Abstract
A series of 61 thiazolidine-2,4-diones bearing a styryl group at position 5 was synthesized in 2–5 steps and their structure was proved by elemental and spectral analyses. The compounds obtained were evaluated in vitro against the promastigote stage of the kinetoplastid parasite Leishmania infantum and the human HepG2 cell line, to determine selectivity indices and to compare their activities with those of antileishmanial reference drugs. The study of structure–activity relationships indicated the potential of some derivatives bearing a nitro group on the phenyl ring, especially when located at the meta position. Thus, among the tested series, compound 14c appeared as a hit compound with good antileishmanial activity (EC50 = 7 µM) and low cytotoxicity against both the hepatic HepG2 and macrophage THP-1 human cell lines (CC50 = 101 and 121 µM, respectively), leading to good selectivity indices (respectively, 14 and 17), in comparison with the reference antileishmanial drug compound miltefosine (EC50 = 3.3 µM, CC50 = 85 and 30 µM, SI = 26 and 9). Regarding its mechanism of action, among several possibilities, it was demonstrated that compound 14c is a prodrug bioactivated, predominantly by L. donovani nitroreductase 1, likely leading to the formation of cytotoxic metabolites that form covalent adducts in the parasite. Finally, compound 14c is lipophilic (measured CHI LogD7.7 = 2.85) but remains soluble in water (measured PBS solubility at pH7.4 = 16 µM), highlighting the antileishmanial potential of the nitrostyrylthiazolidine-2,4-dione scaffold.
Keywords: visceral leishmaniasis, L. infantum, thiazolidinedione ring, nitrostyryl moiety
1. Introduction
Leishmaniasis is a neglected tropical disease, caused by a parasite belonging to the genus Leishmania. The infection is transmitted through a bite of a female Phlebotomine sandfly infected with the protozoan. According to the World Health Organization (WHO), leishmaniasis is the second major tropical parasitic killer disease after malaria [1]. Currently, it affects 12 million people in 98 countries mainly in tropical and sub-tropical regions, and in southern Europe, especially around the Mediterranean area. There are three clinical forms of leishmaniasis: cutaneous (CL), mucocutaneous (MCL), and visceral (VL). The first type is the most common while the third one is the most severe, mainly caused by Leishmania donovani and Leishmania infantum, targeting visceral organs such as the spleen and liver [1]. Currently, there is no vaccine available for any form of human leishmaniasis [1]. Moreover, there is a limited number of available drugs representing major therapeutic limitations including severe side effects and increasing parasite drug-resistance issues.
The available drugs for the treatment of VL include sodium stibogluconate (pentavalent antimonials), miltefosine, pentamidine, paromomycin, and amphotericin B. However, these drugs are outdated, showing serious side effects such as renal or cardiac toxicity, teratogenicity, and parasite resistance. Moreover, today, miltefosine remains the only orally available antileishmanial drug against VL. This matter calls for the discovery of new antileishmanial chemical entities, with both original mechanisms of action and oral route of administration, to improve and facilitate the therapeutic care of the infected populations, especially in endemic developing countries [2,3,4].
Many studies have been performed to evaluate the antileishmanial activity of several nitrogen-containing heterocycles such as pyridines [5], pyrimidines [5], isoxazole [6,7,8], pyrazoles [9,10,11], thiazoles [12], 1,2,4-triazoles [13], 1,3,4-oxadiazoles [14], thiohydantoins [15], quinolines [2,16,17,18], quinazolines [19,20], pyrazolo[3,4-d]pyridine [21,22], pyrazolo[3,4-d]pyridazines [23], and imidazo[1,2-a]pyridines [24,25,26].
Thiazolidinediones (TZDs), also known as Glitazones, are an important class of heterocycles abundantly reported in the literature. Initially, certain derivatives were used as insulin sensitizers for the treatment of Type 2 diabetes mellitus (T2DM) due to their PPAR-γ activation properties [27]. However, the TZDs, clinically used as anti-diabetes mellitus drugs, were later reported to suffer from several serious side effects and thus many of them were withdrawn from the market [28]. However, pioglitazone is still marketed in the USA and Lobeglitazone was recently approved in India for the treatment of Type 2 diabetes.
A literature survey revealed that TZDs possess a very wide range of biological activities, which are included in the reference [29], such as antidiabetic, anti-infectious, anti-inflammatory, analgesic, antioxidant, immunomodulatory, overactive bladder inhibitory and insecticide activities. TZDs also possess inhibitory activity against tyrosinase, hyperlipidemia, and acute liver injury. Furthermore, it was reported that TZDs affected cancer development, progression and metastasis, among which the Raf/MEK/extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K)/AKT, Wnt signal transduction pathways, PIM-1 and PIM-2 protein kinases overexpression, receptor-stimulated genes and peroxisome proliferator-activated receptors (PPARs) signaling cascades which are the most commonly up-regulated in human cancers [29]. It is important to note that the TZD derivative S49076 has reached Phase I clinical trials against solid tumors [30]. On the other hand, the potential of TZD against leishmaniasis has not yet been explored, with the exception of a single study [31].
In the present work, we synthesized a library of sixty-one 5-stryryl-3-substituted thiazolidine-2,4-dione derivatives, in an endeavor to evaluate their in vitro antileishmanial activity.
2. Results and Discussion
2.1. Chemistry
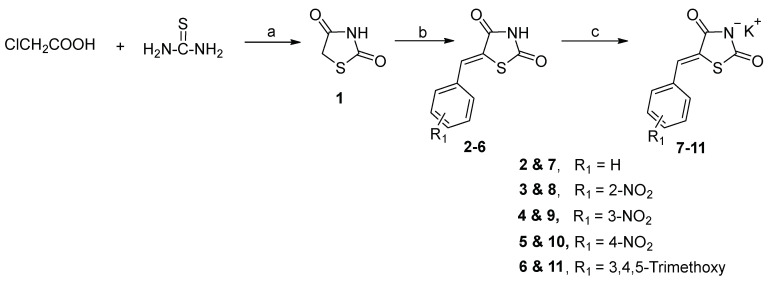
The 1,3-thiazolidine-2,4-dione core heterocycle (1), used as a starting material, was prepared via the reaction of chloroacetic acid with thiourea, as reported in our previous work [29] (Scheme 1).
Scheme 1.
Synthesis of the starting material 1. Reagents and conditions: (a) i. water, 0–5 °C, 20 min, ii. HCl, reflux, 12 h. (b) aromatic aldehyde, anhydrous toluene, cat. piperidinium acetate, reflux, 15 h. (c) KOH/ethanol-dioxane, boiling, 20 min. Yields = 75–90% for 2–6, and 69–90% for 7–11.
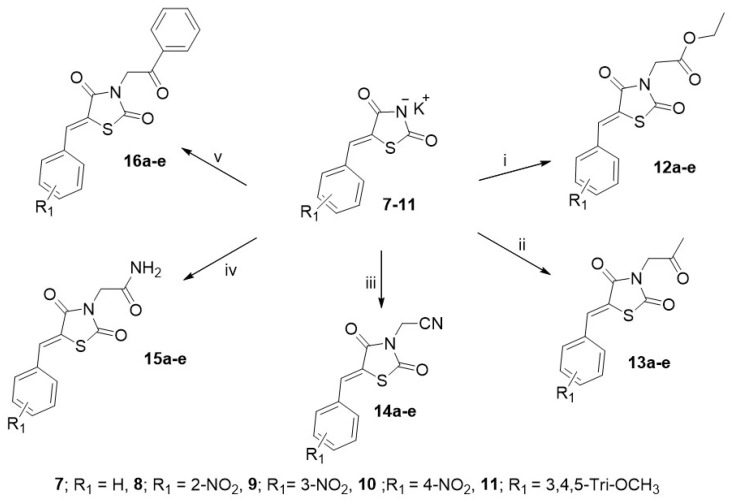
Compound 1 is a versatile heterocycle that allows the synthesis of various thiazolidinediones by virtue of its two reactive centers: (A) the acidic NH hydrogen at position 3 and (B) the active-methylene group at position 5. Thus, when this 5-methylene group was allowed to react, under Knoevenagel reaction conditions, with the aromatic aldehydes; benzaldehyde, o-, m-, p-nitrobenzaldehyde, and 3,4,5-trimethoxy benzaldehyde, the corresponding 5-stryryl-1,3-thiazolidine-2,4-diones 2–6 were obtained (Scheme 1) in 75–90% yields. These latter compounds 2–6, could be easily converted into the corresponding potassium salts 7–11 in 69–90% yields upon treatment with KOH in boiling ethanol or ethanol-dioxane mixture (Scheme 1). The reaction of the nucleophilic N-anion of the latter potassium salts 7–11 with a series of halo-compounds namely ethyl chloroacetate, chloroacetone, chloroacetonitrile, 2-chloroacetamide, and phenacyl bromide in refluxing DMF, resulted in the formation of a series of the corresponding target (Z)-N-substituted 5-styrylthiazolidinediones 12a-e to 16a-e (Scheme 2, Table 1) in 62–95% yields.
Scheme 2.
General synthesis of compounds 12–16. Reagents and conditions: heating in DMF at reflux for 6 h with (i) ethyl chloroacetate, (ii) chloroacetone, (iii) chloroacetonitrile, (iv) 2-chloroacetamide, (v) phenacyl bromide. Yields = 62–95%.
Table 1.
1,3-Thiazolidine-2,4-diones (12a-e to 16a-e).
| Cpd | R1 | Cpd | R1 | Cpd | R1 |
|---|---|---|---|---|---|
| 12a | H | 13e | 3,4,5-Tri-OCH3 | 15d | 4-NO2 |
| 12b | 2-NO2 | 14a | H | 15e | 3,4,5-Tri-OCH3 |
| 12c | 3-NO2 | 14b | 2-NO2 | 16a | H |
| 12d | 4-NO2 | 14c | 3-NO2 | 16b | 2-NO2 |
| 12e | 3,4,5-Tri-OCH3 | 14d | 4-NO2 | 16c | 3-NO2 |
| 13a | H | 14e | 3,4,5-Tri-OCH3 | 16d | 4-NO2 |
| 13b | 2-NO2 | 15a | H | 16e | 3,4,5-Tri-OCH3 |
| 13c | 3-NO2 | 15b | 2-NO2 | ||
| 13d | 4-NO2 | 15c | 3-NO2 |
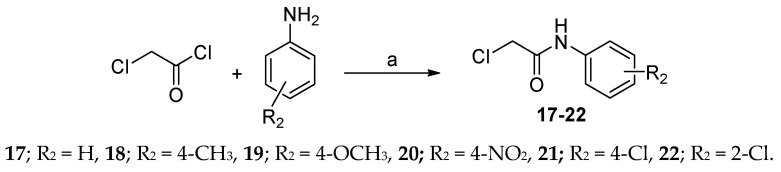
Some 2-chloro-N-arylacetamides (17–22) were prepared by chloroacetylation of the aromatic amines; aniline, 4-methylaniline, 4-methoxyaniline, 4-nitroaniline, 4-chloroaniline and 2-chloroaniline, using chloroacetyl chloride in dry DMF (Scheme 3).
Scheme 3.
Synthesis of N-aryl-2-chloroacetamides 23–28. Reagents and conditions: (a) dry DMF, 0–5 °C, 3 h, then rt, 3 h. Yields = 85–96%.
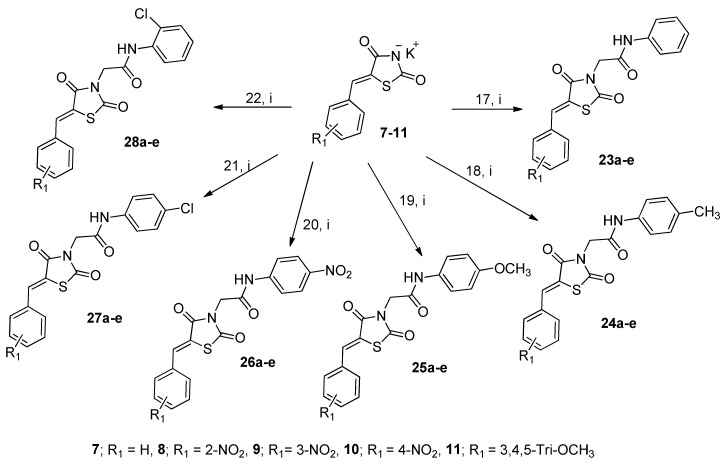
The 2-chloro-N-arylacetamides 17–22 were then reacted via their labile chlorine atoms with the potassium salts 7–11 in boiling DMF, affording another series of 30 new thiazolidine-2,4-dione derivatives 23a-e to 28a-e, as shown in Scheme 4 and Table 2.
Scheme 4.
General synthesis of thiazolidinediones 23a-e to 28a-e. Reagents and conditions: (i) DMF, reflux, 6 h. Yields = 69–94%.
Table 2.
1,3-Thiazolidine-2,4-diones (23a-e to 28a-e).
| Cpd | R1 | Cpd | R1 | Cpd | R1 |
|---|---|---|---|---|---|
| 23a | H | 25a | H | 27a | H |
| 23b | 2-NO2 | 25b | 2-NO2 | 27b | 2-NO2 |
| 23c | 3-NO2 | 25c | 3-NO2 | 27c | 3-NO2 |
| 23d | 4-NO2 | 25d | 4-NO2 | 27d | 4-NO2 |
| 23e | 3,4,5-Tri-OCH3 | 25e | 3,4,5-Tri-OCH3 | 27e | 3,4,5-Tri-OCH3 |
| 24a | H | 26a | H | 28a | H |
| 24b | 2-NO2 | 26b | 2-NO2 | 28b | 2-NO2 |
| 24c | 3-NO2 | 26c | 3-NO2 | 28c | 3-NO2 |
| 24d | 4-NO2 | 26d | 4-NO2 | 28d | 4-NO2 |
| 24e | 3,4,5-Tri-OCH3 | 26e | 3,4,5-Tri-OCH3 | 28e | 3,4,5-Tri-OCH3 |
All the obtained thiazolidinediones were found to possess exclusively a Z-configuration as evidenced by their 1H-NMR spectra. The methylidene =CH- proton of these compounds appeared as a deshielded singlet at 8.25–7.79 ppm. This deshielding is caused by the anisotropic effect of the neighboring C=O group at the 4-position. On the other hand, no signal indicating the presence of the E-isomer was observed. The =CH- proton of the E-isomer should appear around 6.2–6.3 ppm, due to the less deshielding effect of the S atom of the TZD ring [29]. Moreover, the Z-configuration is more thermodynamically stable due to the hydrogen bonding between methylidene hydrogen and the 4-C=O group. The X-ray single crystal analysis of compound 6 is a further unambiguous confirmation of the Z-configuration of 2–6 [32].
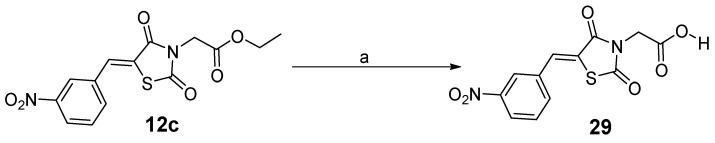
Finally, for discussing structure–activity relationships, the ethyl ester 12c was hydrolyzed into the corresponding carboxylic acid 29 (Scheme 5), following a procedure reported by Maccari et al. [33].
Scheme 5.
Acidic hydrolysis of ethyl ester 12c into 29. Reagents and conditions: (a) heating under reflux in glacial acetic acid and conc. HCl for 4 h. Yield = 90%.
It is of interest to note that the 1H-NMR spectrum of acid 29 did not show the signal corresponding to the carboxylic acid OH group. This could be explained by a rapid exchange of the acidic proton with the deuterium of the deuterated solvent DMSOd6. However, its 13C-NMR spectrum showed the expected 12 carbons which includes the carbonyl carbon of the -COOH group (cf. Supplementary Material).
2.2. In Vitro Assays
All the compounds belonging to the 5-stryryl-1,3-thiazolidine-2,4-dione series (apart from corrosive potassium salts 7–11) were first evaluated in vitro on the promastigote stage of L. infantum, to determine their half-maximal effective concentrations (EC50), and to compare the obtained results with those of some antileishmanial reference drug compounds: amphotericin B (the most potent of all antileishmanial drugs), miltefosine (the unique orally available antileishmanial drug) and fexinidazole sulfone (the active metabolite of fexinidazole). Out of these 61 tested compounds, 22 were found to be insufficiently soluble in the aqueous culture medium to be properly evaluated. Among the 38 remaining molecules, 21 showed moderate to good antileishmanial activity with EC50 values ≤ 30 µM, including 19 nitroaromatic derivatives, as presented in Table 3.
Table 3.
Results of the in vitro evaluations (activity and cytotoxicity on HepG2) of the 21 compounds presenting both good solubility in the culture medium (Schneider’s Drosophila Medium) and antileishmanial EC50 activity values < 30 µM.
| Cpd | Structure | R1 | R2 | Anti-L. infantum Promastigote Activity EC50 (µM) |
HepG2 Cytotoxicity CC50 (µM) |
Selectivity INDEX (SI = CC50/EC50) |
|---|---|---|---|---|---|---|
| 4 |
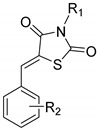
|
-H | 3-NO2 | 28.04 ± 7.15 | 190.26 ± 18.64 | 6.8 |
| 5 | -H | 4-NO2 | 14.60 ± 2.90 | 137.11 ± 1.22 | 9.4 | |
| 12a | -CH2COOEt | -H | 20.55 ± 5.86 | 193.84 ± 8.92 | 9.4 | |
| 12b | -CH2COOEt | 2-NO2 | 24.07 ± 5.14 | 39.35 ± 7.09 | 1.6 | |
| 12c | -CH2COOEt | 3-NO2 | 5.51 ± 1.29 | >125 * | >22.7 | |
| 12d | -CH2COOEt | 4-NO2 | 11.60 ± 2.37 | >125 * | >10.8 | |
| 14c | -CH2CN | 3-NO2 | 7.00 ± 2.96 | 100.77 ± 19.33 | 14.4 | |
| 14d | -CH2CN | 4-NO2 | 24.80 ± 6.09 | 108.52 ± 11.04 | 4.4 | |
| 15c | -CH2CONH2 | 3-NO2 | 13.31 ± 7.75 | >62.5 * | >4.7 | |
| 16b |
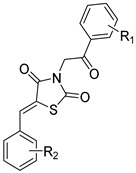
|
-H | 2-NO2 | 5.57 ± 1.28 | 11.04 ± 2.28 | 2.0 |
| 16c | -H | 3-NO2 | 3.22 ± 0.94 | >12.5 * | >3.9 | |
| 23b |
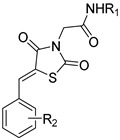
|
-Ph | 2-NO2 | 11.60 ± 3.43 | 22.28 ± 0.59 | 1.9 |
| 24b | 4-MePh- | 2-NO2 | 24.76 ± 6.68 | 30.95 ± 3.97 | 1.2 | |
| 25b | 4-OMePh- | 2-NO2 | 22.32 ± 4.93 | 23.29 ± 2.93 | 1.1 | |
| 26b | 4-NO2Ph- | 2-NO2 | 14.64 ± 2.91 | 30.12 ± 3.94 | 2.1 | |
| 26c | 4-NO2Ph- | 3-NO2 | 7.20 ± 1.48 | >25 * | >3.5 | |
| 26d | 4-NO2Ph- | 4-NO2 | 14.10 ± 2.80 | >25 * | >1.8 | |
| 27b | 4-ClPh- | 2-NO2 | 16.32 ± 3.85 | 34.55 ± 3.03 | 2.1 | |
| 27d | 4-ClPh- | 4-NO2 | 17.30 ± 4.17 | >12.5 * | >0.7 | |
| 27e | 4-ClPh- | 3,4,5-OMe | 5.00 ± 1.98 | 19.35 ± 2.05 | 3.9 | |
| 28b | 2-ClPh- | 2-NO2 | 16.07 ± 4.32 | 22.98 ± 3.20 | 1.4 | |
| 29 |
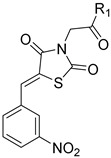
|
-OH | 3-NO2 | >50 | >250 | - |
| Amphotericin B | 0.06 ± 0.02 | 8.80 ± 0.30 | 146.7 | |||
| Miltefosine | 3.30 ± 0.80 | 85.00 ± 8.80 | 25.8 | |||
| Fexinidazole sulfone | 3.10 ± 0.70 | >200 | >64.5 | |||
| Doxorubicin | - | 0.20 ± 0.02 | - | |||
* Compound cytotoxicity could not be evaluated at higher concentrations because of a lack of solubility in the complete MEM culture medium at 37 °C.
Then, the cytotoxicity of these 21 bioactive molecules was evaluated against the human hepatic HepG2 cell line and compared to the one of doxorubicin (chosen as a reference cytotoxic drug compound), by determining their 50% cytotoxic concentrations (CC50), to calculate the corresponding selectivity indices (SI = CC50/EC50). Eight molecules showed low cytotoxicity (CC50 ≥ 50 µM) and 4 hit compounds bearing a nitro group on the benzene ring of the styryl moiety (5, 12c, 12d, 14c) were thus highlighted, presenting good activities (5.5 ≤ EC50 ≤ 14 µM), low cytotoxicities (101 ≤ CC50 ≤ 125 µM) and good selectivity index values (10 ≤ SI ≤ 23), as shown in Table 3. This in vitro biological activity profile appeared rather similar to that of the miltefosine reference drug (EC50 = 3.3 µM, CC50 = 85 µM, SI = 26) but remained far from reaching the high potency of amphotericin B (EC50 = 0.06 µM).
In terms of structure–activity relationships (SARs), it was initially observed that 19 out of the 21 stryrylthiazolidinone derivatives that showed antileishmanial activity (EC50 ≤ 30 µM), had a nitro group on the benzene ring of the styryl moiety (Table 3). The pharmacophore appeared to be a 5-styrylthiazolidin-2,4-dione scaffold, substituted at positions meta or para of the phenyl ring by a nitro group (compounds 4 and 5). Compounds 12a and 27e were the only ones without a nitro group. Compound 27e showed a moderate selectivity index (SI = 3.9), whereas compound 12a was more promising (SI = 9.4), due to its low cytotoxicity, indicating a positive contribution of the ethyloxycarbonylmethyl group at position 3 of the thiazolidine-2,4-dione ring.
All the derivatives bearing a nitro group at the ortho position of the phenyl ring were moderately active and/or selective. Logically, combining an ethyloxycarbonylmethyl group at position 3 of the thiazolidine-2,4-dione ring and a nitro group at position meta of the phenyl ring led to a first hit compound (12c) with good efficacy (EC50 = 5.51 µM) and selectivity (SI > 22.7). Maintaining the meta-nitrostyryl moiety and introducing a cyanomethyl group at position 3 of the thiazolidine-2,4-dione ring led to a second hit compound (14c), showing both activity (EC50 = 7 µM) and selectivity (SI = 14.4). The para-nitro isomers of 12c and 14c; 12d and 14d, were less active and less selective. Among all other substituents introduced at position 3 of the thiazolidine-2,4-dione ring, none allowed any improvement, neither regarding antileishmanial activity, nor cytotoxicity. Introducing a second nitroaromatic moiety on the pharmacophore (compounds 26b-d) was not favorable.
Examining the structure of the two hit compounds 12c and 14c revealed that 12c bears an ester function which is usually quite labile in vivo. Consequently, it was crucial to determine whether its carboxy metabolite was active or not. Thus, the ester compound 12c was subjected to acid hydrolysis to give the corresponding acid 29 (Scheme 5), and this latter was evaluated in vitro (Table 3). Unfortunately, despite being non-cytotoxic (CC50 > 250 µM), carboxylic acid 29 was not active on L. infantum (EC50 > 50 µM), making the ester 12c a poor candidate for further development. Thus, compound 14c was regarded as the best molecule in the series. Accordingly, it was assessed for its cytotoxicity on a second human cell line (THP-1) along with its lipophilicity and aqueous solubility, to evaluate its potential for further hit to lead chemistry (Table 4). Compound 14c was not cytotoxic on the THP-1 cell line (CC50 = 121 µM), and presented a measured logD7.4 value of 2.85, making it a lipophilic molecule. However, 14c showed a PBS (pH 7.4) solubility of 16 µM (logS = 4.8). These complementary parameters were in favor of pursuing the development of this chemical series to search for a new derivative with improved potency.
Table 4.
THP-1 cytotoxicity and physicochemical properties of hit compound 14c.
| Cpd | Structure | THP-1 CC50 (µM) | CHI logD7.4 | PBS Solubility a (log S) |
|---|---|---|---|---|
| 14c |
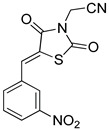
|
121 ± 9 | 2.85 | 4.8 |
| Doxorubicin | 2.2 ± 0.1 | - | ||
| Amphotericin B | 17.6 ± 3 | - | ||
| Miltefosine | 30 ± 4 | - | ||
| Fexinidazole sulfone | >200 | - | ||
a PBS: Phosphate-buffered saline (pH = 7.4).
For decades, antiparasitic (more precisely antiprotozoal) properties were reported for many nitroaromatic compounds, including commercial drug compounds such as metronidazole, tinidazole, secnidazole, ornidazole (anti-T. vaginalis, anti-G. intestinalis, anti-E. histolytica), nifurtimox and benznidazole (anti-T. cruzi) or nitazoxanide (anti-C. parvum). These nitroaromatic compounds were shown to behave as prodrugs which are bioactivated in the parasite cell by nitroreductase enzymes (type 1 and/or 2), leading to lethal cytotoxic metabolites [4,34]. Indeed, nitroaromatics remain attractive chemotypes for the conception of selective and potent novel antiparasitic candidates, such as recently approved fexinidazole (anti-T. brucei gambiense), but also to develop novel antibacterial compounds, as recently illustrated with the approval of antitubercular drug compounds delamanid and pretomanid.
To date, two leishmanial nitroreductases have been characterized: an essential type 1 mitochondrial NTR1 (PMID: 23208716) and a cytosolic type 2 NTR2 (PMID: 27812217), that catalyze the 1-electron reduction in nitroaromatics. To determine if NTR-mediated bio-activation played a role in the mechanism of action of compounds in this series, the potency of 14c was assessed against both wild-type L. donovani (LdBOB)and transgenic L. donovani overexpressing either NTR1 or NTR2. Indeed, parasites overexpressing these nitroreductases were hyper-sensitive to 14c compared to wild-type, most notably promastigotes expressing elevated levels of NTR1 (Table 5). This strongly suggests that 14c is a substrate of Leishmania NTRs, and that bioactivation by these enzymes is a key factor in its antileishmanial activity. However, beyond the bioactivation of these nitroaromatic derivatives by parasitic NTRs, it is possible that stryrylthiazolidin-2,4-diones interact with other antileishmanial targets, such as reported in the literature dealing with inhibition of pteridine reductase 1 [31], perhaps explaining the antileishmanial activity noted with non-nitrated compounds 12a and 27e.
Table 5.
Sensitivity of wild-type and NTR-overexpressing L. donovani promastigotes to compound 14c.
| Cell Line | EC50 Values, nM | ||
|---|---|---|---|
| 14c | Nifurtimox | Delamanid | |
| WT | 22,085 ± 1914 | 3108 ± 223 | 3 ± 0.1 |
| NTR1OE | 3777 ± 313 | 144 ± 10 | 2 ± 0.3 |
| NTR2OE | 18,467 ± 1007 | 163 ± 11 | 0.3 ± 0.001 |
Data represent the weighted mean ± standard deviation for five independent experiments.
3. Material and Methods
3.1. Chemistry
3.1.1. General Methods
The starting materials were purchased from Sigma-Aldrich (Saint Louis, MO, USA). All melting points were determined on a Stuart melting point apparatus SMP3 and are uncorrected. IR spectra were recorded on a Nicolet iS10 FT-IR spectrometer using KBr wafer technique. The 1H-NMR spectra were recorded on a Bruker Avance 400 spectrometer operating at 400 MHz (1H) or 100 MHz (13C). 1H- and 13C-NMR chemical shifts (δ) were reported in parts per million (ppm) and were referenced to the residual proton peaks in the deuterated solvent DMSO-d6 (2.50 ppm for 1H and 39.70 ppm for 13C). Multiplicities are represented by s (singlet), d (doublet), t (triplet), q (quartet), and m (multiplet). Coupling constants (J) are reported in Hertz (Hz). Elemental analyses (C, H, N) were carried out using a Perkin Elmer 240 C Micro analyzer at the Microanalytical Laboratory at Assiut University, and the results obtained were found in good agreement with the theoretical values within ±0.4%. All the compounds involved in biological evaluation showed a level of purity above 95%. Thiazolidine-2,4-dione (1) was prepared according to a literature procedure [31].
3.1.2. Physicochemical Determination of Aqueous Solubility and Partition Coefficient
The solubility of 14c was evaluated by the shake-flask method (N = 2). Briefly, an excess of compound (approx. 2 mg) was stirred in PBS (0.5 mL, 0.15 M, pH 7.4) at 25 °C for 72 h. A 100 µL sample was drawn, any excess material removed by centrifugation, and diluted tenfold with methanol/water 1:1 before HPLC-UV quantification using a pre-established calibration curve (PLRP-S column (Agilent), 3 µm particle size, 100 A porosity, 50 × 2.1 mm internal dimensions). Eluent: isocratic acetonitrile/water/formic acid 80:20:0.1, 0.4 mL/min, detection at 320 nm, 2-µL injections).
Lipophilicity was evaluated by the Chromatographic Hydrophobicity Index (CHI) [35] on a Restek Ultra C18 column (5 µm particle size, 100 A porosity, 50 × 2.1 mm internal dimensions) using a gradient of A: 10 mM ammonium acetate, adjusted to pH 7.4; B: acetonitrile, at a flow rate of 0.300 mL/min and 0.5-µL injections.
The validity of the CHI versus retention time was established on a reference mixture containing theophylline, phenyltetrazole, benzimidazole, acetophenone, propiophenone, butyrophenone and valerophenone. The correlation between CHI and logD was taken from literature data: logD7.4CHI = 0.054 × CHI-1.467 [35].
3.1.3. Organic Synthesis and Product Characterization
General Procedure for the Synthesis of the Potassium 5-Arylidenethiazolidin-2,4-dion-3-ides 7–11
A solution of potassium hydroxide (0.123 g, 2.2 mmol) in ethanol (7 mL) was added to a hot solution of compounds 2–6 (2 mmol) in an ethanol-dioxane mixture (30 mL). The reaction mixture was then heated under stirring for 20 min. The solid products obtained were filtered, washed with ethanol, dried, and crystallized.
(1) Potassium (Z)-5-benzylidenethiazolidine-2,4-dion-3-ide (7). White solid from ethanol-dioxane, yield (69%) mp 320 °C (charring). IR (ν cm−1): 3080 (CH arom.), 3052 (CH arom.), (3016 CH arom.), 1674 (C=O), 1617, 1617, 1548, 1491. 1H-NMR (400 MHz, DMSO) δ (ppm): 7.53 (d, J = 7.5 Hz, 2H, Ph-H), 7.43 (t, J = 7.7 Hz, 2H, Ph-H), 7.34 (s, 1H, -CH=), 7.30 (t, J = 7.3 Hz, 1H, Ph-H). 13C-NMR (100 MHz, DMSO) δ (ppm): 183.27 (C=O), 176.30 (C=O), 136.45 (C), 136.24 (=CH-), 129.47 (2CH), 129.22 (2CH), 128.32 (C), 122.95 (CH). Anal. Calcd. for C10H6KNO2S (243.32): C, 49.36; H, 2.49; N, 5.76; S, 13.18. Found: C, 49.49; H, 2.40; N, 5.69; S, 13.08%.
(2) Potassium (Z)-5-(2-nitrobenzylidene)thiazolidine-2,4-dion-3-ide (8). Yellow solid from ethanol-dioxane, yield (87%), mp 220 °C. IR (ν cm−1): 1691 (C=O), 1616, 1570, 1556, 1542 1473. 1H-NMR (400 MHz, DMSO) δ (ppm): 7.99 (d, J = 8.1 Hz, 1H, Ar-H), 7.79 (s, 1H, -CH=), 7.78–7.76 (m, 1H, Ar-H), 7.56–75.2 (m, 1H, Ar-H), 7.44 (s, 1H, Ar-H). 13C-NMR (100 MHz, DMSO) δ (ppm): 182.39 (C=O), 175.31 (C=O), 149.20 (C), 142.33 (C), 133.75 (CH), 131.06 (C), 129.36 (CH), 129.11 (CH), 124.93 (CH), 116.27 (CH). Anal. Calcd. for C10H5KN2O4S (288.32): C, 41.66; H, 1.75; N, 9.72; S, 11.12. Found: C, 41.53; H, 1.66; N, 9.62; S, 11.33%.
(3) Potassium (Z)-5-(3-nitrobenzylidene)thiazolidine-2,4-dion-3-ide (9). Yellow solid from ethanol-dioxane, yield (85%), mp 300 °C (charring). IR (ν cm−1): 3091 (C-H arom), 3065 (C-H arom.), 2871 (C-H aliph.), 1678 (C=O), 1607, 1545, 1352. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.38 (t, J = 2.0 Hz, 1H, Ar-H), 8.14 (ddd, J = 8.2, 2.3, 0.9 Hz, 1H, Ar-H), 7.97 (d, J = 7.8 Hz, 1H, Ar-H), 7.72 (t, J = 8.0 Hz, 1H, Ar-H), 7.45 (s, 1H, -CH=). 13C-NMR (100 MHz, DMSO) δ (ppm): 182.03 (C=O), 174.57 (C=O), 148.65 (C), 139.40 (=CH-), 138.14 (C), 135.73 (CH), 130.73 (CH), 123.16 (CH), 122.61 (CH), 120.62 (C). Anal. Calcd. for C10H5KN2O4S (288.32): C, 41.66; H, 1.75; N, 9.72; S, 11.12. Found: C, 41.55; H, 1.63; N, 9.68; S, 11.16%.
(4) Potassium (Z)-5-(4-nitrobenzylidene)thiazolidine-2,4-dion-3-ide (10). Yellow solid from ethanol-dioxane, yield (90%), mp 310 °C (charring). IR (ν cm−1): 3107 (C-H arom.), 2897 (C-H aliph.), 2864 (C-H aliph.), 1693 (C=O), 1619 1596, 1577, 1528. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.27 (d, J = 8.8 Hz, 2H, Ar-H), 7.77 (d, J = 8.8 Hz, 2H, Ar-H), 7.40 (s, 1H, -CH=). 13C-NMR (100 MHz, DMSO) δ (ppm): 182.82 (C=O), 174.94 (C=O), 146.25 (C), 143.34 (=CH-), 142.03 (C), 130.15 (2CH), 124.40 (2CH), 120.14 (C). Anal. Calcd. for C10H5KN2O4S (288.32): C, 41.66; H, 1.75; N, 9.72; S, 11.12. Found: C, 41.60; H, 1.71; N, 9.65; S, 11.32%.
General Procedure for the Synthesis of 5-Arylidenethiazolidinediones 12b-e–16b-e
An equimolar mixture (1 mmol) of ethyl chloroacetate (0.123 g, 0.107 mL), and the potassium salts; 7 (0.234 g), 8 (0.288 g), 9 (0.288 g), 10 (0.288 g) or 11 (0.333 g) in DMF (5 mL) was heated under reflux for 6 h. After cooling, the solid products obtained were filtered, washed with water, dried, and recrystallized.
-
(1)
Synthesis of compounds 12b-e
(1) Ethyl ((Z)-5-(2-nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetate (12b). Brownish yellow crystals from ethanol, yield (79%), mp 81–83 °C. IR (ν cm−1): 3068 (C-H arom.), 3009 (C-H arom.), 2983 (C-H aliph.), 2937 (C-H aliph) 1741 (C=O), 1694 (C=O), 1607, 1568. 1568. 1524. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.25–8.23 (m, 2H, (-CH= + Ar-H), 7.92 (t, J = 7.5 Hz, 1H, Ar-H), 7.74–7.78 (m, 2H, Ar-H), 4.52 (s, 2H, CH2), 4.20 (q, J = 7.2 Hz, 2H, CH3CH2), 1.23 (t, J = 7.2 Hz, 3H, CH3CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.12 (C=O), 167.07 (C=O), 164.66 (C=O), 148.25 (C), 135.17 (-CH=), 131.94 (CH), 131.85 (CH), 129.81 (CH), 129.20 (C), 126.02 (CH), 125.42 (CH), 62.18 (OCH2), 42.76 (COCH2), 14.40 (CH3CH2). Anal. Calcd. for C14H12N2O6S (336.32): C, 50.00; H, 3.60; N, 8.33; S, 9.53. Found: C, 49.71; H, 3.61; N, 8.28; S, 9.55%.
(2) Ethyl ((Z)-5-(3-nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetate (12c). Yellow crystals from ethanol, yield (86%), mp 130–132 °C. IR (ν cm−1): 3067 (C-H arom.), 3000 (C-H arom.), 2963 (C-H aliph.), 2939 (C-H aliph.), 1734 (C=O), 1704 (C=O), 1689 (C=O), 1606, 1571, 1533. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.53 (t, J = 1.9 Hz, 1H, Ar-H), 8.34 (ddd, J = 8.3, 2.2, 0.8 Hz, 1H, Ar-H), 8.19 (s, 1H, -CH=), 8.08 (d, J = 7.9 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 4.53 (s, 2H, -CH2-), 4.19 (q, J = 7.2 Hz, 2H, CH2CH3), 1.23 (t, J = 7.2 Hz, 3H, CH2CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.06 (C=O), 166.79 (C=O), 165.10 (C=O), 148.76 (C), 135.93 (-CH=), 134.88 (C), 132.37 (CH), 131.47 (CH), 125.41 (CH), 125.34 (CH), 123.97 (C), 62.19 (OCH2), 42.84 (COCH2), 14.42 (CH3CH2). Anal. Calcd. for C14H12N2O6S (336.32): C, 50.00; H, 3.60; N, 8.33; S, 9.53. Found: C, 49.63; H, 3.51; N, 8.17; S, 9.57%.
(3) Ethyl ((Z)-5-(4-nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetate (12d). Yellow platelets from ethanol, yield (88%), mp 136–138 °C. IR (ν cm−1): 3111 (C-H arom.), 2995 (C-H aliph.), 1738 (C=O), 1698 (C=O), 1607, 1595, 1512. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.35 (d, J = 8.8 Hz, 2H, Ar-H), 8.11 (s, 1H, -CH=), 7.91 (d, J = 8.8 Hz, 2H, Ar-H), 4.53 (s, 2H, -CH2-), 4.19 (q, J = 7.2 Hz, 2H, CH2CH3), 1.22 (t, J = 7.2 Hz, 3H, CH2CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.03 (C=O), 166.93 (C=O), 165.07 (C=O), 148.36 (C), 139.38 (-CH=), 132.03 (C), 131.65 (2CH), 125.25 (C), 124.77 (2CH), 62.20 (O-CH2), 42.84 (CO-CH2), 14.40 (CH3). Anal. Calcd. for C14H12N2O6S (336.32): C, 50.00; H, 3.60; N, 8.33; S, 9.53. Found: C, 49.88; H, 3.51; N, 8.21; S, 9.40%.
-
(2)
Synthesis of compounds 13a-e
These compounds were prepared using 1 mmol of chloroacetone (0.093 g, 0.08 mL) following the above procedure described for 12a-e.
(1) (Z)-5-Benzylidene-3-(2-oxopropyl)thiazolidine-2,4-dione (13a). Pale-yellow crystals from ethanol, yield (80%), mp 139–140 °C. IR (ν cm−1): 3050 (CH arom.), 3017 (CH arom.), 2975 (CH aliph.), 2933 (CH aliph.), 1743 (C=O), 1727 (C=O), 1682 (C=O), 1608, 1597, 1570, 1573. 1H-NMR (400 MHz, DMSO) δ (ppm): 7.96 (s, 1H, -CH=), 7.65–7.63 (m, 2H, Ph-H), 7.57–7.49 (m, 3H, Ph-H), 4.68 (s, 2H, CH2), 2.26 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 200.77 (C=O), 167.30 (C=O), 165.67 (C=O), 133.95 (C=O), 133.27 (-CH=), 131.27 (C), 130.67 (2CH), 129.85 (2CH), 121.30 (C), 50.88 (CH2), 27.34 (CH3). Anal. Calcd. for C13H11NO3S (261.30): C, 59.76; H, 4.24; N, 5.36; S, 12.27. Found: C, 59.56; H, 4.20; N, 5.29; S, 12.21%.
(2) (Z)-5-(2-Nitrobenzylidene)-3-(2-oxopropyl)thiazolidine-2,4-dione (13b). Shiny-buff crystals from ethanol, yield (79%), mp 146–148 °C. IR (ν cm−1): 3102 (CH arom.), 3073 (CH arom.), 2984 (CH aliph.), 2945 (CH aliph.), 2861 (C-H aliph.), 1749 (C=O), 1732 (C=O), 1694 (C=O), 1615, 1603, 1570 and 1528. 1H NMR (400 MHz, DMSO) δ (ppm): 8.26–8.23 (m, 1H, Ar-H), 8.21 (s, 1H, -CH=), 7.94–7.90 (m, 1H, Ar-H), 7.76 (m, 2H, Ar-H), 4.70 (s, 2H, CH2), 2.27 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 200.76 (C=O), 167.10 (C=O), 164.80 (C=O), 148.26 (C), 135.16 (-CH=), 131.79 (CH), 131.47 (CH), 129.82 (CH), 129.28 (C), 126.02 (CH), 125.73 (C), 50.85 (CH2), 27.38 (CH3). Anal. Calcd. for C13H10N2O5S (306.29): C, 50.98; H, 3.29; N, 9.15; S, 10.47. Found: C, 50.81; H, 3.23; N, 9.10; S, 10.40%.
(3) (Z)-5-(3-Nitrobenzylidene)-3-(2-oxopropyl)thiazolidine-2,4-dione (13c). Small lustrous brown crystals from ethanol, yield (88%), mp 188–190 °C. IR (ν cm−1): 3073 (CH arom.), 2992 (CH aliph.), 1756 (C=O), 1729 (C=O), 1686 (C=O), 1604, 1570, 1528. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.52 (t, J = 1.9 Hz, 1H, Ar-H), 8.32 (ddd, J = 8.2, 2.2, 0.8 Hz,1H, Ar-H), 8.15 (s, 1H, -CH=), 8.07 (d, J = 8.0 Hz, 1H, Ar-H), 7.85 (t, J = 8.0 Hz, 1H, Ar-H), 4.71 (s, 2H, -CH2-), 2.27 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 200.73 (C=O), 166.76 (C=O), 165.23 (C=O), 148.76 (C), 135.90 (-CH=), 134.98 (C), 131.92 (CH), 131.45 (CH), 125.28 (CH), 124.29 (C), 50.93 (CH2), 27.52 (CH3). Anal. Calcd. for C13H10N2O5S (306.29): C, 50.98; H, 3.29; N, 9.15; S, 10.47. Found: C, 50.79; H, 3.23; N, 9.11; S, 10.39%.
(4) (Z)-5-(4-Nitrobenzylidene)-3-(2-oxopropyl)thiazolidine-2,4-dione (13d). Yellow crystals from ethanol-dioxane, yield (95%), mp 207–209 °C. IR (ν cm−1): 3014 (CH arom.), 2975 (CH aliph.), 2933 (CH aliph.), 1731 (C=O), 1682 br (C=O), 1608, 1514 and 1407. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.36 (d, J = 8.8 Hz, 2H, Ar-H), 8.08 (s, 1H, -CH=), 7.91 (d, J = 8.8 Hz, 2H, Ar-H), 4.71 (s, 2H, CH2), 2.27 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 200.70 (C=O), 166.82 (C=O), 165.22 (C=O), 148.19 (C), 139.52 (-CH=), 131.60 (C + 2CH), 125.60 (C), 124.78 (2CH), 50.94 (CH2), 27.51 (CH3). Anal. Calcd. for C13H10N2O5S (306.29): C, 50.98; H, 3.29; N, 9.15; S, 10.47. Found: C, 50.91; H, 3.21; N, 9.09; S, 10.43%.
-
(3)
Synthesis of compounds 14a-e
These compounds were prepared using 1 mmol of 2-chloroacetonitrile (0.076 g, 0.064 mL), following the above procedure described for 12a-e.
(1) (Z)-2-(5-Benzylidenethiazolidine-2,4-dion-3-yl)acetonitrile (14a). Brownish-yellow crystals from ethanol, yield (80%), mp 230–232 °C. IR (ν cm−1): 3000 (CH arom.), 2958 (CH arom.), 2252 (CN), 1777 (C=O), 1747 (C=O), 1697 (C=O), 1608, 1571, 1493. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.00 (s, 1H, -CH=), 7.65–7.63 (m, 2H, Ph-H), 7.58–7.50 (m, 3H, Ph-H), 4.81 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 166.87 (C=O), 164.70 (C=O), 134.66 (-CH=), 133.17 (C), 131.44 (CH), 130.74 (2CH), 129.91 (2CH), 120.95 (CH), 115.02 (C), 29.26 (CH2). Anal. Calcd. for C12H8N2O2S (244.27): C, 59.01; H, 3.30; N, 11.47; S, 13.12. Found: C, 59.16; H, 3.27; N, 11.41; S, 13.09%.
(2) (Z)-2-(5-(2-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetonitrile (14b). Yellow crystals from ethanol, yield (85%), mp 119–120 °C. IR (ν cm−1): 3003 (CH arom.), 2963 (CH arom.), 1746 (C=O), 1694 br (C=O), 1619 (C=O), 1619, 1604, 1571. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.26–824 (m, 2H, Ar-H + -CH=), 7.93 (td, J = 7.6, 0.9 Hz, 1H, Ar-H), 7.79–7.72 (m, 2H, Ar-H), 4.82 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 166.73 (C=O), 164.03 (C=O), 148.24 (C), 135.24 (-CH=), 131.94 (CH), 131.90 (CH), 129.75 (CH), 129.22 (C), 126.06 (CH), 125.51 (C), 114.94 (CN), 29.32 (CH2). Anal. Calcd. for C12H7N3O4S (289.27): C, 49.83; H, 2.44; N, 14.53; S, 11.08. Found: C, 49.70; H, 2.36; N, 14.42; S, 11.12%
(3) (Z)-2-(5-(3-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetonitrile (14c). Beige crystals from ethanol-dioxane, yield (95%), mp 173–175 °C. IR (ν cm−1): 3084 (CH arom.), 2998 (CH aliph.), 2957 (CH aliph.), 1751 (C=O), 1688b (C=O), 1608, 1570, 1541. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.52 (t, J = 2.0 Hz, 1H, Ar-H), 8.34 (dt, J = 10.9, 4.3 Hz, 1H, Ar-H), 8.20 (s, 1H, -CH=), 8.07 (d, J = 7.7 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 4.83 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 166.38 (C=O), 164.45 (C=O), 148.80 (C), 135.88 (-CH=), 134.87 (C), 132.23 (CH), 131.52 (CH), 125.43 (CH), 125.29 (CH), 124.10 (C), 114.93 (CN), 29.40 (CH2). Anal. Calcd. for C12H7N3O4S (289.27): C, 49.83; H, 2.44; N, 14.53; S, 11.08. Found: C, 49.80; H, 2.35; N, 14.27; S, 11.12%.
(4) (Z)-2-(5-(4-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetonitrile (14d). Fine brownish-yellow crystals from ethanol-dioxane, yield (87%), mp 193–195 °C. IR (ν cm−1): 3079 (C-H arom.), 3029 (C-H arom.), 2997 (C-H aliph.), 2956 (C-H aliph.), 1753 (C=O), 1698 br (C=O), 1608, 1596 and 1530. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.35 (d, J = 8.8 Hz, 2H, Ar-H), 8.11 (s, 1H, -CH=), 7.89 (d, J = 8.8 Hz, 2H, Ar-H), 4.83 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 166.43 (C=O), 164.43 (C=O), 148.22 (C), 139.37 (C), 131.88 (-CH=), 131.61 (2CH), 125.38 (C), 124.80 (2CH), 114.87 (CH), 29.40 (CH2). Anal. Calcd. for C12H7N3O4S (289.27): C, 49.83; H, 2.44; N, 14.53; S, 11.08. Found: C, 49.70; H, 2.39; N, 14.50; S, 11.20%.
-
(4)
Synthesis of compounds 15a-e
These compounds were prepared using 2-chloroacetamide (1 mmol, 0.093 g), following the procedure described for 12a-e.
(1) (Z)-2-(5-Benzylidenethiazolidine-2,4-dion-3-yl)acetamide (15a). Yellow crystals from ethanol, yield (76%), mp 229–231 °C. IR (ν cm−1): 3365, 3180 (NH2), 3056 (C-H arom.), 2942 (C-H aliph.), 1739 (C=O), 1693 (C=O), 1667 (C=O), 1607, 1573, 1492, 1448.1H-NMR (400 MHz, DMSO) δ (ppm): 7.97 (s, 1H, -CH=), 7.75 (s, 1H, NH), 7.66–7.65 (m, 2H, Ph-H), 7.58–7.51 (m, 3H, Ph-H), 7.34 (s, 1H, NH), 4.25 (s, 2H, CH2) 13C-NMR (100 MHz, DMSO) δ (ppm): 167.58 (C=O), 167.30 (C=O), 165.79 (C=O), 133.72 (-CH=), 133.38 (C), 131.20 (C), 130.62 (2CH), 129.88 (2CH), 121.70 (C), 43.80 (CH2). Anal. calcd. For C12H10N2O3S (262.28): C, 54.95; H, 3.84; N, 10.68; S, 12.22. Found: C, 54.87; H, 3.78; N, 10.60; S, 12.19%
(2) (Z)-2-(5-(2-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (15b). Yellow crystals from ethanol, yield (89%), mp 233–235 °C. IR (ν cm−1): 3441 (N-H), 3315 (N-H), 3181 (N-H), 1747 (C=O), 1678b (C=O), 1607, 1525 and 1474. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.25–8.23 (m, 1H, Ar-H), 8.19 (s, 1H, -CH=), 7.92 (t, J = 7.5 Hz, 1H, Ar-H), 7.78–7.75 (m, 3H, Ar-H + NH), 7.36 (s, 1H, NH), 4.26 (s, 2H, CH2).13C-NMR (100 MHz, DMSO) δ (ppm): 167.35 (C=O), 167.22 (C=O), 165.05 (C=O), 148.30 (C), 135.17 (-CH=), 131.74 (CH), 130.93 (CH), 129.77 (CH), 129.32 (C), 126.08 (C), 126.05 (CH), 43.87 (CH2). Anal. calcd. for C12H9N3O5S (307.28): C, 46.91; H, 2.95; N, 13.68; S, 10.43. Found: C, 46.83; H, 2.90; N, 13.61; S, 10.32%.
(3) (Z)-2-(5-(3-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (15c). Yellow crystals from ethanol-dioxane, yield (87%), mp 256–258 °C. IR (ν cm−1): 3441 (N-H), 3316 (N-H), 3190 (N-H), 3079 (C-H arom), 1747 (C=O), 1678b (C=O),1607, 1525 and 1444. 1H NMR (400 MHz, DMSO) δ (ppm): 8.52 (s, 1H, Ar-H), 8.32 (dd, J = 7.1, 1.1 Hz, 1H, Ar-H), 8.14 (s, 1H, -CH=), 8.07 (d, J = 7.9 Hz, 1H, Ar-H), 7.85 (t, J = 8.0 Hz, 1H, Ar-H), 7.76 (s, 1H, NH), 7.36 (s, 1H, NH), 4.26 (s, 2H, CH2).13C-NMR (100 MHz, DMSO) δ (ppm): 167.18 (C=O), 167.10 (C=O), 165.48 (C=O), 148.77 (C), 135.86 (-CH=), 135.06 (C), 131.46 (2CH), 125.23 (CH), 125.18 (CH), 124.67 (C), 43.95 (CH2). Anal. calcd. for C12H9N3O5S (307.28): C, 46.91; H, 2.95; N, 13.68; S, 10.43. Found: C, 46.85; H, 2.87; N, 13.59; S, 10.39%.
(4) (Z)-2-(5-(4-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (15d). Small shiny yellow crystals from ethanol-dioxane, yield (94%), mp 281–283 °C. IR (ν cm−1): 3432, 3325, 3199 (NH2), 3026 (CH arom.), 2981 (CH aliph.), 2942 (CH aliph.), 1747 (C=O), 1683 br. (C=O), 1694 (C=O), 1607, 1509, 1442. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.36 (d, J = 8.8 Hz, 2H, Ar-H), 8.07 (s, 1H, -CH=), 7.91 (d, J = 8.8 Hz, 2H, Ar-H), 7.76 (s, 1H, NH), 7.36 (s, 1H, NH), 4.26 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.17 (C=O), 167.09 (C=O), 165.47 (C=O), 148.14 (C), 139.62 (=CH-), 131.54 (C), 131.16 (C), 126.00 (C), 124.80 (2CH), 43.97 (CH2). Anal. calcd. for C12H9N3O5S (307.28): C, 46.91; H, 2.95; N, 13.68; S, 10.43. Found: C, 46.97; H, 2.88; N, 13.60; S, 10.36%.
-
(5)
Synthesis of compounds 16b-e
These compounds were prepared using phenacyl bromide (1 mmol, 0.199 g), following the procedure described for 12b-e.
(1) (Z)-5-(2-Nitrobenzylidene)-3-(2-oxo-2-phenylethyl)thiazolidine-2,4-dione (16b). Yellow crystals from ethanol-dioxane, yield (82%), mp 156–158 °C. IR (ν cm−1): 3058 (C-H arom), 2937 (C-H arom.), 2977 (C-H aliph.), 2973 (C-H aliph.), 1746 (C=O), 1693 (C=O), 1682 (C=O), 16,204, 1595, 1570. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.27–8.25 (m, 2H, =CH- + Ar-H), 8.12–8.10 (m, 2H, Ph-H), 7.93 (t, J = 7.5 Hz, 1H, Ar-H), 7.81–7.74 (m, 3H, Ph-H), 7.62 (t, J = 7.7 Hz, 2H, Ar-H), 5.37 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 191.71 (C=O), 167.27 (C=O), 164.97 (C=O), 148.29 (C), 135.19 (-CH=), 134.99 (C), 134.21 (C), 131.82 (CH), 131.73 (C), 129.85 (CH), 129.52 (2CH), 129.29 (CH), 128.82 (2CH), 126.05 (CH), 125.69 (C), 48.46 (CH2). Anal. calcd. for C18H12N2O5S (368.36): C, 58.69; H, 3.28; N, 7.60; S, 8.70. Found: C, 58.60; H, 3.17; N, 7.54; S, 8.66%.
(2) (Z)-5-(3-Nitrobenzylidene)-3-(2-oxo-2-phenylethyl)thiazolidine-2,4-dione (16c). Yellow crystals from ethanol-dioxane, yield (85%), mp 160–162 °C. IR (ν cm−1): 3081 (C-H arom), 3067 (C-H arom.), 2934 (C-H aliph.), 1749 (C=O), 1708 (C=O), 1683 (C=O), 1599, 1535, 1449. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.53 (t, J = 1.9 Hz, 1H, Ar-H), 8.33 (dd, J = 8.2, 2.1 Hz, 1H, Ar-H), 8.18 (s, 1H, -CH=), 8.11–8.08 (m, 3H, Ph-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 7.76 (t, J = 7.0 Hz, 1H Ar-H), 7.61 (t, J = 7.7 Hz, 2H, Ph-H), 5.37 (s, 2H, CH2). 13C-NMR (400 MHz, DMSO) δ (ppm): 191.66 (C=O), 166.91 (C=O), 165.38 (C=O), 148.74 (C), 135.93 (-CH=), 134.99 (C), 134.95 (C), 134.19 (CH), 132.14 (CH), 131.45 (CH), 129.52 (2CH), 128.82 (2CH), 125.34 (CH), 125.29 (CH), 124.23 (C), 48.54 (CH2). Anal. calcd. for C18H12N2O5S (368.36): C, 58.69; H, 3.28; N, 7.60; S, 8.70. Found: C, 58.75; H, 3.22; N, 7.51; S, 8.65%.
(3) (Z)-5-(4-Nitrobenzylidene)-3-(2-oxo-2-phenylethyl)thiazolidine-2,4-dione (16d). Brown crystals from ethanol-dioxane, yield (83%), mp 232–233 °C. IR (ν cm−1): 3073 (C-H arom), 3013 (C-H arom.), 2969 (C-H aliph.), 2933 (C-H aliph.), 1755 (C=O), 1697 (C=O), 1682 (C=O), 1607, 1595, 1517. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.38 (d, J = 8.8 Hz, 2H, Ar-H), 8.14 (s, 1H, -CH=), 8.11 (m, 2H, Ph-H), 7.95 (d, J = 8.8 Hz, 2H, Ar-H), 7.76 (t, J = 7.4 Hz, 1H, Ph-H), 7.62 (t, J = 7.8 Hz, 2H, Ph-H), 5.37 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 191.68 (C=O), 167.02 (C=O), 165.40 (C), 148.25 (CH), 139.54 (C), 135.02 (C), 134.19 (-CH=), 131.87 (CH), 131.66 (2CH), 129.54 (2CH), 128.84 (2CH), 125.59 (C), 124.82 (2CH), 48.58 (CH2). Anal. calcd. for C18H12N2O5S (368.36): C, 58.69; H, 3.28; N, 7.60; S, 8.70: Found: C, 58.59; H, 3.28; N, 7.60; S, 8.70%.
(4) (Z)-3-(2-Oxo-2-phenylethyl)-5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dione (16e). Brownish-yellow crystals from DMF-H2O, yield (62%), mp 187–189 °C. IR (ν cm−1): 3014 (C-H arom), 2969 (C-H aliph.), 2837 (C-H aliph.), 1740 (C=O), 1686b (C=O), 1605, 1577, 1504. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.14 (m, 2H, Ph-H), 8.02 (s, 1H, =CH-), 7.81 (t, J = 8.0 Hz, 1H, Ph-H), 7.66 (t, J = 7.7 Hz, 2H, Ph-H), 7.05 (s, 2H, Ar-H), 5.39 (s, 2H, CH2), 3.91 (s, 6H, 2OCH3), 3.81 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ 191.80 (C=O), 167.49 (C=O), 165.65 (C=O), 153.74 (2C), 140.24 (CH), 134.97 (C), 134.63 (=CH-), 134.24 (C), 129.53 (2CH), 128.78 (2CH), 120.24 (C), 108.25 (2CH), 60.71 (OCH3), 56.53 (2OCH3), 55.37 (CH2). Anal. calcd. for C21H19NO6S (413.44): C, 61.01; H, 4.63; N, 3.39; S, 7.75. Found C, 61.30; H, 4.54; N, 3.35; S, 7.82%.
General Procedure for the Synthesis of Thiazolidine-2,4-dione Derivatives 23b-e–28b-e
-
(1)
Synthesis of compounds 23b-e
A mixture of equimolar amounts of 2-chloro-N-phenylacetamide (17) (1 mmol, 0.169 g) and the potassium salt of 7 (0.234 g), 8 (0.288 g), 9 (0.288 g), 10 (0.288 g) or 11 (0.333 g) in DMF (5 mL) was heated under reflux for 6 hr. After cooling, the solid products obtained were filtered, washed with water, dried, and recrystallized.
(1) (Z)-2-(5-(2-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-phenylacetamide (23b). Yellow crystals from ethanol, yield (85%), mp 186–188 °C. IR (ν cm−1): 3359 (N-H), 3071 (C-H arom.) 2977 (C-H aliph.), 2938 (C-H aliph.),1748 (C=O), 1697 br (C=O), 1617, 1602, 1540, 1498. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.42 (s, 1H, NH), 8.26 (s, 1H, Ar-H), 8.24 (s, 1H, -CH=), 7.95–7.91 (m, 1H, Ar-H), 7.79–7.75 (m, 2H, Ar-H), 7.57 (d, J = 7.7 Hz, 2H, Ph-H), 7.34 (t, J = 7.9 Hz, 2H, Ph-H), 7.09 (t, J = 7.4 Hz, 1H, Ph-H), 4.54 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.37 (C=O), 165.03 (C=O), 164.16 (C=O), 148.30 (C), 138.85 (C), 135.20 (-CH=), 131.80 (C), 131.43 (C), 129.82 (CH), 129.37 (2CH), 129.31 (CH), 126.04 (CH), 125.82 (CH), 124.21 (CH), 119.63 (2CH), 44.60 (CH2). Anal. calcd. for: C18H13N3O5S (383.38): C, 56.39; H, 3.42; N, 10.96; S, 8.36. Found: C, 56.45; H, 3.35; N, 10.89; S, 8.48%.
(2) (Z)-2-(5-(3-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-phenylacetamide (23c). Yellow crystals from ethanol-dioxane, yield 90%, mp 255–257 °C. IR (ν cm−1): 3414 (N-H), 3067 (CH arom.), 2934 (CH aliph.), 1749 (C=O), 1683br (C=O), 1599, 1570, 1484. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.41 (s, 1H, NH), 8.53 (t, J = 1.9 Hz, 1H, Ar-H), 8.33 (dd, J = 8.3, 2.2 Hz, 1H, Ar-H), 8.18 (s, 1H, -CH=), 8.07 (d, J = 7.9 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 7.57 (d, J = 8.3 Hz, 2H, Ph-H), 7.33 (m, 2H, Ph-H), 7.09 (t, J = 7.4 Hz, 1H, Ph-H), 4.55 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.02 (C=O), 165.46 (C=O), 164.12 (C=O), 148.77 (CH), 138.84 (C), 135.90 (-CH=), 135.00 (C), 131.84 (CH), 131.46 (CH), 129.36 (2CH), 125.30 (C),125.25 (CH), 124.40 (CH) 124.21 (C), 119.63 (2CH), 44.66 (CH2). Anal. calcd. for C18H13N3O5S (383.38): C, 56.39; H, 3.42; N, 10.96; S, 8.36. Found: C, 56.42; H, 3.37; N, 11.00; S, 8.41%.
(3) (Z)-2-(5-(4-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-phenylacetamide (23d). Yellow crystals from DMF-H2O, yield (75%), mp 259–261 °C. IR (ν cm−1): 3393 (N-H), 3075 (C-H arom.), 2984 (C-H aliph.), 1753 (C=O), 1697 (C=O), 1682 (C=O), 1609, 1541, 1514. 1H NMR (400 MHz, DMSO) δ (ppm): 10.41 (s, 1H, NH), 8.37 (d, J = 8.8 Hz, 2H, Ar-H), 8.12 (s, 1H, -CH=), 7.93 (d, J = 8.8 Hz, 2H, Ar-H), 7.56 (d, J = 7.6 Hz, 2H, Ph-H), 7.33 (t, J = 7.9 Hz, 2H, Ph-H), 7.09 (t, J = 7.4 Hz, 1H, Ph-H), 4.55 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.10 (C=O), 165.46 (C=O), 164.10 (C=O), 148.20 (C), 139.56 (C), 138.82 (-CH=), 131.61 (2CH), 131.56 (C), 129.37 (2CH), 125.74 (C), 124.81 (2CH), 124.22 (CH), 119.64 (2CH), 44.68 (CH2). Anal. calcd. for: C18H13N3O5S (383.38): C, 56.39; H, 3.42; N, 10.96; S, 8.36. Found: C, 56.46; H, 3.50; N, 11.01; S, 8.45%.
(4) (Z)-N-Phenyl-2-(5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (23e). Pale yellow crystals from DMF-H2O, yield (76%), mp 226–228 °C. IR (ν cm−1): 3264 (N-H), 3147 (C-H arom.), 2992 (C-H aliph.) 2933 (C-H aliph.), 2836 (C-H aliph.),1741 (C=O), 1686 (C=O), 1670 (C=O), 1606, 1579, 1551. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.40 (s, 1H, NH), 7.95 (s, 1H, -CH=), 7.57 (d, J = 7.7 Hz, 2H, Ph-H), 7.33 (t, J = 7.9 Hz, 2H, Ph-H), 7.09 (t, J = 7.4 Hz, 1H, Ph-H), 6.99 (s, 2H, Ar-H), 4.53 (s, 2H, CH2), 3.86 (s, 6H, 2 OCH3), 3.75 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.57 (C=O), 165.73 (C=O), 164.25 (C=O), 153.73 (2C), 140.19 (C), 138.88 (C), 134.31 (-CH=), 129.36 (2CH), 128.82 (C), 124.17 (CH), 120.39 (C), 119.62 (2 CH), 108.21 (2CH), 60.70 (OCH3), 56.52 (2OCH3), 44.48 (CH2). Anal. calcd. for: C21H20N2O6S (428.46): C, 58.87; H, 4.71; N, 6.54; S, 7.48. Found: C, 59.01; H, 4.79; N, 6.63; S, 7.46%.
-
(2)
Synthesis of compounds 24a-e.
These compounds were synthesized using 2-chloro-N-(4-methylphenyl)acetamide (18) (0.184 g, 1 mmol), following the procedure described for 23a-e.
(1) (Z)-2-(5-Benzylidenethiazolidine-2,4-dion-3-yl)-N-(4-methylphenyl) acetamide (24a). Shiny white crystals from ethanol-dioxane, yield (88%), mp 277–279 °C. IR (ν cm−1): 3266 (N-H), 3126 (C-H arom.), 3059 (C-H arom.), 2922 (C-H aliph.), 1745 (C=O), 1695 (C=O), 1659 (C=O), 1608, 1596, 1573. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.31 (s, 1H, NH), 8.00 (s, 1H, -CH=), 7.68–7.66 (m, 2H, Ph-H), 7.60–7.51 (m, 3H, Ph-H), 7.45 (d, J = 8.4 Hz, 2H, Ph-H), 7.13 (d, J = 8.4 Hz, 2H, Ph-H), 4.46 (s, 2H, CH2), 2.26 (s, 3H, CH3).13C-NMR (100 MHz, DMSO) δ (ppm): 167.60 (C=O), 165.79 (C=O), 163.97 (C=O), 136.37 (C), 134.05 (-CH=), 133.34 (C), 133.13 (C), 131.29 (C), 130.68 (2CH), 129.81 (2CH), 129.72 (2CH), (121.49) (C), 119.64 (2CH), 44.49 (CH2), 20.90 (CH3). Anal. Calcd. For C19H16N2O3S (352.41): C, 64.76; H, 4.58; N, 7.95; S, 9.10. Found: C, 64.82; H, 4.49; N, 7.87; S, 9.14%.
(2) (Z)-N-(4-Methylphenyl)-2-(5-(2-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (24b). Yellow crystals from ethanol, yield (83%), mp 181–183 °C. IR (ν cm−1): 3260 (N-H), 3196 (C-H arom.), 3128 (C-H arom.), 3077 (C-H arom.) 2991 (C-H aliph.), 1769 (C=O), 1698 (C=O), 1666 (C=O), 1607, 1551, 1523. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.33 (s, 1H, NH), 8.25 (m, 2H, Ar-H + -CH=), 7.93 (t, J = 7.5 Hz, 1H, Ar-H), 7.77 (t, J = 7.7 Hz, 2H, Ar-H), 7.45 (d, J = 8.4 Hz, 2H, Ar-H), 7.14 (d, J = 8.4 Hz, 2H, Ar-H), 4.52 (s, 2H, CH2), 2.26 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.36 (C=O), 165.04 (C=O), 163.89 (C=O), 148.30 (C), 136.35 (C), 135.18 (CH), 133.17 (C), 131.79 (C), 131.36 (CH), 129.80 (CH), 129.73 (2CH), 129.30 (CH), 126.04 (CH), 125.94 (C), 119.64 (2CH), 44.56 (CH2), 20.90 (CH3). Anal. calcd. for C19H15N3O5S (397.41): C, 57.42; H, 3.80; N, 10.57; S, 8.07. Found: C, 57.52; H, 3.89; N, 10.63; S, 8.17%.
(3) (Z)-N-(4-Methylphenyl)2-(5-(3-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (24c). Yellow crystals from ethanol-dioxane, yield (82%), mp 276–278 °C. IR (ν cm−1): 3264 (N-H), 3071 (CH arom.), 2948 (CH aliph.), 2835 (CH aliph.), 1746 (C=O), 1681 (C=O), 1664 (C=O), 1603, 1570. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.32 (s, 1H, NH), 8.52 (s, 1H, Ar-H), 8.32 (dd, J = 8.2, 1.6 Hz, 1H, Ar-H), 8.16 (s, 1H –CH=), 8.07 (d, J = 7.9 Hz, 1H, Ar-H), 7.85 (t, J = 8.0 Hz, 1H, Ar-H), 7.44 (d, J = 8.4 Hz, 2H, Ar-H), 7.13 (d, J = 8.4 Hz, 2H, Ar-H), 4.53 (s, 2H, CH2), 2.26 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm) 167.02 (C=O), 165.46 (C=O), 163.85 (C=O), 148.75 (C), 136.33 (C), 135.89 (–CH=), 134.99 (C), 133.17 (C), 131.80 (CH), 131.45 (CH), 129.72 (2CH), 125.38 (C) 125.23 (CH), 124.41 (CH), 119.65 (2CH), 44.61 (CH2), 20.90 (CH3). Anal. calcd. for: C19H15N3O5S (397.41): C, 57.42; H, 3.80; N, 10.57; S, 8.07. Found: C, 57.50; H, 3.88; N, 11.06; S, 8.09%.
(4) (Z)-N-(4-Methylphenyl)2-(5-(4-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (24d). Yellow crystals from DMF-H2O, yield (80%), mp 250–252 °C. IR (ν cm−1): 3282 (N-H), 3036 (C-H arom.), 2919 (C-H aliph.), 1750 (C=O), 1704 (C=O), 1676 (C=O), 1611, 1596, 1541. 1H-NMR (400 MHz, DMSO) δ (ppm) 10.32 (s, 1H, NH), 8.36 (d, J = 8.8 Hz, 2H, Ar-H), 8.11 (s, 1H, -CH=), 7.92 (d, J = 8.8 Hz, 2H, Ar-H), 7.44 (d, J = 8.4 Hz, 2H, Ar-H), 7.13 (d, J = 8.4 Hz, 2H, Ar-H), 4.53 (s, 2H, CH2), 2.26 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm) 167.10 (C=O), 165.47 (C=O), 163.83 (C=O), 148.18 (C), 139.56 (C), 136.32 (-CH=), 133.18 (C), 131.60 (2CH), 131.51 (C), 129.72 (2CH), 125.76 (C), 124.80 (2CH), 119.65 (2CH), 44.45 (CH2). 20.89 (CH3). Anal. calcd. for: C19H15N3O5S (397.41): C, 57.42; H, 3.80; N, 10.57; S, 8.07. Found: C, 57.49; H, 3.90; N, 11.06; S, 8.03%.
(5) (Z)-N-(4-Methylphenyl)-2-(5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (24e). Yellow crystals from DMF-H2O, yield (81%), mp 196–198 °C. IR (ν cm−1): 3251 (N-H), 3028 (C-H arom.), 2989 (C-H aliph.) 2934 (C-H aliph.), 2836 (C-H aliph.), 1741 (C=O), 1686 (C=O), 1666 (C=O), 1606, 1579, 1546. H-NMR (400 MHz, DMSO) δ (ppm): 10.31 (s, 1H, NH), 7.94 (s, 1H, -CH=), 7.45 (d, J = 8.4 Hz, 2H, Ar-H), 7.13 (d, J = 8.4 Hz, 2H, Ar-H), 6.98 (s, 2H, Ar-H), 4.51 (s, 2H, CH2), 3.85 (s, 6H, 2OCH3), 3.75 (s, 3H, OCH3), 2.26 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.57 (C=O), 165.73 (C=O), 163.98 (C=O), 153.73 (2C), 140.18 (C), 136.38 (C), 134.28 (-CH=), 133.13 (C), 129.71 (2CH), 128.82 (C), 120.39 (C), 119.63 (2CH), 108.20 (2CH), 60.69 (CH2), 56.51 (OCH3), 44.44 (2 OCH3), 20.89 (CH3). Anal. calcd. for: C22H22N2O6S (442.49): C, 59.72; H, 5.01; N, 6.33; S, 7.25. Found: C, 57.42; H, 3.80; N, 10.57; S, 8.07%.
-
(3)
Synthesis of compounds 25b-e.
These compounds were synthesized using 2-chloro-N-(4-methoxylphenyl)acetamide 19 (1 mmol, 0.2 g), following the procedure described for 23a-e.
(1) (Z)-2-(5-(2-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-(4-methoxyphenyl) acetamide (25b). Brown crystals from ethanol-dioxane, yield (86%), mp 204–205 °C. IR (ν cm−1): 3285 (N-H), 3193 (C-H arom.), 3139 (C-H arom.), 3080 (C-H arom.) 2932 (C-H aliph.), 2835 (C-H aliph.), 1750 (C=O), 1699 (C=O), 1662 (C=O), 1610, 1551, 1520.1H-NMR (400 MHz, DMSO) δ (ppm): 10.27 (s, 1H, NH), 8.25 (d, J = 8.3 Hz, 1H, Ar-H), 8.23 (s, 1H, -CH=), 7.93 (t, J = 7.6 Hz, 1H, Ar-H), 7.77 (t, J = 7.6 Hz, 2H, Ar-H), 7.48 (d, J = 9.0 Hz, 2H, Ar-H), 6.91 (d, J = 9.0 Hz, 2H, Ar-H), 4.51 (s, 2H, CH2), 3.73 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.37 (C=O), 165.04 (C=O), 163.63 (C=O), 155.97 (C), 148.30 (C), 135.19 (CH), 131.97 (C), 131.79 (C), 131. 35 (CH), 129.81 (CH), 129.31 (CH), 126.04 (CH), 125.95 (C), 121.19 (2CH), 114.46 (2CH), 55.63 (OCH3), 44.49 (CH2). Anal. calcd. for: C19H15N3O6S (413.40) C, 55.20; H, 3.66; N, 10.16; S, 7.76. Found: C, 55.27; H, 3.69; N, 10.22; S, 7.78%.
(2) (Z)-2-(5-(3-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-(4-methoxyphenyl) acetamide (25c). Beige crystals from ethanol-dioxane, yield (84%), mp 252–254 °C. IR (ν cm−1): 3258 (N-H), 3069 (CH arom.), 2948 (CH aliph.), 2835 (CH aliph.), 1746 (C=O), 1682 (C=O), 1659 (C=O), 1603, 1533. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.26 (s, 1H, NH), 8.54 (t, J = 1.7 Hz, 1H, Ar-H), 8.34 (dd, J = 8.2, 2.2 Hz, 1H, Ar-H), 8.18 (s, 1H, -CH=), 8.09 (d, J = 7.8 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 7.47 (d, J = 9.1 Hz, 2H, Ar-H), 6.90 (d, J = 9.1 Hz, 2H, Ar-H), 4.51 (s, 2H, CH2), 3.73 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.04 (C=O), 165.48 (C=O), 163.60 (C=O), 155.97 (C), 148.78 (C), 135.89 (-CH=), 135.02 (C), 131.96 (C), 131.79 (CH), 131.47 (CH), 125.30 (CH), 125.25 (CH), 124.45 (C), 121.19 (2CH), 114.46 (2CH), 55.63 (OCH3), 44.56 (CH2). Anal. calcd. for: C19H15N3O6S (413.40) C, 55.20; H, 3.66; N, 10.16; S, 7.76. Found: C, 55.32; H, 3.57; N, 10.12; S, 7.71%.
(3) (Z)-N-(4-Methoxyphenyl)-2-(5-(4-nitrobenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (25d). Yellow crystals from DMF-H2O, yield (83%), mp 254–256 °C. IR (ν cm−1): 3349 (N-H), 3073 (C-H arom.), 2937 (C-H aliph.), 1751 (C=O), 1690 br (C=O), 1611, 1593, 1512. 1H- NMR (400 MHz, DMSO) δ (ppm): 10.26 (s, 1H, NH), 8.36 (d, J = 8.8 Hz, 2H, Ar-H), 8.11 (s, 1H, -CH=), 7.92 (d, J = 8.8 Hz, 2H, Ar-H), 7.47 (d, J = 9.1 Hz, 2H, Ar-H), 6.90 (d, J = 9.1 Hz, 2H, Ar-H), 4.52 (s, 2H, CH2), 3.72 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.10 (C=O), 165.47 (C=O), 163.57 (C=O), 155.98 (C), 148.18 (C), 139.56 (C), 131.95 (2CH), 131.59 (CH), 131.49 (C), 125.77 (C), 124.79 (2CH), 121.20 (2CH), 114.45 (2CH), 55.62 (OCH3), 44.57 (CH2). Anal. calcd. for: C19H15N3O6S (413.40) C, 55.20; H, 3.66; N, 10.16; S, 7.76. Found: C, 55.29; H, 3.59; N, 10.00; S, 7.77%.
(4) (Z)-N-(4-Methoxyphenyl)-2-(5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (25e). Pale yellow crystals from DMF-H2O, yield (77%), mp 215–216 °C. IR (ν cm−1): 3284 (N-H), 3022 (C-H arom.), 2993 (C-H aliph.) 2934 (C-H aliph.), 2837 (C-H aliph.),1741 (C=O), 1686 (C=O), 1663 (C=O), 1606, 1579, 1550. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.25 (s, 1H, NH), 7.95 (s, 1H, -CH=), 7.47 (d, J = 9.1 Hz, 2H, Ar-H), 6.99 (s, 2H, Ar-H), 6.90 (d, J = 9.1 Hz, 2H, Ar-H), 4.49 (s, 2H, CH2), 3.86 (s, 6H, 2OCH3), 3.75 (s, 3H, OCH3), 3.73 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.58 (C=O), 165.75 (C=O), 163.72 (C=O), 155.95 (C), 153.74 (2C), 140.17 (C), 134.25 (-CH=), 132.00 (C), 128.84 (C), 121.17 (2CH), 120.45 (C), 114.45 (2CH), 108.20 (2CH), 60.70 (OCH3), 56.53 (2OCH3), 55.63 (OCH3), 44.38 (CH2). Anal. calcd. for C22H22N2O7S (458.49): C, 57.63; H, 4.84; N, 6.11; S, 6.99. Found: C, 57.57; H, 4.83; N, 6.02; S, 6.90%.
-
(4)
Synthesis of compounds 26a-e.
These compounds were synthesized using 2-chloro-N-(4-nitrophenyl)acetamide 20 (1 mmol, 0.215 g), following the procedure described for 23a-e.
(1) (Z)-2-(5-Benzylidenethiazolidine-2,4-dion-3-yl)-N-(4-nitrophenyl)acetamide (26a). Fluffy off-white needles from DMF-water, yield (89%), mp 270–272 °C. IR (ν cm−1): 3273 (N-H), 3168 (C-H arom.), 3104 (C-H arom.), 2929 (C-H arom.) 2991, 1744 (C=O), 1686 br (C=O), 1619, 1595, 1570. 1H-NMR (400 MHz, DMSO) δ (ppm): 11.03 (s, 1H, NH), 8.25 (d, J = 9.3 Hz, 2H, Ar-H), 8.01 (s, 1H, -CH=), 7.81 (d, J = 9.3 Hz, 2H, Ar-H), 7.67 (d, J = 7.1 Hz, 2H, Ph-H), 7.59–7.51 (m, 3H, Ph-H), 4.61 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.57 (C=O), 165.70 (C=O), 165.41 (C=O), 144.88 (C), 143.07 (C), 134.29 (CH), 133.19 (C), 131.35 (C), 130.70 (2CH), 129.90 (2CH), 125.57 (2CH), 121.31 (CH), 119.49 (2CH), 44.70 (CH2). Anal. Calcd. for: C18H13N3O5S (383.38): C, 56.39; H, 3.42; N, 10.96; S, 8.36. Found: C, 56.30; H, 3.39; N, 10.90; S, 8.30%.
(2) (Z)-2-(5-(2-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-(4-nitroyphenyl) acetamide (26b). Yellow crystals from ethanol, yield (87%), mp 213–215 °C. IR (ν cm−1): 3263 (N-H), 3164 (C-H arom.), 3100 (C-H arom.), 3072 (C-H arom.) 2938 (C-H aliph.), 1750 (C=O), 1679 br (C=O), 1609, 1594, 1568, 1532. 1H NMR (400 MHz, DMSO) δ (ppm): 11.05 (s, 1H, NH), 8.26 (d, J = 9.2 Hz, 2H, Ar-H) 8.25 (s, 2H, -CH= + Ar-H ), 7.94 (t, J = 7.6 Hz, 1H), 7.82 (d, J = 9.2 Hz, 2H, Ar-H), 7.78 (t, J = 7.6 Hz, 2H, Ar-H), 4.62 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.34 (C=O), 165.35 (C=O), 164.96 (C=O), 148.30 (C), 144.87 (CH), 143.10 (C), 135.21 (-CH=), 131.86 (C), 131.68 (CH), 129.82 (CH), 129.29 (C), 126.06 (CH), 125.68 (C), 125.95 (2CH), 119.51 (2CH), 44.80 (CH2). Anal. calcd. for: C18H12N4O7S (428.38): C, 50.47; H, 2.82; N, 13.08; S, 7.48. Found: C, 50.44; H, 2.79; N, 13.01; S, 7.42%.
(3) (Z)-2-(5-(3-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-(4-nitroyphenyl) acetamide (26c). Buff crystals from ethanol-dioxane, yield (89%), mp 264–266 °C. IR (ν cm−1): 3342 (N-H), 3087 (CH arom.), 2943 (CH aliph.), 1751 (C=O), 1709 (C=O), 1686 (C=O), 1616, 1562, 1348. 1H-NMR (400 MHz, DMSO) δ (ppm): 11.04 (s, 1H, NH), 8.52 (t, J = 1.6 Hz, 1H, Ar-H), 8.33 (dd, J = 8.2, 2.1 Hz, 1H, Ar-H), 8.25 (d, J = 9.2 Hz, 2H, Ar-H), 8.18 (s, 1H, -CH=), 8.08 (d, J = 7.8 Hz, 1H, Ar-H), 7.85 (t, J = 8.1 Hz, 1H, Ar-H), 7.81 (d, J = 9.3 Hz, 2H, Ar-H), 4.63 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 166.98 (C=O), 165.38 (C=O), 165.29 (C=O), 148.76 (C), 144.85 (C), 143.08 (C), 135.91 (-CH=), 134.94 (CH), 132.04 (CH), 131.46 (C), 125.57 (2CH), 125.43 (C), 125.27 (CH), 124.24 (CH), 119.49 (2CH), 44.63 (CH2). Anal. calcd. for: C18H12N4O7S (428.38): C, 50.47; H, 2.82; N, 13.08; S, 7.48. Found: C, 50.34; H, 2.78; N, 13.01; S, 7.37%.
(4) (Z)-2-(5-(4-Nitrobenzylidene)thiazolidine-2,4-dion-3-yl)-N-(4-nitroyphenyl) acetamide (26d). Yellow crystals from DMF-H2O, yield (85%), mp 284–285 °C. IR (ν cm−1): 3264 (N-H), 3075 (C-H arom.), 2994 (C-H aliph.), 1756 (C=O), 1698 (C=O), 1609, 1593, 1517. 1H-NMR (400 MHz, DMSO) δ (ppm): 11.03 (s, 1H, NH), 8.36 (d, J = 8.8 Hz, 2H, Ar-H), 8.24 (d, J = 9.2 Hz, 2H, Ar-H), 8.11 (s, 1H, -CH=), 7.92 (d, J = 8.8 Hz, 2H, Ar-H), 7.80 (d, J = 9.3 Hz, 2H, Ar-H), 4.63 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.05 (C=O), 165.38 (C=O), 165.25 (C=O), 148.20 (C), 144.83 (-CH=), 143.08 (C), 139.47 (C), 131.73 (C), 131.62 (2CH), 125.55 (2CH + C), 124.78 (2CH), 119.49 (2CH), 44.84 (CH2). Anal. calcd. for: C18H12N4O7S (428.38): C, 50.47; H, 2.82; N, 13.08; S, 7.48. Found: C, 50.39; H, 2.77; N, 13.18; S, 7.42%.
(5) (Z)-N-(4-Nitrophenyl)-2-(5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (26e). Yellow crystals from DMF-H2O, yield (80%), mp 253–254 °C. IR (ν cm−1): 3282 (N-H), 3091 (C-H arom.), 2940 (C-H aliph.), 2893 (C-H aliph.), 1738 (C=O), 1682 (C=O), 1668, (C=O), 1607, 1596, 1507. 1H-NMR (400 MHz, DMSO) δ (ppm): 11.03 (s, 1H, NH), 8.25 (d, J = 9.3 Hz, 2H, Ar-H), 7.96 (s, 1H, -CH=), 7.82 (d, J = 9.3 Hz, 2H, Ar-H), 6.99 (s, 2H, Ar-H), 4.61 (s, 2H, CH2), 3.86 (s, 6H, 2OCH3), 3.75 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.54 (C=O), 165.65 (C=O), 165.43 (C=O), 153.74 (2C), 144.89 (C), 143.07 (-CH=), 140.24 (C), 134.51 (C), 128.78 (C), 125.58 (2CH), 120.24 (C), 119.48 (2CH), 108.24 (2CH), 60.71 (OCH3), 56.53 (2OCH3), 44.68 (CH2). Anal. calcd. for: C21H19N3O8S (473.46): C, 53.27; H, 4.05; N, 8.88; S, 6.77. Found: C, 53.35; H, 4.00; N, 8.79; S, 6.70%.
-
(5)
Synthesis of compounds 27b-e.
These compounds were synthesized using 2-chloro-N-(4-chlorophenyl)acetamide 21 (1 mmol, 0.204 g), following the procedure described for 23a-e.
(1) (Z)-N-(4-Chlorophenyl)-2-(5-(2-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (27b). Canary yellow crystals from ethanol-dioxane, yield (85%), mp 188–190 °C. IR (ν cm−1): 3258 (N-H), 3123 (C-H arom.), 3073 (C-H arom.) 2992 (C-H aliph.), 2835 (C-H aliph.), 1756 (C=O), 1693 (C=O), 1688 (C=O), 1607, 1570, 1546. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.57 (s, 1H, NH), 8.25 (d, J = 8.5 Hz, 2H, Ar-H + -CH=), 7.93 (t, J = 7.4 Hz, 1H, Ar-H), 7.77 (t, J = 7.5 Hz, 2H, Ar-H), 7.60 (d, J = 8.9 Hz, 2H, Ar-H), 7.39 (d, J = 8.9 Hz, 2H, Ar-H), 4.55 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.35 (C=O), 165.00 (C=O), 164.39 (C=O), 148.29 (C), 137.78 (C), 135.19 (CH), 131.81 (C), 131.48 (CH), 129.80 (CH), 129.29 (C+2CH), 127.81 (C), 126.04 (CH), 125.78 (C), 121.23 (2CH), 44.61 (CH2). Anal. calcd. for: C18H12ClN3O5S (417.02): C, 51.74; H, 2.90; N, 10.06; S, 7.67. Found: C, 51.65; H, 2.86; N, 10.00; S, 7.59%.
(2) (Z)-N-(4-Chlorophenyl)-2-(5-(3-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (27c). Fluffy white crystals from ethanol-dioxane, yield (90%), mp 260–261 °C. IR (ν cm−1): 3349 (N-H), 3075 (CH arom.), 2943 (CH aliph.), 1750 (C=O), 1699br (C=O), 1607 (C=O), 1603, 1570. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.56 (s, 1H, NH), 8.52 (t, J = 1.9 Hz, 1H, Ar-H), 8.34 (ddd, J = 8.3, 2.2, 0.8 Hz, 1H, Ar-H), 8.18 (s, 1H, -CH=), 8.09 (d, J = 7.8 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 7.59 (d, J = 8.9 Hz, 2H, Ar-H), 7.39 (d, J = 8.9 Hz, 2H, Ar-H), 4.55 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.00 (C=O), 165.43 (C=O), 164.35 (C=O), 148.76 (C), 137.77 (C), 135.90 (-CH=), 134.97 (CH), 131.90 (CH), 131.46 (CH), 129.29 (2CH), 127.82 (C), 125.31 (C), 125.25 (CH), 124.34 (C), 121.23 (2CH), 44.66 (CH2). Anal. calcd. for: C18H12ClN3O5S (417.02): C, 51.74; H, 2.90; N, 10.06; S, 7.67. Found: C, 51.62; H, 2.84; N, 9.99; S, 7.55%.
(3) (Z)-N-(4-Chlorophenyl)-2-(5-(4-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (27d). Yellow crystals from DMF-H2O, yield (90%), mp 252–254 °C. IR (ν cm−1): 3276 (N-H), 3120 (C-H arom.), 2994 (C-H aliph.), 1748 (C=O), 1704 (C=O), 1675 (C=O), 1595, 1541, 1491. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.55 (s, 1H, NH), 8.37 (d, J = 8.8 Hz, 2H, Ar-H), 8.12 (s, 1H, -CH=), 7.93 (d, J = 8.8 Hz, 2H, Ar-H), 7.59 (d, J = 8.9 Hz, 2H, Ar-H), 7.39 (d, J = 8.9 Hz, 2H, Ar-H), 4.55 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.08 (C=O), 165.43 (C=O), 164.33 (C=O), 148.20 (C), 139.54 (C), 137.76 (-CH=), 131.61 (4CH), 129.29 (2CH), 127.82 (C), 125.68 (C), 124.80 (2CH), 121.23 (C), 44.68 (CH2). Anal. calcd. for: C18H12ClN3O5S (417.02): C, 51.74; H, 2.90; N, 10.06; S, 7.67. Found: C, 51.68; H, 2.88; N, 10.01; S, 7.66%.
(4) (Z)-N-(4-Chlorophenyl)-2-(5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (27e). Yellow crystals from DMF-H2O, yield (79%), mp 218–219 °C. IR (ν cm−1): 3295 (N-H), 3193 (C-H arom.), 3125 (C-H arom.), 3074 (C-H arom.) 2991 (C-H aliph.), 2838 (C-H aliph.), 1750 (C=O), 1686 (C=O), 1677 (C=O), 1603, 1577, 1507. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.55 (s, 1H, NH), 7.95 (s, 1H, -CH=), 7.59 (d, J = 8.9 Hz, 2H, Ar-H), 7.39 (d, J = 8.9 Hz, 2H, 2Ar-H), 6.99 (s, 2H, Ar-H), 4.53 (s, 2H, CH2), 3.85 (s, 6H, 2 OCH3), 3.74 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.55 (C=O), 165.70 (C=O), 164.48 (C=O), 153.73 (2C), 140.20 (C), 137.81 (C), 134.37 (-CH=), 129.28 (2CH), 128.80 (C), 127.77 (C), 121.21 (2CH), 120.33 (C), 108.22 (2CH), 60.70 (OCH3), 56.52 (2OCH3), 44.49 (CH2). Anal. calcd. for: C21H19ClN2O6S (462.90): C, 54.49; H, 4.14; N, 6.05; S, 6.93. Found: C, 54.42; H, 4.13; N, 6.12; S, 6.98%.
-
(6)
Synthesis of compounds 28a-e.
These compounds were synthesized using 2-chloro-N-(2-chlorophenyl)acetamide 22 (1 mmol, 0.204 g), following the procedure described for 23a-e.
(1) (Z)-2-(5-Benzylidenethiazolidine-2,4-dion-3-yl)-N-(2-chlorophenyl)acetamide (28a). White aggregate crystals from ethanol-dioxane, yield (87%), mp 243–245 °C. IR (ν cm−1): 3260 (N-H), 3020 (C-H arom.) 1747 (C=O), 1694 (C=O), 1668 (C=O), 1608, 1597, 1587. 1H-NMR (400 MHz, DMSO) δ (ppm): 1H NMR (400 MHz, DMSO) δ (ppm): 10.07 (s, 1H, NH), 8.00 (s, 1H, =CH-), 7.71–7.66 (m, 3H, Ar-H), 7.62–7.44 (m, 4H, Ar-H), 7.34 (t, J = 7.5 Hz, 1H, Ar-H), 7.22 (t, J = 7.3, 1H, Ar-H), 4.62 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.56 (C=O), 165.73 (C=O), 164.97 (C=O), 134.68 (C), 134.07 (-CH=), 133.33 (CH), 131.29 (CH), 130.68 (2CH), 130.10 (CH), 129.90 (2CH), 128.02 (CH), 127.20 (C), 126.88 (C), 126.68 (C), 121.48 (CH), 44.31 (CH2). Anal. calcd. for C18H13ClN2O3S (372.82): C, 57.99; H, 3.51; N, 7.51; S, 8.60. Found: C, 57.95; H, 3.40; N, 7.48; S, 8.53%.
(2) (Z)-N-(2-Chlorophenyl)-2-(5-(2-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (28b). Small buff crystals from ethanol-dioxane, yield (80%), mp 174–175 °C. IR (ν cm−1): 3346 (N-H), 3065 (CH arom.), 2934 (CH aliph.), 1751 (C=O), 1703 (C=O), 1697 (C=O), 1616, 1569, 1535. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.09 (s, 1H, NH), 8.25 (m, 2H, Ar-H + -CH=), 7.93 (t, J = 7.6 Hz, 1H, Ar-H), 7.79–7.75 (m, 2H, Ar-H), 7.71 (dd, J = 8.0, 1.1 Hz, 1H, Ar-H), 7.53 (dd, J = 8.0, 1.1 Hz, 1H, Ar-H), 7.35 (td, J = 8.3, 1.2 Hz, 1H, Ar-H), 7.23 (td, J = 7.9, 1.2 Hz, 1H, Ar-H), 4.62 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.33 (C=O), 164.94 (C=O), 164.89 (C=O), 148.31 (C), 135.19 (-CH=), 134.65 (C), 131.80 (CH), 131.37 (CH), 130.11 (CH), 129.80 (CH), 129.30 (C), 128.03 (CH), 127.24 (CH), 127.08 (C), 126.91 (C), 126.51 (C), 126.04 (CH), 125.84 (CH), 44.39 (CH2). Anal. calcd. for C18H12ClN3O5S (417.02): C, 51.74; H, 2.90; N, 10.06; S, 7.67. Found: C, 51.65; H, 2.91; N, 10.00; S, 7.77%.
(3) (Z)-N-(2-Chlorophenyl)-2-(5-(3-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (28c). White crystals from DMF-H2O, yield (69%), mp 240–242 °C. IR (ν cm−1): 3252 (NH), 3068 (C-H arom.), 3013 (C-H arom.), 2914 (C-H aliph.), 1745 (C=O), 1683 (C=O), 1668 (C=O), 1604, 1570, 1533, 1476. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.09 (s, 1H, NH), 8.53 (t, J = 1.9 Hz, 1H, Ar-H), 8.33 (ddd, J = 8.3, 2.2, 0.8 Hz, 1H, Ar-H), 8.18 (s, 1H, =CH-), 8.09 (d, J = 7.9 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 7.86 (t, J = 8.0 Hz, 1H, Ar-H), 7.70 (dd, J = 8.1, 1.5 Hz, 1H, Ar-H), 7.53 (dd, J = 8.0, 1.4 Hz, 1H, Ar-H), 7.34 (td, J = 7.9, 1.4 Hz, 1H, Ar-H), 7.23 (td, J = 7.9, 1.4 Hz, 1H, Ar-H), 4.36 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.00 (C=O), 165.43 (C=O), 164.87 (C=O), 148.78 (C), 135.89 (-CH=), 135.00 (CH), 134.64 (CH), 131.82 (CH), 131.48 (CH), 130.11 (CH), 128.03 (C), 127.26 (C), 126.97 (C), 126.51 (CH), 125.28 (CH), 125.28 (CH), 124.44 (C), 44.45 (CH2). Anal. calcd. for: C18H12ClN3O5S (417.02): C, 51.74; H, 2.90; N, 10.06; S, 7.67. Found: C, 51.64; H, 2.81; N, 10.01; S, 7.59%.
(4) (Z)-N-(2-Chlorophenyl)-2-(5-(4-nitrobenzylidene)thiazolidine-2,4-dion-3-yl) acetamide (28d). Pale yellow crystals from DMF-H2O, yield (89%), mp 242–244 °C. IR (ν cm−1): 3280 (N-H), 3119 (C-H arom.), 3050 (C-H arom.) 2947 (C-H aliph.), 1757 (C=O), 1702 (C=O), 1682 (C=O), 1610, 1592, 1540. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.09 (s, 1H, NH), 8.37 (d, J = 8.8 Hz, 2H, Ar-H), 8.12 (s, 1H, -CH=), 7.93 (d, J = 8.8 Hz, 2H, Ar-H), 7.70 (dd, J = 8.8, 0.7 Hz, 1H, Ar-H), 7.53 (dd, J = 8.0, 1.3 Hz, 1H, Ar-H), 7.34 (td, J = 8.0, 1.3 Hz, 1H, Ar-H), 7.23 (td, J = 7.9, 1.3 Hz, 1H, Ar-H), 4.63 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.07 (C=O), 165.42 (C=O), 164.84 (C=O), 148.20 (C), 139.57 (-CH=), 134.63 (CH), 131.61 (2CH), 131.53 (CH), 130.11 (CH), 128.03 (CH), 127.26 (C), 126.94 (C), 126.52 (C), 125.78 (C), 124.82 (2CH), 44.47 (CH2). Anal. calcd. for: C18H12ClN3O5S (417.02): C, 51.74; H, 2.90; N, 10.06; S, 7.67. Found: C, 51.67; H, 2.80; N, 10.11; S, 7.61%.
(5) (Z)-N-(2-Chlorophenyl)-2-(5-(3,4,5-trimethoxybenzylidene)thiazolidine-2,4-dion-3-yl)acetamide (28e). Pale yellow crystals from DMF-H2O, yield (75%), mp 222–223 °C. IR (ν cm−1): 3251 (N-H), 3047 (C-H arom.), 2938 (C-H aliph.), 2835 (C-H aliph.), 1740 (C=O), 1686 (C=O), 1671 (C=O), 1605, 1578, 1505. 1H-NMR (400 MHz, DMSO) δ (ppm): 10.07 (s, 1H, NH), 7.95 (s, 1H, -CH=), 7.70 (dd, J = 8.1, 1.3 Hz, 1H, Ar-H), 7.53 (dd, J = 8.0, 1.3 Hz, 1H, Ar-H), 7.34 (td, J = 7.9, 1.4 Hz, 1H, Ar-H), 7.23 (td, J = 7.9, 1.4 Hz, 1H, Ar-H), 6.99 (s, 2H, Ar-H), 4.61 (s, 2H, CH2), 3.85 (s, 6H, 2 OCH3), 3.75 (s, 3H, OCH3). 13C-NMR (100 MHz, DMSO) δ (ppm): 167.54 (C=O), 165.68 (C=O), 164.97 (C=O), 153.74 (2C), 140.18 (CH), 134.68 (C), 134.30 (-CH=), 130.26 (CH), 128.83 (CH), 128.02 (C), 127.19 (C), 126.85 (C), 126.46 (CH), 120.41 (C), 108.20 (2CH), 60.80 (OCH3), 56.53 (2OCH3), 44.27 (CH2). Anal. calcd. for: C21H19ClN2O6S (462.90): C, 54.49; H, 4.14; N, 6.05; S, 6.93. Found: C, 54.41; H, 4.09; N, 6.01; S, 6.88%.
Synthesis of (Z)-2-(5-(3-Nitrobenzylidene)thiazolidin-2,4-dion-3-yl)acetic Acid (29)
A mixture of the ester 12c (1.0 g, 3 mmol), glacial AcOH (16 mL) and concentrated HCl (12 N, 6 mL) was heated under reflux for 2 h. After evaporation under reduced pressure, the residue obtained was heated again under reflux in glacial AcOH (16 mL) and concentrated HCl (6 mL) for 2 h. After evaporation till dryness under reduced pressure, the crude product was washed thoroughly with water and recrystallized from ethanol-water to give off-white crystals, 0.8 g, yield 92%, mp 191–193 °C [lit. [35], mp 235 °C]. IR (ν cm−1): 3409 (O-H), 3077 (C-H arom.), 3037 (C-H arom.), 2950 (C-H aliph.), 2784, 2665, 2566 (O-H acid), 1756 (C=O), 1740 (C=O), 1701 (C=O), 1686 (C=O), 1604, 1535. 1H-NMR (400 MHz, DMSO) δ (ppm): 8.50 (t, J = 1.9 Hz, 1H, Ar-H), 8.32 (dd, J = 8.2, 1.5 Hz, 1H, Ar-H), 8.15 (s, 1H, =CH-), 8.06 (d, J = 7.9 Hz, 1H, Ar-H), 7.84 (t, J = 8.0 Hz, 1H, Ar-H), 4.36 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO) δ (ppm): 168.41 (C=O), 166.79 (C=O), 165.24 (C=O), 148.64 (C), 135.84 (=CH-), 134.95 (C), 131.95 (CH), 131.43 (CH), 125.30 (CH), 125.24 (CH), 124.24 (C), 43.25 (CH2). Anal. calcd. for C12H8N2O6S (308.26): C, 46.76; H, 2.62; N, 9.09; S, 10.40. Found: C, 46.85; H, 2.67; N, 10.00; S, 10.46%.
3.2. Biology
3.2.1. In Vitro Antileishmanial Activity on the Promastigote Stage of Leishmania infantum
Luciferase-expressing Leishmania infantum was kindly provided by Professor Thierry Lang of the Institut Pasteur [36]. L. infantum promastigotes were routinely grown at 27 °C in Schneider’s Drosophila Medium (Dominique DUTSCHER SAS, Bernolsheim, France) supplemented with 10% fetal calf serum, 2% human urine, sodium pyruvate 0.04 mM, hemin 0.1 µg/mL, HEPES 5 mM, streptomycin 100 µg/mL, penicillin 100 U/mL, L-glutamine 2 mM (culture medium). The in vitro evaluation of the antileishmanial activity of the tested compound on L. infantum promastigotes forms was assessed according to the Mosmann protocol [37] with slight modifications. Briefly, L. infantum promastigotes in logarithmic phase of growth were incubated at an average density of 1 × 106 parasites/mL in sterile 96-well plates with various concentrations of compound dissolved in DMSO (final concentration less than 0.5% v/v), in duplicate. Appropriate controls treated with DMSO, Miltefosine, Amphotericin B and Fexinidazole sulfone (reference drugs purchased from Sigma-Aldrich, Saint-Louis, MO, USA) were added to each set of experiments. The plates were incubated at 27 °C for 72 h, Then, each plate-well was microscope-analyzed to detect any precipitate formation. Afterward, 20 µL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich, Saint-Louis, MI, USA) solution (5 mg/mL in PBS) were added to each well and incubated at 27 °C for 4 h. The reaction was then stopped by the addition of 100 µL of 10% sodium dodecyl sulfate—50% isopropanol. Plates were shaken vigorously at 400 rpm for 10 min. The absorbance was measured at 570 nm in a TECAN Infinite F-200 spectrophotometer (Tecan Trading AG, Männedorf, Switzerland). An inhibitory concentration of 50% (EC50) was defined as the concentration of drug required to inhibit the metabolic activity of leishmanial promastigote forms by 50% relative to the control. EC50 values were calculated by non-linear regression analysis processed dose-response curves, using TableCurve 2D V5.0 software (Systat Software Inc. (Palo Alto, CA, USA)). EC50 values represent the mean value calculated from at least three separate experiments.
3.2.2. In Vitro Cytotoxicity on the Human Hepatic HepG2 Cell Line
HepG2 cell line (hepatocarcinoma cell line purchased from ATCC, ref HB-8065) was maintained at 37 °C, 5% CO2 with 90% humidity in MEM supplemented with 10% fetal bovine serum, 1% L-glutamine (200 mM) and penicillin (100 U/mL)/streptomycin (100 mg/mL) (complete MEM medium). The tested molecules’ cytotoxicity was assessed according to the method of Mosmann [37] with slight modifications. Briefly, 5 × 103 cells in 100 μL of complete medium were inoculated into each well of 96-well plates and incubated at 37 °C in a humidified 5% CO2. After 24 h incubation, 100 μL of medium with various product concentrations dissolved in DMSO (final concentration less than 0.5% v/v) were added and the plates were incubated for 72 h at 37 °C. Triplicate assays were performed for each sample. Each plate well was then microscope-examined to detect possible precipitate formation before the medium was aspirated from the wells. Then, 100 μL of MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) solution (0.5 mg/mL in medium without FCS) were then added to each well. Cells were incubated for 2 h at 37 °C, following which the MTT solution was removed and DMSO (100 μL) was added to dissolve the resulting blue formazan crystals. Plates were shaken vigorously (700 rpm) for 10 min. The absorbance was measured at 570 nm with 630 nm as reference wavelength using a TECAN Infinite F-200 spectrophotometer (Tecan Trading AG, Männedorf, Switzerland). DMSO was used as blank and doxorubicin (purchased from Sigma Aldrich, Saint Louis, MI, USA) as positive control. Cell viability was calculated as a percentage of control (cells incubated without compound). The 50% cytotoxic concentration (CC50) was determined from the dose–response curve by using the Table Curve 2D v5.0 software (Systat Software Inc.; Palo Alto, CA, USA). CC50 values represent the mean value calculated from three separate experiments.
3.2.3. In Vitro Cytotoxicity on the Human Monocyte THP-1 Cell Line
The undifferentiated THP1 human monocyte cells (acute monocytic leukemia cell line purchased from ATCC, ref TIB-202) were routinely grown in RPMI 1640 medium (Life Technologies, Saint-Aubin, France) supplemented with 10% FCS (Life Technologies, Saint-Aubin, France), 100 U/mL penicillin, 100 µg/mL streptomycin and 2 mM L-glutamine at 37 °C, 5% CO2. The culture was maintained between 3.105 and 8.105 cells/mL. The in vitro evaluation of the cytotoxicity of the tested compound was assessed according to the method of Mosmann [37] with slight modifications. Briefly, 100 μL of THP-1 cells with Phorbol 12-Myristate 13-Acetate (final concentration 50 ng/mL) were seeded in 96-well plates at an average density of 1 × 106 cells/mL and incubated for 24 h at 37 °C, 5% CO2. Wells were washed with RPMI and 100 µL of fresh complete medium was added. Then, 100 µL of various concentrations of compounds dissolved in DMSO (final concentration less than 0.5% v/v) and reference were added in duplicate. The plates were incubated for 72 h at 37 °C, 5% CO2. Each well plate was then microscope-examined to detect possible precipitate formation before the medium was removed from the wells. Then, 100 µL of MTT solution (0.5 mg/mL in medium) was added to each well. Cells were incubated for 2 h at 37 °C. Then, the MTT solution was removed and DMSO (100 µL) was added to dissolve the resulting blue formazan crystals. Plates were shaken vigorously (700 rpm) for 10 min. The absorbance was measured at 570 nm using a TECAN Infinite F-200 spectrophotometer (Tecan Trading AG, Männedorf, Switzerland). DMSO was used as blank and doxorubicin (purchased from Sigma Aldrich) as positive control. Cell viability was calculated as a percentage of control (cells incubated with DMSO). The 50% cytotoxic concentration (CC50) was determined from the dose–response curve by using the TableCurve 2D V5 software Systat Software Inc. (Palo Alto, CA, USA). CC50 values represent the mean value calculated from three separate experiments.
3.2.4. Assessing Antileishmanial Activity of 14c against L. donovani Promastigote Cell Lines Overexpressing NTR1 and NTR2
EC50 values were determined as previously described [24]. Nifurtimox was used as a positive control for activation via NTR1 [38] while delamanid was used as a positive control for activation via NTR2 [39].
4. Conclusions
To conclude, a series of sixty-one 5-stryryl-thiazolidine-2,4-diones was synthesized in 2–5 reaction steps and evaluated in vitro against the promastigote stage of Leishmania infantum. Several nitroaromatic derivatives were active, while the hit derivative proved to be 14c, bearing a nitro group at the meta position of the phenyl ring of the 5-benzylidene moiety and a cyanomethyl function at the 3-position of the thiazolidine-2,4-dione ring. Compound 14c showed similar activity to miltefosine, the only orally available antileishmanial drug compound, and similar cytotoxicity on the human HepG2 and THP-1 cell lines. Because of its nitroaromatic structure, 14c behaves as a prodrug, bioactivated predominantly by the parasite Type 1 nitroreductases, probably generating cytotoxic metabolites in the parasite cell. Finally, hit-molecule 14c is a lipophilic compound. However, it remains soluble in water, allowing further hit-to-lead medicinal chemistry to search for novel antileishmanial candidates that could be orally administered.
Acknowledgments
The authors would like to thank the “Agence Universitaire de la Francophonie AUF” for supporting this project. Also, we are grateful to Pierre Marty (Côte d’Azur University, Nice) and Thierry Lang (Pasteur Institute, Paris) for providing the luciferase-expressing L. infantum strain.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph17070878/s1, S1. Organic synthesis and product characterization of the known compounds used in our study; 1H and 13C spectra of the synthesized compounds.
Author Contributions
Conceptualization: H.E.-K., N.A. and P.V. (Pierre Verhaeghe); formal analysis, O.K., S.H., N.P., C.C.-D., M.L.E., D.F., M.S., S.C. and H.E.-K.; investigation, P.V. (Pierre Verhaeghe), P.V. (Patrice Vanelle), N.A., S.W. and H.E.-K.; writing—review and editing, all authors; project administration, H.E.-K., M.L.E., N.A. and P.V. (Patrice Vanelle). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research was funded by the Agence Universitaire de la Francophonie (AUF), and by the Wellcome trust grants: 203134/Z/16/Z and 218448/Z/19/Z.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization Leishmaniasis. [(accessed on 1 February 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
- 2.Oliveira V.G., Dos Santos Faiões V., Gonçalves G.B.R., Lima M.F.O., Boechat F.C.S., Cunha A.C., de Andrade-Neto V.V., da Silva F.d.C., Torres-Santos E.C., de Souza M.C.B.V. Design, Synthesis and Antileishmanial Activity of Naphthotriazolyl-4-Oxoquinolines. Curr. Top. Med. Chem. 2018;18:1454–1464. doi: 10.2174/1568026618666181002110116. [DOI] [PubMed] [Google Scholar]
- 3.Corpas-López V., Tabraue-Chávez M., Sixto-López Y., Panadero-Fajardo S., Alves de Lima Franco F., Domínguez-Seglar J.F., Morillas-Márquez F., Franco-Montalbán F., Díaz-Gavilán M., Correa-Basurto J., et al. O-Alkyl Hydroxamates Display Potent and Selective Antileishmanial Activity. J. Med. Chem. 2020;63:5734–5751. doi: 10.1021/acs.jmedchem.9b02016. [DOI] [PubMed] [Google Scholar]
- 4.Santos S.S., de Araújo R.V., Giarolla J., Seoud O.E., Ferreira E.I. Searching for Drugs for Chagas Disease, Leishmaniasis and Schistosomiasis: A Review. Int. J. Antimicrob. Agents. 2020;55:105906. doi: 10.1016/j.ijantimicag.2020.105906. [DOI] [PubMed] [Google Scholar]
- 5.Jeddi B., Saberi S., Menéndez J.C., Sepehri S. Synthesis and Biological Evaluation of Tetrahydropyrimidine and Dihydropyridine Derivatives against Leishmania major. Acta Parasitol. 2022;67:255–266. doi: 10.1007/s11686-021-00457-6. [DOI] [PubMed] [Google Scholar]
- 6.Mukhopadhyay S., Barak D.S., Karthik R., Verma S.K., Bhatta R.S., Goyal N., Batra S. Antileishmanial Assessment of Isoxazole Derivatives against L. donovani. RSC Med. Chem. 2020;11:1053–1062. doi: 10.1039/D0MD00083C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trefzger O.S., Barbosa N.V., Scapolatempo R.L., das Neves A.R., Ortale M.L.F.S., Carvalho D.B., Honorato A.M., Fragoso M.R., Shuiguemoto C.Y.K., Perdomo R.T., et al. Design, Synthesis, Antileishmanial, and Antifungal Biological Evaluation of Novel 3,5-Disubstituted Isoxazole Compounds Based on 5-Nitrofuran Scaffolds. Arch. Pharm. 2020;353:1900241. doi: 10.1002/ardp.201900241. [DOI] [PubMed] [Google Scholar]
- 8.Alonso L., Pianoski K.E., Alonso A., Rosa F.A. Antileishmanial Activity of 3,4,5-Trisubstituted Isoxazoles by Interaction with Leishmania amazonensis Plasma Membrane. J. Mol. Struct. 2022;1249:131604. doi: 10.1016/j.molstruc.2021.131604. [DOI] [Google Scholar]
- 9.Mowbray C.E., Braillard S., Speed W., Glossop P.A., Whitlock G.A., Gibson K.R., Mills J.E.J., Brown A.D., Gardner J.M.F., Cao Y., et al. Novel Amino-Pyrazole Ureas with Potent In Vitro and In Vivo Antileishmanial Activity. J. Med. Chem. 2015;58:9615–9624. doi: 10.1021/acs.jmedchem.5b01456. [DOI] [PubMed] [Google Scholar]
- 10.Khan M.F., Alam M.M., Verma G., Akhtar W., Akhter M., Shaquiquzzaman M. The Therapeutic Voyage of Pyrazole and Its Analogs: A Review. Eur. J. Med. Chem. 2016;120:170–201. doi: 10.1016/j.ejmech.2016.04.077. [DOI] [PubMed] [Google Scholar]
- 11.Faria J.V., Vegi P.F., Miguita A.G.C., dos Santos M.S., Boechat N., Bernardino A.M.R. Recently Reported Biological Activities of Pyrazole Compounds. Bioorg. Med. Chem. 2017;25:5891–5903. doi: 10.1016/j.bmc.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues C.A., Dos Santos P.F., da Costa M.O.L., Pavani T.F.A., Xander P., Geraldo M.M., Mengarda A., de Moraes J., Rando D.G.G. 4-Phenyl-1,3-Thiazole-2-Amines as Scaffolds for New Antileishmanial Agents. J. Venom Anim. Toxins Incl. Trop. Dis. 2018;24:26. doi: 10.1186/s40409-018-0163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Süleymanoğlu N., Ustabaş R., Direkel Ş., Alpaslan Y.B., Ünver Y. 1,2,4-Triazole Derivatives with Morpholine; DFT Study and Antileishmanial Activity. Can. J. Phys. 2018;96:719–723. doi: 10.1139/cjp-2017-0710. [DOI] [Google Scholar]
- 14.Espinosa A.V., Costa D.D.S., Tunes L.G., Monte-Neto R.L.D., Grazul R.M., de Almeida M.V., Silva H. Anticancer and Antileishmanial in Vitro Activity of Gold(I) Complexes with 1,3,4-Oxadiazole-2(3H)-Thione Ligands Derived from δ-D-Gluconolactone. Chem. Biol. Drug Des. 2021;97:41–50. doi: 10.1111/cbdd.13757. [DOI] [PubMed] [Google Scholar]
- 15.Camargo P.G., Bortoleti B.T.d.S., Fabris M., Gonçalves M.D., Tomiotto-Pellissier F., Costa I.N., Conchon-Costa I., Lima C.H.d.S., Pavanelli W.R., Bispo M., et al. Thiohydantoins as Anti-Leishmanial Agents: N Vitro Biological Evaluation and Multi-Target Investigation by Molecular Docking Studies. J. Biomol. Struct. Dyn. 2022;40:3213–3222. doi: 10.1080/07391102.2020.1845979. [DOI] [PubMed] [Google Scholar]
- 16.Kieffer C., Cohen A., Verhaeghe P., Hutter S., Castera-Ducros C., Laget M., Remusat V., M’Rabet M.K., Rault S., Rathelot P., et al. Looking for New Antileishmanial Derivatives in 8-Nitroquinolin-2(1H)-One Series. Eur. J. Med. Chem. 2015;92:282–294. doi: 10.1016/j.ejmech.2014.12.056. [DOI] [PubMed] [Google Scholar]
- 17.Pedron J., Boudot C., Hutter S., Bourgeade-Delmas S., Stigliani J.-L., Sournia-Saquet A., Moreau A., Boutet-Robinet E., Paloque L., Mothes E., et al. Novel 8-Nitroquinolin-2(1H)-Ones as NTR-Bioactivated Antikinetoplastid Molecules: Synthesis, Electrochemical and SAR Study. Eur. J. Med. Chem. 2018;155:135–152. doi: 10.1016/j.ejmech.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammill J.T., Sviripa V.M., Kril L.M., Ortiz D., Fargo C.M., Kim H.S., Chen Y., Rector J., Rice A.L., Domagalska M.A., et al. Amino-Substituted 3-Aryl- and 3-Heteroarylquinolines as Potential Antileishmanial Agents. J. Med. Chem. 2021;64:12152–12162. doi: 10.1021/acs.jmedchem.1c00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Horn K.S., Zhu X., Pandharkar T., Yang S., Vesely B., Vanaerschot M., Dujardin J.-C., Rijal S., Kyle D.E., Wang M.Z., et al. Antileishmanial Activity of a Series of N2,N4-Disubstituted Quinazoline-2,4-Diamines. J. Med. Chem. 2014;57:5141–5156. doi: 10.1021/jm5000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumari A., Jaiswal T., Kumar V., Hura N., Kumar G., Babu N.K., Acharya A., Roy P.K., Guchhait S.K., Singh S. Identification of 2-Arylquinazolines with Alkyl-Polyamine Motifs as Potent Antileishmanial Agents: Synthesis and Biological Evaluation Studies. RSC Med. Chem. 2022;13:320–326. doi: 10.1039/D1MD00336D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Mello H., Echevarria A., Bernardino A.M., Canto-Cavalheiro M., Leon L.L. Antileishmanial Pyrazolopyridine Derivatives: Synthesis and Structure-Activity Relationship Analysis. J. Med. Chem. 2004;47:5427–5432. doi: 10.1021/jm0401006. [DOI] [PubMed] [Google Scholar]
- 22.Anand D., Yadav P.K., Patel O.P.S., Parmar N., Maurya R.K., Vishwakarma P., Raju K.S.R., Taneja I., Wahajuddin M., Kar S., et al. Antileishmanial Activity of Pyrazolopyridine Derivatives and Their Potential as an Adjunct Therapy with Miltefosine. J. Med. Chem. 2017;60:1041–1059. doi: 10.1021/acs.jmedchem.6b01447. [DOI] [PubMed] [Google Scholar]
- 23.Jacomini A.P., da Silva M.J.V., Poletto J., Ribeiro G.M., Yokoyama J.T.C., Bidóia D.L., Paula F.R., Nakamura C.V., Sarragiotto M.H., Rosa F.A. Potential Antileishmanial Activity of 4-N-Acylhydrazone Pyrazolo[3,4-d]Pyridazin-7-Ones: Synthesis, In Vitro Biological Evaluations and Computational Studies. J. Braz. Chem. Soc. 2018;29:2657–2668. doi: 10.21577/0103-5053.20180134. [DOI] [Google Scholar]
- 24.Fersing C., Basmaciyan L., Boudot C., Pedron J., Hutter S., Cohen A., Castera-Ducros C., Primas N., Laget M., Casanova M., et al. Nongenotoxic 3-Nitroimidazo[1,2-a]Pyridines Are NTR1 Substrates That Display Potent in Vitro Antileishmanial Activity. ACS Med. Chem. Lett. 2019;10:34–39. doi: 10.1021/acsmedchemlett.8b00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nandikolla A., Srinivasarao S., Kumar B.K., Murugesan S., Aggarwal H., Major L.L., Smith T.K., Sekhar K.V.G.C. Synthesis, Study of Antileishmanial and Antitrypanosomal Activity of Imidazo Pyridine Fused Triazole Analogues. RSC Adv. 2020;10:38328–38343. doi: 10.1039/D0RA07881F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paoli-Lombardo R., Primas N., Bourgeade-Delmas S., Hutter S., Sournia-Saquet A., Boudot C., Brenot E., Castera-Ducros C., Corvaisier S., Since M., et al. Improving Aqueous Solubility and In Vitro Pharmacokinetic Properties of the 3-Nitroimidazo[1,2-a]Pyridine Antileishmanial Pharmacophore. Pharmaceuticals. 2022;15:998. doi: 10.3390/ph15080998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanjan M.J., Mohammed M., Prashantha Kumar B.R., Chandrasekar M.J.N. Thiazolidinediones as Antidiabetic Agents: A Critical Review. Bioorg. Chem. 2018;77:548–567. doi: 10.1016/j.bioorg.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Kumar H., Aggarwal N., Marwaha M.G., Deep A., Chopra H., Matin M.M., Roy A., Emran T.B., Mohanta Y.K., Ahmed R., et al. Thiazolidin-2,4-Dione Scaffold: An Insight into Recent Advances as Antimicrobial, Antioxidant, and Hypoglycemic Agents. Molecules. 2022;27:6763. doi: 10.3390/molecules27196763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Kashef H., Badr G., Abo El-Maali N., Sayed D., Melnyk P., Lebegue N., Abd El-Khalek R. Synthesis of a Novel Series of (Z)-3,5-Disubstituted Thiazolidine-2,4-Diones as Promising Anti-Breast Cancer Agents. Bioorg. Chem. 2020;96:103569. doi: 10.1016/j.bioorg.2020.103569. [DOI] [PubMed] [Google Scholar]
- 30.Burbridge M.F., Bossard C.J., Saunier C., Fejes I., Bruno A., Léonce S., Ferry G., Da Violante G., Bouzom F., Cattan V., et al. S49076 Is a Novel Kinase Inhibitor of MET, AXL, and FGFR with Strong Preclinical Activity Alone and in Association with Bevacizumab. Mol. Cancer Ther. 2013;12:1749–1762. doi: 10.1158/1535-7163.MCT-13-0075. [DOI] [PubMed] [Google Scholar]
- 31.Leite F.H.A., da Silva Santiago P.B.G., Froes T.Q., da Silva Filho J., da Silva S.G., Ximenes R.M., de Faria A.R., Brondani D.J., de Albuquerque J.F.C., Castilho M.S. Structure-Guided Discovery of Thiazolidine-2,4-Dione Derivatives as a Novel Class of Leishmania Major Pteridine Reductase 1 Inhibitors. Eur. J. Med. Chem. 2016;123:639–648. doi: 10.1016/j.ejmech.2016.07.060. [DOI] [PubMed] [Google Scholar]
- 32.Mague J.T., Mohamed S.K., Akkurt M., El-Kashef H., Lebegue N., Albayati M.R. (5Z)-3-(2-Oxopropyl)-5-(3,4,5-Trimethoxybenzylidene)-1,3-Thiazolidine-2,4-Dione. IUCrData. 2016;1:x161959. doi: 10.1107/S2414314616019593. [DOI] [Google Scholar]
- 33.Maccari R., Ottanà R., Curinga C., Vigorita M.G., Rakowitz D., Steindl T., Langer T. Structure-Activity Relationships and Molecular Modelling of 5-Arylidene-2,4-Thiazolidinediones Active as Aldose Reductase Inhibitors. Bioorg. Med. Chem. 2005;13:2809–2823. doi: 10.1016/j.bmc.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 34.Patterson S., Wyllie S. Nitro Drugs for the Treatment of Trypanosomatid Diseases: Past, Present, and Future Prospects. Trends Parasitol. 2014;30:289–298. doi: 10.1016/j.pt.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valkó K.L. Lipophilicity and Biomimetic Properties Measured by HPLC to Support Drug Discovery. J. Pharm. Biomed. Anal. 2016;130:35–54. doi: 10.1016/j.jpba.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Michel G., Ferrua B., Lang T., Maddugoda M.P., Munro P., Pomares C., Lemichez E., Marty P. Luciferase-Expressing Leishmania Infantum Allows the Monitoring of Amastigote Population Size, In Vivo, Ex Vivo and In Vitro. PLoS Negl. Trop. Dis. 2011;5:e1323. doi: 10.1371/journal.pntd.0001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 38.Patterson S., Wyllie S., Stojanovski L., Perry M.R., Simeons F.R.C., Norval S., Osuna-Cabello M., De Rycker M., Read K.D., Fairlamb A.H. The R Enantiomer of the Antitubercular Drug PA-824 as a Potential Oral Treatment for Visceral Leishmaniasis. Antimicrob. Agents Chemother. 2013;57:4699–4706. doi: 10.1128/AAC.00722-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyllie S., Roberts A.J., Norval S., Patterson S., Foth B.J., Berriman M., Read K.D., Fairlamb A.H. Activation of Bicyclic Nitro-Drugs by a Novel Nitroreductase (NTR2) in Leishmania. PLoS Pathog. 2016;12:e1005971. doi: 10.1371/journal.ppat.1005971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and supplementary materials.