined by plaque assay on 293 cells. Plaque assay on HeLa cells confirmed the absence of contaminating replication-competent adenovirus. Negative results obtained in both assays have confirmed that the Ad5CMVhBcl-2 preparation was free from replication-competent adenovirus contamination. As a control, we used an E1A/B deletion, replication-incompetent, recombinant adenovirus vector that encodes the Escherichia coli β-galactosidase reporter gene under the control of the cytomegalovirus promoter (AdCMVLacZ). The number of PFU per preparation was determined by plaque assay on 293 cells. Contamination of the viral preparation was ruled out as previously described.
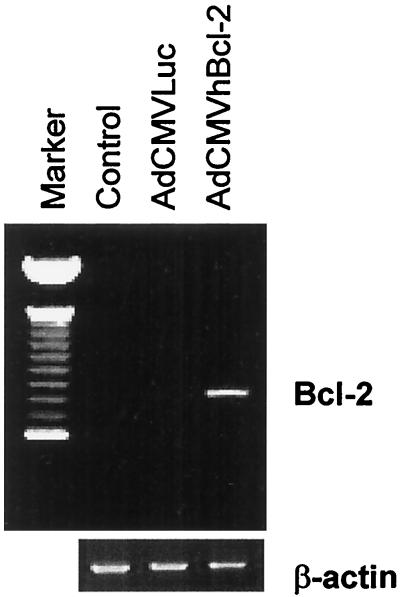
We next sought to demonstrate that AdCMVhBcl-2 could mediate transfer of the Bcl-2 gene to the liver. To this end, experiments were performed with normal male C57BL/6 mice (Jackson Laboratory, Bar Harbor, Maine) 9 to 12 weeks old, weighing 25 to 30 g, and fed on a laboratory diet with water and food ad libitum, in compliance with the University of Alabama at Birmingham Animal Care and Use Committee, which adheres to the National Institutes of Health guidelines for the use of experimental animals. Mice were injected i.v. with AdCMVhBcl-2 plus AdCMVLuc, with a control vector (AdCMVLacZ plus AdCMVLuc) at 109 PFU per vector (total dose, 2 × 109 PFU), or with phosphate-buffered saline (PBS) alone. This delivery schema is known to achieve principally hepatocyte transduction (43, 66). Immunohistochemical staining of the hBcl-2 protein demonstrated transduction of >90% of hepatocytes (data not shown). At 48 h postinjection, total RNA was extracted from the livers and subjected to reverse transcription-PCR. Briefly, total RNA was extracted from snap-frozen liver biopsy specimens with an RNA STAT-60 kit (Tel-Test “B,” Inc., Friendswood, Tex.) according to the manufacturer’s recommendations. Briefly, after homogenization in RNA STAT-60 (1 ml/50 mg of tissue), the samples were incubated for 5 min at room temperature to allow dissociation of nucleoprotein complexes. Next, 0.2 ml of chloroform per ml of RNA STAT-60 was added. After 2 to 3 min at room temperature, the samples were centrifuged at 12,000 × g for 15 min at 4°C. RNA was precipitated with isopropanol at −70°C overnight, washed in 70% ethanol, and resuspended in RNase-free water. The first-strand cDNA synthesis was catalyzed by Moloney murine leukemia virus reverse transcriptase with 15 μg of total RNA and used random hexamer primers. The first-strand cDNA synthesis kit (Pharmacia Biotech, Inc., Milwaukee, Wis.) was used according to the manufacturer’s recommendations. The cDNA was then employed as a template for PCR amplification to generate an hBcl-2-specific fragment (∼590 bp) with the primers AGT GGG ATG CGG GAG ATG TG and GGG GCC GTA CAG TTC CAC AA. As shown in Fig. 1, expression of the Bcl-2 gene could be detected in livers from mice injected with AdCMVhBcl-2 but not in livers from animals treated with the control vector or PBS. Thus, Bcl-2 expression in the liver can be accomplished in vivo after systemic administration of a recombinant adenovirus encoding Bcl-2.
FIG. 1.
hBcl-2 expression in the liver after i.v. AdCMVhBcl-2 administration. Total RNA was extracted from liver biopsy specimens, and samples were analyzed by reverse transcription-PCR (∼700 bp). No expression of hBcl-2 was demonstrated for the groups injected with PBS or AdCMVLuc. Expression was observed in the AdCMVhBcl-2 group at 48 h postinjection.
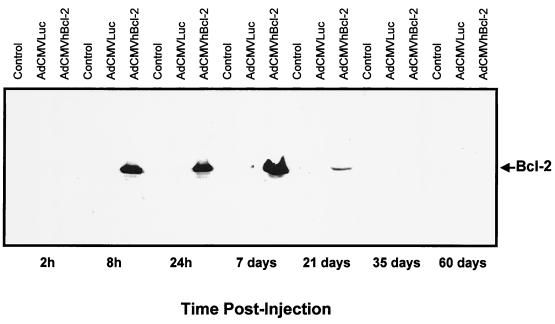
We next sought to confirm the pattern of in vivo liver expression of the hBcl-2 protein encoded by AdCMVhBcl-2 at different times postinjection. For this experiment, we employed an immunoblot analysis of hBcl-2 protein. Fifteen micrograms of total protein from cellular lysate prepared as described elsewhere (Promega luciferase assay system) was size fractionated by sodium dodecyl sulfate (SDS)–12% polyacrylamide gel electrophoresis and electroblotted onto a nitrocellulose membrane. The hBcl-2 protein was detected with the monoclonal antibody mouse anti-hBcl-2 (Oncogene Research Products, Cambridge, Mass.) at a 1:3,000 dilution, followed by the addition of goat anti-mouse horseradish peroxidase antibody. The membrane was developed with Western blot chemiluminescence reagent (DuPont NEN, Boston, Mass.). As a control for equal loading of protein, c-myc was also detected (data not shown). Reference Coomassie blue-stained gels were also run. The molecular mass of Bcl-2 is 25 kDa. As shown in Fig. 2, Bcl-2 protein expression could be detected by Western blotting at 8 h postinjection with a maximum expression by 7 days and was almost undetectable by day 21. No detection of hBcl-2 was demonstrated for the control group or for animals treated with AdCMVLuc.
FIG. 2.
Immunoblot analysis of hBcl-2 protein expression. Adult C57BL/6 mice were injected i.v. with either AdCMVhBcl-2 plus AdCMVLuc or AdCMVLacZ plus AdCMVLuc at 109 PFU per vector (total dose, 2 × 109 PFU). Control animals did not receive a viral injection. At various times postinjection, total protein was extracted from frozen livers. Fifteen micrograms of total protein was size fractionated by SDS–12% polyacrylamide gel electrophoresis. The membrane was developed with Western blot chemiluminescence reagent.
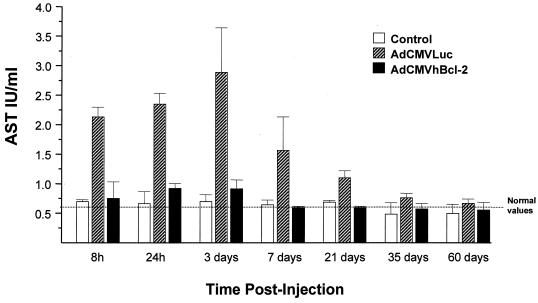
In order to study the hepatotoxic effect of the adenovirus vector, we analyzed liver function after the in vivo gene delivery. Aspartate aminotransferase (AST) is an established marker of hepatic damage after liver injury. Blood samples for AST determinations were obtained at different times postadenovirus administration, and AST was analyzed with a serum analyzer (Amos Seralyzer; Miles Inc., Diagnostics Division, Elkhart, Ind.). Animals from the control group injected with PBS showed normal serum AST levels at all times (Fig. 3). Animals treated with AdCMVhBcl-2 plus AdCMVLuc showed a similar profile in AST levels. In contrast, animals infected with AdCMVLuc plus AdCMVLacZ showed a significant increase in AST levels beginning at 8 h with a peak at 3 days and returned to baseline levels at day 35 postinjection (Fig. 3). Similar results were observed with alanine aminotransferase and lactate dehydrogenase determinations (data not shown). These results demonstrate that adenovirus-mediated gene transfer of the antiapoptotic Bcl-2 gene induces cytoprotection of the adenovirus vector-transduced cells.
FIG. 3.
AST levels after adenovirus-mediated gene transfer. Adult female C57BL/6 mice were injected i.v. with either AdCMVhBcl-2 plus AdCMVLuc or AdCMVLacZ plus AdCMVLuc at 109 PFU per vector (total dose, 2 × 109 PFU). Serum AST levels were measured at various times postinjection. Results are expressed as means ± standard errors of the means for eight animals. Serum AST levels obtained from normal C57BL/6 mice were 0.58 ± 0.08 IU/liter. A significant difference was observed between the AdCMVhBcl-2-plus-AdCMVLuc group and controls or AdCMVLacZ-plus-AdCMVLuc-injected animals (P < 0.01, Student’s t test).
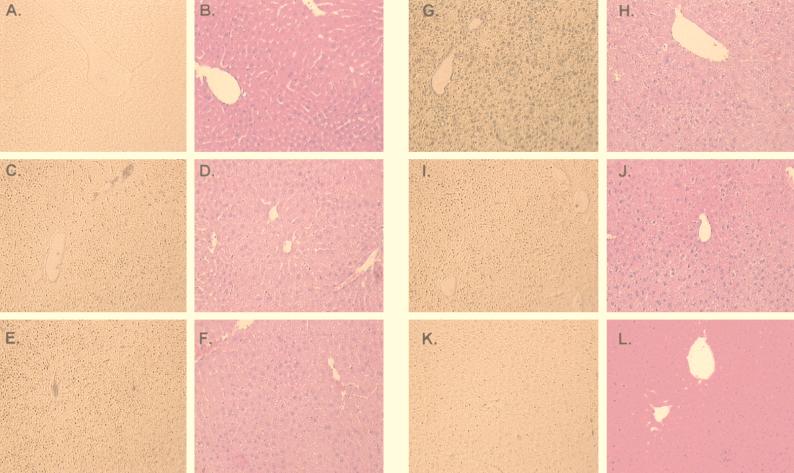
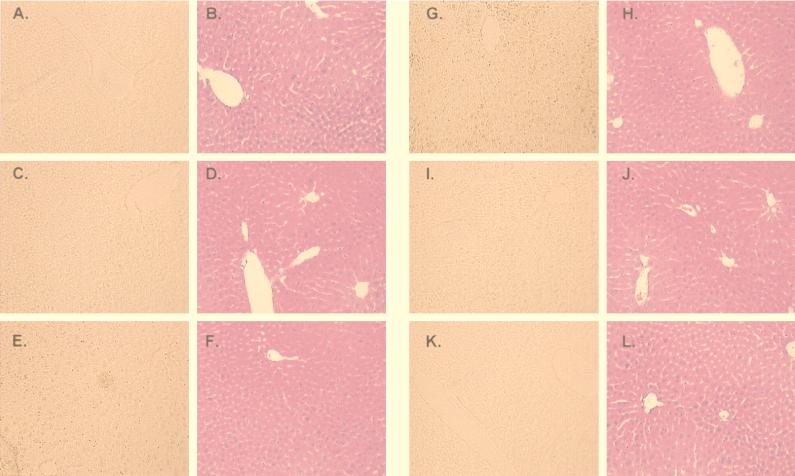
Next we investigated the degree of hepatocyte injury by histological analysis in this experimental model. Liver biopsy specimens for histological assessment were obtained at different times after the systemic administration of the adenovirus vectors. Liver specimens from the median lobe were fixed in 10% formalin and embedded in paraffin. Six-micrometer hematoxylin-and-eosin-stained sections were evaluated at an ×200 magnification by a point-counting method for severity of hepatic injury with an ordinal scale as previously described: grade 0, minimal or no evidence of injury; grade 1, mild injury consisting in cytoplasm vacuolation and focal nuclear pyknosis; grade 2, moderate to severe injury with extensive nuclear pyknosis, cytoplasmic hypereosinophilia, and loss of intercellular borders; grade 3, severe necrosis with disintegration of hepatic cords, hemorrhage, and neutrophil infiltration (7, 27). In this context, control animals injected with PBS did not show any evidence of hepatic injury (grade 0). Consistent with the transaminase levels (Fig. 3), the group injected with AdCMVLuc plus AdCMVLacZ showed moderate to severe injury by day 3 after the systemic administration of the adenovirus vector (grade 2-3) with nuclear pyknosis, cytoplasmic hypereosinophilia, and loss of intercellular borders. Areas of necrosis with disintegration of hepatic cords and neutrophil infiltration were also evident (Fig. 4). The hepatotoxic effect of the adenovirus vector was less evident by day 7 and was resolved by day 35. Animals injected with AdCMVhBcl-2 plus AdCMVLuc showed minimal evidence of injury (grade 0-1) at all the experimental times (Fig. 5). These results suggest that Bcl-2 might protect the host cell against the cytotoxic effects of the adenovirus vector and decrease the inflammatory response secondary to the viral infection.
FIG. 4.
Apoptosis and histological analysis of the liver after coinfection with AdCMVLuc plus AdCMVLacZ. Adult female C57BL/6 mice were injected i.v. with either AdCMVhBcl-2 plus AdCMVLuc or AdCMVLacZ plus AdCMVLuc at 109 PFU per vector (total dose, 2 × 109 PFU). Liver biopsy specimens were obtained at 8 h and 1, 3, 7, and 21 days postinjection. Sections were prepared for apoptosis by in situ histochemical assay for DNA fragmentation (Klenow-FragEL) (A, C, E, G, I, and K). Sections were also stained with hematoxylin and eosin (B, D, F, H, J, and L). Magnification, ×18. (A and B) Control mouse; (C and D) 8 h; (E and F) 1 day; (G and H) 3 days; (I and J) 7 days; (K and L) 21 days.
FIG. 5.
Apoptosis and histological analysis of the liver after coinfection with AdCMVhBcl-2 and AdCMVLuc. Adult female C57BL/6 mice were injected i.v. with either AdCMVhBcl-2 plus AdCMVLuc or AdCMVLacZ plus AdCMVLuc at 109 PFU per vector (total dose, 2 × 109 PFU). Liver biopsy specimens were obtained at 8 h and 1, 3, 7, and 21 days postinjection. Sections were prepared for apoptosis by in situ histochemical assay for DNA fragmentation (Klenow-FragEL) (A, C, E, G, I, and K). Sections were also stained with hematoxylin and eosin (B, D, F, H, J, and L). Magnification, ×18. (A and B) Control mouse; (C and D) 8 h; (E and F) 1 day; (G and H) 3 days; (I and J) 7 days; (K and L) 21 days.
Having demonstrated that Bcl-2 protects the liver against adenovirus vector injury, we wished to examine whether this was the result of reduced apoptosis in the liver cells. To this end, a commercial in situ histochemical assay (Klenow-FragEL; Oncogene) was used to detect the DNA fragmentation characteristic of apoptosis. In this assay, Klenow fragment binds to exposed ends of DNA fragments generated in response to apoptotic signals and catalyzes the template-dependent addition of biotin-labeled and unlabeled deoxynucleotides. Biotinylated nucleotides are detected with a streptavidin-horseradish peroxidase conjugate. Diaminobenzidine reacts with the labeled sample to generate an insoluble colored substrate at the site of DNA fragmentation. Counterstaining with methyl green aids in the morphological evaluation of normal and apoptotic cells. In this regard, nuclei showed condensed chromatin (black) under light microscopy. The results were scored semiquantitatively by averaging the number of apoptotic cells per microscopic field at ×25 magnification. Six fields were evaluated per tissue sample. Apoptotic cells were more evident in samples from animals injected with AdCMVLuc plus AdCMVLacZ (Fig. 4). In contrast, a significantly lower number of apoptotic cells was present in the animals coinjected with AdCMVhBcl-2 and AdCMVLuc (Fig. 5). Thus, these results demonstrate that apoptosis plays a critical role in the clearance of the adenovirus vector at early times postinjection and also demonstrate the ability of hBcl-2 to block the apoptosis process induced by the adenovirus vectors.
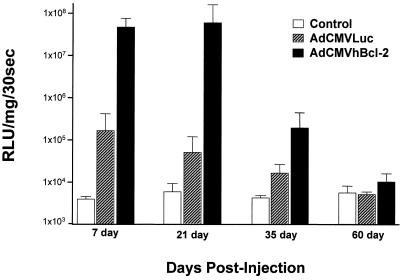
Next we explored the potential biological effect of hBcl-2 in transgene expression. For this experiment, adult male C57BL/6 mice were injected via the tail vein with adenovirus vectors. Animals were challenged with both AdCMVLacZ and AdCMVLuc, or AdCMVhBcl-2 and AdCMVLuc, at 109 PFU per vector per animal. Analysis of luciferase reporter gene expression was assayed as described by the manufacturer (Promega luciferase assay system). Briefly, lysate cells were washed with PBS, and then 400 μl of Promega 1× cell culture lysis reagent was added to the cells. Samples were then incubated on ice for 1 h, after which lysates were collected and spun down at 14,000 rpm for 5 min. Next, 20 μl of supernatant was added to 100 μl of Promega luciferase substrate and analyzed for emitted light. A Lumat LB 9510 luminometer with the parameters described by the manufacturer was used to analyze the light units. Results are expressed as relative light units per milligram of total protein per 30 s. As expected, all uninfected control groups demonstrated an absence of luciferase expression in harvested livers. In the group treated without hBcl-2, an initial high level of gene expression was achieved by day 7 postinfection followed by progressive attenuation in a time-dependent manner such that by day 60 postinfection the luciferase gene expression was at baseline level (Fig. 6). This pattern of nonpersistence of transgene expression is analogous to that described by other authors and reflects, in part, the consequences of the host immune response for the vector-transduced cells (36, 45, 78). However, in situ coexpression of hBcl-2 protein induced a different pattern of transgene expression from that in AdCMVLacZ-plus-AdCMVLuc-treated animals (Fig. 6). In this regard, a 2-log increase of transgene expression by days 7 and 21 and a 1-log increase in transgene expression by day 35 postinjection were noted. In this context, some degree of prolongation of transgene expression was achieved by day 35 posttransfection; however, by day 60 the luciferase expression level was nearly baseline. It thus appeared that, in this organ context, simple ectopic expression of hBcl-2 did confer the desired end of cytoprotection of the vector-infected cell, achieving enhancement and some degree of prolongation of transgene expression.
FIG. 6.
Effect of ectopic expression of hBcl-2 on transgene expression in murine hepatocytes. Adult C57BL/6 mice were injected i.v. with either AdCMVhBcl-2 plus AdCMVLuc or AdCMVLacZ plus AdCMVLuc at 109 PFU per vector (total dose, 2 × 109 PFU). Control animals did not receive a viral injection. At various times postinjection, luciferase activity was determined in harvested frozen livers. Each histogram represents the mean ± standard error of the mean for eight animals. RLU, relative light units.
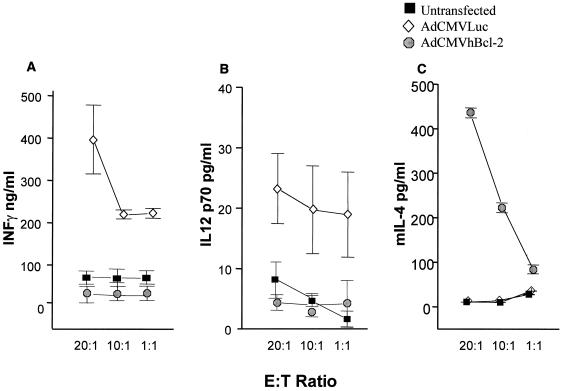
Finally, we investigated the activation of the immune system after in vivo adenovirus administration. To study the cytokine release, interleukin-12 (IL-12) p70, gamma interferon (IFN-γ), and IL-4 levels were evaluated 7 days after i.v. administration of 109 PFU of AdCMVLuc, AdCMVhBcl-2, or PBS to adult C57BL/6 mice. Briefly, cytokine production was evaluated in vitro in response to murine macrophages (antigen-presenting cells [APC]) infected with adenovirus vector (100 PFU/cell). The infection was done for 1 h at 37°C in 1 ml of RPMI 1640 plus 10% heat-inactivated fetal calf serum. Before use as stimulator cells, the APC were γ-irradiated, and 103 APC were mixed with T cells isolated from spleens from mice from the different groups at different effector-to-target ratios. The mixed cells were incubated in 24-well plates for 48 h at 37°C. The supernatants were collected, and the concentrations of IFN-γ, IL-12 p70, and IL-4 were measured by using Quantikine murine enzyme-linked immunosorbent assay (ELISA) kits according to the directions of the manufacturer (R&D Systems, Minneapolis, Minn.). Significantly higher levels of the proinflammatory cytokines IFN-γ and IL-12 p70 were observed for animals transfected with AdCMVLuc (Fig. 7A and B) than for animals injected with AdCMVhBcl-2 or PBS. High levels of IL-4, a cytokine with anti-inflammatory properties, were observed only with animals transfected with AdCMVhBcl-2 (Fig. 7C). These results demonstrate less proinflammatory cytokine release during the initial response to adenovirus in vivo administration when AdCMVhBcl-2 was used.
FIG. 7.
Cytokine profile after in vivo adenovirus administration. IFN-γ (A), IL-12 p70 (B), and murine IL-4 (C) levels were determined by ELISA at day 7 after i.v. adenovirus administration in T-cell culture supernatants from C57BL/6 adult mice treated with 109 PFU of AdCMVLuc or AdCMVhBcl-2 or PBS. Results are expressed as means ± standard deviations for at least three animals.
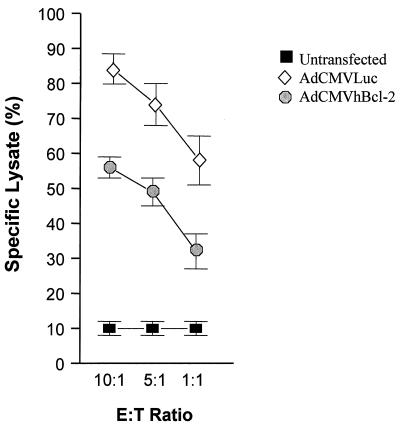
The development of a cytotoxic T-lymphocyte (CTL) response against adenovirus proteins as well as transgene products has been implicated in the transient expression from adenovirus vectors in vivo. In order to analyze the CTL response in our model, CTL activity was estimated 7 days postinfection by measuring the ability of the test cells to induce cytotoxicity of adenovirus-infected C57BL/6 target APC. Briefly, AdCMVLuc- or AdCMVhBcl-2-infected C57BL/6 mouse fibroblasts (10 PFU/cell) were labeled with 20 μCi of sodium [51Cr]chromate (Amersham, Arlington Heights, Ill.) per ml for 1 h at 37°C. The cells were then washed three times with RPMI 1640 medium supplemented with heat-inactivated 10% fetal calf serum. Effector T cells were prepared from spleens of AdCMVLacZ, AdCMVhBcl-2, or PBS groups. These effector cells were then incubated with adenovirus-infected target cells in 96-well plates at different effector-to-target ratios. Supernatants were collected after 24 h, and the amount of released 51Cr was measured with a β-counter (Beckman Instruments, Fullerton, Calif.). Spontaneous release of 51Cr was determined by incubating 51Cr-labeled target cells with medium alone, and maximum release was determined by adding SDS to a final concentration of 0.05%. The percentage of specific 51Cr release was calculated as described elsewhere (79). A significant reduction in the capacity of CTLs to induce lysis was observed for target cells transfected with AdCMVhBcl-2 compared to AdCMVLuc-transfected cells (Fig. 8). Bcl-2 has been reported elsewhere to induce cytoprotection against cytotoxicity induced by T cells (9).
FIG. 8.
Cytotoxic T-cell response after i.v. administration of adenovirus vector. The T-cell cytotoxic responses were determined 7 days after i.v. administration of 109 PFU of AdCMVLuc or AdCMVhBcl-2 or PBS. Results are expressed as means ± standard deviations for at least three animals. A significant difference was observed between the AdCMVhBcl-2 group and the controls or AdCMVLuc-injected animals (P < 0.04). E:T ratio, effector/target cell ratio.
In several reports, the humoral immune response against viral and/or transgene products has been observed to lead to elimination of transduced cells and inability to readminister the adenovirus vector (13, 23, 25). The humoral immune response to the adenovirus vector was analyzed. ELISA plates were coated with guinea pig polyclonal antiadenovirus antibody. Twenty nanograms of AdCMVLuc was added to 96-well plates for 30 min at 4°C and washed three times with PBS. Serum samples obtained at 21 days postadenovirus i.v. administration were diluted 1:100 and incubated for 30 min at 4°C. After washing, peroxidase-conjugated anti-mouse immunoglobulin G1 and immunoglobulin G2a (Southern Biotechnology Associates, Birmingham, Ala.) were added followed by washing and development with tetramethylbenzidine (Sigma, St. Louis, Mo.) substrate. The optical density was determined at 405 nm on a microplate reader (SofMax automatic 96-well microtiter reader). Each sample was assayed in duplicate, and the average optical density reading from the duplicates of each sample was then obtained. At least three mice were tested in each group. ELISA was used in comparison to a standard curve of a commercial antihexon adenovirus antiserum (Accurate Chemical & Scientific Co., Westbury, N.Y.). Antihexon antibody levels were evaluated as not detected (−) or detected as similar to the standard antibody dilution of 1:1,000 (+++), 1:100 (++), or 1:10 (+). No significant differences in neutralizing antibody production were observed between animals treated with AdCMVLuc and those treated with AdCMVhBcl-2 (+++). No antibodies were detected in serum from mice injected with PBS (−) (data not shown).
Apoptosis is a cellular response to viral infection, which has a potential to inhibit viral growth and block the spread within the infected organisms (31, 40, 54, 57). In the case of the liver, the main cellular targets of apoptosis are hepatocytes and sinusoidal lining cells, and the intensity of apoptosis correlates with signs of hepatocyte injury. Lawson et al. proposed that the signal that turns neutrophil sequestration in the liver into full-blown neutrophilic inflammation is apoptosis (35). When apoptosis is blocked, so are neutrophil invasion and the accompanying tissue injury. By contrast, when apoptosis occurs in the liver primed for inflammation, neutrophils migrate toward the dead cells but in the process destroy many others, triggering widespread organ destruction. This phenomenon has been called the neutron bomb effect: that is, the apoptosis of a few hepatocytes seems silent initially but sets off scores of primed neutrophils, which explode with oxyradicals and proteolytic enzymes that kill hundreds of innocent bystander cells (38). In this regard, some viruses, such as herpesviruses, poxviruses, insect baculoviruses, and adenoviruses, are associated with apoptosis of the infected cell even in the absence of an antiviral immune response (37, 71). Viruses have evolved a variety of strategies including modification of the expression of cellular and viral genes that regulate apoptosis to meet their requirement for prolonged survival in the infected cell (1, 10, 11, 19, 81). Adenoviruses encode the E1B–19-kDa protein gene product that effectively blocks apoptosis (8, 18, 44). However, recombinant adenovirus vectors have a deletion of this protein; therefore, they fail to produce a functional E1B–19-kDa protein and induce extensive degradation of host cell and viral DNA, enhanced cytopathic effect, and reduced viral yield when grown in cultured cells (46, 59, 68).
In our model, we have demonstrated an acute severe hepatocyte injury after systemic delivery of adenovirus vector (1 to 3 days postinjection) evaluated by increase in transaminases, proinflammatory cytokines, and apoptosis and by histological analysis of the liver. This acute response was blocked by the expression of Bcl-2. Previous studies have shown that the Bcl-2 gene protects a wide variety of cell types from apoptosis in response to such diverse stimuli as viral infection, ionizing radiation, growth factor deprivation, proinflammatory cytokines, T-cell cytotoxicity, and ischemia (28, 32, 52). The coexpression of Bcl-2 mediated a significant reduction in apoptosis and necrosis following adenovirus-mediated gene transfer, achieving an enhancement of transgene expression (up to 2 logs). These results suggest that apoptosis plays an important role in the early clearance of the adenovirus-infected cells. Thus, transient expression of Bcl-2 at the time of i.v. adenovirus injection was sufficient to enhance and prolong transgene expression.
Recent reports have demonstrated that apoptotic cells induce direct activation of dendritic cells, a critical step in the immune response (2, 50, 51, 73). Therefore, reduced cell damage and apoptosis during the early steps of liver damage after adenovirus transfection would potentially decrease the immunogenicity of the vector-infected cell. Also, cells expressing Bcl-2 have been shown to block cytotoxicity mediated by allospecific CTLs and to resist the cytotoxic effect mediated by perforin and granzymes (6, 9). Therefore, expression of Bcl-2 after adenovirus-mediated gene transfer to the liver would potentially confer protection against the immune system. In accordance with our results, Okuyama et al. showed that Fas-mediated apoptosis is involved in the elimination of gene-transduced hepatocytes with E1/E3-deletion adenovirus vectors (42). Interestingly, Lacronique et al., using hepatocytes from transgenic mice expressing hBcl-2, showed that these hepatocytes could resist the administration of agonistic Fas antibody while normal hepatocytes were killed (34). These observations suggest that expression of Bcl-2 can block the Fas-mediated apoptosis induced by adenovirus vectors (24, 34).
A recent report by Lieber et al. has also described the role of Bcl-2 and IκBM in the persistence of first-generation adenovirus vectors (36). Adenovirus-mediated gene transfer has been shown to activate the transcriptional factor NF-κB, known to induce the expression of proinflammatory cytokines that lead to neutrophil-mediated inflammation (12, 60). These investigators employed transgenic mice that express Bcl-2 (bcl-2tg+/+) with a systemic administration of adenovirus vector expressing IκBM. In this study, they demonstrated that coexpression of Bcl-2 with the inhibitor of NF-κB (IκBM) was necessary for the prolonged adenovirus gene expression. Interestingly, a decrease of the main inflammatory cytokines (IFN-γ and tumor necrosis factor alpha) was observed in the transgenic mice expressing Bcl-2.
Bcl-2 was first discovered by its association with the t(14;18) chromosomal translocations found in non-Hodgkin B-cell lymphomas (63). However, no evidence of neoplasia has been demonstrated for transgenic mice overexpressing Bcl-2 (22, 27). Experiments performed in our laboratory with mice infected with AdCMVhBcl-2 in the liver demonstrated no evidence of liver dysfunction or neoplasia in any organ at long-term follow-up (>18 months).
The transient coexpression of Bcl-2 with a therapeutic gene might be effective in preventing the rapid elimination of hepatocytes transduced with an adenovirus vector. Strategies to prolong the expression of therapeutic genes delivered by adenovirus vector, even in the context of diseases in which transient effects may be sought, are essential requirements for achieving clinical utility.
Acknowledgments
This work was supported in part by grant NIH RO1-CA 72532-01. Guadalupe Bilbao is a USAMRC postdoctoral fellow.
REFERENCES
- 1.Afonso C L, Neilan J G, Kutish G F, Rock D L. An African swine fever virus Bcl-2 homolog, 5-HL, suppresses apoptotic cell death. J Virol. 1996;70:4858–4863. doi: 10.1128/jvi.70.7.4858-4863.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 3.Barr D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay M A. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. [PubMed] [Google Scholar]
- 4.Billon N, van Grunsven L A, Rudkin B B. The CDK inhibitor p21WAF1/Cip1 is induced through a p300-dependent mechanism during NGF-mediated neuronal differentiation of PC12 cells. Oncogene. 1996;13:2047–2054. [PubMed] [Google Scholar]
- 5.Blom W M, De B H, Meijerman I, Kuppen P J, Mulder G J, Nagelkerke J F. Interleukin-2-activated natural killer cells can induce both apoptosis and necrosis in rat hepatocytes. Hepatology. 1999;29:785–792. doi: 10.1002/hep.510290303. [DOI] [PubMed] [Google Scholar]
- 6.Bonnotte B, Favre N, Moutet M, Fromentin A, Solary E, Martin M, Martin F. Bcl-2-mediated inhibition of apoptosis prevents immunogenicity and restores tumorigenicity of spontaneously regressive tumors. J Immunol. 1998;161:1433–1438. [PubMed] [Google Scholar]
- 7.Camargo C A J, Madden J F, Gao W, Selvan R S, Clavien P A. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26:1513–1520. doi: 10.1002/hep.510260619. [DOI] [PubMed] [Google Scholar]
- 8.Chiou S K, Tseng C C, Rao L, White E. Functional complementation of the adenovirus E1B 19-kilodalton protein with Bcl-2 in the inhibition of apoptosis in infected cells. J Virol. 1994;68:6553–6566. doi: 10.1128/jvi.68.10.6553-6566.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu V K, Walsh C M, Liu C C, Reed J C, Clark W R. Bcl-2 blocks degranulation but not fas-based cell-mediated cytotoxicity. J Immunol. 1995;154:2023–2032. [PubMed] [Google Scholar]
- 10.Chou J, Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc Natl Acad Sci USA. 1992;89:3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clem R J, Fechheimer M, Miller L K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- 12.Clesham G J, Adam P J, Proudfoot D, Flynn P D, Efstathiou S, Weissberg P L. High adenoviral loads stimulate NF kappaB-dependent gene expression in human vascular smooth muscle cells. Gene Ther. 1998;5:174–180. doi: 10.1038/sj.gt.3300576. [DOI] [PubMed] [Google Scholar]
- 13.DeMatteo R P, Chu G, Ahn M, Chang E, Burke C, Raper S E, Barker C F, Markmann J F. Immunologic barriers to hepatic adenoviral gene therapy for transplantation. Transplantation. 1997;63:315–319. doi: 10.1097/00007890-199701270-00024. [DOI] [PubMed] [Google Scholar]
- 14.Douglas J T, Curiel D T. Adenoviruses as vectors for gene therapy. Sci Med. 1997;4:44–53. [Google Scholar]
- 15.Fisher K J, Choi H, Burda J, Chen S J, Wilson J M. Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology. 1996;217:11–22. doi: 10.1006/viro.1996.0088. [DOI] [PubMed] [Google Scholar]
- 16.Garcia I, Martinou I, Tsujimoto Y, Martinou J C. Prevention of programmed cell death of sympathetic neurons by the bcl-2 proto-oncogene. Science. 1992;258:302–304. doi: 10.1126/science.1411528. [DOI] [PubMed] [Google Scholar]
- 17.Gooding L R. Regulation of TNF-mediated cell death and inflammation by human adenoviruses. Infect Agents Dis. 1994;3:106–115. [PubMed] [Google Scholar]
- 18.Han J, Wallen H D, Nunez G, White E. E1B 19,000-molecular-weight protein interacts with and inhibits CED-4-dependent, FLICE-mediated apoptosis. Mol Cell Biol. 1998;18:6052–6062. doi: 10.1128/mcb.18.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, Kieff E, Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 20.Hitt M M, Addison C L, Graham F L. Human adenovirus vectors for gene transfer into mammalian cells. In: August J T, editor. Gene therapy. London, United Kingdom: Academic Press; 1997. pp. 138–206. [DOI] [PubMed] [Google Scholar]
- 21.Hockenbery D, Nunez G, Milliman C, Schreiber R D, Korsmeyer S J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss R S, Swanson P E, Knudson C M, Chang K C, Cobb J P, Osborne D F, Zollner K M, Buchman T G, Korsmeyer S J, Karl I E. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- 23.Ilan Y, Droguett G, Chowdhury N R, Li Y, Sengupta K, Thummala N R, Davidson A, Chowdhury J R, Horwitz M S. Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long-term gene expression. Proc Natl Acad Sci USA. 1997;94:2587–2592. doi: 10.1073/pnas.94.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh N, Tsujimoto Y, Nagata S. Effect of bcl-2 on Fas antigen-mediated cell death. J Immunol. 1993;151:621–627. [PubMed] [Google Scholar]
- 25.Jooss K, Yang Y, Wilson J M. Cyclophosphamide diminishes inflammation and prolongs transgene expression following delivery of adenoviral vectors to mouse liver and lung. Hum Gene Ther. 1996;7:1555–1566. doi: 10.1089/hum.1996.7.13-1555. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan J M, St. George J A, Pennington S E, Keyes L D, Johnson R P, Wadsworth S C, Smith A E. Humoral and cellular immune responses of nonhuman primates to long-term repeated lung exposure to Ad2/CFTR-2. Gene Ther. 1996;3:117–127. [PubMed] [Google Scholar]
- 27.Katsumata M, Siegel R M, Louie D C, Miyashita T, Tsujimoto Y, Nowell P C, Greene M I, Reed J C. Differential effects of Bcl-2 on T and B cells in transgenic mice. Proc Natl Acad Sci USA. 1992;89:11376–11380. doi: 10.1073/pnas.89.23.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawahara A, Kobayashi T, Nagata S. Inhibition of Fas-induced apoptosis by Bcl-2. Oncogene. 1998;17:2549–2554. doi: 10.1038/sj.onc.1202192. [DOI] [PubMed] [Google Scholar]
- 29.Kay M A, Meuse L, Gown A M, Linsley P, Hollenbaugh D, Aruffo A, Ochs H D, Wilson C B. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolls J K, Lei D, Odom G, Nelson S, Summer W R, Gerber M A, Shellito J E. Use of transient CD4 lymphocyte depletion to prolong transgene expression of E1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:489–497. doi: 10.1089/hum.1996.7.4-489. [DOI] [PubMed] [Google Scholar]
- 31.Koyama A H, Irie H, Fukumori T, Hata S, Iida S, Akari H, Adachi A. Role of virus-induced apoptosis in a host defense mechanism against virus infection. J Med Investig. 1998;45:37–45. [PubMed] [Google Scholar]
- 32.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. . (Erratum, 3:934.) [DOI] [PubMed] [Google Scholar]
- 33.Kurosawa H, Que F G, Roberts L R, Fesmier P J, Gores G J. Hepatocytes in the bile duct-ligated rat express Bcl-2. Am J Physiol. 1997;272:G1587–G1593. doi: 10.1152/ajpgi.1997.272.6.G1587. [DOI] [PubMed] [Google Scholar]
- 34.Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, Henrion A, Bouscary D, Varlet P, Joulin V, Kahn A. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med. 1996;2:80–86. doi: 10.1038/nm0196-80. [DOI] [PubMed] [Google Scholar]
- 35.Lawson J A, Fisher M A, Simmons C A, Farhood A, Jaeschke H. Parenchymal cell apoptosis as a signal for sinusoidal sequestration and transendothelial migration of neutrophils in murine models of endotoxin and Fas-antibody-induced liver injury. Hepatology. 1998;28:761–767. doi: 10.1002/hep.510280324. [DOI] [PubMed] [Google Scholar]
- 36.Lieber A, He C Y, Meuse L, Himeda C, Wilson C, Kay M A. Inhibition of NF-κB activation in combination with Bcl-2 expression allows for persistence of first-generation adenovirus vectors in the mouse liver. J Virol. 1998;72:9267–9277. doi: 10.1128/jvi.72.11.9267-9277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lieber A, He C Y, Meuse L, Schowalter D, Kirillova I, Winther B, Kay M A. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maher J J, Gores G J. Apoptosis: silent killer or neutron bomb? Hepatology. 1998;28:865–867. doi: 10.1002/hep.510280338. [DOI] [PubMed] [Google Scholar]
- 39.McFadden G, Graham K, Barry M. New strategies of immune modulation by DNA viruses. Transplant Proc. 1996;28:2085–2088. [PubMed] [Google Scholar]
- 40.Meinl E, Fickenscher H, Thome M, Tschopp J, Fleckenstein B. Anti-apoptotic strategies of lymphotropic viruses. Immunol Today. 1998;19:474–479. doi: 10.1016/s0167-5699(98)01309-7. [DOI] [PubMed] [Google Scholar]
- 41.Muruve D A, Barnes M J, Stillman I E, Libermann T A. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 41a.National Institutes of Health. 20 May 1999, last date revised. [Online.] http://www.nih.gov/od/orda/index.htm. [20 May 1999, last date accessed.] National Institutes of Health, Office of Recombinant DNA Activities, Bethesda, Md.
- 42.Okuyama T, Li X K, Funeshima N, Fujino M, Sasaki K, Kita Y, Kosuga M, Takahashi M, Saito H, Suzuki S, Yamada M. Fas-mediated apoptosis is involved in the elimination of gene-transduced hepatocytes with E1/E3-deleted adenoviral vectors. J Gastroenterol Hepatol. 1998;13:S113–S118. [PubMed] [Google Scholar]
- 43.Peeters M J, Patijn G A, Lieber A, Meuse L, Kay M A. Adenovirus-mediated hepatic gene transfer in mice: comparison of intravascular and biliary administration. Hum Gene Ther. 1996;7:1693–1699. doi: 10.1089/hum.1996.7.14-1693. [DOI] [PubMed] [Google Scholar]
- 44.Perez D, White E. E1B 19K inhibits Fas-mediated apoptosis through FADD-dependent sequestration of FLICE. J Cell Biol. 1998;141:1255–1266. doi: 10.1083/jcb.141.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrof B J, Acsadi G, Jani A, Massie B, Bourdon J, Matusiewicz N, Yang L, Lochmuller H, Karpati G. Efficiency and functional consequences of adenovirus-mediated in vivo gene transfer to normal and dystrophic (mdx) mouse diaphragm. Am J Respir Cell Mol Biol. 1995;13:508–517. doi: 10.1165/ajrcmb.13.5.7576685. [DOI] [PubMed] [Google Scholar]
- 46.Pilder S, Logan J, Shenk T. Deletion of the gene encoding the adenovirus 5 early region 1b 21,000-molecular-weight polypeptide leads to degradation of viral and host cell DNA. J Virol. 1984;52:664–671. doi: 10.1128/jvi.52.2.664-671.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin L, Ding Y, Pahud D R, Robson N D, Shaked A, Bromberg J S. Adenovirus-mediated gene transfer of viral interleukin-10 inhibits the immune response to both alloantigen and adenoviral antigen. Hum Gene Ther. 1997;8:1365–1374. doi: 10.1089/hum.1997.8.11-1365. [DOI] [PubMed] [Google Scholar]
- 48.Reed J C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reed J C, Zha H, Aime-Sempe C, Takayama S, Wang H G. Structure-function analysis of Bcl-2 family proteins. Regulators of programmed cell death. Adv Exp Med Biol. 1996;406:99–112. [PubMed] [Google Scholar]
- 50.Rovere P, Manfredi A A, Vallinoto C, Zimmermann V S, Fascio U, Balestrieri G, Ricciardi-Castagnoli P, Rugarli C, Tincani A, Sabbadini M G. Dendritic cells preferentially internalize apoptotic cells opsonized by anti-beta2-glycoprotein I antibodies. J Autoimmun. 1998;11:403–411. doi: 10.1006/jaut.1998.0224. [DOI] [PubMed] [Google Scholar]
- 51.Rovere P, Vallinoto C, Bondanza A, Crosti M C, Rescigno M, Ricciardi-Castagnoli P, Rugarli C, Manfredi A A. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol. 1998;161:4467–4471. [PubMed] [Google Scholar]
- 52.Sato N, Wang S, Li L, Okabe K, Hashimoto M, Yaginuma H, Mikoshiba K, Uchiyama Y, Uetsuki T, Yoshikawa K, Milligan C E, Oppenheim R W. A novel strategy for introducing exogenous bcl-2 into neuronal cells: the Cre/loxP system-mediated activation of bcl-2 for preventing programmed cell death using recombinant adenoviruses. Mol Cell Neurosci. 1998;12:65–78. doi: 10.1006/mcne.1998.0703. [DOI] [PubMed] [Google Scholar]
- 53.Schowalter D B, Tubb J C, Liu M, Wilson C B, Kay M A. Heterologous expression of adenovirus E3-gp19K in an E1a-deleted adenovirus vector inhibits MHC I expression in vitro, but does not prolong transgene expression in vivo. Gene Ther. 1997;4:351–360. doi: 10.1038/sj.gt.3300398. [DOI] [PubMed] [Google Scholar]
- 54.Shen Y, Shenk T E. Viruses and apoptosis. Curr Opin Genet Dev. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu S, Eguchi Y, Kamiike W, Itoh Y, Hasegawa J, Yamabe K, Otsuki Y, Matsuda H, Tsujimoto Y. Induction of apoptosis as well as necrosis by hypoxia and predominant prevention of apoptosis by Bcl-2 and Bcl-XL. Cancer Res. 1996;56:2161–2166. [PubMed] [Google Scholar]
- 56.Song W, Kong H L, Traktman P, Crystal R G. Cytotoxic T lymphocyte responses to proteins encoded by heterologous transgenes transferred in vivo by adenoviral vectors. Hum Gene Ther. 1997;8:1207–1217. doi: 10.1089/hum.1997.8.10-1207. [DOI] [PubMed] [Google Scholar]
- 57.Spriggs M K. One step ahead of the game: viral immunomodulatory molecules. Annu Rev Immunol. 1996;14:101–130. doi: 10.1146/annurev.immunol.14.1.101. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan D E, Dash S, Du H, Hiramatsu N, Aydin F, Kolls J, Blanchard J, Baskin G, Gerber M A. Liver-directed gene transfer in non-human primates. Hum Gene Ther. 1997;8:1195–1206. doi: 10.1089/hum.1997.8.10-1195. [DOI] [PubMed] [Google Scholar]
- 59.Takemori N, Cladaras C, Bhat B, Conley A J, Wold W S. cyt gene of adenoviruses 2 and 5 is an oncogene for transforming function in early region E1B and encodes the E1B 19,000-molecular-weight polypeptide. J Virol. 1984;52:793–805. doi: 10.1128/jvi.52.3.793-805.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taub R. Blocking NF-kappaB in the liver: the good and bad news. Hepatology. 1998;27:1445–1446. doi: 10.1002/hep.510270538. [DOI] [PubMed] [Google Scholar]
- 61.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 62.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Immune responses to transgene-encoded proteins limit the stability to gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–555. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 63.Tsujimoto Y, Gorham J, Cossman J, Jaffe E, Croce C M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985;229:1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- 64.van Ginkel F W, McGhee J R, Liu C, Simecka J W, Yamamoto M, Frizzell R A, Sorscher E J, Kiyono H, Pascual D W. Adenoviral gene delivery elicits distinct pulmonary-associated T helper cell responses to the vector and to its transgene. J Immunol. 1997;159:685–693. [PubMed] [Google Scholar]
- 65.Vaux D L, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci USA. 1996;93:2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vrancken Peeters M J, Perkins A L, Kay M A. Method for multiple portal vein infusions in mice: quantitation of adenovirus-mediated hepatic gene transfer. BioTechniques. 1996;20:278–285. doi: 10.2144/96202rr05. [DOI] [PubMed] [Google Scholar]
- 67.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 68.White E, Grodzicker T, Stillman B W. Mutations in the gene encoding the adenovirus early region 1B 19,000-molecular-weight tumor antigen cause the degradation of chromosomal DNA. J Virol. 1984;52:410–419. doi: 10.1128/jvi.52.2.410-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson J M. Gene therapy for cystic fibrosis: challenges and future directions. J Clin Investig. 1995;96:2547–2554. doi: 10.1172/JCI118318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Worgall S, Leopold P L, Wolff G, Ferris B, Van R N, Crystal R G. Role of alveolar macrophages in rapid elimination of adenovirus vectors administered to the epithelial surface of the respiratory tract. Hum Gene Ther. 1997;8:1675–1684. doi: 10.1089/hum.1997.8.14-1675. [DOI] [PubMed] [Google Scholar]
- 71.Worgall S, Wolff G, Falck-Pedersen E, Crystal R G. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 72.Yamabe K, Shimizu S, Kamiike W, Waguri S, Eguchi Y, Hasegawa J, Okuno S, Yoshioka Y, Ito T, Sawa Y, Uchiyama Y, Tsujimoto Y, Matsuda H. Prevention of hypoxic liver cell necrosis by in vivo human bcl-2 gene transfection. Biochem Biophys Res Commun. 1998;243:217–223. doi: 10.1006/bbrc.1997.7925. [DOI] [PubMed] [Google Scholar]
- 73.Yang L, Matthews R T, Schulz J B, Klockgether T, Liao A W, Martinou J C, Penney J B J, Hyman B T, Beal M F. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyride neurotoxicity is attenuated in mice overexpressing Bcl-2. J Neurosci. 1998;18:8145–8152. doi: 10.1523/JNEUROSCI.18-20-08145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Y, Ertl H C, Wilson J M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 75.Yang Y, Haecker S E, Su Q, Wilson J M. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Genet. 1996;5:1703–1712. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- 76.Yang Y, Jooss K U, Su Q, Ertl H C, Wilson J M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- 77.Yang Y, Xiang Z, Ertl H C, Wilson J M. Upregulation of class I major histocompatibility complex antigens by interferon gamma is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang H G, Bilbao G, Zhou T, Contreras J L, Gomez-Navarro J, Feng M, Saito I, Mountz J D, Curiel D T. Application of a Fas ligand encoding a recombinant adenovirus vector for prolongation of transgene expression. J Virol. 1998;72:2483–2490. doi: 10.1128/jvi.72.3.2483-2490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang H G, Zhou T, Yang P, Edwards C K, Curiel D T, Mountz J D. Inhibition of tumor necrosis factor alpha decreases inflammation and prolongs adenovirus gene expression in lung and liver. Hum Gene Ther. 1998;9:1875–1884. doi: 10.1089/hum.1998.9.13-1875. [DOI] [PubMed] [Google Scholar]
- 80.Zhong L T, Sarafian T, Kane D J, Charles A C, Mah S P, Edwards R H, Bredesen D E. bcl-2 inhibits death of central neural cells induced by multiple agents. Proc Natl Acad Sci USA. 1993;90:4533–4537. doi: 10.1073/pnas.90.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu H, Shen Y, Shenk T. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J Virol. 1995;69:7960–7970. doi: 10.1128/jvi.69.12.7960-7970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]