Abstract
Chimpanzees have been important in studies of human immunodeficiency virus type 1 (HIV-1) pathogenesis and in evaluation of HIV-1 candidate vaccines. However, little information is available about HIV-1-specific cytotoxic T lymphocytes (CTL) in these animals. In the present study, in vitro stimulation of peripheral blood mononuclear cells (PBMC) from infected chimpanzees with HIV-1 Gag peptides was shown to be a sensitive, reproducible method of expanding HIV-1-specific CD8+ effector CTL. Of interest, PBMC from two chimpanzees had CTL activity against Gag epitopes also recognized by major histocompatibility complex class I-restricted CTL from HIV-1-infected humans. The use of peptide stimulation will help to clarify the role of CTL in vaccine-mediated protection and HIV-1 disease progression in this important animal model.
Because chimpanzees are the only nonhuman primates that are readily infectable with human immunodeficiency virus type 1 (HIV-1), these animals have been important for testing HIV-1 vaccine prototypes and for studying AIDS immunopathogenesis. This model for vaccine testing can be expected to grow in importance as it becomes possible to induce AIDS in chimpanzees with selected HIV-1 strains (15). In view of new compelling data implicating the chimpanzee as the natural primary host for HIV-1 (7), studies are likely to intensify to determine why chimpanzees infected with most HIV-1 strains do not develop AIDS, at least within the time periods they have been monitored. With an increasing appreciation for the importance of virus-specific CD8+ cytotoxic T lymphocytes (CTL) in controlling HIV-1 replication, it will be of major importance to monitor HIV-1-specific CTL in chimpanzees to optimize the use of this uniquely important animal model in vaccine and pathogenesis studies.
It has been suggested that CTL may actually contribute to CD4+ T-cell loss and the development of clinical immunodeficiency in HIV-1-infected humans through lysis of infected CD4+ T cells (11, 17). Because, to date, it has proven difficult to detect HIV-1-specific CTL in immunized or infected chimpanzees, some investigators have proposed that the absence of detectable CTL in HIV-1-infected chimpanzees may be related to their failure both to lose CD4+ T cells and to develop immunodeficiency (5). Clearly, it will be important to optimize conditions for detecting HIV-1-specific CTL in chimpanzees. The development of reliable assays would permit assessment of HIV-1-specific CTL in vaccinated chimpanzees and would also clarify the role CTL may play in HIV-1-induced disease.
Four chimpanzees chronically infected for various times were evaluated for the presence of HIV-1-specific CTL in peripheral blood (Table 1). When the present studies began, that is, when peripheral blood was obtained to generate autologous B-cell lines, chimpanzees C-420 and C-424 had been infected with a chimpanzee-passaged strain derived from HIV-1LAI(IIIB) for 15 and 11 months, respectively. Before infection via the cervical route, these two animals had been immunized multiple times with a recombinant canarypox virus vector expressing HIV-1 gp120/TM Env, Gag, and protease (8). The third chimpanzee, C-487, had been infected with the LAV-1b strain for almost 9 years (6), and the fourth animal, C-1196, had been infected with both the LAV-1b strain for 29 months and the subtype E strain 90CR402 for 20 months. During the course of the study, before the assays with the pooled peptides were performed (see below), chimpanzee C-1196 was infected with a third HIV-1 strain, JC499 (4, 16). Actual CTL assays with peripheral blood mononuclear cells (PBMC) from all chimpanzees were begun 2 to 6 months after B-lymphoblastoid cell lines (B-LCL) were generated. At the time these CTL assays were initiated, the level of virion RNA for C-1196 was about 800 copies/ml of plasma whereas that for C-487 was 1.3 × 104 copies/ml. CTL assays were performed over an 18-month period, during which time viral burdens for C-487 fluctuated between 2,900 and 7,300 copies/ml of plasma and those for C-1196 fluctuated between 560 and 4.5 × 105 copies/ml. The latter value for C-1196 was the peak level of plasma virion RNA that occurred 4 weeks after infection with the third HIV-1 strain. No CTL assays with PBMC from C-1196 were performed until 4 months after this peak in viral load occurred. Viral load data were not available for C-420 and C-424 during this study.
TABLE 1.
Infection history of chimpanzees
| Chimpanzee | HIV-1 strain | Routea | Duration of infection (mo)b |
|---|---|---|---|
| C-420c | C90/LAI(IIIB) | Cervical | 21 |
| C-424c | C90/LAI(IIIB) | Cervical | 17 |
| C-487 | LAI(LAV-1b) | Intravenous | 108 |
| C-1196 | LAI(LAV-1b) | Rectal | 31 |
| 90CR402 | Rectal | 22 | |
| JC499 | Intravenous | −2d |
Route of inoculation of HIV-1.
Months infected with indicated HIV-1 strain at the time of the first CTL assay.
C-420 and C-424 were immunized over a period of 22 months with a recombinant canarypox vector expressing HIV-1 gp120/TM, Env, Gag, and protease, beginning 44 months before the first CTL assay was performed.
C-1196 was inoculated with the JC499 strain 2 months after the first CTL assay was performed.
Although C-420 and C-424 were vaccinated prior to their infection with HIV-1, these vaccinations probably had little impact on their CTL responses following infection. On comparison of the vaccinated chimpanzees and unimmunized, control chimpanzees infected with the same HIV-1 strain, vaccination appeared to have no impact on viral load after C-420 and C-424 were infected. HIV-1 was first isolated from PBMC of these chimpanzees at the same time (4 to 6 weeks after challenge) as it was from the controls. Furthermore, during the first months after challenge, both the number of copies of virion RNA per milliliter of plasma and the frequencies of HIV-1 isolation from PBMC by coculture were comparable in these chimpanzees to those for control animals. Although viral burdens for these two animals were not determined during the time that the CTL assays were performed, 3 months after the last CTL assay, virus was isolated from PBMC from both chimpanzees and there were 2,260 and 195 RNA copies/ml of plasma from C-420 and C-424, respectively.
In initial attempts to detect HIV-1-specific CTL from the four HIV-1-infected chimpanzees, freshly isolated PBMC were assessed for the ability to lyse Epstein-Barr virus-transformed autologous B-LCL infected with a recombinant vaccinia virus–HIV-1 gag construct. By this method, no HIV-1 Gag-specific lytic activity was detected (data not shown).
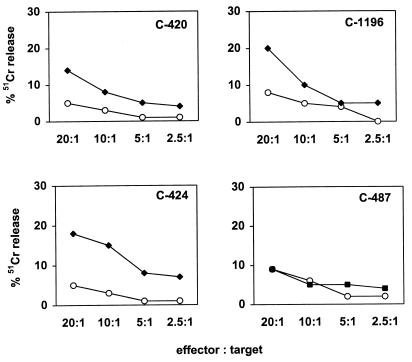
To increase the sensitivity of the assay for detecting HIV-1 Gag-specific CTL, PBMC from these chimpanzees were cultured in vitro with paraformaldehyde-fixed, autologous B-LCL infected with vaccinia virus–HIV-1 gag. After 12 days, these lymphocytes were then used as effector cells to assess their ability to lyse autologous B-LCL infected with vaccinia virus–HIV-1 gag, or, as a control, wild-type vaccinia virus. HIV-1 Gag-specific cytotoxic activity was detected in PBMC of three of the four chimpanzees (Fig. 1). By using this assay with PBMC obtained on five different occasions, CTL activity was detected twice in cells from C-424 and C-1196 and once in cells from C-420. No CTL activity was detected in PBMC from C-487 on these five attempts. The inconsistency in detecting lytic activity suggested that the frequency of Gag-specific effector cells might be at the lower level of detection of the assay being employed.
FIG. 1.
Recombinant vaccinia virus–HIV-1-gag-stimulated PBMC of some HIV-1-infected chimpanzees lyse autologous Gag-expressing B-LCL. PBMC (107) from chimpanzees C-420, C-1196, C-487, and C-424 were cultivated in vitro with paraformaldehyde-fixed, autologous B-LCL infected with vaccinia virus–HIV-1 gag. On day 3 of culture, 20 U of recombinant human interleukin-2 per ml was added to the cultures. On day 12 of culture, the lymphocytes were centrifuged over a Ficoll-diatrizoate gradient and assessed as effector cells in a 51Cr release cytotoxicity assay. Target cells were B-LCL (106) cultured overnight with vaccinia virus–HIV-1 gag (closed squares) or wild-type vaccinia virus (open circles) at a multiplicity of infection of 10 PFU/cell. B-LCL were then washed and labeled with 100 μCi of sodium 51chromate for 1.5 h. After being washed, 104 target cells per well were added to 96-well U-bottom plates in 100-μl volumes. Effector cells were added in another 100-μl volume at various concentrations to give effector/target ratios of 20:1, 10:1, 5:1, and 2.5:1. Plates were incubated at 37°C for 4 h. A 50-μl volume of supernatant was transferred to counting plates, 200 μl of scintillation fluid was added, and the mixture was analyzed in a 1450 microbeta liquid scintillation counter. Spontaneous release varied from 6 to 18%. Specific release was calculated as (experimental release − spontaneous release)/(100% release − spontaneous release) × 100.
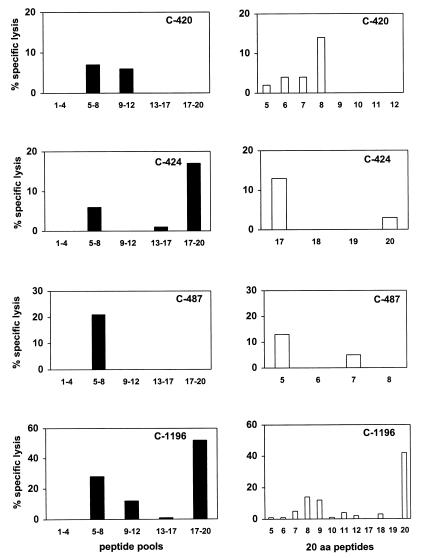
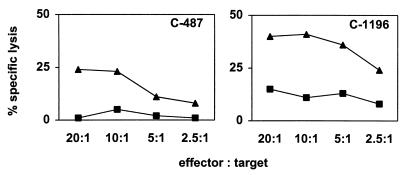
To optimize the sensitivity of the CTL assay, PBMC from the infected chimpanzees were assessed for Gag-specific lysis of autologous cells after in vitro stimulation with Gag peptides. 20-amino-acid peptides, overlapping by 10 amino acids and spanning residues 100 through 310 of HIV-1 Gag, were mixed to create five peptide pools, each containing four peptides. PBMC of the HIV-1-infected chimpanzees were stimulated in vitro for 12 days with each peptide pool and then assessed for cytotoxicity against autologous B-LCL pulsed with the same peptide pools. Using this assay, we detected CTL activity specific for at least one of the peptide pools (left panels of Fig. 2) in PBMC from each of the four chimpanzees. PBMC from chimpanzee C-1196 exhibited cytolytic activity specific for more than one of the peptide pools.
FIG. 2.
HIV-1 Gag peptide-specific cytolytic activity of PBMC from HIV-1-infected chimpanzees. Twenty-amino-acid peptides overlapping by 10 amino acids and spanning the HIV-1 IIIB Gag protein were mixed to make five peptide pools, each containing four peptides. Aliquots of 107 PBMC from the four chimpanzees were cultured with each of the five peptide pools at a concentration of 10 μg/ml for each peptide, resulting in a total concentration of total peptides of 50 μg/ml. On day 3 of culture, 20 U of recombinant human interleukin-2 per ml was added to the cultures. After 12 days of culture, lymphocytes were assessed as effector cells in a 51Cr release CTL assay at an effector/target ratio of 20:1. Epstein-Barr virus-transformed B-LCL were used as target cells. Aliquots of 5 × 105 B-LCL were incubated overnight with either peptide pools (black bars) or individual peptides (white bars) at a concentration of 10 μg/ml and 125 μCi of sodium 51chromate. These cells were then washed and added as targets to the effector cells in 96-well U-bottom plates in a final volume of 200 μl/well. The values shown for percent specific lysis represent the lysis of target cells incubated with the Gag peptide minus lysis of target cells incubated with the control ovalbumin peptide SIINFEKL.
The peptide-stimulated effector cells were then tested for recognition of the individual peptides comprising each pool. As shown in the right panels of Fig. 2, the effector cell activity was, in general, specific for only one of the peptides from each pool. In PBMC from chimpanzee C-1196, CTL activity specific for both peptides 8 and 9 was demonstrated. Since these peptides overlap by 10 amino acids, the effector activity may have been specific for residues common to both peptides. In some instances CTL responses that were specific for B-LCL pulsed with specific peptide pools were detected, but no specific lysis of the targets pulsed with individual peptides was demonstrable. This may simply reflect the low level of CTL activity present in the effector T-cell populations.
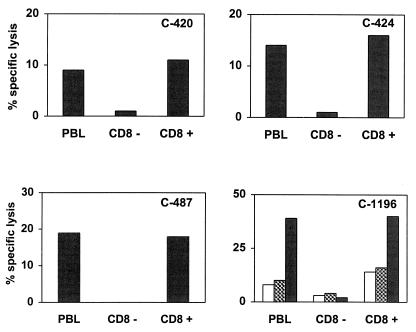
To determine the phenotype of the peptide-specific effector cells, peptide-stimulated PBMC from the four chimpanzees were separated into CD8+-lymphocyte-enriched and CD8+-lymphocyte-depleted populations by using CD8-specific immunomagnetic beads (Dynal Inc., Lake Success, N.Y.). These lymphocyte populations were then used as effector cells in CTL assays. Peptide-specific cytotoxicity was detected when either unfractionated PBMC or the CD8+-T-lymphocyte-enriched cells were used as effectors, but not when CD8+-T-lymphocyte-depleted cells were used (Fig. 3), indicating that the effector cells were CD8+ cells.
FIG. 3.
HIV-1 Gag peptide-specific cytotoxicity of chimpanzee PBMC is mediated by CD8+ T cells. PBMC from chimpanzees C-420, C-424, and C-487 were stimulated with peptides 8, 17, and 5, respectively. PBMC from chimpanzee C-1196 were stimulated with peptides 8 (white bars), 9 (hatched bars), and 20 (black bars). These effector cells were separated into CD8+-lymphocyte-depleted or -enriched populations by using CD8-specific immunomagnetic beads and then used in a 51Cr release cytotoxicity assay with peptide-pulsed autologous B-LCL targets at an effector/target ratio of 20:1. PBL, peripheral blood lymphocytes.
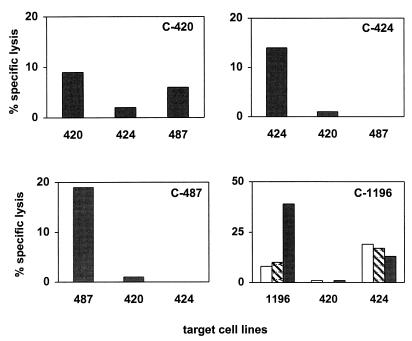
We then sought to confirm that the peptide-stimulated effector cells were T lymphocytes rather than NK cells. Since target cell recognition and lysis by CD8+ T lymphocytes, but not by NK cells, are major histocompatibility complex (MHC) class I restricted, genetic restriction of Gag peptide recognition by these effector lymphocytes was evaluated. Peptide-stimulated effectors from each chimpanzee were assessed for their ability to lyse peptide-pulsed autologous B-LCL as well as peptide-pulsed B-LCL from two other chimpanzees. Since chimpanzee C-1196 possessed effector cells specific for target cells pulsed with three different peptides, all three peptides were used in this assay. As shown in Fig. 4, effector cells from chimpanzees C-424 and C-487 lysed autologous but not allogeneic peptide-pulsed targets. In contrast, effector cells from C-420 and C-1196 lysed peptide-pulsed autologous target cells and one of the two allogeneic target cell populations. This result is consistent with the possibility that the chimpanzees shared one or more MHC class I alleles (3). It remains possible that this allogeneic target cell killing is mediated by NK effector cells. This is, however, unlikely because, in every instance in which allogeneic killing was seen, the effector cells mediated lysis of one but not a second peptide-pulsed allogeneic B-LCL. Therefore, the HIV-1-specific lytic activity detected in PBMC of the HIV-1-infected chimpanzees appeared to be mediated by MHC-restricted CD8+ T cells.
FIG. 4.
Chimpanzee PBMC-mediated HIV-1 Gag peptide-specific cytotoxicity is genetically restricted. PBMC from chimpanzees 420, 424, and 487 were stimulated with peptides 8, 17, and 5, respectively. Effector cells from each of the chimpanzees were assessed for cytotoxicity by using as targets peptide-pulsed autologous B-LCL as well as B-LCL generated from PBMC of two other chimpanzees. Similarly, PBMC from chimpanzee C-1196 were stimulated with peptides 8, 9, and 20 to generate the effector cells. Autologous and allogeneic B-LCL were pulsed with peptides 8 (white bars), 9 (hatched bars), and 20 (black bars) and used as target cells.
These findings suggested that Gag peptide stimulation may be more efficient than vaccinia virus–HIV-1 gag stimulation in expanding Gag peptide-specific effector cells in vitro. To compare directly the effector cells generated by these two methods, PBMC from two infected chimpanzees were stimulated with selected individual peptides or B-LCL infected with vaccinia virus–HIV-1 gag. The effector cells generated during in vitro stimulation were assessed for their ability to lyse autologous B-LCL infected with vaccinia virus–HIV-1 gag or wild-type vaccinia virus. Effector cells generated by peptide stimulation of PBMC from both of the chimpanzees lysed the HIV-1 Gag-expressing targets more efficiently than the effector cells generated by recombinant vaccinia virus stimulation (Fig. 5).
FIG. 5.
Effector cells generated by HIV-1 Gag peptide stimulation of PBMC are more effective than those generated by stimulation of PBMC with recombinant vaccinia virus–HIV-1 gag for lysing target cells infected with vaccinia virus–HIV-1 gag. Aliquots of PBMC from chimpanzees C-487 and C-1196 were stimulated with paraformaldehyde-fixed, autologous B-LCL infected with vaccinia virus–HIV-1 gag. Other aliquots of these PBMC were stimulated with the appropriate HIV-1 Gag peptide. On day 3 of culture, 20 U of recombinant human interleukin-2 per ml was added to the cultures. On day 12 of culture, the lymphocytes were assessed as effector cells in cytotoxicity assays using as target cells 51Cr-labeled autologous B-LCL infected with vaccinia virus–HIV-1 gag. Lysis of the vaccinia virus–HIV-1-gag-infected targets by recombinant vaccinia virus–HIV-1-gag-stimulated PBMC is represented by filled squares, whereas filled triangles show lysis by effectors generated by stimulation of PBMC with HIV-1 Gag peptides.
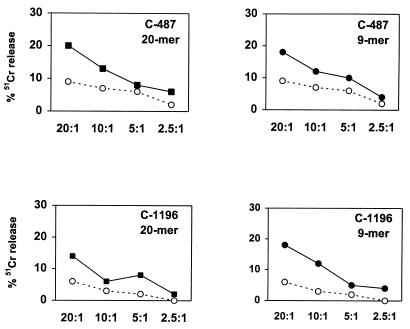
Because of the phylogenetic proximity of chimpanzees and humans, MHC class I alleles of these two species are highly conserved (12). Consistent with this observation, Bertoni et al. (2) and Balla-Jhagjhoorsingh et al. (1) recently reported that several hepatitis B virus and HIV epitopes defined in infected humans were also recognized by CTL found in virus-infected chimpanzees. Thus, we determined whether HIV-1 CTL epitopes are also shared between HIV-1-infected humans and chimpanzees. Several HIV-1 Gag-specific human CTL epitopes within the 20-amino-acid peptides that sensitized chimpanzee B-LCL for lysis have been described. Five such 9-amino-acid peptides were tested for their ability to sensitize chimpanzee B-LCL for CTL lysis. The amino acid sequence of an HLA-B27-restricted p24 peptide (KRWIILGLNK) (13) was located in the 20-amino-acid peptide 17 that was recognized by CTL of chimpanzee C-424 (Fig. 2). Peptides 110E (ISPRTLNAW) (10) and 110G (SPRTLNAWV), which are HLA-B57- and HLA-B7-restricted CTL epitopes, respectively, were within the 20-amino-acid peptide 5 recognized by CTL of chimpanzee C-487, and the HLA-B14-restricted peptides 113C (DLNTMLNTV) (14) and 125P (DRFYKTLRA) (9) were found in the 20-amino-acid peptides 9 and 20 recognized by CTL of chimpanzee C-1196. Therefore, PBMC from these three chimpanzees (C-424, C-487, and C-1196) were stimulated separately in vitro for 12 days with the appropriate 20-amino-acid peptides or the associated human CTL epitope peptides at a concentration of 10 μg/ml. These lymphocytes then were tested for lytic activity against autologous B-LCL pulsed with either the 20-amino-acid peptides, human CTL epitope peptides, or the control peptide. For the effector cells from chimpanzee C-424, no lysis of the target cells pulsed with the human CTL epitope peptide was observed (data not shown). Effector cells from chimpanzee C-487 were able to recognize the HLA-B7-restricted but not the HLA-B57-restricted human CTL epitope, and those from chimpanzee C-1196 were able to recognize one of the two HLA-B14-restricted human CTL epitopes, 113C (Fig. 6).
FIG. 6.
HIV-1 Gag CTL epitopes defined in HIV-1-infected humans are recognized by chimpanzee CTL. PBMC from chimpanzee C-487 were stimulated with 10 μg of the 20-amino-acid peptide 5 or the 9-amino-acid peptide 110G per ml. Similarly, PBMC from chimpanzee C-1196 were stimulated with the 20-amino-acid peptide 9 or the 9-amino-acid peptide 113C. On day 3 of culture, 20 U of recombinant human interleukin-2 per ml was added to the cultures. After 12 days of culture, lymphocytes were assessed as effector cells in a 51Cr release CTL assay at an effector/target ratio of 20:1. Aliquots of 5 × 105 autologous B-LCL were incubated overnight with either the 20-amino-acid peptide (filled squares) or the minimum-epitope 9-amino-acid peptide (filled circles) at a concentration of 10 μg/ml and 125 μCi of sodium 51chromate. Target cells incubated with the ovalbumin peptide SIINFEKL were used as controls (open circles).
These results demonstrated that HIV-1-specific CD8+ CTL are present in HIV-1-infected chimpanzees. In fact, some effector cells shared epitope-specific recognition with human HIV-1-specific CTL. Although the number of chimpanzees evaluated in this study was too small to allow firm quantitative conclusions to be made, the finding that optimal antigen-specific stimulation was needed to expand these cell populations to a detectable level suggests that the frequencies of HIV-1-specific CTL in chimpanzees may be lower than those commonly detected in HIV-1-infected humans. A low CTL frequency would be consistent with relatively low viral load in these animals. However, at least two of the chimpanzees in the present study consistently have had measurable plasma viremia. Chimpanzee C-487 has maintained virion RNA in plasma at a level of at least 3,000 copies/ml for several years, while C-1196 plasma RNA levels have fluctuated between 560 and 4.5 × 105 copies/ml.
The present observations make it impossible to argue that an absence of CTL in HIV-1-infected chimpanzees is responsible for the general lack of disease in these animals. Efforts are likely to intensify to clarify why neither naturally occurring chimpanzee isolates of HIV-1 nor the human strains of HIV-1 used to date are pathogenic in chimpanzees. Measuring virus-specific CTL in infected chimpanzees will become increasingly important in these studies.
Acknowledgments
We thank Bruce Walker, Fred Vogel, and the NIH AIDS Research and Reagent Program for providing the HIV-1 Gag peptides used in this study and Ali Javadian for coordination of blood collection from the chimpanzees.
This work was supported by NIH grants AI-85343 and AI-28147.
REFERENCES
- 1.Balla-Jhagjhoorsingh S S, Koopman G, Mooij P, Haaksma T G M, Teeuwsen V J P, Bontrop R E, Heeney J L. Conserved CTL epitopes shared between HIV-infected human long-term survivors and chimpanzees. J Immunol. 1999;162:2308–2314. [PubMed] [Google Scholar]
- 2.Bertoni R, Sette A, Sidney J, Guidotti L G, Shapiro M, Purcell R, Chisari F V. Human class I supertypes and CTL repertoires extend to chimpanzees. J Immunol. 1998;161:4447–4455. [PubMed] [Google Scholar]
- 3.Cooper S, Kowalski H, Erickson A L, Arnett K, Little A-M, Walker C M, Parham P. The presentation of a hepatitis C viral peptide by distinct major histocompatibility complex class I allotypes from two chimpanzee species. J Exp Med. 1996;183:663–668. doi: 10.1084/jem.183.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis I C, Girard M, Fultz P N. Loss of CD4+ T cells in human immunodeficiency virus type 1-infected chimpanzees is associated with increased lymphocyte apoptosis. J Virol. 1998;72:4623–4632. doi: 10.1128/jvi.72.6.4623-4632.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichberg J W, Zarling J M, Alter H J, Levy J A, Berman P W, Gregory T, Lasky L A, McClure J, Cobb K E, Moran P A, Hu S, Kennedy R C, Chanh T C, Dressman G R. T-cell responses to human immunodeficiency virus (HIV) and its recombinant antigens in HIV-infected chimpanzees. J Virol. 1987;61:3804–3808. doi: 10.1128/jvi.61.12.3804-3808.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fultz P N, Gluckman J-C, Muchmore E, Girard M. Transient increases in numbers of infectious cells in an HIV-infected chimpanzee following immune stimulation. AIDS Res Hum Retroviruses. 1992;8:313–317. doi: 10.1089/aid.1992.8.313. [DOI] [PubMed] [Google Scholar]
- 7.Gao F, Bailes E, Robertson D L, Chen Y, Rodenburg C M, Michael S F, Cummins L B, Arthur L O, Peeters M, Shaw G M, Sharp P M, Hahn B H. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 8.Girard, M., E. van der Ryst, F. Barre-Sinoussi, J. Tartaglia, J. Mahoney, P. Nara, J. Pillot, B. Meinier, R. C. Gallo, A. L. DeVico, and P. N. Fultz. Genital HIV-1 challenge of female chimpanzees immunized with a recombinant canarypox-HIV-1 virus. Unpublished data. [DOI] [PubMed]
- 9.Harrer T, Harrer E, Kalams S A, Barbosa P, Trocha A, Johnson R P, Elbeik T, Feinberg M B, Buchbinder S P, Walker B D. Cytotoxic T lymphocytes in asymptomatic longterm nonprogressing HIV-1 infection. Breadth and specificity of the response and relation to in vivo viral quasispecies in a person with prolonged infection and low viral load. J Immunol. 1996;156:2616–2623. [PubMed] [Google Scholar]
- 10.Johnson R P, Trocha A, Yang L, Mazzara G P, Panicali D L, Buchanan T M, Walker B D. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J Immunol. 1991;147:1512–1521. [PubMed] [Google Scholar]
- 11.Koup R A, Safrit J T, Cao Y, Andrews C A, Wu Y, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune response with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawlor D A, Ward F E, Ennis P D, Jackson A P, Parham P. HLA-A and B polymerase predate the divergence of humans and chimpanzees. Nature. 1988;335:268–271. doi: 10.1038/335268a0. [DOI] [PubMed] [Google Scholar]
- 13.Nixon D F, Townsend A R, Elvin J G, Rizza C R, Gallwey J, McMichael A J. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988;336:484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- 14.Nixon D F, McMichael A J. Cytotoxic T-cell recognition of HIV proteins and peptides. AIDS. 1988;5:1049–1059. [PubMed] [Google Scholar]
- 15.Novembre F, Saucier M, Anderson D C, Klumpp S A, O’Neil S P, Brown II C R, Hart C E, Guenthner P C, Swenson R B, McClure H M. Development of AIDS in a chimpanzee infected with human immunodeficiency virus type 1. J Virol. 1997;71:4086–4091. doi: 10.1128/jvi.71.5.4086-4091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei Q, Fultz P N. Extensive diversification of human immunodeficiency virus type 1 subtype B strains during dual infection of a chimpanzee that progressed to AIDS. J Virol. 1998;72:3005–3017. doi: 10.1128/jvi.72.4.3005-3017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarling J M, Ledbetter J A, Sias J, Fultz P, Eichberg J, Gjerset G, Moran P A. HIV-infected humans, but not chimpanzees, have circulating cytotoxic T lymphocytes that lyse uninfected CD4+ cells. J Immunol. 1990;144:2992–2998. [PubMed] [Google Scholar]