Abstract
Hemotrophic mycoplasmas (HMs) are highly host-adapted and specialized pathogens infecting a wide range of mammals including farm animals, i.e., pigs, cattle, sheep, and goats. Although HMs have been known for over 90 years, we still do not know much about the natural transmission routes within herds. Recently, it has been repeatedly discussed in publications that arthropod vectors may play a role in the transmission of HMs from animal to animal. This is mainly since several HM species could be detected in different potential arthropod vectors by PCR. This review summarizes the available literature about the transmission of bovine, porcine, ovine, and caprine HM species by different hematophagous arthropod vectors. Since most studies are only based on the detection of HMs in potential vectors, there are rare data about the actual vector competence of arthropods. Furthermore, there is a need for additional studies to investigate, whether there are biological vectors in which HMs can multiply and be delivered to new hosts.
Keywords: hemotrophic mycoplasmas, arthropod vectors, transmission
1. Introduction
Hemotrophic mycoplasmas (HMs) are small epicellular, pleomorphic, cell wall-less, and uncultivable bacteria. They parasitize the surface of erythrocytes or even invade them and cause erythrocytic deformity and damage [1,2,3]. HMs are highly adapted to their hosts by virtue of their complex nutritional requirements and the induction of persistent infections [2,4,5]. In 1928, both Schilling and Kikuth identified, for the first time, mouse- and dog-specific HMs (Eperythrozoon coccoides, Haemobartonella canis) [6,7]; since then, species-specific HMs have been described in the blood of several animals. Until now, at least seven species of hemotrophic mycoplasma have been known in livestock, including Mycoplasma suis, M. parvum, ‘Candidatus (Ca.) M. haemosuis’, M. wenyonii, ‘Ca. M. haemobos’, M. ovis, and ‘Ca. M. haemovis’ [8,9,10,11,12,13].
The taxonomic classification of HMs has not been fully clarified and remains unclear, especially because cultivation is not possible. Initially, HMs were classified within two genera, i.e., Eperythrozoon and Haemobartonella within the order Rickettsiales, family Anaplasmataceae. This was followed by a reclassification into the order Mycoplasmatales, family Mycoplasmataceae, genus Mycoplasma due to sequence analyses of the 16S rRNA and RNase P genes [14,15,16,17]. Within the genus Mycoplasma with mostly mucosal-associated bacteria, HMs represent a distinct group of erythrocyte-associated organisms. Due to the lack of in vitro cultivation systems, it remains difficult to fully characterize the taxonomic relationship of HMs. Therefore, HMs have been given the status “incertae sedis” within the family Mycoplasmataceae. In 2018, Gupta and co-workers proposed the reclassification of HMs within a “new” genus Eperythrozoon; however, this proposal has not been implemented in practice [18].
Hemotrophic mycoplasma infections in livestock can cause acute and chronic diseases but also asymptomatic infections. Both types of infections are known as a significant cause of economic loss and welfare concern in the agricultural sector worldwide. The economic impact is on the one hand the result of clinical signs caused by the HMs themselves and on the other hand due to the increased susceptibility to other infectious agents caused by the infection-induced immune dysregulation [1,2,19,20].
Transmission of HMs mainly includes procedures exposing or sharing blood such as iatrogenic procedures or ranking fights [1,5]. Also, transplacental transmission has been described [21,22]. Blood-independent infections by excretions play a minor role under field conditions [23,24]. Because HMs reside on or within erythrocytes, there is considerable potential for livestock HMs to be taken up by a variety of hematophagous arthropod vectors such as flies, midges, mosquitoes, ticks, and lice [5]. However, experimental proof of HM transmission by arthropods is rare. This review aims to analyze and summarize published data on the transmission of HMs by arthropod vectors in livestock.
2. Clinical Signs and Prevalence of Hemotrophic Mycoplasmas in Livestock
Hemotrophic mycoplasma infections in livestock cause significant economic loss in the agricultural sector worldwide. The economic impact is on the one hand the result of the clinical outcomes and performance failure caused by HM infections, and on the other hand due to the increased susceptibility to other pathogens caused by an HM-induced immune dysregulation [2,4,25]. The immune response often fails to eliminate the pathogens leading to persistence and chronicity. Therefore, prophylaxis and therapy are not easy owing to the common persistence of HM infections and their complex pathogenesis [2,4].
The following chapter provides an overview on published data describing the clinical outcomes and prevalences of the individual HMs in farm animals, specifically the three porcine species M. suis, M. parvum, and ‘Ca. M. haemosuis’, the bovine species M. wenyonii, and ‘Ca. M. haemobos’, and the small ruminant HMs M. ovis, and ‘Ca. M. haemovis’ [8,9,10,11,12,13].
2.1. Porcine Hemotrophic Mycoplasmas
To date, three HM species are known to infect pigs. Mycoplasma suis and M. parvum were first described in 1950 by Splitter as Eperythrozoon suis and Eperythrozoon parvum [8]. Recently, the third porcine HM species ‘Ca. M. haemosuis’ was identified in China in 2017 [9].
Most studies deal with M. suis, the longest known and most pathogenic representative of the porcine HMs. Mycoplasma suis causes infectious anemia of pigs (IAP), which can manifest in various degrees of severity, depending on host susceptibility and virulence [26]. Acute infected pigs show high fever, pallor due to hemolytic anemia, and hypoglycemia. Typical skin alterations can range from petechia or urticaria, acro-cyanosis and necrosis, to generalized skin hemorrhages. Furthermore, acute M. suis infections are associated with reproductive disorders in sows including dysgalactia, fertility disorders, and abortion [1,27,28,29,30]. More often, chronic M. suis infections with low-grade bacteriemia occur. Clinical signs in chronically infected animals vary from asymptomatic outcomes to a wide range of clinical conditions including anemia, mild icterus, growth retardation in feeder pigs, or poor reproductive performance in sows [26,27,28,31].
Contrary to M. suis, there are only a few reports regarding M. parvum infections. Although both porcine HM species are genetically remarkably similar and share all coding sequences (CDS) with known functions [32,33], M. parvum seems to be low or non-pathogenic and infections are mostly asymptomatic. Even after splenectomy, infected pigs developed either no or only mild clinical signs such as mild anemia and low-grade fever [32]. However, the significance of asymptomatic M. parvum infections for pig health has not been fully clarified [34]. Two recently published studies found correlations between M. parvum infections and reduced weight gain and slaughter weights in fattening pigs and a reduced number of weaned piglets in clinically healthy sows [35,36].
Recently the third porcine HM species ‘Ca. M. haemosuis’ was detected in pigs with and without clinical signs in China, Korea, and Germany [9,37,38]. Sequencing of the 16S rRNA gene demonstrated that ‘Ca. M. haemosuis’ is similar to the feline HM species ‘Ca. M. turicensis’. Described clinical signs were like those found in M. suis infected pigs and include high fever, reduced feed intake, skin alterations, increased mortality, and decreased daily weight gain [38].
Since HMs cannot yet be cultivated and microscopic detection lacks specificity and sensitivity, only molecular studies are considered. Reports on the molecular detection of porcine HMs in blood samples including wild boars are available from several countries all over the world. The found M. suis detection rates at single animal level are highly variable, ranging from 10% to 53% in Europe (France, Switzerland, and Germany [25,39,40]), from under 2% in piglets to 76% in sows in South America [41,42,43], and from 0.2% to 86% in Asia (Republic of Korea, China, and Japan; [37,44,45]). At herd levels, the detection rates are still higher. Prevalences ranging from 9% in Japan to 100% in Europe have been reported for pigs [39,44].
Data on the distribution of M. parvum infections are rare and mostly on a single animal level. Few PCR-based studies report the occurrence of this species in domestic pigs, among others in South America [35,46], Asia (China, Japan, Republic of Korea, and Thailand [9,44,47,48], and in Europe (Germany) [34]. Additionally, Fernandes et al. detected M. parvum in wild boars in Brazil [49]. Prevalences were determined by Ade and colleagues (2022) in different age groups and production types. Interestingly, they found wide variations in the M. parvum detection rates with rates of 4% in boars, 25% in sows, and 36% in fattening pigs [34]. Potential explanations for these differences include potentially higher biosecurity levels in boar studs, with individually housed animals, and the absence of group vaccinations in boar studs compared with fattening and piglet-producing farms. In the studies in Japan and South Korea, M. parvum determined prevalences ranging from over 2% to 15% in pigs of unspecified age [37,44] were described.
Since ‘Ca. M. haemosuis’ is an emerging pathogen, there are currently only few published data on the occurrence of ‘Ca. M. haemosuis’ in domestic pigs from Asia (China, Republic of Korea, and Thailand) [9,37,48], as well as from Germany [34], with detection rates of 0% in boars and 36% in sows.
2.2. Bovine Hemotrophic Mycoplasmas
At least two distinct HM species were identified in cattle, i.e., M. wenyonii and ‘Ca. M. haemobos’. Mycoplasma wenyonii was first described in a splenectomized calf in 1934 [10] and has since been identified in several countries in cattle, buffalo, and sheep [50,51,52,53,54,55,56]. The second bovine HM species ‘Ca. M. haemobos’ was first reported in Japan [57]. The 16S rRNA gene sequencing revealed that the feline HM species M. haemofelis is the closest relative to ‘Ca. M. haemobos’. The novel bovine HM species was shown to infect cattle, buffalo, and small ruminants [11,52,58,59]. New studies also show a high dynamic of the bovine HM genomes (variable genome sizes, position shifts of genes, the little conserved gene synteny) and strong regional differences, e.g., between Mexican isolates and other American isolates [60].
Various clinical signs have been associated with M. wenyonii and ‘Ca. M. haemobos’ infections in cattle including hemolytic anemia, pyrexia, reproductive disorders, edema, and decreased production outcomes (e.g., decreased milk production, weight loss) [5,56,59,61]. Occasionally, infected cattle die [59]. Chronic infection with M. wenyonii and ‘Ca. M. haemobos’ can cause decreased milk production [62]. Anemia, decreased milk production, infertility, and lameness were also described in cattle co-infected with M. wenyonii and ‘Ca. M. haemobos’ [59,63]. In sheep and goats fever, anorexia, depression, pale oral cavity mucous, eye conjunctiva, hematuria, and lamb mortality were delineated [58]. Although little is known about the pathogenesis of M. wenyonii and ‘Ca. M. haemobos’, some studies suggest that ‘Ca. M. haemobos’ is more pathogenic than M. wenyonii [64]. In addition, it was assumed that clinical signs of HM infections in cattle could be more severe if co-infections with both HM species or other pathogens (e.g., Anaplasma spp.) occur [63].
Detection and prevalences of bovine HMs have been recently and comprehensively reviewed by De Souza Ferreira and Ruegg in 2024 [54]. Both, M. wenyonii and ‘Ca. M. haemobos’ have been detected in cattle in several countries all over the world including Europe (Switzerland, Hungary, France, and Germany), Asia (Japan, China), America (USA, Brazil, and Cuba), New Zealand, and Asia (Japan and China). The found prevalences were highly variable on single animal level, ranging from 0% to 95% for M. wenyonii and from 2% to 97% for ‘Ca. M. haemobos’ [54].
2.3. Ovine Hemotrophic Mycoplasmas
Mainly two HM species, i.e., M. ovis and ‘Ca. M. haemovis’, could be found in small ruminants throughout the world. Mycoplasma ovis can infect sheep, goats, deer, reindeer, and humans. In lambs and young sheep, acute infections can occur with severe hemolytic anemia and decreased exercise tolerance [5,13]. In older animals, M. ovis infections are mostly chronic with absence of or mild clinical signs due to the low pathogenic potential [5,65]. Sheep with chronic infections show mild parasitemia and regenerative anemia, ill thrift, and reduced production outcomes (e.g., poor weight gain, decreased wool and milk production) [5,13,66]. In goats, M. ovis cause a more severe course of disease [66].
‘Candidatus M. haemovis’ was first detected in sheep (mainly yearlings) with fatal hemolytic anemia in Hungary [13,66,67]. In Japan, ‘Ca. M. haemovis’ was also detected in yearlings with mild anemia and in older sheep, co-infected with M. ovis [66,67]. ‘Ca. M. haemovis’ can also affect goats [68].
Ovine HM infections were also detected worldwide. Similar to all other HM species in livestock mentioned before, PCR-based prevalences for the ovine HM representatives also vary considerably [5], mostly on a high level. For example, Urie and co-workers found M. ovis prevalences of 69% to 76% in the USA [69]. In Brazil, a much lower M. ovis prevalence of 39.3% was detected in goats [70] but a higher prevalence was detected in sheep (79%) [71]. In Europe, Hornok and co-workers determined the rate of HM-positive sheep in Hungary to be 52% [13] and Aktas and Ozubek determined the rate of HM-positive animals in Turkey to be 54% [72]. Considerably lower detection rates were found in Tunisia, where only 6% of the tested sheep and 0% of the goats were M. ovis positive [73]. In Asia, HM prevalences (M. ovis and ‘Ca. M. haemovis’) ranging from 36% to 45% were found in goats and sheep [74,75].
3. Vector-Based Transmission of Hemotrophic Mycoplasma in Livestock
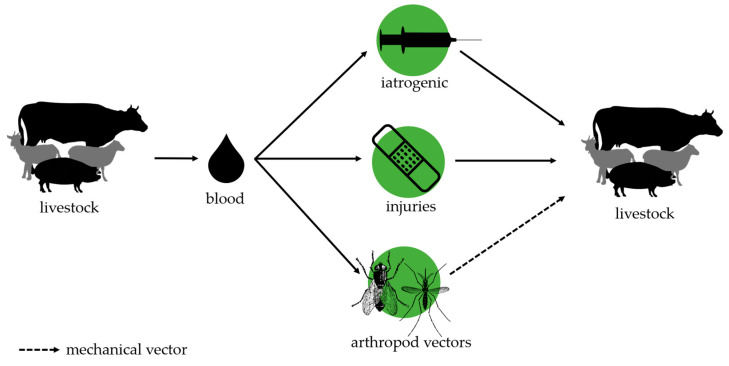
The epidemiology of HM infections in livestock is still insufficiently understood, and many questions remain about possible mechanisms of transmission. Figure 1 gives an overview on the transmission pathways described so far.
Figure 1.
Transmission pathways of hemotrophic mycoplasma in livestock.
Since HMs parasitize mainly in the blood of infected animals, it is generally accepted that blood transferring contact between animals during iatrogenic manipulations, or through small wounds due to technopathies or rank fights, represents the main transmission route. However, high prevalences were not only found in conventional low-space stables but also in extensive farming or in grazing animals. Therefore, there are many discussions about a possible transmission of HMs by arthropod vectors. Because of their capability (i) to reside on or within erythrocytes and (ii) to establish chronic and persistent low-grade infections, there is a permanent and substantial opportunity for all HM species to be taken up by a variety of hematophagous arthropods during suckling on a bacteriemic host. Nevertheless, it remains rather unknown whether HMs are only transported by the arthropod from one host to another (mechanical vector) or if HMs could multiply within arthropod vectors and then be transferred to the next host (biological vectors). In addition, most studies discussing the transmission of HMs by arthropods are based on the detection of the pathogens by PCR methods and, therefore, demonstrate vector potential but fail to prove real vector competence, which means that HMs are actually transmitted. Studies demonstrating the real vector competence of arthropods are mostly from the pre-PCR era. The following chapters summarize the current knowledge on the potential transmission of livestock-associated HMs by arthropod vectors such as flies, mosquitoes, and ticks. Older studies performed in the pre-PCR era mainly combined microscopic methods and animal experiments. All studies now use PCR methods to detect HMs in potential vectors. Table 1 summarizes the current knowledge on flies and mosquitoes as vectors, Table 2 gives an overview about the published studies on the transmission of livestock-associated HM species by ticks.
Table 1.
Known/suspected arthropod vectors for hemotrophic mycoplasma species in livestock (except ticks).
| Vector | Methods | HM Species | Results | Ref. |
|---|---|---|---|---|
|
Aedes aegypti (mosquito) |
Experimental transmission in vivo from infected to uninfected pigs | M. suis |
|
[76] |
|
Aedes camptorhynchus (mosquito) |
Experimental transmission in vivo from infected to uninfected sheep | M. ovis |
|
[77] |
|
Culex annulirostris (mosquito) |
Experimental transmission in vivo from infected to uninfected sheep | M. ovis |
|
[78] |
|
Haematobia irritans (horn fly) |
PCR detection |
M. wenyonii ‘Ca. M. haemobos’ |
|
[55] |
|
Haematopinus suis (pig louse) |
PCR detection and sequence analysis | M. suis |
|
[79] |
|
Haematopinus suis (pig louse) |
Experimental transmission in vivo from infected to uninfected pigs | M. suis |
|
[80] |
|
Haematopinus eurysternus (cattle louse) |
PCR detection | M. wenyonii |
|
[11] |
| Lice, flies, mosquitoes (without further specification) | LAMP and PCR detection | M. wenyonii |
|
[81] |
| Mosquitoes | PCR detection | hemotrophic mycoplasma |
|
[82] |
|
Stomoxys calcitrans (stable flies) |
Experimental transmission in vivo from infected to uninfected pigs | M. suis |
|
[76] |
|
Stomoxys calcitrans (stable flies) |
PCR detection |
M. wenyonii ‘Ca. M. haemobos’ |
|
[55] |
|
Stomoxys calcitrans (stable flies) |
PCR detection | M. wenyonii |
|
[83] |
|
Stomoxys spp. (stable flies) |
PCR detection and sequencing |
M. suis
M. parvum |
|
[84] |
|
Stomoxys spp. (stable flies) |
Experimental transmission in vivo from infected to uninfected sheep | M. ovis |
|
[85] |
| Tabanus bovinus, Tabanus bromius (flies) | PCR detection |
M. wenyonii ‘Ca. M. haemobos’ |
|
[55] |
| Tabanus megalops (flies) | PCR detection | M. wenyonii |
|
[83] |
|
Bovicula ovis
(Mallophaga) |
PCR detection | M. ovis |
|
[86] |
Table 2.
Known/suspected tick vectors for hemotrophic mycoplasma species in livestock.
| Vector | Methods | HM Species | Results | Ref. |
|---|---|---|---|---|
|
Amblyomma sculptum
Amblyomma ovale |
PCR detection |
M. suis
M. parvum |
|
[87] |
| Rhipicephalus microplus | PCR detection and sequence analysis | ‘Ca. M. haemobos’ |
|
[58] |
| Rhipicephalus microplus | PCR detection Mice experiments |
‘Ca. M. haemobos’ |
|
[88] |
|
Haemaphysalis longicornis
Rhipicephalus microplus |
PCR detection and sequence analysis | ‘Ca. M. haemobos’ |
|
[89] |
|
Rhipicephalus microplus
Haemaphysalis bispinosa |
PCR detection | M. wenyonii |
|
[90] |
| Ixodes ricinus | PCR detection | M. wenyonii |
|
[91] |
| Rhipicephalus microplus | PCR detection | M. ovis |
|
[92] |
3.1. Transmission of Hemotrophic Mycoplasmas by Mosquitoes
There are a few studies investigating whether mosquitoes could transmit HMs from animal to animal. Prullage and co-workers utilized the yellow fever mosquito, Aedes aegypti, to determine their capability to transmit M. suis between pigs. For this, mosquitoes were fed on M. suis infected pigs for 5 to 10 min and then transferred immediately or after 7 days to susceptible splenectomized pigs. A successful transmission was accomplished in all used pigs (n = 9) when the transfer of Aedes aegypti was carried out immediately. This indicates a high transmission potential and a role of mosquitoes as mechanical vector. However, none of the pigs became infected when there was a delay before transfer to the susceptible pig, indicating that M. suis ingested in A. aegypti lose their infectivity during the 7-day period of storage in the chamber [76]. Howard (1975) showed that M. ovis was transmitted by immediately transferring feeding Aedes camptorhynchus from infected to non-infected sheep. A transmission of M. ovis was also achieved when the mosquitoes were held for 6 days between feeding on infected sheep and recipient sheep [77]. Another study used Culex annulirostris to investigate a transmission of M. ovis from sheep to sheep by mosquitoes [78]. Mosquitoes were fed on M. ovis infected lambs and then transferred to uninfected animals which then became infected. Additionally, fed mosquitoes were maintained for 6 days and then grounded and extracted. Inoculation of the mosquito extracts led to infections of the lambs, indicating that M. ovis remained infective in mosquitoes for at least 6 days. All in all, the studies suggest that many mosquito species possess substantial competence as a mechanical vector for HMs [78].
Other studies used PCR methods to detect HM species in mosquitoes. Song and co-workers utilized a combination of PCR and LAMP methods to determine the occurrence of M. wenyonii in mosquitoes collected in a stable with clinically healthy but M. wenyonii infected cattle [81]. Overall, 21 out of 26 mosquitoes tested positive for M. wenyonii, suggesting that mosquitoes could serve as mechanical vectors for bovine HMs. Another study of Reagan and co-workers also found M. wenyonii in wild-caught mosquitoes using PCR [82]. However, molecular detection of HMs in mosquitoes collected in HM-positive herds was not consistently successful. Hornok and colleagues failed to detect HM species in Aedes and Culex spp. [55] and Thongmeesee and co-workers were not able to detect ‘Ca. M. haemobos’ in mosquitoes (mostly Culex tritaeniorhynchus) collected by traps and/or sweeping techniques in Thailand on a buffalo farm [83].
3.2. Transmission of Hemotrophic Mycoplasmas by Stomoxys Species
Stable flies can regularly be found in livestock stables, where they feed blood from the farm animals one or two times per day, mostly on more than a single animal. Besides direct harmful effects on their hosts (e.g., restlessness, skin lesions, pain, and stress), Stomoxys spp. are known to be relevant vectors for the transmission of pathogens [84,93]. Several studies could be found in the database investigating the potential of Stomoxys in the transmission of HMs. Prullage and co-workers investigated the ability of Stomoxys calcitrans to transmit M. suis from pig to pig [76]. Stable flies were allowed to feed on M. suis infected pigs and were then transferred immediately to negative recipient pigs. Mycoplasma suis was successfully transferred in 3 out of 15 pigs. However, if the transfer of Stomoxys calcitrans from infected to uninfected pigs was carried out with a delay of 24 h or more, no transmission could be detected. Similar experiments were performed by Overas in 1969 where Stomoxys spp. were utilized to successfully transfer M. ovis from infected to uninfected sheep [85]. Both studies [76,85] indicate that the Stomoxys spp. could have the competence as mechanical vectors for HMs in pigs and sheep. In addition to these older studies, there are some recent PCR-based studies on the occurrence of HM species in stable flies. Although there was no evidence of experimental HM transmission in these studies, the detection of the pathogens in potential vectors nevertheless provides evidence of a vector potential. One study from Thailand was able to detect HM DNA in 41.41% (53/128) of stable flies (Stomoxys calcitrans) at a buffalo farm [83]. Subsequent sequencing of some PCR amplicons revealed M. wenyonii as HM species [83]. Hornok and co-workers collected Stomoxys calcitrans flies in a stable with M. wenyonii and ‘Ca. M. haemobos’ positive animals [55]. Both bovine HM species were detected in the flies. Mycoplasma wenyonii was found in 6/20 Stomoxys samples with a mean copy number of 1.0 × 102. ‘Candidatus M. haemobos’ was detected in 1/20 flies with a mean copy number of 1.0. Another PCR-based study investigated the occurrence of porcine HM species in stable flies. Schwarz and colleagues investigated Stomoxys spp. from a total of 20 farms by PCR with subsequent DNA analysis and found M. parvum and M. suis positive flies in 7 farms [84].
3.3. Transmission of Hemotrophic Mycoplasmas by Tabanidae Species
Two PCR-based studies investigated the potential of Tabanidae spp. as potential vectors for HMs [55,83]. Tabanids are hematophagous ectoparasites, which are seasonally present in many areas. Tabanids are known to be vectors for many animal pathogens [94]. Hornok and colleagues detected M. wenyonii in 2 out of 8 Tabanus bovinus and 5 out of 16 T. bromius samples, collected in an extensively kept cattle herd [55]. ‘Candidatus M. haemobos’ was only found in T. bovinus samples (in two out of eight samples). Mycoplasma wenyonii has also been recorded in Tabanidae in Thailand [83]. Due to the lack of animal experiments, transstadial studies and studies with greater amounts of samples, further data need to be obtained to learn more about the potential of Tabanidae spp. as vectors for HMs.
3.4. Transmission of Hemotrophic Mycoplasmas by Lice
Blood-sucking lice are common ectoparasites of domestic animals and can act as either mechanical or biological vectors of various infectious disease agents [95]. However, there are only a few studies investigating the possible role of hematophagous lice as vectors. Heinritzi performed in vivo transmission experiments in pigs [80]. In this study, it was able to transfer M. suis to uninfected splenectomized pigs by means of Haematopinus suis which had fed on infected pigs for 40 days. Yet, it remains unclear if an infection would have also been successful using unsplenectomized pigs. Recently, another study from Argentina demonstrated the presence of M. suis in lice collected in the field. Haematopinus suis samples (15.3%) originating from both domestic and wild boars tested positive for M. suis, indicating that H. suis could serve at least as a mechanical vector for M. suis [79]. Otherwise, there are also studies in which hemotrophic mycoplasmas could not be detected in lice. Hornok and colleagues examined various hematophagous lice from cattle, goats, and pigs including Haematopinus spp. and Linognathus spp. and were unable to detect HM DNA in any of the ectoparasites examined [96].
3.5. Transmission of Hemotrophic Mycoplasmas by Ticks
For some time there has been considerable interest in ticks as potential vectors for hemotrophic mycoplasma species mainly due to the wide availability of molecular detection methods. The literature research has revealed evidence on the occurrence of HMs in ticks in Asia (China and Malaysia), Brazil and Mexico. Most of the published data are based on the PCR detection of HMs in engorged female ticks. However, the significance of PCR detection from engorged female ticks remains difficult to interpret as blood residues are always found in the sucked ticks, which can originate from infected animals.
In central China, where ‘Ca. M. haemobos’ epidemics have been confirmed, one PCR study demonstrated that 53% of Rhipicephalus microplus ticks were positive for ‘Ca. M. haemobos’ [58]. Interestingly, most of the ticks originated from goats and sheep during suckling but ticks have also been collected from the surrounding grassland. From the grassland-derived B. microplus ticks, 48% were also positive for ‘Ca. M. haemobos’, indicating that these ticks harbor ‘Ca. M. haemobos’ before sucking the blood of goats and sheep and that ‘Ca. M. haemobos’ might be transmitted via the transstadial route from one tick stage to another [58]. In a second study, ticks (Haemaphysalis longicornis and Rhipicephalus microplus) were collected from dogs living in backyard farms sharing their living area with ‘Ca. M. haemobos’ positive sheep and goats. This study could demonstrate for the first time that dogs could be infected with the bovine HM species ‘Ca. M. haemobos’ and that 9% of the H. longicornis and 47.3% R. microplus harbor ‘Ca. M. haemobos’ [89]. ‘Candidatus M. haemobos’ positive ticks could be detected from both positive and negative dogs, and the authors concluded that further studies are necessary to evaluate whether ‘Ca. M. haemobos’ could be transmitted to alternative hosts by R. microplus under natural conditions. In another study, the same research group demonstrated that under experimental conditions, (i) ‘Ca. M. haemobos’ is transmitted from adult ticks to their eggs and larval stages, (ii) larval ticks can transmit ‘Ca. M. haemobos’ to BALB/c mice during feeding, and that (iii) negative larvae could acquire ‘Ca. M. haemobos’ from infected mice [88]. Thus, it could be shown for the first time that R. microplus ticks have vector competence and therefore probably play an important role in the epidemiology of HM infections in livestock farming, especially in pasture farming. Although this study produces very interesting results, it remains unclear if infected larval ticks would have also been able to infect cattle. The possible capacity of Rhipicephalus microplus as a vector for hemotrophic mycoplasmas was further supported by two other studies from Malaysia and Mexico [90,92]. In Malaysia, 36% of R. microplus collected from cattle were M. wenyonii positive but ‘Ca. M. haemobos’ negative [90]. In Mexico, M. ovis was confirmed in Rhipicephalus microplus ticks collected from infected sheep during an acute outbreak [92].
In Brazil, one study demonstrated that Amblyomma sculptum and Amblyomma ovale collected from wild boars also might serve as potential vectors for hemotrophic mycoplasmas [87]. A total of 164 engorged ticks were investigated and 8.69% of these ticks harbored HM, M. suis, or M. parvum as determined by sequence analyses. Also, Ixodes ricinus feeding on a M. wenyonii positive cow tested positive in Bosnia and Herzegowina [91,97]. However, there are also some studies which failed to identify any HM species in ticks collected from infected animals or in the surroundings of HM-positive herds. For example, Hornok and colleagues failed to detect HM DNA in various hard tick species (i.e., Ixodes ricinus, Dermacentor reticulatus, Haemaphysialis inermis, and Dermacentor marginatus) collected in a cattle herd with a known HM history (from cattle and vegetation) [56]. Similarly, Hofmann-Lehmann and colleagues were unable to detect M. wenyonii or ‘Ca. M. haemobos’ DNA in Ixodes ricinus ticks originating from infected dairy cows [11].
4. Conclusions
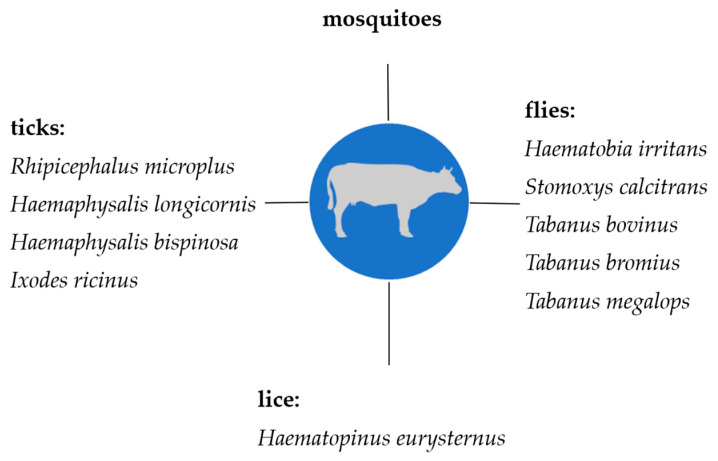
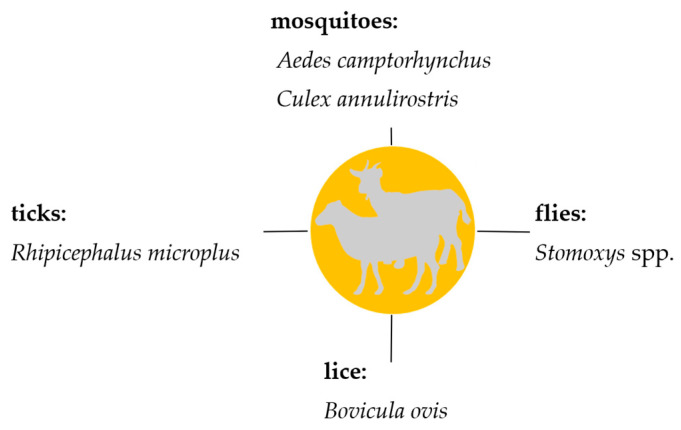
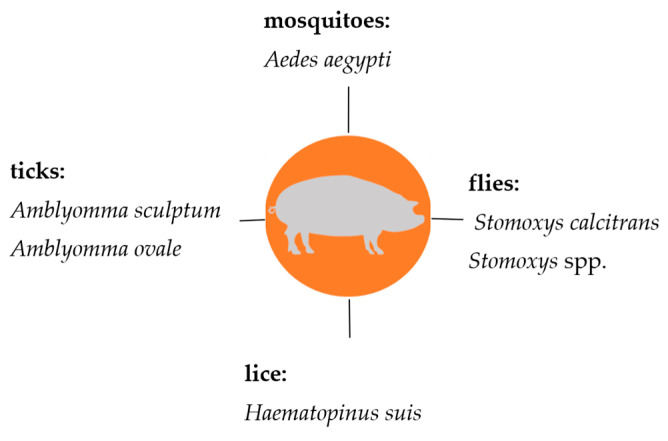
Knowledge on the transmission of hemotrophic mycoplasmas in livestock is very important, in particular, regarding prophylaxis and control strategies which currently mainly comprise biosecurity measures. This review summarizes the current knowledge on hematophagous arthropods as potential vectors for hemotrophic mycoplasmas in livestock. All suspected arthropod vectors described so far are presented in Figure 2 (cattle), Figure 3 (sheep and goats), and Figure 4 (pigs).
Figure 2.
Suspected arthropod vectors for hemotrophic mycoplasmas in cattle described so far.
Figure 3.
Suspected arthropod vectors for hemotrophic mycoplasmas in goats and sheep described so far.
Figure 4.
Suspected arthropod vectors for hemotrophic mycoplasmas in pigs described so far.
During recent years, there has been growing interest and evidence for arthropod-based transmission and different arthropods that might serve as potential vectors for HM species.
Molecular surveys of arthropods suggest that various arthropods should be considered as potential vectors. Only one molecular study demonstrated that ticks are able to transfer HMs from animal to animal [88]. The capacity of hematophagous arthropods as mechanical vector is further supported by older studies which have demonstrated that HM infections can be transmitted from infected animals to uninfected recipients. Currently, there is no evidence that hemotrophic mycoplasma species are able to replicate within arthropod vectors and there is only one study evidencing that HMs can be transmitted transstadial in ticks [88]. Proof of vector competency will require more experimental transmission studies with infected arthropods to gain knowledge about arthropod-dependent animal-to-animal transmission by ticks and other arthropod vectors. Seemingly, the vector competence of other arthropods should also be further investigated, for example, using omics methods. Future studies should also include a potential replication of HMs within arthropods and whether HMs are transmitted transovarially in the hematophagous arthropod species. At the moment, there are little data comparing the transmission of HMs by arthropod vectors in different climate regions and livestock holding systems; nevertheless, effective vector control tools should be integrated into current prophylaxis measures, including treatment against ectoparasites and environmental management, to reduce risks of HM infection [97]. Also, arthropod feeding behavior, survival of HMs in different vectors, and thus, the amount of HMs in arthropod vectors need to be considered to obtain more data on the potential of different arthropod vectors to infect animals.
Author Contributions
Writing—original draft preparation, M.A., K.H. and J.A.; writing—review and editing, J.S., M.R. and L.E.H. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hoelzle L.E. Haemotrophic mycoplasmas: Recent advances in Mycoplasma suis. Vet. Microbiol. 2008;130:215–226. doi: 10.1016/j.vetmic.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 2.Hoelzle L.E., Zeder M., Felder K.M., Hoelzle K. Pathobiology of Mycoplasma suis. Vet. J. 2014;202:20–25. doi: 10.1016/j.tvjl.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Groebel K., Hoelzle K., Wittenbrink M., Ziegler U., Hoelzle L. Mycoplasma suis invades porcine erythrocytes. Infect. Immun. 2009;77:576–584. doi: 10.1128/IAI.00773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoelzle K., Ade J., Hoelzle L.E. Persistence in livestock mycoplasmas—A key role in infection and pathogenesis. Curr. Clin. Microbiol. Rep. 2020;7:81–89. doi: 10.1007/s40588-020-00149-1. [DOI] [Google Scholar]
- 5.Paul B.T., Jesse F.F.A., Chung E.L.T., Che-Amat A., Mohd Lila M.A., Hashi H.A., Norsidin M.J. Review of clinical aspects, epidemiology and diagnosis of haemotropic Mycoplasma ovis in small ruminants: Current status and future perspectives in tropics focusing on Malaysia. Trop. Anim. Health Prod. 2020;52:2829–2844. doi: 10.1007/s11250-020-02357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schilling V. Eperythrozoon coccoides, eine neue durch Splenektomie aktivierbare Dauerinfektion der weissen Maus. Klin. Wochenschr. 1928;7:1853–1855. doi: 10.1007/BF01737905. [DOI] [Google Scholar]
- 7.Kikuth W. Über einen neuen Anämieerreger, Bartonella canis nov. spec. Klin. Wochenschr. 1928;7:1729–1730. doi: 10.1007/BF01738861. [DOI] [Google Scholar]
- 8.Splitter E.J. Eperythrozoon suis n. sp. and Eperythrozoon parvum n. sp., 2 new blood parasites of swine. Science. 1950;111:513–514. doi: 10.1126/science.111.2889.513. [DOI] [PubMed] [Google Scholar]
- 9.Fu Y., Shi T., Xu L., Wei W., Lu F., Zhang X., Yuan X., Li J., Lv J., Fang W. Identification of a novel Hemoplasma species from pigs in Zhejiang province, China. J. Vet. Med. Sci. 2017;79:864–870. doi: 10.1292/jvms.16-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler S., Ellenbogen V. A note on two new blood parasites of cattle, Eperythrozoon and Bartonella. J. Comp. Pathol. Therap. 1934;47:219–221. doi: 10.1016/S0368-1742(34)80027-6. [DOI] [Google Scholar]
- 11.Hofmann-Lehmann R., Meli M.L., Dreher U.M., Gönczi E., Deplazes P., Braun U., Engels M., Schüpbach J.R., Jörger K., Thoma R. Concurrent infections with vector-borne pathogens associated with fatal hemolytic anemia in a cattle herd in Switzerland. J. Clin. Microbiol. 2004;42:3775–3780. doi: 10.1128/JCM.42.8.3775-3780.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neitz W., Alexander R., Du Toit P.J. Eperythrozoon ovis (sp. nov.) infection in sheep. Onderstepoort J. Vet. Sci. Anim. Ind. 1934;3:263–271. [Google Scholar]
- 13.Hornok S., Meli M.L., Erdős A., Hajtós I., Lutz H., Hofmann-Lehmann R. Molecular characterization of two different strains of haemotropic mycoplasmas from a sheep flock with fatal haemolytic anaemia and concomitant Anaplasma ovis infection. Vet. Microbiol. 2009;136:372–377. doi: 10.1016/j.vetmic.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Neimark H., Johansson K.E., Rikihisa Y., Tully J.G. Proposal to transfer some members of the genera Haemobartonella and Eperythrozoon to the genus Mycoplasma with descriptions of ‘Candidatus Mycoplasma haemofelis’, ‘Candidatus Mycoplasma haemomuris’, ‘Candidatus Mycoplasma haemosuis’ and ‘Candidatus Mycoplasma wenyonii’. Int. J. Syst. Evol. Microbiol. 2001;51:891–899. doi: 10.1099/00207713-51-3-891. [DOI] [PubMed] [Google Scholar]
- 15.Neimark H., Johansson K.E., Rikihisa Y., Tully J.G. Revision of haemotrophic Mycoplasma species names. Int. J. Syst. Evol. Microbiol. 2002;52:683. doi: 10.1099/00207713-52-2-683. [DOI] [PubMed] [Google Scholar]
- 16.Tasker S., Helps C.R., Day M.J., Harbour D.A., Shaw S.E., Harrus S., Baneth G., Lobetti R.G., Malik R., Beaufils J.P., et al. Phylogenetic analysis of hemoplasma species: An international study. J. Clin. Microbiol. 2003;41:3877–3880. doi: 10.1128/jcm.41.8.3877-3880.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters I.R., Helps C.R., McAuliffe L., Neimark H., Lappin M.R., Gruffydd-Jones T.J., Day M.J., Hoelzle L.E., Willi B., Meli M., et al. RNase P RNA gene (rnpB) phylogeny of Hemoplasmas and other Mycoplasma species. J. Clin. Microbiol. 2008;46:1873–1877. doi: 10.1128/jcm.01859-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta R.S., Sawnani S., Adeolu M., Alnajar S., Oren A. Phylogenetic framework for the phylum Tenericutes based on genome sequence data: Proposal for the creation of a new order Mycoplasmoidales ord. nov., containing two new families Mycoplasmoidaceae fam. nov. and Metamycoplasmataceae fam. nov. harbouring Eperythrozoon, Ureaplasma and five novel genera. Antonie Van Leeuwenhoek. 2018;111:1583–1630. doi: 10.1007/s10482-018-1047-3. [DOI] [PubMed] [Google Scholar]
- 19.Zachary J., Smith A. Experimental porcine eperythrozoonosis: T-lymphocyte suppression and misdirected immune responses. Am. J. Vet. Res. 1985;46:821–830. [PubMed] [Google Scholar]
- 20.do Nascimento N.C., Guimaraes A.M.S., Dos Santos A.P., Chu Y., Marques L.M., Messick J.B. RNA-Seq based transcriptome of whole blood from immunocompetent pigs (Sus scrofa) experimentally infected with Mycoplasma suis strain Illinois. Vet. Res. 2018;49:49. doi: 10.1186/s13567-018-0546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stadler J., Willi S., Ritzmann M., Eddicks M., Ade J., Hoelzle K., Hoelzle L.E. Detection of Mycoplasma suis in pre-suckling piglets indicates a vertical transmission. BMC Vet. Res. 2019;15:252. doi: 10.1186/s12917-019-2001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niethammer F.M., Ade J., Hoelzle L.E., Schade B. Hemotrophic mycoplasma in Simmental cattle in Bavaria: Prevalence, blood parameters, and transplacental transmission of ‘Candidatus Mycoplasma haemobos’ and Mycoplasma wenyonii. Acta Vet. Scand. 2018;60:74. doi: 10.1186/s13028-018-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ade J., Ritzmann M., Wöstmann C., Eddicks M., Reese S., Hoelzle K., Hoelzle L.E., Stadler J. Update on shedding and transmission routes of porcine haemotrophic mycoplasmas in naturally and experimentally infected pigs. Porc. Health Manag. 2021;7:49. doi: 10.1186/s40813-021-00229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietz S., Mack S.L., Hoelzle K., Becker K., Jannasch C., Stadler J., Ritzmann M., Hoelzle L.E. Quantitative PCR analysis of Mycoplasma suis shedding patterns during experimental infection. Vet. Microbiol. 2014;172:581–585. doi: 10.1016/j.vetmic.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Ritzmann M., Grimm J., Heinritzi K., Hoelzle K., Hoelzle L.E. Prevalence of Mycoplasma suis in slaughter pigs, with correlation of PCR results to hematological findings. Vet. Microbiol. 2009;133:84–91. doi: 10.1016/j.vetmic.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Stadler J., Jannasch C., Mack S.L., Dietz S., Zöls S., Ritzmann M., Hoelzle K., Hoelzle L.E. Clinical and haematological characterisation of Mycoplasma suis infections in splenectomised and non-splenectomised pigs. Vet. Microbiol. 2014;172:294–300. doi: 10.1016/j.vetmic.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Henry S.C. Clinical observations on eperythrozoonosis. J. Am. Vet. Med. Assoc. 1979;174:601–603. [PubMed] [Google Scholar]
- 28.Zinn G., Jesse G., Dobson A. Effect of eperythrozoonosis on sow productivity. J. Am. Vet. Med. Assoc. 1983;182:369–371. [PubMed] [Google Scholar]
- 29.Henderson J., O’hagan J., Hawe S., Pratt M. Anaemia and low viability in piglets infected with Eperythrozoon suis. Vet. Rec. 1997;140:144–146. doi: 10.1136/vr.140.6.144. [DOI] [PubMed] [Google Scholar]
- 30.Strait E.L., Hawkins P.A., Wilson W.D. Dysgalactia associated with Mycoplasma suis infection in a sow herd. J. Am. Vet. Med. Assoc. 2012;241:1666–1667. doi: 10.2460/javma.241.12.1666. [DOI] [PubMed] [Google Scholar]
- 31.Schweighardt H., Fellner A., Pechan P., Leuermann E. Eperythrozoonose beim Schwein-ein Fallbericht. Wien. Tierarztl. Mschr. 1986;73:250–253. [Google Scholar]
- 32.Do Nascimento N.C., dos Santos A.P., Chu Y., Guimaraes A.M., Baird A.N., Weil A.B., Messick J.B. Microscopy and genomic analysis of Mycoplasma parvum strain Indiana. Vet. Res. 2014;45:86. doi: 10.1186/s13567-014-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.do Nascimento N.C., Dos Santos A.P., Chu Y., Guimaraes A.M., Pagliaro A., Messick J.B. Genome Sequence of Mycoplasma parvum (Formerly Eperythrozoon parvum), a Diminutive Hemoplasma of the Pig. Genome Announc. 2013;1 doi: 10.1128/genomeA.00986-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ade J., Hoelzle K., Stadler J., Ritzmann M., Hoelzle L.E. Occurrence of Mycoplasma parvum in German Pigs of Different Age Groups Using a Novel Quantitative Real-Time PCR Assay. Pathogens. 2022;11:1374. doi: 10.3390/pathogens11111374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petri F.A.M., Sonalio K., de Souza Almeida H.M., Ferraz M.E.S., Storino G.Y., de Souza M.R., André M.R., de Oliveira L.G. Porcine hemothropic mycoplasmas infection associated with productive impact in intensive pig production. Porc. Health Manag. 2020;6:33. doi: 10.1186/s40813-020-00171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonalio K., Perles L., Gatto I.R.H., do Amaral R.B., Almeida H.M., Galdeano J.V.B., Vieira R.F., Andre M.R., de Oliveira L.G. Genetic diversity of emerging hemotropic mycoplasmas in domestic pigs from Brazil. Transbound. Emerg. Dis. 2021;68:1162–1174. doi: 10.1111/tbed.13767. [DOI] [PubMed] [Google Scholar]
- 37.Seo M.G., Kwon O.D., Kwak D. Prevalence and phylogenetic analysis of hemoplasma species in domestic pigs in Korea. Parasit. Vectors. 2019;12:378. doi: 10.1186/s13071-019-3638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stadler J., Ade J., Ritzmann M., Hoelzle K., Hoelzle L.E. Detection of a novel haemoplasma species in fattening pigs with skin alterations, fever and anaemia. Vet. Rec. 2020;187:66. doi: 10.1136/vr.105721. [DOI] [PubMed] [Google Scholar]
- 39.Brissonnier M., Normand V., Lebret A., Moalic P.Y., Guyomard A.S., Bachy V., Berton P., Auvigne V., Bouchet F., Boulbria G. Frequency of infection with Mycoplasma suis in gestating sows using qPCR on ten commercial French herds, and impact of the infection on clinical, haematological and biochemical parameters. Porc. Health Manag. 2020;6:13. doi: 10.1186/s40813-020-00152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoelzle K., Engels M., Kramer M.M., Wittenbrink M.M., Dieckmann S.M., Hoelzle L.E. Occurrence of Mycoplasma suis in wild boars (Sus scrofa L.) Vet. Microbiol. 2010;143:405–409. doi: 10.1016/j.vetmic.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Toledo M.A., Leite A.I., Gonçalves L.R., Sousa K.C., Amaral R.B., Silva G.C., Machado R.Z., André M.R. High occurrence of Mycoplasma suis infection in swine herds from non-technified farms in Mossoró, state of Rio Grande do Norte, Northeastern Brazil. Rev. Bras. Parasitol. Vet. 2016;25:414–417. doi: 10.1590/s1984-29612016084. [DOI] [PubMed] [Google Scholar]
- 42.Martins M., Silva L.D., Miranda L.M., Lima C.A.A., Amaral R.B.D., Machado R.Z., André M.R., Braga M., Rosário C., Melo F.A., et al. Molecular detection of Mycoplasma suis in extensive pig production systems in the State of Maranhão, northeast Brazil. Rev. Bras. Parasitol. Vet. 2019;28:306–309. doi: 10.1590/s1984-296120180099. [DOI] [PubMed] [Google Scholar]
- 43.Bordin L.C., Gava D., Sonalio K., Mechler-Dreibi M.L., Zanella J.R.C., Morés N., de Oliveira L.G., Vaz E.K. Investigation of hemotropic Mycoplasmas in fetuses and sows with reproductive failure. Vet. Anim. Sci. 2021;12:100175. doi: 10.1016/j.vas.2021.100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe Y., Fujihara M., Suzuki J., Sasaoka F., Nagai K., Harasawa R. Prevalence of swine hemoplasmas revealed by real-time PCR using 16S rRNA gene primers. J. Vet. Med. Sci. 2012;74:1315–1318. doi: 10.1292/jvms.12-0096. [DOI] [PubMed] [Google Scholar]
- 45.Yuan C.L., Liang A.B., Yao C.B., Yang Z.B., Zhu J.G., Cui L., Yu F., Zhu N.Y., Yang X.W., Hua X.G. Prevalence of Mycoplasma suis (Eperythrozoon suis) infection in swine and swine-farm workers in Shanghai, China. Am. J. Vet. Res. 2009;70:890–894. doi: 10.2460/ajvr.70.7.890. [DOI] [PubMed] [Google Scholar]
- 46.Gatto I.R.H., Sonálio K., Amaral R.B.D., Morés N., Dalla Costa O.A., André M.R., de Oliveira L.G. High frequency and molecular characterization of porcine hemotrophic mycoplasmas in Brazil. Vet. Microbiol. 2019;231:33–39. doi: 10.1016/j.vetmic.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe Y., Fujihara M., Obara H., Nagai K., Harasawa R. Two genetic clusters in swine hemoplasmas revealed by analyses of the 16S rRNA and RNase P RNA genes. J. Vet. Med. Sci. 2011;73:1657–1661. doi: 10.1292/jvms.11-0293. [DOI] [PubMed] [Google Scholar]
- 48.Thongmeesee K., Kamkong P., Thanee S., Wattanapansak S., Kaewthamasorn M., Tiawsirisup S. Molecular detection and genetic analysis of porcine haemoplasmas in commercial pig farms from Thailand reveal a putative novel species. Transbound. Emerg. Dis. 2022;69:e2028–e2040. doi: 10.1111/tbed.14537. [DOI] [PubMed] [Google Scholar]
- 49.Fernandes A.J., Elshafie N.O., Kmetiuk L.B., Ullmann L.S., Brandão A.P.D., Haisi A., van Wilpe Bach R., de Barros-Filho I.R., Araújo Junior J.P., Barbosa D.S., et al. Hemotropic mycoplasmas (hemoplasmas) in wild boars, hunting dogs, and hunters from two Brazilian regions. Transbound. Emerg. Dis. 2022;69:908–912. doi: 10.1111/tbed.14038. [DOI] [PubMed] [Google Scholar]
- 50.dos Santos A.P., Guimaraes A.M., do Nascimento N.C., SanMiguel P.J., Messick J.B. Complete genome sequence of Mycoplasma wenyonii strain Massachusetts. J. Bacteriol. 2012;194:5458–5459. doi: 10.1128/jb.01240-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quiroz-Castañeda R.E., Martínez-Ocampo F., Dantán-González E. Draft genome sequence of Mycoplasma wenyonii, a second hemotropic Mycoplasma species identified in Mexican bovine cattle. Microbiol. Resour. Announc. 2018;7:10–1128. doi: 10.1128/mra.00875-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Díaz-Sánchez A.A., Corona-González B., Meli M.L., Álvarez D.O., Cañizares E.V., Rodríguez O.F., Rivero E.L., Hofmann-Lehmann R. First molecular evidence of bovine hemoplasma species (Mycoplasma spp.) in water buffalo and dairy cattle herds in Cuba. Parasites Vectors. 2019;12:78. doi: 10.1186/s13071-019-3325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nouvel L.X., Hygonenq M.-C., Catays G., Martinelli E., Le Page P., Collin É., Inokuma H., Schelcher F., Citti C., Maillard R. First detection of Mycoplasma wenyonii in France: Identification, evaluation of the clinical impact and development of a new specific detection assay. Comp. Immunol. Microbiol. Infect. Dis. 2019;63:148–153. doi: 10.1016/j.cimid.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 54.De Souza F.L., Ruegg P. Graduate Student Literature Review: Hemotropic mycoplasmas in cattle. J. Dairy. Sci. 2023;107:3185–3196. doi: 10.3168/jds.2023-24120. [DOI] [PubMed] [Google Scholar]
- 55.Hornok S., Micsutka A., Meli M., Lutz H., Hofmann-Lehmann R. Molecular investigation of transplacental and vector-borne transmission of bovine haemoplasmas. Vet. Microbiol. 2011;152:411–414. doi: 10.1016/j.vetmic.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 56.Hornok S., Micsutka A., de Mera I.F., Meli M.L., Gönczi E., Tánczos B., Mangold A.J., Farkas R., Lutz H., Hofmann-Lehmann R. Fatal bovine anaplasmosis in a herd with new genotypes of Anaplasma marginale, Anaplasma ovis and concurrent haemoplasmosis. Res. Vet. Sci. 2012;92:30–35. doi: 10.1016/j.rvsc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 57.Tagawa M., Matsumoto K., Inokuma H. Molecular detection of Mycoplasma wenyonii and ‘Candidatus Mycoplasma haemobos’ in cattle in Hokkaido, Japan. Vet. Microbiol. 2008;132:177–180. doi: 10.1016/j.vetmic.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Shi H., Hu Y., Leng C., Shi H., Jiao Z., Chen X., Peng Y., Yang H., Kan Y., Yao L. Molecular investigation of “Candidatus Mycoplasma haemobos” in goats and sheep in central China. Transbound. Emerg. Dis. 2019;66:22–27. doi: 10.1111/tbed.13021. [DOI] [PubMed] [Google Scholar]
- 59.Hoelzle K., Winkler M., Kramer M.M., Wittenbrink M.M., Dieckmann S.M., Hoelzle L.E. Detection of Candidatus Mycoplasma haemobos in cattle with anaemia. Vet. J. 2011;187:408–410. doi: 10.1016/j.tvjl.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 60.Flores-García D.L., Aguilar-Díaz H., Amaro-Estrada I., Martínez-Ocampo F., Quiroz-Castañeda R.E. An Update of Bovine Hemoplasmas Based on Phylogenetic and Genomics Analysis. Microorganisms. 2022;10:1916. doi: 10.3390/microorganisms10101916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McAuliffe L., Lawes J., Bell S., Barlow A., Ayling R., Nicholas R. The detection of Mycoplasma (formerly Eperythrozoon) wenyonii by 16S rDNA PCR and denaturing gradient gel electrophoresis. Vet. Microbiol. 2006;117:292–296. doi: 10.1016/j.vetmic.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Tagawa M., Yamakawa K., Aoki T., Matsumoto K., Ishii M., Inokuma H. Effect of chronic hemoplasma infection on cattle productivity. J. Vet. Med. Sci. 2013;75:1271–1275. doi: 10.1292/jvms.13-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meli M.L., Willi B., Dreher U.M., Cattori V., Knubben-Schweizer G., Nuss K., Braun U., Lutz H., Hofmann-Lehmann R. Identification, molecular characterization, and occurrence of two bovine hemoplasma species in Swiss cattle and development of real-time TaqMan quantitative PCR assays for diagnosis of bovine hemoplasma infections. J. Clin. Microbiol. 2010;48:3563–3568. doi: 10.1128/JCM.02224-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tagawa M., Matsumoto K., Yokoyama N., Inokuma H. Comparison of the effect of two hemoplasma species on hematological parameters in cattle. J. Vet. Med. Sci. 2010;72:113–115. doi: 10.1292/jvms.09-0304. [DOI] [PubMed] [Google Scholar]
- 65.Deshuillers P.L., Santos A.P., do Nascimento N.C., Hampel J.A., Bergin I.L., Dyson M.C., Messick J.B. Complete genome sequence of Mycoplasma ovis strain Michigan, a hemoplasma of sheep with two distinct 16S rRNA genes. Genome Announc. 2014;2:10–1128. doi: 10.1128/genomea.01235-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tagawa M., Takeuchi T., Fujisawa T., Konno Y., Yamamoto S., Matsumoto K., Yokoyama N., Inokuma H. A Clinical Case of Severe Anemia in a Sheep Coinfected with Mycoplasma ovis andCandidatus Mycoplasma haemovis’ in Hokkaido, Japan. J. Vet. Med. Sci. 2012;74:99–102. doi: 10.1292/jvms.11-0296. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki J., Sasaoka F., Fujihara M., Watanabe Y., Tasaki T., Oda S., Kobayashi S., Sato R., Nagai K., Harasawa R. Molecular identification ofCandidatus Mycoplasma haemovis’ in sheep with hemolytic anemia. J. Vet. Med. Sci. 2011;73:1113–1115. doi: 10.1292/jvms.11-0113. [DOI] [PubMed] [Google Scholar]
- 68.Hornok S., Hajtós I., Meli M., Farkas I., Gönczi E., Meili T., Hofmann-Lehmann R. First molecular identification of Mycoplasma ovis and ‘Candidatus M. haemoovis’ from goat, with lack of haemoplasma PCR-positivity in lice. Acta Vet. Hung. 2012;60:355–360. doi: 10.1556/avet.2012.030. [DOI] [PubMed] [Google Scholar]
- 69.Urie N.J., Highland M.A., Knowles D.P., Branan M.A., Herndon D.R., Marshall K.L. Mycoplasma ovis infection in domestic sheep (Ovis aries) in the United States: Prevalence, distribution, associated risk factors, and associated outcomes. Prev. Vet. Med. 2019;171:104750. doi: 10.1016/j.prevetmed.2019.104750. [DOI] [PubMed] [Google Scholar]
- 70.Machado C.A., Vidotto O., Conrado F.O., Santos N.J., Valente J.D., Barbosa I.C., Trindade P.W., Garcia J.L., Biondo A.W., Vieira T.S. Mycoplasma ovis infection in goat farms from northeastern Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2017;55:1–5. doi: 10.1016/j.cimid.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 71.Souza U.A., Oberrather K., Fagundes-Moreira R., Almeida B.A.d., Valle S.d.F., Girotto-Soares A., Soares J.F. First molecular detection of Mycoplasma ovis (Hemotropic mycoplasmas) from Sheep in Brazil. Rev. Bras. Parasitol. Veterinária. 2019;28:360–366. doi: 10.1590/s1984-29612019022. [DOI] [PubMed] [Google Scholar]
- 72.Aktas M., Ozubek S. A molecular survey of small ruminant hemotropic mycoplasmosis in Turkey, including first laboratory confirmed clinical cases caused by Mycoplasma ovis. Vet. Microbiol. 2017;208:217–222. doi: 10.1016/j.vetmic.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 73.Rjeibi M.R., Darghouth M.A., Omri H., Souidi K., Gharbi M., Rekik M. First molecular isolation of Mycoplasma ovis from small ruminants in North Africa. Onderstepoort J. Vet. Res. 2015;82:e1–e6. doi: 10.4102/ojvr.v82i1.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X., Cui Y., Zhang Y., Shi K., Yan Y., Jian F., Zhang L., Wang R., Ning C. Molecular characterization of hemotropic mycoplasmas (Mycoplasma ovis and ‘Candidatus Mycoplasma haemovis’) in sheep and goats in China. BMC Vet. Res. 2017;13:142. doi: 10.1186/s12917-017-1062-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galon E.M.S., Moumouni P.F.A., Ybanez R.H.D., Macalanda A.M.C., Liu M., Efstratiou A., Ringo A.E., Lee S.-H., Gao Y., Guo H. Molecular evidence of hemotropic mycoplasmas in goats from Cebu, Philippines. J. Vet. Med. Sci. 2019;81:869–873. doi: 10.1292/jvms.19-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prullage J., Williams R., Gaafar S. On the transmissibility of Eperythrozoon suis by Stomoxys calcitrans and Aedes aegypti. Vet. Parasitol. 1993;50:125–135. doi: 10.1016/0304-4017(93)90013-D. [DOI] [PubMed] [Google Scholar]
- 77.Howard G. The experimental transmission of Eperythrozoon ovis by mosquitoes. Parasitology. 1975;71:33. [Google Scholar]
- 78.Daddow K. Culex annulirostris as a vector of Eperythrozoon ovis infection in sheep. Vet. Parasitol. 1980;7:313–317. doi: 10.1016/0304-4017(80)90051-5. [DOI] [Google Scholar]
- 79.Acosta D.B., Ruiz M., Sanchez J.P. First molecular detection of Mycoplasma suis in the pig louse Haematopinus suis (Phthiraptera: Anoplura) from Argentina. Acta Trop. 2019;194:165–168. doi: 10.1016/j.actatropica.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Heinritzi K. Untersuchungen zur Übertragbarkeit von Eperythrozoon suis. Tierarztl. Umsch. 1992;47:588–599. [Google Scholar]
- 81.Song Q., Wang L., Fang R., Khan M.K., Zhou Y., Zhao J. Detection of Mycoplasma wenyonii in cattle and transmission vectors by the loop-mediated isothermal amplification (LAMP) assay. Trop. Anim. Health Prod. 2013;45:247–250. doi: 10.1007/s11250-012-0197-y. [DOI] [PubMed] [Google Scholar]
- 82.Reagan K.L., Clarke L.L., Hawley J.R., Lin P., Lappin M.R. Assessment of the ability of Aedes species mosquitoes to transmit feline Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’. J. Feline Med. Surg. 2017;19:798–802. doi: 10.1177/1098612X16658317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thongmeesee K., Chonglomkrod B., Srisakdi C., Saributr M., Suksai P., Kamkong P., Tiawsirisup S. Molecular detection of Mycoplasma wenyonii and its closely related hemotropic Mycoplasma sp. in blood-sucking flies from a buffalo farm in Chachoengsao province, Thailand. Acta Trop. 2022;235:106647. doi: 10.1016/j.actatropica.2022.106647. [DOI] [PubMed] [Google Scholar]
- 84.Schwarz L., Strauss A., Loncaric I., Spergser J., Auer A., Rümenapf T., Ladinig A. The Stable Fly (Stomoxys calcitrans) as a Possible Vector Transmitting Pathogens in Austrian Pig Farms. Microorganisms. 2020;8:1476. doi: 10.3390/microorganisms8101476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Overas J. Studies on Eperythrozoon ovis infection in sheep. Acta Vet. Scand. 1969;148:38–44. [PubMed] [Google Scholar]
- 86.Ballados-González G.G., Martínez-Hernández J.M., Martínez-Rodríguez P.B., Gamboa-Prieto J., González-Guzmán S., Paredes-Cervantes V., Grostieta E., Becker I., Aguilar-Domínguez M., Vieira R.F. Molecular detection of hemotropic Mycoplasma and Bartonella species in lice from sheep and goats of Mexico. Vet. Parasitol. Reg. Stud. Rep. 2023;44:100921. doi: 10.1016/j.vprsr.2023.100921. [DOI] [PubMed] [Google Scholar]
- 87.Santana M.d.S., Hoppe E.G.L., Carraro P.E., Calchi A.C., de Oliveira L.B., do Amaral R.B., Mongruel A.C.B., Machado D.M.R., Burger K.P., Barros-Batestti D.M. Molecular detection of vector-borne agents in wild boars (Sus scrofa) and associated ticks from Brazil, with evidence of putative new genotypes of Ehrlichia, Anaplasma, and haemoplasmas. Transbound. Emerg. Dis. 2022;69:e2808–e2831. doi: 10.1111/tbed.14632. [DOI] [PubMed] [Google Scholar]
- 88.Shi H., Duan L., Liu F., Hu Y., Shi Z., Chen X., Yang H., Yan B., Yao L. Rhipicephalus (Boophilus) microplus ticks as reservoir and vector of ‘Candidatus Mycoplasma haemobos’ in China. Vet. Parasitol. 2019;274:108929. doi: 10.1016/j.vetpar.2019.108929. [DOI] [PubMed] [Google Scholar]
- 89.Shi H., Li B., Li J., Chen S., Wang L., Bai Z., Zhu L., Yan B., Yao L. Molecular detection of haemophilic pathogens reveals evidence of Candidatus Mycoplasma haemobos in dogs and parasitic ticks in central China. BMC Vet. Res. 2022;18:254. doi: 10.1186/s12917-022-03361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohd Hasan L., Kho K., Koh F., Hassan Nizam Q., Tay S. Molecular evidence of hemoplasmas in Malaysian cattle and ticks. Trop. Biomed. 2017;34:668–674. [PubMed] [Google Scholar]
- 91.Stevanović O., Jurković D., Polkinghorne A., Ćeleš A., Ilić T., Dimitrijević S., Nedić D., Beck R. Molecular detection of Babesia divergens and Mycoplasma wenyonii infection in cattle from Bosnia And Herzegovina. Parasitol. Res. 2020;119:1423–1427. doi: 10.1007/s00436-020-06630-6. [DOI] [PubMed] [Google Scholar]
- 92.Martínez-Hernández J., Ballados-González G., Fernández-Bandala D., Martínez-Soto S., Velázquez-Osorio V., Martínez-Rodríguez P., Cruz-Romero A., Grostieta E., Lozano-Sardaneta Y., Colunga Salas P. Molecular detection of Mycoplasma ovis in an outbreak of hemolytic anemia in sheep from Veracruz, Mexico. Trop. Anim. Health Prod. 2019;51:243–248. doi: 10.1007/s11250-018-1648-x. [DOI] [PubMed] [Google Scholar]
- 93.Schofeld S., Torr S. A comparison of the feeding behaviour of tsetse and stable flies. Med. Vet. Entomol. 2002;16:177–185. doi: 10.1046/j.1365-2915.2002.00361.x. [DOI] [PubMed] [Google Scholar]
- 94.Baldacchino F., Desquesnes M., Mihok S., Foil L.D., Duvallet G., Jittapalapong S. Tabanids: Neglected subjects of research, but important vectors of disease agents! Infect. Genet. Evol. 2014;28:596–615. doi: 10.1016/j.meegid.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 95.Li R., Nie Y., Fu Y.-T., Deng Y.-P., Wang W., Ma P.-P., Liu G.-H. Characterization of the fragmented mitochondrial genome of domestic pig louse Haematopinus suis (Insecta: Haematopinidae) from China. Syst. Parasitol. 2023;100:571–578. doi: 10.1007/s11230-023-10106-3. [DOI] [PubMed] [Google Scholar]
- 96.Hornok S., Hofmann-Lehmann R., de Mera I.F., Meli M.L., Elek V., Hajtós I., Répási A., Gönczi E., Tánczos B., Farkas R. Survey on blood-sucking lice (Phthiraptera: Anoplura) of ruminants and pigs with molecular detection of Anaplasma and Rickettsia spp. Vet. Parasitol. 2010;174:355–358. doi: 10.1016/j.vetpar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 97.Ackermann D., Roden L., Robinson L. 2023 Veterinary Mycoplasmas Research Report. Insight Editing London; London, UK: 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.