Abstract
Cancer, as the foremost challenge among human diseases, has plagued medical professionals for many years. While there have been numerous treatment approaches in clinical practice, they often cause additional harm to patients. The emergence of nanotechnology has brought new directions for cancer treatment, which can deliver anticancer drugs specifically to tumor areas. This article first introduces the application scenarios of nanotherapies and treatment strategies of nanomedicine. Then, the noteworthy characteristics exhibited by biopolymer materials were described, which make biopolymers stand out in polymeric nanomedicine delivery. Next, we focus on summarizing the state-of-art studies of five categories of proteins (Albumin, Gelatin, Silk fibroin, Zein, Ferritin), nine varieties of polysaccharides (Chitosan, Starch, Hyaluronic acid, Dextran, cellulose, Fucoidan, Carrageenan, Lignin, Pectin) and liposomes in the field of anticancer drug delivery. Finally, we also provide a summary of the advantages and limitations of these biopolymers, discuss the prevailing impediments to their application, and discuss in detail the prospective research directions. This review not only helps readers understand the current development status of nano anticancer drug delivery systems based on biopolymers, but also is helpful for readers to understand the properties of various biopolymers and find suitable solutions in this field through comparative reading.
Keywords: drug delivery, nanoformulations, polymer, proteins, polysaccharides
Graphical Abstract
Introduction
Cancer is the second leading cause of death worldwide, with over 30 different types of cancer reported. Common types include breast cancer, lung cancer, liver cancer, prostate cancer, colorectal cancer, and others. Current main treatment modalities for cancer include surgery, chemotherapy, radiation therapy, and immunotherapy, among others. These treatments have shown efficacy in cancer treatment, but they often come with noticeable side effects. Surgical resection may be challenging to completely remove tumor tissue, while chemotherapy delivers anticancer drugs to tumor sites through systemic, non-selective administration, causing harm to healthy tissues and exhibiting low efficacy with the potential for drug resistance.1 In addition, some anticancer drugs exhibit significant cytotoxic effects on cancer cells, such as curcumin,2 doxorubicin, paclitaxel, cisplatin, and vinblastine. However, many of these drugs are hydrophobic in nature, leading to low oral bioavailability, and most of them require renal excretion. Immunotherapy, on the other hand, stimulates the body’s immune system by targeting specific antigens on the surface of cancer cells through antibody binding. However, this treatment approach has limited efficacy in certain patient populations, such as those with autoimmune diseases, and repeated administration may potentially induce immune-related adverse events.3 High dosage and repeated administration are common challenges associated with these treatment modalities, undoubtedly increasing the risk of damage to normal organ tissues. Therefore, novel targeted delivery strategies are needed to improve the treatment of cancer.
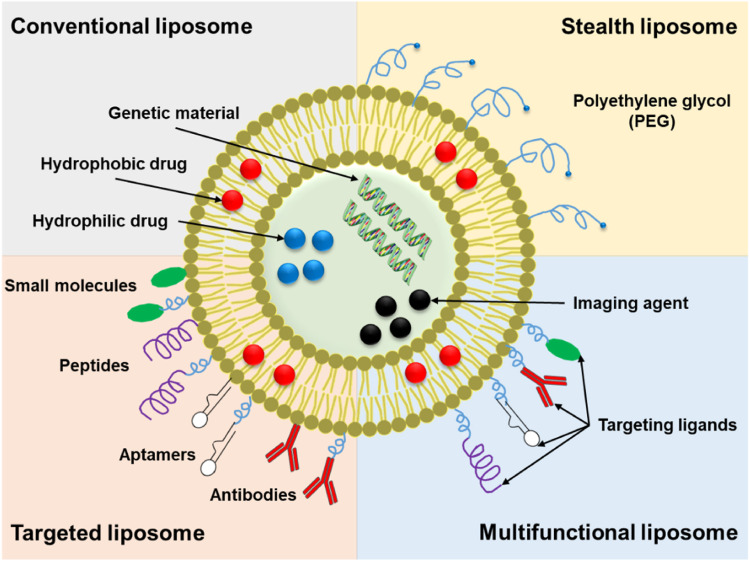
Various delivery system strategies have been developed for cancer treatment, including antibody-based delivery, polymer-based delivery, liposome-based delivery, and protein-based delivery. The antibody-based delivery strategy involves conjugating antibodies as carriers with radioactive drugs or chemotherapeutic agents for radioimmunotherapy or targeted immunotherapy. Currently, FDA-approved targeted radiotherapeutics include Lutathera, Zevalin, and Bexxar.4 Targeted immunotherapy offers a potential solution to reduce the adverse effects of systemic treatments like chemotherapy on patients. The FDA has approved various types of targeted therapy drugs that focus on specific aspects of cancer treatment. These include drugs targeting the tumor vasculature system, such as sorafenib, bevacizumab, and sunitinib. Additionally, there are drugs targeting the tumor immune system, such as nivolumab, pembrolizumab, and atezolizumab, which aim to enhance the body’s immune response against cancer. Furthermore, drugs targeting the tumor microenvironment, including ramucirumab, axicabtagene ciloleucel, and denosumab, are designed to modify the tumor’s surrounding environment.5 The clinical application of antibody-based drug carriers relies heavily on the expression of specific antigens on the tumor surface, while resistance, off-target effects, and toxic side effects such as bone marrow suppression and venous occlusion limit their effectiveness. Polymer-based carriers come in various forms, including polymer micelles (Nanoxel® M, Genexol® PM, SP1049C), polymer-drug conjugates (Oncaspar, Asparlas), dendrimers (DEP®docetaxel, AZD0466), and polymer implants (GLIADEL Wafer). However, polymers are often non-degradable and possess high immunogenicity.6 Liposomes, with a long history as delivery vehicles, have examples like Doxil®, an early FDA-approved liposomal formulation, and Vyxeos®, approved in 2017 for the treatment of acute myeloid leukemia. Liposome preparation is relatively straightforward, but their delivery performance is influenced by size and composition, and they lack targeting specificity.7,8 Proteins, such as the FDA-approved Transdrug®, exhibit low immunogenicity but suffer from poor stability.
Nanotechnology has revolutionized the field of medicine,9,10 particularly in cancer treatment, through the development of nanomedicine. Nanomedicine encompasses a wide range of applications, including prevention, monitoring, diagnosis, and treatment of cancer. Nanoscale materials possess unique properties that enable personalized design and targeted delivery of therapies.11 This technology has overcome many limitations of traditional cancer treatments by offering numerous advantages. Nanoparticles can encapsulate poorly soluble drugs, improving their solubility and efficacy.12 They can also enhance drug circulation time, increasing their bioavailability.13 Through ligand modification, nanoparticles can specifically target tumor sites, overcoming drug resistance and minimizing damage to healthy tissues.14 Additionally, nanomaterials can be engineered to exhibit temperature and pH sensitivity, allowing for controlled drug release. Furthermore, their ability to cross the blood-brain barrier opens new possibilities for treating neurological diseases.15 The emergence of nanomedicine has brought significant advancements to cancer treatment, addressing challenges and improving outcomes for patients.
Nanomedicine predominantly relies on nanomaterials and delivery strategies. In the subsequent section of this paper, we elucidate the applications of nanomaterials, delivery strategies, and the distinctive attributes of various nanomaterials. Following that, the core of this paper (biopolymer-based nanodelivery carriers) is discussed in detail, focusing on the current research status of biopolymer-based delivery carriers (proteins, polysaccharides and liposome), extant challenges, and potential future directions. The article aims to provide assistance and guidance to researchers in this field.
Nanomedicine for Cancer Therapy
Nanomedicines Applications
The clinical use of natural anti-tumor drugs is limited by low water solubility, rapid clearance from the circulation, lack of selectivity and low tissue permeability. Paclitaxel (PTX) and doxorubicin (DOX) are frequently prescribed anticancer medications. PTX exhibits low water solubility, and direct injection can lead to varying degrees of bodily damage, necessitating formulation with 50% ethanol and 50% Cremophor-EL (CrEL) (a polyoxyethylated castor oil derivative) in clinical settings.16 However, CrEL poses a risk of inducing neuropathy and hypersensitivity reactions, necessitating pre-treatment with antihistamines. In contrast, directly injected PTX is highly cytotoxic, leading to side effects such as myelosuppression (neutropenia, anaemia, and thrombocytopenia) and cumulative neurotoxicity (peripheral sensory abnormalities, sensory hypersensitivity, arthralgia, and myalgia). DOX is indicated for the treatment of a wide range of solid tumors, including breast, ovarian, gastric, thyroid and liver cancers, and directly injected DOX can be rapidly distributed to all body tissues, however, DOX and its metabolite, Zoerythromycin, produce free radicals that cause cardiotoxicity, and also have side effects such as myelotoxicity, neuropathy, hypersensitivity reactions, hair loss, and gastrointestinal toxicity.17 In addition to anticancer drugs, nucleic acid-mediated therapies are also effective tools in cancer treatment. For example, siRNA can precisely target and silence almost any gene of interest. However, nucleic acid therapeutics such as siRNA are readily cleaved by endonucleases in the serum and extracellular environment.18
Nanomaterials have garnered significant attention from researchers due to their excellent specific surface area and extremely small volume. When used as drug carriers, they can overcome challenges associated with low bioavailability, non-specific distribution, and poor water solubility of free drugs.19 Abraxane and Opaxio are clinically utilized PTX nanoformulations capable of effectively limiting drug exposure to normal tissues. Particularly, Abraxane utilizes albumin as a drug carrier. The unique characteristics of albumin significantly enhance the pharmacokinetics of PTX and facilitate the drug’s targeting to tumors. Furthermore, Doxil and Myocet are DOX liposome nanoformulations used clinically, which effectively reduce cardiotoxicity by encapsulating DOX in liposomes.17
Due to the rapid growth of tumors, there is an increased demand for oxygen and nutrients, leading to the rapid expansion of the vascular system and increased permeability of the blood vessel walls. Additionally, tumors often lack a functional lymphatic system, which allows for prolonged retention of large molecular substances within the tumor region. This enhanced permeability and retention (EPR) effect in tumors is the main working principle of current nanomedicines.20 By utilizing the EPR effect, nano drugs larger than 8 nm can penetrate blood vessels and permeate the tumor microenvironment, thereby achieving effective drug delivery. Through appropriate modifications of nanomaterials, they can exhibit specific sensitivity to factors such as near-infrared light, pH, temperature, magnetic fields, and ultrasound, enabling responsive drug release. By combining various treatment modalities such as photothermal therapy (PTT), magnetothermal therapy, chemotherapy (CHT), radiation therapy (RT), gene therapy, immunotherapy, photodynamic therapy (PDT), chemical dynamic therapy (CDT), and starvation therapy, targeted controlled release of drugs can be achieved.19 Furthermore, these approaches can be combined with molecular imaging techniques to enable multifunctional treatments, including localized imaging and tracking. However, there is a scarcity of clinically approved nanomedicines. Examples of such approved nanomedicines include Abraxane®, Doxil®, Ontak®, Genexol® and a few others.
Table 1 collates some of the nano-formulations currently FDA-approved for cancer treatment, encompassing those for physical therapy and chemotherapeutic drug treatment. However, clinical utilization of nano-formulations remains limited, primarily due to the low delivery efficiency of nano-administered drugs. Reports indicate that only 0.7% of the injected dose reaches solid tumors, with a concurrent risk of damage to normal tissues.21
Table 1.
FDA-Approved Anticancer Nanomedicines
| Clinical Products | Description | Treating Cancer | Clinical Effect |
|---|---|---|---|
| Abraxane® | Albumin Load PTX | Advanced non-small cell lung cancer (surgery or radiotherapy not an option), metastatic breast cancer (secondary), metastatic pancreatic cancer (primary) | No hypersensitivity |
| NanoTax® | PTX nanoparticles prepared by SCF technology | Malignant tumor of the peritoneum | Reduced systemic exposure and toxicity22 |
| Genexol® | PTX micelles | Breast cancer, locally advanced or metastatic NSCLC | Reduced hypersensitivity and neurotoxic effects |
| Opaxio® | PTX polymer formulations | Glioblastoma | Avoid exposure of normal tissues to high levels of unconjugated active chemotherapy and its associated toxicity (hair loss, infections and cardiac symptoms)23 |
| Paclical® | Paclitaxel micelles | Epithelial ovarian cancer | Allows for higher doses, shorter infusion times, elimination of the need for preoperative medications, and improved patient safety |
| MagForce NanoTherm® | Magnetic Thermal Therapy | Glioblastoma | Lower magneto-thermal conversion efficiency, severe MRI artefacts, susceptibility to tumor leakage |
| AuroShell® | Thermal therapy with near-infrared laser sources | Prostate cancer | Reduce side effects |
| NBTXR3/Hensify® | Crystalline Hafnium Oxide Nanoparticles | Locally advanced squamous cell carcinoma | To improve the anti-tumor efficacy of radiotherapy while reducing its potential side effects, such as damage to surrounding healthy tissue24 |
| Pegasys® | PEG-coupled interferon | Persistent (chronic) infection with hepatitis C virus or hepatitis B virus | Longer half-life |
| Oncaspar® | PEG-coupled asparaginase | Paediatric acute lymphoblastic leukaemia | Hepatotoxicity, pancreatitis, thrombosis, nausea, vomiting and fatigue25 |
| Neulasta® | PEG-modified recombinant methionyl human G-CSF (r-metHuG-CSF) | Non-myeloid malignant tumor | Enhanced activity compared to filgrastim26 |
| Eligard® | PLGA-encapsulated leuprolide | Advanced prostate cancer | Lack of overall safety and tolerability and outbreak drug release27 |
| Kadcyla® | Ado- Trastuzumab Emtansine | Recurrent HER2-positive, metastatic breast cancer | The most common side effects are nausea, fatigue, muscle or joint pain, low levels of platelets in the blood (thrombocytopenia), elevated liver enzyme levels, headache and constipation |
| VYXEOS® | Liposome-encapsulated cytarabine with zorubicin | Acute myeloid leukaemia | Can lead to a severe generalised rash |
| Patisiran/ONPATTRO® | Liposome-encapsulated siRNA | Transthyretin (TTR)-mediated amyloidosis | Back pain, nausea, abdominal pain, dyspnoea. |
| Doxil/ Caelyx® | PEGylated liposome doxorubicin | Metastatic breast cancer, advanced ovarian cancer | Hand-foot syndrome, a sign of idiosyncratic non-IgE-mediated hypersensitivity reaction28,29 |
| Myocet® | Liposome doxorubicin | Treatment of metastatic breast cancer (primary) | May reduce cardiotoxicity associated with doxorubicin treatment and may avoid unwanted toxicity caused by PEG or adriamycin30 |
| Marqibo® | Liposome vincristine | Philadelphia chromosome-negative acute lymphoblastic leukaemia (tertiary) | Higher maximum tolerated dose, superior antitumor activity and delivery of more active drug to the target tissue31 |
| MEPACT® | Liposomal mifamurtide | Osteosarcoma | Well tolerated, but with sequelae such as chills, fever, headache, nausea and myalgia32 |
| Onivyde® | PEGylated liposome irinotecan | Metastatic pancreatic cancer (secondary) | May cause life-threatening neutropenia, diarrhoea33,34 |
| Depocyt® | Liposomal adriamycin | Neoplastic meningitis | Develop serious treatment-related neurological complications35 |
Abbreviations: SCF, supercritical fluid; NSCLC, non-small cell lung cancer; MRI, magnetic resonance imaging.
Tumor Microenvironment and Biobarrier
The tumor microenvironment and biological barriers are two crucial factors that hinder the delivery of nanomedicines. Targeted delivery strategies based on nanotechnology primarily rely on several specific markers of cancer, including (1) Aberrant proliferation signaling pathways; (2) Resistance to cell death; (3) Induction of neovascularization (angiogenesis); (4) Escape from growth inhibitory factors; (5) Activation of invasion and metastasis; (6) Replicative immortality;36 (7) Dysregulated cellular energy and metabolism;2 (8) Evasion of immune destruction. The heterogeneous and unique physiological microenvironment of tumors, such as hypoxia, acidity (pH 6.5–6.9), and elevated glutathione levels, often serve as endogenous stimuli for responsive drug release in nanomedicine targeting. When the drug reaches the tumor site, it senses these heterogeneity-related differences and releases the drug accordingly.
Despite the advantages offered by nanotechnology, the delivery of anticancer drugs based on nanotechnology still needs to overcome various physiological barriers. Firstly, it needs to overcome systemic obstacles such as the mononuclear phagocyte system (or reticuloendothelial system), and clearance by the liver, biliary system, and urinary system, to have a chance to interact with tumor tissue. Secondly, the tumor tissue penetration of the nano delivery system is crucial, with a general requirement that its hydrodynamic size should be smaller than 100 nanometers. Tumor tissue resembles an organ, comprising tumor cells and a heterogeneous tumor stroma, which collectively give rise to a complex and dynamic tumor microenvironment. In addition to the physical barriers conferred by the dense tissue stroma and elevated intra-tumoral pressure, there are also multiple drug resistance mechanisms and immune suppression mediated by tumor-associated cells. Lastly, the uptake of nanodelivered drugs by tumor cells is essential, as the delivered drugs need to cross the cell membrane and reach specific cellular organelles to exert therapeutic effects.37 Additionally, tumor cells exhibit variable uptake of nano-delivery drugs, and since the therapeutic targets of most therapeutic agents are located intracellularly, delivery carriers need to traverse the cell membrane to reach specific organelles for therapeutic efficacy. However, the presence of lysosomes within the cell poses a challenge as they contain various hydrolytic enzymes that may lead to drug degradation or loss of effectiveness.38 Bypassing endosomal pathways and endosomal rupture mechanisms are currently the main strategies employed to address this issue.
Nanomedicine Delivery Strategies
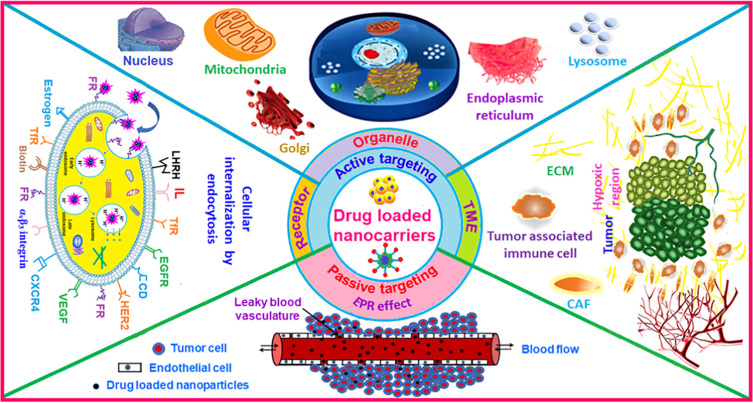
Nano-formulations have been developed to overcome the limitations of free small molecule drug delivery, demonstrating significant potential in enhancing drug bioavailability and reducing drug toxicity. Strategies for delivering nanomedicines to tumors typically involve two main approaches: passive targeting and active targeting, as shown in Figure 1.
Figure 1.
Tumor Targeting Strategies. Reprinted from Advances in Colloid and Interface Science, 296, Dutta B, Barick KC, Hassan PA. Recent advances in active targeting of nanomaterials for anticancer drug delivery, 102509 Copyright 2021, with permission from Elsevier.39
Passive targeting, primarily reliant on the Enhanced Permeation and Retention (EPR) effect, facilitates the accumulation of nanoformulations, typically ranging from 10 to several hundred nanometers in size, within tumors. This effect occurs due to leakage from the tumor vascular system and compromised lymphatic vessels. However, despite its utility, this delivery strategy presents notable drawbacks, as outlined below.40(1) Lack of Specificity. Passive targeted therapeutic approaches to tumors often rely on the variability of tumor tissue from normal tissue. However, this variability is not always apparent, potentially resulting in adverse effects of the drug on normal tissue, such as toxicity. (2) Intra-tumor Heterogeneity. Variability, such as uneven cell density and blood vessel distribution, exists within tumor tissue, influencing the distribution and efficacy of drugs within the tumor. (3) Limited Drug Release. Upon entering tumor tissue, certain targeted drugs or carriers may be influenced by the tumor microenvironment, leading to restricted drug release and reduced therapeutic efficacy. (4) Development of Drug Resistance. Resistance exhibited by certain tumor cells against the targeted drug can diminish its therapeutic effect, thereby reducing overall efficacy. (5) Challenges in controlling drug release and distribution. Passive targeting presents difficulties in accurately controlling the release rate and distribution of drugs within tumor tissue, potentially resulting in inadequate or excessive drug concentration, thereby impacting therapeutic effectiveness and safety. (6) Influence of the Tumor Microenvironment on Therapeutic Effectiveness. Changes in the tumor microenvironment can impact the Enhanced Permeability and Retention (EPR) effect, consequently influencing drug accumulation and therapeutic efficacy.
Active targeting can specifically home in on the tumor area, substantially enhancing therapeutic efficacy. This strategy typically branches into two major directions: surface receptor targeting and targeting the unique pathological environment of the tumor. Following these directions, active targeting strategies can be further classified into three approaches.39 (1) Targeting cancer cell surface receptors. This method involves specific receptors present on the surface of cancer cells, such as the folate receptor, transferrin (Tf) receptor, epidermal growth factor receptor (EGFR), human epidermal growth factor receptor (HER), cluster of differentiation (CD) receptor, integrin receptor, estrogen receptor, among others.41,42 (2) Targeting subcellular organelles. Certain biologically active molecules, such as peptides or nucleic acids, necessitate delivery to specific organelles to attain maximal therapeutic efficacy. Additionally, targeting specific organelles can enhance therapeutic effectiveness and minimize toxicity at lower doses. Currently, the primary subcellular organelles targeted include mitochondria, nucleus, lysosome, endoplasmic reticulum, and Golgi apparatus. (3) Targeting the tumor tissue and microenvironment. Depending on the specific composition of tumor tissues or cells, it is possible to prepare targeting active peptides, such as tumor-homing peptides and cell-penetrating peptides. Tumor homing peptides specifically recognize and adhere to tumor cells/tumor vascular system, and cell-penetrating peptides can target and penetrate the anatomical barrier of the tumor. The tumor microenvironment possesses several unique features, such as low pH, hypoxia, and high glutathione levels. Capitalizing on this heterogeneity, stimulus-responsive nanomedicines with hypoxia-responsive, acid-responsive, GSH-responsive properties, among others, can be designed. Moreover, owing to enhanced angiogenesis in the tumor microenvironment, targeting the vascular endothelial growth factor (VEGF) receptor, highly expressed in this milieu, is achievable.43 Additionally, certain inflammatory mediators are overexpressed in the tumor microenvironment, making them suitable targets for immune cells to mediate immune therapy. Active targeting is able to bind specifically to tumors, deliver drugs selectively, and circumvent the problem of drug resistance, but some of the challenges remain to be solved. Active targeting mainly relies on modifying some characteristic proteins or peptides on the carrier surface, which may be cleared by reticuloendothelial system (RES) and induce local immune responses. In addition, the preparation process is complex and difficult to industrialize.44
Main Parameters of Nanomedicines
The main influencing factors of nanomedicines include size, morphology, surface charge and surface chemistry. These parameters are essential to ensure the efficacy, safety and controllability of nano-formulation design, preparation and application. By regulating and optimising these relevant parameters, the biocompatibility and stability of nano-formulations in organisms can be enhanced, thereby improving the bioavailability of drugs in vivo.
Size
The Size of nano-formulations is a critical factor affecting their bioavailability, as it influences various aspects of their behavior within the body.45 Formulations that are too large can induce inflammatory reactions and peritoneal adhesions, while those that are too small may have insufficient residence time for effective tumor penetration.46 Typically, nanopreparations range in size from 10 to 1000 nm. Nanoparticles smaller than 20–30 nm are rapidly cleared by the excretory system, whereas larger particles (>200 nm) are taken up by the mononuclear phagocytosis system.47 Nanoparticles in the range of 150–300 nm tend to accumulate predominantly in the liver and spleen, while colloids sized between 200 to 400 nm are quickly cleared by the liver.48 In cancer therapy, nano-formulations often rely on the Enhanced Permeability and Retention (EPR) effect of tumors. The gap size for vascular leakage in tumors typically ranges from 100 to 780 nm. Larger particle sizes (60–200 nm) favor prolonged in vivo retention, while smaller sizes (30–50 nm) facilitate penetration into tumor tissue.49 Previous studies have demonstrated that nanocarrier sizes around 50 nm are optimal for enhanced drug delivery and tumor specificity. Designing size-variable nano-formulations can enhance intracellular delivery, promote deep tumor penetration, and improve tumor targeting, thereby optimizing their effectiveness in cancer therapy.48,50
Surface Charge
Surface charge plays a critical role in protein adsorption, with the Zeta point commonly utilized to characterize the charged properties of particles. Positively charged nanocarriers exhibit strong binding to negatively charged proteins via electrostatic attraction, leading to their uptake not only by various plasma proteins but also by phagocytes, ultimately resulting in their excretion from the bloodstream.51 This process reduces the penetration and efficacy of nanomedicines. In contrast, neutral nanoparticles or those with a slightly negative charge demonstrate significantly prolonged circulating half-life. Moreover, the surface charge of nanopreparations is pivotal for cellular internalization. Positively charged liposomes, for instance, have been shown to be more favorable for cellular internalization, albeit this depends on factors such as the growth stage of the tumor and the type of cancer cells.52,53 Designing nanodelivery systems with triggered charge reversal, wherein stimuli such as pH, reactive oxygen species (ROS), enzymes, glutathione (GSH), adenosine triphosphate (ATP), light, and thermal responses are employed, can mitigate the toxic side effects associated with positive surface charge and effectively enhance the delivery efficiency of nanomedicines.54
Morphology
The Morphology of nano-formulations plays a pivotal role in various biological processes, including biological barrier crossing, cell internalization, immune escape, and protein corona formation.55 For instance, non-spherical nanoparticles exhibit a longer circulation time compared to spherical particles, facilitating drug penetration and bioaccumulation at the tumor site.56,57 The cellular uptake of nanoparticles can be modulated by altering the shape of the nanomaterials.58–61 Li Ying et al discovered that the order of cellular uptake of nanoparticles was spherical > cubic > rod > disc.62 Rod-shaped gold nanoparticles demonstrated superior penetration compared to spherical ones.63 Shenshen Cai et al investigated the impact of filamentous micelles versus spherical nanoparticles on drug delivery and found that filamentous micelles had longer in vivo circulation times compared to polyethylene glycolated liposomal particles.64 Marginal dynamics, or the lateral drift of nanoparticles towards the endothelial wall, is a crucial consideration in nanoparticle design. Binding to the vessel wall facilitates particle-cell binding and receptor-ligand interactions in active targeting strategies and enables extravasation through the tumor’s open-window vascular system. Disc particles experience marginalization under blood flow, significantly altering circulation time, biodistribution, and cellular interactions. Nanodiscs exhibit strong binding to the phospholipid bilayer, primarily to the cell membrane, and can be employed for cell membrane tracing.65,66 Ning Wang et al, utilizing erythrocyte membranes coated with disc-shaped mesoporous silica nanoparticles encapsulated with DOX, demonstrated successful drug release in hypoxic environments with enhanced permeability and drug accumulation compared to spherical shapes.67 This in vivo effect attributable to shape differences is correlated with the formation of a protein corona. It was observed that rods and spheres exhibit a shape-dependent relationship with the amount of protein adsorbed, with rods adsorbing a greater quantity of protein.68
Surface Chemistry
Surface chemistry is a pivotal factor influencing the interactions at the nanobio-interface, ultimately determining the pharmacokinetics and tumor targeting of the formulation. Various surface chemistries, such as peptides, natural polysaccharides, and synthetic polymers, exhibit different cellular affinities.69 Studies have revealed that carboxylated surface chemistries exhibit a notable affinity for ovarian cancer cells. Moreover, nanoparticles encapsulated with poly-L-aspartic acid, poly-L-glutamic acid, and hyaluronic acid demonstrate superior tumor targeting compared to conventional polyethylene glycolated (PEG) nanoparticles. Jing Wang et al modified bovine serum albumin-poly(N-3-acrylamidophenylboronic acid) nanoparticles with PEI-PEG copolymer and cRGD peptide, illustrating that surface chemical modification of nanoparticles effectively enhances tumor accumulation and cellular uptake.70 Furthermore, surface chemistry impacts the composition of the protein corona and the quantity of protein. By precisely controlling the surface chemistry of the formulation, modulation of the protein corona and cellular uptake can be achieved.71
Other Parameters
Dispersibility, drug loading, and drug release rate are crucial parameters in nano-formulation design. Dispersibility refers to the extent of uniform distribution of particles in a solution or matrix. Optimal dispersibility ensures stability and homogeneity of the formulation during application. Meanwhile, drug loading and release rate are vital factors affecting the administered dose and therapeutic efficacy. Adequate drug loading ensures the delivery of the intended dosage, while the release rate influences the kinetics of drug release, directly impacting the therapeutic effect. Therefore, careful consideration and optimization of dispersibility, drug loading, and drug release rate are essential for the development of effective nano-formulations.
The pharmacokinetics and pharmacodynamics of drugs are essential parameters for assessing the in vivo behavior of nanomedicines and the mechanism of drug effects. The main parameters and significance of pharmacokinetics are summarized in Table 2. Compared to free drugs, carrier-mediated anticancer drugs or conjugated drugs can achieve optimized pharmacokinetic profiles through parameter tuning, thereby striking a better balance between efficacy and toxicity. (1) Nanomedicines can mitigate the clearance of the mononuclear phagocyte system (MPS) through surface chemical modification. (2) Functionalized nanomedicines can prolong the half-life, enhance the bioavailability, and increase the volume of distribution of the drug. (3) Nanomedicines can exploit the EPR effect or active targeting to access the tumor microenvironment, thereby enhancing drug specificity and reducing toxicity. (4) Encapsulating more therapeutic drugs within nanomedicines can decrease the frequency of required drug dosing intervals and enhance the anticipated pharmacological effects. This leads to a reduction in systemic adverse side effects and an improvement in treatment compliance.72–74
Table 2.
Pharmacokinetic Parameters
| Parameters | Description |
|---|---|
| Cmax | Maximum observed concentration of a drug collected from an animal or human body |
| Cthrough | Means the lowest concentration from the initial moment of administration to the next administration when multiple administrations have reached steady state, and is a reflection of the level of accumulation of the drug |
| Tmax | Time to reach Tmax |
| AUC | Area under the concentration curve, representing the total drug exposure experienced by the subject in the study |
| T1/2 | Half-life, the time for the concentration of the drug to decrease by half |
| MRT | Mean residence time, the average time a drug molecule spends in the body |
| Vd (volume of distribution) | A volume estimated as the ratio of the blood concentration (C) to the amount of drug in the body (D) when the drug is absorbed and distributed to reach a steady state blood concentration. |
| CL (Clearance Rate) | The number of apparent volume of distribution of a drug removed from the body per unit of time, meaning how many volumes of plasma are cleared of the drug per unit of time |
Biopolymer-Based Nanosystems for Cancer Drug Delivery
Cancer nanomedical technology primarily relies on its delivery carriers. The ideal delivery carrier should exhibit good biocompatibility, biodegradability, excellent drug-loading capacity, ease of acquisition, low production costs, and low or no toxicity. Nanomaterials can be primarily categorized into inorganic materials (including metal nanomaterials, carbon nanomaterials, and mesoporous silica nanomaterials) and polymer materials (synthetic polymers and biopolymers).
Inorganic nanomaterials exhibit unique photonic and electromagnetic properties, coupled with their diminutive size. However, there in vivo circulation time is short. Polymers, as macromolecular materials, fulfill the fundamental requirements for serving as delivery carriers. They not only possess exceptional stability but also offer adjustable dimensions. Polymers can be further are subdivided into two categories based on their sources: synthetic polymers and biopolymers. Artificially synthesized polymers, such as polyethyleneimine (PEI), N-(2-hydroxypropyl) methacrylamide (HPMA),75 and polyethylene glycol (PEG), possess high stability. The manufacturing process of these polymers is conducive to commercialization. However, these synthetic polymers often exhibit poor biodegradability and low bioactivity.
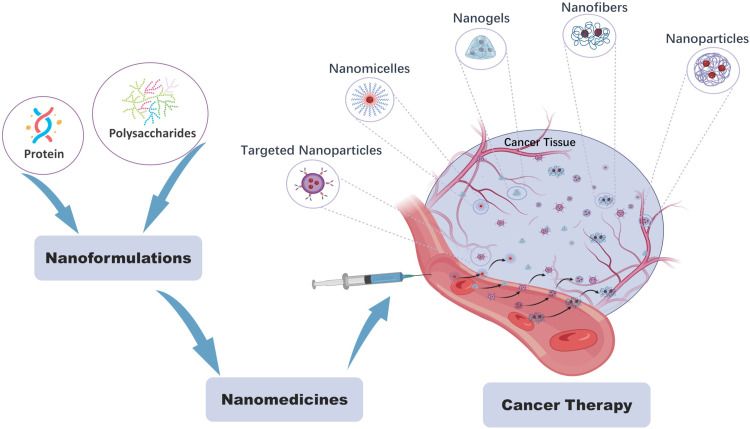
Biopolymers derived from animal, plant, or microbial sources offer advantages as drug delivery carriers in tumor therapy. These biopolymers, such as proteins, polysaccharides, and liposomes, exhibit good biocompatibility, ease of degradation, low toxicity, and easy modifiability. Biopolymers used as delivery carriers can be broadly classified into three categories: proteins (such as albumin, silk fibroin, ferritin, zein, and gelatin), natural polysaccharides (including chitosan, dextran, alginate, hyaluronic acid, pectin, gum, and carrageenan) and liposomes. Based on our research, common forms of biopolymer-based nanocarriers for anticancer drug delivery include nanoparticles, nanogels, nanofibers, nanomicelles, nanocapsules, and nanospheres.76 Current research on biopolymer-based nanocarrier systems for anticancer drug delivery primarily focuses on (1) optimizing preparation processes to improve drug loading efficiency, (2) chemical modification or surface functionalization to enhance targeting and controlled release properties, and (3) combination with chemotherapy or other treatment modalities to enhance therapeutic efficacy. We have provided an overview of the current research status of biopolymer-based nanosystems for cancer drug delivery and prospect future research directions in this field.
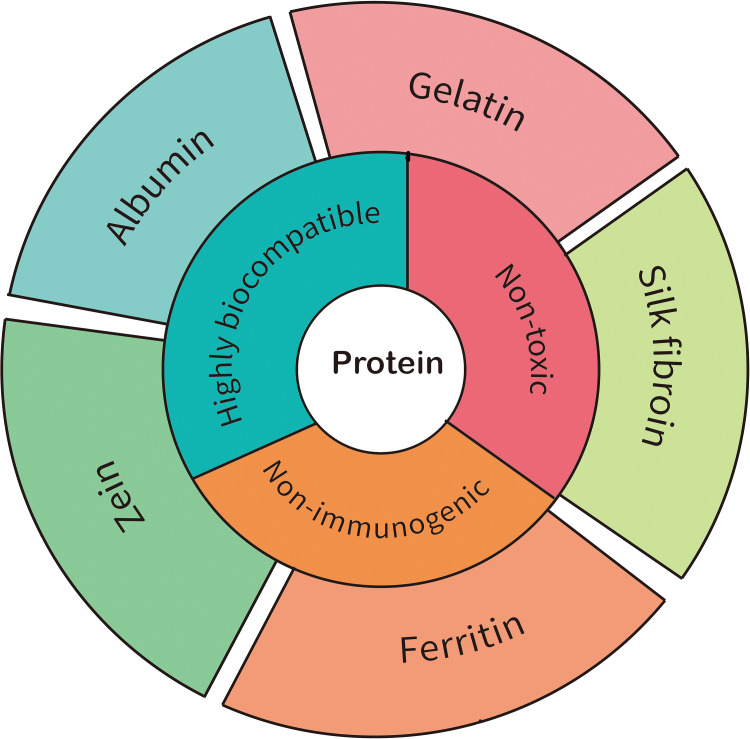
Protein
Proteins, as a type of natural biopolymer, are abundantly present in the human body and play a crucial role in the field of medicine. Their exceptional properties, including excellent biocompatibility, biodegradability, and low immunogenicity, make them highly valuable for various medical applications such as drug delivery, imaging, and protein-based therapies.77 The wide availability of proteins in the human body, combined with their favorable characteristics, has positioned them as essential components for advancing medical interventions and improving patient outcomes in diverse medical domains. Several proteins have been extensively studied and applied for drug delivery purposes, including serum albumin, silk fibroin, gelatin, zein, and ferritin (Figure 2).
Figure 2.
Schematic representation of various members involved in Protein-based nano drug delivery carrier for cancer treatment.
Albumin
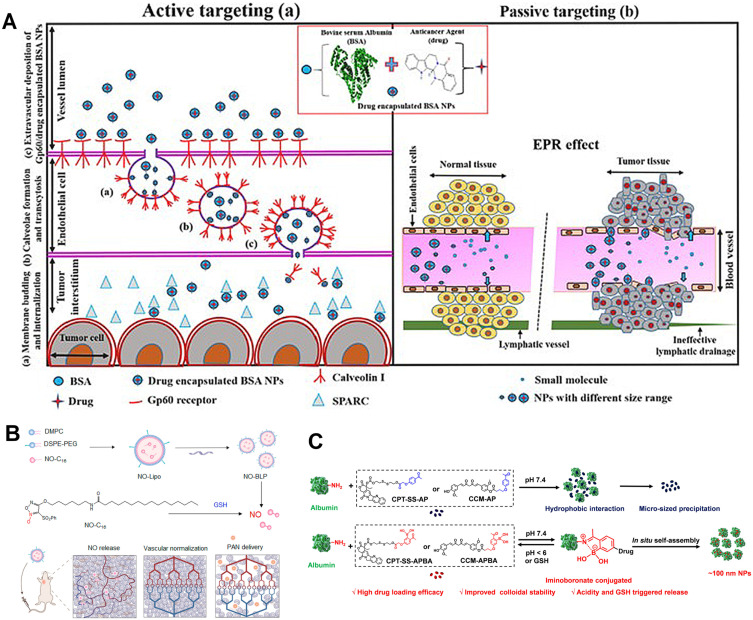
Albumin, the most abundant protein in human blood, exhibits unique physiological characteristics that make it a valuable member of the drug delivery carrier family, especially in the context of cancer treatment. Albumin carries a net negative charge, which gives it excellent water solubility and a relatively long half-life. Its negative charge also allows it to evade clearance by the kidneys through tubular reabsorption. Additionally, albumin possesses inherent binding sites and can accumulate at sites of vascular leakage. The active and passive drug delivery mechanisms for albumin are shown in Figure 3A. Albumin can bind to Gp60 on endothelial cells and thus enter and exit the vasculature by transcytosis. In addition, albumin can bind to SPARC receptors overexpressed in tumor tissues, thereby targeting the tumor tissue. Albumin drug-loaded nanomicrospheres can also enter the tumor microenvironment through the EPR effect. Based on these properties, albumin has been utilized as a carrier for anticancer drug formulations that have found clinical use. One notable example is Abraxane (albumin-bound paclitaxel), which has received FDA approval for the treatment of metastatic breast cancer.78
Figure 3.
The delivery mechanisms and applications of albumin. (A) Mechanisms of albumin accumulation, in which (a) and (b) represent active and passive drug delivery strategies, respectively. Reprinted from International Journal of Biological Macromolecules, 193, Solanki R, Rostamabadi H, Patel S, Jafari SM. Anticancer nano-delivery systems based on bovine serum albumin nanoparticles: a critical review, 528–540, Copyright 2021, with permission from Elsevier.79 (B) Illustration of Designing GSH-responsive nitric oxide-loaded bioinspired lipoprotein system (NO-BLP) to normalize tumor vessels for improving intratumor delivery and chemotherapy of albumin-bound paclitaxel nanoparticles (PAN). Adapted with permission from Wu Y, Xie H, Li Y, et al. Nitric Oxide-Loaded Bioinspired Lipoprotein Normalizes Tumor Vessels To Improve Intratumor Delivery and Chemotherapy of Albumin-Bound Paclitaxel Nanoparticles. Nano Lett. 2023;23(3):939–947. Copyright© 2023 American Chemical Society. 80 (C) Preparation principle of albumin-based nanomedicine. Reprinted from Journal of Controlled Release, 330, Hao L, Zhou Q, Piao Y, Zhou Z, Tang J, Shen Y. Albumin-binding prodrugs via reversible iminoboronate forming nanoparticles for cancer drug delivery, 362-371, Copyright 2021, with permission from Elsevier.81
The successful clinical application of the albumin-bound paclitaxel strategy has inspired researchers to explore additional functionalities of albumin-based drug delivery systems. Some researchers have investigated the surface loading of nitric oxide (NO) donors onto albumin-paclitaxel nanoparticles and incorporated a glutathione-responsive mechanism. This approach aims to achieve tumor vascular normalization while inhibiting tumor cell growth (Figure 3B).80
Albumin itself has the ability to encapsulate drugs within its hydrophobic interior. However, the encapsulation of certain drugs, such as curcumin and camptothecin, is challenging due to their polarity and planar structure. These drugs are often loaded onto albumin through covalent conjugation. To expand the range of anticancer drugs that can be loaded onto albumin and to achieve multifunctionality, researchers have utilized boronic ester functionalization to covalently link albumin with nucleotides, enabling drug release under acidic conditions (Figure 3C).81 In addition to chemical functionalization, albumin can also be radiolabeled to facilitate targeted diagnostic and therapeutic imaging, integrating multiple functionalities into one system.82
Although albumin has gained clinical applications, there are still some aspects that need to be improved. Firstly, although albumin can interact with tumor cells through specific receptors or proteins, its targeting and specificity are still limited. When designing albumin nanocarriers, it is necessary to consider how to improve its specific binding to tumor cells to enhance drug targeting and anti-tumor effects. Secondly, despite its good biocompatibility, albumin may still be cleaved or broken down by enzymes in vivo, leading to drug failure or unstable release rates. Therefore, stable albumin nanocarriers need to be designed to ensure drug stability and long-term sustained release. Finally, albumin has limited loading capacity as a drug carrier, and drug release is affected by temperature, pH value and other factors, which requires precise control of its safety.
Gelatin
Gelatin is derived from the degradation of collagen and consists of residues of glycine, proline, and hydroxyproline. However, the safety limitations of its material source have hindered its clinical applications. Gelatin exhibits excellent biocompatibility, possesses abundant reactive functional groups, and can be easily prepared at a low cost, making it a promising drug delivery carrier. Researchers are still exploring ways to improve its performance through modifications or co-formulation with other polymers.15,83 Mi Zhou et al have developed a size-adjustable and charge-conversion dual-responsive nanocluster called FA-GelDMA@DOX-HMON-NH by combining gelatin with mesoporous silica. It involves the self-assembly of positively charged DOX-HMON-NH within the FA-GelDMA framework. When the nanocluster enters the tumor environment, it responds to acidic conditions and enzymatic degradation. This triggers the release of DOX-HMON-NH, leading to the inhibition of cancer cells.84
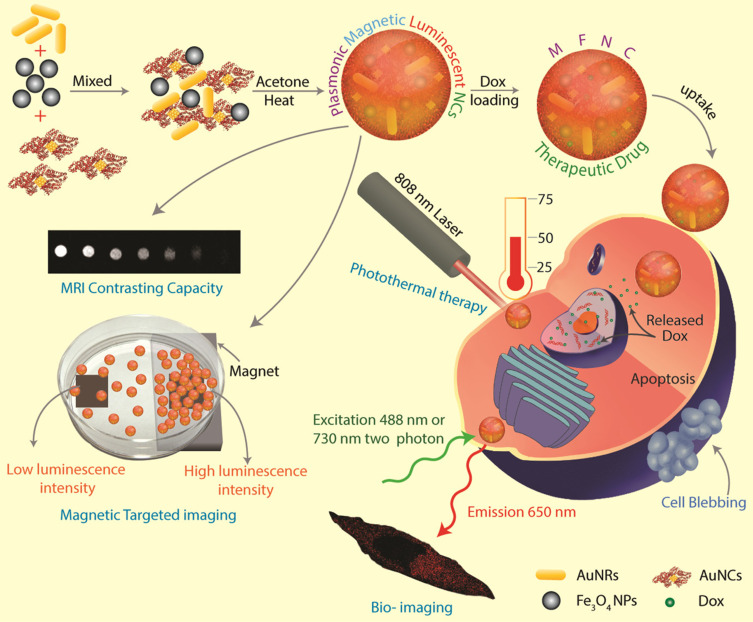
In addition, gelatin exhibits stable physical properties and can serve as a versatile matrix for loading various particles, enabling the construction of multifunctional nanoplatforms. Exploiting this characteristic, researchers have employed gelatin as a matrix to develop a novel nano-therapeutic strategy with photo-responsive and magnetic-responsive properties.85 In this system, respectively as shown in Figure 4, iron oxide nanoparticles, gold nanoclusters, and an anticancer drug, DOX, are loaded into the gelatin matrix, enabling integrated photothermal therapy, chemotherapy, and imaging tracking. The loaded magnetic nanoparticles facilitate targeted delivery of the drug to the tumor site through external magnetic fields, minimizing accumulation in normal tissues. The involvement of gold nanoclusters imparts photo-responsive characteristics, generating heat under near-infrared light irradiation, which further triggers the release of chemotherapy drugs and enables targeted eradication of tumor cells. Successful implementation of photo-responsive and magnetic-responsive properties has been demonstrated in Hela cells, highlighting the versatility and potential of the gelatin-based nano-anticancer delivery system.
Figure 4.
Schematic depiction of preparing MFNCs, their capacity for in vitro MRI contrasting and magnetic targeting, two-photon imaging, plasmonic photothermal therapy, and inducing cell death in cancer cells (Hela), following successful loading and delivery of anticancer drug DOX. Adapted with permission from Pan UN, Khandelia R, Sanpui P, Das S, Paul A, Chattopadhyay A. Protein-Based Multifunctional Nanocarriers for Imaging, Photothermal Therapy, and Anticancer Drug Delivery. ACS Appl. Mater. Interfaces. 2017;9(23):19495–19501. Copyright© 2017 American Chemical Society.85
Gelatin has good biocompatibility and degradability, and is used clinically as a biological scaffold, filler and repair material. In the realm of anticancer drug delivery, gelatin-based nanodelivery systems offer substantial advantages in terms of drug loading and delivery efficiency. The mechanical properties of gelatin can be tailored based on its concentration, fabrication strategy, and cross-linking density, allowing for personalized pharmacokinetics as per specific requirements. However, the use of cross-linking agents introduces potential toxicity concerns, and gelatin sourced from animals may elicit an immune response, posing challenges that must be addressed before clinical translation.86
Silk Fibroin
Silk fibroin is the main component of silk produced by silkworms. It possesses excellent biocompatibility, biodegradability, and mechanical properties. Due to these characteristics, silk fibroin has been approved by the FDA as a medical material and is commonly used for sutures in wound closure. However, there are currently no clinically approved drug products based on silk fibroin.87 Currently, most of the research on silk fibroin-based drug delivery for cancer treatment focuses on the forms of nanospheres88 or nanofibers. Cao et al89 developed a nano-particle for combined sonodynamic therapy and chemodynamic therapy in the treatment of colon cancer. The nano-particle consisted of mesoporous silica loaded with indocyanine green derivative (ID) as the internal component and was enveloped by chondroitin sulfate and regenerated silk fibroin on the outside. Chondroitin sulfate targeted tumor cells, while ID targeted the mitochondria within the tumor cells. Upon entering the tumor microenvironment, the particles were internalized by the tumor cells and targeted the mitochondria. Subsequently, they released Mn2+ which catalyzed the conversion of endogenous hydrogen peroxide into hydroxyl radicals and oxygen, with the oxygen further promoting sonodynamic therapy. In vitro and in vivo experiments confirmed the successful delivery of the constructed nano-delivery system to intestinal epithelial cells in the small intestine. Additionally, the researchers loaded anti-PD-L1 in the nano-particles to enhance their cytotoxic effect on tumor cells. In a mouse model of colon cancer and a subcutaneous colon tumor model, the orally administered nano-delivery system demonstrated superior tumor inhibition under ultrasound irradiation.
Silk proteins serve as carriers in various delivery systems, including sponges, gels, microparticles, and microneedles. However, they suffer from poor mechanical properties and are prone to sudden drug release. In tumor therapy, enhancing efficacy and minimizing off-target effects are primary goals. Unfortunately, silk proteins lack targeting properties, which perpetuates the challenge of off-target effects. Efforts to address these limitations may involve incorporating targeting ligands or modifying the formulation to achieve controlled drug release and improve targeting specificity.90,91
Zein
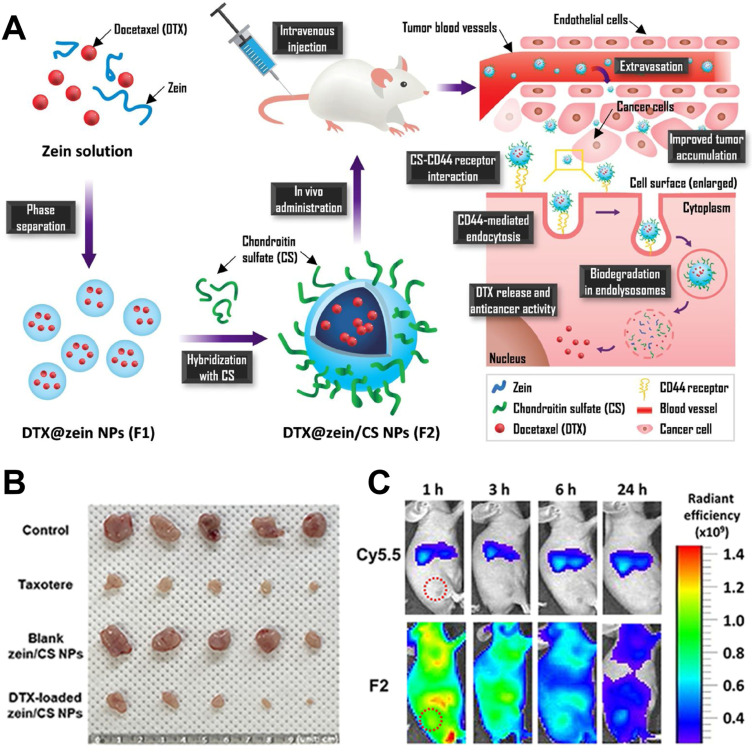
Zein mays albumin is an amphiphilic protein derived from maize cell membranes. It has been approved by the US FDA as a Generally Recognized as Safe (GRAS) excipient and is commonly used as a coating agent for pharmaceuticals. Utilizing Zea mays albumin as a drug delivery carrier for anticancer agents offers high drug loading capacity and low toxicity. However, Zein mays albumin lacks tumor-targeting specificity, and its delivery mainly relies on the enhanced permeability and retention (EPR) effect of tumors, making it susceptible to recognition by macrophages and resulting in short circulation time in the body. Many researchers have attempted to enhance the bioavailability of Zein mays albumin-based delivery systems by combining it with other delivery carriers or targeting ligands.92,93 For instance,94 Figure 5A shows a co-delivered the anticancer drug paclitaxel using a combination of chondroitin sulfate and Zein mays albumin. By utilizing chondroitin sulfate’s ability to target the CD44 receptor, they achieved tumor cell targeting. Furthermore, the association with chondroitin sulfate also enhanced the stability of Zein mays albumin as a drug carrier (Figure 5B and C).
Figure 5.
Mechanisms and therapeutic effects of zein-based nanoparticles. (A) Schematic of zein/CS nanoparticles. (B) In-vivo anti-tumor efficacy. (C) NIRF imaging of PC-3 tumor xenograft mouse model after intravenous injection of Cy5.5 and Cy5.5-labeled zein/CS NPs. Reprinted from Carbohydrate Polymers, 253, Lee HS, Kang N-W, Kim H, et al. Chondroitin sulfate-hybridized zein nanoparticles for tumor-targeted delivery of docetaxel, 117187, Copyright 2021, with permission from Elsevier.94
Ferritin
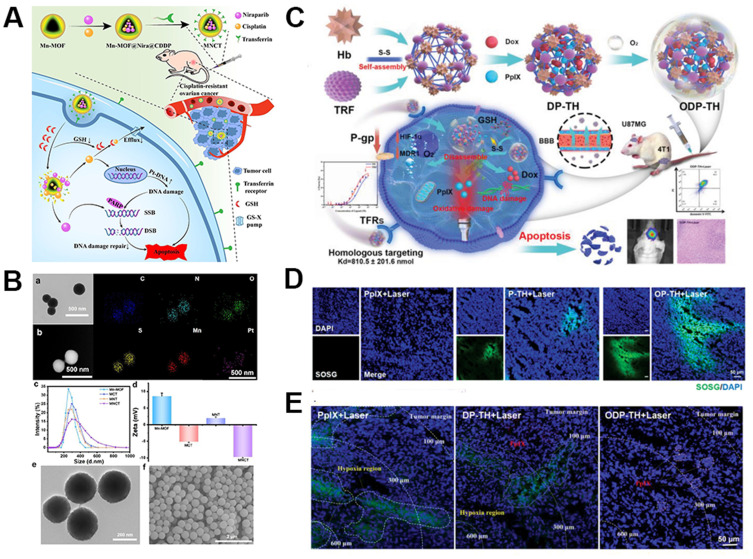
Ferritin is an iron storage protein composed of 24 subunits, and it is found in cells of animals, plants, bacteria, and algae. Depending on the source, ferritin can be categorized as horse spleen ferritin (HoSF), human ferritin (HFt), rat heavy-chain ferritin, Archaeoglobus fulgidus ferritin (AfFtn), and Pyrococcus furiosus ferritin (PfFt), among others. Iron ferritin, as a drug delivery carrier, is derived from its excellent biocompatibility and specific targeting properties. The cage-like structure of ferritin allows for the encapsulation of various small molecules, including metals, drugs, fluorescent molecules, contrast agents,95 etc., with uniform shape. Ferritin can target the transferrin receptor 1 (TfR1) in tumors. Additionally, it can be modified with other targeting ligands or growth factors to enhance its targeting specificity.96 Liu et al97 developed a multi-responsive nano delivery system (Tf-Mn-MOF@Nira@CDDP; MNCT) for the co-delivery of cisplatin (CDDP) and Niraparib (Nira) to treat breast cancer. In this delivery system as schematically depicted in Figure 6A, the drugs were encapsulated within the Mn-MOFs structure, and the outer surface of the Mn-MOFs nanoparticles was modified with ferritin to target cancer cells (Figure 6B). Upon entry into tumor cells, the Mn-MOFs structure disintegrated, releasing the drugs and effectively killing cancer cells. This nano delivery system successfully reversed the resistance of breast cancer cells to cisplatin, enhanced drug targeting, and reduced systemic toxicity of the drugs.
Figure 6.
Delivery strategies and applications of ferritin delivery carriers. (A) Schematic Illustration of the Multitargeted Nanodrug Delivery System Tf-Mn-MOF@Nira@CDDP (MNCT) to Co-Deliver Cisplatin and Niraparib on Cisplatin-Resistant Ovarian Cancer. (B) Characterization of Mn-MOF-based nanoparticles. (a) TEM image of Mn-MOF@Nira@CDDP. Scale bar, 500 nm. (b) HRTEM dark field image and corresponding mapping images of Mn-MOF@Nira@CDDP. Scale bar, 500 nm. (c) Hydration particle size distribution of Mn-MOF, MCT, MNT, and MNCT obtained by DLS. (d) ζ-Potential of Mn-MOF, MCT, MNT, and MNCT obtained by DLS. (e) TEM image of MNCT. Scale bar, 200 nm. (f) SEM image of MNCT. Scale bar, 2 μm. Reprinted with permission from Liu Y, Wang Y, Guan X, et al. Reversal of Cisplatin Resistance in Ovarian Cancer by the Multitargeted Nanodrug Delivery System Tf-Mn-MOF@Nira@CDDP. ACS Appl. Mater. Interfaces. 2023;15(22):26484–26495. Copyright© 2023 American Chemical Society.97 (C) The synthesis and intracellular mechanisms of ODP-TH. (D) CLSM images of SOSG (green) probe evaluating the formation of singlet oxygen after treated with PpIX, P-TH, and OP-TH followed by 650 nm laser for 10 min (100 mW /cm−2). (E) Ex vivo immunofluorescence images of hypoxia area (green) in tumor stained by Hypoxyprobe. Adapted with permission from S-Y W, Y-X Y, Zhang Q, et al. Multifunctional Protein Hybrid Nanoplatform for Synergetic Photodynamic-Chemotherapy of Malignant Carcinoma by Homologous Targeting Combined with Oxygen Transport. Adv. Sci. 2023;10(5):2203742. (Creative Commons CC BY).98
The integration of imaging and therapy in multifunctional drugs is also a development trend in drug delivery systems, aiming to achieve the integration of diagnosis and treatment. Wu et al98 developed a hybrid protein shell by combining ferritin with hemoglobin as schematically depicted in Figure 6C, successfully encapsulating the photosensitizer Pplx and the anticancer drug DOX. This system can improve the hypoxic environment of tumors and achieve controlled drug release in the tumor area. In vitro and in vivo experiments demonstrated that the nanosystem enabled Pplx imaging in the tumor region and achieved targeted accumulation, leading to the slow release of the anticancer drug (Figure 6D and E).
Despite significant progress in the preparation and application of nanoparticles based on ferritin nanocarriers, several challenges remain to be addressed. Firstly, there is a need to enhance the encapsulation efficiency and carrying capacity of ferritin-based nanoparticles. Secondly, improvements are required in the stability of ferritin and the efficiency of cellular uptake of the encapsulated molecules. This entails further exploration of the mechanisms and effects of surface modification of ferritin. Lastly, standardization of the ferritin preparation process is necessary to ensure consistent quality and reproducibility across different studies and applications. Addressing these bottlenecks will contribute to the wider and more effective utilization of ferritin-based nanoparticles in various biomedical applications.96,99
Proteins exhibit a remarkable diversity, and in this study, we highlight five protein-based anticancer drug delivery carriers (Albumin, Gelatin, Silk fibroin, Zein Ferritin), summarizing their applications in Table 3. In general, proteins possess several advantageous characteristics for cancer drug delivery, including non-toxicity, non-immunogenicity, and extended circulation time. Existing commercial products reinforce our belief that there will be a growing number of protein-based delivery carriers advancing towards clinical applications. However, it is important to acknowledge that protein-based delivery carriers also have their limitations, which we have outlined in the Table 4. As novel protein-based drugs make their way towards clinical usage, several aspects warrant further refinement, such as:
Standardization of protein source extraction.
Screening for proteins that can effectively target tumors.
Investigation of the in vivo release mechanisms of protein-based delivery drugs.
In-depth exploration of the structure-function relationships of protein derivative.
Table 3.
Protein-Based Biopolymeric Drug Delivery Systems for Cancer Therapy
| Protein Types | Loaded Drugs | Type of Nanosystems | Application | Aimed Cancer/Cell | Ref |
|---|---|---|---|---|---|
| Albumin | Abraxane | NPs | Chemotherapy | TNBC | [100] |
| Albumin | MnO2 | NPs | Imaging | NCI-H460 | [101] |
| Albumin | CUR | NPs | Diagnosis | Breast cancer | [102] |
| Albumin | DOX | Nanoclusters | Chemotherapy | Malignant bone tumors | [103] |
| Albumin | PTX | NPs | Chemotherapy | Breast cancer | [104] |
| Albumin | DOX | NPs | Chemotherapy | Human pancreatic tumor | [105] |
| Albumin | PTX,4-HPR | NPs | Chemotherapy | Glioma cells | [106] |
| Gelatin | Catalase /siRNA | NPs | Immunotherapy | Melanoma model | [107] |
| Gelatin | ICG/DOX | NPs | Chemo–photothermal therapy | Breast cancer | [108] |
| Gelatin | Cu, polyaniline (PANI) | NGs | PTT/Photoacoustic imaging | A549 | [109] |
| Gelatin | TA | Nanofiber | Chemotherapy | Osteoarthritis | [110] |
| Gelatin | Pba, TPZ | NPs | PDT/Chemotherapy | Tumor-bearing mice | [111] |
| Gelatin | Cisplatin | NPs | Chemotherapy, Imaging | H22 | [112] |
| Silk fibroin | OVA | NPs | Immunotherapy | B16/F10/MB49 | [113] |
| Silk fibroin | MnOx | Nanomotors | SDT /Chemodynamic therapy | Orthotopic colon tumors | [89] |
| Silk fibroin | MnOx, DOX | NPs | PTT/PDT/Chemotherapy | Metastatic breast cancer | [114] |
| Silk fibroin | Rosuvastatin | NPs | Chemotherapy | TNBC | [115] |
| Silk fibroin | DOX | NPs | Chemotherapy | MCF-7 cells | [116] |
| Zein | Docetaxel | NPs | Chemotherapy | PC-3 cells | [94] |
| Zein | CUR | NGs | Chemotherapy | CT26 cells | [117] |
| Zein | Honokiol | NGs | Chemotherapy | Breast cancer | [118] |
| Ferritin | DOX | Nanocage | Chemo-immunotherapy | HCC | [119] |
| Ferritin | Aloe-emodin | Nanocrystals | PDT | HSC-3 cells | [120] |
| Ferritin | PAB /L | NPs | Immunotherapy | TNBC | [121] |
| Ferritin | Camptothecin/Epirubicin | Nanocage | Chemotherapy | Glioma, metastatic liver cancer, and chemo-resistant breast tumors | [122] |
| Ferritin | DOX | NPs | Chemotherapy | Pancreatic cancer | [123] |
Abbreviations: NPs, nanoparticles; TNBC, triple-negative breast cancer; CUR, curcumin; DOX, doxorubicin; PTX, paclitaxel; 4-HPR, fenretinide; ICG, indocyanine green; NGs, nanogels; PTT, photothermal therapy; TA, tannic acid; Pba, pheophorbide a; TPZ, tirapazamine; PDT, photodynamic therapy; OVA, ovalbumin; SDT, sonodynamic therapy; PAB /L, pseudolaric acid B (PAB) \ lapatinib.
Table 4.
Characteristics of Protein Anti-Cancer Drug Delivery Carriers
| Types of Proteins | Advantages | Disadvantages | Targeted |
|---|---|---|---|
| Albumin | Can target tumor KRAS. | In vivo transport mechanism to be studied; Potential immunogenicity. | Targeting the Gp-60 receptor |
| Gelatin | Mild conditions for drug encapsulation; Has a cell-binding site (arginine-glycine-aspartic, RGD). | In vivo transport mechanism to be studied; Potential immunogenicity. | – |
| Silk fibroin | May enhance cancer therapy. | Inhibitory effect on normal cells; Source materials differ significantly in amino acid sequence, morphology, and manufacturing process; Mechanism of drug release unclear; | – |
| Zein | Plant protein with no risk of animal disease transmission; Insoluble alcohol soluble protein. | Lack of biodegradability information. | – |
| Ferritin | Targeted, can target tumor TfR1 receptor; Has a cavity dedicated to loading drugs; Good PH stability. | Low drug loading efficiency; Differences in targeting ability for different tumors. | Targeting transferrin receptor 1 (TfR1) |
Natural Polysaccharides
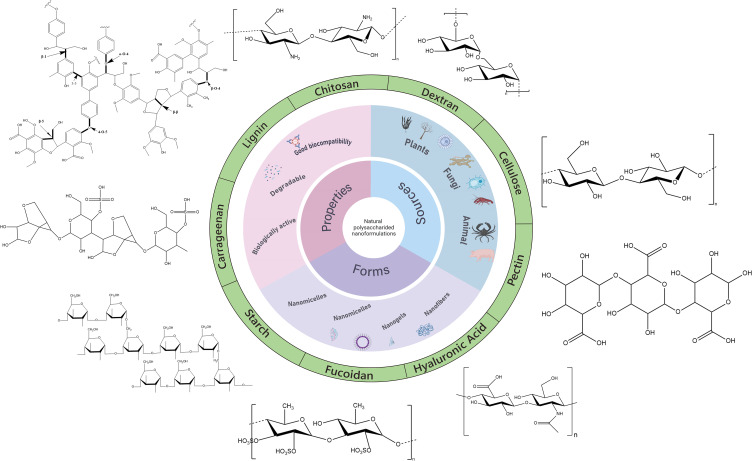
Natural polysaccharides have a wide range of sources, including chitosan, dextran, pullulan, hyaluronic acid, pectin, gum, lignin, carrageenan, and more, in which a few of them are depicted in Figure 7. Due to their non-toxicity, biodegradability, low immunogenicity, excellent biocompatibility,124 rich functional groups, low cost, and easy availability, they serve as excellent carriers for drug delivery and find extensive applications as encapsulants and stabilizers in nanocarriers for drug delivery. Additionally, the inherent targeting ability, antioxidant properties, anti-proliferative effects, and anti-cancer properties of polysaccharides make them ideal carriers for cancer treatment.125 In addition, natural polysaccharides possess abundant functional groups on their surface, such as hydroxyl, amino, and carboxyl groups. Through simple chemical modification or modification, a wide range of derivatives can be obtained, and some derivative groups also exhibit bioadhesive properties, which can enhance drug circulation time and cellular uptake rate.125 Polysaccharides can be utilized not only as drug delivery carriers themselves but also in combination with metal nanoparticles (such as gold nanoparticles, and silver ions), metal oxides (such as iron oxide, copper oxide, and zinc oxide), mesoporous silica, and other delivery systems, forming multifunctional nanocarriers capable of imaging, photothermal therapy, chemotherapy, and other therapeutic modalities.126
Figure 7.
Schematic representation of various members involved in natural polysaccharide-based nano drug delivery carrier for cancer treatment.
Chitosan
Chitosan is a natural linear cationic polysaccharide derived from the exoskeletons of crustaceans. It is composed of N-acetyl-D-glucosamine and D-glucosamine units. Chitosan possesses several characteristics, including mucoid hesiveness, biodegradability, biocompatibility, and low immunogenicity. It also exhibits anti-tumor properties,127 making it a promising nanocarrier for cancer drug delivery.
Although chitosan is non-toxic, its inherent positive charge can disrupt cell membranes. Additionally, chitosan has low water solubility and typically dissolves only under acidic conditions. However, by modifying chitosan, its water solubility can be increased, enhancing drug-loading capacity and improving its bioavailability. There are several derivatives obtained from chitosan modification, including thiolated chitosan (TCS) derivatives, amphiphilic derivatives of chitosan (AD) such as carboxymethyl chitosan (CMC), quaternized derivatives of chitosan (QD) like N, N, N-trimethyl chitosan (TMC), and ethylene glycol-chitosan, among others.
Thiolated chitosan (TCS) is prepared by covalently attaching thiol (-SH) groups primarily to the primary amine or hydroxyl groups of chitosan. It exhibits excellent permeability, cohesion, and bioavailability, and it can also target surface ligands.128 The thiol groups of TCS can form disulfide bonds with cysteine domains of mucin glycoproteins, thereby enhancing its mucoadhesive properties.129,130 Riwang Li et al developed a thermosensitive hydrogel composed of TCS-encapsulated liposomes loaded with curcumin. Through in vitro and in vivo experiments, they demonstrated that the encapsulation of TCS effectively improved the stability of the material and delayed drug release.
Trimethyl chitosan (TMC) is a quaternized derivative of chitosan that possesses a high positive charge. It exhibits improved water solubility,131 mucoadhesive properties, permeability, and drug delivery capabilities compared to traditional chitosan.132 TMC can be further derivatized or grafted to modulate its solubility, cytotoxicity, or cell-targeting abilities. Haiyan Hu et al133 utilized TMC as the drug carrier and employed human serum albumin (HSA) for surface modification of the delivery system. The results demonstrated that the conjugation of HSA effectively enhanced the penetration ability of the carrier and exhibited pH responsiveness, leading to efficient inhibition of tumor cell growth.
Carboxymethyl chitosan (CMC) is an amphiphilic derivative of chitosan and serves as an excellent delivery vehicle for hydrophobic drugs. It exhibits favorable anticancer134 and antitumor properties and possesses pH sensitivity, biodegradability, and low immunogenicity, making it a significant player in the field of anticancer/antitumor drug delivery.135 Researchers have utilized CMC to develop pH-responsive nanomicelles.136 These micelles are self-assembled from CMC, Vitamin E succinate (VES), and the anticancer drug Doxorubicin. CMC imparts pH responsiveness to the nanomicelles, while VES enhances their targeting capability (Figure 8A). The prepared nanomicelles demonstrate significant swelling properties under low pH conditions, allowing for drug release in an acidic environment as shown in Figure 8B. CMC’s abundant carboxyl groups are commonly used for modification and can be utilized to graft targeting ligands. For instance, Yurui Xu et al137 further modified CMC to develop a dual-responsive nanoparticle system. The nanoparticles encapsulated indocyanine green (ICG) and apoptotic peptides functionalized gold nanoparticles (IK-AuNP), imparting photothermal responsiveness to the material. CMC was modified with RGD to achieve tumor targeting and pH-responsive drug release. These nanoparticles were employed for the treatment of oral cancer and demonstrated excellent tumor targeting in an in situ oral cancer mouse model. Additionally, the combination of NIR and the nanoparticles effectively inhibited tumor growth.
Figure 8.
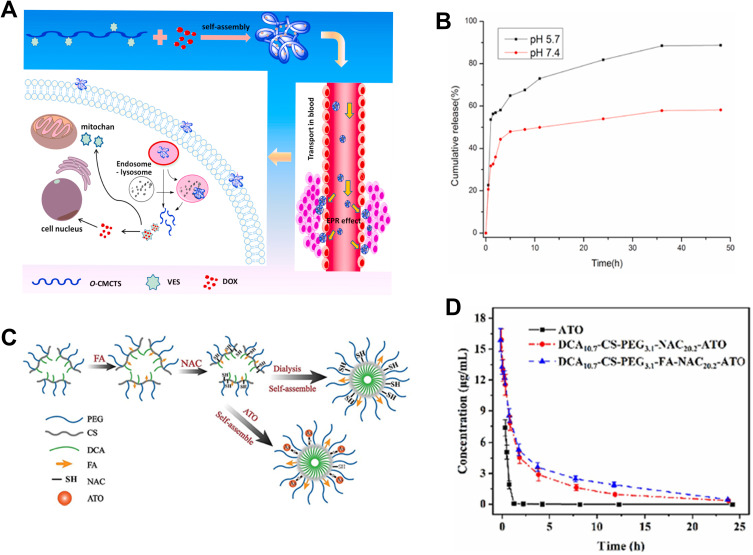
Delivery strategies and applications of chitosan-based delivery carriers. (A) Schematic diagram of the O-CMCTS-VES nanoparticles with EPR effect for anticancer therapy. (B) In vitro release of DOX/O-CMCTS-VES nanoparticles in PBS buffer solution with different pH values. Adapted with permission from Chen X, Gu J, Sun L, et al. Efficient drug delivery and anticancer effect of micelles based on vitamin E succinate and chitosan derivatives. Bioact. Mater. 2021;6(10):3025–3035. (CC BY 4.0).136 (C) Schematic diagram of the preparation of ATO-loaded CS nanodrugs. (D) Pharmacokinetic curves of free ATO, DCA10.7-CS-PEG3.1-NAC20.2-ATO, and DCA10.7-CS-PEG3.1-FA-NAC20.2-ATO. Reprinted from Carbohydrate Polymers, 303, Song X, Wu J, Song W, et al. Thiolated chitosan nanoparticles for stable delivery and smart release of As2O3 for liver cancer through dual actions, 120462, Copyright 2023, with permission from Elsevier.138
Glycol chitosan (GC) is a chitosan derivative that is conjugated with hydrophilic glycol groups, rendering it water-soluble over a wide pH range from acidic to neutral conditions.139 GC is typically prepared by reacting chitosan with ethylene oxide and subsequently deacetylating the chitosan. Modification of chitosan with polyethylene glycol (PEG) can improve its solubility, enhance targeting capability, and reduce interactions between nanoparticles and serum proteins,140 thereby prolonging blood circulation time. Xuejing Zhang et al141 combined the pH-responsive property of chitosan with the thermoresponsive characteristic of di(ethylene glycol) methyl ether methacrylate (PDEGMA) to develop a dual-responsive nanoparticle system. In vitro and in vivo experiments demonstrated that these nanoparticles exhibited dual responsiveness to low pH and elevated physiological temperature.
Shima Bastaki et al142 developed a nano particle system for delivering STAT3/PD-L1 dual-targeted siRNAs by combining two types of chitosan, TMC, and TC, known for their excellent permeability and adhesion properties. The nanoparticles were further coated with TAT peptide and hyaluronic acid (HA). The results demonstrated that these nanoparticles with dual inhibitory effects exhibited significant inhibition against breast cancer and melanoma cell lines. Animal tumor models also confirmed the strong targeted inhibitory effects of these particles on tumors.
Xiaoli Song et al138 designed a CS-based multifunctional delivery system with adjustable thiol content. They achieved CS thiolation using -(N-acetyl-L-cysteine) macromolecule (NAC). To improve drug utilization, CS was further modified with polyethylene glycol (PEG), deoxycholic acid (DC), and folate (FA) as shown in Figure 8C. The study revealed that increasing the proportion of NAC enhanced the drug-loading capacity for As2O3 (ATO). In vitro, experiments demonstrated that the drug release in a normal PBS environment was less than 25%, while it significantly increased in the presence of high glutathione (GSH) levels (Figure 8D). This indicated the selective release properties of the designed drug. Further, in vivo, experiments also confirmed prolonged blood circulation time of modified ATO and improved tumor-targeting inhibitory effects.
Guanqing Yang et al143 synthesized a pH-responsive nanogel by succinylating and methacrylating chitosan (methacrylated succinyl-chitosan, MASCS). The nanogel was used for the delivery of DOX, and in vitro and in vivo experiments demonstrated its pH-responsive behavior, allowing drug release under acidic conditions and exhibiting tumor-targeting properties.
Chitosan, as a biopolymer, possesses various advantageous characteristics such as low pH solubility, cationic nature, targeting ability, and antitumor properties (Table 5). These features make it suitable for applications such as chemotherapy drug/gene delivery, photothermal therapy, and imaging. However, the low solubility of chitosan limits its usage. Therefore, modified chitosan derivatives with improved solubility have been developed. Chitosan and its derivatives exhibit pH-responsive properties, enabling targeted drug release in the acidic tumor microenvironment. However, the safety of chitosan in vivo has been less studied. Chitosan can activate dendritic cells and induce macrophage activation. The potential harm to normal cells and the underlying mechanisms of its in vivo effects remains unclear. These factors contribute to the lack of clinical application of chitosan-based anticancer drugs.
Table 5.
Chitosan-Based Biopolymeric Drug Delivery Systems for Cancer Therapy
| Type of Chitosan | Loaded Drugs | Type of Nanosystems | Zeta Potential (mV) | Loading Content | Encapsulation Efficiency | Diameter (nm) | PDI | Aimed Tumor/Cell | Ref |
|---|---|---|---|---|---|---|---|---|---|
| TMC | siRNA | NPs | ~20 | / | / | ~110 | <0.2 | B16-F10 and 4T1 cancer cells, | [142] |
| TMC | siRNA, BV6 | NPs | 17 | / | / | ~105 | < 0.2 | Breast, colorectal, and melanoma cancer cells | [144] |
| TMC | siRNA | NPs | ~20 | / | / | ~110 | <0.2 | Breast cancer and melanoma | [142] |
| TMC | Bufalin, IR780 | NPs | TIH,8.6 ±0.2; TBH,4.3 ± 0.7 |
IR780,8.53%; BF,9.17% |
IR780=95.74%, BF= 98.16% |
TIH :37.0 ± 3.0, TBH:31.7 ± 3.2 | / | Metastatic breast cancer | [133] |
| CMC | DOX | NPs | −41.5 ± 9.6 | / | / | / | / | Carboxymethyl chitosan | [145] |
| CMC | DOX | NPs | / | 13.7 | 77 | 199.6 ± 3.7 | / | H22 | [146] |
| CMC | MTX, AS1411 | NPs | −0.42 | 13.80% | / | 200 | / | A549, BALB/c mice | [147] |
| CMC | ICG /IK-AuNP | NPs | −17 | 6.47% | 95.52% | 113 | / | Oral cancer | [137] |
| CMC | DOX | NPs | 20~30 | DOX:O-CMCTS-VES 1–10=6.1%, DOX:O-CMCTS-VES 2–10=13.0%, DOX:O-CMCTS-VES 3–10=10.6% |
DOX:O-CMCTS-VES 1–10=64.3%, DOX:O-CMCTS-VES 2–10=74.5%, DOX:O-CMCTS-VES 3–10=39.7% |
208 | <0.5 | HepG2 | [136] |
| CMC | siMDR1, DOX | NPs | / | / | / | / | / | Breast Cancer | [148] |
| CS-PEG | siRNA | NPS | 14.3 | / | / | 127 | 0.29 | Breast and colon cancers | [140] |
| CS-PEG | ICG | Nano-micelles | 11.2 | 89.3%, (3.3 ± 0.2) | – | 143 | 0.15 | – | [149] |
| CS-PEG | DOX | NPs | 34.6 ± 2.6 | 0.11% | 80.40% | 178.5 ± 4.7 | 0.21 ± 0.006 | Breast cancer | [150] |
| CS-PEG | PTX | NGs | (13.6 ± 1.3%) | (76.2 ± 8.5%) | ~170 | / | Breast cancer, MDA-MB-231 | [141] | |
| TC | CUR | NPs | 37.30 ± 0.56 | 3.96 ± 0.32% | 88.75 ± 1.65 | 414.0 ± 7.71 | / | – | [151] |
| TC | Cisplatin | NPs | 22.3 | 70.1% ± 1.2% | 45% ± 0.28% | 265.9 | 0 0.226 | Cervical carcinoma | [152] |
| MSC | DOX | NGs | 30.9 | / | / | / | / | SH-SY5Y, HepG2, H22 | [143] |
| TC | Fe3O4/C6 | NCPs | – | 16.50% | > 80% | 161 ± 3 | 0.081 | A549 cells, HeLa cells | [153] |
Abbreviations: IK-AuNP, apoptotic peptides functionalized gold nanoparticles.
Starch
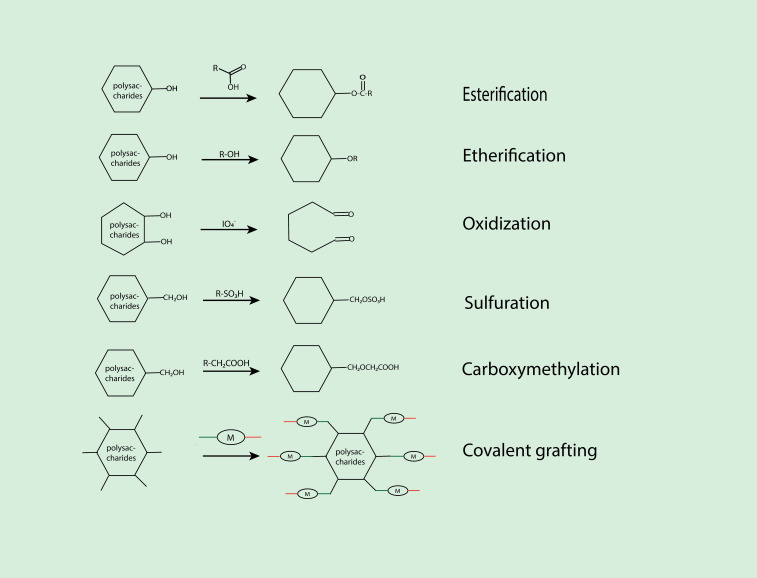
Starch is a source of energy for many plants, such as cereals or grains, roots, tubers, legumes, and fruits. Particularly, grains have a high starch content. Starch, with its basic structural unit being α-D-glucose, can be divided into amylose and amylopectin. Amylose is a linear structure without branching, while amylopectin consists of 24 to 30 glucose residues connected by α-1,4-glycosidic bonds, with branching occurring at α-1,6-glycosidic bonds.154 Starch, as a natural polysaccharide, possesses excellent biocompatibility, degradability, and stability. It is widely available and easily accessible, making it an approved material by the FDA for pharmaceutical applications, particularly as an excipient in drug formulation.155 Researchers prepared hydrogels with starch and bovine serum proteins to be used as carriers for Moringa Oil Lam (MOL), which effectively improved the bioavailability of the drug.156 Despite the numerous advantages of starch as a delivery carrier, it also has limitations such as rapid enzymatic degradation, tendency to aggregate, and swelling properties.157 Research has shown that by chemically modifying starch and introducing various functional groups, its performance can be enhanced. Some examples of chemical modifications include oxidation, amination, aldehydization, quaternization, acetylation, hydroxyethylation, carboxymethylation, and acetylation.
Hydroxyethylated starch (HES) is one of the commonly used modifications of starch. HES is a type of branched starch, prepared by reacting the amylopectin component of corn or potato starch with ethylene oxide, resulting in a hyperbranched structure. Hydroxyethyl starch has unique pharmacological effects, including Improvement of plasma colloid osmotic pressure and hemodynamics, Anti-inflammatory effects, and Influence on coagulation function.158 Based on these characteristics, hydroxyethyl starch is clinically used as a plasma substitute and volume expander for the treatment of conditions such as cerebral ischemia, hypovolemic shock, or arterial occlusive diseases. HES has a structure similar to glycogen, is naturally non-immunogenic, and can be hydrolyzed by α-amylase. With multiple hydroxyl functional groups, it can be utilized for targeted cancer therapy by conjugating anticancer drugs and incorporating responsive chemical linkers. Chan Yu et al159 developed HES-SS-DOX@ICG NPs by conjugating the anticancer drug DOX with HES through disulfide bonds and incorporating the photothermal agent indocyanine green (ICG). These nanoparticles exhibited both light-responsive and chemically-responsive properties. In an H22-tumor-bearing mouse model, it was found that within 14 days, the tumors were eliminated, highlighting their significant clinical potential. By taking advantage of the abundant hydroxyl groups on HES, further modifications can be made to improve its chemical properties. Honglian Wu et al160 carboxylated HES and conjugated it with polydopamine (PDA) through amide bonds, followed by loading the anticancer drug DOX to prepare DOX@HES-PDA NPs. These nanoparticles demonstrated superior antitumor effects compared to traditional delivery vehicles prepared with PEG, such as DOX@PEG-PDA NPs. In a study involving H22 tumor-bearing mice, continuous administration of DOX@HES-PDA and DOX@PEG-PDA for three days resulted in tumor inhibition rates of 73.1% and 63.3%, respectively. Compared to PEG, HES exhibits superior degradation performance and biocompatibility. Additionally, it contains a higher number of hydroxyl functional groups, making it a promising alternative to PEG.
Furthermore, carboxymethylated starch (CMS) is a negatively charged ether derivative of starch that exhibits water solubility and pH sensitivity.157 Under low pH conditions, CMS undergoes protonation and adopts a compact state, inhibiting drug release. Conversely, at high pH, the carboxyl groups become ionized, facilitating drug release. Based on these properties, CMS is commonly used in oral drug delivery systems. The pH sensitivity of carboxymethylated starch is also utilized in cancer treatment. Ranjbar et al161 developed an oral drug delivery system for the treatment of colon cancer. In this system, DOX and 5-fluorouracil (5-FU) were co-loaded onto layered double hydroxide (LDH) nanosheets composed of magnesium and aluminum. The LDH nanosheets were then encapsulated with carboxymethylated starch (CMS). The release efficiency of CMS@LDH(Mg-Al)@DOX, 5-FU, and LDH(Mg-Al)@DOX, 5-FU at different pH values was compared. It was found that in a pH 1.2 environment simulating gastric acid, the drug loaded in LDH(Mg-Al)@DOX, 5-FU was released completely within 120 minutes. In contrast, CMS@LDH(Mg-Al)@DOX, 5-FU exhibited sustained release for 480 minutes, indicating its ability to overcome degradation in acidic conditions. MTT experiments conducted on Caco-2 cells also demonstrated that CMS@LDH(Mg-Al)@DOX, 5-FU exhibited better biocompatibility. Starch can also be modified based on the charge (positive or negative) of the drug being delivered. Most anticancer drugs are hydrophobic and positively charged, but DOX hydrochloride carries a negative charge. To encapsulate such drugs, modifications can be made to transform starch into an anionic carrier. For instance, Ke Li et al162 utilized an amphiphilic cationic starch (CSaSt) and hyaluronic acid to prepare nanoparticles capable of co-delivering docetaxel (DOC) and DOX. Transmission electron microscopy (TEM) images revealed the adsorption of DOX on the surface of DOC MC.
The pre-clinical applications of Starch for cancer therapy are described below and summarized in Table 6. Starch is highly hydrophilic, swells on contact with water and is easily degraded by enzymes, so it does not have drug-carrying properties. However, through modification, the drug-carrying capacity of starch can be enhanced. Several types of modified starch have received FDA approval for use as additives or substrates in granules, capsules, tablets, and other pharmaceutical formulations.163 Nonetheless, utilizing modified starch as a carrier for anticancer drug delivery presents certain challenges that must be addressed. These include the tendency for modified starch to aggregate in water, thereby impacting drug delivery efficiency; potential toxicity associated with the modification process on cellular levels;164 the need for scalable industrial processes for the preparation and processing of modified starch; and the optimization of modified starch as a suitable carrier for targeted anticancer drug delivery.
Table 6.
Starch-Based Biopolymeric Drug Delivery Systems for Cancer Therapy
| Type of Starch | Loaded Drugs | Type of Nanosystems | Diameter (nm) | Drug Release | Aimed Tumor/Cell | Ref |
|---|---|---|---|---|---|---|
| Starch | CG-1521 | NPs | 200 | 120h, 64% at pH 6.0, ~40% at pH 7.4 | Breast cancer | [165] |
| Starch | 5-aminosalicylic acid | NPs | 40 | 50h, >80% at pH 7.4 in 0mM DTT, 98% at pH 7.4 in 20 mM DTT | HeLa | [166] |
| Starch | TPD | NPs | 22.98±4.23 | / | Pancreatic cancer | [167] |
| Starch | DOX | Nanocapsules | 30‒100 | 220h, 13.81 wt% at pH 7.4, 63.14 wt% at pH 5.8 |
/ | [168] |
| Starch | DOX | NPs | 70–200 | 48h, 75% at pH 7.4 | BEL7404 | [169] |
| HES | DOX | NPs | 169.1 ± 16.4 | 24 h, 92% in 2 mM/mL DTT, 38% in 0 mM/mL DTT |
H22, HepG2 | [170] |
| HES | DOX | NPs | 148.1 | 14.0% at PH 5.0, 19.2% at PH 7.4 | TNBC | [171] |
| HES | CD44p, Emodin | Polymer micelles | 154.5 ± 0.9, pH 7.4 125.8 ± 3.6, pH 6.5 |
84h, 70.27±0.03% at pH 7.4, 80.96±0.05% at pH 6.5 | / | [172] |
| HES | CUR | Nanomicelles | 69 | 72h, 25% at pH 7.4, 60% at pH 5.0 | HeLa, Caco-2 | [173] |
| HES | DOX | NPs | 172 | / | TNBC | [174] |
| HES | DOX | Nanomicelles | 59 ± 3.9 | 72h, 70.5% at pH 5.5, 40.9% at pH 5.0, less than 30% at PH 7.4 | Bladder cancer | [159] |
| HES-CHO | DOX, LHRH | Nanomicelles | 51 | 72h, 40.1% at pH 6.8,71.2% at pH 5.5, and less than 30% at PH 7.4 | Prostate | [175] |
| DS | 5-Fu | NPs | 90 | 48h, ~70% at pH 5, ~60% at pH 7, and ~50% at PH 9 | Breast cancer | [176] |
| DS | DOX | NPs | 100 | 72h, 34.25 at pH 5.0,9% at pH 7.4 | HeLa | [177] |
| ARS | Methylene blue | NPs | 173.4 | / | CT-26 | [178] |
| QS | siRNA | NPs | 200–400 | / | NAR | [179] |
| BRE | DOX | NPs | 86±3.6 | 96h, ~46% at pH 4.5, ~14% at pH 7.2, and ~20% at PH 9.0 | MG-63 | [180] |
| AS | Para-Coumaric acid | NPs | 218.97 ±3.07 | 42h, ~80% at pH 5.4, ~20% at pH 7.4 | TNBC | [181] |
| PULL | PTX | NPs | 130 ~ 170 | 72h, 75% at pH 5.4,74% at pH 7.4 | SMMC-772, A549 | [182] |
| TS | CUR | NPs | 16 ~ 30 | 8h, ~70% at pH 5.0, ~60% at pH 7.4 | MCF7, HepG2 | [183] |
Abbreviations: TPD, thienopyrimidine derivative; LHRH, Luteinizing hormone-releasing hormone.
Cellulose
Cellulose is a large molecular polysaccharide composed of D-glucose units linked together by β-1,4-glycosidic bonds. It is the most abundant biopolymer in nature, known for its non-toxicity, biodegradability, and environmentally friendly sourcing. Each structural unit of cellulose contains six hydroxyl groups. The unique polymer chain structure of cellulose forms an extensive network of hydrogen bonds both within and between the chains, making it insoluble in common solvents.184 However, its high degree of functionality makes it easily amenable to surface modification, providing a basis for drug loading.185 Drugs encapsulated within the porous network structure of cellulose spheres exhibit high stability.
Shihao He et al186 utilized a green and efficient sonochemical method to prepare carboxymethyl cellulose nanocapsules with shell modification of folic acid. Due to the overexpression of a protein on the surface of many tumor cell membranes that can specifically recognize and bind to folate (known as folate receptor), folate exhibits strong targeting capability. The surface-loaded folate nanocapsules can be targeted to cancer cells through folate receptor-mediated endocytosis, and the encapsulated drugs can be rapidly released in a reducing environment. The nanocapsules in the presence of a reducing agent exhibit excellent drug release capabilities. Therefore, the stability of the nanocapsules in the body can be adjusted by controlling the degree of cross-linking and the thickness of the nanocapsules without compromising the release of drugs after targeting tumor cells.
Cellulose and its derivatives have shown great promise in biosensors, particularly for enhancing the sensitivity to specific types of cancer through signal amplification methods.187 Nanocellulose-based biosensors exhibit excellent dispersibility and high absorbance capacity, making them suitable for monitoring various biomolecules.188 Bacterial cellulose nanopaper, known for its outstanding optical transparency, thermal properties, mechanical performance, and biodegradability, has been explored for optical biosensing applications.189
Anderson et al190 reported the synthesis of a novel plasmon-exciton nanohybrid utilizing green nanomedicine approaches. They successfully prepared a stable aqueous dispersion of colloidal supramolecular structures by incorporating gold nanoparticles and ZnS semiconductor quantum emitters (ZnS-QEs) onto carboxymethyl cellulose. The excitonic properties of ZnS-QEs enabled in vitro nanodiagnostics for brain cancer cells, while the plasmonic characteristics of gold nanoparticles facilitated photothermal therapy for cancer cell ablation. The proximity between excitons and plasmonic nanoparticles resulted in unique absorption and emission behaviors in the hybrid nanostructures, imparting them with multifunctionality as both diagnostic and therapeutic tools.
Nanocrystalline cellulose (CNC) is produced from different cellulose sources through chemical and mechanical methods. The commonly used approaches include acid or enzymatic hydrolysis for obtaining nanocellulose (chemical methods) and sonication or high-pressure treatment (mechanical methods).191 Chaoqun You et al192 successfully prepared nanocatalysts by conjugating copper ions with gold nanoparticles using CNC as a carrier. This nanocatalyst system can be combined with DOX to generate more H2O2, thereby amplifying the efficacy of chemodynamic therapy. In other studies, researchers have synthesized highly functionalized amorphous cellulose chains known as hairy cellulose nanocrystals (HCNCs) through controlled oxidation of cellulose.193,194 HCNC shares a similar crystal size with CNC but exhibits a larger hydrodynamic size due to its dense protruding polymer structure, along with significant pH and ion-responsive behavior. Sarah A.E. Young et al developed anionic HCNC and combined it with positively charged DOX . The electrostatic adsorption allowed for stable binding of DOX to HCNC, reducing the pronounced nephrotoxic effects of DOX. They quantified the drug desorption by varying conditions such as ionic strength and pH. Compared to other nanoadsorbents, the HCNC they prepared showed a 32-fold increase in DOX adsorption efficiency.195
Furthermore, numerous researchers have explored the modification of cellulose, which is insoluble in water and organic solvents, to create hydrogels with micro-nano structures in combination with other components. For instance, Chenyang Xing et al184 developed a hydrogel by combining black phosphorus (BP) nanosheets with gelled cellulose, enabling its application in photodynamic therapy for superficial tumors. Similarly, Ning et al196 utilized polydopamine, agarose, and fluorescent nanocellulose to create a hydrogel carrier capable of pH-responsive release of paclitaxel.
We have collected and summarized recent studies on the use of cellulose in nanomedicine for cancer treatment, as presented in Table 7. As mentioned earlier, the pH microenvironment of diseased tissue differs from that of normal tissue. Therefore, most drug releases are measured in a weakly acidic environment. However, due to variations in preparation methods, materials, and applications across different studies, there is significant variability in the release data of drugs in vitro. However, all of them have demonstrated significant cytotoxic effects on cancer cells in vitro. Some researchers have applied nanosystems in photothermal therapy, photodynamic therapy, and radiation therapy to achieve better treatment outcomes.
Table 7.
Cellulose-Based Biopolymeric Drug Delivery Systems for Cancer Therapy
| Loaded Drugs | Type of Nanosystems | Encapsulation Efficiency | Loading Content | Diameter (nm) | Drug Release | Ref |
|---|---|---|---|---|---|---|
| CUR | Nanofiber | 99.14% | 1 mg/ml | / | 93h, 50.31% at pH 1.2, 90.72% at pH 5.3, 57.27% at pH 6.8, 74.48% at pH 7.4 | [197] |
| Camptothecin | Nanofiber | 65.28% | 41.40 ± 3.08% | 458.7 | 48h, 63.4% at pH 6.8, 80.2% at pH 7.4 | [198] |
| DOX | NPs | / | 6000 μg | 66 | / | [195] |
| DOX, Au NPs | NPs | / | Au:2.5% DOX:7.71% | 190.1 | 48 h, 98.88% at pH 5.5 | [192] |
| PTX | NPs | 96.8% | 11.50% | 226.9 ± 2.75 | 24h, ~90% at pH 5.5 | [199] |
| Fe3O4 NPs | NPs | / | / | 8.02 | / | [200] |
| MTX/CUR | NPs | 33% and 75% | 3.3% / 7.5% | 530 | 96h, at pH 5.4, MTX and CUR released 56.51% and 53.95%, At pH 7.4, MTX and CUR 52.44% and 47.94% | [201] |
| FA | Nanocapsules | / | 1.2 mg/mL | 200–300 | 48h, 14% in 0mM GSH,25% in 10 μM GSH, 91.2% in 10 mM GSH | [186] |
| DOX | NPs | 94.16 ± 0.05% | 2.11 ± 0.10 wt % | 25 | 48h, 52.0% at pH 5.0 | [202] |
| FA, Cdots, DOX | Nanocrystals | 1.053 mg/mg (27.3%) | 0.15 mg/ml DOX | 179.4 ± 16.1 | 96h, 85.8 ± 1.6% at pH 5.6 | [203] |
| Gold NPs, ZnS-QE | Supramolecular nanoarchitecture | / | / | 75 ± 2 | / | [190] |
Abbreviations: FA, folic acid; Cdots, Carbon dots; ZnS-QE, ZnS semiconductor quantum emitter.
Cellulose and its derivatives have garnered significant attention in the realm of drug delivery; however, several challenges remain unresolved. While cellulose is naturally produced by plants or bacteria, plant-derived compounds may contain traces of endotoxins, necessitating thorough investigations into long-term toxicity, immunogenicity, and pharmacokinetics in vivo, using appropriate animal models.204 To date, much of the research has been confined to in vitro assessments of the drug delivery capabilities of nanocellulose hydrogel-based drug carriers. Additionally, the cost of production presents a noteworthy concern. For instance, bacterial fibers are challenging to industrialize due to their high production costs. Addressing these issues is crucial for the advancement and practical application of cellulose-based drug delivery systems.
Dextran
Dextran is a polysaccharide composed of glucose monomers linked by glycosidic bonds. It is typically extracted from grains and fungi. Dextran is highly soluble in water and biodegradable, exhibiting excellent properties in terms of restricting cell adhesion and diffusion. Research has shown that β-Dextran exhibits immunomodulatory activity. It can be selectively recognized by pattern recognition receptors on certain immune cells,205 including dectin-1, CR3, TLR4, TLR2, scavenger receptors, and lactosylceramide.206,207 Subsequently, it activates immune cells through a series of signaling pathways, promoting both cellular and humoral immunity.208 Therefore, Dextran is an excellent immunostimulant. Dextran and its derivatives have been extensively studied for biomedical applications due to their excellent water solubility, biocompatibility, and biodegradability.209 In addition to serving as carriers for conventional anticancer drug DOX,210 Dextran as a nanocarrier has gained significant attention in drug delivery and immunoadjuvant applications. Currently, it is widely researched as a nanocarrier for tumor vaccines,211 chemotherapy drugs,212 gene therapy,213,214 photodynamic therapy,215 and other modalities. We have collected studies from recent years and presented them in Table 8.
Table 8.
Dextron-Based Biopolymeric Drug Delivery Systems for Cancer Therapy
| Type of Dextron | Loaded Drugs | Type of Nanosystem | Zeta Potential(mV) | Diameter(nm) | PDI | Anti-Tumor Activity | Application | Ref |
|---|---|---|---|---|---|---|---|---|
| CMD | MnO2 | NPs | −23.83 ± 2.11 | 208.3 ± 14.2 | / | The ability to effectively deplete tumor GSH to collapse the tumor antioxidant defense system; Ability to amplify oxidative stress-induced cell necrosis | SDT | [216] |
| DEX | siRNA | Nanodroplets | −13.1±0.8 to 9.82±0.94 | 111.8 ± 1.9 | 0.276 | Dextran-based condensates allow efficient DNA compression and loading with GSH responsiveness, low cytotoxicity, and higher transfection efficiency | Gene Therapy | [217] |
| BFP | SeNPs | Nanotubes | −6.65 | 33 | / | BFP-Se nanomaterials barely affected normal cell growth and significantly inhibited the viability of hepatocellular carcinoma cell lines (HCC) in a dose-dependent manner. Capable of disrupting critical antioxidant systems to promote iron death in tumor cells, BFP-Se was able to target into the tumor environment, increasing tumor suppression from 53% to 81% compared to SeNPs alone | Chemotherapy | [218] |
| Aminated β-glucan | OVA | NPs | 25.4 ± 1.7 | 183.6 ± 0.9 | 0.113 ± 0.022 | Packaging efficiency of more than 80%. Enhanced uptake of antigen by APCs. Promotes macrophage maturation and enhances Th1 and Th2 type immune responses | Immune booster | [219] |
| Acetalated Dextran | Nutlin-3a | NPs | 40±2 | 221±4 | 0.12±0.03 | GM-CSF and CpG-ODN can promote a strong anti-tumor immune response by recruiting and activating APCs in the presence of antigen. NPs can be targeted for delivery to the tumor system | Chemotherapy with immunotherapy | [212] |
| PCL-g-Dex | DOX | Micelles | / | 142.2 | 0.16 | The DOX loading rate can be changed by adjusting the Dox/co-polymer ratio. The micelles released slowly and completely after seven days at pH 5.5, while the DOX release rate was less than 40% at pH 7.4. The drug-loaded micelles were able to kill cancer cells but had little killing effect on normal cells | Chemotherapy | [210] |
| DEAE-DEX | mRNA | NPs | ~20 | 120–210 | / | A suitable molar ratio of DEAE-Dextran: mRNA (3~10) was found, which provides high cellular uptake efficiency, higher transfection rate, and small particle size. | Gene Therapy | [211] |
| SpAcDEX | miRNA-21 oligonucleotide (ATMO-21) | NPs | +35.6 | 175.6 | 0.53 | The ability to target brain cancer cells, thus minimizing damage to normal cells. Inhibits the growth of U87MG cells in a dose-dependent manner. In vivo, tumor model tests demonstrated that the particles have tumor suppressive effects | Gene Therapy | [213] |
| Thiolated Dextran | miRNA | NPs | 20.8 ± 1.2 | 132 ± 8 | 0.174 ± 0.01 | Significantly improve the permeability and retention time of miRNAs. Effectively enhances the uptake of nanoparticles by cancer cells, leading to higher gene silencing and cancer cell apoptosis. | Gene Therapy | [214] |
| Methacrylated Dextran | Synthetic long peptides (SLPs) | Nanogels | +24 | 210 | <0.03 | Nanogels can be internalized by dendritic cells (DCs) and activate these immature cells | Immunotherapy | [220] |
| Dex-b-PLGA | / | NPs | between +20 and −15 | 88 ± 2 | / | Capable of negative charge at physiological pH and a positive charge at slightly acidic pH, thus resisting protein adsorption at neutral pH and enhancing cellular uptake of NPs by cells | / | [221] |
| CMD | ICG | NPs | −13.5 ± 1.7 | 27.5 ± 5.6 | 0.5 | Ability to target macrophages and enable NIR-II fluorescence imaging in a mouse model of subcutaneous pancreatic tumors | Tumor Imaging | [222] |
| Dextran Valproate | Valproic acid | NPs | / | 179 | 0.199 | / | Gene Therapy | [223] |
| Dextran Aldehyde | Quercetin | NPs | −47.4 | 259.7 ± 90.7 | / | NPs are non-toxic to HEK-293T cells and are able to induce histone H3 acetylation, which is rapidly taken up by HEK-3 cells. | molecular targeted therapy | [224] |
| PEG-CMD | Chlorin e6 | Nanobubbles | −54.1±6.7 | 142.3 ± 29.4 | / | / | Sonodynamic Therapy | [225] |
Abbreviations: DEAE-DEX, polysaccharide diethylaminoethylen (DEAE) – Dextran; SpAcDEX, spermine-modified acetalated dextran; PEG-CMD, PEGylated carboxymethyl dextran. BFP, β-glucan.
Due to the presence of numerous active hydroxyl groups on the molecular chain, Dextran can undergo chemical modifications such as phosphorylation, sulfation,226 carboxymethylation,227 and oxidation. Carboxymethyl Dextran (CMD) is a polyanionic polysaccharide with a negative ζ potential. Its functional groups facilitate chemical conjugation and ion complexation with various drugs.209 Xinping Luo et al222 developed a nano-probe called DN-ICG, which consists of a CMD conjugated with indocyanine green (ICG) nanoparticles, for dynamic imaging of macrophages. The nano-probe can specifically target tumor-associated macrophages through the interaction between CMD and a specific receptor (SIGN-R1). After hepatic metabolism, the probe can be detected in the tumor stroma, enabling fluorescence imaging of deep-seated hepatopancreatobiliary cancers. Nastaran Moradi et al228 coated CMD onto superparamagnetic iron oxide nanoparticles and further conjugated anti-CD3 monoclonal antibodies onto the surface of CMD using cyanogen bromide. This approach effectively isolates highly pure CD3+ T lymphocytes from whole blood and can be applied in CAR-T cell therapy. Luisa Pedro et al229 evaluated the safety of superparamagnetic iron nanoparticles for cancer treatment.
These cationic dextran nanoparticles can be used for the delivery of nucleic acids to tumor areas in gene therapy. Cationic dextran nanoparticles can be used to deliver nucleic acids to tumor regions for gene therapy. Chenglong Wang et al217 designed and synthesized two types of amino-containing cationic copolymers based on dextran, which were self-assembled with anionic genes to form nano-droplets. Nanodroplets can enter cells through endocytosis and subsequently undergo reduction-triggered disruption, releasing siRNA and DNA for expression within the cells. Dextran, as a carrier, offers good transfection efficiency while avoiding the higher cytotoxicity associated with commonly used cationic polymers such as polyethylenimine and polypropylenimine. Additionally, modified dextran derivatives, such as aldehyde-modified dextran prepared by Tao Zheng et al213 and thiol-modified dextran prepared by Farnaz Sadat Mirzazadeh Tekie et al214 introduce positively charged groups onto dextran, enabling interaction with negatively charged genes for gene therapy.
Vaccine adjuvants are small molecules, compounds, or macromolecular complexes added to antigens to enhance and modulate their immunogenicity. Dextran, due to its excellent immunomodulatory activity, is highly regarded in the development of immunological adjuvants and cancer vaccines. Jing Wei Jin et al219 synthesized amine-functionalized β-dextran nanoparticles loaded with CpG oligodeoxynucleotides using ion complexation and investigated their synergistic effects as immune enhancers. These nanoparticles achieved APC targeting through specific recognition by dectin-1 and TLR2, leading to enhanced antigen uptake and expression of relevant proteins, ultimately inducing effective Th1 and Th2 immune responses. Neda Kordalivand et al220 conjugated antigenic peptides to methylacrylic acid-modified cationic dextran nanoparticles via disulfide bonds (Figure 9A), enabling triggered release of the antigenic peptides under reducing conditions. C. Siewert et al211 self-assembled messenger RNA (mRNA) with cationic dextran (diethylenimino dextran) to form colloidal nanoparticles, allowing precise control of target selectivity in vivo by controlling the charge ratio. These mRNA vaccines, suitable for intravenous administration, can be used for cancer therapy.
Figure 9.
(A) Schematic representation of the cationic dextran nanogels containing N-(4-(2-(pyridine-2-yldisulfanyl) ethyl)-amidobutyl) methacrylamide as linker (left), and SLP loaded nanogels (right). Reprinted from Journal of Controlled Release, 315, Kordalivand N, Tondini E, Lau CYJ, et al. Cationic synthetic long peptides-loaded nanogels: an efficient therapeutic vaccine formulation for induction of T-cell responses, 114-125, Copyright 2019, with permission from Elsevier.220 (B) The detected selenium content in serum. Reprinted from Chemical Engineering Journal, 446, Cai L, Zhou S, Yu B, et al. The composites of triple-helix glucan nanotubes/selenium nanoparticles target hepatocellular carcinoma to enhance ferroptosis by depleting glutathione and augmenting redox imbalance, 137110, Copyright 2022, with permission from Elsevier.218 (C) The working mechanism of MTNCs for augmented chemo-SDT of cancer. (D and E) TEM/EDS mapping image of MTNCs when exposed to 0 mM and 10 mM of GSH for 30 min (scale bars represent 100 nm). (F) intracellular ROS detection using DCFDA. Reprinted from Carbohydrate Polymers, 273, Um W, P KEK, Song Y, et al. Carboxymethyl dextran-based nanocomposites for enhanced chemo-sonodynamic therapy of cancer, 118488, Copyright 2021, with permission from Elsevier.216
Glutathione (GSH) is an important antioxidant in cells, which inhibits apoptosis by scavenging reactive oxygen species (ROS). In cancer cells, the concentration of GSH is over 100 times higher than in normal tissues.230 Clearing GSH in tumors and inducing ROS can effectively kill cancer cells. Liqin Cai et al218 developed beta-dextran nanotubes loaded with selenium nanoparticles (SeNPs) (BFP-Se) for targeted delivery to cancer cells via the enhanced permeability and retention (EPR) effect. BFP-Se directly reacts with GSH and hydrogen peroxide in cancer cells, clearing GSH while generating ROS. This leads to cell apoptosis and ferroptosis, resulting in cancer cell death. The use of dextran nanotubes improves the bioavailability of SeNPs (Figure 9B). Wooram Um et al216 prepared carboxymethylated dextran-coated nanoparticles with a hydrophilic shell. The nanoparticles have a TiO2 core and an outer MnO2 coating as depicted in Figure 9C. The MnO2 coating acts as a chemical sensitizer to deplete glutathione, while TiO2 acts as a sonosensitizer to generate reactive oxygen species for sonodynamic therapy. The hydrophilic nature of the dextran shell allows prolonged circulation in the bloodstream, thereby enhancing tumor-targeting efficiency (Figure 9D-F).
Dextran is recognized for its anti-protein adsorption, antioxidant, anti-inflammatory, and anti-tumor properties, serving as a potent activator of cellular immunity with strong synergistic effects with anti-cancer antibodies.231 Certain soluble fungal sources of β-glucans, like shiitake polysaccharides from edible mushrooms and Schisandra glycosides from Schisandra chinensis culture filtrates, have been clinically employed in tumor immunotherapy. Additionally, dextran has been observed to scavenge free radicals produced around tumors. However, dextran itself lacks self-assembly ability and exhibits a strong water retention capacity. Its modification can alter its molecular structure and may even induce cytotoxicity, posing a challenge for dextran-based drug carriers.232 Moreover, different sources of dextran possess varying structures and bioactivities, necessitating a clear understanding of the conformational relationships between them.233 Finally, dextran as an immune adjuvant may lead to side effects such as pulmonary edema, platelet dysfunction, cerebral edema, and systemic allergic reactions, which should be carefully considered in the development and use of dextran-based drug delivery systems.234,235
Hyaluronic Acid
Hyaluronic acid (HA) is a linear polysaccharide composed of alternating repeating disaccharide units, including β-1,4-D-glucuronic acid and β-1,3-N-acetyl-D-glucosamine. Unlike other polysaccharide-based delivery carriers, HA is a common polysaccharide found in the human body, abundant in connective tissues, skin, and synovial fluid. It is biodegradable and non-immunogenic as it can be degraded by endogenous hyaluronidases and oxidative species (ROS, RNS).236,237 HA exhibits excellent biocompatibility, degradability, and compatibility with the human immune system. HA is commonly used for surface functionalization of nanocarriers, as it enhances blood circulation time, improves biocompatibility, and actively targets receptors on cancer cells.238 Receptors such as CD44, CD168 (also known as hyaluronic acid-mediated motility receptor, RHAMM), and lymphatic vessel endothelial hyaluronic acid receptor 1 (LYVE-1) are overexpressed in tumors and can be targeted by HA-functionalized nanocarriers.
CD44 is a key receptor of interest that is overexpressed in solid cancers such as prostate cancer, breast cancer, glioblastoma, colon cancer, and cervical cancer. HA is frequently utilized as the active targeting moiety in drug delivery systems, serving as the outer shell of nanocarriers. By specifically binding to CD44 antibodies present on the surface of cancer cells, HA enables targeted delivery to cancer cells. For instance, Lei Zhu et al239 exploited this characteristic and developed HA-encapsulated nanoparticles loaded with GKT831, an inhibitor of NADPH oxidases (NOXs). In breast cancer patient-derived xenograft (PDX) tumors in nude mice, these nanoparticles demonstrated a significantly enhanced therapeutic effect when combined with radiotherapy, highlighting their potential in breast cancer treatment.
RHAMM (CD168) receptor, also known as hyaluronic acid-mediated motility receptor, coexists with CD44 and is expressed in various cancers including breast cancer, ovarian cancer,240 pancreatic cancer,241 and prostate cancer (CaP).242 However, studies have revealed that approximately 23% of cancers overexpress this receptor even in the absence of CD44 expression. As shown in Figure 10A, Chenchen Yang et al243 utilized this characteristic to develop HA nanogels (HAss nanogels) for targeted delivery of DOX. Using an H22 metastatic tumor model as an animal model, fluorescence results demonstrated the overexpression of RHAMM in H22 tumors (Figure 10B and C), and HAss nanogels were observed in the tumor vicinity, indicating the targeting capability of the nanogels towards H22 tumors. Furthermore, it was shown that the HAss nanogels could inhibit lymph node metastasis. HA, as a drug delivery carrier for cancer treatment, is inherently unstable and susceptible to enzymatic degradation in the body. Researchers often modify HA by introducing branching functional groups to enhance its performance. For instance, Xiaoqin Zhang et al244 attached Ppa (a specific moiety) and Dendron branches to the side chains of HA, forming a dendritic three-dimensional structure. This modification effectively prolongs the circulation time of the drug in the bloodstream. Additionally, HA can be functionalized with dopamine (DA) to improve its adhesiveness to target tissues or cells.245
Figure 10.
Delivery strategies and applications of HA-based delivery carriers. (A) The preparation of HAss nanogels and the targeting of HAss nanogels to RHAMM in cancer cell and lymph node which contains cancer cells with RHAMM overexpression. (B) Diagrammatic drawing of establishing lymph node metastases model in mouse and RBITC-HAss nanogels (red) penetrated in metastatic lymph node (left) and normal one (right) at 2 h post-injection ex vivo. The scale bar is 500 μm. (C) Immunofluorescence staining sections of RHAMM (green) expression in tumor, RBITC-HAss nanogels (red) penetrated in the tumor at 24 h post-injection. All cell nuclei are not shown in this figure. The scale bar is 100 μm. Adapted with permission from Yang C, Li C, Zhang P, Wu W, Jiang X. Redox Responsive Hyaluronic Acid Nanogels for Treating RHAMM (CD168) Over-expressive Cancer, both Primary and Metastatic Tumors. Theranostics. 2017;7(6):1719–1734. (https://creativecommons.org/licenses/by-nc/4.0/).243 (D) Graphic illustration of the preparation strategy and the antitumor function of HA-Zein-HNK nanoparticles. (E) The representative images and particle sizes of HA-Zein-HNK at different HA concentrations. (F) Expressions of pro-apoptotic proteins Bax and anti-apoptotic proteins Bcl-2 in 4T1 cells incubation with various HNK formulations. (G) The expressions of Vimentin and E-cadherin in 4T1 cells treated by Zein-HNK, HA-Zein-HNK, and free HNK were examined using western blot. Reprinted from Carbohydrate Polymers, 240, Zhang Q, Wang J, Liu D, et al. Targeted delivery of honokiol by zein/hyaluronic acid core-shell nanoparticles to suppress breast cancer growth and metastasis, 116325, Copyright 2020, with permission from Elsevier.118
Indeed, HA can be combined with various photosensitizers, including Photofrin, Chlorin e6, Indocyanine green, or δ-Aminolevulinic acid (ALA),244 to enhance targeting and maximize the photothermal effect for targeting and killing cancer cells.246 Due to the abundance of functional groups on its surface, HA can facilitate synergistic effects when combined with other targeted drugs. For example, Xiaodan Wang et al247 utilized a ramified glyphosate (GA) based on HA to achieve common targeting of liver cancer cells. The degree of GA ramification influences the release rate of the drug, and the toxicity to HepG2 cells depends on the ramification rate. Furthermore, HA’s inherent negative charge enables it to act as a carrier for various substances, including PEG, PEI, liposomes, proteins, and other polysaccharides, through electrostatic interactions, thereby improving targeting and biocompatibility. For instance, the combination of HA with polyethylenimines (PEI), which are high-molecular-weight delivery carriers limited in their use due to cellular toxicity, not only reduces toxicity but also enhances targeting. In a study by Manoj B. Parmar et al248 a PEI/HA composite carrier was employed for the targeted delivery of small-interfering RNA (siRNA) to inhibit triple-negative breast cancer cells. Moreover, proteins are commonly used as delivery carriers; however, they often exhibit poor stability and tend to aggregate in the body due to electrostatic interactions with plasma proteins. To overcome this challenge, researchers have successfully combined proteins with HA to enhance stability and prevent aggregation, capitalizing on the negative charge of HA.117 As shown in Figure 10D and E, Qi Zhang et al conducted further research based on this foundation and designed a drug delivery system in which Honokiol (HNK) was loaded onto zein and coated with HA to form a core-shell structure (HA-Zein-HNK). In comparison to the bare Zein-HNK nanoparticles without HA coating, the HA-Zein-HNK nanoparticles exhibited a significant decrease in zeta potential from Zein-HNK+18.66 ± 1.6 mV to −34.54 ± 2.8 mV, indicating the formation of a more stable structure. In vitro drug release studies demonstrated that HA-Zein-HNK exhibited sustained release over the same period of time. Evaluation of the anticancer activity of both types of nanoparticles in BALB/c mice bearing 4T1 tumors revealed that HA-coated nanoparticles displayed superior targeting ability and exhibited better antitumor efficacy compared to free HNK and Zein-HNK118 (Figure 10F and G).
HA plays a crucial role in the field of cancer drug delivery due to its unique targeting ability and the presence of abundant functional groups (carboxyl, hydroxyl, and aldehyde end groups) that can be easily chemically modified (Table 9). Unlike other multifunctional nanomaterial delivery systems, HA-based delivery systems offer simpler designs without the need for additional ligands, and HA can achieve both active and passive targeting. Furthermore, the enzymatic and oxidative degradation properties of HA in the body enable responsive activation of drug release in a time- and space-dependent manner. However, there are still many issues that limit its further development.249,250 (1) The targeting ability of HA to CD44 receptors is influenced by its molecular weight. (2) Tumor-targeting properties of HA can be compromised by the presence of enzymes like hyaluronidase in the tumor microenvironment, leading to premature drug release. (3) HA receptors present in hepatic endothelial cells may cause preferential accumulation of hyaluronic acid in the liver, reducing its efficacy at tumor sites. (4) In vivo, HA-based nanoparticles can form a protein corona, affecting their degradation properties and potentially altering their behavior in the body. (5) Modification of HA for drug delivery purposes may alter its binding affinity to receptors, affecting its targeting ability. (6) HA-based drug delivery systems face challenges in industrial-scale production, limiting their widespread application, like the one developed by Alchemia of Australia, has shown promising results in Phase I and Phase II clinical trials, but the phase III clinical trials did not achieve the expected results. Further research and development is needed to address these challenges and improve their clinical efficacy.
Table 9.
HA-Based Biopolymeric Drug Delivery Systems for Cancer Therapy
| Type of HA | Loaded Drugs | Type of Nanosystem | Zeta Potential (mV) | Diameter (nm) | PDI | Drug Release (%) | Aimed Cancer/Cell | Ref |
|---|---|---|---|---|---|---|---|---|
| HA-APMA | DOX | Nanorods | / | length: 50 ± 4, width: 10 ± 2 | / | 48h, <10% % at pH 7.4, ~30% at pH 5.5 without hyaluronidase, 8.5% at pH 7.4,46.8% at pH 5.5 with hyaluronidase | Ovarian cancer cells | [251] |
| HA | CUR | NPs | 18.10±1.08 | 184.1 ± 13.2 | 0.432 | 10h, 16.7% at pH 7.4, 30.6% at pH 5.5 |
Breast cancer | [252] |
| DAHA | DOX | Nanorods | −16.6 | Length: 55.6, Diameter:13.7 | / | 48h, without NIR, 15% at pH 7.4, 35.3% at pH 6.5, 60% at pH 5.5; with NIR radiation 27% at pH 7.4, 52.95% at pH 6.5, 78% at pH 5.5 | Breast cancer, MCF-7 | [253] |
| HA | DOX | Nanocubes | −17.60±0.98 | 149.22 ± 26.15 | / | 72h, 78% at pH 5.5,39% at pH 7.4 | HCC | [254] |
| PEG-HA | MTX | NPs | 338 | 72h, 44.12% at pH 7.4, 87.7% at pH 5 |
MDA-MB-231 and MCF-7 | [255] | ||
| HA/Catechol-modified chitosan | DOX | NPs | −12.7 ± 0.12 | 160 | 0.3 ± 0.01 | / | Oral cancer, HN22 | [256] |
| HA/Lf | Lenalidomide | NPs | 31.7 ± 1.9 | 27.4 ± 2.7 | 0.239 | 12h, 9.5% ± 1.7% at pH 7.4, 12.2% ± 2.9% at pH6.5, 24.4% ± 2.4% at pH 5.5, 19.3% ± 2.9% at pH 4.8 | U87MG | [257] |
| HA-SH | Ovalbumin | Vaccines | −27.23 mV | 175.57 | 0.2 | / | EG.7-OVA tumor-bearing mice | [258] |
| HA-CpG | DOX | NPs | −21.4 | 15.9 | / | / | Glioblastoma | [259] |
| HA, GO | DOX | NPs | −22.45 ± 1.22 | 143.7 ± 3.9 | / | at pH 7.4, GSH 10m, 48h ~28%. Without GSH, PH7.4,48h 10%, PH5.5,48h 25% In the presence of HAase, PH7.4 released ~20%, in the presence of HAase and GSH 10mM, PH7.4 released 45%, in the presence of HAase, PH 5.5 released 55% |
MDA-MB-231 | [260] |
| HA-NH2 | PFOB@IR825-HA-Cy5.5 NPs | NPs | 22.9 ± 1.8 | 80~ 110 | / | / | HT-29 | [261] |
| HA/Liposome | Epalrestat (EPS) and DOX | NPs | −10.9 ± 0.51 | −10.9 ± 0.51 | 0.119 ± 0.048 | / | TNBC | [262] |
| TPGS/hyaluronic acid dual-functionalized PLGA | Paclitaxel | NPs | −19.40 ± 0.79 | 162.30 ±1.80 | 0.15 ± 0.01 | / | TNBC and Melanoma | [263] |
| HA-Zein | CUR | NGs | ~-50 | 200 ~250 | ~0.3 | 4 days released 79% | CT26 | [117] |
| HA-NO3 | PTX/ICG | NPS | 37.9 ± 0.352 | 195.6 ± 3.2 | 0.181 ± 0.012 | / | Breast cancer | [264] |
| HA, Liposome |
DOX | NPS | −28.9 | 165 | 0.15 ± 0.03 | 48h released less than 35% at PH7.4 | Osteosarcoma | [265] |
| HA | DOX | NGs | −30.12 ± 3.12 | 52.4 ± 3.4 | / | After 24 h, 20% and 26% were released in the absence of GSH, pH 7.4 and 5.0, respectively, and 69% and 76% were released in the presence of GSH (10 mM), pH 7.4 and 5.0, respectively. | Human prostate cancer, H22 A549 /NIH3T3 | [243] |
| HA, zein | Honokiol | NGs | −34.54 ± 2.8 | 200 | / | After 48h, Zein-HNK released 80.1% and HA-Zein-HNK released 77.5% | Breast cancer, 4T1 | [118] |
| AHA, HECS | DOX and cisplatin | NGs | −28 | 160 | / | After 24 h, DOX was released 51.2% at PH5.5 and 12.45% at PH7.4, CDDP was released 57.68% at PH5.5 and 25.87% at PH7.4. | Breast cancer | [266] |
| GA, HA | DOX | NGs | −26.4±1.1 | 235.9±4.1 | 0.110±0.042 | The cumulative release time was 48h, 29% at pH 7.4,35% at pH 6.5,51% at PH5.5 | Liver cancer | [247] |
Abbreviations: DAHA, Aldehyde/catechol-functionalized hyaluronic acid; HCC, hepatocellular carcinoma; Lf, lactoferrin; GA, glycyrrhetinic acid; AHA, Aldehyde hyaluronic acid; HECS, hydroxyethyl chitosan; GO, Graphene oxide.
Fucoidan
Fucoidan, a naturally occurring marine polysaccharide with a negative charge, is predominantly found in brown algae species such as marine ribs, myrtle algae, and cyanobacteria. Fucoidan exhibits a wide range of biological activities, including anti-tumor, immune regulation, anticoagulation, antioxidant, anti-angiogenic, and anti-metastatic properties.267 The bioactivity of fucoidan is closely associated with its structural characteristics, including the type, molecular weight, and degree of sulfation of the glucose linkages. Even within the same source, different fucoidan structures can be observed, leading to structural diversity among fucoidan species. As an active anti-cancer molecule, studies have revealed that low-molecular-weight fucoidan with high sulfation exhibits superior anti-cancer effects.268,269 Fucoidan has the ability to specifically target P-selectin receptors, which are expressed in various solid tumors, including scalp cell cancer, bladder cancer, prostate cancer, lung cancer, and breast cancer.270,271 In cancer, it promotes adhesion of tumor cells to activated platelets and endothelial cells, leading to cancer metastasis.270 Leveraging its tumor-targeting properties, researchers have explored the application of fucoidan in tumor drug delivery systems, utilizing it in the form of micelles272 or nanoparticles271 to enhance cellular uptake of anti-cancer drugs and achieve targeted delivery, thereby minimizing damage to healthy organs. Further investigations have demonstrated that fucoidan can induce cancer cell autophagy and premature senescence by modulating specific cellular pathways.273 Wen-Jing Hsu et al274 conducted studies to elucidate the anti-tumor mechanisms of fucoidan and found that it effectively downregulates the MAPK and PI3K/AKT pathways, thereby inhibiting AP-1 and NF-κB signaling pathways in triple-negative breast cancer (TNBC). It is noteworthy that different types of fucoidan exhibit distinct anti-cancer mechanisms. Additionally, researchers have explored the anti-angiogenic effects of fucoidan, particularly focusing on the sulfated algae polysaccharide FP08S2.275 This compound has been shown to inhibit the angiogenic properties of human microvascular endothelial cells (HMEC-1) by blocking the VEGFR08/Erk/VEGF signaling pathway, leading to antimetastatic effects. In vivo, studies using intra-organ tumor xenograft models have further confirmed the anti-tumor activity of fucoidan.
Furthermore, Fucoidan demonstrates its versatility beyond being a carrier for anti-cancer nanopharmaceuticals by its potential in combination with imaging agents for localized tumor tracking. By leveraging Fucoidan’s targeting ability towards P-selectin, researchers have developed a nanogel for injection that serves both as a photothermal therapy agent and an imaging tool as schematically depicted in Figure 11A. In this approach, chlorin e6 (Ce6) and Fucoidan are incorporated into a hydrogel called CFN-gel. When compared to free Ce6, CFN-gel exhibited increased cellular and tumor tissue uptake of Ce6, leading to enhanced fluorescent imaging capabilities (Figure 11B and C). The inherent tumor-targeting ability of Fucoidan allows the gel to concentrate specifically at the tumor site, thereby exerting its anti-tumor effects (Figure 11D). This combination of Fucoidan and imaging agents holds promise for precise and effective tumor imaging and therapy.276
Figure 11.
Delivery strategies and applications of fucoidan-based delivery carriers. (A) Schematic illustration of CFN-gel and its mode of action. (B) NIR fluorescence images of PBS-, free Ce6- or CFN-gel-treated mice. After 5 min and 24 h of intravenous injection of PBS (100 μL), free Ce6 (5 mg Ce6 kg−1), or a CFN-gel (5 mg Ce6 equiv. kg−1), NIR fluorescence images of the HT1080 tumor-bearing mice were obtained. (C) confocal fluorescence microscopic images of tumor sections prepared 24 h post-injection. Nuclei in the tumor sections were stained using DAPI. (D) Left: tumor growth curves of mice. Control group (n = 7), free Ce6 + PDT (n = 5), CFN-gel (n = 7) and CFN-gel + PDT (n = 5). PBS, free Ce6, or Ce6–fucoidan was injected intravenously on day 1. Light illumination (670 nm, 50 mW cm−2, 20 J cm−2) of the tumors was conducted on day 2 for PDT. Right: representative photographs of the mice obtained on day 10. Adapted with permission from Cho MH, Li Y, Lo P-C, Lee H, Choi Y. Fucoidan-Based Theranostic Nanogel for Enhancing Imaging and Photodynamic Therapy of Cancer. Nano-Micro Lett. 2020;12(1):47. https://creativecommons.org/licenses/by-nc/4.0/).276
Fucoidan has antioxidant, anticoagulant, antithrombotic, anti-inflammatory, antiviral, antilipidemic, antimetastatic, antidiabetic and anticancer effects. Although fucoidan extracts have been widely used in various health care products, including food supplements and cosmetics, there is no clinical drug based on fucoidan, mainly because of (1) Fucoidan’s complex structure and challenging purification process contribute to its relatively high cost compared to other polysaccharides.277 Research efforts should focus on developing more efficient and cost-effective methods for isolating and purifying fucoidan. (2) Fucoidan exists in various structural forms, leading to differences in its biological activities, including its anti-tumor effects. Further studies are needed to elucidate the structure-function relationships of different fucoidan variants and their specific effects on cancer cells.278 (3) While fucoidan has demonstrated promising anti-cancer effects in preclinical studies, its safety profile in vivo requires further investigation. Comprehensive toxicity studies are necessary to evaluate potential adverse effects and ensure its safety for clinical use.
Pectin
Pectin is a plant-derived polysaccharide that serves as a major constituent of the plant cell wall, primarily found in citrus fruits, apples, potatoes, and other sources. It is composed mainly of methylated D-galacturonic acid units, forming a cell ring structure. Pectin can be categorized into various types based on its different structural components, including homogalacturonan (HG), Type I Rhamnogalacturonans (RG-I), Type II Rhamnogalacturonans (RG-II), Xylagalacturonan (XGA), and ApioGalacturonan (AGA). Pectin rich in RG-I exhibits notable bioactivity.279 In recent years, pectin has gained significant attention due to its non-toxic nature, biodegradability, excellent biocompatibility, and cost-effectiveness.280 Consequently, it has found extensive applications in various fields, such as food, pharmaceuticals, and cosmetics. Similar to other polysaccharides, pectin possesses a range of beneficial properties, including anti-inflammatory, antioxidant, anti-cancer, and immune regulatory effects. Additionally, it has demonstrated the ability to lower blood sugar and cholesterol levels. These advantageous characteristics have further expanded its utilization in the biomedical field.
Pectin can be further classified into high methoxy pectin (HMP) and low methoxy pectin (LMP) based on the degree of esterification of the semi-lactobacillic acid residues. High methoxy pectin has a degree of esterification greater than 50%, while low methoxy pectin has a lower degree of esterification. LMP has been found to reduce intestinal inflammation, while HMP can alleviate overall and local inflammations.281 The degree of esterification also affects the hydrophobic, viscosity, and molecular weight of the pectin. Researchers have investigated the impact of different degrees of esterification on drug encapsulation and observed that as the degree of esterification increases, the particle size of nanoparticles also increases, the zeta potential decreases, and the efficiency of drug encapsulation improves with better thermal stability.282 However, the excellent water resistance of HMP can lead to poor drainage properties. To overcome this, researchers have discovered that combining HMP with surfactants can enhance drug encapsulation efficiency by introducing hydrophobic groups to the delivery carrier.283
The anticancer efficacy of pectin is primarily achieved through its ability to inhibit tumor cell growth, induce apoptosis (cell death) in tumor cells, and suppress tumor cell migration.284 Pectin can be formulated into various drug preparations, such as films,285 gels,286 microspheres,287 nanoparticles,288 and pearls,289 to facilitate targeted drug delivery and sustained release. These different formulations provide versatile options for the administration of anticancer agents and hold promise for the treatment of cancer.
Pectin is a natural ligand for hepatic cells, and sorafenib (SF) is a standard therapeutic drug for hepatocellular carcinoma (HCC). However, SF has poor water solubility. Jaleh Varshosaz et al290 developed pectin-deoxycholic acid (P-DOCA) copolymeric micelles loaded with SF using pectin. This formulation enabled targeted delivery of SF through intravenous injection. While nanoparticles can enter tumor cells through the EPR effect, their cellular uptake rate is often low. Prolonging the circulation time in the bloodstream is a strategy to increase drug accumulation in tumor tissues. Researchers coated pectin-doxorubicin conjugates (PDC-NPs) with red blood cells in a biomimetic fashion, which effectively improved the stability and cellular uptake of PDC-NPs.283 Moreover, the semi- lactic acid residues of pectin can target the asialoglycoprotein receptors (ASGPRs) on liver cancer cells, thereby enhancing targeted therapy for hepatocellular carcinoma.281
Dietary pectin’s anti-cancer applications are primarily focused on the treatment of colorectal cancer due to its ability to inhibit the growth of harmful bacteria.284 However, pectin exhibits significant swelling characteristics, which can lead to premature and complete drug release, limiting its use.281 The abundant functional groups in pectin, such as hydroxyl, carboxyl, and amide groups, provide possibilities for its modification. In order to enhance the application of pectin in cancer treatment, researchers have explored various modification techniques, including chemical modification,291 ultrasound,292 heat treatment, irradiation,293,294 and enzyme treatment.295 One of the modified pectin derivatives, pH-modified citrus pectin (MCP), has shown excellent anti-cancer efficacy in the treatment of various cancers such as breast cancer, gastric cancer, colorectal cancer, and pancreatic cancer.296
The potential of pectin as an anti-cancer drug delivery system cannot be overlooked; however, there are still some challenges that need to be addressed. The structure of pectin is complex, and pectin extracted from different sources and using different extraction processes can exhibit structural variations.297 These structural differences also lead to variations in functionality. Achieving consistent structural and functional properties of pectin requires further improvement and optimization of the preparation processes. Furthermore, while numerous studies have demonstrated the superiority of pectin and its derivatives in the treatment of colorectal cancer, their effectiveness, mechanisms, and pharmacokinetics in other types of cancer still require further investigation. More research is needed to explore the potential of pectin in these areas.
Carrageenan
Carrageenan, a type of marine anionic polysaccharide predominantly found in red algae, are sulfated polysaccharides composed of alternating 3-β-D-galactopyranose (G-units) and 4-linked α-D-galactopyranose (D-units) or 4-linked 3,6-anhydro-α-D-galactopyranose (DA-units) repeat disaccharide units. Depending on the variations in the position and quantity of DA units and sulfate groups, carrageenan can be classified into several distinct structural types. The three most common types are kappa (κ-), iota (ι-), and lambda (λ-) carrageenan. The solubility of carrageenan is associated with the presence of sulfate groups and cation content, allowing it to form highly viscous solutions in water. As a result, carrageenan is widely used as a thickening and gelling agent in the food and pharmaceutical industries. Due to its remarkable gelling properties, excellent biocompatibility, and degradation characteristics, carrageenan plays a significant role in the field of drug delivery, particularly as an oral drug matrix or in localized drug delivery systems.298 Furthermore, carrageenan exhibits antioxidant, anti-proliferative, and anti-tumor activities, making it useful as an anti-cancer adjuvant, immunostimulant, and antimutagen.299 It can also be formulated into beads300 or microspheres through electrostatic or chemical interactions with drugs for cancer drug delivery purposes.301
The application development of carrageenan in cancer treatment is still in the preclinical research stage, primarily due to the lack of clear understanding regarding its mechanism of action in regulating the tumor microenvironment. Further in-depth investigations are necessary to elucidate its potential role in cancer therapy.302 And studies have suggested that carrageenan may induce inflammation in the gastrointestinal tract, leading to intestinal lesions and impairments in gut barrier function. Additionally, carrageenan’s sulphate groups may interact with proteins in the blood, potentially affecting blood clotting mechanisms and platelet function.303
Lignin
Lignin is a three-dimensional, amorphous, non-linear biopolymer composed of phenolic units, primarily found in the secondary cell walls of plants. It is one of the most abundant natural polymers on Earth, second only to cellulose in terms of phosphorous content. Lignin can be extracted through various processes, resulting in four main types: Kraft lignin (KL), soda lignin, lignosulfonate, and organic solvent lignin.304
Lignin exhibits a wide range of pharmacological properties, including antioxidant, antibacterial, anti-inflammatory, intestinal, immunosuppressive, and anti-tumor activities, which contribute to its increasing importance in the field of medicine.304 Its anti-cancer effects are attributed to its ability to eliminate free radicals from the tumor microenvironment and induce high levels of reactive oxygen species (ROS), resulting in the death of cancer cells. This property also enables lignin to be utilized as a carrier for anti-cancer drugs.305 For instance, Zhou et al306 developed a nanostructure by linking lignin with β-rings as shown in Figure 12A and B, which effectively encapsulated the anti-cancer drug HCPT and demonstrated significant inhibition of Hela cell growth. Furthermore, researchers have leveraged the benzene ring structure present in lignin, which exhibits self-assembly properties through π-π interactions.307 This composite structure demonstrated significantly improved loading capacity for Au nanoparticles and enhanced restorative properties. The anti-cancer effects of the Au/lignin-tannin (TL) composite nanoparticles were evaluated through toxicity trials on HepG2 cells, MCF7 cells (a breast cancer cell line), and A549 cells (a human lung cancer cell line). The results confirmed the anti-cancer activity of the Au/TL composite nanoparticles, highlighting their potential in cancer treatment.
Figure 12.
Delivery strategies and applications of lignin-based delivery carriers (A) Schematic of the Synthesis Route of Au/lignin–tannin particles. (B) SEM and TEM images of Au/LT particles, and size distribution of Au particles. Used with permissions of Royal Society of Chemistry, from Synthesis of Au/lignin–tannin particles and their anticancer application, Jiang Z, Duan J, Guo X, Ma Y, Wang C, Shi B, 23, 18, 2021; permission conveyed through Copyright Clearance Center, Inc..307 (C) Schematic of the Synthesis Route of LG-M(N-DOX)-PEG Copolymer and illustration of the dual pH-responsive LG-M(N-DOX)-PEG NPs. (D)Digital photo of LG-M(N)-PEG solution after the release of lignin for different times at pH = 6.5. (E) In vitro release profiles of DOX from LG-M(N-DOX)-PEG NPs at different pH values (6.5 and 7.2). Adapted with permission from Yang Z, Zhou Z, Li Y, et al. Assessing the Potential of New Lignin-Based pH-Responsive Nanoparticles as Drug Carriers for Cancer Treatment. ACS Sustainable Chem. Eng. 2022;10(32):10590–10603. Copyright© 2022 American Chemical Society.308
Moreover, the surface properties of lignin can be further modified to enhance its performance. Researchers have developed a method to prepare lignin nanoparticles coated with thermogen and modified with mUNO,309 which acts as a double stimulant targeting the TLR7/8 toll-like receptor and as an M2 pattern-like cell marker, respectively. This modified lignin nanoparticle system has the ability to induce polarization of tumor-associated macrophages from the M2 to M1 phenotype. Consequently, it exerts a destructive effect on tumor cells, showcasing its potential for tumor-targeted therapy.
In addition, researchers have performed chemical modification on lignin by introducing hydrazine and β-thiopropionate ester bonds into its structure, imparting pH-responsive properties.308 These modified lignin nanoparticles were then loaded with the anti-cancer drug DOX to investigate their efficacy in cancer treatment, which is schematically depicted in Figure 12C. In vitro experiments demonstrated that the prepared nanoparticles exhibited drug release at pH 6.5 while showing minimal release at pH 7.2 (Figure 12D and E). Furthermore, cell studies revealed that LG-M (N-DOX)-PEG NPs exhibited non-toxicity towards normal cells and inhibited the growth of tumor cells. Compared to free DOX, the nanoparticles exhibited higher cellular uptake rates. In vivo, tumor models also demonstrated that the nanoparticles had superior tumor inhibitory effects compared to free DOX and saline injections alone.
Lignin-based nanoparticles and hydrogels have been explored as promising drug delivery systems due to their biocompatibility, biodegradability, and tunable properties. As research in this area progresses, more efforts are expected to be dedicated to overcoming the challenges associated with lignin-based materials and unlocking their full potential in cancer therapy. Future developments may focus on optimizing lignin extraction methods, elucidating its degradation pathways in vivo, and exploring novel strategies for functionalizing lignin-based drug delivery systems.310
Others
Sodium alginate, a natural polysaccharide derived from seaweed,311 has also been investigated for its potential in the delivery of anti-cancer nanomedicines. Huang et al312 designed a biotinylated nano-micelle system loaded with DOX using the self-crosslinking properties of sodium alginate for the treatment of hepatocellular carcinoma. This nano-micelle system utilized the immune properties of sodium alginate in combination with DOX to synergistically inhibit tumor cells, while the biotin moiety targeted tumor cells. In vitro and in vivo experiments demonstrated that the biotinylated nano-micelles exhibited enhanced targeting efficiency, and the combination of sodium alginate and DOX exhibited improved cytotoxicity against tumor cells. However, research on sodium alginate as a drug delivery carrier for anti-cancer therapy is still relatively limited. This could be attributed to the large molecular weight, complex structure, low bioavailability, and unclear impact on the body’s mechanisms associated with natural sodium alginate. Some studies have suggested that low molecular weight sodium alginate exhibits better biocompatibility compared to high molecular weight sodium alginate. Furthermore, it possesses anti-inflammatory, antioxidant, antimicrobial, and anticancer properties, making it a promising candidate as a novel delivery carrier.313
Plant gums can serve as immunoadjuvants.314 There is a wide variety of natural plant gums that find extensive applications as diluents, adhesives, emulsifiers, thickeners, and more. Common examples of plant gums include gum Arabic (GA), gellan gum (GG), xanthan gum, guar gum, tragacanth gum (TG), gum ghatti (GGH), and cashew tree gum (Anacardium occidentale L.), among Others. Among these, gum Arabic is the most widely used.315 Gum Arabic is a polysaccharide of mixed nature derived from the Acacia tree.316 It exhibits excellent biocompatibility, high water solubility, and abundant functional groups, allowing for drug loading through conjugation. Additionally, the targeting effect of the galactose unit facilitates drug delivery to liver cancer cells.317 Oxidation is a common modification technique for polysaccharides. N. S. El-Sayed et al318 have oxidized gum Arabic, introduced aldehyde groups, and then conjugated 9-aminophenanthrene (9-AA) via Schiff’s base formation to achieve pH-responsive drug release. Composite carriers have shown improved functional characteristics compared to pure delivery vehicles. By combining maize prolamin with gum Arabic. J. Jin et al319 have developed maize prolamin-GA-EGCG composite nanoparticles. The presence of gum Arabic significantly enhances the loading efficiency compared to maize prolamin-EGCG nanoparticles. Z. Feng et al320 have directly incorporated 2-acrylamido-2-methyl-propanesulfonic acid (AMPS) onto xanthan gum, enabling the presence of disulfide bonds in the nanoparticles to achieve stimulus-responsive release of glutathione, thereby achieving targeted drug delivery and controlled release.
In summary, natural polysaccharides exhibit excellent biocompatibility, remarkable biodegradability, low biotoxicity, and an abundance of bioactive functional groups, all of which make them stand out as promising candidates for cancer drug delivery carriers. The rich functional groups of natural polysaccharides have created a great variety of polysaccharide derivatives, and the principles of common modifications of natural polysaccharides are shown in the Figure 13. We have summarized the characteristics of the listed polysaccharides and potential improvement strategies in Table 10. While each type of polysaccharide may have its limitations, effective enhancements can be achieved through chemical modification or modification methods.
Figure 13.
Schematic of the principles of polysaccharide modification methods.
Table 10.
Characterizations of Polysaccharide-Based Nanodelivery Carriers and Modification Methods
| Types of Natural Polysaccharides | Target Receptor | Advantages | Disadvantages | Modification Methods |
|---|---|---|---|---|
| Chitosan | – |
|
|
|
| Starch | – |
|
|
|
| Cellulose | – |
|
|
|
| Dextran | Targeted anti-cancer antibody CR3 |
|
|
|
| Hyaluronic acid | CD44, RHAMM |
|
|
|
| Fucoidan | P-selectin |
|
|
|
| Lignin | – |
|
|
|
| Carrageenan | – |
|
|
|
| Pectin | – |
|
|
|
Liposomes
Liposomes represent a distinct class of biopolymers, exhibiting a bilayered or multilayered spherical vesicular structure primarily composed of amphiphilic phospholipids and cholesterol. Liposomes are clinically recognised as delivery carrier for anticancer drugs, and most of the anticancer nano-formulations currently approved by the FDA are liposome-based. Primary liposomes are mainly composed of phospholipids and cholesterol, and such unmodified liposomes have significant drawbacks, including inefficient loading of hydrophilic drugs, easy leakage of drugs from liposomes, and rapid clearance of liposomes by the RES in the blood circulation.321 In order to prolong the circulation time, hydrophilic polymers coated with polyethylene glycol are effective in avoiding RES clearance, such as Caelyx, Myocet, and Doxil. However, these long-circulating liposomes also exhibit some disadvantages, such as low cellular uptake, the fact that these liposomes are mainly used for passive targeting to the tumor site, poor selectivity in cancer cells, and the limited space for loading the drug inside the liposome. Nowadays, the research on liposome formulations mainly focuses on the direction of improving targeting, drug-carrying performance and overcoming drug resistance, and the main strategy is to carry out surface modification,322 as shown in the Figure 14.
Figure 14.
Liposomes and the way liposomes are modified. Adapted with permission from Nel J, Elkhoury K, Velot É, et al. Functionalized liposomes for targeted breast cancer drug delivery. Bioact. Mater. 2023;24:401–437. https://creativecommons.org/licenses/by-nc/4.0/).322
Surface modification mainly includes, (1) incorporating functional fragments into liposomes; (2) Applying functional coatings on the surface, typically involving polymers such as PEG or targeting ligands like hyaluronic acid; (3) modifying surface charge; (4) conjugating targeted peptides or proteins onto the surface. Additionally, liposome modification may involve dual-carrier systems or combination therapy approaches, further expanding the versatility and efficacy of liposomal formulations. Vidhi M. Shah et al utilized sphingomyelin cholesterol (SPM/Chol) liposome preparation CPD100Li loaded with periwinkle alkaloids alongside an ion carrier (A23187) for treating ovarian cancer.323 Their findings indicated that the presence of A23187 effectively enhanced the stability of the liposomes and prolonged in vivo circulation time. In another study, a hypoxia-sensitive liposome drug delivery system was developed by incorporating a nitroimidazole derivative (a nitroaromatic) into the phospholipid bilayer of liposomes.324 This system exhibited oxygen-dependent drug release, effectively triggering drug release in hypoxic conditions. The system demonstrated efficacy in treating CDX (cell line-derived xenograft) and clinically relevant PDX (patient-derived xenograft) models. PEG is a commonly used modifier for liposome surface modification, which can effectively prolong the cycling time of liposomes but lacks targeting. Yang Wang et al conjugated a pH-responsive cell-penetrating peptide (TH peptide) in tandem with a tumor-targeting RGD motif to form a TH-RGD on the surface of PEGylated liposomes.325 They co-loaded the autophagy-inhibiting drug hydroxychloroquine (HCQ) and anticancer drugs within these liposomes. Their smart delivery system, capable of charge reversal under acidic conditions, demonstrated selective and effective internalization. Polysaccharides exhibit excellent biocompatibility and can effectively improve liposomal carrier stability when used as a coating. The choice of coating can be tailored to specific requirements. For instance, hyaluronic acid possesses tumor-targeting properties and can serve as a targeting coating. Chitosan exhibits pH-responsive characteristics, making it suitable for use as a stimulus-responsive coating.326,327 Carrageenan is capable of resisting gastric acid digestion, aiding liposomes in traversing the gastrointestinal system.328 Additionally, pectin demonstrates anti-tumor activity and enhances liposome stability.329 Cholesterol is a crucial component of most lipid nanocarriers and plays a pivotal role in determining the surface properties of liposomes. Research has demonstrated that smaller particle size can mitigate RES clearance and improve tumor penetration. The analogue cumaric acid (AA) is a cholesterol analogue, and researchers compared DOX-encapsulated AA liposomes (DOX-AALip) with conventional liposomes (DOX-Lip), and found that DOX-AALip exhibited smaller particle size and higher rigidity, which significantly prolonged liposome circulation time, and accumulated more in primary tumors and lung metastases.330
The latest research on liposomes is organized in Table 11. Liposome-based anticancer drugs, while already in clinical use, are not without limitations. These include biological instability, immune responses, lack of tissue specificity and targeting, as well as the complexity and high cost of the preparation process.331 Therefore, there is a pressing need for continued exploration of new and alternative options in cancer treatment.
Table 11.
Liposome Nanodelivery Systems for Cancer Therapy
| Type of Nanosystems | Loaded Drugs | Diameter (nm) | Zeta Potential (mV) | PDI | Functional Modifications | Aimed Cells | Aimed Tumor | Animal Models | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Hypoxia-sensitive liposome | Vinblastine-N-oxide (CPD100) | 142±4.16 | 155.4 ± 4.2 | 0.11±0.005 | A23187 | ES2 cells | Ovarian cancer | Female Swiss Webster mice | [323] |
| Hypoxia-sensitive liposome | DOX | 181 ± 3 | −34.4 ± 1.3 | 0.34 ± 0.07 | Nitroimidazole derivative, | RM-1, FaDu cells | Hypoxic tumor | Cell line and patient-derived xenograft | [324] |
| Liposome | DOX | 92.2±0.4 | −16.3 | 0.21±0.01 | Albumin-binding domain (ABD) | 4T1 | Breast cancer | Mice bearing 4 T1 tumors | [332] |
| PH-sensitive liposome | HCQ, DOX | 121.89±1.59 | pH 7.4, −4.36 ± 0.16, pH 6.5, 12.12 ± 0.08 | – | TH peptide, RGD | B16F10, MCF-7 | – | B16F10 xenograft-bearing mice | [325] |
| GSH- sensitive liposome | DOX | 65.2 ± 5.7 | –14.6 ± 3.3 | – | GSH | BxPC3, Huh7 | HCC\ poorly permeable pancreatic tumors | Mice bearing subcutaneous BxPC3 tumors | [333] |
| MMP sensitive liposome (Cleavable) | DOX | 140 | −17.6 ± 0.6 | 0.059 ± 0.025 | PEG | PC3 cells | – | PC3 cells derived xenograft | [334] |
| Liposome | DOX | – | – | – | Nanobowl | 4T1 | Breast tumor | Orthotopic 4T1 breast tumor mouse model | [335] |
| Dual-layered Au-liposome | – | 72.84±22.49 | −17.7 | – | 64Cu\PEG | 4T1 | – | Orthotopic 4T1 breast tumor mouse model | [336] |
| Targeting liposome | PTX | 112.5±4.1 | −34.4±0.8 | 0.25±0.009 | Ginsenosides | BGC-823 | Gastric cancer | BGC-823 cells derived xenograft | [337] |
| Thermosensitive magnetic liposomes | Irinotecan, Fe3O4 | 193.7 ± 2.3 | 2.3 ± 0.1 | 0.22 0.01 | CET | U87 cell | Brain tumor | U87 cells derived xenograft | [338] |
| PET imaging liposomes | 129.0 ± 2.2 | −16.53 ± 1.3 | – | Glucose- and DOTA-Cu2+ | A431 | – | A431 derived xenograft | [339] | |
| Targeting liposome | DTX | 96.7±4.5 | – | Ginsenoside Rg3 | 4T1 | TNBC | Orthotopic TNBC model | [340] | |
| Liposome | Clodronate | 101.8±24.4 | − 21 ± 5.04 | 0.2 | 4T1 cells | 4T1 breast cancer-bearing mice | – | [341] | |
| Liposomes with targeted | Photochlor (HPPH)\ evofosfamide | 128.7 ± 75.0 | 29.97 ± 3.5 | – | Chitosan oligosaccharide | MDA-MB-231 | TNBC | MDA-MB-231 tumor model | [342] |
| Dual-Targeting liposome | Cabozantinib\ IDO | 115.53±2.35 | 0.14±0.01 | 115.53±2.35 | – | 4T1 | TNBC | TNBC xenograft-bearing mice | [343] |
| Liposome | DOX | 200 | – | – | Holo-Lf | 4T1 | – | 4T1 xenograft-bearing mice | [344] |
| Liposome | ASOs | 150 ± 36 | −6.67 ± 0.18 | 0.0838 | Tumor-penetrating peptide, iRGD | 22Rv1, LNCaP, and VCaP | Bone metastasis model, 4T1 orthotopic tumor model | – | [345] |
| Liposome | DMC | 40 | – | – | Pyrophaeophorbid | CT26 | Mice bearing CT26 tumor | Colorectal cancer | [346] |
| PH-sensitive liposome | UA | 135.4 ± 0.636 | 7.8 | 0.3 | Chitosan | U14cell | [326] | ||
| Targeting liposome | HAPO-1048, IR-1048 | 135.2 | –32.97±2.36 | – | HA | UM-UC-3 | Bladder cancer | Orthotopic BC model | [347] |
| Targeting liposome | Honokiol | 162.6 ± 3.8 | −38.24 | 0.26 ± 0.02 | HA | 4T1 cells | Breast cancer | 4T1 tumor-bearing mouse model | [348] |
Abbreviations: HCQ, hydroxychloroquine; GSH, glutathione; CET, Cetuximab; DTX, docetaxel; TNBC, triple-negative breast cancer; IDO, Indoleamine 2,3-dioxygenase; Holo-Lf, holo-lactoferrin; ASOs, antisense oligonucleotides; HCC, hepatocellular carcinoma; ASOs, antisense oligonucleotides; DMC, demethylcantharidin; UA, ursolic acid.
Summary and Prospect
The medical application of nanotechnology has revitalized medical research, particularly in the field of tumor therapy, by overcoming the limitations of traditional treatment modalities. This has spurred researchers’ enthusiasm for exploring the potential of nanomedicine in cancer treatment. The efficacy of anti-cancer nanomedicines primarily relies on the delivery carrier, and researchers have successfully designed numerous synthetic nanocarriers that exhibit excellent drug-loading capacity and targeting capabilities. Biopolymers, as delivery carriers for tumor-targeted nanodrugs, offer inherent advantages such as biocompatibility, biodegradability, and low toxicity, making them highly promising in this context. However, there are still unresolved challenges associated with the sourcing and purification of biopolymers. Different sources of biopolymers exhibit structural variations that can influence their functional properties. Synthetic biopolymers, designed to mimic natural counterparts, often fall short in terms of functionality. Therefore, the development of an effective purification process is crucial to advance the field of biopolymer-based anti-cancer nanomedicines. We have summarized the characteristics and improvement methods for natural biopolymers (proteins, polysaccharides, liposomes), as detailed in the Table 12.
Table 12.
Characteristics and Improvement Strategies of Biopolymer-Based Nano-Anti-Cancer Delivery Drugs
| Types of Biopolymers | Advantages | Disadvantages | Improvement Strategies |
|---|---|---|---|
| Proteins |
|
|
|
| Natural polysaccharides |
|
|
|
| Liposomes |
|
|
|
Proteins have several advantages as anticancer drug delivery vehicles. Firstly, their natural origin ensures excellent biocompatibility and degradability, thereby reducing the risk of adverse effects during drug delivery. Secondly, certain proteins have innate targeting ability and specificity, enabling them to facilitate targeted drug delivery to tumors tissues or cells, thereby improving efficacy. Finally, the presence of functional groups on proteins enables facile modification, opening avenues for the development of multifunctional therapeutic strategies tailored to specific cancer types or patient needs. There have been successful examples of clinical applications of proteins as delivery vehicles, but so far only one protein has been commercialised for cancer drug delivery. The primary challenge hindering widespread adoption is the inherent instability of proteins, which are susceptible to degradation by enzymes and clearance by the immune system in the body. To address this issue, researchers have explored various strategies to enhance the stability and delivery performance of proteins, including modifications and optimization of the manufacturing processes. However, structural modifications may impact protein functionality and, in some cases, lead to denaturation, reducing binding sites and potentially eliciting immunogenic responses. Additionally, proteins derived from animal sources pose risks of disease transmission.
Natural polysaccharides offer significant potential in oncology therapy due to their diverse properties and interactions with biological systems. These biopolymers, with their antioxidant, anti-inflammatory, and anti-tumor properties, can serve as effective delivery carriers or coatings for anti-cancer drugs. Polysaccharides bind to pattern recognition receptors (PRRs) on cell membranes, such as scavenger receptors (SRs), Toll-like receptors (TLRs), complement receptor 3 (CR3), C-type lectin receptors (CLRs), and mannose receptors (MRs), triggering intracellular signaling cascades that mediate cellular physiological mechanisms, including activation of the immune response.349 Different cancers and tumor microenvironments present unique challenges, and natural polysaccharides can be tailored accordingly. For instance, drug delivery systems for the colon require resistance to absorption and degradation in the upper gastrointestinal tract, making polysaccharides like starch, fiber, and pectin suitable candidates due to their high acid resistance. In contrast, drug delivery systems for the gastrointestinal tract need pH release properties.350 Chitosan, for example, interacts with gastric mucosa via hydrogen bonding, non-covalent bonding, electrostatic interaction, and hydrophobic interaction, enhancing its adhesion to the mucin in gastric mucosa.351 This property enables targeted therapies, ensuring precise delivery of drugs to affected areas. Moreover, certain polysaccharides possess inherent tumor-targeting properties, enhancing delivery efficiency, therapeutic accuracy, and reducing systemic damage.352 Hyaluronic acid and fucoidan, for instance, can target proteins expressed in tumor tissue or activate macrophages in the tumor microenvironment, improving the efficacy of drug delivery systems. Polysaccharides exhibit anticancer properties primarily through scavenging free radicals and modulating the immune microenvironment. Examples include dextran and lignans, which possess antioxidant and immunomodulatory effects. Additionally, most natural polysaccharides carry a negative charge, enabling them to serve as effective delivery carriers by avoiding rapid clearance in vivo. Moreover, their abundance of functional groups allows for further enhancement of their types and functions through modification or branching polymers. Polysaccharides can also be utilized to coat liposomes, enhancing their targeting and stability while conferring specific functions. Examples of polysaccharides used for liposome coating include chitosan, hyaluronic acid, and glucose. This coating enhances the efficacy of liposomal drug delivery systems, providing targeted and stable delivery of therapeutic agents. However, the clinical application of polysaccharide-based anticancer drugs faces several challenges. Firstly, the reliance on the tumor microenvironment’s heterogeneity for polysaccharide-based nanotherapies is hindered by its limited significance, leading to non-specific drug accumulation in normal tissues. Secondly, although polysaccharide-based drugs possess inherent targeting abilities and anticancer properties, the specific mechanisms of action are yet to be elucidated. Thirdly, the potential toxicity of polysaccharide-based drugs to other tissues or organs is uncertain and requires further investigation. Additionally, the degradation and circulation mechanisms of these drugs within the body are not well-defined, lacking reliable data on the absorption or excretion of degraded small molecules. Lastly, limited research on the pharmacokinetics of polysaccharide-based drugs, often confined to preclinical animal models, hinders conclusive evidence of their equivalent therapeutic effects in humans, thereby restricting their clinical application.
Unlike proteins and polysaccharides, liposomes possess a straightforward structure, and their preparation process is amenable to industrialization. The numerous clinical applications of liposomes in nanomedicine highlight their significance in anticancer therapeutics. PEGylated liposomes effectively enhance the circulation duration of liposomes. However, they also disrupt the binding of drugs to cancer cells. Moreover, liposomes lack tumor-targeting capabilities and exhibit instability, resulting in premature drug leakage.
Despite these unresolved issues, the advantages of biopolymers remain significant. Presently, a common strategy involves addressing the limitations of delivery carriers through modification or combining them with other carriers. For example, in the combination of liposome with protein/polysaccharide, liposomes serve to encapsulate the drug, proteins extend the circulation time of liposomes, and polysaccharides enhance the stability of liposomes or improve their targeting capabilities. Similarly, in the combination of protein with polysaccharide, polysaccharides enhance the drug loading and release profile, while proteins aid in targeting enhancement or circulation time improvement. In the future, as research progresses, an increasing number of biopolymer-based nanomedicines are anticipated to be employed in cancer treatment.
Conclusion
This article provides an overview of the current research status and limitations of biopolymer-based nanocarriers for tumor drug delivery. Current nanomedicine delivery strategies primarily rely on the passive targeting strategy of the EPR effect in tumors. However, the delivery efficiency is low, which hinders the clinical application of anticancer nanomedicines. Biopolymers stand out as promising candidates for anticancer nanodrug delivery carriers due to their excellent biocompatibility. Moreover, some biopolymers possess inherent ligands on the surface of human cells, enabling active targeting capabilities. These properties offer potential solutions to overcome the limitations of current delivery strategies based on the EPR effect. The heterogeneity of the tumor microenvironment and the limited differences from normal tissues make solely relying on the EPR effect insufficient for achieving effective targeting.
As with any emerging field of science, the clinical translation of biopolymer-based nanomedicines in cancer therapy faces several challenges and barriers. Looking ahead, the future development of biopolymer-based anticancer drugs can be approached in several ways:
Safety and Standardization: Given their biological origin, ensuring the safety and standardization of the preparation process for biopolymer-based agents is paramount. While liposome-based drugs are relatively simpler to prepare and have seen FDA approval, proteins and natural polysaccharides sourced from diverse origins pose challenges in standardization. Establishing standardized processes not only ensures safety but also facilitates industrial-scale production.
Comprehensive Studies on Efficacy and Toxicology: There is a need for comprehensive studies to assess the efficacy, tissue uptake, pharmacokinetics, and potential toxicological risks of biopolymer-based nanomedicines in vivo. Preclinical studies often rely on mouse models, but variations in the immune system between species can limit the extrapolation of results to clinical settings. Robust data from diverse models are necessary to support clinical applications.
Multimodal Therapy: Multimodal therapy, combining different therapeutic modalities, is a growing trend in nanomedicine. Biopolymer-based nanomedicines should be developed with a multifunctional and multimodal therapeutic approach in mind. Co-delivery of synergistic drug combinations can overcome multidrug resistance mechanisms and enhance therapeutic efficacy compared to monotherapy.353
We believe that with continued advancements and successful clinical applications, biopolymer-based nanomedicines hold promise as effective tools in cancer therapy.
Acknowledgments
This work was funded by the National Key R&D Program of China (2023YFC2410403).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yadav N, Singh D, Rawat M, Sangwan N. Novel archetype in cancer therapeutics: exploring prospective of phytonanocarriers. 3 Biotech. 2022;12(11):324. doi: 10.1007/s13205-022-03372-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FdS F, Agner T, Sayer C, Lona LMF. Curcumin encapsulation in functional PLGA nanoparticles: a promising strategy for cancer therapies. Adv. Colloid Interface Sci. 2022;300:102582. doi: 10.1016/j.cis.2021.102582 [DOI] [PubMed] [Google Scholar]
- 3.Dong S, Guo X, Han F, He Z, Wang Y. Emerging role of natural products in cancer immunotherapy. Acta Pharmaceutica Sinica B. 2022;12(3):1163–1185. doi: 10.1016/j.apsb.2021.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Z-R L, Qiao P. Drug Delivery in Cancer Therapy, Quo Vadis?. Mol Pharmaceut. 2018;15(9):3603–3616. doi: 10.1021/acs.molpharmaceut.8b00037 [DOI] [PubMed] [Google Scholar]
- 5.Kumari S, Advani D, Sharma S, Ambasta RK, Kumar P. Combinatorial therapy in tumor microenvironment: where do we stand?. Biochimica et Biophysica Acta. 2021;1876(2):188585. doi: 10.1016/j.bbcan.2021.188585 [DOI] [PubMed] [Google Scholar]
- 6.Feng L, Liu R, Zhang X, et al. Thermo-Gelling Dendronized Chitosans as Biomimetic Scaffolds for Corneal Tissue Engineering. ACS Appl. Mater. Interfaces. 2021;13(41):49369–49379. doi: 10.1021/acsami.1c16087 [DOI] [PubMed] [Google Scholar]
- 7.Ashrafizadeh M, Delfi M, Zarrabi A, et al. Stimuli-responsive liposomal nanoformulations in cancer therapy: pre-clinical & clinical approaches. J Control Release. 2022;351:50–80. doi: 10.1016/j.jconrel.2022.08.001 [DOI] [PubMed] [Google Scholar]
- 8.Nagayasu A, Uchiyama K, Kiwada H. The size of liposomes: a factor which affects their targeting efficiency to tumors and therapeutic activity of liposomal antitumor drugs. Adv. Drug Delivery Rev. 1999;40(1):75–87. doi: 10.1016/S0169-409X(99)00041-1 [DOI] [PubMed] [Google Scholar]
- 9.Javed R, Ao Q. Nanoparticles in peripheral nerve regeneration: a mini review. J Neurorestoratol. 2022;10(1):1–12. doi: 10.26599/JNR.2022.9040001 [DOI] [Google Scholar]
- 10.Xin Y, Quan L, Zhang H, Ao Q. Emerging Polymer-Based Nanosystem Strategies in the Delivery of Antifungal Drugs. Pharmaceutics. 2023;15(7):1866. doi: 10.3390/pharmaceutics15071866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatia SN, Chen X, Dobrovolskaia MA, Lammers T. Cancer nanomedicine. Nat Rev Cancer. 2022;22(10):550–556. doi: 10.1038/s41568-022-00496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer S, Langhanki J, Krumb M, Opatz T, Bros M, Zentel R. HPMA-Based Nanocarriers for Effective Immune System Stimulation. Macromol biosci. 2019;19(6):1800481. doi: 10.1002/mabi.201800481 [DOI] [PubMed] [Google Scholar]
- 13.Mu W, Chu Q, Liu Y, Zhang N. A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy. Nano-Micro Lett. 2020;12(1):142. doi: 10.1007/s40820-020-00482-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quader S, Kataoka K. Nanomaterial-Enabled Cancer Therapy. Mol Ther. 2017;25(7):1501–1513. doi: 10.1016/j.ymthe.2017.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasmin R, Shah M, Khan SA, Ali R. Gelatin nanoparticles: a potential candidate for medical applications. Nanotechnol Rev. 2017;6(2):191–207. doi: 10.1515/ntrev-2016-0009 [DOI] [Google Scholar]
- 16.Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. J clin oncol. 1990;8(7):1263–1268. doi: 10.1200/JCO.1990.8.7.1263 [DOI] [PubMed] [Google Scholar]
- 17.Giodini L, Re FL, Campagnol D, et al. Nanocarriers in cancer clinical practice: a pharmacokinetic issue. Nanomed Nanotechnol Biol Med. 2017;13(2):583–599. doi: 10.1016/j.nano.2016.07.012 [DOI] [PubMed] [Google Scholar]
- 18.Moghaddam FD, Zare EN, Hassanpour M, et al. Chitosan-based nanosystems for cancer diagnosis and therapy: stimuli-responsive, immune response, and clinical studies. Carbohydr. Polym. 2024;330:121839. doi: 10.1016/j.carbpol.2024.121839 [DOI] [PubMed] [Google Scholar]
- 19.Zhu H, Loh XJ, Ye E, Li Z. Polymeric Matrix-Based Nanoplatforms toward Tumor Therapy and Diagnosis. ACS Mater Lett. 2022;4(1):21–48. doi: 10.1021/acsmaterialslett.1c00558 [DOI] [Google Scholar]
- 20.Khan H, Mirzaei HR, Amiri A, Kupeli Akkol E, Ashhad halimi SM, Mirzaei H. Glyco-nanoparticles: new drug delivery systems in cancer therapy. Semi Cancer Biol. 2021;69:24–42. doi: 10.1016/j.semcancer.2019.12.004 [DOI] [PubMed] [Google Scholar]
- 21.Wilhelm S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nature Rev Mater. 2016;1(5):16014. doi: 10.1038/natrevmats.2016.14 [DOI] [Google Scholar]
- 22.Williamson SK, Johnson GA, Maulhardt HA, et al. A phase I study of intraperitoneal nanoparticulate paclitaxel (Nanotax®) in patients with peritoneal malignancies. Cancer Chemother Pharmacol. 2015;75(5):1075–1087. doi: 10.1007/s00280-015-2737-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seki T, Fang J, Maeda H. Tumor-Targeted Macromolecular Drug Delivery Based on the Enhanced Permeability and Retention Effect in Solid Tumor. In: Lu Y, Mahato RI, editors. Pharmaceutical Perspectives of Cancer Therapeutics. New York, NY: Springer US; 2009. doi: 10.1007/978-1-4419-0131-6_3:doi: 93–120 [DOI] [Google Scholar]
- 24.Alphandéry E, Grand-Dewyse P, Lefèvre R, Mandawala C, Durand-Dubief M. Cancer therapy using nanoformulated substances: scientific, regulatory and financial aspects. Expert Rev Anticancer Ther. 2015;15(10):1233–1255. doi: 10.1586/14737140.2015.1086647 [DOI] [PubMed] [Google Scholar]
- 25.Advani A, Earl M, Douer D, Rytting M, Bleyer A. Toxicities of Intravenous (IV) Pegasparaginase (ONCASPAR®) in Adults with Acute Lymphoblastic Leukemia (ALL). Blood. 2007;110(11):2811. doi: 10.1182/blood.V110.11.2811.281117638850 [DOI] [Google Scholar]
- 26.Morishita M, Leonard RC. Pegfilgrastim; a neutrophil mediated granulocyte colony stimulating factor-expanding uses in cancer chemotherapy. Expert opin biol ther. 2008;8(7):993–1001. doi: 10.1517/14712598.8.7.993 [DOI] [PubMed] [Google Scholar]
- 27.Abulateefeh SR. Long-acting injectable PLGA/PLA depots for leuprolide acetate: successful translation from bench to clinic. Drug Delivery Transl Res. 2023;13(2):520–530. doi: 10.1007/s13346-022-01228-0 [DOI] [PubMed] [Google Scholar]
- 28.Szebeni J, Baranyi L, Savay S, et al. ROLE OF COMPLEMENT ACTIVATION IN HYPERSENSITIVITY REACTIONS TO DOXIL AND HYNIC PEG LIPOSOMES: EXPERIMENTAL AND CLINICAL STUDIES. J Liposome Res. 2002;12(1–2):165–172. doi: 10.1081/LPR-120004790 [DOI] [PubMed] [Google Scholar]
- 29.O’Brien MER, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl(CAELYX™/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–449. doi: 10.1093/annonc/mdh097 [DOI] [PubMed] [Google Scholar]
- 30.Leonard RC, Williams S, Tulpule A, Levine AM, Oliveros S. Improving the therapeutic index of anthracycline chemotherapy: focus on liposomal doxorubicin (Myocet). Breast. 2009;18(4):218–224. doi: 10.1016/j.breast.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 31.Silverman JA, Deitcher SR. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol. 2013;71(3):555–564. doi: 10.1007/s00280-012-2042-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frampton JE. Mifamurtide. Pediatr Drugs. 2010;12(3):141–153. doi: 10.2165/11204910-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 33.Zhang H. Onivyde for the therapy of multiple solid tumors. Onco Targets Ther. 2016;9:3001–3007. doi: 10.2147/OTT.S105587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet (London, England). 2016;387(10018):545–557. doi: 10.1016/S0140-6736(15)00986-1 [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain MC. Neurotoxicity of intra-CSF liposomal cytarabine (DepoCyt) administered for the treatment of leptomeningeal metastases: a retrospective case series. J Neuro-oncol. 2012;109(1):143–148. doi: 10.1007/s11060-012-0880-x [DOI] [PubMed] [Google Scholar]
- 36.Thakkar S, Sharma D, Kalia K, Tekade RK. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: a review. Acta Biomater. 2020;101:43–68. doi: 10.1016/j.actbio.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 37.Feng Q, Tong R. Anticancer nanoparticulate polymer-drug conjugate. Bioeng. Transl. Med. 2016;1(3):277–296. doi: 10.1002/btm2.10033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Li M, Luo Z, Dai L, Guo X, Cai K. Design of nanocarriers based on complex biological barriers in vivo for tumor therapy. Nano Today. 2017;15:56–90. doi: 10.1016/j.nantod.2017.06.010 [DOI] [Google Scholar]
- 39.Dutta B, Barick KC, Hassan PA. Recent advances in active targeting of nanomaterials for anticancer drug delivery. Adv. Colloid Interface Sci. 2021;296:102509. doi: 10.1016/j.cis.2021.102509 [DOI] [PubMed] [Google Scholar]
- 40.Behera A, Padhi S. Passive and active targeting strategies for the delivery of the camptothecin anticancer drug: a review. Environ. Chem. Lett. 2020;18(5):1557–1567. doi: 10.1007/s10311-020-01022-9 [DOI] [Google Scholar]
- 41.Hanafy NAN, Leporatti S, El-Kemary MA. Extraction of chlorophyll and carotenoids loaded into chitosan as potential targeted therapy and bio imaging agents for breast carcinoma. Int J Biol Macromol. 2021;182:1150–1160. doi: 10.1016/j.ijbiomac.2021.03.189 [DOI] [PubMed] [Google Scholar]
- 42.Hanafy NAN, Abdelbadea RH, Abdelaziz AE, Mazyed EA. Formulation and optimization of folate-bovine serum albumin-coated ethoniosomes of pterostilbene as a targeted drug delivery system for lung cancer: in vitro and in vivo demonstrations. Cancer Nanotechnol. 2023;14(1):49. doi: 10.1186/s12645-023-00197-4 [DOI] [Google Scholar]
- 43.Vyas D, Patel M, Wairkar S. Strategies for active tumor targeting-an update. Eur. J. Pharmacol. 2022;915:174512. doi: 10.1016/j.ejphar.2021.174512 [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Cao J, Yuan Z. Strategies and challenges to improve the performance of tumor-associated active targeting. J Mat Chem B. 2020;8(18):3959–3971. doi: 10.1039/D0TB00289E [DOI] [PubMed] [Google Scholar]
- 45.Kohane DS, Tse JY, Yeo Y, Padera R, Shubina M, Langer R. Biodegradable polymeric microspheres and nanospheres for drug delivery in the peritoneum. Int J Med. 2006;77A. doi: 10.1002/jbm.a.30654 [DOI] [PubMed] [Google Scholar]
- 46.Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008;69(1):1–9. doi: 10.1016/j.ejpb.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 47.Desai N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012;14(2):282–295. doi: 10.1208/s12248-012-9339-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma X, Chen X, Yi Z, et al. Size Changeable Nanomedicines Assembled by Noncovalent Interactions of Responsive Small Molecules for Enhancing Tumor Therapy. ACS Appl. Mater. Interfaces. 2022;14(23):26431–26442. doi: 10.1021/acsami.2c04698 [DOI] [PubMed] [Google Scholar]
- 49.MacCuaig WM, Fouts BL, McNally MW, et al. Active Targeting Significantly Outperforms Nanoparticle Size in Facilitating Tumor-Specific Uptake in Orthotopic Pancreatic Cancer. ACS Appl. Mater. Interfaces. 2021;13(42):49614–49630. doi: 10.1021/acsami.1c09379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hou X, Zhong D, Li Y, et al. Facile fabrication of multi-pocket nanoparticles with stepwise size transition for promoting deep penetration and tumor targeting. J Nanobiotechnol. 2021;19(1):111. doi: 10.1186/s12951-021-00854-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature Biotechnol. 2015;33(9):941–951. doi: 10.1038/nbt.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han D, Qi H, Huang K, et al. The effects of surface charge on the intra-tumor penetration of drug delivery vehicles with tumor progression. J Mat Chem B. 2018;6(20):3331–3339. doi: 10.1039/C8TB00038G [DOI] [PubMed] [Google Scholar]
- 53.Wang H-X, Zuo Z-Q, Du J-Z, et al. Surface charge critically affects tumor penetration and therapeutic efficacy of cancer nanomedicines. Nano Today. 2016;11(2):133–144. doi: 10.1016/j.nantod.2016.04.008 [DOI] [Google Scholar]
- 54.Zhang P, Chen D, Li L, Sun K. Charge reversal nano-systems for tumor therapy. J Nanobiotechnol. 2022;20(1):31. doi: 10.1186/s12951-021-01221-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choo P, Liu T, Odom TW. Nanoparticle Shape Determines Dynamics of Targeting Nanoconstructs on Cell Membranes. J Am Chem Soc. 2021;143(12):4550–4555. doi: 10.1021/jacs.1c00850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chauhan VP, Popović Z, Chen O, et al. Fluorescent Nanorods and Nanospheres for Real-Time In Vivo Probing of Nanoparticle Shape-Dependent Tumor Penetration. Angewandte Chemie (International Ed. in English). 2011;50(48):11417–11420. doi: 10.1002/anie.201104449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cong VT, Wang W, Tilley RD, et al. Can the Shape of Nanoparticles Enable the Targeting to Cancer Cells over Healthy Cells?. Cells. 2021;31(32):2007880. doi: 10.1002/adfm.202007880 [DOI] [Google Scholar]
- 58.Liu J, Zhang J, Gao Y, et al. Barrier permeation and improved nanomedicine delivery in tumor microenvironments. Cancer Lett. 2023;562:216166. doi: 10.1016/j.canlet.2023.216166 [DOI] [PubMed] [Google Scholar]
- 59.Shah S, Famta P, Bagasariya D, et al. Nanotechnology based drug delivery systems: does shape really matter?. Int J Pharm. 2022;625:122101. doi: 10.1016/j.ijpharm.2022.122101 [DOI] [PubMed] [Google Scholar]
- 60.Barbero F, Russo L, Vitali M, et al. Formation of the Protein Corona: the Interface between Nanoparticles and the Immune System. Semin Immunopathol. 2017;34:52–60. doi: 10.1016/j.smim.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 61.Guo Y, Zhao S, Qiu H, et al. Shape of Nanoparticles as a Design Parameter to Improve Docetaxel Antitumor Efficacy. Bioconjugate Chem. 2018;29(4):1302–1311. doi: 10.1021/acs.bioconjchem.8b00059 [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Kröger M, Liu WK. Shape effect in cellular uptake of PEGylated nanoparticles: comparison between sphere, rod, cube and disk. Nanoscale. 2015;7(40):16631–16646. doi: 10.1039/C5NR02970H [DOI] [PubMed] [Google Scholar]
- 63.Hsiao PF, Tsai H-C, Peng S, et al. Transdermal delivery of poly(ethylene glycol)-co-oleylamine modified gold nanoparticles: effect of size and shape. Mater Chem Phys. 2019;224:22–28. doi: 10.1016/j.matchemphys.2018.11.060 [DOI] [Google Scholar]
- 64.Geng Y, Dalhaimer P, Cai S, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nature Nanotechnol. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cong VT, Houng JL, Kavallaris M, Chen X, Tilley RD, Gooding JJ. How can we use the endocytosis pathways to design nanoparticle drug-delivery vehicles to target cancer cells over healthy cells?. Chem. Soc. Rev. 2022;51(17):7531–7559. doi: 10.1039/D1CS00707F [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Tekobo S, Tu Y, et al. Permission to Enter Cell by Shape: nanodisk vs Nanosphere. ACS Appl. Mater. Interfaces. 2012;4(8):4099–4105. doi: 10.1021/am300840p [DOI] [PubMed] [Google Scholar]
- 67.Wang N, Li J, Wang J, et al. Shape-directed drug release and transport of erythrocyte-like nanodisks augment chemotherapy. J Control Release. 2022;350:886–897. doi: 10.1016/j.jconrel.2022.09.005 [DOI] [PubMed] [Google Scholar]
- 68.Madathiparambil Visalakshan R, González García LE, Benzigar MR, et al. The Influence of Nanoparticle Shape on Protein Corona Formation. Small. 2020;16(25):2000285. doi: 10.1002/smll.202000285 [DOI] [PubMed] [Google Scholar]
- 69.Correa S, Boehnke N, Barberio AE, et al. Tuning Nanoparticle Interactions with Ovarian Cancer through Layer-by-Layer Modification of Surface Chemistry. ACS Nano. 2020;14(2):2224–2237. doi: 10.1021/acsnano.9b09213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Wu W, Zhang Y, et al. The combined effects of size and surface chemistry on the accumulation of boronic acid-rich protein nanoparticles in tumors. Biomaterials. 2014;35(2):866–878. doi: 10.1016/j.biomaterials.2013.10.028 [DOI] [PubMed] [Google Scholar]
- 71.Kurtz-Chalot A, Villiers C, Pourchez J, et al. Impact of silica nanoparticle surface chemistry on protein Corona formation and consequential interactions with biological cells. Mater Sci Eng C. 2017;75:16–24. doi: 10.1016/j.msec.2017.02.028 [DOI] [PubMed] [Google Scholar]
- 72.Rodallec A, Benzekry S, Lacarelle B, Ciccolini J, Fanciullino R. Pharmacokinetics variability: why nanoparticles are not just magic-bullets in oncology. Crit Rev Oncol/Hematol. 2018;129:1–12. doi: 10.1016/j.critrevonc.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 73.Singh KA, Bahadur S, Yadav D, Dabas H. Pharmaceutical and Pharmacokinetic Aspects of Nanoformulation Based Drug Delivery Systems for Anti-cancer Drugs. Curr. Pharm. Des. 2023;29(24):1896–1906. doi: 10.2174/1381612829666230824144727 [DOI] [PubMed] [Google Scholar]
- 74.Fanciullino R, Ciccolini J, Milano G. Challenges, expectations and limits for nanoparticles-based therapeutics in cancer: a focus on nano-albumin-bound drugs. Crit Rev Oncol/Hematol. 2013;88(3):504–513. doi: 10.1016/j.critrevonc.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 75.Liu X-M, Miller SC, Wang D. Beyond oncology — application of HPMA copolymers in non-cancerous diseases. Adv. Drug Delivery Rev. 2010;62(2):258–271. doi: 10.1016/j.addr.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Delivery Rev. 2008;60(15):1650–1662. doi: 10.1016/j.addr.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 77.Kaltbeitzel J, Wich PR. Protein-based Nanoparticles: from Drug Delivery to Imaging, Nanocatalysis and Protein Therapy. Angew. Chem. Int. Ed. 2023;62(44):e202216097. doi: 10.1002/anie.202216097 [DOI] [PubMed] [Google Scholar]
- 78.Hoogenboezem EN, Duvall CL. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Delivery Rev. 2018;130:73–89. doi: 10.1016/j.addr.2018.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Solanki R, Rostamabadi H, Patel S, Jafari SM. Anticancer nano-delivery systems based on bovine serum albumin nanoparticles: a critical review. Int J Biol Macromol. 2021;193:528–540. doi: 10.1016/j.ijbiomac.2021.10.040 [DOI] [PubMed] [Google Scholar]
- 80.Wu Y, Xie H, Li Y, et al. Nitric Oxide-Loaded Bioinspired Lipoprotein Normalizes Tumor Vessels To Improve Intratumor Delivery and Chemotherapy of Albumin-Bound Paclitaxel Nanoparticles. Nano Lett. 2023;23(3):939–947. doi: 10.1021/acs.nanolett.2c04312 [DOI] [PubMed] [Google Scholar]
- 81.Hao L, Zhou Q, Piao Y, Zhou Z, Tang J, Shen Y. Albumin-binding prodrugs via reversible iminoboronate forming nanoparticles for cancer drug delivery. J Control Release. 2021;330:362–371. doi: 10.1016/j.jconrel.2020.12.035 [DOI] [PubMed] [Google Scholar]
- 82.Hao Y, Zhou J, Tan J, et al. Preclinical evaluation of the safety and effectiveness of a new bioartificial cornea. Bioact. Mater. 2023;29:265–278. doi: 10.1016/j.bioactmat.2023.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou M, Dong J, Huang J, et al. Chitosan-Gelatin-EGCG Nanoparticle-Meditated LncRNA TMEM44-AS1 Silencing to Activate the P53 Signaling Pathway for the Synergistic Reversal of 5-FU Resistance in Gastric Cancer. Adv. Sci. 2022;9(22):2105077. doi: 10.1002/advs.202105077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao R, Ye J, Li X, Wang X. Dual size/charge-switchable and multi-responsive gelatin-based nanocluster for targeted anti-tumor therapy. Int J Biol Macromol. 2023;238:124032. doi: 10.1016/j.ijbiomac.2023.124032 [DOI] [PubMed] [Google Scholar]
- 85.Pan UN, Khandelia R, Sanpui P, Das S, Paul A, Chattopadhyay A. Protein-Based Multifunctional Nanocarriers for Imaging, Photothermal Therapy, and Anticancer Drug Delivery. ACS Appl. Mater. Interfaces. 2017;9(23):19495–19501. doi: 10.1021/acsami.6b06099 [DOI] [PubMed] [Google Scholar]
- 86.Jia X, Fan X, Chen C, et al. Chemical and Structural Engineering of Gelatin-Based Delivery Systems for Therapeutic Applications: a Review. Biomacromolecules. 2024;25(2):564–589. doi: 10.1021/acs.biomac.3c01021 [DOI] [PubMed] [Google Scholar]
- 87.Yu B, Li Y, Lin Y, et al. Research progress of natural silk fibroin and the application for drug delivery in chemotherapies. Front Pharmacol. 2023. 13; doi: 10.3389/fphar.2022.1071868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jaiswal S, Roy R, Dutta SB, et al. Role of Doxorubicin on the Loading Efficiency of ICG within Silk Fibroin Nanoparticles. ACS Biomater. Sci. Eng. 2022;8(7):3054–3065. doi: 10.1021/acsbiomaterials.1c01616 [DOI] [PubMed] [Google Scholar]
- 89.Cao Y, Liu S, Ma Y, et al. Oral Nanomotor-Enabled Mucus Traverse and Tumor Penetration for Targeted Chemo-Sono-Immunotherapy against Colon Cancer. Small. 2022;18(42):2203466. doi: 10.1002/smll.202203466 [DOI] [PubMed] [Google Scholar]
- 90.Rajendra PKM, Nidamanuri BSS, Balan AP, Venkatachalam S, Jawahar N. A review on structure, preparation and applications of silk fibroin-based nano-drug delivery systems. J Nanopart Res. 2022;24(7):141. doi: 10.1007/s11051-022-05526-z [DOI] [Google Scholar]
- 91.Farokhi M, Mottaghitalab F, Reis RL, Ramakrishna S, Kundu SC. Functionalized silk fibroin nanofibers as drug carriers: advantages and challenges. J Control Release. 2020;321:324–347. doi: 10.1016/j.jconrel.2020.02.022 [DOI] [PubMed] [Google Scholar]
- 92.Liu G, Pang J, Huang Y, Xie Q, Guan G, Jiang Y. Self-Assembled Nanospheres of Folate-Decorated Zein for the Targeted Delivery of 10-Hydroxycamptothecin. Ind Eng Chem Res. 2017;56(30):8517–8527. doi: 10.1021/acs.iecr.7b01632 [DOI] [Google Scholar]
- 93.Zhang Q, Li D, Guan S, et al. Tumor-targeted delivery of honokiol via polysialic acid modified zein nanoparticles prevents breast cancer progression and metastasis. Int J Biol Macromol. 2022;203:280–291. doi: 10.1016/j.ijbiomac.2022.01.148 [DOI] [PubMed] [Google Scholar]
- 94.Lee HS, Kang N-W, Kim H, et al. Chondroitin sulfate-hybridized zein nanoparticles for tumor-targeted delivery of docetaxel. Carbohydr. Polym. 2021;253:117187. doi: 10.1016/j.carbpol.2020.117187 [DOI] [PubMed] [Google Scholar]
- 95.Cao R, Tang N, Zhu Y, et al. Dual-ratiometric magnetic resonance tunable nanoprobe with acidic-microenvironment-responsive property to enhance the visualization of early tumor pathological changes. Nano Res. 2023;16(7):10034–10046. doi: 10.1007/s12274-023-5679-x [DOI] [Google Scholar]
- 96.Song N, Zhang J, Zhai J, Hong J, Yuan C, Liang M. Ferritin: a Multifunctional Nanoplatform for Biological Detection, Imaging Diagnosis, and Drug Delivery. Acc. Chem. Res. 2021;54(17):3313–3325. doi: 10.1021/acs.accounts.1c00267 [DOI] [PubMed] [Google Scholar]
- 97.Liu Y, Wang Y, Guan X, et al. Reversal of Cisplatin Resistance in Ovarian Cancer by the Multitargeted Nanodrug Delivery System Tf-Mn-MOF@Nira@CDDP. ACS Appl. Mater. Interfaces. 2023;15(22):26484–26495. doi: 10.1021/acsami.3c05374 [DOI] [PubMed] [Google Scholar]
- 98.S-Y W, Y-X Y, Zhang Q, et al. Multifunctional Protein Hybrid Nanoplatform for Synergetic Photodynamic-Chemotherapy of Malignant Carcinoma by Homologous Targeting Combined with Oxygen Transport. Adv. Sci. 2023;10(5):2203742. doi: 10.1002/advs.202203742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang C, Zhang X, Zhao G. Ferritin Nanocage: a Versatile Nanocarrier Utilized in the Field of Food. Nutrition Med. 2020;10(9):1894. doi: 10.3390/nano10091894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yuan H, Guo H, Luan X, et al. Albumin Nanoparticle of Paclitaxel (Abraxane) Decreases while Taxol Increases Breast Cancer Stem Cells in Treatment of Triple Negative Breast Cancer. Mol Pharmaceut. 2020;17(7):2275–2286. doi: 10.1021/acs.molpharmaceut.9b01221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.R A, Yao Y, Guo X, et al. Precise Cancer Anti-acid Therapy Monitoring Using pH-Sensitive MnO2@BSA Nanoparticles by Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces. 2021;13(16):18604–18618. doi: 10.1021/acsami.1c04310 [DOI] [PubMed] [Google Scholar]
- 102.Xu W, Jiao L, Ye H, et al. pH-responsive allochroic nanoparticles for the multicolor detection of breast cancer biomarkers. Biosens. Bioelectron. 2020;148:111780. doi: 10.1016/j.bios.2019.111780 [DOI] [PubMed] [Google Scholar]
- 103.Kang N-W, Lee J-Y, Kim -D-D. Hydroxyapatite-binding albumin nanoclusters for enhancing bone tumor chemotherapy. J Control Release. 2022;342:111–121. doi: 10.1016/j.jconrel.2021.12.039 [DOI] [PubMed] [Google Scholar]
- 104.Lou X, Zhang D, Ling H, et al. Pure redox-sensitive paclitaxel–maleimide prodrug nanoparticles: endogenous albumin-induced size switching and improved antitumor efficiency. Acta Pharmaceutica Sinica B. 2021;11(7):2048–2058. doi: 10.1016/j.apsb.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kinoshita R, Ishima Y, Chuang VTG, et al. The Therapeutic Effect of Human Serum Albumin Dimer-Doxorubicin Complex against Human Pancreatic Tumors. Pharmaceutics. 2021;13(8):1209. doi: 10.3390/pharmaceutics13081209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin T, Zhao P, Jiang Y, et al. Blood–Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano. 2016;10(11):9999–10012. doi: 10.1021/acsnano.6b04268 [DOI] [PubMed] [Google Scholar]
- 107.Yuan C-S, Teng Z, Yang S, et al. Reshaping hypoxia and silencing CD73 via biomimetic gelatin nanotherapeutics to boost immunotherapy. J Control Release. 2022;351:255–271. doi: 10.1016/j.jconrel.2022.09.029 [DOI] [PubMed] [Google Scholar]
- 108.Chen X, Zou J, Zhang K, et al. Photothermal/matrix metalloproteinase-2 dual-responsive gelatin nanoparticles for breast cancer treatment. Acta Pharmaceutica Sinica B. 2021;11(1):271–282. doi: 10.1016/j.apsb.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.J-W L, Zhou Y, Xu J, et al. Water-Soluble and Degradable Gelatin/Polyaniline Assemblies with a High Photothermal Conversion Efficiency for pH-Switchable Precise Photothermal Therapy. ACS Appl. Mater. Interfaces. 2022;14(47):52670–52683. doi: 10.1021/acsami.2c16480 [DOI] [PubMed] [Google Scholar]
- 110.Chen Y, Xu W, Shafiq M, et al. Injectable nanofiber microspheres modified with metal phenolic networks for effective osteoarthritis treatment. Acta Biomater. 2023;157:593–608. doi: 10.1016/j.actbio.2022.11.040 [DOI] [PubMed] [Google Scholar]
- 111.Lee D, S-y J, Kwon S, Lee Y, Park E, Koo H. Optimized Combination of Photodynamic Therapy and Chemotherapy Using Gelatin Nanoparticles Containing Tirapazamine and Pheophorbide a. ACS Appl. Mater. Interfaces. 2021;13(9):10812–10821. doi: 10.1021/acsami.1c02316 [DOI] [PubMed] [Google Scholar]
- 112.Ding D, Wang J, Zhu Z, et al. Tumor Accumulation, Penetration, and Antitumor Response of Cisplatin-Loaded Gelatin/Poly(acrylic acid) Nanoparticles. ACS Appl. Mater. Interfaces. 2012;4(3):1838–1846. doi: 10.1021/am300138z [DOI] [PubMed] [Google Scholar]
- 113.Elia B, Francesca F, Tiziana A, et al. Trojan-horse silk fibroin nanocarriers loaded with a re-call antigen to redirect immunity against cancer. Journal for ImmunoTherapy of Cancer. 2023;11(1):e005916. doi: 10.1136/jitc-2022-005916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang X, He Q, Sun J, et al. Near-Infrared-Enpowered Nanomotor-Mediated Targeted Chemotherapy and Mitochondrial Phototherapy to Boost Systematic Antitumor Immunity. Adv. Healthcare Mater. 2022;11(14):2200255. doi: 10.1002/adhm.202200255 [DOI] [PubMed] [Google Scholar]
- 115.Yang J, Jia Z, Zhang J, et al. Metabolic Intervention Nanoparticles for Triple-Negative Breast Cancer Therapy via Overcoming FSP1-Mediated Ferroptosis Resistance. Adv. Healthcare Mater. 2022;11(13):2102799. doi: 10.1002/adhm.202102799 [DOI] [PubMed] [Google Scholar]
- 116.Chen Y, Wu H, Yang T, et al. Biomimetic Nucleation of Metal–Organic Frameworks on Silk Fibroin Nanoparticles for Designing Core–Shell-Structured pH-Responsive Anticancer Drug Carriers. ACS Appl. Mater. Interfaces. 2021;13(40):47371–47381. doi: 10.1021/acsami.1c13405 [DOI] [PubMed] [Google Scholar]
- 117.Seok H-Y, Sanoj Rejinold N, Lekshmi KM, Cherukula K, Park I-K, Kim Y-C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: in vitro and in vivo evaluation. J Control Release. 2018;280:20–30. doi: 10.1016/j.jconrel.2018.04.050 [DOI] [PubMed] [Google Scholar]
- 118.Zhang Q, Wang J, Liu D, et al. Targeted delivery of honokiol by zein/hyaluronic acid core-shell nanoparticles to suppress breast cancer growth and metastasis. Carbohydr. Polym. 2020;240:116325. doi: 10.1016/j.carbpol.2020.116325 [DOI] [PubMed] [Google Scholar]
- 119.Chen Y, Zeng L, Zhu H, et al. Ferritin Nanocaged Doxorubicin Potentiates Chemo-Immunotherapy against Hepatocellular Carcinoma via Immunogenic Cell Death. Small Methods. 2023;7(5):2201086. doi: 10.1002/smtd.202201086 [DOI] [PubMed] [Google Scholar]
- 120.Wu M, Ling W, Wei J, et al. Biomimetic photosensitizer nanocrystals trigger enhanced ferroptosis for improving cancer treatment. J Control Release. 2022;352:1116–1133. doi: 10.1016/j.jconrel.2022.11.026 [DOI] [PubMed] [Google Scholar]
- 121.Wu X, Sheng H, Zhao L, et al. Co-loaded lapatinib/PAB by ferritin nanoparticles eliminated ECM-detached cluster cells via modulating EGFR in triple-negative breast cancer. Cell Death Dis. 2022;13(6):557. doi: 10.1038/s41419-022-05007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Z, Zhao Y, Zhang S, et al. Re-engineering the inner surface of ferritin nanocage enables dual drug payloads for synergistic tumor therapy. Theranostics. 2022;12(4):1800–1815. doi: 10.7150/thno.68459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lei Y, Hamada Y, Li J, et al. Targeted tumor delivery and controlled release of neuronal drugs with ferritin nanoparticles to regulate pancreatic cancer progression. J Control Release. 2016;232:131–142. doi: 10.1016/j.jconrel.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 124.Quan L, Xin Y, Wu X, Ao Q. Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering. Polymers. 2022;14(11):2184. doi: 10.3390/polym14112184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rehman A, Jafari SM, Tong Q, et al. Drug nanodelivery systems based on natural polysaccharides against different diseases. Adv. Colloid Interface Sci. 2020;284:102251. doi: 10.1016/j.cis.2020.102251 [DOI] [PubMed] [Google Scholar]
- 126.Mallakpour S, Azadi E, Hussain CM. Recent advancements in synthesis and drug delivery utilization of polysaccharides-based nanocomposites: the important role of nanoparticles and layered double hydroxides. Int J Biol Macromol. 2021;193:183–204. doi: 10.1016/j.ijbiomac.2021.10.123 [DOI] [PubMed] [Google Scholar]
- 127.Li X, Dong W, Nalin AP, et al. The natural product chitosan enhances the anti-tumor activity of natural killer cells by activating dendritic cells. OncoImmunology. 2018;7(6):e1431085. doi: 10.1080/2162402X.2018.1431085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jain S, Nuwal K, Mahmood A, et al. Thiolated chitosan as an improved bioadhesive polymer in drug delivery. In Hasnain MS, Beg S, Nayak AK, editors. Chitosan in Drug Delivery. Academic Press; 2022; 247–276. doi: 10.1016/b978-0-12-819336-5.00013-3 [DOI] [Google Scholar]
- 129.Sudhakar S, Chandran SV, Selvamurugan N, Nazeer RA. Biodistribution and pharmacokinetics of thiolated chitosan nanoparticles for oral delivery of insulin in vivo. Int J Biol Macromol. 2020;150:281–288. doi: 10.1016/j.ijbiomac.2020.02.079 [DOI] [PubMed] [Google Scholar]
- 130.Maria S, Sarwar HS, Sohail MF, et al. Synthesis and characterization of pre-activated thiolated chitosan nanoparticles for oral delivery of octreotide. J Drug Delivery Sci Technol. 2020;58:101807. doi: 10.1016/j.jddst.2020.101807 [DOI] [Google Scholar]
- 131.Sieval AB, Thanou M, Kotze´ AF, Verhoef JC, Brussee J, Junginger HE. Preparation and NMR characterization of highly substitutedN-trimethyl chitosan chloride. Carbohydrate Polymers. 1998;36(2):157–165. doi: 10.1016/S0144-8617(98)00009-5 [DOI] [Google Scholar]
- 132.Mourya VK, Inamdar NN. Trimethyl chitosan and its applications in drug delivery. J Mater Sci Mater Med. 2009;20(5):1057–1079. doi: 10.1007/s10856-008-3659-z [DOI] [PubMed] [Google Scholar]
- 133.Hu H, Qi Q, Dong Z, et al. Albumin coated trimethyl chitosan-based targeting delivery platform for photothermal/chemo-synergistic cancer therapy. Carbohydr. Polym. 2020;241:116335. doi: 10.1016/j.carbpol.2020.116335 [DOI] [PubMed] [Google Scholar]
- 134.Zeng Z, Liu Y, Wen Q, et al. Experimental study on preparation and anti-tumor efficiency of nanoparticles targeting M2 macrophages. Drug Delivery. 2021;28(1):943–956. doi: 10.1080/10717544.2021.1921076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shariatinia Z. Carboxymethyl chitosan: properties and biomedical applications. Int J Biol Macromol. 2018;120:1406–1419. doi: 10.1016/j.ijbiomac.2018.09.131 [DOI] [PubMed] [Google Scholar]
- 136.Chen X, Gu J, Sun L, et al. Efficient drug delivery and anticancer effect of micelles based on vitamin E succinate and chitosan derivatives. Bioact. Mater. 2021;6(10):3025–3035. doi: 10.1016/j.bioactmat.2021.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu Y, Zhang X, Zhou A, et al. A Smart “Energy NanoLock” Selectively Blocks Oral Cancer Energy Metabolism through Synergistic Inhibition of Exogenous Nutrient Supply and Endogenous Energy Production. Adv. Mater. 2023;35(3):2207384. doi: 10.1002/adma.202207384 [DOI] [PubMed] [Google Scholar]
- 138.Song X, Wu J, Song W, et al. Thiolated chitosan nanoparticles for stable delivery and smart release of As2O3 for liver cancer through dual actions. Carbohydr. Polym. 2023;303:120462. doi: 10.1016/j.carbpol.2022.120462 [DOI] [PubMed] [Google Scholar]
- 139.Ryu JH, Yoon HY, Sun I-C, Kwon IC, Kim K. Tumor-Targeting Glycol Chitosan Nanoparticles for Cancer Heterogeneity. Adv. Mater. 2020;32(51):2002197. doi: 10.1002/adma.202002197 [DOI] [PubMed] [Google Scholar]
- 140.Allahyari SE, Hajizadeh F, Zekiy AO, et al. Simultaneous inhibition of CD73 and IL-6 molecules by siRNA-loaded nanoparticles prevents the growth and spread of cancer. Nanomed Nanotechnol Biol Med. 2021;34:102384. doi: 10.1016/j.nano.2021.102384 [DOI] [PubMed] [Google Scholar]
- 141.Zhang X, Niu S, Williams GR, et al. Dual-responsive nanoparticles based on chitosan for enhanced breast cancer therapy. Carbohydr. Polym. 2019;221:84–93. doi: 10.1016/j.carbpol.2019.05.081 [DOI] [PubMed] [Google Scholar]
- 142.Bastaki S, Aravindhan S, Ahmadpour Saheb N, et al. Codelivery of STAT3 and PD-L1 siRNA by hyaluronate-TAT trimethyl/thiolated chitosan nanoparticles suppresses cancer progression in tumor-bearing mice. Life Sci. 2021;266:118847. doi: 10.1016/j.lfs.2020.118847 [DOI] [PubMed] [Google Scholar]
- 143.Yang G, Wang X, Fu S, Tang R, Wang J. pH-triggered chitosan nanogels via an ortho ester-based linkage for efficient chemotherapy. Acta Biomater. 2017;60:232–243. doi: 10.1016/j.actbio.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 144.Nikkhoo A, Rostami N, Farhadi S, et al. Codelivery of STAT3 siRNA and BV6 by carboxymethyl dextran trimethyl chitosan nanoparticles suppresses cancer cell progression. Int J Pharm. 2020;581:119236. doi: 10.1016/j.ijpharm.2020.119236 [DOI] [PubMed] [Google Scholar]
- 145.Yang H, Bremner DH, Tao L, Li H, Hu J, Zhu L. Carboxymethyl chitosan-mediated synthesis of hyaluronic acid-targeted graphene oxide for cancer drug delivery. Carbohydr. Polym. 2016;135:72–78. doi: 10.1016/j.carbpol.2015.08.058 [DOI] [PubMed] [Google Scholar]
- 146.Wang X, Wei B, Cheng X, Wang J, Tang R. 3-Carboxyphenylboronic acid-modified carboxymethyl chitosan nanoparticles for improved tumor targeting and inhibitory. Eur. J. Pharm. Biopharm. 2017;113:168–177. doi: 10.1016/j.ejpb.2016.12.034 [DOI] [PubMed] [Google Scholar]
- 147.Guo X, Zhuang Q, Ji T, et al. Multi-functionalized chitosan nanoparticles for enhanced chemotherapy in lung cancer. Carbohydr. Polym. 2018;195:311–320. doi: 10.1016/j.carbpol.2018.04.087 [DOI] [PubMed] [Google Scholar]
- 148.Peng H, Qiao L, Shan G, et al. Stepwise responsive carboxymethyl chitosan-based nanoplatform for effective drug-resistant breast cancer suppression. Carbohydr. Polym. 2022;291:119554. doi: 10.1016/j.carbpol.2022.119554 [DOI] [PubMed] [Google Scholar]
- 149.Hsu C-W, Hsieh M-H, Xiao M-C, Chou Y-H, Wang T-H, Chiang W-H. pH-responsive polymeric micelles self-assembled from benzoic-imine-containing alkyl-modified PEGylated chitosan for delivery of amphiphilic drugs. Int J Biol Macromol. 2020;163:1106–1116. doi: 10.1016/j.ijbiomac.2020.07.110 [DOI] [PubMed] [Google Scholar]
- 150.Helmi O, Elshishiny F, Mamdouh W. Targeted doxorubicin delivery and release within breast cancer environment using PEGylated chitosan nanoparticles labeled with monoclonal antibodies. Int J Biol Macromol. 2021;184:325–338. doi: 10.1016/j.ijbiomac.2021.06.014 [DOI] [PubMed] [Google Scholar]
- 151.Li R, Lin Z, Zhang Q, et al. Injectable and In Situ-Formable Thiolated Chitosan-Coated Liposomal Hydrogels as Curcumin Carriers for Prevention of In Vivo Breast Cancer Recurrence. ACS Appl. Mater. Interfaces. 2020;12(15):17936–17948. doi: 10.1021/acsami.9b21528 [DOI] [PubMed] [Google Scholar]
- 152.Kousar K, Naseer F, Abduh MS, et al. Green synthesis of hyaluronic acid coated, thiolated chitosan nanoparticles for CD44 targeted delivery and sustained release of Cisplatin in cervical carcinoma. Front Pharmacol. 2023. 13; doi: 10.3389/fphar.2022.1073004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cui X, Dong L, Zhong S, Shi C, Sun Y, Chen P. Sonochemical fabrication of folic acid functionalized multistimuli-responsive magnetic graphene oxide-based nanocapsules for targeted drug delivery. Chem Eng J. 2017;326:839–848. doi: 10.1016/j.cej.2017.06.045 [DOI] [Google Scholar]
- 154.Odeniyi MA, Omoteso OA, Adepoju AO, Jaiyeoba KT. Starch nanoparticles in drug delivery: a review. Polimery w medycynie. 2018;48(1):41–45. doi: 10.17219/pim/99993 [DOI] [PubMed] [Google Scholar]
- 155.Garcia MAVT, Garcia CF, Faraco AAG. Pharmaceutical and Biomedical Applications of Native and Modified Starch: a Review. Starch - Stärke. 2020;72(7–8):1900270. doi: 10.1002/star.201900270 [DOI] [Google Scholar]
- 156.El-Naga HMH A, El-Hashash SA, Yasen EM, Leporatti S, Hanafy NAN. Starch-Based Hydrogel Nanoparticles Loaded with Polyphenolic Compounds of Moringa Oleifera Leaf Extract Have Hepatoprotective Activity in Bisphenol A-Induced Animal Models. Polymers. 2022;14(14):2846. doi: 10.3390/polym14142846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pooresmaeil M, Namazi H. Developments on carboxymethyl starch-based smart systems as promising drug carriers: a review. Carbohydr. Polym. 2021;258:117654. doi: 10.1016/j.carbpol.2021.117654 [DOI] [PubMed] [Google Scholar]
- 158.Wang H, Hu H, Yang H, Li Z. Hydroxyethyl starch based smart nanomedicine. RSC Adv. 2021;11(6):3226–3240. doi: 10.1039/D0RA09663F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao K, Lin M. Combining targeted chemotherapy of hydroxyethyl starch prodrug and photothermal therapy of MoS2 for treatment of bladder cancer. Colloid. Polym. Sci. 2023;301(4):371–381. doi: 10.1007/s00396-023-05065-6 [DOI] [Google Scholar]
- 160.Wu H, Hu H, Wan J, et al. Hydroxyethyl starch stabilized polydopamine nanoparticles for cancer chemotherapy. Chem Eng J. 2018;349:129–145. doi: 10.1016/j.cej.2018.05.082 [DOI] [Google Scholar]
- 161.Ranjbar E, Namazi H, Pooresmaeil M. Carboxymethyl starch encapsulated 5-FU and DOX co-loaded layered double hydroxide for evaluation of its in vitro performance as a drug delivery agent. Int J Biol Macromol. 2022;201:193–202. doi: 10.1016/j.ijbiomac.2021.12.181 [DOI] [PubMed] [Google Scholar]
- 162.Li K, Zhan W, Chen Y, Jha RK, Chen X. Docetaxel and Doxorubicin Codelivery by Nanocarriers for Synergistic Treatment of Prostate Cancer. Front Pharmacol. 2019;10:10. doi: 10.3389/fphar.2019.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao B, Li L, Lv X, et al. Progress and prospects of modified starch-based carriers in anticancer drug delivery. J Control Release. 2022;349:662–678. doi: 10.1016/j.jconrel.2022.07.024 [DOI] [PubMed] [Google Scholar]
- 164.Chavan P, Sinhmar A, Nehra M, et al. Impact on various properties of native starch after synthesis of starch nanoparticles: a review. Food Chem. 2021;364:130416. doi: 10.1016/j.foodchem.2021.130416 [DOI] [PubMed] [Google Scholar]
- 165.Alp E, Damkaci F, Guven E, Tenniswood M. Starch nanoparticles for delivery of the histone deacetylase inhibitor CG-1521 in breast cancer treatment. Int j Nanomed. 2019;14:1335–1346. doi: 10.2147/IJN.S191837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang J, Huang Y, Gao C, Liu M, Zhang X. Fabrication and evaluation of the novel reduction-sensitive starch nanoparticles for controlled drug release. Colloids Surf. B. 2014;115:368–376. doi: 10.1016/j.colsurfb.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 167.Gardouh AR, El-Din AS S, Salem MS, Moustafa Y, Gad S. Starch Nanoparticles for Enhancement of Oral Bioavailability of a Newly Synthesized Thienopyrimidine Derivative with Anti-Proliferative Activity Against Pancreatic Cancer. Drug Des Devel Ther. 2021;15:3071–3093. doi: 10.2147/DDDT.S321962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang J, Li F, Li M, et al. Fabrication and characterization of hollow starch nanoparticles by gelation process for drug delivery application. Carbohydr. Polym. 2017;173:223–232. doi: 10.1016/j.carbpol.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 169.Xiao S, Tong C, Liu X, et al. Preparation of folate-conjugated starch nanoparticles and its application to tumor-targeted drug delivery vector. Chin. Sci. Bull. 2006;51(14):1693–1697. doi: 10.1007/s11434-006-2039-7 [DOI] [Google Scholar]
- 170.Yu C, Liu C, Wang S, et al. Hydroxyethyl Starch-Based Nanoparticles Featured with Redox-Sensitivity and Chemo-Photothermal Therapy for Synergized Tumor Eradication. Cancers. 2019;11(2):207. doi: 10.3390/cancers11020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang C, Wang Q, Wang H, et al. Hydroxyethyl starch-folic acid conjugates stabilized theranostic nanoparticles for cancer therapy. J Control Release. 2023;353:391–410. doi: 10.1016/j.jconrel.2022.11.059 [DOI] [PubMed] [Google Scholar]
- 172.Cheng K, Zhou J, Zhao Y, et al. pH-responsive and CD44-targeting polymer micelles based on CD44p-conjugated amphiphilic block copolymer PEG-b-HES-b-PLA for delivery of emodin to breast cancer cells. Nanotechnology. 2022;33(27):275604. doi: 10.1088/1361-6528/ac5f9a [DOI] [PubMed] [Google Scholar]
- 173.Chen S, Wu J, Tang Q, et al. Nano-micelles based on hydroxyethyl starch-curcumin conjugates for improved stability, antioxidant and anticancer activity of curcumin. Carbohydr. Polym. 2020;228:115398. doi: 10.1016/j.carbpol.2019.115398 [DOI] [PubMed] [Google Scholar]
- 174.Wang C, Wang H, Yang H, et al. Targeting cancer-associated fibroblasts with hydroxyethyl starch nanomedicine boosts cancer therapy. Nano Res. 2023;16(5):7323–7336. doi: 10.1007/s12274-023-5394-7 [DOI] [Google Scholar]
- 175.Zhao K, Li D, Xu W, et al. Targeted hydroxyethyl starch prodrug for inhibiting the growth and metastasis of prostate cancer. Biomaterials. 2017;116:82–94. doi: 10.1016/j.biomaterials.2016.11.030 [DOI] [PubMed] [Google Scholar]
- 176.Xiao S, Liu X, Tong C, et al. Dialdehyde starch nanoparticles as antitumor drug delivery system: an in vitro, in vivo, and immunohistological evaluation. Chin. Sci. Bull. 2012;57(24):3226–3232. doi: 10.1007/s11434-012-5342-5 [DOI] [Google Scholar]
- 177.Wang J, Liu H, Leng F, et al. Autofluorescent and pH-responsive mesoporous silica for cancer-targeted and controlled drug release. Microporous Mesoporous Mater. 2014;186:187–193. doi: 10.1016/j.micromeso.2013.11.006 [DOI] [Google Scholar]
- 178.Park I-K, D-B J, Babu A, et al. In vitro photodynamic therapy of methylene blue-loaded acetyl resistant starch nanoparticles. Biomater Res. 2022;26(1):28. doi: 10.1186/s40824-022-00273-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Amar-Lewis E, Buaron N, Chintakunta R, et al. Quaternized Starch-Based Composite Nanoparticles for siRNA Delivery to Tumors. ACS Appl. Nano Mater. 2021;4(2):2218–2229. doi: 10.1021/acsanm.1c00032 [DOI] [Google Scholar]
- 180.Tmt V, Mondal S, Nguyen VT, et al. Rice starch coated iron oxide nanoparticles: a theranostic probe for photoacoustic imaging-guided photothermal cancer therapy. Int J Biol Macromol. 2021;183:55–67. doi: 10.1016/j.ijbiomac.2021.04.053 [DOI] [PubMed] [Google Scholar]
- 181.Mariadoss AVA, Saravanakumar K, Sathiyaseelan A, Karthikkumar V, Wang M-H. Smart drug delivery of p-Coumaric acid loaded aptamer conjugated starch nanoparticles for effective triple-negative breast cancer therapy. Int J Biol Macromol. 2022;195:22–29. doi: 10.1016/j.ijbiomac.2021.11.170 [DOI] [PubMed] [Google Scholar]
- 182.Huang L, Chaurasiya B, Wu D, et al. Versatile redox-sensitive pullulan nanoparticles for enhanced liver targeting and efficient cancer therapy. Nanomed Nanotechnol Biol Med. 2018;14(3):1005–1017. doi: 10.1016/j.nano.2018.01.015 [DOI] [PubMed] [Google Scholar]
- 183.Saikia C, Das MK, Ramteke A, Maji TK. Controlled release of curcumin from thiolated starch-coated iron oxide magnetic nanoparticles: an in vitro evaluation. Int J Polym Mater Polym Biomater. 2017;66(7):349–358. doi: 10.1080/00914037.2016.1217532 [DOI] [Google Scholar]
- 184.Xing C, Chen S, Qiu M, et al. Conceptually Novel Black Phosphorus/Cellulose Hydrogels as Promising Photothermal Agents for Effective Cancer Therapy. Adv. Healthcare Mater. 2018;7(7):1701510. doi: 10.1002/adhm.201701510 [DOI] [PubMed] [Google Scholar]
- 185.Endes C, Camarero-Espinosa S, Mueller S, et al. A critical review of the current knowledge regarding the biological impact of nanocellulose. J Nanobiotechnol. 2016;14(1):78. doi: 10.1186/s12951-016-0230-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.He S, Zhong S, Meng Q, et al. Sonochemical preparation of folate-decorated reductive-responsive carboxymethylcellulose-based nanocapsules for targeted drug delivery. Carbohydr. Polym. 2021;266:118174. doi: 10.1016/j.carbpol.2021.118174 [DOI] [PubMed] [Google Scholar]
- 187.Yonet-Tanyeri N, Ahlmark BZ, Little SR. Advances in Multiplexed Paper-Based Analytical Devices for Cancer Diagnosis: a Review of Technological Developments. Adv Mater Technol. 2021;6(8):2001138. doi: 10.1002/admt.202001138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kamel S, Khattab T. Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors. 2020;10(6):67. doi: 10.3390/bios10060067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ratajczak K, Stobiecka M. High-performance modified cellulose paper-based biosensors for medical diagnostics and early cancer screening: a concise review. Carbohydr. Polym. 2020;229:115463. doi: 10.1016/j.carbpol.2019.115463 [DOI] [PubMed] [Google Scholar]
- 190.Caires AJ, Mansur HS, Mansur AAP, Carvalho IC, Carvalho SC. A carboxymethylcellulose-mediated aqueous colloidal process for building plasmonic–excitonic supramolecular nanoarchitectures based on gold nanoparticles/ZnS quantum emitters for cancer theranostics. Green Chem. 2021;23(20):8260–8279. doi: 10.1039/D1GC02508B [DOI] [Google Scholar]
- 191.Karimian A, Parsian H, Majidinia M, et al. Nanocrystalline cellulose: preparation, physicochemical properties, and applications in drug delivery systems. Int J Biol Macromol. 2019;133:850–859. doi: 10.1016/j.ijbiomac.2019.04.117 [DOI] [PubMed] [Google Scholar]
- 192.You C, Ning L, Zhang Z, et al. Toxic reactive oxygen species enhanced chemodynamic therapy by copper metal-nanocellulose based nanocatalysts. Carbohydr. Polym. 2022;289:119432. doi: 10.1016/j.carbpol.2022.119432 [DOI] [PubMed] [Google Scholar]
- 193.Sheikhi A, van de Ven TGM. Colloidal aspects of Janus-like hairy cellulose nanocrystalloids. Curr. Opin. Colloid Interface Sci. 2017;29:21–31. doi: 10.1016/j.cocis.2017.02.001 [DOI] [Google Scholar]
- 194.van de Ven TGM, Sheikhi A. Hairy cellulose nanocrystalloids: a novel class of nanocellulose. Nanoscale. 2016;8(33):15101–15114. doi: 10.1039/C6NR01570K [DOI] [PubMed] [Google Scholar]
- 195.Young SAE, Muthami J, Pitcher M, et al. Engineering hairy cellulose nanocrystals for chemotherapy drug capture. Mater Today Chem. 2022;23:100711. doi: 10.1016/j.mtchem.2021.100711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ning L, You C, Zhang Y, Li X, Wang F. Polydopamine loaded fluorescent nanocellulose–agarose hydrogel: a pH-responsive drug delivery carrier for cancer therapy. Compos. Commun. 2021;26:100739. doi: 10.1016/j.coco.2021.100739 [DOI] [Google Scholar]
- 197.Kumari P, Raza W, Meena A. Lemongrass derived cellulose nanofibers for controlled release of curcumin and its mechanism of action. Ind Crops Prod. 2021;173:114099. doi: 10.1016/j.indcrop.2021.114099 [DOI] [Google Scholar]
- 198.Kumari P, Meena A. Application of enzyme-mediated cellulose nanofibers from lemongrass waste for the controlled release of anticancer drugs. Environ. Sci. Pollut. Res. 2021;28(34):46343–46355. doi: 10.1007/s11356-020-08358-3 [DOI] [PubMed] [Google Scholar]
- 199.Song M, Dong S, An X, et al. Erythrocyte-biomimetic nanosystems to improve antitumor effects of paclitaxel on epithelial cancers. J Control Release. 2022;345:744–754. doi: 10.1016/j.jconrel.2022.03.060 [DOI] [PubMed] [Google Scholar]
- 200.Chaabane L, Chahdoura H, Mehdaoui R, et al. Functionalization of developed bacterial cellulose with magnetite nanoparticles for nanobiotechnology and nanomedicine applications. Carbohydr. Polym. 2020;247:116707. doi: 10.1016/j.carbpol.2020.116707 [DOI] [PubMed] [Google Scholar]
- 201.Moghaddam SV, Abedi F, Alizadeh E, et al. Lysine-embedded cellulose-based nanosystem for efficient dual-delivery of chemotherapeutics in combination cancer therapy. Carbohydr. Polym. 2020;250:116861. doi: 10.1016/j.carbpol.2020.116861 [DOI] [PubMed] [Google Scholar]
- 202.Yang X, Jiang X, Yang H, Bian L, Chang C, Zhang L. Biocompatible cellulose-based supramolecular nanoparticles driven by host–guest interactions for drug delivery. Carbohydr. Polym. 2020;237:116114. doi: 10.1016/j.carbpol.2020.116114 [DOI] [PubMed] [Google Scholar]
- 203.Tta D, Grijalvo S, Imae T, Garcia-Celma MJ, Rodríguez-Abreu C. A nanocellulose-based platform towards targeted chemo-photodynamic/photothermal cancer therapy. Carbohydr. Polym. 2021;270:118366. doi: 10.1016/j.carbpol.2021.118366 [DOI] [PubMed] [Google Scholar]
- 204.Ong X-R, Chen AX, Li N, Yang YY, Luo H-K. Nanocellulose: recent Advances Toward Biomedical Applications. Small Science. 2023;3(2):2200076. doi: 10.1002/smsc.202200076 [DOI] [Google Scholar]
- 205.Jin Y, Li P, Wang F. β-glucans as potential immunoadjuvants: a review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine. 2018;36(35):5235–5244. doi: 10.1016/j.vaccine.2018.07.038 [DOI] [PubMed] [Google Scholar]
- 206.Bai J, Ren Y, Li Y, et al. Physiological functionalities and mechanisms of β-glucans. Trends Food Sci Technol. 2019;88:57–66. doi: 10.1016/j.tifs.2019.03.023 [DOI] [Google Scholar]
- 207.Kiron V, Kulkarni A, Dahle D, Vasanth G, Lokesh J, Elvebo O. Recognition of purified beta 1,3/1,6 glucan and molecular signalling in the intestine of Atlantic salmon. Dev. Comp. Immunol. 2016;56:57–66. doi: 10.1016/j.dci.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 208.Su Y, Chen L, Yang F, Cheung PCK. Beta-d-glucan-based drug delivery system and its potential application in targeting tumor associated macrophages. Carbohydr. Polym. 2021;253:117258. doi: 10.1016/j.carbpol.2020.117258 [DOI] [PubMed] [Google Scholar]
- 209.Thambi T, You DG, Han HS, et al. Bioreducible Carboxymethyl Dextran Nanoparticles for Tumor-Targeted Drug Delivery. Adv. Healthcare Mater. 2014;3(11):1829–1838. doi: 10.1002/adhm.201300691 [DOI] [PubMed] [Google Scholar]
- 210.Delorme V, Lichon L, Mahindad H, et al. Reverse poly(ε-caprolactone)-g-dextran graft copolymers. Nano-carriers for intracellular uptake of anticancer drugs. Carbohydr. Polym. 2020;232:115764. doi: 10.1016/j.carbpol.2019.115764 [DOI] [PubMed] [Google Scholar]
- 211.Siewert C, Haas H, Nawroth T, et al. Investigation of charge ratio variation in mRNA – DEAE-dextran polyplex delivery systems. Biomaterials. 2019;192:612–620. doi: 10.1016/j.biomaterials.2018.10.020 [DOI] [PubMed] [Google Scholar]
- 212.Bauleth-Ramos T, Shih T-Y, Shahbazi M-A, et al. Acetalated Dextran Nanoparticles Loaded into an Injectable Alginate Cryogel for Combined Chemotherapy and Cancer Vaccination. Adv. Funct. Mater. 2019;29(35):1903686. doi: 10.1002/adfm.201903686 [DOI] [Google Scholar]
- 213.Zheng T, Wang W, Mohammadniaei M, et al. Anti-MicroRNA-21 Oligonucleotide Loaded Spermine-Modified Acetalated Dextran Nanoparticles for B1 Receptor-Targeted Gene Therapy and Antiangiogenesis Therapy. Adv. Sci. 2022;9(5):2103812. doi: 10.1002/advs.202103812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tekie FSM, Soleimani M, Zakerian A, et al. Glutathione responsive chitosan-thiolated dextran conjugated miR-145 nanoparticles targeted with AS1411 aptamer for cancer treatment. Carbohydr. Polym. 2018;201:131–140. doi: 10.1016/j.carbpol.2018.08.060 [DOI] [PubMed] [Google Scholar]
- 215.Ng KK, Zheng G. Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications. Chem. Rev. 2015;115(19):11012–11042. doi: 10.1021/acs.chemrev.5b00140 [DOI] [PubMed] [Google Scholar]
- 216.Um W, P KEK, Song Y, et al. Carboxymethyl dextran-based nanocomposites for enhanced chemo-sonodynamic therapy of cancer. Carbohydr. Polym. 2021;273:118488. doi: 10.1016/j.carbpol.2021.118488 [DOI] [PubMed] [Google Scholar]
- 217.Chenglong W, Shuhan X, Jiayi Y, Wencai G, Guoxiong X, Hongjing D. Dextran-based coacervate nanodroplets as potential gene carriers for efficient cancer therapy. Carbohydr. Polym. 2020;231:115687. doi: 10.1016/j.carbpol.2019.115687 [DOI] [PubMed] [Google Scholar]
- 218.Cai L, Zhou S, Yu B, et al. The composites of triple-helix glucan nanotubes/selenium nanoparticles target hepatocellular carcinoma to enhance ferroptosis by depleting glutathione and augmenting redox imbalance. Chem Eng J. 2022;446:137110. doi: 10.1016/j.cej.2022.137110 [DOI] [Google Scholar]
- 219.Jin JW, Tang SQ, Rong MZ, Zhang MQ. Synergistic effect of dual targeting vaccine adjuvant with aminated β-glucan and CpG-oligodeoxynucleotides for both humoral and cellular immune responses. Acta Biomater. 2018;78:211–223. doi: 10.1016/j.actbio.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 220.Kordalivand N, Tondini E, Lau CYJ, et al. Cationic synthetic long peptides-loaded nanogels: an efficient therapeutic vaccine formulation for induction of T-cell responses. J Control Release. 2019;315:114–125. doi: 10.1016/j.jconrel.2019.10.048 [DOI] [PubMed] [Google Scholar]
- 221.Li M, Xu Y, Sun J, et al. Fabrication of Charge-Conversion Nanoparticles for Cancer Imaging by Flash Nanoprecipitation. ACS Appl. Mater. Interfaces. 2018;10(13):10752–10760. doi: 10.1021/acsami.8b01788 [DOI] [PubMed] [Google Scholar]
- 222.Luo X, Hu D, Gao D, et al. Metabolizable Near-Infrared-II Nanoprobes for Dynamic Imaging of Deep-Seated Tumor-Associated Macrophages in Pancreatic Cancer. ACS Nano. 2021;15(6):10010–10024. doi: 10.1021/acsnano.1c01608 [DOI] [PubMed] [Google Scholar]
- 223.Kühne M, Lindemann H, Grune C, et al. Biocompatible sulfated valproic acid-coupled polysaccharide-based nanocarriers with HDAC inhibitory activity. J Control Release. 2021;329:717–730. doi: 10.1016/j.jconrel.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 224.Cheng H-W, Chiang C-S, H-Y H, et al. Dextran-modified Quercetin-Cu(II)/hyaluronic acid nanomedicine with natural poly(ADP-ribose) polymerase inhibitor and dual targeting for programmed synthetic lethal therapy in triple-negative breast cancer. J Control Release. 2021;329:136–147. doi: 10.1016/j.jconrel.2020.11.061 [DOI] [PubMed] [Google Scholar]
- 225.Um W, Ko H, You DG, et al. Necroptosis-Inducible Polymeric Nanobubbles for Enhanced Cancer Sonoimmunotherapy. Adv. Mater. 2020;32(16):1907953. doi: 10.1002/adma.201907953 [DOI] [PubMed] [Google Scholar]
- 226.M-K L, Chao C-H, Hsu Y-C, Chang -C-C. Structural sequencing and anti-inflammatory, anti-lung cancer activities of 1,4-α/β-sulfomalonoglucan in Antrodia cinnamomea. Int J Biol Macromol. 2021;170:307–316. doi: 10.1016/j.ijbiomac.2020.12.135 [DOI] [PubMed] [Google Scholar]
- 227.Son S, Rao NV, Ko H, et al. Carboxymethyl dextran-based hypoxia-responsive nanoparticles for doxorubicin delivery. Int J Biol Macromol. 2018;110:399–405. doi: 10.1016/j.ijbiomac.2017.11.048 [DOI] [PubMed] [Google Scholar]
- 228.Moradi N, Muhammadnejad S, Delavari H, Pournoori N, Oghabian MA, Ghafouri H. Bio-conjugation of anti-human CD3 monoclonal antibodies to magnetic nanoparticles by using cyanogen bromide: a potential for cell sorting and noninvasive diagnosis. Int J Biol Macromol. 2021;192:72–81. doi: 10.1016/j.ijbiomac.2021.09.129 [DOI] [PubMed] [Google Scholar]
- 229.Pedro L, Harmer Q, Mayes E, Shields JD. Impact of Locally Administered Carboxydextran-Coated Super-Paramagnetic Iron Nanoparticles on Cellular Immune Function. Small. 2019;15(20):1900224. doi: 10.1002/smll.201900224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–824. doi: 10.1038/nrm2256 [DOI] [PubMed] [Google Scholar]
- 231.Petr S, Josef R, Vaclav V. Glucans as New Anticancer Agents. Anticancer Res. 2019;39(7):3373. doi: 10.21873/anticanres.13480 [DOI] [PubMed] [Google Scholar]
- 232.Petrovici AR, Pinteala M, Simionescu N. Dextran Formulations as Effective Delivery Systems of Therapeutic Agents. Small. 2023;28(3):1086. doi: 10.3390/molecules28031086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Huang G, Huang S. The structure–activity relationships of natural glucans. Phytother Res. 2021;35(6):2890–2901. doi: 10.1002/ptr.6995 [DOI] [PubMed] [Google Scholar]
- 234.Prasher P, Sharma M, Singh SK, et al. Advances and applications of dextran-based nanomaterials targeting inflammatory respiratory diseases. J Drug Delivery Sci Technol. 2022;74:103598. doi: 10.1016/j.jddst.2022.103598 [DOI] [Google Scholar]
- 235.Khan MS, Gowda BHJ, Nasir N, et al. Advancements in dextran-based nanocarriers for treatment and imaging of breast cancer. Int J Pharm. 2023;643:123276. doi: 10.1016/j.ijpharm.2023.123276 [DOI] [PubMed] [Google Scholar]
- 236.Borzacchiello A, Russo L, Malle BM, Schwach-Abdellaoui K, Ambrosio L. Hyaluronic Acid Based Hydrogels for Regenerative Medicine Applications. Biomed Res. Int. 2015;2015:871218. doi: 10.1155/2015/871218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Choi KY, Han HS, Lee ES, et al. Hyaluronic Acid–Based Activatable Nanomaterials for Stimuli-Responsive Imaging and Therapeutics: beyond CD44-Mediated Drug Delivery. Adv. Mater. 2019;31(34):1803549. doi: 10.1002/adma.201803549 [DOI] [PubMed] [Google Scholar]
- 238.Naseer F, Ahmad T, Kousar K, Kakar S, Gul R, Anjum S. Formulation of surface-functionalized hyaluronic acid-coated thiolated chitosan nano-formulation for the delivery of vincristine in prostate cancer: a multifunctional targeted drug delivery approach. J Drug Delivery Sci Technol. 2022;74:103545. doi: 10.1016/j.jddst.2022.103545 [DOI] [Google Scholar]
- 239.Zhu L, Zhao Y, Liu T, et al. Inhibition of NADPH Oxidase-ROS Signal using Hyaluronic Acid Nanoparticles for Overcoming Radioresistance in Cancer Therapy. ACS Nano. 2022;16(11):18708–18728. doi: 10.1021/acsnano.2c07440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Buttermore ST, Hoffman MS, Kumar A, Champeaux A, Nicosia SV, Kruk PA. Increased RHAMM expression relates to ovarian cancer progression. J ovarian Res. 2017;10(1):66. doi: 10.1186/s13048-017-0360-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cheng X-B, Sato N, Kohi S, Koga A, Hirata K. Receptor for Hyaluronic Acid-Mediated Motility is Associated with Poor Survival in Pancreatic Ductal Adenocarcinoma. J Cancer. 2015;6(11):1093–1098. doi: 10.7150/jca.12990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gust KM, Hofer MD, Perner SR, et al. RHAMM (CD168) Is Overexpressed at the Protein Level and May Constitute an Immunogenic Antigen in Advanced Prostate Cancer Disease. Neoplasia. 2009;11(9):956–963. doi: 10.1593/neo.09694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yang C, Li C, Zhang P, Wu W, Jiang X. Redox Responsive Hyaluronic Acid Nanogels for Treating RHAMM (CD168) Over-expressive Cancer, both Primary and Metastatic Tumors. Theranostics. 2017;7(6):1719–1734. doi: 10.7150/thno.18340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhang X, Wu Y, Li Z, et al. Glycodendron/pyropheophorbide-a (Ppa)-functionalized hyaluronic acid as a nanosystem for tumor photodynamic therapy. Carbohydr. Polym. 2020;247:116749. doi: 10.1016/j.carbpol.2020.116749 [DOI] [PubMed] [Google Scholar]
- 245.Lee SY, Yang M, Seo J-H, et al. Serially pH-Modulated Hydrogels Based on Boronate Ester and Polydopamine Linkages for Local Cancer Therapy. ACS Appl. Mater. Interfaces. 2021;13(2):2189–2203. doi: 10.1021/acsami.0c16199 [DOI] [PubMed] [Google Scholar]
- 246.Li J, Xue Y, Tian J, et al. Fluorinated-functionalized hyaluronic acid nanoparticles for enhanced photodynamic therapy of ocular choroidal melanoma by ameliorating hypoxia. Carbohydr. Polym. 2020;237:116119. doi: 10.1016/j.carbpol.2020.116119 [DOI] [PubMed] [Google Scholar]
- 247.Wang X, Gu X, Wang H, Yang J, Mao S. Enhanced delivery of doxorubicin to the liver through self-assembled nanoparticles formed via conjugation of glycyrrhetinic acid to the hydroxyl group of hyaluronic acid. Carbohydr. Polym. 2018;195:170–179. doi: 10.1016/j.carbpol.2018.04.052 [DOI] [PubMed] [Google Scholar]
- 248.Parmar MB, Meenakshi Sundaram DN, Rb KC, et al. Combinational siRNA delivery using hyaluronic acid modified amphiphilic polyplexes against cell cycle and phosphatase proteins to inhibit growth and migration of triple-negative breast cancer cells. Acta Biomater. 2018;66:294–309. doi: 10.1016/j.actbio.2017.11.036 [DOI] [PubMed] [Google Scholar]
- 249.Huang G, Huang H. Application of hyaluronic acid as carriers in drug delivery. Drug Delivery. 2018;25(1):766–772. doi: 10.1080/10717544.2018.1450910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Luo Z, Dai Y, Gao H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharmaceutica Sinica B. 2019;9(6):1099–1112. doi: 10.1016/j.apsb.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhou H, Xu H, Li X, et al. Dual targeting hyaluronic acid - RGD mesoporous silica coated gold nanorods for chemo-photothermal cancer therapy. Mater Sci Eng C. 2017;81:261–270. doi: 10.1016/j.msec.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 252.Yu S, Wang S, Xie Z, et al. Hyaluronic acid coating on the surface of curcumin-loaded ZIF-8 nanoparticles for improved breast cancer therapy: an in vitro and in vivo study. Colloids Surf. B. 2021;203:111759. doi: 10.1016/j.colsurfb.2021.111759 [DOI] [PubMed] [Google Scholar]
- 253.Xu W, Wang J, Qian J, et al. NIR/pH dual-responsive polysaccharide-encapsulated gold nanorods for enhanced chemo-photothermal therapy of breast cancer. Mater Sci Eng C. 2019;103:109854. doi: 10.1016/j.msec.2019.109854 [DOI] [PubMed] [Google Scholar]
- 254.Wang Y, Ma S, Liu X, et al. Hyaluronic acid mediated Fe3O4 nanocubes reversing the EMT through targeted cancer stem cell. Colloids Surf. B. 2023;222:113071. doi: 10.1016/j.colsurfb.2022.113071 [DOI] [PubMed] [Google Scholar]
- 255.Sargazi A, Kamali N, Shiri F, Heidari Majd M. Hyaluronic acid/polyethylene glycol nanoparticles for controlled delivery of mitoxantrone. Artif. Cells Nanomed. Biotechnol. 2018;46(3):500–509. doi: 10.1080/21691401.2017.1324462 [DOI] [PubMed] [Google Scholar]
- 256.Pornpitchanarong C, Rojanarata T, Opanasopit P, Ngawhirunpat T, Patrojanasophon P. Catechol-modified chitosan/hyaluronic acid nanoparticles as a new avenue for local delivery of doxorubicin to oral cancer cells. Colloids Surf. B. 2020;196:111279. doi: 10.1016/j.colsurfb.2020.111279 [DOI] [PubMed] [Google Scholar]
- 257.Pandey A, Singh K, Patel S, Singh R, Patel K, Sawant K. Hyaluronic acid tethered pH-responsive alloy-drug nanoconjugates for multimodal therapy of glioblastoma: an intranasal route approach. Mater Sci Eng C. 2019;98:419–436. doi: 10.1016/j.msec.2018.12.139 [DOI] [PubMed] [Google Scholar]
- 258.Cao F, Yan M, Liu Y, Liu L, Ma G. Photothermally Controlled MHC Class I Restricted CD8+ T-Cell Responses Elicited by Hyaluronic Acid Decorated Gold Nanoparticles as a Vaccine for Cancer Immunotherapy. Adv. Healthcare Mater. 2018;7(10):1701439. doi: 10.1002/adhm.201701439 [DOI] [PubMed] [Google Scholar]
- 259.Catania G, Rodella G, Vanvarenberg K, Préat V, Malfanti A. Combination of hyaluronic acid conjugates with immunogenic cell death inducer and CpG for glioblastoma local chemo-immunotherapy elicits an immune response and induces long-term survival. Biomaterials. 2023;294:122006. doi: 10.1016/j.biomaterials.2023.122006 [DOI] [PubMed] [Google Scholar]
- 260.Yin T, Liu J, Zhao Z, et al. Redox Sensitive Hyaluronic Acid-Decorated Graphene Oxide for Photothermally Controlled Tumor-Cytoplasm-Selective Rapid Drug Delivery. Adv. Funct. Mater. 2017;27(14):1604620. doi: 10.1002/adfm.201604620 [DOI] [Google Scholar]
- 261.Liang X, Fang L, Li X, Zhang X, Wang F. Activatable near infrared dye conjugated hyaluronic acid based nanoparticles as a targeted theranostic agent for enhanced fluorescence/CT/photoacoustic imaging guided photothermal therapy. Biomaterials. 2017;132:72–84. doi: 10.1016/j.biomaterials.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 262.Dong S, Bi Y, Sun X, et al. Dual-Loaded Liposomes Tagged with Hyaluronic Acid Have Synergistic Effects in Triple-Negative Breast Cancer. Small. 2022;18(16):2107690. doi: 10.1002/smll.202107690 [DOI] [PubMed] [Google Scholar]
- 263.Peng T, Huang Y, Feng X, et al. TPGS/hyaluronic acid dual-functionalized PLGA nanoparticles delivered through dissolving microneedles for markedly improved chemo-photothermal combined therapy of superficial tumor. Acta Pharmaceutica Sinica B. 2021;11(10):3297–3309. doi: 10.1016/j.apsb.2020.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Liu R, Xiao W, Hu C, Xie R, Gao H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J Control Release. 2018;278:127–139. doi: 10.1016/j.jconrel.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 265.Chi Y, Yin X, Sun K, et al. Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J Control Release. 2017;261:113–125. doi: 10.1016/j.jconrel.2017.06.027 [DOI] [PubMed] [Google Scholar]
- 266.Wang Y, Qian J, Yang M, et al. Doxorubicin/cisplatin co-loaded hyaluronic acid/chitosan-based nanoparticles for in vitro synergistic combination chemotherapy of breast cancer. Carbohydr. Polym. 2019;225:115206. doi: 10.1016/j.carbpol.2019.115206 [DOI] [PubMed] [Google Scholar]
- 267.Senthilkumar K, Manivasagan P, Venkatesan J, Kim S-K. Brown seaweed fucoidan: biological activity and apoptosis, growth signaling mechanism in cancer. Int J Biol Macromol. 2013;60:366–374. doi: 10.1016/j.ijbiomac.2013.06.030 [DOI] [PubMed] [Google Scholar]
- 268.Cho ML, Lee B-Y, You SG. Relationship between Oversulfation and Conformation of Low and High Molecular Weight Fucoidans and Evaluation of Their in Vitro Anticancer Activity. Molecules. 2011;16(1):291–297. doi: 10.3390/molecules16010291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yang C, Chung D, Shin I-S, et al. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int J Biol Macromol. 2008;43(5):433–437. doi: 10.1016/j.ijbiomac.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 270.Etman SM, Elnaggar YSR, Abdallah OY. Fucoidan, a natural biopolymer in cancer combating: from edible algae to nanocarrier tailoring. Int J Biol Macromol. 2020;147:799–808. doi: 10.1016/j.ijbiomac.2019.11.191 [DOI] [PubMed] [Google Scholar]
- 271.Jafari M, Sriram V, Xu Z, Harris GM, Lee J-Y. Fucoidan-Doxorubicin Nanoparticles Targeting P-Selectin for Effective Breast Cancer Therapy. Carbohydr. Polym. 2020;249:116837. doi: 10.1016/j.carbpol.2020.116837 [DOI] [PubMed] [Google Scholar]
- 272.Guo R, Deng M, He X, et al. Fucoidan-functionalized activated platelet-hitchhiking micelles simultaneously track tumor cells and remodel the immunosuppressive microenvironment for efficient metastatic cancer treatment. Acta Pharmaceutica Sinica B. 2022;12(1):467–482. doi: 10.1016/j.apsb.2021.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Mustafa S, Pawar JS, Ghosh I. Fucoidan induces ROS-dependent epigenetic modulation in cervical cancer HeLa cell. Int J Biol Macromol. 2021;181:180–192. doi: 10.1016/j.ijbiomac.2021.03.110 [DOI] [PubMed] [Google Scholar]
- 274.Hsu W-J, Lin M-H, Kuo T-C, et al. Fucoidan from Laminaria japonica exerts antitumor effects on angiogenesis and micrometastasis in triple-negative breast cancer cells. Int J Biol Macromol. 2020;149:600–608. doi: 10.1016/j.ijbiomac.2020.01.256 [DOI] [PubMed] [Google Scholar]
- 275.Chen H, Cong Q, Du Z, et al. Sulfated fucoidan FP08S2 inhibits lung cancer cell growth in vivo by disrupting angiogenesis via targeting VEGFR2/VEGF and blocking VEGFR2/Erk/VEGF signaling. Cancer Lett. 2016;382(1):44–52. doi: 10.1016/j.canlet.2016.08.020 [DOI] [PubMed] [Google Scholar]
- 276.Cho MH, Li Y, Lo P-C, Lee H, Choi Y. Fucoidan-Based Theranostic Nanogel for Enhancing Imaging and Photodynamic Therapy of Cancer. Nano-Micro Lett. 2020;12(1):47. doi: 10.1007/s40820-020-0384-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wu L, Sun J, Su X, Yu Q, Yu Q, Zhang P. A review about the development of fucoidan in antitumor activity: progress and challenges. Carbohydr. Polym. 2016;154:96–111. doi: 10.1016/j.carbpol.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 278.Bittkau KS, Dörschmann P, Blümel M, et al. Comparison of the Effects of Fucoidans on the Cell Viability of Tumor and Non-Tumor Cell Lines. Marine drugs. 2019;17(8):441. doi: 10.3390/md17080441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zhang H, Chen J, Li J, et al. Extraction and characterization of RG-I enriched pectic polysaccharides from mandarin citrus peel. Food Hydrocoll. 2018;79:579–586. doi: 10.1016/j.foodhyd.2017.12.002 [DOI] [Google Scholar]
- 280.Chandel V, Biswas D, Roy S, Vaidya D, Verma A, Gupta A. Current Advancements in Pectin: extraction, Properties and Multifunctional Applications. Foods. 2022;11(17):2683. doi: 10.3390/foods11172683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kedir WM, Deresa EM, Diriba TF. Pharmaceutical and drug delivery applications of pectin and its modified nanocomposites. Heliyon. 2022;8(9):e10654. doi: 10.1016/j.heliyon.2022.e10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Guo Q, Su J, Shu X, et al. Production and characterization of pea protein isolate-pectin complexes for delivery of curcumin: effect of esterified degree of pectin. Food Hydrocoll. 2020;105:105777. doi: 10.1016/j.foodhyd.2020.105777 [DOI] [Google Scholar]
- 283.Guo Q, Su J, Shu X, et al. Fabrication, structural characterization and functional attributes of polysaccharide-surfactant-protein ternary complexes for delivery of curcumin. Food Chem. 2021;337:128019. doi: 10.1016/j.foodchem.2020.128019 [DOI] [PubMed] [Google Scholar]
- 284.Zhang W, Xu P, Zhang H. Pectin in cancer therapy: a review. Trends Food Sci Technol. 2015;44(2):258–271. doi: 10.1016/j.tifs.2015.04.001 [DOI] [Google Scholar]
- 285.Shahzad A, Khan A, Afzal Z, Umer MF, Khan J, Khan GM. Formulation development and characterization of cefazolin nanoparticles-loaded cross-linked films of sodium alginate and pectin as wound dressings. Int J Biol Macromol. 2019;124:255–269. doi: 10.1016/j.ijbiomac.2018.11.090 [DOI] [PubMed] [Google Scholar]
- 286.Zhang L, Zheng J, Wang Y, et al. Fabrication of rhamnogalacturonan-I enriched pectin-based emulsion gels for protection and sustained release of curcumin. Food Hydrocoll. 2022;128:107592. doi: 10.1016/j.foodhyd.2022.107592 [DOI] [Google Scholar]
- 287.Xu D, Zhao X, Mahsa GC, et al. Controlled release of Lactiplantibacillus plantarum by colon-targeted adhesive pectin microspheres: effects of pectin methyl esterification degrees. Carbohydr. Polym. 2023;313:120874. doi: 10.1016/j.carbpol.2023.120874 [DOI] [PubMed] [Google Scholar]
- 288.Ribeiro IS, Pontes FJG, Carneiro MJM, et al. Poly(ε-caprolactone) grafted cashew gum nanoparticles as an epirubicin delivery system. Int J Biol Macromol. 2021;179:314–323. doi: 10.1016/j.ijbiomac.2021.03.011 [DOI] [PubMed] [Google Scholar]
- 289.Cai R, Pan S, Li R, Xu X, Pan S, Liu F. Curcumin loading and colon release of pectin gel beads: effect of different de-esterification method. Food Chem. 2022;389:133130. doi: 10.1016/j.foodchem.2022.133130 [DOI] [PubMed] [Google Scholar]
- 290.Varshosaz J, Sadri F, Rostami M, Mirian M, Taymouri S. Synthesis of pectin-deoxycholic acid conjugate for targeted delivery of anticancer drugs in hepatocellular carcinoma. Int J Biol Macromol. 2019;139:665–677. doi: 10.1016/j.ijbiomac.2019.07.225 [DOI] [PubMed] [Google Scholar]
- 291.Cheewatanakornkool K, Niratisai S, Manchun S, Dass CR, Sriamornsak P. Thiolated pectin–doxorubicin conjugates: synthesis, characterization and anticancer activity studies. Carbohydr. Polym. 2017;174:493–506. doi: 10.1016/j.carbpol.2017.06.115 [DOI] [PubMed] [Google Scholar]
- 292.Ogutu FO, Mu T-H. Ultrasonic degradation of sweet potato pectin and its antioxidant activity. Ultrason Sonochem. 2017;38:726–734. doi: 10.1016/j.ultsonch.2016.08.014 [DOI] [PubMed] [Google Scholar]
- 293.Zhou Y, Zhao Y, Wang L, Xu L, Zhai M, Wei S. Radiation synthesis and characterization of nanosilver/gelatin/carboxymethyl chitosan hydrogel. Radiat Phys Chem. 2012;81(5):553–560. doi: 10.1016/j.radphyschem.2012.01.014 [DOI] [Google Scholar]
- 294.Ardjoum N, Shankar S, Chibani N, Salmieri S, Lacroix M. In situ synthesis of silver nanoparticles in pectin matrix using gamma irradiation for the preparation of antibacterial pectin/silver nanoparticles composite films. Food Hydrocoll. 2021;121:107000. doi: 10.1016/j.foodhyd.2021.107000 [DOI] [Google Scholar]
- 295.Maxwell EG, Colquhoun IJ, Chau HK, et al. Modified sugar beet pectin induces apoptosis of colon cancer cells via an interaction with the neutral sugar side-chains. Carbohydr. Polym. 2016;136:923–929. doi: 10.1016/j.carbpol.2015.09.063 [DOI] [PubMed] [Google Scholar]
- 296.Emran TB, Islam F, Mitra S, et al. Pectin: a Bioactive Food Polysaccharide with Cancer Preventive Potential. Molecules. 2022;27(21):7405. doi: 10.3390/molecules27217405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Muhidinov ZK, Ikromi KI, Jonmurodov AS, et al. Structural characterization of pectin obtained by different purification methods. Int J Biol Macromol. 2021;183:2227–2237. doi: 10.1016/j.ijbiomac.2021.05.094 [DOI] [PubMed] [Google Scholar]
- 298.Ghanbarzadeh M, Golmoradizadeh A, Homaei A. Carrageenans and carrageenases: versatile polysaccharides and promising marine enzymes. Phytochem Rev. 2018;17(3):535–571. doi: 10.1007/s11101-018-9548-2 [DOI] [Google Scholar]
- 299.Khotimchenko M, Tiasto V, Kalitnik A, et al. Antitumor potential of carrageenans from marine red algae. Carbohydr. Polym. 2020;246:116568. doi: 10.1016/j.carbpol.2020.116568 [DOI] [PubMed] [Google Scholar]
- 300.Sathuvan M, Thangam R, Gajendiran M, et al. κ-Carrageenan: an effective drug carrier to deliver curcumin in cancer cells and to induce apoptosis. Carbohydr. Polym. 2017;160:184–193. doi: 10.1016/j.carbpol.2016.12.049 [DOI] [PubMed] [Google Scholar]
- 301.Jafari H, Atlasi Z, Mahdavinia GR, Hadifar S, Sabzi M. Magnetic κ-carrageenan/chitosan/montmorillonite nanocomposite hydrogels with controlled sunitinib release. Mater Sci Eng C. 2021;124:112042. doi: 10.1016/j.msec.2021.112042 [DOI] [PubMed] [Google Scholar]
- 302.Liu F, Duan G, Yang H. Recent advances in exploiting carrageenans as a versatile functional material for promising biomedical applications. Int J Biol Macromol. 2023;235:123787. doi: 10.1016/j.ijbiomac.2023.123787 [DOI] [PubMed] [Google Scholar]
- 303.Liu J, Zhan X, Wan J, Wang Y, Wang C. Review for carrageenan-based pharmaceutical biomaterials: favourable physical features versus adverse biological effects. Carbohydr. Polym. 2015;121:27–36. doi: 10.1016/j.carbpol.2014.11.063 [DOI] [PubMed] [Google Scholar]
- 304.Karagoz P, Khiawjan S, Marques MPC, Santzouk S, Bugg TDH, Lye GJ. Pharmaceutical applications of lignin-derived chemicals and lignin-based materials: linking lignin source and processing with clinical indication. Biomass Convers. Biorefin. 2023. doi: 10.1007/s13399-023-03745-5 [DOI] [Google Scholar]
- 305.Sathasivam T, Kai J, Sugiarto S, et al. Nano-Strategies for Lignin Biomaterials toward Cancer Therapy. Adv. Healthcare Mater. 2023;2300024. [DOI] [PubMed] [Google Scholar]
- 306.Zhou Y, Han Y, Li G, Yang S, Chu F. Lignin-Based Hollow Nanoparticles for Controlled Drug Delivery: grafting Preparation Using β-Cyclodextrin/Enzymatic-Hydrolysis Lignin. Nanomaterials. 2019;9(7):997. doi: 10.3390/nano9070997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Jiang Z, Duan J, Guo X, Ma Y, Wang C, Shi B. Synthesis of Au/lignin–tannin particles and their anticancer application. Green Chem. 2021;23(18):6945–6952. doi: 10.1039/D1GC01119G [DOI] [Google Scholar]
- 308.Yang Z, Zhou Z, Li Y, et al. Assessing the Potential of New Lignin-Based pH-Responsive Nanoparticles as Drug Carriers for Cancer Treatment. ACS Sustainable Chem. Eng. 2022;10(32):10590–10603. doi: 10.1021/acssuschemeng.2c02209 [DOI] [Google Scholar]
- 309.Figueiredo P, Lepland A, Scodeller P, et al. Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy. Acta Biomater. 2021;133:231–243. doi: 10.1016/j.actbio.2020.09.038 [DOI] [PubMed] [Google Scholar]
- 310.Lee JH, Im JS, Jin X, Kim TM, Choi JW. In Vitro and In Vivo Evaluation of Drug-Encapsulated Lignin Nanoparticles for Release Control. ACS Sustainable Chem. Eng. 2022;10(18):5792–5802. doi: 10.1021/acssuschemeng.1c08529 [DOI] [Google Scholar]
- 311.Zhang H, Wu X, Quan L, Ao Q. Characteristics of Marine Biomaterials and Their Applications in Biomedicine. Mar Drugs. 2022;20(6):372. doi: 10.3390/md20060372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Huang C, Xie T, Liu Y, et al. A Sodium Alginate-Based Multifunctional Nanoplatform for Synergistic Chemo-Immunotherapy of Hepatocellular Carcinoma. Adv. Mater. 2015;35 (33): 2301352. doi: 10.1002/adma.202301352 [DOI] [PubMed] [Google Scholar]
- 313.Wang M, Chen L, Zhang Z. Potential applications of alginate oligosaccharides for biomedicine – a mini review. Carbohydr. Polym. 2021;271:118408. doi: 10.1016/j.carbpol.2021.118408 [DOI] [PubMed] [Google Scholar]
- 314.Mallakpour S, Azadi E, Hussain CM. Chitosan, alginate, hyaluronic acid, gums, and β-glucan as potent adjuvants and vaccine delivery systems for viral threats including SARS-CoV-2: a review. Int J Biol Macromol. 2021;182:1931–1940. doi: 10.1016/j.ijbiomac.2021.05.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Soleimani K, Derakhshankhah H, Jaymand M, Samadian H. Stimuli-responsive natural gums-based drug delivery systems for cancer treatment. Carbohydr. Polym. 2021;254:117422. doi: 10.1016/j.carbpol.2020.117422 [DOI] [PubMed] [Google Scholar]
- 316.Sarika PR, Nirmala RJ. Curcumin loaded gum arabic aldehyde-gelatin nanogels for breast cancer therapy. Mater Sci Eng C. 2016;65:331–337. doi: 10.1016/j.msec.2016.04.044 [DOI] [PubMed] [Google Scholar]
- 317.Sarika PR, James NR, Kumar PRA, Raj DK, Kumary TV. Gum arabic-curcumin conjugate micelles with enhanced loading for curcumin delivery to hepatocarcinoma cells. Carbohydr. Polym. 2015;134:167–174. doi: 10.1016/j.carbpol.2015.07.068 [DOI] [PubMed] [Google Scholar]
- 318.El-Sayed NS, Hashem AH, Kamel S. Preparation and characterization of Gum Arabic Schiff’s bases based on 9-aminoacridine with in vitro evaluation of their antimicrobial and antitumor potentiality. Carbohydr. Polym. 2022;277:118823. doi: 10.1016/j.carbpol.2021.118823 [DOI] [PubMed] [Google Scholar]
- 319.Jin J, Liu C, Tong H, et al. Encapsulation of EGCG by Zein-Gum Arabic Complex Nanoparticles and In Vitro Simulated Digestion of Complex Nanoparticles. Foods. 2022;11(14):2131. doi: 10.3390/foods11142131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Feng Z, Xu J, Ni C. Preparation of redox responsive modified xanthan gum nanoparticles and the drug controlled release. Int J Polym Mater Polym Biomater. 2021;70(14):994–1001. doi: 10.1080/00914037.2020.1767618 [DOI] [Google Scholar]
- 321.L-M M, R-J J, Liu R, et al. Dual-functional drug liposomes in treatment of resistant cancers. Adv. Drug Delivery Rev. 2017;115:46–56. doi: 10.1016/j.addr.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 322.Nel J, Elkhoury K, Velot É, et al. Functionalized liposomes for targeted breast cancer drug delivery. Bioact. Mater. 2023;24:401–437. doi: 10.1016/j.bioactmat.2022.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Shah VM, Nguyen DX, Al Fatease A, et al. Liposomal formulation of hypoxia activated prodrug for the treatment of ovarian cancer. J Control Release. 2018;291:169–183. doi: 10.1016/j.jconrel.2018.10.021 [DOI] [PubMed] [Google Scholar]
- 324.Li Y, Lu A, Long M, Cui L, Chen Z, Zhu L. Nitroimidazole derivative incorporated liposomes for hypoxia-triggered drug delivery and enhanced therapeutic efficacy in patient-derived tumor xenografts. Acta Biomater. 2019;83:334–348. doi: 10.1016/j.actbio.2018.10.029 [DOI] [PubMed] [Google Scholar]
- 325.Wang Y, Shi K, Zhang L, et al. Significantly enhanced tumor cellular and lysosomal hydroxychloroquine delivery by smart liposomes for optimal autophagy inhibition and improved antitumor efficiency with liposomal doxorubicin. Autophagy. 2016;12(6):949–962. doi: 10.1080/15548627.2016.1162930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wang M, Zhao T, Liu Y, et al. Ursolic acid liposomes with chitosan modification: promising antitumor drug delivery and efficacy. Mater Sci Eng C. 2017;71:1231–1240. doi: 10.1016/j.msec.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 327.Hanafy NAN, Sheashaa RF, Moussa EA, Mahfouz ME. Potential of curcumin and niacin-loaded targeted chitosan coated liposomes to activate autophagy in hepatocellular carcinoma cells: an in vitro evaluation in HePG2 cell line. Int J Biol Macromol. 2023;245:125572. doi: 10.1016/j.ijbiomac.2023.125572 [DOI] [PubMed] [Google Scholar]
- 328.Meng X, Fryganas C, Fogliano V, Hoppenbrouwers T. Double-coated nanoliposomes improve the bioavailability of flavanone hesperetin. Food Hydrocoll. 2024;151:109872. doi: 10.1016/j.foodhyd.2024.109872 [DOI] [Google Scholar]
- 329.Hassane Hamadou A, Zhang J, Chao C, Xu B. Stability of rutin using pectin-chitosan dual coating nanoliposomes. LWT. 2022;170:114084. doi: 10.1016/j.lwt.2022.114084 [DOI] [Google Scholar]
- 330.Zhang Y, Wang Y, Zhang H, et al. Replacing cholesterol with asiatic acid to prolong circulation and enhance anti-metastatic effects of non-PEGylated liposomes. J Control Release. 2024;366:585–595. doi: 10.1016/j.jconrel.2024.01.009 [DOI] [PubMed] [Google Scholar]
- 331.Filipczak N, Pan J, Yalamarty SSK, Torchilin VP. Recent advancements in liposome technology. Adv. Drug Delivery Rev. 2020;156:4–22. doi: 10.1016/j.addr.2020.06.022 [DOI] [PubMed] [Google Scholar]
- 332.Li H, Yin D, Liao J, et al. Regulation of protein Corona on liposomes using albumin-binding peptide for targeted tumor therapy. J Control Release. 2023;355:593–603. doi: 10.1016/j.jconrel.2023.02.004 [DOI] [PubMed] [Google Scholar]
- 333.Wang G, Wu B, Li Q, et al. Active Transportation of Liposome Enhances Tumor Accumulation, Penetration, and Therapeutic Efficacy. Small. 2020;16(44):2004172. doi: 10.1002/smll.202004172 [DOI] [PubMed] [Google Scholar]
- 334.Olsman M, Sereti V, Andreassen K, et al. Ultrasound-mediated delivery enhances therapeutic efficacy of MMP sensitive liposomes. J Control Release. 2020;325:121–134. doi: 10.1016/j.jconrel.2020.06.024 [DOI] [PubMed] [Google Scholar]
- 335.Chen Z-J, Yang S-C, Liu X-L, et al. Nanobowl-Supported Liposomes Improve Drug Loading and Delivery. Nano Lett. 2020;20(6):4177–4187. doi: 10.1021/acs.nanolett.0c00495 [DOI] [PubMed] [Google Scholar]
- 336.Jeon M, Kim G, Lee W, Baek S, Jung HN, Im H-J. Development of theranostic dual-layered Au-liposome for effective tumor targeting and photothermal therapy. J Nanobiotechnol. 2021;19(1):262. doi: 10.1186/s12951-021-01010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Hong C, Wang D, Liang J, et al. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics. 2019;9(15):4437–4449. doi: 10.7150/thno.34953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Y-J L, Chuang E-Y, Cheng Y-H, Anilkumar TS, Chen H-A, Chen J-P. Thermosensitive magnetic liposomes for alternating magnetic field-inducible drug delivery in dual targeted brain tumor chemotherapy. Chem Eng J. 2019;373:720–733. doi: 10.1016/j.cej.2019.05.055 [DOI] [Google Scholar]
- 339.Tzror-Azankot C, Betzer O, Sadan T, et al. Glucose-Functionalized Liposomes for Reducing False Positives in Cancer Diagnosis. ACS Nano. 2021;15(1):1301–1309. doi: 10.1021/acsnano.0c08530 [DOI] [PubMed] [Google Scholar]
- 340.Xia J, Zhang S, Zhang R, et al. Targeting therapy and tumor microenvironment remodeling of triple-negative breast cancer by ginsenoside Rg3 based liposomes. J Nanobiotechnol. 2022;20(1):414. doi: 10.1186/s12951-022-01623-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Choi TH, Yoo RJ, Park JY, et al. Development of finely tuned liposome nanoplatform for macrophage depletion. J Nanobiotechnol. 2024;22(1):83. doi: 10.1186/s12951-024-02325-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ding Y, Yang R, Yu W, et al. Chitosan oligosaccharide decorated liposomes combined with TH302 for photodynamic therapy in triple negative breast cancer. J Nanobiotechnol. 2021;19(1):147. doi: 10.1186/s12951-021-00891-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Gu Z, da Silva CG, Ma S, et al. Dual-Targeting Nanoliposome Improves Proinflammatory Immunomodulation of the Tumor Microenvironment. Adv. Healthcare Mater. 2023;12(31):2302046. doi: 10.1002/adhm.202302046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Zhang Z, Yang J, Min Q, et al. Holo-Lactoferrin Modified Liposome for Relieving Tumor Hypoxia and Enhancing Radiochemotherapy of Cancer. Small. 2019;15(6):1803703. doi: 10.1002/smll.201803703 [DOI] [PubMed] [Google Scholar]
- 345.Guan J, Guo H, Tang T, et al. iRGD-Liposomes Enhance Tumor Delivery and Therapeutic Efficacy of Antisense Oligonucleotide Drugs against Primary Prostate Cancer and Bone Metastasis. Adv. Funct. Mater. 2021;31(24):2100478. doi: 10.1002/adfm.202100478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Zhang J, Sun L, Jiang L. et al. Regulation of CTLs/Tregs via Highly Stable and Ultrasound-Responsive Cerasomal Nano-Modulators for Enhanced Colorectal Cancer Immunotherapy. Adv. Sci.;2024. 2400485. doi: 10.1002/advs.202400485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Hong F, Geng X, Min G, et al. Deep NIR-II optical imaging combined with minimally invasive interventional photothermal therapy for orthotopic bladder cancer. Chem Eng J. 2022;449:137846. doi: 10.1016/j.cej.2022.137846 [DOI] [Google Scholar]
- 348.Wang J, Liu D, Guan S, et al. Hyaluronic acid-modified liposomal honokiol nanocarrier: enhance anti-metastasis and antitumor efficacy against breast cancer. Carbohydr. Polym. 2020;235:115981. doi: 10.1016/j.carbpol.2020.115981 [DOI] [PubMed] [Google Scholar]
- 349.Lin Z, Tan X, Zhang Y, Li F, Luo P, Liu H. Molecular Targets and Related Biologic Activities of Fucoidan. Rev. 2020;18(8):376. doi: 10.3390/md18080376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Varum F, Freire AC, Bravo R, Basit AW. OPTICORE™, an innovative and accurate colonic targeting technology. Int J Pharm. 2020;583:119372. doi: 10.1016/j.ijpharm.2020.119372 [DOI] [PubMed] [Google Scholar]
- 351.Wang W, Meng Q, Li Q, et al. Chitosan Derivatives and Their Application in Biomedicine. International Journal of Molecular Sciences. 2020;21(2):487. doi: 10.3390/ijms21020487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Ahmad K, Zhang Y, Chen P, Yang X, Hou H. Chitosan interaction with stomach mucin layer to enhances gastric retention and mucoadhesive properties. Carbohydr. Polym. 2024;333:121926. doi: 10.1016/j.carbpol.2024.121926 [DOI] [PubMed] [Google Scholar]
- 353.Hu Q, Sun W, Wang C, Gu Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Delivery Rev. 2016;98:19–34. doi: 10.1016/j.addr.2015.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]