Abstract
The matrix (M) protein plays an essential role in the assembly and budding of some enveloped RNA viruses. We expressed the human parainfluenza virus type 1 (hPIV-1) M and/or NP genes into 293T cells using the mammalian expression vector pCAGGS. Biochemical and electron microscopic analyses of transfected cells showed that the M protein alone can induce the budding of virus-like particles (vesicles) from the plasma membrane and that the NP protein can assemble into intracellular nucleocapsid-like (NC-like) structures. Furthermore, the coexpression of both the M and NP genes resulted in the production of vesicles enclosing NC-like structures, suggesting that the hPIV-1 M protein has the intrinsic ability to induce membrane vesiculation and to incorporate NC-like structures into these budding vesicles.
Parainfluenza viruses (PIVs) are enveloped viruses with nonsegmentated negative-strand RNA genomes that replicate in the cytoplasm; they belong to the Respirovirus genus of the Paramyxoviridae family in the order Mononegavirales (11). Mature virions consist of the helical nucleocapsid (NC) and the core matrix (M) protein surrounded by the lipid envelope derived from the plasma membrane of the host cell, which contains two glycoprotein spikes, the hemagglutinin-neuraminidase (HN) and fusion (F) proteins. The helical NC contains the viral RNA genome, the nucleoprotein (NP), and the RNA polymerase complex composed of the phosphoprotein (P) and large protein (L) (3). Although the current model for the assembly of PIVs implicates the M protein as the principal component promoting the budding of the virus particle (18), the underlying mechanism is still poorly understood.
Many enveloped viruses are released from infected cells by assembling and budding at the plasma membrane. Since progeny viruses bud from the plasma membrane, three major subviral components must be assembled together: the lipid envelope containing the glycoproteins, the M protein on the inner surface of the lipid bilayer, and the cytosolic helical NC. Previous studies have suggested that the M protein plays a central role in PIV assembly and budding (18). The M protein of PIV is a small (molecular mass, approximately 38 to 41 kDa), basic, amphipathic protein that is a major structural component of the mature virion (11). These properties are common to the matrix proteins of many other enveloped viruses (2, 12), and therefore matrix proteins might play similar roles in the assembly of different viruses. Previous studies have shown that cells expressing either the M gene of vesicular stomatitis virus (13), the gag gene of simian immunodeficiency virus (4, 9), or the gag gene of human immunodeficiency virus (8, 14, 16) induce the formation of vesicles at the plasma membrane, incorporating M proteins. These vesicles pinch off and are released into the culture medium, suggesting that M proteins, in the absence of the other viral proteins, are capable of mediating the formation and release of vesicles and perhaps the virus particles of infected cells.
In the present study, we used the human PIV type 1 (hPIV-1) system to determine (i) if the hPIV-1 M protein, in the absence of the other viral proteins, could induce budding vesicles at the cell surface as in the above-named virus systems and, if so, (ii) whether the coexpression of the hPIV-1 M and NP genes would result in the incorporation of NP into the budding vesicles. To perform these experiments, we used the mammalian expression vector pCAGGS under the control of the chicken β-actin promoter (17) to express the hPIV-1 M and NP genes. We cloned the M (19) and NP genes from viral RNA using the Titan reverse transcriptase-PCR system (Boehringer Mannheim) with primers hybridizing to the noncoding regions of each gene. Efficient production of M and NP was obtained with the expression vector pCAGGS (17) in 293T cells (6). First, we determined whether the expression of the hPIV-1 M gene would induce the release of budding vesicles. The M and NP genes were expressed individually by lipofectamine (GIBCO BRL)-mediated transfection of subconfluent 293T cells plated on poly-d-lysine-coated plates (Biocoat; Collaborative Biomedical Products). After incubation at 37°C for 5 h, the transfection mixture was replaced with Dulbecco’s modified Eagle’s medium (BioWhittaker) supplemented with 10% fetal bovine serum (Atlanta Biologics) and incubated at 33°C overnight. At 24 h posttransfection cells were labeled with methionine-free Dulbecco’s modified Eagle’s medium (ICN Pharmaceutical, Inc.) containing 100 μCi of [35S]methionine (Tran35S-label; ICN Pharmaceutical, Inc.) for 24 h (33°C). Both culture media and cell lysates were harvested at 48 h posttransfection and analyzed for the presence of M and NP.
To concentrate vesicles, the culture media from cells expressing the M or NP gene were first clarified at 10,000 × g (5 min) to remove any detached cells, overlaid on 50% glycerol–phosphate buffered saline, and centrifuged at 190,000 × g for 3 h (4°C). The resulting pellets were resuspended in Laemmli reducing sample buffer. Cell lysates were obtained from transfected cell monolayers after the cells were lysed in cell lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 5 mM EDTA, 0.5% Nonidet P-40), and the cell lysates were then clarified at 9,000 × g for 10 min (4°C). To determine the expression of the M and NP genes, clarified cell lysates were immunoprecipitated with monoclonal antibodies directed against hPIV-1 M (P3B) and NP (P27) (5) and incubated with M-280 Dynabeads (DYNAL) in RIPA buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100) for 1 h (4°C). Immunoprecipitates were washed with RIPA buffer and disrupted in Laemmli reducing sample buffer. 35S-labeled proteins from culture media and cell lysate immunoprecipitates were then separated on 9% polyacrylamide (PA) gels by SDS-PA gel electrophoresis (10).
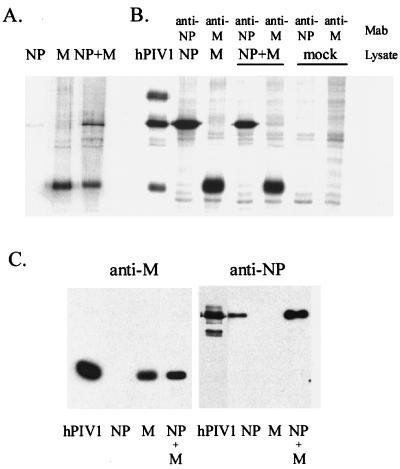
Figure 1A shows proteins in the culture medium of cells transfected with pCAGGS plasmid containing the hPIV-1 NP gene, hPIV-1 M gene, or both. As is evident from this figure, cells transfected with the hPIV-1 M gene released the protein into the culture medium whereas cells expressing the hPIV-1 NP gene released a slight amount of NP into the medium. As will be noted below, the slight amount of released NP might have been derived from dead and lysed cells (see Fig. 2). The cells coexpressing both the M and NP genes revealed abundant amounts of both M and NP in the culture medium. Immunoprecipitation analyses of cell lysates confirmed the abundant cellular expression of the transfected M and NP genes (Fig. 1B). The release of M or NP in the culture supernatants was confirmed by Western blotting with anti-hPIV-1 SDS-denatured M rabbit serum or the anti-hPIV-1 NP monoclonal antibody P27 (Fig. 1C).
FIG. 1.
Expression of the hPIV-1 M and NP genes in 293T cells with the mammalian expression vector pCAGGS. (A) The culture media were clarified, and cellular materials released into the culture media were concentrated by pelleting them through a cushion of 50% glycerol–phosphate-buffered saline. The pellets were resuspended in Laemmli reducing sample buffer, and equal aliquots of all samples were analyzed by SDS-PA gel electrophoresis (9% PA gel). Culture supernatants of cells transfected with the hPIV-1 NP gene alone, the hPIV-1 M gene alone, and the hPIV-1 NP and M genes together are shown. (B) Cell lysates were immunoprecipitated with monoclonal antibodies (MAb) against hPIV-1 M (P3B) or hPIV-1 NP (P27), and immunoprecipitates were resolved in a 9% PA gel. hPIV-1, purified hPIV-1 as a marker. (C) Western blot analysis of proteins released from transfected cells into media. The same samples shown in panel A were transferred to nitrocellulose membranes and reacted with rabbit polyclonal antibody against SDS-denatured hPIV-1 M protein (left column) or with monoclonal antibody against hPIV-1 NP (P27). hPIV-1, 1 μg of purified hPIV-1 used as control.
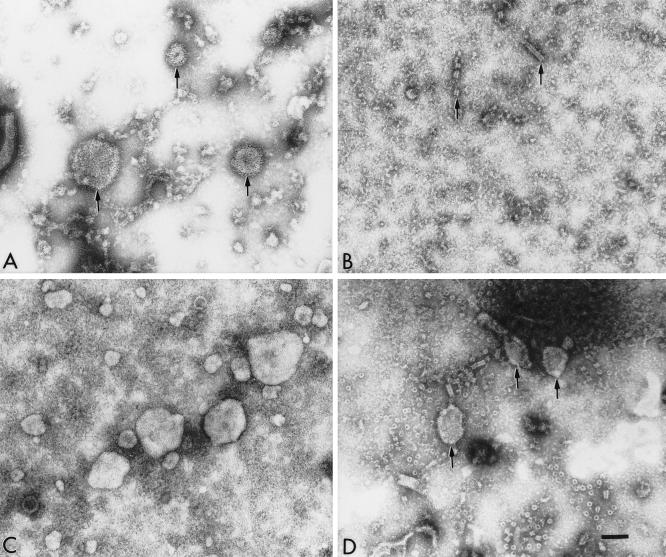
FIG. 2.
Electron micrographs of the components observed in the culture media of cells infected with hPIV-1 (A) or cells expressing the hPIV-1 NP gene (B), the hPIV-1 M gene (C), or the hPIV-1 M and NP genes (D) from pCAGGS plasmids. The clarified culture media of 293T transfected cells were concentrated in Microcons 100 (Amicon) by centrifuging them at 1,000 × g for 25 min. Concentrated media were adsorbed to freshly glow-discharged, carbon-coated grids, negatively stained with 2% phosphotungstic acid, and observed in a Phillips 301 electron microscope operated at 60 kV. The bar represents 100 nm. (A) hPIV-1 virions in culture medium (arrows); (B) occasional NC-like structures (arrows) observed in the culture medium from cells expressing the NP gene; (C) medium from cells expressing the hPIV-1 M gene; (D) medium from cells coexpressing the hPIV-1 M and NP genes (arrows).
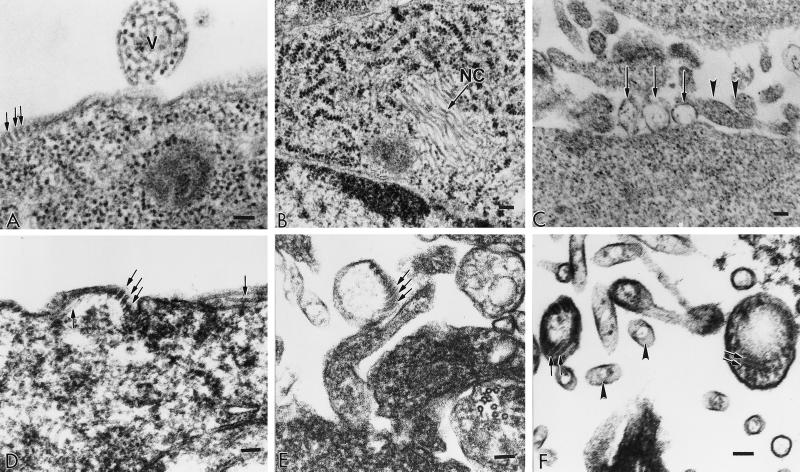
To determine whether the M protein released in the culture medium was secreted as a soluble protein or enclosed in plasma membrane-derived vesicles, the components of the culture medium (Fig. 1A) were examined by electron microscopy. Figure 2A shows material in the supernatant of cells infected with hPIV-1. The supernatant from cells expressing the NP gene (Fig. 2B) contained no structures other than the occasional short NC-like structures. The relative scarcity of these NC-like structures in the medium compared to the abundant expression of the NP gene in the cell (Fig. 1B) and their ubiquity in cells viewed by electron microscopy (Fig. 3B) suggest that they might be released into the medium from occasional lysed cells. The medium from cells expressing the M gene (Fig. 2C) revealed a number of vesicles of different sizes. The medium from cells coexpressing the M and NP genes (Fig. 2D) showed vesicles and NC-like fragments. To further confirm the induction of vesicles by M protein and to determine whether NP was included in these vesicles in cells coexpressing the M and NP genes, cells transfected with the appropriate plasmids were examined by thin-section transmission electron microscopy. The results of this analysis are shown in Fig. 3. Cells infected with hPIV-1 (panel A) showed budding virus particle at the cell surface and NC structures beneath the plasma membrane. Cells expressing the NP gene (panel B) showed bundles of hollow NC-like structures measuring approximately 20 nm. Cells expressing the hPIV-1 M gene (panel C) revealed vesicles budding from the cell surface which are clearly distinguishable from cross-sectioned microvilli. Cells coexpressing both the M and NP genes showed NC-like structures beneath the plasma membrane (panel D), NC-like structures enclosed in vesicles budding from the plasma membrane (panel E), or NC-like structures in vesicles released from the plasma membrane (panel F). These vesicles have dimensions similar to those of the native virus particles (compare panel A with panels C, E, and F but note that panel C is at a lower magnification) but not to those of the cross-sectioned microvilli (panel F). We also determined the densities of the released M and M plus NP vesicles by sucrose gradient analysis. The densities of M vesicles, M plus NP vesicules, and hPIV-1 virus were 1.09, 1.11, and 1.16 g/ml, respectively.
FIG. 3.
Electron micrographs of sections of 293T cells infected with hPIV-1 virus (A) or transfected with pCAGGS plasmids to express the hPIV-1 NP gene (B), the hPIV-1 M gene (C), or both the M and NP genes (D to F). Pellets of cells were fixed in cacodylate-buffered 2.5% glutaraldehyde, postfixed in 1% osmium tetroxide, dehydrated in a graded series of ethanol, and embedded in Spurr embedding medium. Ultrathin sections were cut on a Sorvall MT 6000 ultramicrotome, stained, and examined in a Phillips EM 301 electron microscope. The bar in each panel represents 100 nm. (A) V, budding virus particle; arrows, NC structures beneath the plasma membrane; (B) arrow, bundles of hollow NC-like structures; (C) arrows, vesicles budding from the cell surface; arrowheads, cross-sectioned microvilli; (D) arrows, NC-like structures beneath the plasma membrane; (E) arrows, NC-like structures enclosed in vesicles budding from the plasma membrane; (F) arrows, NC-like structures enclosed in vesicles that have been released from the plasma membrane; arrowheads, cross-sectioned microvilli.
Taken together, the above-described biochemical and electron microscopic experiments suggest that (i) hPIV-1 M protein can induce the formation and release of vesicles but that hPIV-1 NP cannot, (ii) hPIV-1 NP by itself or in conjunction with cellular components can assemble into intracellular NC-like structures, and (iii) coexpression of the M and NP genes leads to the formation of vesicles enclosing NC-like structures. Although induction of budding vesicles from cells expressing the rhabdovirus vesicular stomatitis virus M gene (13) or the retroviral simian immunodeficiency virus and human immunodeficiency virus gag genes (4, 8, 9, 14, 16) has been shown, the present study demonstrates the same phenomenon for the first time in a paramyxovirus. Our present data also confirm earlier observations that NP, when expressed alone in cells, assembles into NC-like structures (1, 7, 15, 20). The novel finding of this study however, is the demonstration that the coexpression of the M and NP genes results in budding vesicles enclosing the NC-like structures. Therefore, we suggest that M protein might interact with NP during virus assembly to incorporate NCs into virus particles. Further analyses by this vesicle system might shed light on the mechanisms governing the assembly of paramyxovirus, including the identification of the M protein domain(s) involved in budding and the role of the cellular cytoskeleton, if any, in this process.
Acknowledgments
This research was supported by NIH grants R01-AI-11949 and R01-AI-38956 from the National Institute of Allergy and Infectious Diseases, support grant CA-21765 from the Cancer Center (CORE), and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital.
REFERENCES
- 1.Buchholz C J, Spehner D, Drillien R, Neubert W J, Homann H E. The conserved N-terminal region of Sendai virus nucleocapsid protein NP is required for nucleocapsid assembly. J Virol. 1993;67:5803–5812. doi: 10.1128/jvi.67.10.5803-5812.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cadd T L, Skoging U, Liljestrom P. Budding of enveloped viruses from the plasma membrane. Bioessays. 1997;19:993–1000. doi: 10.1002/bies.950191109. . (Review.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins P L, Chanock R M, McIntosh K. Parainfluenza viruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1205–1241. [Google Scholar]
- 4.Delchambre M, Gheysen D, Thines D, Thiriart C, Jacobs E, Verdin E, Horth M, Burny A, Bex F. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989;8:2653–2660. doi: 10.1002/j.1460-2075.1989.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshpande K L, Portner A. Structural and functional analysis of Sendai virus nucleocapsid protein NP with monoclonal antibodies. Virology. 1984;139:32–42. doi: 10.1016/0042-6822(84)90327-1. [DOI] [PubMed] [Google Scholar]
- 6.DuBridge R B, Tang P, Hsia H C, Leong P M, Miller J H, Calos M P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fooks A R, Stephenson J R, Warnes A, Dowsett A B, Rima B K, Wilkinson G W. Measles virus nucleocapsid protein expressed in insect cells assembles into nucleocapsid-like structures. J Gen Virol. 1993;74:1439–1444. doi: 10.1099/0022-1317-74-7-1439. [DOI] [PubMed] [Google Scholar]
- 8.Gheysen D, Jacobs E, de Foresta F, Thiriart C, Francotte M, Thines D, De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez S A, Affranchino J L, Gelderblom H R, Burny A. Assembly of the matrix protein of simian immunodeficiency virus into virus-like particles. Virology. 1993;194:548–556. doi: 10.1006/viro.1993.1293. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Lamb R A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1177–1204. [Google Scholar]
- 12.Lenard J. Negative-strand virus M and retrovirus MA proteins: all in a family? Virology. 1996;216:289–298. doi: 10.1006/viro.1996.0064. . (Review.) [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Luo L, Schubert M, Wagner R R, Kang C Y. Viral liposomes released from insect cells infected with recombinant baculovirus expressing the matrix protein of vesicular stomatitis virus. J Virol. 1993;67:4415–4420. doi: 10.1128/jvi.67.7.4415-4420.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo L, Li Y, Kang C Y. Expression of gag precursor protein and secretion of virus-like gag particles of HIV-2 from recombinant baculovirus-infected insect cells. Virology. 1990;179:874–880. doi: 10.1016/0042-6822(90)90159-o. [DOI] [PubMed] [Google Scholar]
- 15.Myers T M, Pieters A, Moyer S A. A highly conserved region of the Sendai virus nucleocapsid protein contributes to the NP-NP binding domain. Virology. 1997;229:322–335. doi: 10.1006/viro.1996.8429. [DOI] [PubMed] [Google Scholar]
- 16.Nermut M V, Hockley D J, Jowett J B, Jones I M, Garreau M, Thomas D. Fullerene-like organization of HIV gag-protein shell in virus-like particles produced by recombinant baculovirus. Virology. 1994;198:288–296. doi: 10.1006/viro.1994.1032. [DOI] [PubMed] [Google Scholar]
- 17.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 18.Peeples M E. Paramyxovirus M proteins: pulling it all together and taking it on the road. In: Kingsbury D W, editor. The paramyxoviruses. New York, N.Y: Plenum Press; 1991. pp. 427–456. [Google Scholar]
- 19.Power U F, Ryan K W, Portner A. Sequence characterization and expression of the matrix protein gene of human parainfluenza virus type 1. Virology. 1992;191:947–952. doi: 10.1016/0042-6822(92)90270-y. [DOI] [PubMed] [Google Scholar]
- 20.Spehner D, Kirn A, Drillien R. Assembly of nucleocapsidlike structures in animal cells infected with a vaccinia virus recombinant encoding the measles virus nucleoprotein. J Virol. 1991;65:6296–6300. doi: 10.1128/jvi.65.11.6296-6300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]