Abstract
Hepatitis C virus (HCV) NS5B protein is the viral RNA-dependent RNA polymerase capable of directing RNA synthesis. In this study, an electrophoretic mobility shift assay demonstrated the interaction between a partially purified recombinant NS5B protein and a 3′ viral genomic RNA with or without the conserved 98-nucleotide tail. The NS5B-RNA complexes were specifically competed away by the unlabeled homologous RNA but not by the viral 5′ noncoding region and very poorly by the 3′ conserved 98-nucleotide tail. A 3′ coding region with conserved stem-loop structures rather than the 3′ noncoding region of the HCV genome is critical for the specific binding of NS5B. Nevertheless, no direct interaction between the 3′ coding region and the HCV NS5A protein was detected. Furthermore, two independent RNA-binding domains (RBDs) of NS5B were identified, RBD1, from amino acid residues 83 to 194, and RBD2, from residues 196 to 298. Interestingly, the conserved motifs of RNA-dependent RNA polymerase for putative RNA binding (220-DxxxxD-225) and template/primer position (282-S/TGxxxTxxxNS/T-292) are present in the RBD2. Nevertheless, the RNA-binding activity of RBD2 was abolished when it was linked to the carboxy-terminal half of the NS5B. These results provide some clues to understanding the initiation of HCV replication.
Hepatitis C virus (HCV) is an enveloped virus that possesses a single-stranded positive-sense RNA genome encoding a polyprotein of approximately 3,000 amino acid residues (7, 20, 29). The conserved 5′ noncoding region (5′NCR) of the HCV genome (4, 13) is highly structured and contributes to the internal ribosome entry site important for the translation initiation of HCV RNA (14, 15, 25, 34, 35, 39). The 3′ noncoding region (3′NCR) consists of a short genotype-specific region and a poly(U)-C(U)n repeat stretch of variable length followed by a highly conserved tail of 98 nucleotides (nt) (12, 21, 30, 37).
Studies to understand the molecular mechanism of HCV replication have been restricted by the lack of a well-established cell culture system, but studies from other positive-sense RNA viruses may provide some clues. Upon flavivirus infection, translation of incoming viral genomic RNA occurs, and replication of the viral RNA begins with the synthesis of minus-strand RNA which then serves as the template for the synthesis of progeny genomic RNA. The replication appears to take place at the perinuclear endoplasmic reticulum and requires virus-encoded proteins NS3 (proteinase/helicase) and NS5 (polymerase) as components of the presumed replicative complex (36). In addition, the 3′ terminus of the flavivirus genomic RNA forms a conserved pseudoknot structure (26). It is generally believed that conserved sequences and structures at the 3′ terminus of viral genomic RNA function as cis-acting signals that interact with viral and cellular proteins to initiate the synthesis of minus-strand RNA during viral replication.
The NS5B protein of HCV is a membrane-associated phosphoprotein (16) that possesses the conserved GDD motif of RNA-dependent RNA polymerase (RdRp) (19). RdRp activity of the HCV NS5B has been demonstrated in vitro, and several amino acid motifs essential for the enzymatic activity were identified (1, 2, 10, 23, 38). Nevertheless, template specificity of the viral RNA on the NS5B RdRp activity was not observed. It is reasonable to propose that following HCV infection, the initiation of minus-strand RNA synthesis depends on an initial recognition and specific binding of the NS5B RNA polymerase or replicative complex to the 3′ terminus of the viral genomic RNA. Many specific sequences as well as structures at the 3′ termini of the genomes of positive-sense RNA viruses have been demonstrated to be important for the synthesis of minus-strand RNA (11, 18, 22, 27, 28). Previous studies also demonstrated a specific binding of encephalomyocarditis virus RNA polymerase to the viral poly(A)-containing 3′NCR (8, 9). However, recent studies indicated that HCV NS5B interacted with the 3′ conserved 98 nt of the viral genome with little specificity (23) and had no clear preference to utilize the 98-nt RNA as a template in RdRp activity assay (38). In this study, by performing a competitive electrophoretic mobility shift assay (EMSA), we have identified conserved stem-loop structures in the 3′ coding region of HCV genomic RNA important for the binding of NS5B RNA polymerase. In addition, two RNA-binding domains of the NS5B RNA polymerase were identified.
Expression of the full-length recombinant HCV NS5B protein.
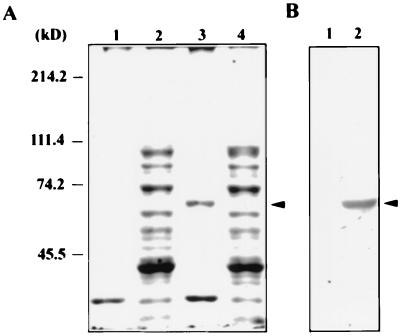
To evaluate the biological functions of HCV RNA polymerase, a full-length NS5B cDNA was obtained from a serum sample of an HCV-infected patient by reverse transcriptase-PCR. The cDNA was cloned into pET15b, which allows expression of the full-length NS5B protein in Escherichia coli. Following induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), a protein with a molecular mass of 67 kDa was detected in the insoluble fraction of the bacterial lysate (Fig. 1A, lane 3). Specificity of the protein as an induced recombinant HCV NS5B protein was confirmed by Western blot analysis using rabbit antibodies against an N-terminal peptide (NH2-MSYTWTGALITPCAAE-COOH) of the NS5B (Fig. 1B) and the serum of an HCV patient (data not shown). Similar to the experience of Yuan et al. (40), our efforts to obtain soluble full-length NS5B protein from E. coli turned out to be unsuccessful regardless of modifications of growth conditions or expression vectors (data not shown). Therefore, the insoluble NS5B protein was recovered from sodium dodecyl sulfate-polyacrylamide gels and was used to examine its biological functions.
FIG. 1.
Expression of HCV NS5B protein in E. coli. (A) Coomassie blue staining. E. coli BL21(DE3) cells were transformed with plasmids pET15b (lanes 1 and 2) and pET15b-NS5B (lanes 3 and 4) and grown at 37°C. Plasmid pET15b-NS5B bears HCV cDNA from nt 7258 to 9030 of the coding region that encodes the full-length NS5B representing the viral polyprotein from amino acid residues 2420 to 3010. Expression and purification of the recombinant NS5B protein were performed essentially according to the procedures described by the manufacturer (Novagen). Cell lysates of soluble (lanes 2 and 4) and insoluble (lanes 1 and 3) fractions were resolved on a sodium dodecyl sulfate–8% polyacrylamide gel. Coomassie blue staining is shown. (B) Western blot analysis. The insoluble fractions of protein lysates from cells transformed with pET15b (lane 1) and pET15b-NS5B (lane 2) were immunoblotted following the procedures previously described (6) with the immunoglobulin G fraction of a rabbit antiserum that was raised against the NS5B peptide NH2-MSYTWTGALITPCAAE-COOH. Arrowheads indicate the IPTG-induced recombinant NS5B protein.
Specific binding of HCV NS5B to the 3′ end of viral genomic RNA.
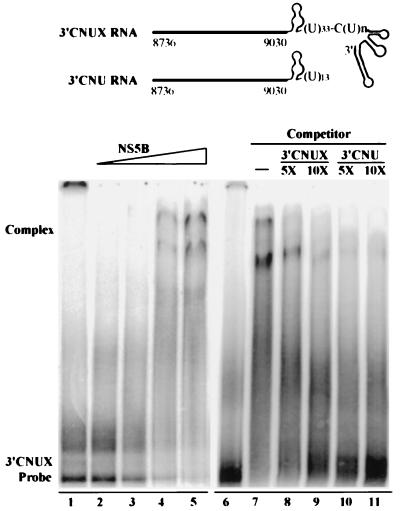
To determine the RNA-binding activity of HCV NS5B, an EMSA was performed as previously described (39). The 3′ HCV RNA designated 3′CNUX, which consists of the coding region of the HCV polyprotein from nt 8736 to 9030 (3′C) and the entire viral 3′NCR, including the genotype-specific region (N), a poly(U)-C(U)n repeat stretch (U), and the conserved 98-nt tail (X), was in vitro synthesized in the presence of [α-32P]UTP and used as a substrate. Results clearly demonstrated an interaction between HCV NS5B protein and the 3′CNUX RNA. The levels of RNA-protein complexes raised were in agreement with increasing amounts of the partially purified NS5B (Fig. 2, lanes 1 to 5). The complexes were diminished in the presence of either the unlabeled 3′CNUX RNA or the 3′CNU RNA that lacks the 3′-terminal 98 nt (Fig. 2, lanes 6 to 11).
FIG. 2.
Complex formation between HCV NS5B protein and the 3′CNUX RNA. (Top) Structures of the HCV 3′-end RNAs, 3′CNUX, and 3′CNU. The heavy line that begins with nt 8736 and ends with nt 9030 represents the 3′ coding region of the HCV polyprotein. The 3′CNUX RNA consists of the 295-nt 3′ coding region and the entire viral 3′NCR encompassing a short genotype-specific region, a (U)33-C(U)n stretch, and a conserved 98-nt tail with stem-loop structure. The 3′CNU RNA contains the 295-nt 3′ coding region and a partial 3′NCR as shown. (Bottom) EMSA. The [α-32P]UTP-labeled 3′CNUX RNA was incubated with increasing amounts (lanes 2 to 5, 0.15, 0.75, 1.5, and 3 ng, respectively) of the partially purified HCV NS5B protein or with 3 ng of the NS5B protein in the presence of various amounts of competitor RNAs as indicated (lanes 7 to 11). Reaction products were resolved in a 4% polyacrylamide gel under nondenaturing conditions. The gel was dried and subjected to autoradiography. Lanes 1 and 6 represent free 3′CNUX RNA probe to which no NS5B was added in the reaction.
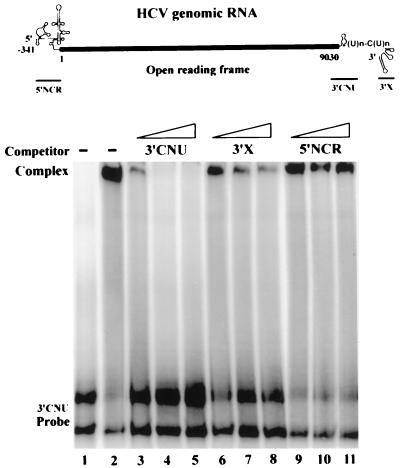
Previous studies using the RNA filter binding assay indicated that the interaction between HCV NS5B protein and a 3′ viral RNA bearing the 98-nt tail was nonspecific (23). Because the 3′NCR of the viral genome is likely to be the entry site for RNA polymerase to initiate genome replication, we attempted to address the question further by performing a competitive EMSA using various fragments of the HCV genomic RNA as competitors. As shown in Fig. 3, the complex formed between radiolabeled 3′CNU RNA and HCV NS5B protein was completely annihilated by a fivefold molar excess of the unlabeled 3′CNU RNA (lane 4). Competition by the 3′ (3′X RNA) conserved 98-nt tail was observed only at a higher dose of competitor, and the competition effect was reduced (lanes 6 to 8). On the other hand, the 5′NCR competitor had no effect at all (lanes 9 to 11). Taken together, these results indicated that the HCV NS5B has a preference to interact with the 3′ viral RNA that contains a region coding for the carboxyl terminus of the HCV polyprotein rather than to interact with the 3′ 98-nt tail or 5′NCR.
FIG. 3.
Preferential binding of NS5B protein to the HCV 3′CNU RNA. (Top) Structures of the HCV genomic RNA and RNA fragments used in the competition analysis. The heavy line represents the coding region of the HCV polyprotein. The 5′NCR contains a highly ordered structure 341 nt in length, and the 3′ NCR consists of the genotype-specific region, a (U)n-C(U)n stretch, and the conserved 98-nt tail. (Bottom) Competitive EMSA. EMSA was performed with HCV NS5B protein and the 32P-labeled 3′CNU RNA in the presence (lanes 3 to 11) or absence (lane 2) of unlabeled competitor RNAs as indicated. Competitor RNAs used were 1- (lanes 3, 6, and 9), 5- (lanes 4, 7, and 10), and 10-fold (lanes 5, 8, and 11) molar excesses to the labeled 3′CNU RNA. Lane 1 represents free 3′CNU RNA probe to which no NS5B protein was added in the reaction. We consistently observed two bands of the 3′CNU RNA probe that should represent the same RNA fragment of different conformations. A single band was detected when the probe was analyzed on a sequencing gel containing 8 M urea (data not shown).
Mapping of the specific NS5B binding sequences on the 3′CNU RNA.
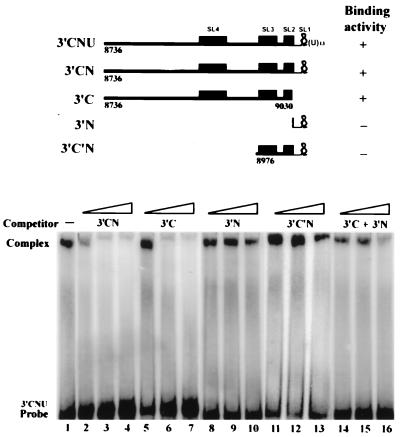
To define more precisely the sequences of 3′CNU RNA essential for the binding of HCV NS5B protein, a series of competition experiments were performed with RNA competitors derived from the 3′CNU RNA as shown in Fig. 4. Both 3′CNU and 3′CN RNAs cover the 3′ region of the HCV genomic RNA that has been predicted to possess four conserved stem-loop structures (data not shown and reference 12), SL1 to SL4. The 3′C and 3′N RNA represent RNA domains of the 3′ coding region and genotype-specific region, respectively, of the 3′CN RNA. The 3′C′N RNA represents a 5′ deletion of the 3′CN RNA up to the beginning of SL3. Interestingly, we found that both 3′CN and 3′C RNA competed efficiently the binding of NS5B to the 3′CNU RNA (Fig. 4, lanes 2 to 7), whereas 3′N and 3′C′N RNA had little effect (lanes 8 to 13), indicating that the coding region 5′ to the SL3 is important for the binding. In addition, although 3′CN RNA competed more effectively than the 3′C RNA (compare lane 2 to lane 5), the presence of both 3′C and 3′N competitors that added together cover the sequences of 3′CN RNA, did not demonstrate a competition effect comparable to the 3′CN RNA (lanes 14 to 16). These indicated that the 3′N RNA must be covalently linked to the 3′C RNA for the binding of NS5B to occur and may imply a role of the SL2 to enhance the interaction between HCV RNA and NS5B. Consistent with the results of the competitive EMSA, we found that among radiolabeled 3′C, 3′N, and 3′C′N RNAs, only 3′C RNA formed complex with NS5B (data not shown). In addition, the formation of 3′C RNA-NS5B complex required a higher dose of NS5B than that of 3′CNU RNA (data not shown).
FIG. 4.
Mapping of the binding domains of NS5B on the HCV 3′CNU RNA. (Top) Structures of the HCV RNAs used in the competitive EMSA and their characteristics in forming a complex with HCV NS5B protein. Nucleotide residues flanking the RNA termini are numbered according to the coding region of HCV polyprotein. RNA fragments that form conserved stem-loop structures (SL) are indicated by filled boxes. SL4 and SL3 contain nt 8875 to 8918 and nt 8980 to 9010, respectively, of the coding region of HCV polyprotein. SL2 contains the 3′-terminal 12 nt of the coding region plus a downstream 11 nt, and SL1 is located within the genotype-specific region (12). Binding activities are indicated by plus and minus signs. (Bottom) Competitive EMSA. The competition analysis was conducted with a gel-purified 3′CNU RNA probe and HCV NS5B protein in the presence of unlabeled 3′CN (lanes 2 to 4), 3′C (lanes 5 to 7), 3′N (lanes 8 to 10), 3′C′N (lanes 11 to 13), and a mixture of 3′C and 3′N RNA (lanes 14 to 16) at 1-, 5-, and 10-fold molar excesses to the [α-32P]UTP-labeled 3′CNU RNA probe. Lane 1 represents the reaction in which no competitor RNA was added.
Mapping of the binding domains of NS5B to the 3′CNU RNA.
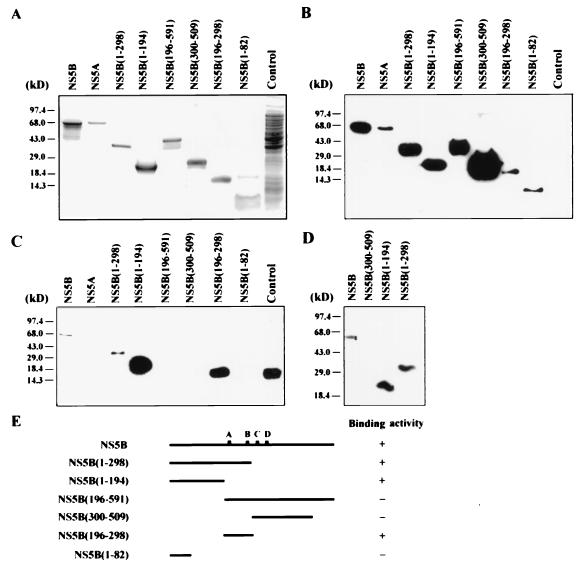
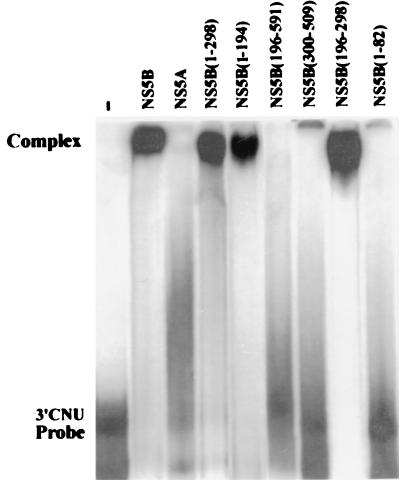
To define domains of the NS5B that conferred RNA binding, recombinant NS5B deletion mutants with His tag were produced as the full-length NS5B. All the mutant proteins were present in the insoluble fractions (data not shown) and were purified by polyacrylamide gel electrophoresis and electroelution as for the full-length NS5B. The purified proteins were analyzed by Coomassie blue staining (Fig. 5A) and Western blot analysis using a monoclonal antibody against the His tag (Fig. 5B). The specificity of the purified proteins was also examined with serum of an HCV patient (data not shown). RNA-binding activities for the NS5B deletion mutants were examined by Northwestern analysis using radiolabeled 3′CNU RNA as the substrate in the presence of 50 mM (Fig. 5C) or 150 mM (Fig. 5D) NaCl. Under the conditions examined, we found that the HCV 3′CNU RNA bound to the deletion mutants NS5B(1–298), NS5B(1–194), and NS5B(196–298) even more strongly than the full-length NS5B protein, but deletion mutants NS5B(196–591), NS5B(300–509), and NS5B(1–82) failed to interact with the HCV RNA. In addition, the HCV NS5A protein that was purified from the insoluble lysate of pET15b-NS5A-transformed cells could not interact directly with the viral 3′CNU RNA (Fig. 5C). Plasmid pET15b-NS5A bears the HCV cDNA from nt 5917 to 7527 of the coding region of the viral polyprotein and encodes the full-length NS5A protein. These results were further confirmed by EMSA. As shown in Fig. 6, the full-length NS5B and deletion mutants NS5B(1–298), NS5B(1–194), and NS5B(196–298) formed complexes with the radiolabeled 3′CNU RNA, whereas no complex was detected with proteins NS5A, NS5B(196–591), NS5B(300–509), and NS5B(1–82). In light of these results, it appeared that HCV NS5B RNA polymerase possesses two independent RNA-binding domains (RBDs), from amino acid residues 83 to 194 (RBD1) and from residues 196 to 298 (RBD2). Nevertheless, the RNA-binding activity of RBD2 is completely annihilated when it is linked to the downstream sequences of the NS5B protein. The reasons why NS5B(196–591) protein failed to interact with HCV RNA are not clear. It is possible that the downstream sequences disrupted a proper folding essential for the binding activity of RBD2. Alternatively, the NS5B(196–591) protein may form a less stable complex with HCV RNA that was not detected under the standard conditions used in this study.
FIG. 5.
Binding of HCV 3′CNU RNA to the recombinant NS5B deletion mutants. Partially purified full-length NS5A and NS5B and NS5B deletion mutants as indicated were analyzed by sodium dodecyl sulfate–15% polyacrylamide gel electrophoresis in Tricine-Tris buffer and stained with Coomassie blue (A). Equal amounts of the recombinant proteins as determined by Bio-Rad protein assay were subjected to Western blot analysis with 6× His monoclonal antibody (ClonTech) (B) and Northwestern analysis as previously described (5) with [α-32P]UTP-labeled 3′CNU RNA in the presence of 50 mM (C) and 150 mM (D) NaCl. The control lanes represent protein lysates prepared from E. coli transformed with the pET15b plasmid. In panel C, a signal at a position beyond the molecular sizes of the recombinant NS5B proteins was detected with the control lysate. Its identity is not known. Panel E shows the structures of the NS5B recombinant proteins and summarizes their characteristics in binding 3′CNU RNA.
FIG. 6.
Complex formation between NS5B deletion mutants and the 3′CNU RNA. An EMSA was performed with [α-32P]UTP-labeled 3′CNU RNA, and equal amounts of the NS5A and NS5B recombinant proteins as indicated. The gel was dried and subjected to autoradiography.
Conclusions.
This study investigated the RNA-binding specificity of the HCV NS5B RdRp. HCV NS5B preferentially interacted with a 3′ viral RNA containing sequences coding for the carboxyl terminus of the HCV polyprotein. The binding domains of NS5B to the HCV RNA were mapped to amino acid residues 83 to 194 and 196 to 298.
Binding of RdRp to viral genome is the key step to initiate replication of positive-sense RNA viruses. Previous studies have demonstrated specific sequences and structures at the 3′ termini of positive-sense RNA viruses involved in the binding of RNA polymerase and/or initiation of replication. Examples include a poly(A)-containing 3′-terminal RNA of encephalomyocarditis virus (8, 9) and a murine coronavirus (22), a non-base-paired 3′ ACC sequence of a tRNA-like structure of turnip yellow mosaic virus (11, 27), a stem-loop structure of turnip crinkle virus (28), and a pseudoknot structure of polioviruses (18). The 3′NCR of HCV genome consists of a short genotype-specific region and a stretch of poly(U)-C(U)n repeats followed by a highly conserved 98-nt tail (12, 21, 30, 37). The conserved 98-nt tail forms a conserved secondary structure (3, 17, 21, 31) and is likely to be involved in the initiation of viral replication. Cellular proteins, including polypyrimidine tract binding protein, were recently demonstrated to specifically interact with the stem-loop structure of the conserved 98 nt (17, 33), but no direct interaction between the 3′ 98-nt tail and 5′NCR of the HCV genomic RNA was indicated (3). In addition, earlier studies with RNA filter binding assay failed to demonstrate the specific interaction between HCV NS5B and a viral RNA containing the 98-nt sequences (23).
In this study, by performing a competitive EMSA, we observed specific complex formation between HCV NS5B protein and the 3′CNU RNA that lacks the 98-nt tail but contains 295 nt upstream and 54 nt downstream from the stop codon of the viral genomic RNA (Fig. 3). The 3′X RNA competed the complex formation poorly, and the 5′NCR competitor had little effect. In addition, the poly(U) stretch in 3′NCR was not essential for the binding but the short genotype-specific region may facilitate the binding of HCV NS5B to the viral RNA (Fig. 4). Nevertheless, the genotype-specific region of 3′NCR did not seem to interact with HCV NS5B by itself (Fig. 4). Interestingly, the binding activity of HCV NS5B to the various 3′-terminal regions of the viral genomic RNA correlated very well with the RdRp activity of the NS5B RNA polymerase. Using various HCV 3′ RNAs as templates and primers, Yamashita et al. (38) demonstrated that the level of in vitro RNA synthesis with the 98-nt RNA was relatively low compared to that of a 480-nt RNA containing 436 nt of the coding region of HCV polyprotein plus a downstream genotype-specific region, whereas a 106-nt poly(U) stretch failed to serve as template/primer. Taken together, these results indicate that the conserved 3′-terminal tail and poly(U)-C(U)n stretch of HCV genome may not be good substrates or templates by themselves for NS5B binding and subsequent RNA replication; a coding region of about 300 nt immediately before the stop codon of the viral genome may play an important role in the viral replication. Highly conserved stem-loop structures have been predicted within the 200-nt region upstream from the U stretch of the HCV genomic RNA (Fig. 4) (12). Whether these conserved structures are involved in the genome replication of HCV remains to be elucidated. However, our results do support the idea that the SL2 made up from sequences of the 3′ coding region and 3′NCR across the stop codon of HCV polyprotein is important for the viral RNA to interact with NS5B (Fig. 4). The binding activity of NS5B to the 3′CN RNA was annihilated when the RNA transcript was divided into two fragments (3′C and 3′N) that disrupted the formation of SL2. In addition, SL4 is required for the RNA-binding activity; HCV RNA transcript 3′C′N in which the SL4 had been deleted failed to interact with NS5B RNA polymerase (Fig. 4).
Sequence analysis has revealed several motifs that are conserved among RdRp (24). Judging from the prediction of secondary structure, it was proposed that four of the motifs, each of which provides one conserved amino acid residue, may constitute a prerequisite polymerase module involved in the template binding and subsequent polymerization (24). Of the four motifs of HCV NS5B, site A (220-DxxxxD-225) and sites C (317-GDD-319) and D (R-345) were predicted to be involved in RNA and nucleoside triphosphate binding, respectively, and possibly catalysis, whereas site B (282-S/TGxxxTxxxNS/T-292) was thought to be important for template/primer position. Nevertheless, previous mutational analysis at these conserved amino acid residues failed to demonstrate their involvement in RNA binding (23). In this study, by performing Northwestern analysis (Fig. 5) and an EMSA (Fig. 6) with deletion mutants, we identified two RNA-binding domains of the HCV NS5B RNA polymerase from amino acid residues 83 to 194 (RBD1) and 196 to 298 (RBD2). Interestingly, consistent with the results of our RNA-binding study, the putative RNA-binding motif (site A) and template/primer position motif (site B) of the polymerase module are located in the RBD2. In addition, several clusters of basic amino acid residues, 151-KGGRKPAR-158, 209-KSKK-212, and 277-RRCR-280, were found in the RNA-binding domains. Whether these clusters play roles in the RNA-binding activity of NS5B has not yet been examined. But the observation that mutations at the cluster 274-CGYRRCR-280 abolished the RdRp activity of a carboxy-terminal truncated NS5B (38) may imply that the RNA-binding activity of the RBD2 is essential for the RdRp activity of NS5B.
Replication of viral genome is a complicated event that requires not only polymerase but also additional viral and cellular factors to form a functional replicative complex. Our RNA-binding studies indicated that there was no direct interaction between HCV NS5A protein and the viral RNA (Fig. 5 and 6). However, preliminary data do suggest there is cross talk between NS5A and NS5B (data not shown). HCV NS5A is a phosphoprotein (32) and was predicted to play roles in HCV replication. NS5A was also found to interact with cellular factors (data not shown). But components that constitute a functional replicative complex to direct replication of HCV genome with specificity are not fully understood. In this report, we have demonstrated a specific interaction between HCV NS5B RNA polymerase and a 3′ region of the viral genomic RNA and identified the interacting domains of the NS5B. The information provided here should aid in understanding the mechanisms of HCV replication.
Nucleotide sequence accession number.
The cDNA sequence encoding the full-length HCV NS5B protein has been deposited in GenBank under accession no. AF145454.
Acknowledgments
We thank Jui-Hung Yen and Ying-Tai Peng for technical assistance. We are grateful to Bon-Chu Chung, Jen-Yang Chen, Shin-Lian Doong, and Won-Bo Wang for helpful discussions and comments.
This work was supported by research grants NSC85-2331-B-002-080-MH, NSC86-2315-B-002-012-MH to S.C.C. from the National Science Council of the Republic of China and DOH87-HR-723 to M.-F.C. from the Department of Health, Executive Yuan of the Republic of China. J.-C. C. was the recipient of a fellowship from the National Science Council (no. 32317D).
REFERENCES
- 1.Al R H, Xie Y, Wang Y, Hagedorn C H. Expression of recombinant hepatitis C virus non-structural protein 5B in Escherichia coli. Virus Res. 1998;53:141–149. doi: 10.1016/s0168-1702(97)00147-0. [DOI] [PubMed] [Google Scholar]
- 2.Behrens S-E, Tomei L, De Francesco R. Identification and properties of the RNA-dependent RNA polymerase of hepatitis C virus. EMBO J. 1996;15:12–22. [PMC free article] [PubMed] [Google Scholar]
- 3.Blight K J, Rice C M. Secondary structure determination of the conserved 98-base sequence at the 3′ terminus of hepatitis C virus genome RNA. J Virol. 1997;71:7345–7352. doi: 10.1128/jvi.71.10.7345-7352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukh J, Purcell R H, Miller R H. Sequence analysis of the 5′ noncoding region of hepatitis C virus. Proc Natl Acad Sci USA. 1992;89:4942–4946. doi: 10.1073/pnas.89.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang M-F, Baker S C, Soe L H, Kamahora T, Keck J G, Makino S, Govindarajan S, Lai M M C. Human hepatitis delta antigen is a nuclear phosphoprotein with RNA-binding activity. J Virol. 1988;62:2403–2410. doi: 10.1128/jvi.62.7.2403-2410.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang M-F, Sun C-Y, Chen C-J, Chang S C. Functional motifs of delta antigen essential for RNA binding and replication of hepatitis delta virus. J Virol. 1993;67:2529–2536. doi: 10.1128/jvi.67.5.2529-2536.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choo Q-L, Richman K H, Han J H, Berger K, Lee C, Dong C, Gallegos C, Coit D, Medina-Selby A, Barr P J, Weiner A J, Bradley D W, Kuo G, Houghton M. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci USA. 1991;88:2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui T, Porter A G. Localization of binding site for encephalomyocarditis virus RNA polymerase in the 3′-noncoding region of the viral RNA. Nucleic Acids Res. 1995;23:377–382. doi: 10.1093/nar/23.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui T, Sankar S, Porter A G. Binding of encephalomyocarditis virus RNA polymerase to the 3′-noncoding region of the viral RNA is specific and requires the 3′-poly(A) tail. J Biol Chem. 1993;268:26093–26098. [PubMed] [Google Scholar]
- 10.De Francesco R, Behrens S-E, Tomei L, Altamura S, Jiricny J. RNA-dependent RNA polymerase of hepatitis C virus. Methods Enzymol. 1996;275:58–67. doi: 10.1016/s0076-6879(96)75006-1. [DOI] [PubMed] [Google Scholar]
- 11.Deiman B A L M, Koenen A K, Verlaan P W G, Pleij C W A. Minimal template requirements for initiation of minus-strand synthesis in vitro by the RNA-dependent RNA polymerase of turnip yellow mosaic virus. J Virol. 1998;72:3965–3972. doi: 10.1128/jvi.72.5.3965-3972.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han J H, Houghton M. Group specific sequences and conserved secondary structures at the 3′ end of HCV genome and its implication for viral replication. Nucleic Acids Res. 1992;20:3520. doi: 10.1093/nar/20.13.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han J H, Shyamala V, Richman K H, Brauer M J, Irvine B, Urdea M S, Tekamp-Olson P, Kuo G, Choo Q-L, Houghton M. Characterization of the terminal regions of hepatitis C viral RNA: identification of conserved sequences in the 5′ untranslated region and poly(A) tails at the 3′ end. Proc Natl Acad Sci USA. 1991;88:1711–1715. doi: 10.1073/pnas.88.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honda M, Brown E A, Lemon S M. Stability of a stem-loop involving the initiator AUG controls the efficiency of internal initiation of translation on hepatitis C virus RNA. RNA. 1996;2:955–968. [PMC free article] [PubMed] [Google Scholar]
- 15.Honda M, Ping L-H, Rijnbrand R C, Amphlett E, Clarke B, Rowlands D, Lemon S M. Structural requirements for initiation of translation by internal ribosome entry within genome-length hepatitis C virus RNA. Virology. 1996;222:31–42. doi: 10.1006/viro.1996.0395. [DOI] [PubMed] [Google Scholar]
- 16.Hwang S B, Park K-J, Kim Y-S, Sung Y C, Lai M M C. Hepatitis C virus NS5B protein is a membrane-associated phosphoprotein with a predominantly perinuclear localization. Virology. 1997;227:439–446. doi: 10.1006/viro.1996.8357. [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Lai M M C. Determination of the secondary structure of and cellular protein binding to the 3′-untranslated region of the hepatitis C virus RNA genome. J Virol. 1997;71:8698–8706. doi: 10.1128/jvi.71.11.8698-8706.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson S J, Konings D A M, Sarnow P. Biochemical and genetic evidence for a pseudoknot structure at the 3′ terminus of the poliovirus RNA genome and its role in viral RNA amplification. J Virol. 1993;67:2961–2971. doi: 10.1128/jvi.67.6.2961-2971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamer G, Argos P. Primary structural comparison of RNA-dependent polymerase from plant, animal and bacterial viruses. Nucleic Acids Res. 1984;12:7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato N, Hijikata M, Ootsuyama Y, Nakagawa M, Ohkohi S, Sugimura T, Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci USA. 1990;87:9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolykhalov A A, Feinstone S M, Rice C M. Identification of a highly conserved sequence element at the 3′ terminus of hepatitis C virus genome RNA. J Virol. 1996;70:3363–3371. doi: 10.1128/jvi.70.6.3363-3371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y-J, Liao C-L, Lai M M C. Identification of the cis-acting signal for minus-strand RNA synthesis of a murine coronavirus: implication for the role of minus-strand RNA in RNA replication and transcription. J Virol. 1994;68:8131–8140. doi: 10.1128/jvi.68.12.8131-8140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohmann V, Korner F, Herian U, Bartenschlager R. Biochemical properties of hepatitis C virus NS5B RNA-dependent RNA polymerase and identification of amino acid sequence motifs essential for enzymatic activity. J Virol. 1997;71:8416–8428. doi: 10.1128/jvi.71.11.8416-8428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poch O, Sauvaget I, Delarue M, Tordo N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989;8:3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds J E, Kaminski A, Kettinen H J, Grace K, Clarke B E, Carroll A R, Rowlands D J, Jackson R J. Unique features of internal initiation of hepatitis C virus RNA translation. EMBO J. 1995;14:6010–6020. doi: 10.1002/j.1460-2075.1995.tb00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi P-Y, Brinton M A, Veal J M, Zhong Y Y, Wilson W D. Evidence for the existence of a pseudoknot structure at the 3′ terminus of the flavivirus genomic RNA. Biochemistry. 1996;35:4222–4230. doi: 10.1021/bi952398v. [DOI] [PubMed] [Google Scholar]
- 27.Singh R N, Dreher T W. Turnip yellow mosaic virus RNA-dependent RNA polymerase: initiation of minus strand synthesis in vitro. Virology. 1997;233:430–439. doi: 10.1006/viro.1997.8621. [DOI] [PubMed] [Google Scholar]
- 28.Song C, Simon A E. Requirement of a 3′-terminal stem-loop in in vitro transcription by an RNA-dependent RNA polymerase. J Mol Biol. 1995;254:6–14. doi: 10.1006/jmbi.1995.0594. [DOI] [PubMed] [Google Scholar]
- 29.Takamizawa A, Mori C, Fuke I, Manabe S, Murakami S, Fujita J, Onishi E, Andoh T, Yoshida I, Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991;65:1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka T, Kato N, Cho M-J, Shimotohno K. A novel sequence found at the 3′ terminus of hepatitis C virus genome. Biochem Biophys Res Commun. 1995;215:744–749. doi: 10.1006/bbrc.1995.2526. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka T, Kato N, Cho M-J, Sugiyama K, Shimotohno K. Structure of the 3′ terminus of the hepatitis C virus genome. J Virol. 1996;70:3307–3312. doi: 10.1128/jvi.70.5.3307-3312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanji Y, Kaneko T, Satoh S, Shimotohno K. Phosphorylation of hepatitis C virus-encoded nonstructural protein NS5A. J Virol. 1995;69:3980–3986. doi: 10.1128/jvi.69.7.3980-3986.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuchihara K, Tanaka T, Hijikata M, Kuge S, Toyoda H, Nomoto A, Yamamoto N, Shimotohno K. Specific interaction of polypyrimidine tract-binding protein with the extreme 3′-terminal structure of the hepatitis C virus genome, the 3′X. J Virol. 1997;71:6720–6726. doi: 10.1128/jvi.71.9.6720-6726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsukiyama-Kohara K, Iizuka N, Kohara M, Nomoto A. Internal ribosome entry site within hepatitis C virus RNA. J Virol. 1992;66:1476–1483. doi: 10.1128/jvi.66.3.1476-1483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Sarnow P, Siddiqui A. Translation of human hepatitis C virus RNA in cultured cells is mediated by an internal ribosome binding mechanism. J Virol. 1993;67:3338–3344. doi: 10.1128/jvi.67.6.3338-3344.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westaway E G. Flavivirus replication strategy. Adv Virus Res. 1987;33:45–90. doi: 10.1016/s0065-3527(08)60316-4. [DOI] [PubMed] [Google Scholar]
- 37.Yamada N, Tanihara K, Takada A, Yorihuzi T, Tsutsumi M, Shimomura H, Tsuji T, Date T. Genetic organization and diversity of the 3′ noncoding region of the hepatitis C virus genome. Virology. 1996;223:255–261. doi: 10.1006/viro.1996.0476. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita T, Kaneko S, Shirota Y, Qin W, Nomura T, Kobayashi K, Murakami S. RNA-dependent RNA polymerase activity of the soluble recombinant hepatitis C virus NS5B protein truncated at the C-terminal region. J Biol Chem. 1998;273:15479–15486. doi: 10.1074/jbc.273.25.15479. [DOI] [PubMed] [Google Scholar]
- 39.Yen J-H, Chang S C, Hu C-R, Chu S-C, Lin S-S, Hsieh Y-S, Chang M-F. Cellular proteins specifically bind to the 5′-noncoding region of hepatitis C virus RNA. Virology. 1995;208:723–732. doi: 10.1006/viro.1995.1204. [DOI] [PubMed] [Google Scholar]
- 40.Yuan Z-H, Kumar U, Thomas H C, Wen Y-M, Monjardino J. Expression, purification, and partial characterization of HCV RNA polymerase. Biochem Biophys Res Commun. 1997;232:231–235. doi: 10.1006/bbrc.1997.6249. [DOI] [PubMed] [Google Scholar]