Abstract
Avian pathogenic Escherichia coli (APEC) causes colibacillosis, one of the main diseases leading to economic losses in industrial poultry farming due to high morbidity and mortality and its role in the condemnation of chicken carcasses. This study aimed to isolate and characterize APEC obtained from necropsied chickens on Brazilian poultry farms. Samples from birds already necropsied by routine inspection were collected from 100 batches of broiler chickens from six Brazilian states between August and November 2021. Three femurs were collected per batch, and characteristic E. coli colonies were isolated on MacConkey agar and characterized by qualitative PCR for minimal predictive APEC genes, antimicrobial susceptibility testing, and whole genome sequencing to identify species, serogroups, virulence genes, and resistance genes. Phenotypic resistance indices revealed significant resistance to several antibiotics from different antimicrobial classes. The isolates harbored virulence genes linked to APEC pathogenicity, including adhesion, iron acquisition, serum resistance, and toxins. Aminoglycoside resistance genes were detected in 79.36% of isolates, 74.6% had sulfonamide resistance genes, 63.49% showed β-lactam resistance genes, and 49.2% possessed at least one tetracycline resistance gene. This study found a 58% prevalence of avian pathogenic E. coli in Brazilian poultry, with strains showing notable antimicrobial resistance to commonly used antibiotics.
Keywords: avian pathogenic Escherichia coli, colibacillosis, Brazilian poultry, antimicrobial resistance, antimicrobial resistance genes
1. Introduction
In 2023, the global chicken meat production was 102.389 million tons [1]. Brazil is among the largest producers and exporters of chicken meat in the world. According to the Brazilian Association of Animal Protein (ABPA), in 2023, Brazil produced 14.833 million tons of chicken meat, making it the second largest producer in the world. Of this production, 65.35% was destined for the domestic market and 34.650% was destined for exports. Brazil exported 5.139 million tons of chicken meat; hence, it is considered the largest exporter of chicken meat in the world [1].
The bacteria Escherichia coli was first described in 1884 by the German microbiologist and pediatrician Theodor Escherich [2]. It has a cosmopolitan distribution, and its various serotypes are intestinal inhabitants found in large numbers in most animals, including humans [3,4,5]. E. coli strains can be classified according to their antigenic structure. Thus, there are approximately 180 O, 80 K, and 60 H antigens. The O antigen determines the serogroup; the addition of H antigens and sometimes K antigens determines the serotype [5,6,7].
Colibacillosis is a disease of local or systemic manifestation, usually associated with avian pathogenic Escherichia coli (APEC), although not every case of colibacillosis is necessarily caused by APEC [8]. Colibacillosis is one of the most important diseases in poultry production because it leads to significant economic losses due to carcass condemnation and other conditions, such as colisepticemia, peritonitis, pneumonia, pleuropneumonia, airsacculitis, pericarditis, cellulitis, coligranuloma, panophthalmos, omphalitis, oophoritis, osteomyelitis, salpingitis, swollen head syndrome (SCI), and synovitis [5,9,10].
Primary APEC infections are related to management failures, such as high ammonia concentrations, high population density, temperature fluctuations, and other environmental changes that affect birds. When secondary, APEC infections can be associated with respiratory viruses such as Newcastle disease, Infectious Bronchitis Virus, and avian Metapneumovirus or with bacteria such as Mycoplasma gallisepticum and Mycoplasma synoviae [5,8,9].
Different virulence and pathogenicity factors are used by APEC strains to cause colibacillosis in birds. APEC strains utilize different virulence and pathogenicity factors to establish an infection and cause disease in birds. The main virulence factors include proteins that facilitate adhesion and invasion, elements involved in toxin production, secretion systems, and mechanisms for antibiotic resistance [5,11]. A set of five genes have been identified and considered the minimal virulence predictors for an isolate to cause colibacillosis, distinguishing APEC from avian fecal Escherichia coli (AFEC). These genes are hlyF (putative avian hemolysin), iutA (aerobactin siderophore receptor gene), iss (episomal enhanced serum survival gene), iroN (salmochelin siderophore receptor gene), and ompT (outer membrane protease gene) [3,10,12].
Several antimicrobial agents from different classes are used for the treatment of colibacillosis, including β-lactams (penicillins, cephalosporins), aminoglycosides, lincosamides, tetracyclines, sulfonamides, quinolones, and fluoroquinolones [5,13,14]. Currently, many of the antimicrobials used in poultry production are also utilized in human medicine, a fact that raises concerns about the potential transfer of antimicrobial resistance genes between animals and humans [15].
Antimicrobial agents can be natural, semisynthetic, or synthetic substances, which act by inhibiting or killing microorganisms and are used in the treatment of different types of infections caused by bacteria, viruses, fungi, and parasites [16]. Furthermore, antibiotics have been widely used as growth promoters and as metaphylactic agents in animal production. Such practices, however, increase selective pressure and may favor the development of resistance to these substances [14,17,18,19].
Antimicrobial resistance can be naturally developed or acquired [20,21]. Resistance can arise through mutation processes, in which genes, normally present in the bacterial genome, mutate to a form that renders the antibiotic ineffective. Genes encoding antimicrobial resistance can be transferred between bacteria through classical mechanisms of horizontal transfer, such as conjugation, transformation, and transduction, as well as other pathways, like membrane vesicles [14,20,21,22,23,24].
The emergence of resistant and multiresistant bacteria to antibiotics is a growing and worldwide health problem, encouraging the search for alternatives that replace the use of antibiotics. Disinfectants are a good alternative for controlling the growth of microorganisms, and they are used in the food industry, in the agricultural industry, in health establishments, in homes, and in pharmaceutical products [25,26]. Different disinfectants such as formaldehyde, quaternary ammonium compounds (QACs), hydrogen peroxide, and sodium hypochlorite are commonly used on poultry farms [27,28].
Given the importance and role of Brazilian poultry farming, the economic impacts caused by APEC infection, and the challenges of antimicrobial resistance, the characterization of APEC strains is important to understand the pathogenesis of colibacillosis and to develop effective prevention and control strategies. Therefore, this study aims to determine the prevalence of Escherichia coli in broiler chickens from six Brazilian states and characterize the isolates obtained from the femurs of necropsied birds.
2. Materials and Methods
2.1. Sample Collection
Femur samples were collected between August and November 2021 from chicken carcasses (Gallus gallus domesticus) necropsied in the field for sanitary inspection and provided for the present study. The necropsied birds were between 13 and 32 days old. A total of 100 batches were evaluated in 6 different Brazilian states. Samples came from the states of Paraná (n = 30), Santa Catarina (n = 15), Rio Grande do Sul (n = 15), São Paulo (n = 10), Minas Gerais (n = 10), and Ceará (n = 20), which represent approximately 80% of the chicken meat production in Brazil [1].
Anamnesis and clinical evaluation of the batches were performed, and batches were selected based on a history of respiratory problems, as well as clinical manifestations associated with the respiratory tract, such as sneezing, rattling, and nasal discharge.
For each batch, three femurs were collected for E. coli research. The femurs were chosen for the isolation of E. coli because they were collected intact, which reduced the chances of bone marrow contamination. Additionally, there was a high probability of being highly pathogenic, as they were circulating in the bone marrow. The samples were received and processed at the Laboratory of Applied Virology (LVA) of the Federal University of Santa Catarina (UFSC).
All biological samples assessed in this study were donated by farms subject to regular inspection routines, eliminating the need for approval from an ethics board as they were residual samples collected in routine of health surveillance services—Consultation with the Ethics Committee on the Use of Animals (no 4434190521/Federal University of Santa Catarina).
2.2. Escherichia coli Isolation
For E. coli isolation, femurs were processed aseptically, and the bone marrow was collected with a swab, which was suspended in saline buffer. The swabs were inoculated in MacConkey agar and incubated at 37 °C for 24 h. Typical E. coli colonies (pink, precipitated colonies) were isolated. Isolates were preserved in a repository and biobank in Luria–Bertani (LB) broth (KASVI, Marbella, Spain) with glycerol (5:1), and maintained at −80 °C for subsequent genomic DNA extraction.
2.3. APEC Molecular Confirmation
The femur isolates were subjected to qualitative polymerase chain reaction (PCR) to identify APEC strains. Genomic DNA extraction was performed using an adapted phenol–chloroform method [29].
Cryopreserved colonies were grown in Luria–Bertani (LB) broth for 6 h at 37 °C, then cultured on MacConkey agar, and incubated for 24 h at 37 °C. A colony was selected and inoculated again in 25 mL of LB broth for 18 h at 37 °C. After incubation, the medium was centrifuged (700× g for 10 min), the supernatant was discarded, and the pellet was washed twice with PBS 1× pH 7.2. The sample was centrifuged again, and the pellet was separated into a microtube. For sample lysis, the pellet was suspended in 200 μL of lysis buffer (10 mM Tris HCl pH 7.4, 10 mM NaCl, 25 mM ethylenediaminetetraacetic acid (EDTA) pH 8, 1% sodium dodecyl sulfate (SDS)), containing 26.2 μL of Proteinase K, and it was incubated in a thermoblock at 56 °C for 30 min. For DNA extraction, an equal volume of equilibrated phenol was added, homogenized by inversion, and centrifuged for 10 min at 14,000 ×g at room temperature. The aqueous phase was transferred to a new microtube, and the same volume of a phenol–chloroform solution (1:1) was added, homogenized by inversion, and centrifuged for 10 min at 14,000× g at 4 °C. The aqueous phase was transferred to a new microtube, and an equal volume of chloroform was added, homogenized by inversion, and centrifuged for 10 min at 14,000× g at 4 °C. The aqueous phase was transferred to a new microtube, and to each tube, 2.5 times the total volume of ice-cold 100% ethanol was added. The microtubes were kept at −20 °C for 1 h. Tubes were centrifuged for 30 min at 14,000× g at 4 °C. The supernatant was discarded, and the pellet was washed twice with 500 μL of ice-cold 70% ethanol and centrifuged for 10 min at 14,000× g at 4 °C. The pellet was dried in a thermoblock at 37 °C. Finally, the pellet was suspended in 50 μL of DNase- and RNase-free water (QIAGEN, Inc., Valencia, Spain, EUA). DNA was quantified by optical density using NanoVue™ spectrophotometry and stored at −20 °C.
For the qualitative PCR reactions, the genes (iroN, ompT, hlyF, iss, and iutA) were used as minimum virulence predictors of APEC (Table 1). The reagents for amplifying the five gene targets were used at the following concentrations: 2 mM magnesium chloride, 0.25 mM deoxyribonucleotide phosphates (dNTPs), 0.3 μM of each primer, 1 U of GoTaq® DNA Polymerase (Promega, Madison, WI, USA), 1× Green GoTaq® Reaction Buffer (Promega, Madison, WI, USA), 3 μL of sample, and sterile ultrapure water to complete 25 μL. The reactions were performed in a thermocycler (TECHNE Flexigene, Burlington, VT, USA), using the following cycling parameters: 94 °C for 2 min; 35 cycles of 94 °C for 30 s, 63 °C for 30 s, 68 °C for 3 min; and a final cycle of 72 °C for 10 min [12].
Table 1.
Target gene, sequence of primers, amplicon size, and reference.
| Target Gene | Primer Sequence | Amplicon Size | Reference |
|---|---|---|---|
| iroN | 5′-AAGTCAAAGCAGGGGTTGCCCG-3′ 5′-GATCGCCGACATTAAGACGCAG-3′ |
667 bp | [30] |
| ompT | 5′-TCATCCCGGAAGCCTCCCTCACTACTAT-3′ 5′-TAGCGTTTGCTGCACTGGCTTCTGATAC-3′ |
496 bp | [12] |
| hlyF | 5′-GGCCACAGTCGTTTAGGGTGCTTACC-3′ 5′-GGCGGTTTAGGCATTCCGATACTCAG-3′ |
450 bp | [12] |
| iss | 5′-CAGCAACCCGAACCACTTGATG-3′ 5′-AGCATTGCCAGAGCGGCAGAA-3′ |
323 bp | [30] |
| iutA | 5′-GGCTGGACATCATGGGAACTGG-3′ 5′-CGTCGGGAACGGGTAGAATCG-3′ |
302 bp | [12] |
The samples were subjected to horizontal agarose gel electrophoresis at 1%, using GelRed as a DNA intercalating agent. The sizes of the amplicons were determined by comparison with a low-molecular-weight (LMW) marker.
2.4. Antimicrobial Sensitivity Testing
The disk diffusion method was used to perform the antibiotic sensitivity test, following the Kirby–Bauer (KB) methodology [31]. Eleven antibiotics (Laborclin) were tested: Nalidixic Acid (30 μg), Ampicillin (10 μg), Azithromycin (15 μg), Ceftiofur (30 μg), Ceftriaxone (30 μg), Enrofloxacin (5 μg), Gentamicin (10 μg), Lincomycin/Spectinomycin (109 µg), Nitrofurantoin (300 µg), Norfloxacin (10 µg), and Sulfamethoxazole/Trimethoprim (25 µg). Isolated strains were inoculated in tubes containing LB broth and incubated at 37 °C for 18 h. After incubation, diluted cultures were swabbed onto plates containing Mueller–Hinton agar (HIMEDIA, Mumbai, India). Antimicrobial disks were added, and the plates were incubated for 18 h at 37 °C. The plates were read by measuring the diameter of the inhibition zones using a vernier. A resistance pattern of the inhibition zone was considered according to the VET01S Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 6th Edition, and M100 Performance Standards for Antimicrobial Susceptibility Testing, 33rd Edition, established by the Clinical and Laboratory Standards Institute (CLSI) [32,33].
2.5. Whole Genome Sequencing
For sequencing, genomic DNA libraries were prepared using an Illumina DNA Prep—Nextera kit (Illumina, Inc., San Diego, CA, USA) and quantified with a Collibri Library Quantification Kit (Invitrogen Inc., Carlsbad, CA, USA) following the manufacturer’s recommendations. A Nextseq system (Illumina) was employed to generate raw reads based on 300 cycles, in a paired-end sequencing configuration (2 × 150 bp reads).
Raw data obtained from the MiSeq platform (Illumina) were processed using the Phred quality score. Reads with a Q score below 20 were excluded from the analyses, and adapters or segments with poor quality were also discarded.
After quality control (QC), genome assembly was performed using the company’s proprietary pipeline, oneshotWGS v1.9. OneshotWGS v1.9 integrates a set of bioinformatics tools commonly used by the scientific community. One of these programs is the A5 assembly, which includes additional steps for adapter trimming, quality filtering, and error correction to generate scaffolds [34]. Chimeric segments were removed at the end of the assembly steps (best assembly). Assembly statistics (total scaffolds, GC content percentage, N50, L50, etc.) were determined using QUAST 5.2.0 software [35].
Sequencing and genome assembly data were deposited in the National Center for Biotechnology Information (NCBI) database under Bioproject accession number PRJNA917297.
Genome annotation was performed using g Prokka 1.14.6 software [36]. For this step, custom proprietary (Neoprospecta, Florianópolis, Brasil) databases were used, consisting of curated gene sequences obtained from public databases such as Pfam, GenBank, nt/nt, etc. A similar protocol was applied for virulence and resistance genes.
2.6. In Silico Analysis
2.6.1. Species Confirmation
In silico confirmation of the species was conducted, and the best assembly was used for the species identification process, implemented by the company’s pipeline module, neogSpecies. This module, written in Python 3.11.9, applied an Average Nucleotide Identity (ANI) analysis to estimate genome species (cutoff: 97%). ANI is the standard method for defining a prokaryotic species [37].
2.6.2. Serogroup Identification
Serogroup determination utilized all genome sequencing data from the sample, and the Sorotypefinder 2.0 program (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4508402/ (accessed on 20 May 2023)) was employed for serogroup prediction.
2.6.3. Virulence, Antimicrobial, and Disinfectant Resistance Gene Detection
The prediction of virulence genes was carried out using the virulencefinder 2.0 program (https://pubmed.ncbi.nlm.nih.gov/24574290/ (accessed on 20 May 2023)) with a minimum identity of 90%, based on the data obtained from whole genome sequencing.
The presence of antimicrobial resistance genes was assessed with complete genome sequencing data, using the Abricate 1.0.1 program (https://github.com/tseemann/abricate (accessed 24 May 2023)), with the Resfinder database version (accessed 24 May 2023), applying a minimum coverage and identity of 80% [38].
3. Results
3.1. Batch History
From the batches analyzed, 61% had a history of respiratory problems, septicemia, and/or mortality, with unknown etiology. During sample collection, 42% of the batches exhibited clinical signs of respiratory symptoms, such as rales, sneezing, nasal discharge and discharge, infraorbital sinus enlargement, and swollen head.
Among the batches from the Southern states (Paraná, Santa Catarina, and Rio Grande do Sul), 13.3% used antibiotics during the bird housing phase. The drugs used were ciprofloxacin, sulfachlorpyridazine+trimethoprim, and florfenicol. In the Southeast Region (Minas Gerais and São Paulo), only one batch (5%) showed clinical signs and was treated with ciprofloxacin on the day of collection.
3.2. Escherichia coli Isolation and APEC Confirmation
A total of 63 isolates characteristic of E. coli were obtained from the femurs, and these isolates were subjected to whole genome sequencing. Through sequencing, all 63 isolates (100%) were identified as Escherichia coli.
Using qualitative PCR, out of the 63 E. coli isolates, 58 (92%) exhibited between three and five of the genes considered minimum virulence predictors for APEC strains, thus confirming their classification as APEC. Among the 63 analyzed isolates, 40 (63.4%) exhibited all five genes, 14 (22.2%) had four genes, 4 (6.3%) had three genes, 4 (6.3%) had one to two genes, and in one batch, none of the five genes were detected (Table 2). Regarding APEC isolates, 96.5% carried the ompT and iss genes, 93.1% had hlyF, 94.8% contained the iutA gene, and 89.6% harbored iroN.
Table 2.
Place of origin of batches according to state and genes detected in each batch.
| Brazilian State | Batch Number | Genes Detected |
|---|---|---|
| Santa Catarina | 1 | ompT , iutA, iss, iroN, hlyF |
| 4 | ompT , iutA, iss, iroN, hlyF | |
| 5 | ompT , iss, iroN, hlyF | |
| 6 | ompT , iutA, iss, iroN, hlyF | |
| 7 | iutA , iss, hlyF | |
| Rio Grande do Sul | 18 | ompT , iutA, iss, iroN, hlyF |
| 19 | ompT , iutA, iss, iroN, hlyF | |
| 20 | ompT , iutA, iss, iroN, hlyF | |
| 23 | ompT , iutA, iss, hlyF | |
| 24 | ompT , iutA, iss, hlyF | |
| 26 | ompT , iutA, iss, iroN, hlyF | |
| 28 | ompT , iutA, iss, iroN, hlyF | |
| 29 | ompT , iroN, hlyF | |
| 30 | ompT , iutA, iss, iroN, hlyF | |
| Paraná | 31 | ompT , iutA, iss, iroN, hlyF |
| 33 | ompT , iutA, iss, iroN, hlyF | |
| 35 | ompT , iutA, iss, iroN, hlyF | |
| 38 | ompT , iutA, iss, iroN, hlyF | |
| 39 | ompT , iutA, iss, iroN, hlyF | |
| 40 | iutA , iss, iroN, hly | |
| 41 | ompT , iutA, iss, iroN, hlyF | |
| 43 | ompT , iutA, iss, hlyF | |
| 45 | ompT , iutA, iss, iroN, hlyF | |
| 46 | ompT , iutA, iroN, hlyF | |
| 48 | ompT , iutA, iss, hlyF | |
| 49 | ompT , iutA, iss, iroN, hlyF | |
| 51 | ompT , iutA, iss, iroN, hlyF | |
| 52 | ompT , iutA, iss, iroN, hlyF | |
| 54 | ompT , iutA, iss, iroN, hlyF | |
| 55 | ompT , iutA, iss, iroN, hlyF | |
| 56 | ompT , iutA, iss, iroN, hlyF | |
| 57 | ompT , iutA, iss, iroN, hlyF | |
| 58 | ompT , iutA, iss, iroN, hlyF | |
| 59 | ompT , iutA, iss, iroN, hlyF | |
| Minas Gerais | 61 | ompT , iutA, iss, iroN, hlyF |
| 65 | ompT , iutA, iss, iroN, hlyF | |
| 66 | ompT , iutA, iss, iroN, hlyF | |
| 67 | ompT , iutA, iss, iroN, hlyF | |
| 69 | ompT , iutA, iss, iroN | |
| 75 | ompT , iutA, iss, iroN, hlyF | |
| 76 | ompT , iutA, iss, iroN, hlyF | |
| 77 | hlyF | |
| 78 | hlyF | |
| 80 | ompT , iutA, iss, iroN, hlyF | |
| São Paulo | 62 | ompT , iutA, iss, iroN, hlyF |
| 63 | ompT , iutA, iss, iroN, hlyF | |
| 64 | ompT , iutA, iss, iroN, hlyF | |
| 68 | ompT , iutA, iss, iroN | |
| 70 | ompT , iutA, iss, iroN, hlyF | |
| 71 | ompT , iutA, iss, iroN, hlyF | |
| 72 | ompT , iutA, iss, iroN, hlyF | |
| 74 | ompT , iutA, iss, iroN | |
| 79 | ompT | |
| Ceará | 82 | ompT , hlyF |
| 83 | ompT , iutA, iss, iroN, hlyF | |
| 87 | ompT , iutA, iss | |
| 88 | ompT , iutA, iss, iroN, hlyF | |
| 89 | ompT , iutA, iss, hlyF | |
| 90 | ompT , iutA, iss, hlyF | |
| 93 | ompT , iutA, iss, hlyF | |
| 95 | - | |
| 98 | ompT , iss, iroN, hlyF | |
| 99 | ompT , iutA, iss, iroN, hlyF |
3.3. Phenotypic Antimicrobial Resistance
The obtained diameters were compared with the cutoff points established by the CLSI [32,33].
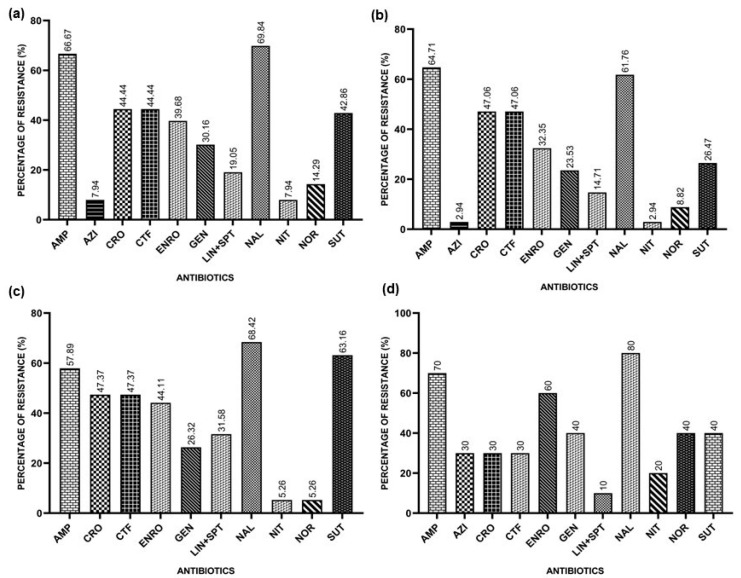
The overall resistance rates found were 66.67% for Ampicillin, 7.94% for Azithromycin, 44.44% for Ceftriaxone, 44.44% for Ceftiofur, 39.68% for Enrofloxacin, 30.16% for Gentamicin, 19.95% for Lincomycin/Spectinomycin, 69.84% for Nalidixic Acid, 7.94% for Nitrofurantoin, 14.29% for Norfloxacin, and 42.86% for Sulfamethoxazole/Trimethoprim.
In the Southern Region, the resistance rates found for E. coli isolates were 64.71% for Ampicillin, 2.94% for Azithromycin, 47.06% for Ceftriaxone, 47.06% for Ceftiofur, 32.35% for Enrofloxacin, 23.53% for Gentamicin, 14.71% for Lincomycin/Spectinomycin, 61.76% for Nalidixic Acid, 2.94% for Nitrofurantoin, 8.82% for Norfloxacin, and 26.47% for Sulfazotrim.
In the Southeast Region, the results found for E. coli isolates indicate a resistance to Ampicillin of 57.89%, 47.37% for Ceftriaxone, 47.37% for Ceftiofur, 44.11% for Enrofloxacin, 26.32% for Gentamicin, 31.58% for Lincomycin/Spectinomycin, 68.42% for Nalidixic Acid, 5.26% for Nitrofurantoin, 5.26% for Norfloxacin, and 63.16% for Sulfazotrim. All isolates were sensitive to Azithromycin.
In the Northeast Region, the resistance indices found that the E. coli isolates indicate a resistance to Ampicillin of 70%, 30% for Azithromycin, 30% for Ceftiofur, 30% for Ceftriaxone, 60% for Enrofloxacin, 40% for Gentamicin, 10% for Lincomycin/Spectinomycin, 80% for Nalidixic Acid, 20% for Nitrofurantoin, 40% for Norfloxacin, and 40% for Sulfazotrim.
Figure 1 represents the general resistance rates and the rates for each region.
Figure 1.
Resistances presented by APEC isolates against the tested antimicrobials: Ampicillin (AMP), Azithromycin (AZI), Ceftiofur (CFT), Ceftriaxone (CRO), Enrofloxacin (ENRO), Gentamicin (GEN), Lincomycin/Spectinomycin (LIN+SPT), Nalidixic Acid (NAL), Nitrofurantoin (NIT), Norfloxacin (NOR), and Sulfazotrim (SUT). (a) Resistances presented by APEC isolates against the tested antimicrobials in Brazil; (b) resistances presented by the APEC isolates from the Southern Region; (c) resistances presented by APEC isolates from the Southeast Region; (d) resistances presented by APEC isolates from the Northeast Region.
3.4. Serogroups
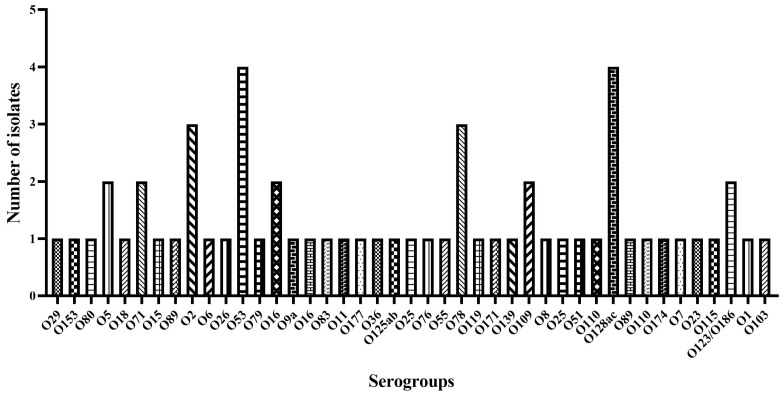
Whole genome sequencing was used to determine E. coli serogroups. It was possible to identify the serotype of a total of 60 isolates (92%), distributed among 40 serogroups (Figure 2). The predominant serogroups were O128 and O53, both with a frequency of 6.8%. Serogroups O78 and O16 occurred with a frequency of 5.1%, while O2, O25, O5, O110, O71, and O109 occurred with a frequency of 3.4%. The remaining serogroups appeared at a lower frequency (1.7%).
Figure 2.
E. coli serogroup distribution using the program Sorotypefinder 2.0.
3.5. Detection and Distribution of Genes Associated with Virulence
The sequenced isolates carried between 4 and 27 virulence genes, and the prevalence of these genes ranged from 1.58% to 100%. Of the sequenced isolates, 88% harbored at least one gene related to adhesion (eae, papA_F11, papA_F19, papA_F20, papC, hra, iha, lpfA, and tsh), while 93.65% harbored at least one gene related to iron acquisition systems (chuA, fyuA, ireA, irp2, iroN, iucC, iutA, sitA). Regarding serum resistance factors, 96.82% of the isolates harbored at least one virulence gene (iss, kpsE, kpsMII, kpsMIII_K98, kpsMII_K1, kpsMII_K5, neuC, ompT, traT). All isolates (100%) carried at least one gene encoding toxins (astA, cma, cvaC, hlyE, hlyF, usp, vat). Table 3 covers the virulence genes found and their prevalence.
Table 3.
Prevalence of virulence genes in Escherichia coli isolates.
| Gene, Operon, or Region | Description | Prevalence (%) |
|---|---|---|
| Adhesins | ||
| eae | Intimin | 1.58 |
| papA_F11 | Major pilin subunit F11 | 3.17 |
| papA_F19 | Major pilin subunit F19 | 1.58 |
| papA_F20 | Major pilin subunit F20 | 11.11 |
| papC | Pilus associated with pyelonephritis | 23.80 |
| hra | Heat-resistant agglutinin | 69.84 |
| iha | Adherence protein | 12.69 |
| lpfA | Long polar fimbriae | 60.31 |
| tsh | Temperature-sensitive hemagglutinin | 34.92 |
| Invasins | ||
| ibeA | Invasion of brain endothelium | 1.58 |
| Serum resistance factors | ||
| cvaC | Structural genes of colicin V operon (Microcin ColV) | 30.15 |
| kpsE | Capsule polysaccharide export inner-membrane protein | 11.11 |
| kpsMII | ABC-type polysaccharide/polyol phosphate export system permease; Group 3 capsule | 4.76 |
| kpsMIII_K98 | Polysialic acid transport protein; Group 2 capsule | 1.58 |
| kpsMII_K1 | Polysialic acid transport protein; Group 2 capsule | 1.58 |
| kpsMII_K5 | Polysialic acid transport protein; Group 2 capsule | 3.17 |
| neuC | Polysialic acid capsule biosynthesis protein | 3.17 |
| traT | Outer membrane protein complement resistance | 82.53 |
| Iron acquisition systems | ||
| chuA | Heme receptor gene (E. coli haem utilization) | 41.26 |
| fyuA | Siderophore receptor | 31.74 |
| ireA | Siderophore receptor | 20.63 |
| irp2 | Iron repressible protein (yersiniabactin synthesis) | 31.74 |
| iucC | Aerobactin synthetase | 53.96 |
| sitA | Iron transport protein | 61.90 |
| Toxins | ||
| astA | EAST-1 heat-stable toxin | 39.68 |
| cma | Structural gene for CoIM activity | 23.80 |
| hlyE | Avian E. coli haemolysin | 77.77 |
| usp | Uropathogenic-specific protein (bacteriocin) | 3.17 |
| vat | Vacuolating autotransporter toxin | 15.87 |
| Other virulence factors | ||
| air | Enteroaggregative immunoglobulin repeat protein | 12.69 |
| pic | Serin protease autotransporter | 17.46 |
| eilA | Salmonella HilA homolog | 12.69 |
| espA | Type III secretion system | 1.58 |
| espB | Secreted protein B | 1.58 |
| espF | Type III secretion system | 1.58 |
| espJ | Prophage-encoded type III secretion system effector | 1.58 |
| etpD | Type II secretion protein | 1.58 |
| etsC | Putative type I secretion outer membrane protein | 73.01 |
| capU | Hexosyltransferase homolog | 1.58 |
| cba | Colicin B | 3.17 |
| cea | Colicin E1 | 17.46 |
| celb | Endonuclease colicin E2 | 4.76 |
| cia | Colicin ia | 30.15 |
| cib | Colicin ib | 17.46 |
| cif | Type III secreted effector | 1.58 |
| gad | Glutamate decarboxylase | 100 |
| mchB | Microcin H47 part of colicin H | 1.58 |
| mchC | MchC protein | 1.58 |
| mchF | ABC transporter protein MchF | 23.80 |
| mcmA | Microcin M part of colicin H | 1.58 |
| nleA | Non-LEE encoded effector A | 1.58 |
| nleB | Non-LEE encoded effector B | 1.58 |
| tccP | Tir-cytoskeleton coupling protein | 1.58 |
| terC | Tellurium ion resistance protein | 100 |
| tir | Translocated intimin receptor protein | 1.58 |
| yfcV | Fimbrial protein | 6.34 |
The terC (Tellurium ion resistance protein) and gad (Glutamate decarboxylase) genes were detected in 100% of the isolates.
Genes encoding adhesins are related to processes such as adhesion, motility, biofilm formation, and survival in macrophages. Of the isolates, 69.84% harbored the hra gene, 60.31% harbored the lpfA gene, 23.8% harbored the pap gene, and 34.92% harbored the tsh gene.
The ability to resist serum is one of the factors related to APEC strains; 96.82% of the isolates harbored genes related to serum resistance, of which 82.53% harbored the traT gene.
Genes related to iron acquisition were detected in 59 isolates, with the most prevalent being chuA (41.26%), fyuA (31.74%), iucC (53.96%), irp2 (31.74%), and sitA (61.90).
The cvaC gene was detected in 30.15% of the isolates and the hlyE gene was detected in 77.77% of the samples.
3.6. Detection and Distribution of Antimicrobial and Disinfectant Resistance Genes
Out of the analyzed isolates, all of them showed at least one antimicrobial resistance gene (ARG), and all of them (100%) contained the formA gene, a formaldehyde resistant gene. The resistance genes detected in 20% or more of the isolates were sul2 (60.31%), sitABCD (57.14%), sul1 (52.38%), ant(3″)-Ia (50.79%), qacE (50.79%), aac(3)-VIa (36.5%), aph(6)-Id (31.74%), tet(A) (32.74%), tet(B) (20.63%), aadA2 (20.63%), and aph( 3″)-Ib (20.63%).
In this study, 78.1% of the APEC isolates harbored at least one of the aminoglycoside resistance genes (aac(3)-IId, aac(3)-IVa, aac(3)-VIa, aadA12, aadA2, ant(2″)-Ia, ant(3″)-Ia, aph(3′)-Ia, aph(3″)-Ib, aph(4)-Ia, and aph(6)-Idii). At least one of the predicted sulfonamide resistance genes (sul1, sul2, and sul3) was found in 73.4% of the isolates.
Similarly, at least one predicted β-lactam resistance gene was found in 64.06% of the isolates, including blaCMY-2, blaCTX-M-1, blaCTX-M-15, blaCTX-M-164, blaCTX-M-2, blaCTX-M-55, blaCTX-M-8, blaSHV-12, blaTEM-106, blaTEM-141, blaTEM-1A, and blaTEM-1B.
For tetracycline resistance genes, tet(A), tet(B), and tet(D), 48% of the isolates harbored at least one tetracycline resistance gene.
Table 4 covers the antimicrobial classes, the antimicrobial resistance genes found, and their prevalence.
Table 4.
Prevalence of antimicrobial resistance genes (ARGs) in Escherichia coli isolates.
| Antimicrobial Class | Antimicrobial Resistance Gene | Prevalence (%) |
|---|---|---|
| Aminoglycosides | aac(3)-IId | 4.76 |
| aac(3)-IVa | 3.17 | |
| aac(3)-VIa | 36.5 | |
| aadA12 | 1.58 | |
| aadA2 | 20.63 | |
| ant(2″)-Ia | 7.93 | |
| ant(3″)-Ia | 50.79 | |
| aph(3 ′ )-Ia | 20.63 | |
| aph(3″)-Ib | 31.74 | |
| aph(4)-Ia | 3.17 | |
| aph(6)-Id | 31.74 | |
| Beta-lactams | blaCMY-2 | 9.52 |
| blaCTX-M-1 | 3.17 | |
| blaCTX-M-15 | 1.58 | |
| blaCTX-M-164 | 1.58 | |
| blaCTX-M-2 | 17.46 | |
| blaCTX-M-55 | 9.52 | |
| blaCTX-M-8 | 12.69 | |
| blaSHV-12 | 1.58 | |
| blaTEM-106 | 1.58 | |
| blaTEM-141 | 6.34 | |
| blaTEM-1A | 7.93 | |
| blaTEM-1B | 14.28 | |
| Trimethoprim | dfrA1 | 7.93 |
| dfrA12 | 4.76 | |
| dfrA14 | 4.76 | |
| dfrA15 | 4.76 | |
| dfrA7 | 3.17 | |
| dfrA8 | 3.17 | |
| Phenicoles | catA1 | 3.17 |
| cmlA1 | 9.52 | |
| floR | 19.04 | |
| Lincosamides | lnu(A) | 4.76 |
| lnu(F) | 7.93 | |
| Colistin | mcr-1.5 | 1.58 |
| mcr-9 | 1.58 | |
| Macrolides | mph(A) | 1.58 |
| mph(B) | 1.58 | |
| Quinolones | qnrA1 | 1.58 |
| qnrB19 | 11.11 | |
| qnrS1 | 6.34 | |
| Sulfonamides | sul1 | 52.38 |
| sul2 | 60.31 | |
| sul3 | 7.93 | |
| Tetracyclines | tet(A) | 31.74 |
| tet(B) | 20.63 | |
| tet(D) | 1.58 | |
| Others | fosA | 6.25 |
| fosA3 | 1.5625 | |
| qacE | 50.79 | |
| sitABCD | 57.14 | |
| formA (Genbank Acc, No, X73835) | 100 |
Among the resistance genes detected, those present in more than 20% of the samples from the Southern Region were aac(3)-VIa (35.29%), aadA2 (26.47%), ant(3″)-Ia (50%), aph(6)-Id (29.41%), blaCTX-M-2 (20.58%), sul1 (47.05%), sul2 (47.05%), and tet(A) (29.41%).
In the Southeast Region, the most prevalent genes among the batches were aac(3)-Vla (36.84%), ant(3″)-Ia (52.63%), aph(3″)-Ib (21.05%), aph(6)-Id (31.57%), sul1 (63.15%), sul2 (73.68%), tet(A) (31.57%), and tet(B) (26.31%).
In the Northeast Region, the resistance genes detected in more than 20% of the samples were aac(3)-Via (40%), aadA2 (20%), ant(3″)-Ia (50%), aph(3″)-Ib (40%), aph(3′)-Ia (20%), aph(6)-Id (40%), blaTEM-1 (20%), blaTEM-1B (20%), dfrA12 (20%), floR (40%), qnrB19_1 (20%), sul1 (50%), sul2 (80%), tet(A) (40%), and tet(B) (30%).
Regarding resistance genes associated with disinfectants, 100% of the strains harbored a formaldehyde resistance gene (formA), 50.79% of the APEC strains harbored the qacE gene, and 57.14% of the isolates harbored the sitABCD gene.
4. Discussion
E. coli was isolated from the femurs of chickens from different regions of Brazil. Qualitative analysis by PCR showed that 92% of the isolates tested were characterized as APECs based on the presence of at least three of the five minimal predictors of virulence [12]. In comparison, a study in Poland demonstrated that 43% (124/290) of all tested strains were characterized as APEC; in northern Egypt, 51.85% (28/54) of the batches were positive for APEC, while in Nepal, 90% (45/50) of colibacillosis isolates were identified as APECs [39,40,41].
The percentage of genes in the APEC isolates was 96.5% for ompT, 96.5% for iss, 93.1% for hlyF, 94.8% for iutA, and 89.6% for iroN. Other studies conducted in Brazil, involving chickens and turkeys, reported similar values. In these studies, the prevalence of APEC in the isolates was 58.6% and 84.34%, with a gene frequency of 98.8% for iroN, 96.3% for iss, 81.5% for iutA, and 100% for hlyF and ompT [42,43]. A study conducted in Egypt found a gene prevalence of 93.3% for iss and 46.6% for iutA [40].
Similar to other pathogenic Escherichia coli strains, avian pathogenic E. coli (APEC) harbor a wide variety of virulence genes that distinguish them from commensal strains. Among these, the ten genes most frequently associated with APEC strains are iss, tsh, iroN, ompT, iutA, cvaC, hlyF, iucD, papG allel (II/III), and papC [44]. These genes are located on chromosomes or plasmids, such as the ColV and ColBM plasmids. Virulence factors commonly associated with APEC strains include adhesins, toxins, iron acquisition mechanisms, invasins, and plasmids [5,45,46].
In this study, all isolates (100%) harbored the resistance genes tetC (Tellurium ion resistance protein) and gad (Glutamate decarboxylase). Tellurite, a Tellurium oxyanion, is toxic to bacteria due to its oxidative capability. However, Escherichia coli shows high resistance to tellurite, and among the genes associated with this resistance are terB, terC, terF, terX, and terY3 [47]. Previous studies have described the presence of the terC gene in all E. coli isolates analyzed [48,49].
The enzyme glutamate decarboxylase (gad) transforms glutamate (Glu) and a proton into gamma-aminobutyric acid (GABA) and carbon dioxide, with pyridoxal 5′-phosphate (PLP) acting as a cofactor [50,51]. The gad system is a common mechanism found in bacteria that allows them to survive and adapt to acidic environments [51]. A previous study described the presence of the gad gene in 100% of E. coli isolates tested [48].
In addition to the five genes used as minimum predictors, various studies describe other genes associated with APEC, including iucD, hlyE, irp2, papC, cva/cvi, and tsh [5,44,45]. In this study, the pap gene was detected in 23.80% of the isolates, and previous studies described its presence in 15% of systemic isolates and 26% of cellulitis isolates [52]. The trat gene was detected in 82.53% of the isolates tested in this study. Similarly, previous studies detected the traT gene in 82% of systemic isolates and in 62.29% of colibacillosis isolates [46,52].
It is essential to note that the use of the five virulence genes alone is insufficient to determine the virulence potential of strains. The correlation between the presence of two plasmid markers, APEC hlyF and ompT, which are among the most conserved plasmids, with multilocus sequence typing (MLST) or serogrouping can be used to identify highly virulent APEC strains [8]. In the present study, the isolates were obtained from the bone marrow of femurs. To reach the femur, the agent must have the ability to enter the bloodstream, which indicates the potential of the bacteria to be highly pathogenic. Additionally, PCR screening with the five minimum predictors was used, along with the identification of the serogroups of these isolates [12].
Different O serogroups have been associated with colibacillosis; however, the most common ones linked to these cases, found in various studies worldwide, are O78, O2, O1, O18, O35, O36, O109, O115, and O111 [5,45]. In this study, the combined prevalence of these most prevalent serogroups represents a total of 18.7% of the classified isolates in this study.
Serogroups O128 and O53 are not commonly associated with cases of colibacillosis; however, they were the most prevalent among the isolates analyzed in this study. Previous studies characterized isolates from broilers with a history of respiratory symptoms, pericarditis, perihepatitis, and airsacculitis as belonging to serogroup O128 [53,54]. In another study that used samples from birds with lesions characteristic of colibacillosis, 8.9% of the isolates evaluated belonged to serogroup O53 [55]. These data demonstrate the diversity of serogroups among APEC strains. Among the factors that can influence the geographical variation in these serogroups, the genetic diversity of bacterial strains and exposure to various sources of infection are highlighted [5,45].
The resistance of Escherichia coli to different classes of antimicrobials has been described. APEC is often resistant to tetracyclines, sulfonamides, ampicillin, and streptomycin. However, this resistance profile varies according to the geographic location and bird characteristics [5,11].
For this study, the antibiotics most widely used in poultry farming were selected, with some of them also employed in human treatment, such as ceftriaxone, nitrofurantoin, and sulfamethoxazole/trimethoprim. All (100%) E. coli isolates were resistant to at least one antibiotic, displaying diverse antibiotic resistance profiles. Sixty percent of the strains exhibited resistance to more than three classes of antibiotics, categorizing them as multidrug-resistant. In Brazil, multidrug resistance rates of 54.6%, 78.9%, 94.2%, and 71% have been observed in APEC isolated from cases of airsacculitis, cellulitis, commercial poultry, and chicken carcasses, respectively [56,57,58,59]. Studies conducted in China, Poland, and Thailand reported multidrug resistance rates of 100%, 81.1%, and 80%, respectively [60,61,62].
In this study, the most prevalent resistance genes among the isolates belong to the classes of aminoglycosides, sulfonamides, β-lactams, and tetracyclines. In veterinary medicine, aminoglycosides are widely used to treat bacterial infections in various animal species, including birds. These antibiotics are effective against a variety of Gram-negative bacteria, such as Escherichia coli, Salmonella, and Pseudomonas aeruginosa [63]. Gentamicin is one of the most commonly used aminoglycosides in veterinary medicine. A high presence of aminoglycoside resistance genes was reported in this study, similar to the high rates described previously in Pakistan [64].
Resistance to sulfonamides can be explained by their extensive use in the treatment of infections caused by Gram-negative bacilli and their wide availability on the market [65]. Previous studies described prevalences of sulfonamide resistance genes in 70% and 89.3% of isolates, rates close to those described in this work [14,64]. Another study described the sulfonamide resistance genes, sul1 and sul2, in 6.3% and 25.3% of isolates, respectively [62].
Due to their wide availability and affordable cost, tetracyclines are a class of antibiotics commonly used in veterinary medicine to treat a variety of bacterial infections in animals. They are effective against a wide range of bacteria, including both Gram-positive and Gram-negative bacteria, and are commonly used to treat respiratory, urinary tract, and skin infections in animals [65]. In Jordan, 90.7% of APEC isolates harbored at least one tetracycline resistance gene (tet(A) and tet(B)) [14], while another study demonstrated that 16.4% of isolates from cases of colibacillosis harbored tetracycline resistance genes (tet(A)) [46].
Beta-lactams are widely used in veterinary medicine. One of the main factors involved in high rates of resistance to beta-lactams is the production of extended-spectrum beta-lactamases (ESBLs) by Enterobacteriaceae species [66,67]. This study indicated a prevalence of 64.06% of isolates harboring at least one beta-lactam resistance gene. Here, the presence of the blaTEM gene was found in 43.3% of isolates. Similarly, studies conducted in Thailand and Jordan described its presence in 72.9% and 43.3% of isolates, respectively [14,62].
Constant exposure to low concentrations of disinfectant residues can result in increased bacterial tolerance. This increased tolerance may lead to greater bacterial adaptive resistance to antibiotics and the disinfectants themselves, enhancing the ability of bacteria to survive various environmental stresses [68,69]. Studies suggest a relationship between the use of disinfectants and the transfer of antimicrobial resistance genes [70,71,72]. In this study, three genes associated with resistance to disinfectants used in the production chain were found, namely formaldehyde, quaternary ammonium compounds (QACs), and hydrogen peroxide.
A study described that certain strains of E. coli have a formaldehyde resistance mechanism that involves enzymatic degradation of the compound by a variant of the enzyme present on a plasmid [73]. A study conducted in Germany described how phenotypic resistance to formaldehyde was associated with the presence of this gene [74]. In this study, all analyzed strains contained a formaldehyde dehydrogenase gene (formA).
QACs are widely used in the poultry industry due to their relatively low toxicity, good antibacterial properties, and non-irritating, non-corrosive, and reasonably effective properties in the presence of organic matter [75]. The qacE gene is widely spread in Gram-negative bacteria, mainly in Enterobacteriaceae. In this study, 50.79% of APEC strains contained the qacE gene. The presence of the qacEΔ1 gene, described as a qacE deletion mutation, has been reported in some studies and appears with a relatively high frequency in isolates [75,76,77].
The SitABCD operon, initially described in Salmonella enterica, consists of four distinct regions. The sitA gene encodes a periplasmic binding protein that captures manganese and iron. The sitB gene encodes the ATP-binding component, providing energy for ion transport across the cell membrane. The sitC gene encodes a permease that facilitates the active transport of ions across the membrane. Finally, the sitD gene encodes the inner-membrane component of the system, aiding in the transportation and incorporation of ions into the bacterial cytoplasm. Collectively, these genes form an ABC transport system that imparts resistance to the bactericidal effects of hydrogen peroxide and plays a crucial role in the uptake and homeostasis of manganese and iron in bacterial cells [78]. In the current study, 57.14% of the isolates harbored the sitABCD gene. In cases of avian mortality associated with colibacillosis, it was observed that the gene sitABCD was present in 71.3% of the bacterial isolates [79]. This suggests a potential role of the SitABCD operon in the survival and adaptation of Escherichia coli under conditions associated with colibacillosis in poultry.
5. Conclusions
This study identified the prevalence of avian pathogenic Escherichia coli (APEC) in 58% of the analyzed broiler chicken batches, encompassing commercial poultry-producing regions in Brazil. Additionally, it demonstrated a broad diversity of serogroups distributed throughout the country. These findings underscore the widespread occurrence of pathogenic E. coli strains in broiler chicken populations, emphasizing the need for continued monitoring and research to better understand and manage these bacterial infections in the poultry industry.
In this study, all isolates examined harbored at least one virulence gene associated with the pathogenicity of APEC. The majority of the isolates carried genes related to adhesion, iron acquisition systems, and serum resistance factors. Additionally, all isolates demonstrated the presence of genes associated with toxin production.
The characterization of the antimicrobial resistance profile revealed a significant presence of multidrug-resistant strains, not only to antibiotics commonly used in animal production but also to antibiotics frequently employed in human infection treatment.
In the broader context, antimicrobial resistance is recognized as a global issue tied to the concept of One Health, which integrates environmental, human health, and animal health considerations. Integrated strategies, grounded in the One Health approach and addressing all three domains, emerge as potential solutions to combat antimicrobial resistance.
These results highlight the importance of monitoring APEC serotypes and resistances through pharmacovigilance. Surveillance plays a critical role in both evaluating the effectiveness of interventions against this challenge and investigating events, ultimately aiming at the identification and prevention of antimicrobial resistance.
Acknowledgments
The authors thank the Bioinformatics Laboratory of the Department of Microbiology, Immunology, and Parasitology (MIP) of the Federal University of Santa Catarina (UFSC) for their help analyzing the sequencing data; the team who worked on the sample collection, especially Josias Rodrigo Vogt and Antonio Junior de Lima Neto; and the poultry companies that provided biological samples for this study.
Author Contributions
Conceptualization, G.F. and G.B.C.S.; methodology, G.F. and G.V.T.P.; software, V.B.F.; validation, G.F., G.V.T.P., B.P.S. and M.D.; formal analysis, V.B.F. and G.V.T.P.; investigation, G.V.T.P., B.P.S., M.D., M.A.E., G.B.C.S., E.C.M. and D.S.M.S.; resources, G.F.; data curation, V.B.F., G.V.T.P. and G.F.; writing—original draft preparation, G.V.T.P. and G.F.; writing—review and editing, G.F., M.D. and M.A.E.; visualization, G.F.; supervision, G.F.; project administration, G.F.; funding acquisition, G.F. and G.B.C.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The genome sequencing and assembly data were deposited in the NCBI database with a Bioproject accession number (PRJNA917297).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by Santa Catarina Research Foundation (Fundação de Amparo à Pesquisa e Inovação of Santa Catarina, FAPESC, Santa Catarina, Brazil/grant nº 2022TR002036/ FAPESC 30/2022), CAPES (Coordination for the Improvement of Higher Education Personnel, Brazil), CNPq (National Council for Scientific and Technological Development, Brazil, protocol 408073/2023-3 and research fellowship number 304434/2023-0), and UFSC (Federal University of Santa Catarina). The APC was funded by Santa Catarina Research Foundation (Fundação de Amparo à Pesquisa e Inovação of Santa Catarina, FAPESC, Santa Catarina, Brazil), Federal University of Santa Catarina (UFSC) Project SIGPEX 201917940, and by Project SIGPEX 202021165/Master fellowship of Giulia Von Tönnemann Pilati.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.ABPA (Brazilian Animal Protein Association) Annual Report 2024. APBA; Guarulhos, Brazil: 2024. [Google Scholar]
- 2.Blount Z.D. The Unexhausted Potential of E. coli. Elife. 2015;4:e05826. doi: 10.7554/eLife.05826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreira A.J.P., Knöbl T.K. Colibacilose. In: Berchieri Júnior A., Nepomuceno Silva E., Di Fábio J., Sesti L., Zuanaze M.A.F., editors. Doenças das Aves. Fundação APINCO de Ciência e Tecnologia Avícolas; Campinas, Brazil: 2009. pp. 667–692. [Google Scholar]
- 4.Yu D., Banting G., Neumann N.F. A Review of the Taxonomy, Genetics, and Biology of the Genus Escherichia and the Type Species Escherichia coli. Can. J. Microbiol. 2021;67:553–571. doi: 10.1139/cjm-2020-0508. [DOI] [PubMed] [Google Scholar]
- 5.Nolan L.K., Vaillancourt J., Barbieri N.L., Logue C.M. Colibacillosis. In: Swayne D.E., Boulianne M., Logue C.M., McDougald L.R., Nair V., Suarez D.L., editors. Diseases of Poultry. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2020. pp. 770–830. [Google Scholar]
- 6.Orskov I., Orskov F., Jann B., Jann K. Serology, Chemistry, and Genetics of O and K Antigens of Escherichia coli. Bacteriol. Rev. 1977;41:667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fratamico P.M., DebRoy C., Liu Y., Needleman D.S., Baranzoni G.M., Feng P. Advances in Molecular Serotyping and Subtyping of Escherichia coli. Front. Microbiol. 2016;7:644. doi: 10.3389/fmicb.2016.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson T.J., Miller E.A., Flores-Figueroa C., Munoz-Aguayo J., Cardona C., Fransen K., Lighty M., Gonder E., Nezworski J., Haag A., et al. Refining the Definition of the Avian Pathogenic Escherichia coli (APEC) Pathotype through Inclusion of High-Risk Clonal Groups. Poult. Sci. 2022;101:102009. doi: 10.1016/j.psj.2022.102009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dho-Moulin M., Fairbrother J.M. Avian Pathogenic Escherichia coli (APEC) Vet. Res. 1999;30:299–316. [PubMed] [Google Scholar]
- 10.La Ragione R.M., Woodward M.J. Virulence Factors of Escherichia coli Serotypes Associated with Avian Colisepticaemia. Res. Vet. Sci. 2002;73:27–35. doi: 10.1016/S0034-5288(02)00075-9. [DOI] [PubMed] [Google Scholar]
- 11.Kathayat D., Lokesh D., Ranjit S., Rajashekara G. Avian Pathogenic Escherichia coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies. Pathogens. 2021;10:467. doi: 10.3390/pathogens10040467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson T.J., Wannemuehler Y., Doetkott C., Johnson S.J., Rosenberger S.C., Nolan L.K. Identification of Minimal Predictors of Avian Pathogenic Escherichia coli Virulence for Use as a Rapid Diagnostic Tool. J. Clin. Microbiol. 2008;46:3987–3996. doi: 10.1128/JCM.00816-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landoni M.F., Albarellos G. The Use of Antimicrobial Agents in Broiler Chickens. Vet. J. 2015;205:21–27. doi: 10.1016/j.tvjl.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim R.A., Cryer T.L., Lafi S.Q., Basha E.A., Good L., Tarazi Y.H. Identification of Escherichia coli from Broiler Chickens in Jordan, Their Antimicrobial Resistance, Gene Characterization and the Associated Risk Factors. BMC Vet. Res. 2019;15:159. doi: 10.1186/s12917-019-1901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall B.M., Levy S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Organization for Animal Health (OIE) OIE Annual Report on Antimicrobial Agents Intended for Use in Animals. 5th ed. WOAH; Paris, France: 2021. [Google Scholar]
- 17.Callaway T.R., Edrington T.S., Rychlik J.L., Genovese K.J., Poole T.L., Jung Y.S., Bischoff K.M., Anderson R.C., Nisbet D.J., Rd B. Ionophores: Their Use as Ruminant Growth Promotants and Impact on Food Safety. Ionophores Impact on Food Safety 43. Curr. Issues Intestig. Microbiol. 2003;4:43–51. [PubMed] [Google Scholar]
- 18.Gambi L., Crippa C., Lucchi A., De Cesare A., Parisi A., Manfreda G., Pasquali F. The Resistome of Commensal Escherichia coli Isolated from Broiler Carcasses “Produced without the Use of Antibiotics”. Poult. Sci. 2022;101:101770. doi: 10.1016/j.psj.2022.101770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roth N., Käsbohrer A., Mayrhofer S., Zitz U., Hofacre C., Domig K.J. The Application of Antibiotics in Broiler Production and the Resulting Antibiotic Resistance in Escherichia coli: A Global Overview. Poult. Sci. 2019;98:1791–1804. doi: 10.3382/ps/pey539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McVey S., Kennedy M., Chengappa M.M. Veterinary Microbiology. 3rd ed. Guanabara Koogan; Bela Vista, Brazil: 2016. [Google Scholar]
- 21.Reygaert W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018;4:482–501. doi: 10.3934/microbiol.2018.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collett S.R., Smith J.A., Boulianne M., Owen R.L., Gingerich E., Singer R.S., Johnson T.J., Hofacre C.L., Berghaus R.D., Stewart-Brown B. Principles of disease prevention, diagnosis, and control. In: Swayne D.E., Boulianne M., Logue C.M., McDougald L.R., Nair V., Suarez D.L., editors. Diseases of Poultry. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2020. pp. 1–78. [Google Scholar]
- 23.MacGowan A., Macnaughton E. Antibiotic Resistance. Medicine. 2017;45:622–628. doi: 10.1016/j.mpmed.2017.07.006. [DOI] [Google Scholar]
- 24.Torres-Barceló C. The Disparate Effects of Bacteriophages on Antibiotic-Resistant Bacteria. Emerg. Microbes Infect. 2018;7:1–12. doi: 10.1038/s41426-018-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mc Carlie S., Boucher C.E., Bragg R.R. Molecular Basis of Bacterial Disinfectant Resistance. Drug Resist. Updates. 2020;48:100672. doi: 10.1016/j.drup.2019.100672. [DOI] [PubMed] [Google Scholar]
- 26.Tong C., Hu H., Chen G., Li Z., Li A., Zhang J. Disinfectant Resistance in Bacteria: Mechanisms, Spread, and Resolution Strategies. Environ. Res. 2021;195:110897. doi: 10.1016/j.envres.2021.110897. [DOI] [PubMed] [Google Scholar]
- 27.Bermudez A.J., Stewart-Brown B. Disease Prevention and Diagnostic. In: Saif Y.M., Fadly A.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E., editors. Diseases of Poultry. Iowa State University Press; Ames, IA, USA: 2003. pp. 17–55. [Google Scholar]
- 28.Jaenisch F.R.F., Kuchiishi S.S., Coldebella A. Atividade Antibacteriana de Desinfetantes Para Uso Na Produção Orgânica de Aves. Ciência Rural. 2010;40:354–358. doi: 10.1590/S0103-84782010000200020. [DOI] [Google Scholar]
- 29.Wilson K. Preparation of Genomic DNA from Bacteria. Curr. Protoc. Mol. Biol. 2001;56 doi: 10.1002/0471142727.mb0204s56. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Siek K.E., Giddings C.W., Doetkott C., Johnson T.J., Nolan L.K. Characterizing the APEC Pathotype. Vet. Res. 2005;36:241–256. doi: 10.1051/vetres:2004057. [DOI] [PubMed] [Google Scholar]
- 31.Bauer A.W., Kirby W.M.M., Sherris J.C., Turck M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966;45:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 32.Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. CLSI (Clinical and Laboratory Standards Institute); Malvern, PA, USA: 2023. [Google Scholar]
- 33.Performance Standards for Antimicrobial Susceptibility Testing. CLSI (Clinical and Laboratory Standards Institute); Malvern, PA, USA: 2023. [Google Scholar]
- 34.Coil D., Jospin G., Darling A.E. A5-Miseq: An Updated Pipeline to Assemble Microbial Genomes from Illumina MiSeq Data. Bioinformatics. 2015;31:587–589. doi: 10.1093/bioinformatics/btu661. [DOI] [PubMed] [Google Scholar]
- 35.Gurevich A., Saveliev V., Vyahhi N., Tesler G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seemann T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 37.Richter M., Rosselló-Móra R., Oliver Glöckner F., Peplies J. JSpeciesWS: A Web Server for Prokaryotic Species Circumscription Based on Pairwise Genome Comparison. Bioinformatics. 2016;32:929–931. doi: 10.1093/bioinformatics/btv681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zankari E., Hasman H., Cosentino S., Vestergaard M., Rasmussen S., Lund O., Aarestrup F.M., Larsen M.V. Identification of Acquired Antimicrobial Resistance Genes. J. Antimicrob. Chemother. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subedi M., Luitel H., Devkota B., Bhattarai R.K., Phuyal S., Panthi P., Shrestha A., Chaudhary D.K. Antibiotic Resistance Pattern and Virulence Genes Content in Avian Pathogenic Escherichia coli (APEC) from Broiler Chickens in Chitwan, Nepal. BMC Vet. Res. 2018;14:113. doi: 10.1186/s12917-018-1442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Awad A.M., El-Shall N.A., Khalil D.S., El-Hack M.E.A., Swelum A.A., Mahmoud A.H., Ebaid H., Komany A., Sammour R.H., Sedeik M.E. Incidence, Pathotyping, and Antibiotic Susceptibility of Avian Pathogenic Escherichia coli among Diseased Broiler Chicks. Pathogens. 2020;9:114. doi: 10.3390/pathogens9020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilczyński J., Stępień-Pyśniak D., Wystalska D., Wernicki A. Molecular and Serological Characteristics of Avian Pathogenic Escherichia coli Isolated from Various Clinical Cases of Poultry Colibacillosis in Poland. Animals. 2022;12:1090. doi: 10.3390/ani12091090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoepers P. Dissertation Thesis. Universidade Federal de Uberlândia; Santa Monica, Brazil: 2016. Caracterização de Escherichia coli Isoladas de Perus. [Google Scholar]
- 43.De Carli S., Ikuta N., Lehmann F.K.M., Da Silveira V.P., De Melo Predebon G., Fonseca A.S.K., Lunge V.R. Virulence Gene Content in Escherichia coli Isolates from Poultry Flocks with Clinical Signs of Colibacillosis in Brazil. Poult. Sci. 2015;94:2635–2640. doi: 10.3382/ps/pev256. [DOI] [PubMed] [Google Scholar]
- 44.Ovi F., Zhang L., Nabors H., Jia L., Adhikari P. A Compilation of Virulence-Associated Genes That Are Frequently Reported in Avian Pathogenic Escherichia coli (APEC) Compared to Other E. coli. J. Appl. Microbiol. 2023;134:lxad014. doi: 10.1093/jambio/lxad014. [DOI] [PubMed] [Google Scholar]
- 45.Mehat J.W., van Vliet A.H.M., La Ragione R.M. The Avian Pathogenic Escherichia coli (APEC) Pathotype Is Comprised of Multiple Distinct, Independent Genotypes. Avian Pathol. 2021;50:402–416. doi: 10.1080/03079457.2021.1915960. [DOI] [PubMed] [Google Scholar]
- 46.Newman D.M., Barbieri N.L., de Oliveira A.L., Willis D., Nolan L.K., Logue C.M. Characterizing Avian Pathogenic Escherichia coli (APEC) from Colibacillosis Cases, 2018. PeerJ. 2021;9:e11025. doi: 10.7717/peerj.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen T.T.H., Kikuchi T., Tokunaga T., Iyoda S., Iguchi A. Diversity of the Tellurite Resistance Gene Operon in Escherichia coli. Front. Microbiol. 2021;12:681175. doi: 10.3389/fmicb.2021.681175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tofani S., Albini E., Blasi F., Cucco L., Lovito C., Maresca C., Pesciaroli M., Orsini S., Scoccia E., Pezzotti G., et al. Assessing the Load, Virulence and Antibiotic-Resistant Traits of ESBL/Ampc E. coli from Broilers Raised on Conventional, Antibiotic-Free, and Organic Farms. Antibiotics. 2022;11:1484. doi: 10.3390/antibiotics11111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jonare L., Östlund E., Söderlund R., Hansson I., Aspán A., Jansson D.S. Core Genome Multilocus Sequence Typing (CgMLST) Confirms Systemic Spread of Avian Pathogenic Escherichia coli (APEC) in Broilers with Cellulitis. Vet. Microbiol. 2023;282:109755. doi: 10.1016/j.vetmic.2023.109755. [DOI] [PubMed] [Google Scholar]
- 50.De Biase D., Tramonti A., Bossa F., Visca P. The Response to Stationary-Phase Stress Conditions in Escherichia coli: Role and Regulation of the Glutamic Acid Decarboxylase System. Mol. Microbiol. 1999;32:1198–1211. doi: 10.1046/j.1365-2958.1999.01430.x. [DOI] [PubMed] [Google Scholar]
- 51.Hou C.Y., Ahn C., Cho B.-K., Kang T.J. Selection of Escherichia coli Glutamate Decarboxylase Active at Neutral PH from a Focused Library. Biotechnol. Bioprocess. Eng. 2018;23:473–479. doi: 10.1007/s12257-018-0258-9. [DOI] [Google Scholar]
- 52.de Oliveira A.L., Newman D.M., Sato Y., Noel A., Rauk B., Nolan L.K., Barbieri N.L., Logue C.M. Characterization of Avian Pathogenic Escherichia coli (APEC) Associated with Turkey Cellulitis in Iowa. Front. Vet. Sci. 2020;7:380. doi: 10.3389/fvets.2020.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khafagy A., Eid S., Mohammed R. Phenotypic Characterization of Escherichia coli Isolated from Broiler Chicken. Suez Canal Vet. Med. J. SCVMJ. 2019;24:1–12. doi: 10.21608/scvmj.2019.66688. [DOI] [Google Scholar]
- 54.Casalino G., Dinardo F.R., D’Amico F., Bozzo G., Bove A., Camarda A., Lombardi R., Dimuccio M.M., Circella E. Antimicrobial Efficacy of Cinnamon Essential Oil against Avian Pathogenic Escherichia coli from Poultry. Animals. 2023;13:2639. doi: 10.3390/ani13162639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim Y.B., Yoon M.Y., Ha J.S., Seo K.W., Noh E.B., Son S.H., Lee Y.J. Molecular Characterization of Avian Pathogenic Escherichia coli from Broiler Chickens with Colibacillosis. Poult. Sci. 2020;99:1088–1095. doi: 10.1016/j.psj.2019.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barros M.R., da Silveira W.D., de Araújo J.M., Costa E.P., Oliveira A.A.D.F., Santos A.P.D.S., Silva V.A.S., Mota R.A. Resistência Antimicrobiana e Perfil Plasmidial de Escherichia coli Isolada de Frangos de Corte e Poedeiras Comerciais No Estado de Pernambuco. Pesqui. Veterinária Bras. 2012;32:405–410. doi: 10.1590/S0100-736X2012000500008. [DOI] [Google Scholar]
- 57.Barbieri N.L., Ald O., Tejkowski T.M., Pavanelo D.B., Rocha D.A. Genotypes and Pathogenicity of Cellulitis Isolates Reveal Traits That Modulate APEC Virulence. PLoS ONE. 2013;8:72322. doi: 10.1371/journal.pone.0072322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cardoso A., Tessari E., Luciano R. Resistência Antimicrobiana de Escherichia coli Isolada de Aves Comerciais. Biológico. 2019;81:1–8. doi: 10.31368/1980-6221v81a10013. [DOI] [Google Scholar]
- 59.Cyoia P.S., Koga V.L., Nishio E.K., Houle S., Dozois C.M., De Brito K.C.T., De Brito B.G., Nakazato G., Kobayashi R.K.T. Distribution of ExPEC Virulence Factors, BlaCTX-M, FosA3, and Mcr-1 in Escherichia coli Isolated from Commercialized Chicken Carcasses. Front. Microbiol. 2019;10:153–159. doi: 10.3389/fmicb.2018.03254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Afayibo D.J.A., Zhu H., Zhang B., Yao L., Abdelgawad H.A., Tian M., Qi J., Liu Y., Wang S. Isolation, Molecular Characterization, and Antibiotic Resistance of Avian Pathogenic Escherichia coli in Eastern China. Vet. Sci. 2022;9:319. doi: 10.3390/vetsci9070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Racewicz P., Majewski M., Biesiada H., Nowaczewski S., Wilczyński J., Wystalska D., Kubiak M., Pszczoła M., Madeja Z.E. Prevalence and Characterisation of Antimicrobial Resistance Genes and Class 1 and 2 Integrons in Multiresistant Escherichia coli Isolated from Poultry Production. Sci. Rep. 2022;12:6062. doi: 10.1038/s41598-022-09996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomrongsuwannakij T., Narinthorn R., Mahawan T., Blackall P.J. Molecular and Phenotypic Characterization of Avian Pathogenic Escherichia coli Isolated from Commercial Broilers and Native Chickens. Poult. Sci. 2022;101:101527. doi: 10.1016/j.psj.2021.101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Duijkeren E., Schwarz C., Bouchard D., Catry B., Pomba C., Baptiste K.E., Moreno M.A., Rantala M., Ružauskas M., Sanders P., et al. The Use of Aminoglycosides in Animals within the EU: Development of Resistance in Animals and Possible Impact on Human and Animal Health: A Review. J. Antimicrob. Chemother. 2019;74:2480–2496. doi: 10.1093/jac/dkz161. [DOI] [PubMed] [Google Scholar]
- 64.Azam M., Mohsin M., Johnson T.J., Smith E.A., Johnson A., Umair M., Saleemi M.K., Sajjad-ur-Rahman Genomic Landscape of Multi-Drug Resistant Avian Pathogenic Escherichia coli Recovered from Broilers. Vet. Microbiol. 2020;247:108766. doi: 10.1016/j.vetmic.2020.108766. [DOI] [PubMed] [Google Scholar]
- 65.Palma E., Tilocca B., Roncada P. Antimicrobial Resistance in Veterinary Medicine: An Overview. Int. J. Mol. Sci. 2020;21:1914. doi: 10.3390/ijms21061914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pitout J.D.D. Multiresistant Enterobacteriaceae: New Threat of an Old Problem. Expert. Rev. Anti Infect. Ther. 2008;6:657–669. doi: 10.1586/14787210.6.5.657. [DOI] [PubMed] [Google Scholar]
- 67.Krizman M., Avgustin J.A., Zdovc I., Golob M., Trkov M., Ciglenecki U.J., Biasizzo M., Kirbis A. Antimicrobial Resistance and Molecular Characterization of Extended-Spectrum β-Lactamases and Other Escherichia coli Isolated from Food of Animal Origin and Human Intestinal Isolates. J. Food Prot. 2017;80:113–120. doi: 10.4315/0362-028X.JFP-16-214. [DOI] [PubMed] [Google Scholar]
- 68.Chen B., Han J., Dai H., Jia P. Biocide-Tolerance and Antibiotic-Resistance in Community Environments and Risk of Direct Transfers to Humans: Unintended Consequences of Community-Wide Surface Disinfecting during COVID-19. Environ. Pollut. 2021;283:117074. doi: 10.1016/j.envpol.2021.117074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rozman U., Pušnik M., Kmetec S., Duh D., Šostar Turk S. Reduced Susceptibility and Increased Resistance of Bacteria against Disinfectants: A Systematic Review. Microorganisms. 2021;9:2550. doi: 10.3390/microorganisms9122550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Itzek A., Zheng L., Chen Z., Merritt J., Kreth J. Hydrogen Peroxide-Dependent DNA Release and Transfer of Antibiotic Resistance Genes in Streptococcus gordonii. J. Bacteriol. 2011;193:6912–6922. doi: 10.1128/JB.05791-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang X., Xu Y., Li Y., Zhang K., Liu L., Wang H., Tian J., Ying H., Shi L., Yu T. Characterization and Horizontal Transfer of QacH -Associated Class 1 Integrons in Escherichia coli Isolated from Retail Meats. Int. J. Food Microbiol. 2017;258:12–17. doi: 10.1016/j.ijfoodmicro.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Kampf G. Biocidal Agents Used for Disinfection Can Enhance Antibiotic Resistance in Gram-Negative Species. Antibiotics. 2018;7:110. doi: 10.3390/antibiotics7040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kümmerle N., Feucht H.H., Kaulfers P.M. Plasmid-Mediated Formaldehyde Resistance in Escherichia coli: Characterization of Resistance Gene. Antimicrob. Agents Chemother. 1996;40:2276–2279. doi: 10.1128/AAC.40.10.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roedel A., Vincze S., Projahn M., Roesler U., Robé C., Hammerl J.A., Noll M., Al Dahouk S., Dieckmann R. Genetic but No Phenotypic Associations between Biocide Tolerance and Antibiotic Resistance in Escherichia coli from German Broiler Fattening Farms. Microorganisms. 2021;9:651. doi: 10.3390/microorganisms9030651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ibrahim W.A., Marouf S.A., Erfan A.M., Nasef S.A., El Jakee J.K. The Occurrence of Disinfectant and Antibiotic-Resistant Genes in Escherichia coli Isolated from Chickens in Egypt. Vet. World. 2019;12:141. doi: 10.14202/vetworld.2019.141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li T., Castañeda C.D., Miotto J., McDaniel C., Kiess A.S., Zhang L. Effects of in Ovo Probiotic Administration on the Incidence of Avian Pathogenic Escherichia coli in Broilers and an Evaluation on Its Virulence and Antimicrobial Resistance Properties. Poult. Sci. 2021;100:100903. doi: 10.1016/j.psj.2020.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zou L., Meng J., McDermott P.F., Wang F., Yang Q., Cao G., Hoffmann M., Zhao S. Presence of Disinfectant Resistance Genes in Escherichia coli Isolated from Retail Meats in the USA. J. Antimicrob. Chemother. 2014;69:2644–2649. doi: 10.1093/jac/dku197. [DOI] [PubMed] [Google Scholar]
- 78.Sabri M., Léveillé S., Dozois C.M. A SitABCD Homologue from an Avian Pathogenic Escherichia coli Strain Mediates Transport of Iron and Manganese and Resistance to Hydrogen Peroxide. Microbiology. 2006;152:745–758. doi: 10.1099/mic.0.28682-0. [DOI] [PubMed] [Google Scholar]
- 79.Lozica L., Villumsen K.R., Li G., Hu X., Maljković M.M., Gottstein Ž. Genomic Analysis of Escherichia Coli Longitudinally Isolated from Broiler Breeder Flocks after the Application of an Autogenous Vaccine. Microorganisms. 2022;10:377. doi: 10.3390/microorganisms10020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The genome sequencing and assembly data were deposited in the NCBI database with a Bioproject accession number (PRJNA917297).