Gadolinium-based contrast agents (GBCAs) are an essential tool in clinical MRI. Millions of doses are administered annually to patients worldwide.1 However, free gadolinium, the basis of GBCAs, is extremely toxic in only small amounts. Gadolinium has an atomic radius similar to that of calcium and can interfere with calcium-dependent processes such as voltage-gated calcium channels or calcium-activated enzymes.1 To reduce toxicity, GBCAs are composed of gadolinium in chelated form. Following GBCA administration, presumably the gadolinium–chelate complex is excreted intact by the kidneys in patients with normal renal function. Each of the 9 Food and Drug Administration–approved MRI contrast agents has its own specific chelate, leading to variation in stabilities of the gadolinium–chelate complex. GBCAs are generally classified as either linear or macrocyclic based on chelate structure. An ionic, macrocyclic structure has the highest kinetic and thermodynamic stabilities.1
Recent reports describe gadolinium deposition in the brain following exposure to GBCAs, even in patients with normal renal function,2 recognized as dentate nucleus and globus pallidus hyperintensity on unenhanced T1-weighted images, which correlated with the number of prior GBCA doses.2 Autopsy studies of adult decedents using inductively coupled plasma mass spectroscopy (ICP-MS) confirmed the presence of trace amounts of gadolinium in the brain, bone, and skin following as little as 1 dose of a GBCA.3,4 ICP-MS is a technique that accurately measures total gadolinium levels within tissue; however, information concerning localization of gadolinium is limited to biopsy site selection. The highest reported gadolinium levels in the brain have been in the dentate nucleus.3,4
Here we use laser ablation ICP-MS (LA-ICP-MS) to demonstrate the distribution of gadolinium deposition throughout the cerebellum. The decedent was 17 years of age at death. The formerly healthy patient presented to the hospital in status epilepticus, the proximal cause of death. Prior to death, the patient underwent 4 MRI scans with GBCA administration. For 2 of the MRI examinations, hospital records document administration of gadopentetate dimeglumine. For the other 2 examinations, hospital records do not indicate the specific GBCA administered and the patient could have received either gadopentetate dimeglumine or gadodiamide. In addition, records from 10 years ago did not include contrast dose or patient’s weight at time of scan; however, departmental policy was to use body weight–adjusted dosing. The patient’s renal function was normal (creatinine values 0.5–0.7 mg/dL). The 4 GBCA doses were administered over a 10-week period with the last dose administered 84 days before death.
On MRI obtained prior to the last dose of GBCA (figure, A), no hyperintense signal was noted within the dentate nucleus, which previous studies suggest becomes visible only after approximately 5 GBCA doses.2 Despite the lack of T1-weighted hyperintensity within the dentate nucleus on MRI, heavy deposition of gadolinium was found within the dentate nucleus on the corresponding LA-ICP-MS gadolinium distribution map (figure, C) in addition to gadolinium deposition throughout the cerebellar cortex. Interestingly, higher levels of gadolinium deposition were noted within the folia depths, which may be related to cerebellar microvasculature as arterial ramifications are present within the bases of the folia, a site that demonstrates selective vulnerability during ischemic states.5 Using ICP-MS, total gadolinium concentration within the dentate/peridentate white matter was 1.01 μg/g, similar to previous reports. For example, McDonald et al.3 reported 0.1–58.8 μg/g of gadolinium within the dentate nuclei of patients receiving 4–29 doses of a linear GBCA.
Figure. Distribution map of gadolinium deposition within the cerebellum.
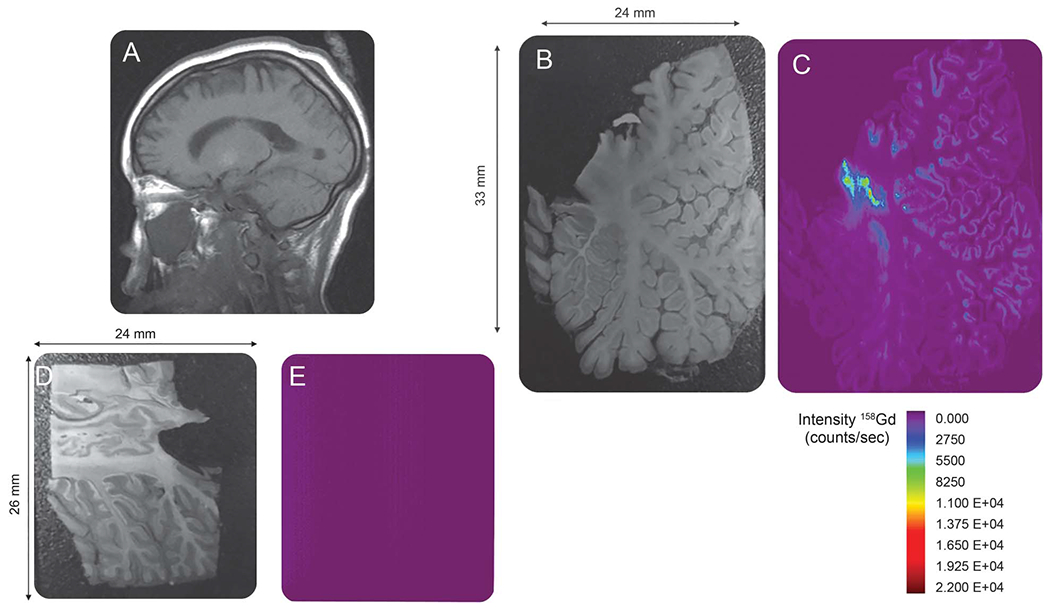
(A) Sagittal image through the level of the dentate nucleus prior to the last dose of gadolinium-based contrast agent (GBCA). No hyperintense signal is demonstrated within the dentate nucleus. (Note that the globus pallidus demonstrates T1 shortening due to hypoxic ischemic injury.) (B) Autopsy section through the cerebellum. (C) Laser ablation inductively coupled plasma mass spectroscopy (LA-ICP-MS) gadolinium distribution map corresponding to the autopsy section in (B) demonstrates heavy deposition of gadolinium within the dentate nucleus. In addition, there is gadolinium deposition throughout the cerebellar cortex, particularly within the depths of the cerebellar folia. (D) Autopsy section through the cerebellum of the control patient not exposed to GBCA administration. (E) LA-ICP-MS gadolinium distribution map corresponding to the autopsy section in (D). The structure of the cerebellum is not seen and the signal intensity is equal to background as no gadolinium is detected in the control specimen.
As a control, the figure, E, shows a LA-ICP-MS gadolinium distribution map of a 4-year-old patient not exposed to GBCA administration. Cause of death was status epilepticus and respiratory failure. Quantification of total gadolinium within the control sample was below the limits of quantification. Employing the National Institute of Standards and Technology 600-series standard reference materials as calibration standards, the limit of detection for our technique was 0.075 μg/g.
Here we demonstrate gadolinium deposition throughout the cerebellum of a child following 4 GBCA administrations. Further studies should address several important questions. The form of gadolinium present within tissue as either chelated or released is unknown. Similar studies with autopsy specimens of patients receiving exclusively macrocyclic GBCAs are needed as prior reports have demonstrated that gadolinium retention is dependent on GBCA class. Macrocyclic GBCAs (in contrast to linear GBCAs) have not been shown to cause T1-signal hyperintensity in the dentate nucleus,6 which led to the recommendation by the NIH to preferentially use macrocyclic agents.7 The potential clinical significance of gadolinium retention within the brain is unknown; further research is needed to identify any long-term clinical consequences (e.g., possibly subtle signs/symptoms of dentate nucleus dysfunction). While definitive studies could extend over decades, we recommend taking a cautious stance and considering use of higher stability GBCAs. For patients undergoing contrast-enhanced MRI examinations, the potential for gadolinium deposition vs risks associated with withholding a well-indicated MRI examination should be included in a risks/benefits analysis.
Disclosure:
D. Roberts: consultant and advisory board member for Guerbet, research support from Guerbet. C. Welsh, D. LeBel II, and W. Davis report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
Contributor Information
Donna R. Roberts, Medical University of South Carolina, Charleston.
Cynthia A. Welsh, Medical University of South Carolina, Charleston.
David P. LeBel, II, Medical University of South Carolina, Charleston.
W. Clay Davis, National Institute of Standards and Technology, Gaithersburg, MD.
References
- 1.Idee JM, Port M, Raynal I, Schaefer M, Le Greneur S, Corot C. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam Clin Pharmacol 2006;20:563–576. [DOI] [PubMed] [Google Scholar]
- 2.Kanda T, Oba H, Toyoda K, Kitajima K, Furui S. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol 2016;34:3–9. [DOI] [PubMed] [Google Scholar]
- 3.McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015;275:772–782. [DOI] [PubMed] [Google Scholar]
- 4.Murata N, Gonzalez-Cuyar LF, Murata K, et al. Macrocyclic and other nongroup 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol 2016;51:447–453. [DOI] [PubMed] [Google Scholar]
- 5.Nonaka H, Akima M, Hatori T, Nagayama T, Zhang Z, Ihara F. The microvasculature of the human cerebellar meninges. Acta Neuropathol 2002;104:608–614. [DOI] [PubMed] [Google Scholar]
- 6.Radbruch A, Weberling LD, Kieslich PJ, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 2015;275:783–791. [DOI] [PubMed] [Google Scholar]
- 7.Malayeri AA, Brooks KM, Bryant LH, et al. National Institutes of Health Perspective on reports of gadolinium deposition in the brain. J Am Coll Radiol 2016;13:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]


