Abstract
Recently the mouse cytomegalovirus (MCMV) genome was cloned as an infectious bacterial artificial chromosome (BAC) (M. Messerle, I. Crnković, W. Hammerschmidt, H. Ziegler, and U. H. Koszinowski, Proc. Natl. Acad. Sci. USA 94:14759–14763, 1997). The virus obtained from this construct is attenuated in vivo due to deletion of viral sequences and insertion of the BAC vector. We reconstituted the full-length MCMV genome and flanked the BAC vector with identical viral sequences. This new construct represents a versatile basis for construction of MCMV mutants since virus generated from the construct loses the bacterial sequences and acquires wild-type properties.
Human cytomegalovirus (HCMV) is a ubiquitous pathogen that can cause severe disease in immunologically immature and immunocompromised patients (5). The strict species specificity of HCMV precludes investigation of the HCMV infection in an animal host. Infection of the mouse with mouse cytomegalovirus (MCMV) is a valuable in vivo model for studying various aspects of CMV pathogenesis (13, 14). Fast and efficient mutagenesis procedures for MCMV are desirable in order to analyze the role of CMV genes during the disease course in vivo. However, current mutagenesis schemes are difficult, laborious, and time-consuming since CMV replicates rather slowly and mutagenesis relies on rare recombination events in eukaryotic cells (12, 15, 18, 27, 28). Recently, we reported on a new approach for the generation of herpesvirus mutants that is based on homologous recombination in Escherichia coli (21). To this end, we cloned the MCMV genome as an infectious bacterial artificial chromosome (BAC) in E. coli. Mutagenesis of the MCMV BAC plasmid in E. coli allows one to introduce any kind of mutation (including deletion, insertion, and point mutation) into the MCMV genome.
The original MCMV BAC plasmid, pSM3, and the corresponding genome of recombinant virus MC96.73 (21) contain a deletion of a nonessential genome region, extending from nucleotide (nt) 209756 to nt 217934, of MCMV strain Smith (24) and comprising open reading frames m151 to m158. Deletion of this genome region was required for integration of BAC vector sequences (21). Although the deleted genes are nonessential for replication in cell culture, they probably play a role during the disease course in vivo. For example, a spontaneous MCMV mutant (4), as well as engineered MCMV recombinants (6, 8, 11, 19) with genome deletions, showed wild-type (wt)-like replication in vitro but were all attenuated in vivo. In addition, the BAC vector sequences remained inserted in the recombinant MC96.73 genome and could cause unpredictable effects in vivo.
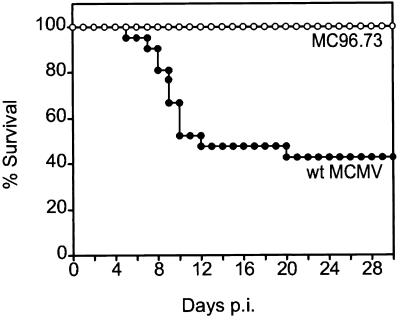
The MCMV BAC virus MC96.73 is attenuated in vivo.
To test the virulence of the recombinant virus MC96.73, newborn BALB/c mice were infected with virus MC96.73 or wt MCMV and monitored for 30 days. Infection with wt MCMV resulted in a mortality rate of 60%, whereas all MC96.73-infected mice survived (Fig. 1). Thus, as expected, the recombinant virus MC96.73 is less virulent than wt MCMV and of only limited value for mutagenesis and in vivo studies.
FIG. 1.
The recombinant MCMV MC96.73 is attenuated in vivo. Newborn BALB/c mice were infected intraperitoneally with 100 PFU of wt MCMV (21 animals; solid circles) or MC96.73 virus (14 animals; open circles). Mortality of mice was monitored for 30 days postinfection, and survival rates were determined.
Construction of the full-length MCMV BAC plasmid pSM3fr.
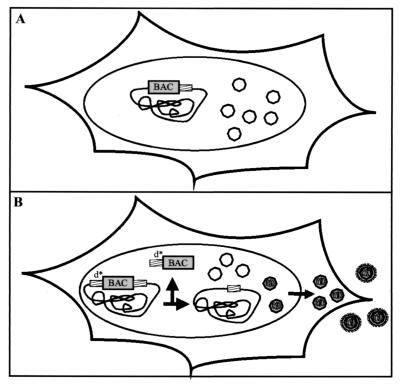
To reconstitute recombinant viruses with biological properties similar to those of the wt we decided to (i) reinsert the missing MCMV sequences into the MCMV BAC plasmid pSM3 and (ii) to make it possible to excise the BAC vector sequences from the recombinant virus genome. Reinsertion of the missing sequences with the newly established mutagenesis technique (21) should be possible since there are no size constraints for an MCMV BAC plasmid in E. coli. Recombination can be used to delete a genomic region located between homologous sequences. To eliminate all nonviral sequences from the recombinant genome the BAC vector was therefore flanked with short identical viral sequences (Fig. 2), in order to generate a substrate for homologous recombination which is selectively used during virus reconstitution in cells.
FIG. 2.
Schema for excision of the BAC vector from the viral genome in eukaryotic cells. (A) The MCMV BAC genome has an overlength due to the BAC vector insertion. Results obtained with other DNA viruses (2, 3) suggest that there is a relatively tight constraint on the length of DNA that can be encapsidated into virions. The BAC-containing genomes probably exceed this limit and are poorly packaged. (B) Insertion of the duplicate sequence d* should lead to excision of the BAC vector since the duplicated sequences (short identical viral sequences) (hatched boxes) serve as a target site for homologous recombination. The resulting unit length genomes will be preferentially packaged. This will lead to accumulation of a virus progeny with the wt genome.
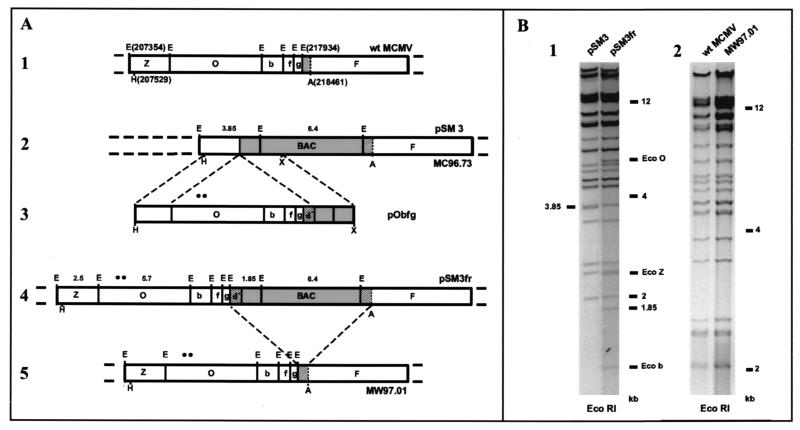
To reintroduce the missing sequences the recombination plasmid pObfg was generated. For its construction plasmid HindIII E′ΔB, which contained the 17-kbp HindIII-BamHI subfragment of the MCMV HindIII E′ fragment (20) cloned in pACYC184 (7) was digested with AvrII and BamHI, releasing a 6.1-kbp fragment, and an oligonucleotide adapter (5′-CTA GCG GCC GCT TAA TTA AGG ATC CAC TAG TAA GCT T-3′) containing NotI, SpeI, and HindIII sites (underlined) was inserted. Then, a 2-kbp NotI-XbaI fragment derived from plasmid pKSO-gpt (21) and carrying a part of the BAC vector was inserted between the NotI and SpeI sites of the adapter. Finally, the complete insert was excised as a 12.9-kbp HindIII fragment and transferred into the HindIII site of plasmid pMBO96 (23), resulting in shuttle plasmid pObfg. Plasmid pObfg provided the EcoRI fragments O, b, f, and g flanked by sequences homologous to the integration site in plasmid pSM3 that were required for homologous recombination (Fig. 3A, maps 2 and 3). Note that plasmid pObfg also contains a 527-bp MCMV EcoRI-AvrII fragment (nt 217934 to 218461; Fig. 3A, map 3) that is already present in its authentic position to the right of the BAC vector in plasmid pSM3 (Fig. 3A, map 2). Integration of the missing sequences by homologous recombination between recombination plasmid pObfg and BAC plasmid pSM3 leads to flanking of the BAC vector with identical sequences in the same orientation (see Fig. 2 and Fig. 3A, map 4).
FIG. 3.
Construction (A) and analysis (B) of full-length MCMV BAC plasmid pSM3fr and of the MW97.01 virus genome. (A) The top line (map 1) represents the right-terminal region of the wt MCMV genome with the EcoRI (E) fragments indicated (nucleotide positions are given in parentheses). Homologous recombination in E. coli between MCMV BAC plasmid pSM3 (map 2) and recombination plasmid pObfg (map 3) leads to reinsertion of the missing MCMV sequences and to the duplication of a 527-bp sequence (duplicate sequence d*) which is already present in its authentic position to the right of the BAC vector (hatched boxes), resulting in full-length MCMV BAC plasmid pSM3fr (map 4). Excision of the BAC vector by homologous recombination after transfection of the full-length MCMV BAC plasmid pSM3fr into eukaryotic cells results in the MW97.01 virus genome (map 5), whose EcoRI map is identical to that of wt MCMV (map 1). Two silent point mutations in the inserted MCMV sequences (indicated by solid circles) allow differentiation of the MW97.01 and wt MCMV genomes. Additional restriction enzyme sites indicated are AvrII (A), HindIII (H), and XbaI (X). (B) Structural analysis of BAC plasmids pSM3 and pSM3fr (panel 1) and of wt MCMV and reconstituted MW97.01 virus genomes (panel 2). Panel 1, the ethidium bromide-stained agarose gel shows EcoRI-digested DNA of BAC plasmids pSM3 and pSM3fr. The EcoRI O (Eco O), Z (Eco Z), and b (Eco b) fragments, a 3.85-kbp fragment, and size markers are indicated. Panel 2, EcoRI restriction analysis of virus genomes isolated from virions of recombinant virus MW97.01 and wt MCMV is shown.
Homologous recombination in E. coli was performed by a two-step replacement procedure (1, 21, 23), resulting in MCMV BAC plasmid pSM3fr (Fig. 3A, map 4). To confirm the successful insertion, the EcoRI restriction pattern of plasmid pSM3fr was analyzed (Fig. 3B, panel 1). In comparison to the EcoRI restriction pattern of the parental BAC plasmid pSM3, a 3.85-kbp EcoRI fragment was lacking in the full-length MCMV BAC plasmid pSM3fr, as expected (see Fig. 3A, maps 2 and 4, and Fig. 3B, panel 1). Additional fragments of 5.7, 2.5, 1.3, 0.7, and 0.53 kbp correspond to EcoRI fragments O, Z, b, f, and g (Fig. 3B, panel 1; the latter two bands are not shown). The 1.85-kbp EcoRI fragment in BAC plasmid pSM3fr results from the duplicate sequence d* and part of the BAC vector sequence (see Fig. 3A, map 4). These results demonstrate the successful reconstitution of the complete MCMV sequence and the introduction of the duplicate sequence d* in plasmid pSM3fr. pSM3fr was stable in E. coli CBTS since expression of the RecA protein is stringently controlled (16) and large regions of homologous sequences are required for homologous recombination in this bacterial strain (1).
Generation of wt genomes by excision of the BAC vector from the MCMV BAC genome.
After transfection of the MCMV BAC plasmid into eukaryotic cells we expected homologous recombination via the duplicated sequences leading to excision of the vector sequences and generation of a wt genome (see Fig. 2 and Fig. 3A, maps 4 and 5). During construction of the original MCMV BAC plasmid pSM3 we had observed that overlength genomes are not stable in cells (22), suggesting that overlength genomes are poorly packaged into viral capsids. Similar observations have been made for other DNA viruses. An overlength of more than 5% over the adenovirus wt genome leads to unstable genomes (2), and Epstein-Barr virus preferentially packages genomes within a very narrow size range (3). Thus, we expected that even when rare recombination events occur at the created target site, preferential packaging of unit length genomes should lead to an accumulation of viruses with the wt genome.
For reconstitution of virus progeny approximately 1.5 μg of MCMV BAC plasmid pSM3fr was transfected into mouse embryonic fibroblasts (MEF) by using the calcium phosphate precipitation method (17). About 5 days posttransfection plaque formation was observed. To allow homologous recombination via the duplicate sequence d* (see Fig. 3A, maps 4 and 5) virus progeny obtained after transfection was passaged on MEF. Supernatant of these cultures was harvested when 100% cytopathic effect was observed in the cultures, and an aliquot (1/20) of the supernatant was used to infect new cell monolayers. The recombinant virus MW97.01 was isolated after the fifth passage and DNA of the MW97.01 virus was prepared from purified virions as previously described (9). Digestion of MW97.01 DNA with EcoRI revealed a genome structure identical to that of wt MCMV (Fig. 3B, panel 2). For reinsertion of the EcoRI fragments O, b, f, and g we had used sequences originating from the HindIII E′ fragment of MCMV strain K181 (10, 20), because a sequence polymorphism between the MCMV strains Smith and K181 within these fragments proves that the MW97.01 virus is derived from the MCMV BAC plasmid pSM3fr and cannot represent a contamination with the MCMV wt strain Smith. To this end, a part of the 3′ region of gene m152 (nt 209913 to 210400) was amplified by PCR by using primers m209913-932 (5′-GAC TCA CAC ACA GAG GCT GC-3′) and m210400-381 (5′-GTA CTC CAT CTT CTT CAT GG-3′) and DNA of virus MW97.01 and MCMV strain Smith and of plasmid HindIII E′ (20) as a template. The same primers were used for sequencing of the PCR products. A polymorphism at MCMV nt 210010 (G→A) and 210190 (G→A) in strain K181-derived sequences of the MW97.01 virus and plasmid HindIII E′ confirmed the origin of the MW97.01 virus.
Rapid excision of BAC vector sequences and accumulation of wt genomes.
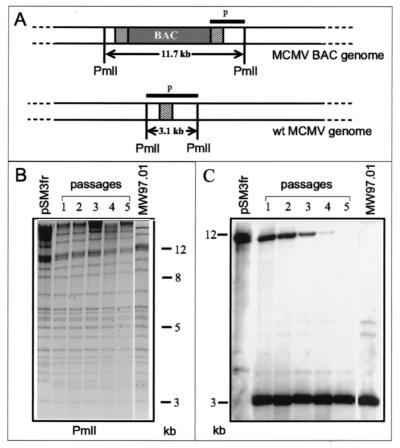
To examine how efficient wt genomes are generated and accumulate in eukaryotic cells after transfection of the MCMV BAC plasmid pSM3fr, viral DNA collected after each virus passage was analyzed by Southern blotting. Total DNA was isolated from cells transfected with BAC plasmid pSM3fr (Fig. 4B, passage 1) and from cells infected with reconstituted viruses after each of the second to fifth passages (Fig. 4B, passages 2 to 5). The isolated DNAs and DNAs of plasmid pSM3fr and of virus MW97.01 were digested with the restriction enzyme PmlI (Fig. 4B), and Southern blot analysis was performed with probe p (nt 217673 to 220817; see Fig. 4A), which detects an 11.7-kbp band in BAC vector-containing genomes and a 3.1-kbp band in the reconstituted wt genomes (Fig. 4A). Remarkably, the 3.1-kbp band was already present with high abundance in the progeny of transfected cells (Fig. 4C, passage 1), indicating that excision of the BAC vector occurs rapidly and quite frequently. The 11.7-kbp band gradually diminished in the DNA preparations (Fig. 4C), confirming that BAC-containing genomes have a propagation disadvantage compared to wt genomes. The gradual loss of the BAC vector shown in Fig. 4C was consistently seen in three separate reconstitution experiments (data not shown).
FIG. 4.
Excision of the BAC vector sequences from reconstituted virus genomes. (A) Structural organization of the viral genomes before and after vector excision. Probe p detects fragments of 11.7 kbp (before vector excision) and 3.1 kbp (after vector excision) in PmlI-digested viral DNA. The duplicated sequences are indicated as hatched boxes. (B) Ethidium bromide-stained agarose gel of PmlI-digested viral DNA isolated from cells transfected with plasmid pSM3fr (first passage) and from cells infected with reconstituted virus after the second to fifth passages. PmlI-digested DNAs of BAC plasmid pSM3fr and MW97.01 virus were used as controls for the presence of the BAC vector-containing fragment and the reconstituted wt genome fragment, respectively. (C) Southern blot analysis of the viral DNAs with probe p.
To test whether excision of the BAC vector sequences occurs reproducibly, five independent transfections with BAC plasmid pSM3fr were performed. Total DNA was isolated from infected cells collected after each of the first and the fifth passages of the recombinant viruses. PCR with primers b (5′-GCC CGC CTG ATG AAT GCT C-3′) and g (5′-GGA TAC TCA GCG GCA GTT TGC-3′), which bind in the BAC vector sequences and in the MCMV EcoRI g fragment, was performed to see whether the isolated DNA still contained genomes with the BAC vector. The presence of a 1,950-bp PCR fragment indicated the presence of BAC-containing genomes. Southern analysis of the PCR products was done with a BAC-specific probe (a 600-bp BamHI-EcoRI fragment from the BAC vector pKSO) (21) to detect even minor amounts of the amplified products. In none of the five experiments was a signal found in the DNA preparations isolated from recombinant viruses collected in the fifth passage, whereas positive signals were detected in those collected in the first passage (data not shown). Thus, the BAC-containing genomes had reproducibly disappeared during five virus passages.
Next we asked whether excision of the BAC vector via the duplicated sequences was accurate. PCR was performed with DNA obtained from cells that were infected with recombinant virus in the fifth passage and with DNA of virus MW97.01 by using primers f (5′-GGT TAC TGG ATG GGT ACG AG-3′) and g (see above), which bind to viral sequences flanking the excision site. A 590-bp fragment was amplified after successful excision of the BAC vector. Sequencing of the PCR products was done with primer f. The sequences of all PCR products obtained from the reconstituted viruses were identical to the corresponding sequence of the MCMV strain Smith (data not shown). Thus, excision of the BAC vector from the reconstituted genomes occurs with high accuracy and the devised strategy can be used for reproducible excision of the BAC vector sequences from the recombinant MCMV genome.
In vivo growth properties of the recombinant virus MW97.01.
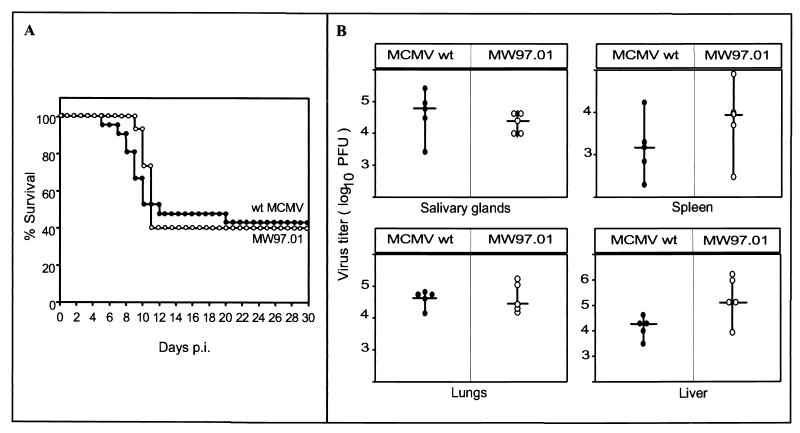
The recombinant virus MW97.01 has a genome structure indistinguishable from that of wt MCMV. However, minor genome alterations, i.e., point mutations that might have accumulated by passage of the MCMV BAC in E. coli, should escape detection by restriction enzyme analysis. Therefore, we examined the virulence of the MW97.01 virus in vivo as a sensitive test for the biological properties of the recombinant virus. Newborn mice are highly susceptible to MCMV (25), and infection of such mice can reveal even minor differences between the recombinant virus MW97.01 and wt MCMV. Newborn BALB/c mice were infected with 100 or 1,000 PFU of the MW97.01 virus or wt MCMV. The viruses showed remarkably similar degrees of virulence in vivo (Fig. 5). While infection with 100 PFU resulted in a mortality rate of about 60% for mice infected with either MW97.1 virus or wt MCMV (Fig. 5A), infection with 1,000 PFU led to 100% mortality by day 17 (data not shown). To analyze whether the identical mortality rates are reflected by the virus titers in organs of infected mice, BALB/c mice were infected with 100 PFU of the MW97.01 virus or wt MCMV, and virus titers were determined 12 days postinfection. The virus titers in organs of MW97.01- and wt MCMV-infected mice (determined by standard plaque assay as previously described [26]) showed comparable levels in all organs tested (liver, spleen, lungs, and salivary glands; Fig. 5B). We concluded from these data that the examined biological properties in vivo of the recombinant virus MW97.01 are comparable to those of wt MCMV.
FIG. 5.
Biological properties of the BAC-derived recombinant virus MW97.01 in vivo. Newborn BALB/c mice were infected intraperitoneally with 100 PFU of wt MCMV (21 animals; solid circles) or MW97.01 virus (15 animals; open circles). (A) Mortality of mice was monitored for 30 days postinfection (p.i.), and survival rates were determined. (B) Virus titers in organs of five animals infected with wt MCMV or MW97.01 virus were determined 12 days p.i., and median values (horizontal bars) were calculated.
In this communication we report on three observations relevant for the general use of infectious herpesvirus BACs. First, mutagenesis of herpesvirus genomes in bacteria can be combined with subsequent recombination procedures in eukaryotic cells that operate independently from each other and result in virus genomes free of any bacterial vector sequences. Second, MCMV BAC genomes, although lacking a biological pressure in E. coli, remain remarkably stable as bacterial plasmids. Third, reconstitution of full-length genomes by recombination of fragments from two different MCMV strains results in a virus with wt properties. The genome of the virus MW97.01 can be distinguished from those of both parents by defined nucleotide exchanges. The newly generated MCMV BAC plasmid pSM3fr will serve as the basis for easy and rapid construction of MCMV mutants to study CMV pathogenesis in vivo.
Acknowledgments
This work was supported by grants of the Bundesministerium für Bildung und Forschung (BMBF) and the Deutsche Forschungsgemeinschaft (SFB 455, projects A2 and A7).
We thank Stefanie Eichler for excellent technical assistance.
REFERENCES
- 1.Angulo A, Messerle M, Koszinowski U H, Ghazal P. Enhancer requirement for murine cytomegalovirus growth and genetic complementation by the human cytomegalovirus enhancer. J Virol. 1998;72:8502–8509. doi: 10.1128/jvi.72.11.8502-8509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bett A J, Prevek L, Graham F L. Packaging capacity and stability of human adenovirus type 5 vectors. J Virol. 1993;67:5911–5921. doi: 10.1128/jvi.67.10.5911-5921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloss T A, Sugden B. Optimal lengths of DNAs encapsidated by Epstein-Barr virus. J Virol. 1994;68:8217–8222. doi: 10.1128/jvi.68.12.8217-8222.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boname J M, Chantler J K. Characterization of a strain of murine cytomegalovirus which fails to grow in the salivary glands of mice. J Gen Virol. 1992;73:2021–2029. doi: 10.1099/0022-1317-73-8-2021. [DOI] [PubMed] [Google Scholar]
- 5.Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. New York, N.Y: Lippincott-Raven; 1996. pp. 2493–2523. [Google Scholar]
- 6.Cavanaugh V J, Stenberg R M, Staley T L, Virgin IV H W, MacDonald M R, Paetzold S, Farrell H E, Rawlinson W D, Campbell A E. Murine cytomegalovirus with a deletion of genes spanning HindIII-J and -I displays altered cell and tissue tropism. J Virol. 1996;70:1365–1374. doi: 10.1128/jvi.70.3.1365-1374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang A C Y, Cohen S. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crnković-Mertens I, Messerle M, Milotić I, Szepan U, Kuĉić N, Krmpotić A, Jonjić S, Koszinowski U H. Virus attenuation after deletion of the cytomegalovirus Fc receptor gene is not due to antibody control. J Virol. 1998;72:1377–1382. doi: 10.1128/jvi.72.2.1377-1382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebeling A, Keil G M, Knust E, Koszinowski U H. Molecular cloning and physical mapping of murine cytomegalovirus DNA. J Virol. 1983;47:421–433. doi: 10.1128/jvi.47.3.421-433.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott R, Clark C, Jaquish D, Spector D. Transcription analysis and sequence of the putative murine cytomegalovirus DNA polymerase gene. Virology. 1991;185:169–186. doi: 10.1016/0042-6822(91)90765-4. [DOI] [PubMed] [Google Scholar]
- 11.Farrell H E, Vally H, Lynch D M, Fleming P, Shellam G R, Scalzo A A, Davis-Poynter N J. Inhibition of natural killer cells by a cytomegalovirus MHC class I homologue in vivo. Nature. 1997;386:510–514. doi: 10.1038/386510a0. [DOI] [PubMed] [Google Scholar]
- 12.Greaves R F, Brown J M, Vieira J, Mocarski E S. Selectable insertion and deletion mutagenesis of the human cytomegalovirus genome using the Escherichia coli guanosine phosphoribosyl transferase (gpt) gene. J Gen Virol. 1995;76:2151–2160. doi: 10.1099/0022-1317-76-9-2151. [DOI] [PubMed] [Google Scholar]
- 13.Ho M. Cytomegalovirus: biology and infection. New York, N.Y: Plenum Press; 1982. [Google Scholar]
- 14.Hudson J B. The murine cytomegalovirus as a model for study of viral pathogenesis and persistent infections. Arch Virol. 1979;62:1–29. doi: 10.1007/BF01314900. [DOI] [PubMed] [Google Scholar]
- 15.Kemble G, Duke G, Winter R, Spaete R. Defined large-scale alterations of the human cytomegalovirus genome constructed by cotransfection of overlapping cosmids. J Virol. 1996;70:2044–2048. doi: 10.1128/jvi.70.3.2044-2048.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kempkes B, Pich D, Zeidler R, Sugden B, Hammerschmidt W. Immortalization of human B lymphocytes by a plasmid containing 71 kilobase pairs of Epstein-Barr virus DNA. J Virol. 1995;69:231–238. doi: 10.1128/jvi.69.1.231-238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 18.Manning W C, Mocarski E S. Insertional mutagenesis of the murine cytomegalovirus genome: one prominent α gene (ie2) is dispensable for growth. Virology. 1988;167:477–484. [PubMed] [Google Scholar]
- 19.Manning W C, Stoddart C A, Lagenaur L A, Abenes G B, Mocarski E S. Cytomegalovirus determinant of replication in salivary glands. J Virol. 1992;66:3794–3802. doi: 10.1128/jvi.66.6.3794-3802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercer J A, Marks J R, Spector D H. Molecular cloning and restriction endonuclease mapping of the murine cytomegalovirus genome (Smith strain) Virology. 1983;129:94–106. doi: 10.1016/0042-6822(83)90398-7. [DOI] [PubMed] [Google Scholar]
- 21.Messerle M, Crnković I, Hammerschmidt W, Ziegler H, Koszinowski U H. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc Natl Acad Sci USA. 1997;94:14759–14763. doi: 10.1073/pnas.94.26.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messerle, M. Unpublished data.
- 23.O’Connor M, Pfeifer M, Bender W. Construction of large DNA segments in Escherichia coli. Science. 1989;244:1307–1312. doi: 10.1126/science.2660262. [DOI] [PubMed] [Google Scholar]
- 24.Rawlinson W D, Farrell H E, Barrell B G. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996;70:8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddehase M J, Balthesen M, Rapp M, Jonjić S, Pavić I, Koszinowski U H. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J Exp Med. 1994;179:185–193. doi: 10.1084/jem.179.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddehase M J, Weiland F, Münch K, Jonjić S, Lüske A, Koszinowski U H. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985;55:264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spaete R R, Mocarski E S. Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc Natl Acad Sci USA. 1987;84:7213–7217. doi: 10.1073/pnas.84.20.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vieira J, Farrell H E, Rawlinson W D, Mocarski E S. Genes in the HindIII J fragment of the murine cytomegalovirus genome are dispensable for growth in cultured cells: insertion mutagenesis with a lacZ/gpt cassette. J Virol. 1994;68:4837–4846. doi: 10.1128/jvi.68.8.4837-4846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]