Abstract
Recombinant interleukin-16 (rIL-16) has been found to inhibit human immunodeficiency virus type 1 (HIV-1) replication in acutely or endogenously infected CD4+ T cells. However, the effect of rIL-16 on HIV-1 replication in antigen-presenting cells (APCs) is still unknown. We show here a potent HIV-suppressive activity of rIL-16 in acutely infected monocyte-derived macrophages and dendritic cells determined by the levels of viral RNA transcripts or of viral reverse transcriptase in culture supernatants. The observed effect was dependent on the presence of rIL-16 early after infection and could not be induced by a 24-h treatment of cells with the cytokine prior to infection. Using macrophage-tropic and dually tropic primary isolates, we also showed that the addition of rIL-16 to cell cultures only during the infection period was effective in blocking virus entry and reducing proviral DNA levels in APCs. However, the anti-HIV activity of rIL-16 could not be linked to the induction of virus-suppressive concentrations of β-chemokines or to the inhibition of HIV-enhancing cytokines. These findings establish a critical role for rIL-16 in protecting APCs against HIV-1 infection and lend further support to its potential use in the treatment of HIV disease.
Interleukin-16 (IL-16) is a pleiotropic cytokine inducing chemoattractant activity in CD4+ T cells, monocytes, and eosinophils (6, 7). The cytokine is synthesized mainly by CD8+ lymphocytes as a precursor molecule which is then cleaved and secreted as a 17-kDa protein upon cell activation (8). Monomeric IL-16 aggregates into a tetrameric form which is essential for the cytokine to interact directly with and to cross-link its receptor, the CD4 antigen (9). A recombinant form of IL-16 (rIL-16), corresponding to the C-terminal 130 amino acid residues, has been cloned and found to inhibit human immunodeficiency virus type 1 (HIV-1) replication in acutely (5) and endogenously (3) infected CD4+ lymphocytes. However, the majority (>90%) of the rIL-16 produced in a bacterial expression system has been characterized as inactive monomers and dimers (5, 13), possibly due to incorrect folding and/or a lack of stability. This could explain the need for high concentrations of exogenously added rIL-16 (>5 μg/ml) to exert HIV-suppressive activity in infected peripheral blood mononuclear cell (PBMC) cultures (3, 5, 13).
Macrophages and dendritic cells are key antigen-presenting cells (APCs) which express surface CD4 molecules and are susceptible to HIV-1 infection. These APCs are believed to be among the first cells to be infected by HIV-1 in patients, to act as reservoirs for virus dissemination, and to be key players in the pathogenesis of HIV-1 infection (15, 18, 23). Although the HIV-suppressive activity of rIL-16 in CD4+ lymphocytes has been well studied (3, 5, 13, 31), no information on the capacity of this cytokine to regulate HIV-1 replication in APCs is yet available. To address this issue, monocyte-derived macrophages (MDMs) and monocyte-derived dendritic cells (MDDCs) were generated in a 7-day culture period from adherent monocytes (24) in RPMI 1640 containing 10% heat-inactivated human AB serum or in the same medium supplemented with 1,000 U of granulocyte-macrophage colony-stimulating factor (kindly provided by Sandoz Pharma, Basel, Switzerland) per ml, 10 ng of IL-4 per ml, and 200 U of tumor necrosis factor alpha (TNF-α) (R&D Systems Europe Ltd., Abingdon, United Kingdom) per ml, as previously described (1, 30). At the end of the differentiation period, >90% of MDMs were CD14+, and MDDCs were found to represent mature dendritic cells as judged by morphologic (adherent cells with fine membrane projections) and phenotypic (CD14−, CD3−, high levels of CD80 and CD86, >40% CD83+, and >60% CD4+) criteria. These two cell types were then acutely infected by a 2-h exposure to different HIV-1 isolates (macrophage-tropic [M-tropic] HIV-1Ba-L, M-tropic primary isolate HIV-1CHR-4, or the dually tropic primary isolate HIV-1CHR-1) at a dose corresponding to 10,000 cpm of reverse transcriptase activity/106 cells (3, 13) and were treated with rIL-16 either before, during, or after infection. We show here a potent HIV-suppressive activity of the recombinant cytokine in both types of APCs and that the inhibition of virus entry is one of the mechanisms mediating this antiviral effect.
Effect of IL-16 on HIV-1Ba-L replication in APCs.
In a series of experiments, we compared the effects of rIL-16 (1 μg/ml), produced in our laboratory as an endotoxin-free protein (less than 0.125 endotoxin unit/10 μg of protein) containing 5 to 7% of the homotetrameric form (3, 13), and macrophage inflammatory protein 1α (MIP-1α) (0.2 μg/ml; R&D Systems) on HIV-1Ba-L replication in acutely infected MDMs and MDDCs. Following a 12- to 15-day period of culture in the continuous presence of the tested cytokines, supernatants were titrated to determine the levels of viral reverse transcriptase as previously described (13). Results shown in Table 1 demonstrate the potent HIV-suppressive activity of rIL-16 in both cell populations and the lack of a significant effect of MIP-1α on virus replication. Furthermore, the specificity of the IL-16 effect was also confirmed in two of these presented experiments by the absence of any virus-suppressive activity of a 1-μg/ml concentration of an irrelevant recombinant protein, rat IL-15 (kindly provided by J. Khalife, Institut Pasteur de Lille, France), that was expressed and purified in a system identical to that of rIL-16. Four additional experiments performed to compare the effects of 0.3, 1, and 3 μg of rIL-16 per ml in MDMs indicated that the highest concentration induced 91 to 100% inhibition (mean ± standard error of the mean [SEM], 95% ± 2.5%) of viral reverse transcriptase levels, whereas the lowest concentration tested had no reproducible inhibitory activity (0 to 32%) on HIV-1 replication. Similar dose-response effects were observed in acutely infected MDDCs. Nevertheless, it remains evident that the virus-suppressive activity of rIL-16 in APCs is detectable at a 10-fold-lower concentration than that needed to induce comparable effects in acutely infected PBMCs (5, 13). This may be explained by the lower level of CD4 expression in MDMs and MDDCs than in T cells. Alternative explanations, including a higher efficiency of signal transduction in APCs, cannot be ruled out. Moreover, since one of the IL-16 batches used in a previous study on PBMCs (13) was also employed in the present work, the higher sensitivity of APCs to the virus-inhibiting activity of rIL-16 could not be attributed to differences in the homotetrameric contents of different cytokine preparations. This was further verified by evaluating the activity of a second batch of 1 μg of rIL-16 per ml in acutely infected PBMCs and MDMs originating from the same donor, resulting in 18 and 77% inhibitions of HIV-1 replication, respectively. On the other hand, low (nanomolar) concentrations of IL-16 have been reported to protect against HIV-1 infection following the transfection of Jurkat cells with IL-16 cDNA (31). These findings strongly suggest that the cytokine produced by human cells is present mainly in a biologically active tetrameric form, in contrast to the bacterially derived IL-16. Moreover, rIL-16 may not be the most active form of the naturally secreted molecule since a smaller polypeptide, resulting from proteolytic processing of CD8+ cell lysates, has been recently identified (4, 29). In this context, it is worthwhile to point out that commercially available enzyme-linked immunosorbent assay kits (3) did not detect endogenously secreted natural IL-16 in the supernatants of noninfected or HIV-1-infected MDMs and MDDCs tested throughout a culture period of 10 days in four independent experiments.
TABLE 1.
IL-16 inhibits HIV-1Ba-L replication in MDMs and MDDCs
| Cell typea and donor | Reverse transcriptase level (cpm/50 μl) in cultures treated withb:
|
||
|---|---|---|---|
| RPMI 1640 | IL-16 | MIP-1α | |
| MDMs | |||
| 1 | 47,834 | 13,499 (72) | 57,170 (−20) |
| 2 | 78,075 | 28,165 (64) | 51,879 (33) |
| 3 | 37,848 | 3,702 (90) | 38,837 (−3) |
| 4 | 46,898 | 3,602 (92) | 26,635 (43) |
| 5 | 85,636 | 21,859 (74) | ND |
| Mean ± SEM | 59,258 ± 9,464 | 14,165 ± 4,882 (78 ± 5) [0.0012] | 43,630 ± 6,850 (13 ± 15) [0.3638] |
| MDDCs | |||
| 1 | 16,483 | 3,738 (83) | ND |
| 2 | 10,822 | 0 (100) | ND |
| 3 | 16,440 | 354 (98) | 14,530 (13) |
| 4 | 34,510 | 3,747 (89) | 18,250 (47) |
| 5 | 20,871 | 3,386 (84) | 24,381 (−17) |
| 6 | 22,301 | 976 (96) | 26,051 (−17) |
| Mean ± SEM | 20,237 ± 3,294 | 2,033 ± 724 (92 ± 3) [0.0016] | 20,803 ± 2,680 (6 ± 15) [0.6021] |
MDMs and MDDCs from healthy donors were infected in vitro with HIV-1Ba-L and then cultured in the absence (RPMI 1640) or the presence of IL-16 (1 μg/ml) or MIP-1α (0.2 μg/ml).
Supernatants from infected cultures were harvested and assayed on days 12 to 15 postinfection. Numbers in parentheses are percent inhibition; numbers in brackets are P values calculated by Student’s t test for paired data. ND, not determined.
Effect of IL-16 occurs at the level of HIV-1 mRNA expression.
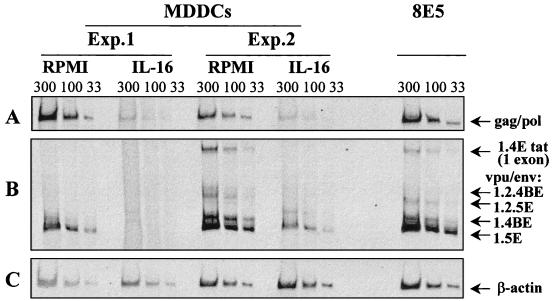
We evaluated the effect of rIL-16 on virus mRNA levels in HIV-1Ba-L-infected MDMs and MDDCs. Thus, following 10 to 12 days of culture in the absence or presence of rIL-16 (3 μg/ml), total cellular RNA was extracted from infected cells with RNAzol (Bioprobe Systems, Montreuil, France) and subjected to a single step of reverse transcription (RT)-PCR to detect HIV-1 unspliced Gag or Pol mRNA with the primer pair GAG06-GAG04 (20) and singly spliced mRNA with the primer pair BSS-KPNA (19), as previously described (3). Cell equivalence was evaluated with β-actin primers (16). Representative results for MDDCs from two donors are shown in Fig. 1. HIV-1 mRNA expression levels of each of the three tested concentrations were normalized to the corresponding levels of β-actin by calculating the ratio of the HIV-1 band volume over that of β-actin by using imaging systems (Image Master 1D Prime; Pharmacia Biotech, Uppsala, Sweden), and then the mean percent inhibition of HIV-1 expression was calculated. The HIV-1 Gag and Pol mean band volumes (in densitometric units) for RPMI 1640- and IL-16-treated cultures were, respectively, 34,292 and 3,131 in experiment 1 and 28,764 and 6,660 in experiment 2 (Fig. 1A). Correcting for the intensities of the β-actin bands shown in Fig. 1C (mean band volumes for RPMI 1640- and IL-16-treated cultures were, respectively, 20,166 and 18,497 in experiment 1 and 31,039 and 38,692 in experiment 2), the levels of inhibition of unspliced HIV-1 transcripts by rIL-16 were 96% in experiment 1 and 81% in experiment 2. Similarly, the expression of the various forms of intermediate, singly spliced mRNA (Fig. 1B) was dramatically lower in IL-16-treated cultures (mean band volumes of the five transcripts were 2,071 in experiment 1 and 24,243 in experiment 2) than in untreated cultures (mean band volumes were 34,283 in experiment 1 and 111,061 in experiment 2), and the level of inhibition was 82% in both experiments. Identical results were obtained with RNAs from untreated and IL-16-treated MDMs (data not shown). Experiments were then performed to evaluate whether a 24-h treatment of primary MDMs or MDDCs with rIL-16 prior to infection could result in the suppression of HIV-1Ba-L replication. Results from three separate experiments demonstrated reverse transcriptase levels on day 8 postinfection in the supernatants of IL-16-pretreated MDMs (mean ± SEM, 15,439 ± 1,824) and MDDCs (9,082 ± 896) that were identical to those in non-pretreated cells (14,786 ± 1,964 for MDMs and 9,624 ± 1,534 for MDDCs). Thus, stimulation of APCs with IL-16 before HIV-1 infection does not appear to induce cellular factors capable of repressing virus promoter activity. Such a mechanism has been reported to be activated in IL-16-pretreated lymphocytes but not in monocytoid cell lines (17).
FIG. 1.
Viral mRNA expression in HIV-1Ba-L-infected MDDCs cultured for 12 days in the absence or presence of rIL-16 (3 μg/ml). In two separate experiments (Exp.), RNA samples (33, 100, and 300 ng) were subjected to RT-PCR amplifications with primer pair GAG06-GAG04 to detect Gag or Pol unspliced mRNA (A) and with primer pair BSS-KPNA to detect intermediate-size singly spliced viral transcripts (B). These mRNAs were named according to the exons they contain and the proteins they produce (19): 1.4E Tat (exons 1 and 4E), 1.2.4BE Vpu/Env (exons 1, 2, and 4BE), 1.2.5E Vpu/Env (exons 1, 2, and 5E), 1.4BE Vpu/Env (exons 1 and 4BE), and 1.5E Vpu/Env (exons 1 and 5E). Constitutively expressed β-actin mRNA in the same samples was amplified (C). An equivalent amount of RNA from the 8E5 cell line was used as a positive control of the RT-PCR amplification. RT-PCR products were resolved on a 4 to 5% nondenaturing polyacrylamide gel, visualized under UV light after ethidium bromide staining, and photographed.
Effect of IL-16 on the replication of HIV-1 primary isolates.
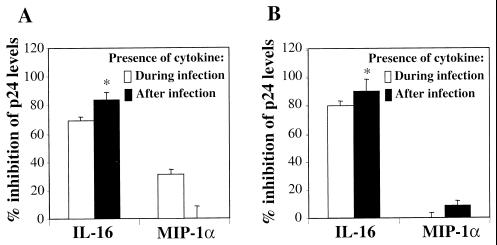
The abilities of rIL-16 and MIP-1α to inhibit the replication of M-tropic and dually tropic primary isolates in acutely infected MDMs and MDDCs was then studied in parallel under conditions in which either cytokine was the only one present during the 2-h period of infection. Results shown in Fig. 2 demonstrate that p24 levels in supernatants of MDMs infected with either M-tropic (CHR-4 [Fig. 2A]) or dually tropic (CHR-1 [Fig. 2B]) primary isolates were highly reduced (mean, 84 to 91%) in cultures maintained after infection, in the presence of rIL-16 (1 μg/ml). Identical results were observed in MDDCs infected with the same primary isolates (data not shown). In contrast to the effect of rIL-16, no detectable virus-suppressive activity on either of the tested isolates by MIP-1α (0.2 μg/ml [Fig. 2]) was observed; the addition of rIL-16 (1 μg/7.5 × 105 cells) to MDMs only during the 2-h infection period resulted in a significant inhibition (mean, 69 to 80%) of virus replication. This effect, though highly significant, was of lower magnitude than that observed in cultures maintained in the presence of IL-16 immediately after infection and throughout the 10- to 12-day culture period (Fig. 2). The presence of MIP-1α (0.2 μg/7.5 × 105 cells) during the period of infection with M-tropic (CHR-4) but not dually tropic (CHR-1) HIV strains presented a weak but significant inhibitory effect on subsequent viral replication. A higher concentration of MIP-1α (0.5 μg/7.5 × 105 cells), used in two other experiments, resulted in a higher (50 and 55%) inhibitory activity on M-tropic HIV-1 replication (data not shown). This is consistent with the capacity of MIP-1α to block the binding of HIV-1 to CCR5, the coreceptor for M-tropic HIV-1 strains (2), but not to CXCR4, the coreceptor used by dually tropic primary isolates to enter MDMs (27). Taken together, these results indicate that the HIV-suppressive effect of IL-16 in APCs is also evident at the level of virus entry, in contrast to the suggested activity on CD4+ lymphocytes (31). One possible explanation for this difference is that T cells express much higher levels of CD4 than MDMs or MDDCs, which render competitive inhibition of virus binding to lymphocytes by rIL-16 less efficient. A second explanation could be that the cross-linking of CD4 by rIL-16 on APCs, but not on T lymphocytes (6), results in immediate downregulation of CD4 expression, sufficient to inhibit virus entry. Alternatively, treatment with rIL-16 either during or early after infection may block proviral DNA formation in APCs but not in T cells (31). Experiments presented below were carried out to address some of these issues.
FIG. 2.
Effects of rIL-16 (1 μg/ml) and MIP-1α (0.2 μg/ml) on replication of primary HIV-1 isolates in MDMs. Cytokines were added either during the 2-h infection period or continuously after infection with the M-tropic primary isolate HIV-1CHR-4 (A) or with the dually tropic primary isolate HIV-1CHR-1 (B). The levels of viral p24 were quantified 10 to 12 days postinfection and are presented as percent inhibition (mean ± SEM) of p24 release from six and five separate experiments with CHR-4 and CHR-1, respectively. ∗, significantly different level of inhibition from that observed with the cytokine present during the infection period only (P < 0.05, Student’s t test for paired data).
Effect of IL-16 on proviral DNA levels.
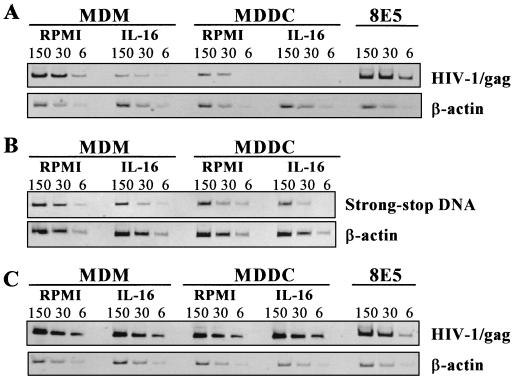
To determine whether the observed effects of rIL-16 are also mediated during early postentry events, we evaluated the proviral DNA levels in cytokine-treated and nontreated HIV-1Ba-L-infected MDMs and MDDCs. A semiquantitative PCR with primer pair GAG06-GAG04 (20) was used to amplify viral DNA from total cellular DNA that was extracted 16 h after the infection period. DNA samples (6 to 150 ng) were subjected to 25 to 40 repeated rounds of amplification with AmpliTaq Gold DNA polymerase (Perkin-Elmer, Norwalk, Conn.) by following the manufacturer’s protocol. We found that rIL-16 (3 μg/well) added only during the 2-h infection period resulted in 72 and 93% inhibition of HIV-1 Gag proviral DNA in MDMs and MDDCs, respectively (Fig. 3A). Similarly, the level of HIV-1 strong-stop DNA, analyzed by PCR with primer pair M667-AA55 (28), was also found to be highly reduced when the infection was carried out in the presence of rIL-16 (3 μg/ml [Fig. 3B]). The mean ratios of strong-stop DNA band volume (in densitometric units) over that of β-actin were, respectively, 0.51 and 0.13 in untreated and IL-16-treated MDMs (74% mean inhibition). In MDDCs, these ratios were 0.25 (RPMI 1640) and 0.07 (IL-16), with 72% inhibition of virus DNA levels. This further confirms that the observed inhibition of the proviral DNA by rIL-16 is mediated mostly at the step of virus entry. Moreover, the lack of 100% inhibition of viral DNA in cells infected in the presence of rIL-16 (3 μg/ml) may well be attributable to the low level of the homotetrameric form (approximately 150 ng/3 μg of protein) in the preparation. Higher concentrations of the active form of IL-16 seem to be necessary to achieve complete saturation of surface CD4 molecules on 7.5 × 105 cells/well. On the other hand, the addition of rIL-16 (3 μg/ml) for 16 h after the infection period had no inhibitory effect (>12%) on proviral DNA formation in both cell populations studied, as shown by representative results from four identical experiments (Fig. 3C). Therefore, the suppression of HIV-1 replication by rIL-16, when added to freshly infected cells, is not mediated at the level of proviral DNA formation. However, the findings of the capacity of rIL-16, when present during the infection period, to inhibit proviral DNA levels and subsequent viral replication suggest either a competitive inhibition of HIV-1 binding to the CD4 molecule or a rapid and dramatic internalization of this receptor in APCs. The latter possibility was excluded by the findings in three separate experiments testing unmodified CD4 surface expression, in both types of APCs, following a 15-min or 2-h incubation with rIL-16. Taken together, these findings strongly suggest an IL-16-mediated competitive inhibition of virus binding to the CD4 molecule as a major pathway of inhibition of HIV-1 infection in APCs.
FIG. 3.
Effect of rIL-16 on the HIV-1Ba-L proviral DNA levels in MDMs and MDDCs. Cells (7.5 × 105) were infected for 2 h in the absence or presence of 3 μg of rIL-16 (A and B) or were infected in the absence of cytokine and then cultured with or without rIL-16 (3 μg/ml) (C). DNA was extracted 16 h postinfection, and various concentrations (6, 30, and 150 ng) were subjected to PCR amplification with primer pair GAG04-GAG06 to detect HIV-1 Gag (A and C) and primer pair M667-AA55 to detect HIV-1 strong-stop DNA (B). Cell equivalence was evaluated by using β-actin-specific primers. Results shown are PCR products migrated on 6% acrylamide gels from one of two to four identical experiments. Also shown is the PCR amplification of DNA from the 8E5 cell line, used as a positive control.
Effect of IL-16 is not mediated by a change in the balance of released cytokines.
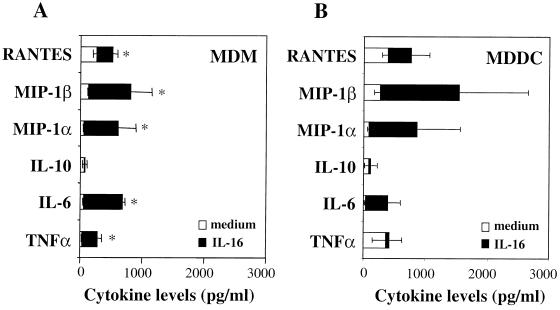
The question of whether the HIV-1-suppressive activity of rIL-16 is mediated by the regulation of the endogenous cytokine network was addressed by evaluating the levels of secreted cytokines and chemokines. Following the 2-h period of infection with HIV-1Ba-L, MDMs and MDDCs were cultured in the absence or presence of rIL-16 (3 μg/ml), and supernatants were collected 1, 2, or 3 days later. Peak cytokine release was generally detected 2 days after treatment with rIL-16; results from eight and five independent experiments with MDMs and MDDCs, respectively, are shown in Fig. 4. The addition of rIL-16 to freshly infected MDMs induced weak but significant increases in the release of RANTES (regulated upon activation, normal T cell expressed and secreted), MIP-1α, MIP-1β, IL-6, and TNF-α, but not of IL-10 (Fig. 4A). On the other hand, IL-16-stimulated MDDCs did not show a significant increase in the release of any of the tested cytokines (Fig. 4B). Therefore, it is evident that neither the induction of high levels of β-chemokines nor the inhibition of the release of HIV-enhancing cytokines (IL-6 and TNF-α) could account for the IL-16-mediated suppression of HIV-1 replication in APCs.
FIG. 4.
Profiles of released cytokines in HIV-1Ba-L-infected MDM and MDDC cultures that were maintained in the absence (medium) or in the presence of rIL-16 (3 μg/ml). Culture supernatants were collected 2 days postinfection, and the levels of different cytokines were assayed by commercially available enzyme-linked immunosorbent assay kits. Values are means ± SEMs of five to eight separate experiments. ∗, significantly different values from those of unstimulated cultures (P < 0.05, Wilcoxon matched-pairs test).
The binding of IL-16 to CD4 on APCs results in the steric inhibition of HIV-1 entry and the repression of virus transcription in infected cells. However, the mechanisms involved appear to be different from those mediating the cytokine effect on lymphocytes (7, 31). In this respect, IL-16-induced signaling in lymphocytes is dependent on the CD4-associated tyrosine kinase p56lck (9, 21), whereas monocytes/macrophages are known to lack this Src tyrosine kinase (9, 10). Recently, rIL-16 was found to activate the stress-activated protein kinase in CD4+ macrophages (14). This difference in the signaling cascade between lymphocytes and macrophages could partly explain the observed differences in the effects of IL-16 on the two cell populations. Alternatively, rIL-16 may repress the activation of different transcription factors that either are cell type specific or are required for HIV-1 transcription in one cell population but not in the other (11, 22, 26). Thus, the interaction of IL-16 with its CD4 receptor on different cell types may not necessarily result in similar modulations of cell function and could depend on the nature of the signal transduction pathways induced in a defined CD4+ cell. This is substantiated by a recent study demonstrating different effects of rIL-16 as measured by changes in receptor expression and cytokine release, on the two populations of APCs (12). Although mRNA expression of CCR5 and CXCR4 was found to be significantly downregulated in IL-16-treated MDMs, minimal or no stable effects could be noted in MDDCs. Similarly, regulation of surface expression of costimulatory molecules (CD80 and CD86) in MDMs was consistently observed following a 24-h treatment with rIL-16; however, no such effect could be detected in IL-16-treated MDDCs (12). Based on these findings, it is possible to envisage an important role of IL-16 in regulating certain immune functions of macrophages and to suggest that the HIV-suppressive activity of this cytokine in MDMs, but not in MDDCs, may be partly linked to its capacity to downregulate HIV-1 coreceptor expression. Finally, the abilities of the chemoattractant cytokine to control virus infection and/or replication in several cell targets, to restore antigen-specific proliferation, and to expand populations of CD4+ lymphocytes (25) strongly support its being considered a component of immune-based therapies in HIV disease.
Acknowledgments
This work was supported by research grants (no. 97088 and 98016) from the Agence National de la Recherche sur le SIDA in France.
We are grateful to J. Khalife, T. Idziorek, O. Billaut-Mulot, and M. Loyens for help in the cloning and purification of rIL-16.
REFERENCES
- 1.Akridge R E, Reed S G. Interleukin-12 decreases human immunodeficiency virus type 1 replication in human macrophage cultures reconstituted with autologous peripheral blood mononuclear cells. J Infect Dis. 1996;173:559–564. doi: 10.1093/infdis/173.3.559. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR-5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Amiel C, Darcissac E, Truong M J, Dewulf J, Loyens M, Mouton Y, Capron A, Bahr G M. Interleukin-16 (IL-16) inhibits human immunodeficiency virus replication in cells from infected subjects, and serum IL-16 levels drop with disease progression. J Infect Dis. 1999;179:83–91. doi: 10.1086/314550. [DOI] [PubMed] [Google Scholar]
- 4.Baier M, Bannert N, Werner A, Lang K, Kurth R. Molecular cloning, sequence, expression, and processing of the interleukin 16 precursor. Proc Natl Acad Sci USA. 1997;94:5273–5277. doi: 10.1073/pnas.94.10.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baier M, Wermer A, Bannert N, Metzner K, Kurth R. HIV suppression by interleukin-16. Nature. 1995;378:563. doi: 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 6.Center D M, Kornfeld H, Cruikshank W W. Interleukin 16 and its function as a CD4 ligand. Immunol Today. 1996;17:476–481. doi: 10.1016/0167-5699(96)10052-i. [DOI] [PubMed] [Google Scholar]
- 7.Center D M, Kornfeld H, Cruikshank W W. Interleukin 16. Int J Biochem Cell Biol. 1997;29:1231–1234. doi: 10.1016/s1357-2725(97)00053-8. [DOI] [PubMed] [Google Scholar]
- 8.Chupp G L, Wright E A, Wu D, Vallen-Mashikian M, Cruikshank W W, Center D M, Kornfeld H, Berman J S. Tissue and T cell distribution of precursor and mature IL-16. J Immunol. 1998;161:3114–3119. [PubMed] [Google Scholar]
- 9.Cruikshank W W, Kornfeld H, Center D M. Signaling and functional properties of interleukin-16. Int Rev Immunol. 1998;16:523–540. doi: 10.3109/08830189809043007. [DOI] [PubMed] [Google Scholar]
- 10.Geleziunas R, Morin N, Wainberg M A. Mechanisms of reduction of CD4 receptor expression on the surface of HIV-1 infected cells. CR Acad Sci Ser III. 1996;319:653–662. . (In French.) [PubMed] [Google Scholar]
- 11.Henderson A J, Calame K L. CCAAT/enhancer binding protein (C/EBP) sites are required for HIV-1 replication in primary macrophages but not CD4+ T cells. Proc Natl Acad Sci USA. 1997;94:8714–8719. doi: 10.1073/pnas.94.16.8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermann, E., E. Darcissac, T. Idziorek, A. Capron, and G. M. Bahr. Recombinant interleukin-16 selectively modulates surface receptor expression and cytokine release in macrophages and dendritic cells. Immunology, in press. [DOI] [PMC free article] [PubMed]
- 13.Idziorek T, Khalife J, Billaut-Mulot O, Hermann E, Aumercier M, Mouton Y, Capron A, Bahr G M. Recombinant human interleukin-16 inhibits HIV-1 replication and protects against activation-induced cell death. Clin Exp Immunol. 1998;112:84–91. doi: 10.1046/j.1365-2249.1998.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krautwald S. IL-16 activates the SAPK signaling pathway in CD4+ macrophages. J Immunol. 1998;160:5874–5879. [PubMed] [Google Scholar]
- 15.Langhoff E, Terwilliger E F, Bos H J, Kalland K H, Poznansky M C, Bacon O M L, Haseltine W A. Replication of human immunodeficiency virus type 1 in primary dendritic cell cultures. Proc Natl Acad Sci USA. 1991;88:7998–8002. doi: 10.1073/pnas.88.18.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legoux P, Minty C, Delpech B, Minty A J, Shire D. Simultaneous quantitation of cytokine mRNAs in interleukin-1β stimulated U373 human astrocytoma cells by a polymerisation chain reaction method involving co-amplification with an internal multi-specific control. Eur Cytokine Netw. 1992;3:553–563. [PubMed] [Google Scholar]
- 17.Maciaszek J W, Parada N A, Cruikshank W W, Center D M, Kornfeld H, Viglianti G A. IL-16 represses HIV-1 promoter activity. J Immunol. 1997;158:5–8. [PubMed] [Google Scholar]
- 18.Meltzer M S, Skillman D R, Gomatos P J, Kalter D C, Gendelman H E. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- 19.Neumann M, Harrison J, Sartarelli M, Hadziyannis E, Erfle V, Felber B K, Pavlakis G N. Splicing variability in HIV type 1 revealed by quantitative RNA polymerase chain reaction. AIDS Res Hum Retroviruses. 1994;10:1531–1542. doi: 10.1089/aid.1994.10.1531. [DOI] [PubMed] [Google Scholar]
- 20.Piatak M, Jr, Luk K-C, Williams B, Lifson J D. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. BioTechniques. 1993;14:70–81. [PubMed] [Google Scholar]
- 21.Ryan T C, Cruikshank W W, Kornfeld H, Collins T L, Center D M. The CD4-associated tyrosine kinase p56lck is required for lymphocyte chemoattractant factor-induced T lymphocyte migration. J Biol Chem. 1995;270:17081–17086. doi: 10.1074/jbc.270.29.17081. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan P L, Sheline C T, Cannon K, Voz M L, Pazin M J, Kadonaga J T, Jones K A. Activation of the HIV-1 enhancer by the LEF-1 HMG protein on nucleosome-assembled DNA in vitro. Genes Dev. 1995;9:2090–2104. doi: 10.1101/gad.9.17.2090. [DOI] [PubMed] [Google Scholar]
- 23.Stoler M H, Eskin T A, Benn S, Angerer R C, Angerer L M. Human T-cell lymphotropic virus type III infection of the central nervous system. A preliminary in situ analysis. JAMA. 1986;256:2360–2364. [PubMed] [Google Scholar]
- 24.Verani A, Pesenti E, Polo S, Tresoldi E, Scarlatti G, Lusso P, Siccardi A G, Vercelli D. CXCR4 is a functional coreceptor for infection of human macrophages by CXCR4-dependent primary isolates. J Immunol. 1998;161:2084–2088. [PubMed] [Google Scholar]
- 25.Viglianti G A, Parada N A, Maciaszek J W, Kornfeld H, Center D M, Cruikshank W W. IL-16 anti-HIV-1 therapy. Nat Med. 1997;3:938. doi: 10.1038/nm0997-938. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z, Engel J D. Human T cell transcription factor GATA-3 stimulates HIV-1 expression. Nucleic Acids Res. 1993;21:2831–2836. doi: 10.1093/nar/21.12.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi Y, Rana S, Turner J D, Gaddis N, Collman R G. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:772–777. doi: 10.1128/jvi.72.1.772-777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Center D M, Wu D M, Cruikshank W W, Yuan J, Andrews D W, Kornfeld H. Processing and activation of pro-interleukin-16 by caspase-3. J Biol Chem. 1998;273:1144–1149. doi: 10.1074/jbc.273.2.1144. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L J, Tedder T F. CD14+ blood monocytes can differentiate into functionally mature CD83 dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou P, Goldstein S, Devadas K, Tewari D, Notkins A L. Human CD4+ cells transfected with IL-16 cDNA are resistant to HIV-1 infection: inhibition of mRNA expression. Nat Med. 1997;3:659–664. doi: 10.1038/nm0697-659. [DOI] [PubMed] [Google Scholar]