Abstract
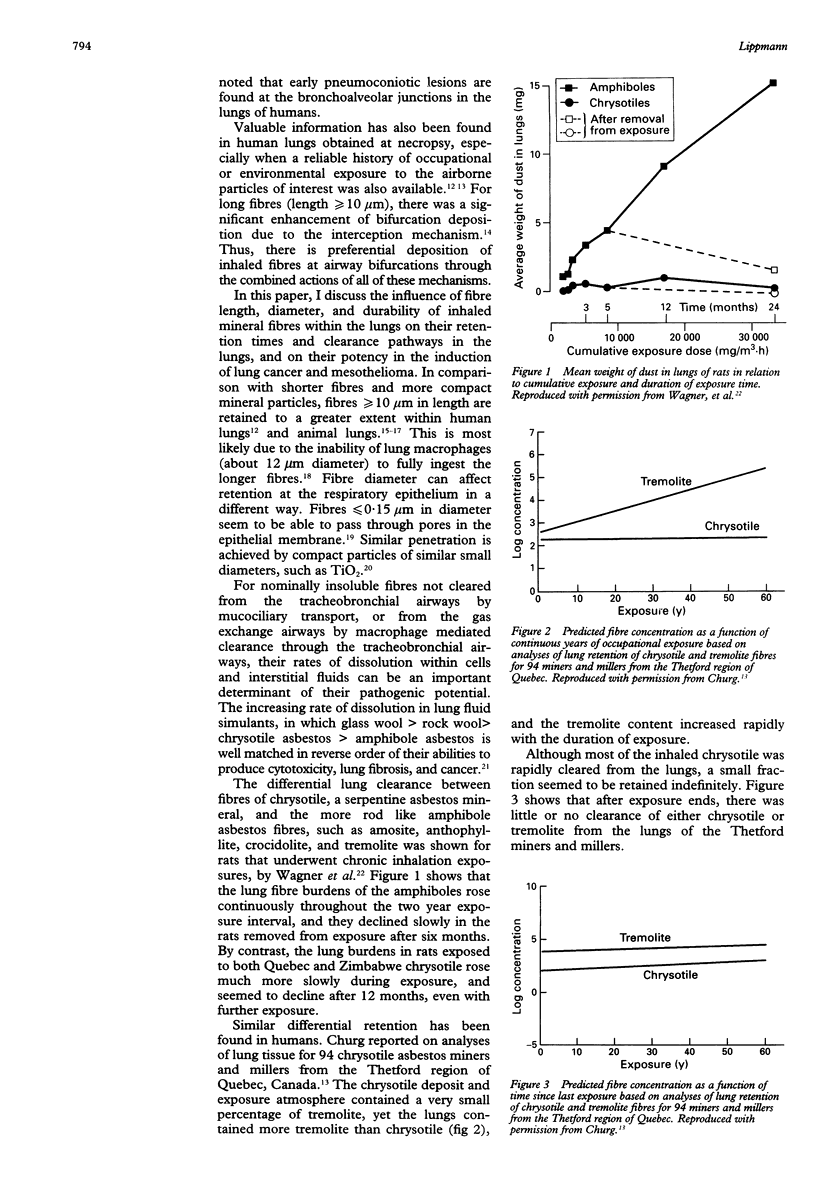
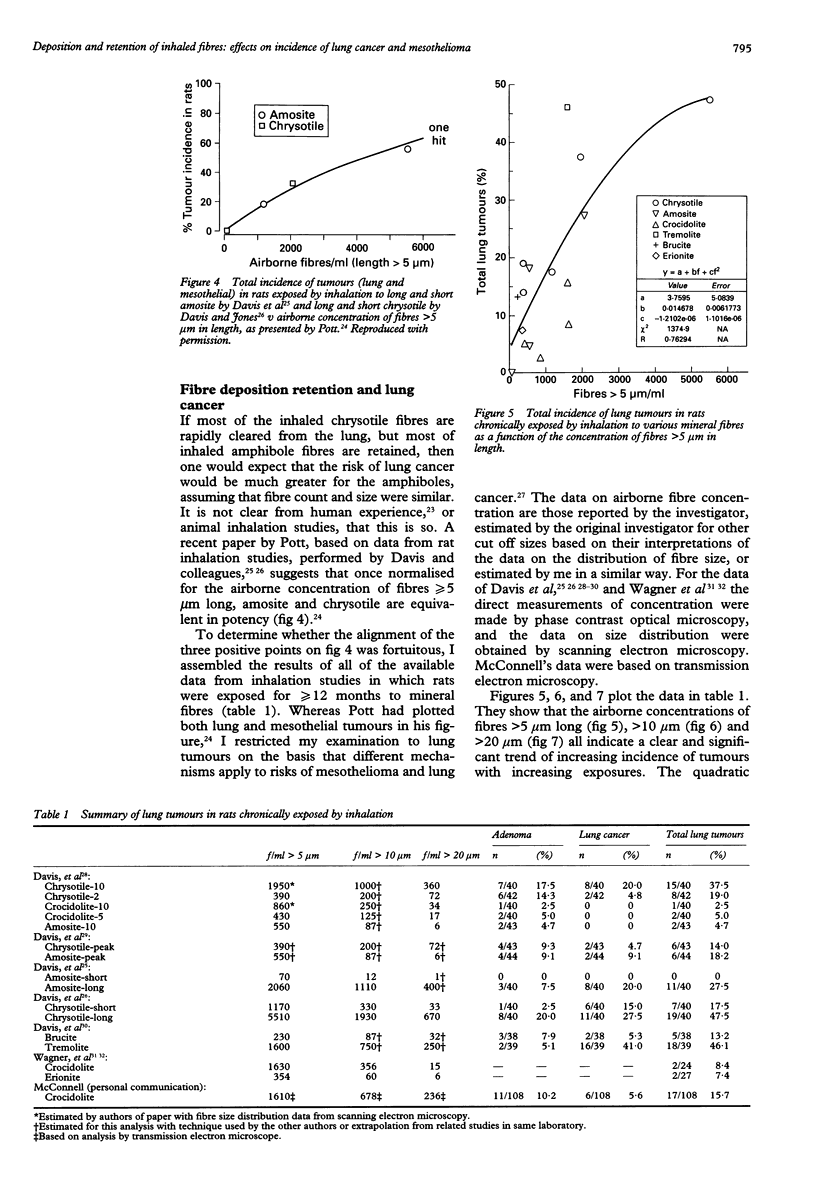
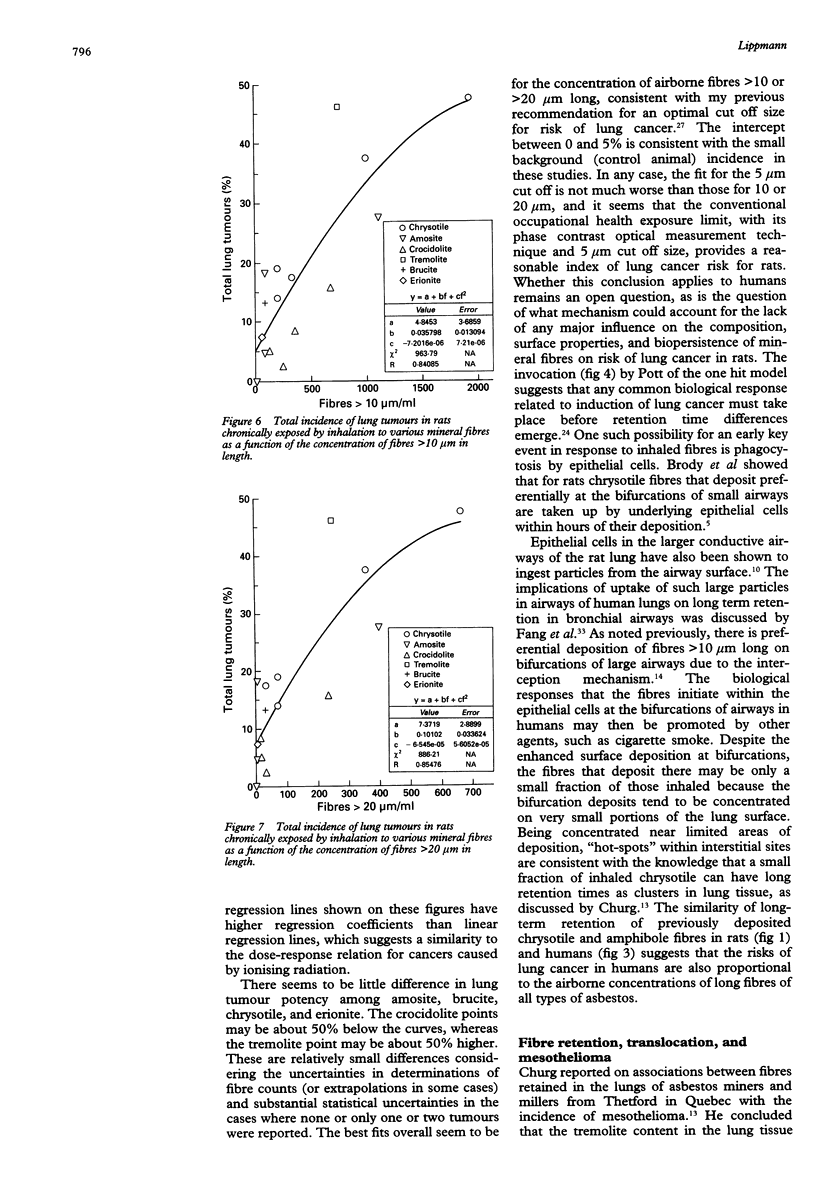
A review of the literature on chronic inhalation studies in which rats were exposed to mineral fibres at known fibre number concentrations was undertaken to examine the specific roles of fibre length and composition on the incidences of both lung cancer and mesothelioma. For lung cancer, the percentage of lung tumours (y) could be described by a relation of the form y = a + bf + cf2, where f is the concentration of fibre numbers and a, b, and c are fitted constants. The correlation coefficients for the fitted curves were 0.76 for > 5 microns f/ml, 0.84 for > 10 microns f/ml, and 0.85 for > 20 microns f/ml. These seemed to be independent of fibre type. It has been shown that brief inhalation exposures to chrysotile fibre produces highly concentrated fibre deposits on bifurcations of alveolar ducts, and that many of these fibres are phagocytosed by the underlying type II epithelial cells within a few hours. Churg has shown that both chrysotile and amphibole fibres retained in the lungs of former miners and millers do not clear much with the years since last exposure. Thus, lung tumours may be caused by that small fraction of the inhaled fibres that are retained in the interstitium below small airway bifurcations where clearance processes are ineffective. By contrast, for mesothelioma, the (low) tumour yields seemed to be highly dependent upon fibre type. Combining the data from various studies by fibre type, the percentage of mesotheliomas was 0.6% for Zimbabwe (Rhodesian) chrysotile, 2.5% for the various amphiboles as a group, and 4.7% for Quebec (Canadian) chrysotile. This difference, together with the fact that Zimbabwe chrysotile has 2 to 3 orders of magnitude less than tremolite than Quebec chrysotile, provides support for the hypothesis that the mesotheliomas that have occurred among chrysotile miners and millers could be largely due to their exposures to tremolite fibres. The chrysotile fibres may be insufficiently biopersistent because if dissolution during translocation from their sites of deposition to sites where more durable fibres can influence the transformation or progression to mesothelioma.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briant J. K., Lippmann M. Particle transport through a hollow canine airway cast by high-frequency oscillatory ventilation. Exp Lung Res. 1992 May-Jun;18(3):385–407. doi: 10.3109/01902149209031692. [DOI] [PubMed] [Google Scholar]
- Brody A. R., Hill L. H., Adkins B., Jr, O'Connor R. W. Chrysotile asbestos inhalation in rats: deposition pattern and reaction of alveolar epithelium and pulmonary macrophages. Am Rev Respir Dis. 1981 Jun;123(6):670–679. doi: 10.1164/arrd.1981.123.6.670. [DOI] [PubMed] [Google Scholar]
- Brody A. R., Roe M. W. Deposition pattern of inorganic particles at the alveolar level in the lungs of rats and mice. Am Rev Respir Dis. 1983 Oct;128(4):724–729. doi: 10.1164/arrd.1983.128.4.724. [DOI] [PubMed] [Google Scholar]
- Churg A. Deposition and clearance of chrysotile asbestos. Ann Occup Hyg. 1994 Aug;38(4):625-33, 424-5. doi: 10.1093/annhyg/38.4.625. [DOI] [PubMed] [Google Scholar]
- Churg A., Tron V., Wright J. L. Effects of cigarette smoke exposure on retention of asbestos fibers in various morphologic compartments of the guinea pig lung. Am J Pathol. 1987 Nov;129(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Davis J. M., Addison J., Bolton R. E., Donaldson K., Jones A. D., Miller B. G. Inhalation studies on the effects of tremolite and brucite dust in rats. Carcinogenesis. 1985 May;6(5):667–674. doi: 10.1093/carcin/6.5.667. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Addison J., Bolton R. E., Donaldson K., Jones A. D., Smith T. The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br J Exp Pathol. 1986 Jun;67(3):415–430. [PMC free article] [PubMed] [Google Scholar]
- Davis J. M., Beckett S. T., Bolton R. E., Collings P., Middleton A. P. Mass and number of fibres in the pathogenesis of asbestos-related lung disease in rats. Br J Cancer. 1978 May;37(5):673–688. doi: 10.1038/bjc.1978.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. M., Beckett S. T., Bolton R. E., Donaldson K. The effects of intermittent high asbestos exposure (peak dose levels) on the lungs of rats. Br J Exp Pathol. 1980 Jun;61(3):272–280. [PMC free article] [PubMed] [Google Scholar]
- Davis J. M., Jones A. D. Comparisons of the pathogenicity of long and short fibres of chrysotile asbestos in rats. Br J Exp Pathol. 1988 Oct;69(5):717–737. [PMC free article] [PubMed] [Google Scholar]
- Fang C. P., Wilson J. E., Spektor D. M., Lippmann M. Effect of lung airway branching pattern and gas composition on particle deposition in bronchial airways: III. Experimental studies with radioactively tagged aerosol in human and canine lungs. Exp Lung Res. 1993 May-Jun;19(3):377–396. doi: 10.3109/01902149309064353. [DOI] [PubMed] [Google Scholar]
- Gore D. J., Patrick G. A quantitative study of the penetration of insoluble particles into the tissue of the conducting airways. Ann Occup Hyg. 1982;26(1-4):149–161. [PubMed] [Google Scholar]
- Hesterberg T. W., Miiller W. C., McConnell E. E., Chevalier J., Hadley J. G., Bernstein D. M., Thevenaz P., Anderson R. Chronic inhalation toxicity of size-separated glass fibers in Fischer 344 rats. Fundam Appl Toxicol. 1993 May;20(4):464–476. doi: 10.1006/faat.1993.1057. [DOI] [PubMed] [Google Scholar]
- Lippmann M. Asbestos exposure indices. Environ Res. 1988 Jun;46(1):86–106. doi: 10.1016/s0013-9351(88)80061-6. [DOI] [PubMed] [Google Scholar]
- Lippmann M., Schlesinger R. B. Interspecies comparisons of particle deposition and mucociliary clearance in tracheobronchial airways. J Toxicol Environ Health. 1984;13(2-3):441–469. doi: 10.1080/15287398409530509. [DOI] [PubMed] [Google Scholar]
- Morgan A., Holmes A., Davison W. Clearance of sized glass fibres from the rat lung and their solubility in vivo. Ann Occup Hyg. 1982;25(3):317–331. doi: 10.1093/annhyg/25.3.317. [DOI] [PubMed] [Google Scholar]
- Pott F. Asbestos use and carcinogenicity in Germany and a comparison with animal studies. Ann Occup Hyg. 1994 Aug;38(4):589-600, 420. doi: 10.1093/annhyg/38.4.589. [DOI] [PubMed] [Google Scholar]
- Stirling C., Patrick G. The localisation of particles retained in the trachea of the rat. J Pathol. 1980 Aug;131(4):309–320. doi: 10.1002/path.1711310403. [DOI] [PubMed] [Google Scholar]
- Wagner J. C., Berry G., Skidmore J. W., Timbrell V. The effects of the inhalation of asbestos in rats. Br J Cancer. 1974 Mar;29(3):252–269. doi: 10.1038/bjc.1974.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. C., Skidmore J. W., Hill R. J., Griffiths D. M. Erionite exposure and mesotheliomas in rats. Br J Cancer. 1985 May;51(5):727–730. doi: 10.1038/bjc.1985.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit D. B., Hartsky M. A. Species comparisons of proximal alveolar deposition patterns of inhaled particulates. Exp Lung Res. 1990 Mar-Apr;16(2):83–99. doi: 10.3109/01902149009087874. [DOI] [PubMed] [Google Scholar]


