Abstract
Existing research on the precise link between dietary niacin intake and erectile dysfunction (ED) is scarce. Thus, this study aimed to investigate the potential association between dietary niacin intake and the risk of ED. Multivariate logistic regression and restricted cubic splines (RCSs) were used to examine the relationship between dietary niacin intake and ED. Subgroup interaction analysis was performed to assess the impact of different subgroups on the study outcomes. In addition, 1:1 propensity score matching (PSM) was employed to adjust for potential confounding factors, ensuring the reliability of the results. The analyzed data were collected from the 2001–2004 National Health and Nutrition Examination Survey (NHANES) in the USA. The study encompassed 3184 adults, among whom 863 participants were identified as having ED. Following adjustments for potential confounders, the findings revealed that higher niacin intake, specifically in the highest tertile, was associated with a decreased risk of ED compared to that in the lowest tertile, showing an odds ratio (OR) of 0.56 (95% confidence interval [CI]: 0.37–0.85). Analysis of dose–response curves illustrated a negative correlation between dietary niacin intake and the risk of ED. Subgroup and interaction analyses fortified the consistency of these results. Furthermore, PSM corroborated the validity of the findings. This study suggests an inverse association between dietary niacin intake and the risk of ED. However, establishing a cause-and-effect relationship remains elusive, and defining the safe threshold of niacin intake to prevent ED requires further investigation.
Keywords: dietary niacin, erectile dysfunction, National Health and Nutrition Examination Survey, propensity score matching
INTRODUCTION
Erectile dysfunction (ED), a prevalent multifactorial disorder, is defined as the inability to achieve or maintain an erection sufficient for satisfactory sexual performance.1 An epidemiological survey highlights a roughly 19% prevalence of ED among adult males aged 20 years and older in the USA.2 In addition, the prevalence of ED tends to rise with age.3 The contributors to ED vary, stemming from psychological, neurological, hormonal, arterial, to cavernous damage.4 However, there is a general consensus that underlying vascular causes, particularly atherosclerosis, primarily lead to ED.5 Previous studies indicate ED as a potential risk indicator for cardiovascular disease (CVD),6 sharing vascular endothelial dysfunction as a common pathophysiological basis with CVD.5 Moreover, the likelihood of developing ED may increase with obesity, diabetes, hypertension, and hypercholesterolemia.7,8,9 Lifestyle adjustments can impact the risk of ED, with smoking and alcohol consumption showing positive associations, while regular, moderate physical activity might reduce the risk.4
Niacin, also known as nicotinic acid or Vitamin B3, serves as the precursor to nicotinamide adenine dinucleotide (NAD) and NAD phosphate (NADP), pivotal in energy metabolism and redox reactions.10 Studies indicate that niacin supplementation can regulate abnormal lipid metabolism, enhance vascular endothelial function, and demonstrate antioxidant and anti-inflammatory properties.11,12 Human bodies acquire niacin through both endogenous and exogenous pathways. Dietary niacin primarily originates from various meat products, animal organs, seafood, and diverse grains.13,14 Several studies have revealed that higher dietary niacin intake levels aid in reducing or delaying the risk of dyslipidemia.15 In a study on the correlation between dietary niacin intake and hypertension in Chinese adults, when dietary niacin intake fell below 15.6 mg per day, the risk of hypertension gradually declined with increasing niacin consumption. Conversely, when dietary niacin intake exceeded 15.6 mg per day, the results were contradictory.16
Severe niacin deficiency in humans results in pellagra, characterized by dermatitis, dementia, diarrhea, and potentially death.17 Contrastingly, excessive niacin intake can lead to skin flushing, gastrointestinal disorders, abnormal liver function, and insulin resistance.18,19,20 However, most adverse effects associated with excessive niacin intake stem from overconsumption of dietary niacin supplements or high doses of niacin medication. Adverse events associated with excessive dietary niacin intake are rarely reported. Notably, a recent cross-sectional study has highlighted that increased dietary niacin consumption elevates the risk of developing diabetes.21
Considering the aforementioned points, niacin demonstrates protective effects on the vascular endothelium and harbors antioxidant and anti-inflammatory properties. The pathogenesis of ED could potentially be linked to vascular endothelial dysfunction, inflammation, and oxidative stress. Hence, we hypothesize that dietary niacin might offer assistance in preventing and treating ED. However, there is a scarcity of studies investigating this aspect. As a result, this study delves into utilizing data from the National Health and Nutrition Examination Survey (NHANES) to investigate the potential relationship between dietary niacin intake and the risk of ED.
PARTICIPANTS AND METHODS
Study population
The NHANES, conducted by the Centers for Disease Control and Prevention (CDC), stands as an ongoing survey program, scrutinized and sanctioned by the National Health Statistics Research Council Center in the USA. Its primary objective is to assess the health and nutritional well-being of both adults and children across the USA. Employing a stratified multistage sampling technique, the study aims to comprehensively evaluate the health and nutritional status of the American population. Studies involving human participants are reviewed and approved by the National Center for Health Statistics (NCHS) Ethics Review Committee (Approval No. Protocol #98-12). Each participant involved in the survey provided written informed consent before participation.
Given that data on erectile function questionnaires were only available from 2001 to 2004, this study utilized data from the 2001 to 2004 NHANES, encompassing 4116 males aged ≥20 years who completed an erectile ability questionnaire. To ensure accuracy, several exclusion criteria were applied: (1) individuals lacking dietary niacin data (n = 241); (2) participants with a history of prostate cancer (n = 101); and (3) those with incomplete survey details on body mass index (BMI; n = 93), educational level (n = 1), smoking status or drinking status (n = 8), poverty-to-income ratio (PIR; n = 199), marital status (n = 2), recreational activities (n = 1), CVD status (n = 1), and hypertension status (n = 3). In addition, 282 participants with unreliable data on total daily energy intake, consuming <800 kcal or >4200 kcal per day, were excluded.22 Ultimately, the study included a total of 3184 participants. Figure 1 provides a participant flowchart for visual reference.
Figure 1.
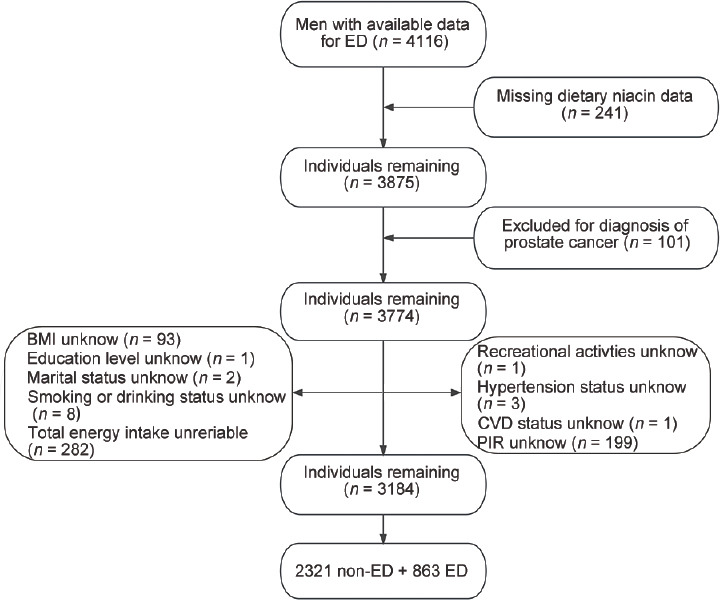
The selection process of the National Health and Nutrition Examination Survey (NHANES) 2001–2004. ED: erectile dysfunction; BMI: body mass index; PIR: poverty-to-income ratio; CVD: cardiovascular disease.
Exposure variable
In this study, dietary niacin intake emerged as the primary exposure variable. Trained diet interviewers utilized the NHANES computer-assisted diet interview (CADI) system to gather data on dietary intake. Information regarding individual levels of niacin intake in the diet was acquired from NHANES diet interview days 1 and 2 questionnaires, specifically designed to document the total dietary intake of the participants over two distinct 24-h periods. The initial day of the diet interview was conducted face to face, while the subsequent day was conducted via telephone 3–10 days later. Each mobile examination center (MEC) diet interview room adhered to standardized measurement guidelines, aiding respondents in reporting the quantity and size of food consumed. The United States Department of Agriculture (USDA)’s Food and Nutrient Database for Dietary Studies (FNDDS) supplied the nutritional value of all diet items, offering detailed nutritional profiles for each food reported in NHANES. Dietary niacin intake was calculated by averaging the results of 2 days of dietary interviews; if only the data from day 1 were available, that value was used. This study exclusively focused on niacin obtained from food sources and excluded supplements.
Outcome variable
ED was assessed using a direct and uncomplicated questionnaire developed by the Massachusetts Male Aging Study (MMAS),23 which asked, “how would you describe your ability to get and maintain an erection sufficient for satisfactory intercourse?” Respondents could choose from four options: “always or almost always”, “usually”, “sometimes”, or “never”. Research has shown that the outcomes of this direct ED questionnaire align with those of the International Index of Erectile Function (IIEF).23 In this questionnaire, individuals who reported being “sometimes able” or “never able” to maintain an erection were categorized as having ED, while those who reported being “usually able” or “always or almost always able” were classified as not having ED. Supplementary Figure 1 (320KB, tif) shows a flowchart outlining the selection criteria for ED.
Covariates
In addition to investigating the primary outcome variables, this study explored potential confounding factors that might influence the relationship between dietary niacin intake and ED. These factors included age, race (Caucasian, African American, or others), marital status (married/living with a partner or single/divorced/widowed), educational level (less than high school, high school or equivalent, or college or above), BMI (<30.00 kg m−2 or ≥30.00 kg m−2), PIR (≤1.30, 1.31–3.50, or >3.50), smoking status (never, former, or now), drinking status (never, former, or now), recreational physical activity (vigorous, moderate, or no activity), hypercholesterolemia (yes or no), CVD (yes or no), hypertension (yes or no), diabetes (yes or no), testosterone levels (low, normal, or unknown), and total daily energy intake.
Statistical analyses
To counterbalance the influence of NHANES’s complex multi-stage sampling design, we applied appropriate sample weights following NHANES guidelines and conducted weighted analyses to enhance data accuracy. Demographic characteristics are presented as weighted mean ± standard error (s.e.) for continuous variables and weighted percentages (%) for categorical variables. Subsequently, a Student’s t-test and a Chi-square test were employed to assess baseline features based on ED status. Weighted logistic regression models were used to determine the adjusted odds ratios (aORs) and their respective 95% confidence intervals (CI) concerning ED across niacin intake tertiles. We constructed four weighted logistic regression models: model 1 without variable adjustment; model 2 adjusting for age and race; model 3 adjusting for age, race, PIR, BMI, marital status, education, smoking status, drinking status, recreational activities, and total daily energy intake; and model 4 additionally adjusted for hypertension, diabetes, hypercholesterolemia, CVD, and testosterone levels. We employed weighted restricted cubic splines (RCSs) to elucidate the dose–response relationship between dietary niacin intake and the risk of ED. Subsequently, we stratified the participants by age, race, BMI, smoking status, drinking status, recreational activity, hypertension, diabetes, hypercholesterolemia, and CVD and conducted interaction analyses to explore potential differential associations among subgroups. In addition, sensitivity analyses were performed by excluding participants using medications potentially affecting erectile function, such as phosphodiesterase type 5 (PDE5) inhibitors,24 sex hormones,25 corticosteroids,25 antidepressants,26 and antipsychotics.27 Furthermore, we conducted 1:1 propensity score matching (PSM) to mitigate differences in the baseline characteristics among participants and re-analyzed the PSM data to validate the findings. Statistical analyses were conducted using R software (R4.2.3; http://www.R-project.org; The R Foundation, Vienna, Austria). A bilateral P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Table 1 shows the inclusion of 3184 participants in this study. The prevalence of ED was 27.1%, revealing significant differences in various demographic and health factors among groups. Significant differences in age, marital status, education level, BMI, PIR, smoking status, drinking status, recreational activities, hypercholesterolemia, CVD, hypertension, diabetes, testosterone level, and total energy intake were observed (all P < 0.05). Those participants with ED exhibited a higher average age and were predominantly Caucasian. The mean daily dietary niacin intake among participants was 26.8 (s.e.: 0.3) mg per day. Notably, the non-ED group displayed a significantly higher mean daily dietary niacin intake (27.6 [s.e.: 0.4] mg per day) compared to the ED group (23.4 [s.e.: 0.5] mg per day; P < 0.001).
Table 1.
Baseline characteristics of the study population before propensity score matching
| Characteristic | Total | ED | P | |
|---|---|---|---|---|
|
| ||||
| No | Yes | |||
| Patients, n (%) | 3184 (100.0) | 2321 (72.9) | 863 (27.1) | |
| Age (year), mean (s.e.) | 45.4 (0.4) | 41.6 (0.4) | 61.5 (0.6) | <0.0001 |
| Race, n (%) | 0.01 | |||
| Caucasian | 1750 (55.0) | 1227 (73.4) | 523 (79.7) | |
| African American | 569 (17.9) | 450 (10.4) | 119 (7.4) | |
| Others | 865 (27.2) | 644 (16.2) | 221 (12.9) | |
| Marital status, n (%) | <0.0001 | |||
| Married/living with a partner | 2253 (70.8) | 1598 (68.3) | 655 (78.5) | |
| Single/divorced/widowed | 931 (29.2) | 723 (31.7) | 208 (21.5) | |
| Education level, n (%) | <0.0001 | |||
| Less than high school | 849 (26.7) | 517 (13.2) | 332 (27.9) | |
| High school or equivalent | 786 (24.7) | 605 (26.2) | 181 (24.5) | |
| College or above | 1549 (48.7) | 1199 (60.7) | 350 (47.6) | |
| BMI (kg m−2), n (%) | <0.001 | |||
| <30.00 | 2278 (71.6) | 1691 (72.3) | 587 (62.7) | |
| ≥30.00 | 906 (28.5) | 630 (27.7) | 276 (37.3) | |
| PIR, n (%) | <0.001 | |||
| ≤1.30 | 732 (23.0) | 515 (16.6) | 217 (16.7) | |
| 1.31–3.50 | 1258 (39.5) | 869 (32.6) | 389 (43.7) | |
| >3.50 | 1194 (37.5) | 937 (50.8) | 257 (39.6) | |
| Smoking status, n (%) | <0.0001 | |||
| Never | 1286 (40.4) | 1027 (45.3) | 259 (29.8) | |
| Former | 1049 (33.0) | 616 (26.1) | 433 (48.1) | |
| Now | 849 (26.7) | 678 (28.6) | 171 (22.2) | |
| Drinking status, n (%) | <0.0001 | |||
| Never | 211 (6.6) | 152 (7.5) | 59 (7.2) | |
| Former | 648 (20.4) | 366 (13.3) | 282 (30.2) | |
| Now | 2325 (73.0) | 1803 (79.2) | 522 (62.7) | |
| Recreational activity, n (%) | <0.0001 | |||
| Vigorous | 1097 (34.5) | 949 (45.3) | 148 (18.2) | |
| Moderate | 895 (28.1) | 600 (26.9) | 295 (39.6) | |
| Inactivity | 1192 (37.4) | 772 (27.7) | 420 (42.2) | |
| Hypercholesterolemia, n (%) | <0.0001 | |||
| No | 1998 (62.8) | 1547 (66.1) | 451 (47.4) | |
| Yes | 1186 (37.3) | 774 (33.9) | 412 (52.6) | |
| CVD, n (%) | <0.0001 | |||
| No | 2761 (86.7) | 2161 (94.6) | 600 (71.2) | |
| Yes | 423 (13.3) | 160 (5.5) | 263 (28.8) | |
| Hypertension, n (%) | <0.0001 | |||
| No | 1909 (60.0) | 1588 (69.5) | 321 (41.3) | |
| Yes | 1275 (40.0) | 733 (30.5) | 542 (58.7) | |
| Diabetes, n (%) | <0.0001 | |||
| No | 2747 (86.3) | 2136 (94.0) | 611 (71.9) | |
| Yes | 437 (13.7) | 185 (6.0) | 252 (28.1) | |
| Testosterone, n (%) | 0.001 | |||
| Low | 80 (2.5) | 41 (1.6) | 39 (4.6) | |
| Normal | 430 (13.5) | 332 (12.8) | 98 (11.8) | |
| Unknown | 2674 (84.0) | 1948 (85.6) | 726 (83.7) | |
| Total energy (kcal), mean (s.e.) | 2395.2 (25.1) | 2460.7 (28.3) | 2116.8 (43.1) | <0.0001 |
| Dietary niacin (mg), mean (s.e.) | 26.79 (0.3) | 27.60 (0.4) | 23.40 (0.5) | <0.0001 |
BMI: body mass index; PIR: poverty-to-income ratio; CVD: cardiovascular disease; PSM: propensity score matching; s.e.: standard error; ED: erectile dysfunction
Associations between niacin intake and ED
The dose–response curve analysis of RCS demonstrated a decrease in the risk of ED with an increase in dietary niacin intake (P for overall < 0.001; P for nonlinearity = 0.453; Figure 2). Table 2 illustrates that weighted multivariate logistic regression analysis identified an inverse association between daily dietary niacin intake and the risk of ED. In model 1 (OR: 0.97; 95% CI: 0.96–0.98; P < 0.0001), model 2 (OR: 0.98; 95% CI: 0.97–0.99; P < 0.001), and model 3 (OR: 0.98; 95% CI: 0.97–0.99; P = 0.01), this association remained significant. Notably, in model 4, the significance persisted even after adjusting for all covariates (OR: 0.98; 95% CI: 0.97–0.99; P = 0.01). In addition, we transformed dietary niacin intake into a categorical variable (tertiles) for further analysis. In model 4, after adjusting for all potential covariates, participants in the highest tertile (T3) of daily dietary niacin intake exhibited a 56% lower risk of ED compared to those in the lowest tertile (T1; T3 vs T1, OR: 0.56; 95% CI: 0.37–0.85; P = 0.01, P for trend = 0.009).
Figure 2.
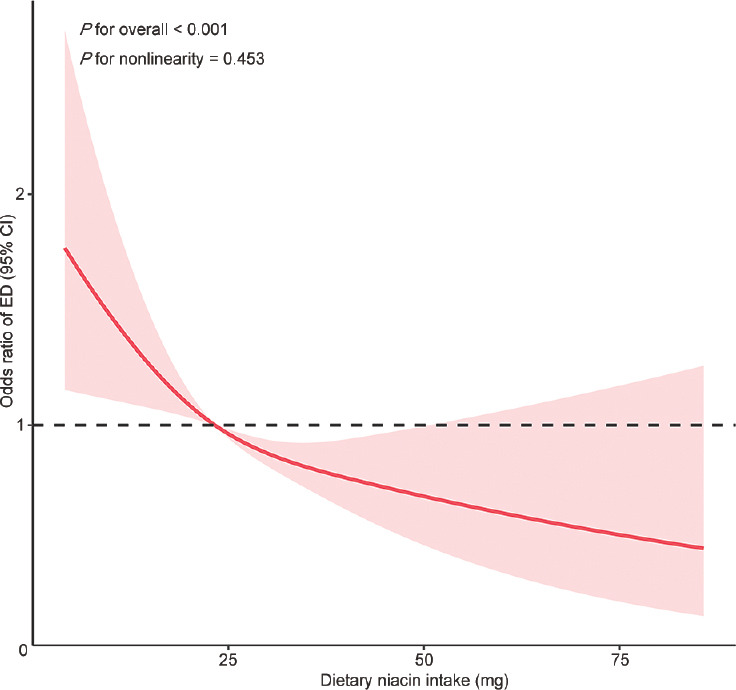
Dose–response relationship analysis between dietary niacin intake and erectile dysfunction before PSM. RCS regression was adjusted for age, race, marital status, education level, PIR, BMI categories, recreational activity, smoking status, drinking status, total daily energy intake, CVD, hypercholesterolemia, hypertension, diabetes, as well as testosterone levels (Model 4). The red solid line represents ORs, and red-shaded region represents 95% CI. PSM: propensity score matching; ED: erectile dysfunction; CI: confidence interval; RCS: restricted cubic spline; BMI: body mass index; PIR: poverty-to-income ratio; CVD: cardiovascular disease; OR: odds ratio.
Table 2.
Association of dietary niacin intake and erectile dysfunction risk before propensity score matching
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Dietary niacin | 0.97 (0.96–0.98) | <0.0001 | 0.98 (0.97–0.99) | <0.001 | 0.98 (0.97–0.99) | 0.01 | 0.98 (0.97–0.99) | 0.01 |
| Stratified by dietary niacin tertiles | ||||||||
| Tertile 1 | 1 | 1 | 1 | 1 | ||||
| Tertile 2 | 0.66 (0.49–0.88) | 0.01 | 0.67 (0.48–0.93) | 0.02 | 0.69 (0.50–0.96) | 0.03 | 0.67 (0.46–0.99) | 0.05 |
| Tertile 3 | 0.43 (0.32–0.58) | <0.0001 | 0.51 (0.37–0.71) | <0.001 | 0.58 (0.41–0.82) | 0.01 | 0.56 (0.37–0.85) | 0.01 |
| P for trend | <0.0001 | <0.001 | 0.004 | 0.009 | ||||
Model 1: unadjusted; Model 2: adjusted for age and race; Model 3: adjusted for age, race, marital status, education level, PIR, BMI categories, recreational activity, smoking status, drinking status, and total daily energy intake; Model 4: additionally adjusted for CVD, hypercholesterolemia, hypertension, diabetes, as well as testosterone levels. OR: odds ratio; CI: confidence interval; BMI: body mass index; PIR: poverty-to-income ratio; CVD: cardiovascular disease
Subgroup analyses
We conducted stratified analyses to evaluate the stability of the association between dietary niacin intake and ED across various subgroups (Figure 3). On adjusting for covariates, our findings indicated no significant differences in dietary niacin intake concerning ED within any subgroups (all P for interaction > 0.05). Specifically, the relationship between dietary niacin intake and the risk of ED remained consistent across subgroups based on age, race, BMI category, recreational activity, smoking status, drinking status, hypertension, diabetes, hypercholesterolemia, and CVD.
Figure 3.
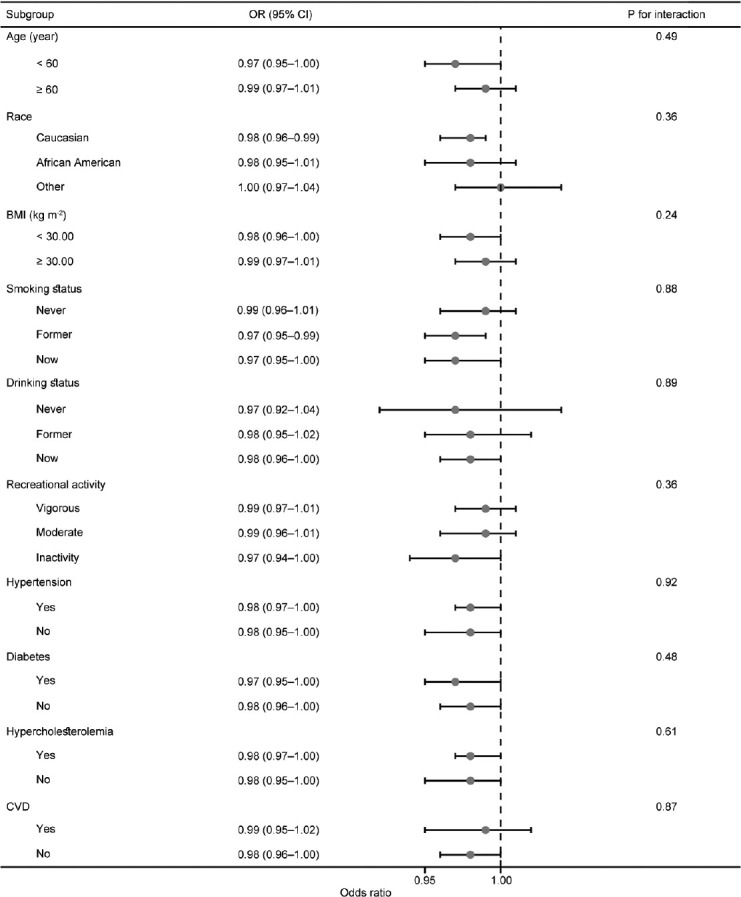
Association between dietary niacin intake and erectile dysfunction in different subgroups before PSM. Analyses were adjusted for age, race, marital status, education level, PIR, BMI categories, recreational activity, smoking status, drinking status, total daily energy intake, CVD, hypercholesterolemia, hypertension, diabetes, as well as testosterone levels. OR: odds ratio; CI: confidence interval; BMI: body mass index; CVD: cardiovascular disease; PSM: propensity score matching; PIR: poverty-to-income ratio.
Sensitivity analysis
In the sensitivity analysis shown in Table 3, the logistic regression outcomes remained consistent even after excluding medications that could potentially influence ED, such as PDE5 inhibitors, sex hormones, cortisol hormones, antidepressants, and antipsychotic medications. In model 4, following adjustment for all covariates, in comparison to the lowest tertile of dietary niacin intake, the OR for ED was 0.66 (95% CI: 0.44–0.98; P = 0.04) for the second tertile and 0.57 (95% CI: 0.37–0.89; P = 0.02) for the highest tertile (P for trend = 0.008).
Table 3.
Sensitivity analyses before propensity score matching
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Dietary niacin | 0.97 (0.96–0.98) | <0.0001 | 0.98 (0.96–0.99) | <0.001 | 0.98 (0.97–0.99) | 0.01 | 0.98 (0.97–0.99) | 0.02 |
| Stratified by dietary niacin tertiles | ||||||||
| Tertile 1 | 1 | 1 | 1 | 1 | ||||
| Tertile 2 | 0.63 (0.46–0.86) | 0.01 | 0.65 (0.46–0.93) | 0.02 | 0.68 (0.48–0.95) | 0.03 | 0.66 (0.44–0.98) | 0.04 |
| Tertile 3 | 0.43 (0.32–0.59) | <0.0001 | 0.53 (0.37–0.76) | 0.001 | 0.61 (0.42–0.87) | 0.01 | 0.57 (0.37–0.89) | 0.02 |
| P for trend | <0.0001 | <0.001 | 0.003 | 0.008 | ||||
Sensitivity analyses excluded participants taking medications that could affect erectile function. Model 1: unadjusted; Model 2: adjusted for age and race; Model 3: adjusted for age, race, marital status, education level, PIR, BMI categories, recreational activity, smoking status, drinking status, and total daily energy intake; Model 4: additionally adjusted for CVD, hypercholesterolemia, hypertension, diabetes, as well as testosterone levels. OR: odd ratio; CI: confidence interval; BMI: body mass index; PIR: poverty-to-income ratio; CVD: cardiovascular disease
PSM analysis
We implemented a 1:1 PSM approach to mitigate the impact of differing baseline characteristics among participants. Following PSM, both the ED and non-ED groups comprised 863 participants each. Supplementary Table 1 illustrates the post-PSM baseline characteristics of the study population. On re-examining the outcomes of PSM, the dose–response curve analysis of RCS reaffirmed the negative linear correlation between dietary niacin intake and ED (P for overall < 0.001; P for nonlinearity = 0.678; Supplementary Figure 2 (979.3KB, tif) ). In addition, the weighted multivariate logistic regression outcomes following PSM indicated a significant decrease in the risk of ED associated with higher dietary niacin intake (Supplementary Table 2). Subgroup analysis post-PSM revealed no influence of different subgroups on the study’s results (Supplementary Figure 3 (713.7KB, tif) ). Moreover, sensitivity analyses once again confirmed the stability of the findings (Supplementary Table 3).
Supplementary Table 1.
Baseline characteristics of the study population after propensity score matching
| Characteristic | Total | ED | P | |
|---|---|---|---|---|
|
| ||||
| No | Yes | |||
| Patients, n (%) | 1726 | 863 (50.00) | 863 (50.00) | |
| Age (year), mean (s.e.) | 58.24 (0.5) | 55.55 (0.5) | 61.50 (0.6) | <0.0001 |
| Race, n (%) | 0.24 | |||
| Caucasian | 997 (57.8) | 474 (76.0) | 523 (79.7) | |
| African American | 248 (14.4) | 129 (8.4) | 119 (7.4) | |
| Others | 481 (27.9) | 260 (15.6) | 221 (12.9) | |
| Marital status, n (%) | 0.8 | |||
| Married/living with a partner | 1312 (76.0) | 657 (77.8) | 655 (78.5) | |
| Single/divorced/widowed | 414 (24.0) | 206 (22.2) | 208 (21.5) | |
| Education level, n (%) | 0.06 | |||
| Less than high school | 609 (35.3) | 277 (20.3) | 332 (27.9) | |
| High school or equivalent | 391 (22.7) | 210 (27.3) | 181 (24.5) | |
| College or above | 726 (42.1) | 376 (52.4) | 350 (47.6) | |
| BMI (kg m−2), n (%) | 0.93 | |||
| <30.00 | 1160 (67.2) | 573 (63.1) | 587 (62.7) | |
| ≥30.00 | 566 (32.8) | 290 (37.0) | 276 (37.3) | |
| PIR, n (%) | 0.05 | |||
| ≤1.30 | 411 (23.8) | 194 (16.7) | 217 (16.7) | |
| 1.31–3.50 | 733 (42.5) | 344 (36.2) | 389 (43.7) | |
| >3.50 | 582 (33.7) | 325 (47.1) | 257 (39.6) | |
| Smoking status, n (%) | 0.1 | |||
| Never | 534 (30.9) | 275 (31.0) | 259 (29.8) | |
| Former | 781 (45.3) | 348 (41.1) | 433 (48.1) | |
| Now | 411 (23.8) | 240 (27.9) | 171 (22.2) | |
| Drinking status, n (%) | 0.18 | |||
| Never | 104 (6.0) | 45 (6.7) | 59 (7.2) | |
| Former | 509 (29.5) | 227 (24.1) | 282 (30.2) | |
| Now | 1113 (64.5) | 591 (69.2) | 522 (62.7) | |
| Recreational activity, n (%) | 0.4 | |||
| Vigorous | 318 (18.4) | 170 (22.1) | 148 (18.2) | |
| Moderate | 596 (34.5) | 301 (37.7) | 295 (39.6) | |
| Inactivity | 812 (47.1) | 392 (40.2) | 420 (42.2) | |
| Hypercholesterolemia, n (%) | 0.46 | |||
| No | 915 (53.0) | 464 (50.1) | 451 (47.4) | |
| Yes | 811 (47.0) | 399 (49.9) | 412 (52.6) | |
| CVD, n (%) | 0.002 | |||
| No | 1316 (76.3) | 716 (82.9) | 600 (71.2) | |
| Yes | 410 (23.8) | 147 (17.1) | 263 (28.8) | |
| Hypertension, n (%) | 0.01 | |||
| No | 747 (43.3) | 426 (48.6) | 321 (41.3) | |
| Yes | 979 (56.7) | 437 (51.4) | 542 (58.7) | |
| Diabetes, n (%) | 0.004 | |||
| No | 1304 (75.6) | 693 (81.0) | 611 (71.9) | |
| Yes | 422 (24.5) | 170 (19.0) | 252 (28.1) | |
| Testosterone, n (%) | 0.25 | |||
| Low | 70 (4.1) | 31 (3.4) | 39 (4.6) | |
| Normal | 224 (13.0) | 126 (14.3) | 98 (11.8) | |
| Unknown | 1432 (83.0) | 706 (82.4) | 726 (83.7) | |
| Total energy (kcal), mean (s.e.) | 2183.98 (27.8) | 2239.09 (40.5) | 2116.81 (43.1) | 0.06 |
| Dietary niacin (mg), mean (s.e.) | 24.53 (0.3) | 25.49 (0.4) | 23.37 (0.5) | 0.002 |
BMI: body mass index; PIR: poverty income ratio; CVD: cardiovascular disease; s.e.: standard error; ED: erectile dysfunction
Supplementary Table 2.
Association of dietary niacin intake and erectile dysfunction risk after propensity score matching
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Dietary niacin | 0.98 (0.97–0.99) | 0.003 | 0.98 (0.97–1.00) | 0.01 | 0.98 (0.97–1.00) | 0.01 | 0.98 (0.97–1.00) | 0.02 |
| Stratified by dietary niacin quartiles | ||||||||
| Tertile 1 | 1 | 1 | 1 | 1 | ||||
| Tertile 2 | 0.85 (0.58–1.24) | 0.38 | 0.81 (0.56–1.20) | 0.28 | 0.82 (0.55–1.23) | 0.31 | 0.82 (0.53–1.27) | 0.31 |
| Tertile 3 | 0.59 (0.44–0.81) | 0.002 | 0.61 (0.44–0.83) | 0.003 | 0.59 (0.41–0.84) | 0.01 | 0.58 (0.39–0.86) | 0.01 |
| P for trend | 0.002 | 0.004 | 0.02 | 0.012 | ||||
Model 1: unadjusted; Model 2: adjusted for age and race, marital status, education level, PIR, and BMI categories; Model 3: adjusted for age, race, marital status, education level, PIR, BMI categories, recreational activity, smoking, drinking status, and total daily energy intake; Model 4: additionally adjusted for CVD, hypercholesterolemia, hypertension, diabetes, as well as testosterone levels. OR: odd ratio; CI: confidence interval; BMI: body mass index; PIR: poverty income ratio; CVD: cardiovascular disease
Supplementary Table 3.
Sensitivity analyses after propensity score matching
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Dietary niacin | 0.98 (0.97–0.99) | 0.01 | 0.98 (0.97–1.00) | 0.02 | 0.99 (0.97–1.00) | 0.03 | 0.98 (0.97–1.00) | 0.02 |
| Stratified by dietary niacin quartiles | ||||||||
| Tertile 1 | 1 | 1 | 1 | 1 | ||||
| Tertile 2 | 0.86 (0.60–1.24) | 0.41 | 0.86 (0.60–1.23) | 0.39 | 0.90 (0.61–1.34) | 0.58 | 0.87 (0.57–1.33) | 0.45 |
| Tertile 3 | 0.59 (0.43–0.82) | 0.003 | 0.61 (0.43–0.86) | 0.003 | 0.66 (0.46–0.96) | 0.03 | 0.57 (0.37–0.89) | 0.02 |
| P for trend | 0.002 | 0.006 | 0.023 | 0.016 | ||||
Sensitivity analyses excluded participants taking medications that could affect erectile function. Model 1: unadjusted; Model 2: adjusted for age and race, marital status, education level, PIR, and BMI categories; Model 3: adjusted for age, race, marital status, education level, PIR, BMI categories, recreational activity, smoking, drinking status, and total daily energy intake; Model 4: additionally adjusted for CVD, hypercholesterolemia, hypertension, diabetes, as well as testosterone levels. OR: odd ratio; CI: confidence interval; BMI: body mass index; PIR: poverty income ratio; CVD: cardiovascular disease
DISCUSSION
This study is an exploration into the association between niacin intake and ED. With this nationally representative study, we found a strong association between dietary niacin intake and ED. Both univariate and multivariate logistic regression analyses underscored the link between higher niacin intake and a reduced risk of ED. When transitioning niacin intake from continuous to categorical, heightened dietary niacin significantly lowered the risk of ED compared to lower intake levels. In addition, a comprehensive dose–response analysis underscored a consistent, inverse linear relationship between dietary niacin and ED. Subgroup analyses further fortified the stability of this association across diverse subgroups. Moreover, the meticulous re-examination following 1:1 PSM reinforced the consistency and reliability of the study outcomes.
The pathophysiology of ED encompasses a multifaceted interplay of factors. A normal penile erection relies on the harmonious functioning of neural integrity, a robust vascular system, and healthy cavernous tissue. Conventionally, ED has been categorized into organic, psychogenic, and mixed types based on its etiology. However, this classification might present limitations due to the prevalent mixed nature of ED cases. Hence, as per the recommendations of the European Association of Urology (EAU) sexual and reproductive health guidelines, ED can be dichotomized into two categories: primary organic ED and primary psychogenic ED.28 In a European cross-sectional study involving 2009 patients with ED, a substantial 86.2% of patients with ED were classified under primary organic ED.29
Primary organic ED primarily stems from vascular, endocrine, or pharmaceutical causes, with vascular issues being the predominant factor.30 The erection process hinges on the synthesis of NO by endothelial NO synthase (eNOS).31 Within the smooth muscle of the cavernous body, NO activation of the guanosine cyclase elevates cyclic guanosine monophosphate (cGMP) levels, including smooth muscle hyperpolarization and relaxation by opening potassium channels and inhibiting calcium channels, culminating in penile erection.31 Hence, NO synthesized by the vascular endothelium serves as the chief mediator of erection.32 Nevertheless, dysfunction in vascular endothelium curtails NO production, thereby contributing to ED.33 Hormonal disorders notably impact vascular endothelial dysfunction, with testosterone playing a pivotal role.34 Testosterone exerts a catabolic effect on the expression and activity of hydrolases involved in cGMP degradation and upregulates PDE5, the principle enzyme responsible for cGMP degradation.35 These actions positively influence NO synthesis. Conversely, testosterone deficiency prompts increased production of endothelin-1, a potent vasoconstrictor, exacerbating cellular hypoxia and prompting apoptosis.36 Consequently, testosterone deficiency exacerbates vascular endothelial dysfunction and diminishes NO synthesis by the vascular endothelium, culminating in ED. Intriguingly, research indicates that NO inhibits Leydig cell conversion of cholesterol to pregnenolone, ultimately reducing testosterone production.37
Oxidative stress is believed to have a significant impact on ED. Prior studies have highlighted the role of oxidative stress-induced nitric oxide synthase (NOS)-dependent endothelial dysfunction in the initiation and progression of diabetic ED.38 High levels of reactive oxygen species (ROS) accompanying oxidative stress can interact with NO, forming peroxynitrite, subsequently reducing the available NO.39 Moreover, peroxynitrite and superoxide can elevate endothelial cell apoptosis rates,40 leading to endothelial damage and a further decline in available NO. Therefore, strategies aimed at repairing endothelial dysfunction and employing antioxidant therapy hold promise for preventing and treating ED.
Niacin, an essential nutrient derived from dietary sources, fulfills crucial bodily requirements. Nicotinic acid, a precursor of NAD (NADP), and reduced glutathione (GSH), play a pivotal role in cellular processes. GSH, an intracellular nonprotein mercaptan, is instrumental in preserving the intracellular redox balance and shielding cells against oxidative stress.41 Dietary niacin intake contributes to elevating GSH levels within the human body. A study by Wu et al.42 showed that niacin can mitigate vascular inflammation and enhance endothelial function by augmenting vascular GSH, a vital agent in clearing ROS generated by myeloperoxidase from neutrophils within the blood vessel walls. Previous research indicates that increased dietary niacin intake correlates with enhanced endothelial function and reduced vascular and systemic oxidative stress in middle-aged and older adults.43 In addition, findings from Ganji et al.44 suggest that niacin reduces vascular inflammation by inhibiting endothelial cell ROS production to reduce subsequent LDL oxidation and inflammatory cytokine production, thereby reducing the risk of atherosclerosis. Current preclinical evidence further reinforces the notion that niacin shields blood vessel cells from oxidative stress triggered by diverse stressors.45,46 Considering these findings, it is hypothesized that niacin may influence the risk of ED by improving vascular endothelial function and diminishing oxidative stress.
In this observational study, we evaluated the impact of dietary niacin intake on ED by examining dietary data from the study population. Through a series of statistical analyses, we found that a higher dietary niacin intake is associated with a reduced risk of ED. To further explore potential causality, we recommend that future studies employ a longitudinal study design combined with interventions for dietary niacin. In addition, conducting mechanistic studies would be beneficial in elucidating the biological pathways through which niacin might influence erectile function.
The study has several advantages and limitations. One key advantage lies in its extensive sample size, which allows for a representation of characteristics mirroring those of the national population. Concurrently, appropriate sampling weights were considered during the analysis to mitigate oversampling bias, bolstering the reliability of our conclusions. However, the study does have limitations. Primarily, being a nutritional and epidemiological cross-sectional study, it cannot establish a causal relationship between dietary niacin intake and the development of ED. Moreover, it lacks measurements of serum or blood levels of niacin in humans. Secondly, the ED diagnosis in NHANES relies predominantly on a questionnaire format, which not only diminishes accuracy but also constrains our ability to further categorize ED. Finally, the data on ED in NHANES are confined to the period from 2001 to 2004, curtailing our ability to ascertain the applicability of our findings to the current population.
CONCLUSIONS
In this observational, population-based study, there seems to be an inverse association between dietary niacin intake and the risk of ED. However, the cause-and-effect relationship remains unclear, and the safe threshold of niacin intake to prevent ED remains unknown.
AUTHOR CONTRIBUTIONS
WLL, WZ, and MEL designed the study; performed statistical analysis; interpreted the analysis, wrote, revised, and reviewed the manuscript; and supervised all processes. CZ and HXW wrote and revised the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
Selection criteria for erectile dysfunction.
Dose–response relationship analysis between dietary niacin intake and erectile dysfunction after PSM. RCS regression was adjusted for age, race, marital status, education level, PIR, BMI categories, recreational activity, smoking status, drinking status, total daily energy intake, CVD, hypercholesterolemia, hypertension, diabetes, as well as testosterone levels (Model 4). The red solid line represents ORs, and red-shaded region represents 95% CI. ED: erectile dysfunction; CI: confidence interval; CVD: cardiovascular disease; PSM: propensity score matching; RCS: restricted cubic spline; OR: odds ratio.
Association between dietary niacin intake and erectile dysfunction in different subgroups after PSM. Analyses were adjusted for age, race, marital status, education level, PIR, BMI categories, recreational activity, smoking, drinking status, total daily energy intake, CVD, hypercholesterolemia, hypertension, diabetes, as well as testosterone levels. OR: odds ratio; CI: confidence interval; BMI: body mass index; CVD: cardiovascular disease; PSM: propensity score matching.
ACKNOWLEDGMENTS
We sincerely appreciate the investigators who conducted the original NHANES study and the USA National Center for Health Statistics for sharing the data.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.NIH Consensus Conference. Impotence. NIH consensus development panel on impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 2.Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ Urologic diseases in America project. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med. 2006;166:207–12. doi: 10.1001/archinte.166.2.207. [DOI] [PubMed] [Google Scholar]
- 3.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, et al. Incidence of erectile dysfunction in men 40 to 69 years old:longitudinal results from the Massachusetts male aging study. J Urol. 2000;163:460–3. [PubMed] [Google Scholar]
- 4.Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802–13. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 5.Solomon H, Man JW, Jackson G. Erectile dysfunction and the cardiovascular patient:endothelial dysfunction is the common denominator. Heart. 2003;89:251–3. doi: 10.1136/heart.89.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banks E, Joshy G, Abhayaratna WP, Kritharides L, Macdonald PS, et al. Erectile dysfunction severity as a risk marker for cardiovascular disease hospitalisation and all-cause mortality:a prospective cohort study. PLoS Med. 2013;10:e1001372. doi: 10.1371/journal.pmed.1001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corona G, Rastrelli G, Filippi S, Vignozzi L, Mannucci E, et al. Erectile dysfunction and central obesity:an Italian perspective. Asian J Androl. 2014;16:581–91. doi: 10.4103/1008-682X.126386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponholzer A, Temml C, Mock K, Marszalek M, Obermayr R, et al. Prevalence and risk factors for erectile dysfunction in 2869 men using a validated questionnaire. Eur Urol. 2005;47:80–6. doi: 10.1016/j.eururo.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Roumeguère T, Wespes E, Carpentier Y, Hoffmann P, Schulman CC. Erectile dysfunction is associated with a high prevalence of hyperlipidemia and coronary heart disease risk. Eur Urol. 2003;44:355–9. doi: 10.1016/s0302-2838(03)00306-3. [DOI] [PubMed] [Google Scholar]
- 10.Kirkland JB, Meyer-Ficca ML. Niacin. Adv Food Nutr Res. 2018;83:83–149. doi: 10.1016/bs.afnr.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Jin FY, Kamanna VS, Kashyap ML. Niacin accelerates intracellular ApoB degradation by inhibiting triacylglycerol synthesis in human hepatoblastoma (HepG2) cells. Arterioscler Thromb Vasc Biol. 1999;19:1051–9. doi: 10.1161/01.atv.19.4.1051. [DOI] [PubMed] [Google Scholar]
- 12.Meyers CD, Kamanna VS, Kashyap ML. Niacin therapy in atherosclerosis. Curr Opin Lipidol. 2004;15:659. doi: 10.1097/00041433-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Gasperi V, Sibilano M, Savini I, Catani MV. Niacin in the central nervous system:an update of biological aspects and clinical applications. Int J Mol Sci. 2019;20:974. doi: 10.3390/ijms20040974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laskowski W, Górska-Warsewicz H, Kulykovets O. Meat, meat products and seafood as sources of energy and nutrients in the average polish diet. Nutrients. 2018;10:1412. doi: 10.3390/nu10101412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim C, Park K. Dietary niacin intake and risk of dyslipidemia:a pooled analysis of three prospective cohort studies. Clin Nutr. 2022;41:2749–58. doi: 10.1016/j.clnu.2022.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Liu M, Zhou C, He P, Zhang Y, et al. Evaluation of dietary niacin and new-onset hypertension among Chinese adults. JAMA Netw Open. 2021;4:e2031669. doi: 10.1001/jamanetworkopen.2020.31669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegyi J, Schwartz RA, Hegyi V. Pellagra:dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1–5. doi: 10.1111/j.1365-4632.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- 18.McKenney JM, Proctor JD, Harris S, Chinchili VM. A comparison of the efficacy and toxic effects of sustained- versus immediate-release niacin in hypercholesterolemic patients. JAMA. 1994;271:672–7. [PubMed] [Google Scholar]
- 19.Minto C, Vecchio MG, Lamprecht M, Gregori D. Definition of a tolerable upper intake level of niacin:a systematic review and meta-analysis of the dose-dependent effects of nicotinamide and nicotinic acid supplementation. Nutr Rev. 2017;75:471–90. doi: 10.1093/nutrit/nux011. [DOI] [PubMed] [Google Scholar]
- 20.Montastier E, Beuzelin D, Martins F, Mir L, Marqués MA, et al. Niacin induces miR-502-3p expression which impairs insulin sensitivity in human adipocytes. Int J Obes. 2019;43:1485–90. doi: 10.1038/s41366-018-0260-5. [DOI] [PubMed] [Google Scholar]
- 21.Ke P, Jiang H, Dowling R, Zhong L, Ke L, et al. Relationship between dietary niacin intake and diabetes mellitus in the National Health and Nutrition Examination Survey (NHANES) 2003-2018. Eat Weight Disord. 2022;27:2425–34. doi: 10.1007/s40519-021-01347-6. [DOI] [PubMed] [Google Scholar]
- 22.Banna JC, McCrory MA, Fialkowski MK, Boushey C. Examining plausibility of self-reported energy intake data:considerations for method selection. Front Nutr. 2017;4:45. doi: 10.3389/fnut.2017.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derby CA, Araujo AB, Johannes CB, Feldman HA, McKinlay JB. Measurement of erectile dysfunction in population-based studies:the use of a single question self-assessment in the massachusetts male aging study. Int J Impot Res. 2000;12:197–204. doi: 10.1038/sj.ijir.3900542. [DOI] [PubMed] [Google Scholar]
- 24.Mitidieri E, Cirino G, d’Emmanuele di Villa Bianca R, Sorrentino R. Pharmacology and perspectives in erectile dysfunction in man. Pharmacol Ther. 2020;208:107493. doi: 10.1016/j.pharmthera.2020.107493. [DOI] [PubMed] [Google Scholar]
- 25.Nieschlag E, Vorona E. Mechanisms in endocrinology:medical consequences of doping with anabolic androgenic steroids:effects on reproductive functions. Eur J Endocrinol. 2015;173:R47–58. doi: 10.1530/EJE-15-0080. [DOI] [PubMed] [Google Scholar]
- 26.Montejo AL, Prieto N, de Alarcón R, Casado-Espada N, de la Iglesia J, et al. Management strategies for antidepressant-related sexual dysfunction:a clinical approach. J Clin Med. 2019;8:1640. doi: 10.3390/jcm8101640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumontaud M, Korchia T, Khouani J, Lancon C, Auquier P, et al. Sexual dysfunctions in schizophrenia:beyond antipsychotics. A systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2020;98:109804. doi: 10.1016/j.pnpbp.2019.109804. [DOI] [PubMed] [Google Scholar]
- 28.Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, et al. European Association of Urology guidelines on sexual and reproductive health-2021 update:male sexual dysfunction. Eur Urol. 2021;80:333–57. doi: 10.1016/j.eururo.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Pozzi E, Fallara G, Capogrosso P, Boeri L, Belladelli F, et al. Primary organic versus primary psychogenic erectile dysfunction:findings from a real-life cross-sectional study. Andrology. 2022;10:1302–9. doi: 10.1111/andr.13212. [DOI] [PubMed] [Google Scholar]
- 30.Diehm N, Borm AK, Keo HH, Wyler S. Interdisciplinary options for diagnosis and treatment of organic erectile dysfunction. Swiss Med Wkly. 2015;145:w14268. doi: 10.4414/smw.2015.14268. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal A, Nandipati KC, Sharma RK, Zippe CD, Raina R. Role of oxidative stress in the pathophysiological mechanism of erectile dysfunction. J Androl. 2006;27:335–47. doi: 10.2164/jandrol.05136. [DOI] [PubMed] [Google Scholar]
- 32.Burnett AL. Nitric oxide in the penis:physiology and pathology. J Urol. 1997;157:320–4. [PubMed] [Google Scholar]
- 33.Andersson KE. Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol. 2003;170:S6–14. doi: 10.1097/01.ju.0000075362.08363.a4. [DOI] [PubMed] [Google Scholar]
- 34.Kałka D, Biernikiewicz M, Gebala J, Sobieszczańska M, Jakima S, et al. Diagnosis of hypogonadism in patients treated with low energy shock wave therapy for erectile dysfunction:a narrative review. Transl Androl Urol. 2020;9:2786–96. doi: 10.21037/tau-20-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang XH, Morelli A, Luconi M, Vignozzi L, Filippi S, et al. Testosterone regulates PDE5 expression and in vivo responsiveness to tadalafil in rat corpus cavernosum. Eur Urol. 2005;47:409–16. doi: 10.1016/j.eururo.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Moreland RB. Pathophysiology of erectile dysfunction:the contributions of trabecular structure to function and the role of functional antagonism. Int J Impot Res. 2000;12(Suppl 4):S39–46. doi: 10.1038/sj.ijir.3900576. [DOI] [PubMed] [Google Scholar]
- 37.Del Punta K, Charreau EH, Pignataro OP. Nitric oxide inhibits leydig cell steroidogenesis. Endocrinology. 1996;137:5337–43. doi: 10.1210/endo.137.12.8940355. [DOI] [PubMed] [Google Scholar]
- 38.Tuncayengin A, Biri H, Onaran M, Sen I, Tuncayengin O, et al. Cavernosal tissue nitrite, nitrate, malondialdehyde and glutathione levels in diabetic and non-diabetic erectile dysfunction. Int J Androl. 2003;26:250–4. doi: 10.1046/j.1365-2605.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- 39.Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992;5:834–42. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 40.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite:the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 41.Forman HJ, Zhang H, Rinna A. Glutathione:overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu BJ, Yan L, Charlton F, Witting P, Barter PJ, et al. Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids. Arterioscler Thromb Vasc Biol. 2010;30:968–75. doi: 10.1161/ATVBAHA.109.201129. [DOI] [PubMed] [Google Scholar]
- 43.Kaplon RE, Gano LB, Seals DR. Vascular endothelial function and oxidative stress are related to dietary niacin intake among healthy middle-aged and older adults. J Appl Physiol. 2014;116:156–63. doi: 10.1152/japplphysiol.00969.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganji SH, Qin S, Zhang L, Kamanna VS, Kashyap ML. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis. 2009;202:68–75. doi: 10.1016/j.atherosclerosis.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 45.Huang PH, Lin CP, Wang CH, Chiang CH, Tsai HY, et al. Niacin improves ischemia-induced neovascularization in diabetic mice by enhancement of endothelial progenitor cell functions independent of changes in plasma lipids. Angiogenesis. 2012;15:377–89. doi: 10.1007/s10456-012-9267-z. [DOI] [PubMed] [Google Scholar]
- 46.Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101:20B–6B. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Selection criteria for erectile dysfunction.
Dose–response relationship analysis between dietary niacin intake and erectile dysfunction after PSM. RCS regression was adjusted for age, race, marital status, education level, PIR, BMI categories, recreational activity, smoking status, drinking status, total daily energy intake, CVD, hypercholesterolemia, hypertension, diabetes, as well as testosterone levels (Model 4). The red solid line represents ORs, and red-shaded region represents 95% CI. ED: erectile dysfunction; CI: confidence interval; CVD: cardiovascular disease; PSM: propensity score matching; RCS: restricted cubic spline; OR: odds ratio.
Association between dietary niacin intake and erectile dysfunction in different subgroups after PSM. Analyses were adjusted for age, race, marital status, education level, PIR, BMI categories, recreational activity, smoking, drinking status, total daily energy intake, CVD, hypercholesterolemia, hypertension, diabetes, as well as testosterone levels. OR: odds ratio; CI: confidence interval; BMI: body mass index; CVD: cardiovascular disease; PSM: propensity score matching.


