Abstract
The nodavirus flock house virus (FHV) has a bipartite, positive-sense, RNA genome that encodes the catalytic subunit of the RNA replicase and the viral capsid protein precursor on separate genomic segments (RNA1 and RNA2, respectively). RNA1 can replicate autonomously when transfected into permissive cells, allowing study of the kinetics of RNA1 replication in the absence of either RNA2 or capsid proteins. However, RNA1 replication ceases ca. 3 days after transfection despite the presence of replication-competent RNA. We examined this inhibition by inducing the expression of RNA1 in cells from a cDNA copy that was under the control of a hormone-regulated RNA polymerase II promoter. This system reproduced the shutoff of RNA replication when DNA-templated primary transcription was turned off. Continued primary transcription partially alleviated the shutoff and maintained the rate of RNA replication for several days at a steady-state level approximately one-third that of the peak rate. After shutoff, RNA replication could be restored by transferring the resulting intracellular RNA to fresh cells or by reinducing primary transcription, indicating that cessation of replication occurred despite the competence of both the viral RNA and the cytoplasmic environment. These data suggest that there is a mechanism by which replication is shut off at late times after transfection, which may reflect the natural endpoint of the replicative cycle.
Late after infection of a host cell with a cytocidal virus, macromolecular synthesis (both host and virus specific) shuts down. Often the cell is dying, while the virus has shifted from a biosynthetic phase into an assembly phase. In the case of the positive-strand RNA viruses, this entails the encapsidation of the viral genomic RNA into virions. These inevitably competing events complicate the analysis and interpretation of the kinetics of RNA synthesis at late times after infection. However, many of these complications can be circumvented by using flock house virus (FHV), a member of the Nodaviridae, as a model system for the study of RNA replication. This approach takes advantage of the nodavirus divided genome, which naturally separates the replicative and packaging functions onto two different positive-sense RNA molecules, RNA1 and RNA2, respectively (39–41).
Both genomic RNAs have cap zero structures at their 5′ ends, and their 3′ ends, which are not polyadenylated, are blocked either by an unusual secondary structure or by an undetermined covalent modification (3, 18, 32). RNA1 (3,107 nucleotides [nt]), encodes protein A, the catalytic subunit of the viral RNA-dependent RNA polymerase (RNA replicase), while RNA2 (1,400 nt) encodes the capsid protein precursor, protein α, which is autocatalytically cleaved into the proteins (β and γ) found in the mature virion (30). The genomic segments (RNA1 and RNA2) are copackaged into virions, and both are required for infectivity (35, 41, 55).
The RNA replicase catalyzes replication of both genomic RNAs via negative-strand intermediates (1, 4, 29). It is not known whether host cell factors are required for replicase activity. However, the ability of FHV RNA to replicate in insect (29), mammalian (4), plant (54), and yeast (48) cells despite a natural host range that is limited to insects (53), suggests that any such host factors must be functionally conserved in all of these disparate intracellular environments. During RNA replication, a small subgenomic RNA (RNA3) is synthesized from RNA1, probably by internal initiation on the RNA1 negative strand. RNA3 contains the 3′ terminal 387 nt of RNA1 (3, 32) and encodes two small proteins (B1 and B2) of unknown function (3, 25, 26) but is not packaged into virions (25).
In general, the replication of positive-sense RNA involves at least three well-recognized mechanistic steps: parental RNA is first translated to generate active replicase and then copied by this enzyme to produce negative strands, which in turn are used as templates for the synthesis of positive-sense progeny RNAs. According to the model developed for bacteriophage Qβ replicase (7–11, 23), the progeny RNAs feed back both as mRNA for synthesis of more replicase and as template for further negative-strand synthesis, resulting in initial hyperbolic kinetics of RNA replication. However, the bulk of the RNA products accumulate during the linear phase of the reaction, when the replication rate is constant, because the concentrations of enzyme and template that are involved in replication have reached steady states. Later, RNA replication slows when the feedback is no longer sufficient to maintain the steady-state concentrations of functional enzyme and negative strand template. In Qβ-infected bacterial cells, some progeny RNA molecules are prevented from feeding back into the reaction by encapsidation into phage particles and others by the formation of double-stranded RNA (23).
Guarino and Kaesberg (33) studied the kinetics of nodavirus RNA replication in cell extracts prepared at various times postinfection (p.i.) from Drosophila melanogaster cells infected with black beetle virus (BBV), the RNA1 of which shares 99% sequence identity with RNA1 from FHV (17, 18). BBV RNA replication conformed rather well to the general pattern established for Qβ. After a 3-h lag period, RNA replication increased exponentially until 10 h p.i., then increased at a slower linear rate until 48 h p.i., and finally decreased by 72 h p.i. As the viral yield at each time point was not determined in these experiments, it is unclear to what extent the kinetics of BBV replication were influenced by continuous removal of positive-strand template RNAs by packaging into progeny virions (33).
Neither RNA2 nor the capsid proteins it encodes are required for replication of nodavirus RNA1, which replicates autonomously in the absence of RNA2 because it encodes the entire viral contribution to the RNA replicase (29). When transfected into susceptible cells, RNA1 reaches its peak rate of synthesis after about 17 h, replicates vigorously for at least 24 h, and attains levels comparable to those of the rRNAs (1, 4, 29). Under these conditions, RNA3 is synthesized in greater abundance than in the presence of RNA2 (29) because replication of RNA2 specifically downregulates RNA3 synthesis (63).
Nodavirus RNA replication in mammalian cells can also be initiated by the use of cDNA-based transcription systems that reconstruct many features of the natural situation (1–3, 5, 6, 34). Transcription of full-length cDNA clones of FHV RNA1 (19) by T7 RNA polymerase expressed from a recombinant vaccinia virus (VV) (27) results in abundant cytoplasmic RNA replication (1–3, 5, 38). Synthesis of primary transcripts with authentic termini was essential for optimum replication because the presence of extraneous nucleotides at either the 5′ or the 3′ end was detrimental (1, 5). This was accomplished by placing the promoter in the transcription plasmids precisely at the 5′ end of the viral cDNA and including a cDNA copy of the antigenomic ribozyme from hepatitis delta virus (HDV) (46) immediately downstream of the FHV cDNA; the resulting transcription products self-cleaved to yield authentic 3′ ends (1–3, 5). Abundant RNA1 replication was also obtained on infection of susceptible cells by using VV recombinants containing the FHV RNA1 cDNA positioned between the VV 7.5k promoter and the HDV ribozyme (6). In addition, we have achieved the replication of primary transcripts made in the nucleus of cells from a transcription plasmid that retains the general structure described above but contains the FHV RNA1 cDNA downstream of a constitutive RNA polymerase II (pol II) promoter (34).
The potential use of nodavirus RNA replication to amplify and express foreign mRNAs focused our interest on the longevity of RNA1 replication in the absence of RNA2. In the current study, we observed that RNA1 replication ceased 3 days after transfection, despite the presence of a high intracellular concentration of replication-competent RNA. This inhibition was also observed when we used a plasmid containing an inducible promoter to launch RNA1 replication from the nucleus of DNA-transfected cells. After RNA replication had stopped, it could be restored either by transferring the resulting intracellular RNA to fresh cells or by reinducing primary transcription in the original plasmid-transfected culture, indicating that replication ceased despite the competence of both the viral RNA and the cytoplasmic environment. This shutoff may represent the natural endpoint of RNA replication, functionally equivalent to the late decline in the rates of RNA replication observed in vivo with Qβ and BBV (23, 33).
MATERIALS AND METHODS
Cells and viruses.
EcR-CHO cells, a subline of CHO cells that stably express a heterodimeric ecdysone-retinoid X receptor (EcR/RXR) were purchased from Invitrogen (Carlsbad, Calif.). The cells were grown in Ham’s F-12 medium (GIBCO/BRL) supplemented with 10% fetal bovine serum (HyClone, Logan, Utah), penicillin-streptomycin (50 U/ml and 50 μg/ml, respectively), 2 mM l-glutamine, and 250 μg of Zeocin (Invitrogen) per ml. Baby hamster kidney (BHK21) cells were grown as described previously (1). VV-FHV recombinant vF1 contained full-length cDNA of FHV RNA1 between the 7.5k VV promoter and a cDNA copy of the antigenomic ribozyme of HDV (6). By autolytic cleavage of the primary transcripts, the ribozyme generated RNA that terminated at the authentic 3′ nucleotide.
Plasmids.
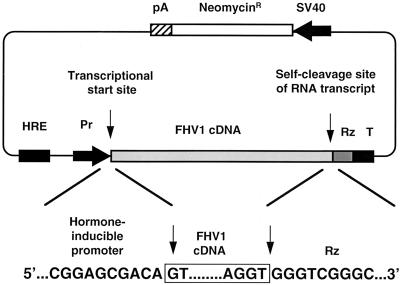
Plasmids pIND and pIND-lacZ, which contain a hormone-regulated pol II promoter, were purchased from Invitrogen, and plasmid FHV1[1,0] was as described previously (3). Plasmid pIND-FHV1[0,0] (shown schematically in Fig. 4) was constructed from pIND and FHV1[1,0] by PCR and conventional cloning techniques to put the FHV1 cDNA under the control of the inducible promoter (51). It contained a pol II promoter located downstream of a hormone response element (42), followed by the FHV RNA1 cDNA. As in previous FHV transcription plasmids (1–3, 5, 34), the promoter was resected to place the 5′ terminus of the RNA1 cDNA at the transcriptional initiation site, and the 3′ end of the FHV1 cDNA was juxtaposed with the cleavage site of the HDV antigenomic ribozyme (46). The nucleotide sequences of the promoter-cDNA-ribozyme junctions were confirmed experimentally and are shown in Fig. 4. The T7 transcriptional terminator (49) was also retained in pIND-FHV1[0,0], as it appeared to facilitate the ribozyme-mediated cleavage (34).
FIG. 4.
Schematic diagram of plasmid designed to direct inducible FHV RNA1 replication. The transcriptional start site and self-cleavage site of the RNA transcript are indicated by vertical arrows. The nucleotide sequence of the junctions between the promoter, cDNA, and ribozyme are shown below the plasmid diagram. HRE, hormone response elements; Pr, minimal RNA pol II promoter; Rz, HDV antigenomic ribozyme; T, T7 terminator.
Isolation of FHV RNA1 expressed from a VV-FHV recombinant.
To provide a convenient source of molecularly cloned, replication-competent FHV RNA1, BHK21 cells were infected at 28°C with the VV-FHV recombinant vF1 (6) at a multiplicity of infection of 10. Total cellular RNA was isolated at 24 h p.i., diluted 25-fold, and amplified by a second 24-h replicative passage in fresh BHK21 cells (3).
RNA transfection of cells.
Monolayers containing 2 × 106 EcR-CHO cells were transfected with this mixture of cellular and FHV RNAs by using Lipofectin (10 μg) and OptiMEM (1 ml) (both from GIBCO/BRL) as previously described (1). Transfected cells were incubated at 28°C for the times indicated in the figure legends. For the RNA passage experiments, total cellular RNA was isolated from transfected cells as described previously (34); for each sample, 4% of this mixture of viral and cellular RNAs was then transfected into a fresh monolayer of EcR-CHO cells as described above.
Protein labeling, extraction, and analysis.
For protein labeling, transfected cells were preincubated for 30 min in modified Eagle minimum essential medium lacking methionine and cysteine (ICN). The products of protein synthesis were then metabolically labeled for 3 h by incorporation of [35S]methionine-[35S]cysteine (Tran35S label; 50 μCi/ml; ICN) in Met-Cys-free medium. Labeled proteins were extracted, resolved by electrophoresis on a sodium dodecyl sulfate (SDS)–12.5% polyacrylamide gel, and visualized by autoradiography (1).
Plasmid transfection and hormone treatment of cells.
Monolayers containing 2 × 106 EcR-CHO cells were transfected with pIND-FHV1 (2.5 μg) by using Lipofectamine (14 μg) (GIBCO/BRL) and OptiMEM (1 ml) as described earlier (34). After transfection, the cells were incubated at 37°C for 6 h, the transfection mixture was replaced with complete F-12 medium, and incubation was continued at 37°C for an additional 18 h. The steroid hormone muristerone A (Invitrogen) was then added to the medium to a final concentration of 1 μM, and the cells were shifted to 28°C, the permissive temperature for FHV RNA replication. Incubation was continued at 28°C with daily replenishment of medium containing muristerone A for the times indicated in the text. In the hormone removal experiments, the medium containing muristerone A was removed, the cells were washed twice with phosphate-buffered saline, complete F-12 medium lacking muristerone A was added, and the incubation was continued at 28°C as above. Transfection efficiency was estimated to be approximately 10% at 28°C by transfection of EcR-CHO cells with reporter plasmid pIND-lacZ and subsequent induction with muristerone A, as described previously (34).
RNA labeling, extraction, and analysis.
The products of RNA replication were labeled by metabolic incorporation of [3H]uridine in the presence of 20 μg of actinomycin D per ml to inhibit DNA-dependent RNA synthesis (1). After incubation at 28°C for 2 h, total cellular RNAs were extracted with guanidine thiocyanate (RNAgents; Promega Biotec) (16), as described previously (34). Samples corresponding to the RNA from 3.3 × 105 cells were analyzed by electrophoresis on denaturing formaldehyde-agarose gels and visualized by fluorography (34). For quantitation of RNA replication, triplicate samples of the 3H-labeled RNA replication products from 1.7 × 105 cells were precipitated with trichloroacetic acid, and the combined levels of RNAs 1 and 3 were determined by scintillation spectrometry (3). The resulting counts per minute were averaged for each triplicate set and plotted as a function of the incubation time; error bars represent the range of values obtained. Each experiment was repeated a minimum of three times, and the results of representative experiments are shown in the figures.
Primer extension.
RNA samples from 2.7 × 105 cells were analyzed by primer extension, using oligonucleotide primers that were complementary to nt 85 to 69 of FHV RNA1 or nt 98 to 80 of RNA3. Primer extension products were labeled by incorporation of [α-35S]dATP, resolved on a 6% polyacrylamide sequencing gel alongside dideoxy sequencing ladders generated with the same primers on a pIND-FHV1 template, and visualized by autoradiography as described previously (3). Where indicated, FHV RNA levels in different samples were normalized to the amount of 18S rRNA present in each sample as determined by primer extension (34). A primer specific for FHV RNA3 (complementary to nt 2819 to 2801 of RNA1) was mixed with a primer complementary to nt 82 to 65 of Rattus norvegicus 18S rRNA (57) and extended by using reverse transcriptase and [α-35S]dATP. The relative amounts of total RNA present in each sample were quantitated by densitometric scanning of the resulting autoradiographs and normalized to the abundance of 18S rRNA.
RESULTS
Kinetics of RNA replication in cells transfected with FHV RNA1.
When transfected into insect (Drosophila line 1) or vertebrate (BHK21) cells, nodavirus RNA1 replicates autonomously for at least 24 h (29), reaching levels comparable to those of rRNAs (4). As part of our continuing efforts to understand and harness nodavirus RNA replication, we examined the process over longer time periods to determine the duration of replication. A subline of Chinese hamster ovary (CHO) cells (see Materials and Methods) was transfected with molecularly cloned, replication-competent FHV RNA1. At 24-h intervals, transfected cells were pulse-labeled with [3H]uridine in the presence of actinomycin D to inhibit DNA-dependent RNA synthesis. Under these conditions, uridine incorporation specifically measures the activity of the RNA replicase as it synthesizes FHV RNAs 1 and 3 (see Fig. 6B), so we could quantitate RNA replication simply by measuring uridine incorporation (Fig. 1, lower panel). The peak rate of RNA replication occurred within the first 24 h posttransfection, a finding consistent with previous observations of the replication of FHV RNA1 in Drosophila cells (29) and Nodamura virus RNA1 in BHK21 cells (1). However, on further incubation, the rate of RNA replication diminished so that after 3 days the reaction was undetectable. In view of the self-supporting nature of RNA1 replication, this cessation was surprising. Examination of the transfected cultures by phase-contrast microscopy showed no overt signs of cytopathology during the 5-day course of this experiment. Similar results were obtained upon transfection of BHK21 cells with FHV RNA1, although the kinetics of the shutoff were delayed by several days (data not shown).
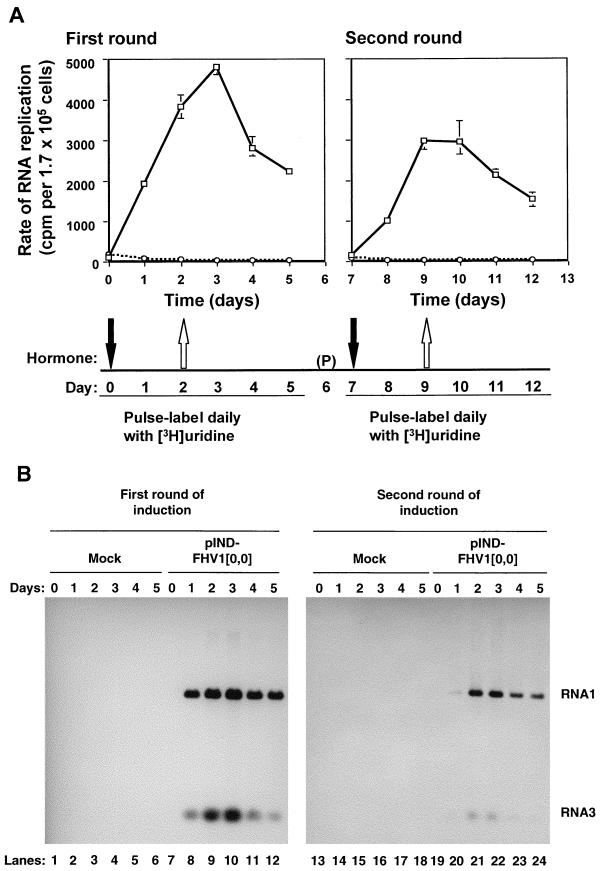
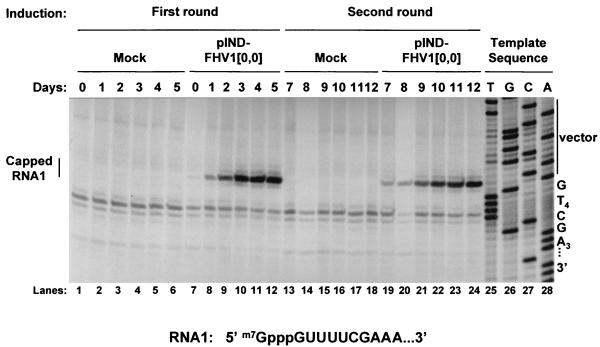
FIG. 6.
Analysis of RNA replication in cells transfected with pIND-FHV1[0,0] in response to two pulses of hormone treatment. Cells were transfected with pIND-FHV1[0,0] (□) or mock transfected (○) and treated with hormone as described for Fig. 5, except that the hormone was removed from parallel dishes after 48 h, and incubation was continued in its absence for the times indicated. The cells were passaged, “(P)”, onto fresh plates on day 6, and a second round of hormone addition and removal was performed. During each round of induction, cells were pulse-labeled in the presence of actinomycin D at daily intervals for 5 days, as in Fig. 1. First round, days 0 to 5 (left panels); second round, days 8 to 13 (right panels). Labeled RNAs were isolated and analyzed as follows. In panel A, RNAs were acid precipitated in triplicate, quantitated by scintillation spectrometry, and normalized to rRNA levels as described for Fig. 1. In panel B, RNAs were normalized to rRNA levels as described above and analyzed by electrophoresis on denaturing formaldehyde-agarose gels; the fluorographs are shown. Lanes 1 to 6 and 13 to 18, mock-transfected cells; 7 to 12 and 19 to 24, cells transfected with plasmid pIND-FHV1[0,0].
FIG. 1.
Kinetics of FHV RNA1 replication in RNA-transfected cells. (Lower panel) EcR-CHO cells were transfected with FHV RNA1 and incubated at 28°C. At 24-h intervals, cells were pulse-labeled for 2 h with [3H]uridine in the presence of actinomycin D. Total cellular RNAs were isolated. Labeled RNAs were then acid precipitated and quantitated by scintillation spectrometry. The rate of RNA replication (counts per minute per 1.7 × 105 cells) was expressed as a function of incubation time. Results were normalized to the amount of rRNA present in each sample as described in Materials and Methods. (Middle panel) The relative abundance of FHV RNA1 in the total RNA pool isolated at each time point was determined by primer extension with an RNA1-specific primer and quantitated by densitometric analysis as described in Materials and Methods. The amounts are expressed as percentages of the maximum (day 2) value. (Upper panel) A portion (4%) of the total cellular RNAs isolated at each time point shown in the lower panel were transfected into a fresh monolayer of EcR-CHO cells and incubated at 28°C. At 24 h posttransfection, RNAs were labeled, isolated, and analyzed as described for the lower panel.
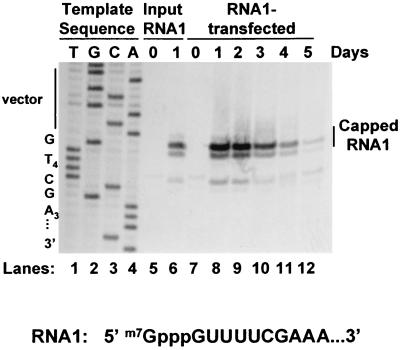
We considered the possibility that the failure to maintain RNA replication over the longer time period might result from defects in the quantity or functional quality of the RNAs produced by replication. The level of RNA1 that accumulated during the course of this experiment was measured by primer extension (Fig. 2) and, after normalization to the levels of rRNA in each sample, quantitated by densitometry as described in Materials and Methods (Fig. 1, middle panel). A family of three primer extension products was detected as previously described, with the major product corresponding to capped RNA1 (Fig. 2) (34). Clearly, RNA1 molecules with intact 5′ termini were still present in these cells even on day 5, more than 48 h after RNA replication had ceased. Furthermore, after several days of replication, there was no evidence for the accumulation of uncapped RNA1, which would have yielded a 1-nt shorter primer extension product (3, 20). A similar examination of the 3′ termini was not possible because these ends are blocked to chemical modification (18, 32). However, the observation that removal of 5 nt from the 3′ end of FHV RNA1 prevented its replication (3), coupled with the replicability of the extracted RNA in fresh cells (Fig. 1, upper panel), suggests that at least some of these molecules must have intact 3′ ends.
FIG. 2.
Primer extension analysis of RNA1 isolated from transfected cells. Equivalent amounts of total cellular RNAs isolated at each time point during the experiment shown in the lower panel of Fig. 1 (RNA1-transfected lanes) were analyzed by primer extension with an RNA1-specific primer as described in Materials and Methods. The RNA1 stock originally used to transfect cells in Fig. 1, lower panel, was analyzed in parallel as a control (Input RNA1 lanes). Products were separated by electrophoresis on a 6% polyacrylamide sequencing gel alongside a dideoxynucleotide sequencing ladder generated by using the same primer with plasmid pIND-FHV1[0,0] as template; an autoradiograph is shown. The sequencing lanes were labeled with the complement of the terminating dideoxynucleotide to reflect the sequence of the viral positive strand. The sequence of the 5′ terminus of RNA1 is shown. The position of the primer extension product that corresponds to capped RNA1 is indicated. The FHV RNA1 sequence in vF1 lacks nt C6 so the primer extension product corresponding to capped RNA1 comigrates with nt 1 of the sequencing ladder shown in lanes 1 to 4 rather than migrating 1 nt more slowly as it does in Fig. 7, where C6 is present.
Quantitative analysis of the primer extension products (Fig. 1, middle panel) revealed that although the rate of RNA replication was maximal within the first 24 h after transfection, the accumulation of RNA1 peaked at 48 h and then progressively declined as replication ceased. After 48 h, RNA1 decayed with an apparent half-life of approximately 24 h, so that on day 5 only 10% as much RNA1 was detected by primer extension as on day 2. Similar results were obtained for RNA3 (data not shown). The question remained whether this decline in RNA accumulation was the cause of the replicative shutoff or simply an effect thereof (e.g., the result of normal turnover of this RNA in the absence of further synthesis).
To address this point further, we tested whether the accumulated replication products could direct a second round of replication in fresh monolayers of CHO cells. Samples corresponding to 4% aliquots of the six daily RNA harvests from the first round of replication were transfected into fresh cells and assayed for RNA replication 24 h later (Fig. 1, upper panel). This experiment yielded a qualitative rather than quantitative picture of replicative fitness because replication of transfected RNA was linear over only a narrow range of RNA concentrations (data not shown). Nonetheless, the results showed clearly that the cells in which replication had ceased still contained RNA that could initiate autonomous replication in naive cells, indicating that the shutoff could not be attributed to any deficiency of the RNA template.
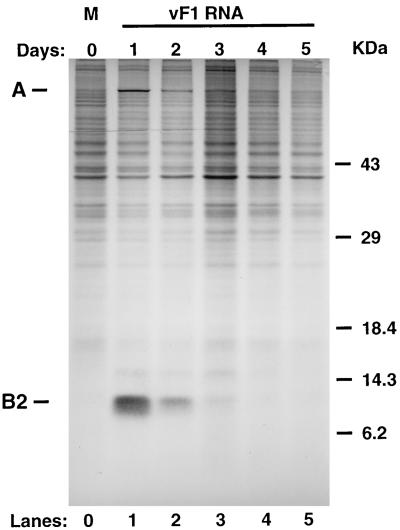
We next examined the kinetics of protein synthesis after RNA transfection. Cells were transfected with FHV RNA1 as for Fig. 1, and total cellular proteins were labeled at 24-h intervals by metabolic incorporation of [35S]methionine and [35S]cysteine. The results of SDS-polyacrylamide gel electrophoresis analysis of the labeled proteins are shown in Fig. 3. At 24 h after transfection with RNA1, three new proteins were detected above the background of cellular protein synthesis: protein A, encoded by RNA1, and proteins B1 and B2, encoded by RNA3. The positions of proteins A and B2 are indicated in Fig. 3; protein B1 is the minor band migrating slightly slower than B2, with an apparent Mr of 14,000. Synthesis of these proteins declined to background levels over the next 48 h, with kinetics that approximated those of the replicative shutoff, despite the continued presence of the corresponding messages. Furthermore, the primer extension product pattern provides no evidence for the accumulation of uncapped RNA even at late times after transfection (Fig. 2), suggesting that these messages retain the 5′ cap structure required for recognition by the translation machinery. No decrease in overall cellular protein synthesis was observed (compare lanes 1 and 5 in Fig. 3). Similarly, the viral proteins were visible by Coomassie blue staining 24 h after transfection, then disappeared with a half-life very comparable to the decrease in the rate of protein synthesis seen in Fig. 3, with no corresponding decrease in staining of cellular proteins (data not shown). This showed that the replicase protein made from these RNAs did not persist indefinitely in the absence of further protein synthesis. However, the question still remained whether the disappearance of the replicase was the cause or an effect of the replicative shutoff.
FIG. 3.
Analysis of FHV protein synthesis. Cells were transfected with authentic FHV RNA1 as indicated in the legend to Fig. 1. At 24-h intervals, the cells were labeled for 3 h by metabolic incorporation with [35S]Met-[35S]Cys. Labeled proteins were separated on an SDS–12.5% polyacrylamide gel and visualized by autoradiography. Lanes: 0, mock transfected; 1 to 5, transfected with FHV RNA1. The migration pattern of molecular mass standards is indicated at right in kilodaltons.
Development of an inducible plasmid-driven RNA replication system.
In order to address the question of cause and effect and to further examine the role played by the intracellular environment in the regulation of RNA replication, we developed a system in which replication was initiated by the synthesis of DNA-templated primary transcripts. We showed previously that FHV RNA1 transcribed from a constitutive RNA pol II promoter could initiate autonomous cytoplasmic RNA replication in plasmid-transfected mammalian cells (34). Replacement of the constitutive promoter with an inducible promoter enabled us to use this system to further study replicative shutoff because it allowed temporal separation of RNA replication from primary transcription.
We constructed plasmid pIND-FHV1[0,0] (shown schematically in Fig. 4), which contained a hormone-responsive promoter (42) upstream of the FHV RNA1 cDNA. As in previous FHV transcription plasmids (1, 34), the 5′ terminus of the RNA1 cDNA was positioned exactly at the transcriptional initiation site, and the 3′ end of the FHV1 cDNA was juxtaposed with the cleavage site of the HDV antigenomic ribozyme (46).
This plasmid was designed for use with the ecdysone-inducible expression system (42), which relies on the ability of a hormone receptor, in the presence of its steroid hormone ligand, to bind to a specific DNA sequence on the target plasmid and transactivate transcription by cellular RNA pol II. In the work described here, the hormone receptor was provided by a subline of Chinese hamster ovary (CHO) cells engineered to stably express it (EcR-CHO cells). Wild-type CHO cells can support the replication of nodavirus RNAs (4), and the results shown in Fig. 1 and 2 confirm that the EcR-CHO cells also support FHV RNA replication.
The inducible system reproduces the replicative shutoff.
Upon transfection of EcR-CHO cells with plasmid pIND-FHV1[0,0], followed by treatment with the steroid hormone muristerone A, RNA replication was induced and then subsequently shutoff when the hormone was removed (Fig. 5, dotted line), a result reminiscent of the behavior observed after transfection with authentic RNA1. Surprisingly, the replicative shutoff was partially alleviated if the hormone was present continuously (Fig. 5, solid line), indicating that continued primary transcription prolonged the duration of RNA replication.
FIG. 5.
RNA replication in CHO cells transfected with pIND-FHV1[0,0]. Cells were transfected with plasmid pIND-FHV1[0,0] and incubated at 37°C for 24 h. The cells were shifted to 28°C and treated with muristerone A for 24 h. Thereafter, hormone was replenished daily in one set of dishes (□) and removed from a parallel set by washing and replacing with complete medium that lacked the hormone (○). At daily intervals, cells were pulse-labeled with [3H]uridine in the presence of actinomycin D. Labeled RNAs were isolated and quantitated as described for Fig. 1. The rate of RNA replication (in counts per minute per 1.7 × 105 cells) was expressed as a function of time after hormone addition.
When hormone treatment was limited to a 24-h pulse, the peak rate of RNA replication was reached during the first 24 h (Fig. 5, dotted line), as seen after RNA1 transfection (Fig. 1, lower panel) (3, 29). After removal of the inducing hormone, this rate diminished progressively over the course of the next 3 days, returning to background levels by day 5 (Fig. 5, dotted line). In contrast, in the continuous presence of the hormone the rate of RNA replication continued to increase for an additional 48 h and then diminished but did not shut off entirely (Fig. 5, solid line). Instead, the rate of RNA replication was maintained at approximately one-third the peak rate for at least 8 days (data not shown).
RNA replication can be induced repeatedly.
One possible explanation for the failure to maintain long-term RNA replication after hormone removal was that the process had perturbed the intracellular environment in such a way that it was no longer able to support RNA replication. To examine this possibility, we tested whether a second round of RNA replication could be achieved by reinducing primary transcription after shutoff. Cells were either transfected with pIND-FHV1[0,0] (Fig. 6A, solid lines) or else mock transfected (Fig. 6A, dotted lines) and then treated with muristerone A as before. At daily intervals, cells in parallel dishes were pulse-labeled with [3H]uridine in the presence of actinomycin D, beginning immediately before hormone addition and continuing for 6 days. The hormone was removed after 2 days and incubation and labeling were continued as before. At 6 days after the initial hormone treatment began, the cells were passaged to minimize overcrowding and again treated with muristerone A for 2 days beginning on day 7. Day 7 was chosen to ensure that the first round of replication had been completely shut off before reinduction, allowing clear temporal distinction between the products of the two rounds. As before, sister monolayers were labeled daily with [3H]uridine in the presence of actinomycin D. Labeled replication products were quantitated by acid precipitation, and the results are shown in Fig. 6A.
In the first round of hormone treatment (Fig. 6A, left panel), the rate of RNA replication peaked within 72 h of induction and decreased progressively after hormone removal, so that by day 7 (5 days after hormone withdrawal), the rate of RNA replication had returned to background levels (Fig. 6A, right panel). However, RNA replication could be restored by renewed hormone treatment of these cultures (Fig. 6A, right panel). No restoration of replication occurred in the absence of renewed hormone treatment (data not shown), suggesting that reinduction was not simply the result of cellular metabolic changes caused by passaging of the cells. These results were particularly informative because they established that even after the shutoff of the first round of RNA replication, the intracellular environment was able to support the replication of new primary transcripts. The rate of RNA replication was reproducibly somewhat lower in the second round than in the first (compare the left and right panels of Fig. 6A), but since the decrease correlated approximately with the extent of cell dilution during passaging (data not shown), we attribute it to enrichment of the culture with cells that had escaped transfection.
Agarose gel analysis of the RNAs labeled during the two rounds of hormone-induced replication (Fig. 6B) showed the presence of FHV RNAs 1 and 3, the expected products of RNA synthesis, and the absence of detectable levels of aberrant RNA species such as defective interfering RNAs that might have accumulated during replication and contributed to the replicative shutoff. The patterns of RNA products made during the two rounds of replication were similar (Fig. 6B), and the rate of RNA replication was higher in the first round than in the second, as predicted by the rate measurements shown in Fig. 6A.
The accumulated levels of these RNAs were analyzed by primer extension, using primers specific for RNA1 (Fig. 7) or RNA3 (data not shown). As seen after RNA1 transfection and subsequent replication (Fig. 2), the major primer extension products corresponded to capped RNAs 1 and 3, and no evidence for the accumulation of uncapped RNA was detected. In uninduced cells, low levels of RNA1 were detected (Fig. 7, lane 7), but this RNA accumulated to high levels only after hormone treatment and the onset of RNA replication (Fig. 7, lanes 8 to 12).
FIG. 7.
Primer extension analysis of RNAs from the two-round induction experiment. Equivalent amounts of total cellular RNAs isolated at each time point in Fig. 6 were analyzed by primer extension with an RNA1-specific primer as described in the legend to Fig. 2. An autoradiograph of the sequencing gel is shown. The position of the primer extension product that corresponds to capped RNA1 (which migrates as 1 nt longer than the predicted size for the product from uncapped FHV RNA1) is indicated; the sequence of the 5′ terminus of RNA1 is shown.
The results in Fig. 7 show that most of the RNA1 present on day 5 disappeared within the next 2 days, as its rate of replenishment by replication diminished (compare lanes 12 and 19 of Fig. 7). By day 7, the level of RNA1 had fallen nearly to uninduced levels (compare lanes 7 and 19 of Fig. 7). However, as with the experiments shown in Fig. 1 and 2, it appeared that the replicative shutoff preceded the disappearance of the RNA rather than being caused by it. For example, similar levels of RNA1 were detected during the onset of replicative shutoff in each round (days 3 to 5 and 10 to 12 and lanes 10 to 12 and 22 to 24, respectively, in Fig. 7), suggesting that the decreases in RNA replication during these time periods did not result from loss of the RNA. These results suggest, therefore, that the disappearance of the RNA replication products is an effect rather than the cause of the shutoff of replication.
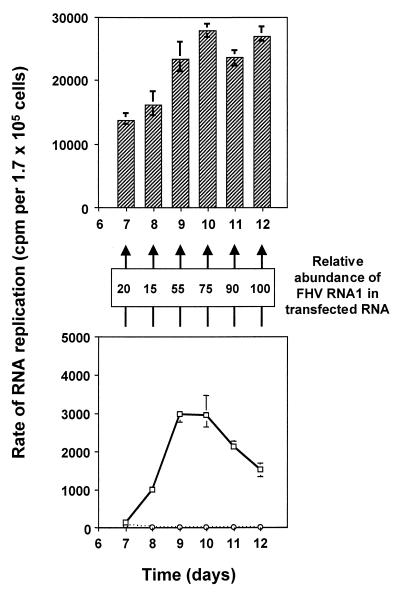
To test this conclusion further, we examined whether the RNA isolated from cells during the two rounds of RNA replication retained the ability to direct its own replication. The RNAs harvested at days 7 to 12 from the experiment shown in Fig. 6 and 7 were transfected into fresh cells and assayed for RNA replication after 24 h. [3H]uridine-labeled RNAs were quantitated as described for Fig. 1 (top panel), and the results are shown in the top panel of Fig. 8; the righthand panel from Fig. 6A is reproduced in the bottom panel of Fig. 8 for the sake of clarity. RNAs isolated at each time point were competent for replication in naive cells, even at the onset of the second round, when the rate of replication had returned nearly to background levels. These results indicate that the replicative shutoff occurs even in the presence of replication-competent RNA in an intracellular environment that is capable of supporting further RNA replication.
FIG. 8.
Replication in naive cells of RNAs isolated from pIND-FHV1 transfected cells after shutoff. (Lower panel) Reproduction of Fig. 6A (right panel): solid line, cells transfected with pIND-FHV1[0,0]; dotted line, mock transfected cells. (Middle panel) The relative abundance of FHV RNA1 in the total RNA pool isolated at each time point was determined by primer extension with an RNA1-specific primer, followed by densitometric analysis, as described in Materials and Methods. The amounts are expressed as percentages of the maximum (day 12) value. (Upper panel) Portions (4%) of the total RNAs isolated at each time point in Fig. 6A (right panel) were transfected into fresh monolayers of EcR-CHO cells. After 24 h, the products of RNA replication were labeled, analyzed, and normalized as described in Fig. 1.
DISCUSSION
In general, the events involved in the replication of viral positive-strand RNA genomes via negative-strand intermediates can be divided into four phases on the basis of their kinetic properties, which have been studied extensively by using bacteriophage Qβ, both in vitro (7–11) and in vivo (23). During the first, or lag, phase of Qβ RNA replication in vivo, the input positive-sense viral RNA is used predominantly as a template for translation; the RNA concentration remains essentially constant, and the replicase concentration increases until sufficient enzyme has accumulated for the onset of RNA replication. The lag phase is followed by a hyperbolic phase characterized by rapid increases in the rates of synthesis of both RNA strands (positive and negative polarity) and of the replicase itself. At this time, the rate of replication increases so that it exceeds the translation rate, eventually saturating the machineries of translation and replication. The positive-strand RNAs can bind to ribosomes, to the replicase, or to the viral coat protein, while the negative strands bind exclusively to the replicase (23).
The reaction next enters a linear phase, when RNA replication and protein synthesis slow to constant rates due to saturation of the replicase and the ribosomes, respectively, with free viral RNA. For Qβ, positive-strand synthesis is stimulated at the expense of the negative strand when a factor required for the latter process becomes limiting (23, 56), so that the bulk of the positive-strand progeny are produced during this phase of the reaction. No further increase in the replicase concentration is observed, as viral protein synthesis shifts to the production of the coat protein. Late in replication only a tiny fraction of the RNA product appears to be translated or replicated. In the final phase, the rates of synthesis decline concomitantly with packaging of the progeny positive strands into the viral coat protein (23).
In the experiments presented here, we examined the transition from the early and intermediate phases of FHV RNA replication to the final stage, where the reaction slowed progressively and eventually stopped. This stage of nodavirus RNA1 replication has not been examined previously because most earlier analyses were performed over shorter time periods and it was not possible to temporally separate RNA replication from primary transcription until the development of the inducible cDNA transcription system (3, 34, 48).
Two lines of evidence suggest that the observed inhibition is specific for actively replicating viral RNAs and does not represent a general transcriptive inhibition. The first is provided by the gross pattern of cellular protein synthesis, which did not change qualitatively or quantitatively over the course of the experiment shown in Fig. 3, while viral protein synthesis was shut off. Clearly, this result demonstrates that functional levels of cellular mRNAs were maintained, because cellular protein synthesis would diminish in the absence of continued transcription. The reinduction experiment shown in Fig. 6A provides further evidence of this specificity. In that case, switching primary transcription back on at a time when replication had ceased resulted in restoration of replication, showing that de novo transcription was still active in these cells.
Strikingly, synthesis of the FHV replicase diminished with kinetics that approximated the replicative shutoff, despite the continued presence of the RNA that encodes it (Fig. 1 to 3). This observation supports the hypothesis, posed by the Qβ model, that replication stops when progeny RNAs can no longer feed back into the replicative cycle as templates for translation or replication. However, we observed a late decline in the rate of RNA replication with an RNA that does not encode virion structural proteins, suggesting that for FHV the block to feedback of progeny RNAs can be separated from packaging into virions.
What other reasons could account for the diminishing feedback at this point in the replicative cycle? The results presented above allowed us to eliminate several plausible explanations. For example, the accumulated RNA1 maintained its intrinsic message and template activities, as demonstrated by its ability to replicate anew when transferred to naive cells (Fig. 1 and 8). This refuted the idea that the replicative shutoff was due to the accumulation of deleterious mutations (error catastrophe, as defined in reference 44) which might result from error-prone RNA replication. These results exonerated both the quantity and the quality of the RNA1 from being responsible for mediating shutoff, and they focused our attention instead on the ability of the intracellular environment to support replication.
In cells where it had previously been shut off, replication resumed only when cDNA transcription was reinduced (Fig. 6 and 7), while any changes in cellular metabolism due to cell passaging failed to restore replication. These results are not consistent with the presence of an inhibitor of replication or depletion of a necessary cellular host factor. Instead, they established that the cytoplasmic environment remained hospitable after replication had ceased and was therefore not responsible for the shutoff.
This conclusion was further supported by the observation that the shutoff could be partially alleviated when FHV RNA1 was replenished continuously by transcription of a nuclear cDNA (Fig. 5). Could this be due to some intrinsic difference between the primary transcripts exported from the nucleus and the progeny of cytoplasmic RNA replication? None of the predicted covalent differences (cap structure, 3′ end) are expected to alter the translation or replication of the RNA. However, the fates of the two RNAs are markedly different. The pathway by which pol II transcripts are transported from the nucleus evolved to export spliced mRNA and deliver it to the cytoplasmic translation machinery (14, 15, 36). Therefore, the nuclear transcripts most likely are exported and translated to yield fresh replicase that can initiate a new replication cycle in spite of the block to translational and/or replicative feedback that is imposed on the products of cytoplasmic replication.
The question still remains how the progeny RNA can be prevented from feeding back into the reaction. One possibility is that at the later stages of replication, the RNA products are sequestered away from ribosomes and/or the replicase, perhaps in membranous compartments. The FHV replicase is known to be membrane associated, and intact membranes are required for RNA replication (33, 52, 60, 61). Perhaps the later products of RNA replication are released into a separate membrane-bound compartment, where they are no longer accessible as templates for either translation or replication. The observation that most of the FHV capsid protein is assembled into provirions within a few minutes of synthesis, yet encapsidation of newly synthesized RNA occurs very slowly (30), indicates that a substantial pool of unencapsidated progeny RNA exists. A membrane-associated progeny RNA pool has been observed in poliovirus-infected cells and may be the site of virion assembly in the cell (12, 13, 47, 58). The recent observation that poliovirus RNA replication and RNA packaging are coupled, perhaps as a result of direct interaction between the replication machinery and the capsid proteins (43), lends credence to the proposal. This model does not exclude the possibility that RNA replication could result in local depletion of a factor still abundant in the cytoplasm as a whole, resulting in replicative shutoff at the initial site. In that case, primary transcription products establishing new sites of replication might have access to a fresh supply of the limiting factor.
Alternatively, it is possible that the shutoff is the consequence of a replication-specific host response mechanism, such as the interferon response, apoptosis, or homology-dependent gene silencing. However, during shutoff, no evidence for a global inhibition of protein or RNA synthesis, such as occurs in apoptosis or sometimes occurs during an interferon response (21, 22, 28, 31, 37, 62), was detected (Fig. 3 and 6). In addition, the morphologies of cells transfected with RNA1 or FHV1 plasmids when observed by light or electron microscopy were inconsistent with the cellular alterations observed during apoptosis (data not shown). Recent reports have suggested that some animal cells may have mechanisms of gene silencing (24, 45, 50) that resemble the phenomena discovered in plants and fungi (reviewed in reference 59), although no analogous phenomenon has yet been reported for mammalian cells. Nonetheless, the intriguing observation that interference with the expression of selected genes in Caenorhabditis elegans is initiated by sequence-specific double-stranded RNA (24) raises the possibility that the shutoff of FHV RNA replication reported here might be the result of a similar silencing mechanism in which double-stranded RNA is involved. The latter possibility remains to be tested.
In summary, the work presented here indicates that FHV RNA1 replication is inhibited despite the continued presence of replication-competent RNA in a cytoplasmic environment that retains the ability to support further RNA replication. Further work will be required to determine the exact mechanism of the replicative shutoff.
ACKNOWLEDGMENTS
We thank the members of the L. A. Ball and G. W. Wertz laboratories for many helpful discussions during the course of this work and for critical examination of the manuscript.
This work was supported by Public Health Service grant R01 AI18270.
REFERENCES
- 1.Ball L A. Cellular expression of a functional nodavirus RNA replicon from vaccinia virus vectors. J Virol. 1992;66:2335–2345. doi: 10.1128/jvi.66.4.2335-2345.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball L A. Replication of the genomic RNA of a positive-strand RNA animal virus from negative-sense transcripts. Proc Natl Acad Sci USA. 1994;91:12443–12447. doi: 10.1073/pnas.91.26.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball L A. Requirements for the self-directed replication of flock house virus RNA 1. J Virol. 1995;69:720–727. doi: 10.1128/jvi.69.2.720-727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball L A, Amann J M, Garrett B K. Replication of nodamura virus after transfection of viral RNA into mammalian cells in culture. J Virol. 1992;66:2326–2334. doi: 10.1128/jvi.66.4.2326-2334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball L A, Li Y. cis-Acting requirements for the replication of flock house virus RNA2. J Virol. 1993;67:3544–3551. doi: 10.1128/jvi.67.6.3544-3551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball L A, Wohlrab B, Li Y. Nodavirus RNA replication: mechanism and harnessing to vaccinia virus recombinants. Arch Virol. 1994;9(Suppl.):407–416. doi: 10.1007/978-3-7091-9326-6_40. [DOI] [PubMed] [Google Scholar]
- 7.Biebricher C K, Eigen M. Kinetics of RNA replication by Qβ replicase. In: Domingo E, Holland J J, Ahlquist P, editors. RNA genetics. I. RNA-directed virus replication. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 1–21. [Google Scholar]
- 8.Biebricher C K, Eigen M, Gardiner W C., Jr Kinetics of RNA replication. Biochemistry. 1983;22:2544–2559. doi: 10.1021/bi00279a036. [DOI] [PubMed] [Google Scholar]
- 9.Biebricher C K, Eigen M, Gardiner W C., Jr Kinetics of RNA replication: competition and selection among self-replicating RNA species. Biochemistry. 1985;24:6550–6560. doi: 10.1021/bi00344a037. [DOI] [PubMed] [Google Scholar]
- 10.Biebricher C K, Eigen M, Gardiner W C., Jr Kinetics of RNA replication: plus-minus asymmetry and double-strand formation. Biochemistry. 1984;23:3186–3194. doi: 10.1021/bi00309a012. [DOI] [PubMed] [Google Scholar]
- 11.Biebricher C K, Eigen M, Luce R. Kinetic analysis of template-instructed and de novo RNA synthesis by Qβ replicase. J Mol Biol. 1981;148:391–410. doi: 10.1016/0022-2836(81)90183-2. [DOI] [PubMed] [Google Scholar]
- 12.Bienz K, Egger D, Pfister T. Characteristics of the poliovirus replication complex. Arch Virol. 1994;9(Suppl.):147–157. doi: 10.1007/978-3-7091-9326-6_15. [DOI] [PubMed] [Google Scholar]
- 13.Bienz K, Egger D, Pfister T, Troxler M. Structural and functional characterization of the poliovirus replication complex. J Virol. 1992;66:2740–2747. doi: 10.1128/jvi.66.5.2740-2747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang D D, Sharp P A. Regulation by HIV Rev depends upon recognition of splice sites. Cell. 1989;59:789–795. doi: 10.1016/0092-8674(89)90602-8. [DOI] [PubMed] [Google Scholar]
- 15.Chapon C, Legrain P. A novel gene, spp91-1, suppresses the splicing defect and the pre-mRNA nuclear export in the prp9-1 mutant. EMBO J. 1992;11:3279–3288. doi: 10.1002/j.1460-2075.1992.tb05406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Dasgupta, R. 17 January 1994, submission date. Sequence of flock house virus genomic RNA for protein A, protein B1, and protein B2, accession no. X77156. [Online.] http://www.ncbi.nlm.nih.gov. [23 July 1999, last date accessed.]
- 18.Dasmahapatra B, Dasgupta R, Ghosh A, Kaesberg P. Structure of the black beetle virus genome and its functional implications. J Mol Biol. 1985;182:183–189. doi: 10.1016/0022-2836(85)90337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasmahapatra B, Dasgupta R, Saunders K, Selling B, Gallagher T, Kaesberg P. Infectious RNA derived from transcription from cloned cDNA copies of the genomic RNA of an insect virus. Proc Natl Acad Sci USA. 1986;83:63–66. doi: 10.1073/pnas.83.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davison A J, Moss B. Structure of vaccinia virus early promoters. J Mol Biol. 1989;210:749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- 21.Deckwerth T L, Johnson E M., Jr Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol. 1993;123:1207–1222. doi: 10.1083/jcb.123.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Der S D, Yang Y-L, Weissmann C, Williams B R G. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eigen M, Biebricher C K, Gebinoga M, Gardiner W C. The hypercycle. Coupling of RNA and protein biosynthesis in the infection cycle of an RNA bacteriophage. Biochemistry. 1991;30:11005–11018. doi: 10.1021/bi00110a001. [DOI] [PubMed] [Google Scholar]
- 24.Fire A, Xu S-Q, Montgomery M K, Kostas S A, Driver S E, Mello C C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 25.Friesen P D, Rueckert R R. Black beetle virus: messenger RNA for protein B is a subgenomic viral RNA. J Virol. 1982;42:986–995. doi: 10.1128/jvi.42.3.986-995.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friesen P D, Rueckert R R. Early and late functions in a bipartite RNA virus: evidence for translational control by competition between viral mRNAs. J Virol. 1984;49:116–124. doi: 10.1128/jvi.49.1.116-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gale M, Jr, Katze M G. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher T M, Friesen P D, Rueckert R R. Autonomous replication and expression of RNA1 from black beetle virus. J Virol. 1983;46:481–489. doi: 10.1128/jvi.46.2.481-489.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher T M, Rueckert R R. Assembly-dependent maturation cleavage in provirions of a small icosahedral insect ribovirus. J Virol. 1988;62:3399–3406. doi: 10.1128/jvi.62.9.3399-3406.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonalons E, Barrachina M, Garcia-Sanz J A, Celada A. Translational control of MHC class II I-A molecules by IFN-γ. J Immunol. 1998;161:1837–1843. [PubMed] [Google Scholar]
- 32.Guarino L A, Ghosh A, Dasmahapatra B, Dasgupta R, Kaesberg P. Sequence of the black beetle virus subgenomic RNA and its location in the viral genome. Virology. 1984;139:199–203. doi: 10.1016/0042-6822(84)90342-8. [DOI] [PubMed] [Google Scholar]
- 33.Guarino L A, Kaesberg P. Isolation and characterization of an RNA-dependent RNA polymerase from black beetle virus-infected Drosophila melanogaster cells. J Virol. 1981;40:379–386. doi: 10.1128/jvi.40.2.379-386.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson K L, Ball L A. Replication of flock house virus RNAs from primary transcripts made in cells by RNA polymerase II. J Virol. 1997;71:3323–3327. doi: 10.1128/jvi.71.4.3323-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishna N K, Schneemann A. Formation of an RNA heterodimer upon heating of nodavirus particles. J Virol. 1999;73:1699–1703. doi: 10.1128/jvi.73.2.1699-1703.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Legrain P, Rosbach M. Some cis- and trans-acting mutants for splicing target pre-mRNA to the cytoplasm. Cell. 1989;57:573–583. doi: 10.1016/0092-8674(89)90127-x. [DOI] [PubMed] [Google Scholar]
- 37.Levy-Strumpf N, Deiss L P, Berissi H, Kimchi A. DAP-5, a novel homolog of eukaryotic translation initiation factor 4G isolated as a putative modulator of gamma interferon-induced programmed cell death. Mol Cell Biol. 1997;17:1615–1625. doi: 10.1128/mcb.17.3.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Ball L A. Nonhomologous RNA recombination during negative-strand synthesis of flock house virus RNA. J Virol. 1993;67:3854–3860. doi: 10.1128/jvi.67.7.3854-3860.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Longworth J F, Carey G P. A small RNA virus with a divided genome from Heteronychus arator (F.) [Coleoptera: Scarabaeidae] J Gen Virol. 1976;33:31–40. doi: 10.1099/0022-1317-33-1-31. [DOI] [PubMed] [Google Scholar]
- 40.Newman J F E, Brown F. Evidence for a divided genome in Nodamura virus, an arthropod-borne picornavirus. J Gen Virol. 1973;21:371–384. [Google Scholar]
- 41.Newman J F E, Brown F. Further physicochemical characterization of Nodamura virus. Evidence that the divided genome occurs in a single component. J Gen Virol. 1977;38:83–95. doi: 10.1099/0022-1317-38-1-83. [DOI] [PubMed] [Google Scholar]
- 42.No D, Yao T-P, Evans R M. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc Natl Acad Sci USA. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nugent C I, Johnson K L, Sarnow P, Kirkegaard K. Functional coupling between replication and packaging of poliovirus RNA. J Virol. 1999;73:427–435. doi: 10.1128/jvi.73.1.427-435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orgel L E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc Natl Acad Sci USA. 1963;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pal-Bahdra M, Bahdra U, Birchler J A. Cosuppression in Drosophila: gene silencing of alcohol dehydrogenase by white-Adh transgenes is Polycomb dependent. Cell. 1997;90:479–490. doi: 10.1016/s0092-8674(00)80508-5. [DOI] [PubMed] [Google Scholar]
- 46.Perrotta A T, Been M D. A pseudoknot-like structure required for efficient self-cleavage of hepatitis delta virus RNA. Nature. 1991;350:434–436. doi: 10.1038/350434a0. [DOI] [PubMed] [Google Scholar]
- 47.Pfister T, Pasamontes L, Troxler M, Egger D, Bienz K. Immunocytochemical localization of capsid-related particles in subcellular fractions of poliovirus-infected cells. Virology. 1992;188:676–684. doi: 10.1016/0042-6822(92)90522-q. [DOI] [PubMed] [Google Scholar]
- 48.Price B D, Rueckert R R, Ahlquist P. Complete replication of an animal virus and maintenance of expression vectors derived from it in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:9465–9470. doi: 10.1073/pnas.93.18.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg A H, Lade B N, Chui D, Lin S-W, Dunn J J, Studier F W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56:125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- 50.Ruiz F, Vayssie L, Klotz C, Sperling L, Madeddu L. Homology-dependent gene silencing in Paramecium. Mol Biol Cell. 1998;9:931–943. doi: 10.1091/mbc.9.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 52.Saunders K, Kaesberg P. Template-directed RNA polymerase from black beetle virus-infected Drosophila melanogaster cells. Virology. 1985;147:373–381. doi: 10.1016/0042-6822(85)90139-4. [DOI] [PubMed] [Google Scholar]
- 53.Scotti P D, Dearing S, Mossop D W. Flock house virus: a nodavirus isolated from Costelytra zealandica (White) (Coleoptera: Scarabaeidae) Arch Virol. 1983;75:181–189. doi: 10.1007/BF01315272. [DOI] [PubMed] [Google Scholar]
- 54.Selling B H, Allison R F, Kaesberg P. Genomic RNA of an insect virus directs synthesis of infectious virions in plants. Proc Natl Acad Sci USA. 1990;87:434–438. doi: 10.1073/pnas.87.1.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selling B H, Rueckert R R. Plaque assay for black beetle virus. J Virol. 1984;51:251–253. doi: 10.1128/jvi.51.1.251-253.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sumper M, Luce R. Evidence for de novo production of self-replicating and environmentally adapted RNA structures by bacteriophage Qβ replicase. Proc Natl Acad Sci USA. 1975;72:162–166. doi: 10.1073/pnas.72.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torczynski R M, Bollon A P, Fuke M. The complete nucleotide sequence of the rat 18S ribosomal RNA gene and comparison with the respective yeast and frog genes. Nucleic Acids Res. 1983;11:4879–4890. doi: 10.1093/nar/11.14.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Troxler M, Egger D, Pfister T, Bienz K. Intracellular localization of poliovirus RNA by in situ hybridization at the ultrastructural level using single-stranded riboprobes. Virology. 1992;191:687–697. doi: 10.1016/0042-6822(92)90244-j. [DOI] [PubMed] [Google Scholar]
- 59.Voinnet O, Vain P, Angell S, Baulcombe D C. Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell. 1998;95:177–187. doi: 10.1016/s0092-8674(00)81749-3. [DOI] [PubMed] [Google Scholar]
- 60.Wu S-H, Kaesberg P. Synthesis of template-sense, single-stranded flockhouse virus RNA in a cell-free replication system. Virology. 1991;183:392–396. doi: 10.1016/0042-6822(91)90153-3. [DOI] [PubMed] [Google Scholar]
- 61.Wu S-X, Ahlquist P, Kaesberg P. Active complete in vitro replication of nodavirus RNA requires glycerophospholipid. Proc Natl Acad Sci USA. 1992;89:11136–11140. doi: 10.1073/pnas.89.23.11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zakeri Z, Quaglino D, Latham T, Woo K, Lockshin R A. Programmed cell death in the tobacco hornworm, Manduca sexta: alteration in protein synthesis. Microscopy Res Tech. 1996;34:192–201. doi: 10.1002/(SICI)1097-0029(19960615)34:3<192::AID-JEMT2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 63.Zhong W, Rueckert R R. Flock house virus: down-regulation of subgenomic RNA3 synthesis does not involve coat protein and is targeted to synthesis of its positive strand. J Virol. 1993;67:2716–2722. doi: 10.1128/jvi.67.5.2716-2722.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]