Abstract
Background
This article aims to compare clinical efficacy of newly introduced PowerScope 2 appliance with Forsus FRD in the treatment of Skeletal Class II malocclusion.
Methods
This randomized controlled trial studied 40 patients at two centers (20 patients at each center, divided into two groups of 10 patients each as Group 1: Forsus FRD and Group 2: PowerScope 2 appliance. The skeletal, dental, soft tissue, and airway changes were noted at T0 (pretreatment), T1 (preappliance therapy), and T2 (postappliance therapy). Acoustic Pharyngometry (Eccovision® Acoustic Pharyngometer) was done to ascertain changes in mean airway volume and area with both modalities noninvasively. Treatment duration and chairside time in appliance installation and removal were noted. Patient comfort was compared using a customized questionnaire survey.
Results
A significant change was observed in skeletal, dental, soft tissue, and airway parameters after treatment with both modalities (p-value <0.05 for all) with no significant intergroup difference (p-value >0.05). Chairside time spent in appliance insertion was significantly lower with PowerScope 2 appliance (10.33 min) as compared to the Forsus FRD appliance (18.28 min) (p-value <0.05). Visual Analogue Scale scores for parameters such as problems in mastication, problems in speech, and problems in oral hygiene maintenance were significantly lower with PowerScope 2 appliance as compared to the Forsus FRD appliance (p-value <0.05).
Conclusions
Although both modalities are effective in the management of Class II malocclusion, the PowerScope 2 appliance scores better in terms of lesser chairside time in appliance installation and better patient comfort.
Keywords: PowerScope 2, Forsus FRD, Class II, Randomized controlled trial
Introduction
The fixed functional appliances such as Herbst, Jasper Jumper, Forsus Fatigue Resistant Device (Forsus FRD), and so on, are a treatment of choice in patients who report toward the end of their active skeletal growth or during the later stages of puberty with a minimal amount of mandibular growth anticipated in the future. These appliances, fixed to the dentition by various attachments, can only be removed by the orthodontist, unlike the removable appliances that can be removed by the patients at will. They are therefore used in noncompliant patients to improve the duration of appliance wear and achieve the desired skeletal changes toward the fag end of growth. These appliances are often termed noncompliant Class II correctors due to the reasons quoted above.1,2
The Forsus FRD (3M Unitek Corp., Monrovia, California, USA) is the most commonly used and time-tested fixed functional appliance that is prescribed to harness the remaining mandibular growth in the later stages of puberty. It brings about Class II correction by a combination of skeletal growth and dentoalveolar movement. Despite various advantages such as being fatigue resistant (delivers constant force throughout treatment) and permitting anteroposterior and some amount of lateral mandibular movements; limitations such a laceration of buccal mucosa, frequent debonding of canine brackets, and oral hygiene maintenance have also been reported. A larger inventory is required to select the correct size of the appliance for the patient out of various size options available. This may increase the chairside time and cause operator inconvenience.3, 4, 5, 6
The PowerScope 2 (American Orthodontics, Sheboygan, Wis), a recently introduced Class II corrector, is an improvement over the earlier introduced PowerScope appliance. This appliance incorporates several modifications to the original PowerScope appliance to bring about a more effective Class II correction. PowerScope 2 incorporates a sturdier wire attachment nut to reduce appliance breakage during jaw movements and markings/visual indicators for more controlled appliance activation. For quick selection, faster installation and to reduce inventory, it is preassembled with attachment nuts and available as a one-size-fits-all appliance. It provides a wire-to-wire installation with attachments placed mesial to the first molar in the maxillary arch and distal to the canine in the mandibular arch.7
A few studies are available in the literature, which compare the treatment efficacy, and patient and operator convenience with PowerScope appliance as compared to other fixed functional appliances such as Forsus FRD, but the majority of them are case reports/case series and retrospective record-based studies that are placed lower in the hierarchy of evidence and better quality evidence (i.e., RCTs, systematic reviews, and meta-analysis published in reputed journals) are lacking in the present literature.3,5,6,8 Good quality evidence is further limited on the newly introduced PowerScope 2 appliance.
Therefore, it was prudent to conduct a study to evaluate the clinical efficacy of the PowerScope 2 appliance in the treatment of skeletal Class II malocclusion and compare the treatment effects with Forsus FRD, which is often considered a gold standard noncompliant Class II corrector.5 The null hypothesis was that there is no significant difference in treatment effects between PowerScope 2 and Forsus FRD appliance.
Aim
The aim of this study was to compare the clinical efficacy of PowerScope 2 versus Forsus FRD appliance in the treatment of Skeletal Class II malocclusion.
Objectives
-
1.The primary objective was:
-
a)to compare changes in skeletal, dental, soft tissues, and upper airway parameters between the study groups.
-
a)
-
2.The secondary objectives were to compare the following variables between the study groups:
-
a)to compare treatment duration between the study groups,
-
b)to compare the chairside time taken for appliance insertion and removal between the study groups,
-
c)patient comfort and operator convenience, and
-
d)frequency of breakage of the appliance and bracket debonding.
-
a)
Material and methods
Study settings
This multicenter randomized controlled trial (RCT) was carried out at the Departments of Orthodontics and Dentofacial Orthopedics of two premier institutions of the Armed Forces imparting Post Graduate training in different dental specialties.
The study protocol was duly approved by the Institutional Ethical Committee (IEC). Written informed consent was obtained from the parents of the participants for inclusion of data as well as images in the study.
Inclusion criteria
-
1.
Class II malocclusion (ANB>4°)
-
2.
Molar relation Class II/Half cusp Class II
-
3.
Overjet ≥ 4 mm
-
4.
Positive visual treatment objective (VTO)
-
5.
Cervical vertebrae maturation index (CVMI) Stage III–V
-
6.
No missing or supernumerary teeth
Exclusion criteria
-
1.
Systemic diseases affecting bone metabolism
-
2.
Patients with neuromuscular disorders and Temporomandibular Joint (TMJ) problems
-
3.
Insufficient teeth/diseased periodontal apparatus to support the appliance
-
4.
Patients whose parents did not give consent to participate in study
-
5.
History of orthodontic treatment/trauma to jawbones
Study sample
The sample size was calculated based on an earlier study.9 The maximum sample worked out to be six in each group at each center. However, 20 patients (10 in each group) were recruited at each center (total of 40 patients) to increase the reliability of the study and cater to the possibility of drop-outs during the study.
Randomization
The selected patients at each center were allotted to either treatment modality using block randomization and divided into two groups:
Group 1 consisted of 10 patients treated with Forsus FRD appliance (standard treatment modality).
Group 2 consisted of 10 patients treated with PowerScope 2 appliance (newer treatment modality) (Fig. 1).
Fig. 1.
PowerScope™ 2 kit comprising of (A) Hex head driver with magnetic sleeve; (B) PowerScope™ 2 appliance right and left side; (C) Crimpable shims.
The bracket prescription used for both modalities was 0.018″ MBT Pre-adjusted Edgewise appliance with standard protocol for correction of malocclusion. The archwire sequence used was 0.016″ NiTi, 0.016″ ×0.022″ NiTi, 0.016″ ×0.022″ Stainless Steel (SS), and 0.017″ ×0.025″ SS archwires. PowerScope 2 or Forsus FRD appliances were placed after completion of leveling and alignment on 0.017″ ×0.025″ SS archwires as per standard manufacturer instructions.
The correct size of Forsus FRD was selected using the measurement guide provided with the kit. The measurement was done with the patient in centric occlusion from the distal end of the lower canine bracket to the distal end of the upper molar tube. The appliance was fixed to the headgear tube using an L-pin available with the kit on both sides. In the lower arch, the push rod was placed distal to the canine bracket using the circular loop on the push rod. In the PowerScope 2 appliance, the attachments screws were placed mesial to the maxillary molar and distal to the mandibular canine when the patients were in 0.017″ ×0.025″ SS arch-wire utilizing the Hex head driver with magnetic sleeves provided with the kit. The patients in both groups were reviewed at a 4-week interval, and appliance activation was done as per protocol. No adjunct modalities for Class II treatment such as head-gear, implants, or Class II elastics were utilized during the entire treatment.
The treatment records included complete case sheets, intraoral and extraoral photographs, lateral cephalogram, orthopantomogram (OPG), study models, and Acoustic Pharyngometry (AP). The radiographs were obtained using the Planmeca proline XC unit, M/s Planmeca Oy Finland.
The skeletal, dental, soft tissue, and airway changes were noted at T0 (pretreatment), T1 (preappliance therapy), and T2 (postappliance therapy) and compared for changes between T1 and T2. All cephalograms were manually traced, and the parameters studied are summarized in Supplementary Tables 1–3.
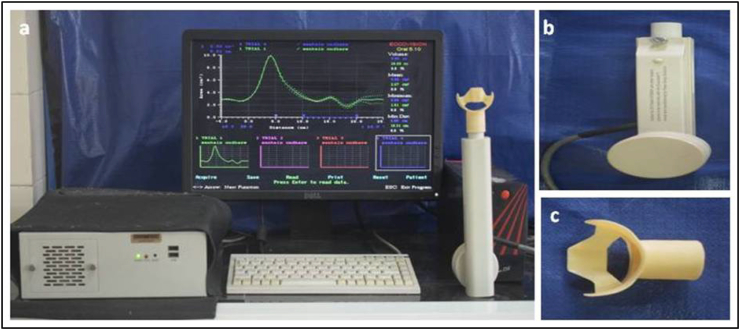
The AP (Eccovision® Acoustic Pharyngometer) was done to ascertain changes in mean airway volume and area with both modalities noninvasively at T1 and T2 (Fig. 2, Fig. 3).
Fig. 2.
(a) Acoustic Pharyngometry work station, (b) wave tube, (c) mouthpiece.
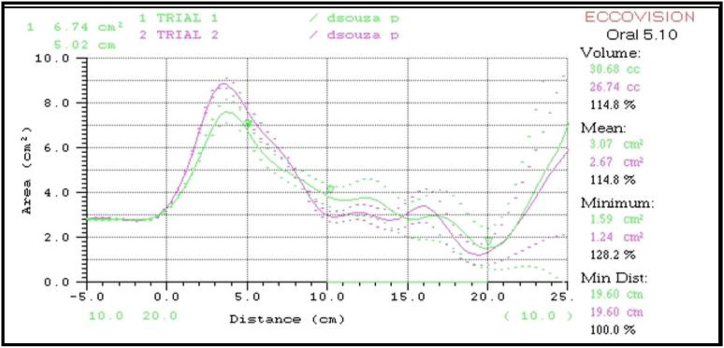
Fig. 3.
Acoustic Pharyngogram showing mean volume, mean area and minimum distance (Trail 1: Purple line – T1 Prefunctional, Trail 2: Green line- T2 Postfunctional).
The treatment duration was considered as the time period between appliance placement and removal, that is, the time required for correction of molar relation from Class II to Class I. Clinical time taken for appliance placement and removal was noted for each patient using a stopwatch and entered in the case sheets against each appointment. The frequencies of breakage of appliance/bracket debonding were also noted in the case sheets.
At the end of treatment, the patients were interviewed regarding the comfort and ease of using appliances using a customized questionnaire survey. The questionnaire was in English; however, necessary help was provided to the patients in the language known to them. The survey questionnaire utilized a Visual Analogue Scale (VAS) with a score of 1–10 (Score 1: minimum problem/discomfort and Score 10: maximum problem/discomfort).
The data were collected and compiled in MS Excel sheets at both centers. The compiled data were subjected to appropriate statistical analysis.
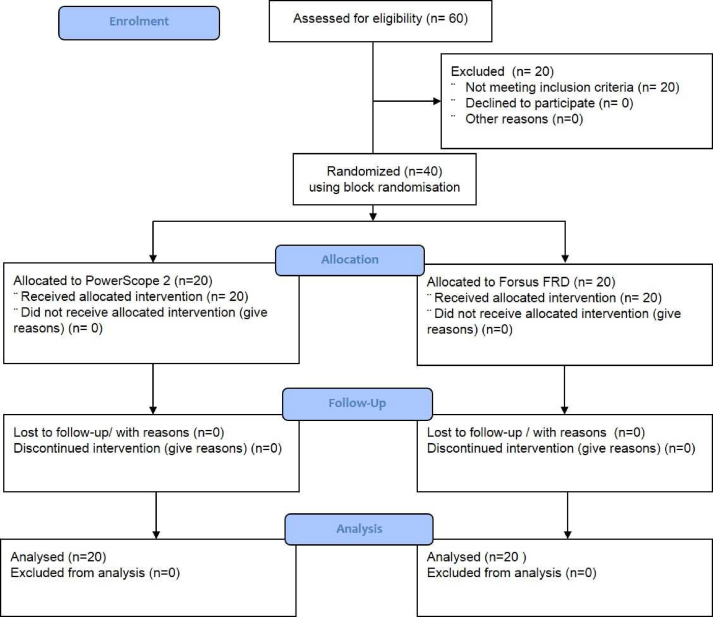
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the trial is given below:
Statistical analysis
The entire data were statistically analyzed using Statistical Package for Social Sciences (SPSS version 24.0, IBM Corporation, USA) for MS Windows. The intergroup statistical comparison of the distribution of categorical variables was tested using the chi-square test or Fisher's exact probability test if more than 20% of cells have expected frequency less than 5. The intergroup statistical comparison of means of continuous variables was done using independent sample t-test. The intragroup statistical comparison of means of continuous variables was done using paired t-test. The underlying normality assumption was tested before subjecting the study variables to t-test. The p-values less than 0.05 were considered to be statistically significant.
Results
Demographic parameters
The study sample (n = 40) consisted of 20 patients in each group (Supplementary Table 4). The mean age in Groups 1 and 2 was 14.8 and 14.4 years, respectively (p-value >0.05). Group 1 had 10 males and 10 females, whereas Group 2 had 8 males and 12 females (p-value >0.05) (Supplementary Tables 5 and 6).
Changes in skeletal parameters posttreatment
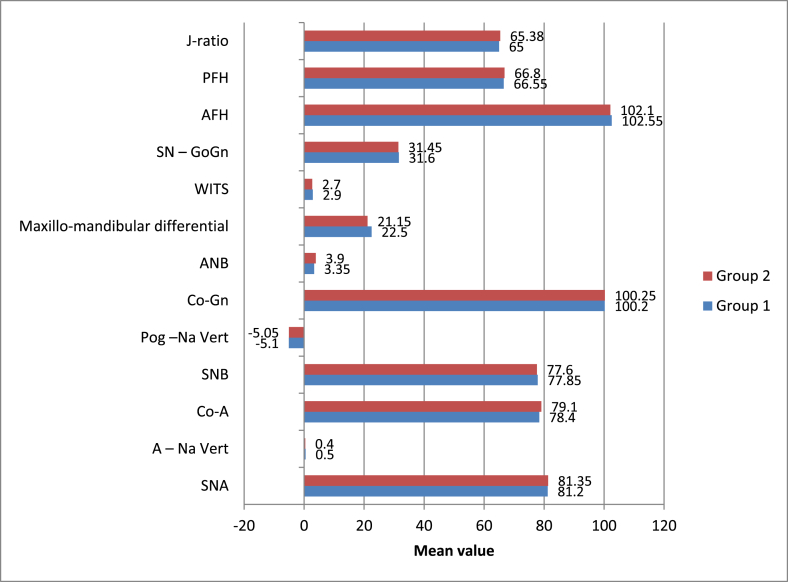
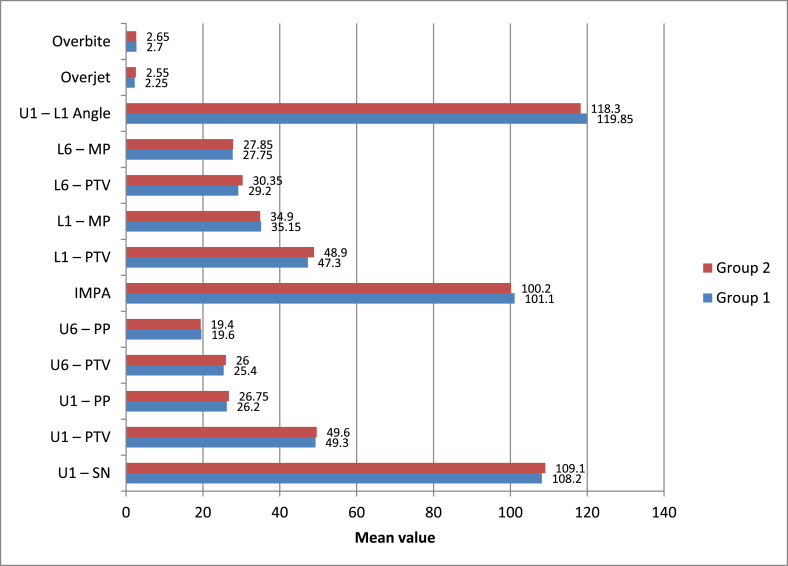
A statistically significant change was observed in all skeletal parameters after treatment with both modalities (p-value <0.05 for all) except Co-A, which showed no significant change at T2 (p-value >0.05). However, no statistically significant intergroup difference was observed (p-value >0.05) (Supplementary Tables 7–9, Fig. 4).
Fig. 4.
Intergroup comparison of means of skeletal parameters studied.
Changes in dental parameters posttreatment
A statistically significant change was observed after treatment in all dental parameters with both modalities (p-value <0.05 for all) except L6-MP, which showed no significant change at T2 (p-value >0.05). However, no statistically significant intergroup difference was observed (p-value >0.05) (Supplementary Tables 10–12, Fig. 5).
Fig. 5.
Intergroup comparison of means of dental parameters studied.
Changes in soft tissue parameters posttreatment
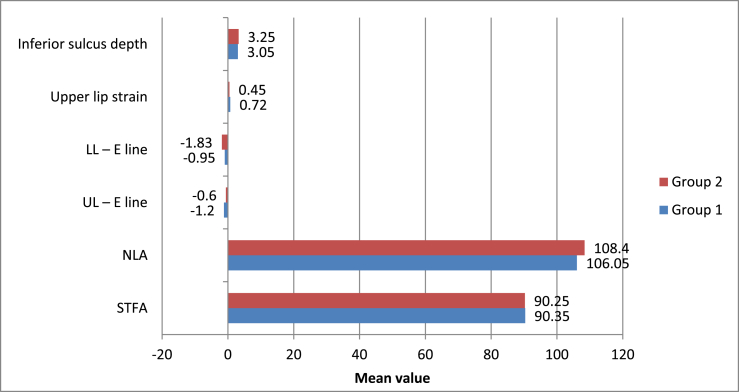
A statistically significant change was observed after treatment in all soft tissue parameters with both modalities (p-value <0.05 for all). However, no statistically significant intergroup difference was observed (p-value >0.05) (Supplementary Tables 13–15, Fig. 6).
Fig. 6.
Intergroup comparison of means of soft tissue parameters studied.
Changes in airway parameters posttreatment
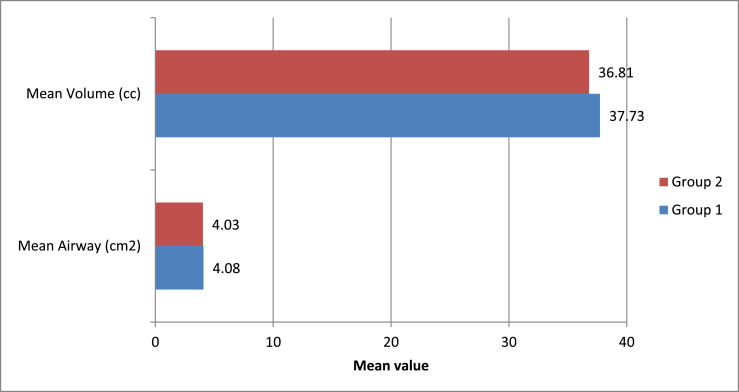
A statistically significant change was observed in all airway parameters (area and volume of the upper airway) with both modalities (p-value <0.05 for all). However, no statistically significant intergroup difference was observed (p-value >0.05) (Supplementary Tables 16–18, Fig. 7).
Fig. 7.
Intergroup comparison of means of airway parameters studied.
Intergroup comparison of means of patient comfort parameters studied
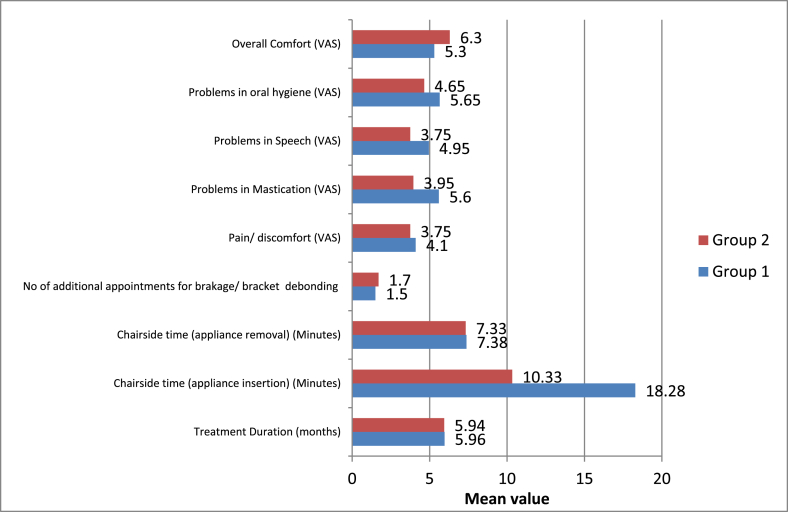
No statistically significant intergroup difference was in parameters such as treatment duration, chairside time in appliance removal, the number of additional appointments for breakage/bracket debonding and pain/discomfort after treatment with both modalities (p-value >0.05) (Supplementary Table 19, Fig. 8).
Fig. 8.
Intergroup comparison of means of patient comfort parameters studied.
However, the chairside time spent in appliance insertion was significantly lower with PowerScope 2 appliance (10.33 min) as compared to the Forsus FRD appliance (18.28 min) (p-value <0.05) (Supplementary Table 19, Fig. 8).
The Visual Analogue Scale (VAS) scores for parameters such as problems in mastication, problems in speech and problems in oral hygiene maintenance were significantly lower with PowerScope 2 appliance as compared to the Forsus FRD appliance (p-value <0.05) (Supplementary Table 19, Fig. 8).
The Visual Analogue Scale (VAS) score for overall comfort during treatment was significantly higher with PowerScope 2 appliance as compared to the Forsus FRD appliance (p-value <0.05) (Supplementary Table 19, Fig. 8).
Discussion
The results of the present study show that both the appliances used in the study did not produce a statistically significant restraint effect on the sagittal growth of the maxilla. There was no statistically significant difference between both study groups in this aspect. These results are in concurrence line with earlier studies,3,10,11 which reported no inhibitory effect on maxillary growth by the fixed functional appliances. These findings contradict a study12 that observed a significant restraint effect of Forsus FRD on maxillary growth. Another study3 observed some restraining effects of both these appliances on maxillary sagittal growth, which was more with PowerScope than with Forsus FRD appliance.
In the present study, both appliances produced significant forward movement of the mandible and resulted in improvement in Class II relation and overjet reduction. The intergroup difference was however insignificant. These findings are similar to earlier literature,13 which observed no significant difference in sagittal growth between both modalities. However, a study observed a greater amount of sagittal mandibular growth with Forsus FRD compared to PowerScope.3 Contrary to this, another study observed greater sagittal growth with PowerScope compared to Forsus FRD.14 These differences may be attributed to the difference in study designs and various patient/operator-related factors, which may be overcome by a large-scale prospective study with a larger sample size.
In the present study, both appliances produced significant distal movement of the maxillary molars and mesial movement of mandibular molars as well as retroclination of maxillary and proclination of mandibular incisors, which resulted in the achievement of Class I molar relation and reduction of overjet in conjunction with the skeletal changes. Both modalities caused mild extrusion of upper molars and an increase in lower anterior facial height. The interincisal angle was increased with both treatment modalities. However, no statistically significant difference was observed between the two treatment modalities. These findings are similar to earlier literature.15, 16, 17, 18, 19, 20, 21, 22, 23 Some studies have3,14 observed greater mesial movement of the mandibular incisors and molars with PowerScope compared to Forsus FRD and attributed this difference to the mode of attachment of the PowerScope 2 appliance which is directly on the wire and may result in greater dento alveolar effects. The authors also observed that the skeletal movement for Class II correction was more with PowerScope as compared to Forsus FRD. Varghese et al,14 observed greater extrusion of both maxillary and mandibular molars with PowerScope as compared to Forsus FRD, which is in contradiction to the results of the present study which found no significant difference between both modalities.
In the present study, both modalities brought about improvement in the soft tissue facial profile, nasolabial angle, the relation of upper and lower lips to the E line, reduction in lip strain, and inferior sulcus depth. However, no statistically significant difference was observed between both modalities. These findings are similar to earlier literature comparing both modalities.3,13,14
The improvement in airway with both modalities was assessed with AP which is a noninvasive and nonionizing modality based on the acoustic reflection technique for assessment of upper airway dimensions. Upper airway area and volume were compared between both modalities that showed some improvement in the upper airway with both modalities, but no statistically significant intergroup difference was observed. Though various studies have studied airway improvement with various fixed functional appliances including Forsus FRD,24,25 there are not enough data on the newly introduced PowerScope 2 appliance and comparison between the two modalities used in this study. We recommend further research in this regard to substantiate the finding of the present study.
The present study observed significantly reduced chairside time with PowerScope 2 appliance as seen by reduced time in installation of the appliance as compared to Forsus FRD. The treatment duration was however similar to both modalities. The numbers of breakages resulting in additional appointments were similar with both modalities. However, the visual analogue scores for patient comfort, oral hygiene maintenance, speech, and mastication were better with PowerScope 2 appliance as compared to Forsus FRD. A study14 comparing these two modalities, however, observed more patient discomfort with PowerScope which is contrary to the findings of the present study. The installation time was significantly lower with PowerScope in the above study, which concurs with the findings of the present study. The above study also observed greater breakage with PowerScope attributing it to being a wire-to-wire installation. The treatment time observed was also greater with PowerScope in the above study attributed to frequent breakages. These differences may be attributed to the use of PowerScope 2 in the present study against the original PowerScope in the above study. The new patient and operator-friendly features incorporated in the PowerScope 2 may have contributed to these differences.
Conclusions
The following conclusions can be drawn from the findings of this multicenter RCT:
-
1.
Both PowerScope 2 and Forsus FRD are equally effective in the management of Class II malocclusion. The improvement in skeletal, dental, soft tissue and airway parameters studied among Class II patients reporting at the end of pubertal growth were similar in the two groups.
-
2.
The total duration of Class II correction along with the number of breakages/brackets debonding was similar in the two groups studied and was not statistically significant.
-
3.
The chairside time required for installation of the appliance was recorded to be lower with PowerScope 2 as compared to the Forsus FRD appliance.
-
4.
The patient-related factors such as patient comfort, oral hygiene maintenance, speech, and mastication were better with PowerScope 2 appliance as compared to the Forsus FRD appliance.
-
5.
Further large-scale studies utilizing PowerScope 2 appliance will help the clinician in selecting the correct treatment modality.
Disclosure of competing interest
The authors have none to declare.
Acknowledgement
This paper is based on Armed Forces Medical Research Committee Project No. 5151 (A&B)/2019 granted and funded by the office of the Directorate General Armed Forces Medical Services and Defence Research Development Organization, Government of India.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mjafi.2023.03.011.
Appendix A. Supplementary data
The following are the supplementary data to this article.
References
- 1.Dhiman I., Dhiman P. Powerscope - non-compliance class II corrector- a review. Int J Curr Res. 2017;9(7):57–62. [Google Scholar]
- 2.Patel H.P., Moseley H.C., Noar J.H. Cephalometric determinants of successful functional appliance therapy. Angle Orthod. 2002;72:410–417. doi: 10.1043/0003-3219(2002)072<0410:CDOSFA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Arora V., Sharma R., Chowdhary S. Comparative evaluation of treatment effects between two fixed functional appliances for correction of Class II malocclusion: a single-center, randomized controlled trial. Angle Orthod. 2018;88:259–266. doi: 10.2319/071717-476.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunay E.A., Arun T., Nalbantgil D. Evaluation of the immediate dentofacial changes in late adolescent patients treated with Forsus FRD. Eur J Dermatol. 2011;5:423–432. [PMC free article] [PubMed] [Google Scholar]
- 5.George A.S., Ganapati Durgekar S. Skeletal and dentoalveolar contributions during Class II correction with Forsus™ FRD appliances: quantitative evaluation. J Orofac Orthop. 2022;83(2):87–98. doi: 10.1007/s00056-021-00297-z. [DOI] [PubMed] [Google Scholar]
- 6.Heinrichs D.A., Shammaa I., Martin C., Razmus T., Gunel E., Ngan P. Treatment effects of a fixed intermaxillary device to correct class II malocclusions in growing patients. Prog Orthod. 2014;15(1):45. doi: 10.1186/s40510-014-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malhotra A., Negi K.S., Kaundal J.R., Negi N., Mahajan M., Chainta D. Cephalometric evaluation of dentoskeletal and soft tissue changes with powerscope Class II corrector. J Indian Orthod Soc. 2018;52:167–173. [Google Scholar]
- 8.Keerthi V.N., Kanya S.D., Babu K.P., Mathew A., Kumar A.N. Early prevention and intervention of Class II division 1 in growing patients. J Int Soc Prev Community Dent. 2016;6:79–83. doi: 10.4103/2231-0762.181191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antony T., Amin V., Hegde S., Hegde S., Shetty D., Khan M.B. The evaluation and clinical efficiency of powerscope: an original research. J Int Soc Prev Community Dent. 2018;8:264–270. doi: 10.4103/jispcd.JISPCD_48_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinig N., Goz G. Clinical application and effects of the Forsus spring. A study of a new Herbst hybrid. J Orofac Orthop. 2001;62:436–450. doi: 10.1007/s00056-001-0053-6. [DOI] [PubMed] [Google Scholar]
- 11.Jones G., Buschang P.H., Kim K.B., Oliver D.R. Class II nonextraction patients treated with the Forsus fatigue resistant device versus intermaxillary elastics. Angle Orthod. 2008;78:332–338. doi: 10.2319/030607-115.1. [DOI] [PubMed] [Google Scholar]
- 12.Franchi L., Baccetti T., McNamara J.A., Jr. Treatment and posttreatment effects of acrylic splint herbst appliance therapy. Am J Orthod Dentofacial Orthop. 1999;115:429–438. doi: 10.1016/s0889-5406(99)70264-7. [DOI] [PubMed] [Google Scholar]
- 13.Shalu S., Rai R., Sudhakar S.S. Comparison of dental effects of Forsus fatigue resistant device and powerscope treatment –A cephalometric study. Ann R.S.C.B. 2021;25:20667–20676. [Google Scholar]
- 14.Varghese R.M., Subramanian A.K., Sreenivasagan S. Comparison of dentoskeletal changes in skeletal class II cases using two different fixed functional appliances: Forsus fatigue resistant device and powerscope class II corrector—a clinical study. J Int Oral Health. 2021;13(3):234–244. [Google Scholar]
- 15.Franchi L., Alvetro L., Giuntini V., Masucci C., Defraia E., Baccetti T. Effectiveness of comprehensive fixed appliance treatment used with the Forsus fatigue resistant device in Class II patients. Angle Orthod. 2011;81(4):678–683. doi: 10.2319/102710-629.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gohilot A., Pradhan T., Keluskar K.M. Comparison of dentoskeletal and soft tissue changes seen in class II division I malocclusion using Forsus fatigue resistant device, Churro Jumper and Herbst Appliance – a randomized clinical trial. Orthod Cyber J. 2013;1:1–14. [Google Scholar]
- 17.Perinetti G., Primozic J., Furlani G., Franchi L., Contardo L. Treatment effects of fixed functional appliances alone or in combination with multibracket appliances: a systematic review and meta-analysis. Angle Orthod. 2015;85(3):480–492. doi: 10.2319/102813-790.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tandon R., Chandra P., Rohmetra A., Singh V.A., Sonal Management of Class II malocclusion with Powerscope appliance: report of two cases. Asian J Oral Health Allied Sci. 2019;9(1):6–9. [Google Scholar]
- 19.Paul R., Gupta M., Golccha V., Yadav D., Sharma I., Sharma S.K. PowerScope as a Class II corrector in a noncompliant patient – a case report. J Contemp Orthod. 2020;4(2):12–15. [Google Scholar]
- 20.Pacha M.M., Fleming P.S., Johal A. Complications, impacts and success rates of different approaches to treatment of Class II malocclusion in adolescents: a systematic review and meta-analysis. Am J Orthod Dentofacial Orthop. 2020;158(4):477–494. doi: 10.1016/j.ajodo.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien K., Wright J., Conboy F., et al. Effectiveness of treatment for Class II malocclusion with the Herbst or Twinblock appliances: a randomized controlled trial. Am J Orthod Dentofacial Orthop. 2003;124(2):128–137. doi: 10.1016/s0889-5406(03)00345-7. [DOI] [PubMed] [Google Scholar]
- 22.Papadopoulos M.A. Mosby; St Louis: 2006. Orthodontic Treatment of the Class II Non-compliant Patient: Current Principles and Techniques; pp. 9–17. [Google Scholar]
- 23.Kalra A., Swami V., Bhosale V. Treatment effects of “PowerScope” fixed functional appliance – a clinical study. Folia Med (Plovdiv) 2021;63(2):253–263. doi: 10.3897/folmed.63.e52892. [DOI] [PubMed] [Google Scholar]
- 24.Celikoglu M., Buyuk S.K., Ekizer A., Unal T. Pharyngeal airway effects of Herbst and skeletal anchored Forsus FRD EZ appliances. Int J Pediatr Otorhinolaryngol. 2016 Nov;90:23–28. doi: 10.1016/j.ijporl.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Ganesh G., Tripathi T. Effect of fixed functional appliances on pharyngeal airway dimensions in Skeletal Class II individuals - a scoping review. J Oral Biol Craniofac Res. 2021;11(4):511–523. doi: 10.1016/j.jobcr.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.