Abstract
Centaurea thracica (Janka) Hayek is a plant common in southern Bulgaria. The inflorescences were collected during June and September 2021, while their seeds were obtained in September 2021. The chemical and lipid composition of the inflorescences during the vegetation process of the plant were established. A significant decrease in total proteins (from 8.7 to 7.4%), glyceride oils (2.0–1.7%), and ash (4.5–4.2%) content was observed, while the amount of carbohydrates (72.3–77.2%) and fibers (28.7–35.8%) increased. During the vegetation of the plant, the content of oleic and linoleic acids increased up to 2–3 times, while the level of palmitic acid decreased. The lipids from the seeds were rich in oleic (53.0%) and palmitic (36.2%) acids. The tocopherol content in the oils of the inflorescences during vegetation increased from 58 to 110 mg/kg, and the content in the oil from the seeds was 260 mg/kg. The phospholipid content decreased during vegetation, and differences were observed in the composition between the inflorescences and the seeds. The high content of oleic acid, linoleic acid, tocopherols, and phospholipids determine the nutritional and biological value of the oils isolated from Centaurea thracica, and contribute to their potential use in various directions.
Keywords: Centaurea thracica, chemical composition, lipid composition, fatty acids, bioactive components, lipid indices
1. Introduction
The genus Centaurea includes a large number of species (more than 700) belonging to the Asteraceae family [1]. Members of the genus are only found north of the equator, primarily in the Eastern Hemisphere, the Middle East, and surrounding regions. A particularly rich diversity of these species is observed mainly in Eastern Anatolia and the South Caucasus, followed by the Mediterranean region and the Balkan Peninsula [2,3]. In the Bulgarian flora, the genus Centaurea is represented by more than 75 species and is the richest in endemics [4,5]. It consists of diverse representatives with different growth cycles (annual, biennial, or perennial plants). Some of them are traditionally used for raw consumption or as medicinal plants due to their bioactive properties [6,7,8,9,10].
The genus Centaurea is one of the most important of the Asteraceae family [11]. The main species are widely used by people to treat various diseases [12]. Studies conducted with extracts of various Centaurea species have shown that they have various vital activities such as enzyme inhibitory activity and anti-hemorrhoidal, anti-cancer, and anti-diabetic effects. They also have anti-inflammatory, analgesic, antioxidant, antimicrobial, and antimutagenic properties [13,14,15,16,17,18,19,20].
Plants of the genus Centaurea are pharmaceutically valuable due to their secondary metabolites such as phenols and flavonoids, which have positive effects on human health. These substances are responsible for many biological activities, including the overall effect of purifying the body [17,21,22,23,24]. According to Sokovic et al. [25] and Tešević et al. [26], the biological activity is mostly associated with the presence of flavonoids (apigenin, isokaempferide, hispidulin, eupatorin, cirsimaritin, santoflavone, and salvigenin) as well as sesquiterpene lactones (germacranolides, eudesmanolides, elemanolides, and guaianolides). Cnicin is the major sesquiterpene lactone for many Centaurea species and it is responsible for cytotoxic activities.
The lipids contain mainly acylglycerols, which are accompanied by minor amounts of phosphatides, sterols, fat-soluble vitamins, waxes, hydrocarbons, etc. The type and amount of fatty acids in acylglycerols, as well as the position and distribution of these fatty acids in them, determine the chemical, physical, and functional properties of lipids. Clinical and epidemiological studies show that many chronic diseases, such as cardiovascular, cancer, and degenerative diseases, are associated with increased consumption of saturated and trans fatty acids, while intake of monounsaturated and polyunsaturated fatty acids is associated with better health [27,28,29,30,31,32]. Plant lipids contain more long-chain unsaturated fatty acids in their molecule, which easily undergo oxidative changes under the action of atmospheric oxygen. Tocopherols belong to the microcomponents of lipids, and are one of the most important biologically active substances in them. In plants, α-tocopherol is biosynthesized via β- or γ-tocopherol [33]. Tocopherols are one of the most important antioxidants and lipid stabilizers against oxidation. They exert their antioxidant effect through multiple biochemical and biophysical mechanisms, including the deactivation of active free radicals. α-Tocopherol has a stronger antioxidant effect than γ-tocopherol, but it can also act as a “pro-oxidant” under certain conditions [34]. The lipids are an important nutrient, a major source of energy, and transport fat-soluble vitamins.
The fatty acid composition can serve for evaluation of the quality and nutritional properties of the oil. The fatty acid composition of aerial parts from six species of Centaurea—C. balsamita, C. calolepis, C. carduiformis subsp. carduiformis, C. cariensis subsp. maculiceps, C. cariensis subsp. microlepis, and C. iberica—was studied. The main fatty acid identified in the oils of all species was linoleic acid (C18:2), except C. cariensis subsp. microlepis. Other predominant fatty acids in the oils isolated from Centaurea species are palmitic (C16:0), linolenic (C18:3), oleic (C18:1), and stearic (C18:0) acids [35]. The significant amounts of linoleic and linolenic acids found in oils isolated from plants of the genus Centaurea make Centaurea oil a valuable food, i.e., it can be a good source of essential fatty acids that are extremely important in the formation of cell membranes, especially of nervous tissue.
According to some authors, palmitic acid is the main predominant saturated fatty acid in the lipids isolated from C. salicifolia subsp. abbreviata and C. babylonica (from 29.37 to 30.49%) [36]. The data are in agreement with Yildirim et al. [37], who also reported that it is the major fatty acid present in some oils from plants in the genus Centaurea.
In recent years, there has been a growing interest in new, previously unexplored plant oils that could be a source of valuable biologically active substances. All this created interest to investigate the chemical composition of the inflorescences of Centaurea thracica, as well as the lipid composition of acylglycerols from its inflorescences and seeds, in order to evaluate the possibility of its use as a new alternative source of different biologically active components.
2. Results and Discussion
2.1. Chemical Composition of Centaurea thracica Inflorescences
The changes in the main indicators determining the chemical composition of the inflorescences of Centaurea thracica—content of protein, oil, carbohydrates, moisture, mineral substances, and crude fiber—during the vegetation process of the plant (inflorescences collected in June and September) were established.
The data on the content of the main components in the composition of the inflorescences collected in June and September from Centaurea thracica are presented in Table 1.
Table 1.
Changes in the total chemical composition of Centaurea thracica inflorescences.
| Indicators | June | September |
|---|---|---|
| Proteins, % | 8.7 ± 0.1 * | 7.4 ± 0.2 |
| Oil, % | 2.0 ± 0.1 | 1.7 ± 0.1 |
| Carbohydrates, % | 72.3 ±0.6 | 77.2 ± 0.7 |
| Crude fibers, % | 28.7 ± 0.4 | 35.8 ± 0.3 |
| Ash, % | 4.5 ± 0.1 | 4.2 ± 0.1 |
| Moisture, % | 12.5 ± 0.1 | 9.5 ± 0.1 |
| Energy value **, kJ/100 g (kcal/100 g) | 1453 (342) | 1503 (354) |
* The samples were analyzed in triplicate (n = 3), and the results were expressed as mean and standard deviation. ** The energy value (EV) in kJ/100 g (kcal/100 g) was calculated as follows: EV = % proteins × 17 (4.0) + % carbohydrates × 17 (4.0) + lipids × 38 (9.0).
The inflorescences collected in June from Centaurea thracica have a higher protein content (8.7%) and oil content (2.0%) compared to those from September (7.4% and 1.7%). The obtained results for protein content correlate with those of some medicinal and edible plants, for which proteins range from 1.30 to 11.56% [38,39]. A lower carbohydrate content was found in the inflorescences in June (about 72.3%), which increased with the development of the plant by about 5.0%. A higher fiber content is observed in the inflorescences from the month of September (35.8%) compared to their content in those from the month of June (28.7%). The fiber content is close to the results obtained by Vishwakarma and Dubey, who studied various medicinal and aromatic plants and found that their fiber content varied from 0.90 to 28.59% [40]. The inflorescences of Centaurea thracica contain mineral salts, and their amount decreases in the process of vegetation of the flower from 4.5 to 4.2%. The content of mineral substances in the inflorescence from the month of September is lower than the data for ash of seeds of the species Centaurea karduchorum Boiss (5.93%) [41]. A trend in decreasing moisture content from 12.5 to 9.5% was observed in the last investigated inflorescence.
Generally, the aerial parts of the plants at the beginning of their development mainly consist of water, proteins, and carbohydrates. In the process of vegetation, a decrease in the amount of water and accumulation of the dry matter is observed, as well as the favored formation of reserve non-nitrogen substances, such as sugars, starch, and cellulose. In this regard, the highest increase was observed in the amounts of the carbohydrates, including fibers in the inflorescences from the examined plants.
The increase in carbohydrate content in plants during vegetation is primarily due to photosynthesis. During this period, the plant actively synthesizes, uses, and stores carbohydrates to support its development and ensure survival through less favorable conditions. The decrease in protein content in the plants during vegetation is primarily due to the redistribution of nitrogen to support the growth of plants’ parts other than the inflorescence.
The optimal ratio of the carbohydrates and proteins in plants for edible purposes varies depending on the dietary needs. A balanced diet typically includes a mix of foods with varying carbohydrate-to-protein ratios. For overall dietary balance, aiming for an average ratio in the range of 4:1 to 7:1 might be suitable, but this can vary based on individual nutritional needs. This ratio in the inflorescence in June was 8:1, and in those from September was 10:1.
Data on the chemical composition of the inflorescences of Centaurea thracica studied by us are close to the literature data on protein content (10.93%), ash (5.93%), and fiber (32.58%) in the plant Centaurea karduchorum Boiss grown in Turkey [41].
The results obtained by us are higher than the literature data for protein content (1.60–2.89%), lipids (0.80–1.39%), mineral substances (1.20–1.56%), and of fiber (14.8–17.2%) when studying the bud of the genus Centaurea during three stages of its development (tight bud, mature bud, and fully open) [42].
The inflorescences in September of Centaurea thracica have a higher content of carbohydrates, which determines, at a great extent, the increase in the energy value compared to that of the inflorescences in June (from 1453 kJ/100 g (342 kcal/100 g) to 1503 kJ/100 g (354 kcal/100 g)). The energy value of Centaurea thracica inflorescences is close to that of herbs or medicinal plants, which have an average energy value of 314 kcal/100 g. The energy value of the inflorescences in June is close to the literature data on the energy value of lentils and potatoes (1449 and 1463 kJ/100 g), while this value of inflorescences in September is similar to that of corn (1577 kJ/100 g) [43].
2.2. Lipid Composition of Oil Isolated from Inflorescences and Seeds of Centaurea thracica
The lipid composition of the glyceride oils isolated from the inflorescences and seeds of Centaurea thracica was investigated, i.e., fatty acid composition of the oils, tocopherol content, and the content and composition of the phospholipid fraction.
2.2.1. Fatty Acid Composition
Table 2 presents the fatty acid composition of acylglycerols isolated from inflorescences and seeds of Centaurea thracica.
Table 2.
Fatty acid composition of glyceride oils isolated from Centaurea thracica.
| Fatty Acids % | Inflorescences | Seeds | ||
|---|---|---|---|---|
| June | September | |||
| C 4:0 | butyric | 3.4 ± 0.2 * | - ** | - |
| C 6:0 | caproic | 3.1 ± 0.1 | 0.7 ± 0.2 | - |
| C 8:0 | caprylic | - | 0.1 ± 0.0 | 0.2 ± 0.02 |
| C 10:0 | capric | - | 0.2 ± 0.05 | 0.1 ± 0.0 |
| C 12:0 | lauric | 2.9 ± 0.2 | 1.1 ± 0.1 | 0.1 ± 0.0 |
| C 14:0 | myristic | 2.5 ± 0.1 | 2.5 ± 0.15 | 0.2 ± 0.05 |
| C 14:1 | myristoleic | 0.5 ± 0.1 | 0.8 ± 0.2 | 0.1 ± 0.0 |
| C 15:0 | pentadecanoic | 1.0 ± 0.2 | 0.4 ± 0.1 | 0.2 ± 0.05 |
| C 15:1 | pentadecenoic | 0.5 ± 0.1 | 0.1 ± 0.0 | - |
| C 16:0 | palmitic | 32.6 ± 0.5 | 21.6 ± 0.6 | 36.2 ± 0.2 |
| C 16:1 | palmitoleic | 3.8 ± 0.1 | 11.7 ± 0.2 | - |
| C 17:0 | heptadecanoic | 0.9 ± 0.1 | 0.5 ± 0.06 | 0.3 ± 0.05 |
| C 17:1 | heptadecenoic | 0.8 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.0 |
| C 18:0 | stearic | 4.9 ± 0.3 | 4.5 ± 0.2 | 3.7 ± 0.4 |
| C 18:1 | oleic | 13.7 ± 0.5 | 30.9 ± 0.7 | 53.0 ± 0.5 |
| C 18:2 (n-6) | linoleic | 13.8 ± 0.4 | 20.3 ± 0.3 | 1.4 ± 0.2 |
| C 18:3 (n-3) | linolenic | 3.6 ± 0.3 | 0.7 ± 0.1 | 1.1 ± 0.1 |
| C 20:0 | arachidic | 3.5 ± 0.5 | 0.2 ± 0.05 | - |
| C 20:1 | gadoleic | 0.4 ± 0.1 | 0.1 ± 0.0 | - |
| C 20:2 (n-6) | eicosadienoic | 0.5 ± 0.2 | 0.2 ± 0.05 | 0.4 ± 0.1 |
| C 22:0 | behenic | 2.9 ± 0.3 | 1.5 ± 0.1 | 0.5 ± 0.1 |
| C 22:1 | erucic | 0.3 ± 0.1 | - | - |
| C 22:2 (n-6) | docosadienoic | - | - | 0.7 ± 0.1 |
| C 20:5 (n-3) | eicosapentaenoic | - | - | 0.1 ± 0.0 |
| C 23:0 | tricosanoic | 1.0 ± 0.2 | 0.4 ± 0.1 | - |
| C 24:0 | lignoceric | 2.8 ± 0.4 | 1.0 ± 0.2 | 0.2 ± 0.1 |
| C 24:1 | nervonic | - | - | 0.2 ± 0.05 |
| C 22:6 (n-3) | docosahexaenoic | 0.6 ± 0.1 | 0.1 ± 0.0 | 1.0 ± 0.2 |
| Σ n-6 | 14.3 | 20.5 | 2.5 | |
| Σ n-3 | 4.2 | 0.8 | 2.2 | |
| Ratio of n-6/n-3 | 3.4 | 26.6 | 1.14 | |
| Iodine value, g I2/100 g | 47.2 | 66.5 | 53.2 | |
* The samples were analyzed in triplicate (n = 3), and the results are expressed as mean and standard deviation. **—not detected, Limit of detection is 0.02%, Limit of Quantitation is 0.05%.
The composition of the oil from the inflorescences of Centaurea thracica contains 23 fatty acids, and the oil from seeds contains 20 fatty acids. Palmitic acid (32.6%) predominates in the composition of the oil from the inflorescences collected in June, followed by linoleic (13.8%) and oleic acid (13.7%), which are in comparable quantities. There is a relatively high content of lower saturated fatty acids—butyric (C4:0—3.4%) and caproic (C6:0—3.1%)—as well as higher saturated fatty acids—stearic acid (C18:0–4.9%), arachidic (C20:0—3.5%), behenic (C22:0—2.9%), and lignoceric (C24:0—2.8%). The oil also contains a larger amount of the essential linolenic acid (C18:3—3.6%) compared to traditional sunflower oil (0–0.3%), but its amount is close to that in soybean oil (4.5–11.0%) [44].
In the composition of the oil from the inflorescences collected in September, oleic acid (C18:1—30.9%) predominates, followed by palmitic acid (C16:0—21.6%) and linoleic acid (C18:2—20.3%). During the vegetation process, a significant increase in the amount of oleic (from 13.7 to 30.9%) and linoleic (from 13.8 to 20.3%) acids was observed. A threefold increase in the content of palmitoleic acid (C16:1—from 3.8 to 11.7%) and a decrease in the amount of the saturated fatty acids were found, as follows: arachidic acid (C20:0—from 3.5 to 0.2%), behenic (C22:0—from 2.9 to 1.5%), and lignoceric (C24:0—from 2.8 to 1.0%), as well as linolenic acid (C18:3—from 3.6 to 0.7%).
The glyceride oil from the seeds of Centaurea thracica is rich in oleic (53.0%) and palmitic (36.2%) acids. Linoleic and linolenic acids are represented in insignificant amounts, 1.4% and 1.1%, respectively. The presence of the essential docosahexaenoic acid in the amount of 1.0% is also noticeable in the seed oil. The content of stearic acid is 3.7%, and the remaining fatty acids are represented in insignificant amounts—from 0.1 to 0.7%. The fatty acid composition of Centaurea thracica seed oil is close to that of palm–oleic oil, in which palmitic (38.0–43.5%) and oleic (39.9–46.0%) acids predominate [44].
In the study of seeds from various species of plants of the genus Centaurea, different data on the fatty acid composition were reported. The obtained results are different from the data by other authors regarding the composition of oils from different species of plants of the genus Centaurea (Centaurea albonitens and C. balsamita), in which the main acids are linoleic (49.94–47.75%), oleic (30.36–30.07%), palmitic (8.98–10.64%), and stearic (5.89–6.41%) acids [45]. In the literature, there are data on the fatty acid composition of glyceride oils isolated from the bud of the genus Centaurea during three stages of its development (tight bud, mature bud, and fully open). It was established that the main fatty acids were oleic (from 9.04 to 26.4%), palmitic (from 24.8 to 30.0%), and linoleic (from 12.8 to 20.7%) acids [42]. This composition is close to the fatty acid composition of the oil isolated from the inflorescences collected in September of Centaurea thracica.
The analyzed oils were rich of palmitic acid and our results are close to the reported by Aktumsek et al. [36], who found that palmitic acid was the main one in the oils isolated from C. salicifolia subsp. abbreviata and C. babylonica (from 29.37 to 30.49%). The results are in agreement with Yildirim et al. [37], who reported that the palmitic acid was also the major one in some oils from plants of the genus Centaurea.
There are some differences in the fatty acid composition of the oils obtained from the three investigated Centaurea species (C. patula, C. pulchella, and C. tchihatcheffii), where the content of linolenic (C18:3—24.01–33.62%), linoleic (C18:2—18.92–34.55%), palmitic (C16:0—13.22–21.02%), and oleic (C18:1—1.44–11.30%) acids were established [46]. The presence of the essential docosahexaenoic acid was also found in an amount of 0.56% to 0.97%, which is similar to its content in the oils of the unripe inflorescences and the seeds of Centaurea thracica in the present study.
The fatty acid compositions of six species of Centaurea—C. balsamita, C. calolepis, C. carduiformis subsp. carduiformis, C. cariensis subsp. maculiceps, C. cariensis subsp. microlepis, and C. iberica—were also determined. According to Tekeli et al. [35], the major fatty acid identified in all species was linoleic acid (C18:2—31.23–40.60%), except for C. cariensis subsp. microlepis (23.92%). Other predominant fatty acids in Centaurea oil are palmitic (C16:0—17.83–25.31%), linolenic (C18:3—8.54–27.36%), oleic (C18:1—8.65–27.22%), and stearic (C18:0—2.93–7.18%) acids. The Centaurea oil can be a good source of essential fatty acids, and the high content of linoleic acid, which is the main fatty acid of the polyunsaturated fatty acids, makes it a valuable food.
The n-6/n-3 ratio was found to vary widely from 1.14 in the seed oil to 26.6 in the oil isolated from the inflorescences collected in September. It can be seen that in the oil isolated from the seeds, the amounts of n-3 and n-6 are almost the same, while in the oils isolated from the inflorescences, the content of n-6 significantly exceeds that of n-3. The established n-6/n-3 ratio of the seed oil is close to that obtained by a previous study on the fatty acid composition of glyceride oils isolated from the bud of the genus Centaurea at three stages of its development (n-6/n-3 is 0.65–1.00) [42]. The higher ratio of n-6/n-3 in the oil from the inflorescences collected in September is due to the high content of linoleic acid (20.3%) and the insignificant amount of linolenic acid (0.7%). These changes can be explained, as follows, by the different biosynthesis’ stages of the fatty acids: saturated fatty acids predominate in the first stage of development of the plants, and after that, the content of the polyunsaturated fatty acids increases. These ratio values indicate that the studied oils have a lower content of n-3 fatty acids, which is a characteristic of the most vegetable oils.
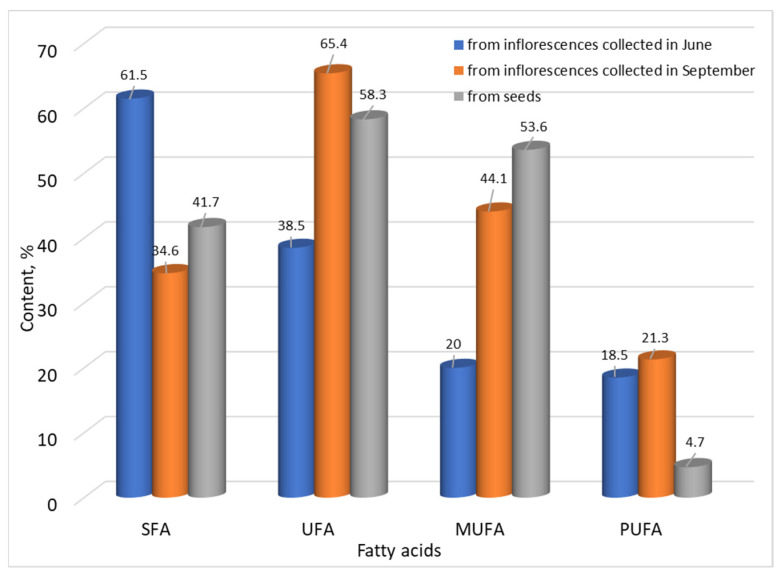
Figure 1 shows the ratio of saturated, unsaturated, monounsaturated, and polyunsaturated fatty acids in the oil isolated from inflorescences at different stages of vegetation and seeds of Centaurea thracica.
Figure 1.
Content of saturated (SFA), unsaturated (UFA), monounsaturated (MUFA), and polyunsaturated (PUFA) fatty acids in oil isolated from inflorescences at different stages of vegetation and seeds of Centaurea thracica.
The amount of saturated fatty acids predominates in the oil isolated from the inflorescences in June (61.5%), while in the oils isolated from inflorescences collected in September and from the seeds, the amount of the unsaturated fatty acids is the highest—65.4% and 58.3%, respectively. The main representative of saturated fatty acids is palmitic acid (21.6–36.2%), followed by stearic acid (3.7–4.9%). The quantities of monounsaturated fatty acids in the oils from inflorescences in September (44.1%) and from the seeds (53.6%) were significantly higher than those of polyunsaturated fatty acids—21.3% and 4.7%, respectively—while in the oil from the inflorescences in the month of June, the ratio of monounsaturated (20.0%) to polyunsaturated (18.5%) fatty acids is almost 1:1. The main representative of monounsaturated fatty acids is oleic acid (13.7–53.0%), while the polyunsaturated ones are linoleic (1.4–20.3%) and linolenic (0.7–3.6%) acids.
The results of our research differ from other studies regarding the fatty acid composition of oils isolated from six Centaurea species, where polyunsaturated fatty acids predominate (41.02–58.80%), and the amount of monounsaturated fatty acids varies from 9.43% to 28.56% [35]. The content of saturated fatty acids in the oils from inflorescences and seeds that we studied is close to that obtained by studying the fatty acid composition of glyceride oils isolated from the bud of the genus Centaurea during the three stages of its development (SFA—37.0–49.0%), but the amounts of MUFA and PUFA fatty acids differ significantly from our results (MUFA from 10.0 to 27.0%, and PUFA from 32.0 to 42.0%) [42].
The obtained results differ compared with the data about the composition of oils from different species of Centaurea plants (Centaurea albonitens and C. balsamita) reported by Peker and Baştürk [45], in which polyunsaturated fatty acids predominate (50.11% and 47.96%, respectively), monounsaturated are found to be 31.22% for both species, and saturated fatty acids are found to be 17.25% and 18.81%, respectively. These oils, compared to the ones we studied, have a significantly lower content of saturated fatty acids (2–3 times) and a higher content of polyunsaturated fatty acids (from 2 to 10 times).
The iodine value, which is an indicator of the degree of unsaturation of fatty acids in the oils, is relatively low (47.2–66.5 g I2/100 g), and this is due to the high content of saturated fatty acids in the studied oils. Their iodine value is close to that of palm–olein oil (≥56 g I2/100 g) [44]. For that reason, the investigated oils can be classified as non-drying oils, which are characterized using an iodine value between 50 and 100 g I2/100 g.
Based on the data about fatty acid composition of both oils (from seeds and inflorescences) of Centaurea thracica, we determined the following indicators which are related to the evaluation of the benefits of the oil for human health and which are the criteria for their therapeutic effect: the ratio between polyunsaturated and saturated fatty acids, and the atherogenic and thrombogenic index.
Table 3 presents the data regarding the ratio between polyunsaturated and saturated fatty acids, and the atherogenic and thrombogenic index.
Table 3.
Ratio of polyunsaturated and saturated fatty acids (PUFA/SFA), and the atherogenic and thrombogenic indices of oils from inflorescences and seeds.
| Oils From: | PUFA/SFA | Atherogenic Index | Thrombogenic Index |
|---|---|---|---|
| Inflorescences in June | 0.30 ± 0.05 * | 1.20 ± 0.2 | 1.33 ± 0.05 |
| Inflorescences in September | 0.62 ± 0.02 | 0.50 ± 0.1 | 0.82 ± 0.02 |
| Seeds | 0.11 ± 0.01 | 0.64 ± 0.1 | 1.13 ± 0.03 |
* The samples were analyzed in triplicate (n = 3), and the results were expressed as mean and standard deviation.
The ratio of polyunsaturated and saturated fatty acids has an important role in determining the various properties of cell membranes that help maintain normal cell metabolism. The recommended minimum ratio of polyunsaturated and saturated fatty acids is 0.45, and the optimal ratio, which leads to a reduction in the risk of cardiovascular diseases, is 1.0–1.5 [47]. For inflorescence oils, it is 0.30 and 0.61, respectively, which is close to the recommended minimum ratio, but that for seed oil is significantly lower than the optimal value, which is a result of the low content of polyunsaturated fatty acids. The PUFA/SFA ratio of the investigated Centaurea thracica inflorescences and seed oils was much lower than the same ratio of soybean oil (4.39), corn oil (4.10), sesame oil (2.94), and was close to that of palm oil (0.18) [47].
The atherogenic index shows the relationship between the sum of the main saturated fatty acids, which are considered to be pro-atherogenic (they cause an increase in the level of cholesterol in the blood because they are easily deposited on the walls of the arteries) and the main unsaturated fatty acids, which have an anti-atherogenic effect [48]. The oil from the inflorescences collected in June has a twice higher atherogenic index (1.20) than the oils isolated from the inflorescences in September and the seeds (0.50 and 0.64). Plant oils are characterized by different values of the atherogenic index: palm oil—0.88; olive oil—0.14; and sunflower oil—0.07. Anti-atherogenic lipids inhibit the accumulation of plaque and reduce the levels of esterified fatty acids and cholesterol, thus preventing the occurrence of coronary diseases [49,50].
The thrombogenic index determines the tendency to induce thrombogenesis in blood vessels. The values of this index for the studied oils ranged from 0.82 to 1.33, while for other plant oils, they were as follows: palm oil—1.74; olive oil—0.32; and sunflower oil—0.28 [50].
Atherogenic and thrombogenic indices lower than 1.0 are considered to be indicative of better antiatherogenic and antithrombogenic properties of lipids [51]. The values of these indices for the oil isolated from the inflorescences in September were lower than 1.0, while those collected in June were slightly over 1.0 but lower than 1.5.
Based on the results, the oil from the inflorescences and seeds of Centaurea thracica may eventually find application in food, cosmetic, and pharmaceutical products.
2.2.2. Content of Tocopherols
The results regarding the tocopherol content and individual composition of tocopherol fraction are presented in Table 4.
Table 4.
Tocopherol content and individual tocopherol composition of oils from inflorescences and seeds of Centaurea thracica.
| Content | Inflorescences | Seeds | |
|---|---|---|---|
| June | September | ||
| Tocopherols, mg/kg | 58 ± 5 * | 110 ± 5 | 260 ± 10 |
| Individual tocopherol composition | |||
| α-Tocopherol, % of total tocopherol content | 100 ± 0 | 100 ± 0 | 100 ± 0 |
* The samples were analyzed in triplicate (n = 3), and the results were expressed as mean and standard deviation. Limit of detection is 15 mg/kg.
The highest content of tocopherols was observed in the oil isolated from the seeds—260 mg/kg. The total content of tocopherols in the oil of the inflorescences increases about 2 times during their growing season—110 and 58 mg/kg, respectively. The content of tocopherols in the examined oils is close to that of palm kernel oil (0–260 mg/kg) and that of grape seed oil (240–410 mg/kg), but it is significantly lower than that of traditional sunflower oil (440–1520 mg/kg) [44].
Significantly higher values for the content of tocopherols were obtained by Peker and Baştürk, who examined oils from different species of plants of the genus Centaurea: Centaurea albonitens—1689 mg/kg, and C. Balsamita—1186 mg/kg [45].
The amount of tocopherols in the studied oils is close to that of Camellia sinensis seed oil (210 mg/kg) [52] and rapeseed oil (190 mg/kg) [53].
The presence of only one representative of tocopherols (α-isomer) was found in the investigated oils from inflorescences and seeds of Centaurea thracica. The tocopherol composition matches that of oils isolated from Centaurea albonitens and C. balsamita, in which α-tocopherol is also predominant. The individual tocopherol composition of Centaurea thracica inflorescences and seed oil is close to the composition of sunflower and safflower oil, which mainly contain α-tocopherol [44].
The results obtained by us differ from those in the study of the tocopherol composition of oils isolated from the bud of the genus Centaurea during three stages of its development. Fernandes et al. [42] found that during the inflorescence’s development of plants of the genus Centaurea, the tocopherol content in the isolated oil decreased from 3.0 to 2.4 mg/100 g dry weight, and their oils contained all four major representatives of tocopherols (α-, β-, γ-, and δ-tocopherol), with α-tocopherol being predominant.
2.2.3. Phospholipid Content and Individual Phospholipid Composition
The results regarding the total phospholipid content and the individual phospholipid composition of the lipids isolated from the inflorescences and seeds of Centaurea thracica are given in Table 5.
Table 5.
Individual phospholipid composition of lipids isolated from inflorescences and seeds of Centaurea thracica.
| Phospholipids % (from Total Phospholipids) | Inflorescences | Seeds | |
|---|---|---|---|
| June | September | ||
| Phosphatidylcholine | 6.6 ± 0.2 * | 23.9 ± 0.5 | 9.1 ± 0.5 |
| Phosphatidylinositol | 14.6 ± 0.3 | 10.4 ± 0.4 | 25.0 ± 1.5 |
| Phosphatidylethanolamine | 6.9 ± 0.5 | 13.9 ± 0.4 | 31.8 ± 1.1 |
| Sphingomyelin | 7.3 ± 0.1 | 7.6 ± 0.5 | 11.3 ± 1.1 |
| Phosphatidylserine | 12.5 ± 0.4 | 4.7 ± 0.1 | 7.0 ± 1.0 |
| Lysophosphatidylcholine | 6.1 ± 0.1 | 9.4 ± 0.4 | 2.8 ± 0.4 |
| Lysophosphatidylethanolamine | 2.5 ± 0.3 | 2.0 ± 0.2 | 2.5 ± 0.6 |
| Monophosphatidylglycerol | - ** | 1.0 ± 0.1 | 5.6 ± 0.5 |
| Diphosphatidylglycerol | 21.4 ± 0.4 | 7.3 ± 0.3 | 4.9 ± 0.2 |
| Phosphatidic acids | 22.1 ± 0.1 | 19.8 ± 0.4 | - |
| Total phospholipid content in the oil % | 0.70 ± 0.10 | 0.35 ± 0.05 | 0.30 ± 0.04 |
* The samples were analyzed in triplicate (n = 3), and the results were expressed as mean and standard deviation; **—not detected, Limit of detection is 0.02%, Limit of Quantitation is 0.05%.
The phospholipid content decreased twice during inflorescence development, from 0.70% to 0.35%, while the amount of phospholipids in lipids from the seeds was found to be 0.30%. The higher percentage of phospholipids in the lipids isolated from the inflorescence in June is due to the fact that they are mainly synthesized in the initial stage of vegetation. Their phospholipid content is similar to that in sunflower, linseed, corn, soybean, and rapeseed oils (0.7–1.0%) [54].
In the phospholipid fraction of lipids from the inflorescences and seeds of Centaurea thracica, almost all major classes of phospholipids were identified. In the phospholipids isolated from the inflorescences in June, phosphatidic acids (22.1%), and diphosphatidylglycerol (21.4%) predominate. This is followed by phosphatidylinositol (14.6%) and phosphatidylserine (12.5%). The remaining classes of phospholipids are represented in almost the same amount (6–7%), with the exception of lysophosphatidylethanolamine, which is 2.5%. During inflorescence development, there was a sharp increase in the amount of phosphatidylcholine (from 6.6 to 23.9%), and to a lesser extent phosphatidylethanolamine (from 6.9 to 13.9%) and lysophosphatidylcholine (from 6.1 to 9.4%), at the expense of reducing the amount of diphosphatidylglycerol (from 21.4 to 7.3%), phosphatidylserine (from 12.5 to 4.7%), phosphatidic acids (from 22.1 to 19.8%), and of phosphatidylinositol (from 14.6 to 10.4%). The presence of monophosphatidylglycerol (1.0%) was also found in a minimal amount. Lysophosphatidylcholine and lysophosphatidylethanolamine are considered to be breakdown products of other classes of phospholipids. Phosphatidic acids can be considered both as degradation products and as a stage in the biosynthesis of the remaining phospholipids. For this reason, their amount is greater in the lipids isolated from the inflorescence during the maturation period.
The phospholipid composition of lipids isolated from seeds is qualitatively similar to that of Centaurea thracica inflorescence from both June and September, but quantitatively different. The main representatives of the phospholipids isolated from the seeds were found to be phosphatidylethanolamine (31.8%) and phosphatidylinositol (25.0%), followed by sphingomyelin (11.3%), phosphatidylcholine (9.1%), and phosphatidylserine (7.0%). The amount of monophosphatidylglycerol and diphosphatidylglycerol was 5.6% and 4.9%, respectively, and lysoforms were 2.8% and 2.5%. No phosphatidic acids were identified in the seed phospholipids. The phospholipid composition of seed lipids was closer to that of most plant lipids, in which phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol predominate.
3. Materials and Methods
3.1. Materials
The inflorescences and seeds of the plant Centaurea thracica (Janka) Hayek, common in southern Bulgaria, were investigated. The aerial part was collected during the vegetation period (during flowering and seed forming) in the vicinity of the town of Tsarevo, a floristic region near the sandy shore of the Black Sea (Popski Plazh locality), from a population with a high number of the species. The plant material was collected from natural habitats (meadows) from the coastal area. The climatic conditions of the area in June 2021 were as follows: the average temperature was 20.3 °C, and the average rainfall was 69 mm. The average temperature and the average rainfall of the area in September 2021 were 19.3 °C and 49 mm. The flowering inflorescences from Centaurea thracica were collected in June 2021, and the mature inflorescences and seeds were obtained in September 2021. Approximately 500 g of fresh plant material were collected randomly. The inflorescences and seeds were air-dried and kept at room temperature in the dark before they were subjected to analysis after grinding.
3.2. Chemical Composition
The lipids were isolated by a Soxhlet extraction with n-hexane, and the oil content was determined [55]. The protein content was determined according to AOAC (2016) after mineralization and distillation of the samples [56]. Total carbohydrates were calculated by the following formula: 100 − (weight in grams [protein + lipids + moisture + ash] in 100 g of dry seeds) [57]. The content of fibers, ash, and moisture were determined according to AOAC (2016) [56]. The energy value (EV) in kJ/100 g (kcal/100 g) was calculated as follows:
| EV = % proteins × 17 (4.0) + % carbohydrates × 17 (4.0) + lipids × 38 (9.0). |
3.3. Lipid Composition
3.3.1. Fatty Acids
The fatty acid composition was determined after transesterification of the oil with methanol (with the presence of sulfuric acid) and the following determination with gas chromatography (GC) [58,59]. The conditions of the GC (Agilent (Santa Clara, CA, USA) 8860) are as follows: 70 °C (1 min), at 6 °C/min to 180 °C (0 min), and at 5 °C/min to 250 °C; split ratio—50:1; temperatures of the injector and detector (flame ionization detector) were 270 °C and 300 °C; column—capillary DBFastFAME (30 m × 0.25 mm × 0.25 μm (film thickness)); and the carrier gas was nitrogen. The identification of FAMEs was performed by the retention time of a standard FAME mix (37 components, Supelco, Bellefonte, PA, USA).
3.3.2. Tocopherols
Total and individual tocopherol composition was determined according to ISO 9936 [60], with slight modifications. Tocopherols were determined on an Merck-Hitachi (Merck, Darmstadt, Germany) high-performance liquid chromatograph instrument with a fluorescent detection (295 nm of excitement and 330 nm of emission) using Nucleosil Si 50–5 column (250 mm × 4 mm). The mobile phase was hexane:dioxane, 96:4 (v/v), and the flow rate was 1 mL/min. A 2% solution of the oil in hexane was prepared, and 20 μL were injected into the instrument. The tocopherols were identified by comparing the retention times with those of the standards (reference individual pure tocopherols—α-, β-, γ-, and δ-tocopherols with purity ≥ 98% from Merck (Darmstadt, Germany)). Tocopherol content was calculated on the base of the tocopherol peak areas in the sample vs. the tocopherol peak area of a standard tocopherol solution.
3.3.3. Phospholipids
The individual phospholipids were isolated by two-dimensional thin-layer chromatography [61]. The obtained phospholipid spots were scraped, placed in tubes, and mineralized with a mixture of perchloric acid and sulfuric acid, 1:1 (v/v). After that, a water solution of ammonium molybdate and a reducing reagent were added to the samples, and the tubes were kept for 10 min in a boiling water bath in order for the reaction to take place. The quantification was carried out spectrophotometrically at 700 nm. The phospholipid content in the sample was calculated as a percentage of the phosphorus [62].
3.3.4. Iodine Value
The iodine value (IV) was determined according to the procedure by AOCS (1999), as follows [63]:
| IV = (90 × % Oleic acid + 181 × % Linoleic acid + 274 × % Linolenic acid)/ 100, g I2/100 g. |
3.3.5. Index of Atherogenicity and Thrombogenicity
The index of atherogenicity (IA) and thrombogenicity (IT) were calculated according to the formulae given by Ulbricht and Southgate [50]:
| IA = (C12:0 + 4 × C14:0 + C16:0)/(∑MUFA + ∑PUFA) | (1) |
| IT = (C14:0 + C16:0 + C18:0)/[(0.5 × ∑MUFA) + (0.5 × ∑n-6 PUFA) + (3 × ∑n-3 PUFA) + (∑n-3 PUFA/∑n-6 PUFA)] | (2) |
where ∑MUFA—amount of the monounsaturated fatty acids, ∑PUFA—polyunsaturated fatty acids, ∑n-6 PUFA—polyunsaturated fatty acids (n-6), ∑n-3 PUFA—polyunsaturated fatty acids (n-3), C12:0—lauric acid, C14:0—myristic acid, C16:0—palmitic acid, and C18:0—stearic acid.
3.4. Statistical Analyses
All the analyses were performed in triplicates (n = 3). The results are represented as mean and standard deviation (SD).
4. Conclusions
For the first time in Bulgaria, detailed research was conducted on the chemical and lipid composition of inflorescences and seeds from Centaurea thracica. The results obtained for the general chemical composition of the inflorescences during vegetation show a notable decrease in total proteins, glyceride oils, and ash content, while the amount of carbohydrates and fibers increased. A higher content of carbohydrates was observed in the plants collected in September, which determined the increase in the energy value of these inflorescences. In the composition of the oil isolated from the inflorescences in June, saturated fatty acids predominated, while in the oils isolated from the inflorescences collected in September and from the seeds, unsaturated fatty acids prevailed. The main representatives of the saturated fatty acids were palmitic and stearic acids, of monounsaturated fatty acids was oleic acid, and of polyunsaturated fatty acids were linoleic and linolenic acids. During the vegetation of the plant, the content of oleic and linoleic acids increased about 2–3 times, while the level of palmitic acid decreased. All examined oils were a rich source of essential fatty acids (oleic, linoleic, and docosahexaenoic acids). The values of the indices of atherogenicity and thrombogenicity described relatively good antiatherogenic and antithrombogenic properties of the studied oils, as well as the values of the ratio PUFA/SFA confirmed their possible nutritional and biological properties. The tocopherol content in the oils of the inflorescences increased, while the phospholipids decreased during vegetation.
The high content of essential fatty acids, tocopherols, and phospholipids define the inflorescences and the seeds of Centaurea thracica as a good alternative source of biologically active substances with health benefits. The current study involves preliminary examinations on the abovementioned components in the plant. For that reason, there is a need for determining the biological activity of the plant extracts, in which basis can be made conclusions on their potential use in various areas. Apart from that, a detailed examination on the lipid composition of this plant is also a priority because it can give information on its potential utilization in the development of numerous nutritional supplements and pharmaceutical products for the prevention of some chronic diseases.
Author Contributions
Conceptualization, P.S., R.M., D.G., A.B. and G.A.; methodology, O.T., Z.P., G.A. and M.A.-R.; validation, O.T. and G.A.; formal analysis, Z.P., G.A., O.T., M.A.-R., T.M. and D.G.; investigation, Z.P., O.T., G.A., D.G. and T.R.; resources, P.S., R.M., K.T. and T.R.; data curation, G.A. and O.T.; writing—original draft preparation, G.A., D.G. and O.T.; writing—review and editing P.S., R.M., A.B., K.T., Z.P., M.A.-R., T.M., T.R. and V.P.; visualization, G.A. and O.T.; supervision, P.S. and R.M.; project administration, P.S. and R.M.; funding acquisition, P.S. and R.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The author Venelin Petkov is employed by the company Bristol Myers Squibb. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This study is financed by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project № BG-RRP-2.004-0001-C01.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ertas A., Gören A.C., Boga M., Demirci S., Kolak U. Chemical composition of the essential oils of three Centaurea species growing wild in Anatolia and their anticholinesterase activities. J. Essent. Oil Bear. Plants. 2014;17:922–926. doi: 10.1080/0972060X.2014.886164. [DOI] [Google Scholar]
- 2.Wagenitz G. Floristic connection between the Balkan peninsula and the Near East as exemplified by the genus Centaurea L. In: Jordanov D., editor. Problems of the Balkan Flora and Vegetation. Publishing House Bulgarian Acad. Sci.; Sofia, Bulgaria: 1975. pp. 223–228. [Google Scholar]
- 3.Wagenitz G., Hellwig F.H. Evolution of characters and phylogeny of Centaureinae. In: Hind D.J.N., Beentje H.J., editors. Compositae. Systematics. Proceedings of the International Compositae Conference. Volume 1. Royal Botanic Gardens; Kew, UK: 1996. pp. 491–510. [Google Scholar]
- 4.Greuter W. In: Med-Checklist. A Critical Inventory of Vascular Plants of the Circum-Mediterranean Countries. Dicotyledones (Compositae) Greuter W., von Raab-Straube E., editors. Volume 2. OPTIMA Secretariat; Palermo, Italy: Med-Checklist Trust of OPTIMA; Geneve, Switzerland: Euro+Med Plantbase Secretariat; Berlin, Germany: 2008. p. 798. [Google Scholar]
- 5.Velchev V., editor. Atlas of the Endemic Plants in Bulgaria. Bulgarian Academy of Sciences; Sofia, Bulgaria: 1992. [Google Scholar]
- 6.Pieroni A., Janiak V., Dürr C.M., Lüdeke S., Trachsel E., Heinrich M. In vitro antioxidant activity of non-cultivated vegetables of ethnic Albanians in southern Italy. Phytother. Res. 2002;16:467–473. doi: 10.1002/ptr.1243. [DOI] [PubMed] [Google Scholar]
- 7.Della A., Paraskeva-Hadjichambi D., Hadjichambis A.C. An ethnobotanical survey of wild edible plants of Paphos and Larnaca countryside of Cyprus. J. Ethnobiol. Ethnomed. 2006;2:34. doi: 10.1186/1746-4269-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubacey T., Haggag E., El-Toumy S., Ahmed A., El-Ashmawy I., Youns M. Biological activity and flavonoids from Centaurea alexanderina leaf extract. J. Pharm. Res. 2012;5:3352–3361. [Google Scholar]
- 9.Csupor D., Widowitz U., Blazsó G., Laczkó-Zöld E., Tatsimo J.S.N., Balogh Á., Boros K., Dankó B., Bauer R., Hohmann J. Anti-inflammatory activities of eleven Centaurea species occurring in the Carpathian basin. Phyther. Res. 2013;27:540–544. doi: 10.1002/ptr.4754. [DOI] [PubMed] [Google Scholar]
- 10.Tekeli Y., Zengin G., Aktumsek A., Mehmet S., Torlak E. Antibacterial activities of extracts from twelve Centaurea species from Turkey. Arch. Biol. Sci. 2011;63:685–690. doi: 10.2298/ABS1103685T. [DOI] [Google Scholar]
- 11.Zengin G., Locatelli M., Carradori S., Mocan A.M., Aktumsek A. Total phenolics, flavonoids, condensed tannins content of eight Centaurea species and their broad inhibitory activities against cholinesterase, tyrosinase, α-amylase and α-glucosidase. Not. Bot. Horti Agrobo. Cluj-Napoca. 2016;44:195–200. doi: 10.15835/nbha44110259. [DOI] [Google Scholar]
- 12.Ceyhan Güvensen N., Keskin D., Güneş H., Kesik Oktay M., Yıldırım H. Antimicrobial property and antiproliferative activity of Centaurea babylonica (L.) L. on human carcinomas and cervical cancer cell lines. Ann. Agric. Environ. Med. 2019;26:290–297. doi: 10.26444/aaem/108563. [DOI] [PubMed] [Google Scholar]
- 13.Baytop T. Therapy with Medicinal Plants in Turkey (Past and Present) 2nd ed. Nobel Tıp Basımevi, Publication of the Istanbul University; Istanbul, Turkey: 1999. pp. 348–349. [Google Scholar]
- 14.Sezik E., Yeşilada E., Honda G., Takaishi Y., Takeda Y., Tanaka T. Traditional medicine in Turkey X. Folk medicine in central Anatolia. J. Ethnopharmacol. 2001;75:95–115. doi: 10.1016/S0378-8741(00)00399-8. [DOI] [PubMed] [Google Scholar]
- 15.Kargıoglu M., Cenkci S., Serteser A., Evliyaoglu N., Konuk M., Kok M.S., Bagcı Y. An ethnobotanical survey of inner-West Anatolia, Turkey. Hum. Ecol. 2008;36:763–777. doi: 10.1007/s10745-008-9198-x. [DOI] [Google Scholar]
- 16.Uysal A., Zengin G., Mollica A., Gunes E., Locatelli M., Yilmaz T., Aktumsek A. Chemical and biological insights on Cotoneaster integerrimus: A new (-) -epicatechin source for food and medicinal applications. Phytomedicine. 2016;23:979–988. doi: 10.1016/j.phymed.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Korga A., Jozefczyk A., Zgorka G., Homa M., Ostrowska M., Burdan F., Dudka J. Evaluation of the phytochemical composition and protective activities of methanolic extracts of Centaurea borysthenica and Centaurea daghestanica (Lipsky) Wagenitz on cardiomyocytes treated with doxorubicin. Food Nutr. Res. 2017;61:1344077. doi: 10.1080/16546628.2017.1344077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zengin G., Zheleva-Dimitrova D., Gevrenova R., Nedialkov P., Mocan A., Ćirić A., Glamočlija J., Soković M., Aktumsek A., Mahomoodally M.F. Identification of phenolic components via LC–MS analysis and biological activities of two Centaurea species: C. drabifolia subsp. drabifolia and C. lycopifolia. J. Pharm. Biomed. Anal. 2018;149:436–441. doi: 10.1016/j.jpba.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 19.Labed F., Masullo M., Mirra V., Nazzaro F., Benayache F., Benayache S., Piacente S. Amino acid-sesquiterpene lactone conjugates from the aerial parts of Centaurea pungens and evaluation of their antimicrobial activity. Fitoterapia. 2019;133:51–55. doi: 10.1016/j.fitote.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Lockowandt L., Pinela J., Roriz C.L., Pereira C., Abreu R.M.V., Calhelha R.C., Alves M.J., Barros L., Bredol M., Ferreira I.C. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crops Prod. 2019;128:496–503. doi: 10.1016/j.indcrop.2018.11.059. [DOI] [Google Scholar]
- 21.Khanavi M., Taheri M., Rajabi A., Fallah-Bonekohal S., Baeeri M., Mohammadirad A., Amin G., Abdollahi M. Stimulation of hepatic glycogenolysis and inhibition of gluconeogenesis are the mechanisms of antidiabetic effect of Centaurea bruguierana ssp. belangerana. Asian J. Anim. Vet. Adv. 2012;7:1166–1174. doi: 10.3923/ajava.2012.1166.1174. [DOI] [Google Scholar]
- 22.Esmaeili A., Mousavi Z., Shokrollahi M., Shafaghat A. Antioxidant activity and isolation of luteoline from Centaurea behen L. grown in Iran. J. Chem. 2013;2013:620305. doi: 10.1155/2013/620305. [DOI] [Google Scholar]
- 23.Aktumsek A., Zengin G., Guler G.O., Cakmak Y.S., Duran A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013;55:290–296. doi: 10.1016/j.fct.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Mężyńska M., Brzóska M.M. Review of polyphenol-rich products as potential protective and therapeutic factors against cadmium hepatotoxicity. J. Appl. Toxicol. 2019;39:117–145. doi: 10.1002/jat.3709. [DOI] [PubMed] [Google Scholar]
- 25.Sokovic M., Ciric A., Glamoclija J., Skaltsa H. Biological activities of sesquiterpene lactones isolated from the genus Centaurea L. (Asteraceae) Curr. Pharm. Des. 2017;23:2767–2786. doi: 10.2174/1381612823666170215113927. [DOI] [PubMed] [Google Scholar]
- 26.Tešević V., Aljančić I., Milosavljević S., Vajs V., Đorđević I., Jadranin M., Menković N., Matevski V. Secondary metabolites of three endemic Centaurea L. species. J. Serb. Chem. Soc. 2014;79:1355–1362. doi: 10.2298/JSC140318048T. [DOI] [Google Scholar]
- 27.Das U.N. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: But, why and how? Prostaglandins Leukot. Essent. Fatty Acids. 2000;63:351–362. doi: 10.1054/plef.2000.0226. [DOI] [PubMed] [Google Scholar]
- 28.Grundy S.M. What is the desirable ratio of saturated, polyunsaturated, and monounsaturated fatty acids in the diet? Am. J. Clin. Nutr. 1997;66:988S–990S. doi: 10.1093/ajcn/66.4.988S. [DOI] [PubMed] [Google Scholar]
- 29.Larsson S.C., Kumlin M., Ingelman-Sundberg M., Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 30.Slattery M.L., Potter J.D., Duncan D.M., Berry T.D. Dietary fats and colon cancer: Assessment of risk associated with specific fatty acids. Int. J. Cancer. 1997;73:670–677. doi: 10.1002/(SICI)1097-0215(19971127)73:5<670::AID-IJC10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 31.Von Schacky C., Angerer P., Kothny W., Theisen K., Mudra H. The effect of dietary ω-3 fatty acids on coronary atherosclerosis: A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 1999;130:554–562. doi: 10.7326/0003-4819-130-7-199904060-00003. [DOI] [PubMed] [Google Scholar]
- 32.Lemaitre R.N., King I.B., Mozaffarian D., Sotoodehnia N., Rea T.D., Kuller L.H., Tracy R.P., Siscovick D.S. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults. The cardiovascular health study. Circulation. 2006;114:209–215. doi: 10.1161/CIRCULATIONAHA.106.620336. [DOI] [PubMed] [Google Scholar]
- 33.Furua T., Yoshikawa T., Kimura T., Kaneko H. Production of tocopherols by cell culture of safflower. Phytochemistry. 1987;26:2741–2747. doi: 10.1016/S0031-9422(00)83582-7. [DOI] [Google Scholar]
- 34.Kamal-Eldin A., Andersson R. A multivariate study of the correlation between tocopherol content and fatty acid composition in vegetable oils. J. Am. Oil Chem. Soc. 1997;74:375–380. doi: 10.1007/s11746-997-0093-1. [DOI] [Google Scholar]
- 35.Tekeli Y., Sezgin M., Aktumsek A., Ozmen Guler G., Aydin Sanda M. Fatty acid composition of six Centaurea species growing in Konya, Turkey. Nat. Prod. Res. 2010;24:1883–1889. doi: 10.1080/14786411003754314. [DOI] [PubMed] [Google Scholar]
- 36.Aktumsek A., Zengin G., Guler G.O., Cakmak Y.S., Duran A. Assessment of the antioxidant potential and fatty acid composition of four Centaurea L. taxa from Turkey. Food Chem. 2013;141:91–97. doi: 10.1016/j.foodchem.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 37.Yildirim N., Sunar S., Agar G., Bozari S., Aksakal O. Biochemical and molecular characterization of some Centaurea species growing in the Eastern Anatolia region of Turkey. Biochem. Genet. 2009;47:850–859. doi: 10.1007/s10528-009-9284-9. [DOI] [PubMed] [Google Scholar]
- 38.Sekeroglu N., Ozkutlu F., Deveci M., Dede O., Yilmaz N. Evaluation of some Wild Plants Aspect of Their Nutritional Values Used as Vegetable in Eastern Black Sea Region of Turkey. Asian J. Plant Sci. 2006;5:185–189. doi: 10.3923/ajps.2006.185.189. [DOI] [Google Scholar]
- 39.Tuncturk M., Celen E., Tuncturk R. Nutrient content of three edible wild plants from Polygonaceae family. Oxid. Commun. 2017;40:327–334. [Google Scholar]
- 40.Vishwakarma K.L., Dubey V. Nutritional analysis of indigenous wild edible herbs used in eastern Chhattisgarh, India. [(accessed on 8 November 2023)];Emir. J. Food. Agric. 2011 23:554–560. Available online: https://ejfa.me/index.php/journal/article/view/1289. [Google Scholar]
- 41.Tunçtürk R., Tunçtürk M., Nohutcu L. Study on chemical composition of Centaurea karduchorum Boiss. species from endemic plants of Eastern Anatolia/Turkey. Curr. Pers. MAPs. 2019;2:47–52. doi: 10.38093/cupmap.585945. [DOI] [Google Scholar]
- 42.Fernandes L., Pereira J.A., Saraiva J.A., Ramalhosa E., Casal S. Phytochemical characterization of Borago officinalis L. and Centaurea cyanus L. during flower development. Food Res. Int. 2019;123:771–778. doi: 10.1016/j.foodres.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 43.FAO . Food Balance Sheets, A Handbook. Food and Agriculture Organization of the United Nations; Rome, Italy: 2001. pp. 60–65. Food Composition Tables, Annex I. [Google Scholar]
- 44.Codex Alimentarius, International Food Standards. Standard for named Vegetable Oils. Food and Agriculture Organization of the United Nations and World Health Organization; Codex Alimentarius Commission; Rome, Italy: 1999. Adopted in 1999, Revised in 2001, 2003, 2009, 2017, 2019. Amended in 2005, 2011, 2013, 2015, 2019, 2021, 2022, 2023. [Google Scholar]
- 45.Peker S., Baştürk A. Volatile compounds, fatty acid composition and antioxidant activity of Centaurea albonitens and Centaurea balsamita seeds growing in Van, Turkey. Eur. J. Nutr. Food Saf. 2020;11:187–199. doi: 10.9734/ejnfs/2019/v11i430161. [DOI] [Google Scholar]
- 46.Zengin G., Cakmak Y.S., Guler G.O., Aktumsek A. In vitro antioxidant capacities and fatty acid compositions of three Centaurea species collected from Central Anatolia region of Turkey. Food Chem. Toxicol. 2010;48:2638–2641. doi: 10.1016/j.fct.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 47.Kang M.J., Shin M.S., Park J.N., Lee S.S. The effects of polyunsaturated: Saturated fatty acids ratios and peroxidizability index values of dietary fats on serum lipid profiles and hepatic enzyme activities in rats. Br. J. Nutr. 2005;94:526–532. doi: 10.1079/BJN20051523. [DOI] [PubMed] [Google Scholar]
- 48.Cottin S.C., Sander T.A., Hall W.L. The differential effects of EPA and DHA on cardiovascular risk factors. Proc. Nutr. Soc. 2011;70:215–231. doi: 10.1017/S0029665111000061. [DOI] [PubMed] [Google Scholar]
- 49.Hooper L., Thompson R., Harrison R., Summerbell C., Ness A., Moore H., Worthington H., Durrington P., Higgins J., Capps N., et al. Risks and benefits of omega-3 fats for mortality, cardiovascular disease, and cancer: Systematic review. Br. Med. J. 2006;332:752–760. doi: 10.1136/bmj.38755.366331.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ulbricht T.L.V., Southgate D.A.T. Coronary heart disease: Seven dietary factors. Lancet. 1991;338:985–992. doi: 10.1016/0140-6736(91)91846-M. [DOI] [PubMed] [Google Scholar]
- 51.Wołoszyn J., Haraf G., Okruszek A., Wereńska M., Goluch Z., Teleszko M. Fatty acid profiles and health lipid indices in the breast muscles of local Polish goose varieties. Poult. Sci. 2020;99:1216–1224. doi: 10.1016/j.psj.2019.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahari M.A., Amooi M. Tea seed oil: Extraction, compositions, applications, functional and antioxidant properties. Acad. J. Med. Plants. 2013;1:68–79. doi: 10.13140/RG.2.1.3147.8644. [DOI] [Google Scholar]
- 53.Gunstone F.D., Harwood J.L., Dijkstra A.J. The Lipid Handbook with CD-ROM. CRC Press; Boca Raton, FL, USA: 2007. [Google Scholar]
- 54.Popov A., Ilinov P. Chemistry of Lipids. Science and Art; Sofia, Bulgaria: 1986. p. 290. [Google Scholar]
- 55.Oilseeds Determination of Oil Content (Reference Method) ISO; Geneva, Switzerland: 2014. [Google Scholar]
- 56.AOAC . Association of Official Analytical Chemist (AOAC) 2016, Official Methods of Analysis. 20th ed. AOAC International; Arlington, VA, USA,: 2016. [Google Scholar]
- 57.Food and Agriculture Organization of the United Nations . Food Energy—Methods of Analysis and Conversion Factors. Food and Agriculture Organization of the United Nations; Rome, Italy: 2003. FAO Food and Nutrition Paper 77, Report of a Technical Workshop; [Google Scholar]
- 58.Animal and Vegetable Fats and Oils. Gas Chromatography of Fatty Acid Methyl Esters—Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. ISO; Geneva, Switzerland: 2014. [Google Scholar]
- 59.Animal and Vegetable Fat and Oils. Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. ISO; Geneva, Switzerland: 2017. [Google Scholar]
- 60.Animal and Vegetable Fats and Oils. Determination of Tocopherol and Tocotrienol Contents by High-Performance, Liquid Chromatography. ISO; Geneva, Switzerland: 2016. [Google Scholar]
- 61.Schneiter R., Daum G. Analysis of yeast lipids. In: Xiao W., editor. Methods in Molecular Biology. 2nd ed. Humana Press Inc.; Totowa, NJ, USA: 2006. pp. 75–84. [DOI] [PubMed] [Google Scholar]
- 62.Animal and Vegetable Fats and Oils. Determination of Phosphorus Content. Part 1: Colorimetric Method. ISO; Geneva, Switzerland: 2014. [Google Scholar]
- 63.AOCS . American Oil Chemists Society (AOCS), Official Methods and Recommended Practices of the American Oil Chemists Society. 7th ed. AOCS Press; Champaign, IL, USA: 2022. Calculated Iodine Value. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.