Abstract
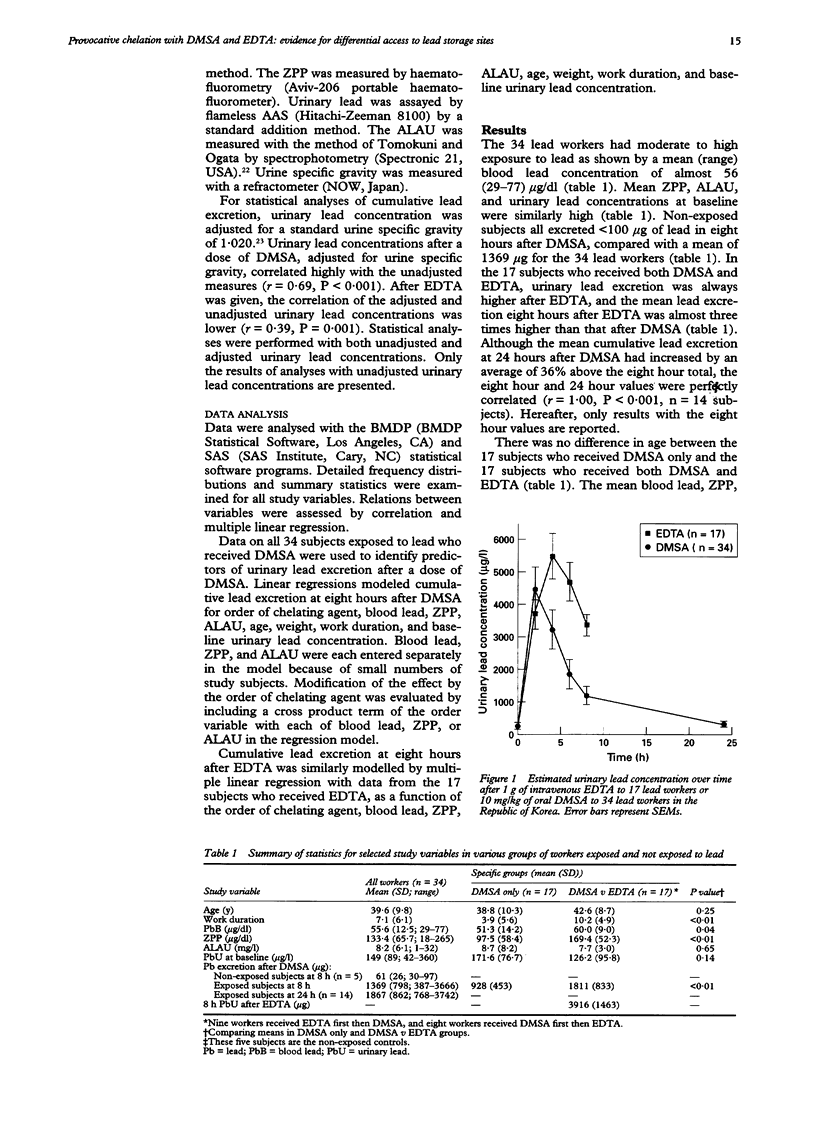
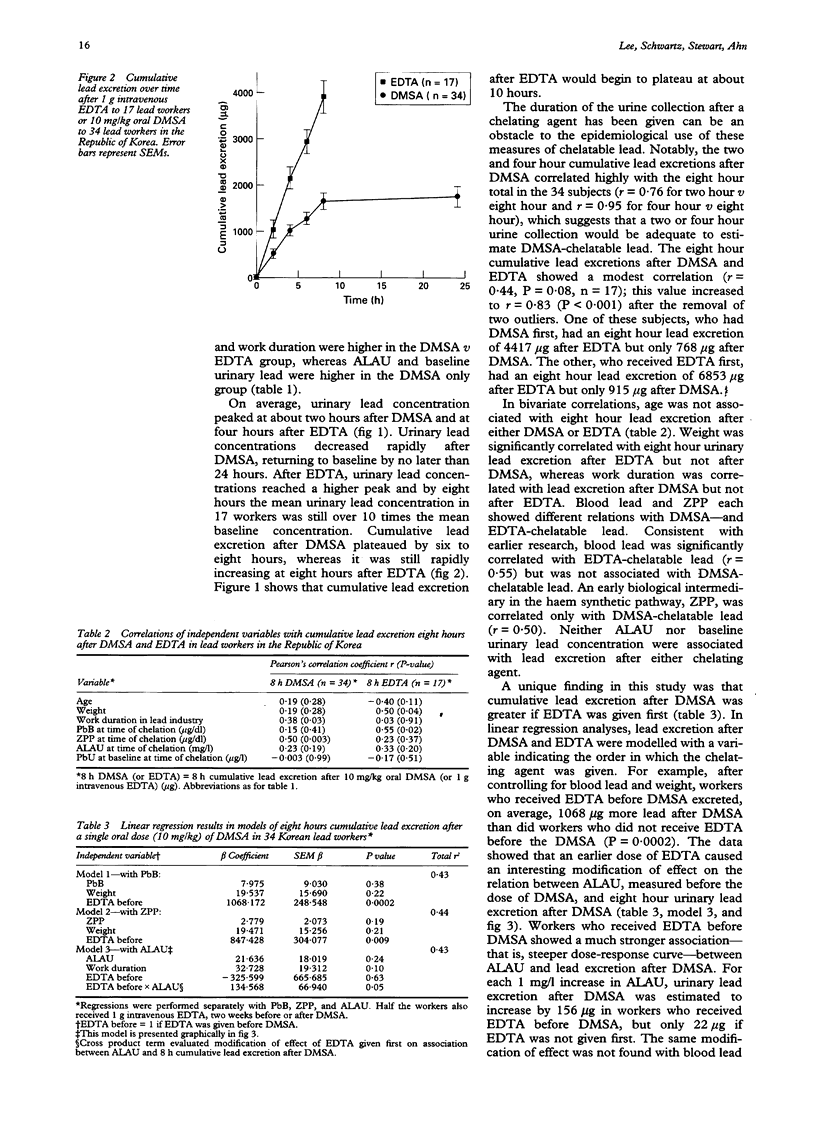
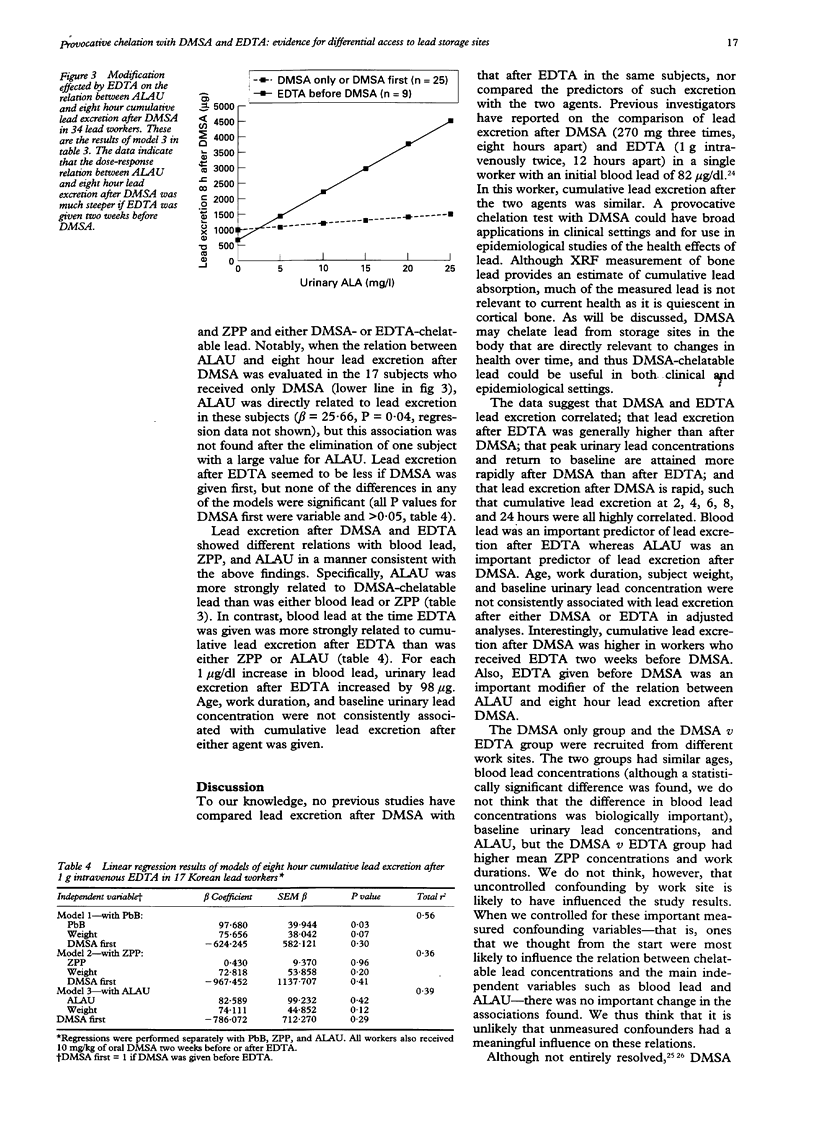
OBJECTIVES--To validate a provocative chelation test with 2,3-dimercaptosuccinic acid (DMSA) by direct comparison with the standard ethylene diamine tetraacetic acid (EDTA) test in the same subjects; and to compare and contrast the predictors of lead excretion after DMSA with those after EDTA. A metal chelating agent given orally, DMSA may mobilise and enhance the excretion of lead from the storage sites in the body that are most directly relevant to the health effects of lead. A provocative chelation test with DMSA could thus have wide potential application in clinical care and epidemiological studies. METHODS--34 male lead workers in the Republic of Korea were given a single oral dose of 10 mg/kg DMSA, urine was collected over the next eight to 24 hours, and urine volume and urinary lead concentration determined at 0, 2, 4, 6, 8, and 24 hours. Either two weeks before or two weeks after the dose of DMSA 17 of these workers also received 1 g intravenous EDTA followed by an eight hour urine collection with fractionation at 0, 2, 4, 6, and 8 hours. RESULTS--Urinary lead concentration peaked at two hours after DMSA and four hours after EDTA. Lead excretion after DMSA was less than after EDTA, and cumulative excretion after DMSA plateaued at six to eight hours. The two hour and four hour cumulative lead excretions after DMSA were highly correlated with the eight hour total (r = 0.76 and 0.95). In multiple linear regression analyses, blood lead was found to be an important predictor of EDTA-chelatable lead, whereas urinary aminolevulinic acid (ALAU) was associated with DMSA-chelatable lead. Notably, lead excretion after DMSA was greatly increased if EDTA was given first. An earlier dose of EDTA also modified the relation between ALAU and DMSA-chelatable lead in that workers who received EDTA before DMSA showed a much steeper dose-response relation between these two measures. CONCLUSIONS--The predictors of lead excretion after DMSA and EDTA are different and an earlier dose of EDTA may increase lead excretion after a subsequent dose of DMSA. The results suggest that two hour or four hour cumulative lead excretion after DMSA may provide an estimate of lead in storage sites that are most directly relevant to the health effects of lead.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessio L., Castoldi M. R., Monelli O., Toffoletto F., Zocchetti C. Indicators of internal dose in current and past exposure to lead. Int Arch Occup Environ Health. 1979 Sep;44(2):127–132. doi: 10.1007/BF00386746. [DOI] [PubMed] [Google Scholar]
- Aposhian H. V., Aposhian M. M. meso-2,3-Dimercaptosuccinic acid: chemical, pharmacological and toxicological properties of an orally effective metal chelating agent. Annu Rev Pharmacol Toxicol. 1990;30:279–306. doi: 10.1146/annurev.pa.30.040190.001431. [DOI] [PubMed] [Google Scholar]
- Aposhian H. V., Maiorino R. M., Dart R. C., Perry D. F. Urinary excretion of meso-2,3-dimercaptosuccinic acid in human subjects. Clin Pharmacol Ther. 1989 May;45(5):520–526. doi: 10.1038/clpt.1989.67. [DOI] [PubMed] [Google Scholar]
- Apostoli P., Porru S., Duca P., Ferioli A., Alessio L. Significance and validity of a shortened lead chelation test. J Occup Med. 1990 Nov;32(11):1124–1129. doi: 10.1097/00043764-199011000-00016. [DOI] [PubMed] [Google Scholar]
- Araki S., Aono H., Murata K. Mobilisation of heavy metals into the urine by CaEDTA: relation to erythrocyte and plasma concentrations and exposure indicators. Br J Ind Med. 1986 Sep;43(9):636–641. doi: 10.1136/oem.43.9.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S., Murata K., Aono H. Subclinical cervico-spino-bulbar effects of lead: a study of short-latency somatosensory evoked potentials in workers exposed to lead, zinc, and copper. Am J Ind Med. 1986;10(2):163–175. doi: 10.1002/ajim.4700100207. [DOI] [PubMed] [Google Scholar]
- Barry P. S. A comparison of concentrations of lead in human tissues. Br J Ind Med. 1975 May;32(2):119–139. doi: 10.1136/oem.32.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry P. S., Mossman D. B. Lead concentrations in human tissues. Br J Ind Med. 1970 Oct;27(4):339–351. doi: 10.1136/oem.27.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batuman V., Landy E., Maesaka J. K., Wedeen R. P. Contribution of lead to hypertension with renal impairment. N Engl J Med. 1983 Jul 7;309(1):17–21. doi: 10.1056/NEJM198307073090104. [DOI] [PubMed] [Google Scholar]
- Batuman V., Maesaka J. K., Haddad B., Tepper E., Landy E., Wedeen R. P. The role of lead in gout nephropathy. N Engl J Med. 1981 Feb 26;304(9):520–523. doi: 10.1056/NEJM198102263040905. [DOI] [PubMed] [Google Scholar]
- Bentur Y., Brook J. G., Behar R., Taitelman U. Meso-2,3-dimercaptosuccinic acid in the diagnosis and treatment of lead poisoning. J Toxicol Clin Toxicol. 1987;25(1-2):39–51. doi: 10.3109/15563658708992612. [DOI] [PubMed] [Google Scholar]
- Chisolm J. J., Jr Evaluation of the potential role of chelation therapy in treatment of low to moderate lead exposures. Environ Health Perspect. 1990 Nov;89:67–74. doi: 10.1289/ehp.908967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersson J. O., Schütz A., Ahlgren L., Haeger-Aronsen B., Mattsson S., Skerfving S. Lead in finger-bone analysed in vivo in active and retired lead workers. Am J Ind Med. 1984;6(6):447–457. doi: 10.1002/ajim.4700060608. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta D. A. Mobilization of lead over the course of DMSA chelation therapy and long-term efficacy. J Pharmacol Exp Ther. 1988 Jul;246(1):84–91. [PubMed] [Google Scholar]
- Cory-Slechta D. A., Weiss B., Cox C. Mobilization and redistribution of lead over the course of calcium disodium ethylenediamine tetraacetate chelation therapy. J Pharmacol Exp Ther. 1987 Dec;243(3):804–813. [PubMed] [Google Scholar]
- Craswell P. W., Price J., Boyle P. D., Heazlewood V. J., Baddeley H., Lloyd H. M., Thomas B. J., Thomas B. W., Williams G. M. Chronic lead nephropathy in Queensland: alternative methods of diagnosis. Aust N Z J Med. 1986 Feb;16(1):11–19. doi: 10.1111/j.1445-5994.1986.tb01107.x. [DOI] [PubMed] [Google Scholar]
- Fournier L., Thomas G., Garnier R., Buisine A., Houze P., Pradier F., Dally S. 2,3-Dimercaptosuccinic acid treatment of heavy metal poisoning in humans. Med Toxicol Adverse Drug Exp. 1988 Nov-Dec;3(6):499–504. doi: 10.1007/BF03259898. [DOI] [PubMed] [Google Scholar]
- Graziano J. H., Leong J. K., Friedheim E. 2,3-Dimercaptosuccinic acid: a new agent for the treatment of lead poisoning. J Pharmacol Exp Ther. 1978 Sep;206(3):696–700. [PubMed] [Google Scholar]
- Graziano J. H., Siris E. S., LoIacono N., Silverberg S. J., Turgeon L. 2,3-Dimercaptosuccinic acid as an antidote for lead intoxication. Clin Pharmacol Ther. 1985 Apr;37(4):431–438. doi: 10.1038/clpt.1985.67. [DOI] [PubMed] [Google Scholar]
- Greenberg G. N., Levine R. J. Urinary creatinine excretion is not stable: a new method for assessing urinary toxic substance concentrations. J Occup Med. 1989 Oct;31(10):832–838. doi: 10.1097/00043764-198910000-00008. [DOI] [PubMed] [Google Scholar]
- Hansen J. P., Døssing M., Paulev P. E. Chelatable lead body burden (by calcium-disodium EDTA) and blood lead concentration in man. J Occup Med. 1981 Jan;23(1):39–43. doi: 10.1097/00043764-198101000-00017. [DOI] [PubMed] [Google Scholar]
- Hu H., Pepper L., Goldman R. Effect of repeated occupational exposure to lead, cessation of exposure, and chelation on levels of lead in bone. Am J Ind Med. 1991;20(6):723–735. doi: 10.1002/ajim.4700200603. [DOI] [PubMed] [Google Scholar]
- Liang Y. Y., Marlowe C., Waddell W. J. Disposition of [14C]dimercaptosuccinic acid in mice. Fundam Appl Toxicol. 1986 Apr;6(3):532–540. [PubMed] [Google Scholar]
- Maiorino R. M., Akins J. M., Blaha K., Carter D. E., Aposhian H. V. Determination and metabolism of dithiol chelating agents: X. In humans, meso-2,3-dimercaptosuccinic acid is bound to plasma proteins via mixed disulfide formation. J Pharmacol Exp Ther. 1990 Aug;254(2):570–577. [PubMed] [Google Scholar]
- Maiorino R. M., Aposhian M. M., Xu Z. F., Li Y., Polt R. L., Aposhian H. V. Determination and metabolism of dithiol chelating agents. XV. The meso-2,3-dimercaptosuccinic acid-cysteine (1:2) mixed disulfide, a major urinary metabolite of DMSA in the human, increases the urinary excretion of lead in the rat. J Pharmacol Exp Ther. 1993 Dec;267(3):1221–1226. [PubMed] [Google Scholar]
- Marcus A. H. The body burden of lead: comparison of mathematical models for accumulation. Environ Res. 1979 Jun;19(1):79–90. doi: 10.1016/0013-9351(79)90036-7. [DOI] [PubMed] [Google Scholar]
- O'Flaherty E. J. Physiologically based models for bone-seeking elements. IV. Kinetics of lead disposition in humans. Toxicol Appl Pharmacol. 1993 Jan;118(1):16–29. doi: 10.1006/taap.1993.1004. [DOI] [PubMed] [Google Scholar]
- Somervaille L. J., Chettle D. R., Scott M. C., Aufderheide A. C., Wallgren J. E., Wittmers L. E., Jr, Rapp G. R., Jr Comparison of two in vitro methods of bone lead analysis and the implications for in vivo measurements. Phys Med Biol. 1986 Nov;31(11):1267–1274. doi: 10.1088/0031-9155/31/11/008. [DOI] [PubMed] [Google Scholar]
- Tomokuni K., Ogata M. Simple method for determination of urinary -aminolevulinic acid as an index of lead exposure. Clin Chem. 1972 Dec;18(12):1534–1538. [PubMed] [Google Scholar]
- Vitale L. F., Joselow M. M., Wedeen R. P., Pawlow M. Blood lead - an inadequate measure of occupational exposure. J Occup Med. 1975 Mar;17(3):155–156. [PubMed] [Google Scholar]
- Yokoyama K., Araki S., Aono H. Reversibility of psychological performance in subclinical lead absorption. Neurotoxicology. 1988 Fall;9(3):405–410. [PubMed] [Google Scholar]


