Abstract
Human T-cell leukemia virus type 1 (HTLV-1) Tax is thought to play a pivotal role in immortalization of T cells. We have recently shown that the expression of Tax protected the mouse T-cell line CTLL-2 against apoptosis induced by interleukin-2 (IL-2) deprivation and converted its growth from being IL-2 dependent to being IL-2 independent. In this study, we demonstrate that constitutive expression of bcl-xl but not bcl-2, bcl-xs, bak, bad, or bax was associated with apoptosis resistance after IL-2 deprivation in CTLL-2 cells that expressed Tax. Transient-transfection assays showed that bcl-x promoter was transactivated by wild-type Tax. Similar effects were observed in mutant Tax retaining transactivating ability through NF-κB. Deletion or substitution of a putative NF-κB binding site identified in the bcl-x promoter significantly decreased Tax-induced transactivation. This NF-κB-like element was able to form a complex with NF-κB family proteins in vitro. Furthermore, Tax-induced transactivation of the bcl-x promoter was also diminished by the mutant IκBα, which specifically inhibits NF-κB activity. Our findings suggest that constitutive expression of Bcl-xL induced by Tax through the NF-κB pathway contributes to the inhibition of apoptosis in CTLL-2 cells after IL-2 deprivation.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia (22, 34). HTLV-1 encodes a 40-kDa regulatory protein, Tax, that accelerates viral gene expression (6, 13, 15, 39, 41). This protein is thought to be responsible for the transforming features of HTLV-1. This conclusion is supported by several lines of evidence, such as in vitro transformation of rodent fibroblasts and human T cells (1, 27, 46) and in vivo induction of a variety of mesenchymal tumors and leukemia in transgenic mice carrying the tax gene (20, 29).
Various mechanisms of T-cell leukemogenesis by HTLV-1 Tax have been proposed. Some of them relate to the transcriptional activator function of this protein. Tax activates the transcription of not only HTLV-1 itself but also a number of cellular genes in trans. This transactivation by Tax is mediated through interaction with transcriptional factors CREB, NF-κB, and serum response factor (14, 42–44, 56). Thus, the expression of a variety of target genes related to cellular activation and growth is enhanced by Tax. These include genes for interleukin-2 (IL-2), IL-6, granulocyte-macrophage colony-stimulating factor, the α chain of IL-2 receptor, Fra-1, c-Myc, c-Fos, and c-Jun (36, 55). Aberrant expression of these proteins could be involved in the dysregulated proliferation of HTLV-1-infected cells.
Recent studies support another hypothesis; that Tax may induce cell cycle promotion through protein-protein interaction. Suzuki et al. (45) demonstrated that Tax binds and inactivates p16INK4a, a negative regulatory molecule of the cell cycle. Tax may also directly associate with cyclin D, which is important in cell cycle transition from G1 to S phase (30).
Tax also influences cell apoptosis. We and others have previously shown that human T-cell lines expressing Tax were resistant to apoptosis signals triggered through Fas receptors (2, 9). A similar resistance to apoptosis was observed in T cells derived from transgenic mice carrying the tax gene (25). Resistance to cell apoptosis is one of the mechanisms of autoimmunity and is also an important requirement for the immortalization of T cells (7, 33). Other investigators, however, have reported opposite observations; Tax induces apoptosis of rat fibroblasts and a human T-cell line under certain conditions (7, 53). The precise mechanisms of the Tax effect on apoptosis are not well understood.
We have recently shown that the expression of Tax in a mouse T-cell line CTLL-2 converted its growth from being IL-2 dependent to being IL-2 independent. Not only promotion of cell cycle but also prevention of apoptosis occurred in these cells in the absence of IL-2 (23). In the present study, we investigated the mechanisms by which Tax prevents apoptosis. We demonstrated (i) that constitutive expression of Bcl-xL, one of the molecules protecting cells against apoptosis, is associated with resistance of CTLL-2/Tax transfectants to apoptosis induced by IL-2 deprivation and (ii) that Tax transactivates bcl-xl through the NF-κB pathway. This is the first report that indicates the involvement of Bcl-xL in HTLV-1 Tax-induced inhibition of apoptosis in T cells.
MATERIALS AND METHODS
Plasmids.
Plasmids containing wild-type tax (pβMT-2Tax), mutant tax genes (pβTax703 and pβTaxM22) driven by a β-actin promoter, and its control plasmid (pHβAPr-1-neo) were used (21, 26). Wild-type Tax encoded in pβMT-2Tax is effective in the activating NF-κB, CArG, and CRE sites. On the other hand, the Tax mutant encoded by pβTax703 can activate the NF-κB site but not the CArG or the CRE site, while the Tax mutant encoded by pβTaxM22 can activate the CArG and CRE site but hardly the NF-κB site (40, 44). We also used the mouse bcl-x promoter-driven luciferase (Luc) reporter plasmid pGL2-3.2, which contained a 3.2-kbp fragment upstream of the bcl-x gene (19). pGL2(1160), pGL2(848), pGL2(822), and pGL2(631) were newly generated by insertion of DNA fragments of the bcl-x promoter at the base positions of −1160 to −75, −848 to −75, −822 to −75, and −631 to −75 from the translation initiation ATG of the bcl-x gene, respectively, into the basic pGL2 vector containing a Luc reporter gene (Promega, Madison, Wis.). These DNA fragments were amplified by PCR with DNA templates extracted from CTLL-2 cells and four sense primers, (5′-CCGCTCGAGGAGGTCTCCACTGTGGGAGC-3′, 5′-CCGCTCGAGGGAAGTCCCTTTAGGGTTTC-3′, 5′-CTCATCTAGGGCTGGTACTT-3′, and 5′-CCGCTCGAGTGGTCGATGGAGGAACCAGG-3′) and one antisense primer (5′-GAAGATCTAGCGATTCTCTTCCAGGATC-3′). pGLκBM is a mutant of pGL2(848), carrying CC-to-GG mutations at positions of −841 and −840 within the NF-κB binding site, which were generated by PCR by using primers with the mutation. Plasmid integrity was confirmed by DNA sequencing. Another Luc reporter plasmid, WT-Luc, containing five tandem repeats of 21-bp Tax-inducible CREs of HTLV-1 LTR originated from 21WT-CAT (16), was kindly provided by J. Fujisawa (Kansai Medical University, Osaka, Japan). pcDNA3-IκBαM was constructed by cloning an IκBαM-coding region in pBluescript SK(−)-IκBα S32A/S36A, a kind gift from J. Inoue (Tokyo University, Tokyo, Japan), into pcDNA3 (Invitrogen, Carlsbad, Calif.) with the cytomegalovirus promoter. pSV–β-Galactosidase Vector (Promega) was also used.
Cell lines and transfections.
The mouse IL-2-dependent T-cell line CTLL-2 (18) was maintained in RPMI 1640 medium with 10% fetal calf serum (FCS) (RPMI–10% FCS), 50 μM 2-mercaptoethanol and 1 nM human recombinant IL-2 (hrIL-2; Ajinomoto, Yokohama, Japan) at 37°C and 5% CO2 CTLL-2 cell clones permanently expressing wild-type and mutant HTLV-I Tax were established by neomycin-selection after gene transfection as described below. CTLL-2 cells (107) were resuspended in 0.7 ml of FCS-free RPMI containing 10 μg of wild-type or mutant Tax expression plasmids and electroporated by use of a Gene Pulser (Bio-Rad, Hercules, Calif.) at 320 V and 950 μF. Then, the cells were resuspended in 20 ml of RPMI–10% FCS and 1 nM hrIL-2. After 48 h, the cells were selected in 24-well plates in G418-containing medium (0.4 mg/ml) for 3 weeks. Wells were screened for Tax expression by Western blot analysis, and cells in the wells positive for Tax were further cloned by the limiting-dilution method. In this study, we used two or three randomly selected clones for each group of CTLL-2/Tax transfectants. We also used an IL-2-independent T-cell line, Jurkat, which originated from human acute lymphocytic leukemia (38).
Western blot analysis.
Expressions of HTLV-1 Tax, Bcl-xL, and α-tubulin in CTLL-2 cell clones were analyzed by Western blot assays as described previously (23). Briefly, cell lysates prepared from CTLL-2 (5 × 106 cells) were subjected to electrophoresis under reduced conditions on a discontinuous 12.5% polyacrylamide gel with sodium dodecyl sulfate, and the proteins in the gel were transferred to polyvinylidene difluoride membranes. The blots were first incubated with a human serum containing anti-Tax antibodies, rabbit antiserum specific for Bcl-xL (Transduction Laboratories, Lexington, Ky.), or mouse monoclonal antibody to α-tubulin (Oncogene, Cambridge, Mass.), then rinsed, and finally incubated with second antibodies conjugated with horseradish peroxidase. Sites of antibody binding were visualized by using ECL Western blotting detection system (Amersham International Plc, Little Chalfont, Buckinghamshire, United Kingdom).
RPA.
We analyzed the levels of bcl-xl, bcl-xs, bak, bax, bcl-2, and bad gene mRNAs in CTLL-2 cells cultured with or without IL-2 for 48 h by RNase protection assay (RPA) with the RPA II kit (Ambion, Austin, Tex.) as described previously (24). Cells were lysed in Isogen (Nippon Gene, Toyama, Japan) solution, and total cellular RNA was extracted by separation over CHCl4 and precipitation with isopropanol. The total RNA (20 μg) was hybridized with a mixture of 32P-labeled antisense RNA probes (3 × 104 cpm) overnight at 42°C in a hybridization buffer containing 80% formamide and then treated with RNase A/T1. The resultant protected RNAs were separated on a 5% acrylamide gel with 8 M urea and visualized by autoradiography. As internal controls, the mRNA levels of a ribosomal protein (L32) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were analyzed. RNA probes used for this assay were synthesized by in vitro transcription with templates included in an mAPO-2 multiprobe template set (PharMingen, San Diego, Calif.).
Transient-transfection assay.
To evaluate the ability of Tax to activate transcription of bcl-x, 106 Jurkat cells were cotransfected by the DEAE-dextran method with 4 μg of a reporter plasmid pGL2-3.2 or control WT-Luc, 1 μg of pSV–β-galactosidase containing the bacterial β-galactosidase gene driven by the simian virus 40 (SV40) promoter, and an effector plasmid containing wild-type or mutant Tax at a 1:2 molar ratio with the reporter plasmids. After 48 h of incubation, cells were harvested, and Luc activity in the cellular lysates was measured with a luminometer (Lumat; Berthold & Co.). All Luc assays were performed in triplicates. At the same time, the activity of β-galactosidase in the lysates was measured as described previously (37). Luc activity was normalized with β-galactosidase activity.
EMSA.
Binding activity of NF-κB proteins to NF-κB elements was examined by electrophoretic mobility shift assay (EMSA) as described previously (23). In brief, 10 μg of nuclear extracts prepared from 107 of CTLL-2/Tax transfectants cultured with or without IL-2 for 48 h were preincubated with a binding buffer containing 1 μg of poly(dI-dC). They were then incubated with approximately 20,000 cpm of 32P-labeled oligonucleotide probes containing NF-κB like elements for 20 min at room temperature. The DNA-protein complex was separated on a 5% polyacrylamide gel and visualized by autoradiography. To examine the specificity of the NF-κB-like element of the probes, unlabeled competitor oligonucleotides were preincubated with nuclear extracts for 20 min on ice prior to incubation with probes. The probes or competitors utilized were prepared by annealing the sense and antisense synthetic oligonucleotides as follows: NF-κB-like element in the bcl-x promoter, 5′-cgatAAAGGGACTTCCAAGat-3′ and 3′-taTTTCCCTGAAGGTTCtagc-5′; NF-κB-like mutant element, 5′-cgatAAACCGACTTCCAAGat-3′ and 3′-taTTTGGCTGAAGGTTCtagc-5′; typical NF-κB element of SV40 early promoter, 5′-cgatAGAGGGGACTTTCCGat-3′ and 3′-taTCTCCCCTGAAAGGCtagc-5′; and typical CRE sequence of T-cell receptor α promoter, 5′-cgatCCATGACGTCATGGat-3′ and 3′-taGGTACTGCAGTACCtagc-5′. (Lowercase letters signify nonsense nucleotides inserted to make cohesive ends, which increases the radiolabeling efficiency.) To identify NF-κB proteins in the DNA-protein complex by EMSA, antibodies specific for various NF-κB family proteins, including NF-κB p65, NF-κB p50, c-Rel, RelB, and NF-κB p52 (Santa Cruz Biotechnology, Santa Cruz, Calif.), were used to elicit supershift and/or inhibition of the bands. These antibodies were incubated with the nuclear extracts for 1 h on ice prior to incubation with radiolabeled probes in EMSA.
RESULTS
Constitutive expression of bcl-x mRNA and Bcl-xL protein in CTLL-2 cells stably transfected with HTLV-1 Tax.
We previously established CTLL-2 clones stably transfected with various Tax plasmids such as wild-type Tax (WT), mutant Tax retaining ability to activate NF-κB (703), or mutant Tax deficient in activating the NF-κB site (M22) (23). By using these clones, we demonstrated that CTLL-2 clones transfected with WT Tax (CTLL-2/WT) and mutant Tax 703 (CTLL-2/703) but not the parental CTLL-2 or CTLL-2 clones transfected with Tax M22 (CTLL-2/M22) or control vector (CTLL-2/Vec) were resistant to apoptosis induced by IL-2 deprivation (23).
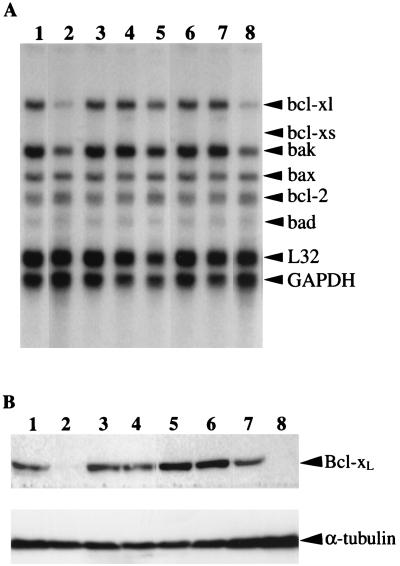
To dissect the molecular mechanisms of Tax-induced inhibition of apoptosis in CTLL-2 cells, we determined the mRNA-levels of several apoptosis-related genes, including bcl-xl, bcl-xs, bak, bax, bcl-2, and bad in CTLL-2/Tax transfectants, by using RNase protection assays. A representative result is shown in Fig. 1A. In a clone of CTLL-2/Vec (CTLL-2/Vec-#22), the mRNA level of bcl-xl, which is known to inhibit apoptosis, was markedly decreased after 48 h of IL-2 deprivation. Expression of bak slightly decreased by IL-2 deprivation, but none of the other members of the bcl family tested was significantly affected by IL-2 deprivation. Bcl-xs mRNA, a spliced variant of bcl-xl (19), was not detected in any of these CTLL-2 cells regardless of the presence of IL-2. In contrast, clones of CTLL-2/WT and CTLL-2/703 (CTLL-2/WT-#34 and CTLL-2/703-#23, respectively) showed a constitutive expression of bcl-xl even in the absence of IL-2. However, the induction of bcl-xl mRNA in a CTLL-2/M22 clone (CTLL-2/M22-#23) was dependent on the presence of IL-2. Similar results were obtained by using another set of independent clones of CTLL-2/Tax transfectants.
FIG. 1.
Constitutive expression of bcl-xl mRNA and Bcl-xL protein in CTLL-2/Tax transfectants in the absence of IL-2. (A) RNase protection assays with specific probes for bcl-xl, bcl-xs, bak, bax, bcl-2, and bad genes were performed by using 20 μg of total RNA samples extracted from CTLL-2/Vec-#22 (lanes 1 and 2), CTLL-2/WT-#34 (lanes 3 and 4), CTLL-2/703-#23 (lanes 5 and 6), and CTLL-2/M22-#23 (lanes 7 and 8) after 48 h of culture in the presence (lanes 1, 3, 5, and 7) or absence (lanes 2, 4, 6, and 8) of 1 nM IL-2. Similar results were obtained with another set of independent clones. (B) Western blot analysis of Bcl-xL (top) and α-tubulin (bottom) expression in CTLL-2/Tax transfectants. In each lane, we used 50 μg of total cell lysates prepared from a similar set of CTLL-2 clones as described in panel A. The arrangement of lanes is similar to that of panel A. Specific protein bands are indicated by arrows. Similar results were obtained with another set of independent clones.
Constitutive expression of the Bcl-xL protein in stable CTLL-2 transfectants with Tax was also confirmed by Western blot analysis. As shown in Fig. 1B, CTLL-2/WT-#34 and CTLL-2/703-#23 but not CTLL-2/Vec or CTLL-2/M22-#23 expressed detectable levels of Bcl-xL protein after 48 h of IL-2 deprivation. The levels of α-tubulin, a cytoskeletal protein as an internal control, was comparable among these clones. The protein levels of Bak in CTLL-2/WT clones did not differ from those of CTLL-2/Vec clones in the absence of IL-2 (data not shown). These results suggested that the constitutive expression of Bcl-xL in CTLL-2/WT and CTLL-2/703 clones in the absence of IL-2 may contribute to Tax-induced resistance to apoptosis. We then explored the mechanisms of Bcl-xL induction by HTLV-1 Tax.
Transactivation of bcl-x promoter by HTLV-1 Tax.
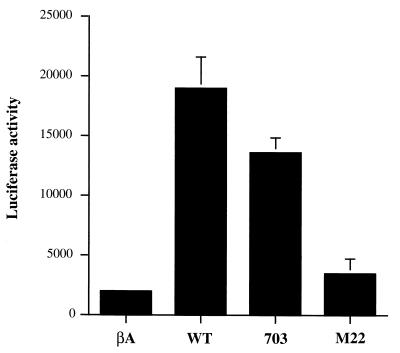
Using transient-transfection assays, we then assessed whether Tax can enhance bcl-x promoter activity. In these assays, HTLV-1-negative IL-2-independent human T-cell line Jurkat but not CTLL-2 cells were used since CTLL-2 cells cannot survive in the absence of IL-2 after transfection with plasmids. Jurkat cells were cotransfected with Tax effector plasmids and a Luc reporter plasmid (pGL2-3.2) containing a 3.2-kbp segment of 5′ flanking region of the mouse bcl-x gene in the absence of IL-2 (19). As shown in Fig. 2, the Luc activities of Jurkat cells transfected with WT Tax or Tax 703 were seven to nine times higher than those of cells transfected with the control vector. However, Luc activity in cells transfected with Tax M22 plasmid was almost equivalent to that in control cells. These results indicated that both WT Tax and Tax 703 transactivates bcl-x promoter activity, presumably through the NF-κB pathway.
FIG. 2.
Transactivation of bcl-x promoter by Tax. Jurkat cells (106) were cotransfected with 2 μg of the Luc reporter plasmid pGL2-3.2 driven by the bcl-x promoter, 1 μg of pSV–β-galactosidase, and 2 μg of Tax-expressing plasmids or its control vector pHβAPr-1-neo (βA). The Tax-expressing plasmids utilized were pβMT-2Tax (WT), pβTax703 (703), and pβTaxM22 (M22). Luciferase activities were normalized with β-galactosidase activity. Data represent the mean ± the standard deviation (SD) of three independent experiments.
Mapping of HTLV-1 Tax-responsive regions in the bcl-x promoter.
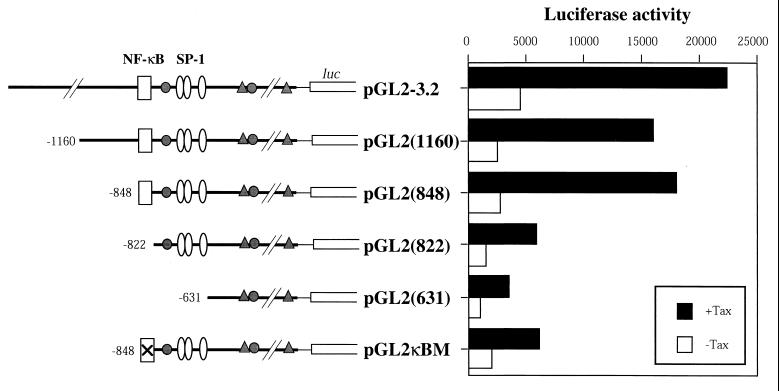
We next mapped Tax-responsive elements in the mouse bcl-x promoter. Several consensus motifs for the binding of transcription factors such as SP-1, Ets-1, and GATA-1 have been identified (19). We also identified an NF-κB binding motif (GGGACTTCC) similar to the one in the immunoglobulin promoter, which was located at positions −848 to −840 of the bcl-x promoter in an inverted direction. To examine the contributions of these sites for Tax-induced transactivation, a series of Luc reporter constructs with several fragments of different lengths of 5′ deleted bcl-x promoters were prepared and transiently transfected into Jurkat cells along with Tax expression plasmid or control plasmid (Fig. 3). The highest level of Tax-induced Luc activity was observed in cells transfected with the longest construct, pGL2-3.2. The two deletion constructs, pGL2(1160) and pGL2(848), also responded to Tax significantly. All three constructs contained both SP-1 and NF-κB binding site-like elements. However, the Tax-induced Luc activity was markedly reduced in cells transfected with pGL2(822), which had the SP-1 but not the NF-κB site. The shortest construct pGL2(631), which lacked both the NF-κB and the SP-1 sites, elicited minimal responses to Tax. Furthermore, a mutated NF-κB-Luc plasmid, pGLκBM, which had the same length as pGL2(848) but had a CC-to-GG mutation at positions −841 and −840 within the NF-κB motif, exhibited markedly reduced levels of Tax-induced transactivation as observed with pGL2(822). These results suggest that the NF-κB site located at positions −848 to −840 in the bcl-x gene promoter region is one of the major responsive elements of Tax-induced transactivation of bcl-x.
FIG. 3.
Mapping of HTLV-1 Tax-responsive region in the bcl-x promoter. A series of Luc reporter plasmids based on pGL2 vectors containing fragments of 5′ flanking region of the bcl-x gene were cotransfected into Jurkat cells with WT Tax plasmid pβMT-2Tax (solid bars) or its control plasmid (open bars), together with reference plasmid pSV–β-galactosidase. Luciferase activities normalized with β-galactosidase activities after 48 h of incubation are indicated. Constructs of the reporter plasmids utilized are schematically illustrated in the left panel. Fragments of the 5′ flanking region of bcl-x gene are represented by heavy lines, and the number at the 5′ end of each construct indicate the 5′-most end base positions from the translation initiation codon of the bcl-x gene. The locations of the binding sites for transcription factors NF-κB (□), SP-1 ( ), Ets-1 ( ) and GATA-1 ( ), identified by using a transcription factor database (1a), are also indicated. pGL2κBM contained mutations within the NF-κB site (⊠) as described in Materials and Methods. Similar results were obtained in two independent experiments.
Binding of NF-κB family proteins to the NF-κB-like element in bcl-x promoter.
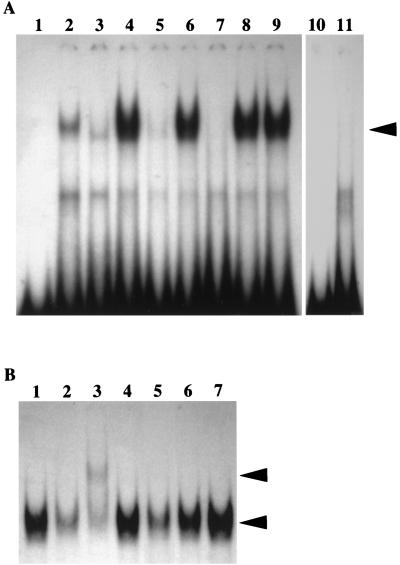
We further examined whether NF-κB family proteins can actually bind to the putative NF-κB element identified in the bcl-x promoter by EMSA. The synthetic oligonucleotide containing the NF-κB-site at positions of −848 to −840 in the bcl-x promoter was used as a probe. As shown in Fig. 4A, a clear shifted band was observed when the probes were incubated with nuclear extracts of CTLL-2/WT-#34 cultured in the presence or the absence of IL-2. However, the binding activity of nuclear extracts of CTLL-2/Vec-#22 was dependent on the presence of IL-2. Similar results were observed with parental CTLL-2 cells (data not shown). Complex formation was competed by 100-fold excess cold probe or unlabeled oligonucleotides with a typical NF-κB consensus sequence of SV40 early promoter but not by oligonucleotides with a mutant NF-κB-like sequence of the bcl-x promoter or other unrelated competitors such as CRE sequence. When the mutant NF-κB-like sequence was used as a probe, complex formation was not observed with nuclear extracts from CTLL-2/WT-#34 cultured in the absence of IL-2. When anti-NF-κB p50 antibody was preincubated with nuclear factors of CTLL-2/WT-#34, the bands formed with the probes supershifted in the EMSA, whereas anti-NF-κB p65 and anti-RelB partially inhibited the complex formation (Fig. 4B). A similar pattern of supershift was also observed when a typical NF-κB consensus sequence was used as a probe (data not shown). These results indicated that p65 and p50 subunits of NF-κB and RelB can bind the NF-κB like element identified in the bcl-x promoter.
FIG. 4.
Binding of NF-κB family proteins to 32P-labeled oligonucleotide probes in EMSA. (A) Nuclear extracts (10 μg) from CTLL-2/Vec-#22 (lanes 2 and 3) and CTLL-2/WT (lanes 4 to 9) cultured in the presence (lanes 2 and 9) or absence (lanes 3 to 8) of IL-2 were incubated with (lanes 5 to 8) or without (lanes 2 to 4 and 9) a 100-fold excess of competitors and then incubated with probes containing the NF-κB-like element in the bcl-x promoter. The DNA-protein complexes were separated on a 5% polyacrylamide gel and visualized by autoradiography. Competitors used included unlabeled probe (lane 5), mutant NF-κB like element (lane 6), a typical NF-κB sequence (lane 7), and a typical CRE sequence (lane 8). Lane 1 represents the band of probes alone. Radiolabeled probes containing a mutant NF-κB-like element were also incubated without (lane 10) or with (lane 11) nuclear extracts from CTLL-2/WT cultured in the absence of IL-2. Specific bands are indicated by arrows. (B) Nuclear extracts (10 μg) from IL-2-untreated CTLL-2/WT were preincubated without (lane 1) or with antibodies specific for NF-κB family proteins, including NF-κB p65 (lane 2), NF-κB p50 (lane 3), c-Rel (lane 4), RelB (lane 5), NF-κB p52 (lane 6), and control rabbit antiserum (lane 7), and then incubated with radiolabeled probe containing the NF-κB-like element in the bcl-x promoter. The lower arrow indicates NF-κB–DNA complex alone, while the upper arrow indicates a supershifted band with an anti-NF-κB p50 antibody. Similar results were obtained with at least three independent CTLL-2/WT clones.
Suppression of Tax-induced enhancement of bcl-x promoter activity by blocking NF-κB pathway.
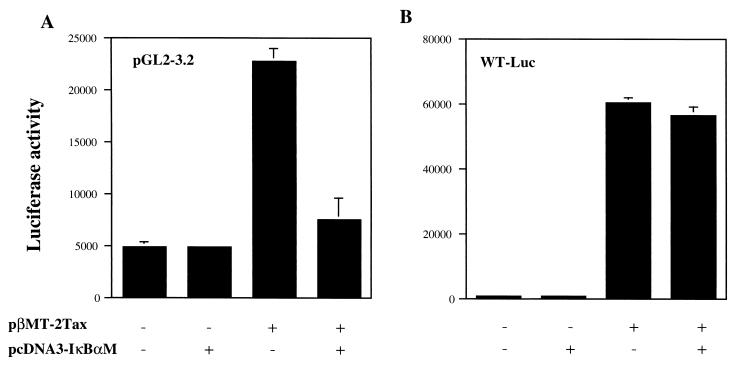
We then examined whether blocking of the NF-κB pathway can affect Tax-induced transactivation of bcl-xl by transient assays by using a super-repressor form of IκBα mutant (IκBαM) that specifically suppresses NF-κB activity. IκBαM contains serine to alanine mutations at residues 32 and 36, which abolish phosphorylation sites by IκB kinase. The IκBαM expression vector was cotransfected into Jurkat cells along with the WT Tax plasmid and the pGL2-3.2. As shown in Fig. 5A, the elevated Luc activity in response to WT Tax was markedly suppressed by cotransfection with IκBαM but not with the control plasmid. In contrast, the Luc activity of another Luc reporter plasmid, WT-Luc, which can be activated by Tax through the CRE pathway but not through the NF-κB pathway (16), was not affected by IκBαM (Fig. 5B). These results confirmed that the NF-κB pathway was involved in Tax-induced transactivation of the bcl-x promoter in these cells.
FIG. 5.
Involvement of NF-κB pathway in Tax-induced transactivation of bcl-x promoter. (A) Jurkat cells were cotransfected with 4 μg of bcl-xl promoter–Luc reporter plasmid pGL2-3.2, 1 μg of pSV–β-galactosidase, and various combinations of 2 μg of pβMT-2Tax and 2 μg of pcDNA3-IκBαM as indicated. The total plasmid amount for transfection was always adjusted up to 9 μg by adding control plasmids pHβAPr-1-neo or pcDNA3. The Luc activity of the samples after 48 h of incubation was normalized to the β-galactosidase activity. Data represent the mean ± the SD of three independent experiments. (B) Control experiments with reporter plasmids without potential NF-κB binding sites. Jurkat cells were transfected with 4 μg of HTLV-LTR (21 bp)-Luc reporter plasmid WT-Luc, 2 μg of pβMT-2Tax, 1 μg of pSV–β-galactosidase, and 2 μg of pcDNA3-IκBαM or pcDNA3. The luciferase activity was indicated as described in panel A.
Involvement of NF-κB pathway in constitutive bcl-xl promoter activity in CTLL-2/Tax transfectants.
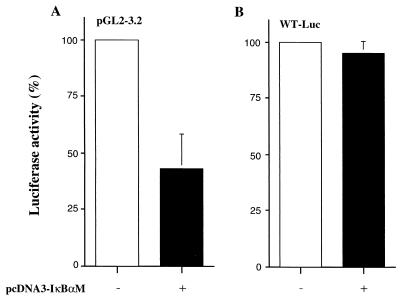
Finally, we examined involvement of the NF-κB pathway in Tax-induced constitutive expression of bcl-xl in CTLL-2 cells. CTLL-2/WT-#34 were transfected with the pGL2-3.2 in the presence or absence of IκBαM in IL-2-free conditions. As shown in Fig. 6A, the bcl-x promoter activity in CTLL-2/WT-#34 was partially inhibited by transfection with IκBαM but not with control plasmid. The Luc activity of WT-Luc lacking potential NF-κB sites was not affected by IκBαM in these cells (Fig. 6B). Similar results were obtained with at least three independent CTLL-2/WT clones. These results confirmed the involvement of the NF-κB pathway in Tax-induced transactivation of bcl-xl in CTLL-2 cells, as well as in Jurkat cells.
FIG. 6.
Inhibition of constitutive activation of bcl-xl promoter in CTLL-2/Tax transfectants by IκBαM. (A) CTLL-2/WT clone cells were transfected with pGL2-3.2, pSV–β-galactosidase, and either pcDNA3-IκBαM (solid bar) or pcDNA3 (open bar). Luc activity after 48 h of incubation in the absence of IL-2 was normalized to β-galactosidase activity and indicated as the percentage of activity in the IκBαM(−) sample. Data represent the mean ± the SD of three independent experiments. (B) Control experiments with the WT-Luc reporter plasmid instead of pGL2-3.2.
DISCUSSION
In this study, we demonstrated that HTLV-1 Tax transactivates the bcl-x promoter through NF-κB. Tax-mediated constitutive expression of Bcl-xL in CTLL-2 cells expressing Tax is presumed to prevent these cells from apoptosis induced by IL-2 deprivation. This can be one of the molecular mechanisms of apoptosis resistance shown in our previous study, which demonstrated that Tax converted IL-2-dependent growth of CTLL-2 to IL-2-independent growth (23).
RNA protection assays showed that bcl-xl, but not other members of bcl family tested, was constitutively expressed in CTLL-2/WT and CTLL-2/703 clones in the absence of IL-2 (Fig. 3A). Constitutive expression of Bcl-xL protein was also observed in these cells (Fig. 3B). Bcl-xL has been shown to inhibit apoptosis of several types of cells including T lymphocytes (4, 32), and overexpression of Bcl-xL protects CTLL-2 cells against apoptosis after IL-2 deprivation (3). Therefore, Tax-induced constitutive expression of Bcl-xL can be the underlying molecular mechanism of resistance of CTLL-2 cells expressing Tax to apoptosis after IL-2 deprivation. Other investigators, however, reported that the otherwise IL-3-dependent B cells could grow independently of IL-3 when they expressed both Tax and c-Myc but not Tax or c-Myc alone (28). bcl-xl induction by Tax was not observed in these cells. Such variation in the ability of Tax to induce bcl-xl among different cell types implies a requirement of cellular mediators in this phenomenon.
Although Bcl-2 is also known to protect cells against apoptosis (10, 11), the expression of bcl-2 did not correlate with Tax-induced resistance to apoptosis of CTLL-2 in the present system. This finding is consistent with that of previous reports demonstrating that Tax did not induce bcl-2 expression in human endothelial cells and a pro-B cell line (28, 31). Expression of bax, an accelerator of apoptosis, was not significantly affected by Tax in the present study. However, other investigators reported that Tax repressed bax transcription (5). In general, the Bcl-2/Bax and Bcl-xL/Bax ratios are thought to be important determinants of apoptosis (35). Our results of Tax-induced activation of bcl-xl, together with the finding of Tax-induced repression of bax by others, suggest that Tax inhibits apoptosis by increasing the Bcl-xL/Bax ratio in HTLV-1-infected cells.
Tax is known to activate NF-κB by activating IκB kinases and other related molecules (8, 17, 47, 54). Involvement of the NF-κB pathway in Tax-induced transactivation of bcl-xl was strongly suggested by our results that the bcl-x promoter was activated by mutant Tax preferentially activating NF-κB (Fig. 3) and that an NF-κB-like element at positions −848 to −840 from the translation initiation codon of bcl-x gene was responsive for Tax-induced activation of the bcl-x promoter (Fig. 4). The binding of NF-κB family proteins, including NF-κB p65, NF-κB p50, and RelB, to the NF-κB-like element that we identified in the mouse bcl-x promoter was also confirmed in in vitro (Fig. 4B). Similar NF-κB binding sequences were also found in the human bcl-x promoter (12). Furthermore, the Tax-mediated bcl-x promoter activation was profoundly inhibited by a super-repressor mutant of IκBα (Fig. 5 and 6) or by introduction of mutations in the NF-κB-like element in the mouse bcl-x promoter constructs. These findings suggest that the NF-κB-like element is one of the major responsive regions of Tax-induced bcl-x promoter activation.
NF-κB activation is known to inhibit apoptosis induced by tumor necrosis factor alpha or immunoglobulin G cross-linking in several types of cells unrelated to HTLV-1 (48, 49, 51). A few reports suggested the involvement of inhibitor of apoptosis protein (50) and IEX-1L (52), although these could be a part of multiple mechanisms and may also vary among different cell types. The molecular mechanisms of NF-κB-induced inhibition of apoptosis, apart from the bcl-xl induction demonstrated in the present study, remain to be clarified.
In conclusion, we demonstrated in the present study that Tax enhanced the expression of Bcl-xL at least through the NF-κB pathway, and that this was associated with cellular resistance to apoptosis. Tax-mediated Bcl-xL induction may be one of the underlying mechanisms of inhibition of apoptosis of HTLV-1 infected cells, which might contribute to the pathogenesis of HTLV-1 infection.
ACKNOWLEDGMENTS
We thank K. Matumoto (Osaka Red Cross Center, Osaka, Japan); J. Fujisawa (Kansai Medical University, Osaka, Japan); and J. Inoue (Tokyo University, Tokyo, Japan) for providing pHβAPr-1-neo; pβMT-2Tax, pβTax703, pβTaxM22, and WT-Luc; and IκBαM, respectively. We also thank F. G. Issa, Word-Medex, Sydney, Australia, for the careful reading and editing of the manuscript.
This work was supported in part by grants from the Agency of Science and Technology of Japan, and Core Research for Evolutional Science and Technology of Japan Science and Technology Corporation.
REFERENCES
- 1.Akagi T, Ono H, Shimotohno K. Characterization of T cells immortalized by Tax1 of human T-cell leukemia virus type 1. Blood. 1995;86:4243–4249. [PubMed] [Google Scholar]
- 1a.Akiyama, Y. 12 May 1998, posting date. TFSEARCH. [Online.] http: //pdap1.trc.rwcp.or.jp/research/db/TFSEARCH.html. [1 February 1999, last date accessed.]
- 2.Arai M, Kannagi M, Matsuoka M, Sato T, Yamamoto N, Fujii M. Expression of FAP-1 (Fas-associated phosphatase) and resistance to Fas-mediated apoptosis in T cell lines derived from human T cell leukemia virus type 1-associated myelopathy/tropical spastic paraparesis patients. AIDS Res Hum Retroviruses. 1998;14:261–267. doi: 10.1089/aid.1998.14.261. [DOI] [PubMed] [Google Scholar]
- 3.Boise L H, McShan C L, Thompson C B. Introduction of the cell survival gene bcl-xLimproves the viability of CTLL-2 cells without affecting their IL-2 proliferative response. Implications for the development of bioassays. J Immunol Methods. 1996;191:143–148. doi: 10.1016/0022-1759(96)00011-7. [DOI] [PubMed] [Google Scholar]
- 4.Boise L H, Minn A J, Noel P J, June C H, Accavitti M A, Lindsten T, Thompson C B. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 5.Brauweiler A, Garrus J E, Reed J C, Nyborg J K. Repression of bax gene expression by the HTLV-1 Tax protein: implications for suppression of apoptosis in virally infected cells. Virology. 1997;231:135–140. doi: 10.1006/viro.1997.8509. [DOI] [PubMed] [Google Scholar]
- 6.Chen I S, Cann A J, Shah N P, Gaynor R B. Functional relation between HTLV-II x and adenovirus E1A proteins in transcriptional activation. Science. 1985;230:570–573. doi: 10.1126/science.2996140. [DOI] [PubMed] [Google Scholar]
- 7.Chlichlia K, Moldenhauer G, Daniel P T, Busslinger M, Gazzolo L, Schirrmacher V, Khazaie K. Immediate effects of reversible HTLV-1 tax function: T-cell activation and apoptosis. Oncogene. 1995;10:269–277. [PubMed] [Google Scholar]
- 8.Chu Z L, DiDonato J A, Hawiger J, Ballard D W. The Tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IκB kinases containing IKKα and IKKβ. J Biol Chem. 1998;273:15891–15894. doi: 10.1074/jbc.273.26.15891. [DOI] [PubMed] [Google Scholar]
- 9.Copeland K F, Haaksma A G, Goudsmit J, Krammer P H, Heeney J L. Inhibition of apoptosis in T cells expressing human T cell leukemia virus type I Tax. AIDS Res Hum Retroviruses. 1994;10:1259–1268. doi: 10.1089/aid.1994.10.1259. [DOI] [PubMed] [Google Scholar]
- 10.Cory S. Regulation of lymphocyte survival by the bcl-2 gene family. Annu Rev Immunol. 1995;13:513–543. doi: 10.1146/annurev.iy.13.040195.002501. [DOI] [PubMed] [Google Scholar]
- 11.Deng G, Podack E R. Suppression of apoptosis in a cytotoxic T-cell line by interleukin 2-mediated gene transcription and deregulated expression of the protooncogene bcl-2. Proc Natl Acad Sci USA. 1993;90:2189–2193. doi: 10.1073/pnas.90.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon E P, Stephenson D T, Clemens J A, Little S P. Bcl-Xshortis elevated following severe global ischemia in rat brains. Brain Res. 1997;776:222–229. doi: 10.1016/s0006-8993(97)01040-8. [DOI] [PubMed] [Google Scholar]
- 13.Felber B K, Paskalis H, Kleinman-Ewing C, Wong-Staal F, Pavlakis G N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985;229:675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- 14.Fujii M, Niki T, Mori T, Matsuda T, Matsui M, Nomura N, Seiki M. HTLV-1 Tax induces expression of various immediate early serum responsive genes. Oncogene. 1991;6:1023–1029. [PubMed] [Google Scholar]
- 15.Fujisawa J, Seiki M, Kiyokawa T, Yoshida M. Functional activation of the long terminal repeat of human T-cell leukemia virus type I by a trans-acting factor. Proc Natl Acad Sci USA. 1985;82:2277–2281. doi: 10.1073/pnas.82.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujisawa J, Toita M, Yoshimura T, Yoshida M. The indirect association of human T-cell leukemia virus tax protein with DNA results in transcriptional activation. J Virol. 1991;65:4525–4528. doi: 10.1128/jvi.65.8.4525-4528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geleziunas R, Ferrell S, Lin X, Mu Y, Cunningham E T, Jr, Grant M, Connelly M A, Hambor J E, Marcu K B, Greene W C. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase alpha (IKKα) and IKKβ cellular kinases. Mol Cell Biol. 1998;18:5157–65. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillis S, Smith K A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977;268:154–156. doi: 10.1038/268154a0. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 19.Grillot D A, Gonzalez-Garcia M, Ekhterae D, Duan L, Inohara N, Ohta S, Seldin M F, Nunez G. Genomic organization, promoter region analysis, and chromosome localization of the mouse bcl-x gene. J Immunol. 1997;158:4750–4757. [PubMed] [Google Scholar]
- 20.Grossman W J, Kimata J T, Wong F H, Zutter M, Ley T J, Ratner L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1995;92:1057–1061. doi: 10.1073/pnas.92.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunning P, Leavitt J, Muscat G, Ng S Y, Kedes L. A human β-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci USA. 1987;84:4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita K I, Shirakawa S, Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwanaga Y, Tsukahara T, Ohashi T, Tanaka Y, Arai M, Nakamura M, Ohtani K, Koya Y, Kannagi M, Yamamoto N, Fujii M. Human T-cell leukemia virus type 1 Tax protein abrogates interleukin-2 dependence in a mouse T-cell line. J Virol. 1999;73:1271–1277. doi: 10.1128/jvi.73.2.1271-1277.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiefer M C, Brauer M J, Powers V C, Wu J J, Umansky S R, Tomei L D, Barr P J. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995;374:736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- 25.Kishi S, Saijyo S, Arai M, Karasawa S, Ueda S, Kannagi M, Iwakura Y, Fujii M, Yonehara S. Resistance to fas-mediated apoptosis of peripheral T cells in human T lymphocyte virus type I (HTLV-1) transgenic mice with autoimmune arthropathy. J Exp Med. 1997;186:57–64. doi: 10.1084/jem.186.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto K, Akashi K, Shibata H, Yutsudo M, Hakura A. Single amino acid substitution (58Pro→Ser) in HTLV-1 tax results in loss of ras cooperative focus formation in rat embryo fibroblasts. Virology. 1994;200:813–815. doi: 10.1006/viro.1994.1248. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto K, Shibata H, Fujisawa J I, Inoue H, Hakura A, Tsukahara T, Fujii M. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J Virol. 1997;71:4445–4451. doi: 10.1128/jvi.71.6.4445-4451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazaki T, Liu Z J, Taniguchi T. Selective cooperation of HTLV-1-encoded p40tax-1with cellular oncoproteins in the induction of hematopoietic cell proliferation. Oncogene. 1996;12:2403–2408. [PubMed] [Google Scholar]
- 29.Nerenberg M, Hinrichs S H, Reynolds R K, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987;237:1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- 30.Neuveut C, Low K G, Maldarelli F, Schmitt I, Majone F, Grassmann R, Jeang K T. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol Cell Biol. 1998;18:3620–3632. doi: 10.1128/mcb.18.6.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicot C, Astier-Gin T, Guillemain B. Activation of Bcl-2 expression in human endothelial cells chronically expressing the human T-cell lymphotropic virus type I. Virology. 1997;236:47–53. doi: 10.1006/viro.1997.8720. [DOI] [PubMed] [Google Scholar]
- 32.Noel P J, Boise L H, Green J M, Thompson C B. CD28 costimulation prevents cell death during primary T cell activation. J Immunol. 1996;157:636–642. [PubMed] [Google Scholar]
- 33.Petropoulos C J, Givol I, Hughes S H. Comparative analysis of the structure and function of the chicken c-myc and v-myc genes: v-myc is a more potent inducer of cell proliferation and apoptosis than c-myc. Oncogene. 1996;12:2611–2621. [PubMed] [Google Scholar]
- 34.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed J C, Zha H, Aime-Sempe C, Takayama S, Wang H G. Structure-function analysis of Bcl-2 family proteins. Regulators of programmed cell death. Adv Exp Med Biol. 1996;406:99–112. [PubMed] [Google Scholar]
- 36.Rosenblatt J D, Miles S, Gasson J C, Prager D. Transactivation of cellular genes by human retroviruses. Curr Top Microbiol Immunol. 1995;193:25–49. doi: 10.1007/978-3-642-78929-8_2. [DOI] [PubMed] [Google Scholar]
- 37.Rosenthal N. Identification of regulatory elements of cloned genes with functional assays. Methods Enzymol. 1987;152:704–720. doi: 10.1016/0076-6879(87)52075-4. [DOI] [PubMed] [Google Scholar]
- 38.Schneider U, Schwenk H U, Bornkamm G. Characterization of EBV-genome negative “null” and “T” cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1977;19:521–526. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- 39.Seiki M, Inoue J, Takeda T, Hikikoshi A, Sato M, Yoshida M. The p40x of human T-cell leukemia virus type I is a trans-acting activator of viral gene transcription. Jpn J Cancer Res. 1985;76:1127–1131. [PubMed] [Google Scholar]
- 40.Smith M R, Greene W C. Identification of HTLV-1 tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. . (Errata, 5:150, 1991; 9:2324, 1995.) [DOI] [PubMed] [Google Scholar]
- 41.Sodroski J G, Rosen C A, Haseltine W A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984;225:381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- 42.Sun S C, Elwood J, Beraud C, Greene W C. Human T-cell leukemia virus type I Tax activation of NF-κ B/Rel involves phosphorylation and degradation of Iκ Bα and RelA (p65)-mediated induction of the c-relgene. Mol Cell Biol. 1994;14:7377–7384. doi: 10.1128/mcb.14.11.7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki T, Fujisawa J I, Toita M, Yoshida M. The trans-activator tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc Natl Acad Sci USA. 1993;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki T, Hirai H, Fujisawa J, Fujita T, Yoshida M. A trans-activator Tax of human T-cell leukemia virus type 1 binds to NF-κ B p50 and serum response factor (SRF) and associates with enhancer DNAs of the NF-κ B site and CArG box. Oncogene. 1993;8:2391–2397. [PubMed] [Google Scholar]
- 45.Suzuki T, Kitao S, Matsushime H, Yoshida M. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4Aand counteracts its inhibitory activity towards CDK4. EMBO J. 1996;15:1607–1614. [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka A, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci USA. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uhlik M, Good L, Xiao G, Harhaj E W, Zandi E, Karin M, Sun S C. NF-κB-inducing kinase and IκB kinase participate in human T-cell leukemia virus I Tax-mediated NF-κB activation. J Biol Chem. 1998;273:21132–21136. doi: 10.1074/jbc.273.33.21132. [DOI] [PubMed] [Google Scholar]
- 48.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 49.Wang C Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 50.Wang C Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 51.Wu M, Lee H, Bellas R E, Schauer S L, Arsura M, Katz D, FitzGerald M J, Rothstein T L, Sherr D H, Sonenshein G E. Inhibition of NF-κB/Rel induces apoptosis of murine B cells. EMBO J. 1996;15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 52.Wu M X, Ao Z, Prasad K V, Wu R, Schlossman S F. IEX-1L, an apoptosis inhibitor involved in NF-κB-mediated cell survival. Science. 1998;281:998–1001. doi: 10.1126/science.281.5379.998. [DOI] [PubMed] [Google Scholar]
- 53.Yamada T, Yamaoka S, Goto T, Nakai M, Tsujimoto Y, Hatanaka M. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J Virol. 1994;68:3374–3379. doi: 10.1128/jvi.68.5.3374-3379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin M J, Christerson L B, Yamamoto Y, Kwak Y T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. HTLV-I Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida M, Suzuki T, Fujisawa J, Hirai H. HTLV-1 oncoprotein tax and cellular transcription factors. Curr Top Microbiol Immunol. 1995;193:79–89. doi: 10.1007/978-3-642-78929-8_4. [DOI] [PubMed] [Google Scholar]
- 56.Zhao L J, Giam C Z. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc Natl Acad Sci USA. 1992;89:7070–7074. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]