Abstract
We investigated the prognostic importance of noninvasive factors in predicting sperm retrieval failure in idiopathic nonobstructive azoospermia (iNOA). We studied 193 patients with nonobstructive azoospermia who underwent microsurgical testicular sperm extraction. The Chi-square test and Mann–Whitney U tests for clinical parameters and seminiferous tubule distribution were used for between-group comparisons. A logistic regression analysis was conducted to identify predictors of retrieval failure. Area under the receiver operating characteristic curve for each variable was evaluated, and the net clinical benefit was calculated using a clinical decision curve. Patients with iNOA had a lower sperm retrieval rate than those with known causes. Moreover, testicular volume was an independent factor affecting sperm extraction outcomes (odds ratio = 0.79, P < 0.05). The testicular volume cut-off value was 6.5 ml (area under the curve: 0.694). The patients with iNOA were categorized into two groups on the basis of the distribution of seminiferous tubules observed. The sperm retrieval rate and testicular volume were significantly different between the groups with a uniform or heterogeneous tubule distribution. There was also a significant association between a uniform tubule distribution and testicular volume. In conclusion, a testicular volume of more than 6.5 ml effectively predicts microsurgical testicular sperm extraction failure due to a uniform tubule distribution in patients with iNOA.
Keywords: idiopathic nonobstructive azoospermia, microsurgical testicular sperm extraction, sperm retrieval failure, testicular volume
INTRODUCTION
Azoospermia, which is the most severe manifestation of male infertility, accounts for approximately 10%–15% of all infertility cases.1 Approximately 60% of all azoospermia cases are nonobstructive. Azoospermia can present with a wide range of etiologies, including congenital factors (e.g., cryptorchidism, Klinefelter syndrome, and other chromosomal abnormalities), as well as acquired factors, such as testicular inflammation, trauma, and local radiotherapy.1,2 In addition, certain idiopathic factors contribute to the development of nonobstructive azoospermia (NOA). Currently, the most effective method for obtaining sperm from patients with NOA is microsurgical testicular sperm extraction (mTESE), which can subsequently be combined with mature intracytoplasmic sperm injection technology to facilitate pregnancy. This approach allows some patients with NOA to have children.3 However, patients with idiopathic NOA (iNOA) have a lower sperm retrieval rate (SRR) than those with a clear preoperative etiology.4,5,6
Although meticulous microscopic manipulation can enhance the SRR and minimize testicular damage,7,8 a postoperative decline in testosterone concentrations is unavoidable and carries a risk of erectile dysfunction.9 Therefore, before performing mTESE in patients with iNOA, a comprehensive evaluation is required to predict the success rate of sperm extraction and reduce unnecessary surgical trauma. This study aimed to investigate the predictive value of noninvasive preoperative examinations for failed sperm retrieval in iNOA patients.
PARTICIPANTS AND METHODS
Study population
A total of 193 NOA cases were collected between December 2016 and November 2023 from the Department of Andrology and Sexual Medicine of the First Affiliated Hospital of Fujian Medical University (Fuzhou, China). The inclusion criteria were as follows: (1) meeting the criteria for azoospermia set by the World Health Organization Laboratory Manual for the Examination and Processing of Human Semen10 (i.e., no sperm found in at least three semen samples before and after centrifugation [3000g, 15 min; Centrifuge 5425, Eppendorf, Hamburg, Germany]); (2) normal semen volume, pH value, and seminal plasma biochemical results; (3) no seminal duct obstruction identified on a physical examination, scrotal ultrasound, or transrectal ultrasound; and (4) complete clinical data, such as age, medical history, physical examinations, related laboratory tests, surgical data, and assisted reproduction data. The exclusion criteria were as follows: (1) an unmarried status; (2) obstructive azoospermia; (3) hypogonadotropic hypogonadism; or (4) severe female infertility factors, such as anovulation, bilateral tubal factors, hormonal and immune infertility, and ovarian failure.
Data collection followed the principles outlined in the Declaration of Helsinki. All patients agreed to provide anonymized information and tissue samples for the investigation by signing an informed consent form. The Medical Ethics Committee of the First Affiliated Hospital of Fujian Medical University reviewed and approved the study (Fujian Medical University Fuyi Ethical Medical Research, Approval No. [2020]375).
Observational index
The patients’ demographic and clinical parameters, such as age, medical history (history of cryptorchidism surgery, radiotherapy or chemotherapy, mumps, or orchitis), and testicular volume (TV) were collected. The TV was evaluated using a color Doppler ultrasound examination (GE LOGIQ Fortis; GE HealthCare, Chicago, IL, USA), which was performed by a color ultrasound technician (Dr. Yong Zhuang, Department of Ultrasound Medicine of the First Affiliated Hospital of Fujian Medical University) with more than 10 years of experience. The final TV was calculated as the product of three diameter lines (upper, lower, and anteroposterior) and then multiplied by 0.71. Clinical indicators included serum concentrations of sex hormones (follicle-stimulating hormone, luteinizing hormone, prolactin, testosterone, estradiol, and inhibin B) and semen analysis results. The serum concentrations of sex hormones were examined between 8:00 a.m. and 10:00 a.m. The patients were required to abstain from sex for 3–7 days before the semen examination.
Acquired NOA was defined as having a medical history of orchitis and mumps. iNOA was defined as the absence of chromosomal abnormalities, such as Klinefelter syndrome and Y-chromosome microdeletions, a history of testicular surgery, and testicular inflammation.
Surgical approaches
In all patients, mTESE was performed by the same team of andrologists. After successful anesthesia, the patient was placed in the supine position. The center of the perineum was routinely disinfected and covered with towels, and the procedure was initiated in the larger testicle. A 2.0-cm incision was made in the middle and upper scrotum. Moreover, the layers were successfully cut to reach the tunica vaginalis cavity, and the testis was extruded. The epididymis, vas deferens, and spermatic veins were examined for abnormalities, and the TV was measured and recorded. A transverse incision was made in the median testis under microscopic assistance (Zeiss S88; Carl Zeiss, Oberkochen, Germany). Testicular tissue was divided into upper and lower halves and separated along the testicular lobules. Suspected seminiferous tubules were harvested and cut into pieces under an inverted microscope (CKX31; Olympus, Tokyo, Japan). Our team of experienced andrologists and embryologists with more than 10 years of expertise conducted an extensive search for sperm. The testicular tissue was shredded and initially examined by an embryologist who used a microscope in the operating room. Subsequently, the samples were promptly transferred to an embryology laboratory on another floor of the same building for further detailed sperm retrieval by another senior embryologist. This embryologist reviewed the specimens again the next day to prevent oversight.
The timing of termination depended on the successful retrieval of suitable sperm, while further dissection may have compromised the blood supply to the testis. The tunica albuginea incision was closed with intermittent sutures using a 5–0 absorbable thread under microscopic guidance to close the thecal cavity. The testes were further included in the scrotum, and the incision was closed after confirming the absence of active bleeding. The decision to perform contralateral testicular surgery was made on the basis of the success of obtaining sufficient sperm and the patient’s condition and requirements. When necessary, a similar procedure was performed on the contralateral testicle. During the operation, a few testicular seminiferous tubules suspected to have sperm were collected, fixed with Bouin’s solution, and sent for a pathological examination and Johnson’s scoring.
Statistical analyses
SPSS software, version 26.0 (IBM Corp., Armonk, NY, USA) was used for statistical data processing. Groups were compared using the Chi-square test. Data are expressed as the mean ± standard deviation (s.d.) or median (interquartile range), and enumeration data are expressed as cases or percentages. The Shapiro–Wilk test was used to determine the normality of data distribution, and Levene’s test was used for variance uniformity. The Student’s t-test was used for comparisons with a normal distribution and homogeneity of variance. The Kolmogorov–Smirnov and Mann–Whitney U tests nonparametric rank-sum tests were used to compare nonnormally distributed measurement and count data. Univariate and multivariate logistic regression analyses were used to identify independent risk factors for sperm retrieval failure. In addition, a univariate logistic regression analysis was used to identify independent risk factors for seminiferous tubule distribution during mTESE. The predicted results were evaluated using the area under the receiver operating characteristic curve. Image rendering was performed using GraphPad Prism 9.5 (GraphPad Software Inc., San Diego, CA, USA). Decision curve analysis (DCA) was calculated using R version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria). Statistical tests were two-sided, and P < 0.05 was considered statistically significant.
RESULTS
A total of 193 patients with NOA who underwent mTESE were enrolled, and the overall SRR was 28.0% (54/193). The patients were categorized on the basis of the underlying pathogenesis, which included Klinefelter syndrome (29 patients), Y-chromosome microdeletions (11 patients), a history of surgical correction for cryptorchidism (30 patients), acquired NOA (10 patients), and iNOA (113 patients). The SRRs for these subgroups were 51.7% (15/29), 36.4% (4/11), 40.0% (12/30), 60.0% (6/10), and 15.0% (17/113), respectively. The SRR in patients with iNOA was significantly lower than that in patients without iNOA (15.0% vs 46.3% P < 0.001).
The iNOA subgroup (113 patients) was stratified according to sperm retrieval outcomes (Table 1). Sperm retrieval was successful in 17 (SRR: 15.0%) patients. These patients had a significantly lower TV than those in whom sperm retrieval failed (P < 0.05; Table 1). Supplementary Table 1 shows the results of logistic regression analyses for sperm retrieval failure at mTESE. A univariate analysis through binary logistic regression modeling showed a significant difference in successful sperm retrieval for TV (odds ratio [OR] = 0.79, 95% confidence interval [CI]: 0.66–0.95, P = 0.012). TV, follicle-stimulating hormone concentrations, and testosterone concentrations were analyzed using multivariate logistic regression. This analysis showed that TV remained negatively associated with successful sperm retrieval (OR = 0.78, 95% CI: 0.63–0.95, P = 0.016, r2 = 0.135; Supplementary Table 2). The area under the curve was calculated on the basis of TV to quantify the discrimination of mTESE outcomes. The area under the curve for TV was 0.694 (95% CI: 0.563–0.825; Figure 1). Using the receiver operating characteristic curve, the cut-off value for TV was 6.5 ml, with a sensitivity of 62.5%, specificity of 70.6%, and Youden index of 0.331.
Table 1.
Comparison of the clinical parameters for microsurgical testicular sperm extraction outcomes
| Characteristic | Success (n=17), median (IQR) | Failure (n=96), median (IQR) | P |
|---|---|---|---|
| Age (year) | 29.0 (26.5–32.0) | 29.50 (28.0–32.0) | 0.569 |
| Testis volume (ml) | 5.00 (3.50–8.93) | 8.00 (6.00–10.00) | 0.010 |
| PRL (mIU l−1) | 314.10 (256.78–553.05) | 296.15 (210.65–418.58) | 0.166 |
| FSH (IU l−1) | 21.65 (10.76–27.40) | 20.43 (12.28–26.26) | 0.766 |
| LH (IU l−1) | 9.84 (7.83–13.00) | 8.52 (6.03–12.32) | 0.096 |
| E2 (pmol l−1) | 85.95 (66.41–124.91) | 87.64 (62.40–118.22) | 0.942 |
| Testosterone (nmol l−1) | 11.91 (7.76–15.56) | 13.95 (10.13–20.27) | 0.182 |
| Inhibin B (pg ml−1) | 13.30 (11.55–30.35) | 16.70 (12.63–24.03) | 0.650 |
IQR: interquartile range; PRL: prolactin; FSH: follicle-stimulating hormone; LH: luteinizing hormone; E2: estradiol
Supplementary Table 1.
Predictors of sperm retrieval failure via univariate logistic regression
| Characteristic | OR (95% CI) | P |
|---|---|---|
| Age (year) | 0.96 (0.83–1.12) | 0.606 |
| Testis volume (ml) | 0.79 (0.66–0.95) | 0.012 |
| PRL (mIU l−1) | 1.00 (1.00–1.00) | 0.328 |
| FSH (IU l−1) | 1.00 (0.96–1.04) | 0.884 |
| LH (IU l−1) | 1.08 (1.00–1.17) | 0.061 |
| E2 (pmol l−1) | 1.00 (0.99–1.01) | 0.820 |
| Testosterone (nmol l−1) | 0.95 (0.88–1.03) | 0.194 |
| Inhibin B (pg ml−1) | 1.00 (0.97–1.02) | 0.856 |
OR: odds ratio; CI: confidence interval; PRL: prolactin; FSH: follicle-stimulating hormone; LH: luteinizing hormone; E2: estradiol
Supplementary Table 2.
Predictors of sperm retrieval failure via multivariate logistic regression
| Characteristic | OR (95% CI) | P |
|---|---|---|
| Testis volume (ml) | 0.78 (0.63–0.95) | 0.016 |
| FSH (IU l−1) | 0.97 (0.92–1.03) | 0.386 |
| Testosterone (nmol l−1) | 0.97 (0.89–1.05) | 0.432 |
OR: odds ratio; CI: confidence interval; FSH: follicle-stimulating hormone
Figure 1.
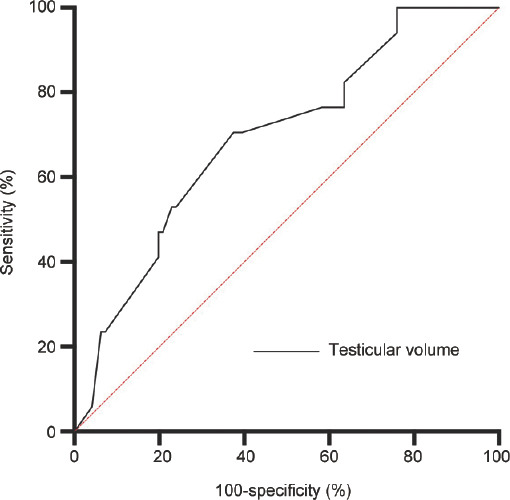
ROC curve analysis of testicular volume as a predictor of mTESE sperm retrieval outcomes in patients with iNOA. The diagonal area under the ROC curve (red line, line segment from 0 to 1) was 0.5. ROC: receiver operating characteristic; mTESE: microsurgical testicular sperm extraction; iNOA: idiopathic nonobstructive azoospermia.
Before mTESE sperm retrieval in patients with iNOA, TV was assessed, leading to more positive outcomes, as indicated by DCA findings. A positive net benefit of failed sperm retrieval was observed for TV values >6.5 ml. No net benefit was associated with sperm retrieval in the absence of a biomarker when the likelihood of retrieval was 15%. When the threshold value was <40%, the net benefit was improved by detecting TV before mTESE (Figure 2).
Figure 2.
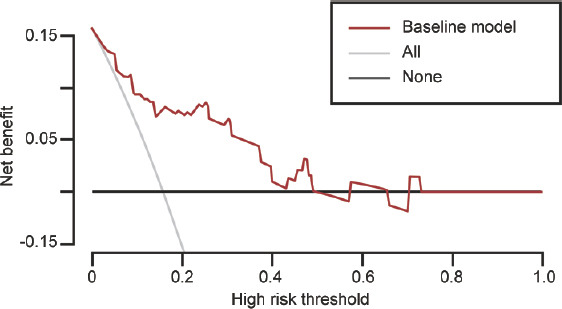
DCA of the model. The horizontal and vertical coordinates represent the threshold probability and net benefit, respectively. The graph contains three types of lines: TV (baseline model; red line), and the gray and black lines represent two extreme examples. All samples on the horizontal line are negative, none are intervened, and no benefits are gained. DCA: decision curve analysis; TV: testicular volume.
On the basis of the observed distribution of seminiferous tubules during mTESE, patients with iNOA were classified into two categories: those with a uniform distribution, indicating similar tubule diameters, and those with a heterogeneous distribution. In the second category, most tubules had similar diameters, although considerably enlarged diameters were occasionally observed. The SRR was significantly lower in patients with a uniform distribution than that in those with a heterogeneous distribution (3.2% vs 29.4%, P < 0.001). Patients with a heterogeneous distribution of seminiferous tubules had a significantly lower TV than those with a uniform distribution (P < 0.001; Table 2). Further univariate binary logistic regression analysis showed a significant difference in the uniform distribution of seminiferous tubules for TV (OR = 0.78, 95% CI: 0.65–0.85, P < 0.001; Supplementary Table 3). Of the 113 patients with iNOA, 48 had a TV <6.5 ml, and approximately 35.4% (17/48) showed a uniform distribution of seminiferous tubules. Of the remaining 65 patients with a TV >6.5 ml, a uniform distribution of seminiferous tubules was observed in 69.2% (45/65). A significant association was observed between a TV <6.5 ml and the proportion of patients with iNOA who had a uniform distribution of seminiferous tubules (P < 0.001). Figure 3 illustrates distinct variations in seminiferous tubule morphology. Specifically, Figure 3a exhibits well-developed seminiferous tubules in the smaller testis, while Figure 3b displays consistently thin seminiferous tubules in the larger testis. Notably, the testis affected by Klinefelter syndrome demonstrates a complete absence of sperm, and Figure 3d shows seminiferous tubules displaying due to azoospermia factor c region (AZFc) region deletion.
Table 2.
Comparison of the clinical parameters for the distribution of seminiferous tubules
| Characteristic | Uniform distribution (n=62), median (IQR) | Heterogeneous distribution (n=51), median (IQR) | P |
|---|---|---|---|
| Age (year) | 30.00 (28.00–32.25) | 29.00 (27.00–31.00) | 0.137 |
| Testis volume (ml) | 8.52 (6.00–12.00) | 6.00 (4.00–8.00) | <0.001 |
| PRL (mIU l−1) | 279.90 (212.30–412.07) | 322.40 (223.40–439.45) | 0.320 |
| FSH (IU l−1) | 20.49 (11.61–28.36) | 19.77 (12.22–25.49) | 0.917 |
| LH (IU l−1) | 8.33 (4.88–12.72) | 9.32 (7.35–12.36) | 0.095 |
| E2 (pmol l−1) | 79.22 (59.46–116.93) | 96.05 (66.33–124.70) | 0.222 |
| Testosterone (nmol l−1) | 13.36 (10.18–20.87) | 13.97 (8.72–18.27) | 0.539 |
| Inhibin B (pg ml−1) | 16.40 (12.10–24.40) | 16.50 (13.10–27.70) | 0.854 |
IQR: interquartile range; PRL: prolactin; FSH: follicle-stimulating hormone; LH: luteinizing hormone; E2: estradiol
Supplementary Table 3.
Predictors of a uniform distribution of seminiferous tubules via univariate logistic regression
| Characteristic | OR (95% CI) | P |
|---|---|---|
| Age (year) | 0.92 (0.82–1.02) | 0.116 |
| Testis volume (ml) | 0.78 (0.65–0.85) | <0.001 |
| PRL (mIU l−1) | 1.00 (1.00–1.00) | 0.390 |
| FSH (IU l−1) | 0.99 (0.96–1.02) | 0.521 |
| LH (IU l−1) | 1.05 (0.98–1.12) | 0.210 |
| E2 (pmol l−1) | 1.00 (1.00–1.01) | 0.461 |
| Testosterone (nmol l−1) | 0.97 (0.92–1.02) | 0.199 |
| Inhibin B (pg ml−1) | 1.00 (0.99–1.02) | 0.996 |
OR: odds ratio; CI: confidence interval; PRL: prolactin; FSH: follicle-stimulating hormone; LH: luteinizing hormone; E2: estradiol
Figure 3.
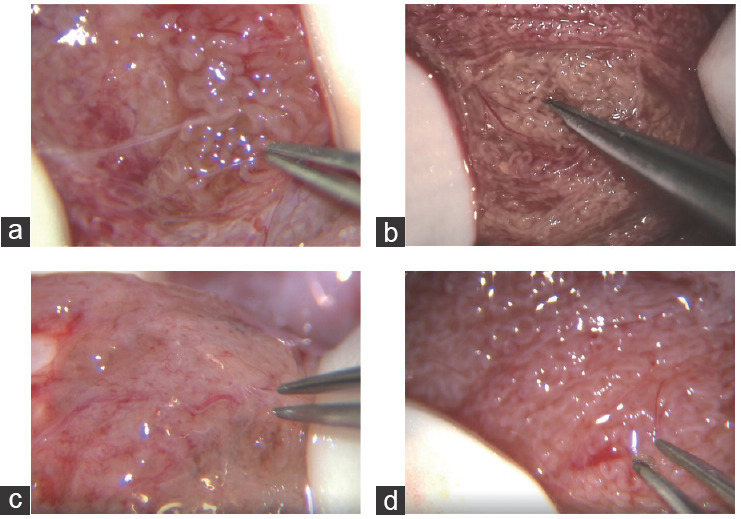
Different etiological types of seminiferous tubules. (a) Successful sperm retrieval in a patient with iNOA. The seminiferous tubules are atrophic and occasionally well-developed. (b) Failed sperm retrieval in a patient with iNOA. All seminiferous tubules are atrophic. (c) Failed sperm retrieval in a patient with Klinefelter syndrome. All seminiferous tubules are atrophic. (d) Sperm retrieval failure in a patient with AZFc region deletion. The seminiferous tubules are generally thin, and no sperm can be seen within them. iNOA: idiopathic nonobstructive azoospermia; AZFc: azoospermia factor c region.
DISCUSSION
This study showed a lower SRR in patients with iNOA than that in patients without iNOA. The primary objective of this study was to develop a predictive model of the value of noninvasive preoperative examinations for failed sperm retrieval in patients with iNOA before performing mTESE. By effectively predicting which patients are likely to experience a failed sperm extraction through a preoperative noninvasive examination, unnecessary mTESE procedures can be avoided, optimizing the cost–benefit ratio.
Previous studies have proposed using the serum inhibin B-to-anti-Müllerian hormone (AMH) ratio and serum AMH levels as potential predictors of successful sperm extraction in patients with iNOA6,11,12 AMH concentrations provide useful insight into the function of Sertoli cells. Sertoli cells in patients with iNOA who experience sperm retrieval failure may be less developed than those in patients with normal AMH levels. However, limited research has been conducted on the prediction of mTESE outcomes, specifically in patients with iNOA.
We successfully established a prediction model using logistic regression. We obtained the cut-off value using the receiver operating characteristic curve and evaluated the model using the DCA curve. Our study showed that surgical-side TV was an effective predictor of sperm retrieval failure.
Previously, Zhang et al.13 found that patients with iNOA who had a TV <5 ml were more likely to have successful sperm retrieval than those with a TV >5 ml. These authors suggested that this finding might have been due to the large TV associated with an unknown congenital genetic disorder or complete spermatogenic arrest. Moreover, Bryson et al.14 found that when the TV was <2 ml, obtaining sperm through mTESE remained possible in 56% of cases. Although a smaller TV indicates fewer Leydig cells and seminiferous tubules, we found that the seminiferous tubules in patients with iNOA who had a larger TV tended to be uniformly hyalinized during surgery.
Furthermore, our study showed that among preoperative noninvasive factors, only TV showed effective predictive capability for the distribution of seminiferous tubules. This finding suggests that patients with iNOA who have larger testes are more likely to show a uniform distribution of seminiferous tubules. In contrast, patients with occasionally enlarged seminiferous tubules present with greater difficulty in sperm retrieval. Consequently, obtaining sperm from small testes is easier for surgeons, whereas larger testes pose greater difficulties.15
Our study has some limitations, such as the limited sample size, single-center design, and lack of a control group and external validation. According to the DCA, the preoperative measurement of TV improved the net benefit of failed sperm retrieval. However, a large TV is not a reason to refuse mTESE inpatients with iNOA; it only predicts the difficulty of surgery and some possible outcomes. Multicenter, large-scale, prospective studies are required to confirm our conclusions.
The prediction of mTESE outcomes is gradually becoming more refined, and patients’ information should be obtained before surgery. Therefore, classifying patients with NOA according to the cause, including idiopathic causes, is necessary. There is a need to establish a reasonable clinical model for different causes, strengthen communication with patients and their family members, and achieve accurate diagnosis and treatment.
AUTHOR CONTRIBUTIONS
HX, SXT and HLZ contributed to the concept and design of the study. SXT, YLD, QC, PY, HLH, XC, and HLZ conducted the study. HX, SXT, RJY, and HLZ participated in data collection, analysis, and interpretation and made important subject matter revisions to the manuscript. HX and HLZ made major revisions to the manuscript. All authors were involved in revising the manuscript, and read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
We would like to thank Dr. Yong Zhuang, Department of Ultrasound Medicine of the First Affiliated Hospital of Fujian Medical University (Fuzhou, China), for his contribution to the color Doppler ultrasound examination in this study.
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Cocuzza M, Alvarenga C, Pagani R. The epidemiology and etiology of azoospermia. Clinics (Sao Paulo) 2013;68(Suppl 1):15–26. doi: 10.6061/clinics/2013(Sup01)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, et al. Male infertility. Lancet. 2021;397:319–33. doi: 10.1016/S0140-6736(20)32667-2. [DOI] [PubMed] [Google Scholar]
- 3.Silber SJ, van Steirteghem A, Nagy Z, Liu J, Tournaye H, et al. Normal pregnancies resulting from testicular sperm extraction and intracytoplasmic sperm injection for azoospermia due to maturation arrest. Fertil Steril. 1996;66:110–7. doi: 10.1016/s0015-0282(16)58396-4. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Ma Y, Zou S, Wang S, Qiu J, et al. Comparison and outcomes of nonobstructive azoospermia patients with different etiology undergoing microTESE and ICSI treatments. Transl Androl Urol. 2019;8:366–73. doi: 10.21037/tau.2019.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao S, Yang X, Xiao X, Yin S, Guan Y, et al. Outcomes and affecting factors for ICSI and microTESE treatments in nonobstructive azoospermia patients with different etiologies:a retrospective analysis. Front Endocrinol (Lausanne) 2022;13:1006208. doi: 10.3389/fendo.2022.1006208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng CY, Liu DF, Zhao LM, Lin HC, Mao JM, et al. Development of a predictive model for increasing sperm retrieval success by microdissection testicular sperm extraction in patients with nonobstructive azoospermia. Asian J Androl. 2023;25:598–603. doi: 10.4103/aja2022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen CF, Ohl DA, Fode M, Jørgensen N, Giwercman A, et al. Microdissection testicular sperm extraction versus multiple needle-pass percutaneous testicular sperm aspiration in men with nonobstructive azoospermia:a randomized clinical trial. Eur Urol. 2022;82:377–84. doi: 10.1016/j.eururo.2022.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Major N, Edwards KR, Simpson K, Rogers M. An examination of predictive markers for successful sperm extraction procedures:a linear model and systematic review. Asian J Androl. 2023;25:38–42. doi: 10.4103/aja202221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliveld J, van Wely M, Meißner A, Repping S, van der Veen F, et al. The risk of TESE-induced hypogonadism:a systematic review and meta-analysis. Hum Reprod Update. 2018;24:442–54. doi: 10.1093/humupd/dmy015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th edition. Switzerland: WHO Press; 2010. [Google Scholar]
- 11.Pozzi E, Raffo M, Negri F, Boeri L, Saccà A, et al. Anti-Müllerian hormone predicts positive sperm retrieval in men with idiopathic non-obstructive azoospermia-findings from a multi-centric cross-sectional study. Hum Reprod. 2023;38:1464–72. doi: 10.1093/humrep/dead125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu HY, Zhang HX, Xiao Z, Qiao J, Li R. Regulation of anti-Müllerian hormone (AMH) in males and the associations of serum AMH with the disorders of male fertility. Asian J Androl. 2019;21:109–14. doi: 10.4103/aja.aja_83_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Xi Q, Zhang X, Zhang H, Jiang Y, et al. Prediction of microdissection testicular sperm extraction outcome in men with idiopathic nonobstruction azoospermia. Med (Baltim) 2020;99:e19934. doi: 10.1097/MD.0000000000019934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryson CF, Ramasamy R, Sheehan M, Palermo GD, Rosenwaks Z, et al. Severe testicular atrophy does not affect the success of microdissection testicular sperm extraction. J Urol. 2014;191:175–8. doi: 10.1016/j.juro.2013.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouker A, Halouani L, Kharouf M, Latrous H, Makni M, et al. Step-by-step loupes-mTESE in non-obstructive azoospermic men, a retrospective study. Basic Clin Androl. 2019;29:11. doi: 10.1186/s12610-019-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


