Abstract
The unique Gag polyprotein of the replication-defective virus responsible for murine AIDS (MAIDS) induces B-cell activation, proliferation, and differentiation, including immunoglobulin class switch-recombination to immunoglobulin E (IgE). Secretion of IgE normally requires the serial induction of interleukin 4 (IL-4), engagement of the IL-4 receptor, activation of signal transducer and activator of transcription (STAT) 6, and induction of Iɛ germline transcripts as a prelude to switching. Remarkably, expression of IgE is equivalent in normal and IL-4-deficient mice with MAIDS (Morawetz et al., J. Exp. Med. 184:1651–1661, 1996). To understand this anomaly, we studied mice with a null mutation of STAT6. Lymphoproliferation and immunodeficiency, the hallmarks of MAIDS, developed with comparable kinetics and degree in normal and mutant mice. In addition, serum IgE levels were indistinguishable in mice of either genotype. We conclude that B cells from mice with MAIDS activate unique IL-4- and STAT6-independent signaling pathways for B-cell activation and differentiation.
A murine retrovirus-induced immunodeficiency syndrome, designated murine AIDS (MAIDS), develops following infection of susceptible mice with a replication-defective virus that encodes only a variant Pr60gag polyprotein (1, 9, 15). The syndrome is characterized by progressive lymphoproliferation and severe immune defects associated with enhanced susceptibility to infection. The mechanisms by which the defective virus Gag induces disease are not known but may involve aberrant activation of extra- or intracellular signaling pathways.
Although type 2 cytokines (interleukin 4 [IL-4] and IL-10) were once considered possible driving forces in this disorder (2), recent results have shown that MAIDS develops normally in mice deficient in expression of IL-4 (12) and/or IL-10 (14). Unexpectedly, IL-4-deficient mice with MAIDS had levels of immunoglobulin E (IgE) in serum comparable to those in wild-type mice (13). Because IL-4 was previously thought to be an absolute requirement for induction of IgE, this suggested that B cells from IL-4-deficient infected mice were responding to signals mimicking those activated by engagement of the IL-4 receptor (IL-4R).
The IL-4R consists of the IL-4Rα chain and either of two additional molecules, the IL-2Rγ common chain or the IL-13Rα chain (5, 16). Like other members of the hematopoietin receptor family, IL-4Rs do not encode either tyrosine or serine-threonine kinases; however, binding of IL-4 to the receptor complexes results in activation of the Janus family tyrosine kinases, Jak-1 and Jak-3, activation of two distinct signaling pathways by phosphorylation of an insulin receptor substrate (IRS) designated IRS-1 or 4PS (8), and activation of the signal transducer and activator of transcription, STAT6 (7). Phosphorylated IRS-1 interacts with the p85 regulatory subunit of phosphatidylinositol 3-kinase (PI-3K) and mediates proliferative and antiapoptotic responses to stimulation with IL-4 (20). Phosphorylated STAT6 translocates to the nucleus, binds to γ-activated sequences, and activates transcription of several genes, including CD23 and the IgE germline sequence, Iɛ (17). Studies of mice deficient in STAT6 due to gene targeting in embryonic cells showed that STAT6 is critical for induction of T helper 2 responses and production of IgE following immunization with anti-IgD or infection with a nematode (10, 18). Proliferative responses to IL-4 were moderately to severely depressed in these mice, depending on the assay system (10, 18).
To determine the importance of STAT6 to induction of MAIDS and expression of IgE in association with this disease, we infected STAT6−/− (referred to hereafter by genotype only) mice with LP-BM5 murine leukemia virus (MuLV). The STAT6-deficient mice used in this study derived from targeted D3 ES cells injected into BALB/c blastocysts. Heterozygous knockout mice were crossed to generate mice homozygous for the Stat6 mutation (10). These mice were crossed to C57BL/6 (B6) mice and intercrossed. Progeny homozygous for H-2b and the Stat6 mutation were identified and crossed. Progeny homozygous for the Fv1b allele of B6 were selected and intercrossed for infection with stocks of the LP-BM5 virus mixture prepared from the G6 clone of SC-1 cells (4).
At 6 and 10 weeks after infection, wild-type, +/−, and −/− mice were found to have equivalent levels of lymphadenopathy and splenomegaly (Table 1). Fluorescence-activated cell sorter (FACS) (Fig. 1) and histopathologic studies (not shown) revealed that mice of all three genotypes had comparably advanced disease at these time points.
TABLE 1.
Development of MAIDS in STAT6 knockout micea
| STAT6 genotypeb | Wks after infection | Tissue wt (mg)
|
Histopathologyd | |
|---|---|---|---|---|
| Spleenc | Lymph nodesc | |||
| +/+ | 0 | 90 | <10 | N |
| 6 | 257 | 90 | 1 | |
| 10 | 525 | 223 | 2 | |
| +/− | 0 | 100 | <10 | N |
| 6 | 325 | 168 | 1 | |
| 10 | 428 | 258 | 1–2 | |
| −/− | 0 | 90 | <10 | N |
| 6 | 215 | 105 | 1 | |
| 10 | 662 | 474 | 2 | |
Data are for three mice per data point and are representative of three experiments involving two to three mice compared at each time point.
Mice of the indicated STAT6 genotypes were infected with LP-BM5 virus mixture and killed at the indicated time points.
Spleens and lymph nodes were weighed and material was saved for histopathology.
Histopathologic diagnoses were as follows: N, normal; 1, definitive evidence of MAIDS with germinal center expansion and proliferation of immunoblasts and plasmacytoid cells; 2, more advanced MAIDS with fusions of germinal centers, early loss of lymphoid architecture, and further expansions of blast cells.
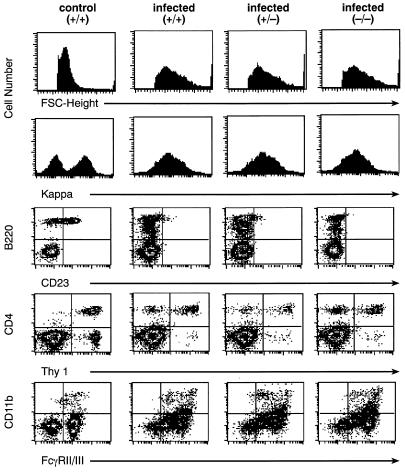
FIG. 1.
FACS analyses of spleen cells from mice infected with LP-BM5 viruses for 8 weeks. Cells were stained with the indicated antibodies to cell surface antigens, and 2 × 105 viable cells, as determined by size and exclusion of propidium iodide, were evaluated. Similar results were obtained with mice infected for 8 to 12 weeks. FSC, forward angle scatter.
In the FACS profiles, blast populations—indicated by increased forward angle scatter—were seen along with reduced expression of immunoglobulin kappa light chain and CD45R (B220) as signs of B-cell activation in infected +/+, +/−, and −/− mice; profiles of spleen cells from uninfected +/− and −/− mice were indistinguishable from those of +/+ mice (11). Interestingly, expression of CD23, a gene regulated by IL-4 and STAT6, was almost completely inhibited in all infected mice. The unusual Thy-1− CD4+ T-cell population expanded in MAIDS (6) was readily detected. Expression of the IgG FcR was greatly increased, and the size of the CD11b+ population was expanded. Finally, mice of each genotype showed almost identical profiles for CD69, CD25, CD62L, CD44, NK1.1, GR-1, CD43, CD24a, IgD, Ia, and TCR-α/β (data not shown).
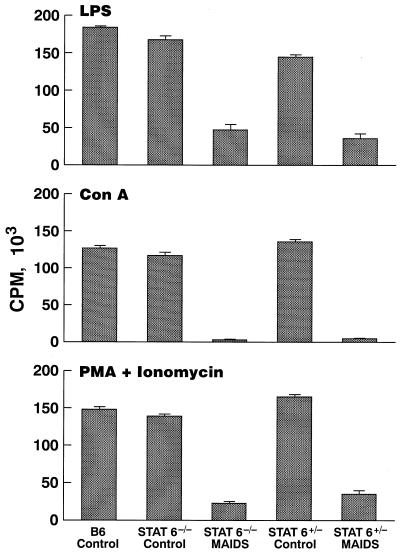
Immunodeficiency of increasing severity is a second prominent feature of MAIDS (9, 15). Studies of proliferative responses to lipopolysaccharide (LPS), concanavalin A (ConA), and phorbol myristate acetate (PMA) plus ionomycin at 8 weeks after infection showed similarly pronounced defects in −/− and +/− mice (Fig. 2). It has been suggested that altered expression of cytokines may contribute to this phenotype (3, 19). Semiquantitative reverse transcription-PCR analyses of BM5def, Iɛ, and cytokine transcripts demonstrated comparable levels of p12def expression and germline ɛ induction (data not shown).
FIG. 2.
Proliferative responses of spleen cells to stimulation with mitogens or PMA plus ionomycin. Cells (2 × 105) from spleens of uninfected mice or mice infected for 8 weeks were cultured for 48 h in triplicate in 96-well plates with ConA (2 μg/ml), LPS (20 μg/ml), or PMA (20 ng/ml) plus ionomycin (250 ng/ml). Cultures were pulsed with [3H]thymidine for the last 6 h of culture. Data are average values ± 1 standard error of the mean for three mice per time point. Similar results were obtained in a second experiment. CPM, counts per minute.
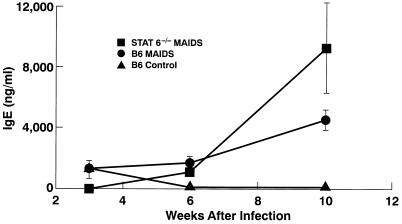
We used an enzyme-linked immunosorbent assay (ELISA) (13) to measure serum IgE levels in sera from mice infected for 3 to 10 weeks (Fig. 3). Sera of uninfected +/+ mice contained 5 ng of IgE/ml, while sera of uninfected −/− mice contained less than 1 ng/ml. At 10 weeks after infection, the levels of IgE in serum were statistically similar among all three groups of mice (data not shown). Digestion circularization-PCR analyses of spleens from infected −/− and +/+ mice demonstrated the presence of the predicted 550-bp PCR fragment that hybridized with a specific Cɛ membrane region probe (not shown), indicating that expression of IgE was a consequence of deletional switch recombination.
FIG. 3.
IgE levels in serum in mice infected with LP-BM5 MuLV. Sera obtained at the indicated time points were assayed for IgE levels by ELISA. Data are the averages ± 1 standard error of the mean for three mice per time point.
These results provide unequivocal evidence that STAT6 is not required for induction of MAIDS or for IgE switch recombination and secretion as part of this disorder. These findings exclude any possibility that IL-4-independent induction of IgE in MAIDS can be ascribed to an IL-4-like activity of the BM5-defective virus and demonstrate the involvement of a STAT6-independent pathway. It will be important to determine whether alterations in other transcription factors binding to the cis-controlling elements of the IgE locus are responsible for driving IL-4- and STAT6-independent high-level IgE expression.
Acknowledgments
We thank Brenda Marshall for excellent editorial assistance.
M.J.G. is a Scholar of the Leukemia Society of America. This work was supported in part by contract N01-AI-45203 at MA Bioservices, Inc. (Rockville, Md.), by NIH grant AI40171, and by a gift from the Mathers Foundation.
REFERENCES
- 1.Chattopadhyay S K, Sengupta D N, Fredrickson T N, Morse III H C, Hartley J W. Characteristics and contributions of defective, ecotropic, and mink cell focus-inducing viruses involved in a retrovirus-induced immunodeficiency syndrome of mice. J Virol. 1991;65:4232–4241. doi: 10.1128/jvi.65.8.4232-4241.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gazzinelli R T, Makino M, Chattopadhyay S K, Snapper C M, Sher A, Hügin A W, Morse H C., III CD4+ subset regulation in viral infection. J Immunol. 1992;148:182–188. [PubMed] [Google Scholar]
- 3.Giese N A, Giese T, Morse H C., III Murine AIDS is an antigen-driven disease: requirements for major histocompatibility complex class II expression and CD4+ T cells. J Virol. 1994;68:5819–5824. doi: 10.1128/jvi.68.9.5819-5824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartley J W, Fredrickson T N, Yetter R A, Makino M, Morse H C., III Retrovirus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J Virol. 1989;63:1223–1231. doi: 10.1128/jvi.63.3.1223-1231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilton D J, Zhang J-G, Metcalf D, Alexander W S, Nicola N A, Wilson T A. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci USA. 1996;93:497–501. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes K L, Morse III H C, Makino M, Hardy R R, Hayakawa K A. A unique subset of normal murine CD4+ T cells lacking Thy-1 is expanded in a murine retrovirus-induced immunodeficiency syndrome, MAIDS. Eur J Immunol. 1990;20:2783–2787. doi: 10.1002/eji.1830201237. [DOI] [PubMed] [Google Scholar]
- 7.Hou J, Schindler U, Henzel W J, Ho T C, Brasseur M, McKnight S L. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994;265:1701–1705. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- 8.Johnson J A, Wang L-M, Hanson E P, Sun X-J, White M F, Oakes S A, Pierce J H, O’Shea J J. Interleukins 1, 4, 7, and 15 stimulate tyrosine phosphorylation of insulin receptor substrates 1 and 2 in T cells. J Biol Chem. 1995;270:28527–28530. doi: 10.1074/jbc.270.48.28527. [DOI] [PubMed] [Google Scholar]
- 9.Jolicoeur P. Murine acquired immunodeficiency syndrome (MAIDS): an animal model to study the AIDS pathogenesis. FASEB J. 1991;5:2398–2405. doi: 10.1096/fasebj.5.10.2065888. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan M H, Schindler U, Smiley S T, Grusby M J. Stat6 is required for mediating responses to IL-4 and for the development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 11.Klinman D M, Morse H C., III Characteristics of B cell proliferation and activation in murine AIDS. J Immunol. 1989;142:1144–1149. [PubMed] [Google Scholar]
- 12.Morawetz R A, Doherty T M, Giese N A, Hartley J W, Müller W, Kühn R, Rajewsky K, Coffman R, Morse H C., III Resistance to murine acquired immunodeficiency syndrome (MAIDS) Science. 1994;265:264–267. doi: 10.1126/science.8023146. [DOI] [PubMed] [Google Scholar]
- 13.Morawetz R A, Gabriele L, Rizzo L V, Noben-Trauth N, Kühn R, Rajewsky K, Müller W, Doherty T M, Finkelman F, Coffman R L, Morse H C., III Interleukin (IL)-4-independent immunoglobulin class switch to immunoglobulin (Ig)E in the mouse. J Exp Med. 1996;184:1651–1661. doi: 10.1084/jem.184.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morawetz R A, Giese N A, Gabriele L, Rothman P, Horak I, Ozato K, Morse H C., III Relationship of cytokines and cytokine signaling to immunodeficiency disorders in the mouse. Braz J Med Biol Res. 1998;31:61–67. doi: 10.1590/s0100-879x1998000100008. [DOI] [PubMed] [Google Scholar]
- 15.Morse H C, III, Chattopadhyay S K, Makino M, Fredrickson T N, Hügin A W, Hartley J W. Retrovirus-induced immunodeficiency in the mouse: MAIDS as a model for AIDS. AIDS. 1992;6:607–621. doi: 10.1097/00002030-199207000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Mosley B, Beckmann M P, March C J, Idzerda R L, Gimpel S D, vandenBos T, Friend D, Alpert A, Anderson D, Jackson J, Wignall J M, Smith C, Gallis B, Sims J E, Urdal D, Widmer M B, Cosman D, Park L S. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989;59:335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- 17.Reichel M, Nelson B H, Greenberg P D, Rothman P B. The IL-4 receptor α-chain cytoplasmic domain is sufficient for activation of JAK-1 and STAT6 and the induction of IL-4-specific gene expression. J Immunol. 1997;158:5860–5867. [PubMed] [Google Scholar]
- 18.Shimoda K, vanDeursen J, Sangster M Y, Sarawar S R, Carson R T, Tripp R A, Chu C, Quelle F W, Nosaka T, Vignali D A A, Doherty P C, Grosveld G, Paul W E, Ihle J N. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 19.Uehara S, Hitoshi Y, Numata F, Makino M, Howard M, Mizuochi T, Takatsu K. An IFN-γ-dependent pathway plays a critical role in the pathogenesis of murine immunodeficiency syndrome induced by LP-BM5 murine leukemia virus. Int Immunol. 1994;6:1937–1947. doi: 10.1093/intimm/6.12.1937. [DOI] [PubMed] [Google Scholar]
- 20.Zamorano J, Wang H Y, Wang L-M, Pierce J H, Keegan A D. IL-4 protects cells from apoptosis via the insulin receptor substrate pathway and a second independent signaling pathway. J Immunol. 1996;157:4926–4934. [PubMed] [Google Scholar]