Abstract
Background
Cervical ripening is crucial for induction. However, its influencing factors, mechanistic understanding, and effective risk stratification are still challenging. Recent research suggested that microorganisms and their metabolites in vaginal spaces correlate to preterm birth. However, it remains unclear whether the cervicovaginal metabolome is related to the natural physiological process of cervical maturation.
Objective
We aimed to analyze the cervicovaginal metabolome in women with favorable induction cervix and those unfavorable for induction when delivering at term.
Study design
Cervicovaginal swabs were collected between 40 and 41 weeks gestation from the following 2 different groups of patients: Ripe group (n = 25) which was favorable for the induction cervix and Unripe group which was unfavorable for the induction cervix (n = 25). Samples were tested using untargeted metabolomics analysis and analyzed by a bioinformatics platform. The correlation analysis between the metabolome and the previously acquired microbiome was also performed.
Results
A total of 629 metabolites were identified in cervicovaginal fluid. The cervicovaginal metabolome was significantly different between the women with the ripe cervix and those with the unripe cervix, especially within each stratum of the same CST. Metabolites within the amino acid, carbohydrate, and dipeptide pathways may play a role in this distinction. Thirty-four metabolites were significantly upregulated, and the remaining fourteen were significantly downregulated in the Unripe group with an unripe cervix unfavorable for induction. Statistical modeling identified Arachidonic Acid and Nicotinate associated with the risk of cervical maturation disorder (AUC 0.87) in negative ion mode. A combination of Choline and d-Mannose identified a risk of cervical maturation disorder (AUC 0.80) in positive ion mode, improved by Lactobacillus relative abundance (AUC 0.89).
Conclusion
These data suggested that the cervicovaginal space was metabolically active during pregnancy and significantly altered among the women with the mature and immature cervix. Combining the genera-level phylotypes and metabolites could build better cervix maturity prediction models. By using cervicovaginal fluid samples, we demonstrated the potential of multi-data type integration for developing composite models toward understanding the contribution of the vaginal environment to the remodeling of cervix during term pregnancy.
Keywords: Cervicovaginal metabolome, Cervical ripening, Pregnancy
1. Introduction
The cervix plays a crucial role in maintaining pregnancy and ensuring the safe and, timely delivery of a fetus. Cervical ripening is necessary for normal birth because it allows the fetus to pass through the cervix and vagina. This process refers to three phases of softening, ripening, and dilation that begin before the onset of labor contractions [1]. Premature cervical ripening can lead to preterm birth. Failure or disruption of cervical ripening during pregnancy results in various obstetric complications and often leads to drug treatment and induction or even cesarean delivery [2,3]. The process of cervical ripening is influenced by complicating factors that are still unclear.
Emerging data suggest an association between cervical microbes and the vaginal microenvironment [[4], [5], [6]]. The optimal and non-optimal cervicovaginal microbes have an important effect on the health of the reproductive tract. A disorder microbial community of the vagina named bacterial vaginosis (BV) has been related to poor obstetrical outcomes such as spontaneous preterm birth (sPTB) [7,8], and poor health outcomes such as the increased transmission of sexually transmitted infections including chlamydia, gonorrhea [9] and HIV [10,11]. Numerous researchers have found that the composition of the mother's microbiota, particularly the mother's vaginal microbiome, significantly correlated with sPTB. Microorganisms and their products can induce a local inflammatory response in gestational tissues (acute chorioamnionitis), leading to preterm labor [[12], [13], [14], [15]].
The cervix is between a sterile uterine microenvironment and a microbial-rich vaginal environment [16,17]. Cervical remodeling that has a short second-trimester cervical length has been well established as a risk factor for sPTB. Moreover, the relationship between the cervicovaginal microbial communities and the short cervix has been examined. The mixed anaerobic bacterial community or Lactobacillus iners has been associated with cervical shortening and subsequent sPTB [[18], [19], [20]]. Besides the vaginal microbiotas, the metabolome of vaginal microbiota also has been found to correlate with the reproductive tract health [5,21]. Although the field of pregnancy metabolomics is still emerging, existing research has shown that the cervicovaginal space is metabolically active during pregnancy, with a distinct cervicovaginal metabolome in sPTB and term birth women [19,22,23]. These studies suggest that metabolites may provide a lens into mechanisms underlying premature cervical remodeling.
Previously, we found that the vaginal microbiome significantly differed between the women with a mature cervix and those with an immature cervix [24]. In addition, several bacterial taxa at different levels were found to be different in these two groups, and the Lactobacillus profile seemed positive while the CST IV type seemed negative for the cervix ripening. It is unknown whether bacterial metabolites play a role in delayed cervical ripening. Therefore, the study's objective was to investigate whether there is a difference in cervicovaginal metabolome in women with favorable induction cervix and those with unfavorable for induction when delivering at term.
2. Materials and methods
2.1. Study population and sample collection
In this study, we conducted a prospective cohort study involving 500 low-risk nulliparous women with singleton pregnancies, 428 women delivered before 40 + 5 weeks and 22 women who were premature rupture of membranes were excluded, thus, 50 women remained in the study. From this cohort, biospecimens were obtained from 50 participants, from whom the case and control groups were derived (Fig. 1). The study protocol was approved by the Institutional Review Board of the Guangzhou Women and Children's Medical Center (approval number #202057401). Informed consent was obtained from all individual participants included in the study.
Fig. 1.
Flowchart illustrating women enrolled in this study. Abbreviation: PROM, premature rupture of membranes.
The inclusion criteria for this study were as follows: low-risk nulliparous women at gestational age between 40 weeks 0 days and 40 weeks 6 days, determined by calculating from the date of the first day of the last menstrual period and confirmed by ultrasound scan. These women had a live singleton fetus in cephalic presentation and no contraindication to vaginal delivery. Additionally, they had no planned cesarean delivery and were free from any condition that would indicate the need for delivery before 40 weeks 5 days, such as hypertensive disorders of pregnancy or suspected fetal growth restriction. Exclusion criteria included women who were in labor, experienced premature rupture of membranes, had vaginal bleeding, had family history of diabetes, or had non-reassuring fetal status requiring immediate delivery. All the participants in our study were no smokers. Women who had engaged in vaginal activity within 24 h prior to specimen collection were also excluded.
The cases, labeled as "Ripe,” were defined as women with a Bishop Score of ≥6 (n = 25). Controls, labeled as "Unripe”, were defined as women with a Bishop Score between 0 and 4 (n = 25). Cervicovaginal biospecimens were collected under direct vision by the same attending obstetrician using a sterile Dacron swab from the posterior vaginal fornix before the digital vaginal examination (Bishop Scoring). The samples were collected in 0.5 mL phosphate-buffered saline, immediately frozen in liquid nitrogen, and stored at −80 °C for future use. Based on the preliminary analysis of the microbiome using 16S ribosomal RNA gene high-throughput sequencing (the data has been submitted for publication), the same set of samples was further subjected to metabolomic analysis in this study.
2.2. Sample preparation and untargeted metabolomics analysis
Metabolomics analysis was performed by Shanghai Applied Protein Technology Co. Ltd. Samples of 100 μL were extracted with 400 μL of cold methanol/acetonitrile/water (2:2:1, v/v). Analyses were performed using an Ultrahigh pressure liquid chromatography (1290 Infinity LC, Agilent Technologies) coupled to a quadrupole time-of-flight (AB Sciex TripleTOF 6600) with an electrospray ionization source operating in positive and negative ion modes. In order to be detected by mass spectrometry, substances need to be ionized. Depending on the properties or structural characteristics of the compounds, some substances tend to acquire a positive charge during ionization, while others tend to acquire a negative charge. Positive ions can be detected in positive ion mode, while negative ions can be detected in negative ion mode. Therefore, for untargeted metabolomics analysis, it is necessary to pay attention to the results obtained from both modes.
2.3. Data processing and statistical analysis
The raw MS data were converted to MzXML files using ProteoWizard MSConvert and then importing them into XCMS software [25,26]. After normalized to total peak intensity, the processed data were analyzed by R [27]. Statistical analysis included univariate analysis and multivariate analysis. Univariate statistical analysis included Student's t-test or Wilcoxon test and variance multiple analysis or Kruskal-Wallis test, Fisher's exact test. Multivariate statistical analysis included principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA), and orthogonal partial least squares discriminant analysis (OPLS-DA). The resulting P-values were corrected by false discovery rates calculated using the Benjamini-Hochberg (BH) method. The calculation of adjusted p-values in the Benjamini-Hochberg procedure involves comparing each individual p-value to a critical value or threshold. The critical value is determined based on the desired false discovery rate (FDR) control. The 16S RNA gene sequencing data was from our previous research, which has been submitted for publication. Prediction analyses with stepwise logistic regression were conducted on the relationship between the cervicovaginal microbiome and metabolome in R version 3.4.1.
3. Results
3.1. Demographic characteristics of the study population
A total of 50 age-matched individuals (25 each for ripe and unripe groups) were enrolled in the metabolic profiling study (Fig. 1), which was also conducted in the matched 16S RNA gene sequencing analysis previously (under review). The general demographic and clinical characteristics of the study participants were summarized in Table 1. The age, height, weight, body mass index, gestational age, nulliparity, and birth weight of neonates between the two groups showed no statistical difference (Table 1). The samples were classified into community state types (CST) based on the previously obtained microbiome composition (Table S1).
Table 1.
Clinical and demographic characteristics of participants with ripe and unripe cervix [24].
| Ripe group | Unripe group | P | CI (95 %) | |
|---|---|---|---|---|
| Age at conception in years; Median (IQR) | 30 (26–32.75) | 29 (26–31.75) | 0.696 | 27.9–30.3 |
| Gestational Age(days); Median (IQR) | 286 (285–286) | 286 (285–286) | 0.637 | 39.5–40.6 (2.5–8.1) |
| Height at conception in cm mean (IQR) | 160.97 (157.00–165.00) | 161.23 (157.75–165.00) | 0.539 | (-45.8) −55.2 |
| Weight at conception in kilograms mean (IQR) | 68.56 (63.00–73.75) | 68.84 (63.75–75.00) | 0.894 | 49.9–89.0 |
| BMI at conception; Median (IQR) | 26.38 (25.31–27.84) | 26.04 (24.81–27.73) | 0.697 | 15.3–36.8 |
3.2. Quality control of UHPLC-Q-TOF/MS analysis
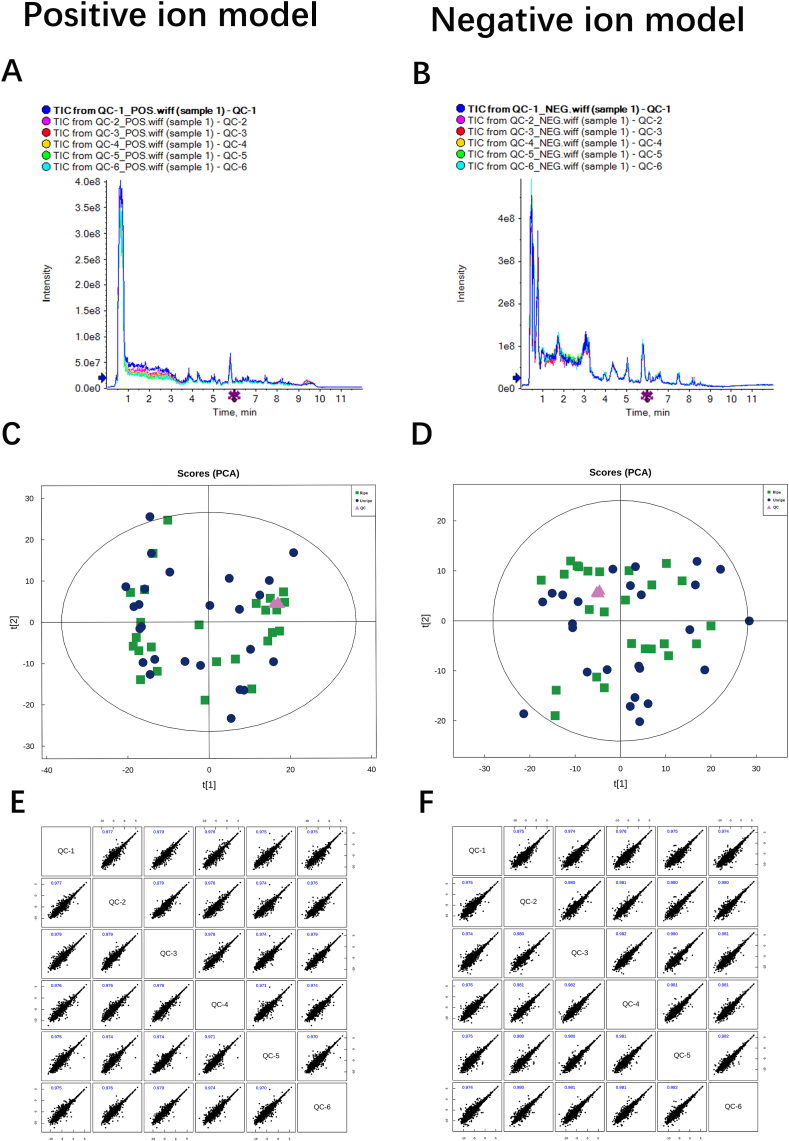
As shown by Fig. 2 and S1, the instrumental error and experiment repeatability were evaluated by several analyses using the quality control (QC) samples and reflected mild instrument volatility and good repeatability. These results indicated that the data were reliable and could be used for subsequent analysis.
Fig. 2.
Quality control (QC) of UHPLC-Q-TOF/MS analysis. A and B Total ion chromatograms in positive and negative ion modes for QC samples. C and D Principal component analysis (PCA) score charts in the positive and negative ion modes. Group Ripe with a mature cervix is shown as green squares, group Unripe with immature cervix is shown as blue dots, and QC samples are shown as a red triangle. E and F Pearson correlation map of QC samples for the positive and negative ion modes.
3.3. Cervicovaginal metabolome
A total of 629 metabolites were identified in the cervicovaginal samples combined with positive (372) and negative (257) ion modes with ≥ level 2 annotation (Fig. S2).
3.4. Multivariate analysis of UHPLC-Q-TOF/MS data
The OPLS-DA showed that the metabolomic profiles of the Ripe group were separated from the Unripe group for both positive (R2X = 0.286, R2Y = 0.641, Q2 = 0.132, Fig. 3A) and negative ion modes (R2X = 0.203, R2Y = 0.709, Q2 = 0.321, Fig. 3B). This suggested that the cervix showed significant differences in the biochemical metabolites at different cervical maturity during late pregnancy. The crossvalidation of the permutation test plot proved that the OPLS-DA model was reliable (Fig. 3C and D), as the line plot intercepted Y-axis below zero, and all permutated values were lower than the original values at the right. We also analyzed the possibility that the metabolomic profile differed by the cervix mature degree within each stratum of cervicovaginal CST. The CST types had got from our previous research from the microbiome analysis (Table S1). Considering the CST types, metabolite profiles clustered better within each stratum of the CST rather than directly according to the cervix mature degree (Fig. S3). Significant differences in the cervicovaginal metabolome between the cervical maturity women (Ripe) and immaturity women (Unripe) within common microbial communities were obvious.
Fig. 3.
Orthogonal partial least square-discriminate analysis (OPLS-DA) score charts and cross-validation test in the positive and negative ion modes. A and B Score plot of OPLS-DA in the positive and negative ion modes. C and D Cross-validation graph of permutation test results of the OPLS-DA model in the positive and negative ion modes.
3.5. Univariate analysis of UHPLC-Q-TOF/MS data
Volcano plots were created according to the p-value (<0.05) and fold change (>1.5 or < 0.67) that showed the dysregulated metabolites (Fig. 4). The results also indicated that both ion modes significantly separated the Ripe and Unripe groups.
Fig. 4.
Volcano map based on fold change (FC) analysis and statistical test. A and B Volcano plot in the positive and negative ion modes. Each point in the volcano plot represents a metabolite. Black dots represent metabolites with no significant difference. The red and blue dots in the figures are differential metabolites with P < 0.05 and FC > 1.5 or FC < 0.6. Differentially abundant metabolites of different categories are individually color coded.
Based on the difference between the Ripe and Unripe groups of the OPLS-DA model, variable values were set at variable importance for the projection (VIP) > 1 and P < 0.05 to select those metabolites with obvious discrimination potential. In total, 21 differential metabolites in positive ion mode (Table S2) and 27 in negative ion mode (Table S3) were identified in the cervicovaginal fluid between two groups.
The level of metabolites was used to perform hierarchical clustering of each group of samples to evaluate the rationality of metabolites and to intuitively display the relationship between samples and the differences in the expression of metabolites in different samples. As the metabolic heatmap shown in Fig. 5, compared with the Ripe group, the metabolites of the Unripe group changed significantly. The differences in these metabolites may be related to cervical ripening.
Fig. 5.
The metabolite heatmaps between ripe and unripe groups. A Negative ion mode; B Positive ion mode. The ordinate represents the metabolites that are significantly differently expressed and the abscissa is the sample's information. Red represents significantly up-regulated metabolites, blue represents significantly down-regulated metabolites, and the white part represents no quantitative information on the metabolites.
The top 5 upregulated metabolites in positive ion mode were Choline, Ser-Pro, Tyramine, 4-Aminobenzoate, and Larixinic Acid. Conversely, the top 5 downregulated metabolites in positive ion mode were Thr-Arg, Thioetheramide-PC, Creatine, Pro-Arg, and l-Carnitine (Fig. 6 A). The top 5 upregulated metabolites in negative ion mode were 2-hydroxy-butanoic acid, 2-Hydroxy-3-methyl butyric acid, Xanthine, Nicotinate, and Deoxyinosine. Conversely, the top 5 downregulated metabolites in negative ion mode were Salicylic acid, Stearic acid, Azelaic acid, (R)-2-Hydroxycaprylic acid, and 3-Phosphoserine (Fig. 6B).
Fig. 6.
Histogram of significantly differentially detected metabolites in positive ion mode (A) and negative ion mode (B). The x-axis shows the fold change; the y-axis corresponds to the differential metabolites. Differentially abundant metabolites of different categories are individually font color coded. Green and red indicate decreased and increased differential metabolites compared between ripe and unripe, respectively.
Spearman correlation analysis was performed to investigate the correlation between different metabolites in Ripe and Unripe groups (Fig. 7). The two compounds with the most significant differential fold change were 2-hydroxy-butanoic acid and choline (Fig. 6), which were significantly correlated with 9 and 15 different metabolites, respectively.
Fig. 7.
Spearman correlation analysis of microbiota and metabolites (A) and Hierarchical clustering heatmap of differentially expressed microbiota and metabolites (B). The correlation coefficients were depicted in blue and red, where red represents a positive correlation and blue or green represents a negative correlation. The darker the color, the stronger the correlation. P Value reflects the significant level of correlation, 0.01 < P < 0.05, expressed by *, P < 0.01, expressed by **.
3.6. Metabolic pathway analysis
To further determine which metabolic and signal transduction pathways were significantly affected, we performed a KEGG pathway analysis of the differential metabolites. The results showed that 10 pathways were significantly altered, and membrane transport was highly altered (Fig. 8). It was worth noting that the differential metabolite choline significantly affected four pathways, including ABC transporters, bile secretion, glycine, serine, and threonine metabolism, and Choline metabolism in cancer (Fig. 8). The metabolites related to carbohydrate metabolisms, including fructose, mannose, and galactose metabolism in women with an unripe cervix, revealed upregulation (Fig. 8). Three metabolites related to amino acid metabolism were upregulated in the Unripe group, while two were downregulated (Fig. 8).
Fig. 8.
Metabolic pathway analysis. (A) Analyses of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment. The x-axis shows the enrichment factor; the y-axis corresponds to the KEGG pathway. The size of the dot represents the number of metabolites mapped to the reference pathways. The color of the dot represents the P-value. (B) A pathway-based analysis of metabolic differences upon comparing Unripe group with immature cervix and Ripe group with mature cervix. The differential abundance score captures the average, gross changes for all metabolites in a pathway. A score of 1 indicates all measured metabolites in the pathway increase. The differential color of pathway is mapped into corresponding category of pathway hierarchy.
3.7. Relationship between cervicovaginal metabolites and previous microbiota
In our previous study, we observed significant differences in the relative abundance of Lactobacillus, Pseudoxanthomonas, TX1A-55, Agromyces, Arthrobacter, and bacterium YC-ZSS-LKJ57 between the Ripe and Unripe groups (Fig. S4). To explore the relationship between these different cervicovaginal genera and associated metabolites, a spearman correlation analysis was conducted. The results were presented in a heatmap (Fig. 7). We found a strong correlation between Lactobacillus and 2-hydroxy-butanoic acid (−0.59), 2-Hydroxy-3-methyl butyric acid (−0.58), Pro-Arg (0.51), and Thr-Arg (0.71).
3.8. Developing cervix maturity degree models using genera-level phylotypes, and metabolites
Exploratory statistical modeling was undertaken to gain insight into which metabolites and bacteria influence the risk of cervix maturity prediction. Using genera-level phylotypes (Fig. S4) alone for prediction estimates and stepwise logistic regression, only Lactobacillus remained significant. The ROC curve area for this microbiota model was 0.76. The accuracy, specificity, and sensitivity for the Lactobacillus model were 0.70, 0.92, and 0.48, respectively (Fig. 9A).
Fig. 9.
Receiver operating characteristic (ROC) curves areas and AUC for composite models for cervix maturity predictions. A Model using the phylotypes alone. B Model using the metabolites alone. C Model combined the metabolite and microbiota composite.
Considering metabolites alone, A composite of 2 significant metabolites (positive ion mode: Choline and d-Mannose, AUC 0.80; negative ion mode: Arachidonic Acid and Nicotinate, AUC 0.87) respectively in two ion modes were retained in the model that could predict cervix maturity (Fig. 9 B and C). The accuracy, specificity, and sensitivity for the 2-metabolites model in positive ion mode were 0.70, 0.72, and 0.68, respectively, and 0.82, 0.84, 0.80, respectively.
We further assessed the metabolite composite model by combining it with the phylotypes to assess prediction performance. The stepwise logistic regression selected Lactobacillus to the metabolite model could improve its performance (AUC 0.89) in the positive ion mode model (Fig. 9D). The accuracy, specificity, and sensitivity of the 2-metabolite combined Lactobacillus model were 0.80, 0.88, and 0.72, respectively. However, no phylotypes were selected through the selection in the negative model.
4. Discussion
4.1. Principal findings
Our data demonstrated that 48 out of 561 differential metabolites were identified in the cervicovaginal samples between ripe cervix group and unripe cervix group, 34 metabolites were significantly upregulated, while the remaining 14 were significantly downregulated in the unripe cervix group. Statistical modeling showed Arachidonic Acid and Nicotinate associated with the risk of delayed cervical maturation (AUC 0.87) in negative ion mode. A combination of Choline and d-Mannose identified a risk for delayed cervical maturation (AUC 0.80) in positive ion mode, improved by Lactobacillus relative abundance (AUC 0.89).
Arachidonic acid (AA) is a polyunsaturated fatty acid that serves as a precursor for the synthesis of various bioactive lipid compounds, including prostaglandins [28]. The precise regulation of Prostaglandin synthesis and activity is crucial for successful reproductive outcomes. Arachidonic Acid acts as a key component in the production of Prostaglandins, enabling their involvement in ovulation, fertilization, implantation, pregnancy maintenance, and labor initiation [29]. Nicotinate, also known as nicotinic acid or niacin, is a form of vitamin B3 [30,31]. Nicotinate, as a form of vitamin B3, does not directly participate in cervical ripening or prostaglandin production. However, maintaining appropriate nutritional status, including sufficient intake of essential vitamins, can support overall reproductive health [32]. Choline is an essential nutrient that plays a vital role in various physiological processes in the body [33]. When it comes to cervical health, choline indirectly contributes to maintaining the integrity and function of cervical cells. Choline indirectly supports cervical health by contributing to the proper structure and function of cervical cells [34,35]. Ensuring an adequate intake of choline through a balanced diet can help promote optimal cervical function and overall reproductive well-being. d-Mannose is a sugar that is commonly used as a dietary supplement for urinary tract health [36]. While its direct effects on the cervix are not well-established, maintaining a healthy urinary tract environment with d-Mannose may indirectly contribute to a healthy cervical environment by reducing the risk of urinary tract infections and potential complications associated with bacterial infections [36,37]. Arachidonic acid may play an important role in pregnancy complications such as preeclampsia. First, arachidonic acid, as an inflammatory modulator, may influence the pathogenesis of preeclampsia by regulating prostaglandin levels and the synthesis of other bioactive lipids [38]. Second, the elevated level of PGT in placental tissue in patients with preeclampsia may promote the proliferation of trophoblast cells and arachidonic acid synthesis may be closely related to this process [39]. Therefore, the metabolism and action of arachidonic acid may affect trophoblast function and thus the development of preeclampsia.
In summary, substances like arachidonic acid, nicotinate, choline, and d-Mannose are discussed about cervical health and reproductive outcomes, and their roles in cervical ripening vary. Arachidonic acid is involved in prostaglandin synthesis and cervical ripening, while nicotinate supports overall reproductive health. Choline indirectly contributes to cervical health by maintaining the integrity and function of cervical cells. d-Mannose primarily focuses on urinary tract health, indirectly promoting a healthy cervical environment by reducing the risk of urinary tract infections. By in-depth study of the mechanism of action of arachidonic acid in reproductive health and pregnancy complications, its potential role in diseases such as preeclampsia can be better understood, providing more insights for future treatment and prevention measures. These studies will help uncover the key role of arachidonic acid in reproductive diseases and provide new ideas for the development of treatments for these diseases.
4.2. Results in the context of what is known
Several investigators investigated the metabolome in cervicovaginal fluid and its association with preterm birth [18,23,26,[40], [41], [42], [43]]. Some of the discriminatory metabolites identified in our research have been previously reported [18,23,26,40,41,43] and have biologically plausible roles in mediating vaginal health and disease [42]. For example, increased vaginal levels of biogenic amines are associated with activation of pro-inflammatory pathways, which contribute to reduced barrier integrity of epithelia [44]. At the same time, some metabolites were reported for the first time in the cervicovaginal space that might correlate to the cervix ripening (Fig. 6, Fig. 8).
Membrane transport and carbohydrate metabolisms were the top two significantly altered pathways, and all metabolites in these two pathways were upregulated in women with cervix immaturity (Fig. 8). Glycogen is deposited in large amounts when highly estrogenic states and metabolized to lactic acid in the epithelial cells [45]. These activities were related to the membrane transport and carbohydrate metabolism pathways. Microbiota uses glycogen as a substrate and is thought to support lactobacilli colonization [46,47] during a healthy pregnancy. The significant upregulation of carbohydrate metabolism was also reported in women with preterm delivery [23]. It has been reported that down-regulation of carbohydrate metabolism in women destined to have a term delivery may represent a ‘quiescent state’ in the CV space [23]. These findings indicated that women with cervix immaturity used more active energy, whereas those with cervix maturity benefited from the nature of birth and appeared to maintain a relatively steady energy state regulation in late pregnancy. The cervix remodeling before delivery involves the coordination of immune cell activities for degradation of collagen structure in the extracellular matrix presented as the physiological inflammation [47]. Sialic acid is a ubiquitous cell-signaling molecule with essential roles in immunity [48]. A significant increase among women with preterm birth compared to term birth has been reported, while our results revealed a significantly decreased number of women with cervical immaturity compared to cervical maturity (Fig. 6). Its notable increase among women with cervix maturity may represent enhanced immunization activities. Overall, a relatively steady energy metabolic state and active immune cell activities seemed to present in the women with cervical maturity favorable for induction compared to the women with cervical immaturity unfavorable for induction.
The upregulated metabolites numbers of amino acid metabolism pathway were relatively more in women with unripe cervix than in women with a ripe cervix. The observation supported our prior results that a significantly high frequency of vaginal CST IV was observed in women whose cervix was immature. It has been reported that anaerobic organisms preferentially utilize amino acids as carbon and nitrogen energy sources, in contrast to Lactobacillus species, thereby leading to an increased abundance of amino acid catabolites [43,49]. Alternatively, anaerobes may produce more significant amounts of amino acids, converting them to biogenic amines (such as tyramine presented in Fig. 6) or other metabolites. Similar to the results of the metabolome in the cervicovaginal fluid of preterm birth study [42], when comparing ripe cervix to unripe cervicovaginal microbiota, metabolites in the amino acid super pathway were both significantly upregulated and downregulated. This observation may reflect physiological shifts to maintain homeostasis in response to distinct microenvironments. Therefore, metabolites could provide metabolomic equipoise by increasing some metabolites to counteract those decreased metabolites.
Among the different significant metabolites, most dipeptides decreased among the women with an unripe cervix, consistent with the report that dipeptides decreased among the women with preterm birth [23] (Fig. 6). The decrease in dipeptides may represent alterations in proteases and their inhibitors among women with cervix immaturity, as the expression change of proteases and protease inhibitors have been detected before spontaneous labor [50,51]. This may not be conducive to collagen degradation leading to cervical maturation disorders.
The 2-hydroxy-butanoic acid significantly increased in the women with unripe cervix and strongly correlated with Lactobacillus. The previous study found that 2-hydroxy-butanoic acid is primarily produced in l-threonine metabolism or glutathione synthesis as a coproduct of protein metabolism associated with microbiota [52]. It regulated the expression of genes involved in inflammation, oxidative stress, cell proliferation, and DNA damage process [38]. So, the 2-hydroxy-butanoic acid might play a role in the cervix remodeling process by regulating the inflammation and oxidative stress under the Lactobacillus dysfunctional state, which is revealed as the unripe cervix that is unfavorable for induction.
Choline, betaine, and carnitine were involved in the trimethylamine synthesis pathway [53]. Choline and betaine were higher in the unripe group compared with the ripe group, while carnitine was converse. It was consistent with the result that choline and betaine were higher in Lactobacillus iners and mixed dysbiotic groups [18], as the main CST types of cervix immaturity group were CST III (Lactobacillus iners) and CSTIV (Diverse microbes and higher proportion of anaerobes) according to our previous study. Simultaneously, choline was the second-highest fold change metabolite at 8.2. Our previous animal study also found that prolonged nicotine gestation inhibited cervical ripening in pregnant rats [3]. A high-choline diet has also been found to exacerbate cardiac dysfunction and myocardial fibrosis [54]. As the main component of the cervix is collagen, a high concentration of choline in the cervix inhibited cervical ripening. The fibrogenic effect of choline and nicotine activates the cholinergic anti-inflammatory pathway, which is vital in regulating local and systemic inflammation.
A simpler model comprising Arachidonic Acid and Nicotinate was the best predictor in negative ion mode. A composite model including two metabolites, Choline and d-Mannose, plus the inclusion of Lactobacillus, was useful in positive ion mode. However, the highest fold change metabolite 2-hydroxy-butanoic acid was not included in the models, which might be due to the correlation of the metabolite to Lactobacillus.
4.3. Research implications
The results of this study revealed a relationship between the metabolome and cervical maturity, and proposed a predictive model for assessing cervical maturity. This has significant implications for understanding the mechanisms underlying cervical maturity and predicting associated physiological processes. Further research and validation of this model could provide a simple and effective clinical approach for evaluating cervical maturity and implementing appropriate interventions. These findings hold potential to enhance obstetric care and optimize maternal healthcare.
4.4. Strengths and limitations
Our results first uncovered a correlation between metabolites and cervical maturation degree. To improve the reliability and generalizability of our findings, we need to validate these results with a larger sample size and diverse populations. The assessment of host defense peptides will also enhance our understanding of the functional roles of the identified microbial taxa and their impact on immune and inflammatory status. Furthermore, including data on diet, environment, and social stressors will provide valuable insights into the complex interactions between microbiota and host health. Considering these additional factors is crucial in future studies to strengthen the scientific significance of our research.
5. Conclusion
In summary, we shared the difference in the cervicovaginal metabolome in women with favorable induction cervix and those unfavorable for induction at term delivery. We have developed 2-metabolites combined (Choline and d-Mannose) with Lactobacillus and 2-metabolites (Arachidonic Acid and Nicotinate) statistical models in positive and negative ion modes that may predict the unripe cervix risk. The metabolites we found to be associated with Lactobacillus may be helpful as microbiome cultivation approaches are developed to direct the composition of the vaginal microbiome. Future research should incorporate multiple omics techniques to focus on larger prospective cohorts and mechanisms mediating microbiota-immune interactions in cervical remodeling and ripening.
Funding statement
This study was supported by the Natural Science Foundation of Guangdong Province, China (2023A1515010112, 2021A1515010597), and the NSFC-Guangdong Joint Fund Project (U1901210).
Disclosure statement
The authors report no conflict of interest.
Condensation
This was the first study to uncover cervicovaginal metabolome differences between the women with favorable cervix for induction and those with unfavorable cervix and revealed that the vaginal environment contributed to cervical remodeling during term pregnancy.
A Glance.
- A.Why was this study conducted?
- •Despite being extensively studied, the role of vaginal metabolome in the process of cervical remodeling remains unclear.
- •The differences between vaginal metabolome at different cervical maturity were analyzed during late pregnancy to make up for the lack of research in this field.
- B.What are the key findings?
- •The cervicovaginal metabolites of Choline and d-Mannose identified cases of delayed cervical maturation (AUC 0.80), which was improved by Lactobacillus relative abundance (AUC 0.89).
- C.What does this add to what is known?
- •Delayed cervical maturity could be predicted by detecting vaginal microbiome and metabolome.
Data availability statement
All the relevant data are included in the manuscript and the supplementary document. The raw metabolomic data in this paper have been deposited in the MetaboLights of EMBL-EBI (https://www.ebi.ac.uk/metabolights/) under accession number MTBLS10143.
CRediT authorship contribution statement
Ping Chen: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Tingting Hu: Writing – original draft, Methodology. Zheng Zheng: Methodology, Investigation, Data curation. Robert E. Garfield: Writing – review & editing, Supervision. Jinying Yang: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34166.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Word R.A., Li X.H., Hnat M., Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin. Reprod. Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 2.Nallasamy S., Mahendroo M. Distinct roles of cervical epithelia and stroma in pregnancy and parturition. Semin. Reprod. Med. 2017;35:190–200. doi: 10.1055/s-0037-1599091. [DOI] [PubMed] [Google Scholar]
- 3.Yang J., Shi S.Q., Shi L., Liu H., Fang D., Garfield R.E. Nicotine treatment prolongs gestation and inhibits cervical ripening in pregnant rats. Am. J. Obstet. Gynecol. 2014;210:76 e71–e77. doi: 10.1016/j.ajog.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Nam K.H., Kim Y.T., Kim S.R., Kim S.W., Kim J.W., Lee M.K., Nam E.J., Jung Y.W. Association between bacterial vaginosis and cervical intraepithelial neoplasia. J Gynecol Oncol. 2009;20:39–43. doi: 10.3802/jgo.2009.20.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilhan Z.E., Laniewski P., Thomas N., Roe D.J., Chase D.M., Herbst-Kralovetz M.M. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine. 2019;44:675–690. doi: 10.1016/j.ebiom.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitra A., MacIntyre D.A., Marchesi J.R., Lee Y.S., Bennett P.R., Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. 2016;4:58. doi: 10.1186/s40168-016-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillier S.L., Nugent R.P., Eschenbach D.A., Krohn M.A., Gibbs R.S., Martin D.H., Cotch M.F., Edelman R., Pastorek J.G., 2nd, Rao A.V. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N. Engl. J. Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 8.Leitich H., Bodner-Adler B., Brunbauer M., Kaider A., Egarter C., Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am. J. Obstet. Gynecol. 2003;189:139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 9.Wiesenfeld H.C., Hillier S.L., Krohn M.A., Landers D.V., Sweet R.L. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin. Infect. Dis. 2003;36:663–668. doi: 10.1086/367658. [DOI] [PubMed] [Google Scholar]
- 10.Atashili J., Poole C., Ndumbe P.M., Adimora A.A., Smith J.S. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eastment M.C., McClelland R.S. Vaginal microbiota and susceptibility to HIV. AIDS. 2018;32:687–698. doi: 10.1097/QAD.0000000000001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews W.W., Hauth J.C., Goldenberg R.L. Infection and preterm birth. Am. J. Perinatol. 2000;17:357–365. doi: 10.1055/s-2000-13448. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsson B., Mattsby-Baltzer I., Andersch B., Bokstrom H., Holst R.M., Wennerholm U.B., Hagberg H. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet. Gynecol. Scand. 2003;82:120–128. doi: 10.1034/j.1600-0412.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 14.Novy M.J., Duffy L., Axthelm M.K., Sadowsky D.W., Witkin S.S., Gravett M.G., Cassell G.H., Waites K.B. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod. Sci. 2009;16:56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 15.Romero R., Hassan S.S., Gajer P., Tarca A.L., Fadrosh D.W., Bieda J., Chaemsaithong P., Miranda J., Chaiworapongsa T., Ravel J. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2014;2:18. doi: 10.1186/2049-2618-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinturache A.E., Gyamfi-Bannerman C., Hwang J., Mysorekar I.U., Jacobsson B., Preterm Birth International C. Maternal microbiome - a pathway to preterm birth. Semin. Fetal Neonatal Med. 2016;21:94–99. doi: 10.1016/j.siny.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Racicot K., Cardenas I., Wunsche V., Aldo P., Guller S., Means R.E., Romero R., Mor G. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J. Immunol. 2013;191:934–941. doi: 10.4049/jimmunol.1300661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flaviani F., Hezelgrave N.L., Kanno T., Prosdocimi E.M., Chin-Smith E., Ridout A.E., von Maydell D.K., Mistry V., Wade W.G., Shennan A.H., Dimitrakopoulou K., Seed P.T., Mason A.J., Tribe R.M. Cervicovaginal microbiota and Metabolome predict preterm birth risk in an ethnically diverse cohort. JCI Insight. 2021;6 doi: 10.1172/jci.insight.149257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerson K.D., McCarthy C., Elovitz M.A., Ravel J., Sammel M.D., Burris H.H. Cervicovaginal microbial communities deficient in Lactobacillus species are associated with second trimester short cervix. Am. J. Obstet. Gynecol. 2020;222:491 e491–e491 e498. doi: 10.1016/j.ajog.2019.11.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kindinger L.M., Bennett P.R., Lee Y.S., Marchesi J.R., Smith A., Cacciatore S., Holmes E., Nicholson J.K., Teoh T.G., MacIntyre D.A. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. 2017;5:6. doi: 10.1186/s40168-016-0223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan S., Morgan M.T., Fiedler T.L., Djukovic D., Hoffman N.G., Raftery D., Marrazzo J.M., Fredricks D.N. Metabolic signatures of bacterial vaginosis. mBio. 2015;6 doi: 10.1128/mBio.00204-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghartey J., Anglim L., Romero J., Brown A., Elovitz M.A. Women with symptomatic preterm birth have a distinct cervicovaginal metabolome. Am. J. Perinatol. 2017;34:1078–1083. doi: 10.1055/s-0037-1603817. [DOI] [PubMed] [Google Scholar]
- 23.Ghartey J., Bastek J.A., Brown A.G., Anglim L., Elovitz M.A. Women with preterm birth have a distinct cervicovaginal metabolome. Am. J. Obstet. Gynecol. 2015;212:776 e771–e776 e712. doi: 10.1016/j.ajog.2015.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen P., Hu T., Zheng Z., R E.G., Yang J. Characteristics of cervicovaginal microflora at different cervical maturity during late pregnancy: a nested case-control study. PLoS One. 2024;19 doi: 10.1371/journal.pone.0300510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benton H.P., Ivanisevic J., Mahieu N.G., Kurczy M.E., Johnson C.H., Franco L., Rinehart D., Valentine E., Gowda H., Ubhi B.K., Tautenhahn R., Gieschen A., Fields M.W., Patti G.J., Siuzdak G. Autonomous metabolomics for rapid metabolite identification in global profiling. Anal. Chem. 2015;87:884–891. doi: 10.1021/ac5025649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blazenovic I., Kind T., Ji J., Fiehn O. Software tools and Approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites. 2018;8 doi: 10.3390/metabo8020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team . 2022. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- 28.Utiger R.D. Prostaglandins. 2023 https://www.britannica.com/science/prostaglandin [Google Scholar]
- 29.Mayoral Andrade G., Vasquez Martinez G., Perez-Campos Mayoral L., Hernandez-Huerta M.T., Zenteno E., Perez-Campos Mayoral E., Martinez Cruz M., Martinez Cruz R., Matias-Cervantes C.A., Meraz Cruz N., Romero Diaz C., Cruz-Parada E., Perez-Campos E. Molecules and Prostaglandins related to embryo tolerance. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.555414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Department of health & human Services, niacin. 2022. https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional/
- 31.Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference, Intakes its Panel on Folate, the National Academies Collection: Reports Funded by National Institutes of Health. National Academies Press (US) Copyright © 1998. National Academy of Sciences; Washington (DC): 1998. report. [DOI] [Google Scholar]
- 32.Xie N., Zhang L., Gao W., Huang C., Huber P.E., Zhou X., Li C., Shen G., Zou B. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Targeted Ther. 2020;5:227. doi: 10.1038/s41392-020-00311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Department of Health & Human Services . 2022. Choline.https://ods.od.nih.gov/factsheets/Choline-HealthProfessional/ [Google Scholar]
- 34.Derbyshire E., Obeid R., Schön C. Habitual Choline intakes across the childbearing years: a review. Nutrients. 2021;13 doi: 10.3390/nu13124390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korsmo H.W., Jiang X., Caudill M.A. Choline: exploring the growing science on its benefits for moms and babies. Nutrients. 2019;11 doi: 10.3390/nu11081823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper T.E., Teng C., Howell M., Teixeira-Pinto A., Jaure A., Wong G. D-mannose for preventing and treating urinary tract infections. Cochrane Database Syst. Rev. 2022;8 doi: 10.1002/14651858.CD013608.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kyriakides R., Jones P., Somani B.K. Role of D-Mannose in the prevention of recurrent urinary tract infections: evidence from a systematic review of the literature. Eur Urol Focus. 2021;7:1166–1169. doi: 10.1016/j.euf.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Zheng N., Gu Y., Hong Y., Sheng L., Chen L., Zhang F., Hou J., Zhang W., Zhang Z., Jia W., Li H. Vancomycin pretreatment attenuates acetaminophen-induced liver injury through 2-hydroxybutyric acid. J Pharm Anal. 2020;10:560–570. doi: 10.1016/j.jpha.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang H., Lei D., Chen T., Liu Y., Fan C. The enzyme 15-hydroxyprostaglandin dehydrogenase inhibits a shift to the mesenchymal pattern of trophoblasts and decidual stromal cells accompanied by prostaglandin transporter in preeclampsia. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms24065111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auray-Blais C., Raiche E., Gagnon R., Berthiaume M., Pasquier J.-C. Metabolomics and preterm birth: what biomarkers in cervicovaginal secretions are predictive of high-risk pregnant women? Int. J. Mass Spectrom. 2011;307:33–38. doi: 10.1016/j.ijms.2011.02.009. [DOI] [Google Scholar]
- 41.Borgogna J.C., Shardell M.D., Santori E.K., Nelson T.M., Rath J.M., Glover E.D., Ravel J., Gravitt P.E., Yeoman C.J., Brotman R.M. The vaginal metabolome and microbiota of cervical HPV-positive and HPV-negative women: a cross-sectional analysis. BJOG. 2020;127:182–192. doi: 10.1111/1471-0528.15981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerson K.D., Liao J., McCarthy C., Burris H.H., Korem T., Levy M., Ravel J., Elovitz M.A. A non-optimal cervicovaginal microbiota in pregnancy is associated with a distinct metabolomic signature among non-Hispanic Black individuals. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-02304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliver A., LaMere B., Weihe C., Wandro S., Lindsay K.L., Wadhwa P.D., Mills D.A., Pride D.T., Fiehn O., Northen T., de Raad M., Li H., Martiny J.B.H., Lynch S., Whiteson K. Cervicovaginal microbiome composition Is associated with metabolic profiles in healthy pregnancy. mBio. 2020;11 doi: 10.1128/mBio.01851-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson T.M., Borgogna J.L., Brotman R.M., Ravel J., Walk S.T., Yeoman C.J. Vaginal biogenic amines: biomarkers of bacterial vaginosis or precursors to vaginal dysbiosis? Front. Physiol. 2015;6:253. doi: 10.3389/fphys.2015.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boskey E.R., Cone R.A., Whaley K.J., Moench T.R. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 2001;16:1809–1813. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 46.Spear G.T., French A.L., Gilbert D., Zariffard M.R., Mirmonsef P., Sullivan T.H., Spear W.W., Landay A., Micci S., Lee B.H., Hamaker B.R. Human alpha-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J. Infect. Dis. 2014;210:1019–1028. doi: 10.1093/infdis/jiu231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yellon S.M. Immunobiology of cervix ripening. Front. Immunol. 2019;10:3156. doi: 10.3389/fimmu.2019.03156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varki A., Gagneux P. Multifarious roles of sialic acids in immunity. Ann. N. Y. Acad. Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeoman C.J., Yildirim S., Thomas S.M., Durkin A.S., Torralba M., Sutton G., Buhay C.J., Ding Y., Dugan-Rocha S.P., Muzny D.M., Qin X., Gibbs R.A., Leigh S.R., Stumpf R., White B.A., Highlander S.K., Nelson K.E., Wilson B.A. Comparative genomics of Gardnerella vaginalis strains reveals substantial differences in metabolic and virulence potential. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heng Y.J., Di Quinzio M.K., Liong S., Permezel M., Rice G.E., Georgiou H.M. Temporal investigation of matrix metalloproteinases and their inhibitors in human cervicovaginal fluid in late pregnancy and labor. Reprod. Sci. 2012;19:55–63. doi: 10.1177/1933719111413299. [DOI] [PubMed] [Google Scholar]
- 51.Heng Y.J., Di Quinzio M.K., Permezel M., Rice G.E., Georgiou H.M. Cystatin A protease inhibitor and cysteine proteases in human cervicovaginal fluid in term pregnancy and labor. Am. J. Obstet. Gynecol. 2011;204:254 e251–e257. doi: 10.1016/j.ajog.2010.10.912. [DOI] [PubMed] [Google Scholar]
- 52.Sousa A.P., Cunha D.M., Franco C., Teixeira C., Gojon F., Baylina P., Fernandes R. Which role plays 2-Hydroxybutyric Acid on insulin resistance? Metabolites. 2021;11 doi: 10.3390/metabo11120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fennema D., Phillips I.R., Shephard E.A. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-Mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab. Dispos. 2016;44:1839–1850. doi: 10.1124/dmd.116.070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shuai W., Wen J., Li X., Wang D., Li Y., Xiang J. High-choline diet exacerbates cardiac dysfunction,fibrosis, and inflammation in a mouse model of heart failure with preserved ejection fraction. J. Card. Fail. 2020;26:694–702. doi: 10.1016/j.cardfail.2020.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the relevant data are included in the manuscript and the supplementary document. The raw metabolomic data in this paper have been deposited in the MetaboLights of EMBL-EBI (https://www.ebi.ac.uk/metabolights/) under accession number MTBLS10143.