Abstract
Background: Syphilis remains a significant global public health issue, and female sex workers (FSWs) are highly vulnerable to the etiological agent of this disease. This study aimed to describe the prevalence of exposure to Treponema pallidum, as well as the vulnerability factors among FSWs in the state of Pará, Brazilian Amazon. Methods: A cross-sectional, retrospective study involving 360 FSWs from five cities in Pará was conducted from 2005 to 2007. Blood samples were collected for treponemal and non-treponemal testing, and epidemiological information was obtained through interviews. Results: The exposure rate to T. pallidum was 37.7% (136/360), and the majority of FSWs had serological results indicating past exposure (21.1%). Among the FSWs exposed to T. pallidum, most of them were single, aged 23 to 42 years old, had less than 8 years of schooling, and had a family income of between 1 and 3 minimum wages. They reported using condoms during sexual intercourse and had no history of sexually transmitted infection (STI). Furthermore, many of the FSWs exposed to T. pallidum reported having more than 20 sexual partners per month, and had partners from other Brazilian states, but not from other countries. An age over 42 years and a reduced level of education were factors associated with exposure to T. pallidum. Finally, a high rate of exposure to T. pallidum among FSWs in the Brazilian state of Pará (from 2005 to 2007) was detected. In later years, epidemiological studies conducted with FSWs recorded that this rate remained high. Measures to control, treat, and prevent syphilis among FSWs were necessary between 2005 and 2007, and they are still imperative today. Actions related to educational programs and STI control, treatment, and prevention measures contained in Brazilian policies aimed at women’s health have not changed the vulnerability scenario of FSWs regarding their exposure to T. pallidum, even after 16 years, and must be reviewed and adapted to the conditions of the Brazilian Amazon.
Keywords: Treponema pallidum, sexually transmitted infections, female sex workers, epidemiology, public health
1. Introduction
Syphilis is a systemic sexually transmitted infection (STI) caused by the bacterium Treponema pallidum subspecies pallidum (T. pallidum). Despite being curable, syphilis still represents a significant public health problem worldwide, given the high prevalences recorded in poor and developing countries and the increasing incidence detected in the population of men who have sex with men in developed countries [1,2]. T. pallidum can be transmitted from one person to another during sex (anal, vaginal, or oral) without a condom, through direct contact with primary and secondary syphilis lesions, or blood transfusion. Another form of transmission of this pathogen occurs from an infected mother to their child during pregnancy or childbirth [2].
The prevalence and incidence of syphilis has increased among men and women in different parts of the world [3,4,5]. A high prevalence of syphilis has been recorded in men and women in Latin America and the Caribbean (around 1.3%), and the highest incidence rate of this STI was observed in women and men in the Americas (5.3 cases per 1000 in men and women, which equates to over 3 million incident cases). In Brazil, there has been a significant upward trend in the prevalence of syphilis in its various forms from 2007 to 2017, representing a major public health risk in various municipalities located in the five Brazilian regions, showing that the challenge of reducing or even eliminating syphilis is still very difficult [5].
In this context, female sex workers (FSWs), as well as transgender women and transvestites, are considered key populations for acquiring syphilis and other STIs due to their high social and economic vulnerability, considering the high levels of stigma, violence, and criminalization. In Brazil, there are few epidemiological studies on syphilis in FSWs. In 2016, a multicenter study conducted with FSWs working in 12 Brazilian cities indicated a syphilis prevalence of 8.5% [6]. In three cities in the south of Brazil, the syphilis prevalence recorded among FSWs was 19.7% [7]. On the other hand, a high prevalence of syphilis has been detected in studies conducted with FSWs in the north of Brazil—36.1% in the cities of Belém, Macapá, and Rio Branco [8]; 14.1% in the municipalities of Augusto Corrêa, Barcarena, and Belém [9]; 41.1% in municipalities and riverside communities in the Marajó Archipelago [10]; and 36.9% in municipalities crossed by major highways in Pará [11]. Unprotected sex, multiple sexual partners, early initiation into sex work, illicit drug use, limited knowledge about STIs, and reduced monthly income have been associated with syphilis in this key population [12,13]. Therefore, investments in healthcare, health promotion, prevention, and social assistance for these women should be a priority for managers in Brazilian municipalities and states. Treating a disease like syphilis that affects different populations, such as pregnant women and FSWs, requires effective and integrated measures from healthcare services at all levels of complexity.
Since 1984, the Ministry of Health has developed women’s health assistance programs in Brazil, which have included educational, preventive, diagnostic, treatment, and recovery actions, encompassing women’s care in gynecological clinics, prenatal, childbirth, and postpartum care, menopause, family planning, STIs, and cervical and breast cancer, as well as other needs identified from the population profile of women [14,15]. However, there are still several gaps and challenges to be overcome regarding women’s health, such as adolescent women’s health, occupational health, mental health, infectious diseases, and the inclusion of gender perspective [15].
These challenges are even more difficult to overcome in the northern region of Brazil, a rural and socioeconomically underdeveloped area with limited transportation infrastructure and inadequate healthcare services, where poverty, malnutrition, domestic and urban violence, sex work, and the use and trafficking of illicit drugs are commonly observed and recorded at high levels [16]. Thus, this study describes the prevalence and factors associated with the exposure to T. pallidum in FSWs working in municipalities in the state of Pará, in the Brazilian Amazon.
2. Materials and Methods
2.1. Study Characteristics and Ethical Aspects
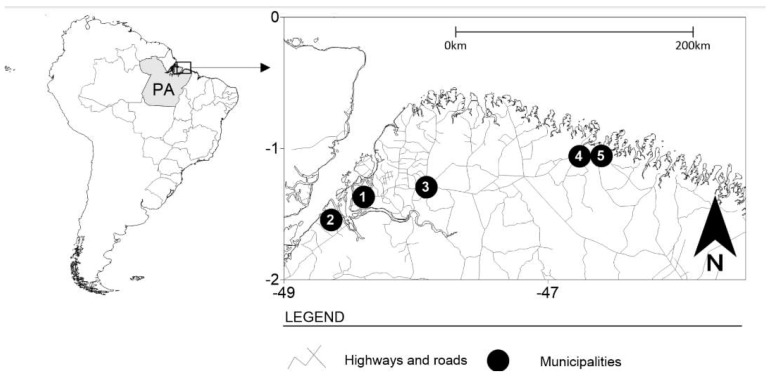
The present study was cross-sectional, retrospective, descriptive, and analytical, involving FSWs from five municipalities in the state of Pará, northern Brazil—Belém (the capital), Bragança, Augusto Corrêa, Barcarena, and Castanhal (Figure 1). These municipalities are areas of an intense flow of people and the circulation of products with many historical, cultural, and tourist attractions that stand out in the context of the Amazon region, and, at the same time, they also have records of sexual trade in the state of Pará [10,11,12,13,16,17]. This study was approved by the Human Research Ethics Committee of the HEMOPA Foundation, under number 12/2005.
Figure 1.
The geographic location of the municipalities where personal information and biological samples were collected from FSWs in northern Brazil. Points 1 to 5 are municipalities in the state of Pará (PA)—Belém (1), Barcarena (2), Castanhal (3), Bragança (4), and Augusto Corrêa (5).
2.2. Study Design and Sampling
This study involved FSWs from January 2005 to August 2007. A non-probabilistic (convenience) sample was utilized to collect data from participants, with enrollment taking place four times a week at their workplaces (such as bars, streets, strip clubs, etc.), determined through sample size analysis. Women who identified as cisgender and engaged in sexual activities in exchange for money in the selected locations for at least six months were included in the study. Those who self-identified as transgender, women under the influence of alcohol or drugs during data collection, and those unable to respond to the epidemiological questionnaire were excluded from the study. Initially, a survey of prostitution venues in the evaluated municipalities was conducted, followed by approaching FSWs who played a leadership role in enhanced participant recruitment. They were invited to participate in the study after the presentation of the study.
All FSWs were informed about the study objectives and were invited to participate. Those who agreed to take part in the research signed an Informed Consent Form and provided epidemiological information. The refusal rate to participate in the study was 10.3%. The determination of the sample size was based on the estimated prevalence of syphilis in women in Belém, Pará (33.0%) at the time of the study. The sample error (ε) assumed in the calculation was 5%, and a test power of 80% was established, resulting in a minimum sample size of 320 FSWs.
2.3. Laboratory Tests and Interpretations
From each FSW, a sample of peripheral blood (5 mL) was collected using a vacuum collection system into tubes containing EDTA as an anticoagulant. Plasma was separated using centrifugation (9500 rpm for 15 min) and was stored at −20 °C until use at the Virology Laboratory of the Institute of Biological Sciences at the Federal University of Pará. FSW samples were examined for the detection of anti-Treponema pallidum antibodies (immunoglobulin M and immunoglobulin G) using the enzyme-linked immunosorbent assay (ELISA; Eti-Treponema Plus—DiaSorin, Cypress, CA, USA) according to the manufacturer’s instructions. The use of the ELISA to detect treponemal antibodies is considered to be a treponemal test, as it involves the detection of specific anti-treponemal antibodies in a sample [18]. FSWs with non-reactive results, as determined using the ELISA, were classified as not being exposed to T. pallidum, and no procedure or test was performed. All FSWs with reactive results according to the ELISA were classified as being exposed to T. pallidum (i.e., they have been exposed in the past or recently to T. pallidum) and had biological samples collected to perform additional tests. Reactive samples according to the ELISA were tested using the rapid plasma reagin (RPR-Brás-Laborclin, Paraná, Brazil). RPR is considered a non-treponemal test, as it detects antibodies that are not specific for T. pallidum, but that are found in patients with syphilis. All samples were tested pure (1:1) and in titrations (≥1:2) to eliminate the possibility of the prozone phenomenon in the execution of this laboratory test. The positivity of the sample in the treponemal test (ELISA) was considered as demonstrating an exposure to T. pallidum (outcome). All FSW samples were examined 15 to 30 days after collection.
2.4. Data Collection and Statistical Analysis
All FSWs completed an interviewer-administered questionnaire consisting of questions about sociodemographic, behavioral, drug use, and clinical/health outcome variables. These data were fed into an Excel database and were converted to STATA format for all procedures and statistical analyses. Significant associations between the outcome (with or without exposure to T. pallidum) and epidemiological information (sociodemographic, behavioral, drug use, and health/clinical variables) were assessed using the Chi-square test. The latter used a 0.05 significance level for the type I error. The data were analyzed using STATA 17 (StataCorp®, College Station, TX, USA).
3. Results
The study included 360 FSWs, with a mean age of 36.2 years (ranging from 15 to 71 years), with the majority (61.1%; 220/360) falling within the age range of 23 to 42 years. Regarding marital status, most participants were single (71.4%; 257/360), had less than 8 years of education (72.5%; 261/360), and had a family income of between 1 and 3 minimum wages (55.6%; 200/360).
The majority of study participants reported using condoms in all sexual encounters in the past 12 months (55.8%; 201/360), stated no history of STIs (78.1%; 281/360), but admitted to using illicit drugs (51.7%; 186/360) and having more than 20 sexual partners per month (54.4%; 196/360). Furthermore, 58.0% (209/360) reported having had partners from other states in Brazil, and 43.9% (158/360) had previously had partners from other countries (Table 1).
Table 1.
Demographic, socioeconomic, behavioral, and health characteristics of FSWs in the Brazilian state of Pará, from January 2005 to August 2007, related to exposure to T. pallidum.
| Characteristics | Total (n = 360) |
No Exposure (n = 224) |
With Exposure * (n = 136) |
p-Value |
|---|---|---|---|---|
| N | n (%) | n (%) | ||
| Age groups (years) | <0.01 | |||
| 15–22 | 112 | 86 (76.8) | 26 (23.2) | |
| 23–42 | 220 | 131 (59.5) | 89 (40.5) | |
| >42 | 28 | 7 (25.0) | 21 (75.0) | |
| Marital status | 0.09 | |||
| Single | 257 | 153 (59.5) | 104 (40.5) | |
| Married | 56 | 35 (62.5) | 21 (37.5) | |
| Widow/divorced | 47 | 36 (76.6) | 11 (23.4) | |
| Education level | 0.02 | |||
| <8 years of study | 261 | 153 (58.6) | 108 (41.4) | |
| >8 years of study | 99 | 71 (71.7) | 28 (28.3) | |
| Family income (in minimum wage) | 0.07 | |||
| Less than 1 | 131 | 89 (67.9) | 42 (32.1) | |
| From 1 to 3 | 200 | 114 (57.0) | 86 (43.0) | |
| More than 3 | 29 | 21 (72.4) | 8 (27.6) | |
| Condom use | 0.27 | |||
| Yes | 201 | 120 (59.7) | 81 (40.3) | |
| No | 159 | 104 (65.4) | 55 (34.6) | |
| STI # history | 0.06 | |||
| Yes | 79 | 42 (53.2) | 37 (46.8) | |
| No | 281 | 182 (64.8) | 99 (35.2) | |
| Use of illicit drugs | 0.13 | |||
| Yes | 186 | 126 (67.7) | 60 (32.3) | |
| No | 174 | 98 (56.3) | 76 (43.7) | |
| Number of partners (monthly) | 0.45 | |||
| <20 | 164 | 106 (64.6) | 58 (35.4) | |
| ≥20 | 196 | 118 (60.2) | 78 (39.8) | |
| Partners from other states of Brazil | 0.19 | |||
| Yes | 209 | 124 (59.3) | 85 (40.7) | |
| No | 85 | 60 (70.6) | 25 (29.4) | |
| Do not know | 66 | 40 (60.6) | 26 (39.4) | |
| Partners from other countries | 0.83 | |||
| Yes | 158 | 97 (61.4) | 61 (38.6) | |
| No | 202 | 127 (62.9) | 75 (37.1) |
* Treponemal test positive (ELISA) = exposure to T. pallidum. # STI = sexually transmitted infection.
Overall, the prevalence of exposure to T. pallidum was 37.7% among FSWs. The rates of past exposure (21.1%) were higher than those detected for recent exposure (16.7%) to T. pallidum (Table 2). Among the FSWs exposed to T. pallidum, the majority of them were single, aged 23 to 42 years old, had less than 8 years of schooling, and had a family income of between 1 and 3 minimum wages. They reported using condoms during sexual intercourse, and had no history of STI. Furthermore, many of the FSWs exposed to T. pallidum reported having more than 20 sexual partners per month, and had partners from other Brazilian states, but not from other countries. Only two factors associated with exposure to T. pallidum were detected here—being aged over 42 years and having a reduced level of education (less than eight years of study). Other sociodemographic, economic, behavioral, and health factors were not associated with exposure to T. pallidum (Table 1).
Table 2.
Prevalence of exposure to T. pallidum (etiological agent of syphilis) among female sex workers in the state of Pará, Brazilian Amazon.
| Prevalence (Serological Markers) | % (Positive/Total) | 95% Confidence Intervals |
|---|---|---|
| Exposure to Treponema pallidum (IgG * + IgM **) | 37.7 (136/360) | 32.8–42.8 |
| Past exposure (IgG *) | 21.1 (76/360) | 16.9–25.3 |
| Recent exposure (IgM **) | 16.7 (60/360) | 12.8–20.5 |
* IgG = Immunoglobulin G; ** IgM = Immunoglobulin M.
The rates of past infections were higher than those detected for recent infections with T. pallidum. The overall prevalence of recent infections was 16.7%. In this study, nine (2.5%) FSWs showed positive results for RPR (with low titers) and negative results for ELISA. These cases were considered cross-reactions and were interpreted as false-positive results (i.e., FSWs are also susceptible to infection with T. pallidum). The majority of FSWs (59.7%) participating in this study had negative serology for anti-T. pallidum in both serological tests (Table 2).
Furthermore, significant associations were observed between syphilis infection and several demographic and behavioral factors among the FSWs. These factors included age groups, with a higher proportion of infections being found among those aged 23–42 years (65.5%, p < 0.001), and marital status, with a higher proportion of infections being detected among single individuals (76.5%, p = 0.144). Additionally, a significant association was found between syphilis infection and education level, with a higher proportion of infections being found among individuals with less than 8 years of study (79.4%, p = 0.007). These findings highlight the importance of considering socio-demographic factors in the prevention and control of syphilis among FSWs.
4. Discussion
The present study documented a high prevalence of exposure to T. pallidum (37.7%) among FSWs working in five municipalities in the Brazilian state of Pará, including the state capital, from 2005 to 2007, indicating the historical vulnerability of these women to T. pallidum. High rates of syphilis were recorded among FSWs after 2007. In the Marajó archipelago, state of Pará (2015–2017), a high prevalence of syphilis (41.1%) was recorded among FSWs in 25 locations (7 municipalities and 18 riverside communities), the majority of whom were young (18 to 30 years old), which differs from the findings in this study [10]. A high prevalence of syphilis (36.9%) was also recorded among FSWs who worked on the road system in the state of Pará from 2015 to 2016 [11]. This demonstrates that the history of syphilis and the profile of FSWs working in this Brazilian state in the Brazilian Amazon are very worrying; an increase in the number of syphilis cases and younger FSWs with syphilis has been reported.
The prevalence of exposure to T. pallidum among FSWs in the present study (including both new and old cases) was much higher than that found in other regions of Brazil, such as sex workers in Curitiba from 2010 to 2019 (1.0%) [19]; in 12 Brazilian cities in 2016 (8.5%) [6]; and in Tubarão, Laguna, and Imbituba, Southern Brazil (19.7%) in the year 2009 [7,20]. Our results demonstrate that exposure to T. pallidum in the state of Pará is much higher than that found in other populations of sex workers and non-sex workers, such as among residents of peri-urban islands in Belém, Pará, from 2020 to 2021 (5.9%) [21]; among recyclable waste collectors in Central Brazil from 2014 to 2016 (7.91%) [22]; and among manual sugarcane cutters (2.5%) in the Midwest and northeast regions of Brazil in 2016 [23].
Among Latin American countries, the prevalence of syphilis among FSWs in the present study was also higher than that found among FSWs in Lima, Peru (3.4%) [24]; in Argentina between 2006 and 2009 (22.4%) [25]; and in Colombia between 2001 and 2002 (10.3%) [26]. It is worth noting that in Argentina between 2000 and 2002, the prevalence of syphilis in FSWs was 45.7% [27]. However, in 1999, the prevalence was 2.4% in Venezuela [28]. Compared to other countries, the prevalence of syphilis found in Pará is much higher than that observed among FSWs in Ethiopia from 2019 to 2021 (6.2% and 11.3%) [29,30]; in China from 2013 to 2021 (1.8%, 1.73%, and 4.41%) [31,32,33]; in Kurdistan, west of Iran, from 2019 to 2020 (1.0%) [34]; in Togo in 2017 (0.8%) [35]; in Cameroon from 2015 to 2016 (8.3%) [36]; in the Middle East and North Africa in 2018 (12.7%) [37]; in Sudan and Uganda from 2015 to 2017 (7.3% and 9.2%) [38,39]; in Moscow, Russia, from 2017 to 2018 (13.9%) [40]; and in the Sino–Vietnam border area from 2016 to 2021 (8.8%) [41]. However, the prevalence of syphilis in the present study was like that found in Bangladesh at 38.2% [42] and was relatively higher than that observed in FSWs in Malawi in 2019 (9.7%) [43].
Additionally, a low level of education and a high age of FSWs were associated with exposure to T. pallidum in this study. The predictive role of lower educational levels, low monthly income, and longer involvement in the sex trade (representing older age) as factors associated with syphilis point to the role of adverse socioeconomic determinants and, specifically, social marginalization. This has been recorded in epidemiological studies on syphilis and STIs conducted in Brazil and other countries in South America [9,11,13,28,44,45]. Overall, the risk factors associated with syphilis here are clear indicators of the health vulnerability of these women in the Brazilian Amazon and, consequently, reflect the failure or absence of actions related to educational programs and measures of control, treatment, and prevention of STIs that are contained in the National Policy for Comprehensive Women’s Health Care, guiding women’s health care actions from 2004 to 2007 in Brazil [14,15]. Unfortunately, studies conducted on syphilis and other STIs among FSWs in the Brazilian Amazon after 2007 have reinforced the historical invisibility of FSWs about the National Policy for Comprehensive Women’s Health Care (PNAISM) and the National Plan for Women’s Policies (PNPM) in Brazil [8,9,10,11,44,45]. An example of this is the absence of any indicator (so far) to record, monitor, and track the living and health conditions of FSWs in Brazil.
This study has several limitations that should be considered. The sample size could have been larger. However, due to the significant social stigma surrounding FSWs worldwide, recruiting participants for studies aimed at promoting physical and mental health is very challenging, and the refusal rate is very high. Many women report feeling ashamed, and their families are unaware of their actual work, making it difficult to even deliver test results for treatment, as they do not provide their data correctly. Another relevant limitation is the origin of the data. As the interview data are self-reported, some information, such as drug use or sex-related risks behaviors, may contain response or recall bias. Additionally, the study used a convenience sample and may not establish a causal relationship. Although the information on exposure to T. pallidum and the profile of FSWs in this study pertains to the years 2005 to 2007, and the regional scenario of this pathogen and syphilis is probably different today, this study can serve as a basis for evaluating the dynamics of this STI over time, being an important tool for evaluating the effectiveness of public health policies and tackling the spread of T. pallidum.
5. Conclusions
A high rate of exposure to T. pallidum among FSWs in the Brazilian state of Pará (from 2005 to 2007) was detected. In later years, epidemiological studies conducted with FSWs recorded that this rate remained high. Measures to control, treat, and prevent syphilis among FSWs were necessary between 2005 and 2007 and, unfortunately, are imperative today. Actions related to educational programs and STI control, treatment, and prevention measures contained in Brazilian policies aimed at women’s health have not changed the vulnerability scenario of FSWs regarding exposure to T. pallidum, even after 16 years. The actions to be carried out in this group of vulnerable women must consider different aspects, from the level of education and economic capacity to risk behaviors for acquiring STI and the trauma caused by the violence experienced.
Acknowledgments
The authors thank all the individuals who took part in the study, Executive Secretariat of Public Health of the State of Pará, CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior), and the Federal University of Pará.
Author Contributions
All authors contributed to the research development. T.M.d.S.C., D.O.d.A. and L.F.A.M. were involved in the research conceptualization; T.M.d.S.C., P.d.S.d.O.d.C.L., D.O.d.A., J.C.M. and R.N.M.F. conducted data curation; T.M.d.S.C., P.d.S.d.O.d.C.L., D.O.d.A., F.A.M.d.C. and L.M.d.S. conducted methodology; R.R.d.S.F., A.B.O.-F. and L.F.A.M. were involved in writing the original draft; L.F.A.M. was involved in supervision; J.C.M., R.N.M.F., R.R.d.S.F., R.V.L. and A.B.O.-F. were responsible for formal analysis; R.R.d.S.F., R.V.L. and A.B.O.-F. were responsible for investigation; T.M.d.S.C., D.O.d.A. and L.F.A.M. were involved in reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Human Research Committee for Ethics in Research of the Ethics Committee of the Foundation Hemotherapy and Hematology Center of Pará (HEMOPA) (PA), Brazil (protocol number 12/2005). All participants were included in the study after providing written consent.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Funding Statement
This study was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Ministry of Education—Brazil—Grant code 001. L.F.A.M. is a CNPq Grantee (#314209/2021-2). The publication of this article was supported by Public Notice PAPQ, PROPESP/FADESP of the Federal University of Pará.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pan American Health Organization (PAHO) Epidemiological Review of Syphilis in the Americas. [(accessed on 29 April 2024)]. Available online: https://iris.paho.org/bitstream/handle/10665.2/56085/PAHOCDEHT220009_eng.pdf?sequence=1&isAllowed=y.
- 2.World Health Organization (WHO) Syphilis. [(accessed on 29 April 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/syphilis.
- 3.Marques Dos Santos M., Lopes A.K.B., Roncalli A.G., Lima K.C. Trends of syphilis in Brazil: A growth portrait of the treponemic epidemic. PLoS ONE. 2020;15:e0231029. doi: 10.1371/journal.pone.0231029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kojima N., Klausner J.D. An Update on the Global Epidemiology of Syphilis. Curr. Epidemiol. Rep. 2018;5:24–38. doi: 10.1007/s40471-018-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuboi M., Evans J., Davies E.P., Rowley J., Korenromp E.L., Clayton T., Taylor M.M., Mabey D., Chico R.M. Prevalence of syphilis among men who have sex with men: A global systematic review and meta-analysis from 2000–2020. Lancet Glob Health. 2021;9:e1110–e1118. doi: 10.1016/S2214-109X(21)00221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira-Júnior O.D.C., Guimarães M.D.C., Damacena G.N., de Almeida W.D.S., de Souza-Júnior P.R.B., Szwarcwald C.L., Brazilian FSW Group Prevalence estimates of HIV, syphilis, hepatitis B and C among female sex workers (FSW) in Brazil, 2016. Medicine. 2018;97((Suppl. S1)):S3–S8. doi: 10.1097/MD.0000000000009218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuelter-Trevisol F., Custódio G., Silva A.C., Oliveira M.B., Wolfart A., Trevisol D.J. HIV, hepatitis B and C, and syphilis prevalence and coinfection among sex workers in Southern Brazil. Rev. Soc. Bras. Med. Trop. 2013;46:493–497. doi: 10.1590/0037-8682-1364-2013. [DOI] [PubMed] [Google Scholar]
- 8.Machado L.F.A., Monteiro J.C., Siravenha L.Q., Mota M.P., Souza M.C., Santos A.S.D., Moreira M.R.C., Laurentino R.V., Oliveira-Filho A.B., Queiroz M.A.F., et al. Treponema pallidum among Female Sex Workers: A Cross-Sectional Study Conducted in Three Major Cities in Northern Brazil. Pathogens. 2021;10:923. doi: 10.3390/pathogens10080923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Souza R.L., Dos Santos Madeira L.D.P., Pereira M.V.S., da Silva R.M., de Luna Sales J.B., Azevedo V.N., Feitosa R.N.M., Monteiro J.C., Ishak M.d.O.G., Ishak R., et al. Prevalence of syphilis in female sex workers in three countryside cities of the state of Para, Brazilian Amazon. BMC Infect. Dis. 2020;20:129. doi: 10.1186/s12879-020-4850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coelho E.C., Souza S.B., Costa C.C.S., Costa L.M., Pinheiro L.M.L., Machado L.F.A., Silva-Oliveira G.C., Martins L.C., Frade P.C.R., Oliveira-Filho A.B. Treponema pallidum in female sex workers from the Brazilian Marajo Archipelago: Prevalence, risk factors, drug-resistant mutations and coinfections. Trans. R. Soc. Trop. Med. Hyg. 2021;115:792–800. doi: 10.1093/trstmh/traa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalcante N.D.S., Lima H.R.R., Tabosa D.F., Barbosa E.D.S.S., Costa N.P.D.S., Costa L.M.D., Frade P.C.R., Martins L.C., Silva-Oliveira G.C., de Oliveira-Filho A.B. Syphilis in female sex workers: An epidemiological study of the highway system of the state of Para, northern Brazil. Rev. Soc. Bras. Med. Trop. 2019;18:e20180064. doi: 10.1590/0037-8682-0064-2018. [DOI] [PubMed] [Google Scholar]
- 12.Khezri M., Shokoohi M., Mirzazadeh A., Karamouzian M., Sharifi H., Haghdoost A., Baral S.D. Early sex work initiation and its association with condomless sex and sexually transmitted infections among female sex workers in Iran. Int. J. STD AIDS. 2020;31:671–679. doi: 10.1177/0956462420913431. [DOI] [PubMed] [Google Scholar]
- 13.Zoni A.C., González M.A., Sjögren H.W. Syphilis in the most at-risk populations in Latin America and the Caribbean: A systematic review. Int. J. Infect. Dis. 2013;17:e84–e92. doi: 10.1016/j.ijid.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Brazilian Ministry of Health—Department of Strategic Programmatic Actions. [(accessed on 29 April 2024)]; Available online: https://conselho.saude.gov.br/ultimas_noticias/2007/politica_mulher.pdf.
- 15.Monitoring and Follow-Up of the National Policy for Comprehensive Attention to Women’s Health (PNAISM) [(accessed on 29 April 2024)]; Available online: https://www.gov.br/mdh/pt-br/navegue-por-temas/politicas-para-mulheres/arquivo/central-de-conteudos/publicacoes/publicacoes/2015/pnaism_pnpm-versaoweb.pdf.
- 16.Oliveira-Filho A.B., Silva F.Q., Santos F.J.A., Cardoso Y.M.N., Di Miceli J.F.F., Resque R.L., Silva-Oliveira G.C., Martins L.C., Pinheiro L.M.L., Machado L.F.A., et al. Prevalence and risk factors for HIV-1 infection in people who use illicit drugs in northern Brazil. Trans. R. Soc. Trop. Med. Hyg. 2020;114:213–221. doi: 10.1093/trstmh/trz106. [DOI] [PubMed] [Google Scholar]
- 17.Brazilian Institute of Geography and Statistics (IBGE) [(accessed on 29 April 2024)]; Available online: https://cidades.ibge.gov.br/brasil/pa/panorama.
- 18.Brazilian Ministry of Health—Technical Manual for the Diagnosis of Syphilis [(accessed on 3 June 2024)]; Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/s/sifilis/publicacoes/manual-tecnico-para-o-diagnostico-da-sifilis.pdf.
- 19.Braga L.P., Szwarcwald C.L., Damacena G.N., de Souza-Júnior P.R.B., Dourado I., de Brito A.M., Grangeiro A., Guimarães M.D.C. Health vulnerabilities in female sex workers in Brazil, 2016. Medicine. 2022;101:e30185. doi: 10.1097/MD.0000000000030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva B.G.D., Ferreira L.H., Ribeiro C.E.L., Raboni S.M. HIV, syphilis, hepatitis B and C in key populations: Results of a 10-year cross-sectional study, Southern Brazil. Einstein. 2022;6:eAO6934. doi: 10.31744/einstein_journal/2022AO6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinho E.C.C., da Silva Galvão J.J., Ramos A.M.P.C., Aben-Athar C.Y.U.P., da Silva R.A.R., Cunha C.L.F., Botelho E.P., Ferreira G.R.O.N. Social and individual vulnerability factors associated with syphilis among populations living on islands in the Brazilian Amazon. BMC Infect. Dis. 2024;24:23. doi: 10.1186/s12879-023-08955-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardoso W.M., Motta-Castro A.R.C., Weis-Torres S.M.D.S., Bandeira L.M., Higa Júnior M.G., Puga M.A.M., Barbieri A.R., Fitts S.M.F. High prevalence of syphilis among recyclable waste collectors in Central Brazil. Rev. Soc. Bras. Med. Trop. 2024;12:e007022024. doi: 10.1590/0037-8682-0283-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Castro Rocha D.F.N., da Cunha Rosa L.R., de Almeida Silva C., de Oliveira B.R., Martins T.L.S., Martins R.M.B., de Matos M.A., Carneiro M.A.d.S., Soares J.P., Silva A.C.d.O.e., et al. Epidemiology of HIV, syphilis, and hepatitis B and C among manual cane cutters in low-income regions of Brazil. BMC Infect. Dis. 2018;18:546. doi: 10.1186/s12879-018-3439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung P., Osias E., Konda K.A., Calvo G.M., Reyes-Díaz E.M., Vargas S.K., Goldbeck C., Klausner J.D. High Lifetime Prevalence of Syphilis in Men Who Have Sex with Men and Transgender Women Versus Low Lifetime Prevalence in Female Sex Workers in Lima, Peru. Sex. Transm. Dis. 2020;47:549–555. doi: 10.1097/OLQ.0000000000001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pando M.L., Reynaga E., Coloccini R.S., Rodríguez Fermepín M., Kochel T., Montano S.M., Marone R., Avila M.M. Prevalence of HIV infection and Treponema pallidum in Argentine female sex workers. Rev. Panam. Salud Publica. 2011;30:303–308. [PubMed] [Google Scholar]
- 26.Mejia A., Bautista C.T., Leal L., Ayala C., Prieto F., de la Hoz F., Alzate M.L., Acosta J., Sanchez J.L. Syphilis infection among female sex workers in Colombia. J. Immigr. Minor. Health. 2009;11:92–98. doi: 10.1007/s10903-007-9081-7. [DOI] [PubMed] [Google Scholar]
- 27.Pando M.A., Berini C., Bibini M., Fernández M., Reinaga E., Maulen S., Marone R., Biglione M., Montano S.M., Bautista C.T., et al. Prevalence of HIV and other sexually transmitted infections among female commercial sex workers in Argentina. Am. J. Trop. Med. Hyg. 2006;74:233–238. doi: 10.4269/ajtmh.2006.74.233. [DOI] [PubMed] [Google Scholar]
- 28.Camejo M.I., Mata G., Díaz M. Prevalence of hepatitis B, hepatitis C and syphilis in female sex workers in Venezuela. Rev. Saude Publica. 2003;37:339–344. doi: 10.1590/S0034-89102003000300012. [DOI] [PubMed] [Google Scholar]
- 29.Tura J.B., Ayalew J., Moreda A.B., Lulseged S., Rameto M.A., Debel L.N., Bedassa B.B., Wariso F.B., Belihu W.B., Gutema E.A., et al. Prevalence of syphilis and associated factors among female sex workers in Ethiopia: Findings from a multilevel analysis of a national bio-behavioral survey. BMC Public Health. 2023;23:809. doi: 10.1186/s12889-023-15745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wondmagegn M., Wondimeneh Y., Getaneh A., Ayalew G. Seroprevalence of Hepatitis B Virus, Hepatitis C Virus, Syphilis and Associated Factors Among Female Sex Workers in Gondar Town, Northwest Ethiopia. Infect. Drug Resist. 2022;14:5915–5927. doi: 10.2147/IDR.S380952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi L., Luo J., Chen Y., Chen L., Hu H., Qiu T., Liu X., Xu X., Chen Y., Zhang Z., et al. Prevalence of syphilis and chlamydia trachomatis infection among female sex workers in Jiangsu, China: Results from a multicenter cross-sectional and venue-based study. Front. Public Health. 2022;31:1018724. doi: 10.3389/fpubh.2022.1018724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu L., Wu G., Lu R., Zhu H., Qiu H., Jing D., Ye M. Changing trends of HIV, syphilis, HCV infections and behavioural factors among female sex workers in Chongqing, China: Findings from six serial surveillance surveys. BMJ Open. 2020;10:e036654. doi: 10.1136/bmjopen-2019-036654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie T., Wang G., Sun Q. Prevalence and Predictors of Syphilis in Female Sex Workers in Eastern China: Findings from Six Consecutive Cross-Sectional Surveys. J. Multidiscip Healthc. 2021;16:853–860. doi: 10.2147/JMDH.S305492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mafakheri Bashmaq S., Ahmadi A., Mohsenpour B., Rahmani K., Arasteh M., Shams Alizadeh N., Babahajian A., Advay S., Abbaszadeh A. Prevalence of HIV, HBV, HCV, HPV and syphilis among female sex workers in Kurdistan, west of Iran. Caspian J. Intern. Med. 2024;15:38–45. doi: 10.22088/cjim.15.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekouevi D.K., Bitty-Anderson A.M., Gbeasor-Komlanvi F.A., Konu Y.R., Sewu E.K., Salou M., Dagnra C.A. Low prevalence of syphilis infection among key populations in Togo in 2017: A national cross-sectional survey. Arch Public Health. 2019;5:39. doi: 10.1186/s13690-019-0365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grosso A., Bowring A.L., Njindam I.M., Decker M.R., Lyons C., Rao A., Tamoufe U., Fako G.H., Fouda G., Levitt D., et al. Sexually Transmitted Infection Risks and Symptoms Heightened among Female Sex Workers who Started Selling Sex before the Age of 18 in Five Cities in Cameroon. AIDS Behav. 2024;28:898–906. doi: 10.1007/s10461-023-04196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chemaitelly H., Weiss H.A., Smolak A., Majed E., Abu-Raddad L.J. Epidemiology of Treponema pallidum, Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and herpes simplex virus type 2 among female sex workers in the Middle East and North Africa: Systematic review and meta-analytics. J. Glob. Health. 2019;9:020408. doi: 10.7189/jogh.09.020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hakim A.J., Bolo A., Werner M., Achut V., Katoro J., Caesar G., Lako R., Taban A.I., Wesson J., Okiria A.G. High HIV and syphilis prevalence among female sex workers in Juba, South Sudan. PLoS ONE. 2020;15:e0239543. doi: 10.1371/journal.pone.0239543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okiria A.G., Achut V., McKeever E., Bolo A., Katoro J., Arkangelo G.C., Ismail Michael A.T., Hakim A.J. High HIV and syphilis prevalence among female sex workers and sexually exploited adolescents in Nimule town at the border of South Sudan and Uganda. PLoS ONE. 2023;18:e0266795. doi: 10.1371/journal.pone.0266795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernier A., Rumyantseva T., Reques L., Volkova N., Kyburz Y., Maximov O., Derrienic E., Guschin A., Bouscaillou J., Luhmann N., et al. HIV and other sexually transmitted infections among female sex workers in Moscow (Russia): Prevalence and associated risk factors. Sex. Transm. Infect. 2020;96:601–607. doi: 10.1136/sextrans-2019-054299. [DOI] [PubMed] [Google Scholar]
- 41.Liang B., Zhang F., Ou Y., Zhang P., Bao L., Mo S., Nong A., Wei D., Wu Z., Xie H., et al. Prevalence, Trends and Correlates of HIV, Syphilis and HCV Infection Among Chinese Local and Cross-border Migrant Female Sex Workers in the Sino-Vietnam Border Area of Guangxi, 2016–2021. AIDS Behav. 2024;28:1257–1269. doi: 10.1007/s10461-023-04153-6. [DOI] [PubMed] [Google Scholar]
- 42.Bint Harun K.U.H., Kawser M., Nabi M.H., Mitra D.K. Factors associated with the malnutrition inflammation score (MIS) among hemodialysis patients in Dhaka city: A cross-sectional study in tertiary care hospitals. Porto Biomed. J. 2024;9:243. doi: 10.1097/j.pbj.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bossard C., Chihana M., Nicholas S., Mauambeta D., Weinstein D., Conan N., Nicco E., Suzi J., Oconnell L., Poulet E., et al. HIV, sexual violence, and termination of pregnancy among adolescent and adult female sex workers in Malawi: A respondent-driven sampling study. PLoS ONE. 2022;17:e0279692. doi: 10.1371/journal.pone.0279692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.da Costa L.M., Raiol N.C., Lisboa B.L.A., Frade P.C.R., Blandtt L.D.S., Silva-Oliveira G.C., Machado L.F.A., Martins L.C., Oliveira-Filho A.B. Prevalence and Risk Factors for Human Immunodeficiency Virus Infection Among Female Sex Workers: Distinct Offers of Sexual Services in a Municipality of the Brazilian Amazon. AIDS Res. Hum. Retroviruses. 2019;35:826–832. doi: 10.1089/aid.2019.0032. [DOI] [PubMed] [Google Scholar]
- 45.Frade P.C.R., Raiol N.C., da Costa L.M., Pinheiro L.M.L., Silva-Oliveira G.C., Pinho J.R.R., Lemos J.A.R., Martins L.C., Oliveira-Filho A.B. Factors associated with exposure to hepatitis B virus in female sex workers from the Marajo Archipelago, northern Brazil. Int. J. STD AIDS. 2019;30:1127–1128. doi: 10.1177/0956462419868641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.